Pemeriksaan oksimetri nadi untuk kecacatan jantung kongenital yang kritikal
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient sampling | Prospective multicenter study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Switzerland Setting: 4 hospitals in Zurich: 3 maternity hospitals and the Division of Cardiology of the University Children’s Hospital Study period: 1‐year period (from May 13, 2003, to May 12, 2004) Inclusion criteria: all newborn infants from 35 weeks' gestation Exclusion criteria: Premature infants below 35 weeks' gestation Infants with a respiratory disorder Live birth cohort, n = 3663 (401 infants excluded according to exclusion criteria) N screened: 3262 (89%) (1764 at the University Hospital, 1011 at the Zollikerberg Hospital, 487 at the Triemli Hospital) Gestational age: median: 39 weeks (range 35 to 42) Prevalence of CCHD: 3.7 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with the Nellcor NPB‐40 handheld pulse oximeter and the Nellcor Max‐N Oximax adhesive sensors. Screening protocol: Site of testing: right or left foot Test timing: within 24 hours (in 48 cases [1%], pulse oximetry was performed too early in part because of immediate postnatal transfer to the cardiology unit or because patients were discharged before 6 hours of age; in 255 cases (8%), pulse oximetry was performed after 12 hours; 2959 measurements [91%] were performed at between 6 and 12 hours) Oxygen saturation: functional Threshold: < 95% Measurement did not exceed 2 minutes. If saturation was below 95%, a senior house officer performed a full clinical examination of the newborn. If the infant had saturation below 90% or any signs suggestive of a CHD, echocardiography was performed immediately. In the case of an asymptomatic newborn with borderline values (90% to 94%), a second measurement was performed 4 to 6 hours later. | ||
| Target condition and reference standard(s) | Target condition: Congenital heart disease was defined as the presence of a gross structural abnormality of the heart or intrathoracic great vessels that is actually or potentially of functional significance. Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography (complete M‐mode, 2‐dimensional, and Doppler echocardiograms were performed either at the University Hospital with an Acuson 128XP/10 [Siemens, Erlangen, Germany] with a 7.5‐mHz transducer, or at the University Children’s Hospital with a Sonos 5500 [Philips, Amsterdam, Netherlands], both equipped with all Doppler modalities) Reference standard used for negative pulse oximetry results: not stated | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analysed: 3262) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Saudi Arabia Setting: neonatology department of King Abdel‐Aziz Specialist Hospital Study period: 6‐month period (January 2004 to July 2004) Inclusion criteria: asymptomatic newborns Exclusion criteria: Those admitted to the neonatal intensive care unit at birth N screened: 5211 Prevalence of CCHD: 3.7 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a Digioxi PO 920 pulse oximeter (Digicare Biomedical Technology, West Palm Beach, FL, USA). Screening protocol: Site of testing: right upper and lower limbs Test timing: longer than 24 hours. Average age at screening was 31.7 hours. Oxygen saturation: fractional Threshold: ≤ 94% "Any infant who had an oxygen saturation < 90% from either limb was examined by echocardiography. Saturations between 90% and 94% were verified by three readings; if they persisted in this range, echocardiography was also done." | ||
| Target condition and reference standard(s) | Target condition: congenital heart disease Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: cardiology service of the only pediatric hospital in the region to identify patients who had received a diagnosis of CHD after discharge from the well‐baby nursery | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n = 5211) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Retrospective observational study | ||
| Patient characteristics and setting | Country: Australia Setting: Royal Prince Alfred Hospital (tertiary maternity hospital delivering over 5000 newborns a year) Study period: 42‐month period (from April 2008 to December 2011) Inclusion criteria: all newborns (routine neonatal examination) Exclusion criteria: not stated Live birth cohort, n = 19,765 N screened: 18,801 (95.1%) (648 had been admitted to the nursery and did not qualify for screening, 316 missed) Prevalence of CCHD: 0.2 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a Masimo Radical 5 portable oximeter (Masimo Corporation, Irvine, CA, USA) with a reusable probe with disposable Coban tape (1‐inch self‐adherent wrap, manufactured by 3M, Australia). Screening protocol: Site of testing: post‐ductal (foot) Test timing: longer than 24 hours (between 24 and 72 hours of life) Oxygen saturation: functional Threshold: < 95% "If the post‐ductal saturation was 95% or more, the result was assigned as a pass. Readings between 90% and 95% led to a repeat saturation measurement in the next 1–2 hours. If the post‐ductal saturation remained below 95% on repeat testing, the newborn was referred for review and examination by a senior neonatal paediatrician. If the saturation was less than 90%, at any time, the newborn was referred for review by a senior neonatal paediatrician without waiting for a repeat test." | ||
| Target condition and reference standard(s) | Target condition: congenital heart disease Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: database of the Heart Centre for Children at Children’s Hospital at Westmead for any newborns undergoing cardiac surgery or catheter intervention in the first year of life | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: 316 missed (not performed by the resident, performed but not recorded, screening not completed before early discharge or owing to compliance issues when the new protocol was originally introduced) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Unclear | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Sweden Setting: 5 maternity units in West Götaland Study period: July 2004 to March 31, 2007 Inclusion criteria: all newborn infants Exclusion criteria: Admitted to neonatal special care units Live birth cohort, n = 46,963 (7064 excluded owing to rolling start of study or admission to neonatal intensive care) Eligible, n = 39,899 (Östran n = 13,455, Mölndaln n = 8953, Trollhättan n = 7019, Borås n = 5382, Skövde n = 5090) N screened: 38,429 (flowchart page 4) Prevalence of CCHD: 0.7 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a pulse oximeter Radical SET, version 4 (average time set on 8 seconds) with multisite LNOP YI sensors, Masimo, Irvine, CA, USA Screening protocol: Site of testing: pre‐ductal (palm of right hand) and post‐ductal (either foot) Test timing: longer than 24 hours Oxygen saturation: functional Threshold: < 95% "When both pre‐ductal and post‐ductal oxygen saturation was < 95% or the difference between the two measurements was > 3% (≥ 2 standard deviations of interobserver measurement variability) the baby was provisionally considered to be screening positive, but a repeat measurement was performed. Babies with three repeated positive measurements were supposed to have an echocardiogram performed the same day according to the study protocol, but with some babies scheduled for early discharge only two pulse oximetry screenings were managed before the discharge examination was performed. Babies were considered screening positive until a measurement not fulfilling screening positive criteria was obtained. If saturation ≤ 90% the newborn was referred for an echocardiogram the same day." | ||
| Target condition and reference standard(s) | Target condition: congenital heart disease Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: mortality data of the National Board of Forensic Medicine (information on all deaths due to undiagnosed cardiovascular malformations in children younger than 1 year in Sweden born during the study) | ||
| Flow and timing | Duration of follow‐up: not stated Inconclusive results: 73 (results for only 1 site [34], oxygen saturation < 90% but not optimal [39]) N analyzed: 39,821 | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | No | ||
| Was there at least 28 days of appropriate follow up? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicenter study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: United Kingdom Setting: 6 obstetrical units in the West Midlands Study period: February 2008 to January 2009 Inclusion criteria: asymptomatic newborns (gestation > 34 weeks) Study includes newborns in whom congenital heart defects were suspected antenatally after midtrimester ultrasonography. Exclusion criteria: Newborns with symptoms suggestive of cardiac disease that were detected before screening Livebirth cohort, n = 26,513 (3768 missed, 2005 declined, 685 ineligible) N screened: 20,055 (75.6%) Prevalence of CCHD: 1.2 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with the Radical‐7 pulse oximeter with reusable probe LNOP Y1 (Masimo, Irvine, CA, USA). Screening protocol: Site of testing: pre‐ductal (right hand) and post‐ductal (either foot in non‐specified order) Test timing: within 24 hours (median age at testing of 12.4 hours for the full cohort) Oxygen saturation: functional Threshold: < 95% "A saturation of less than 95% in either limb or a difference of more than 2% between the limb saturation readings (if both were ≥ 95%) was judged to be abnormal. Clinical examination was expedited if an abnormal test result was obtained. If this examination was unremarkable, oximetry was repeated 1to 2 hours later. If abnormalities of the cardiovascular system were detected with expedited examination, or saturations remained abnormal during a second test, the newborn were classified as test positive." | ||
| Target condition and reference standard(s) | Target condition: critical congenital heart defects (ie, death or requiring invasive intervention before 28 days) All infants with hypoplastic left heart, pulmonary atresia with intact ventricular septum, simple transposition of the great arteries, or interruption of the aortic arch All infants dying or requiring surgery within the first 28 days of life with coarctation of the aorta, aortic valve stenosis, pulmonary valve stenosis, tetralogy of Fallot, pulmonary atresia with ventricular septal defect, or total anomalous pulmonary venous connection Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical follow‐up, use of cardiology databases and congenital anomaly registries | ||
| Flow and timing | Duration of follow‐up: up to 12 months Loss to follow‐up: none (n analyzed: 20,055) | ||
| Comparative | |||
| Notes | Funding: National Institute for Health Research Health Technology Assessment (NIHR HTA) program (project number 06/06/03) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Cross‐sectional prospective study | ||
| Patient characteristics and setting | Country: Mexico Setting: Department of Neonatology, UMAE 48‐Instituto Mexicano del Seguro Social (IMSS), León, Gto Study period: July 2010 to April 2011 Inclusion criteria: newborns > 6 hours of age in whom no CHD was suspected; only tested consecutive newborns who were available during the working hours of investigators Exclusion criteria: newborns with lung disease no informed consent N screened: 1037 Gestational age: mean (SD): 38.9 (1.1) weeks Prevalence of CCHD: 1.9 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a Rad‐5 handheld pulse oximeter with multisite sensor. Screening protocol: Site of testing: left lower extremity (post‐ductal) Test timing: within 24 hours (mean age at pulse oximetry screening 12 hours ‐ range 6 to 48 hours) Oxygen saturation: functional Threshold: < 95% Measurement was taking during 2 minutes until the reading remained the same in 2 determinations. | ||
| Target condition and reference standard(s) | Target condition: critical congenital heart disease (no definition included) Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical records of all follow‐up at 6 months | ||
| Flow and timing | Duration of follow‐up: 6 months Loss to follow‐up: none (n analyzed: 1037) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Retrospective observational study | ||
| Patient characteristics and setting | Country: United Kingdom Setting: neonatal intensive care unit, Northwick Park Hospital, Harrow, Middlesex (level‐2 neonatal unit without on‐site access to pediatric echocardiography) Study period: September 1, 2011, to August 31, 2013 Inclusion criteria: all newborns admitted to the neonatal unit during the study period Exclusion criteria: Antenatal diagnosis of CCHD Admitted to neonatal intensive care unit after birth Live birth cohort, n = 11,233 (973 neonatal unit admissions) N screened: 10,260 Gestational age: not stated Prevalence of CCHD: 0.2 per 1000 live births | ||
| Index tests | Type of pulse oximeter not stated Screening protocol: Site of testing: both pre‐ductal and post‐ductal Test timing: within 24 hours Oxygen saturation: not stated Threshold: ≤ 95% (or pre‐ductal and post‐ductal difference > 3%) | ||
| Target condition and reference standard(s) | Target condition: critical congenital heart disease defined as CHD resulting in death or requiring surgical intervention or therapeutic catheterization within the first 28 days of life Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: National Congenital Heart Disease Audit | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 10,260) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Retrospective observational study | ||
| Patient characteristics and setting | Country: USA Setting: 4 Yale–New Haven Health System hospitals in Connecticut Study period: January 1 and December 31, 2014 Inclusion criteria: all newborns delivered during the study period Exclusion criteria: Live‐born infants who died before CCHD screening Antenatal screening Live birth cohort, n = 10,589 (171 [1.6%] underwent an echocardiogram before screening, and 98 [0.9%] were not screened; 96 were missed in error and parents refused in 2 instances) N screened: 10,320 Gestational age: 9584 (90.5%) were term (> 37 weeks) Prevalence of CCHD: 0 per 1000 live births | ||
| Index tests | Type of pulse oximeter not stated Screening protocol: Site of testing: both pre‐ductal and post‐ductal Test timing: longer than 24 hours Oxygen saturation: not stated Threshold: < 95% | ||
| Target condition and reference standard(s) | Target condition: critical congenital heart disease defined as structural defect associated with hypoxemia in the newborn period that requires surgical intervention before 1 year and, without intervention, can lead to significant morbidity and mortality Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiogram Reference standard used for negative pulse oximetry results: follow‐up | ||
| Flow and timing | Of 10,316 infants with negative pulse oximetry at the time of birth, possible to review postdischarge records of only 52.1% (n = 5367) | ||
| Comparative | |||
| Notes | Study was supported in part by a National Heart, Lung, and Blood Institute Medical Student Research Fellowship, National Institutes of Health (award T35HL007649; to Ms Klausner), and by grant UL1 TR001863 from the National Center for Advancing Translational Science at the National Institutes of Health and the NIH Roadmap for Medical Research. Funded by the National Institutes of Health (NIH) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | No | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: USA Setting: well infant nurseries at 2 hospitals (New York) Study period: from May 1998 to November 1999 Inclusion criteria: all asymptomatic newborns Exclusion criteria: Admitted to neonatal special care units (infants who did manifest any of these clinical findings: cyanosis, tachypnea [respiratory rate: 60/min], grunting, flaring, retraction, murmur, active precordium, or diminished pulses) N screened: 11,281 (8642 at hospital A, 2639 at hospital B) Prevalence of CCHD: 0.4 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with an Ohmeda Medical pulse oximeter. Screening protocol: Site of testing: post‐ductal Test timing: longer than 24 hours. Timing of oximetry determination was linked to state‐mandated metabolic screening (24 hours of age) at hospital A. At hospital B, screening was performed immediately before discharge as part of a series of discharge procedures (average length of stay for vaginal delivery: 56.9 hours; for cesarean section: 103.2 hours) Oxygen saturation: functional Threshold: ≤ 95% "single determination of post‐ductal saturation" | ||
| Target condition and reference standard(s) | Target condition: critical congenital cardiovascular malformation (CCVM) defined as a lesion that would likely require surgical correction during the first month of life Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical follow‐up and New York State Congenital Malformations Registry (CMR) | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed = 11,281) | ||
| Comparative | |||
| Notes | Study was supported by a cooperative agreement from the Centers for Disease Control and Prevention. Oximeters were provided by Ohmeda Medical. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicenter study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Norway Setting: 14 hospitals with obstetrical departments and pediatric services and neonatal special or intensive care units (50% of all deliveries in Norway) Study period: 1‐year period (2005 to 2006) Inclusion criteria: healthy newborns Exclusion criteria: Prenatal diagnosis Live birth cohort, n = 57,959 (not screened: 7951 [14%]; 224 of 7951 newborns [3%] had CHD) N screened: 50,008 (86%) Prevalence of CCHD: 0.7 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a pulse oximeter type RAD‐5v (Masimo Corporation, Irvine, CA) with a multisite reusable sensor (LNOP YI). Screening protocol: Site of testing: post‐ductal (foot) Test timing: within 24 hours (first day) Oxygen saturation: functional Threshold: < 95% "The probe was attached for at least 2 minutes, until a stable value was obtained. Retest if the result of pulse‐ox was < 95%." | ||
| Target condition and reference standard(s) | Target condition: congenital heart defects Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical follow‐up | ||
| Flow and timing | Duration of follow‐up: 6 months after the last infants were born Loss to follow‐up: none (n analyzed: 50,008) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective observational study | ||
| Patient characteristics and setting | Country: United Kingdom Setting: Tertiary Neonatal Unit from the Royal Gwent Hospital, Newport (3700 deliveries a year) Study period: 2 years (from January 2007 to December 2009) Inclusion criteria: all newborns at 35 weeks' gestation and above who were admitted to the postnatal ward Exclusion criteria: Admitted to the neonatal intensive care unit Antenatal diagnosis of CHD Births on weekends and holidays Live birth cohort, n = 9613 N screened: 6329 (65.8%) Gestational age of newborn infants included, range 35 to 42 weeks Prevalence of CCHD: 1.3 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a Nellcor NPB 40 pulse‐oximeter (Pleasanton, CA) and a reusable OXI‐A/N saturation probe. Screening protocol: Site of testing: post‐ductal (foot) Test timing: longer than 24 hours (all newborns were greater than 6 hours of age at the time of examination) Oxygen saturation: functional Threshold: < 95% "Newborns with saturation readings < 95% had a repeat reading taken on the other leg after thirty minutes and if still < 95%, a further repeat reading after one hour. If the reading remained < 95%, it was considered abnormal." | ||
| Target condition and reference standard(s) | Target condition: CCHD Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: regional pediatric cardiology database and local death records | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 6369) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Retrospective observational study | ||
| Patient characteristics and setting | Country: Turkey Setting: Bursa Sevket Yilmaz Training and Research Hospital Study period: between January 2014 and December 2014 Inclusion criteria: asymptomatic newborns Exclusion criteria: Referred within first 24 hours of life or admitted to neonatal intensive care unit Perinatal CCHD Live birth cohort, n = 10,200 (excluded: hospitalized = 1100, referred = 890, perinatal CCHD = 2) N screened: 8208 Gestational age: not stated Prevalence of CCHD: 1 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a Nellcor pulse oximeter. Screening protocol: Site of testing: both pre‐ductal and post‐ductal Test timing: longer than 24 hours Oxygen saturation: functional Threshold: Screening test was considered positive in newborns whose saturation with pulse oximetry was less than or equal to 95% and/or who had a difference < 3% between right lower and right extremities. | ||
| Target condition and reference standard(s) | Target condition: CCHD defined as congenital heart disease requiring catheter‐based or surgical intervention within the first month of life, or causing high mortality and morbidity in the first weeks of life Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: echocardiography | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 8208) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: United Kingdom Setting: Sunderland Royal Hospital Study period: from April 1, 1999, to March 31, 2001 Inclusion criteria: asymptomatic newborn without signs of respiratory or cardiac illness Exclusion criteria: Admitted to neonatal care units Live birth cohort, n = 6166 (540 excluded: 447 neonatal unit, 5 no consent, 88 newborns missed) N screened: 5626 (91%) Prevalence of CCHD: 1.6 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a radiometer Oxi machine. Screening protocol: Site of testing: post‐ductal (foot) Test timing: within 24 hours (after the age of 2 hours and before discharge) Oxygen saturation: fractional Threshold: < 95% "Any baby who did not achieve a post‐ductal fractional saturation of at least 95% was clinically examined by the midwife. If no suspicions were raised by the examination, a second saturation measurement was performed an hour or two later. If either the examination or the repeat saturation measurement were not satisfactory, an echocardiogram was performed." | ||
| Target condition and reference standard(s) | Target condition: congenital cardiac malformation Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: Regional Perinatal Mortality Survey and Northern Congenital Abnormality Survey & Diagnostic Database at Freeman Hospital (referral hospital) | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 5626) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicenter study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Germany Setting: primary, secondary, and tertiary care (34 neonatal/obstetrical departments in Saxony) Study period: 2‐year period (from July 2006 to June 2008) Inclusion criteria: full‐term and post‐term neonates (gestational age ≥ 37 weeks) Normal routine clinical examination Informed parental consent Exclusion criteria: Antenatal diagnosis/suspicion of congenital heart disease Livebirth cohort, n = 48,348 (excluded: 6108 newborns [72 clinical or prenatal diagnosis of CCHD; 6036 other]) N eligible for pulse oximetry screening: 42,240 (n = 727 [91%] did not receive pulse oximetry screening, mainly because of early discharge after birth) N screened: 41,445 (85.7%) Prevalence of CCHD: 0.4 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a great variety of devices (no further information). Screening protocol: Site of testing: post‐ductal (foot) Test timing: longer than 24 hours Oxygen saturation: functional Threshold: ≤ 95% "The study protocol included repeated SpO2 measurements after 1 hour if the initial value was < 96%." | ||
| Target condition and reference standard(s) | Target condition: CCHD Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: Saxonian perinatal and neonatal registries | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: 3 for violation of study protocol (n analyzed: 41,442) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | No | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Italy Setting: Perrino Hospital (referral center of the area) Study period: from May 1, 2000, to November 30, 2004 Inclusion criteria: term newborns with uncomplicated neonatal courses Exclusion criteria: Infants who were symptomatic (ie, heart murmur, severe cyanosis) Prenatal diagnosis of critical congenital cardiovascular malformation N screened: 5292 Prevalence of CCHD: 0.6 per 1000 live births | ||
| Index tests | Type of pulse oximeter not stated Screening protocol: Site of testing: post‐ductal (foot) Test timing: longer than 24 hours Oxigen saturation: functional Threshold: ≤ 95% "Post‐ductal saturation (SpO2) and the monitoring of oxymetry values were evaluated for two minutes in each newborn." | ||
| Target condition and reference standard(s) | Target condition: CCHD defined as lesions requiring surgical correction or interventional procedures during the first month of life Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical follow‐up | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 5292) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: USA Setting: large public hospital (Parkland Health and Hospital System [PHHS]) in Dallas, TX, which serves a primarily indigent Hispanic population) Study period: from March 1, 2006, to February 28, 2007 Inclusion criteria: Term and late preterm neonates who did not have major malformations Gestational age criteria: ≥ 35 weeks Birth weight: ≥ 2100 grams Exclusion criteria: Admitted to neonatal intensive care unit (NICU) Respiratory distress and/or cyanosis before 4 hours of age Live birth cohort, n = 16,432 (excluded: 66 [0.4%]; 11 had CHD) N screened: 15,233 (99.6%) Prevalence of CCHD: 0.1 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a Nellcor N‐395 (Boulder, CO) pulse oximeter. Screening protocol: Site of testing: post‐ductal (foot) Test timing: within 24 hours (4 hours after delivery) Oxygen saturation: functional Threshold: < 96% "On the day of discharge, the 4‐hour pulse oximetry result was made available to the provider. A pulse oximetry result of 96% was considered normal and was not repeated. For neonates who failed to achieve 96% on the 4‐hour screen, a follow‐up pulse oximetry reading was performed by either the nursing staff or the medical provider by using the procedure described above. When the discharge pulse oximetry reading was < 96%, echocardiography was performed." | ||
| Target condition and reference standard(s) | Target condition: CCHD including cyanotic defects such as tetralogy of Fallot, pulmonary atresia, truncus arteriosus, transposition of the great vessels, total anomalous pulmonary venous return, and tricuspid atresia, as well as left‐sided obstructive lesions, including coarctation of the aorta, critical aortic stenosis, interrupted aortic arch, and hypoplastic left heart syndrome Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical follow‐up | ||
| Flow and timing | Duration of follow‐up: not stated Follow‐up information not available for 19 (0.1%) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | No | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | No | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Retrospective observational study | ||
| Patient characteristics and setting | Country: United Kingdom Setting: level 3 Neonatal Unit of Birmingham Women’s Hospital Study period: from April 1, 2010, to July 31, 2013 Inclusion criteria: screening is part of routine practice Exclusion criteria: Antenatal diagnosis of CCHD N screened: 25,859 Prevalence of CCHD: 0.6 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a handheld oximeter with a reusable probe. Screening protocol: Site of testing: post‐ductal (foot) and pre‐ductal (right hand) Test timing: within 24 hours Oxygen saturation: functional Threshold: < 95% "A saturation result of < 95% in either limb or a difference of > 2% between the readings (if both were ≥ 95%) was considered abnormal. Following an abnormal first test, an initial assessment was performed. If this was unremarkable, oximetry was repeated 1to 2 hours later. If the saturations remained abnormal on second testing, or if there were concerns following the initial assessment, newborns were classified as test positive and were admitted to the neonatal unit for further assessment." | ||
| Target condition and reference standard(s) | Target condition: CCHD Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: the Regional Cardiac Centre database at Birmingham Children’s Hospital, the Regional Congenital Anomaly Register, and the local mortality database | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 25,859) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective multicenter study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Poland Setting: 51 neonatal units in the Mazovian province of Poland as part of the POLKARD 2006 to 2008 program Study period: 1 year (from January 16, 2007, to January 31, 2008) Inclusion criteria: Protocol B: asymptomatic newborns at ≥ 34 weeks' gestation Exclusion criteria: Circulatory symptoms or coexisting diseases Prenatal diagnosis Live birth cohort, n = 55,944 (in 2611 newborns, the test could not be performed owing to technical problems [equipment failure, absence of trained staff due to holiday], 340 no consent, 1295 newborns with symptoms) N screened: 51,698 (92.4%) Prevalence of CCHD: 0.4 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with Novametrix, Nellcor, and Masimo pulse oximeters. Screening protocol: Site of testing: post‐ductal (foot) Test timing: within 24 hours (between the 2nd and 24th hours of life) Oxygen saturation: functional Threshold: < 95% "The measurement was carried out by specially trained nurses for 2 to 3 min on the infant’s lower extremity between the 2nd and 24th hour of life after normalisation of the plethysmographic curve of the pulse oximeter." | ||
| Target condition and reference standard(s) | Target condition: CCHD defined as requiring an interventional procedure or cardiac surgery in the first month of life Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical follow‐up or based on data from the Mazovian Centre of Public Health | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 51,698) | ||
| Comparative | |||
| Notes | Funding: Ministry of Health in Poland | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective observational study | ||
| Patient characteristics and setting | Country: South Africa Setting: Mowbray Maternity Hospital (MMH), a busy level‐2 maternity hospital in the Western Cape Province, SA Study period: May 19 to September 19, 2014 Inclusion criteria: All neonates > 6 hours old with no clinical signs of cardiovascular disease were eligible. Exclusion criteria: "unwell" infants, those < 6 hours old, those born to mothers < 14 years of age or unable to give informed verbal consent (owing to illness, illiteracy, or language barriers); all infants with a prenatal diagnosis of CHD or any signs of CHD, including a heart murmur (≥ 3/6) or significant dysmorphic features Livebirth cohort, n = 2256 (1220 mothers not approached) N screened: 1001 (44%) Prevalence of CCHD: 1 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with Nellcor pulse oximeters. Screening protocol: Site of testing: right hand and any foot Test timing: longer than 24 hours Oxygen saturation: functional Threshold: < 95% | ||
| Target condition and reference standard(s) | Target condition: CCHD, which leads to death or needs surgical intervention before 28 days Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: not stated | ||
| Flow and timing | Duration of follow‐up: no physical follow‐up Loss to follow‐up: none (n analyzed: 1001) | ||
| Comparative | |||
| Notes | Study was funded in part by the School of Child and Adolescent Health Research Committee, Department of Paediatrics, Red Cross War Memorial Children’s Hospital and University of Cape Town. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | No | ||
| Did all patients receive a reference standard? | No | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Prospective multicenter study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: China Setting: 18 hospitals Study period: from August 1, 2011, to November 30, 2012 Inclusion criteria: all consecutive newborns (irrespective of gestational age or neonatal intensive care unit status) Exclusion criteria: Prenatally diagnosed major CHD Livebirth cohort, n = 130,282 (not screened: 9575 [3571 incomplete screening data, 1450 lack of consent, 2496 transfer to superior hospital, 27 prenatally diagnosed major CHD, 2031 symptomatic newborns]) N screened: 120,707 (92.7%) Prevalence of CCHD: 1.2 per 1000 live births | ||
| Index tests | Pulse oximetry was performed with a RAD‐5V / Multisite reusable sensor (LNOP YI, Masimo). Screening protocol: Site of testing: pre‐ductal (right hand) and post‐ductal (foot) Test timing: longer than 24 hours Oxygen saturation: functional Threshold: < 95% "The clinician repeated pulse oximetry testing 4 hours later if the first pulse oximeter oxygen saturation measurement was between 90% and 95%. Screening was deemed positive if an SpO2 of less than 95% was obtained both on the right hand and on either foot on two measures, separated by 4 hours; a difference between the two extremities was more than 3% on two measures, separated by 4 hours; or any measure was less than 90%." | ||
| Target condition and reference standard(s) | Target condition: CCHD Reference standard(s): Reference standard used for positive pulse oximetry results: echocardiography Reference standard used for negative pulse oximetry results: clinical follow‐up and parents’ feedback | ||
| Flow and timing | Duration of follow‐up: clinical examination at 6 weeks of age at the hospital "The Children’s Hospital of Fudan University provided help with further confirmation of diagnosis for all affected babies from the participating hospitals. All cases of congenital heart disease were followed up by telephone review at least 1 year of age." Loss to follow‐up: none (n analyzed: 120,707) | ||
| Comparative | |||
| Notes | Funding: Key Clinical Research Project sponsored by Ministry of Health, Shanghai Public Health Three‐Year Action Plan, sponsored by Shanghai Municipal Government, and National Basic Research Project of China. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Prospective study with consecutive enrollment of participants | ||
| Patient characteristics and setting | Country: Italy Setting: Agostino Gemelli General Hospital Study period: 2 years (from 2009 to 2010) Inclusion criteria: all newborns admitted to the nursery. These newborns by definition were considered healthy or were under observation for maternal disease, mild prematurity, or low birth weight. Exclusion criteria: Newborns with syndrome Total number of newborn infants included: N screened: 5750 Prevalence of CCHD: 0.2 per 1000 births | ||
| Index tests | Pulse oximetry was performed with an Ohmeda 3900 pulse oximeter. Screening protocol: Site of testing: post‐ductal (foot) Test timing: longer than 24 hours Oxygen saturation: functional Threshold: < 95% "The measurement was performed by a professional nurse in all newborns admitted to the nursery, between the 48th and 72nd hours of life, before discharge. The probe detector was placed on one of the two legs, making sure that the newborn was quiet and with warm ends. The measurement was performed in presence of stable, continuous and free of artefacts pulse wave, for at least 3 minutes. In case of positive screening, a second check was carried out by medical staff after 15 to 30 min." | ||
| Target condition and reference standard(s) | Target condition: CCHD defined as severe cardiac alterations that require cardiac surgery during the first year of life Reference standard(s): Reference standard used for positive pulse oximetry results: electrocardiographic and echocardiography (echocardiograph ‘‘HP Sonos 4500, Agilent Technologies’’ [Andover, MA], a multifrequency probe [5 to 12 MHz], suitable for study of the neonatal heart. Evaluation was performed by 2‐dimensional analysis [2‐D], analysis of M‐mode, and Doppler ultrasound). Reference standard used for negative pulse oximetry results: not stated | ||
| Flow and timing | Duration of follow‐up: not stated Loss to follow‐up: none (n analyzed: 5751) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Pulse oximetry | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Were all patients included in the analysis? | Yes | ||
| Was there at least 28 days of appropriate follow up? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
CCHD: critical congenital heart defect.
CHD: congenital heart defect.
NICU: neonatal intensive care unit.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study includes only information about positive results. Investigators did not follow infants who passed the screen once they left the hospital. | |
| Health Technology Assessment report on already included study | |
| Case‐control design | |
| Study includes only information about positive results. | |
| Different population (not asymptomatic newborns) | |
| Different outcome (not accuracy) | |
| Out‐of‐hospital births | |
| Different outcome (not accuracy) | |
| Study includes only information about positive results. | |
| Out‐of‐hospital births | |
| Study did not include enough information for construction of a 2 × 2 table. Study includes data contained in Richmond 2002 (study included). | |
| Study provides a partial 2 × 2 diagnostic table from which estimation of sensitivity was not possible. | |
| Different outcome (not accuracy) | |
| Different outcome (not accuracy) | |
| Preliminary study | |
| Study includes only information about positive results. | |
| Journal club | |
| Different population (not asymptomatic newborns) | |
| Different index test (combined pulse oximetry and perfusion index) | |
| Different outcome (not accuracy) | |
| Study includes only information about positive results. Definition of test positive was not given. | |
| No ability to determine CCHD outcomes | |
| Study includes only information about positive results. | |
| Study provides a partial 2 × 2 diagnostic table. No ability to determine CCHD outcomes | |
| Case report | |
| Different outcome (not accuracy) |
CCHD: critical congenital heart defect.
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 All studies Show forest plot | 21 | 457202 |
| Test 1  All studies. | ||
| 2 Primary analysis (threshold < 95% or ≤ 95%) Show forest plot | 19 | 436758 |
| Test 2  Primary analysis (threshold < 95% or ≤ 95%). | ||
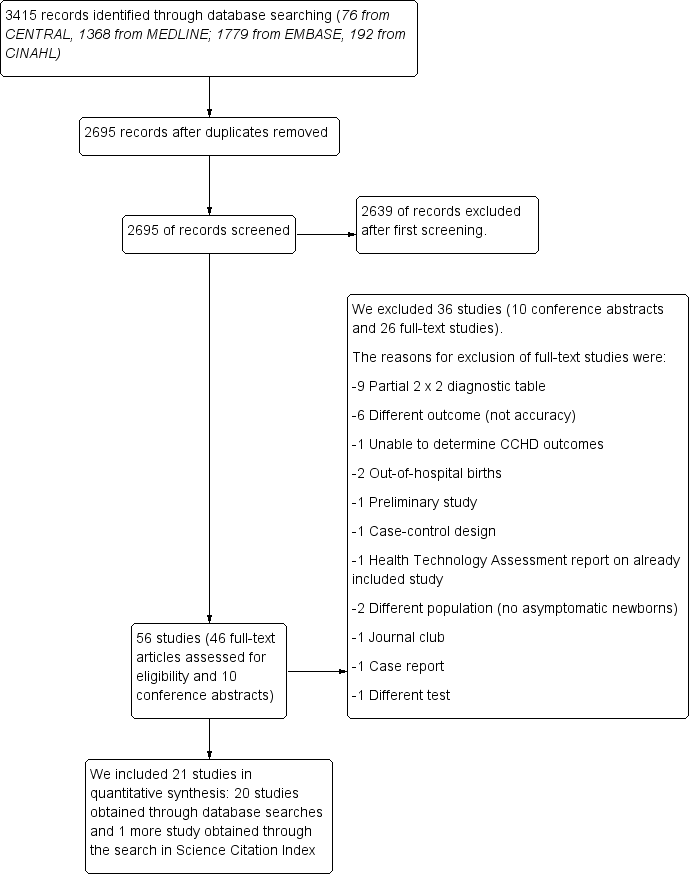
Flow of studies through the screening process. CCHD: critical congenital heart defect.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Forest plot of sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (blue square) and its 95% confidence interval (black horizontal line). Studies are ordered by ascending specificity.

Summary ROC plot for pulse oximetry using a threshold lower than or lower than or equal to 95% (n = 19 studies). The solid circle corresponds to the summary estimate of sensitivity and specificity, and is shown with a 95% prediction region (dashed line).

Primary analysis (threshold < 95% or ≤ 95%).
| Should pulse oximetry be used to diagnose CCHD in asymptomatic newborns? | ||||||||
| Patient or population: asymptomatic newborns at the time of pulse oximetry screening | ||||||||
| Setting: hospital births | ||||||||
| Index test: pulse oximetry | ||||||||
| Reference test: Reference standards were both diagnostic echocardiography (echocardiogram) and clinical follow‐up in the first 28 days of life, including postmortem findings and mortality and congenital anomaly databases to identify false‐negative patients. | ||||||||
| Studies: We included prospective or retrospective cohorts and cross‐sectional studies. We excluded case reports and studies of case‐control design. | ||||||||
| Threshold | Summary accuracy (95% CI) | Number of participants (diseased /non‐diseased) Number of studies | Prevalence median (range) | Implications (in a cohort of 10,000 newborns tested [95% CI]) | Certainty of the evidence (GRADE) | |||
| Prevalence 0.6 per 1000 | Prevalence 0.1 per 1000 | Prevalence 3.7 per 1000 | ||||||
| 95% (less than or less than or equal to) | Sensitivity 76.3% (69.5 to 82.0) Specificity 99.9% (99.7 to 99.9) | 436,758 (345/436,413) 19 studies | 0.6 per 1000 (0.1 to 3.7) | True positives (newborns with CCHD) | 5 (4 to 5) | 1 (1 to 1) | 28 (26 to 30) | LOW* ⊕⊕⊝⊝ |
| False negatives (newborns incorrectly classified as not having CCHD) | 1 (1 to 2) | 0 (0 to 0) | 9 (7 to 11) | |||||
| True negatives (newborns without CCHD) | 9980 (9966 to 9987) | 9985 (9971 to 9992) | 9949 (9935 to 9956) | HIGH ⊕⊕⊕⊕ | ||||
| False positives (newborns incorrectly classified as having CCHD) | 14 (7 to 28) | 14 (7 to 28) | 14 (7 to 28) | |||||
| CCHD: critical congenital heart defect; CI: confidence interval. Sensitivity: *We have downgraded certainty of the evidence from high to low because the low number of CCHD cases included in the review (serious imprecision) and secondly, there was a serious risk of differential verification bias (ie, diagnosis was established by echocardiography in test positive cases however test negatives were usually confirmed by clinical follow‐up or by accessing congenital malformation registries and mortality databases)." | ||||||||
| Certainty of the evidence (Balshem 2011) | ||||||||
| Study | Population | Index test | Reference standard(s) | |||||
| Antenatal diagnosis of CHD | Pulse oximeter | Limb | Test timing | Oxygen saturation | Threshold | Positive pulse oximetry | Negative pulse oximetry | |
| Arlettaz 2006 | included | Nellcor NPB‐40 | post‐ductal | within 24 hours | functional | < 95% | echocardiography | NA |
| Bakr 2005 | excluded | Digioxi PO 920 | pre‐ductal and post‐ductal | longer than 24 hours | fractional | ≤ 94% | echocardiography | cardiology database |
| Bhola 2014 | included | Masimo Radical 5 | post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | cardiology database |
| De‐Wahl 2009 | excluded | Radical SET v4 | pre‐ductal and post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | mortality data |
| Ewer 2011 | included | Radical‐7 | pre‐ductal and post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up, cardiology database & congenital registry |
| Gomez‐Rodriguez 2015 | excluded | Radical‐5 | post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up |
| Jones 2016 | excluded | NA | pre‐ductal and post‐ductal | within 24 hours | NA | ≤ 95% | echocardiography | National Congenital Heart Disease Audit |
| Klausner 2017 | excluded | NA | pre‐ductal and post‐ductal | longer than 24 hours | NA | < 95% | echocardiography | clinical follow‐up |
| Koppel 2003 | excluded | Ohmeda Medical | post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | clinical follow‐up & congenital registry |
| Meberg 2008 | excluded | RAD‐5v | post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up |
| Oakley 2015 | excluded | Nellcor NPB 40 | post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | cardiology database & mortality data |
| Ozalkaya 2016 | excluded | Nellcor | pre‐ductal and post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | echocardiography |
| Richmond 2002 | included | Oxi machine | post‐ductal | within 24 hours | fractional | < 95% | echocardiography | mortality data & congenital registry |
| Riede 2010 | excluded | NA | post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | congenital registry |
| Rosati 2005 | excluded | NA | post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | clinical follow‐up |
| Sendelbach 2008 | excluded | Nellcor N‐395 | post‐ductal | within 24 hours | functional | < 96% | echocardiography | clinical follow‐up |
| Singh 2014 | excluded | NA | pre‐ductal and post‐ductal | within 24 hours | functional | < 95% | echocardiography | mortality data & congenital registry & cardiology database |
| Turska 2012 | excluded | Novametrix, Nellcor & Masimo | post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up and Public Health registries |
| Van Niekerk 2016 | excluded | Nellcor | pre‐ductal and post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | NA |
| Zhao 2014 | excluded | RAD‐5V | pre‐ductal and post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | clinical follow‐up |
| Zuppa 2015 | excluded | Ohmeda 3900 | post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | NA |
| NA: not available | ||||||||
| N | Sensitivity (95% CI) | Relative sensitivity P value | False‐positive rate (FPR) (95% CI) | Relative FPR P value | |
| Antenatal diagnosis | |||||
| Included | 4 | 86.3% (71.8 to 94.0) | 0.071 | 0.46% (0.13 to 1.59) | 0.231 |
| Excluded | 15 | 74.1% (65.7 to 81.1) | 0.10% (0.05 to 0.21) | ||
| Test timing | |||||
| Longer than 24 hours | 11 | 73.6% (62.8 to 82.1) | 0.393 | 0.06% (0.03 to 0.13) | 0.027 |
| Within 24 hours | 8 | 79.5% (70.0 to 86.6) | 0.42% (0.20 to 0.89) | ||
| Limb | |||||
| Foot only | 11 | 81.2% (70.9 to 88.4) | 0.197 | 0.13% (0.05 to 0.31) | 0.718 |
| Foot and right hand | 8 | 71.2% (58.5 to 81.3) | 0.17% (0.06 to 0.46) | ||
| Risk of bias ("flow and timing") | |||||
| Unclear risk of bias | 9 | 77.8% (64.1 to 87.3) | 0.937 | 0.05% (0.02 to 0.12) | 0.016 |
| Low risk of bias | 10 | 77.3% (68.8 to 84.0) | 0.34% (0.17 to 0.66) |
| Test | No. of studies | No. of participants |
| 1 All studies Show forest plot | 21 | 457202 |
| 2 Primary analysis (threshold < 95% or ≤ 95%) Show forest plot | 19 | 436758 |


