Smanjenje doze inhalacijskih kortikosteroida u terapiji astme kod odraslih osoba
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: randomised controlled trial Total duration of study: 24 weeks. 'Run‐in' period: 8 weeks. All participants received salmeterol/fluticasone propionate combination (SFC) at a dose of 50/250 μg twice daily. Number of study centres and locations: 124 centres (no locations specified) Study setting: not stated Date of study: not stated | |
| Participants | Enrolled (N): 603 Randomised (n): 475 (SFC 50/250, n = 159; SFC 50/100, n = 157; FP 250, n = 159) Analysed (n): 464 (SFC 50/250, n = 154; SFC 50/100, n = 156; FP 250, n = 154) Withdrawals (n): 63 Median age (range), years: SFC 50/250, 46.5 (18‐81); SFC 50/100, 43.0 (18‐75); FP 250 42.0 (18‐77) Age range, years: 18‐81 Gender (% female): SFC 50/250, 48.1; SFC 50/100, 46.2; FP 250 51.3 Severity of condition: well controlled on step 2 or 3. Mean % predicted prebronchodilator FEV1 (SD) as follows: SFC 50/250, 87.8 (18.2); SFC 50/100, 91.2 (17.8); FP 250, 90.8 (17.2) Diagnostic criteria: Asthma control was assessed using the GOAL definitions of 'well controlled' and 'total control'. Baseline lung function (mean morning PEF (SD), L/min): SFC 50/250, 465.6 (113.2); SFC 50/100, 467.9 (111.2); FP 250, 463.7 (105.1) Smoking history, % smokers or ex‐smokers: SFC 50/250, 24.7; SFC 50/100, 21.3; FP 250, 16.2 Inclusion criteria: aged ≥18 years; documented history of asthma (≥ 6 months) well controlled with current treatment (ICS at a dose of CFC beclomethasone dipropionate or equivalent and a long‐acting beta2‐agonist at recommended dose) at a stable dose for ≥ 4 weeks before initial clinic visit (V1); respiratory tract infection, with acute exacerbation requiring emergency department treatment/hospitalisation or use of oral/parenteral steroids, within 4 weeks of V1; any change in asthma maintenance treatment within 4 weeks Exclusion criteria: smoking history ≥ 10 pack‐years; respiratory tract infection Details of criteria for stepping down treatment: All participants received SFC 50/250 μg twice daily and were randomised to remain on SFC 50/250 or move to 1 of the 2 step‐down treatment arms if their asthma was assessed as 'well controlled' over the last 2 weeks of the run‐in period; asthma control was assessed according to GOAL definitions (see Bateman 2004). | |
| Interventions | Intervention 1: SFC 50/100 μg twice daily Intervention 2: FP 250 μg twice daily (not relevant to review) Comparison: SFC 50/250 μg twice daily Concomitant medications: Short‐acting bronchodilators (previously used as rescue medication) and antihistamines were permitted, provided they had been used for at least 4 weeks. Excluded medications: All previous asthma medications were discontinued at entry into the run‐in period, except short‐acting bronchodilators (previously used as rescue medication) and antihistamines, provided they had been used for at least 4 weeks. | |
| Outcomes | Primary outcomes: mean morning PEF over the first 12 weeks of randomised treatment Secondary outcomes: mean morning PEF over the last 12 weeks of randomised treatment; daily symptoms; use of short‐acting bronchodilator as rescue medication; FEV1; asthma control based on GOAL definitions of total control and 'well‐controlled' (see Bateman 2004) | |
| Notes | Funding for trial: not stated Notable conflicts of interest of trial authors: Three of the trial authors had received sponsorship and had attended advisory boards for various pharmaceutical companies, including AstraZeneca, GlaxoSmithKline and Boehringer‐Ingelheim; 3 authors are employees of GlaxoSmithKline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Study reported as double blind |
| Blinding of outcome assessment (detection bias) | Low risk | Study reported as double blind |
| Incomplete outcome data (attrition bias) | Low risk | Data provided for all randomised individuals. We note that study authors reported lung function results only for the per‐protocol population, whereas they reported all other outcomes for the intent‐to‐treat population. |
| Selective reporting (reporting bias) | High risk | Study authors reported the primary outcome for the per‐protocol data set on the basis that this is a non‐inferiority study. Furthermore, the primary outcome considers lung function only over the first 12 weeks of treatment; a secondary outcome assessed lung function in the full analysis set but considered only the second 12 weeks of treatment. All in all, findings were quite confusing and inconsistent. This trial was not reported as registered, and we cannot source a protocol. |
| Other bias | High risk | The protocol suggests that only participants whose condition was well controlled within the last 2 weeks of the run‐in period would go on to randomisation; however, it appears that a relatively high proportion of participants whose asthma was not controlled were included in the full analysis set. Results of this study are not well reported, and as the study does not appear to have been prospectively registered, and a protocol was not cited, it is difficult to ascertain whether selective outcome reporting occurred. Study sponsorship is not reported, although several authors worked for GSK. Key exclusion criteria of poor control according to ACQ were not defined or reported. A large proportion of poorly controlled randomised participants were not included in the primary outcome analysis (but were included in the secondary outcome analysis). Reporting was confusing. |
| Methods | Study design: randomised controlled trial, multi‐centre, open label Total duration of study: 2‐week run‐in period; 12‐week treatment period 'Run‐in' period: 2‐week run in period, during which participants remained on their existing doses of ICS ('high‐dose' budesonide 400 μg twice daily, beclomethasone 400 μg twice daily or beclomethasone 500 μg twice daily via a pMDI with a spacer; or 'low‐dose' budesonide or beclomethasone 200 μg twice daily) Number of study centres and locations: UK Study setting: primary care Withdrawals: 147/631 (23%) randomised participants withdrew during the treatment period Date of study: not stated | |
| Participants | N: 631 patients were randomised after a 2‐week run‐in period. Mean age (range), years: budesonide OD: 44.1 (16.5‐80.2); budesonide BID: 45.7 (16.7‐77.0); no ICS dose change: 40.9 (16.2‐80.2) Gender M/F, n: budesonide OD: 100/128; budesonide BID: 90/101; no ICS dose change: 100/112 Severity of condition: baseline mean morning PEFR (SD), L/min: controlled on step 2. Budesonide OD: 437.2 (106.5); budesonide BID: 447.4 (111.3); no ICS dose change: 445.8 (100.9) Diagnostic criteria: mild, well controlled Baseline lung function ‐ mean morning PEFR (SD), L/min: budesonide OD: 437.2 (106.5); budesonide BID: 447.4 (111.3); no ICS dose change: 445.8 (100.9) Smoking history: not stated Inclusion criteria: aged ≥ 16 years; documented diagnosis of asthma (currently stable); asthma considered by physician to be well controlled (as per BTS guidelines); receiving 200 μg twice daily (low dose) or 400/500 μg twice daily (high dose) budesonide or beclomethasone (via a pMDI ± spacer) for 6 months before entry; patients on the higher dose of steroid were required to have used a large volume spacer for a minimum of 4 weeks before entry Exclusion criteria: pregnant, at risk of pregnancy, breast feeding, brittle asthma, night shift workers. Within 3 months: any increase in total daily inhaled steroid dose; exacerbation resulting in hospitalisation or requiring nebulisation, oral/injectable/rectal steroids, beta blockers, sodium cromoglycate, sodium nedocromil, any unlicensed medication or fluticasone propionate. Within 1 week before the study: Patients were not permitted to have taken theophylline (or derivatives), any long‐acting bronchodilators, ipratropium/oxitropium bromide or ketotifen. Details of criteria for stepping‐down treatment: Participants were eligible for randomisation if their diary cards showed that they had no nocturnal wakening due to asthma in the previous 7 nights, and if they fulfilled 3 of the following criteria: asthma symptoms of no more than mild severity experienced on 3 or fewer days of the previous 7 days; using ≤ 1 puff per day of inhaled bronchodilator on a maximum of 5 of the last 7 days; circadian variation in PEFR < 20% in the previous 7 days; morning PEFR ≥ 80% or predicted or best (if this value was greater than predicted) on 5 of the 7 previous days | |
| Interventions | Intervention: Participants on an initial high dose of ICS (budesonide 400 μg twice daily or beclomethasone 400 μg twice daily or beclomethasone 500 μg twice daily delivered via a pMDI and a spacer device) were randomised to receive budesonide 200 μg twice daily via a turbuhaler or 400 μg once daily (i.e. both groups represent a halving of the initial ICS dose). Participants on an initial low dose of ICS (budesonide or beclomethasone 200 μg twice daily) were randomised to receive budesonide 100 μg twice daily via a turbuhaler or 200 μg once daily (i.e. both groups represent a halving of the initial ICS dose). Comparison: No change in initial dose of budesonide or beclomethasone. Concomitant medications: Each patient was given terbutaline (Bricanyl) turbuhaler 500 μg prn for rescue mediation during the run‐in and throughout the study. Excluded medications: See exclusion criteria. | |
| Outcomes | Primary outcomes: morning PEFR recorded by diary cards (recorded at baseline, and at 4, 8 and 12 weeks) Secondary outcomes: evening PEFR, proportion of symptom‐free days/nights, proportion of beta2‐agonist‐free days/nights, sleep disturbance (all recorded via diary cards) quality of life (Juniper Asthma Quality of Life Questionnaire (Juniper 1993); PEFR measured at clinic visits; asthma severity measured at clinic visits; asthma control) | |
| Notes | Funding for trial: not stated; likely Astra Pharmaceuticals Notable conflicts of interest of trial authors: not stated. One study author was an employee of Astra Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) | High risk | The study was open label. |
| Blinding of outcome assessment (detection bias) | High risk | The study was open label, and it does not appear that outcome assessors were blinded to treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete primary outcome data were reported, but the number of participants for whom data were missing was similar across OD/BD/pMDI groups. |
| Selective reporting (reporting bias) | High risk | No study protocol available. The data for high‐dose and low‐dose groups were pooled and were not presented individually. Study authors stated that separate data were not presented individually because no significant differences between the 2 dose groups were found for any of the analyses performed. |
| Other bias | Unclear risk | Unclear risk of bias: This is a complicated study, and some changes in inhaler device appear to have occurred at the same time as changes in dose. Participants entered the run‐in period on their existing dose of ICS ('high' or 'low') and were later randomised to remain on their existing dose, or step down to half the dose in 1 of 2 different formats (half the dose twice daily, or the same dose but only once daily). No data were reported for the run‐in period. Unclear risk of bias: Funding for the study is not reported. The paper has industry authors, and the company manufactures products that seem to match the products reported upon. Funding for the study is not declared, but one study author is employed by Astra. |
| Methods | Study design: randomised controlled trial, double‐blind, parallel group Total duration of study: 1 year 'Run‐in' period: 1 month Number of study centres and locations: general practices in Western and Central Scotland Study setting: primary care (general practice) Withdrawals: 24/130 participants in the stepdown group and 22/129 in the control group discontinued the intervention. Analyses were performed on all randomised participants. Date of study: The study was performed between May 1999 and October 2001. | |
| Participants | N: 259 participants were randomised. Mean age (SD), years: step‐down 52.8, (14.5); control 55 (15.2) Age range: 18‐86 years Gender (M/F), n: step‐down, 54/76; control, 54/75 Severity of condition: controlled on high‐dose ICS (at least 1000 μg BDP) plus possibly other drugs (steps 2‐4) Baseline lung function ‐ % predicted pre‐salbutamol FEV1 (SD), L/min: step‐down, 80.3 (19.2); control, 80.1 (18.6) Smoking history ‐ current/former/never, n: step‐down, 16/44/70; control, 17/49/63 Inclusion criteria: aged ≥ 18 years; diagnosis of asthma ≥ 1 year; treated with ≥ 800 μg inhaled BDP (or budesonide or fluticasone propionate at equivalent dosage) Exclusion criteria: required oral corticosteroids or attended general practice or hospital within 2 months; inability to use peak flow meter; treatment with immunosuppressive drugs; serious illness; alcohol, substance or drug misuse; pregnancy; participation in other research within the past 6 months Details of criteria for stepping down treatment: stable asthma (i.e. good control) assessed at end of run‐in period and at 3, 6, 9 and 12 months. Good control was defined as an asthma morbidity score ≤ 2, no visits to general practice or hospital since previous visit and peak flow ≥ target flow on 8 of the previous 14 days; if peak flow data were missing, the first two criteria were used. | |
| Interventions | Intervention: step‐down ‐ 50% reduction in ICS dose Comparison: no change in ICS dose Concomitant medications: Reliever inhalers were permitted. 36.9% of the step‐down group and 30.2% of the control group were receiving a concomitant LABA. Excluded medications: immunosuppresive drugs | |
| Outcomes | Primary outcomes: proportion of participants experiencing an asthma exacerbation, asthma control (short asthma morbidity score (Rimmington 1997); scores ranged from 0 (perfect control) to 8 (very poor control)) Secondary outcomes: adverse events, health‐related quality of life (EuroQoL and St George's Respiratory Questionnaire), annual corticosteroid dose | |
| Notes | Funding for trial: NHS R&D Programme on Asthma Management Notable conflicts of interest of trial authors: Study authors had received funding, and various pharmaceutical companies including GlaxoSmithKline provided the study inhalers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Well‐described randomisation with computer‐generated randomisation stratified by centre |
| Allocation concealment (selection bias) | Low risk | Computer‐allocated randomisation sequence; randomisation code withheld from investigators until study completion |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded to treatment allocation via use of identical inhaler packs. |
| Blinding of outcome assessment (detection bias) | Low risk | Randomisation code was maintained blind until the end of the study. |
| Incomplete outcome data (attrition bias) | Low risk | Primary outcome data were reported for all participants as intention to treat. Some data for health status secondary outcome measures were missing (not explained), but the number of participants for whom data were missing was similar in both treatment groups. Lung function was not reported during or at the end of the treatment period. |
| Selective reporting (reporting bias) | High risk | Protocol was not available. It is not clear why study authors did not present lung function as, according to the Methods section, participants did monitor lung function for 2 weeks before each visit. Detailed adverse event data were not presented. |
| Other bias | Low risk | Study medication was provided by industry, but study was funded by NHS R&D programme on asthma development. No industry was involved in authorship of the paper. |
| Methods | Study design: randomised controlled trial, double‐blind, parallel group Total duration of study: 3 months 'Run‐in' period: no run‐in (this is a follow‐up extension to a previous study) Number of study centres and locations: Firestone Regional Chest and Allergy Clinic at St Joseph's Hospital and the McMaster University Medical Centre in Hamilton, Canada Study setting: secondary care (asthma clinic) Withdrawals: All 28 participants completed the study Date of study: not reported | |
| Participants | N: 28. A subgroup of 14 participants were relevant to this review. Mean age: not reported. Mean age in parent study was ˜ 42 years (Juniper 1990). Age range: not reported Gender: not reported Severity of condition: controlled on step 2 (mild to moderate: approximately half of participants were 'steroid dependent') Baseline lung function: Individual participant data were reported. At entry to initial study, all participants had airway hyper‐responsiveness to methacholine (PC20 < 8.0 mg/mL) and symptomatic asthma. Smoking history: not reported Inclusion criteria and exclusion criteria: successful completion of previous study Details of criteria for step‐down treatment: not reported | |
| Interventions | Intervention: a halving of the budesonide dose in steroid‐dependent participants (n = 6) Comparison: no change in budesonide dose among steroid‐dependent participants (n = 8) Concomitant medications: Bronchodilator medication was permitted (long‐acting vs short‐acting not specified). Excluded medications: not reported | |
| Outcomes | Primary outcomes: airway responsiveness to methacholine (measured with a standardised tidal breathing protocol); clinical asthma severity (i.e. asthma control assessed via asthma severity questionnaire). The questionnaire comprised 6 items: awakened at night by symptoms; awakened in the morning by symptoms; limitation of normal daily activities; sputum; use of bronchodilator more than 4 times per day; FEV1 prebronchodilator < 70% predicted (One point was scored for each of the first 5 items that had been positive on ≥ 1 day during the previous week; 1 point was scored for reduced spirometry; therefore, the maximum asthma severity score (i.e. worst control) was 6). Secondary outcomes: bronchodilator use; allergen exposure score; upper respiratory tract infection score | |
| Notes | Funding for trial: Funding was not reported. Notable conflicts of interest of trial authors: Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) | Low risk | The study was reported as double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | The study was reported as double‐blind. |
| Incomplete outcome data (attrition bias) | Low risk | All 28 randomised participants completed the study, and it appears that data were reported for all 28 participants. |
| Selective reporting (reporting bias) | High risk | Study authors state, "During analysis, it was found that all the outcomes in the two reduction groups were very similar, and also, the outcomes in the two groups in whom steroids were not reduced were very similar. Therefore, for simplicity, the data have been combined and are presented as two groups, reduced and maintained". No protocol was available; no prespecified analysis plan was prepared. Group data were combined as described above. |
| Other bias | Low risk | None identified |
| Methods | Study design: randomised controlled, double‐blind, double‐dummy, parallel group Total duration of study: 14 weeks 'Run‐in' period: 2 weeks Number of study centres and locations: 16 centres (8 each in UK and Belgium) Study setting: not stated Withdrawals: 5 participants (CIC 160 μg, n = 4; FP 250 μg, n = 1) Date of study: October 2004 to July 2005 | |
| Participants | N: 111 randomised Mean age, years: CIC 160 μg OD: 43; FP 250 μg BID: 46 Age range, years: 18‐75 Gender M/F, n: CIC 160 μg OD: 28/30; FP 250 μg BID: 30/23 Severity of condition: controlled on step 2 Baseline lung function ‐ mean (SD) FEV1, L: CIC 160 μg OD: 3.272 (0.869); FP 250 μg BID: 3.146 (0.823) Smoking history ‐ non‐smoker/ex‐smoker/current smoker, n: CIC 160 μg OD: 38/18/2; FP 250 μg BID: 34/18/1 Inclusion criteria: male and female patients aged 17–75 years; diagnosis of asthma as defined by American Thoracic Society guidelines for at least 6 months, but otherwise in good health; FEV1 ≥ 90% of predicted; maintained asthma control over previous 3 months using fluticasone propionate 250 μg twice daily, or equivalent, with short‐acting bronchodilator use as rescue medication only Exclusion criteria: concomitant severe disease, such as a lower respiratory tract infection; chronic obstructive pulmonary disease or other relevant lung diseases; more than 1 emergency care visit or hospitalisation due to asthma exacerbations in the previous year; or clinically relevant abnormal laboratory values suggesting an Details of criteria for step‐down treatment: Participants were randomised to step‐down (for eligibility, see inclusion and exclusion criteria). | |
| Interventions | Intervention: ciclesonide 160 μg OD (i.e. ˜ 50% reduction according to GINA 2016) Comparison: fluticasone propionate 250 μg BID (i.e. no change) Concomitant medications: short‐acting bronchodilator used as rescue medication only Excluded medications: See exclusion criteria. | |
| Outcomes | Primary outcomes: efficacy ‐ percentage of days with asthma control (defined as days without asthma symptoms and without rescue medication use); asthma symptom‐free days; rescue medication‐free days; and nocturnal awakening‐free days. Safety ‐ adverse events Secondary outcomes: efficacy ‐ FEV1; forced vital capacity (FVC); PEF from spirometry; PEF from participant diaries measured on a Mini‐Wright PEF meter; asthma symptom scores from participant diaries (sum scores based on a 9‐point scale, with 0 indicating no symptoms); use of rescue medication; number of participants with an asthma exacerbation; and time to onset of the first asthma exacerbation. Safety ‐ vital signs (blood pressure and pulse rate); standard laboratory tests (including haematology, blood chemistry and urinalysis); and number of participants with oral candidiasis | |
| Notes | Funding for trial: This study was funded and sponsored by ALTANA Pharma AG, a member of the Nycomed Group. Notable conflicts of interest of trial authors: Editorial assistance for preparation of the manuscript was provided by Nathan Price‐Lloyd, PhD, Medicus International, which was funded by ALTANA Pharma AG, a member of the Nycomed Group. Study authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) | Low risk | The study was reported as double‐blind, double‐dummy. |
| Blinding of outcome assessment (detection bias) | Low risk | The study was reported as double‐blind, double‐dummy. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analyses were performed for safety analyses and comprised all randomised participants. Some data for lung function analyses were missing, but only from 3 participants in the step‐down group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available; however, the range of outcomes seems fairly comprehensive. |
| Other bias | Low risk | None identified |
| Methods | Study design: randomised controlled, double‐blind, double‐dummy, parallel group Total duration of study: 14 weeks 'Run‐in' period: 4 weeks Number of study centres and locations: 18 pulmonology practices Study setting: pulmonology outpatient practices Withdrawals: none reported Date of study: November 1996 to October 1997 | |
| Participants | N: 150 Mean (SD) age, years: 400 μg/day BDP: 43 (15); 1000 μg/day BDP: 42 (15) Age range: not reported Gender ‐ M/F, n: 400 μg/day BDP: 22/50; 1000 μg/day BDP: 30/48 Severity of condition: step 2 Baseline lung function ‐ mean (SE) FEV1, L: 400 μg/day BDP: 2.77 (0.09); 1000 μg/day BDP: 2.85 (0.09) Smoking history: not reported Inclusion criteria and exclusion criteria, allowable range: age 18‐75 years; use of inhaled steroids for ≥ 3 months (BDP 1000 mg or BUD 800‐1000 mg); use of β2‐agonists on demand (≥ 1 puffs/d); reversible airflow obstruction assessed within the last 2 years; change in FEV ≥ 12%; change in PEF ≥ 20%; bronchial hyper‐responsiveness to inhaled histamine (PC20 FEV1 ≥ 4 mg/mL); baseline FEV1 ≥ 60% of predicted; variability of baseline FEV1 during run‐in period ≤ 15% Details of criteria for step‐down treatment: Participants were randomised to step‐down (for eligibility, see inclusion and exclusion criteria). | |
| Interventions | Intervention: hydrofluoroalkane beclomethasone 400 μg/day (i.e. < 50% dose reduction) Comparison: chlorofluorocarbon beclomethasone 1000 μg/day. Concomitant medications: not reported; likely that use of short‐acting bronchodilators as rescue medication was permitted Excluded medications: none specified | |
| Outcomes | Primary outcomes: Efficacy ‐ morning peak flow; Safety ‐ adverse events Secondary outcomes: evening peak flow, FEV1, concentration of inhaled histamine causing a 20% decline in FEV1, frequency of β2‐agonist use, daily asthma symptom score (0 represents no symptoms; 5 represents severe symptoms); and sleep disturbance score. Safety ‐ oropharyngeal candidiasis; reported hoarseness; clinical laboratory tests (i.e. haematology, serum chemistry, urine analysis); and vital signs (i.e. sitting pulse rate, blood pressure, ECG) | |
| Notes | Funding for trial: 3M Medica (Borken, Germany) Notable conflicts of interest of trial authors: not reported; however, several study authors were employees of 3M Medica | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) | Low risk | The study was reported as double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | The study was reported as double‐blind. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data appear complete. Data appear to be reported for all randomised participants. |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Reporting of safety results appears to be fairly selective (SAEs not reported, details of individual AEs not reported). |
| Other bias | Low risk | None identified |
Abbreviations: BDP, beclomethasone dipropionate; BID, twice daily; BTS, British Thoracic Society; CFC, chlorofluorocarbon; CIC, ciclesonide; ECG, electrocardiogram; FEV1, forced expiratory volume in 1 second; FP, fluticasone propionate; FVC, forced vital capacity; GOAL, Gaining Optimal Asthma Control study; ICS, inhaled corticosteroid; LABA, long‐acting beta agonist; NHS, National Health Service; OD, once daily; PC20, provocative concentration that produces a 20% reduction in FEV1 from baseline value; PEF, peak expiratory flow; PEFR, peak expiratory flow rate; pMDI, pressurised metered‐dose inhaler; QoL, quality of life; R&D, research and development; SD, standard deviation; SE, standard error; SFC, salmeterol formoterol combination; UK, United Kingdom.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong intervention | |
| Wrong patient population | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong study design | |
| Wrong route of administration | |
| Wrong comparator | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong study design | |
| Wrong intervention | |
| Wrong comparator | |
| Wrong intervention | |
| Wrong intervention | |
| Wrong route of administration | |
| Wrong intervention | |
| Wrong intervention |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.16, 21.09] |
| Analysis 1.1  Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 1 Exacerbation requiring OCS. | ||||
| 2 Asthma control Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 2 Asthma control. | ||||
| 3 All‐cause SAEs Show forest plot | 2 | 742 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.25, 6.25] |
| Analysis 1.3  Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 3 All‐cause SAEs. | ||||
| 4 Steroid‐related AEs Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.54] |
| Analysis 1.4  Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 4 Steroid‐related AEs. | ||||
| 5 Juniper AQLQ score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.5 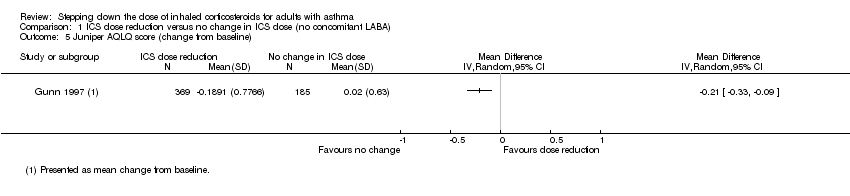 Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 5 Juniper AQLQ score (change from baseline). | ||||
| 6 Lung function, PEFR morning (L/min) Show forest plot | 3 | 875 | Mean Difference (IV, Random, 95% CI) | ‐5.98 [‐19.47, 7.51] |
| Analysis 1.6 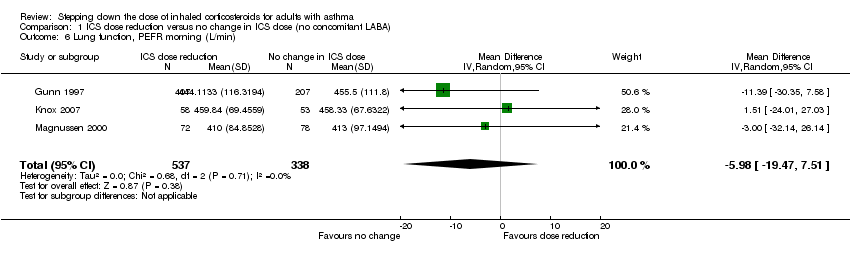 Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 6 Lung function, PEFR morning (L/min). | ||||
| 7 Lung function, FEV1 (L) Show forest plot | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| Analysis 1.7 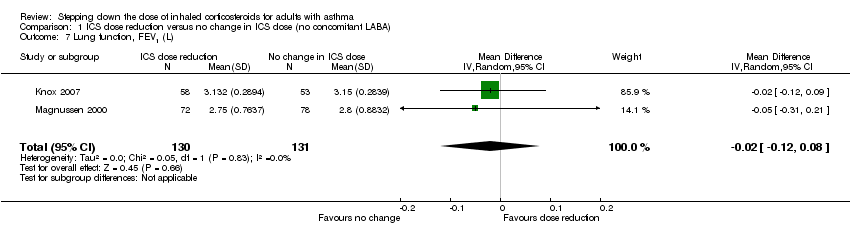 Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 7 Lung function, FEV1 (L). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.08] |
| Analysis 2.1  Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 1 Exacerbation requiring OCS. | ||||
| 2 Asthma control (short asthma morbidity score), change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.2 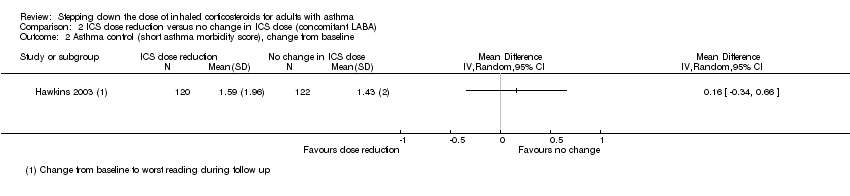 Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 2 Asthma control (short asthma morbidity score), change from baseline. | ||||
| 3 Asthma control (Asthma Severity Questionnaire) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 3 Asthma control (Asthma Severity Questionnaire). | ||||
| 4 All‐cause SAEs Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.33] |
| Analysis 2.4 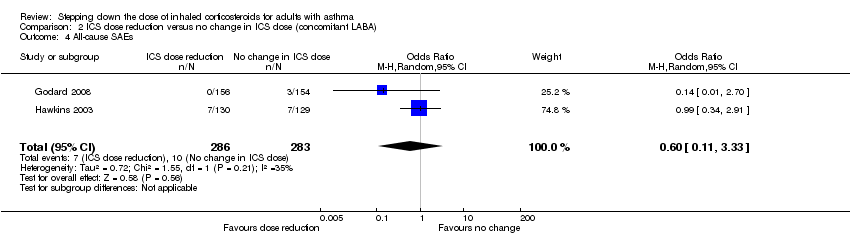 Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 4 All‐cause SAEs. | ||||
| 5 EuroQoL score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 5 EuroQoL score (change from baseline). | ||||
| 6 St. George's Respiratory Scale score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 6 St. George's Respiratory Scale score (change from baseline). | ||||
| 7 Lung function, PEFR morning (L/min) (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 7 Lung function, PEFR morning (L/min) (change from baseline). | ||||
| 8 Lung function, reduction in FEV1 (% predicted, change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.8 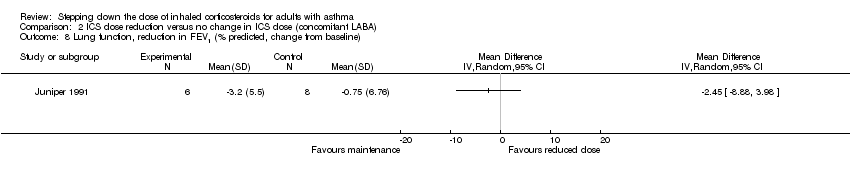 Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 8 Lung function, reduction in FEV1 (% predicted, change from baseline). | ||||
| 9 Exacerbation requiring hospitalisation Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 4.06 [0.45, 36.86] |
| Analysis 2.9  Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 9 Exacerbation requiring hospitalisation. | ||||
| 10 Exacerbation requiring ED visit Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.10 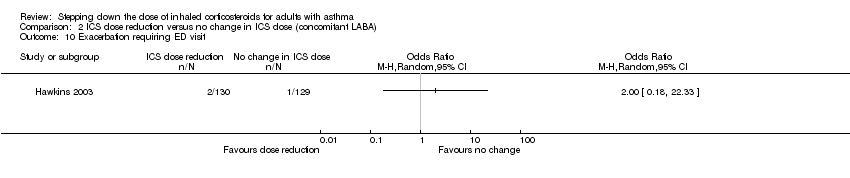 Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 10 Exacerbation requiring ED visit. | ||||
| 11 Mortality Show forest plot | 1 | 310 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.11  Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 11 Mortality. | ||||
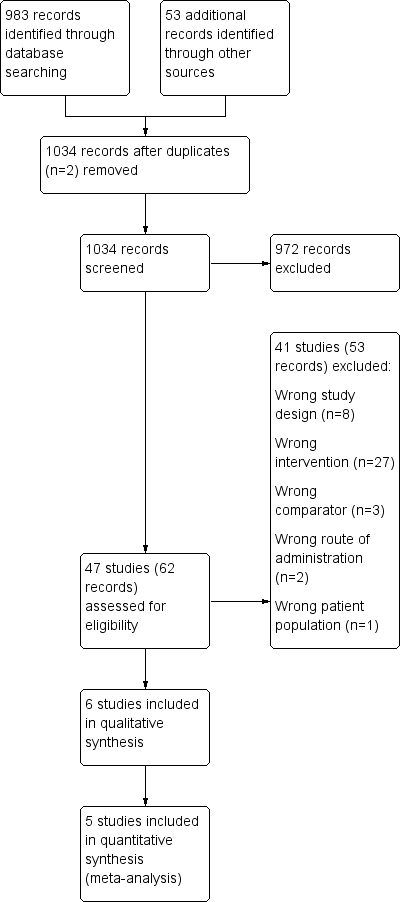
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
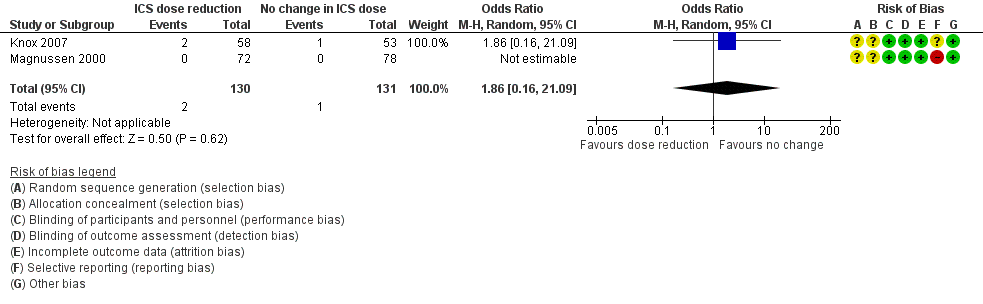
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.1 Exacerbation requiring OCS.
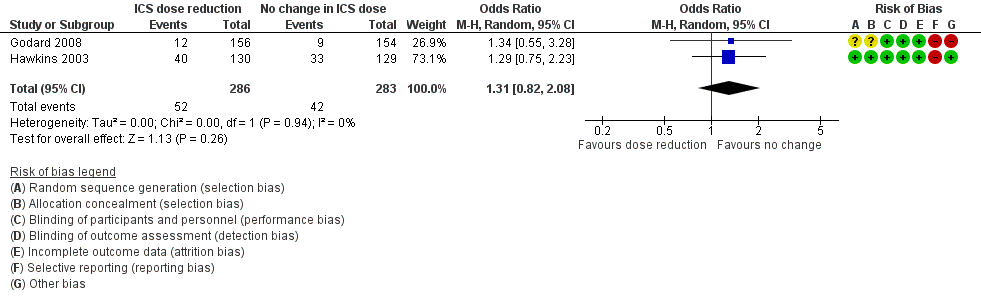
Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.1 Exacerbation requiring OCS.
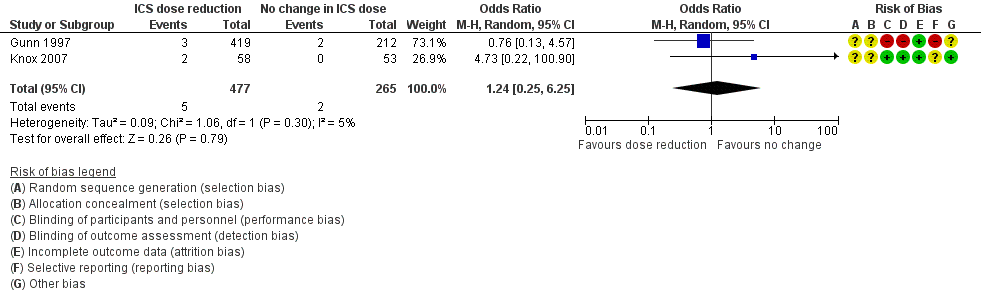
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.3 All‐cause SAEs.
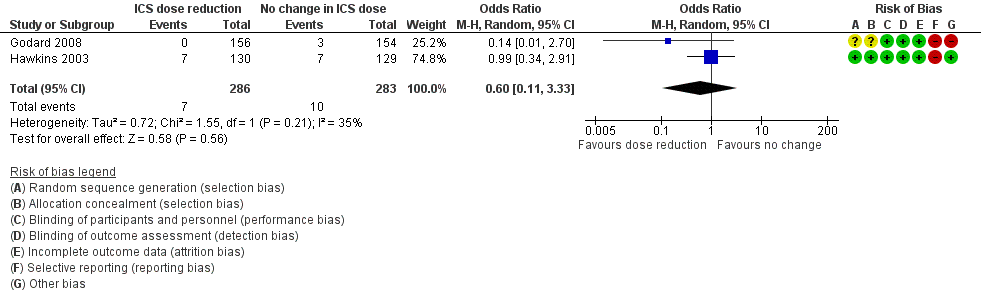
Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.4 All‐cause SAEs.
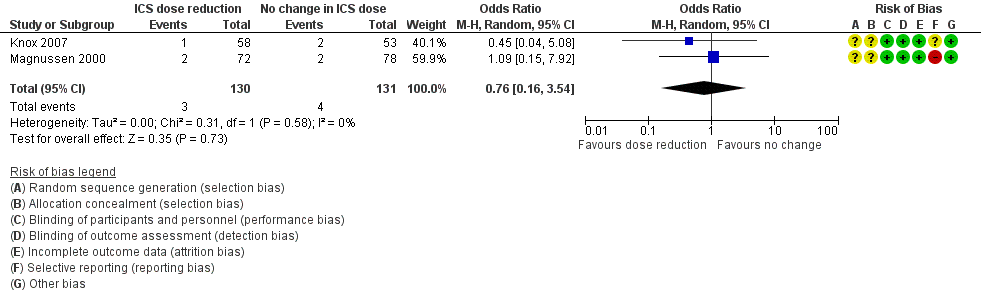
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.4 Steroid‐related AEs.

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.7 Lung function, FEV1 (L).
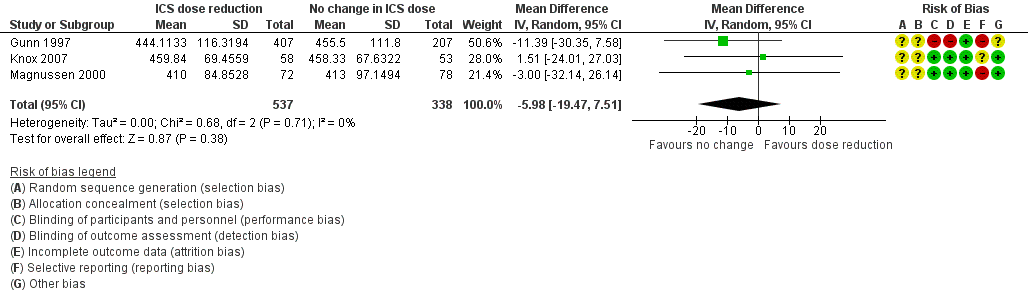
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.6 Lung function, PEFR morning (L/min).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.9 Exacerbation requiring hospitalisation.

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 1 Exacerbation requiring OCS.
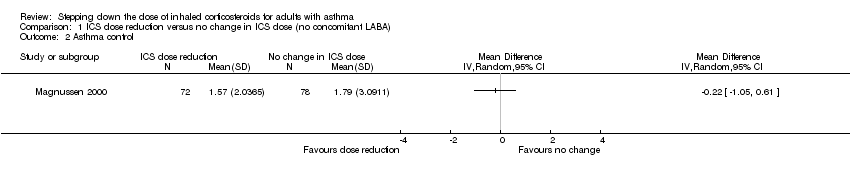
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 2 Asthma control.
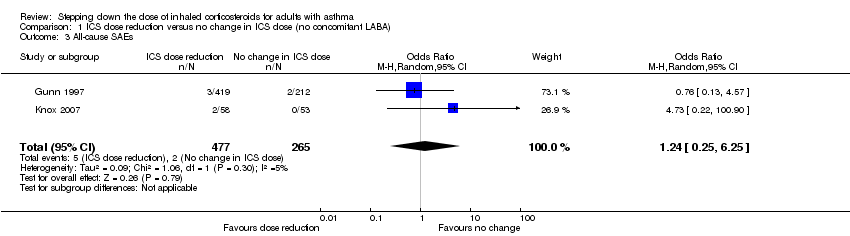
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 3 All‐cause SAEs.
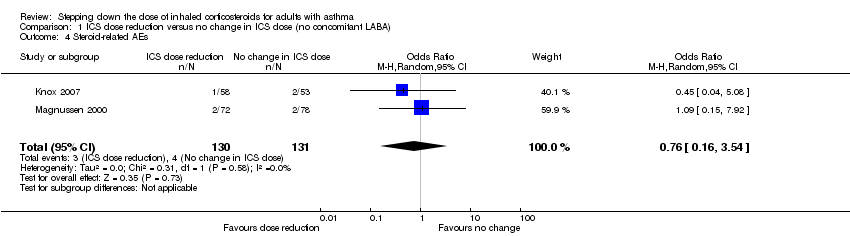
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 4 Steroid‐related AEs.
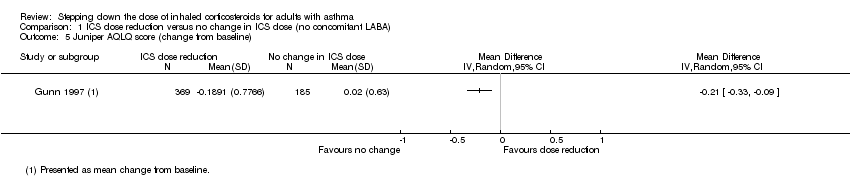
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 5 Juniper AQLQ score (change from baseline).

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 6 Lung function, PEFR morning (L/min).

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 7 Lung function, FEV1 (L).
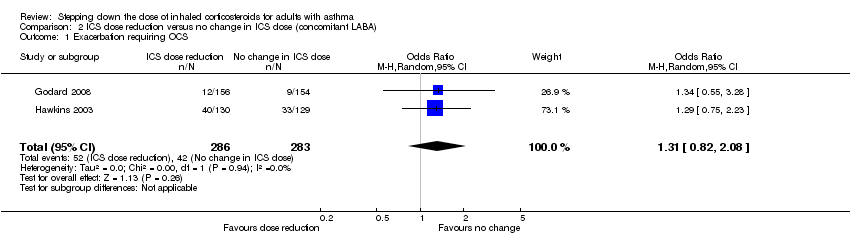
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 1 Exacerbation requiring OCS.
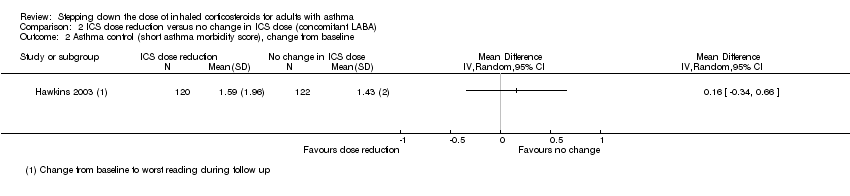
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 2 Asthma control (short asthma morbidity score), change from baseline.
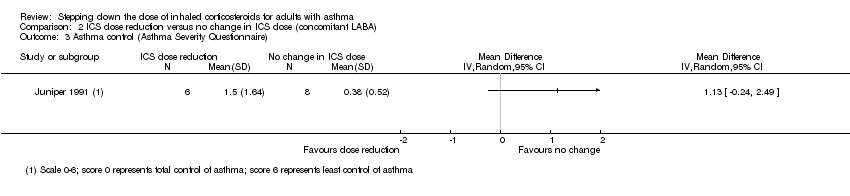
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 3 Asthma control (Asthma Severity Questionnaire).
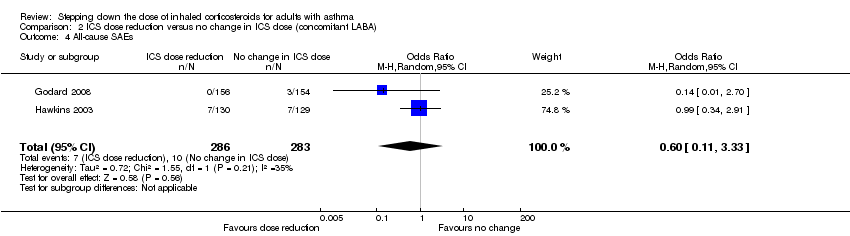
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 4 All‐cause SAEs.
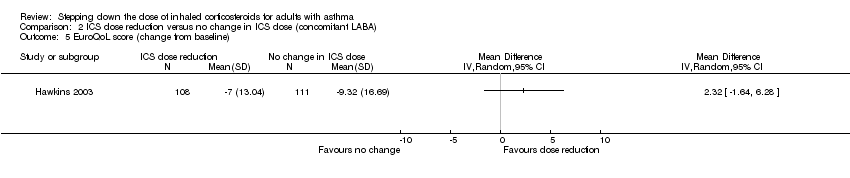
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 5 EuroQoL score (change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 6 St. George's Respiratory Scale score (change from baseline).
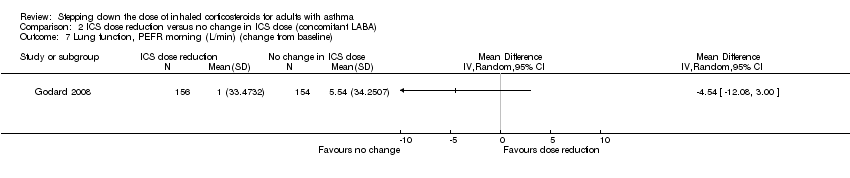
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 7 Lung function, PEFR morning (L/min) (change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 8 Lung function, reduction in FEV1 (% predicted, change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 9 Exacerbation requiring hospitalisation.
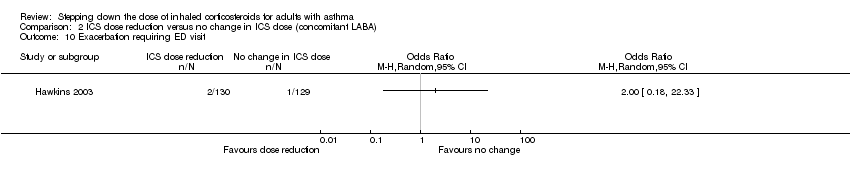
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 10 Exacerbation requiring ED visit.

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 11 Mortality.
| ICS dose reduction compared with no change in ICS dose (no concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (no concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 8 per 1000 | 14 per 1000 | OR 1.86 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Asthma control | Mean asthma control score in the no change in ICS dose group was 1.79. | MD 0.22 lower | ‐ | 150 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| All‐cause SAEs | 8 per 1000 | 9 per 1000 | OR 1.24 | 742 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Steroid‐related AEs | 31 per 1000 | 23 per 1000 | OR 0.76 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Health‐related quality of life (change from baseline) | Mean change from baseline in health‐related quality of life for the no change in ICS dose group was 0.02. | MD 0.21 lower | ‐ | 554 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence); MCID is 0.5 for AQLQ |
| Lung function, FEV1 (L) | Mean FEV1 in the no change in ICS dose group was 3.15 litres. | MD 0.02 litres lower | ‐ | 261 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Exacerbations requiring hospitalisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported by included studies |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). dThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (representative of specialist centres) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded twice for risk of bias (selective reporting and lack of blinding (subjective outcome)) and once for indirectness (single study representative of one setting and drug regimen). fThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ICS dose reduction compared with no change in ICS dose (concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 148 per 1000 | 186 per 1000 | OR 1.31 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring OCS (low‐quality evidence) |
| Asthma control (short asthma morbidity score) | Mean asthma control score was 1.43. | MD 0.16 higher | ‐ | 242 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to asthma control (low‐quality evidence) |
| All‐cause SAEs | 35 per 1000 | 22 per 1000 | OR 0.60 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to all‐cause SAEs (low‐quality evidence) |
| Steroid‐related AEs ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| St. George's Respiratory Scale score (change from baseline) Score 0‐100. 100 = greatest impact of chest disease on life; MCID is 4 units. | Mean change from baseline in HRQoL score was 7.4.c | MD 0.13 higher | ‐ | 229 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to HRQoL (low‐quality evidence) |
| Exacerbation requiring hospitalisation | 4 per 1000 | 14 per 1000 | OR 4.06 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring hospitalisation (low‐quality evidence) |
| Lung function, reduction in FEV1 (% predicted, change from baseline) | Mean change from baseline in % predicted FEV1 was ‐0.75%. | MD 2.45 lower | ‐ | 14 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to lung function (very low‐quality evidence) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cNote that study authors reported the change to the lowest SGRQ score during follow‐up. dThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (single study representative of one setting or drug regimen) and once for imprecision (wide CI). AE, adverse event; CI, confidence interval; FEV1, forced expiratory volume in one second; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; HRQoL, health‐related quality of life; ICS, inhaled corticosteroid; LABA, long‐acting beta agonist; MCID, minimum clinically important difference; MD, mean difference; OCS, oral corticosteroid; OR, odds ratio; RCT, randomised controlled trial; RR, risk ratio; SAE, serious adverse event. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.16, 21.09] |
| 2 Asthma control Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 All‐cause SAEs Show forest plot | 2 | 742 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.25, 6.25] |
| 4 Steroid‐related AEs Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.54] |
| 5 Juniper AQLQ score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Lung function, PEFR morning (L/min) Show forest plot | 3 | 875 | Mean Difference (IV, Random, 95% CI) | ‐5.98 [‐19.47, 7.51] |
| 7 Lung function, FEV1 (L) Show forest plot | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.08] |
| 2 Asthma control (short asthma morbidity score), change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Asthma control (Asthma Severity Questionnaire) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 All‐cause SAEs Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.33] |
| 5 EuroQoL score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 St. George's Respiratory Scale score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Lung function, PEFR morning (L/min) (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Lung function, reduction in FEV1 (% predicted, change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Exacerbation requiring hospitalisation Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 4.06 [0.45, 36.86] |
| 10 Exacerbation requiring ED visit Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Mortality Show forest plot | 1 | 310 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

