Administración de líquidos para la hiperbilirrubinemia neonatal no conjugada
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: randomised controlled trial. Study grouping: parallel group. | |
| Participants | Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: healthy term breastfed infants with severe hyperbilirubinaemia. Exclusion criteria: "We excluded haemolytic disease (ABO or Rh incompatibility and a positive Coomb's test), G6PD deficiency, direct hyperbilirubinaemia, infection, dehydration, and prolonged jaundice persisting beyond 14 days of life." Pretreatment: "No major differences between the two groups." | |
| Interventions | IV fluid supplementation:
No fluid supplementation:
| |
| Outcomes | Serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
| |
| Identification | Sponsorship source: none stated. Country: Jordan. Setting: NICU in 2 medical centres. Comments: study conducted between September 2008 and October 2009. Author's name: Hazem A Al‐Masri. Institution: Royal Medical Services. Email: [email protected]. Address: Department of Pediatrics, Royal Medical Services, Jordan. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "Infants were divided randomly into two groups." Authors did not state methods of sequence generation as they only described in a simple statement that "infants were divided randomly..." |
| Allocation concealment | Unclear risk | No statements on methods of randomisation or party involved sequence generation and allocation (or both) to enable an assessment of whether the 2 steps were performed independently from each other. |
| Blinding of participants and personnel | High risk | Not stated if blinding occurred but due to nature of intervention (IV drip) as compared to oral feeding only in control group, it is highly unlikely that the researchers were blinded. |
| Blinding of outcome assessors | Unclear risk | Not stated if the laboratory staff knew group that participants were allocated to. |
| Incomplete outcome data | Low risk | Results for all 40 infants in each group analysed and presented in Table 2. |
| Selective outcome reporting | Low risk | Major outcomes specified in methods, namely serum bilirubin at different time points and number of infants who required exchange transfusion reported in sufficient detail in results. |
| Selective outcome reporting | Low risk | Major outcomes specified in methods, namely serum bilirubin at different time points and number of infants who required exchange transfusion reported in sufficient detail in results. |
| Other sources of bias | Low risk | None identified. |
| Methods | Study design: randomised controlled trial. Study grouping: parallel group. | |
| Participants | Baseline characteristics IV fluid supplementation:
Oral fluid supplementation:
Inclusion criteria: healthy term infants (≥ 37 weeks' gestation) admitted to the NICU with TSB level ≥ 300 μmol/L and conjugated serum bilirubin levels ≤ 15% of the TSB. Exclusion criteria: unwell infants (e.g. septicaemia, feed intolerance, kernicterus), major congenital malformation, conjugated hyperbilirubinaemia > 15% of TSB levels, or prolonged jaundice persisting beyond 14 days of life. Pretreatment: no significant differences between groups in mean birth weight, mean gestational age, ethnic distribution, gender distribution, places of birth, modes of delivery, and types of feeding. No significant differences in proportion with birth trauma, ABO incompatibility, abnormal blood film, G6PD deficiency, herbal intake for breastfeeding mothers, and clinical dehydration. Mean total indirect serum bilirubin on admission were not significantly different. The infants in the enteral group were significantly older on admission than those in IV group (P = 0.02). | |
| Interventions | IV fluid supplementation:
Oral fluid supplementation:
| |
| Outcomes | Serum bilirubin
Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion (when the serum bilirubin level remained > 340 µmol/L after commencement of phototherapy)
Number of infants with abnormal neurological signs
Number of infants with vomiting
Number of infants with abdominal distension
| |
| Identification | Sponsorship source: not stated. Country: Malaysia. Setting: NICU of a university hospital. Comments: study conducted between 1 October 1999 and 30 September 2000. Author's name: Boo NY. Institution: Universiti Kebangsaan Malaysia. Email: [email protected]. Address: Department of Paediatrics, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Cheras, Kuala Lumpur, 56000, Malaysia. | |
| Notes | Rate of decrease in bilirubin reported was indirect and not TSB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "assignment into groups were based on information previously inserted randomly into sealed envelopes, which were then numbered sequentially." Unclear from the statements what method was used to generate the random sequence. |
| Allocation concealment | Unclear risk | Quote: "Assignment into groups was based on information previously inserted randomly into sealed envelopes, which were then numbered sequentially." Not stated if envelopes used were opaque (therefore, the contents will not be visible) although they were sealed and numbered sequentially. |
| Blinding of participants and personnel | High risk | Although not clearly stated in paper, blinding appeared highly unlikely as the way of administering fluid differed between the 2 groups, 1 receiving IV supplementation while another receiving enteral supplementation. |
| Blinding of outcome assessors | Low risk | Authors stated that the laboratory technicians had no knowledge of which fluid regimen was given to which infant. |
| Incomplete outcome data | Low risk | All 54 randomised infants were included in analyses. |
| Selective outcome reporting | Low risk | Appeared that all major outcomes as specified in the methods were reported in sufficient detail in the results. We were unable to obtain trial protocol to assess whether there were any further outcomes that were not reported. |
| Other sources of bias | Low risk | None identified. |
| Methods | Study design: randomised controlled trial. Study grouping: parallel group. | |
| Participants | Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: delivered between 38 and 41 weeks' gestation following an uneventful pregnancy with TSB > 308 μmol/L (18 mg/dL) to < 375 μmol/L (22 mg/dL). Exclusion criteria: major congenital malformation, haemolytic disease (Rh or ABO incompatibility and a positive Coombs' test), infection (congenital or acquired), G6PD deficiency, dehydration, conjugated hyperbilirubinaemia > 15% of TSB levels, prolonged jaundice persisting beyond 14 days of life. Pretreatment: no significant differences between groups noted. | |
| Interventions | IV fluid supplementation:
No fluid supplementation:
| |
| Outcomes | Serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
| |
| Identification | Sponsorship source: none stated. Country: Iraq. Setting: neonatal unit of a teaching hospital. Comments: study conducted from 2 January 2010 to 31 December 2010. Author's name: Easa ZO. Institution: author's affiliation not stated in paper. Email: not provided in paper. Address: not provided in paper. | |
| Notes | Interventions: text under Methods stated that, "neonates were assigned randomly to two groups, either the breast‐fed or formula‐fed with IV fluid (non‐supplemented group; N=32), or breast‐fed or formula fed in addition to IV fluid (supplemented group; N=32)". Based on abstract and whole manuscript, review author believed that there was a typographical error where the statement should have READ (mistake corrected in bold) "neonates were assigned randomly to two groups, either the breast‐fed or formula‐fed WITHOUT IV fluid (non‐supplemented group; N=32), or breast‐fed or formula fed in addition to IV fluid (supplemented group; N=32)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "assigned randomly to two groups." Unclear if allocation sequence was genuinely randomised as authors only stated that "neonates were assigned randomly to two groups." |
| Allocation concealment | Unclear risk | No information on how and where randomisation was performed to enable an assessment of whether random sequence was generated independently from allocation. |
| Blinding of participants and personnel | High risk | No mention of blinding in study. As intervention group received IV drip compared to control group (no IV drip), it is highly unlikely that the research personnel were blinded to intervention. |
| Blinding of outcome assessors | Unclear risk | Not stated if laboratory personnel who tested outcome measures (serum bilirubin) were blinded to the participant's allocation to either intervention or control. |
| Incomplete outcome data | Low risk | All 64 infants initially randomised were analysed. |
| Selective outcome reporting | Low risk | Main outcomes which researchers stated in methodology (serum bilirubin levels) were presented adequately in mean and SD for an appropriate length of time (while infants still receiving phototherapy until TSB declined to < 14 mg/dL). Numbers of infants requiring exchange transfusion also described. |
| Other sources of bias | Low risk | None identified. |
| Methods | Study design: randomised controlled trial. Study grouping: parallel group. | |
| Participants | Baseline characteristics IV fluid supplementation in addition to breastfeeding on‐demand:
Breastfeeding on‐demand without fluid supplementation:
Inclusion criteria: "These neonates were all Iranian race, healthy, breast‐fed, delivered between 38 and 41 weeks of gestation, following an uneventful pregnancy and had a total serum bilirubin (TSB) between 17 and 24.9 mg/dl (291 to 426 μmol/L)." Exclusion criteria: major congenital malformation, haemolytic disease (Rh or ABO incompatibility and a positive Coombs' test), infection (congenital or acquired), G6PD deficiency, dehydration, conjugated hyperbilirubinaemia > 15% of the TSB levels, and prolonged jaundice persisting beyond 14 days of life. Pretreatment: no statistical differences in baseline TSB at time of enrolment between groups (P = 0.17). | |
| Interventions | IV fluid supplementation in addition to breastfeeding on‐demand:
Breastfeeding on‐demand without fluid supplementation:
| |
| Outcomes | Serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
Duration of phototherapy
Rate of decrease in serum bilirubin
| |
| Identification | Sponsorship source: no information provided. Country: Iran. Setting: NICU. Comments: article identified via the reference list of an included article (Easa 2013). Study conducted between March and October 2003. Author's name: Iranpour R. Institution: Department of Pediatrics, Isfahan University of Medical Sciences, Isfahan, Iran. Email: not provided. Address: Correspondence to: Dr Iraj Haghshenas, Pediatric Department, Al‐Zahra Hospital, Isfahan, Iran. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Sequence generation not described as there was only a simple statement that, "neonates were assigned randomly" so it is unclear if allocation sequence was genuinely randomised. |
| Allocation concealment | Unclear risk | No information given to enable an assessment of whether random sequence was generated independently from allocation. |
| Blinding of participants and personnel | High risk | Although not clearly stated, blinding appeared highly unlikely as 1 group received IV fluid while the other did not. |
| Blinding of outcome assessors | Unclear risk | No mention if laboratory personnel who measured serum bilirubin levels were blinded to allocation received. |
| Incomplete outcome data | Low risk | All 60 infants initially randomised were included in analyses. |
| Selective outcome reporting | High risk | Authors reported only serum bilirubin at different time points. Key clinical outcomes, such as infants with symptoms of bilirubin encephalopathy or kernicterus, infants who required exchange transfusion, or infants who developed adverse effects from IV infusion such as phlebitis were not reported. |
| Other sources of bias | Low risk | None identified. |
| Methods | Study design: randomised controlled trial. Study grouping: parallel group. | |
| Participants | Baseline characteristics Fluid supplementation:
No fluid supplementation:
Inclusion criteria: term (≥ 37 weeks' gestation) neonates presenting with severe non‐haemolytic hyperbilirubinemia (TSB > 308 mmol/L (18 mg/dL). Exclusion criteria: infants with TSB > 427 mmol/L (25 mg/dL), acute bilirubin encephalopathy (kernicterus), evidence of haemolysis, obvious signs of dehydration (i.e. sunken fontanel, reduced skin turgor, dry mucosa, tachycardia, delayed capillary refill, excessive weight loss), or major congenital malformations, and receiving IV fluids for any reason. Pretreatment: no major differences between groups in baseline characteristics. | |
| Interventions | Fluid supplementation:
No fluid supplementation:
| |
| Outcomes | Serum bilirubin
Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion
Duration of phototherapy
Number of infants with bilirubin encephalopathy
Frequency of breastfeeding in the first 3 days of hospital stay
| |
| Identification | Sponsorship source: not stated. Country: India. Setting: tertiary level NICU. Comments: study period: September 2003 to June 2004. Author's name: Dr Praveen Kumar. Institution: Department of Pediatrics, Postgraduate Institute of Medical Education & Research, Chandigarh, India. Email: [email protected]. Address: Department of Pediatrics, PGIMER, Chandigarh 160012, India. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "Assignment to the various groups was based on stratified block randomisation with variable block size." Methods of random sequence generation clearly stated. |
| Allocation concealment | Low risk | Researchers used serially numbered opaque envelopes to assign groups. Envelopes only opened after enrolment. |
| Blinding of participants and personnel | High risk | In paragraph 2 under discussion, authors stated that, "they could not do complete blinding." It is noted that due to the study design, blinding would be impossible due to different management instituted to groups in which 1 group received IV drip in addition to oral feed as compared to the other group which only received oral feeding. |
| Blinding of outcome assessors | Low risk | In paragraph 2 of discussion (page 783), authors stated that, "the laboratory personnel performing the serum bilirubin and other biochemical investigations were sent coded samples and were unaware of the group allocation." |
| Incomplete outcome data | Low risk | All 54 infants enrolled were included in analyses. Number of infants with serum bilirubin levels at 4, 8, and 24 hours were lower than number at inclusion as some infants required exchange transfusion and their serum bilirubin readings were not included. |
| Selective outcome reporting | Low risk | All major outcomes, including predefined primary outcomes of the number of infants receiving exchange transfusion; duration of phototherapy; % drop in serum bilirubin at 4, 8, and 24 hours of study; and number of infants with bilirubin encephalopathy were reported in sufficient details. |
| Other sources of bias | Low risk | None identified. |
| Methods | Study design: randomised controlled trial. Study grouping: parallel group. | |
| Participants | Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: term neonates, well‐hydrated, aged 2‐10 days who had indirect non‐haemolytic jaundice with serum bilirubin ≥ 308 μmol/L (18 mg/dL). Exclusion criteria: jaundice in first day after birth, any manifestations of kernicterus, any form of haemolysis, dehydration, infants with symptoms of dehydration, congenital malformation, taking antibiotics, direct bilirubin > 15% of the total bilirubin, exchange transfusion if it was performed soon after admission (page 2525, column 1, paragraph 2). Pretreatment: no significant differences between groups in terms of gender, age on admission, weight, and serum bilirubin on admission. | |
| Interventions | IV fluid supplementation:
No fluid supplementation:
| |
| Outcomes | Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
Duration of phototherapy
| |
| Identification | Sponsorship source: no information provided. Country: India. Setting: NICU of a hospital (level unclear). Comments: conducted over 6‐month period but the authors did not state the month and year it was conducted. Author's name: Patel M (first author). Institution: Civil Hospital, Ahmedabad, India. B. J. Medical College. Email: not provided. Address: Department of Pediatrics, B. J. Medical College, Asarwa, Ahmedabad, 380016, Gujarat, India (address for all 3 study authors). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "By using a random number table, simple randomisation was performed." Methods of sequence generation clearly stated. |
| Allocation concealment | Unclear risk | No relevant information to enable an assessment of whether allocation was performed independently from random sequence generation. |
| Blinding of participants and personnel | High risk | Although not mentioned in text, it is highly unlikely that research personnel were blinded due to nature of intervention which was IV fluid compared to enteral feeding only in control group. |
| Blinding of outcome assessors | Unclear risk | Not stated if laboratory personnel were blinded to the group allocation. |
| Incomplete outcome data | Low risk | All infants (42 in each group) appeared to have been analysed and their results presented. |
| Selective outcome reporting | High risk | Rates of decrement of bilirubin in first 6 and first 12 hours presented as means (without SD) as seen in Table 3. Anticipate difficulty obtaining this additional data from authors as corresponding author was not named and there was no email address included. Data for number of infants who required exchange transfusion were adequately reported. Several infants required exchange transfusion but, despite this, there was no report on numbers who developed bilirubin encephalopathy. |
| Other sources of bias | Low risk | None identified. |
| Methods | Study design: randomised controlled trial. Study grouping: parallel group. | |
| Participants | Baseline characteristics IV fluid supplementation:
No fluid supplementation:
Inclusion criteria: term, healthy neonate, aged 2‐28 days, serum total bilirubin ≥ 308 μmol/L (18 mg/dL). Exclusion criteria: jaundice in first day after birth, any manifestations of kernicterus, any form of haemolysis, dehydration, any congenital malformation, treatment with antibiotics, direct bilirubin > 15% of total bilirubin, exchange transfusion if performed soon after admission. Pretreatment: no major differences in the baseline characteristics between groups. | |
| Interventions | IV fluid supplementation:
No fluid supplementation:
| |
| Outcomes | Rate of decrease of serum bilirubin
Number of infants who required exchange transfusion (criteria not stated)
| |
| Identification | Sponsorship source: not stated. Country: Iran. Setting: Paediatric ward of a hospital (level unclear). Comments: study period: October 2007 to April 2008. Some outcome data reported in a manner that was insufficient for pooling in meta‐analysis. Awaiting reply from study authors. Author's name: Associate Professor Farhad Heydarian. Institution: Paediatrics Ward, Ghaem Hospital, Ahmad Abad Ave, Mashhad, Iran. Email: [email protected]. Address: Paediatrics Ward, Ghaem Hospital, Ahmad Abad Ave, Mashhad, Iran. | |
| Notes | Outcome 'rate of decrease in serum bilirubin' was incompletely reported, as authors only reported median with a single figure given as "confidence interval." We are awaiting further information from study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "By using a random number table, simple randomisation was performed." |
| Allocation concealment | Unclear risk | Unclear how allocation carried out, in particular, whether allocation performed independently from sequence generation. |
| Blinding of participants and personnel | High risk | Blinding to care personnel very unlikely as 1 group of infants received additional IV fluid while another group did not. |
| Blinding of outcome assessors | Unclear risk | Unclear whether assessors of laboratory outcomes were blinded to allocation status of infants. |
| Incomplete outcome data | Low risk | Appeared that all 100 infants randomised included in report for major outcomes of number of infants who required exchange transfusion and rate of decrease in serum bilirubin in first 24 hours. |
| Selective outcome reporting | High risk | The outcome 'Rate of decrease of serum bilirubin in the first 24 hours after admission' was incompletely reported, as authors only provided median figures with a single figure given for confidence interval (we are waiting study author's response to our request for these data). Study authors did not include any data on adverse events of IV fluids such as thrombophlebitis. Number of infants with bilirubin encephalopathy not reported. |
| Other sources of bias | Low risk | None identified. |
G6PD: glucose‐6‐phosphate dehydrogenase; NICU: neonatal intensive care unit; SD: standard deviation; TSB: total serum bilirubin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Letter to editor commenting on findings of an included study (Saeidi 2009). Excluded on basis of study design. | |
| Evaluated 2 different types of intravenous fluid supplementation (hypotonic vs isotonic fluid), which was not within our prespecified comparison list. Excluded on basis of intervention. | |
| A randomised controlled trial that evaluated 2 different levels of fluid in small‐for‐gestational age babies. Inclusion criteria of infants did not include hyperbilirubinaemia and phototherapy. Excluded on basis of participant population. | |
| 3‐arm randomised controlled trial that compared effects of 3 regimen of fat emulsion in total parenteral nutrition for preterm infants. Excluded on basis of intervention. | |
| Randomised controlled trial that compared 2 regimens of parenteral‐enteral nutrition combination for very‐low‐birth‐weight infants. Excluded on basis of intervention. | |
| Non‐randomised trial that compared effects of water supplementation to no supplementation on bilirubin level for breastfed infants. Excluded on basis of study design. | |
| Randomised controlled trial that compared effects of early introduction of hypocaloric feeding versus no early feeding for preterm infants. Excluded on basis of intervention. | |
| Comparative trial that evaluated effects of 2 different milk formulae (whey‐predominant versus casein‐hydrolysate) in newborn infants. Trial did not compare 2 different levels of fluid. Excluded on basis of intervention. | |
| Randomised controlled trial that evaluated effects of 2 levels of fluid (1 starting with 60 mL/kg/day on day 1 and increasing to 150 mL/kg/day over the first week and the other receiving 20% less fluid over same period). Population enrolled were preterm infants in general and not infants with hyperbilirubinaemia or on phototherapy. Excluded on basis of participant population. | |
| Randomised controlled trial that compared frequent vs usual on‐demand breastfeeding regimen at reducing serum bilirubin of healthy term infants. Infants were healthy at birth and not infants with hyperbilirubinaemia who were undergoing phototherapy. Excluded on basis of population. | |
| Randomised controlled trial that examined effects of early parenteral nutrition on prevention of jaundice in term and near‐term infants. Excluded on basis of intervention. | |
| 4‐arm randomised controlled trial that compared effects of 4 interventions for term newborn infants with severe hyperbilirubinaemia. Intervention involved a combination of continuing breastfeeding or starting formula feeding and starting or not starting phototherapy, and thus they were not related to fluid level. Excluded on basis of intervention. | |
| Survey on practice of giving supplementary feeds to newborn infants and occurrence of jaundice. Excluded on basis of study design. | |
| Randomised controlled trial comparing supplementation of formula or maltodextrin solution to breastfeeding term infants. Excluded on basis of intervention. | |
| 4‐arm randomised controlled trial comparing feeding very‐low‐birth‐weight infants using 4 different feed regimens comprising different combinations of glucose solution, water, or saline and different timings of feed commencement. Excluded on basis of intervention. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Single‐centre, parallel randomised controlled trial (Turkey). |
| Participants | 250 Healthy term infants with hyperbilirubinaemia. |
| Interventions | Study group: intravenous fluid supplementation in addition to breastfeeding (125 infants) during phototherapy. Control group: breastfeeding without fluid supplementation during phototherapy. |
| Outcomes | Mean indirect serum bilirubin level at time of admission to neonatal intensive care unit and at 4, 8, 12, 24, and 48 hours after commencement of phototherapy, duration of phototherapy, duration of hospitalisation. |
| Notes | Insufficient information from the published abstract to merit inclusion, awaiting full‐text. |
| Methods | Single‐centre, parallel randomised controlled trial (Iran). |
| Participants | Inclusion criteria: gestational age > 38 weeks, age > 24 hours on admission, serum level of total bilirubin > 15 mg/dL, indirect hyperbilirubinaemia. Exclusion criteria: fluid therapy required for conditions other than jaundice, preterm and low birth weight neonates, haemolytic jaundice, sepsis, prolonged jaundice. |
| Interventions | Study group: intravenous fluids containing dextrose 10% equal amounts of maintenance with sodium 30 mEq/L and potassium 20 mEq/L plus phototherapy. Control group: phototherapy with no intravenous fluid. |
| Outcomes | Serum bilirubin level on admission and at 6, 12, and 24 hours postadmission, duration of hospitalisation. |
| Notes | Study completed in 2012 according to the Iranian Registry of Clinical Trials. Study appeared not to have been published in full or in part according to our searches. Awaiting further information from the study author. |
| Methods | Single‐centre, parallel randomised controlled trial (Germany). |
| Participants | Preterm infants < 33 weeks' gestation undergoing phototherapy for hyperbilirubinaemia. |
| Interventions | Study group: extra intravenous fluid intake of 20% of total fluid demand per 24 hours of saline 0.9% during each 2‐hour period of phototherapy (12 hours total per day). Extra fluid intake was interrupted during 12‐hours break of phototherapy. Control group: previous fluid regimen, as intravenous fluid was given constantly, without a specific guideline according to extra fluid intake. |
| Outcomes | Highest TSB level within 1 week after the commencement of phototherapy. No other outcomes provided. |
| Notes | Study completed in 2009 according to the ClinicalTrials.gov. The study appeared not to have been published in full or in part according to our searches. Awaiting further information from the study author. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of infants with bilirubin encephalopathy Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 1 Number of infants with bilirubin encephalopathy. | ||||
| 2 Serum bilirubin (μmol/L) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 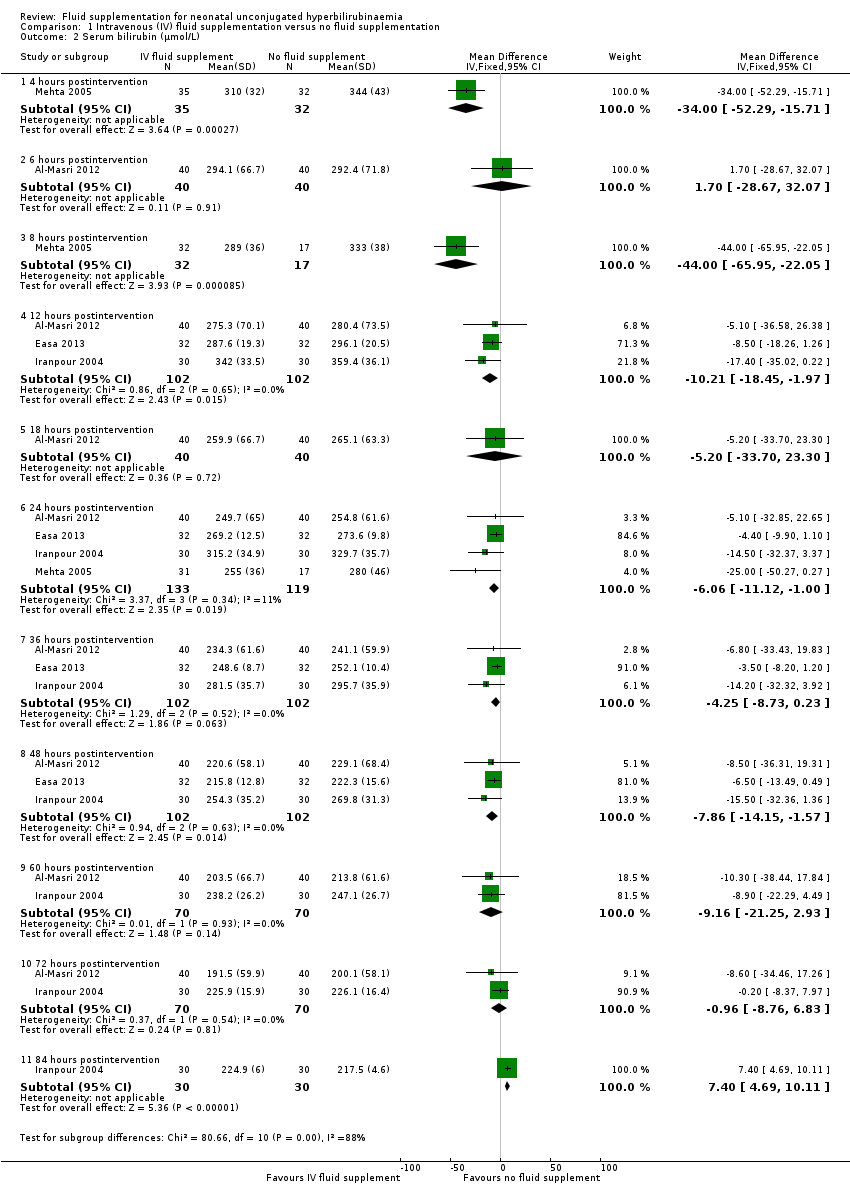 Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 2 Serum bilirubin (μmol/L). | ||||
| 2.1 4 hours postintervention | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐52.29, ‐15.71] |
| 2.2 6 hours postintervention | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐28.67, 32.07] |
| 2.3 8 hours postintervention | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐44.0 [‐65.95, ‐22.05] |
| 2.4 12 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐10.21 [‐18.45, ‐1.97] |
| 2.5 18 hours postintervention | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐33.70, 23.30] |
| 2.6 24 hours postintervention | 4 | 252 | Mean Difference (IV, Fixed, 95% CI) | ‐6.06 [‐11.12, 1.00] |
| 2.7 36 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐4.25 [‐8.73, 0.23] |
| 2.8 48 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐7.86 [‐14.15, ‐1.57] |
| 2.9 60 hours postintervention | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐21.25, 2.93] |
| 2.10 72 hours postintervention | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐8.76, 6.83] |
| 2.11 84 hours postintervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 7.40 [4.69, 10.11] |
| 3 Difference in serum bilirubin (%) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 3 Difference in serum bilirubin (%). | ||||
| 3.1 0‐4 hours of study | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 10.5 [6.66, 14.34] |
| 3.2 0‐8 hours of study | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [7.49, 18.51] |
| 3.3 0‐24 hours of study | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [1.11, 14.89] |
| 4 Rate of change of serum bilirubin (μmol/L/hour) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.21, 1.21] |
| Analysis 1.4 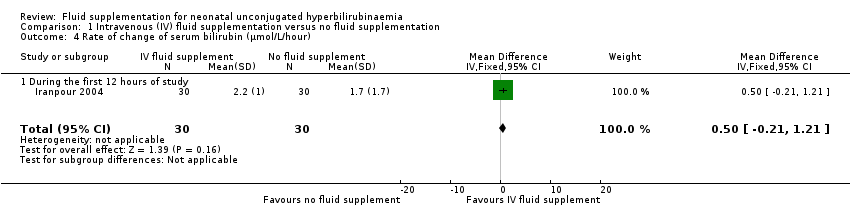 Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 4 Rate of change of serum bilirubin (μmol/L/hour). | ||||
| 4.1 During the first 12 hours of study | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.21, 1.21] |
| 5 Duration of phototherapy (hours) Show forest plot | 3 | 218 | Mean Difference (IV, Fixed, 95% CI) | ‐10.70 [‐15.55, ‐5.85] |
| Analysis 1.5  Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 5 Duration of phototherapy (hours). | ||||
| 6 Number of infants who required exchange transfusion Show forest plot | 6 | 462 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.21, 0.71] |
| Analysis 1.6  Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 6 Number of infants who required exchange transfusion. | ||||
| 7 Frequency of breastfeeding per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 7 Frequency of breastfeeding per day. | ||||
| 7.1 Day 1 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.62, 2.02] |
| 7.2 Day 2 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.43, 2.23] |
| 7.3 Day 3 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.40, 2.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of infants with abnormal neurological signs Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.1  Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 1 Number of infants with abnormal neurological signs. | ||||
| 2 Serum bilirubin (μmol/L) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐21.58, 43.58] |
| Analysis 2.2  Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 2 Serum bilirubin (μmol/L). | ||||
| 2.1 4 hours postphototherapy | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐21.58, 43.58] |
| 3 Rate of change of serum bilirubin (μmol/L/hour) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.55, 4.15] |
| Analysis 2.3  Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 3 Rate of change of serum bilirubin (μmol/L/hour). | ||||
| 3.1 During first 4 hours of admission | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.55, 4.15] |
| 4 Number of infants who required exchange transfusion Show forest plot | 1 | 54 | Risk Difference (IV, Fixed, 95% CI) | 0.11 [‐0.12, 0.34] |
| Analysis 2.4 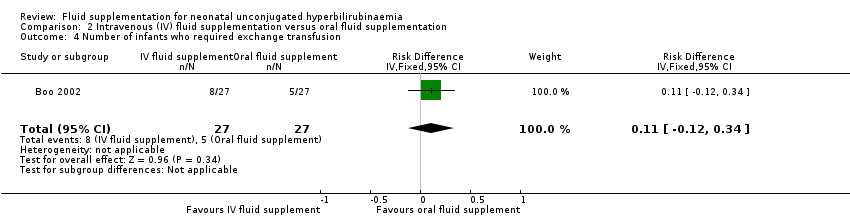 Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 4 Number of infants who required exchange transfusion. | ||||
| 5 Number of infants with feed intolerance: vomiting Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.5  Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 5 Number of infants with feed intolerance: vomiting. | ||||
| 6 Number of infants with feed intolerance: abdominal distension Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.6  Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 6 Number of infants with feed intolerance: abdominal distension. | ||||

Study flow diagram.
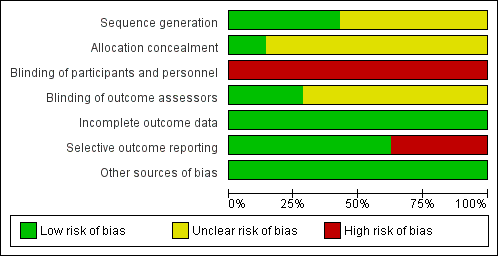
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
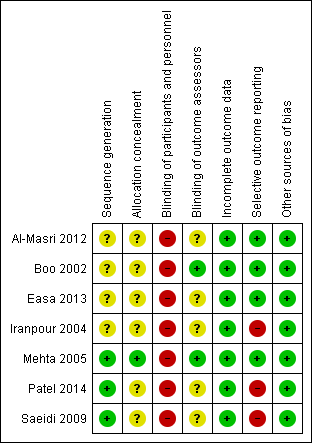
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
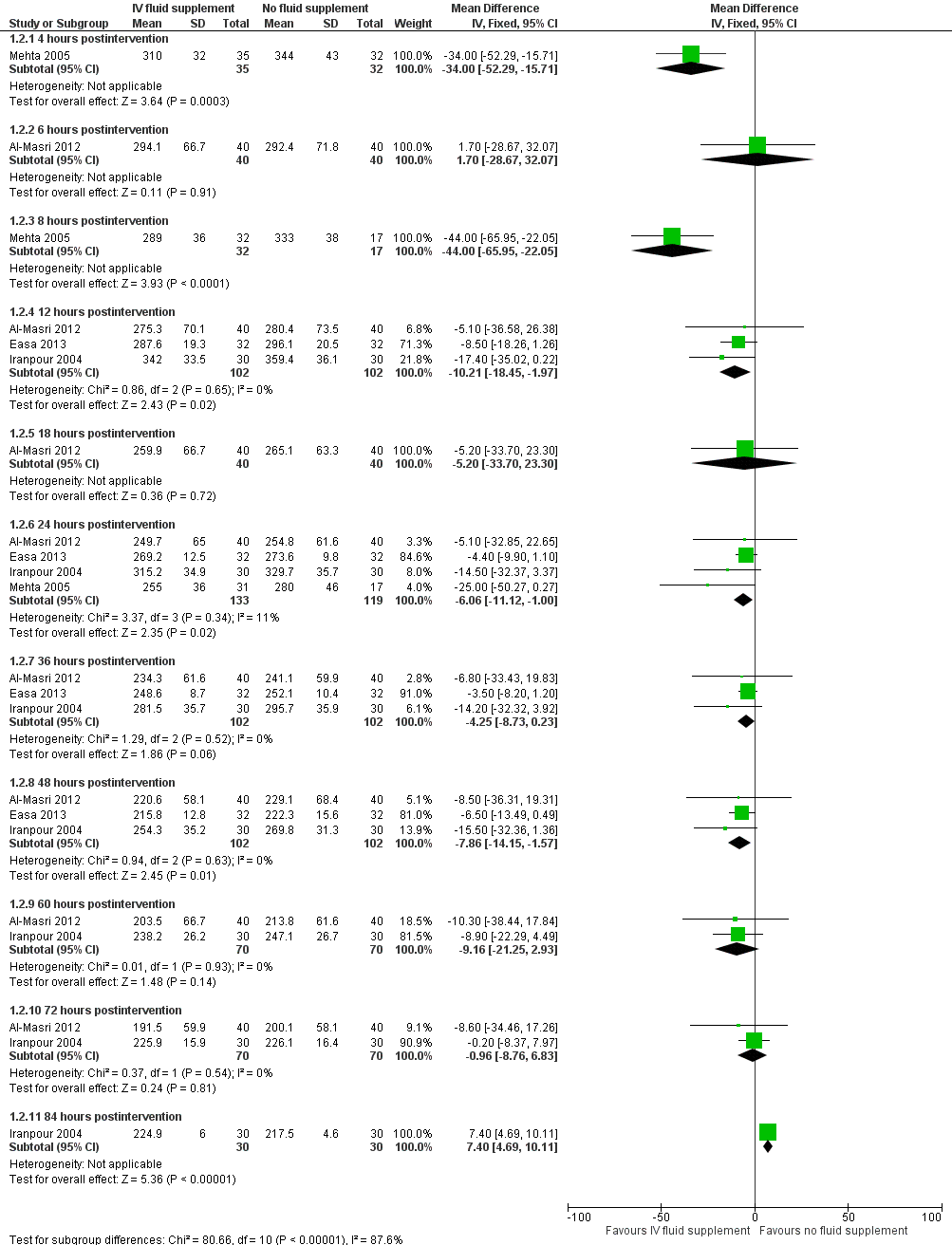
Forest plot of comparison: 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, outcome: 1.2 Serum bilirubin (μmol/L).
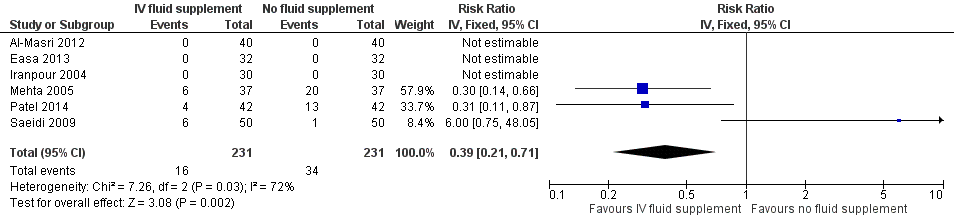
Forest plot of comparison: 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, outcome: 1.6 Number of infants who required exchange transfusion.

Forest plot of comparison: 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, outcome: 1.7 Frequency of breastfeeding per day.
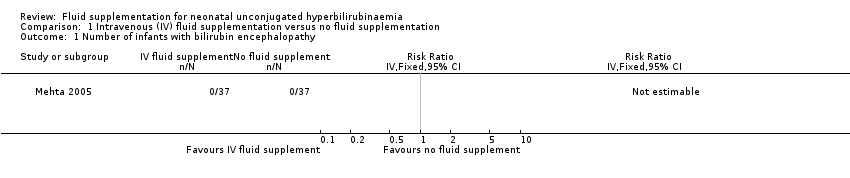
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 1 Number of infants with bilirubin encephalopathy.
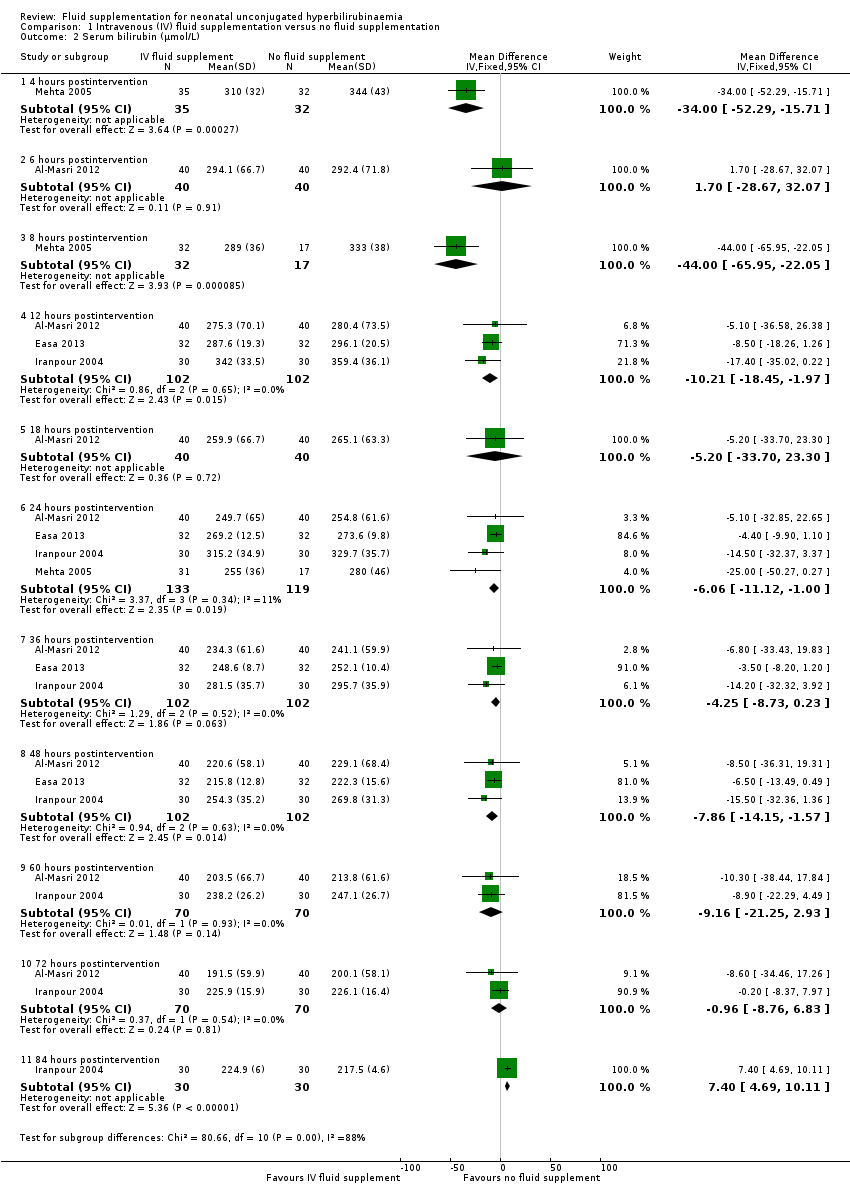
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 2 Serum bilirubin (μmol/L).

Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 3 Difference in serum bilirubin (%).
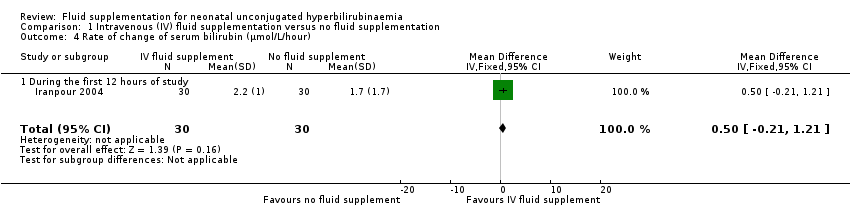
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 4 Rate of change of serum bilirubin (μmol/L/hour).
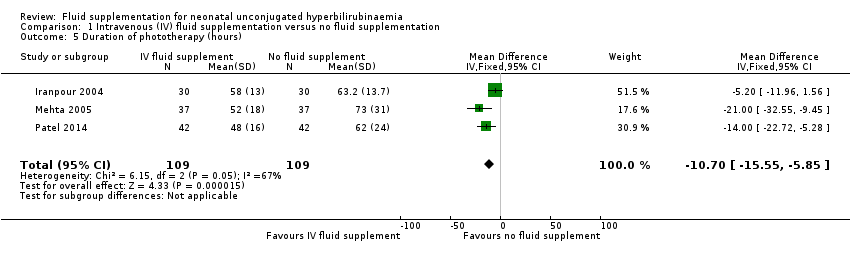
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 5 Duration of phototherapy (hours).
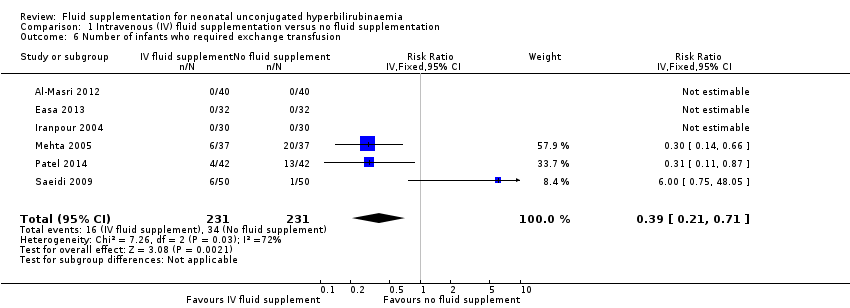
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 6 Number of infants who required exchange transfusion.
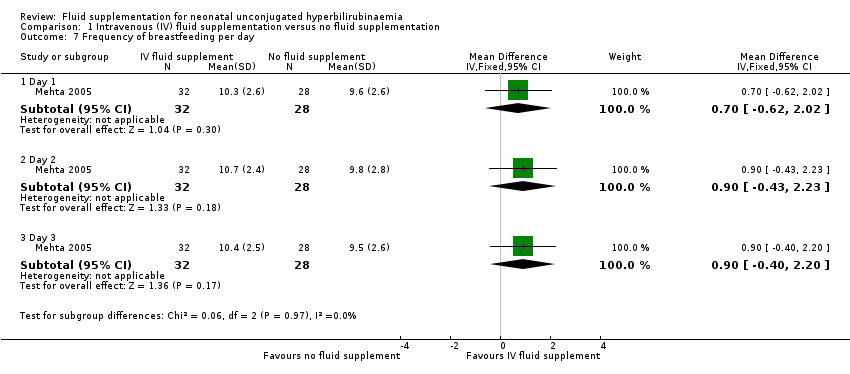
Comparison 1 Intravenous (IV) fluid supplementation versus no fluid supplementation, Outcome 7 Frequency of breastfeeding per day.
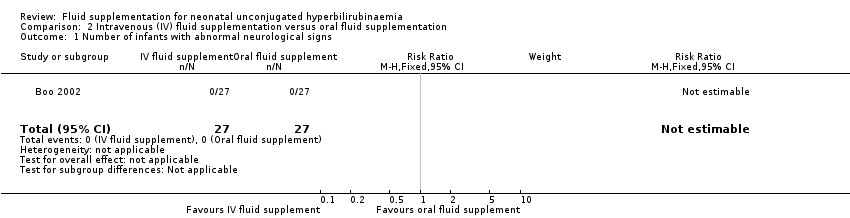
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 1 Number of infants with abnormal neurological signs.
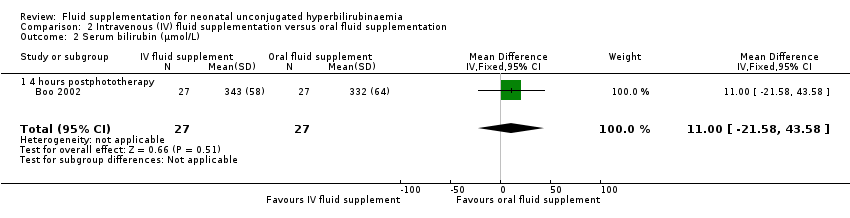
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 2 Serum bilirubin (μmol/L).

Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 3 Rate of change of serum bilirubin (μmol/L/hour).
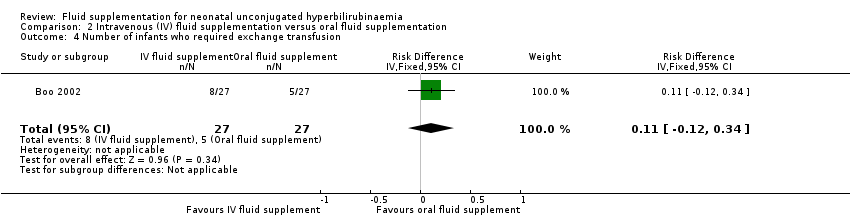
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 4 Number of infants who required exchange transfusion.
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 5 Number of infants with feed intolerance: vomiting.
Comparison 2 Intravenous (IV) fluid supplementation versus oral fluid supplementation, Outcome 6 Number of infants with feed intolerance: abdominal distension.
| Intravenous fluid supplementation versus no fluid supplementation for neonatal unconjugated hyperbilirubinaemia | ||||||
| Patient or population: newborn infants with unconjugated hyperbilirubinaemia undergoing phototherapy Setting: neonatal intensive care unit Intervention: intravenous fluid supplementation Comparison: no fluid supplementation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with no fluid supplementation | Risk with intravenous fluid supplementation | |||||
| Incidence of acute bilirubin encephalopathy | Study population | Not estimable | 74 (1 RCT) | ‐ | Effects not estimable as there were no reported cases of bilirubin encephalopathy in either group. | |
| 0 per 1000 | 0 per 1000 | |||||
| Bilirubin level (μmol/L): 4 hours postintervention | The mean serum bilirubin 4 hours postintervention was 344 μmol/L | The mean serum bilirubin 4 hours postintervention in the intervention group was 34 μmol/L lower (52.29 lower to 15.71 lower) | ‐ | 67 | ⊕⊕⊝⊝ | ‐ |
| Proportion of infants who required exchange transfusion | Study population | RR 0.39 | 462 | ⊕⊕⊝⊝ | ‐ | |
| 147 per 1000 | 57 per 1000 | |||||
| Frequency of breastfeeding per day: day 3 | The mean frequency of breastfeeding per day on day 3 was 9.5 times per day | The mean frequency of breastfeeding per day on day 3 in the intervention group was 0.9 more feeds (0.4 fewer to 2.2 more) | ‐ | 60 | ⊕⊕⊕⊝ | Although the study had high risk of bias in blinding of personnel, breastfeeding frequency on‐demand was considered unlikely to be affected. The study evaluated breastfeeding frequencies on days 1, 2, and 3. Data on day 3 were chosen because compared to the earlier period, it most closely reflected the breastfeeding frequency on discharge. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Although widely used, serum bilirubin is only a surrogate to the relevant clinical outcomes, namely, bilirubin encephalopathy or kernicterus. Quality of evidence downgraded one level on the basis of indirectness. b There is at least one unpublished study that was registered in ClinicalTrials.gov that included similar outcomes (NCT01550627). Quality of evidence downgraded one level on the basis of suspected publication bias. This outcome was also assessed in a published study for which we are yet to acquire full‐text (Demirsoy 2011). Both of these studies are currently placed under Studies awaiting classification. c Moderate degree of heterogeneity was present, as indicated by an I² statistic of 72%, which was mainly due to one included study with effect estimates in opposite direction to the other studies. Quality of evidence downgraded one level on the basis of inconsistency. d There are two unpublished studies (NCT01550627; IRCT2013022711145N5) identified from ClinicalTrials.gov, and one published study that we are yet to acquire full‐text (Demirsoy 2011) that are likely to assess this outcome. All three studies are placed under Studies awaiting classification. Quality of evidence downgraded one level on the basis of suspected publication bias. e Quality of evidence downgraded one level due to imprecision, as reflected by wide 95% CIs for the estimate. | ||||||
| Intravenous fluid versus oral fluid supplementation for neonatal unconjugated hyperbilirubinaemia | ||||||
| Patient or population: newborn infants with unconjugated hyperbilirubinaemia undergoing phototherapy Setting: neonatal intensive care unit Intervention: intravenous fluid supplementation Comparison: oral fluid supplementation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with oral fluid supplementation | Risk with intravenous fluid supplementation | |||||
| Number of infants with abnormal neurological signs | Study population | Not estimable | 54 | ‐ | Not estimable as there were no cases reported in either group. | |
| 0 per 1000 | 0 per 1000 | |||||
| Bilirubin level (μmol/L): 4 hours postphototherapy | The mean serum bilirubin 4 hours postphototherapy was 332 μmol/L | The mean serum bilirubin 4 hours postphototherapy in the intervention group was 11 μmol/L higher (21.58 lower to 43.58 higher) | ‐ | 54 | ⊕⊕⊕⊝ | ‐ |
| Rate of change in bilirubin (μmol/L/hour): during the first 4 hours of admission | The mean rate of decrease of serum bilirubin (μmol/L/hour) during the first 4 hours of admission was 10.4 μmol/L/hour | The mean rate of decrease of serum bilirubin (μmol/L/hour) during the first 4 hours of admission in the intervention group was 0.8 μmol/L/hour higher (2.55 lower to 4.15 higher) | ‐ | 54 | ⊕⊕⊕⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Although widely used, serum bilirubin is only a surrogate to the relevant clinical outcomes, namely, bilirubin encephalopathy or kernicterus. Quality of evidence downgraded one level on the basis of indirectness. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of infants with bilirubin encephalopathy Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Serum bilirubin (μmol/L) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 4 hours postintervention | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐52.29, ‐15.71] |
| 2.2 6 hours postintervention | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐28.67, 32.07] |
| 2.3 8 hours postintervention | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐44.0 [‐65.95, ‐22.05] |
| 2.4 12 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐10.21 [‐18.45, ‐1.97] |
| 2.5 18 hours postintervention | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐33.70, 23.30] |
| 2.6 24 hours postintervention | 4 | 252 | Mean Difference (IV, Fixed, 95% CI) | ‐6.06 [‐11.12, 1.00] |
| 2.7 36 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐4.25 [‐8.73, 0.23] |
| 2.8 48 hours postintervention | 3 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐7.86 [‐14.15, ‐1.57] |
| 2.9 60 hours postintervention | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐21.25, 2.93] |
| 2.10 72 hours postintervention | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐8.76, 6.83] |
| 2.11 84 hours postintervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 7.40 [4.69, 10.11] |
| 3 Difference in serum bilirubin (%) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 0‐4 hours of study | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 10.5 [6.66, 14.34] |
| 3.2 0‐8 hours of study | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [7.49, 18.51] |
| 3.3 0‐24 hours of study | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [1.11, 14.89] |
| 4 Rate of change of serum bilirubin (μmol/L/hour) Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.21, 1.21] |
| 4.1 During the first 12 hours of study | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.21, 1.21] |
| 5 Duration of phototherapy (hours) Show forest plot | 3 | 218 | Mean Difference (IV, Fixed, 95% CI) | ‐10.70 [‐15.55, ‐5.85] |
| 6 Number of infants who required exchange transfusion Show forest plot | 6 | 462 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.21, 0.71] |
| 7 Frequency of breastfeeding per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Day 1 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.62, 2.02] |
| 7.2 Day 2 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.43, 2.23] |
| 7.3 Day 3 | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.40, 2.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of infants with abnormal neurological signs Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serum bilirubin (μmol/L) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐21.58, 43.58] |
| 2.1 4 hours postphototherapy | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐21.58, 43.58] |
| 3 Rate of change of serum bilirubin (μmol/L/hour) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.55, 4.15] |
| 3.1 During first 4 hours of admission | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.55, 4.15] |
| 4 Number of infants who required exchange transfusion Show forest plot | 1 | 54 | Risk Difference (IV, Fixed, 95% CI) | 0.11 [‐0.12, 0.34] |
| 5 Number of infants with feed intolerance: vomiting Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number of infants with feed intolerance: abdominal distension Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

