Повязки и средства наружного применения для лечения пролежней
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT; unit of randomisation unclear (unclear if > 1 wound per person) | |
| Participants | ˜24 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Comfeel Plus: hydrocolloid‐alginate, combination of 2 groups randomised to treatment in the debridement and granulation phases; n = 12 (probably). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at about 7 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear who the outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none ‐ i.e. no missing data |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Unclear risk | Unit of randomisation unclear and unit of analysis unclear ‐ assumed the participant was analysed ("cases"); no details on the ratio of ulcers:participants |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) | |
| Participants | 50 participants with pressure ulcers. PU Stage: not stated and no indication apart from mean depth (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Comfeel Ulcus (not in BNF): 1 week washout with saline gauze; then hydrocolloid sheet and, if appropriate, hydrocolloid paste (7) and powder (1 ulcer); dressings changed when necessary; n = total 50 (number per group not reported). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but of unclear importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data: not reported by group and very unclear overall ‐ possibly 9/50 (18%) missing (1 died, 2 protocol violations, 2 results missing, 3 discontinued for surgery, 1 adverse event) |
| Selective reporting (reporting bias) | High risk | Inadequate – reported incompletely (e.g. P value > 0.05) |
| Other bias | Low risk | Unit of randomisation ulcer and unit of analysis ulcer ‐ 6/50 participants had 2 pressure ulcers (2 participants had 1 ulcer assigned to each group); ulcer:person = 60/56 overall = 1.12 |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) | |
| Participants | 12 participants with pressure ulcers. PU Stage: 3 (n = 7); 4 (n = 5) overall; data per group not stated (PU classification: NPUAP) | |
| Interventions | Group 1: standard care (all advanced dressings): hydrocolloid (fibrous hydrocolloid) dressing, a foam dressing or an alginate dressing (all non‐silver); n = 6. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 26 (6 months) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: low |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 1/6 (17%) withdrew from treatment and received other treatment; 0/6 died (PU slow to heal). Group 2 ‐ 6/6 (100%) withdrew from treatment and received other treatment. 2/6 (33%) died during the trial (1 recurrence of black slough, 1 ulcer too small to continue treatment, 1 foam embedded in granulation tissue, 1 deterioration, 1 participant refusal, 1 difficulty with applying treatment) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 60 participants with pressure ulcers. PU Stage: II/III (acceptable); 71% and 79% Stage II (PU classification: Stirling) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex; n = 31. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 4 (30 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ not stated. Allocation concealment inadequate ‐ evidence that researchers knew the sequence. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 22/31 (71%) withdrew (8 discharged, 2 died, 2 adverse incident, 2 participant request, 2 dressing unsuitable, 2 wound deteriorated, 1 lack of progress, 2 dressing rolling). Group 2 ‐ 18/29 withdrew (62%) (5 discharged, 6 died, 3 adverse incident, 2 participant request, 1 dressing unsuitable, 1 wound deteriorated) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 40 participants with pressure ulcers. PU Stage: II and III (Stages I, IV, V excluded); proportions not stated (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex: concurrent standard pressure‐relieving devices and cushions in community as appropriate; n = 20. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but of unclear importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 10/20 (50%) withdrawn (2 wound deteriorated, 2 overgranulation, 2 discomfort, 4 unrelated to wound (2 died, 2 had respite care)). Group 2 ‐ 2/20 (10%) (2 for reasons unrelated to wound (1 died, 1 admitted to hospital)) |
| Selective reporting (reporting bias) | High risk | Inadequate – outcome included in methods section but not results |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (> 1 wound per person, unclear how assessed) | |
| Participants | 50 participants with pressure ulcers. PU Stage: II (non‐blanching erythema +/‐ superficial damage) and III (PU classification: Torrance) | |
| Interventions | Group 1: foam dressing ‐ Lyofoam; n = 26. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment adequate ‐ independent 3rd party allocates and retains schedule. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 7/26 (27%) (2 died, 5 withdrew; 2 reasons NS, 2 improved, 1 deteriorated). Group 2 ‐ 9/24 (38%) (2 died, 7 withdrew, 2 reason NS, 1 improved, 4 deteriorated) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ stated that protocol allowed > 1 per wound person, but no evidence that this happened |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 29 participants with pressure ulcers. PU Stage: II and III (involving loss of skin) proportions not stated (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex: Granuflex E; additional support therapy for immobile participants; n = 16. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 4/16 (25%) (3 wound deterioration, 1 wound/dressing‐related problems). Group 2 ‐ 3/13 (23%) (1 wound deterioration, 1 wound/dressing‐related problems, 1 discharged from hospital) |
| Selective reporting (reporting bias) | High risk | Inadequate – outcome included in methods section but not results |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) | |
| Participants | 76 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex; n = 38. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 2/38 (5%) (2 dropped out due to deterioration). Group 2 ‐ 5/38 (13%) (5 dropped out due to deterioration in the wound) |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Unclear risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ probably 1 ulcer per person |
| Other bias | Unclear risk | PU classification unclear |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) | |
| Participants | 110 participants with pressure ulcers. PU Stage: III and IV; stage III proportions = group 1: 82.7% and group 2: 71.4% (PU classification: Yarkony) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM Extra Thin: note different HC; hydrocolloid paste for deep ulcers. Prior treatment with mainly HC; n = 53. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ other. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ all analysed, though 16/53 (30%) did not complete treatment (8 died and 8 withdrew (2 transfer to another unit, 3 local infection, 3 PU impairment)). Group 2 ‐ all analysed, though 17/57 (30%) did not complete treatment (11 died and 6 withdrew (1 transfer to another unit, 1 worsening health status, 1 local infection, 3 PU impairment)) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (selected ulcer) ‐ one ulcer selected |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT (letter to journal); participants randomised (unclear if > 1 wound per person) | |
| Participants | 43 participants with pressure ulcers. PU Stage: II and III (description available); stratified then randomised; proportions not stated (PU classification: not stated) | |
| Interventions | Group 1: hydrogel dressing ‐ poly HEMA: Hydron dressing; n = 27. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 2/27 (7%) (both died). Group 2 ‐ 3/16 (19%) (1 died, 2 did not complete treatment (1 poor response, 1 adverse event)) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ one ulcer implied (e.g. "52% of group 1 had complete healing of the study ulcer") |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; ulcers randomised (> 1 wound per person, other selection of wound) | |
| Participants | ||
| Interventions | Group 1: hydrogel dressing ‐ Transorbent dressing; n = 77. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 10 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 19/77 (25%) (11 unable to follow, 5 died, 3 other; overall 19 participants did not complete first 3 weeks of trial or missed 2 sequential visits ). Group 2 ‐ 12/63 (19%) (4 unable to follow, 5 died, 3 other; overall 19 participants did not complete first 3 weeks of trial or missed 2 sequential visits) |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | High risk | Unit of randomisation ulcer and unit of analysis person (1 ulcer/person) ‐ ulcers randomised (stratified), but one II, III or IV ulcer was selected (implied at the beginning), at the discretion of the (unblinded) investigator at each centre |
| Other bias | Unclear risk | Some discrepancy between text and table in the number of participants |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) | |
| Participants | 36 participants with pressure ulcers. PU Stage: II, III and IV (proportions not stated) (PU classification: not stated) | |
| Interventions | Group 1: protease‐modulating dressing ‐ Fibracol (90% collagen, 10% alginate (from suppliers' website)); n = 24. Grouped intervention category: protease‐modulating dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data: Group 1 ‐ 116 total enrolled, 80 evaluable and interim analysis on 36 (not stated). Group 2 ‐ 116 total enrolled, 80 evaluable and interim analysis on 36 (not stated) |
| Selective reporting (reporting bias) | High risk | Inadequate – outcome included in methods section but not results |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ one ulcer implied (e.g. "participants stratified before randomisation according to pressure ulcer location and size") |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) | |
| Participants | 72 participants with pressure ulcers. PU Stage: II (59.5% and 65%; P = 0.59), and shallow III (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM CGF; n = 37. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ if > 1, authors selected highest grade PU then largest ulcer |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 37 participants with pressure ulcers. PU Stage: III only (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Varihesive (not in BNF): ulcers cleaned with saline; Varihesive paste used for deep ulcers/high exudate for HC group only; n = 19. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ other. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) | High risk | Missing data: 6 participants excluded overall (4 protocol violations) ‐ not given by group. Additionally, discontinuations: Group 1: 6 (32%) (because of death due to unrelated cause, deterioration in general condition, discharge from hospital, protocol violations, lack of efficacy). Group 2: 8 (44%) (because of deaths due to unrelated cause, discharge from hospital, transfer to another centre), i.e. similar rate missing in both groups; high rate – more than control event rate. "Eight (44.4%) and six (31.6%) patients in the collagenase and hydrocolloid groups, respectively, discontinued the study prematurely. Reasons for discontinuation in the collagenase group were: death due to unrelated cause (n = 3), discharge from the hospital (n = 3) and transfer to another centre (n = 3). Reasons for discontinuation in the hydrocolloid group included death due to unrelated cause (n = 1), deterioration of the patient’s general condition (n = 1), discharge from the hospital (n = 1), protocol violation (n = 2) and lack of efficacy (n = 1)", i.e. discrepancy between total number missing and sum of reasons for group 2 ‐ but 44% corresponds to 8 participants. |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ same number of ulcers as participants in table |
| Other bias | Unclear risk | Paste used for hydrocolloid group only |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) | |
| Participants | 70 participants with pressure ulcers. PU stage: II (69% and 44%) and III (PU classification: NS). | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM CGF (not BNF); n = 33. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ not stated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ Overall 24/94 (26%) (12 died from causes unrelated to PU, 5 discharged from hospital, 5 lost to follow‐up, 1 colonised with MRSA, 1 participant's ulcer progressed to Stage 4. Equivalent number dropped from each group). Group 2 ‐ Overall 24/94 (26%) (12 died from causes unrelated to PU, 5 discharged from hospital, 5 lost to follow‐up, 1 colonised with MRSA, 1 participant's ulcer progressed to Stage 4. Equivalent number dropped from each group) |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Low risk | Unit of randomisation ulcer and unit of analysis ulcer ‐ approx 1.5 ulcer:person ratio = 48/33 and 49/37 |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; unit of randomisation unclear (> 1 wound per person, all followed) | |
| Participants | 90 participants with pressure ulcers. PU Stage: I and II (54% and 56%) (results separate); stage I is ulceration or skin breakdown limited to superficial epidermal and dermal layer ‐ probably corresponds to grade II? (PU classification: Enis and Sarmiento). | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM; n = 49 overall. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8.5 (60 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear ‐ no information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 4/67 (6%) excluded from the authors' analysis (3 wounds' size increased by more than 10% per day and 1 decreased by more than 25% per day). Group 2 ‐ 2/62 (3%) excluded from the authors' analysis (1 wound's size increased by more than 10% per day and 1 decreased by more than 25% per day). |
| Selective reporting (reporting bias) | High risk | Inadequate – reported incompletely |
| Other bias | High risk | Unit of randomisation unclear and unit of analysis ulcer ‐ Overall ulcer:person ratio = 67/49 and 62/41 (1.52) |
| Other bias | Unclear risk | Extraction from a graph |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high/very high |
| Methods | RCT; wards randomised (> 1 wound per person, all followed) | |
| Participants | 52 participants with pressure ulcers. PU Stage: II (87% and 79%) and III (with acceptable definition) (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM; n = 27. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at approx 11 (assumed from mean + SD) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | High risk | Unit of randomisation ward and unit of analysis ulcer ‐ each ward assigned one or other treatment regimen |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 65 participants with pressure ulcers. PU Stage: 2 (77% and 83%) and 3 (PU classification: NPUAP) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM: twice‐weekly. Standard nursing care. No ancillary non‐protocol treatments; n = 30. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability adequate ‐ no suggestion of problems. Rating: low |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 5/30 (17%) (1 withdrew consent, 3 died, 2 hospitalised). Group 2 ‐ 6/35 (17%) (2 died, 1 hospitalised, 2loss to follow‐up). |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Low risk | Adequate ‐ well‐conducted study |
| ALL‐DOMAIN RISK OF BIAS | Low risk | Rating: low |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 52 participants with pressure ulcers. PU Stage: I (33%; 36%) and II (58%, 64%) (stratified and results separate). Shea I defined as "Limited to epidermis, exposing dermis; includes a red area" (PU classification: Shea). | |
| Interventions | Group 1: hydrogel dressing ‐ hydrocolloid adhesive dressing (description "hydrocolloid adhesive dressings absorb water and low molecular weight components from ulcer secretions, so they swell to produce a jelly"). No concomitant antibiotic, steroid or antisuppressant treatments allowed. No debridement needed during treatment. All other concomitant treatments the same; n = 16. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | Sequence generation adequate ‐ random number tables. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability adequate ‐ no suggestion of problems. Rating: low |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none. Group 3 ‐ none |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Unclear risk | Unit of randomisation person and unit of analysis ulcer ‐ probably participants randomised; if > 1 ulcer then same treatment within participant; < 1.2 ulcer:person = 18/16, 21/19 and 19/17 |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear/low |
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) | |
| Participants | 168 participants with pressure ulcers. PU Stage: 1 grade I (excluded from analysis), 187 II to IV (II: 54% and 64%; III: 40% and 30%; IV: 5.7% and 6.2%) (PU classification: Shea) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Comfeel (unspecified); n = 88. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ vague statement about central randomisation. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 24/88 (27%) (6 withdrew because of local complications (mainly necrosis), 18 withdrew for reasons unconnected with treatment (mainly death, transfer to another ward, discharge from hospital)). Group 2 ‐ 14/80 (17.5%) (4 withdrew because of local complications (mainly necrosis), 10 withdrew for reasons unconnected with treatment (mainly death, transfer to another ward, discharge from hospital)) |
| Selective reporting (reporting bias) | High risk | Inadequate ‐ analysis methods differed from those of other trials |
| Other bias | Unclear risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ study says, "in cases of multiple ulcers, only one sore per patient was evaluated". Not stated how many this applied to |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT (translation); participants randomised (only 1 wound per person) | |
| Participants | 141 participants with pressure ulcers. PU Stage: I (23% and 21%), II and III (44% and 38%) and IV (34% and 41%) (PU classification: not stated) | |
| Interventions | Group 1: topical ‐ sugar plus povidone iodine: sugar 70 g/100 g and povidone iodine 3 g/100 g; ointment applied directly on the wound or applied on a sheet of gauze and then applied on the wound once or twice a day; n = 72. Grouped intervention category: sugar plus povidone iodine | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ random number tables. Allocation concealment adequate ‐ central randomisation with contact details or list held independently. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 27/72 (38%) (withdrew (1 because of adverse effects)). Group 2 ‐ 29/69 (42%) (withdrew (1 because of adverse effects)). |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 27 participants with pressure ulcers. PU Stage: 1 (24% and 25% of ulcers), 2 (68% and 71%) and 3 (results separate, but best to combine) (PU classification: NPUAP) | |
| Interventions | Group 1: hydrogel dressing ‐ Elastogel (not in BNF); n = 15. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear ‐ no information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 0; i.e. no missing data (no details) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | High risk | Unit of randomisation person and unit of analysis ulcer ‐ for combination of stages I and II and III, ulcer:person ratio = 25/15 (1.7) and 24/12 (2.0) |
| Other bias | Unclear risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 38 participants with pressure ulcers. PU Stage: II (58% overall) and III (PU classification: Enterstomal Therapy) | |
| Interventions | Group 1: foam dressing ‐ Epi‐Lock (not in BNF); n = 24. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 24 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ not stated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 11/24 (45%) and (5 staff‐requested removal, 1 participant‐requested removal, 1 special bed treatment, 4 reactions to treatment). Group 2 ‐ 6/14 (43%) (2 died, 1 staff‐requested removal, 1 participant‐requested removal, 1 surgery, 1 reaction to treatment). |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 32 participants with pressure ulcers. PU Stage: III and IV: median for both groups was IV (PU classification: not stated) | |
| Interventions | Group 1: hydrogel dressing ‐ amorphous hydrocolloid (hydrogel, Coloplast) ‐ in Cochrane Review as hydrogel; n = 17. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 9/17 (53%) (5 other illness, 2 deaths, 1 missing schedule, 1 wish to cease participation). Group 2 ‐ 11/15 (73%) (6 insufficient effect of treatment, 3 other illness, 1 death, 1 wish to cease participation) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (unclear if > 1 wound per person) | |
| Participants | 38 participants with pressure ulcers. PU Stage: 2 (PU classification: EPUAP) | |
| Interventions | Group 1: soft polymer dressing ‐ Mepilex Border; n = 18. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ envelopes not said to be opaque. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 1/18 ? (6%) (unclear if other withdrawals) (1 died during the study (so missing), 1 had hip fracture). Group 2 ‐ 1/20? (5%) (unclear about withdrawals) (1 died (but unclear when and not listed by authors as missing); 1 developed symptoms of heart disorder). |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ implies 1 per person |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 10 participants with pressure ulcers. PU Stage: II (30%) and III (PU classification: not stated) | |
| Interventions | Group 1: hydrogel dressing ‐ Flexigel (not in BNF); n = 5. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 0. i.e. no missing data (no details) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 24 participants with pressure ulcers. PU Stage: IV (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM: complete debridement first. New necrosis led to a change to alginate or collagenase (4/12; 33%); n = 12. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at probably 16 weeks; time to complete healing reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 1/12 (8%); 4/12 (33%) changed treatment (1 failed to comply with weekly inspection, so dropped; changed treatment for new necrosis). Group 2 ‐ 1/12 (8%) changed treatment (changed treatment for new necrosis) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ reported incompletely as ‘significant’ or P value < 0.05 |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (all ulcers analysed as a whole) ‐ 2/24 (8%) participants had 2 ulcers ‐ but participants analysed; ratio ulcers:participants = 13/12 (1.08) in each group |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high/very high |
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) | |
| Participants | 87 participants with pressure ulcers. PU Stage: II (60% and 76%) and III (% of available cases) (PU classification: Shea) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Tegasorb (not in BNF): dressing scheduled to be changed every 7 days; if there was necrotic tissue it was debrided; n = 100 ulcers randomised (total), number of participants not stated, but available cases 87 total. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment inadequate ‐ alternation. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ overall 13/100 (13%) ulcers excluded from the analysis (intercurrent medical events (n = 11) and 2 had protocol violations). Group 2 ‐ overall 13/100 (13%) ulcers excluded from the analysis (intercurrent medical events (n = 11) and 2 had protocol violations) |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Low risk | Unit of randomisation ulcer and unit of analysis ulcer ‐ 22/87 (25%) participants had 2 ulcers |
| Other bias | Unclear risk | 25% had 2 ulcers ‐ not treated as paired data |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high/very high |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 80 participants with pressure ulcers. PU Stage: 2‐4 (proportion not stated) (PU classification: NPUAP) | |
| Interventions | Group 1: protease‐modulating dressing ‐ Promogran: hydropolymer secondary dressing; preparation phase included hydrogel; n = 40. Grouped intervention category: protease modulating dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 0 (all appear to be covered). Group 2 ‐ 0 |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 20 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) | |
| Interventions | Group 1: basic wound contact dressing ‐ paraffin gauze (Jelonet); n = 9. Grouped intervention category: basic dressing | |
| Outcomes | Primary outcomes: proportion completely healed at could choose (IPD) e.g. Results given at 8 (reviewer choice) weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 3/9 (33%) (2 elected to have wounds surgically repaired and withdrew; 1 transferred to acute hospital). Group 2 ‐ 0. Group 3 ‐ 1/6 (17%) (1 transferred to acute hospital). |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | High risk | Unit of randomisation person and unit of analysis ulcer ‐ IPD reported per ulcer (but only 2/16 (12.5%) participants had 2 ulcers); ≤ 1.2 ulcer:person = 9/9, 6/5 and 7/6 |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; nursing module (cluster)s randomised (> 1 wound per person, all followed) | |
| Participants | 15 participants with pressure ulcers. PU Stage: I (22% and 50%) and II, results separately for II. Inclusion criteria state all should have break in skin (PU classification: Enis and Sarmiento) | |
| Interventions | Group 1: foam dressing ‐ self adhesive PU dressing; n = 7 (5 grade II). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 1.5 (12 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ other. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 1/16 dropped from analysis but group unclear (1 unanticipated transfer to nursing home). Group 2 ‐ 1/16 dropped from analysis but group unclear (1 unanticipated transfer to nursing home). |
| Selective reporting (reporting bias) | Low risk | Inadequate – reported incompletely (e.g. P value > 0.05) |
| Other bias | High risk | Unit of randomisation nursing module (cluster) and unit of analysis ulcer ‐ 4/15 (27%) participants had 2 ulcers each (2 participants had different treatments for their 2 ulcers); < 1.3 ulcer:person ratio = 9/7 and 10/8 |
| Other bias | Unclear risk | Results not adjusted for clustering. Unclear if grades I and II are subgroups in this classification |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 17 participants with pressure ulcers. PU Stage: not stated (PU classification: not stated) | |
| Interventions | Group 1: collagenase‐containing ointment ‐ collaganese: ointment applied with wooden applicator and covered with a dry dressing; n = 5. Grouped intervention category: collagenase ointment | |
| Outcomes | Primary outcomes: proportion completely healed at 4 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none. Group 3 ‐ none |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Unclear risk | Unit of randomisation person and unit of analysis results for both people and ulcers ‐ we used the results for the participants, but unclear what was meant by healing => unclear risk of bias |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) | |
| Participants | 34 participants with pressure ulcers. PU Stage: III (PU classification: not stated) | |
| Interventions | Group 1: combination intervention ‐ other: non‐adherent + saline gauze + foam (Allevyn) dressing; n = 16. Grouped intervention category: mixed advanced and basic dressings | |
| Outcomes | Primary outcomes: proportion completely healed at 26 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ "sealed envelopes". Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 11/16 (69%) (1 death due to unrelated cause, other withdrawals related to morbidity). Group 2 ‐ 13/18 (72%) (3 deaths due to unrelated causes, other withdrawals related to morbidity) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ largest ulcer selected |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) | |
| Participants | 36 participants with pressure ulcers. PU Stage: 2 (PU classification: NPUAP) | |
| Interventions | Group 1: foam dressing ‐ Allevyn Thin: no secondary dressing; n = 20. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 4 weeks; time to complete healing reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ other. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 6/20 (30%) (3 died, 1 developed wound infection, 1 developed an abscess unrelated to the study wound, 1 ineligible for other reasons). Group 2 ‐ 3/16 (19%) (2 died, 1 asked to be discharged) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ largest ulcer selected |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) | |
| Participants | 10 participants with pressure ulcers. PU Stage: 3 (PU classification: EPUAP) | |
| Interventions | Group 1: protease‐modulating dressing ‐ Suprasorb C: with Suprasorb P as secondary dressing; n = 5. Grouped intervention category: protease‐modulating dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 3 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 0 i.e. no missing data (clearly stated) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ largest ulcer selected |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 58 participants with pressure ulcers. PU Stage: III (92% and 80%) and IV (PU classification: not stated) | |
| Interventions | Group 1: alginate dressing ‐ type not stated (standard care); n = 26 (missing data added). Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment adequate ‐ serially‐numbered opaque sealed envelopes. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded to interventions (clear description) |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 1/26 (4%); Group 2 ‐ 7/32 (22%). Reasons for 'missingness' across both groups: 3 died, 3 experienced general deterioration, 1 experienced device‐related deterioration and 1 asked to withdraw: i.e. differential missing data rates; high differential rate – likely to change effect estimate |
| Selective reporting (reporting bias) | Low risk | Adequate – outcome measured but not necessarily analysed for a good reason |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 124 ulcers, participants with pressure ulcers. PU Stage: 2 and 3 (control: 96%, group A: 85.3%, group B: 100% and group C: 60%) (PU classification: EPUAP) | |
| Interventions | Group 1: hydrogel dressing ‐ Intrasite Gel: saline cleansing, hydrogel and PU (secondary) dressing; n = 25 ulcers. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 5 (36 days) weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 15/115 (13%) overall (loss to follow‐up). Group 2 ‐ 15/115 (13%) overall (loss to follow‐up). Group 3 ‐ 15/115 (13%) overall (loss to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | High risk | Unit of randomisation person and unit of analysis ulcer ‐ multiple PUs per person treated with the same interventions. 140 ulcers in 100 persons across both groups. Unit of analysis issue |
| Other bias | Unclear risk | Data extracted from graph |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (> 1 wound per person, likely slowest healing wound selected) | |
| Participants | 124 participants with pressure ulcers. PU Stage: 3 and 4 (PU classification: NPUAP) | |
| Interventions | Group 1: hydrogel dressing ‐ carboxymethylcellulose vehicle gel (as placebo) + saline gauze; n = 31. Grouped intervention category: advanced dressing Group 3: ineligible intervention ‐ 300 µg / g of growth factor in vehicle gel + saline gauze Group 4: ineligible intervention ‐ 100 µg / g of growth factor in vehicle gel, twice daily + saline gauze Results available separately ‐ numbers calculated from % ‐ but results from groups 2‐4 were combined ( n = 93). Grouped intervention category: ineligible ‐ growth factor gel | |
| Outcomes | Primary outcomes: proportion completely healed at 16 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data: Group 1 ‐ unclear but may be 0. Group 2 ‐ unclear but may be 1 (1 participant with 100 microg bid discontinued). i.e. similar rate missing in both groups; unclear rate |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ ulcer selected that was likely to be the slowest healing |
| Other bias | Unclear risk | Results calculated from percentages |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) | |
| Participants | 12 participants with pressure ulcers. PU Stage: 2 and 3 (proportions not stated) (PU classification: EPUAP) | |
| Interventions | Group 1: hydrogel dressing ‐ DuoDERM Hydrogel (not in BNF): with OpSite Flexigrid secondary dressing; n = 6. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ no information. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data: Group 1 ‐ 0 (implied). Group 2 ‐ 0 (implied) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Unclear risk | Unit of randomisation person and unit of analysis unclear ‐ 1 ulcer per person implied |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; ulcers randomised (> 1 wound per person, all followed) | |
| Participants | 48 participants with pressure ulcers. PU Stage: II and III (41% and 70% grade III) (PU classification: Shea). All participants had chronic illness (focal cerebral disorders, spinal chord disorders, neurological disorders, cardiac disease, diabetes) | |
| Interventions | Group 1: vapour‐permeable dressing: polyurethane adhesive dressing; vapour‐permeable; n = unclear number randomised, but overall 48 participants in analysed population. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: complete healing not reported; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ other. Baseline comparability inadequate ‐ baseline characteristics different between arms. Rating: high |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data: Group 1 ‐ 13/50 (26%) ulcers missing (number participants missing not reported) (Overall, the "Most frequent causes of dropout were: death, hospitalisation, and inability to comply with protocol for pressure relief" ‐ no more information). Group 2 ‐ 10/50 (20%) ulcers missing (number participants missing not reported) (Overall, the "Most frequent causes of dropout were: death, hospitalisation, and inability to comply with protocol for pressure relief" ‐ no more information) |
| Selective reporting (reporting bias) | High risk | Comment: inadequate – reported incompletely (results given only for grade II ulcers and "not significantly different" for grade III ulcers) |
| Other bias | Low risk | Unit of randomisation ulcer and unit of analysis ulcer; > 6 people had 2 or more ulcers; 6 people had 2 ulcers assigned to different treatments; 77/48 (1.6) ulcers: people in available case analysis. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (> 1 wound per person, largest selected) | |
| Participants | 40 participants with pressure ulcers. PU Stage: II (11 and 15%) and III (PU classification: AHCPR) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM CGF (not BNF); n = 20. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Other evidence for no blinding |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 6/20 (30%) (2 adverse effects (both due to dressing), 1 death, 2 increased ulcer size, 1 unable to tolerate dressing). Group 2 ‐ 8/20 (40%) (1 participant request, 3 loss to follow‐up, 2 adverse effects (1 related to dressing), 1 death, 1 infection). |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (selected ulcer) ‐ largest ulcer selected |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT (abstract); not stated randomised (unclear if > 1 wound per person) | |
| Participants | 74 participants with pressure ulcers. PU Stage: 3 (PU classification: NPUAP) | |
| Interventions | Group 1: combination intervention ‐ "primary nonadherent silicone dressing and foam dressing"; n = 44. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data: Group 1 ‐ none stated. Group 2 ‐ none stated |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Unclear risk | Unit of randomisation not stated and unit of analysis person (unclear if > 1 ulcer analysed) ‐ implies 1 per person |
| Other bias | Unclear risk | Unclear if the trial was stopped early because of the results |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 37 participants with pressure ulcers. PU Stage: 2 (39% and 45%), 3 (50% and 45%) and 4 (11% and 9%) (PU classification: EPUAP) | |
| Interventions | Group 1: resin salve ‐ resin salve: Norway spruce salve mixed with butter between gauze; n = 21. Grouped intervention category: antimicrobial | |
| Outcomes | Primary outcomes: proportion completely healed at 26 (6 months) weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ other. Allocation concealment unclear ‐ other. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 8/21 (38%) (3 deaths, 2 admissions to operative treatment, 1 allergic skin reaction, 1 misdiagnosis, 1 participant‐based refusal without any specific cause). Group 2 ‐ 7/16 (44%) (4 deaths, 2 participant‐based refusal without any specific cause, 1 participant‐based refusal because of randomisation to control group) |
| Selective reporting (reporting bias) | High risk | Inadequate ‐ other. Time to event outcome excluded dropouts |
| Other bias | Low risk | Unit of randomisation person and unit of analysis results for both people and ulcers ‐ 3/21 (14%) and 2/16 (12.5%) participants had > 1 ulcer; study analysis seemed to require that all ulcers in a person should heal; ulcers:person ratio = 27/21 (1.3) and 18/16 (1.1) |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 34 participants with pressure ulcers. PU Stage: II (non‐blanching erythema and superficial damage ‐ may be closer to NPUAP I; 33% and 30%) and III (PU classification: Torrance) | |
| Interventions | Group 1: hydrogel dressing ‐ Aquagel (not in BNF); n = 17. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 3/17 (18%) (3 died ). Group 2 ‐ 2/17 (12%) (2 died) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | High risk | Unit of randomisation person and unit of analysis ulcer ‐ ulcer:person ratio = 20/17 (1.2) and 18/17 (1.1) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 99 participants stratified by wound. PU Stage: II and III (61% and 54% grade II) (PU classification: Stirling) | |
| Interventions | Group 1: hydrocolloid dressing ‐ Granuflex: cleansed using 0.9% saline as necessary; n = 49. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 6 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ "sealed envelopes". Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ('open label') and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 1/49 (2%) and some may have died (reason not stated; overall 5 participants died). Group 2 ‐ 2/50 (4%) and some may have died (reason not stated; overall 5 participants died) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, other selection of wound) | |
| Participants | 41 participants with pressure ulcers. PU Stage: II (50% and 43%), III (38% and 50%) and IV (13% and 7%) (PU classification: not stated) | |
| Interventions | Group 1: hydrogel dressing ‐ Carrosyn Gel Wound Dressing (contains Acemannan hydrogel ‐ from aloe vera); n = 22. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 10 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear ‐ vague |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 6/22 (27%) (4 died (not attributed to treatment), 1 showed deterioration and was terminated from study, 1 participant hospitalised). Group 2 ‐ 5/19 (26%) (2 died (not attributed to treatment), 1 showed deterioration and was terminated from study, 1 participant hospitalised, 1 protocol violation) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ 1 per person; NS how selected |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 41 participants with pressure ulcers. PU Stage: III (55% and 52%) or IV (PU classification: not stated) | |
| Interventions | Group 1: hydrocolloid with or without alginate ‐ DuoDERM with or without Sorbasan: calcium alginate filler given as needed if the wound was highly exudative. Dressing changed every 7 d; n = 20. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 12 weeks; time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ "opaque envelopes". Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded to interventions – deduced from interventions |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 4/20 (20%) (1 died, 3 hospitalised). Group 2 ‐ 6/21 (29%) (2 died, 2 hospitalised, 2 dropped out for non‐study‐related reasons) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ unclear if selected |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT (abstract); participants randomised (unclear if > 1 wound per person) | |
| Participants | 11 participants with pressure ulcers. PU Stage: II only (no subcutaneous involvement) (PU classification: not stated) | |
| Interventions | Group 1: topical ‐ enamel matrix protein; n = 6. Grouped intervention category: enamel matrix protein | |
| Outcomes | Primary outcomes: proportion completely healed at 8 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded ("open label") and no evidence that outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data: Group 1 ‐ none stated. Group 2 ‐ none stated |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Unclear risk | Unit of randomisation person and unit of analysis person (unclear if > 1 ulcer analysed) ‐ implies 1 per person |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, ulcer chosen at random) | |
| Participants | 39 participants with pressure ulcers. PU Stage: II (100% and 90%) and III (Shea ‐ must have a break in the skin for inclusion) (PU classification: Shea) | |
| Interventions | Group 1: hydrocolloid dressing ‐ DuoDERM; n = 18. Grouped intervention category: advanced dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 26 weeks (6 months); time to complete healing reported (Kaplan Meier plot included) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation unclear ‐ “randomised”. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ 2/18 (11%) (1 hospitalised, 1 withdrew consent). Group 2 ‐ 3/21 (14%) (3 died) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ full results reported |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) ‐ ulcer chosen at random (by coin toss) |
| Other bias | Low risk | Adequate ‐ no suggestion of problems |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: high |
| Methods | RCT; participants randomised (> 1 wound per person, all followed) | |
| Participants | 27 participants with pressure ulcers. PU Stage: II and III (96% III in both groups) (PU classification: AHCRQ) | |
| Interventions | Group 1: honey ‐ unprocessed gauze impregnated (dressing): semi‐permeable adhesive secondary dressing; n = 15. Grouped intervention category: antimicrobial | |
| Outcomes | Primary outcomes: proportion completely healed at 5 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ computer‐generated. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability adequate ‐ no suggestion of problems. Rating: unclear |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded to interventions – clear description |
| Incomplete outcome data (attrition bias) | High risk | Missing data: Group 1 ‐ 0. Group 2 ‐ 1/12 (8%) (1 died) |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ reported incompletely as ‘significant’ or P value < 0.05 |
| Other bias | High risk | Unit of randomisation person and unit of analysis ulcer ‐ ulcer:person ratio: 25/15 (1.7) and 26/12 (2.2) |
| Other bias | Unclear risk | Only available case analysis reported |
| ALL‐DOMAIN RISK OF BIAS | High risk | Rating: very high |
| ALL‐DOMAIN RISK OF BIAS 2 | High risk | |
| Methods | RCT; participants randomised (only 1 wound per person) | |
| Participants | 24 participants with pressure ulcers. PU Stage: 2 and 3 (PU classification: NPUAP) | |
| Interventions | Group 1: protease‐modulating dressing ‐ Fibroquel: collagen plus polyvinylpyrrolidone + zinc oxide paste cleansing; n = 12. Grouped intervention category: protease‐modulating dressing | |
| Outcomes | Primary outcomes: proportion completely healed at 3 weeks; time to complete healing not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Sequence generation adequate ‐ random number tables. Allocation concealment unclear ‐ no information on allocation concealment. Baseline comparability unclear ‐ baseline difference but unclear of importance. Rating: unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear who outcome assessor was |
| Incomplete outcome data (attrition bias) | Low risk | Missing data: Group 1 ‐ none. Group 2 ‐ none. i.e. no missing data (clearly stated) |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Low risk | Unit of randomisation person and unit of analysis person (1 ulcer/person) |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| ALL‐DOMAIN RISK OF BIAS | Unclear risk | Rating: unclear |
AHRQ: US Agency for Healthcare Research and Quality
BNF: British National Formulary
HC: hydrocolloid
IPD: individual participant data
NPWT: negative pressure wound therapy
NS: not stated
RCT: randomized controlled trial
UV: ultraviolet
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible intervention | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible patient population | |
| Ineligible type of healing outcome | |
| Comparison of two interventions in the same class | |
| Ineligible patient population | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible intervention | |
| Ineligible patient population | |
| Ineligible study design | |
| Ineligible outcomes | |
| Comparison of two interventions in the same class | |
| Ineligible patient population | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible indication | |
| Comparison of two interventions in the same class | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible study design | |
| Ineligible patient population | |
| Mixed intervention | |
| Ineligible indication | |
| Ineligible patient population | |
| Ineligible outcomes | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Mixed intervention | |
| Comparison of two interventions in the same class | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible indication | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible intervention | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible type of healing outcome | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible study design | |
| Comparison of two interventions in the same class | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible outcomes | |
| Ineligible study design | |
| Editorial | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Comparison of two interventions in the same class | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Mixed intervention | |
| Ineligible study design | |
| Mixed intervention | |
| Mixed intervention | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible patient population | |
| Ineligible intervention | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible intervention | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible type of healing outcome | |
| Ineligible outcomes | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible intervention | |
| Comparison of two interventions in the same class | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible intervention | |
| Mixed intervention | |
| Ineligible intervention | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible patient population | |
| Mixed intervention | |
| Ineligible study design | |
| Mixed intervention | |
| Mixed intervention | |
| Ineligible type of healing outcome | |
| Ineligible outcomes | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible patient population | |
| Ineligible indication | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible patient population | |
| No results | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible study design | |
| Dressings/topical agents not the only difference between interventions (nursing care was also different) | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Protocol only and review still not published | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible study design | |
| Confounded ‐ selection into phase 2 of trial on basis of results | |
| Mixed intervention | |
| Ineligible patient population | |
| Mixed intervention | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible intervention | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible intervention | |
| Mixed intervention | |
| Ineligible intervention | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible intervention | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible intervention | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible indication | |
| Ineligible type of healing outcome | |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible intervention | |
| Ineligible patient population | |
| Ineligible patient population | |
| Ineligible patient population | |
| Comparison of two interventions in the same class | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible study design | |
| Comparison of two interventions in the same class | |
| Ineligible outcomes | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible outcomes | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible patient population | |
| Ineligible patient population | |
| Mixed intervention | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible outcomes | |
| Ineligible patient population | |
| Comparison of two interventions in the same class | |
| Ineligible study design | |
| Ineligible intervention | |
| Ineligible type of healing outcome | |
| Ineligible outcomes | |
| Ineligible type of healing outcome | |
| Confounded ‐ selection into phase 2 of trial on basis of results | |
| Ineligible patient population | |
| Ineligible study design | |
| Mixed intervention | |
| Mixed intervention | |
| Ineligible outcomes | |
| Ineligible outcomes | |
| Ineligible patient population | |
| Ineligible outcomes | |
| Ineligible patient population | |
| Ineligible type of healing outcome | |
| Ineligible type of healing outcome | |
| Ineligible study design | |
| Ineligible type of healing outcome | |
| Ineligible patient population | |
| Ineligible intervention | |
| Ineligible study design |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | |
| Methods | RCT pilot study; Duration 3 months |
| Participants | 30 eligible participants with pressure ulcers randomised in a ratio of 1:1 |
| Interventions | Treatment group: indirect moxibustion for 30 min before application of a dressing, 1 session daily, 5 sessions weekly for 4 weeks Control group will only receive a dressing, applied in the same way as in the treatment group |
| Outcomes | Primary outcomes: wound surface area (WSA) and proportion of ulcers healed within trial period |
| Starting date | registered 7/12/2013 |
| Contact information | |
| Notes | Protocol only |
| Trial name or title | ISCRCTN57842461 study reported to be registered |
| Methods | RCT; participants randomised Duration 8 weeks |
| Participants | 820 participants with at least 1 grade II pressure ulcer will be recruited from primary health care |
| Interventions | Polyurethane foam and hydrocolloid dressings |
| Outcomes | Primary outcome: percentage of wounds healed after 8 weeks. Secondary outcomes will include cost‐effectiveness, as |
| Starting date | Not stated |
| Contact information | |
| Notes | Protocol only; trial not on ClinicalTrials.gov |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Interventions vs saline gauze Show forest plot | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 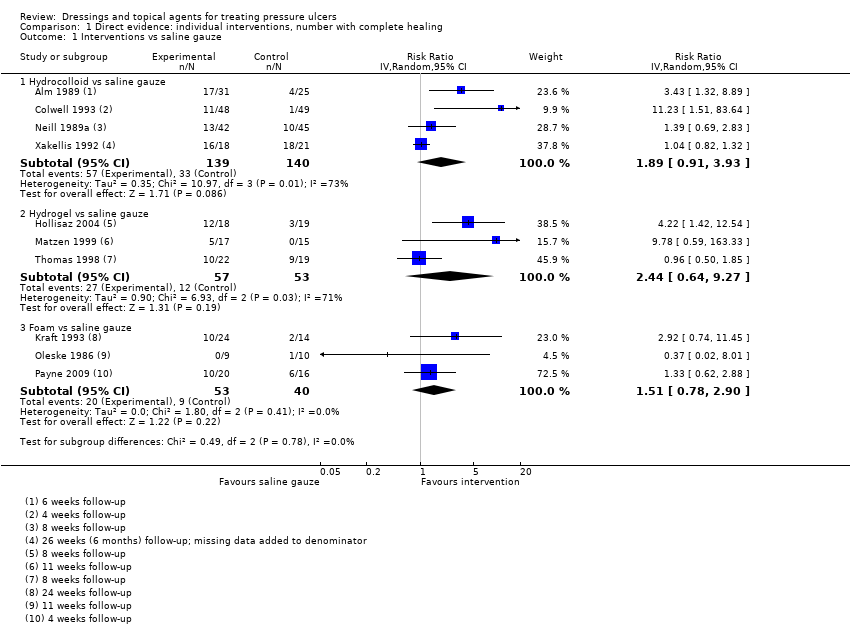 Comparison 1 Direct evidence: individual interventions, number with complete healing, Outcome 1 Interventions vs saline gauze. | ||||
| 1.1 Hydrocolloid vs saline gauze | 4 | 279 | Risk Ratio (IV, Random, 95% CI) | 1.89 [0.91, 3.93] |
| 1.2 Hydrogel vs saline gauze | 3 | 110 | Risk Ratio (IV, Random, 95% CI) | 2.44 [0.64, 9.27] |
| 1.3 Foam vs saline gauze | 3 | 93 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.78, 2.90] |
| 2 Interventions vs hydrocolloid Show forest plot | 13 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2 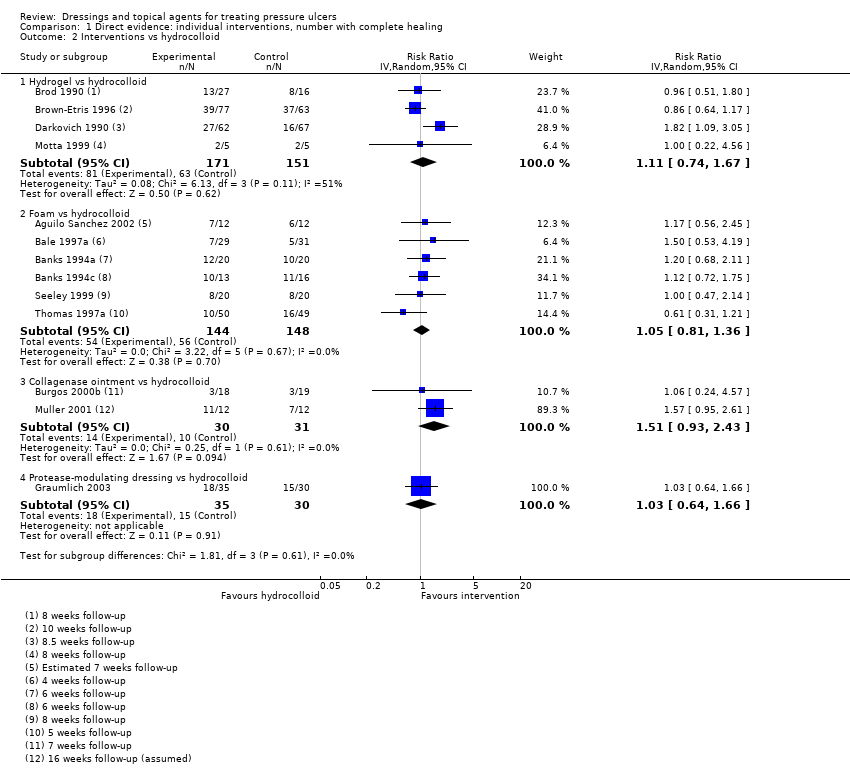 Comparison 1 Direct evidence: individual interventions, number with complete healing, Outcome 2 Interventions vs hydrocolloid. | ||||
| 2.1 Hydrogel vs hydrocolloid | 4 | 322 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.74, 1.67] |
| 2.2 Foam vs hydrocolloid | 6 | 292 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.81, 1.36] |
| 2.3 Collagenase ointment vs hydrocolloid | 2 | 61 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.93, 2.43] |
| 2.4 Protease‐modulating dressing vs hydrocolloid | 1 | 65 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.64, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intervention 1 vs intervention 2 Show forest plot | 18 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1 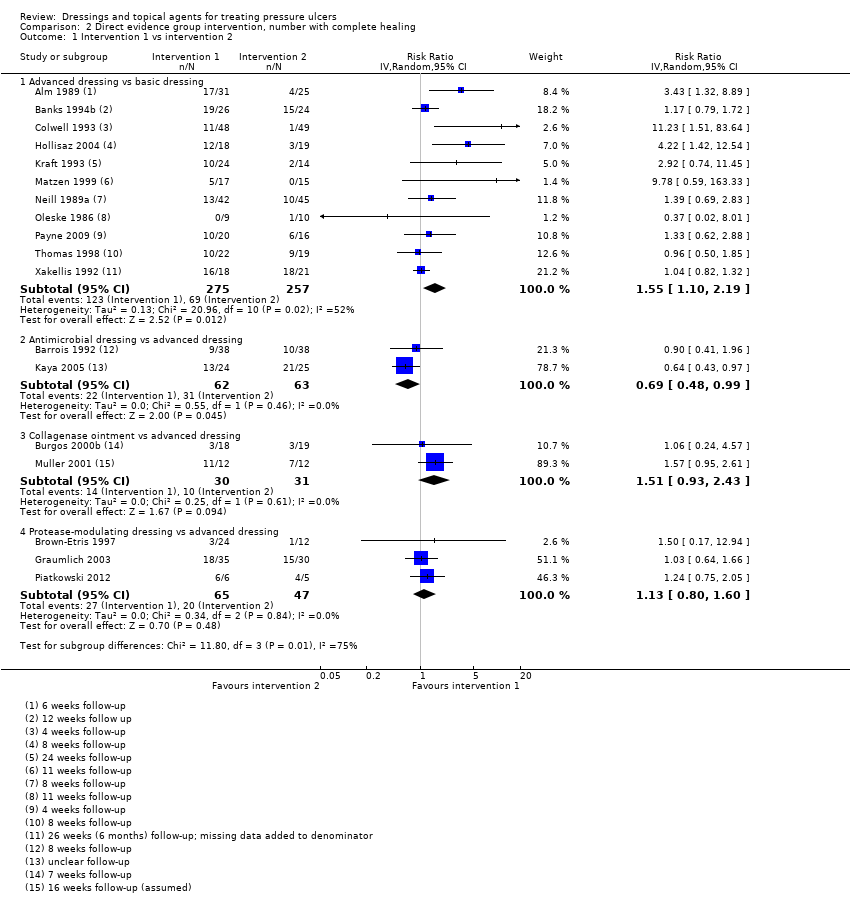 Comparison 2 Direct evidence group intervention, number with complete healing, Outcome 1 Intervention 1 vs intervention 2. | ||||
| 1.1 Advanced dressing vs basic dressing | 11 | 532 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.10, 2.19] |
| 1.2 Antimicrobial dressing vs advanced dressing | 2 | 125 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.48, 0.99] |
| 1.3 Collagenase ointment vs advanced dressing | 2 | 61 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.93, 2.43] |
| 1.4 Protease‐modulating dressing vs advanced dressing | 3 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.80, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐healing (survival analysis) Show forest plot | 7 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Direct evidence: individual interventions, time‐to‐healing data, Outcome 1 Time‐to‐healing (survival analysis). | ||||
| 1.1 Hydrocolloid versus saline gauze | 2 | 95 | Hazard Ratio (Fixed, 95% CI) | 1.75 [1.00, 3.05] |
| 1.2 Hydrogel versus hydrocolloid | 1 | 43 | Hazard Ratio (Fixed, 95% CI) | 1.30 [0.54, 3.13] |
| 1.3 Protease‐modulating versus hydrocolloid | 1 | 65 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.67, 2.65] |
| 1.4 Collagenase ointment versus hydrocolloid | 1 | 24 | Hazard Ratio (Fixed, 95% CI) | 2.59 [1.01, 6.62] |
| 1.5 Foam versus saline gauze | 1 | 36 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.42, 3.00] |
| 1.6 Hydrocolloid +/‐ alginate versus ineligible: radiant heat | 1 | 41 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.23, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐healing (survival analysis) Show forest plot | 5 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1 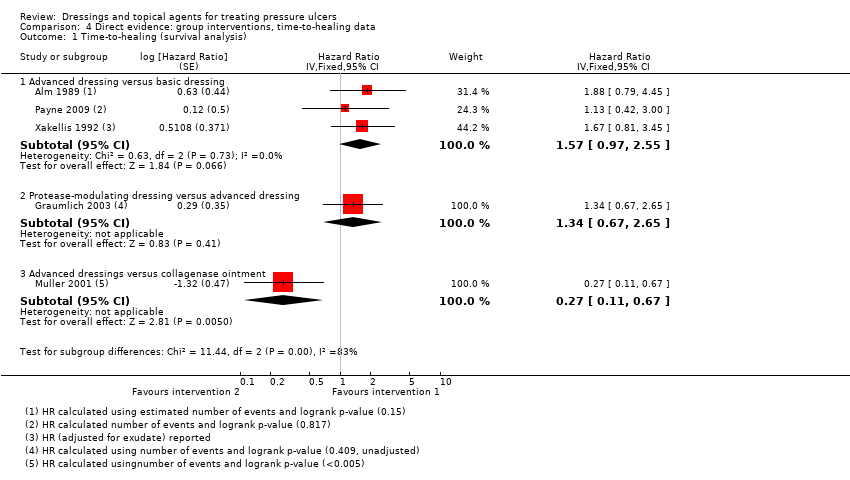 Comparison 4 Direct evidence: group interventions, time‐to‐healing data, Outcome 1 Time‐to‐healing (survival analysis). | ||||
| 1.1 Advanced dressing versus basic dressing | 3 | Hazard Ratio (Fixed, 95% CI) | 1.57 [0.97, 2.55] | |
| 1.2 Protease‐modulating dressing versus advanced dressing | 1 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.67, 2.65] | |
| 1.3 Advanced dressings versus collagenase ointment | 1 | Hazard Ratio (Fixed, 95% CI) | 0.27 [0.11, 0.67] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intervention 1 vs intervention 2 Show forest plot | 4 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Direct evidence ‐ non‐network comparisons, Outcome 1 Intervention 1 vs intervention 2. | ||||
| 1.1 Sugar + povidone iodine vs lysosyme | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Enamel matrix protein vs propylene glycol alginate | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Honey vs ethoxy‐diaminoacridine +nitrofurazone dressings | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Resin salve vs hydrocolloid or hydrocolloid silver dressing | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
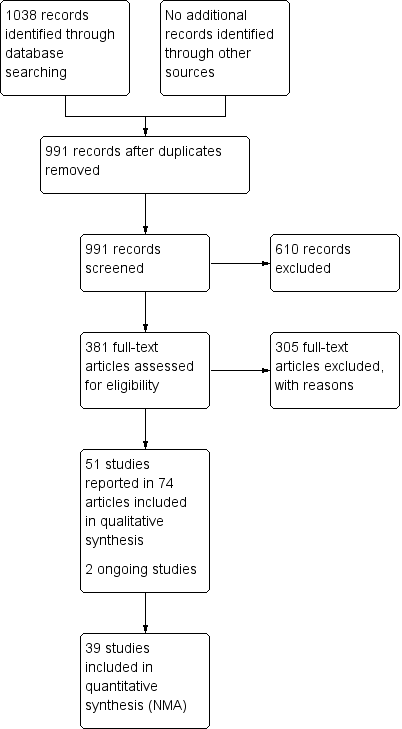
Study flow diagram

Network diagram ‐ individual interventions, by risk of bias (3 categories)
Key: green = low/unclear; yellow = high; red = very high overall risk of bias for the contrast. The number of studies for each contrast is given in Table 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
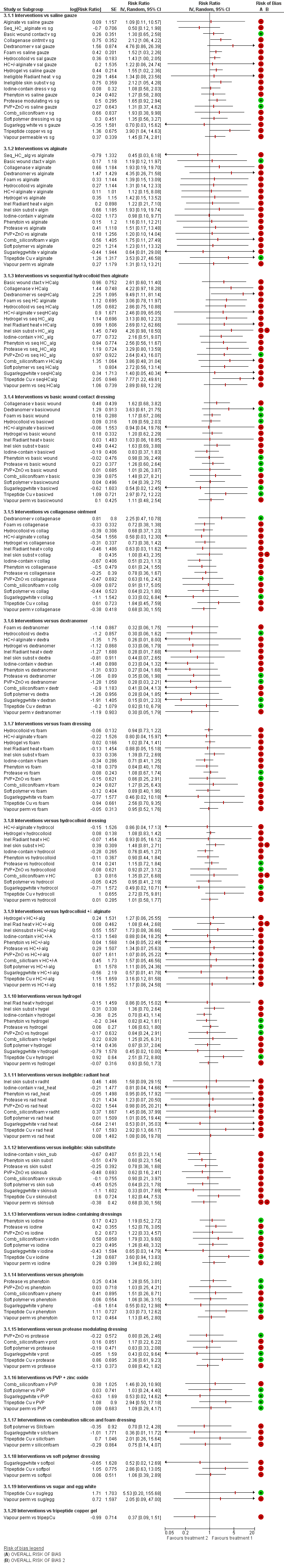
NMA results: individual intervention 1 versus individual intervention 2
Key for overall risk of bias for the contrast: green = low/unclear; one red = high; two reds = very high

Rankograms for each intervention ‐ individual network

Funnel plot ‐ individual network
Key to interventions: 1: saline gauze; 2: alginate dressing; 3: sequential hydrocolloid alginate dressings; 4: basic wound contact dressing; 5: collagenase ointment; 6: dextranomer; 7: foam dressing; 8: hydrocolloid dressing; 9: hydrocolloid +/‐ alginate (hydrocolloid dressing with/without alginate filler); 10: hydrogel dressing; 11: ineligible radiant heat; 12: ineligible skin substitute; 13: iodine‐containing dressing; 14: phenytoin; 15: protease‐modulating dressing; 16: PVP + zinc oxide 17: silicone + foam dressing; 18: soft polymer dressing; 19: sugar + egg white; 20: tripeptide copper gel; 21: vapour‐permeable dressing
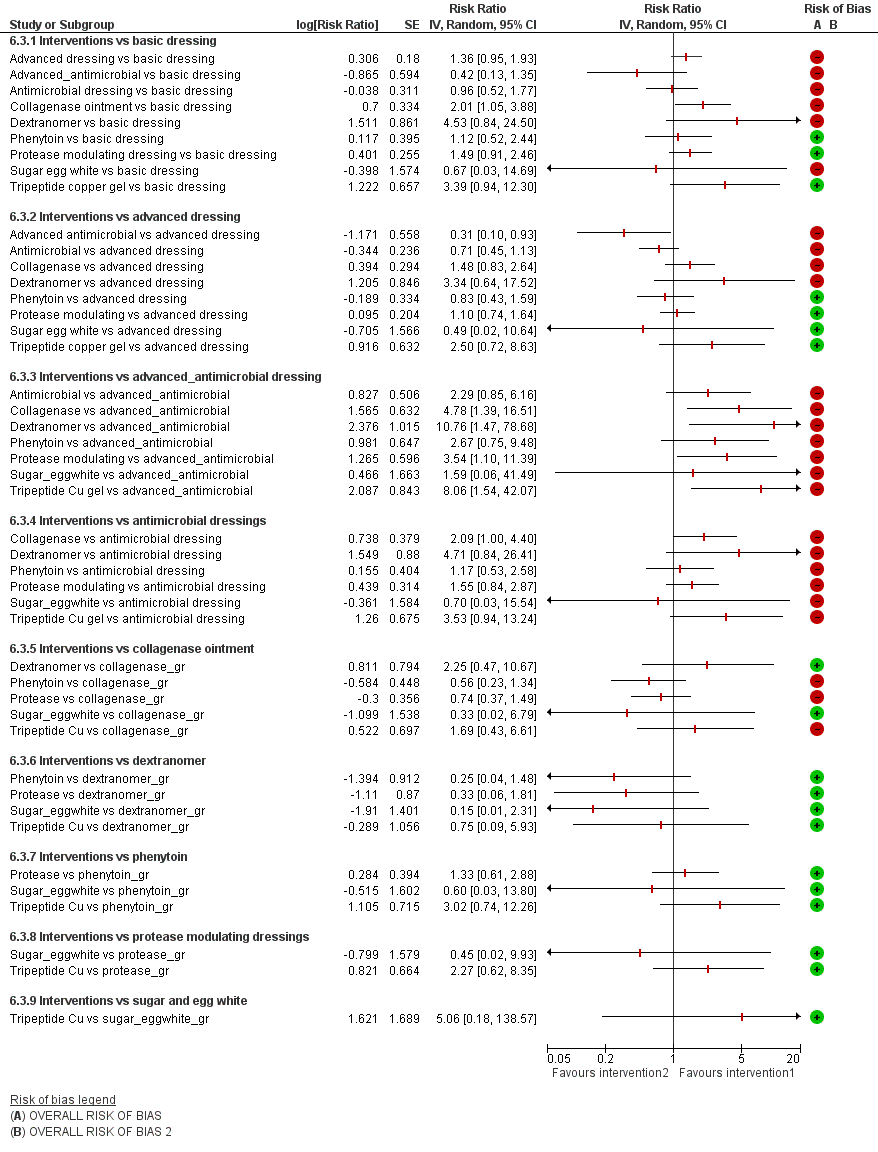
Intervention 1 versus intervention 2 ‐ group network
Key for overall risk of bias for the contrast: green = low/unclear; one red = high; two reds = very high

Rankograms combined ‐ group network

Funnel plot ‐ group network
Key to interventions: 1: basic dressing; 2: advanced dressing; 3: advanced or antimicrobial dressing; 4: antimicrobial dressing;
5: collagenase ointment; 6: dextranomer; 7: phenytoin; 8: protease‐modulating dressing; 9: sugar + egg white; 10: tripeptide copper gel
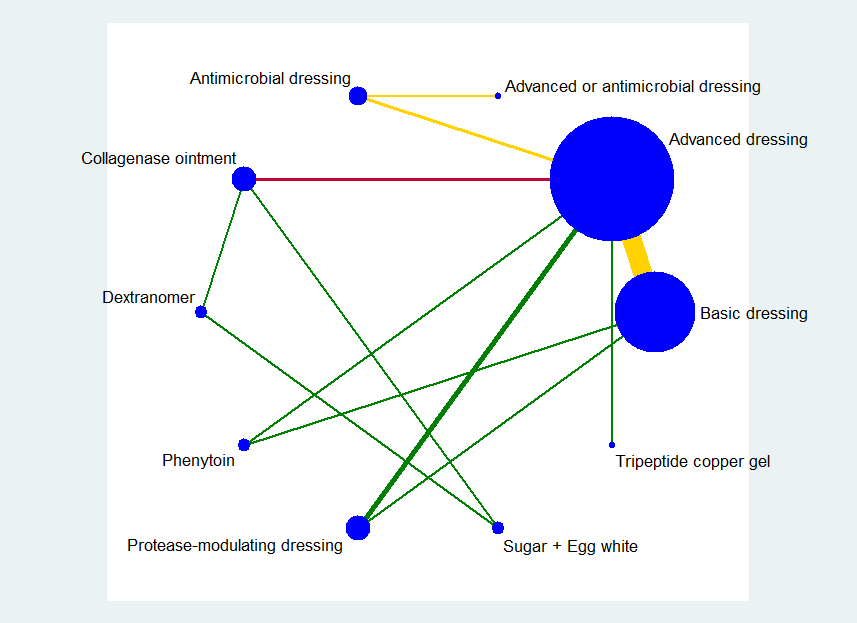
Key: green = low/unclear risk of bias; yellow = high risk of bias; red = very high overall risk of bias for the contrast. The number of studies for each contrast is given in Table 4.
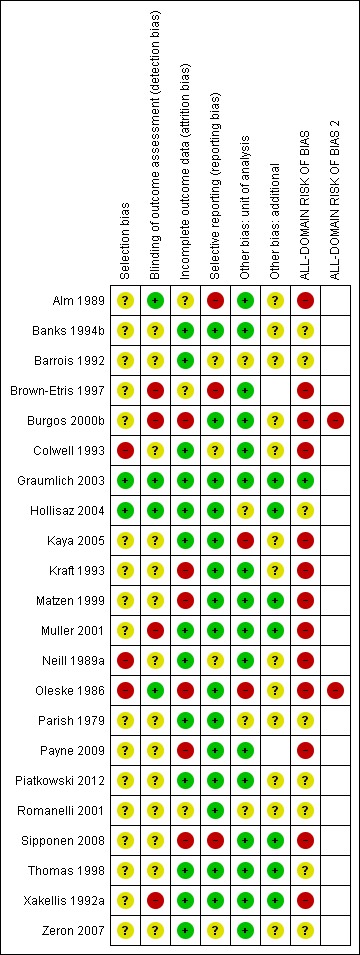
Risk of bias summary ‐ group network: review authors' judgements about each risk of bias item for each included study

Group network ‐ rankograms
Network diagram ‐ all interventions
Key: red = isolated interventions; blue = ineligible interventions joined to only one eligible intervention. Line and node weights not to scale
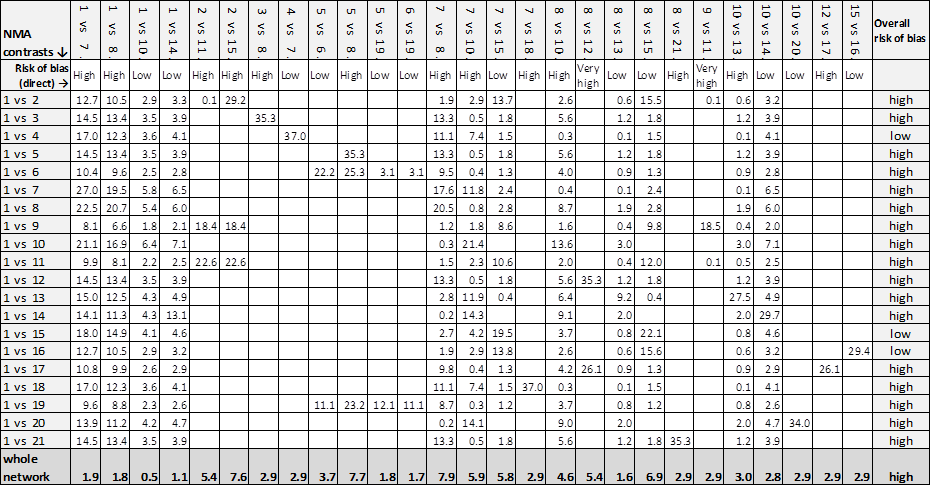
Contributions matrix ‐ interventions versus saline gauze (independent network)
Key: 1 = saline gauze dressing; 2 = alginate dressing; 3 = sequential hydrocolloid alginate dressings; 4 = basic wound contact dressing; 5 = collagenase ointment; 6 = dextranomer; 7 = foam dressing; 8 = hydrocolloid dressing; 9 = hydrocolloid +/‐ alginate (hydrocolloid with/without alginate filler); 10 = hydrogel dressing; 11 = ineligible intervention: radiant heat; 12 = ineligible intervention: skin substitute; 13 = iodine‐containing dressing; 14 = phenytoin; 15 = protease‐modulating dressing; 16 = PVP + zinc oxide; 17 = silicone + foam dressing; 18 = soft polymer dressing; 19 = sugar + egg white; 20 = tripeptide copper gel; 21 = vapour‐permeable dressing.

Rankograms combined ‐ individual network
Key to interventions: 1: saline gauze; 2: alginate dressing; 3: sequential hydrocolloid alginate dressings; 4: basic wound contact dressing; 5: collagenase ointment; 6: dextranomer; 7: foam dressing; 8: hydrocolloid dressing; 9: hydrocolloid +/‐ alginate (hydrocolloid dressing with/without alginate filler); 10: hydrogel dressing; 11: ineligible radiant heat; 12: ineligible skin substitute; 13: iodine‐containing dressing; 14: phenytoin; 15: protease‐modulating dressing; 16: PVP + zinc oxide
17: silicone + foam dressing; 18: soft polymer dressing; 19: sugar + egg white; 20: tripeptide copper gel; 21: vapour‐permeable dressing

Comparison 1 Direct evidence: individual interventions, number with complete healing, Outcome 1 Interventions vs saline gauze.

Comparison 1 Direct evidence: individual interventions, number with complete healing, Outcome 2 Interventions vs hydrocolloid.

Comparison 2 Direct evidence group intervention, number with complete healing, Outcome 1 Intervention 1 vs intervention 2.

Comparison 3 Direct evidence: individual interventions, time‐to‐healing data, Outcome 1 Time‐to‐healing (survival analysis).

Comparison 4 Direct evidence: group interventions, time‐to‐healing data, Outcome 1 Time‐to‐healing (survival analysis).

Comparison 5 Direct evidence ‐ non‐network comparisons, Outcome 1 Intervention 1 vs intervention 2.
| NMA evidence for individual network: proportion with complete healing ‐ interventions versus saline gauze | ||||
| Patient or population: people with pressure ulcers Settings: hospital, community or care home, or combinations | ||||
| Contrasts: interventions versus saline gauze | Relative effect | Anticipated absolute effects* (95% CI) ‐ from median of saline gauze control groups in direct evidence | Certainty (quality) of | |
| Median CGR | With interventions | |||
| Alginate dressings | RR 1.09 | 157 per 1000 | 171 per 1000 (17 to 1000) | ⊕⊝⊝⊝ |
| 14 more people healed per 1000 (140 fewer to 1000 more) | ||||
| Sequential hydrocolloid alginate dressings | RR 0.50 | 157 per 1000 | 78 per 1000 (1.9 to 31.2) | ⊕⊝⊝⊝ |
| 79 fewer people healed per 1000 (138 fewer to 155 more) | ||||
| Basic wound contact dressings | RR 1.30 | 157 per 1000 | 204 per 1000 (102 to 407) | ⊕⊕⊝⊝ |
| 47 more people healed per 1000 (55 fewer to 250 more) | ||||
| Collagenase ointment | RR 2.12 | 157 per 1000 | 333 per 1000 (166 to 663) | ⊕⊕⊝⊝ |
| 176 more people healed per 1000 (9 more to 506 more) | ||||
| Dextranomer | RR 4.76 | 157 per 1000 | 747 per 1000 (135 to 1000) | ⊕⊝⊝⊝ |
| 590 more people healed per 1000 (22 fewer to 1000 more) | ||||
| Foam dressings | RR 1.52 | 157 per 1000 | 239 per 1,000 (162 to 353) | ⊕⊕⊝⊝ |
| 82 more people healed per 1,000 (5 more to 196 more) | ||||
| Hydrocolloid dressing | RR 1.22 | 157 per 1000 | 192 per 1,000 (9 to 1000) | ⊕⊝⊝⊝ |
| 35 more people healed per 1,000 (148 fewer to 1000 more) | ||||
| Hydrocolloid dressings | RR 1.43 | 157 per 1000 | 225 per 1000 (157 to 322) | ⊕⊝⊝⊝ |
| 68 more people healed per 1000 (from 0 fewer to 165 more) | ||||
| Hydrogel | RR 1.55 | 157 per 1000 | 243 per 1000 (160 to 371) | ⊕⊝⊝⊝ |
| 86 more people healed per 1000 (from 3 more to 214 more) | ||||
| Iodine‐containing dressings | RR 1.08 | 157 per 1000 | 170 per 1000 (91 to 316) | ⊕⊝⊝⊝ |
| 13 more people healed per 1000 (from 66 fewer to 159 more) | ||||
| Phenytoin | RR 1.27 | 157 per 1000 | 199 per 1000 (91 to 440) | ⊕⊝⊝⊝ |
| 42 more people healed per 1000 (from 66 fewer to 283 more) | ||||
| Protease‐modulating dressings | RR 1.65 | 157 per 1000 | 259 per 1,000 (144 to 462) | ⊕⊕⊕⊝ |
| 102 more people healed per 1000 (from 13 fewer to 305 more) | ||||
| Polyvinylpyrrolidone + zinc oxide | RR 1.31 | 157 per 1000 | 206 per 1,000 (58 to 732) | ⊕⊕⊝⊝ |
| 49 more people healed per 1000 (from 99 fewer to 575 more) | ||||
| Combination silicone foam dressings | RR 1.93 | 157 per 1000 | 303 per 1,000 (60 to 1,000) | ⊕⊝⊝⊝ |
| 146 more people healed per 1000 (from 97 fewer to 1,000 more) | ||||
| Soft polymer dressings | RR 1.35 | 157 per 1000 | 212 per 1,000 (86 to 517) | ⊕⊝⊝⊝ |
| 55 more people healed per 1000 (from 71 fewer to 360 more) | ||||
| Sugar + egg white | RR 0.70 | 157 per 1000 | 110 per 1000 (5 to 1,000) | ⊕⊝⊝⊝ |
| 47 fewer people healed per 1000 (from 152 fewer to 1000 more) | ||||
| Tripeptide copper gel | RR 3.90 | 157 per 1000 | 612 per 1000 (163 to 1000) | ⊕⊝⊝⊝ |
| 455 more people healed per 1000 (6 more to 1000 more) | ||||
| Vapour‐permeable dressings | RR 1.45 | 157 per 1000 | 228 per 1000 | ⊕⊝⊝⊝ |
| 71 more people healed per 1000 (from 39 fewer to 283 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparator group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1Majority of evidence at high risk of bias (downgraded once); imprecision: very wide CI (crosses 0.75 and 1.25) (downgraded twice). | ||||
| Study characteristic | Studies in the individual | Studies in both individual and group networks | Studies in the group network only | Studies included in neither network | |
| Publication (all others were full published papers) | Conference abstracts (5) | ||||
| Multiple interventions | > 2 arms (4) | Nussbaum 1994; Ramos‐Torrecillas 2015 (4 arms) | |||
| Unit of randomisation (all other studies randomised individuals) | Ulcers randomised (5) | ||||
| Cluster‐randomised (2) | |||||
| not stated (3) | |||||
| Funding | Industry funding (18) | Banks 1994c; Belmin 2002; Brod 1990; Brown‐Etris 2008; Hondé 1994; Motta 1999 | Banks 1994a; Burgos 2000b; Colwell 1993; Kraft 1993; Neill 1989a; Payne 2009; Piatkowski 2012; Thomas 1998 | ||
| Mixed industry and non industry (2) | |||||
| Non industry (10) | Brown‐Etris 1997; Hollisaz 2004; Kaya 2005; Muller 2001; Oleske 1986; Xakellis 1992 | ||||
| Not stated (21) | Aguilo Sanchez 2002; Bale 1997a; Brown‐Etris 1996; Darkovich 1990; Meaume 2003; Price 2000; Seeley 1999; Seeley 1999; Thomas 1997a; Thomas 2005 | Alm 1989; Banks 1994b; Barrois 1992; Matzen 1999; Parish 1979; Romanelli 2001; Zeron 2007 | |||
| Setting | Community only (6) | ||||
| Hospital only (20) | Bale 1997a; Banks 1994c; Belmin 2002; Hondé 1994; Sopata 2002 | Alm 1989; Burgos 2000b; Colwell 1993; Kaya 2005; Muller 2001; Oleske 1986; Piatkowski 2012; Zeron 2007 | Gorse 1987; Imamura 1989; Nisi 2005; Nussbaum 1994; Ramos‐Torrecillas 2015; Yapucu Güneş 2007 | ||
| Hospital and other setting (7) | Price 2000 (hospital and community); Darkovich 1990 (hospital and care home); Brown‐Etris 1996 (hospital and community and care home) | Banks 1994b (hospital and community); Kraft 1993; Neill 1989a (hospital and care home); Payne 2009 (hospital and community and care home) | Ashby 2012 (hospital and community) | ||
| Care home and community (5) | |||||
| Care home only (5) | |||||
| Not stated (7) | |||||
| Follow‐up time | < 6 weeks (8) | Oleske 1986; Parish 1979; Payne 2009; Piatkowski 2012; Zeron 2007 | |||
| 6 to 8 weeks (25) | Aguilo Sanchez 2002; Banks 1994c; Belmin 2002; Brod 1990; Brown‐Etris 2008; Hondé 1994; Meaume 2003; Motta 1999; Price 2000; Seeley 1999; Sopata 2002; Thomas 1997a; | Alm 1989; Banks 1994a; Barrois 1992; Brown‐Etris 1997; Graumlich 2003; Hollisaz 2004; Neill 1989a; Romanelli 2001 | Imamura 1989; Nisi 2005; Nussbaum 1994; Sebern 1986; Van De Looverbosch 2004 | ||
| > 8 to 12 weeks (10) | Banks 1994b; Burgos 2000b; Colwell 1993; Matzen 1999; Thomas 1998 | ||||
| ≥ 16 weeks (7) | |||||
| Unclear (1) | |||||
| Mean age | < 65 years (8) | ||||
| Not stated (1) | |||||
| Physical conditions | Spinal cord injuries (4) | ||||
| Other (2) | Sopata 2002 (advanced cancer) | Parish 1979 ("chronically ill or physically disabled") | |||
| Ulcer grade | Mainly Stage 2 (17) | Bale 1997a; Thomas 1997a (Stirling); Brown‐Etris 2008 (classification not stated); Darkovich 1990 (Enis and Sarmiento); Hondé 1994 (Shea); Meaume 2003 (EUPAP) | Colwell 1993 (classification not stated); Graumlich 2003; Kaya 2005; Payne 2009 (NPUAP); Hollisaz 2004; Neill 1989a; Xakellis 1992 (Shea); Kraft 1993 (Enterstomal Therapy); Oleske 1986 (Enis and Sarmiento) | Gorse 1987; Van De Looverbosch 2004 (classification not stated) | |
| Mainly Stage 3 (15) | Belmin 2002 (Yarkony); Seeley 1999 (AHCPR); Thomas 1997a (Stirling); Serena 2010 (NPUAP); Brown‐Etris 1996; Motta 1999; Price 2000; Thomas 2005 (classification not stated) | Burgos 2000b (classification not stated); Piatkowski 2012 (EPUAP) | Sipponen 2008 (EPUAP) | Ashby 2012; Ramos‐Torrecillas 2015; (EPUAP classification); Yapucu Güneş 2007 (AHCRQ); Payne 2004 (classification not stated) | |
| Mainly Stage 4 (2) | Matzen 1999; Muller 2001 (classification not stated) | ||||
| Other (12) | Banks 1994c (II/III); Sopata 2002 II/III (Torrance); Brod 1990 (II/III) (classification not stated) | Banks 1994a (II/III); Brown‐Etris 1997 (II/III/IV) (classification not stated); Banks 1994b (Torrance II/III); Romanelli 2001 (II/III); Zeron 2007(2/3) (NPUAP) | Nisi 2005 (2‐4); Rees 1999 (3/4) (NPUAP); Sebern 1986 (II/III) (Shea); Imamura 1989 (II/III/IV) (classification not stated) | ||
| Not stated (5) | |||||
| Ulcer duration (other studies had ≥ 3 months) | < 3 months (16) | Banks 1994c (median 7 days); Belmin 2002; Brown‐Etris 1996; Brown‐Etris 2008; Meaume 2003; Motta 1999; Seeley 1999; Sopata 2002 (mean 2.5 weeks); Thomas 1997a | Banks 1994a; Burgos 2000b; Colwell 1993; Graumlich 2003; Hollisaz 2004; Kraft 1993; Payne 2009 | ||
| ≥ 3 months (6) | |||||
| Not stated/unclear (29) | Aguilo Sanchez 2002; Bale 1997a; Brod 1990; Darkovich 1990; Hondé 1994; Price 2000; Thomas 2005 | Banks 1994b; Barrois 1992; Brown‐Etris 1997; Kaya 2005; Matzen 1999; Muller 2001; Neill 1989a; Oleske 1986; Parish 1979; Piatkowski 2012 (> 4 weeks); Romanelli 2001; Thomas 1998; Xakellis 1992; Zeron 2007 | Gorse 1987; Imamura 1989; Nisi 2005; Nussbaum 1994 (> 6 weeks); Sebern 1986; Van De Looverbosch 2004 (> 1 month); Yapucu Güneş 2007 | ||
| Contrast/comparison | Number | RR (95% CI) direct evidence Random‐effects (inverse variance) Heterogeneity statistics | NMA results (consistency assumption) RR (95% CI) |
| Hydrocolloid dressing versus saline gauze dressing | 4 (279) | 1.89 (0.91 to 3.93) Tau² = 0.35; P = 0.01; I² = 73% | 1.43 (1.00 to 2.05) |
| Hydrogel versus saline gauze dressing | 3 (110) | 2.44 (0.64 to 9.27) Tau² = 0.90; P = 0.03; I² = 71% | 1.55 (1.02 to 2.36) |
| Foam dressings versus saline gauze dressing | 3 (93) | 1.51 (0.78 to 2.90) P = 0.41; I² = 0% | 1.52 (1.03 to 2.26) |
| Phenytoin versus saline gauze dressing | 1 (40) | 3.02 (0.97 to 9.35) | 1.27 (0.58 to 2.80) |
| Hydrogel versus hydrocolloid dressings | 4 (322) | 1.11 (0.74 to 1.67) Tau² = 0.08; P = 0.11; I² = 51% | 1.08 (0.83 to 1.42) |
| Foam dressing versus hydrocolloid dressing | 6 (292) | 1.05 (0.81 to 1.36) Tau² = 0.00; P = 0.67; I² = 0% (Stata: 1.05 (0.73 to 1.23)) | 1.07 (0.82 to 1.38) |
| Collagenase ointment versus hydrocolloid dressing | 2 (61) | 1.51 (0.93 to 2.43) P = 0.61; I² = 0% | 1.48 (0.81 to 2.69) |
| Iodine‐containing dressing versus hydrocolloid dressing | 1 (76) | 0.90 (0.41 to 1.96) | 0.76 (0.45 to 1.27) |
| Protease‐modulating dressing versus hydrocolloid dressing | 1 (65) | 1.03 (0.64 to 1.66) | 1.15 (0.72 to 1.84) |
| Vapour‐permeable dressing versus hydrocolloid dressing | 1 (72) | 1.01 (0.69 to 1.47) | 1.01 (0.58 to 1.77) |
| Hydrocolloid dressing 4 weeks then alginate dressing 4 weeks versus hydrocolloid dressing (Belmin 2002) | 1 (110) | 0.35 (0.10 to 1.25) | 0.35 (0.09 to 1.33) |
| Ineligible intervention: skin substitute versus hydrocolloid dressing (Hondé 1994) | 1 (168) | 1.48 (0.95 to 2.32) | 1.48 (0.81 to 2.71) |
| Foam dressing versus hydrogel (Sopata 2002) | 1 (38) | 1.11 (0.80 to 1.54) | 0.98 (0.71 to 1.36) |
| Tripeptide copper versus hydrogel | 1 (12) | 2.50 (0.76 to 8.19) | 2.51 (0.72 to 8.80) |
| Iodine‐containing dressing versus hydrogel | 1 (49) | 0.64 (0.43 to 0.97) | 0.70 (0.43 to 1.14) |
| Phenytoin versus hydrogel (Hollisaz 2004) | 1 (39) | 0.71 (0.41 to 1.24) | 0.82 (0.42 to 1.61) |
| Foam dressing versus protease‐modulating dressing | 1 (10) | 0.82 (0.49 to 1.38) | 0.93 (0.57 to 1.49) |
| Alginate dressing versus protease‐modulating dressing (Brown‐Etris 1997) | 1 (36) | 0.67 (0.08 to 5.75) | 0.30 (0.07 to 1.25) |
| PVP + zinc oxide versus protease‐modulating dressing | 1 (24) | 0.80 (0.28 to 2.27) | 0.80 (0.26 to 2.46) |
| Soft polymer dressing versus foam dressing | 1 (38) | 0.89 (0.45 to 1.75) | 0.89 (0.40 to 1.96) |
| Combination silicone‐foam dressing versus ineligible intervention: skin substitute (Serena 2010) | 1 (74) | 0.91 (0.22 to 3.77) | 0.90 (0.21 to 3.97) |
| Hydrocolloid with/without alginate filler versus ineligible intervention: radiant heat (Thomas 2005) | 1 (41) | 0.92 (0.41 to 2.06) | 0.92 (0.37 to 2.27) |
| Collagenase ointment versus dextranomer | 1 (12) | 0.35 (0.05 to 2.26) (Stata 0.44 (0.10 to 2.02)) | 0.44 (0.09 to 2.13) |
| Collagenase ointment versus sugar + egg white | 1 (10) | 3.00 (0.15 to 59.89) (Stata 3.00 (0.15 to 59.79)) | 3.00 (0.15 to 61.59) |
| Dextranomer versus sugar + egg white | 1 (12) | 6.75 (0.44 to 102.80) | 6.75 (0.43 to 105.99) |
| Foam dressing versus basic wound contact dressing | 1 (50) | 1.17 (0.79 to 1.72) | 1.17 (0.67 to 2.06) |
| Alginate dressing versus ineligible intervention: radiant heat (Price 2000) | 1 (58) | 0.82 (0.15 to 4.55) | 0.82 (0.14 to 4.77) |
| NMA evidence for group network: proportion with complete healing ‐ interventions versus basic dressings | ||||
| Patient or population: people with pressure ulcers Settings: hospital, community or care home, or combinations | ||||
| Contrasts: interventions versus basic dressing | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) ‐ from median of basic dressing control groups in direct evidence | Certainty (quality) of the | |
| Median CGR | With interventions | |||
| Advanced dressings versus basic dressing | RR 1.36 | 191 per 1000 | 260 per 1000 | ⊕⊝⊝⊝ |
| 69 more people healed per 1000 (from 10 fewer to 178 more) | ||||
| Advanced + antimicrobial dressing versus basic dressing | RR 0.42 | 191 per 1000 | 80 per 1000 | ⊕⊝⊝⊝ |
| 111 fewer people healed per 1000 (from 67 more to 166 fewer) | ||||
| Antimicrobial dressing versus basic dressing | RR 0.96 | 191 per 1000 | 183 per 1000 | ⊕⊝⊝⊝ |
| 8 fewer people healed per 1000 (from 92 fewer to 147 more) | ||||
| Collagenase ointment versus basic dressing | RR 2.01 | 191 per 1000 | 384 per 1000 | ⊕⊕⊝⊝ |
| 193 more people healed per 1000 (from 10 more to 550 more) | ||||
| Dextranomer versus basic dressing | RR 4.53 | 191 per 1000 | 865 per 1000 | ⊕⊝⊝⊝ |
| 674 more people healed per 1000 (from 31 fewer to 1,000 more) | ||||
| Phenytoin versus basic dressing | RR 1.12 | 191 per 1000 | 214 per 1000 | ⊕⊝⊝⊝ |
| 23 more people healed per 1000 (from 92 fewer to 275 more) | ||||
| Protease‐modulating dressing versus basic dressing | RR 1.49 | 191 per 1000 | 285 per 1000 | ⊕⊕⊕⊝ |
| 94 more people healed per 1000 (from 17 fewer to 279 more) | ||||
| Sugar + egg white versus basic dressing | RR 0.67 (0.03 to 14.69) | 191 per 1000 | 128 per 1000 | ⊕⊝⊝⊝ |
| 63 fewer people healed per 1000 (from 185 fewer to 1000 more) | ||||
| Tripeptide copper gel versus basic dressing | RR 3.39 | 191 per 1000 | 647 per 1000 | ⊕⊕⊝⊝ |
| 456 more people healed per 1000 (from 11 fewer to 1000 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1Majority of evidence at high risk of bias (downgraded once); inconsistency: heterogeneity in direct evidence (downgraded once); imprecision: wide CI (downgraded once). | ||||
| Individual intervention | Group intervention (studies in final network) |
| Basic wound contact dressing | Basic dressing (12 studies) |
| Saline gauze dressing | |
| Polyvinylpyrrolidone | |
| Hydrocolloid dressing | Advanced dressing (19 studies) |
| Foam dressing | |
| Hydrogel dressing | |
| Soft polymer dressing | |
| Alginate dressing | |
| Vapour‐permeable dressing | |
| Combination silicone foam dressing | |
| Hydrocolloid with/without alginate | |
| Standard care as described in Ashby 2012 | |
| Honey | Antimicrobial dressing (3 studies) |
| Iodine‐containing dressing | |
| Ethoxy diaminoacridine + nitrofurazone dressing | |
| Resin salve | |
| Hydrocolloid or hydrocolloid silver dressing | Advanced or antimicrobial dressing (1 study) |
| Protease‐modulating dressing | Protease‐modulating dressing (4 studies) |
| Collagenase ointment | Collagenase ointment (3 studies) |
| Dextranomer | Dextranomer (1 study) |
| Phenytoin topical | Phenytoin topical (1 study) |
| Sugar + egg white | Sugar + egg white (1 study) |
| Tripeptide copper gel | Tripeptide copper gel (1 study) |
| Contrast/comparison | Number | RR (95% CI) direct evidence Random‐effects (inverse variance) Heterogeneity statistics | NMA results (consistency assumption) RR (95% CI) |
| Advanced dressings versus basic dressings (Alm 1989; Banks 1994b; Colwell 1993; Hollisaz 2004; Kraft 1993; Matzen 1999; | 11 (532) | 1.55 (1.10 to 2.19) Tau² = 0.13; P = 0.02; I² = 52% | 1.36 (0.95 to 1.93) |
| Phenytoin versus basic dressing | 1 (40) | 3.02 (0.97 to 9.35) | 1.12 (0.52 to 2.44) |
| Protease‐modulating dressing versus basic dressing (Zeron 2007) | 1 (24) | 1.25 (0.44 to 3.55) | 1.49 (0.91 to 2.46) |
| Antimicrobial dressings versus advanced dressings (Barrois 1992; Kaya 2005) | 2 (125) | 0.69 (0.48 to 0.99) Tau² = 0.00; P = 0.46; I² = 0% | 0.71 (0.45 to 1.13) |
| Collagenase ointment versus advanced dressings | 2 (61) | 1.51 (0.93 to 2.43) Tau² = 0.00; P = 0.61 ; I² = 0% | 1.48 (0.83 to 2.64) |
| Phenytoin versus advanced dressing | 1 (39) | 0.71 (0.41 to 1.24) | 0.83 (0.43 to 1.59) |
| Protease‐modulating dressing versus advanced dressing (Brown‐Etris 1997; Graumlich 2003; Piatkowski 2012) | 3 (112) | 1.13 (0.80 to 1.60) Tau² = 0.00; P = 0.84 ; I² = 0% (Stata: 1.12 (0.79 to 1.59)) | 1.10 (0.74 to 1.64) |
| Tripeptide copper gel versus advanced dressing | 1 (12) | 2.50 (0.76 to 8.19) | 2.50 (0.72 to 8.63) |
| Antimicrobial dressing versus advanced antimicrobial dressing (Sipponen 2008) | 1 (37) | 2.29 (0.91 to 5.77) | 2.29 (0.85 to 6.16) |
| Collagenase versus dextranomer | 1 (12) | 0.35 (0.05 to 2.26) (Stata 0.44 (0.10 to 2.02)) | 0.44 (0.09 to 2.11) |
| Collagenase ointment versus sugar + egg white (part of 3‐arm trial) (Parish 1979) | 1 (10) | 3.00 (0.15 to 59.89) (Stata 3.00 (0.15 to 59.79)) | 3.00 (0.15 to 61.18) |
| Dextranomer versus sugar + egg white (part of 3‐arm trial) (Parish 1979) | 1 (12) | 6.75 (0.44 to 102.80) | 6.75 (0.43 to 105.22) |
| TOTAL | 22 (959) |
| Group intervention | Mean rank | SUCRA | Probability at | Rank at maximum |
| Basic dressing | 7.3 | 0.3 | 30.6 | 8 |
| Advanced dressing | 5.2 | 0.5 | 38.8 | 5 |
| Advanced ‐ antimicrobial dressing | 9.4 | 0.1 | 57.0 | 10 |
| Antimicrobial dressing | 7.4 | 0.3 | 31.9 | 8 |
| Collagenase ointment | 3.3 | 0.7 | 39.4 | 3 |
| Dextranomer | 2 | 0.9 | 55.3 | 1 |
| Phenytoin | 6.5 | 0.4 | 20.5 | 7 |
| Protease‐modulating dressing | 4.6 | 0.6 | 31.2 | 4 |
| Sugar + egg white | 7 | 0.3 | 36.5 | 10 |
| Tripeptide copper gel | 2.3 | 0.9 | 36.2 | 2 |
| Intervention | Number of | Number of participants | In joined | Number of studies | Number of participants |
| Alginate dressings | 2 | 38 | Y | 2 | 38 |
| Basic wound contact dressings | 2 | 33 | Y | 1 | 24 |
| Collagenase‐containing ointment | 3 | 35 | Y | 3 | 35 |
| Combination dressing: non‐adherent + saline gauze + foam | 1 | 16 | N | ||
| Combination dressing: silicone + foam | 1 | 44 | Y | 1 | 44 |
| Dextranomer | 1 | 7 | Y | 1 | 7 |
| Enamel matrix protein | 1 | 6 | N | ||
| Ethoxy‐diaminoacridine plus nitrofurazone dressing | 1 | 26 | N | ||
| Foam dressings | 13 | 266 | Y | 13 | 266 |
| Gauze saline dressings | 11 | 245 | Y | 10 | 233 |
| Honey | 1 | 25 | N | ||
| Hydrocolloid dressings | 22 | 791 | Y | 21 | 715 |
| Hydrocolloid or hydrocolloid silver dressing | 1 | 16 | N | ||
| Hydrocolloid with or without alginate filler | 1 | 20 | Y | 1 | 20 |
| Hydrocolloid‐alginate sequential dressings | 1 | 57 | Y | 1 | 57 |
| Hydrogel | 12 | 335 | Y | 10 | 279 |
| Ineligible intervention: graft + conventional dressing | 1 | 18 | N | ||
| Ineligible intervention: growth factor + hyaluronic acid | 1 | 40 | N | ||
| Ineligible intervention: growth factor | 2 | 152 | N | ||
| Ineligible intervention laser | 1 | 7 | N | ||
| Ineligible intervention: NPWT (only included in group analysis) | 1 | 6 | N | ||
| Ineligible intervention: povidone iodine + paraffin soaked gauze | 1 | 40 | N | ||
| Ineligible intervention: radiant heat | 2 | 53 | Y | 2 | 53 |
| Ineligible intervention: skin substitute | 2 | 110 | Y | 2 | 110 |
| Ineligible intervention: ultrasound + ultraviolet | 1 | 6 | N | ||
| Ineligible intervention: whirlpool + chloramine dressing | 1 | 52 | N | ||
| Iodine‐containing dressings | 2 | 62 | Y | 2 | 62 |
| Lysosyme ointment | 1 | 69 | N | ||
| Phenytoin topical | 1 | 21 | Y | 1 | 21 |
| Polyvinylpyrrolidone + zinc oxide | 1 | 12 | Y | 1 | 12 |
| Propylene glycol alginate | 1 | 5 | N | ||
| Protease‐modulating dressings | 5 | 116 | Y | 4 | 76 |
| Resin salve | 1 | 16 | N | ||
| Soft polymer dressing | 1 | 18 | Y | 1 | 18 |
| Standard care (only included in group analysis) | 1 | 6 | N | ||
| Sugar + egg white | 1 | 5 | Y | 1 | 5 |
| Sugar + povidone iodine | 1 | 72 | N | ||
| Tripeptide copper + Opsite | 1 | 6 | Y | 1 | 6 |
| Vapour‐permeable dressings | 2 | 57 | Y | 1 | 35 |
| NMA ⇩ | Contributions from each direct evidence contrast | Overall risk |
| 1 vs 2 | 74.5% 1 vs 2 + 5.5% 1 vs 7 + 7.2% 1 vs 8 + 5.5% 2 vs 7 + 7.2% 2 vs 8 | high |
| 1 vs 3 | 27.1% 1 vs 2 + 2.0% 1 vs 7 + 2.6% 1 vs 8 + 31.8% 2 vs 4 + 2.0% 2 vs 7 + 2.6% 2 vs 8 + 31.8% 3 vs 4 | high |
| 1 vs 4 | 39.8% 1 vs 2 + 2.9% 1 vs 7 + 3.9% 1 vs 8 + 46.6% 2 vs 4 + 2.9% 2 vs 7 + 3.9% 2 vs 8 | high |
| 1 vs 5 | 39.8% 1 vs 2 + 2.9% 1 vs 7 + 3.9% 1 vs 8 + 46.6% 2 vs 5 + 2.9% 2 vs 7 + 3.9% 2 vs 8 | high |
| 1 vs 6 | 26.1% 1 vs 2 + 1.9% 1 vs 7 + 2.5% 1 vs 8 + 30.6% 2 vs 5 + 1.9% 2 vs 7 + | high |
| 1 vs 7 | 37.8% 1 vs 2 + 13.4% 1 vs 7 + 3.7% 1 vs 8 + 41.4% 2 vs 7 + 3.7% 2 vs 8 | low |
| 1 vs 8 | 40.8% 1 vs 2 + 3.0% 1 vs 7 + 9.3% 1 vs 8 + 3.0% 2 vs 7 + 43.8% 2 vs 8 | low |
| 1 vs 9 | 23.6% 1 vs 2 + 1.7% 1 vs 7 + 2.3% 1 vs 8 + 27.6% 2 vs 5 + 1.7% 2 vs 7 + | high |
| 1 vs 10 | 39.8% 1 vs 2 + 2.9% 1 vs 7 + 3.9% 1 vs 8 + 2.9% 2 vs 7 + 3.9% 2 vs 8 + 46.9% 2 vs 10 | low |
| Whole network | 8.7% 1 vs 2 + 2.1% 1 vs 7 + 1.6% 1 vs 8 + 14.9% 2 vs 4 + 19.6% 2 vs 5 + | high |
| Key to interventions: 1 = basic dressing; 2 = advanced dressing; 3 = advanced +/‐ antimicrobial dressing; Risk of bias for direct contrasts: Low ‐ 1 vs 7; 1 vs 8; 2 vs 7; 2 vs 8; 2 vs 10; 5 vs 6; 5 vs 9; 6 vs 9. | ||
| Common heterogeneity estimate within each loop | Common heterogeneity estimate for network: tau² (network) = 0.0435 | ||||
| Loop | Ratio of RR (90% CI) | P value | Loop heterogeneity tau² (loop) | Ratio of RR (90% CI) | P value |
| Saline gauze ‐ hydrogel ‐ phenytoin | 3.90 (1.19 to 12.77) | 0.059 | 0.000 | 3.75 (90%CI 1.14 to 12.29) | 0.067 |
| Saline gauze ‐ foam ‐ hydrogel | 1.64 (0.27 to 9.99) | 0.651 | 0.512 | 1.21 (90%CI 0.56 to 2.63) | 0.682 |
| Hydrocolloid ‐ hydrogel ‐ iodine containing dressing | 1.26 (0.44 to 3.61) | 0.721 | 0.084 | 1.28 (90%CI 0.59 to 2.75) | 0.602 |
| Foam ‐ hydrocolloid ‐ protease‐modulating | 1.25 (0.66 to 2.35) | 0.562 | 0 | 1.24 (90%CI 0.66 to 2.35) | 0.572 |
| Foam ‐ hydrocolloid ‐ hydrogel | 1.13 (0.7 to 1.83) | 0.675 | 0.016 | 1.16 (90%CI 0.77 to 1.75) | 0.548 |
| Saline gauze ‐ foam ‐ hydrocolloid | 1.04 (0.45 to 2.41) | 0.936 | 0.084 | 1.04 (90%CI 0.55 to 1.96) | 0.919 |
| Saline gauze ‐ hydrocolloid ‐ hydrogel | 1.01 (0.33 to 3.11) | 0.993 | 0.244 | 1.09 (90%CI 0.62 to 1.89) | 0.808 |
| Contrast | Direct evidence RR (95% CI) | Indirect evidence RR (95% CI) | RR direct/RR indirect (90% CI) | P value | tau² |
| Foam versus saline gauze | 1.52 (0.73 to 3.16) | 1.54 (0.95 to 2.49) | 0.99 (90% CI 0.47 to 2.05) | 0.973 | 0.22 |
| Hydrocolloid versus saline gauze | 1.41 (0.88 to 2.26) | 1.49 (0.87 to 2.56) | 0.95 (90% CI 0.53 to 1.69) | 0.876 | 0.22 |
| Hydrogel versus saline gauze | 1.67 (0.86 to 3.22) | 1.52 (0.91 to 2.54) | 1.10 (90% CI 0.57 to 2.12) | 0.820 | 0.22 |
| Phenytoin versus saline gauze | 3.01 (0.93 to 9.71) | 0.29 (0.05 to 1.51) | 10.06 (90% CI 1.35 to 75.13) | 0.059 | 0.15 |
| Hydrocolloid versus foam | 0.95 (0.68 to 1.32) | 0.91 (0.57 to 1.44) | 1.04 (90% CI 0.65 to 1.68) | 0.881 | 0.23 |
| Hydrogel versus foam | 0.90 (0.51 to 1.58) | 1.10 (0.72 to 1.68) | 0.81 (90% CI 0.45 to 1.47) | 0.568 | 0.23 |
| Protease‐modulating dressing versus foam | 1.22 (0.61 to 2.41) | 0.94 (0.46 to 1.92) | 1.29 (90% CI 0.56 to 2.94) | 0.614 | 0.22 |
| Hydrogel versus hydrocolloid | 1.10 (0.76 to 1.59) | 1.07 (0.68 to 1.68) | 1.02 (90% CI 0.63 to 1.67) | 0.935 | 0.24 |
| Iodine‐containing dressing versus hydrocolloid | 0.90 (0.36 to 2.19) | 0.68 (0.35 to 1.33) | 1.31 (90% CI 0.51 to 3.32) | 0.638 | 0.22 |
| Protease‐modulating dressing versus hydrocolloid | 1.02 (0.53 to 1.97) | 1.32 (0.63 to 2.76) | 0.78 (90% CI 0.34 to 1.77) | 0.614 | 0.22 |
| Iodine‐containing dressing versus hydrogel | 0.64 (0.35 to 1.16) | 0.84 (0.32 to 2.15) | 0.77 (90% CI 0.30 to 1.95) | 0.639 | 0.22 |
| Phenytoin versus hydrogel | 0.71 (0.38 to 1.34) | 7.18 (0.68 to 75.47) | 0.10 (90% CI 0.01 to 0.74) | 0.059 | 0.15 |
| Contrast | Design 1 | NMA results for design 1 | Design 2 | (NMA results for design 2 | NMA results |
| Foam versus saline gauze | 7 vs 1 (3 studies) | 1.53 (0.71 to 2.22) | NA | NA | 1.52 (1.03 to 1.85) |
| Hydrocolloid versus saline gauze | 8 vs 1 (4 studies) | 1.50 (0.9 to 1.92) | NA | NA | 1.43 (1.00 to 1.70) |
| Hydrogel versus saline gauze | 10 vs 1 (2 studies; heterogeneity) | 1.16 (0.51 to 1.73) | 10 vs 1 vs 14 (one 3‐arm study) | 4.22 (1.26 to 7.65) | 1.55 (1.02 to 1.91) |
| Phenytoin versus saline gauze | NA | NA | 14 vs 1 vs 10 | 3.02 (0.86 to 5.56) | 1.28 (0.58 to 1.88) |
| Hydrocolloid versus collagenase | 8 vs 5 (2 studies) | 0.68 (0.35 to 0.95) | NA | NA | 0.68 (0.37 to 0.91) |
| Hydrocolloid versus foam | 8 vs 7 (6 studies) | 0.96 (0.67 to 1.13) | NA | NA | 0.94 (0.73 to 1.07) |
| Hydrogel versus foam | 10 vs 7 (1 study) | 0.90 (0.48 to 1.22) | NA | NA | 1.02 (0.74 to 1.2) |
| Protease‐modulating versus foam | 15 vs 7 (1 study) | 1.22 (0.58 to 1.76) | NA | NA | 1.08 (0.67 to 1.36) |
| Hydrogel versus hydrocolloid | 10 vs 8 (4 studies) | 1.11 (0.74 to 1.35) | NA | NA | 1.09 (0.83 to 1.24) |
| Iodine‐containing dressing versus hydrocolloid | 13 vs 8 (1 study) | 0.90 (0.35 to 1.43) | NA | NA | 0.76 (0.45 to 0.97) |
| Protease‐modulating versus hydrocolloid | 15 vs 8 (1 study) | 1.03 (0.5 to 1.46) | NA | NA | 1.15 (0.72 to 1.45) |
| Iodine‐containing dressing versus hydrogel | 13 vs 10 (1 study) | 0.64 (0.33 to 0.9) | NA | NA | 0.70 (0.43 to 0.88) |
| Phenytoin versus hydrogel | NA | NA | 14 vs 10 vs 1 | 0.71 (0.33 to 1.04) | 0.82 (0.42 to 1.14) |
| Loop | Ratio of RR (90% CI) | P value | Loop heterogeneity ‐ tau² |
| Basic dressing ‐ advanced dressing ‐ phenytoin | 3.04 (0.71 to 13.06) | 0.210 | 0.085 |
| Basic dressing ‐ advanced dressing ‐ protease‐modulating dressing | 1.36 (0.39 to 4.73) | 0.682 | 0.091 |
| Contrast | Direct evidence RR (95% CI) | Indirect evidence RR (95% CI) | RR direct/RR indirect (90% CI) | P value | Tau² |
| Advanced dressing versus basic dressing | 1.41 (0.96 to 2.09) | 1.10 (0.33 to 3.73) | 1.28 (90% CI 0.44 to 3.76) | 0.705 | 0.221791 |
| Phenytoin versus basic dressing | 3.02 (0.97 to 9.38) | 0.24 (0.06 to 1.02) | 12.51 (90% CI 1.87 to 83.55) | 0.029 | 0.048414 |
| Protease‐modulating dressing versus basic dressing | 1.25 (0.4 to 3.87) | 1.60 (0.87 to 2.95) | 0.78 (90% CI 0.27 to 2.3) | 0.707 | 0.221734 |
| Phenytoin versus advanced dressing | 0.71 (0.41 to 1.25) | 8.94 (0.96 to 83.43) | 0.08 (90% CI 0.01 to 0.53) | 0.029 | 0.048424 |
| Protease‐modulating dressing versus advanced dressing | 1.13 (0.71 to 1.79) | 0.88 (0.27 to 2.92) | 1.28 (90% CI 0.44 to 3.76) | 0.706 | 0.221781 |
| Contrast | Design 1 | NMA results for design 1 | Design 2 | NMA results for design 2 | NMA results |
| Advanced dressing versus basic dressing | 2 vs 1 (10 studies; heterogeneity) | 1.21 (0.88 to 1.67) | 2 vs 1 vs 7 (1 study) | 4.22 (1.41 to 12.68) | 1.36 (0.95 to 1.93) |
| Phenytoin versus basic dressing | NA | NA | 7 vs 1 vs 2 (1 study) | 3.02 (0.96 to 9.44) | 1.12 (0.52 to 2.44) |
| Protease‐modulating dressing versus basic dressing | 8 vs 1 (1 study) | 1.25 (0.44 to 3.59) | NA | NA | 1.49 (0.91 to 2.46) |
| Phenytoin versus advanced dressing | NA | NA | 7 vs 2 vs 1 (1 study) | 0.71 (0.4 to 1.27) | 0.83 (0.43 to 1.59) |
| Protease‐modulating dressing versus advanced dressing | 8 vs 2 (3 studies) | 1.12 (0.78 to 1.62) | NA | NA | 1.10 (0.74 to 1.64) |
| Intervention | Mean rank | SUCRA | Probability | Rank at maximum |
| Saline gauze | 16.3 | 0.2 | 16.2 | 17 |
| Alginate dressing | 12.4 | 0.4 | 10.8 | 19 |
| Sequential hydrocolloid alginate dressings | 18.6 | 0.1 | 34.6 | 21 |
| Basic wound contact dressing | 12.4 | 0.4 | 11.6 | 15 |
| Collagenase ointment | 6.9 | 0.7 | 13.5 | 6 |
| Dextranomer | 3.5 | 0.9 | 40.8 | 1 |
| Foam dressing | 10.3 | 0.5 | 15.0 | 10 |
| Hydrocolloid dressing | 11.6 | 0.5 | 18.6 | 11 |
| Hydrocolloid with/without alginate filler | 11.9 | 0.5 | 12.4 | 21 |
| Hydrogel | 9.9 | 0.6 | 14.9 | 10 |
| Ineligible intervention ‐ radiant heat | 11.4 | 0.5 | 12.8 | 20 |
| Ineligible intervention ‐ skin substitute | 6.6 | 0.7 | 15.0 | 4 |
| Iodine‐containing dressing | 15.3 | 0.3 | 13.4 | 17 |
| Phenytoin | 12.6 | 0.4 | 9.4 | 16 |
| Protease‐modulating dressing | 9.3 | 0.6 | 11.8 | 8 |
| PVP + zinc oxide | 11.8 | 0.5 | 8.1 | 20 |
| Silicone + foam dressing | 8.9 | 0.6 | 10.0 | 2 |
| Soft polymer dressing | 11.9 | 0.5 | 7.7 | 16 |
| Sugar + egg white | 14.4 | 0.3 | 31.8 | 21 |
| Tripeptide copper gel | 3.7 | 0.9 | 25.3 | 1 |
| Vapour‐permeable dressing | 11.4 | 0.5 | 8.9 | 13 |
| Contrast | Study | Risk ratio (95% CI) | Hazard ratio (95% CI) | Median times to healing |
| Hydrocolloid versus saline gauze | (6 weeks) | 3.43 (1.32 to 8.89) | 1.88 (0.80 to 4.45) | |
| Xakellis 1992 | 1.04 (0.82 to 1.32) | 1.67 (0.81 to 3.45) | 9 days versus 11 days | |
| Meta‐analysis of 2 studies in 95 participants (Alm 1989; Xakellis 1992) ‐ selected | Meta‐analysis: 1.72 (0.54 to 5.47) I² = 82%, P = 0.02 | Meta‐analysis: 1.75 (95% CI 1.00 to 3.05 I² = 0%, P = 0.84 | ||
| Hydrogel versus hydrocolloid | Brod 1990 (8 weeks) | 1.11 (0.74 to 1.67) | 1.30 (0.54 to 3.13) | 32 days versus 42 days (P = 0.56); Kaplan‐Meier curves crossing |
| Protease‐modulating dressing versus hydrocolloid | Graumlich 2003 (8 weeks) | 1.03 (0.64 to 1.66) | 1.34 (0.67 to 2.65) | 4 weeks and 7 weeks (estimated from Kaplan‐Meier plot); protease‐modulating dressing had more healing from 5 weeks |
| Collagenase ointment versus hydrocolloid | Muller 2001 (16 weeks ‐ probably) | 1.57 (0.95 to 2.61) | 2.58 (1.00 to 6.65) | |
| Hydrocolloid +/‐ alginate versus ineligible: radiant heat | Thomas 2005 (12 weeks) | 0.92 (0.41 to 2.06) | 0.64 (0.23 to 1.77) | > 90 days and 70 days (estimated from Kaplan‐Meier plot); radiant heat had consistently more healing at all time points |
| Foam versus saline gauze | Payne 2009 (4 weeks) | 1.33 (0.62 to 2.88) | 1.12 (0.42 to 3.01) |
| Risk ratio (95% CI) intervention versus saline gauze | Mean rank (of 21 interventions unless otherwise stated) | |||||
| Intervention | Original | Sensitivity analysis 1. Very high risk of bias | Sensitivity analysis 2. Complete case | Original saline 16.3 | Sensitivity analysis 1. Very high risk of bias | Sensitivity analysis 2. Complete case saline = 16.3 |
| Alginate dressing | 1.10 (0.11 to 10.57) | 1.14 (0.11 to 11.45) | 1.08 (0.11 to 10.69) | 12.4 | 11 | 12.6 |
| Sequential hydrocolloid | s0.50 (0.12 to 1.99) | 0.52 (0.12 to 2.20) | 0.51 (0.12 to 2.1) | 18.6 | 15.8 | 18.5 |
| Basic wound contact dressing | 1.30 (0.65 to 2.59) | 1.33 (0.61 to 2.93) | 1.44 (0.76 to 2.73) | 12.4 | 10.6 | 11.1 |
| Collagenase ointment | 2.11 (1.06 to 4.21) | 2.35 (1.02 to 5.44) | 2.01 (0.98 to 4.12) | 6.9 | 5.2 | 7.4 |
| Dextranomer | 4.75 (0.86 to 26.34) | 5.29 (0.87 to 32.26) | 4.51 (0.79 to 25.88) | 3.5 | 2.9 | 3.8 |
| Foam dressing | 1.52 (1.03 to 2.26) | 1.56 (1 to 2.43) | 1.45 (0.97 to 2.15) | 10.3 | 9 | 11.2 |
| Hydrocolloid dressing | 1.43 (1 to 2.05) | 1.50 (0.99 to 2.26) | 1.47 (1.02 to 2.12) | 11.6 | 9.7 | 11.1 |
| Hydrocolloid with/without | 1.23 (0.06 to 24.86) | not in network | 1.33 (0.06 to 27.37) | 11.9 | not in network | 11.5 |
| Hydrogel dressing | 1.55 (1.02 to 2.36) | 1.74 (1.09 to 2.77) | 1.56 (1.02 to 2.37) | 9.9 | 7.5 | 9.8 |
| Ineligible: radiant heat | 1.34 (0.08 to 23.53) | 1.39 (0.07 to 25.76) | 1.62 (0.09 to 29.28) | 11.4 | not in network | 10.2 |
| Ineligible: skin substitute | 2.12 (1.05 to 4.28) | not in network | 1.92 (0.91 to 4.02) | 6.6 | 9.8 | 7.6 |
| Iodine‐containing dressing | 1.08 (0.58 to 2.02) | 1.19 (0.6 to 2.38) | 1.13 (0.58 to 2.18) | 15.3 | 12.1 | 14.7 |
| Phenytoin | 1.28 (0.58 to 2.81) | 1.66 (0.71 to 3.89) | 1.3 (0.57 to 2.96) | 12.6 | 8.6 | 12.4 |
| Protease‐modulating dressing | 1.64 (0.92 to 2.93) | 1.71 (0.89 to 3.26) | 1.63 (0.89 to 2.97) | 9.3 | 7.9 | 9.4 |
| PVP + ZnO | 1.32 (0.37 to 4.64) | 1.37 (0.36 to 5.19) | 1.30 (0.35 to 4.78) | 11.8 | 10.2 | 11.9 |
| Combined silicone foam dressing | 1.93 (0.37 to 9.92) | not in network | 1.74 (0.33 to 9.32) | 8.9 | not in network | 9.7 |
| Soft polymer dressing | 1.35 (0.56 to 3.27) | 1.39 (0.53 to 3.63) | 1.29 (0.52 to 3.23) | 11.9 | 10.2 | 12.3 |
| Sugar + egg white | 0.70 (0.03 to 15.6) | 0.78 (0.03 to 18.33) | 0.67 (0.03 to 15.11) | 14.4 | 12 | 14.6 |
| Tripeptide copper gel | 3.88 (1.03 to 14.56) | 2.78 (0.90 to 8.58) | 3.89 (1.01 to 15) | 3.7 | 4.8 | 3.8 |
| Vapour‐permeable dressing | 1.44 (0.74 to 2.8) | 1.51 (0.70 to 3.25) | 1.48 (0.72 to 3.04) | 11.4 | 9.5 | 11 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Interventions vs saline gauze Show forest plot | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Hydrocolloid vs saline gauze | 4 | 279 | Risk Ratio (IV, Random, 95% CI) | 1.89 [0.91, 3.93] |
| 1.2 Hydrogel vs saline gauze | 3 | 110 | Risk Ratio (IV, Random, 95% CI) | 2.44 [0.64, 9.27] |
| 1.3 Foam vs saline gauze | 3 | 93 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.78, 2.90] |
| 2 Interventions vs hydrocolloid Show forest plot | 13 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Hydrogel vs hydrocolloid | 4 | 322 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.74, 1.67] |
| 2.2 Foam vs hydrocolloid | 6 | 292 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.81, 1.36] |
| 2.3 Collagenase ointment vs hydrocolloid | 2 | 61 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.93, 2.43] |
| 2.4 Protease‐modulating dressing vs hydrocolloid | 1 | 65 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.64, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intervention 1 vs intervention 2 Show forest plot | 18 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Advanced dressing vs basic dressing | 11 | 532 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.10, 2.19] |
| 1.2 Antimicrobial dressing vs advanced dressing | 2 | 125 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.48, 0.99] |
| 1.3 Collagenase ointment vs advanced dressing | 2 | 61 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.93, 2.43] |
| 1.4 Protease‐modulating dressing vs advanced dressing | 3 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.80, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐healing (survival analysis) Show forest plot | 7 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Hydrocolloid versus saline gauze | 2 | 95 | Hazard Ratio (Fixed, 95% CI) | 1.75 [1.00, 3.05] |
| 1.2 Hydrogel versus hydrocolloid | 1 | 43 | Hazard Ratio (Fixed, 95% CI) | 1.30 [0.54, 3.13] |
| 1.3 Protease‐modulating versus hydrocolloid | 1 | 65 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.67, 2.65] |
| 1.4 Collagenase ointment versus hydrocolloid | 1 | 24 | Hazard Ratio (Fixed, 95% CI) | 2.59 [1.01, 6.62] |
| 1.5 Foam versus saline gauze | 1 | 36 | Hazard Ratio (Fixed, 95% CI) | 1.13 [0.42, 3.00] |
| 1.6 Hydrocolloid +/‐ alginate versus ineligible: radiant heat | 1 | 41 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.23, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time‐to‐healing (survival analysis) Show forest plot | 5 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Advanced dressing versus basic dressing | 3 | Hazard Ratio (Fixed, 95% CI) | 1.57 [0.97, 2.55] | |
| 1.2 Protease‐modulating dressing versus advanced dressing | 1 | Hazard Ratio (Fixed, 95% CI) | 1.34 [0.67, 2.65] | |
| 1.3 Advanced dressings versus collagenase ointment | 1 | Hazard Ratio (Fixed, 95% CI) | 0.27 [0.11, 0.67] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intervention 1 vs intervention 2 Show forest plot | 4 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Sugar + povidone iodine vs lysosyme | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Enamel matrix protein vs propylene glycol alginate | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Honey vs ethoxy‐diaminoacridine +nitrofurazone dressings | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Resin salve vs hydrocolloid or hydrocolloid silver dressing | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |

