Augmentation de la dose d'antipsychotique ou changement d'antipsychotique en cas de non‐réponse dans le traitement de la schizophrénie
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomisation: randomised | |
| Participants | Diagnosis: schizophrenia, schizoaffective disorder or schizophreniform disorder (DSM‐III‐R). 58 participants entered the double‐blind phase, but only 29 participants were of interest for the purpose of the present review*. | |
| Interventions | 1. Antipsychotic dose increase: fluphenazine 80 mg/day. N = 16*. 2. Antipsychotic switching: haloperidol 20 mg/day. N = 13*. 3. Antipsychotic dose maintenance: fluphenazine 20 mg/day. N = 18*. Rescue medication: benztropine, no further details. | |
| Outcomes | Global state: clinically relevant response (defined as CGI‐I ≤ 2 = at least much improved). Mental state: overall mental state (BRPS total score), negative symptoms (modified SANS). Unable to use: Adverse effects: extrapyramidal symptoms (modified SAS, no mean). | |
| Notes | *58 non‐responders entered the double‐blind phase and were randomised to the three treatment options (fluphenazine 20 mg/day, fluphenazine 80 mg/day and haloperidol 20 mg/day). Data were presented for 47 of 58 initially randomised participants (fluphenazine 20 mg/day, N = 18; fluphenazine 80 mg/day, N = 16; and haloperidol 20 mg/day, N = 13). For the purpose of the review, we were interested in the comparison between fluphenazine 80 mg/day, N = 16 and haloperidol 20 mg/day, N = 13; i.e. 29 participants in total. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Nonresponders were then randomly assigned to double‐blind treatment for...", "Subjects were stratified |
| Allocation concealment (selection bias) | Unclear risk | No details were presented. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "...double‐blind treatment..." (p. 310); no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "...double‐blind treatment..." (p. 310); no further details. |
| Incomplete outcome data (attrition bias) | Unclear risk | Outcome not addressed. Data were presented for 81% (47/58) of all randomised participants. |
| Selective reporting (reporting bias) | High risk | SAS was used but scores were available only for two items, not total. |
| Other bias | Low risk | No obvious risk for other bias. |
Scales
BPRS: Brief Psychiatric Rating Scale
CGI‐I: Clinical Global impression‐Improvement
SANS: Scale for the Assessment of Negative Symptoms
SAS: Simpson Angus Scale
Diagnostic Tools
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, third edition, revised
Others
mg: milligram
N: number
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: not randomised | |
| Allocation: randomised, no further details | |
| Allocation: not indicated | |
| Allocation: randomised, no further details | |
| Allocation: double‐blind phase: randomised; open‐label phase: not randomised. | |
| Allocation: randomised, no further details | |
| Allocation: randomised, no further details | |
| Allocation: randomised, no further details | |
| Allocation: randomised and concealed; "a random number table", "sequentially numbered, opaque, sealed envelopes" (p. 195). | |
| Allocation: randomised and concealed; "with a computerized schedule", "the person who generated the randomization schedule was not involved in determining subject eligibility, administering treatment, or determining outcome" (p. 14). | |
| Allocation: randomised, no further details | |
| Allocation: randomised, no further details | |
| Allocation: randomised, no further details. | |
| Allocation: not randomised. | |
| Allocation: randomised and concealed; "interactive voice response system", "the precise response criterion was withheld from research staff but defined a priori in the Institutional Review Board (IRB) Supplement, and early responder/non‐responder status was identified and treatment randomization was implemented using an interactive voice response (IVR) system" (p583). | |
| Allocation: randomised, no further details | |
| Allocation: randomised, no further details. | |
| Allocation: randomised, no further details. | |
| Allocation: randomised, no further details. | |
| Allocation: randomised, no further details. | |
| Allocation: randomised; "randomised on a centralized basis" (p. 82). | |
| Allocation: randomised, no further details. | |
| Allocation: not randomised; randomisation to treatment algorithms, switching of all non‐responders. | |
| Allocation: randomised, no further details | |
| Allocation: randomised; "a computerized random‐number‐generating program" (p. 1375). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: clinically relevant response – as defined by trial Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.17, 15.99] |
| Analysis 1.1 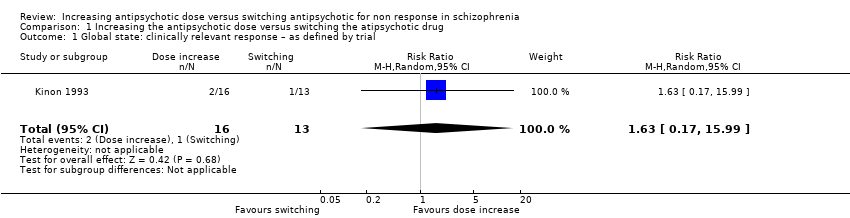 Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 1 Global state: clinically relevant response – as defined by trial. | ||||
| 2 Mental state: general mental state ‐ average endpoint score (BPRS total, high =poor) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐4.20, 8.20] |
| Analysis 1.2 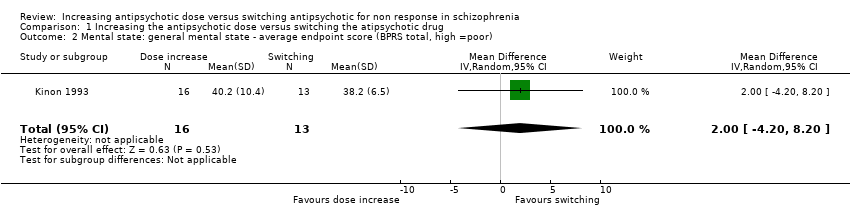 Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 2 Mental state: general mental state ‐ average endpoint score (BPRS total, high =poor). | ||||
| 3 Mental state: negative symptoms ‐ average endpoint score (SANS, high = poor) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐12.56, 19.36] |
| Analysis 1.3 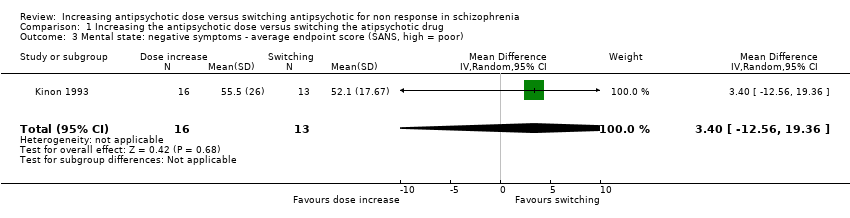 Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 3 Mental state: negative symptoms ‐ average endpoint score (SANS, high = poor). | ||||

Study flow diagram for trial selection up to March 2017

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 1 Global state: clinically relevant response – as defined by trial.
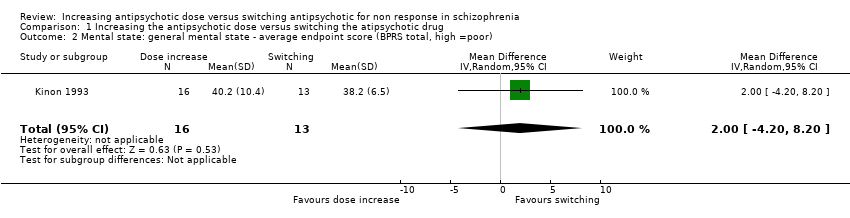
Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 2 Mental state: general mental state ‐ average endpoint score (BPRS total, high =poor).

Comparison 1 Increasing the antipsychotic dose versus switching the atipsychotic drug, Outcome 3 Mental state: negative symptoms ‐ average endpoint score (SANS, high = poor).
| Methods | Randomisation: random |
| Participants | Diagnosis: people with schizophrenia, schizoaffective disorder or schizophreniform disorder N > 450 |
| Interventions | All participants firstly receive treatment with one antipsychotic drug for at least 2 weeks. Those participants who do not at least minimally improve after 2 weeks, are considered non‐responders and are randomised to: 1. increasing the dose of the initial antipsychotic drug above the officially recommended dose range; or 2. switching the initial antipsychotic drug to another one with a different receptor profile; or 3. continuing treatment with the initial antipsychotic drug and at the same, initial dose (within the officially recommended dose range). |
| Outcomes | Response (defined as PANSS or BPRS decrease ≥ 50%)* Relapse Leaving the study early due to any reason Leaving the study early due to side effects General mental state: average change in general mental state scores Adverse effects: at least one adverse effect; clinically important general adverse effects; sudden and unexpected death Service use: time in hospital Quality of life All outcomes by time ‒ short term (up to 12 weeks), medium term (13 to 26 weeks) and long term (over 26 weeks) |
| Notes | *Primary outcome of interest |
| Increasing the antipsychotic dose compared to switching the antipsychotic drug for non responsein schizophrenia | ||||||
| Patient or population: patients with non response in schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Switching the atipsychotic drug | Increasing the antipsychotic dose | |||||
| Global state: Clinically relevant response – as defined by trial | 77 per 1000 | 125 per 1000 | RR 1.63 | 29 | ⊕⊝⊝⊝ | |
| Leaving the study early: Tolerability ‒leaving the study early due to side effects | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Leaving the study early: Acceptability ‒leaving the study early due to any reason | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome, |
| General mental state ‒BPRS total score at endpoint* | The mean general mental state ‒ BPRS total score at endpoint in the control groups was | The mean general mental state ‐ BPRS total score at endpoint in the intervention groups was | 29 | ⊕⊝⊝⊝ | Data for prespecified outcome: Clinically important change were not reported. | |
| Adverse effects ‒at least one adverse effect | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Service use ‒time in hospital | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| Quality of life ‒average change in quality of life | See comment | See comment | Not estimable | 0 | See comment | No studies reported on this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision: total (cumulative) sample size was just 29 participants and 95% confidence interval around the estimate of effect included no effect and appreciable benefit and appreciable harm; thus, very serious imprecision was present. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: clinically relevant response – as defined by trial Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.17, 15.99] |
| 2 Mental state: general mental state ‐ average endpoint score (BPRS total, high =poor) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐4.20, 8.20] |
| 3 Mental state: negative symptoms ‐ average endpoint score (SANS, high = poor) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐12.56, 19.36] |

