Środki odkażające w oparzeniach
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: not reported (until epithelialisation) | |
| Participants | Inclusion criteria: partial‐thickness burn wounds < 24 h post‐injury with TBSA between 10%‐40% and aged 5‐60 years Exclusion criteria: chemical & electrical burns, multiple trauma and serious comorbidity Participants: 69 hospital patients Mean age (years): 27.9 vs 26.2 years Male participants: 67.6% vs 68.6% Burn type: fire 73.5% vs 74.3%; hot liquid 14.7% vs 20%; other 11.8% vs 5.7% Burn degree: NR Burn size (%TBSA): NR Burn location: NR | |
| Interventions | Intervention arm 1: SSD cream, covered with cotton gauze, changed every other day. N = 34 Intervention arm 2: Silver nylon dressing (Agicoat) covered with cotton gauze, wetted regularly with sterile water, changed every 7 days. N = 35 Cointerventions: fentanyl analgesia as required | |
| Outcomes | Primary outcome: wound healing rate (mean time to complete healing) Secondary outcome: resource use (total hospitalisation cost) | |
| Notes | SD for wound healing and hospitalisation data extrapolated from graph Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "sixty‐nine burn wounds patients were included and randomised (the random number generator was used) into two groups and given burn wound treatment with 1% AgSD or Agicoat®" Comment: unclear what random‐number generator was used but acceptable |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sixty‐nine burn wounds patients were included and randomised (the random number generator was used) into two groups and given burn wound treatment with 1% AgSD or Agicoat®" Comment: no information on allocation concealment |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “both clinicians and patients or relatives were aware of the treatment procedure (open label design)” Comment: open label design and no mention of blinded outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote “all patients remained in the study” Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | No specific quote Comment: no report of VAS or resource use, which were listed as assessed outcomes. Also many outcomes had to be extrapolated from graphs |
| Other bias | Unclear risk | No evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel‐group RCT (stratified randomisation) Unit of randomisation: participant Unit of analysis: participant Duration: 4 weeks for most outcomes, until epithelialisation for wound healing | |
| Participants | Inclusion criteria: second‐degree burns, 20% to 60% TBSA Exclusion criteria: superficial (first‐degree) or full‐thickness (third‐degree burns); pregnancy; "significant” comorbidities: pre‐existing heart disease; renal disease; diabetes Participants: 163 hospital patients (unclear if inpatient or outpatient) Mean age (years): 27.4 vs. 31.8 Male participants: 29/52 vs 25/52 Burn type: NR Burn degree and size (%TBSA): mix of 20% ‐40% TBSA (12 vs 15 superficial; 13 vs 17 deep dermal) and > 40%‐60% TBSA (10 vs 6 superficial; 14 vs 14 deep dermal) (also stratified in the analysis) Burn location: NR Note participant characteristic data refers to analysed participants not the total number randomised (substantial difference) | |
| Interventions | Intervention arm 1: nano‐crystalline silver hydrogel (50 ppm), applied topically on alternate days. N = 52 Intervention arm 2; SSD cream (DISILVA, 1%), applied topically on alternate days. N = 52 Cointerventions: unspecified dressing | |
| Outcomes | Primary outcome: wound healing ‐ proportion of wounds completely healed by 4 weeks (reported only for deep dermal burns) Primary outcome: wound healing ‐ time (days) to complete wound healing | |
| Notes | Funding: Department of Science & Technology, West Bengal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Simple randomization sequence was generated by computer software” Comment: unclear what “simple” means in this context but computer‐generated randomisation sequences generally regarded as low risk |
| Allocation concealment (selection bias) | Unclear risk | Quote: “After allocation of patients into two different groups, SSD and AgNP gel were administered topically…” Comment: no detail on allocation concealment |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “this study was designed as an open‐label prospective, parallel group, randomized controlled trial.....Clinical assessments of burn wound were done on every week till 4th week and on completion of treatment.” Comment: open label trial with no mention of blinding assessors |
| Incomplete outcome data (attrition bias) | High risk | Quote: “Data for evaluation were obtained for 54 patients on SSD (2° deep‑dermal cases 27) and 52 (2° deep‑dermal cases 31) on AgNP treatment” Comment: 163 randomised, 57 lost to follow‐up. Similar numbers in each arm (30 vs. 27) but no reasons given |
| Selective reporting (reporting bias) | Unclear risk | Quote: “As shown in Table 4, considering deep‑dermal burn wounds only, the differences in treatment outcome at 4 weeks was statistically highly significant (P = 0.003) in favor of AgNP treatment. However, at 4 weeks, only 4 cases in AgNP arm had achieved complete wound healing compared to none in the SSD arm, and this was not a statistically significant difference [Table 5]. However, 25 had achieved 50% wound healing compared to 13 on SSD, and this was statistically significant (P = 0.001).” Comment: proportion of wounds healed completely by 4 weeks given for deep dermal wounds only. No explanation of why analysis would be stratified |
| Other bias | Low risk | Comment: no other issues identified, but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: any age, TBSA >10% up to 40% Exclusion criteria: systemic diseases e.g. diabetes, or malignancy, vitamin deficient and immunosuppressed; electrical, chemical and radiation burns Participants: 100 patients from tertiary hospital Mean age (years): NR (comparable between groups) Male participants: NR (comparable between groups) Burn type: NR Burn degree NR (severity comparable between groups) Burn size (%TBSA): NR (severity comparable between groups, see inclusion criteria) Burn location: NR (comparable between groups) | |
| Interventions | Intervention arm 1: Aloe vera cream every 3rd day. N = 50 Intervention arm 2: framycetin cream every 3rd day. N = 50 Cointerventions: NR | |
| Outcomes | Wound healing (mean time to healing) | |
| Notes | Reported in abstract form only Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation to intervention was done by block randomization of 8 subjects." Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation to intervention was done by block randomization of 8 subjects." Comment: no information on whether allocation to treatment groups was concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote "Blinded randomized controlled trial." Comment: not clear who was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | No specific quote Comment: reported in abstract only and unclear whether there was any or significant attrition |
| Selective reporting (reporting bias) | Unclear risk | No specific quote; reported in abstract only; unclear if all planned outcomes were reported |
| Other bias | Unclear risk | Abstract only, unclear if any additional sources of bias |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 2 months (follow‐up) | |
| Participants | Inclusion criteria: 10‐50 years, 1st‐ or 2nd‐degree burn less than 50% TBSA Exclusion criteria: immunocompromised people; patients on chemotherapy, with renal or liver failure or with asthma Participants: 78 hospital patients Mean age (years): 34.5 vs 28.5 years Male participants: 21/37 vs 23/41 Burn type: NR Burn degree: 1st‐degree 21/37 vs 21/41; 2nd 16/37 vs 20/41 Burn size (%TBSA): < 10% 0 vs 2; 11%‐20% 7 vs 12; 21%‐30% 13 vs 10; 31%‐40% 8 vs 6; 41%‐50% 9 vs 11 Burn location: NR | |
| Interventions | Intervention arm 1: pure undiluted honey; wounds dressed daily with sterile gauze and cotton dressing applied. N = 37 Intervention arm 2: SSD; wounds dressed daily with sterile gauze and cotton dressing applied. N = 41 Cointerventions: All stabilised and given IV antibiotics (ampicillin, gentamicin, metronidazole) for minimum 10 days in 2nd‐degree and 5 days in 1st‐degree, wounds cleaned | |
| Outcomes | Primary outcome: wound healing (mean time to wound healing) | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote “after taking consent patients were randomly attributed to two study groups” Comment: no information on how randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote “after taking consent patients were randomly attributed to two study groups” Comment: no information on whether allocation of study treatment was concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Wound was assessed at third and seventh day and at the time of completion of study. Final outcome was measured after 2 months of follow‐up, in terms of complete and incomplete recovery." Comment: no information on whether assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but outcome data on time to healing reported for all 78 randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes other than "complete recovery" were not prespecified so it is unclear whether all outcomes assessed were fully reported |
| Other bias | Unclear risk | Comment: no specific quote, no evidence of other bias but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: < 12 years old, superficial thermal burn, < 50% TBSA Exclusion criteria: NR Participants: 64 hospital patients (children) Mean age (years): NR Male participants: 23/32 vs 25/32 Burn type: 56 wet burns, 8 dry burns Burn degree: NR/NA Burn size (%TBSA): < 10% 5 vs 3; 11%‐20% 2 vs 5; 21%‐30% 7 vs 8; 31%‐40% 16 vs 15; 41%‐50% 2 vs 1 Burn location: 12 facial, 20 extremities, 21 trunk and abdomen | |
| Interventions | Intervention arm 1: honey dressing (changed twice daily) N = 32 Intervention arm 2: SSD (dressing changed twice daily) N = 32 Cointervention: Thorough bath, twice daily with tap water and soap; followed by sponging and peeling away dead skin. | |
| Outcomes | Primary outcome: Wound healing (mean time to healing) Secondary outcome: Adverse events | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “two groups…. were formed and patients assigned to it randomly” Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Unclear risk | Quote: “two groups were formed and patients assigned to it randomly” Comment: no information on whether the allocation of participants to interventions was concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Culture swabs were taken from the burnt surface on admission, before any treatment was instituted and repeated after 48 h and, thereafter, every 72 h until the wound healed" Comment: no information on whether assessment of healing was conducted by assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Wound healing took 10 days in 26 patients belonging to group A, while in 6 patients it took 2 weeks or more to heal.....Wound healing took 3 weeks or more in 19 patients belonging to group B." Comment: it appears that all participants (64 randomised) completed the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but the outcomes assessed were not prespecified so it is unclear whether all outcomes assessed were fully reported; the balance of probabilities is that they were. |
| Other bias | Unclear risk | No specific quote but no evidence of other source of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: Greece Parallel‐group RCT (stratified by burn thickness) Unit of randomisation: participant Unit of analysis: participant Duration: 18 days | |
| Participants | Inclusion criteria: thermal burns with TBSA < 15% and need for hospitalisation but no need of surgical operation Exclusion criteria: cancer or diabetes Participants: 217 randomised (3 excluded for needing surgery) hospital patients Mean age (years): 42.6 vs 42.7 Male participants: 60/104 vs 71/107 Burn type: flame 57 vs 56; scald 50 vs 48 Burn degree: deep partial‐thickness: 50 vs 52; superficial 54 vs 55 (stratified randomisation) Burn size (%TBSA): NR; surface area 10.26 (4.37) vs 9.89 (4.89) (cm2) Burn location: NR | |
| Interventions | Intervention arm 1: moist exposed burn ointment (MEBO) applied twice per day. No dressings were used. N = 104 Intervention arms 2: povidone iodine applied twice per day plus bepanthenol cream applied twice daily after 3rd or 4th day (according to degree of epithelialisation). No dressings were used. N = 107 Cointerventions: burns were lightly debrided by antiseptic in the shower every second day | |
| Outcomes | Primary outcome: infections Secondary outcome: adverse events Secondary outcome: resource use (length of hospital stay) Secondary outcome: cost associated with resource use Secondary outcome: pain (VAS) | |
| Notes | Funding: most resources provided by Regional General Hospital of Athens "Georgios Gennimatas" (Greece) Department of Plastic Surgery, Microsurgery and Burn Center (equipment, stock medicines (except MEBO), and personnel) MEBO provided by MEBO International Group Company (MEBO medicines, China) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly, alteration [sic] was used of permuted 20 sub‐blocks of sizes from 1‐3 for deep partial thickness burns group and 25 sub‐blocs of the same size for the superficial partial burn groups." Comment: does not state how randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation was carried out by the staff of outpatient reception desk of the Clinic. Patient Envelopes were provided for patients requiring treatment allocation in each group. These were numbered sequentially and a list was provided with the envelopes and completed with the trial number and patient name. The date when the envelope was opened (i.e., the date of randomization) was added." Comment: the envelopes were sequentially numbered but not said to be sealed or opaque, and it's not known what the reception staff knew about the participants |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Blinding was made only for persons evaluating treatment outcomes in order to eliminate classification bias." This was not the case for pain "Blinding the treatments was not possible because Povidone iodine has a characteristic color and odor" Comment: outcome assessors were blinded to treatment allocation except for pain outcomes where participants were the assessors |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "211 (214 randomized) patients, aged between 18‐75 years were prospectively selected. Three patients were excluded because of violation of the inclusion criteria (need of surgical operation). The flow of the participants is described in Figure 1..... We did have loss of contact for the pain measurement (9th day and after) for 3 patients recovered earlier than 8th day (1 for the MEBO group and 2 for the old therapy group). These censored observations were imputed by the Method of Last Observation Carried Forward, with decreased risk of bias because the censoring occurred near the end of the follow‐up period" Comment: Figure one shows all randomised participants included in analysis; the number of participants affected by censoring was low. |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but primary outcomes and other outcomes specified and reported fully |
| Other bias | Low risk | Comment: no specific quote but no evidence of other sources of bias and detailed reporting of methods |
| Methods | Country where data collected: USA Parallel‐group RCT (stratified by TBSA and age) Unit of randomisation: participant Unit of analysis: participant Duration: 21 days | |
| Participants | Inclusion criteria: age ≥ 2 months; superficial, mid‐dermal, or mixed partial‐thickness burns, 5%‐40% TBSA, within 36 h of enrolment. Randomisation stratified by TBSA (5%‐20% or > 20% ‐40%) and age (0‐3 years or ≥ 4 years) Exclusion criteria: pregnancy; electrical, chemical, or frostbite burns; areas of burn likely to require excision/grafting; antibiotic use in 2 days prior to burn injury; evidence of inhalation injury; fractures and/or neurological injury. Participants: 84 hospital or clinic outpatients (unclear if some inpatients also included) Mean age (years): 29.4 vs 24.0 years Male participants: 27/42 vs 30/40 Burn type: scald 27/42 vs 18/40; flash 9/42 vs 13/40; flame 4/42 vs 8/40; contact, 0 vs 1; other 2 vs 0 Burn degree: superficial and mid‐dermal (N = NR) Burn size (%TBSA): 12.0% vs 10.8% (superficial 4.8% vs 4.2%; mid‐dermal BSA 8.8% vs. 8.1%) Burn location:NR | |
| Interventions | Intervention arm 1: silver hydrofibre dressing (AQUACEL Ag Hydrofiber, 1.2% weight ionic silver). Dressing overlapped wounds by 4‐5 cm. Applied in hospital/clinic on day 1 and every 2‐3 days for 21 days. Dressing covered with gauze and retention dressings. (N = 42) Intervention arm 2: SSD cream (Silvadene, 1%). 1/16” (1.6 mm) thick application. Outer dressing and dressing changes per package insert but “at least once daily”. Home dressing changes permitted between clinic visits. (N = 42) Cointerventions: procedural medications & opiates where indicated | |
| Outcomes | Primary outcome: wound healing (proportion of participants with full epithelialisation) Primary outcome: infection Secondary outcome: resource use (frequency of dressing changes) Secondary outcome: pain (VAS) | |
| Notes | Patient characteristic data refers to participants included in analysis, not numbers randomised (2 participants from 1 group excluded) Funding: ConvaTec, a BristolMyers Squibb company (manufacturer of silver dressing) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were assigned randomly to a protocol of care that included either AQUACEL® Ag dressing or silver sulfadiazine. The randomization schedule was stratified by extent of burns (5% to 20% or _20% to 40% of TBSA) and age (0–3 years or 4 years and older)” Comment: no details on how randomisation schedule was produced |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were assigned randomly to a protocol of care that included either AQUACEL® Ag dressing or silver sulfadiazine” Comment: no information on allocation concealment is mentioned |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “Study treatment was not blinded”; “Outcomes were measured at every in‐clinic dressing change until study completion or premature study discontinuation” Comment: Blinding in relation to clinical outcome assessment was not mentioned. Healthcare cost analysis was performed by an independent group but no mention of blinding. Participants weren't blinded and outcomes were assessed at in‐clinic dressing change when group assignment would have been apparent based on the dressing. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “In the AQUACEL® Ag dressing group, all 42 patients were included in the safety and intent‐to‐treat analyses. In the silver sulfadiazine group, 40 of 42 patients were included in the safety and intent‐to‐treat analyses because 2 patients did not receive study treatment. Comment: although there was incomplete data for pain and long‐term follow‐up all participants were accounted for in the ITT wound healing analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: No direct quote but the outcomes to be assessed were not prespecified in the methods so it is unclear whether they were fully reported |
| Other bias | Unclear risk | Comment: No direct quote but no evidence of other sources of bias although high levels of manufacturer involvement were noted |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (until healing) | |
| Participants | Inclusion criteria: second‐degree burn wounds (superficial or deep); in hospital within 0.5‐12 h Exclusion criteria: NR Participants: 191 hospital patients Mean age (years): (35 ± 12) vs (30 ± 9) vs (32 ± 11) Male participants: 42/65 vs 36/63 vs 35/63 Burn type: NR Burn degree: superficial 31 vs 33 vs 32; deep 34 vs 30 vs 31 Burn size (%TBSA): superficial: 38.3 ± 18.1 vs 22.5 ± 10.2 vs 28.3 ± 8.6; deep 10.1 ± 2.2 vs 6.3 ± 3.2 vs 8.2 ± 1.6) Burn location:NR | |
| Interventions | Intervention arm 1: silver nanoparticle dressing, changed every day (N = 65) Intervention arm 2: 1% SSD cream, changed every day (N = 63) Intervention arm 3: Vaseline gauze, changed every day (N = 63) Cointerventions: wounds cleaned with 0. 5% iodophor | |
| Outcomes | Primary outcome: wound healing (mean time to wound healing) | |
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Comment: result section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | Comment: The whole process of conducting this RCT was not clear |
| Methods | Country where data collected: Phillipines Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (until healing of partial‐thickness burns and readiness for skin grafting in full‐thickness burns) | |
| Participants | Inclusion criteria: aged > 4 months with TBSA > 15%, admitted within 24 h of burn injury Exclusion criteria: inhalation injury, known hypersensitivity to sulfonamides, known methemoglobinemia during the pre‐burn period Participants: 60 participants with moderate or severe burns Mean age (years): 30 (11.5) vs 24 (14.6) Male participants: 16/30 vs 20/30 Burn type: NR Burn degree: partial and full‐thickness Burn size (%TBSA): partial‐thickness 22% vs 30%; full‐thickness 5.6% vs 2.1% Burn location: face, perineum, trunk, extremities (proportions not reported) | |
| Interventions | Intervention arm 1: SSD (Flammazine) changed 2‐3 times daily for open dressings on face or perineum; daily on trunk and extremities (closed dressings) Intervention arm 2: SSD plus cerium nitrate (Flammacerium) changed 2‐3 times daily for open dressings on face or perineum; daily on trunk and extremities (closed dressings) Cointerventions: fluid and electrolyte resuscitation, wound cleansing with skin cleanser soap and water or normal saline | |
| Outcomes | Primary outcome: wound healing (partial‐thickness burns only) Primary outcome: infection and septicaemia Secondary outcome: mortality Secondary outcome: adverse events | |
| Notes | Data extraction and 'Risk of bias' assessment undertaken by translators from Portuguese Funding unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "20 patients.... were assigned consecutively to receive either SSD‐CN or SSD alone, according to a pre‐established randomized sequence" Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "20 patients.... were assigned consecutively to receive either SSD‐CN or SSD alone, according to a pre‐established randomized sequence" Comment: no information on whether allocation was concealed adequately |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The gross appearance of the burn wound was noted in all patients...... overall responses to therapy were rated in terms of wound bacterial count and time for epithelialization of partial thickness wounds or readiness of full thickness burns to accept skin grafts". Comment: no indication whether outcome assessment was performed in a blinded fashion |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes were reported. |
| Other bias | Unclear risk | It is not clear if the groups were similar regarding relevant characteristics at baseline |
| Methods | Country where data collected: USA Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days + | |
| Participants | Inclusion criteria: age: 2 months–18 years; enrolment: < 36 h post‐injury; burn severity: superficial to mid‐dermal, TBSA 1%‐40% Exclusion criteria: electrical or chemical burns; deep or full‐thickness burns; previous antimicrobial or enzymatic debridement; death likely within study period; enrolment in a previous study; pregnancy Participants: 24 children attending a paediatric hospital; mixture of inpatients and outpatients Mean age (months): 22.8 vs 43.0 Burn size (%TBSA): TBSA 1%‐10% (stated as being “comparable” between treatment arms) All other characteristics NR | |
| Interventions | Arm 1: SSD cream (Silvadene, 10 mg) 1/16” (1.6 mm) thickness every 2‐3 days Arm 2: silver hydrogel (SilvaSorb), 1/16” (1.6 mm) thickness every 2‐3 days Cointerventions: initial blister fluid drainage. Cream/gel covered with non‐adherent dressing, rolled gauze and Elasti‐net. Participants or parents were allowed to change wound dressings in outpatient cases. | |
| Outcomes | Primary outcome: wound healing: time to complete wound healing (mean time to (full) re‐epithelialisation) Primary outcome: wound healing: proportion of wounds completely healed during follow‐up ((full) re‐epithelialisation at 21 days Secondary outcome: adverse events Secondary outcome: resource use (number of dressing chances) Secondary outcome: pain (during dressing changes, measured using the Wong‐Baker Faces Pain Scale/observational pain assessment scale in infants or toddlers) | |
| Notes | Funding: Drexel University School of Medicine by Medline Industries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote “Patients were randomly assigned to a protocol of care that included either SSD cream or SilvaSorb Gel” Comment: no further details on method of randomisation |
| Allocation concealment (selection bias) | High risk | Quote: ”patients were randomly assigned to a protocol of care… without blinding of the physician investigator or other medical personnel to the type of treatment” Comment: states that physicians and other personnel were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: ”patients were randomly assigned to a protocol of care … without blinding of the physician investigator or other medical personnel to the type of treatment” Comment: mentions (unblinded) physicians as investigators, no mention of any independent assessors |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 24 participants enrolled, mean/median/SD data for 4 stated outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Quote: “Study endpoints that were recorded included the following…” Comment: wording implies that there may have been other end points, though data are given for the stated endpoints |
| Other bias | Low risk | No direct quote. no evidence of other sources of bias and study methods reasonably well reported |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days + | |
| Participants | Inclusion criteria: age 20‐40; fresh burn wound; total burn < 10% TBSA; no infection in wound; non‐joint site Exclusion criteria: NR Participants 104 hospital patients Burn degree and size: superficial 2nd‐degree 7.4 ± 1.6cm2; deep 2nd‐degree 7.7 ± 1.4cm2 vs superficial 2nd‐degree 7.1 ± 1.5cm2; deep 2nd‐degree 7.3 ± 1.3cm2 All other characteristics NR | |
| Interventions | Intervention arm 1: ionic silver dressing combined with hydrogel, changed every other day to 7 days and then covered with hydrogel. N = 52 Intervention arm 2: 1% SSD, changed every other day. N = 52 Cointerventions: anti‐infection treatment and nutrition support | |
| Outcomes | Primary outcome: wound healing (proportion completely healed) Primary outcome: infection (detection rate of wound bacteria) Secondary outcome: adverse events | |
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“This prospective randomised trial was conducted according to the random number table” Comment: a random component in the sequence generation process was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no mention of blinding of key study personnel used |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | No unit of analyses issues but reporting not sufficient to determine if other risks |
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: people attending ED with partial skin thickness burns Exclusion criteria: pregnancy, steroid or immunosuppressive therapy, diabetes, antibiotic therapy, iodine allergy; burns with more than 6 h between injury and admission, facial and perineal burns, burns > 10% TBSA; infected burns Participants: 213 people attending ED Mean age (years): NR; proportion children < 12 years 20.5 vs 20.7; detailed age breakdown also reported Male participants: NR distribution equal between groups; female:male ratio 1:1 vs 1.1.2 Burn type: steam/hot liquid 67 vs 80; flame/fumes 14 vs 10; hot object 15 vs 12; other 6 vs 9 Burn degree: NR Burn size (%TBSA): Mean NR. < 1%, 73 vs 87; 1%‐2%, 21 vs 15; 2%‐3%, 4 vs 4; 3%‐4%, 3 vs 3; 4%‐5%, 0 vs 2; > 5% 1 vs 0 Burn location: trunk and neck 11 vs 14; shoulder and proximal arms 5 vs 6; elbow and forearm 21 vs 19; wrists and hands 38 vs 42; thigh, knee and lower leg 19 vs 14; ankle and foot 8 vs 16 | |
| Interventions | Intervention arm 1: 0.5% chlorhexidine acetate BP (N = 102) Intervention arm 2: lnadine (rayon dressing with 10% povidone iodine ointment) (n = III) as required; application of cold soaks using refrigerated sterile water/saline; cleansed with Hibidil (0.25 per cent chlorhexidine gluconate in sterile aqueous solution). Blisters deroofed only if large and tense. Dressings covered with gauze and crepe bandage. Upper limb injuries were elevated in a sling. | |
| Outcomes | Primary outcome: infection (bacterial culture positive and clinical evidence) Secondary outcome: pain Secondary outcome: resource use (hospital visits) | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 213 patients who attended the Accident and Emergency Department, Royal Victoria Infirmary, Newcastle upon Tyne with partial skin thickness bums were entered into a prospective randomized (random permuted block allocation) single blind trial." Comment: insufficient information on how the randomisation sequence was derived. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A total of 213 patients who attended the Accident and Emergency Department, Royal Victoria Infirmary, Newcastle upon Tyne with partial skin thickness bums were entered into a prospective randomized (random permuted block allocation) single blind trial." Comment: no information on whether the allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "All patients were reviewed in the clinic 3 days later in the first instance and subsequently every 5 days. A data sheet was prepared for each patient and data recorded during the change of dressing according to a predetermined grading system relating to the description of the wound and/or dressings and clinical parameters". Comment: no information on whether outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no specific quote but no information on whether all patients were involved in most analyses; children were specifically excluded from assessment of pain and a total of 24% of participants were not included for this outcome |
| Selective reporting (reporting bias) | High risk | Quote: "Mean scores for pain and wound characteristics were calculated for each patient." Comment: it was not clear whether these (and dressing performance) were planned as the only assessed outcomes; the outcomes that they planned to assess appear to be listed on the datasheet (fig 1) ‐ this includes healing, which is not properly reported (e.g. "there were no differences in the other parameters") |
| Other bias | Unclear risk | Comment: no specific quote, no evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: Up to 14 days | |
| Participants | Inclusion criteria: people with partial‐thickness burns covering < 10% TBSA Exclusion criteria: burns to face and hands Participants: 32 individuals with burns (no further information) Mean age (years): 2.6 (includes 0 adults) versus 20.6 (includes 5 adults) Male participants: NR Burn type: scald 25, flame 6, contact 1 (numbers approximately equal between groups) Burn degree: partial‐thickness Burn size (%TBSA): 1.8 ± 0.8 vs 2.3 ± 0.6 Burn location: NR | |
| Interventions | Intervention arm 1: silver‐impregnated porcine xenograft (E‐Z Derm) N = 16 Intervention arm 2: petroleum gauze (Jelonet) N = 16 Cointerventions: NR | |
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events (need for surgery) | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization to either the E‐Z Derm or Jelonet groups was by drawing a card from a sealed envelope." Comment: unclear how the randomisation process was designed and implemented so unclear if truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization to either the E‐Z Derm or Jelonet groups was by drawing a card from a sealed envelope." Comment: unclear whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "All of the burns in both groups were assessed for the following: I. The need for surgical intervention to achieve healing............2. The time to spontaneous healing was noted in those patients not requiring surgical treatment. 3. Laboratory reports of significant growths of pathogenic microorganisms on culture of superficial wound swabs" Comment: no indication that assessment was carried out in a blinded manner |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all randomised participants appeared to be included in the analysis (based on tables) |
| Selective reporting (reporting bias) | Low risk | Quote: "All of the burns in both groups were assessed for the following: I. The need for surgical intervention to achieve healing, indicated by clinical evidence of an increase in burn depth and lack of evidence of spontaneous healing by 10‐14 days. 2. The time to spontaneous healing was noted in those patients not requiring surgical treatment. 3. Laboratory reports of significant growths of pathogenic microorganisms on culture of superficial wound swabs." Comment: specified outcomes were properly reported. |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: Germany RCT with intra‐individual design Unit of randomisation: burn Unit of analysis: burn Duration: 21 days | |
| Participants | Inclusion criteria: 2 partial‐thickness burn wounds of comparable size, location and prior treatment, ≤ 3 days from injury; TBSA ≤ 50%; wound area between 36 cm2 ‐300 cm2; upper body injuries needed to both occur on wither ventral or dorsal side Exclusion criteria: infected wounds at study onset, wounds in the axillary or inguinal region, deep body folds or a distinctive adipose tissue region Participants: 43 participants with 2 comparable burns Mean age (years): NR Male participants: NR Burn type: NR Burn degree: partial‐thickness Burn size (%TBSA): 11.1 ± 7.7 (79.2 cm2 vs 77.3 cm2) Burn location: NR | |
| Interventions | Intervention arm 1: polyvinylpyrrolidone iodine liposome hydrogel (Repithel) (3% PVP‐iodine, 3% phospholipin 90 H liposome). Applied once daily as 2 mm layer covered with paraffin gauze dressing. N = 43 Intervention arm 2: SSD (10 mg/g). Applied once daily as 2 mm layer covered with paraffin gauze dressing. N = 43 Cointerventions: no additional topical treatments | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: adverse events | |
| Notes | Funding: Mundipharma GmbH (manufacturer) This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization list was prepared by the statistics department from Mundipharma GmbH, using the EDP program Rancode 3.6.” Comment: computer‐generated randomisation list is classed as low in terms of risk‐of‐bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “After written informed consent was obtained, patients were enrolled and the 2 burn wounds to be assessed were randomized to treatment with the liposome PVP‐I hydrogel Repithel or the silver‐sulfadiazine cream Flammazine.” Comment: no explicit mention of allocation concealment |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “A limitation to this study is the fact that, due to the characteristic coloring of PVP‐I, this was not a blinded study." Comment: unblinded study |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: “Forty‐three patients comprised the full analysis set (intent‐to‐treat) and 39 patients completed the study per protocol. Protocol violations were wounds older than 3 days in 2 patients and lack of comparability of wounds or a full‐thickness (degree IIb/III) burn wound in 1 patient each.” Comment: no unexplained loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Quote: “The clinical assessment of study wounds included inflammation (secretion, reddening, coating) and healing tendency (very good, good, moderate, none).” Comment: some uncertainty about the above statement – the word “included” implies there may possibly have been more outcomes assessed. |
| Other bias | Unclear risk | Comment: it was unclear whether the analysis took account of the intra‐individual design |
| Methods | Country where data collected: China Parallel‐group RCT (multicentre) Unit of randomisation: participant Unit of analysis: burn Duration: 20 days | |
| Participants | Inclusion criteria: men and women aged 18‐65 years with burn wounds unhealed 3 weeks after injury (residual burn wounds) Exclusion criteria: serious complications of heart, liver, kidney or blood system (blood production or bleeding issues); serious complications, shock or serious systemic infection; uncontrolled diabetes, pregnancy or breast feeding, allergy to solver ions; other reason unable to complete observation period Participants: 111 participants randomised, 98 analysed with 166 burns Mean age (years): NR Male participants: NR Burn type: NR (residual wound) Burn degree: NR Burn size (%TBSA): NR | |
| Interventions | Intervention arm 1: nanocrystalline silver dressing (Acticoat) changed once daily where redness, swelling and high levels of exudate, otherwise every 3 days. Auxilliary dressing over intervention dressing. (83 burns analysed) Intervention arm 2: SSD (5 g per 80 cm2) changed once daily. (83 burns analysed) Cointerventions: washing/rinsing of wounds with sterile water | |
| Outcomes | Primary outcome: wound healing Primary outcome: change in infection status Secondary outcome: adverse events | |
| Notes | Data extracted from English language publication; 2 additional Chinese language publications Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A multi‐center, randomized experimental design is adopted, with blinding and positive parallel control. The clinical trial was done in four burn centers throughout the country at the same time with the same experimental design. The observing doctor hands out the dressing to every patient according to the time that they come to the hospital and to a randomized serial number." Comment: no information on how the randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A multi‐center, randomized experimental design is adopted, with blinding and positive parallel control. The clinical trial was done in four burn centers throughout the country at the same time with the same experimental design. The observing doctor hands out the dressing to every patient according to the time that they come to the hospital and to a randomized serial number." Comment: insufficient information on whether the allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Standards for the healing of wound: the wound healed was determined by inspection by two doctors." Comment: no information on whether the outcome assessors were blinded (although the trial is described as blinded) |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Altogether 111 patients were enrolled in this group, in the process of the trial, 13 patients were dropped out of the study. Among them two patients were dropped out because of silver allergy. Eight were removed because they left to their local clinic before the wound healed, therefore we do not have their related records. Three patients were dropped because of liver dysfunction. The remaining 98 patients who were included in the statistical analysis had altogether 166 residual wounds" Comment: 13/111 participants were not included in the analysis. The event rate was high so although there is potential for differential missing data the impact on the effect estimate was probably small. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "This study is to investigate the efficacy and safety of nanocrystalline silver (Acticoat) in the treatment of burn wounds, and to assess the clinical value of this dressing." Comment: no specification of how efficacy and safety was to be assessed so difficult to determine if all planned outcomes were reported. However a statistical analysis for wound healing rate was pre‐specified and presented |
| Other bias | High risk | Comment: unit of analysis issues arising from randomisation at the participant level and analysis at the level of the burn (multiple burns for some participants) |
| Methods | Country where data collected: Canada Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (duration of healing up to mean 26 days) | |
| Participants | Inclusion criteria: age > 1 year; full‐thickness burns; < 24 h post injury Exclusion criteria: prior topical antibiotic treatment, pregnant, allergic to sulfa drugs Participants: 121 analysed, N randomised unclear Mean age (years): 31 ± 21 vs 33 ± 25 Male participants: NR Burn type: flame 35 vs 38; scald 8 vs 20; electrical contact 3 vs 1; other 8 vs 8 Burn degree: full‐thickness Burn size (%TBSA): full‐thickness 13 ± 16 vs 10 ± 11 Burn location: perineal 10 vs 9 (9 vs 5 full‐thickness); inhalation injury 10 vs 16 (ventilator 7 vs 9) | |
| Interventions | Intervention arm 1: SSD (1%) plus chlorhexidine digluconate (0.2%) cream (Silvazine); "buttered on to wound and/or wound dressed with "buttered" cotton gauze. 54 participants Intervention arm 2: SSD (1%) (Flamazine) buttered on to wound and/or wound dressed with "buttered" cotton gauze. 67 participants Cointerventions: antibiotics as appropriate; daily bathing with non‐antibacterial soap and wound debridement, wound excision as appropriate | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: mortality (overall, infection‐related) Secondary outcome: adverse events | |
| Notes | A list of exclusions are presented that appear likely to account for post‐randomisation withdrawals, number randomised unclear Funding: British Columbia Professional Firefighters Association; Smith & Nephew Canada An additional paper (Snelling 1991) reported additional participants but it appeared that these participants were not randomised to the intervention groups and so are not reported here. The reference is provided as a secondary citation for the study. Funding: British Columbia Professional Firefighters Association and Smith and Nephew Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to receive either Silvazine or Flamazine”. Comment: no detail on randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to receive either Silvazine or Flamazine”. Comment: no detail on allocation concealment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “Wounds were cultured with a swab once or twice weekly with twice weekly cultures being taken from most patients whose wounds involved more than 10 per cent of the body surface. Surface cultures were obtained at each culture session. Full‐thickness burn wound biopsies were also obtained, and examined for histological evidence of bacterial invasion into dermis or fat and quantitative bacterial counts determined.” Comment: no information on blinding of assessors |
| Incomplete outcome data (attrition bias) | High risk | “Patients who did not survive for 7 days, who had all eschar excised before day 7, who were discharged before day 7 or who went on to heal all of what was initially diagnosed as the full‐thickness component of the burn wound were excluded from the study group.” Comment: excluded participants would more usually be handled as part of an ITT population. As such, their exclusion is a potential source of bias. |
| Selective reporting (reporting bias) | Unclear risk | Quote: “The clinical assessment of study wounds included inflammation (secretion, reddening, coating) and healing tendency (very good, good, moderate, none).” Comment: some uncertainty about the above statement – the word “included” implies there may possibly have been more outcomes assessed |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant (one wound per participant) Duration: until healed | |
| Participants | Inclusion criteria: fresh burn wound; total burn 10%‐20% TBSA; no other serious injury; no other major diseases (including cancer, brain disease; heart disease; kidney disease; haematological system disease; and infection); admitted to hospital within 24 h of injury Exclusion criteria: NR Participants 76 hospital patients Male/female: 44/76 (24/38 vs 20/38) Age: 18‐58 (36.8 ± 14.2) (36.5 ± 11.8 vs 36.8 ± 13.2 %TBSA: 15.2 (4.3) Burn degree: superficial: 19 vs 22; deep 19 vs 16 All burns were located around knee areas | |
| Interventions | Intervention arm 1: nano‐silver dressing (N =38) Intervention arm 2: ordinary sterile gauze (N = 38) Co‐interventions: human epidermal growth factor was coated on the surface of the wound; dressing was changed every other day | |
| Outcomes | Primary outcome: wound completely healed Primary outcome: infection ‐ bacterial positive rate at different time points Secondary outcome: adverse events; scar hyperplasia | |
| Notes | Paper in Chinese; data extraction and 'Risk of bias' assessment performed by one review author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomised table was used" Comment: not clear how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details to indicate whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no details of outcome assessment were given |
| Incomplete outcome data (attrition bias) | Low risk | Comment: result section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear based on paper; protocol not obtained |
| Other bias | Unclear risk | Comment: no specific quote, no evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: Iran RCT with intra‐individual design Unit of randomisation: burn Unit of analysis: burn Duration: 24 days | |
| Participants | Inclusion criteria: 2 comparable second‐degree ("same site") burns e.g. on hands or feet with similar areas Exclusion criteria: electrical or chemical burns, diabetes, pregnancy, immunodeficiency, kidney disease Participants: 30 participants with 2 comparable burns Mean age (years): 33 (± 11) Male participants: 25/30 Burn type: NR Burn degree: 2nd degree Burn size (%TBSA): 19.8 ± 7.9 Burn location: 26 burns on right and left hand, 2 on right and left foot, 2 on right or left hand | |
| Interventions | Intervention arm 1: 0.5% A vera cream produced from powder applied twice daily. 30 burns Intervention arm 2: SSD (concentration not explicitly stated) applied twice daily. 30 burns Cointerventions: wound cleaning with water and saline; dressings; fluid resuscitation; "other treatment protocols"; oral nutrition; occasional amino acid infusions; blood products | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection | |
| Notes | Funding: Mazandaran University, Iran This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Each patient had one burn treated with topical SSD and one treated with aloe cream, randomly.” Comment: no further details on randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Each patient had one burn treated with topical SSD and one treated with aloe cream, randomly.” Comment: no further details on allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: ”At the time of each dressing, the wound was observed clinically for signs of infection, size, and rate and nature of epithelialization by an expert surgeon. In this study, the “B” part of the body was treated with SSD and the “A” part was treated with aloe cream. Patients and nursing staff were blinded to the procedure.” Comment: no mention of blinding of the surgeon/assessors |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Finally, 30 patients were enrolled in this study.” Comment: 30 participants included in outcome reporting |
| Selective reporting (reporting bias) | Unclear risk | “At the time of each dressing, the wound was observed clinically for signs of infection, size, and rate and nature of epithelialization by an expert surgeon.” Comment: results of visual infection checks not reported (though the study does report on microbial swab contamination) |
| Other bias | Unclear risk | Comment: unclear whether analysis took into account the intra‐individual design |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR (until healing) | |
| Participants | Inclusion criteria: people with deep second‐degree burn wounds 1%‐12% TBSA and aged 16‐70 Exclusion criteria: NR Participants: 115 hospital patients Mean age (years): NR Male participants: 84/115 Burn type: NR Burn degree: second‐degree Burn size (%TBSA): NR (about 100 cm2) Burn location: NR | |
| Interventions | Intervention arm 1: Moist burn ointment (MEBO) every 6 h. N = 31 Intervention arm 2: 0.25% iodophor every 6 h. N = 24 Intervention arm 3: 1% Rivanol every 6 hs. N = 22 Intervention arm 4: SSD every 6 h. N = 38 Cointerventions: antibiotics for 3‐10 days | |
| Outcomes | Primary outcome: wound healing Secondary outcome: cost | |
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: NR Exclusion criteria: NR Participants: 277 hospital patients with superficial, deep or residual burn wounds Mean age (years): 30.3 (range 5‐74) Male participants: NR Burn type: NR Burn degree: superficial 46 vs 16; deep 89 vs 32; residual 68 vs 26 Burn size (%TBSA): 3.4 ± 0.6 (range 0.1‐6.0) Burn location: trunk and limbs | |
| Interventions | Intervention arm 1: carbon fibre dressing changed daily Intervention arm 2: 0.5% iodine gauze changed daily Cointerventions: NR | |
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events | |
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear |
| Methods | Country where data collected: China Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: NR (until healing) | |
| Participants | Inclusion criteria: second‐degree burns (superficial or deep) within 72 h of injury; TBSA ≤ 60% Exclusion criteria: general infection, pregnancy, patients with serious heart, kidney or liver disease (AST > 1.5; ALT > 1.5); "mental disease" Participants: 120 hospital patients Mean age (years): NR Male participants: 99/120 Burn type: NR Burn degree: second‐degree; superficial/deep 80/40 Burn size (%TBSA): NR about 100 cm2 Burn location: NR | |
| Interventions | Intervention arm 1: 0.1% silver nitrate changed every other day Intervention arm 2: 1% SSD changed every other day Cointerventions: wound cleansing with isotonic saline; treatment duration 14 days for superficial wounds, 28 days for deep wounds | |
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events | |
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear including whether the analysis took account of the intra‐individual design |
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 3 months' follow‐up | |
| Participants | Inclusion criteria: partial‐thickness (superficial thermal) burn, < 40% TBSA Exclusion criteria: NR Participants: 100 hospital patients Mean age (years): 25.2 vs 26.4 Male participants: 23 vs 25 Burn type: flame 43 vs 39; scald 7 vs 11 Burn degree: NR Burn size (%TBSA): 14.5 (10‐40) vs 15.6 (10.5‐40) Burn location: NR | |
| Interventions | Intervention arm 1: honey applied in quantity 16 mL‐30 mL on alternate days after saline wash. Wound covered with dry gauze Intervention arm 2: mafenide acetate‐impregnated gauze over wound after saline wash. Changed daily. Cointerventions: wound cleansing with saline; 1% lidocaine before biopsy | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were allocated at random” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were allocated at random” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The wounds were inspected every two days until healing…..the amount of discharge, any foul smell, the type of granulation tissue and signs of healing, and the time taken for healing were noted. The wounds were observed for evidence of infection, excessive exudate, or leakage until healing…" Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “two groups of 50 randomly allocated patients” Comment: no withdrawals reported and Tables 2 and 3 suggest that all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Quote: “a clinical and histochemical comparison of burns treated with honey dressing and with mafenide acetate in order to assess their wound healing rates” Comment: all stated outcomes of interest were reported |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: Pakistan Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: NR | |
| Participants | Inclusion criteria: partial‐thickness burns in 2 different parts of the body (same site, e.g. right and left abdomen) occurred within 24 h of treatment initiation. TBSA < 40% Exclusion criteria: diabetes, pregnancy, immunodeficiency, kidney diseases; electrical and chemical burns Participants: 150 hospital patients Mean age (years): 28 ± 16 Male participants: 67/150 Burn type: NR Burn degree: NR Burn size (%TBSA): 22.7 ± 8.5 (10‐38) Burn location: NR but same site/equivalent) | |
| Interventions | Intervention arm 1: honey applied directly to wound twice daily; dressing changed twice daily Intervention arm 2: SSD applied daily Cointerventions: fluid resuscitation, oral nutrition, occasional IV infusion of amino acids and blood products | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection | |
| Notes | Funding: NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Each patient had one burn site treated with honey and one treated with topical SSD, randomly” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Each patient had one burn site treated with honey and one treated with topical SSD, randomly” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “wound was observed clinically for signs of infection, size, and rate and nature of epithelialization by an expert surgeon…. Patients and nursing staff were blinded to the procedure” Comment: nursing staff were blinded but unsure whether the inspecting surgeon was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “150 patients were enrolled in this study” Comment: no withdrawals reported and Table 2 suggests that all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Quote: “rate of burn wound healing” Comment: all stated outcomes of interest were reported |
| Other bias | Unclear risk | Comment: it was unclear whether the analysis took account of the intra‐individual design of the study |
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 6 weeks' treatment; follow‐up at 6 months | |
| Participants | Inclusion criteria: superficial and partial‐thickness burns, TBSA < 15% Exclusion criteria: deep burns; any medical illness beginning before or after injury Participants: 50 surgical hospital outpatients Mean age (years): 27.4 Male participants: NR (both men and women were included) Burn type: NR Burn degree: NR Burn size (%TBSA): NR Burn location: NR | |
| Interventions | Intervention arm 1: pure honey applied once daily after wound cleansing with normal saline. N = 25 Intervention arm 2: 1% SSD cream once daily. N = 25 Cointerventions: wound cleansing with normal saline; sterile gauze dressings | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: costs Secondary outcome: adverse events | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... 50 patients were selected for the study. They were randomly assigned to two groups" Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "... 50 patients were selected for the study. They were randomly assigned to two groups" Comment: no information on whether the allocations to treatment were adequately concealed |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "At the time of change of dressing details regarding the condition of the wound such as signs of wound infection, condition of surrounding unburned tissues, discharge, smell, necrotic tissue and state of epithelialization was noted. Swabs for bacterial density and cultures were also obtained regularly. Subjective factors such as pain and local irritation were recorded regularly. Allergies or other side effects were noted in both groups." Comment: appears that blinded assessment could not have occurred as observations were undertaken when dressings were changed |
| Incomplete outcome data (attrition bias) | Low risk | Quote: " In group I treated with honey, 52% (n=13) of the patients had all the burns healed after 2 weeks and 100% (n=25) got cured after 4 weeks. In group II treated with 1% silver sulfadiazine, 20% (n=5) of the patients had their burns healed after 2 weeks, 60% (n=15) after 4 weeks and 100% (n=25) were cured by the end of 6 weeks of the treatment." Comment: results reported for all 50 randomised participants |
| Selective reporting (reporting bias) | Low risk | Quote: "The effectiveness of the two modalities of treatment was judged on the basis of three criteria: 1. Wound healing. 2. Pain relief. 3. Time taken for the wound to get sterile." Comment: all 3 prespecified outcomes were fully reported |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: aged 4‐62 years, superficial‐dermal, mid‐dermal or deep‐dermal burns 10%‐40% TBSA Exclusion criteria: people with chemical or electrical burns, superficial burns, full‐thickness burns or burns involving > 40% TBSA Participants: 80 Mean age (years): Male participants: 54/80 Burn type: NR (not chemical or electrical) Burn degree: superficial 18 vs 12, mid‐dermal 6/8, deep‐dermal 16/20 Burn size (%TBSA): 10%‐15% 18 vs 12; 16%‐25% 14 vs 20; 26%‐40% 8 vs 8 Burn location: NR | |
| Interventions | Intervention arm 1: natural, unprocessed honey‐gauze dressings every other day Intervention arm 2: SSD dressings (SSD cream covered with occlusive dressing) every other day | |
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding source NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were allotted at random in two different groups” Comment: in addition, it was reported in the abstract that the design was “a quasiexperimental study” The method for generating the random sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The patients were allotted at random in two different groups”. Comment: there was no information on whether allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no quote but no information on blinding reported |
| Incomplete outcome data (attrition bias) | Low risk | Coment: ITT analysis was not reported, but since no drop‐outs were reported and all the randomised participants completed the study, ITT analysis was assumed to have been done and to be acceptable |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the important outcome measures stated in the methods section were reported in the results |
| Other bias | Unclear risk | Insufficient reporting to determine the risk of other sources of bias |
| Methods | Country where data collected: Thailand Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: partial‐thickness burns < 25% TBSA Exclusion criteria: NR Participants: 50 people attending burns unit Mean age (years): 38 ± 25 vs 26 ± 27 Male participants: NR Burn type: flame 14 vs 12; scald 9 vs 12; electrical 1 vs 1; chemical 1 vs 0 Burn degree: NR Burn size (%TBSA): 15 ± 7 vs 15 ± 5 Burn location: NR | |
| Interventions | Intervention arm 1: silver‐coated dressing moistened with sterile water (Acticoat), covered with dry dressing. Inner gauze moistened twice daily and silver dressing changed every 3 days Intervention arm 2: SSD and dry gauze dressing changed twice daily Cointerventions: 2 tabs of acetaminophen (paracetamol) (500 mg/tab) before dressing changes | |
| Outcomes | Primary outcome: wound infection Secondary outcome: pain | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Fifty patients were identified and randomized into 2 groups” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Fifty patients were identified and randomized into 2 groups” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “A swab of wounds was sent for routine culture and sensitivity twice a week. Wounds were observed daily by an experienced burn surgeon for signs of infection such as erythema, induration, purulent discharge and malodor. Swabs were processed by the laboratory and returned results of 1+, 2+, or 3+ bacterial growth, corresponding to light, medium, or heavy growth on the culture plate ” Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: “Fifty patients were identified and randomized into 2 groups” Comment: no direct quotes on any withdrawals or whether outcome data was used for all 50 patients |
| Selective reporting (reporting bias) | High risk | Quote: “Patients were also reviewed for documentation of efficacy of treatment including day of burn wound closure, pain scores, type of cultured organisms, wound colonization and infection, surgical procedures and mortality between both groups” Comment: no information on day of wound burn closure |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: Thailand Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: partial‐thickness burn (superficial second‐degree) within 24 h of enrolment and < 15% of TBSA Exclusion criteria: concomitant trauma, chemical and electrical burns, and serious comorbidity were excluded Participants: 70 people attending outpatient burns unit Mean age (years): 34.9 vs 42.3 years Male participants: 5 (42.9%) vs 17 (48.6%) Burn type: flame 8 vs 7/scalded 27 vs 28 Burn degree: 2nd‐degree Burn size (%TBSA): NR Burn location: NR | |
| Interventions | Intervention arm 1: hydrofibre dressing coated with ionic silver (Aquacel Ag) with 1 cm overlap, covered with a layer of plain gauze, changed every 3 days. N = 35 Intervention arm 2: SSD and gauze dressing, changed daily. N = 35 Cointerventions: wound cleansing with saline, blisters removed | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: resource use | |
| Notes | Funding: Faculty of Medicine Siriraj Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomized by computer and assigned into two groups according to the burn wound treatment” Comment: computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomized by computer and assigned into two groups according to the burn wound treatment” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote “Dressings were evaluated …..on postburn day 1 and then every 3 days until the wound healed. At each evaluation after the dressing was removed, the burn wound was inspected for wound healing and change in depth and infection……Burn wounds were also observed daily by the experienced burn surgeon. After each burn dressing change in both groups, the performance characteristic photograph and questionnaire were recorded." Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: “Seventy patients were enrolled in the study and randomly assigned into two groups” Comment: no direct quotes on any withdrawals or whether outcome data was used for all 70 participants |
| Selective reporting (reporting bias) | Low risk | Quote: “The primary endpoint of this study was time‐to‐wound healing, defined as spelling [sic] of the wound. Secondary endpoints included pain assessment by patients’ pain scores during wound dressing…... Total dressing cost was divided into hospital charges including hospital fee, dressing cost and pain medication and transportation cost …for each hospital visit.” Comment: all stated outcomes of interest were reported |
| Other bias | Low risk | Comment: no direct quotes but no evidence of additional sources of bias with reasonable level of reporting |
| Methods | Country where data collected: Iran Intra‐individual RCT Unit of randomisation: burn Unit of analysis: burn Duration: 30 days | |
| Participants | Inclusion criteria: aged 16–65 years, diagnosed by the same expert emergency burn physician based on the presentation of two same sites of second‐degree burns. The burn should have occurred within 24 h before the beginning of treatment, second‐degree burn on 2 sides of the same person's body, and with < 15% TBSA Exclusion criteria: People with epilepsy, diabetes, immunodeficiency disease, electrical and chemical burns, known allergy and sensitivity to either AEO or SSD, or pregnant women were excluded from the study Participants: 49 randomised; 45 analysed Mean age (years): 39.9 (SD 15.6) Male participants: NR but "most participants were women" Burn type: scalds 30; flame 14; contact 1 (analysed participants only) Burn degree: second‐degree Burn size (%TBSA): 3.7 (SD 2.4; range 1‐13) (analysed participants only) Burn location: 44% involved lower limbs (analysed participants only) | |
| Interventions | Intervention arm 1: Arnebia euchroma ointment (AEO) Intervention arms 2: SSD Cointerventions: after admission and primary preparation, the wounds were washed with normal saline or sterile water and dried with sterile gases | |
| Outcomes | Primary outcome: proportion of wounds healed at day 13 and mean time to wound healing (re‐epithelialisation) Primary outcome: signs of clinical infection rated on 6‐point scale from 0 = absent to 5 = all components present Secondary outcome: adverse events defined as erythema, edema, infection, inflammation, and general wound appearance Secondary outcome: pain and itching during first 15 minutes of dressing change measured using a 10‐point VAS | |
| Notes | Funding: grant (118‐92) from Mazandaran University of Medical Sciences, Sari, Iran This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "These areas were randomly assigned to AEO treatment and the opposite site was treated with conventional treatment with SSD cream. A simple coin‐based randomization was performed for each patient after enrolment by the blinded staff nurse." Comment: the randomisation sequence was generated by an acceptable method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "These areas were randomly assigned to AEO treatment and the opposite site was treated with conventional treatment with SSD cream. A simple coin‐based randomization was performed for each patient after enrolment by the blinded staff nurse." Comment: not clear whether the allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The general condition of the wound areas were first observed and evaluated by the expert emergency burn physician and the Burn unit special nurse prior to utilization of topical agents. Thereafter, before each dressing, the wounds were assessed by same team who were unaware of the assigned treatment to each side and the ointment applied on the wounds for treatment." Comment: appears that outcome assessment was performed by individuals blinded to treatment allocation and separate from those applying dressings |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "a total of 51 eligible patients were registered. Forty‐nine of them signed the consent form and were randomly allocated sequentially to the two sides and two treatment groups. Four patients were lost to follow up. Therefore, 45 patient's results were eligible for data analysis..... In addition, 1 patient in both groups needed bilateral skin graft on the day of 11th according to the plastic surgeon's decision. Furthermore, 2 patients in the SSD group needed skin graft from days 11–14, but their treatment area on the opposite area with AEO healed after 5 and 7 days, respectively" Comment: of the 49 randomised participants 4 were not included in the analysis; each participant was lost from both groups equally; all other participants' data were included in the analysis for each group |
| Selective reporting (reporting bias) | Unclear risk | Comment: the outcomes to be assessed were not defined in the methods section so it is not clear whether all planned outcomes were fully reported |
| Other bias | Unclear risk | Comment: there is no evidence of additional sources of bias; it is not clear whether the paired data were accounted for in the analysis |
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: people with blistered burns Exclusion criteria: burns on face, hands or feet or injury > 12 h before attendance Participants: 51 people attending the ED Mean age (years): children 3.4 ± 3 vs 2.4 ± 3; adults 39 ± 20 vs 40 ± 18 Male participants: 10 vs 12 Burn type: scald 23 vs 22; other 2 vs 4 Burn degree: NR Burn size (%TBSA): 1.83 ± 1.5 vs 1.58 ± 1 Burn location: NR | |
| Interventions | Intervention arm 1: paraffin gauze impregnated with chlorhexidine (Bactigras), covered by an absorbent dressing. N = 25 Intervention arm 2: plastic film (Opsite). N = 26 Cointerventions: removal of large blisters prior to treatment | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: pain | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A consecutive series of patients with blistered burns who attended the A/E Department were randomly selected to receive either a standard dressing or a plastic film." Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A consecutive series of patients with blistered burns who attended the A/E Department were randomly selected to receive either a standard dressing or a plastic film." Comment: no information on whether the allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The endpoint taken was when the wounds were dry and epithelialised, needing only a dry protective dressing. Bias was minimised by having a specific endpoint and using the confirmatory judgement of assessors not directly involved in trial." Comment: it appears that the assessors were blinded to treatment allocation for the outcome of healing. However it is unclear whether the assessments of pain (by participants) and infection (by healthcare professionals) were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Fig. 1 shows that most of the patients' wounds had healed within sixteen or seventeen days" Comment: Figure 1 and the table which accompanies it show cumulative healing for all 51 randomised participants. |
| Selective reporting (reporting bias) | Low risk | Quote: ".....the following parameters were studied: the rate of healing, the rate of infection, and the degree of pain and social inconvenience." Comment: data were reported on all the prespecified parameters although it was not clear that planned methods for data management were followed |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: China Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: 28 days | |
| Participants | Inclusion criteria: deep partial second‐degree burn wounds < 60% TBSA; age 18‐65; presented within 24 h of injury Exclusion criteria: complications; other disease; pregnancy; multiple trauma or serious comorbidity Participants: 20 participants with 2 comparable burns Mean age (years): NR Male participants: 12/20 Burn type: NR Burn degree: 2nd degree Burn size (%TBSA): 24.1 ± 0.2 Burn location: NR | |
| Interventions | Intervention arm 1: sodium hypochlorite (Dermacyn) changed every other day. N = 20 burns Intervention arm 2: SSD changed every other day. N = 20 burns Cointerventions: NR | |
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events | |
| Notes | Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it was not stated how the randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | The whole process of conducting this RCT was not clear, including whether the paired data were accounted for in the analysis |
| Methods | Country where data collected: Netherlands Parallel‐group RCT (multicentre) Unit of randomisation: participant Unit of analysis: participant Duration: 21 days; follow‐up to 12 months | |
| Participants | Inclusion criteria: adults (aged > 18 years) with acute facial burns (thermal or electrical injuries involving face including scalp, ears and jaw line); neck included only if facial burn extended into it Exclusion criteria: facial burns < 0.25% TBSA; hospitalised for < 72 h; started with topical treatment before admission; unable to consent Participants: 154 (179 originally randomised) participants from 3 dedicated burns centres Mean age (years): 41.9 ± 16.9 vs 41.3 ± 14.5 Male participants: 64 vs 61 Burn type: scald 4 vs 3; flame 70 vs 60; contact 1 vs 2; electrical 2 vs 4; other 1 vs 7 Burn degree: NR Burn size (%TBSA): median 9.8 (IQR 5.0‐19.4) vs 9.3 (4.5‐17.0); facial 3.0 (2.0‐4.5) vs 3.0 (2.0‐4.5) Burn location: facial | |
| Interventions | Intervention arm 1: SSD 10 mg/g plus cerium nitrate 22 mg/g (Flammacerium) at admission and once daily for 48‐72 h. Wounds were then washed daily with chlorhexidine, rinsed with water and left open. N = 78 Intervention arm 2: SSD 10 mg/g (Flammazine) once daily, covered with plain gauze dressing and a fixation dressing until healed. N = 76 Cointerventions: treatment protocols in clinical practice in Dutch burn centres. Washing with chlorhexidine gluconate (Hibiscrub and rinsing with water) | |
| Outcomes | Primary outcome: wound healing secondary outcome: pain secondary outcome: mortality | |
| Notes | Funding: Dutch Burns Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To this end, an allocation sequence was developed according to center using mixed randomization (M.N.). Prespecified inequality ranged from two to four, and block sizes varied from four to 11. Randomization sequences were generated with a random numbers table." Comment: an appropriate method was used to generate the randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed from the physician enrolling patients, and subversion was prevented by using nontransparent envelopes." Comment: Does not specifically state sealed envelopes but appears to be appropriate allocation concealment |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "It was not possible to guarantee blinding of the observers to treatment allocation because of the presence and/or involvement in clinical care of most observers. The data analysts (I.O. and M.B.) were blinded." Comment: stated that assessors could not be guaranteed to be blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No specific quote but all randomised participants were accounted for in comprehensive flow diagram. There were 25 post‐randomisation exclusions for clearly documented reasons mostly related to protocol violations. These were balanced between the groups. 4 deaths occurred (3 vs 1) but these participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Quote: "Primary outcomes were number of patients requiring surgical intervention and time to complete wound healing......Secondary outcomes consisted of wound colonization, pain, and aesthetic and functional aspects." Comment: all specified outcomes were fully reported |
| Other bias | Low risk | Comment: no specific quote but no other sources of bias identified and good level of reporting |
| Methods | Country where data collected: Thailand Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: partial‐thickness burn, less than 24 h post‐burn injury, TBSA < 15% Exclusion criteria: pregnancy, immunocompromised patients and hypersensitivity to treatments used Participants: 65 Mean age (years): 42.31 ± 23.49 vs 31.03 ± 19.76 Male participants: 15 vs 21 Burn type: flame 8 (23%) vs 18 (60%)/dcald 27 (77%) vs 10 (33%)/other (chemical, contact burn 0 (0%) vs 2 (7%) Burn degree: NR Burn size (%TBSA): 2.77 ± 0.41 vs 7.93 ± 1.8 Burn location: upper limb 31% vs 53%/lower limb 46% vs 33%/hand 11% vs 3%/other 12% vs 11% | |
| Interventions | Intervention arm 1: 1% SSD (1% AgSD) covered with dry gauze dressing changed every day until complete wound closure. N = 35 Intervention arm 2: Alginate silver dressing (Askina Calgitrol Ag) changed every 5 days until complete wound closure. N = 30 Cointerventions: none reported | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Sixty‐five patients were identified and randomised into two groups” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Sixty‐five patients were identified and randomised into two groups” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “clinical assessment was evaluated by two experienced burn surgeons” Comment: no information on whether outcome assessors were blinded as to allocation of treatment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Sixty‐five patients were identified and randomised into two groups” Comment: no withdrawals reported and Table 2 suggested that all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Quote: “pain scores, number of wound dressing change, nursing time and time of burn wound healing” Comment: all stated outcomes of interest were reported |
| Other bias | Low risk | Comment: no direct quotes but no evidence of additional sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 14 days | |
| Participants | Inclusion criteria: thermal second‐degree burns < 5% TBSA, which occurred in preceding 48 h with no other injuries Exclusion criteria: renal, hepatic, endocrine, cardiovascular or cerebrovascular disease, pregnancy, drug/alcohol abuse and concurrent use of antibiotics, steroids or immunosuppressive drugs Participants: 120 people with burns (setting NR) Mean age (years): 33.6 ± 13.4 vs 37.4 ± 12.7 Male participants: 21 (37.5) vs 25 (45.5) Burn type: hot water, steam 24 (42.9) vs 23 (41.8)/fire 22 (39.3) vs 18 (32.7)/hot liquid 5 (8.9) vs 10 (18.2)/hot object 2 (3.6) vs 3 (5.5)/chemical substance 3 (5.4) vs 1 (1.8) Burn degree: second Burn size (%TBSA): 2.48 ± 1.45 vs 2.38 ± 1.42 Burn location: NR | |
| Interventions | Intervention arm 1: herbal cream (A vera gel, Lavandula stoechas essential oil, Pelargonium roseum essential oil), 5 g for each 10 cm² of burn area applied once daily. Sterile gauze used to cover wound and then bandaged. N = 60 Intervention arm 2: SSD 1% cream. Following cleansing and debridement with antimicrobial solution, 5 g for each 10 cm² of burn area applied once daily. Sterile gauze used to cover wound and then bandaged. N = 60 Cointerventions: cleansing and debridement with antimicrobial solution before randomised treatment period; analgesia | |
| Outcomes | Primary outcome: infection Secondary outcome: pain | |
| Notes | Funding: Baqiyatallah University of Medical Sciences, Iran. Herbal creams were provided by Barij Essence Pharmaceutical Co; 3 authors are described as members of this company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were randomized in a double‐blind manner” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants were randomized in a double‐blind manner” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “Patients were evaluated for the severity of pain, frequency of skin dryness and infection” Comment: no information on whether outcome assessors were blinded as to allocation; balance of probabilities based on quote is that assessment was unblinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “From the initial 120 patients…9 were excluded due to study protocol violation…Data from 111 completers (n=56 in the herbal cream and 55 in the SSD group) were included in the final analysis” Comment: reasons for withdrawals were reported ‐ study protocol violation; numbers excluded were not high |
| Selective reporting (reporting bias) | Low risk | Quote: “Patients were evaluated for the severity of pain, frequency of skin dryness and infection” Comment: all stated outcomes of interest were reported |
| Other bias | Low risk | Comment: no direct quotes but no evidence of additional sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: UK Parallel group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: burns less than 5% TBSA (averaging under 1%) suitable for outpatient treatment Exclusion criteria: those needing inpatient treatment, facial burns, hand burns managed in bags and those whose treatment was to be continued elsewhere Participants: 196 outpatients Mean age (years): < 5 years: 21 vs 24; 5‐14 years: 7 vs 9; > 14 years: 64 vs 71 Male participants: 49 vs 64 Burn type: NR Burn degree: NR Burn size (%TBSA): < 1% Burn location: NR | |
| Interventions | Intervention arm 1: hydrocolloid material covered with cotton gauze overlaid with cotton wool and secured with crepe bandage or adhesive tape. Dressing inspected on 3rd or 4th day and then changed weekly unless dressing contaminated or adverse symptoms developed Intervention arm 2: chlorhexidine‐impregnated tulle‐gras dressing covered with cotton gauze overlaid with cotton wool and secured with crepe bandage or adhesive tape. Dressing inspected on 3rd or 4th day and then changed weekly unless dressing contaminated or adverse symptoms developed Cointerventions: NR | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were allocated randomly to one of two treatment groups” Comment: no further information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were allocated randomly to one of two treatment groups” Comment: no further information to indicate concealment of allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote "at each inspection of the wound, its progress towards healing was noted" Comment: no indication that outcome assessment was blinded but unclear |
| Incomplete outcome data (attrition bias) | High risk | Quote: “119 of the 196 patients were followed to complete healing” Comment: details were given on why the excluded participants' data were not included |
| Selective reporting (reporting bias) | Low risk | Comment: no direct quotes but all stated outcomes of interest were reported |
| Other bias | Unclear risk | Comment: no direct quotes but no evidence of additional sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: Netherlands Parallel‐group RCT Unit of randomisation: burns Unit of analysis: burns Duration: NR | |
| Participants | Inclusion criteria: second‐degree burns up to 10% TBSA Exclusion criteria: Aged > 18 years; dermatological diseases and/or pre‐existent poly‐neuropathy Participants: 60 outpatients with 72 burns Mean age (years): 46.5 ± 15.6 vs 34 ± 14.2 Male participants: 19 vs 20 Burn type: scald 19 vs 19; contact 8 vs 2; flame 5 vs 7 Burn degree: all second‐degree Burn size (%TBSA): NR (cm² 151.2 ± 109.6 vs 134.7 ± 99) Burn location: hands 2 vs 6; arms 11 vs 13; thorax 2 vs 2; abdomen 4 vs 2; thighs 18 vs 8; feet 1 vs 3 | |
| Interventions | Intervention arm 1: SSD cream (Flammazine) changed daily. N = 30 Intervention arm 2: polyhexanide‐containing bio‐cellulose dressing (Suprasorb X+PHMB) changed every 2nd or 3rd day. N = 30 Cointerventions: | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: costs | |
| Notes | Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Suitable patients were assigned to one of the treatment groups, using computer generated randomization." Comment: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A prospective, randomized, controlled single center study was designed to evaluate clinical efficacy of a polyhexanide containing bio‐cellulose dressing (group B) compared to a silver‐sulfadiazine cream (group A) in sixty partial‐thickness burn patients." Comment: no information on whether the allocations to treatment were adequately concealed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Wound healing was documented using standardized digital photographs, which were assessed by two experienced wound specialists, that were blinded for the treatment." Comment: blinded outcome assessment documented although pain assessment probably not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but although all planned outcomes were reported in some cases the data were only presented graphically |
| Other bias | Unclear risk | Comment: there is potential for unit of analysis issues as they analyse 72 wounds on 60 participants and 2 of the participants had more than one treatment. The data were not useful to our analysis. No other sources of bias were identified and methods were well reported |
| Methods | Country where data collected: Brazil Parallel‐group RCT Unit of randomisation: participant Unit of analysis: burn Duration: NR | |
| Participants | Inclusion criteria: second‐degree burns 1%‐20% TBSA Exclusion criteria: NR Participants: 125 Mean age (years): NR Male participants: NR Burn type: NR Burn degree: second‐degree Burn size (%TBSA): mean 4% Burn location: NR | |
| Interventions | Intervention group 1: merbromin 2% N = 25 Intervention group 2: sodium salicylate 2% N = 25 Intervention group: zinc sulfadiazine 2% N = 25 Intervention group 4: sodium salicylate 2% + zinc sulfadiazine 2% N = 25 Intervention group 5: collagenase 0.6 μg/g + chloramphenicol 1% N = 25 Cointerventions: surgical debridement under general anaesthesia; occlusive dressings after topical application | |
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR. Study reported in Portuguese; data extraction and risk of bias provided by two translators. Although the unit of analysis is stated to be "burns" it appears that there was only one burn per participant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Time to wound healing was analysed by an observer who was blinded to the participant's treatment group |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | all proposed outcomes were reported |
| Other bias | Unclear risk | Unclear whether the groups had similar baseline characteristics |
| Methods | Country where data collected: Germany Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: 24 h | |
| Participants | Inclusion criteria: aged 18‐80 years with 2nd‐degree partial‐thickness burn > 3% TBSA and at least two 10 cm² symmetrical or similar areas for comparison. Abbreviated Burn severity Index score no higher than 10 Exclusion criteria: NR Participants: 30 people with burns presenting at burn department of trauma centre Mean age (years): median 42 Male participants: 22/30 Burn type: scald 12, contact 7, flame 11 Burn degree: 2nd Burn size (%TBSA): median 18 (range 6‐36) Burn location: trunk 9, thigh 11, lower leg 5, arm 5 | |
| Interventions | Intervention arm 1: SSD (Flammazine); gauze Intervention arm 2: octenidine gel, gauze Cointerventions: initial disinfection with Octinisept and removal of blisters; preparation for treatment with synthetic skin substitute | |
| Outcomes | Secondary outcome: pain | |
| Notes | Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomized, non‐blinded, clinical study was conducted" Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The prospective, randomized, clinical study was performed.... . Patients needed to have symmetrical or similar burned areas close to each other for comparability. Burns were randomly selected, one area was treated with Flammazine1/gauze, another area in the same patient was treated with Octenidine‐Gel1/ gauze as initial antiseptic treatment." Comment: no information on whether the treatment allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "the patient was instructed to mark his/her pain on a visual analogue scale". Comment: it was not clear if the participant was blinded. So unclear whether assessment was |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All enrolled participants completed the study." Comment: all randomised participants/burns included in the analysis |
| Selective reporting (reporting bias) | Low risk | Quote: "In this study we compared the feasibility and practicability, with focusing on pain scores, time of wound bed preparation and quality of the wound site" Comment: individual patient data were reported for the planned outcomes; a paired analysis is required to analyse these |
| Other bias | Unclear risk | Comment: it was unclear whether the analysis took into account the intra‐individual study design |
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 60 days | |
| Participants | Inclusion criteria: partial‐thickness burns involving between 5% and 40% TBSA Exclusion criteria: NR Participants: 50 adults and children with partial‐thickness burns Mean age (years): range 18 months‐50 years) Male participants: 21/50 Burn type: NR Burn degree: second‐degree (partial‐thickness) Burn size (%TBSA): surface area Burn location: NR | |
| Interventions | Intervention arm 1: pure unprocessed, undiluted honey applied once daily, covered with cotton sterilized gauze Intervention arms 2: layer of 1% SSD cream applied once daily Cointerventions: general management including initial debridement and wound excision were the same in both groups The wounds were cleansed with normal saline and thorough debridement done | |
| Outcomes | Primary outcome: wound healing (epithelialisation) Primary outcome: infection (culture positive) Secondary outcome: pain (VAS 1‐10) and time to pain‐free status Secondary outcome: cost per dressing per %TBSA | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The cases were divided into two groups randomly by consecutive sampling method, in equal numbers." Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The cases were divided into two groups randomly by consecutive sampling method, in equal numbers." Comment: no information on whether the allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "At the time of change of dressing, details regarding the condition of the wound such as signs of infection, condition of the surrounding tissue, discharge, smell, presence of necrotic tissue, and degree of epithilialisation were noted." Comment: unclear if this assessment was performed by personnel/assessors blinded to the allocation: since the interventions clearly differ then it may be unlikely that assessment could be blinded if it was performed by those changing the dressings |
| Incomplete outcome data (attrition bias) | Low risk | No direct quote but all participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | The primary and secondary outcomes were not defined in the methods section so it is difficult to assess if all planned outcomes were reported. |
| Other bias | Unclear risk | No evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: Pakistan Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: treatment duration until healing (longest 60 days); 2 months' follow‐up | |
| Participants | Inclusion criteria: 2nd‐degree burns presenting within 24 h of injury and TBSA < 25% Exclusion criteria: corrosive, electrical or chemical burns; history of diabetes, hypertension, epilepsy or kidney disease; pregnancy Participants: 50 people attending the ED and admitted to burns unit Mean age (years): 30.2 (15‐65); no significant difference between groups Male participants: 17 vs 9 Burn type: flame 16 vs 11; scald 9 vs 14 Burn degree: Burn size (%TBSA): 13.6 ± 4.7 (6‐25); no significant difference between groups Burn location: NR | |
| Interventions | Intervention arm 1: A vera gel twice daily. N = 25 Intervention arm 2: 1% SSD twice daily. N = 25 Cointerventions: 3rd generation cephalosporins; fluid resuscitation, shock prevention/treatment; wound cleansing with Pyodine scrub and normal saline | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: pain | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifty patients with second degree burns were randomized (consecutive sampling method) into 2 groups." Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Fifty patients with second degree burns were randomized (consecutive sampling method) into 2 groups." Comment: no information on whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "At the time of change of dressing details regarding the condition of the wound such as signs of wound infection, condition of surrounding unburned tissues, discharge, smell, necrotic tissue and state of epithelialisation was noted by on every 3rd day. ...... The patients and attendants were given information regarding the Aloe Vera gel and SSD cream. Tape method was used to measure length and width of the wound and then these measurements were multiplied i.e. Area (in centimetre square) = length x width." Comment: outcome assessment done at time of dressing change making it unblinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Among 25 patients treated with Aloe dressing, 24 patients had complete recovery while 1 had incomplete. In the SSD group, out of 25 patients, 19 patients had complete recovery and 6 had hypertrophic scar formation or the development of contractures" Comment: all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Patients were also reviewed for documentation of efficacy of treatment including time required for healing (epithelialization), pain scores, type of cultured organisms, wound colonization and infection, cost of treatment and mortality between both groups." Comment: all the planned outcomes were reported adequately |
| Other bias | Unclear risk | Comment: No evidence of other sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: USA Parallel‐group RCT (multicentre) Unit of randomisation: participant Unit of analysis: participant Duration: 21 days + | |
| Participants | Inclusion criteria: aged at least 5 years; had a thermal burn within 36 h of enrolment; 2.5%‐20% of TBSA (burns covering between 3% and 25% of TBSA, allowing for up to 10% of TBSA to be third‐degree burn); only second‐degree burn area treated as per study protocol Exclusion criteria: chemical or electrical burn; clinically‐infected burn; treatment of the burn with an active agent before study entry, and pregnancy; necrotising leukocytic vasculitis or pyoderma gangrenosa, diagnosed illness (e.g. HIV/AIDS, cancer, severe anaemia); corticosteroid use; other immunosuppressants/chemotherapy in past 30 days; known allergy/hypersensitivity to components; physical/mental condition meaning not expected to comply Participants: 101 participants at 10 centres Mean age (years) (SE): 37.0 (18.1) vs 39.2 (18.2) Male participants: 36/41 Burn type: scald n=17 vs 9, flash 17 vs 16, flame 13 vs 19, contact 2 vs 4; other 0 vs 3 Burn degree: second‐degree Burn size (%TBSA): mean partial‐thickness burn size values used within the analysis, 5.64% vs 4.93%, Burn location: NR | |
| Interventions | Intervention arm 1: silver soft silicone foam (Mepilex Ag). Dressing changes every 5‐7 days (3–5 days during the acute phase) depending on the status of the burn. Additional light bandage as needed to ensure fixation Intervention arm 2: SSD cream applied once or twice daily to a thickness of approximately 2 mm, then covered with a gauze pad and gauze wrap or other fixation Cointerventions: wound cleansing; sharp debridement at baseline | |
| Outcomes | Primary outcome: wound healing Secondary outcome: adverse events Secondary outcome: pain Secondary outcome: costs | |
| Notes | Funding: Molnlycke Health Care educational grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Enrolled subjects were assigned randomly to a treatment regimen that included either SSD or MAg. This was achieved through the use of sealed envelopes that were opened at the time of randomization. The randomization schedules were designed to ensure that equal numbers of patients were assigned to each treatment group at all participating centers.” Comment: no information on how randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This was achieved through the use of sealed envelopes that were opened at the time of randomization." Comment: although use of sealed envelopes was reported there is insufficient information to determine if the allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The study treatment was not blinded. .... Observation of dressings in both groups continued until 21 days postburn or until full reepithelialization occurred, alternative therapy for infection was initiated, or significant change in burn depth required surgical intervention. Sharp debridement was carried out at baseline visit only. Outcomes were measured at every scheduled visit: ie, days 0 (at inclusion in study), 7, 14, 21, and 35 (1 day) until study discontinuation." Comment: the outcome assessment did not appear to be blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all except 2 randomised participants were included in analyses with the exception of cost assessment where analysis of fewer participants was prespecified |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but outcomes were specified in detail and all were reported adequately |
| Other bias | Low risk | Comment: no evidence of other sources of bias and well reported |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: superficial thermal burns < 40% TBSA Exclusion criteria: NR Participants: 104 participants attending burns unit Mean age (years): 28.5 (3.2) vs 26.7 (4.1) (information provided by author to Jull et al (Jull 2015). (range 1‐65 years) Male participants: 82/104 (42 vs 40) Burn type: thermal Burn degree: NR (superficial) Burn size (%TBSA): mean NR. most participants had 21%‐30% or 30%‐40%; mean 26.5 vs 27.2 Burn location: NR | |
| Interventions | Intervention arm 1: 15 mL‐30 mL honey applied directly to wound, covered with gauze and bandaged, changed daily. N = 52 Intervention arm 2: SSD soaked gauze that was changed daily. N = 52 Cointerventions: washed with normal saline | |
| Outcomes | Primary outcome: wound healing Secondary outcomes: pain and selected AE reported qualitatively | |
| Notes | Funding NR. Information on allocation method, allocation concealment, blinding, mean TBSA, mean time to healing and standard deviation for mean time to healing were provided by the author to Jull et al. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “the cases were allotted at random to two groups” Comment: no further information to indicate how randomisation sequence was generated. Study author information that the sequence was generated by the "chit method", which is a method of drawing lots however the detail provided by the study authors was minimal and not sufficient to reassure us that the sequence was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: “the cases were allotted at random to two groups” Comment: study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered, sealed envelopes but envelopes may not have been opaque |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "In both groups culture and sensitivity determinations were performed on swabs taken from the surface at the time of admission. This was repeated on days 7 and 21 in all cases or untIl the wound healed. The time required for complete healing was noted in both groups." Comment: information provided by the author to Jull et al stated that outcomes assessors were blinded but data analysts were not. So still unclear. Additionally honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all randomised participants were included in the analysis (shown in tables) |
| Selective reporting (reporting bias) | Unclear risk | No specific quote but although the stated outcomes were all reported some were reported only qualitatively |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias, but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: partial‐thickness burns < 40% TBSA Exclusion criteria: Participants: 92 people attending a general hospital Mean age (years): 42.8 (3‐65) Male participants: 44 Burn type: NR Burn degree: NR (partial‐thickness) Burn size (%TBSA): 22.7 (15‐35) groups 22.8 vs 22.6 Burn location: NR | |
| Interventions | Intervention arm 1: honey‐impregnated gauze prepared by dipping sterile gauze in unprocessed and undiluted honey, covered with pad and bandage, changed on alternate days unless signs of infection Intervention arm 2: bio‐occlusive, moisture‐permeable polyurethane dressing (OpSite) kept in place until day 8 if no sign of infection, leakage etc Cointerventions: washed with normal saline | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection | |
| Notes | Funding NR; information on allocation method, allocation concealment, blinding, mean TBSA, mean time to healing and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After initial management, patients were allotted at random to two groups." Comment: no further information on methods of sequence generation; study author information that the sequence was generated by the "chit method", which is a method of drawing lots however the detail provided by the authors was minimal and not sufficient to reassure us that the sequence was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After initial management, patients were allotted at random to two groups." Comment: study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered, sealed envelopes but not known whether these were opaque |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "In both groups bacterial culture and sensitivity determinations were performed from swabs taken from the surface of the wound.... until the wound healed. The time required for complete healing was noted in both groups." Study author provided a statement to Jull et al that outcome assessors were blinded Comment: despite author information that assessors were blinded, honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but the outcomes cited were subsequently reported |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but all randomised participants were included in the analysis (shown in tables) |
| Other bias | Unclear risk | Comment: no specific quote but no evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: partial‐thickness burns less than 40% TBSA within 6 h of burn Exclusion criteria: NR Participants: 64 Mean age (years): 25 vs 24.6 (3‐62; 60 aged 21‐30) Male participants: 28 vs 15 Burn type: scald n = 25 vs 18, flame 12 vs 4, contact burn 3 vs 2 Burn degree: NR (partial‐thickness) Burn size (%TBSA): 18.5% vs 19.4% Burn location: NR | |
| Interventions | Intervention arm 1: dry gauze dipped into unprocessed honey and applied to wound, covered with an absorbent dressing that was changed alternate days. Changed more often if signs of infection. N = 40 Intervention arm 2: amniotic membrane ‐ no other details of dressing given, after day 8 dressing was changed on alternate days, changed more often if signs of infection. N = 24 Cointerventions: washed with normal saline | |
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by study author to Jull et al (Jull 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After initial treatment, patients were allotted to the two groups at random." Comment: no further information on methods of sequence generation in study report but study author provided information that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After initial treatment, patients were allotted to the two groups at random." Comment: no further information on whether allocation was adequately concealed in study report but author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The following observations were recorded in all patients: leakage of exudate from the dressing, skin reactions, infection and time for wound healing. Pain was assessed during the change of dressing in both groups, by two separate observers." Comment: no indication as to whether the assessments were conducted by observers blinded to treatment allocation, author provided information to Jull et al (Jull 2015) that outcome assessors were blinded but honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all randomised participants included in analysis (based on table) |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but stated outcomes were all reported |
| Other bias | Unclear risk | Quote: "The honey‐impregnated gauze was prepared by dipping sterile gauze in unprocessed and undiluted honey. The gauze was applied to the wound and then covered with an absorbent dressing. These wounds were inspected every 2 days until healed. In contrast the patients treated with amniotic membrane had a first wound inspection on day 8, when the dressing was changed and then every second day until healed." Comment: unclear if differing observation times influenced outcomes |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days | |
| Participants | Inclusion criteria: partial‐thickness burns < 40% TBSA, presenting within 6 h of injury Exclusion criteria: NR Participants: 100 Mean age (years): 28.2 vs 27.5 (range age 5‐59 years) Male participants: 29 vs 28 Burn type: scald n = 17 vs 15, flame 23 vs 22, contact 7 vs 12, explosives 2 vs 1, chemical 1 vs 0 Burn degree: NR (partial‐thickness) Burn size (%TBSA): 16.5 vs 17.2% (range 10‐40) Burn location: NR | |
| Interventions | Intervention arm 1: 15 mL to 30 mL undiluted and unprocessed honey, dry gauze applied on top and covered with bandage, inspected on alternate days. N = 50 Intervention arm 2: autoclaved potato‐peel dressing, dry gauze and bandage applied, changed alternate days or earlier if signs of infection, or excessive exudate or leakage. N = 50 Cointerventions: washed with normal saline | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection | |
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by study author to Jull et al (Jull 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the initial management, patients were allotted at random to two groups." Comment: no indication how the randomisation sequence was generated. Study author provided information to Jull et al (Jull 2015) that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After the initial management, patients were allotted at random to two groups." Comment: no further information on whether allocation was adequately concealed in study report but study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered, sealed envelopes but not known whether these were opaque |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The wounds were inspected every 2 days until healed." Comment: no indication as to whether outcome was determined by a blinded observer in study report; study author provided information to Jull et al that outcome assessors were blinded but honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all randomised participants were included in analysis (tables) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes cited in methods were all reported |
| Other bias | Unclear risk | No specific quote but no evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: TBSA burnt < 40% | |
| Interventions | Intervention arm 1: pure, unprocessed, undiluted, honey, covered with gauze, changed every 2nd day Intervention arm 2: soframycin (90 participants), Vaseline‐impregnated gauze (90 participants), OpSite (90 participants), sterile gauze (90 participants) or left exposed (90 participants). “Dressings were replaced on alternative days, except in the case of OpSite, which was continued until the wounds healed... sterile linen changed at frequent intervals.” Frequency of dressing change is not mentioned with respect to the sterile gauze group | |
| Outcomes | Primary outcome: wound healing | |
| Notes | Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After initial treatment, the cases were divided at random into a study group treated with honey dressing and a control group treated with conventional dressing” Comment: method of generating the random sequence not reported. Study author provided information that the sequence was generated by the “chit method”, which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated, but study author provided information that allocation concealment was by means of sequentially‐numbered, sealed envelopes, although it is not clear whether the envelopes were opaque |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not stated in study report, but study author responded to request for further information from Jull et al by stating the investigators and outcome assessors were blinded. How blinding was achieved was not described in the response and honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) | Low risk | Appears that all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine whether there is a risk of outcomes being selectively reported |
| Other bias | Unclear risk | Comment: no specific quote but there was no evidence of other bias but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 30 days | |
| Participants | Inclusion criteria: superficial thermal burns less than 40% TBSA within 6 h of burn Exclusion criteria: NR Participants: 50 people attending burns unit Mean age (years): 25.2 vs 26.4 Male participants: 14 vs 13 Burn type: flame 23/22, scalds 2/3, TBSA 14.5%/15.6% Burn degree: NR Burn size (%TBSA): 14.5 vs 15.6 Burn location: NR | |
| Interventions | Intervention arm 1: 16 mL‐30 mL unprocessed honey, dry gauze applied on top and covered with bandage; honey changed alternate days Intervention arm 2: SSD impregnated gauze, changed daily Cointerventions: washed with normal saline | |
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, mean time to healing and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the initial management, patients were allocated at random to two groups." Comment: no indication how the randomisation sequence was generated but study author provided information to Jull et al that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After the initial management, patients were allocated at random to two groups." Comment: no indication in study report whether the allocation was adequately concealed. Study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The wounds were observed for evidence of infection, excessive exudate or leakage until the wounds healed. The times taken for healing of the wounds were recorded in both groups." Comment: no indication if observers were blinded in study report; author provided information to Jull et al that outcome assessors were blinded but honey is known to cause discolouration of periwound skin making blinded outcome assessment very difficult; therefore judgement unclear |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all randomised participants were included in the analysis (tables) |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but the specified outcomes of interest were all reported |
| Other bias | Low risk | Comment: no specific quote but there was no evidence of other bias but reporting insufficient to be certain |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 21 days | |
| Participants | Inclusion criteria: less than 40% TBSA burn, hospitalised within 6 h post‐burn Exclusion criteria: Participants: 100 people attending burns unit Mean age (years): 26.5 ± 1 vs 25.2 ± 2 Male participants: 52 Burn type: NR Burn degree: NR Burn size (%TBSA): 22.5 ± 3 vs 23.4 ± 1; full‐thickness 3.2 +/‐2 vs 4.7 +/‐1% Burn location: NR | |
| Interventions | Intervention arm 1: 15 mL‐30 mL unprocessed honey, dry gauze applied on top and covered with bandage, changed every 2 days. N = 50 Intervention arm 2: SSD impregnated gauze changed every 2 days. N = 50 Cointerventions: washed with normal saline | |
| Outcomes | Primary outcome: wound healing Primary outcome: infection (resolution) Secondary outcome: resource use (hospital stay) | |
| Notes | Funding NR Information about allocation method, allocation concealment, blinding, and standard deviation for mean time to healing provided by author to Jull et al (Jull 2015) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were allotted at random to two groups," Comment: no indication how the randomisation sequence was generated but author provided information to Jull et al that the sequence was generated by the "chit method", which is a method of drawing lots however the information provided was minimal and lacked detail to sufficiently reassure us that the method was truly random |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were allotted at random to two groups," Comment: no indication in study report whether the allocation was adequately concealed. Study author provided information to Jull et al that allocation concealment was by means of sequentially‐numbered sealed envelopes, although it is not clear whether the envelopes were opaque |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The wounds were observed for evidence of infection, excessive exudate, or leakage until they healed." Comment: no indication that observers were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Thus, in all the patients in this group, the wounds healed by day 21..... In the group treated with sulphur sulphadiazine, the wounds healed in 4 patients by day 7, in 22 patients by 14 day, and in 24 patients by day 21 (mean, 17.2 days)." Comment: it is clear that all participants randomised to the honey group were included in the analysis but not that all of those in the SSD group were, although no attrition is reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but it was not clear which outcomes the authors planned to assess and therefore whether they were all reported fully |
| Other bias | Low risk | Comment: no specific quote but no evidence of other bias |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: unclear (burn?) Duration: 4 weeks | |
| Participants | Inclusion criteria: deep partial‐thickness thermal burn injury covering 2.5%‐25% TBSA (third‐degree areas were not to exceed 10% TBSA). aged 5‐65 years; at least one isolated burn area not on head or face with deep partial 2nd‐degree burn from 1%‐10% TBSA Exclusion criteria: burns older than 36 h, clinically infected; treated with active agent before study entry (SSD allowed up to 24 h before randomisation); dermatologic disorders or necrotising processes; underlying diseases such as HIV/AIDS, cancer, severe anaemia, insulin‐dependent diabetes, systemic glucocorticoid use except occasional prednisolone < 10 mg/d; immunosuppressive agents, radiation or chemotherapy in previous 30 days; known allergy/sensitivity to the products; pregnancy; previous participation in this (or other study within 1 month) Participants: 158 randomised participants (total number of burns > 200) Mean age (years): 36.2 (range 5.2‐65.5; only 5 < 12 years). No difference between groups Male participants: 55 vs 57 Burn type: scald 30 vs 41; flash 8 vs 7; flame 32 vs 31; contact 1 vs 3 Burn degree & TBSA: 2nd‐degree superficial partial 4.48% vs 4.29%; deep partial‐thickness 6.28% vs 5.18%; third‐degree 0.345% vs 0.317%. enrolled study site: 2.72 vs 2.64 Burn location: arm 52 vs 53, buttock 6 vs 7, hand 41 vs 42, leg 29 vs 35, thigh 24 vs 31, trunk 26 vs 27, other 43 vs 46 | |
| Interventions | Intervention arm 1: absorbent foam silver dressing (Mepilex Ag) changed every 5‐7 days; gauze wrap as secondary dressing. N = 73 Intervention arm 2: SSD 1% cream; gauze pad and wrap as secondary dressing. N = 85 Cointerventions: debrided and/or cleansed according to standard practice | |
| Outcomes | Primary outcome: wound healing primary outcome: infection Secondary outcome: adverse event Secondary outcome: pain | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled subjects were assigned randomly using a block design, with block sizes varying between 2.4 and 6 (in Viedoc, Pharma Consulting Group, Uppsala, Sweden) to either SSD or Mepilex Ag. Subjects were consecutively allocated to the treatment at each center and given a subject code, depending on which strata they belonged to." Comment: randomisation sequence computer‐generated using blocking design |
| Allocation concealment (selection bias) | Low risk | Quote: "Enrolled subjects were assigned randomly using a block design, with block sizes varying between 2.4 and 6 (in Viedoc, Pharma Consulting Group, Uppsala, Sweden) to either SSD or Mepilex Ag. Subjects were consecutively allocated to the treatment at each center and given a subject code, depending on which strata they belonged to." Comment: allocation conducted remotely by consecutive allocation of codes within stratified design |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "In addition, the investigator was required to make a subjective assessment of healing at each weekly assessment before cleansing and/or debridement. Percentage of the burn healed since baseline was to be performed by a blinded observer." Comment: assessment of healing was conducted by an assessor blinded to the treatment allocation; it's not clear whether assessment of other outcomes was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: ".....158 patients were randomized, and 153 patients were subjected to at least one treatment and were included in the ITT population, 71 (46%) of them randomized to Mepilex Ag and 82 (54%) randomized to SSD. Thirteen patients (8%) discontinued before the study ended, 5 (7%) of them from the Mepilex Ag group and 8 (10%) from the SSD group. One patient withdrew consent from the SSD group, while the other 12 discontinued because of other reasons (Fig. 1)." Comment: all participants were accounted for and the proportion who discontinued was low and low relative to the event rate for healing |
| Selective reporting (reporting bias) | Low risk | Quote: "The primary end point was time to healing (defined as 95% epithelialisation by visual inspection). The secondary end points were percentage of burns epithelialised/healed, numbers of burns healed or not at each visit (not at baseline), number of study burns requiring a skin graft, and number of dressing changes. Additional outcomes were measured assessing the tolerability and performance of the dressings on wound and periwound status, including pain and experience of use of the dressings." Comment: the defined outcomes were all fully reported |
| Other bias | Unclear risk | Comment: it was unclear how the designated burn was chosen in participants with multiple burns. However it was clear that there were no unit of analysis issues. |
| Methods | Country where data collected: Singapore Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 26 days | |
| Participants | Inclusion criteria: thermal 1st‐ or 2nd‐degree burns, < 30% TBSA, within 24 h of admission with no prior antibiotics or topical treatment for burn Exclusion criteria: diabetes mellitus and terminal patients Participants: 38 patients at 2 community hospitals Mean age (years): 18 vs 25.2 Male participants: 11 vs 11 Burn type: thermal 18 vs 17; electrical 2 vs 1 Burn degree: 1st 9 vs 5; 2nd 11 vs 13 Burn size (%TBSA): 8 vs 11.1 Burn location: NR | |
| Interventions | Intervention arm 1: Aloe vera Linn. mucilage dressings changed twice daily Intervention arm 2: SSD dressings changed twice daily Cointerventions: intravenous fluid 6 vs 6; antibiotics 12 vs 12, analgesia 13 vs 13, tetanus 2 vs 1, sedatives 2 vs 2, other 2 vs 0 | |
| Outcomes | Primary outcome: wound healing secondary outcome: adverse events | |
| Notes | No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were designated to receive Aloe vera Linn., mucilage or silver sulfadiazine for topical treatment of their burns by stratified randomization selection based on two prognostic factors...." Comment: unclear how randomisation sequence was derived |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were designated to receive Aloe vera Linn., mucilage or silver sulfadiazine for topical treatment of their burns by stratified randomization selection based on two prognostic factors...." Comment: unclear whether treatment allocations were adequately concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Each patient was assessed daily for healing, side effects and satisfaction with the treatment" Comment: no information on whether assessment was conducted in a blinded fashion |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no specific quote but all randomised participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote |
| Other bias | Unclear risk | Comment: no evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: burn Duration: NR | |
| Participants | Inclusion criteria: < 5% TBSA, presented up to 24 h post burn Exclusion criteria: burns to face, neck, axilla; chemical and electrical burns Participants: 50 participants with 54 burns Mean age (years): NR; children 10/18 vs 7/16 vs 7/16 Male participants: NR; ratios 2:1 vs 1:1.3 vs 1:1.3 no significant difference between groups Burn type: scalds 95% vs 56% vs 88%; no significant difference between groups Burn degree: NR (minor) Burn size (%TBSA): 0.84 vs 0.94 vs 0.79; no significant difference between groups Burn location: NR | |
| Interventions | Intervention arm 1: chlorhexidine tulle‐gras. N = 18 Intervention arm 2: hydrocolloid (granuflex). N = 16 Intervention arm 3: hydrocolloid + SSD. N = 16 Cointerventions: NR | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain | |
| Notes | Funding: Convatec/Squibb supplied granuflex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of three treatment groups after obtaining informed consent" Comment: no information on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of three treatment groups after obtaining informed consent". Comment: no information on whether allocation concealment was adequate |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "During dressing changes the healing progress of the wound was noted..." Comment: no information on whether observers were blinded; balance of probabilities would be not |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: no specific quote but unclear whether all randomised participants were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no specific quote but outcomes mentioned in early part of text are reported in findings |
| Other bias | High risk | Comment: unit of analysis issues as randomisation was at the participant level whilst analysis was at the level of burn wounds (some participants had multiple burns) |
| Methods | Country where data collected: USA Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: completion of treatment (max 14 days) | |
| Participants | Inclusion criteria: partial‐thickness burn injuries requiring topical wound care that, in the opinion of the observer, would not go on to require surgical excision and grafting. The wounds had to involve two areas far enough apart so as not to create interference of the treatments. Wounds of similar sizes were chosen, but not specifically measured. Exclusion criteria: NR Participants: 14 people attending a hospital/burn centre Mean age (years): 41 (25‐68) Male participants: 13/14 Burn type: 12 flame, 2 scalding (both arms same cause) Burn degree: NR partial‐thickness Burn size (%TBSA): 14.6% (4.5–27) Burn location: upper extremities 8 vs 8, lower extremities 4 vs 6, trunk 2 vs 0 | |
| Interventions | Intervention arm 1: Acticoat‐ silver‐impregnated membrane applied wet and left in place; moistened and change of overlying dry gauze dressings every 6 h. 14 burns Intervention arm 2: SSD ‐ applied and removed then dressed with a dry gauze dressings twice daily. 14 burns Cointerventions: NR | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain Secondary outcome: adverse events | |
| Notes | Funding NR This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by assignment using random drawing of sealed envelopes from a box with equal numbers of treatment and control envelopes. According to the protocol, the patient’s most left and/or upper‐most wound was labelled as wound #1, and the patient’s most right and/ or lower‐most wound was labelled as wound #2. Wound #1 was assigned randomly to one of the treatment algorithms, and wound #2 was assigned to the alternate algorithm." Comment: randomisation appeared adequate |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by assignment using random drawing of sealed envelopes from a box with equal numbers of treatment and control envelopes. According to the protocol, the patient’s most left and/or upper‐most wound was labelled as wound #1, and the patient’s most right and/ or lower‐most wound was labelled as wound #2. Wound #1 was assigned randomly to one of the treatment algorithms, and wound #2 was assigned to the alternate algorithm." Comment: it was unclear how well the allocation system was concealed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "endpoint for dressings both in the inpatient and outpatient setting was based on the clinical judgment of the attending physicians at the Burn Center" Comment: unclear whether the outcome assessor was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Fourteen patients were enrolled .....Four patients continued in the study until completion of treatment" Comment: very high proportion of participants did not complete treatment |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes were not clearly specified in methods section so difficult to determine if all assessed outcomes reported |
| Other bias | Unclear risk | Comment: unclear whether the analysis adjusted for intra‐individual design |
| Methods | Country where data collected: UK Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR | |
| Participants | Inclusion criteria: partial‐thickness burns manageable through outpatients Exclusion criteria: burns requiring grafting, > 48 h post‐burn injury, sensitive to dressings, burn on face or hand joints, burn infected, receiving treatment other than first aid or more suited to alternative treatments Participants: 98 people presenting at ED/outpatient care. Other characteristics NR but "no statistically significant differences with regard to patient demographics and physical characteristics" (refers to participants included in analysis only) | |
| Interventions | Intervention arm 1: hydrocolloid dressing (Granuflex) Intervention arm 2: paraffin gauze impregnated with 0.5% chlorhexidine acetate (Bactigras) Cointerventions: cleaned with sodium chloride solution and allowed to dry | |
| Outcomes | Secondary outcome: pain Secondary outcome: resource use | |
| Notes | Funded by ConvaTec Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Written, informed consent of the patients was obtained and witnessed, and the patients were randomly allocated to either Granuflex E or Bactigras" Comment: no further information on how the randomisation sequence was produced |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Written, informed consent of the patients was obtained and witnessed, and the patients were randomly allocated to either Granuflex E or Bactigras" Comment: no further information on whether allocation was adequately concealed |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "At each follow‐up attendance the following details were noted: 1. Reason for dressing change. 2. Ease of removal. 3. Wound appearance. 4. Pain while dressing was in situ. 5. Pain on dressing removal or application. 6. Analgesia or antibiotics administered. When the wound had completely healed a final evaluation was made, the quality of healing with regard to re‐epithelialization and cosmetic results was noted. The dressing was rated by both the investigator and the patient." Comment: it appeared that the investigator was not blinded to treatment allocation and also performed the assessment of outcome |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Out of a total of 98 patients involved, 31 patients were withdrawn. Of these, 22 patients were lost to follow‐up, two patients requested withdrawal and there was one protocol violation." Comment: a large number of participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but outcomes were not fully prespecified so difficult to determine if all planned outcomes were reported |
| Other bias | Unclear risk | Comment: no evidence of other sources of bias but reporting insufficient to be certain |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: burn Unit of analysis: burn Duration: 14 days | |
| Participants | Inclusion criteria: total burn < 30% TBSA, deep partial second‐degree burn wounds, > one month treatment; residual wound < 10% TBSA, single wound < 5 cm x 5 cm Exclusion criteria: no general infection or complications Participants: 60 hospital patients each with 2 burns Mean age (years): 39 ± 13 (range 18‐65) Male participants: NR Burn type: NR Burn degree: NR Burn size (%TBSA): NR; size 18 ± 8 cm2 vs 15 ± 10 cm2 Burn location: NR | |
| Interventions | Intervention arm 1: FLAMIGEL (hydrogel dressing) covered with cotton gauze, changed every day to 7 days, then every other day to 14 days. N = 60 burns Intervention arm 2: iodophor gauze covered with cotton gauze, changed every day to 7 days, then every other day to 14 days. N = 60 burns Cointerventions: | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain | |
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “This prospective randomised trial was conducted according to the random number table” Comment: a random component in the sequence generation process was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no mention of blinding of key study personnel used |
| Incomplete outcome data (attrition bias) | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | Reporting insufficient to determine whether the intra‐individual design was adjusted for or other risks |
| Methods | Country where data collected: USA Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: | |
| Participants | Inclusion criteria: superficial partial‐thickness burns, 0‐4 days post thermal injury < 25% TBSA, aged 11‐80 years Exclusion criteria: burn on face, ears or scalp; allergic to silver Participants: 24 participants attending a wound management centre Mean age (years): 33.8 vs 33.9 Male participants: 18/24 Burn type: NR Burn degree: NR (superficial partial‐thickness) Burn size (%TBSA): NR (area burned 1103.10 ± 1086.10 cm² vs 753.70 ± 934.30 cm²) Burn location: NR | |
| Interventions | Intervention arm 1: Aquacel Ag plus standard care Intervention arm 2: SSD plus standard care Cointerventions: whirlpool wound cleansing for 15 mins using hexaclorophene + selective debridement followed by wound dressing as per arm and 2nd dressing | |
| Outcomes | Primary outcome: wound healing Secondary outcome: pain | |
| Notes | Funding NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty‐four subjects (18 men and 6 women) who sustained superficial partial‐thickness burns and who were between the ages of 19 and 53 years, and with time of injury from 0 to 4 days, were randomly assigned into a control group (silver sulfadiazine) and experimental group (Aquacel Ag)." Comment: no further information on how the randomisation sequence was produced |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Twenty‐four subjects (18 men and 6 women) who sustained superficial partial‐thickness burns and who were between the ages of 19 and 53 years, and with time of injury from 0 to 4 days, were randomly assigned into a control group (silver sulfadiazine) and experimental group (Aquacel Ag)." Comment: no information on whether treatment allocation concealment was adequate |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Wound measurements were assessed at the time of the initial examination and every 4 days subsequently until the area was re‐epithelialized 100%. To ensure objectivity, the burn area was measured digitally with the software program Aspyra (AspyraLLC; Blue Springs, Missouri) in order to prevent discrepancies in wound measurements. Length and width of wounds were assessed based on a clock face with length from 12 to 6 o'clock and width 3 to 9 o'clock based on anatomic position......In addition, pain, utilizing the standard 0‐ to 10‐point scale, was assessed at the conclusion of each treatment session" Comment: digital methods were used to assess wound healing but it was unclear if the assessors were blinded to treatment allocation; assessment of pain was also unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote "Twenty‐four subjects (18 men and 6 women) who sustained superficial partial‐thickness burns and who were between the ages of 19 and 53 years, and with time of injury from 0 to 4 days, were randomly assigned into a control group (silver sulfadiazine) and experimental group (Aquacel Ag)." Comment: no withdrawals were reported but it was unclear whether all randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote, all outcomes mentioned in paper were reported in table |
| Other bias | Unclear risk | Comment: frequency of additional treatments and if they differed between groups is not reported |
| Methods | Country where data collected: Iran Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: 20 days | |
| Participants | Inclusion criteria: participants with second‐degree burns (depth 0.2‐5.0 mm) up to 40% TBSA; aged 15‐55, referred during first 24 h following injury, negative culture on admission Exclusion criteria: participants with underlying conditions such as diabetes, chronic renal or hepatic diseases, and those with simultaneous burns, trauma, and skin lacerations were excluded Participants: 30 individuals with superficial or deep partial‐thickness burns Mean age (years): 24.8 (11.9) Male participants: 21/30 Burn type: direct fire or oil: 26 Burn degree: partial‐thickness burns; deep partial‐thickness 6/10 vs 11/20 Burn size (%TBSA): surface area Burn location: NR | |
| Interventions | Intervention arm 1: olea ointment which contains 33.4% honey, 33.3% olive oil, and 33.3% sesame oil. After washing the wound with normal saline solution, 3–5 mm thick layer of Olea ointment was applied over the wound and closed dressing was performed every day Intervention arms 2: 1.5 mm‑thick layer of acetate mafenide ointment (8.5%) every 12 h, Cointerventions: debridement as required | |
| Outcomes | Primary outcome: wound healing (development of granulation tissue) Primary outcome: infection (development of positive culture after 7 days) Secondary outcome: adverse events: need for surgical debridement | |
| Notes | Funding: Kurdistan University of Medical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "30 available patients .....who were divided into two groups using simple randomized method and table of random numbers" Comment: table of random numbers used to generate randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: "30 available patients .....who were divided into two groups using simple randomized method and table of random numbers" Comment: unclear whether allocation sequence was adequately concealed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the microbiologist and pathologist were blinded to the treatment groups. To assess the outcomes, the burn wounds were evaluated daily after a week of intervention by a pathologist and a microbiologist for the formation of granulation tissues, debridement (using scalpel), and wound culture results" Comment: blinded outcome assessment for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "If they had positive culture, they were excluded from the study and treated by routine treatment for bacterial strains. However, the excluded patients were entered in the analysis." Comment: all participants appear to be included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no specific quote but not clear that all the outcomes assessed were specified in the methods |
| Other bias | Low risk | Comment: does not appear to be any additional source of bias |
| Methods | Country where data collected: China Parallel‐group RCT (intra‐individual) Unit of randomisation: burn Unit of analysis: burn Duration: 14 days | |
| Participants | Inclusion criteria: paediatric superficial second‐degree burn wounds within 24 h Exclusion criteria: prior treatment; other disease Participants: 40 children with burns divided into 2 areas for treatment Mean age (years): 4.5 ± 2.2 (2‐6) Male participants: 22/40 Burn type: NR Burn degree: second‐degree Burn size (%TBSA): 3.85 ± 1.27 (3‐5) Burn location: neck or front trunk | |
| Interventions | Intervention arm 1: Aquacel‐Ag, covered by 20 layers gauze; took off the outer layer at Day 3; changed new dressing at Day 7 with debridement Intervention arm 2: SD‐Ag, covered by 20 layers gauze; took off the outer layer at Day 3; changed new dressing at Day 7 with debridement Cointerventions: cleaned the wound with water; 5% chlorhexidine acetate for 2 min then cleaned with water again | |
| Outcomes | Primary outcome: wound healing | |
| Notes | Funding NR Article in Chinese, extracted and assessed for risk of bias by one review author, discussed with a second review author This was a "split‐body" or "intra‐individual" design where a person with two wounds had one wound randomised to each treatment. It was not clear whether the analysis took account of this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: a random component in the sequence generation process was not reported in detail |
| Allocation concealment (selection bias) | Unclear risk | Comment: it did not state how randomisation sequence was allocated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Comment: results section and tables show that all participant data were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: protocol not obtained, based on paper only |
| Other bias | Unclear risk | Note: the abstract stated that “two burn wound areas of similar size were selected from each patient”; in main text, it showed that these two burn areas actually was one wound, just divided to two same‐sized parts; this increases the chance of "carry‐over" from one test site to another. It was unclear whether the analysis adjusted for this intra‐individual design. |
AE: adverse event(s); AEO: Arnebia euchroma ointment; ALT amino alanine transferase; AST: aspartate amino transferase; A vera: Aloe vera; ED: Emergency Department; ITT: intention‐to‐treat; IV: intravenous; NR: not reported; SSD: silver sulfadiazine; TBSA: total body surface area; VAS: visual analogue scale;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Ineligible intervention: antiseptic combined with SSD | |
| Ineligible intervention: chlorhexidine rinse followed by SSD treatment | |
| Ineligible intervention: chlorhexidine cleansing then SSD treatment for deeper burns | |
| Ineligible study design: quasi‐randomised trial | |
| Ineligiblestudy design: quasi‐randomised trial | |
| Antiseptic agent did not differ between groups | |
| Ineligible study design: quasi‐randomised trial | |
| Ineligible study design: study designed only to test moisture‐absorption properties of dressings over short time period | |
| Ineligible population ‐ post‐surgical burn wounds | |
| Ineligible intervention ‐ comparison of non‐antiseptic with a mixture of antiseptic and non‐antiseptic agents | |
| Ineligible study design: quasi‐randomised trial | |
| Ineligible population: minority burns patients | |
| Ineligible study design: quasi‐randomised trial | |
| Ineligible was not the only systematic difference between the groups: other agents included in sprays | |
| Ineligible agent did not differ between groups | |
| Ineligible study design: quasi‐randomised trial | |
| Ineligible intervention: combination of SSD and chlorhexidine | |
| Ineligiblepopulation ‐ residual burns were a minority of participants | |
| Ineligible study design: quasi‐randomised trial | |
| Ineligible population (post‐surgery burns) | |
| Ineligible intervention: additional treatments given in both comparison groups | |
| Ineligible population: burn wounds a minority of included participants | |
| Antiseptic was not only systematic difference between groups | |
| Antiseptic agent did not differ between groups | |
| Wrong population: post‐surgical burn wounds | |
| Antiseptic agent did not differ between groups | |
| Antiseptic was not only systematic difference between groups | |
| Ineligible study design: agents under investigation only used for short period to assess blood and urine levels | |
| Ineligible study design: quasi‐randomised trial |
RCT: randomised controlled trial; SSD: silver sulfadiazine
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Country where data collected: NR Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: unclear |
| Participants | Inclusion criteria: irrespective of age with deep‐dermal burn wound; admitted to hospital less than 3 days after burn; the burn wounds were not to be operated on Exclusion criteria: burn wound involved the head and face region; history of allergy to dressing composed of ionic silver; serious infective wound; required emergency surgery Participants: 10 individuals; no details of participant characteristics were reported |
| Interventions | Intervention arm 1: silver‐impregnated antimicrobial dressing combined with a granulocyte macrophage colony‐stimulating factor (GM‐CSF) gel Intervention arms 2: gauze dressing combined with GM‐CSF gel Cointerventions: the GM‐CSF gel was applied in both arms |
| Outcomes | Primary outcome: no primary outcomes were reported Secondary outcome: pain Secondary outcome: resource use ‐ time to debridement complete; no outcomes currently have evaluable data |
| Notes | Funding: NR Reported as abstract only; study author contact not yet established Appears to be assessing time to debridement rather than the outcomes of this review |
| Methods | Country where data collected: China Parallel‐group RCT Unit of randomisation: participant Unit of analysis: participant Duration: NR |
| Participants | Inclusion criteria: participants with deep partial‐thickness burn wounds Exclusion criteria: NR Participants: 366 participants |
| Interventions | Intervention arm 1: gauze with iodophor Intervention arms 2: recombinant human granulocyte‐macrophage colony‐stimulating factor (rhGM‐CSF) gel Cointerventions: NR |
| Outcomes | Primary outcome: complete wound healing time Secondary outcome: adverse events |
| Notes | Paper in Chinese. Awaiting obtaining full text and translator assessment; details here based on English abstract |
| Methods | Country where data collected: India Parallel‐group RCT Unit of randomisation: participant Unit of analysis: unclear Duration: at least 2 weeks |
| Participants | Patients with burns (N = 17) |
| Interventions | Azadirachta indica (formulation unclear) SSD (formulation unclear) |
| Outcomes | Wound area? (ulcer score) Healing? (healing without deformity) Adverse events? (scar‐related events reported) |
| Notes | Abstract assessed; full text unobtainable |
| Methods | Country where data collected: NR Parallel‐group RCT Unit of randomisation: unclear Unit of analysis: unclear Duration: unclear |
| Participants | Inclusion criteria: children with second‐degree burns Exclusion criteria: NR Participants: 27 children in the intervention group, control group Mean age (years): 5 (range 4 months‐14 years) Male participants: 16 Burn type: NR Burn degree: second Burn size (%TBSA): NR Burn location: NR |
| Interventions | Intervention arm 1: alginate with embedded anti‐bacterial enzyme system Intervention arm 2: NR Cointerventions: NR |
| Outcomes | Primary outcome: wound healing Primary outcome: infection Secondary outcome: pain Secondary outcome: adverse events(?) |
| Notes | Reported in abstract form only, very limited information |
| Methods | Country where data collected: China Intra‐individual RCT Unit of randomisation: burn area Unit of analysis: burn area Duration: NR |
| Participants | Inclusion criteria: participants with deep or superficial partial‐thickness facial burn wounds Exclusion criteria: NR Participants: 25 participants, 10 with deep partial‐thickness and 15 with superficial partial‐thickness facial burns |
| Interventions | Intervention arm 1: silver hydrocolloid Intervention arms 2: biological dressing (porcine xenoderm [sic]) Cointerventions: NR |
| Outcomes | Primary outcome: wound healing time Primary outcome: infection Secondary outcome: resource use, possibly also pain |
| Notes | Paper in Chinese. Awaiting obtaining full text and translator assessment; details here based on English abstract |
NR: not reported; RCT; randomised controlled trial; SSD: silver sulfadiazine
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (hazard ratio) Show forest plot | 3 | 259 | Hazard Ratio (Random, 95% CI) | 1.25 [0.94, 1.67] |
| Analysis 1.1  Comparison 1 Silver dressings versus topical antibiotics, Outcome 1 Wound healing (hazard ratio). | ||||
| 2 Wound healing (mean time to healing) Show forest plot | 10 | 1085 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐4.96, ‐1.70] |
| Analysis 1.2  Comparison 1 Silver dressings versus topical antibiotics, Outcome 2 Wound healing (mean time to healing). | ||||
| 3 Wound healing (risk ratio) up to 28 days Show forest plot | 5 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.00, 1.37] |
| Analysis 1.3  Comparison 1 Silver dressings versus topical antibiotics, Outcome 3 Wound healing (risk ratio) up to 28 days. | ||||
| 4 Infection (up to 4 weeks or NR) Show forest plot | 4 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.49] |
| Analysis 1.4  Comparison 1 Silver dressings versus topical antibiotics, Outcome 4 Infection (up to 4 weeks or NR). | ||||
| 5 Adverse events (14‐28 days) Show forest plot | 6 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.18] |
| Analysis 1.5  Comparison 1 Silver dressings versus topical antibiotics, Outcome 5 Adverse events (14‐28 days). | ||||
| 6 Withdrawals due to adverse events (21 days or NR) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.6 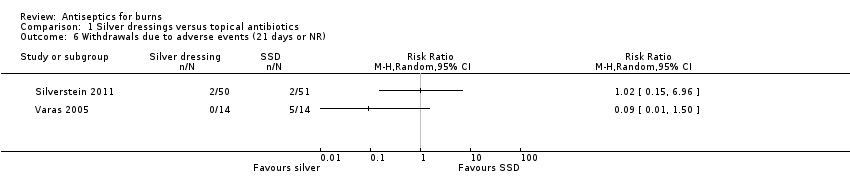 Comparison 1 Silver dressings versus topical antibiotics, Outcome 6 Withdrawals due to adverse events (21 days or NR). | ||||
| 7 Pain at dressing change (up to 28 days or NR) Show forest plot | 5 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.92, ‐0.49] |
| Analysis 1.7 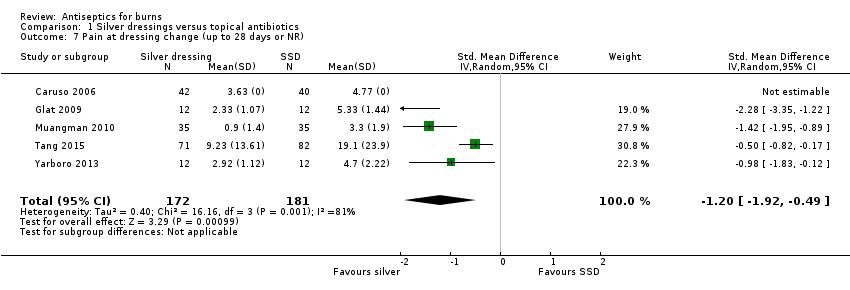 Comparison 1 Silver dressings versus topical antibiotics, Outcome 7 Pain at dressing change (up to 28 days or NR). | ||||
| 8 Pain (time/follow‐up not specified) Show forest plot | 3 | 135 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.06, ‐1.27] |
| Analysis 1.8  Comparison 1 Silver dressings versus topical antibiotics, Outcome 8 Pain (time/follow‐up not specified). | ||||
| 9 Mortality (21 days or NR) Show forest plot | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.64] |
| Analysis 1.9 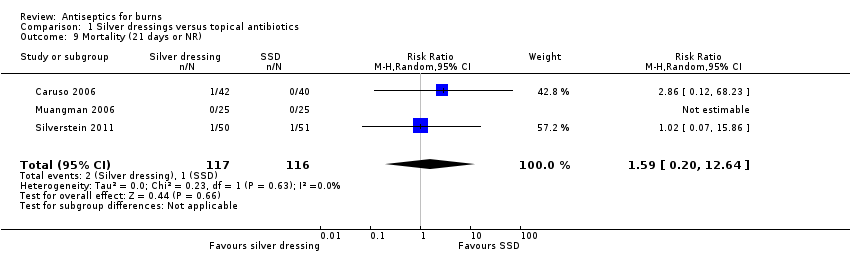 Comparison 1 Silver dressings versus topical antibiotics, Outcome 9 Mortality (21 days or NR). | ||||
| 10 Resource use (number of dressings) (up to 28 days or NR) Show forest plot | 6 | 446 | Mean Difference (IV, Random, 95% CI) | ‐7.56 [‐12.09, ‐3.04] |
| Analysis 1.10  Comparison 1 Silver dressings versus topical antibiotics, Outcome 10 Resource use (number of dressings) (up to 28 days or NR). | ||||
| 11 Costs (21 days or NR) Show forest plot | 4 | 261 | Mean Difference (IV, Random, 95% CI) | ‐117.18 [‐280.02, 45.67] |
| Analysis 1.11  Comparison 1 Silver dressings versus topical antibiotics, Outcome 11 Costs (21 days or NR). | ||||
| 12 Cost‐effectiveness/wound healed (21 days) Show forest plot | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐384.71 [‐503.66, ‐265.75] |
| Analysis 1.12  Comparison 1 Silver dressings versus topical antibiotics, Outcome 12 Cost‐effectiveness/wound healed (21 days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (hazard ratio) Show forest plot | 5 | 580 | Hazard Ratio (Random, 95% CI) | 2.45 [1.71, 3.52] |
| Analysis 2.1 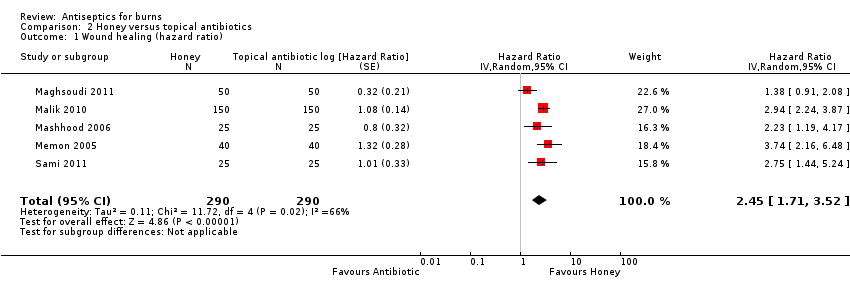 Comparison 2 Honey versus topical antibiotics, Outcome 1 Wound healing (hazard ratio). | ||||
| 2 Wound healing (risk ratio) (up to 60 days) Show forest plot | 6 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.99, 2.76] |
| Analysis 2.2  Comparison 2 Honey versus topical antibiotics, Outcome 2 Wound healing (risk ratio) (up to 60 days). | ||||
| 3 Wound healing (mean time to healing) Show forest plot | 6 | 712 | Mean Difference (IV, Random, 95% CI) | ‐3.79 [‐7.15, ‐0.43] |
| Analysis 2.3  Comparison 2 Honey versus topical antibiotics, Outcome 3 Wound healing (mean time to healing). | ||||
| 4 Incident infection (up to 24 days) Show forest plot | 4 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.08, 0.34] |
| Analysis 2.4  Comparison 2 Honey versus topical antibiotics, Outcome 4 Incident infection (up to 24 days). | ||||
| 5 Persistent positive swabs (up to 21 days) Show forest plot | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.05, 0.19] |
| Analysis 2.5  Comparison 2 Honey versus topical antibiotics, Outcome 5 Persistent positive swabs (up to 21 days). | ||||
| 6 Adverse events (time points between 21 days and 6 weeks) Show forest plot | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.97] |
| Analysis 2.6  Comparison 2 Honey versus topical antibiotics, Outcome 6 Adverse events (time points between 21 days and 6 weeks). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 3 | 210 | Mean Difference (IV, Random, 95% CI) | ‐7.79 [‐17.96, 2.38] |
| Analysis 3.1  Comparison 3 Aloe vera vs topical antibiotics, Outcome 1 Wound healing (mean time to healing). | ||||
| 2 Infection (time points between 14 days and 2 months) Show forest plot | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.26, 3.34] |
| Analysis 3.2  Comparison 3 Aloe vera vs topical antibiotics, Outcome 2 Infection (time points between 14 days and 2 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 2 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐2.76, 1.83] |
| Analysis 4.1 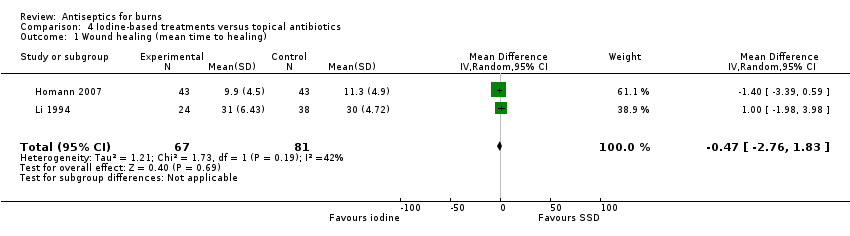 Comparison 4 Iodine‐based treatments versus topical antibiotics, Outcome 1 Wound healing (mean time to healing). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 2 | 204 | Mean Difference (IV, Random, 95% CI) | ‐3.49 [‐4.46, ‐2.52] |
| Analysis 5.1  Comparison 5 Silver‐based antiseptics versus non‐antimicrobial, Outcome 1 Wound healing (mean time to healing). | ||||
| 2 Positive swab (21 days) Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.95] |
| Analysis 5.2  Comparison 5 Silver‐based antiseptics versus non‐antimicrobial, Outcome 2 Positive swab (21 days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (hazard ratio) Show forest plot | 2 | 164 | Hazard Ratio (Fixed, 95% CI) | 2.86 [1.60, 5.11] |
| Analysis 6.1 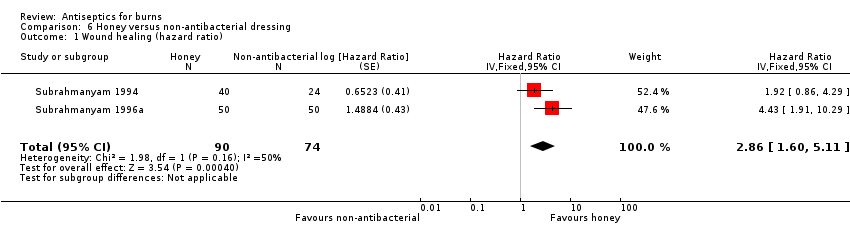 Comparison 6 Honey versus non‐antibacterial dressing, Outcome 1 Wound healing (hazard ratio). | ||||
| 2 Wound healing (mean time to healing) Show forest plot | 4 | 1156 | Mean Difference (IV, Random, 95% CI) | ‐5.32 [‐6.30, ‐4.34] |
| Analysis 6.2  Comparison 6 Honey versus non‐antibacterial dressing, Outcome 2 Wound healing (mean time to healing). | ||||
| 3 Persistent positive swabs (up to 30 days) Show forest plot | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.06, 0.40] |
| Analysis 6.3 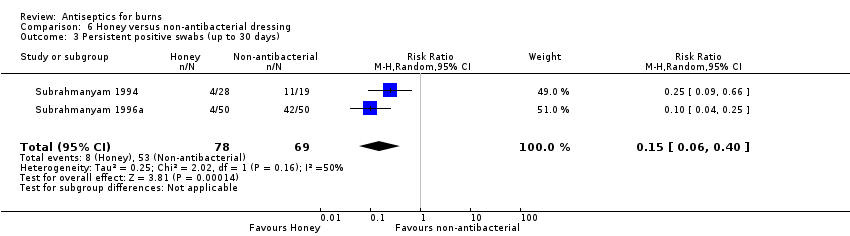 Comparison 6 Honey versus non‐antibacterial dressing, Outcome 3 Persistent positive swabs (up to 30 days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Chlorhexadine versus non‐antibacterial dressing, Outcome 1 Wound healing (mean time to healing). | ||||
| 2 Infection (up to 30 days) Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.54, 2.27] |
| Analysis 7.2  Comparison 7 Chlorhexadine versus non‐antibacterial dressing, Outcome 2 Infection (up to 30 days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.1 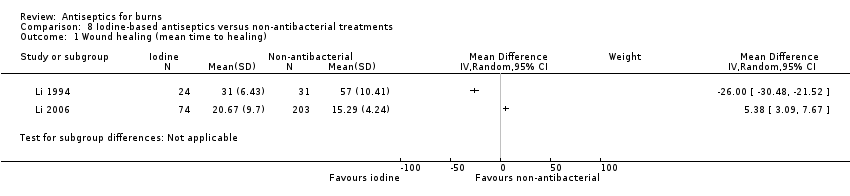 Comparison 8 Iodine‐based antiseptics versus non‐antibacterial treatments, Outcome 1 Wound healing (mean time to healing). | ||||
| 2 Costs (duration 18 days +) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 Iodine‐based antiseptics versus non‐antibacterial treatments, Outcome 2 Costs (duration 18 days +). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (short‐term or unclear) Show forest plot | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.99] |
| Analysis 9.1 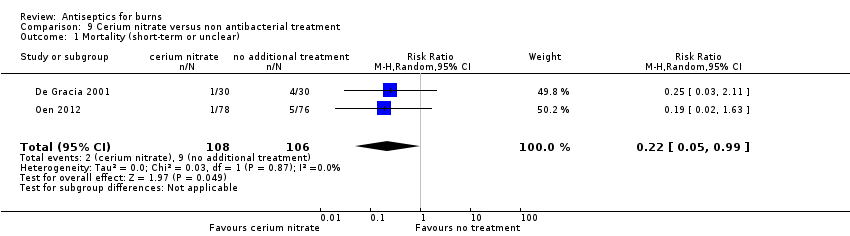 Comparison 9 Cerium nitrate versus non antibacterial treatment, Outcome 1 Mortality (short‐term or unclear). | ||||

PRISMA flow diagram

Network of included treatment types
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Silver dressings versus topical antibiotics, Outcome 1 Wound healing (hazard ratio).
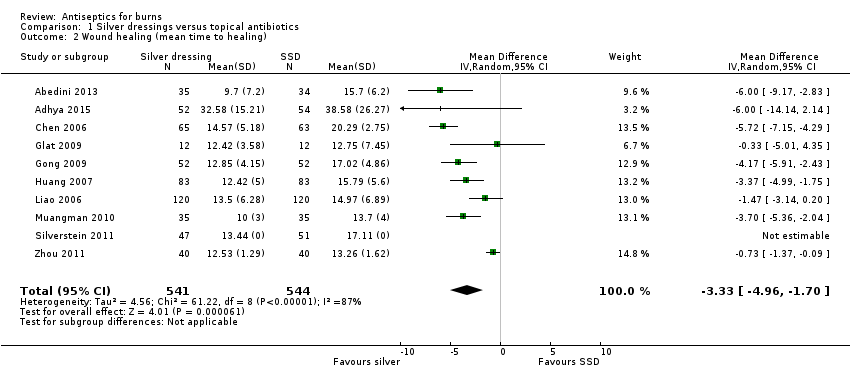
Comparison 1 Silver dressings versus topical antibiotics, Outcome 2 Wound healing (mean time to healing).
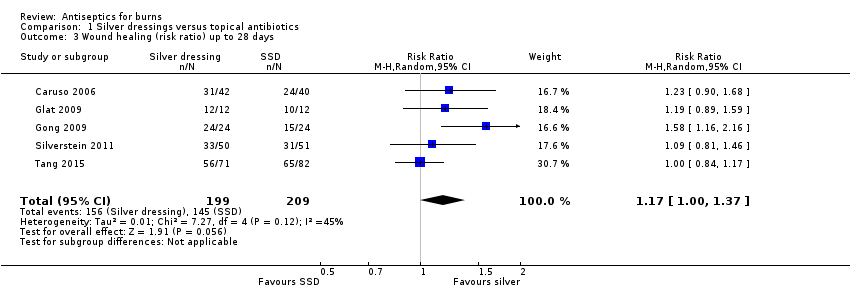
Comparison 1 Silver dressings versus topical antibiotics, Outcome 3 Wound healing (risk ratio) up to 28 days.
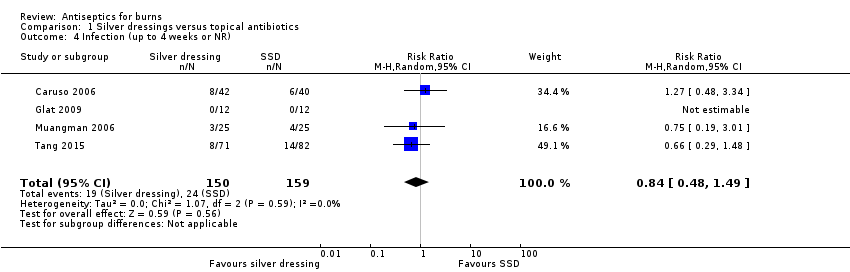
Comparison 1 Silver dressings versus topical antibiotics, Outcome 4 Infection (up to 4 weeks or NR).

Comparison 1 Silver dressings versus topical antibiotics, Outcome 5 Adverse events (14‐28 days).
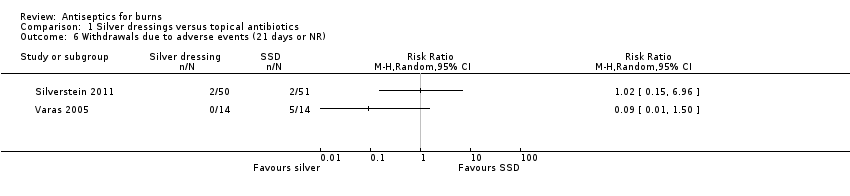
Comparison 1 Silver dressings versus topical antibiotics, Outcome 6 Withdrawals due to adverse events (21 days or NR).
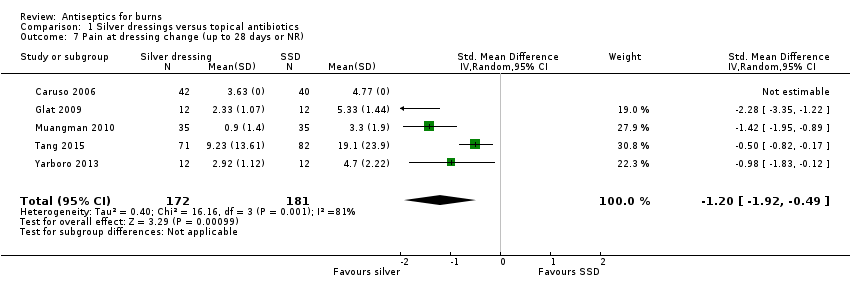
Comparison 1 Silver dressings versus topical antibiotics, Outcome 7 Pain at dressing change (up to 28 days or NR).
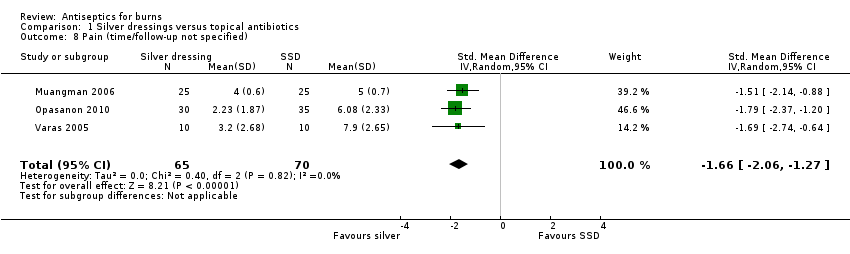
Comparison 1 Silver dressings versus topical antibiotics, Outcome 8 Pain (time/follow‐up not specified).

Comparison 1 Silver dressings versus topical antibiotics, Outcome 9 Mortality (21 days or NR).
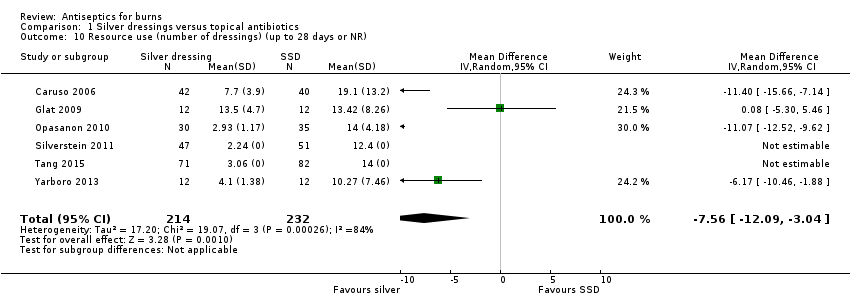
Comparison 1 Silver dressings versus topical antibiotics, Outcome 10 Resource use (number of dressings) (up to 28 days or NR).

Comparison 1 Silver dressings versus topical antibiotics, Outcome 11 Costs (21 days or NR).
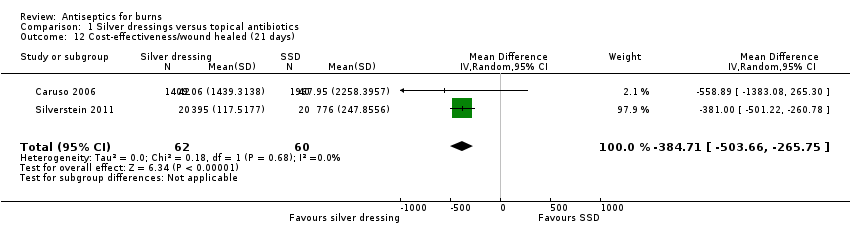
Comparison 1 Silver dressings versus topical antibiotics, Outcome 12 Cost‐effectiveness/wound healed (21 days).

Comparison 2 Honey versus topical antibiotics, Outcome 1 Wound healing (hazard ratio).

Comparison 2 Honey versus topical antibiotics, Outcome 2 Wound healing (risk ratio) (up to 60 days).

Comparison 2 Honey versus topical antibiotics, Outcome 3 Wound healing (mean time to healing).
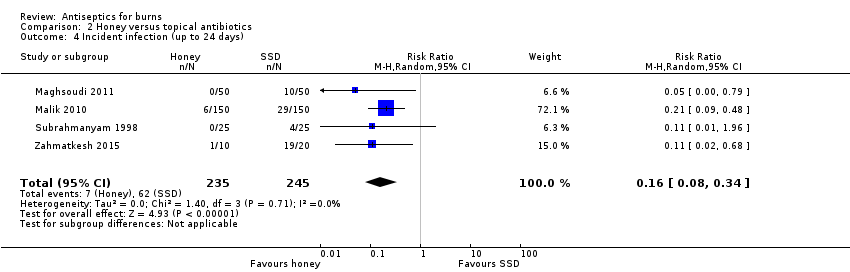
Comparison 2 Honey versus topical antibiotics, Outcome 4 Incident infection (up to 24 days).
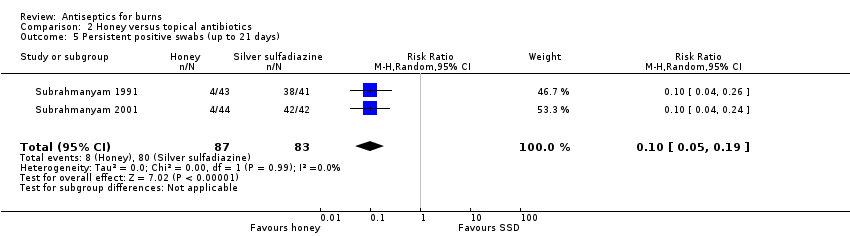
Comparison 2 Honey versus topical antibiotics, Outcome 5 Persistent positive swabs (up to 21 days).

Comparison 2 Honey versus topical antibiotics, Outcome 6 Adverse events (time points between 21 days and 6 weeks).

Comparison 3 Aloe vera vs topical antibiotics, Outcome 1 Wound healing (mean time to healing).

Comparison 3 Aloe vera vs topical antibiotics, Outcome 2 Infection (time points between 14 days and 2 months).
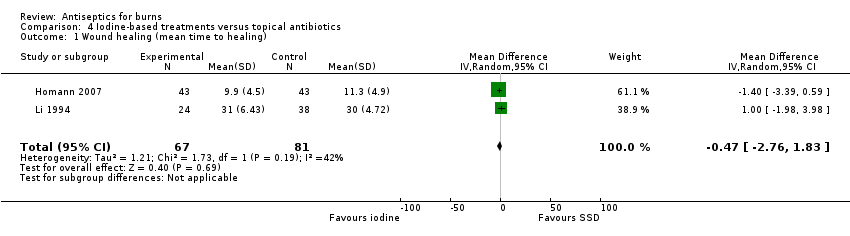
Comparison 4 Iodine‐based treatments versus topical antibiotics, Outcome 1 Wound healing (mean time to healing).

Comparison 5 Silver‐based antiseptics versus non‐antimicrobial, Outcome 1 Wound healing (mean time to healing).
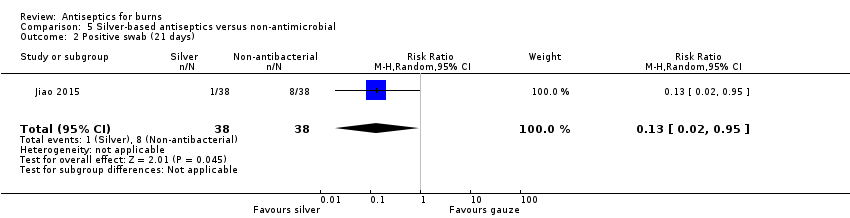
Comparison 5 Silver‐based antiseptics versus non‐antimicrobial, Outcome 2 Positive swab (21 days).
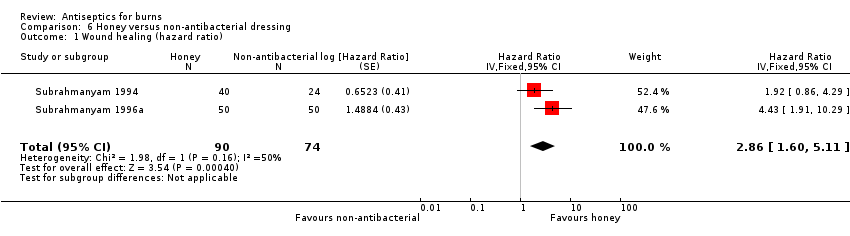
Comparison 6 Honey versus non‐antibacterial dressing, Outcome 1 Wound healing (hazard ratio).

Comparison 6 Honey versus non‐antibacterial dressing, Outcome 2 Wound healing (mean time to healing).
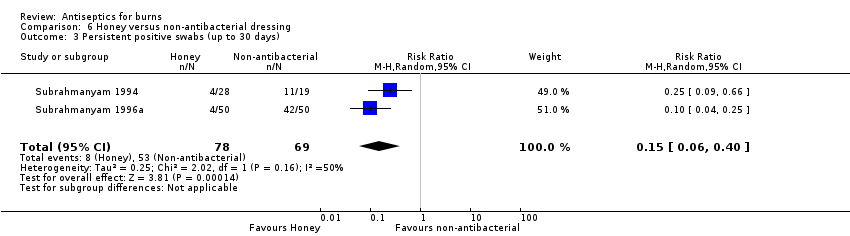
Comparison 6 Honey versus non‐antibacterial dressing, Outcome 3 Persistent positive swabs (up to 30 days).
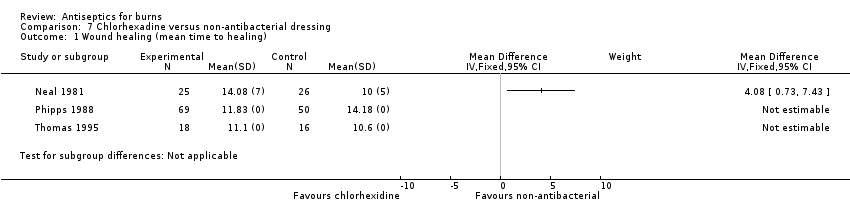
Comparison 7 Chlorhexadine versus non‐antibacterial dressing, Outcome 1 Wound healing (mean time to healing).

Comparison 7 Chlorhexadine versus non‐antibacterial dressing, Outcome 2 Infection (up to 30 days).
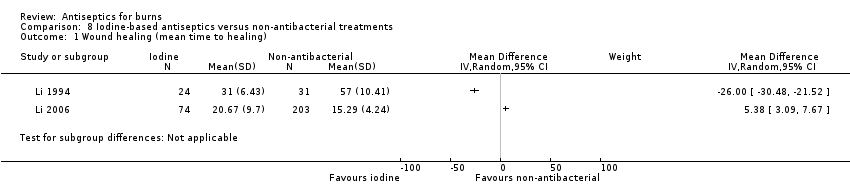
Comparison 8 Iodine‐based antiseptics versus non‐antibacterial treatments, Outcome 1 Wound healing (mean time to healing).

Comparison 8 Iodine‐based antiseptics versus non‐antibacterial treatments, Outcome 2 Costs (duration 18 days +).

Comparison 9 Cerium nitrate versus non antibacterial treatment, Outcome 1 Mortality (short‐term or unclear).
| Silver‐based antiseptics versus topical antibiotics | ||||||
| Patient or population: people with burns Intervention: silver‐based antiseptics (primarily dressings) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with SSD | Risk with silver dressings | |||||
| Wound healing: time to complete healing (time‐to‐event data) | 739 per 1000 | 813 per 1000 (717 to 894) | HR 1.25 | 259 | ⊕⊕⊝⊝ | Only three studies provided sufficient data for an HR; this showed that, on average, there is no clear difference in the 'chance' of healing in burns treated with silver‐based antiseptic dressings compared with SSD. HR calculated using standard methods for two trials |
| Risk difference: 74 more burns healed per 1000 with silver dressings than with SSD (22 fewer to 155 more) | ||||||
| Wound healing (mean time to healing) | The mean time to wound healing was 11.92 days | The mean time to wound healing in the intervention group was 3.33 days shorter (4.96 fewer to 1.70 fewer) | MD ‐3.33 days (‐4.96 to ‐1.70) | 1085 | ⊕⊕⊝⊝ | Silver may, on average, slightly improve mean time to healing compared with SSD |
| Wound healing (number of healing events) | 784 per 1000 | 917 per 1000 (784 to 1000) | RR 1.17 (1.00 to 1.37) | 408 (5 RCTs) | ⊕⊕⊝⊝ | There may be little difference in the number of healing events over short‐term follow‐up (up to 28 days) compared with SSD |
| Risk difference: 133 more burns healed per 1000 with silver dressings than with SSD (0 more to 290 more) | ||||||
| Infection | 151 per 1000 | 127 per 1000 | RR 0.84 | 309 | ⊕⊝⊝⊝ | It is uncertain whether silver‐containing antiseptics increase or reduce the risk of infection compared with use of SSD as evidence is very low certainty |
| Risk difference: 24 fewer participants with adverse events per 1000 with silver dressings than with SSD (78 fewer to 71 more) | ||||||
| Adverse events | 227 per 1000 | 195 per 1000 | RR 0.86 | 440 | ⊕⊕⊝⊝ | There may be little or no difference in the number of adverse events in participants treated with silver dressings compared with SSD |
| Risk difference: 34 fewer participants with adverse events per 1000 with silver dressings than with SSD (86 fewer to 29 more). | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Not downgraded for risk of selection bias and detection bias because most participants were in a study at low risk of bias; downgraded twice for serious imprecision due to low numbers of participants and wide confidence intervals. | ||||||
| Honey versus topical antibiotics | ||||||
| Patient or population: people with burns Intervention: honey Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with topical antibiotics | Risk with honey | |||||
| Wound healing: time to complete healing (time‐to‐event data): honey versus SSD or mafenide acetate | 641 per 1000 | 919 per 1000 (827 to 973) | HR 2.45 | 580 | ⊕⊕⊕⊝ | Burns treated with honey probably have a greater 'chance' of healing compared with SSD or mafenide acetate. HR calculated using standard methods for all trials |
| Risk difference: 278 more burns healed per 1000 with honey than with topical antibiotics (185 more to 332 more). | ||||||
| Wound healing (mean time to healing): honey versus SSD | The mean time to wound healing was 15.53 days | The mean time to wound healing was 3.79 days fewer (7.15 fewer to 0.43 fewer) | MD ‐3.79 (‐7.15 to ‐0.43) | 712 | ⊕⊝⊝⊝ Very low2 | It is uncertain what the effect of honey is on mean time to wound healing compared with SSD |
| Wound healing (number of healing events): honey versus SSD | 434 per 1000 | 946 per 1000 (499 to 1000) | RR 2.18 (1.15 to 4.13) | 318 (4 RCTs) | ⊕⊕⊝⊝ | There may, on average, be more healing events in burns treated with honey compared with SSD over short‐term follow‐up (maximum 21 days) |
| Risk difference: 512 more burns healed per 1000 with honey than with SSD (65 more to 1358 more) | ||||||
| Incident infection: honey versus SSD or mafenide acetate | 135 per 1000 | 22 per 1000 | RR 0.16 | 480 | ⊕⊝⊝⊝ Very low4 | It is uncertain if fewer burns treated with honey may become infected compared with those treated with SSD or mafenide acetate |
| Risk difference: 113 fewer infections (positive swabs in 3 RCTs) per 1000 with honey compared with topical antibiotics (124 fewer to 89 fewer) | ||||||
| Peristent infection: honey versus SSD | 964 per 1000 | 98 per 1000 (48 to 183) | RR 0.10 (0.05 to 0.19) | 170 (2 RCTs) | ||
| Risk difference: 867 fewer persistently positive swabs per 1000 with honey compared with topical antibiotics (961 to 781) | ||||||
| Adverse events: honey versus SSD | 16 per 1000 | 3 per 1000 | RR 0.20 | 250 | ⊕⊝⊝⊝ | It is uncertain whether fewer participants treated with honey experience adverse events compared with those treated with SSD |
| Risk difference: 13 fewer participants with adverse events per 1000 with honey compared with SSD (16 fewer to 48 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision. A post‐hoc sensitivity analysis excluding a study with an intra‐individual design made no material difference to the analysis. | ||||||
| Aloe Vera versus topical antibiotics | ||||||
| Patient or population: people with burns Intervention: Aloe Vera Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with topical antibiotics | Risk with Aloe Vera | |||||
| Wound healing (number of healing events): Aloe Vera versus SSD | 389 per 1000 | 548 per 1000 | RR 1.41 | 38 | ⊕⊕⊝⊝ | It is unclear whether Aloe Vera may alter the number of healing events compared with SSD; confidence intervals are wide, spanning both benefits and harms so clear differences between treatments are not apparent |
| Risk difference: 159 more burns healed per 1000 with Aloe Vera than with SSD (117 fewer to 719 more) | ||||||
| Wound healing (mean time to healing): Aloe Vera versus SSD or framycetin | The mean time to wound healing was 21.25 days | The mean time to wound healing was 7.79 days shorter (17.96 shorter to 2.38 longer) | MD ‐7.79 (‐17.96 to 2.38) | 210 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in mean time to healing between Aloe Vera and SSD or framycetin. No data were contributed by the trial using framycetin |
| Infection: Aloe Vera versus SSD | 36 per 1000 | 34 per 1000 | RR 0.93 | 221 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in infection incidence between Aloe Vera and SSD |
| Risk difference: 3 fewer infections per 1000 with Aloe Vera than with SSD (27 fewer to 85 more) | ||||||
| Adverse events | No trial reported evaluable adverse event data for this comparison | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for very serious imprecision. | ||||||
| Iodine versus topical antibiotics | ||||||
| Patient or population: people with burns Intervention: iodine‐based treatments Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with topical antibiotics | Risk with iodine‐based treatments | |||||
| Wound healing (mean time to healing) | The mean time to wound healing was 20.07 days | The mean time to wound healing in the intervention group was 0.47 days shorter (2.76 shorter to 1.83 longer) | MD ‐0.47 (‐2.76 to 1.83) | 148 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in mean time to wound healing between iodine‐based antiseptic treatments and SSD |
| Infection | No study reported evaluable data for infection | |||||
| Adverse events | 350 per 1000 | 301 per 1000 | RR 0.86 | 40 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the proportion of participants with adverse events between iodine‐based antiseptic treatments and SSD |
| Risk difference: 48 fewer participants with adverse events per 1000 with iodine‐based treatments than with SSD (227 fewer to 385 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for detection bias in one trial accounting for 61% of the analysis weight and twice for imprecision due to low participant numbers and confidence intervals that cross the line of no effect; one study also had an intra‐individual design, which may not have been accounted for in the analysis, this is taken account of in the double downgrading for imprecision. | ||||||
| Silver versus non‐antibacterial | ||||||
| Patient or population: people with burns Intervention: silver‐based interventions (dressings) Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐antibacterial dressing | Risk with silver dressing | |||||
| Wound healing (number of healing events): silver xenograft vs petroleum gauze | 500 per 1000 | 565 per 1000 | RR 1.13 | 32 | ⊕⊕⊝⊝ | There may be little or no difference between silver xenograft and petroleum gauze |
| Risk difference: 65 more burns healed per 1000 with silver xenograft compared with petroleum gauze (205 fewer to 580 more) | ||||||
| Wound healing (mean time to healing): silver nanoparticle vs Vaseline gauze | The mean time to wound healing was 15.87 days | The mean time to wound healing in the silver nanoparticle group was 3.49 days shorter (4.46 shorter to 2.52 shorter) compared with gauze | MD ‐3.49 (‐4.46 to ‐2.52) | 204 | ⊕⊕⊕⊝ | The mean time to wound healing is probably slightly shorter in the group treated with silver nanoparticle dressing compared with Vaseline gauze |
| Infection | No study reported evaluable data for this comparison | |||||
| Adverse events | No study reported evaluable data for this comparison | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision as fragile confidence intervals cross the line of no effect. | ||||||
| Honey versus non‐antibacterial | ||||||
| Patient or population: people with burns Intervention: honey Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐antibacterial dressing | Risk with honey | |||||
| Wound healing: time to complete healing (time‐to‐event data) | 1000 per 1000 | 1000 per 1000 (1000 to 1000) | HR 2.86 | 164 | ⊕⊕⊕⊝ | The 'chance' of healing is probably somewhat greater in participants treated with honey compared with unconventional non‐antibacterial treatments |
| Risk difference: 0 difference burns healed per 1000 with honey compared with non‐antibacterial treatments (0 to 0) | ||||||
| Wound healing (mean time to healing) | The mean time to wound healing was 14.05 days | The mean time to wound healing in the intervention group was 5.32 days shorter (6.30 shorter to 4.34 shorter) | MD ‐5.32 (‐6.30 to ‐4.34) | 1156 | ⊕⊕⊕⊕ | Participants treated with honey, on average, have a shorter mean time to healing compared with those treated with a range of treatments without antibacterial properties, including unconventional treatments |
| Infection (incident) | 370 per 1000 | 174 per 1000 | RR 0.47 | 92 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the incidence or persistence of wound infection in participants treated with honey compared with a range of treatments without antimicrobial properties |
| Risk difference: 196 fewer incident infections (persistently positive swabs) per 1000 with honey compared with non‐antibacterial treatments (285 fewer to 7 fewer) | ||||||
| Infection (persistent) | 768 per 1000 | 115 per 1000 | RR 0.15 (0.06 to 0.40) | 147 of 164 randomised (2 RCTs) | ⊕⊝⊝⊝ | |
| Risk difference: 653 fewer persistent infections (persistently positive swabs) per 1000 with honey compared with non‐antibacterial treatments (722 fewer to 461 fewer) | ||||||
| Adverse events | One study reported that there were no events in either intervention group; other studies did not report data that clearly related to the number of participants who experienced adverse events in each group | 239 (3 RCTs) | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the incidence of adverse effects between participants treated with honey and those treated with a range of alternative non‐antimicrobial therapies | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision due to low numbers of participants. | ||||||
| Chlorhexidine versus non‐antibacterial | ||||||
| Patient or population: people with burns Intervention: chlorhexidine Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐antibacterial dressing | Risk with biguanides | |||||
| Wound healing: time to complete healing (time‐to‐event data): chlorhexidine versus polyurethane | 1000 per 1000 | 1000 per 1000 (1000 to 1000) | HR 0.71 | 51 | ⊕⊕⊝⊝ | There may be some difference in the 'chance' of healing for chlorhexidine compared with polyurethane but CIs span benefit and harm so a clear difference between treatments is not apparent |
| Risk Difference: 0 difference per 1000 for chlorhexidine compared with polyurethane (0 to 0) | ||||||
| Wound healing (mean time to healing): chlorhexidine versus non‐antibacterial | The mean time to wound healing ‐ chlorhexidine versus polyurethane was 10 days | The mean time to wound healing ‐ chlorhexidine versus polyurethane in the intervention group was 4.08 days longer (0.73 longer to 7.43 longer) | MD 4.08 (0.73 to 7.43) | 51 153 participants in 2 RCTs did not have evaluable data | ⊕⊕⊝⊝ | The mean time to wound healing may be slightly longer in burns treated with chlorhexidine compared with polyurethane; data from 2 additional RCTs comparing chlorhexidine with hydrocolloid lacked measures of variance |
| Infection: chlorhexidine versus no antimicrobial/no additional antimicrobial | 179 per 1000 | 184 per 1000 | RR 1.11 (0.54 to 2.27) | 172 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the incidence of infection between participants treated with chlorhexidine either alone or in addition to SSD and participants treated with no antimicrobial or SSD alone |
| Risk Difference: 15 more infections per 1000 with chlorhexidine compared with non‐antibacterial treatments (64 fewer to 178 more) | ||||||
| Adverse events: chlorhexidine versus hydrocolloid | 102 per 1000 | 20 per 1000 | RR 0.20 | 98 | ⊕⊝⊝⊝ | It is uncertain whether there is a difference in the number of participants with adverse effects between chlorhexidine and a hydrocolloid dressing |
| Risk Difference: 82 fewer participants with adverse events with chlorhexidine compared with hydrocolloid (100 fewer to 66 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision due to wide confidence intervals, which cross the line of no effect, and fragility due to small numbers of participants. | ||||||
| Iodine‐based treatments versus non‐antibacterial | ||||||
| Patient or population: people with burns Intervention: iodine‐based treatments Setting: hospitals and burn clinics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐antibacterial treatments | Risk with iodine‐based treatments | |||||
| Wound healing (number of healing events): iodophor versus hydrogel | 700 per 1000 | 119 per 1000 | RR 0.17 | 120 | ⊕⊕⊝⊝ | There may be a smaller number of healing events at 26 days in participants treated with iodophor compared with those treated with hydrogel |
| Risk difference: 581 fewer wounds healed per 1000 at 14 days with iodophor treatment compared with hydrogel (644 fewer to 462 fewer) | ||||||
| Wound healing (mean time to healing): iodine gauze versus carbon fibre | The mean time to wound healing) ‐ iodine gauze versus carbon fibre was 15.29 days | The mean time to wound healing) ‐ iodine gauze versus carbon fibre in the intervention group was 5.38 days longer (3.09 longer to 7.67 longer) | MD 5.38 (3.09 to 7.67) | 277 | ⊕⊝⊝⊝ | The clinical heterogeneity between these studies, both in terms of interventions and comparators, combined with the wide divergence in effects meant that they could not meaningfully be pooled. It is very uncertain what the effect of iodine compared with non‐antibacterial dressings/topical treatments is on mean time to wound healing |
| Wound healing (mean time to healing): iodophor versus MEBO | The mean time to wound healing) ‐ iodophor versus MEBO was 57 days | The mean time to wound healing) ‐ iodophor versus MEBO in the intervention group was 26 days shorter (30.48 shorter to 21.52 shorter) | MD ‐26.00 (‐30.48 to ‐21.52) | 55 | ||
| Infection: iodine gauze versus MEBO | 58 per 1000 | 75 per 1000 | RR 1.30 | 211 | ⊕⊕⊝⊝ | There may be little or no difference in the incidence of infection in participants treated with iodine gauze compared with those treated with MEBO |
| Risk difference: 17 more infections per 1000 with iodine gauze compared with MEBO (31 fewer to 151 more) | ||||||
| Adverse effects: iodine gauze versus MEBO | 106 per 1000 | 75 per 1000 | RR 0.71 | 211 | ⊕⊕⊝⊝ | There may be little or no difference in the incidence of adverse effects in participants treated with iodine gauze compared with those treated with MEBO |
| Risk difference: 31 fewer participants with adverse events with iodine gauze compared with MEBO (74 fewer to 73 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision due to wide confidence intervals and fragility due to small numbers of participants and uncertainty about the analysis of an intra‐individual design. | ||||||
| Comparison | Number of studies | Number of participants |
| Antiseptics versus topical antibiotics | ||
| Silver vs SSD | 16 | 1368 |
| Honey vs SSD or mafenide acetate | 11 | 856 |
| Aloe Vera vs SSD or framycetin | 5 | 338 |
| Iodine vs SSD | 2 | 158 |
| Sodium hypochlorite vs SSD | 1 | 20 |
| Chlorhexidine or polyhexanide (biguanides) vs SSD | 2 | 115 |
| Octenidine vs SSD | 1 | 30 |
| Ethacridine lactate (Rivanol) vs SSD | 1 | 115 |
| Merbromin vs zinc sulfadiazine | 1 | 125 |
| Arnebia euchroma vs SSD | 1 | 49 |
| Antiseptics versus alternative antiseptics | ||
| Chlorhexidine vs iodine | 1 | 213 |
| Iodine vs ethacridine lactate | 1 | 115 |
| Antiseptics versus non‐antibacterial | ||
| Silver vs non‐antibacterial | 3 | 299 |
| Honey vs non‐antibacterial | 3 | 256 |
| Chlorhexidine vs non‐antibacterial | 5 | 516 |
| Iodine vs non‐antibacterial | 4 | 663 |
| Ethacridine lactate vs non‐antibacterial | 1 | 115 |
| Cerium nitrate vs non‐antibacterial | 2 | 214 |
| Merbromin vs non‐antibacterial | 1 | 125 |
| SSD: silver sulfadiazine | ||
| Comparison | Study | Number participants /wounds | Duration | Time to wound healing (days) (mean (SD)) | Difference in means (days) (95% CI) | Proportion of wounds healed | Risk ratio (for longest time point) or Hazard Ratio |
| Antiseptic versus topical antibiotic | |||||||
| Silver hydrofibre Silver sulfadiazine (SSD) | Silver 35 SSD 34 | Until healing | Silver 9.7 (7.2) SSD 15.7 (6.2) | ‐6.00 (‐9.17 to ‐2.83) | ‐ | ‐ | |
| Silver hydrofibre Silver sulfadiazine | Silver 42 SSD 40 | 21 days | Median Silver 16 SSD 17 | ‐ | Silver 31 SSD 24 | HR 1.67 (0.76 to 3.65) RR 1.23 (0.60 to 1.68) | |
| Silver hydrofibre Silver sulfadiazine | Silver 35 SSD 35 | NR | Silver 10 (3) SSD 13.7 (4) | ‐3.70 (‐5.36 to ‐2.04) | ‐ | ‐ | |
| Silver hydrogel Silver sulfadiazine | Silver 84 SSD 79 (analysed silver 54, SSD 52) | 4 weeks/until healing | Silver 38.58 (26.27) SSD 32.58 (15.21) | 6.00 (‐2.14 to 14.14) | Deep dermal wounds only reported | ‐ | |
| Silver hydrogel Silver sulfadiazine | Silver 12 SSD 12 | 21 days+ | Silver 12.42 (3.58) SSD 12.75 (7.45) (participants followed up after 21 days when binary data reported) | ‐0.33 (‐5.01 to 4.35) | Silver 12 SSD 10 | HR 1.03 (0.44 to 2.39) RR 1.19 (0.89 to 1.59) | |
| Silver hydrogel Silver sulfadiazine | Silver 52 SSD 52 | 21 days+ | Silver 12.85 (4.15) SSD 17.02 (4.86) (participants followed up after 21 days when binary data reported) | ‐4.17 (‐5.91 to ‐2.43) | Silver 52/52 (day 21) SSD 43/52 (day 21) | RR 1.58 (1.16 to 2.16) | |
| Silver foam Silver sulfadiazine | Silver 50 SSD 51 | 21 days | Silver 13.44 (N = 47) SSD 17.11 (N = 51) Reported as NS | ‐ | 1 week Silver 16 SSD 10 3 weeks Silver 33 SSD 31 | RR 1.09 (0.81 to 1.46) | |
| Silver foam Silver sulfadiazine | Silver 71 SSD 82 | 4 weeks | Silver 56/71 (median 15 days) SSD 65/82 (median 16 days) | ‐ | 28 days Silver 56 SSD 65 | HR 1.22 (0.88 to 1.70) favouring silver RR 1.00 (0.84 to 1.17) | |
| Silver foam Silver sulfadiazine | 24 participants randomised; group allocation unclear | NR | ‐ | ‐ | ‐ | ‐ | |
| Silver foam Silver sulfadiazine | 40 participants; part of each burn randomised to treatments | 14 days | Silver 12.53 (± 1.29) SSD 13.26 (± 1.62) | ‐0.73 (‐1.37 to 0.09) | ‐ | ‐ | |
| Nanocrystalline silver Silver sulfadiazine Vaseline gauze | Silver 65 SSD 63 Vaseline gauze 63 | Until healing | Silver 14.57 (5.18) SSD 20.29 (2.75) Vaseline 18.03 (5.1) | Silver vs SSD ‐5.72 (‐7.15 to ‐4.29) Silver vs Vaseline ‐3.49 (‐4.46 to ‐2.52) | ‐ | ‐ | |
| Nanocrystalline silver Silver sulfadiazine | 98 participants with 166 burns 83 burns in each group | 20 days | Silver 12.42 (5.40) SSD 15.79 (5.60) | ‐3.37 (‐4.49 to ‐1.75) | ‐ | ‐ | |
| Nanocrystalline silver Silver sulfadiazine | Silver 25 SSD 25 | NR | ‐ | ‐ | ‐ | ‐ | |
| Nanocrystalline silver Silver sulfadiazine | 14 participants with 2 burn areas; 14 burn areas in each group | NR | ‐ | ‐ | ‐ | ‐ | |
| Silver nitrate Silver sulfadiazine | 120 participants with 2 burn areas; 120 burn areas in each group | Until healing | Silver 13.5 (6.28) SSD 14.97 (6.89) | ‐1.47 (‐3.14 to 0.20) | ‐ | ‐ | |
| Silver alginate Silver sulfadiazine | Silver 30 SSD 35 | NR | ‐ | ‐ | ‐ | ‐ | |
| Honey Silver sulfadiazine | Honey 37 SSD 41 | NR (2 months' follow‐up) | Honey 18.16 (SD ‐) SSD 32.68 (SD ‐) | ‐ | ‐ | ‐ | |
| Honey Silver sulfadiazine | Honey 32 SSD 32 | 21 days | ‐ | ‐ | Honey 10 days 26 ≥ 14 days 32 SSD ≥ 3 weeks 19 unclear if all healed | RR 1.67 (1.25 to 2.22) | |
| Honey Silver sulfadiazine | 150 participants with 2 burns; 150 burns in each group | 24 days | Honey 13.47 (4.06) SSD 15.62 (4.40) | ‐2.15 (‐3.11 to ‐1.19) | 10 days Honey 30 SSD 13 14 days Honey 122 SSD 80 19 days Honey 140 SSD 90 21 days Honey 142 SSD 111 24 days Honey 142 SSD 121 | HR 2.93 (2.23 to 3.86) | |
| Honey Silver sulfadiazine | Honey 25 SSD 25 | 6 weeks | ‐ | ‐ | 2 weeks Honey 13 SSD 5 4 weeks Honey 25 SSD 15 6 weeks Honey 25 SSD 25 | HR 2.23 (1.19 to 4.19) | |
| Honey Silver sulfadiazine | Honey 40 SSD 40 | 46 days | Honey 15.3 (SD ‐) SSD 20.0 (SD ‐) | ‐ | Honey Day 16: 20 Day 26: 32 Day 30: 40 SSD Day 20: 16 Day 36: 34 Day 46: 40 | HR 3.75 (2.18 to 6.45) | |
| Honey Silver sulfadiazine | Honey 52 SSD 52 | 15 days | Honey 9.4 (2.3) SSD 17.2 (3.2)* *Jull 2015 author contact | ‐7.77 (‐8.84 to ‐6.70) | Honey 87% (42) SSD 10% (5) | RR 8.40 (3.61 to 19.53) | |
| Honey Silver sulfadiazine | Honey 25 SSD 25 | 21 days | Honey 4.92 (3.61) SSD 8.22 (8.31)* *Jull 2015 author contact | ‐3.30 (‐6.85 to 0.25) | Honey 25 SSD 21 | RR 1.19 (0.99 to 1.43) | |
| Honey Silver sulfadiazine | Honey 50 SSD 50 | 21 days | Honey 15.4 (3.2) SSD 17.2 (4.3)* *SD from Jull 2015 author contact | ‐1.80 (‐3.29 to 0.31) | Honey 50 SSD 24 | RR 2.06 (1.55 to 2.75) | |
| Honey Silver sulfadiazine | Honey 25 SSD 25 | 60 days | ‐ | ‐ | Days 5‐10 Honey 14 SSD 3 Days 11‐15 Honey 6 SSD 2 Days 16‐20 Honey 3 SSD 7 Days 21‐30 Honey 1 SSD 8 Days 31‐40 Honey 1 SSD 3 Days 41‐50 Honey 0 SSD 1 Days 51‐60 Honey 0 SSD 1 | HR 2.73 (1.43 to 5.24) | |
| Honey Mafenide acetate | Honey 50 Mafenide acetate 50 | 30 days | ‐ | ‐ | Day 7 Honey 42 Mafenide 36 Day 10 Honey 46 Mafenide 38 Day 15 Honey 48 Mafenide 40 Day 21 Honey 50 Mafenide 42 Day 30 Honey 50 Mafenide 50 | HR 1.38 (0.91 to 2.09) | |
| Honey (olea) Mafenide acetate | Honey 10 Mafenide acetate 20 | 20 days | Development of granulation tissue: median Honey: 12 (range 10.3‐13.6) Madenide: 17 (range 13.3‐20.6) Not all participants developed this | ‐ | Proportion of participants with granulation tissue at day 20 Honey 8/10 Mafenide 16/20 | ‐ | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 30 SSD 30 | 24 days | Aloe Vera 15.9 (2) SSD 18.73 (2.65) | ‐2.85 (‐4.04 to ‐1.66) | ‐ | ‐ | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 60 SSD 60 | 14 days | ‐ | ‐ | ‐ | ‐ | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 25 SSD 25 | Until healing/ 2 months | Aloe Vera 11 (4.18) SSD 24.24 (11.16) | ‐13.24 (‐17.91 to ‐8.57) | ‐ | ‐ | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 20 SSD 18 | 26 days | ‐ | Aloe Vera 55% (11) SSD 39% (7) | RR 1.41 (0.70 to 2.85) | ||
| Aloe Vera Framycetin | Aloe Vera 50 Framycetin:50 | NR | Aloe Vera 18 (SD ‐) Framycetin: 30.9 (SD ‐) | ‐ | ‐ | ‐ | |
| Povidone iodine Silver sulfadiazine | 43 participants each with 2 comparable burns; 43 burns in each group | 21 days | Povidone iodine 9.9 (4.5) SSD 11.3 (4.9) | ‐1.40 (‐3.39 to 0.59) | ‐ | ‐ | |
| Iodophor Moist exposed burn ointment (MEBO) Ethacridine lactate Silver sulfadiazine | Iodophor 24 MEBO 31 Ethacridine lactate 22 SSD 38 | Until healing | MEBO 57 (10.41) Iodophor 31 (6.43) Ethacridine lactate 32 (4.98) SSD 30 (4.72) | Iodophor vs SSD: 1.00 (‐1.95 to 3.98) Ethacridine vs SSD 2.00 (‐0.57 to 4.57) Iodophor vs MEBO ‐26.0 (‐30.48 to ‐21.52) Ethacridine vs MEBO ‐25.00 (‐29.21 to ‐20.79) Iodophor vs ethacridine 2.00 (‐0.57 to 4.57) | ‐ | ‐ | |
| Sodium hypochlorite Silver sulfadiazine | 20 participants with 2 burns (20 burns/group) | 28 days | Sodium hypochlorite 20.0 (2.7) SSD 22.1 (3.0) | ‐2.10 (‐3.87 to 0.33) | ‐ | ‐ | |
| Octenidine Silver sulfadiazine | 30 participants with 2 burn areas; 30 burns in each group | 24 hours | ‐ | ‐ | ‐ | ‐ | |
| Polyhexanide Silver sulfadiazine | Polyhexanide 30 with 38 burns SSD 30 with 34 | NR | Polyhexanide 10 (‐) SSD 10 (‐) | ‐ | ‐ | ‐ | |
| Arnebia euchroma Silver sulfadiazine | 49 participants with 2 burns (49 burns/group) | 36 days | A euchroma 13.9 (5.3) SSD 17.5 (6.9) | ‐3.60 (‐6.41 to ‐1.06) | Day 7 A euchroma 3 SSD 0 Day 10 A euchroma 14 SSD 8 Day 13 A euchroma 24 SSD 13 Day 15 A euchroma 29 SSD 24 Day 20 A euchroma 41 SSD 35 Day 25 A euchroma 42 SSD 38 Day 30 A euchroma 45 SSD 43 Day 36 A euchroma 45 SSD 45 | HR 1.42 (0.91 to 2.21) | |
| Antiseptic versus alternative antiseptic | |||||||
| Iodine Chlorhexidine | Iodine 111 Chlorhexidine 102 | NR | Iodine 9.48 (5.43) Chlorhexidine 11.69 (8.09) | 2.21 (0.34 to 4.08) | ‐ | ‐ | |
| Antiseptic versus non‐antibacterial treatment | |||||||
| Nanocrystalline silver Vaseline gauze | Silver 38 Gauze 38 | 30 days | Silver 8.8 (2.3) Gauze 12.3 (2.8) | ‐3.50 (‐4.65 to 2.35) | ‐ | ‐ | |
| Silver xenograft Petroleum gauze | Silver 16 Gauze 16 | 14 days | Silver 12.9 (1.4) N = 9 Gauze 12.5 (2.7) N = 8 | 0.40 (‐1.68 to 2.48) | Silver 9/16 Gauze 8/16 | RR 1.13 (0.59 to 2.16) | |
| Honey Polyurethane film | Honey 46 Polyurethane 46 | NR | Honey 10.8 (3.93) Polyurethane 15.3 (2.98)* *Jull 2015 author contact for SD | ‐4.50 (‐5.93 to ‐3.07) | ‐ | ‐ | |
| Honey gauze Amniotic membrane | Honey gauze 40 Amniotic 24 | 30 days | Honey 9.4 (2.52) Amniotic 17.5 (6.66)* *Jull 2015 author contact for SD | ‐8.10 (‐10.88 to ‐5.32) | Day 10 Honey 23 Amniotic 4 Day 15 Honey 33 Amniotic 14 Day 20 Honey 38 Amniotic 20 Day 25 Honey 40 Amniotic 21 Day 30 Honey 40 Amniotic 24 | HR 1.80 (1.09 to 2.98) | |
| Honey Potato peel | Honey 50 Potato peel 50 | 21 days | Honey 10.4 (2.2) Potato peel 16.2 (2.3) *Jull 2015 author contact for SSD | ‐5.80 (‐6.88 to ‐4.92) | 7 days Honey 20 Potato peel 4 10 days Honey 36 Potato peel 12 15 days Honey 50 Potato peel 40 21 days Honey 50 Potato peel 50 | HR 2.37 (1.53 to 3.67) | |
| Honey "Conventional dressing" | Honey 450 "Conventional dressing" 450 | NR | Honey: 8.8 (SD 2.1) "Conventional dressing": 13.5 (SD 4.1) *Jull 2015 author contact | ‐4.70 (‐5.13 to ‐4.27) | ‐ | ‐ | |
| Silver sulfadiazine + chlorhexidine Silver sulfadiazine alone | SSD + chlorhexidine 54 assessed SSD only 67 assessed Unclear if additional post‐randomisation exclusions | Until healing (26 days) | ‐ | ‐ | ‐ | ‐ | |
| Chlorhexidine Polyurethane | Chlorhexidine 25 Polyurethane 26 | 30 days | Chlorhexidine 14.08 (7) Polyurethane 10 (5) | 4.08 (0.73 to 7.43) | Chlorhexidine Day 5: 1 Day 10: 8 Day 15: 19 Day 20: 21 Day 25: 22 Day 30: 25 Polyurethane Day 5: 4 Day 10: 17 Day 15: 22 Day 20: 23 Day 25: 23 Day 30: 26 | HR 0.71 (0.39 to 1.29) | |
| Chlorhexidine Hydrocolloid | Chlorhexidine 104 Hydrocolloid 92 | NR | Chlorhexidine 69 analysed 11.83 (‐) Hydrocolloid 50 analysed 14.18 (‐) Not statistically significant | ‐ | ‐ | ‐ | |
| Chlorhexidine tulle‐gras Hydrocolloid Hydrocolloid + SSD | Chlorhexidine tulle‐gras 18 Hydrocolloid 16 Hydrocolloid + SSD 16 | NR | Chlorhexidine 11.1 (‐) Hydrocolloid 10.6 (‐) Hydrocolloid SSD 14.2 (‐) | ‐ | ‐ | ‐ | |
| Chlorhexidine Hydrocolloid | Chlorhexidine 49 Hydrocolloid 49 | NR | Median Chlorhexidine 12 Hydrocolloid 12 P = 0.89; based on 67 participants | ‐ | ‐ | ‐ | |
| Povidone iodine + Bepanthenol Moist exposed burn ointment (MEBO) | Povidone iodine + Bepanthenol 107 MEBO 104 | 18 days | ‐ | ‐ | ‐ | ‐ | |
| Iodine gauze Carbon‐fibre dressing | Iodine gauze 74 Carbon‐fibre dressing 203 | NR | Calculated using method in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c) Iodine 20.67 (9.7) Carbon 15.29 (4.24) | 5.38 (3.09 to 7.67) | ‐ | ‐ | |
| Iodophor gauze Hydrogel | 60 participants with burn wounds; 60 burn areas/group | 14 days | ‐ | ‐ | Day 7 Iodophor 4 Hydrogel 10 Day 14 Iodophor 7 Hydrogel 42 | RR 0.17 (0.08 to 0.34) | |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone | CN + SSD 30 SSD 30 | Until healing/ readiness for grafting | CN + SSD 17.2 (8.3) N = 29 SSD 25.1 (19.4) N = 30 Partial‐thickness areas only; time to graft readiness reported for full‐thickness areas (CN + SSD 13.6 (11.3) SSD 24.6 (11.4)) | ‐ | ‐ | ‐ | |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone | CN + SSD 78 SSD 76 | 21 days | Median (IQR) for participants not requiring surgery CN + SSD 11.0 (7‐15) SSD 9.0 (5.0‐15.75) (13 vs 15 required surgery) | ‐ | ‐ | ‐ | |
| Merbromin Sodium salicylate Zinc sulfadiazine Sodium salicylate + zinc sulfadiazine Collagenase + chloramphenicol | Merbromin 25 Sodium salicylate 25 Zinc sulfadiazine 25 Sodium salicylate + zinc sulfadiazine 25 Collagenase + chloramphenicol 25 | NR | Merbromin 11.32 (3.99) Sodium salicylate 15.00 (8.00) Zinc sulfadiazine 11.08 (4.69) Sodium salicylate + zinc sulfadiazine 14.8 (7.61) Collagenase + chloramphenicol 12.32 (5.92) | Merbromin vs sodium salicylate ‐3.68 (‐7.18 to ‐0.18) Merbromin vs zinc sulfadiazine ‐3.48 (‐6.85 to ‐0.11) | ‐ | ‐ | |
| CN: cerium nitrate; MEBO: moist exposed burn ointment; NR: not reported; NS: not significant; SD: standard deviation; SSD: silver sulfadiazine aChen 2006 assessed the following relevant comparisons between antiseptic (silver) and non‐antibacterial (Vaseline gauze) and between silver and SSD bLi 1994 assessed the following relevant comparisons between two antiseptics (ethacridine lactate and iodophor), between ethacridine lactate and a non‐antibacterial treatment (MEBO) and between iodophor and MEBO. dPiccolo‐Daher 1990 assessed the following relevant comparisons: Merbromin vs sodium salicylate and Merbromin vs zinc sulfadiazine; other comparisons were not relevant to the review | |||||||
| Comparison | Study | Number participants/burns | Duration | Measure reported | Reported data | RR (95% CI) |
| Antiseptic versus topical antibiotic | ||||||
| Silver hydrofibre Silver sulfadiazine | Silver 35 SSD 34 | Until healing | ‐ | ‐ | ‐ | |
| Silver hydrofibre Silver sulfadiazine | Silver 42 SSD 40 | 21 days | Participants with wound infection | Silver 8/42 SSD 6/40 | 1.27 (0.48 to 3.34) | |
| Silver hydrofibre Silver sulfadiazine | Silver 35 SSD 35 | NR | ‐ | ‐ | ‐ | |
| Silver hydrogel Silver sulfadiazine | Silver 84 SSD 79 (analysed silver 54, SSD 52) | 4 weeks/until healing | ‐ | ‐ | ‐ | |
| Silver hydrogel Silver sulfadiazine | Silver 12 SSD 12 | 21 days+ | Participants with wound infection | Silver 0 SSD 0 | ‐ | |
| Silver hydrogel Silver sulfadiazine | Silver 52 SSD 52 | 21 days+ | ‐ | ‐ | ‐ | |
| Silver foam Silver sulfadiazine | Silver 50 SSD 51 | 21 days | ‐ | ‐ | ‐ | |
| Silver foam Silver sulfadiazine | Silver 71 SSD 82 | 4 weeks | Participants with new signs of inflammation | Silver 8/71 SSD 14/82 | 0.66 (0.29 to 1.48) | |
| Silver foam Silver sulfadiazine | 24 participants randomised; group allocation unclear | NR | ‐ | ‐ | ‐ | |
| Silver foam Silver sulfadiazine | 40 participants; part of each burn randomised to treatments | 14 days | ‐ | ‐ | ‐ | |
| Nanocrystalline silver Silver sulfadiazine | Silver 65 SSD 63 Vaseline gauze 63 | Until healing | ‐ | ‐ | ‐ | |
| Nanocrystalline silver Silver sulfadiazine | 98 participants with 166 burns 83 burns in each group | 20 days | Bacterial clearance rates | ‐ | ‐ | |
| Nanocrystalline silver Silver sulfadiazine | Silver 25 SSD 25 | NR | Participants with wound infection | Silver 3/25 SSD 4/25 | 0.75 (0.19 to 3.01) | |
| Nanocrystalline silver Silver sulfadiazine | 14 participants with 2 burn areas; 14 burn areas in each group | NR | ‐ | ‐ | ‐ | |
| Silver nitrate Silver sulfadiazine | 120 participants with 2 burns; 120 burns in each group | Until healing | ‐ | ‐ | ‐ | |
| Silver alginate Silver sulfadiazine | Silver 30 SSD 35 | NR | ‐ | ‐ | ‐ | |
| Honey Silver sulfadiazine | Honey 37 SSD 41 | NR (2 months' follow‐up) | ‐ | ‐ | ‐ | |
| Honey Silver sulfadiazine | Honey 32 SSD 32 | 21 days | ‐ | ‐ | ‐ | |
| Honey Silver sulfadiazine | 150 participants with 2 burns; 150 burns in each group | 24 days | Burns with wound infection | Honey 6/150 SSD 29/150 | 0.21 (0.09 to 0.48) | |
| Honey Silver sulfadiazine | Honey 25 SSD 25 | 6 weeks | Time to achieve negative wound cultures | Honey 3 weeks SSD 5 weeks | ‐ | |
| Honey Silver sulfadiazine | Honey 40 SSD 40 | 46 days | ‐ | ‐ | ‐ | |
| Honey Silver sulfadiazine | Honey 52 SSD 52 | 15 days | Persistent infections (positive cultures) | Honey 4/43 SSD 38/41 | 0.10 (0.04 to 0.26) | |
| Honey Silver sulfadiazine | Honey 25 SSD 25 | 21 days | Participants with wound infection | Honey 0/25 SSD 4/25 | 0.11 (0.01 to 1.96) | |
| Honey Silver sulfadiazine | Honey 50 SSD 50 | 21 days | Persistent infections (positive cultures) | Honey 4/44 SSD 42/42 | 0.10 (0.04 to 0.24) | |
| Honey Silver sulfadiazine | Honey 25 SSD 25 | 42 days | Persistent infections (positive cultures); participants becoming culture negative. Details of isolated organisms | Week 1 Honey 17/20 SSD 11/22 Week 2 Honey 20/20 SSD 16/22 Week 3 Honey 20/20 SSD 19/22 Week 4 Honey 20/20 SSD 21/22 Week 6 Honey 20/20 SSD 22/22 | Not estimable at week 6 | |
| Honey Mafenide acetate | Honey 50 Mafenide acetate 50 | 30 days | New infections Day 7 New infections Day 21 | Honey 2/50 Mafenide 2/50 Honey 0/50 Mafenide 10/50 | 0.05 (0.00 to 0.79) | |
| Honey (olea) Mafenide acetate | Honey 10 Mafenide acetate 20 | 20 days | Infections (positive cultures) Day 7 | Honey 1/10 SSD 19/20 | 0.11 (0.02 to 0.68) | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 30 SSD 30 | 24 days | Participants with wound infection | Aloe Vera 0 SSD 0 | ‐ | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 60 SSD 60 | 14 days | Participants with wound infection | Aloe Vera 1 SSD 0 | 2.95 (0.12 to 70.82) | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 25 SSD 25 | Until healing/ 2 months | Participants with wound infection | Aloe Vera 3 SSD 4 | 0.75 (0.19 to 3.01) | |
| Aloe Vera Silver sulfadiazine | Aloe Vera 20 SSD 18 | 26 days | ‐ | ‐ | ‐ | |
| Aloe Vera Framycetin | Aloe Vera 50 Framycetin 50 | NR | Grade of infection | Lower in Aloe Vera | ‐ | |
| Povidone iodine Silver sulfadiazine | 43 participants each with 2 comparable burns; 43 burns in each group | 21 days | ‐ | ‐ | ‐ | |
| Iodophor Moist exposed burn ointment (MEBO) Ethacridine lactate Silver sulfadiazine | Iodophor 24 MEBO 31 Ethacridine lactate 22 SSD 38 | Until healing | ‐ | ‐ | ‐ | |
| Sodium hypochlorite Silver sulfadiazine | 20 participants with 2 burns (20 burns/group) | 28 days | ‐ | ‐ | ‐ | |
| Octenidine Silver sulfadiazine | 30 participants with 2 burn areas; 30 burns in each group | 24 hours | ‐ | ‐ | ‐ | |
| Polyhexanide Silver sulfadiazine | Polyhexanide 30 with 38 burns SSD 30 with 34 burns | NR | ‐ | ‐ | ‐ | |
| Arnebia euchroma Silver sulfadiazine | 49 participants with 2 burns (49 burns/group) | 36 days | Infection score between 0 and 5; 1 point for each symptom of infection; 45 burns analysed/group | A euchroma 0: 37/45 1: 7/45 2: 1/45 3: 0/45 SSD 0: 31/45 1: 11/45 2: 2/45 3: 1/45 | ‐ | |
| Antispetic versus alternative antiseptic | ||||||
| Iodine Chlorhexidine | Iodine 111 Chlorhexidine 102 | NR | Systemic antibiotics prescribed for clinical/bacteriological signs of infection | Iodine 4/111 Chlorhexidine 4/102 | 1.09 (0.28 to 4.24) | |
| Antispetic versus non‐antibacterial treatment | ||||||
| Nanocrystalline silver Vaseline gauze | Nanocrystalline silver 38 Vaseline gauze 38 | 21 days | "Positive for bacteria" | Silver 1/38 Gauze 8/38 | 0.13 (0.02 to 0.95) | |
| Silver xenograft Petroleum gauze | Silver xenograft 16 Petroleum gauze 16 | 14 days | Rate of infection Bacterial colonisation | "No difference" Details of organisms reported | ‐ | |
| Honey Polyurethane film | Honey 46 Polyurethane film 46 | NR | Infection on day 8 | Honey 8 Polyurethane 17 | 0.47 (0.23 to 0.98) | |
| Honey gauze Amniotic membrane | Honey gauze 40 Amniotic membrane 24 | 30 days | Persistent infection at 7 days | Honey 4/28 Amniotic 11/19 | 0.25 (0.09 to 0.66) | |
| Honey Potato peel | Honey 50 Potato peel 50 | 21 days | Persistent infection at 7 days | Honey 4/40 Potato 42/42 | 0.10 (0.04 to 0.25) | |
| Honey "Conventional dressing" | Honey 450 "Conventional dressing" 450 | NR | ‐ | ‐ | ‐ | |
| Silver sulfadiazine + chlorhexidine Silver sulfadiazine alone | SSD + chlorhexidine 54 assessed SSD 67 assessed Unclear if additional post‐randomisation exclusions | Until healing (26 days) | Infection incidence | Chlorhexidine 10/54 SSD alone 12/67 | 1.03 (0.48 to 2.21) | |
| Chlorhexidine Polyurethane | Chlorhexidine 25 Polyurethane 26 | 30 days | Proven infection | Chlorhexidine 2/25 Polyurethane 1/26 | 2.08 (0.20 to 21.52) | |
| Chlorhexidine Hydrocolloid | Chlorhexidine 104 Hydrocolloid 92 | NR | ‐ | ‐ | ‐ | |
| Chlorhexidine tulle‐gras Hydrocolloid Hydrocolloid + SSD | Chlorhexidine tulle‐gras 18 Hydrocolloid 16 Hydrocolloid + SSD 16 | NR | Percentage of wounds with bacteria and pathogenic bacteria at baseline and post treatment | ‐ | ‐ | |
| Chlorhexidine Hydrocolloid | Chlorhexidine 49 Hydrocolloid 49 | NR | ‐ | ‐ | ‐ | |
| Povidone iodine + Bepanthenol Moist exposed burn ointment (MEBO) | Povidone iodine + Bepanthenol f107 MEBO 104 | 18 days | New infections | Iodine 8/107 MEBO 6/104 | 1.30 (0.47 to 3.61) | |
| Iodine gauze Carbon‐fibre dressing | Iodine gauze 74 Carbon‐fibre dressing 203 | NR | ‐ | ‐ | ‐ | |
| Iodophor gauze/ Hydrogel | 60 participants with burns wounds; 60 burn areas/group | 14 days | Bacterial presence reported | No difference between groups | ‐ | |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone | CN + SSD 30 SSD 30 | Until healing/ readiness for grafting | Bacterial cultures at baseline Resolved New 2/13 Total post‐treatment Sepsis by day 5 Sepsis after day 5 | CN + SSD 17/30 SSD 11/30 CN + SSD 16/17 SSD 8/11 CN + SSD 2/13 SSD 3/19 CN + SSD 3 SSD 6 CN + SSD 1 SSD 1 (both recovered) CN + SSD 0 SSD 3 (1 died) | RR post‐treatment infection 0.50 (0.14 to 1.82) RR Sepsis 0.25 (0.03, 2.11) RR new infection 0.97 (0.19 to 5.04) RR resolution 1.29 (0.88 to 1.89) | |
| Cerium nitrate + silver sulfadiazine Silver sulfadiazine alone | CN +SSD 78 SSD 76 | 21 days | ‐ | ‐ | ‐ | |
| Merbromin Sodium salicylate Zinc sulfadiazine Sodium salicylate + zinc sulfadiazine Collagenase + chloramphenicol | Merbromin 25 Sodium salicylate 25 Zinc sulfadiazine 25 Sodium salicylate + zinc sulfadiazine 25 Collagenase + chloramphenicol 25 | NR | ‐ | ‐ | ‐ | |
| CN: cerium nitrate; MEBO: moist exposed burn ointment; NR: not reported; SSD: silver sulfadiazine aChen 2006 also assessed a relevant comparison between antiseptic (silver) and non‐antibacterial (Vaseline gauze) bLi 1994 also assessed relevant comparisons between two antiseptics (ethacridine lactate and iodophor), between ethacridine lactate and a non‐antibacterial treatment (MEBO) and between iodophor and MEBO. | ||||||
| Study ID | Number participants/burns | Duration | Adverse events | Pain Means (SD) | Mortality | Quality of life | Resource use Means (SD) | Costs: Means (SD) Difference in means (95% CI) |
| Antiseptic versus topical antibiotic | ||||||||
| Silver versus SSD | ||||||||
| Silver hydrofibre 35 SSD 34 | Until healing | ‐ | Doses of fentanyl silver: 3.3 (1.9) SSD 10.3 (4.2) SD extrapolated from graph | ‐ | ‐ | ‐ | Costs of antibiotics, analgesics, dressings, accommodation, nursing/visiting (USD) Silver 26,000 (20,000) SSD 38,000 (30,000) Data extrapolated from graph MD ‐12,000 (‐24,065.99 to 65.99) | |
| Silver hydrofibre 42 SSD 40 | 21 days | All Silver 20 SSD 18 RR 1.06 (0.66 to 1.69) Serious Silver 8 SSD 8 RR 0.95 (0.40 to 2.29) | Participants aged > 4 years (69%) VAS score during dressing changes Silver 3.63 SSD 4.77 P = 0.003 | Silver 1 SSD 0 | Dressing changes/day Silver 0.5 (0.1) SSD 1.2 (0.5) Total dressing changes Silver 7.7 (3.9) SSD 19.1 (13.2) MD ‐11.40 (‐15.66 to ‐7.14) | Cost of nursing time (USD) Silver 14.30 SSD 21.90 Costs of study dressings Silver 684 SSD 398 Cost of all dressings (USD) Silver 845.5 SSD 759.6 Total care Silver 1040 (856.66) SSD 1180 (792.18) MD ‐140 (‐4.96.92 to 216.92) Cost effectiveness /patient healed (USD) Silver 1409.06 (1050.41‐1857.58) SSD 1967.95 (1483.06‐2690.22) MD ‐558.89 (‐1383.08 to 265.30) ICER ‐1019.21 (‐6320.59 to 4054.32) | ||
| Silver hydrofibre 35 SSD 35 | NR | ‐ | Pain during dressing Day 1: Silver 4.1 (2.1) SSD 6.1 (2.3) Day 3 Silver 2.1 ( 1.8) SSD 5.2 (2.1) Day 7 Silver 0.9 (1.4) SSD 3.3 (1.9) MD ‐1.42 (‐1.95 to ‐0.89) | ‐ | ‐ | ‐ | Total cost (USD) Silver 52 (29) SSD 93 (36) MD ‐ 41.00 (‐56.31 to ‐25.69) Hospital cost Silver 43 (28) SSD 57 (SD 25) Travel cost: Silver 9 (4) SSD 36 (SD 14) | |
| Silver hydrogel 84 SSD 79 analysed Silver 54 SSD 52 | 4 weeks/until healing | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Silver hydrogel 12 SSD 12 | 21 days+ | Silver 0 SSD 0 | Pain during dressing changes (Wong‐Baker Faces Pain Scale observational pain assessment scale in infants or toddlers) Silver 2.33 (1.07) SSD 5.33 (1.44) ‐2.28 (‐3.35, ‐1.22) | ‐ | ‐ | Number of dressing changes (over 21 days) Silver 13.50 (4.70) SSD 13.42 (8.26) MD 0.08 (‐5.30 to 5.46) | ‐ | |
| Silver hydrogel 52 SSD 52 | 21 days+ | During dressing: Silver no significant damage to granulation SSD damage to granulation | Silver no pain during dressing SSD pain during dressing | ‐ | ‐ | ‐ | ‐ | |
| Silver foam 50 SSD 51 | 21 days | 2 associated withdrawals in each group Other events reported Silver 16 SSD 10 RR 0.75 (0.48, 1.16) | Dressing application (week 1) Silver 19.1 SSD 40.0 During wear silver 22.0 SSD 35.5 Dressing removal: reported as NS | Silver 1 SSD 1 | ‐ | Mean number of dressing changes over 3 weeks (SD NR) Silver 2.24 (N = 47) SSD 12.4 Mean time to discharge Silver 5.62 d SSD 8.31 d | Total costs (USD) Silver 309 (144) SSD 514 (282) Average C‐E Silver 395 (344‐450) SSD 776 (659‐892) ICER ‐1688 Based on 20 participants | |
| Silver foam 71 SSD 82 | 4 weeks | Silver 4 participants with 5 events SSD 7 participants with 7 events RR 0.66 (0.20, 2.16) | Baseline Silver 35.3 (22.4) SSD 42.9 (25.8) Week 4 Before dressing removal silver 6.78 (12.95) SSD 11.0 (17.3) MD ‐4.22 (‐9.03 to 0.59) During dressing removal silver 9.23 (13.61) SSD 19.1 (23.9) After dressing removal silver 9.41 (17.33) SSD 15.8 (19.7) MD ‐6.39 (‐12.26 to ‐0.52) | ‐ | ‐ | Total number of dressing changes silver 3.06 SSD 14.0 Per week silver 1.36 SSD 5.67 SD NR | ‐ | |
| Silver foam SSD 24 participants randomised; group allocation unclear | NR | ‐ | Mean after each treatment Silver 2.92 (1.12) SSD 4.70 (2.22) MD ‐0.98 (‐1.83 to ‐0.12) | ‐ | ‐ | Number of treatments required: Silver 4.10 (1.38) SSD 10.27 (7.46) MD ‐6.17 (‐10.46 to ‐1.88) | ‐ | |
| Silver foam SSD 40 participants; part of each burn randomised to each treatment | 14 days | No serious events | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chen 2006a (nanoparticle) | Silver nanoparticle) 65 SSD 63 Vaseline gauze 63 | Until healing | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 98 participants with 166 burns Nanocrystalline silver 83 burns SSD 83 burns | 20 days | No local allergic or systemic symptoms. No side effects related to silver dressing | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Nanocrystalline silver 25 SSD 25 | NR | ‐ | Silver 4 (± 0.6) SSD 5 (± 0.7) MD ‐1.51 (‐2.14 to ‐0.88) | Silver 0 SSD 0 | ‐ | ‐ | ‐ | |
| Nanocrystalline silver SSD 14 participants with 2 burn areas; 14 burn areas/group | NR | Withdrawals due to pain/infection silver 0 SSD 5/10 after 4.8 d (0‐8) | Silver 3.2 (2.68) SSD 7.9 (2.65) Paired data for 10 participants ‐1.69 (‐2.74 to ‐0.64) | ‐ | ‐ | ‐ | ‐ | |
| Silver nitrate SSD 120 participants with 2 burns; 120 burns/ group | Until healing | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Silver alginate 30 SSD 35 | NR | ‐ | Silver 2.23 (1.87) SSD 6.08 (2.33) MD ‐1.79 (‐2.37 to ‐1.20) | ‐ | ‐ | Nursing time (min) Silver 8.47 (6.16) SSD 13.29 (4.19) Dressing changes Silver 2.93 (1.17) SSD 14.00 (4.18) MD ‐11.07 (‐12.52 to ‐9.62) | ‐ | |
| Honey versus topical antibiotics | ||||||||
| Honey 37 SSD 41 | NR (2 months' follow‐up) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey 32 SSD 32 | 21 days | Contractures or over‐granulation reported in 3 vs 5 cases | Pain reported as "worse" for honey group | ‐ | ‐ | ‐ | ‐ | |
| Honey SSD 150 participants with 2 burns; 150 burns/group | 24 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey 25 SSD 25 | 6 weeks | Honey no allergy or side effects SSD 2 participants irritation/burning (mild) | Pain free 1 week honey 9 SSD 4 2 weeks honey 20 SSD 11 3 weeks honey 25 SSD 18 4 weeks honey 25 SSD 25 | ‐ | ‐ | ‐ | Cost per % of TBSA affected Honey 0.75 Rupees for 5 mL SSD 10 Rupees for 2 g ointment SD NR | |
| Honey 40 SSD 40 | 46 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey 52 SSD 52 | 15 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey 25 SSD 25 | 21 days | SSD 4 participants required skin grafting | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey 50 SSD 50 | 21 days | No irritation allergy or other side effects. Need for skin grafting Honey 4 SSD 11 | Subjective relief of pain better in honey group | Hospital stay days Honey 22.0 (1.2) SSD 32.3 (2.0) | ||||
| Honey 25 SSD 25 | 60 days | ‐ | Time to complete relief of pain (mean) Honey 12 days SSD 16.8 days Up to 5 days Honey 9 SSD1 6‐12 days Honey 9 SSD 11 13‐21 days Honey 7 SSD 11 22‐26 days Honey 0 SSD 2 | Amount used per dressing per % burn Honey 5 gm (sic) SSD 2 gm (sic) Based on adult participants | Cost per dressing per % burn Honey 2.40 Rs SSD 4.92 Rs | |||
| Honey 50 Mafenide acetate 50 | 30 days | Honey 0 Mafenide 0 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey (olea) 10 Mafenide acetate 20 | 20 days | Need for surgical debridement Honey 0/10 Mafenide 13/20 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Aloe Vera versus topical antibiotics | ||||||||
| Aloe Vera 30 SSD 30 | 24 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Aloe Vera 60 SSD 60 | 14 days | ‐ | Changes from baseline Day 2 Aloe Vera 2.61 (1.55) SSD 1.19 (2.25) Day 7 Aloe Vera 5.13 (2.82) SSD 3.78 (2.83) Day 14 Aloe Vera 5.68 (3.2) SSD 4.54 (2.83) MD 1.14 (0.02 to 2.26) | ‐ | ‐ | ‐ | ‐ | |
| Aloe Vera 25 SSD 25 | Until healing/ 2 months | ‐ | Time to being pain free reported differently for groups | ‐ | ‐ | ‐ | Cost/%TBSA Aloe Vera 2.40 Rs SSD 4.92 Rs SD NR | |
| Aloe Vera 20 SSD 18 | 26 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Aloe Vera 50 Framycetin 50 | NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Iodine versus SSD | ||||||||
| Povidone iodine Hydrogel SSD 43 participants each with 2 comparable burns; 43 burns in each group | 21 days | 20 participants with events. 6 systemic and considered unrelated to study interventions Iodine 6 (5 pain) SSD 7 (5 pain) | ‐ | ‐ | ‐ | ‐ | ‐ | |
| MEBO 31 Iodophor 24 Ethacridine lactate 22 SSD 38 | Until healing | ‐ | ‐ | ‐ | ‐ | ‐ | All RMB (Chinese Yuan) MEBO 1836 (542.35) Iodophor 621 (130.83) Ethacridine 598 (125.43) SSD 674 (191.50) Ethacridine vs SSD MD ‐76.00 (‐1.56.34 to 4.34) Iodophor vs Ethacridine MD 23 (‐51.07 to 97.07) Ethacridine vs MEBO MD ‐1238 (‐1435.98 to ‐1040.02) Iodophor vs SSD MD ‐53.00 (‐133.29 to 27.29) Iodophor vs MEBO MD ‐1215 (‐1412.96 to ‐1017.04) | |
| Other antiseptics versus topical antibiotics | ||||||||
| 20 participants with 2 burns (20 burns/group) | 28 days | No serious events in either group. | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Octenidine SSD 30 participants with 2 burn areas; 30 burns in each group | 24 hours | ‐ | Median VAS Octenidine 3 (1‐6) SSD 6 (3‐8) | ‐ | ‐ | ‐ | ‐ | |
| Polyhexanide 30 with 38 burns SSD 30 with 34 burns | NR | ‐ | Graph data Baseline Polyhexanide Change 7.8 Between 1.2 SSD Change 8 Between 3 Day 1 Polyhexanide Change 4.2 Between 0.8 SSD Change 6 Between 2.6 Day 3 Polyhexanide Change 2.2 Between 0.2 SSD Change 5 Between 1.8 Day 5 Polyhexanide Change 1.4 Between 0.1 SSD Change 4 Between 1 Day 7 Polyhexanide Change 0.8 Between 0.1 SSD Change 3 Between 0.8 Day 10 Polyhexanide Change 0.2 Between 0.5 SSD Change 2 Between 0.5 Day 14 Polyhexanide Change 0 Between 0 SSD Change 1.4 Between 0 SD NR | ‐ | ‐ | ‐ | Costs/day (EUR) Materials Polyhexanide 5.14 SSD 6.96 Personnel Polyhexanide 9.63 SSD 9.63 Total Polyhexanide 14.77 SSD 16.59 SD NR | |
| Arnebia euchroma SSD 49 participants with 2 burns; 49 burns in each group | 36 days but up to 10 days for secondary outcomes | Specific complications such as burning, pain, itching, warming , allergic reactions and requiring skin graft. Scores reported for itching and warming. Skin graft risk A euchroma 2.2% (2.2 to 6.7) SSD 6.7% (0.9 to 14.3) | Pain scores reported graphically for days 1, 3, 5 and 10 for minutes 1, 5 and 15 after dressing. Graphs appeared to show overlapping CI but P reported < 0.05 (CI could not be extracted) | ‐ | ‐ | ‐ | ‐ | |
| Antiseptics versus alternative antiseptics | ||||||||
| Iodine 111 Chlorhexidine 102 | NR | ‐ | Pain at rest Iodine (N = 84) 9.18 (15.11) Chlorhexidine (N = 78) 11.44 (14.27) MD 2.26 (‐2.26 to 6.78) Pain on dressing removal Iodine (N = 92) 6.66 (11.06) Chlorhexidine (N = 84) 8.75 (15.84) MD 2.09 (‐2.00 to 6.18) | ‐ | ‐ | Number hospital visits (N unclear) Iodine 2.64 (1.45) Chlorhexidine 3.03 (1.62) MD 0.25 (‐.0.02 to 0.52) | ‐ | |
| Antispetic versus non‐antibacterial treatment | ||||||||
| Nanocrystalline silver 38 Vaseline gauze 38 | 30 days | Scar hyperplasia reported; no other data | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Silver xenograft 16 Petroleum gauze 16 | 14 days | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey 46 Polyurethane film 46 | NR | Honey 4 noted Polyurethane 6 noted Not clear all events were reported/basis of reported events | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Honey gauze 40 Amniotic membrane 24 | 30 days | Honey 4/40 Amniotic 5/24 Not clear all events were reported/basis of reported events | Numbers with pain evaluated with 4‐point scale None/mild Honey 33/40 Amniotic 13/24 Moderate/severe Honey 7/40 Amniotic 11/24 | ‐ | ‐ | ‐ | ‐ | |
| Honey 50 Potato peel 50 | 21 days | "Allergy or other side effects were not observed in any patient of either group" | "Subjective relief of pain was the same in both groups" | ‐ | ‐ | ‐ | ‐ | |
| Honey 450 "Conventional dressing" 450 | NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| SSD + chlorhexidine 54 assessed SSD only 67 assessed Unclear if additional post‐randomisation exclusions | Until healing (26 days) | ‐ | Pain sufficient to stop treatment Chlorhexidine 1/54 SSD alone 0/67 | Chlorhexidine 3/54 SSD alone 4/67 RR 0.93 (0.22 to 3.98) Infection‐related chlorhexidine 3/54 SSD alone 0/67 | ‐ | ‐ | ‐ | |
| Chlorhexidine 25 Polyurethane 26 | 30 days | ‐ | Qualitative data only (chlorhexidine perceived as more painful) | ‐ | ‐ | ‐ | ‐ | |
| Chlorhexidine 104 Hydrocolloid 92 | NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chlorhexidine tulle‐gras 18 Hydrocolloid 16 Hydrocolloid + SSD 16 | NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chlorhexidine 49 Hydrocolloid 49 | NR | Chlorhexidine 1 Hydrocolloid 5 Denominator unclear | VAS (summed for each visit) Chlorhexidine (N = 31) Hydrocolloid (N = 36) P = 0.284 | ‐ | ‐ | Number dressings Chlorhexidine 2.8 Hydrocolloid 2.61 SD NR | ‐ | |
| Povidone iodine + Bepanthenol 107 MEBO 104 | 18 days | "Complications" Iodine 8 MEBO 11 RR 1.30 (0.47 to 3.61) | Median pain scores reported graphically. Analgesia requirements also reported | ‐ | ‐ | Reduction of hospital stay (subtracted from a standard length of stay (10 days)) Iodine ‐3.01 (2.02) MEBO ‐3.63 (2.19) MD 0.62 (0.05 to 1.19) | Costs of hospital stay including medicines and examinations and the visits and treatments after discharge 2006 (EUR) Total MEBO 529.66 (172.75) Total iodine 566.21 (151.45) MD 36.55 (‐7.33 to 80.43) ICERs reported per day of hospitalisation and per day of recovery gained. Total/hospitalisation day gained ‐58.95E (‐63.10, ‐55.09) (favours MEBO) | |
| Iodine gauze 74 Superficial 16 Deep 32 Residual 26 Carbon‐fibre dressing 203 Superficial 46 Deep 89 Residual 68 | NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 60 participants with burn wounds; 60 burn areas/group (Iodophor gauze/ hydrogel) | 14 days | ‐ | Dressing change pain Iodophor 43 wounds caused evident pain (VAS score 3‐6) Hydrogel 37 wounds caused mild pain (VAS 1‐3) | ‐ | ‐ | ‐ | ‐ | |
| Cerium nitrate + SSD 30 SSD 30 | Until healing/ readiness for grafting | ‐ | ‐ | CN + SSD 1/30 SSD 4/30 RR 0.25 (0.03 to 2.11) | ‐ | Days of hospitalisation CN + SSD 23.3 (11.4) SSD 30.7 (22.7) MD ‐7.40 (‐16.49 to 1.69) | ‐ | |
| Cerium nitrate + SSD 78 SSD 76 | 21 days | ‐ | Mean (SEM) CN + SSD 0.6 (0.2) SSD 1.2 (0.4) MD Procedural Mean (SEM) CN + SSD 1.3 (0.3) SSD 1.6 (0.5) MD ‐0.60 (‐0.70 to ‐0.50) | CN SSD 1 SSD 5 RR 0.19 (0.02 to 1.63) | ‐ | ‐ | ‐ | |
| Multiple comparisons Merbromin 25 Sodium salicylate 25 Zinc sulfadiazine 25 Sodium salicylate + zinc sulfadiazine 25 Collagenase + chloramphenicol 25 | NR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C‐E: cost‐effectiveness; CN: cerium nitrate; ICER: incremental cost‐effectiveness ratio; MEBO: moist exposed burn ointment; NR: not reported; NS: not significant; SD: standard deviation; SEM: standard error of mean; SSD: silver sulfadiazine; TBSA: total body surface area; VAS: visual analogue scale aChen 2006 also assessed a relevant comparison between antiseptic (silver) and non‐antibacterial (Vaseline gauze) bLi 1994 also assessed relevant comparisons between two antiseptics (ethacridine lactate and iodophor), between ethacridine lactate and a non‐antibacterial treatment (MEBO) and between iodophor and MEBO. | ||||||||
| Comparison | Number trials & study detail | Number participants | Wound healing evidence | Wound healing: certainty of the evidence | Infection evidence | Infection: certainty of the evidence | Adverse events evidence | Adverse events: certainty of the evidence |
| Sodium hypochlorite versus SSD | 1 trial | Trial N = 20 Intra‐individual design | Mean time to healing MD 2.1 (3.87 to 0.33) | Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Chlorhexidine or polyhexanide (biguanides) versus SSD | 2 trials | Trial N = 110 participants with 126 burns; 106 burns relevant to comparison | ‐ | ‐ | ‐ | ‐ | ||
| Octenidine versus SSD | 1 trial | Trial N = 30 Intra‐individual design | ‐ | ‐ | ‐ | ‐ | ||
| Ethacridine lactate versus SSD | 1 trial | Trial N = 115 Relevant to comparison: 60 | Mean time to healing MD 2.0 (‐0.57 to 4.57) | Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Merbromin versus zinc sulfadiazine | 1 trial | Trial N = 125 Relevant to comparison: 50 | Mean time to healing MD ‐3.48 (‐6.85 to ‐0.11) | Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Arnebia euchroma versus SSD | 1 trial | Trial N = 49 Intra‐individual design | HR 1.42 (0.91 to 2.21) Mean time to healing MD ‐3.60 (95% ‐6.41 to ‐1.06) | Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Chlorhexidine versus Iodine‐based | 1 trial | Trial N = 213 | Mean time to healing MD 2.21 (0.34 to 4.08) | Low (Downgraded once for reporting bias and once for imprecision) | RR 1.09 (0.28 to 4.24) | Very low (downgraded once for risk of reporting bias and twice for imprecision) | ‐ | ‐ |
| Ethacridine lactate versus iodophor | 1 trial | Trial N = 115 Relevant to comparison: 46 | Mean time to healing MD ‐1.0 (‐4.31 to 2.31) | Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Ethacridine lactate versus non‐antibacterial (MEBO) | 1 trial | Trial N = 115 Relevant to comparison: 53 | Mean time to healing MD ‐25.00 (‐29.1 to ‐20.79) | Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| Cerium nitrate versus non‐antibacterial | 2 trials Oen 2012 | Trial N = 214 Reporting wound healing: 214 Reporting infection: 60 | No evaluable data | ‐ | RR 0.50 (0.14 to 1.82) | Low (downgraded twice for imprecision) | ‐ | ‐ |
| Merbromin versus sodium salicylate | 1 trial | Trial N = 125 Relevant to comparison: 50 | Mean time to healing MD ‐3.68 (‐7.18 to ‐0.18) | Low (downgraded twice for imprecision) | ‐ | ‐ | ‐ | ‐ |
| HR: hazard ratio; MD: mean difference; N: number; RR: risk ratio | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (hazard ratio) Show forest plot | 3 | 259 | Hazard Ratio (Random, 95% CI) | 1.25 [0.94, 1.67] |
| 2 Wound healing (mean time to healing) Show forest plot | 10 | 1085 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐4.96, ‐1.70] |
| 3 Wound healing (risk ratio) up to 28 days Show forest plot | 5 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.00, 1.37] |
| 4 Infection (up to 4 weeks or NR) Show forest plot | 4 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.49] |
| 5 Adverse events (14‐28 days) Show forest plot | 6 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.18] |
| 6 Withdrawals due to adverse events (21 days or NR) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Pain at dressing change (up to 28 days or NR) Show forest plot | 5 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.92, ‐0.49] |
| 8 Pain (time/follow‐up not specified) Show forest plot | 3 | 135 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.06, ‐1.27] |
| 9 Mortality (21 days or NR) Show forest plot | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.64] |
| 10 Resource use (number of dressings) (up to 28 days or NR) Show forest plot | 6 | 446 | Mean Difference (IV, Random, 95% CI) | ‐7.56 [‐12.09, ‐3.04] |
| 11 Costs (21 days or NR) Show forest plot | 4 | 261 | Mean Difference (IV, Random, 95% CI) | ‐117.18 [‐280.02, 45.67] |
| 12 Cost‐effectiveness/wound healed (21 days) Show forest plot | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐384.71 [‐503.66, ‐265.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (hazard ratio) Show forest plot | 5 | 580 | Hazard Ratio (Random, 95% CI) | 2.45 [1.71, 3.52] |
| 2 Wound healing (risk ratio) (up to 60 days) Show forest plot | 6 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.99, 2.76] |
| 3 Wound healing (mean time to healing) Show forest plot | 6 | 712 | Mean Difference (IV, Random, 95% CI) | ‐3.79 [‐7.15, ‐0.43] |
| 4 Incident infection (up to 24 days) Show forest plot | 4 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.08, 0.34] |
| 5 Persistent positive swabs (up to 21 days) Show forest plot | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.05, 0.19] |
| 6 Adverse events (time points between 21 days and 6 weeks) Show forest plot | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 3 | 210 | Mean Difference (IV, Random, 95% CI) | ‐7.79 [‐17.96, 2.38] |
| 2 Infection (time points between 14 days and 2 months) Show forest plot | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.26, 3.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 2 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐2.76, 1.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 2 | 204 | Mean Difference (IV, Random, 95% CI) | ‐3.49 [‐4.46, ‐2.52] |
| 2 Positive swab (21 days) Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (hazard ratio) Show forest plot | 2 | 164 | Hazard Ratio (Fixed, 95% CI) | 2.86 [1.60, 5.11] |
| 2 Wound healing (mean time to healing) Show forest plot | 4 | 1156 | Mean Difference (IV, Random, 95% CI) | ‐5.32 [‐6.30, ‐4.34] |
| 3 Persistent positive swabs (up to 30 days) Show forest plot | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.06, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Infection (up to 30 days) Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.54, 2.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound healing (mean time to healing) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Costs (duration 18 days +) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality (short‐term or unclear) Show forest plot | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.99] |

