Screening auf kritische angeborene Herzfehler mit Pulsoximetrie
Appendices
Appendix 1. Searches performed
| Date: March 2017 | Search strategy | Hits retrieved |
| Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) in the Cochrane Library | (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW) AND (Congenital Heart Defects OR Heart Valve Diseases OR tetralogy near fallot* OR cyanotic near heart OR congenital near heart OR congenital near cardiac OR aortic near coarctation OR valve near diseases OR hypoplastic near syndrome OR pulmonary near atresia OR interruption of the aortic arch OR valve near stenosis OR pulmonary near atresia) AND (oximetry OR pulse near oximetr* OR oxygen near saturation OR O2 near saturation) | 76 |
| MEDLINE via PubMed (1966 to current) | (Infant, Newborn[MeSH] OR neonate* OR infant* OR newborn)* AND (Heart Defects, Congenital[MeSH] OR Heart Valve Diseases[MeSH] OR tetralogy fallot* OR cyanotic heart OR congenital heart OR congenital cardiac OR aortic coarctation OR valve diseases OR hypoplastic syndrome OR pulmonary atresia OR interruption of the aortic arch OR valve stenosis OR pulmonary atresia) AND (oximetry[MeSH] OR oximetry OR pulse oximetr* OR oxygen saturation OR O2 saturation) | 1368 |
| Embase via Ovid (1980 to current) | (exp Infant OR exp Newborn OR neonat*.mp OR infant*.mp OR newborn*.mp) AND ((exp congenital heart malformation/) OR (exp valvular heart disease/) OR (tetralogy adj3 fallot*).mp OR (cyanotic adj3 heart).mp OR (congenital adj3 heart).mp OR (congenital adj3 cardiac).mp OR (aortic adj3 coarctation).mp OR (valve adj3 diseases).mp OR (hypoplastic adj3 syndrome).mp OR (pulmonary adj3 atresia).mp OR (interruption of the aortic arch).mp OR (valve adj3 stenosis).mp) AND (exp oximetry OR (pulse adj3 oximetr*).mp OR (oxygen adj3 saturation).mp OR (O2 adj3 saturation).mp) | 1779 |
| CINAHL (1982 to current) | TX (Infant, Newborn OR neonate* OR infant* OR newborn*) AND TX (Congenital Heart Defects OR Heart Valve Diseases OR tetralogy fallot* OR cyanotic heart OR congenital heart OR congenital cardiac OR aortic coarctation OR valve diseases OR hypoplastic syndrome OR pulmonary atresia OR interruption of the aortic arch OR valve stenosis OR pulmonary atresia) AND TX (oximetry OR pulse oximetr* OR oxygen saturation OR O2 saturation) | 192 |
| TOTAL before de‐duplication | 3415 | |
| TOTAL after de‐duplication | 2695 | |
Appendix 2. QUADAS 2
| Item | Criteria for assessment |
| Domain 1: Patient selection | |
| Describe methods of patient selection (prior testing, presentation, intended use of index test and setting). | |
| A. Risk of bias | |
| Was a consecutive or random sample of patients enrolled? | "Yes" if described enrolling a consecutive or random sample of newborns before discharge from hospital "No" if criteria for "yes" not achieved "Unclear" if the study did not describe the method of enrollment |
| Did the study avoid inappropriate exclusions? | "Yes" if exclusions were detailed and review authors reached consensus on the appropriateness of any exclusion "No" if inappropriate exclusions were reported (eg, if cases with antenatally diagnosed congenital heart disease were excluded) "Unclear" if insufficient information was provided |
| Could selection of patients have introduced bias? | A judgment of low, high, or unclear risk of bias was based on a balanced assessment of responses to the above signaling questions. |
| B. Concerns about applicability | |
| Is there concern that the included patients do not match the review question? | A judgment of low, high, or unclear concern about applicability was made on the basis of how closely the sample matches an asymptomatic newborn population screened for CCHD. |
| Domain 2: Index test | |
| Describe the index test and how it was conducted and interpreted. | |
| A. Risk of bias | |
| Were the index test results interpreted without knowledge of results of the reference standard? | "Yes" if pulse oximetry was conducted and interpreted before the echocardiogram or clear temporal pattern to the order of testing that precludes the need for formal blinding (eg, echocardiogram, clinical follow‐up, and inclusion in congenital anomaly registries are always posterior to index test) "No" if reference standard results were available to those who conducted or interpreted the pulse oximetry "Unclear" if insufficient information was provided |
| If a threshold was used, was it prespecified? | "Yes" if a threshold was prespecified "No" if trial authors selected a cutoff value based on analysis of collected data "Unclear" if insufficient information was provided |
| Could the conduct or interpretation of the index test have introduced bias? | A judgment of low, high, or unclear risk of bias was based on a balanced assessment of responses to the above signaling questions. |
| B. Concerns about applicability | |
| Is there concern that the index test, its conduct, or its interpretation differ from the review question? | A judgment of low, high, or unclear concern about applicability was based on a balanced assessment of information detailed under "index test" description. |
| Domain 3: Reference standard | |
| Describe the reference standard(s) and how they were conducted and interpreted. | |
| A. Risk of bias | |
| Is the reference standard likely to correctly classify the target condition? | "Yes" if the study used an appropriate reference standard (diagnostic echocardiography and clinical follow‐up in the first 28 days of life, including postmortem findings and mortality and congenital anomaly databases to identify false‐negative patients) "No" if the study did not use an appropriate reference standard "Unclear" if the reference standard used was not clearly specified |
| Were the reference standard results interpreted without knowledge of results of the index test? | "Yes" if the person undertaking the reference test did not know the results of the pulse oximetry "No" if pulse oximetry results were available to those who conducted or interpreted the echocardiogram "Unclear" if insufficient information was provided |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | A judgment of low, high, or unclear risk of bias was based on a balanced assessment of responses to the above signaling questions. |
| B. Concerns about applicability | |
| Is there concern that the target condition as defined by the reference standard does not match the question? | A judgment of low, high, or unclear concern about applicability was based on the possibility of reference standards mixing both critical and non‐critical congenital heart disease. |
| Domain 4: Flow and timing | |
| Describe any patients who did not receive the index test and/or reference standard(s) or who were excluded from the two‐by‐two table (refer to flow diagram), and describe the time interval and any interventions between index test and reference standard(s). | |
| A. Risk of bias | |
| Was at least 28 days of appropriate follow‐up provided? | "Yes" if follow‐up was at least 28 days "No" if follow‐up was less than 28 days "Unclear" if insufficient information was provided |
| Did all patients receive a reference standard? | "Yes" if the study specifically stated that all patients received echocardiogram, clinical follow‐up, or confirmation by mortality and congenital anomaly databases (for both positive and negative pulse oximetry results) "No" if some negative pulse oximetry participants were lost to follow‐up without any confirmation "Unclear" if insufficient information was provided |
| Were all patients included in the analysis? | "Yes" if the study had no withdrawals or withdrawals were clearly described "No" if the number of patients contributing to the two‐by‐two tables did not match the number of patients recruited and no reasons for exclusions were described "Unclear" if information was not enough to establish the flow of participants |
| Could the patient flow have introduced bias? | A judgment of low, high, or unclear risk of bias was based on a balanced assessment of responses to the above signaling questions. |
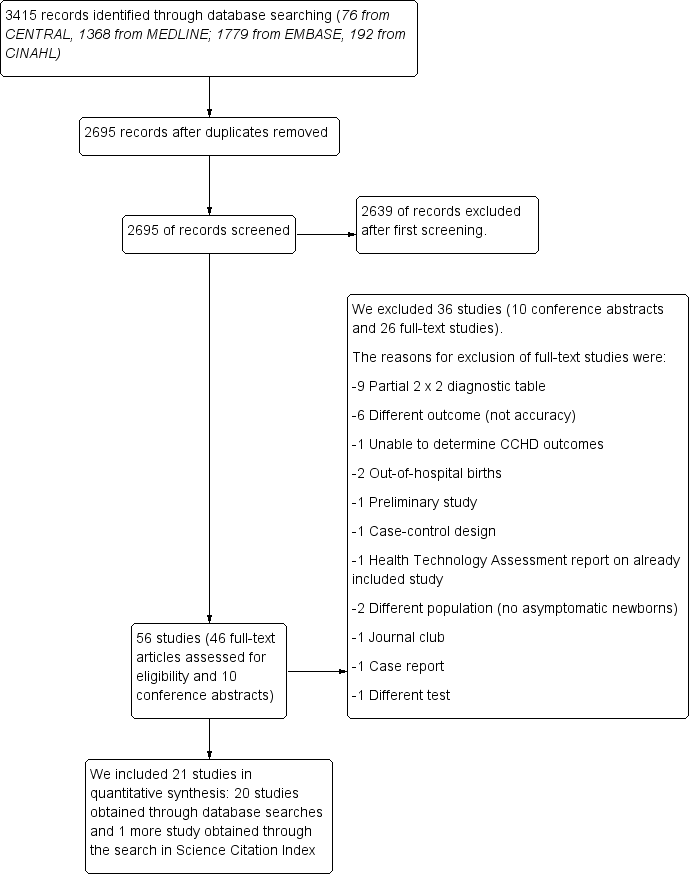
Flow of studies through the screening process. CCHD: critical congenital heart defect.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Forest plot of sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (blue square) and its 95% confidence interval (black horizontal line). Studies are ordered by ascending specificity.

Summary ROC plot for pulse oximetry using a threshold lower than or lower than or equal to 95% (n = 19 studies). The solid circle corresponds to the summary estimate of sensitivity and specificity, and is shown with a 95% prediction region (dashed line).

Primary analysis (threshold < 95% or ≤ 95%).
| Should pulse oximetry be used to diagnose CCHD in asymptomatic newborns? | ||||||||
| Patient or population: asymptomatic newborns at the time of pulse oximetry screening | ||||||||
| Setting: hospital births | ||||||||
| Index test: pulse oximetry | ||||||||
| Reference test: Reference standards were both diagnostic echocardiography (echocardiogram) and clinical follow‐up in the first 28 days of life, including postmortem findings and mortality and congenital anomaly databases to identify false‐negative patients. | ||||||||
| Studies: We included prospective or retrospective cohorts and cross‐sectional studies. We excluded case reports and studies of case‐control design. | ||||||||
| Threshold | Summary accuracy (95% CI) | Number of participants (diseased /non‐diseased) Number of studies | Prevalence median (range) | Implications (in a cohort of 10,000 newborns tested [95% CI]) | Certainty of the evidence (GRADE) | |||
| Prevalence 0.6 per 1000 | Prevalence 0.1 per 1000 | Prevalence 3.7 per 1000 | ||||||
| 95% (less than or less than or equal to) | Sensitivity 76.3% (69.5 to 82.0) Specificity 99.9% (99.7 to 99.9) | 436,758 (345/436,413) 19 studies | 0.6 per 1000 (0.1 to 3.7) | True positives (newborns with CCHD) | 5 (4 to 5) | 1 (1 to 1) | 28 (26 to 30) | LOW* ⊕⊕⊝⊝ |
| False negatives (newborns incorrectly classified as not having CCHD) | 1 (1 to 2) | 0 (0 to 0) | 9 (7 to 11) | |||||
| True negatives (newborns without CCHD) | 9980 (9966 to 9987) | 9985 (9971 to 9992) | 9949 (9935 to 9956) | HIGH ⊕⊕⊕⊕ | ||||
| False positives (newborns incorrectly classified as having CCHD) | 14 (7 to 28) | 14 (7 to 28) | 14 (7 to 28) | |||||
| CCHD: critical congenital heart defect; CI: confidence interval. Sensitivity: *We have downgraded certainty of the evidence from high to low because the low number of CCHD cases included in the review (serious imprecision) and secondly, there was a serious risk of differential verification bias (ie, diagnosis was established by echocardiography in test positive cases however test negatives were usually confirmed by clinical follow‐up or by accessing congenital malformation registries and mortality databases)." | ||||||||
| Certainty of the evidence (Balshem 2011) | ||||||||
| Study | Population | Index test | Reference standard(s) | |||||
| Antenatal diagnosis of CHD | Pulse oximeter | Limb | Test timing | Oxygen saturation | Threshold | Positive pulse oximetry | Negative pulse oximetry | |
| Arlettaz 2006 | included | Nellcor NPB‐40 | post‐ductal | within 24 hours | functional | < 95% | echocardiography | NA |
| Bakr 2005 | excluded | Digioxi PO 920 | pre‐ductal and post‐ductal | longer than 24 hours | fractional | ≤ 94% | echocardiography | cardiology database |
| Bhola 2014 | included | Masimo Radical 5 | post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | cardiology database |
| De‐Wahl 2009 | excluded | Radical SET v4 | pre‐ductal and post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | mortality data |
| Ewer 2011 | included | Radical‐7 | pre‐ductal and post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up, cardiology database & congenital registry |
| Gomez‐Rodriguez 2015 | excluded | Radical‐5 | post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up |
| Jones 2016 | excluded | NA | pre‐ductal and post‐ductal | within 24 hours | NA | ≤ 95% | echocardiography | National Congenital Heart Disease Audit |
| Klausner 2017 | excluded | NA | pre‐ductal and post‐ductal | longer than 24 hours | NA | < 95% | echocardiography | clinical follow‐up |
| Koppel 2003 | excluded | Ohmeda Medical | post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | clinical follow‐up & congenital registry |
| Meberg 2008 | excluded | RAD‐5v | post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up |
| Oakley 2015 | excluded | Nellcor NPB 40 | post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | cardiology database & mortality data |
| Ozalkaya 2016 | excluded | Nellcor | pre‐ductal and post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | echocardiography |
| Richmond 2002 | included | Oxi machine | post‐ductal | within 24 hours | fractional | < 95% | echocardiography | mortality data & congenital registry |
| Riede 2010 | excluded | NA | post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | congenital registry |
| Rosati 2005 | excluded | NA | post‐ductal | longer than 24 hours | functional | ≤ 95% | echocardiography | clinical follow‐up |
| Sendelbach 2008 | excluded | Nellcor N‐395 | post‐ductal | within 24 hours | functional | < 96% | echocardiography | clinical follow‐up |
| Singh 2014 | excluded | NA | pre‐ductal and post‐ductal | within 24 hours | functional | < 95% | echocardiography | mortality data & congenital registry & cardiology database |
| Turska 2012 | excluded | Novametrix, Nellcor & Masimo | post‐ductal | within 24 hours | functional | < 95% | echocardiography | clinical follow‐up and Public Health registries |
| Van Niekerk 2016 | excluded | Nellcor | pre‐ductal and post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | NA |
| Zhao 2014 | excluded | RAD‐5V | pre‐ductal and post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | clinical follow‐up |
| Zuppa 2015 | excluded | Ohmeda 3900 | post‐ductal | longer than 24 hours | functional | < 95% | echocardiography | NA |
| NA: not available | ||||||||
| N | Sensitivity (95% CI) | Relative sensitivity P value | False‐positive rate (FPR) (95% CI) | Relative FPR P value | |
| Antenatal diagnosis | |||||
| Included | 4 | 86.3% (71.8 to 94.0) | 0.071 | 0.46% (0.13 to 1.59) | 0.231 |
| Excluded | 15 | 74.1% (65.7 to 81.1) | 0.10% (0.05 to 0.21) | ||
| Test timing | |||||
| Longer than 24 hours | 11 | 73.6% (62.8 to 82.1) | 0.393 | 0.06% (0.03 to 0.13) | 0.027 |
| Within 24 hours | 8 | 79.5% (70.0 to 86.6) | 0.42% (0.20 to 0.89) | ||
| Limb | |||||
| Foot only | 11 | 81.2% (70.9 to 88.4) | 0.197 | 0.13% (0.05 to 0.31) | 0.718 |
| Foot and right hand | 8 | 71.2% (58.5 to 81.3) | 0.17% (0.06 to 0.46) | ||
| Risk of bias ("flow and timing") | |||||
| Unclear risk of bias | 9 | 77.8% (64.1 to 87.3) | 0.937 | 0.05% (0.02 to 0.12) | 0.016 |
| Low risk of bias | 10 | 77.3% (68.8 to 84.0) | 0.34% (0.17 to 0.66) |
| Test | No. of studies | No. of participants |
| 1 All studies Show forest plot | 21 | 457202 |
| 2 Primary analysis (threshold < 95% or ≤ 95%) Show forest plot | 19 | 436758 |


