Diminution de la dose de corticoïdes inhalés pour les adultes asthmatiques
Résumé scientifique
Contexte
L'asthme est une pathologie des voies respiratoires affectant plus de 300 millions d'adultes et d'enfants dans le monde. Les directives nationales et internationales recommandent de titrer la dose de corticoïdes inhalés (CI) jusqu'à obtenir un contrôle des symptômes avec la dose la plus faible possible car un usage à long terme et à des doses plus élevées de CI comporte des risques d'événements indésirables systémiques. Pour les patients dont les symptômes asthmatiques sont contrôlés avec des doses modérées ou plus élevées de CI, il pourrait être possible de réduire cette dose sans compromettre le contrôle des symptômes.
Objectifs
Évaluer les preuves concernant la diminution du traitement à base de CI chez les adultes ayant un asthme bien contrôlé et recevant déjà des CI à des doses modérées ou élevées.
Stratégie de recherche documentaire
Nous avons identifié des essais dans le registre spécialisé du groupe Cochrane sur les voies respiratoires et effectué une recherche dans ClinicalTrials.gov (www.ClinicalTrials.gov) et au travers du système d'enregistrement des essais de l'Organisation Mondiale de la Santé (OMS) (www.who.int/ictrp/en/). Nous avons effectué des recherches dans toutes les bases de données depuis leur création, sans aucune restriction concernant la langue. Nous avons également effectué des recherches dans les références bibliographiques des études incluses et des revues pertinentes. La recherche la plus récente a été effectuée en juillet 2016.
Critères de sélection
Nous avons inclus des essais contrôlés randomisés (ECR) d'une durée d'au moins 12 semaines et nous avons exclu les essais croisés. Nous avons recherché des études portant sur des adultes (âgés de plus de ≥ 18 ans) dont l'asthme avait été bien contrôlé pendant un minimum de trois mois avec au moins une dose modérée de CI. Nous avons exclu les études ayant recruté des participants atteints de n'importe quelle autre comorbidité respiratoire.
Nous avons inclus les essais comparant une réduction de la dose de CI par rapport à l'absence de changement de la dose de CI chez les personnes souffrant d'asthme bien contrôlé qui a) ne prenaient pas de bêta 1‐agoniste à action prolongée (BAAP, comparaison 1) de manière conjointe ; et b ) qui prenaient des BAAP de manière conjointe (comparaison 2).
Recueil et analyse des données
Deux auteurs de la revue ont indépendamment passé au crible les résultats de recherche pour les études à inclure, extrait les données quant aux critères de jugement prédéfinis et évalué le risque de biais des études incluses ; ceux‐ci ont résolu les désaccords au travers de discussions avec un troisième auteur de la revue. Nous avons analysé les données dichotomiques en tant que rapports de cotes (RC) en utilisant les participants à l'étude comme unité d'analyse et nous avons analysé les données continues sous forme de différences moyennes (DM). Nous avons utilisé un modèle à effets aléatoires. Nous avons évalué tous les critères de jugement en utilisant le système GRADE (Grades of Recommendation, Assessment, Development and Evaluation) et nous avons présenté les résultats dans des tableaux « Résumé des résultats ».
Résultats principaux
Nous avons inclus six études, qui randomisaient un total de 1654 participants (réduction de la dose de CI, sans usage conjoint de BALA (comparaison 1) : n = 892 participants, trois ECR ; réduction de la dose de CI avec usage conjoint de BALA (comparaison 2) : n = 762 participants, trois ECR). Toutes les études incluses étaient des ECR présentant une conception parallèle et ayant comparé une dose fixe de CI par rapport à une réduction de 50 à 60 % de la dose de CI chez des participants adultes présentant un asthme bien contrôlé. La durée de la période de traitement allait de 12 à 52 semaines (durée moyenne de 21 semaines ; durée médiane de 14 semaines). Deux études ont été réalisées dans un environnement de soins primaires, deux ont été réalisées dans un contexte de soins secondaires et deux essais n'ont pas rapporté d'information quant au contexte.
La méta‐analyse était limitée par le faible nombre d'études ayant fourni des données pour chaque comparaison, et par l'hétérogénéité des critères de jugement rapportés dans les études incluses. Nous avons trouvé que la qualité des preuves synthétisées était faible ou très faible pour la plupart des critères de jugement pris en compte en raison d'un risque de biais (principalement une notification sélective), d'imprécisions et de résultats indirects. Bien que nous n'ayons pas trouvé de différence statistiquement significative ou de différence cliniquement pertinente entre les groupes en ce qui concerne un quelconque critère de jugement principal ou secondaire pris en compte dans cette revue, les données étaient insuffisantes pour exclure des effets bénéfiques ou délétères.
Conclusions des auteurs
La solidité des preuves n'est pas suffisante pour déterminer si la réduction de la dose de CI apporte un net bénéfice (en termes de réduction des effets indésirables) ou des préjudices supplémentaires (en termes de réduction de l'efficacité du traitement) pour les personnes adultes ayant un asthme bien contrôlé. Le faible nombre d'études pertinentes et la variété des mesures des résultats a limité le nombre de méta‐analyses que nous avons pu réaliser. Des ECR supplémentaires bien conçus et de plus longue durée sont nécessaires pour orienter la pratique clinique en ce qui concerne les stratégies de « réduction de la dose de CI » chez les patients ayant un asthme bien contrôlé.
PICO
Résumé simplifié
Diminution de la dose de corticoïdes inhalés pour les adultes asthmatiques
Contexte
L'asthme est une pathologie des voies respiratoires affectant plus de 300 millions d'adultes et d'enfants dans le monde. Les directives nationales et internationales recommandent d'augmenter la dose de corticoïdes inhalés (CI) par paliers pour rétablir un contrôle des symptômes en utilisant la dose plus faible possible, car un usage à long terme et à des doses élevées de CI comporte des risques d'effets secondaires. Pour les patients dont les symptômes asthmatiques sont contrôlés avec des doses modérées ou plus élevées de CI, il pourrait être possible de réduire la dose sans que le contrôle des symptômes ne soit affecté.
Question de la revue
Nous avons recherché des études (d'une durée minimale de 12 semaines) portant sur les personnes ayant un asthme bien contrôlé et comparant l'effet de la réduction de la dose de CI au maintien de celle‐ci. Les études devaient inclure des adultes âgés de 18 ans ou plus, dont l'asthme était bien contrôlé avec une dose moyenne de CI pendant un minimum de trois mois. Cela nous a également intéressé de déterminer si la prise d'autres médicaments inhalés pour l'asthme (les bêta agonistes à longue durée d'action ‐ BALA) pourrait avoir une influence sur les résultats. Deux auteurs de la revue ont passé au crible les résultats des recherches de façon indépendante et ont déterminé quelles études étaient pertinentes pour l'inclusion dans cette revue. Les informations pertinentes issues de ces études ont également été récoltées de manière indépendante par deux auteurs de la revue.
Résultats
Nous avons trouvé six études pertinentes pour cette revue. Dans l'ensemble, nous n'avons trouvé aucune différence entre les groupes (réduction de la dose de CI en comparaison avec le maintien de la dose de CI) en termes de crises d'asthme, de contrôle de l'asthme, de qualité de vie ou d'effets secondaires. Que des BALA aient été utilisés en même temps ou pas ne semblait pas affecter les résultats. Cependant, nous avons évalué la qualité des preuves comme étant faible ou très faible en raison du nombre limité d'études trouvées et parce que des problèmes étaient présents dans la manière dont les études ont été rapportées. Cela signifie que nous ne pouvons pas être certains de nos résultats ; des études supplémentaires sont nécessaires afin d'étudier ce sujet.
Conclusions
En conclusion, les preuves actuelles ne sont pas suffisamment bonnes pour établir si les patients peuvent réduire leur dose de CI sans perdre le contrôle de leur asthme. On ignore également si la diminution de la dose de CI permettrait de réduire la survenue d'effets secondaires. Des études supplémentaires sont nécessaires pour répondre à cette question.
Authors' conclusions
Summary of findings
| ICS dose reduction compared with no change in ICS dose (no concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (no concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 8 per 1000 | 14 per 1000 | OR 1.86 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Asthma control | Mean asthma control score in the no change in ICS dose group was 1.79. | MD 0.22 lower | ‐ | 150 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| All‐cause SAEs | 8 per 1000 | 9 per 1000 | OR 1.24 | 742 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Steroid‐related AEs | 31 per 1000 | 23 per 1000 | OR 0.76 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Health‐related quality of life (change from baseline) | Mean change from baseline in health‐related quality of life for the no change in ICS dose group was 0.02. | MD 0.21 lower | ‐ | 554 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence); MCID is 0.5 for AQLQ |
| Lung function, FEV1 (L) | Mean FEV1 in the no change in ICS dose group was 3.15 litres. | MD 0.02 litres lower | ‐ | 261 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Exacerbations requiring hospitalisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported by included studies |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). dThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (representative of specialist centres) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded twice for risk of bias (selective reporting and lack of blinding (subjective outcome)) and once for indirectness (single study representative of one setting and drug regimen). fThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ICS dose reduction compared with no change in ICS dose (concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 148 per 1000 | 186 per 1000 | OR 1.31 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring OCS (low‐quality evidence) |
| Asthma control (short asthma morbidity score) | Mean asthma control score was 1.43. | MD 0.16 higher | ‐ | 242 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to asthma control (low‐quality evidence) |
| All‐cause SAEs | 35 per 1000 | 22 per 1000 | OR 0.60 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to all‐cause SAEs (low‐quality evidence) |
| Steroid‐related AEs ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| St. George's Respiratory Scale score (change from baseline) Score 0‐100. 100 = greatest impact of chest disease on life; MCID is 4 units. | Mean change from baseline in HRQoL score was 7.4.c | MD 0.13 higher | ‐ | 229 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to HRQoL (low‐quality evidence) |
| Exacerbation requiring hospitalisation | 4 per 1000 | 14 per 1000 | OR 4.06 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring hospitalisation (low‐quality evidence) |
| Lung function, reduction in FEV1 (% predicted, change from baseline) | Mean change from baseline in % predicted FEV1 was ‐0.75%. | MD 2.45 lower | ‐ | 14 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to lung function (very low‐quality evidence) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cNote that study authors reported the change to the lowest SGRQ score during follow‐up. dThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (single study representative of one setting or drug regimen) and once for imprecision (wide CI). AE, adverse event; CI, confidence interval; FEV1, forced expiratory volume in one second; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; HRQoL, health‐related quality of life; ICS, inhaled corticosteroid; LABA, long‐acting beta agonist; MCID, minimum clinically important difference; MD, mean difference; OCS, oral corticosteroid; OR, odds ratio; RCT, randomised controlled trial; RR, risk ratio; SAE, serious adverse event. | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Asthma is a condition of the airways affecting adults and children. The number of diagnoses worldwide is estimated at more than 300 million (Global Asthma Network 2014; Partridge 2006). During an asthma attack (exacerbation), narrowing of the airways and excess mucus production occurs, causing symptoms of chest tightness, wheezing and breathlessness. Lung function tests typically show airflow obstruction with a low peak expiratory flow rate (PEFR), low forced expiratory volume in one second (FEV1) and a low FEV1/forced vital capacity (FVC) ratio (SIGN/BTS 2016). Lung function abnormalities improve and function may return to normal with treatment. Variability in measures of airflow is the hallmark of asthma.
Exacerbations of asthma can be triggered by environmental stimuli. In immunoglobulin E (IgE)‐mediated asthma (which may account for half of asthma cases) (Pearce 1999), indoor inhaled allergens such as house dust mite, cat and dog are often implicated (Custovic 2012). Other recognised environmental stimuli include air pollutants such as ozone and fine particulates, active and passive exposure to tobacco smoke (Xepapadaki 2009), industrial chemicals such as phthalates (Jaakkola 2008), isocyanates (Fisseler‐Eckhoff 2011), viral infections and cold air.
Description of the intervention
Acute episodes of asthma are treated with reliever therapy, usually a short‐acting beta2‐agonist (SABA). Inhaled corticosteroids (ICS) are used widely as first‐line therapy for patients with asthma that is uncontrolled on reliever therapy alone (SIGN/BTS 2016). Inhaled corticosteroids, which effectively relieve symptoms and prevent asthma exacerbations (Adams 2005; Adams 2008), are preferable to treatment by the oral route, as they lead to lower systemic absorption and fewer side effects. However, economic and social factors may contribute to non‐compliance with inhaler‐based therapies in some low‐ and middle‐income countries (GINA 2016). A variety of devices are available for delivery of ICS at differing doses and particle sizes. Generally, ICS are taken twice daily, although some newer preparations are taken once daily. For patients with persistent asthma, ICS are often taken alongside a long‐acting beta2 agonist (LABA), sometimes via a combination inhaler. ICS should be commenced at a dose appropriate to disease severity and control. National and international guidelines recommend titrating up the dose of ICS to gain symptom control at the lowest possible dose. Long‐term use of higher doses of ICS carries risk of systemic adverse events (i.e. side effects caused by the action of the steroid at sites other than the intended target ‐ the airways) (Lipworth 1999); however, lower doses of up to 800 mcg per day of beclomethasone dipropionate are considered tolerable (SIGN/BTS 2016). For patients whose asthma symptoms are controlled on moderate or higher doses of ICS, it may be possible to reduce the dose of ICS without compromising symptom control (Hawkins 2003).
How the intervention might work
ICS offer effective treatment for asthma owing to their anti‐inflammatory and decongestive effects on bronchial airways (Tse 1984). LABA function by decreasing bronchial hyperreactivity to physical and chemical stimuli and by relaxing bronchial smooth muscle (Lipworth 1992). Guidelines for asthma treatment focus on achieving, then maintaining, control while balancing the risks associated with long‐term medication (Bateman 2008). Once asthma control is achieved (e.g. as per GINA 2016 criteria), guidelines recommend 'stepping down' treatment to the lowest possible dose of ICS (SIGN/BTS 2016). These recommendations are based on known risks of systemic adverse effects (e.g. loss of bone density in adults, growth retardation in children) associated with long‐term use of high‐dose ICS (Colice 2006; Lipworth 1999; SIGN/BTS 2016).
Why it is important to do this review
Patients with persistent asthma are generally treated with a high dose of ICS or with a combination of ICS and LABA (Ducharme 2010). Two separate Cochrane reviews (Ahmad 2015; Kew 2015) have synthesised the evidence for removing the LABA from the ICS/LABA combination when treating children and adults with asthma. Stepping down the dose of ICS may reduce the likelihood of unwanted side effects, particularly the systemic side effects of steroid use (Colice 2006; SIGN/BTS 2016). Indeed, current British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN) guidelines recommend that ICS should be titrated to the lowest possible dose at which effective asthma control is maintained (SIGN/BTS 2016). However, debate continues regarding the best protocol for stepping down ICS treatment, particularly with respect to the lowest acceptable dose of ICS and the rate of down‐titration (Rogers 2012). Therefore, synthesis of the evidence for 'stepping down ICS therapy' is important. Finally, ICS are among the most widely prescribed repeat medications and thus account for a substantial proportion of drug spending in the United Kingdom and in other countries (NHS 2013). Any strategy to reduce the use of ICS may thus represent an important cost‐saving measure.
Objectives
To evaluate the evidence for stepping down ICS treatment in adults with well‐controlled asthma who are already receiving a moderate or high dose of ICS.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomised controlled trials (RCTs) of at least 12 weeks' duration. We included studies reported as full text, those published as abstract only and unpublished data. We did not exclude studies on the basis of language or blinding.
Types of participants
We included adults (aged ≥ 18 years) whose asthma was well controlled for a minimum of three months on at least a moderate dose of ICS (i.e. a dose of at least 400 mcg beclomethasone dipropionate (BDP) or equivalent) (SIGN/BTS 2016). We classified asthma control according to predefined criteria, for example, as per the criteria described in GINA 2016 (i.e. daily symptoms twice or less often per week, use of rescue inhaler twice or less often per week, no nocturnal symptoms and no limitation to daily activities), or as per the asthma control questionnaire (i.e. a score less than 1.5). We excluded participants who had the following comorbidities/characteristics: chronic obstructive pulmonary disease (COPD), bronchiectasis or any other respiratory comorbidity.
If studies enrolled adults and adolescents (aged 10 to 17 years) (WHO 2014), and data were not reported separately, we included the study if the mean age of participants in the intervention and comparator groups was 18 years or older.
Types of interventions
We included trials that compared the following.
-
Reduction in the dose of ICS versus no change in the dose of ICS, in people with asthma whose condition was well controlled on at least a moderate dose of any ICS, but who were not taking a concomitant LABA.
-
Reduction in the dose of ICS versus no change in the dose of ICS, in people with asthma whose condition was well controlled on at least a moderate dose of any ICS and who were taking a concomitant LABA.
For both comparisons, a different ICS could be used in the intervention and comparator groups, provided both groups used the same beclomethasone dipropionate (BDP) equivalent dose of ICS (≥ 400 mcg) before randomisation. We excluded studies in which treatment with ICS was stopped, as this relates to a different clinical question. We included studies that permitted use of short‐acting reliever medications, provided they were not part of the randomised treatment.
For the latter comparison (patients taking a concomitant LABA), several studies included participants who used combination (ICS/LABA) inhalers; we excluded studies in which randomised treatment included a concurrent dose reduction of both ICS and LABA, because this strategy relates to a different clinical question. We also excluded studies if randomised treatment involved a step‐down to single inhaler therapy (i.e. 'single inhaler maintenance and reliever therapy' (SMART)) with a lower dose of ICS, because this also relates to a different clinical question that is addressed in another review (Kew 2013).
Types of outcome measures
Primary outcomes
-
Exacerbations requiring oral corticosteroids
-
Asthma control (measured on a validated scale; preferred measure is the Asthma Control Questionnaire (ACQ) score)
-
All‐cause serious adverse events
-
Steroid‐related adverse events
Secondary outcomes
-
Health‐related quality of life (measured on a validated scale; preferred measure is the Asthma Quality of Life Questionnaire (AQLQ) score)
-
Lung function indices (preferred measure is trough FEV1)
-
Exacerbations requiring hospitalisation
-
Exacerbations requiring an emergency department visit
-
Mortality
Reporting one or more of these outcomes in a trial was not an inclusion criterion.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Group's Trials Search Co‐ordinator. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and via handsearches of respiratory journals and meeting abstracts (Appendix 1). We searched all records in the Cochrane Airways Group Specialised Register using the search strategy presented in Appendix 2.
We also conducted a search of ClinicalTrials.gov (https://clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/). We searched all databases from their inception to July 2016, and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
On 4 October 2016, we searched for errata and retractions from included studies published in full text on PubMed.
Data collection and analysis
Selection of studies
Two review authors (DE, NH) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. Two review authors (DE, NH or IC) independently retrieved and screened the full‐text reports/publications to identify studies for inclusion, and to identify and record reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, we consulted a third review author (PM). We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram and the Characteristics of excluded studies table.
Data extraction and management
We used a data collection form to collect information on study characteristics and outcome data after piloting the form on at least one study included in the review. Two review authors (DE, NH) extracted the following study characteristics from included studies in duplicate.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, dates of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria and details of criteria for stepping down treatment (clinical, e.g. symptoms, lung function, exacerbation history; airway responsiveness, e.g. mannitol challenge; inflammatory biomarkers, e.g. exhaled nitric oxide).
-
Interventions: intervention, comparison, concomitant medications, excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, time points reported.
-
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (IC, DE) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving a third review author (PM). One review author (DE) transferred data into Cochrane's statistical software, Review Manager 2014. We double‐checked that data were entered correctly by comparing data presented in the systematic review against study reports. A second review author (NH) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (DE, PM) independently assessed risk of bias for each study using Cochrane's tool for assessing risk of bias (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (IC or NH).
We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in a 'Risk of bias table'. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from that observed for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias table'.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assesment of bias in conducting the systematic review
We conducted the review according to this published protocol and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs), and continuous data as mean differences (MDs). We entered data presented as a scale with a consistent direction of effect. When included studies reported dichotomous data as risk ratios (RRs) or hazard ratios (HRs), we calculated and presented the ORs.
We undertook meta‐analyses only when this was meaningful (i.e. when treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A vs placebo and drug B vs placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
When the duration of studies included in an analysis varied by more than three months, we performed sensitivity analyses to examine whether study duration influenced the treatment effect. If an influence of study duration was apparent, we re‐expressed ORs as a variety of numbers needed to treat (NNTs) across a range of assumed control risks (control group risks are likely to vary in studies of different duration) (Higgins 2011).
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. the number of participants admitted to hospital at least once rather than the number of admissions per participant). We planned to also analyse exacerbations leading to admission or to a course of oral steroids as rate ratios and time to event data, if these data were presented.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as "abstract only"). When this was not possible, and missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity (i.e. I2 ≥ 50%), we reported this and explored possible causes by performing prespecified subgroup analysis.
Assessment of reporting biases
As we included only six studies, we were not able to pool more than 10 trials to create a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model and planned to perform a sensitivity analysis using a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using all of the outcomes listed above (Types of outcome measures), with the exception of mortality and exacerbations requiring an emergency department visit. We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions and GRADEpro (GRADEproGDT) software (http://www.guidelinedevelopment.org/). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid readers' understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Rate of dose reduction (e.g. 25% dose reduction vs 50% dose reduction).
-
Separate inhaler therapy versus combination inhaler therapy (i.e. ICS/LABA).
We planned to use the following primary outcomes in subgroup analyses.
-
Exacerbations requiring oral corticosteroids.
-
Asthma control.
We used the formal test for subgroup interactions provided in Review Manager 2014.
Sensitivity analysis
We planned to carry out the following sensitivity analyses.
-
Unpublished data (i.e. no peer‐reviewed full‐text publication available).
-
Studies at unclear or high risk of bias for blinding.
-
Fixed‐effect versus random‐effects models.
-
Duration of included studies (e.g. short term (less than three months) vs longer term (more than three months)).
-
Studies at high risk of any other bias versus those at low risk of any other bias.
Results
Description of studies
The Characteristics of included studies table presents details of the included studies. We reported in the Characteristics of excluded studies table reasons for exclusion of studies considered during review of full‐text articles.
Results of the search
We identified 983 records by performing electronic searches of bibliographic databases and an additional 53 records by searching clinicaltrials.gov. Of a total of 1034 records (two duplicates removed), we excluded most (n = 972) upon screening titles and abstracts. We examined full‐text articles of the remaining 62 records and excluded 53 records (reporting 41 studies), primarily because the intervention did not meet the criteria for inclusion in this review (n = 27 studies). The interventions considered were typically complex and difficult to separate from other components; this resulted in a high rate of exclusions at full‐text review stage. Other reasons for exclusion at this stage included 'wrong study design' (n = 8), 'wrong comparator' (n = 3), 'wrong route of administration' (n = 2) and 'wrong patient population' (n = 1). The remaining 11 records reported the findings of six studies, which we included in this review. Figure 1 depicts the flow of information through the different stages of this systematic review.
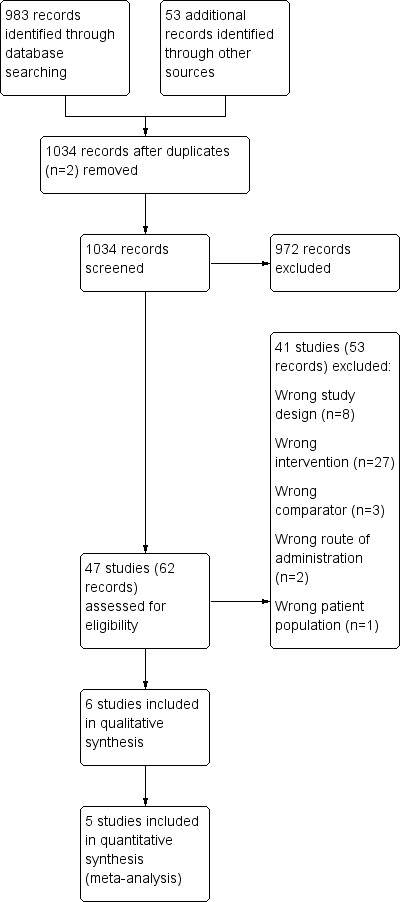
Study flow diagram.
Included studies
Six studies met the inclusion criteria and contributed data to the analyses (Godard 2008; Gunn 1997; Hawkins 2003; Juniper 1991; Knox 2007; Magnussen 2000). The six included studies randomised a total of 1654 participants (ICS dose reduction, no concomitant LABA: n = 892; ICS dose reduction, concomitant LABA: n = 762). The largest (Gunn 1997) and smallest (Juniper 1991) studies included 631 and 28 participants, respectively. All included studies were reported as full peer‐reviewed articles.
Methods
All included studies were RCTs with a parallel design that compared a fixed dose of ICS versus a reduced dose of ICS. Two studies included three arms (Godard 2008; Gunn 1997), and in one of these, only two out of three arms were relevant to this review (Godard 2008). Five studies were performed as double‐blind, and one study was open‐label (Gunn 1997). Five studies reported a run‐in period (duration two to eight weeks), and one study was an extension of a previous 12‐month study (Juniper 1991). Duration of the treatment period ranged from 12 to 52 weeks (mean duration 21 weeks; median duration 14 weeks). Outcome data were reported at the last time point reported for each study. Most studies were performed in Europe (Godard 2008; Gunn 1997; Hawkins 2003; Knox 2007; Magnussen 2000), and one study in Canada (Juniper 1991). Two studies were conducted in the setting of primary care (Gunn 1997; Hawkins 2003), two were conducted in the secondary care setting (Juniper 1991; Magnussen 2000) and two reported no information on setting (Godard 2008; Knox 2007).
Participants
We included studies that recruited adult participants aged ≥ 18 years or in which most participants were adults. When reported, the age range of participants across included studies was 16.2 to 86 years (Godard 2008; Gunn 1997; Hawkins 2003; Knox 2007); in the two studies for which the age range was not reported, the mean age of participants was approximately 40 years (Juniper 1991; Magnussen 2000). Participants in the included studies had asthma that was generally well controlled by regular preventive therapy (i.e. step 2 of the BTS/SIGN guidelines; SIGN/BTS 2016) (Gunn 1997; Juniper 1991; Knox 2007; Magnussen 2000), with the use of high‐dose ICS (≥ 1000 μg BDP) (Hawkins 2003) or with an add‐on therapy (i.e. step 3 of the BTS/SIGN guidelines) (Godard 2008). When reported, most participants were non‐smokers (Godard 2008; Hawkins 2003; Knox 2007).
Interventions
All included studies compared a 50% to 60% reduction in dose of ICS versus no change in ICS dose. In terms of the type and baseline dose of ICS, studies included a variety of comparisons: fluticasone propionate (FP) 250 μg twice daily versus ciclesonide 160 μg once daily (representing a 50% reduction according to Global Initiative for Asthma (GINA) guidelines) (Knox 2007); a 50% reduction in dose of any ICS (as used before the study) (Hawkins 2003); salmeterol/fluticasone combination (SFC) 50/100 μg twice daily versus no change (SFC 50/250 μg twice daily) (Godard 2008); a 50% reduction in dose of budesonide versus no change in budesonide dose (any dose) (Juniper 1991); and chlorofluorocarbon beclomethasone 1000 μg/day versus hydrofluoroalkane beclomethasone 400 μg/day (< 50% reduction) (Magnussen 2000). The study comparison reported by Gunn and colleagues (Gunn 1997) was as follows: Participants on an initial high dose of ICS (budesonide 400 μg twice daily or beclomethasone 400 μg twice daily or beclomethasone 500 μg twice daily delivered via a pressurised metered‐dose inhaler (pMDI) and spacer device) were randomised to receive budesonide 200 μg twice daily via a Turbohaler, or 400 μg once daily (i.e. both groups represent a halving of the initial ICS dose). Participants on an initial low dose of ICS (budesonide or beclomethasone 200 μg twice daily) were randomised to receive budesonide 100 μg twice daily via a Turbuhaler, or 200 μg once daily (i.e. both groups represent a halving of the initial ICS dose). There was no change in initial dose of budesonide or beclomethasone in the control group (Gunn 1997).
Inhaler devices varied across studies but were consistent between intervention and control groups in at least three of the six included studies. One study used a Diskus dry powder inhaler (Godard 2008); another used the Autohaler, a breath‐actuated metered‐dose inhaler (MDI) (Magnussen 2000); one study used a hydrofluoroalkane MDI (Knox 2007); another did not report the device used (Juniper 1991); one study permitted the use of an MDI or a dry powder inhaler as long as the same device was used throughout the study (Hawkins 2003); and another used the Turbohaler for participants in the intervention group and an MDI for those in the comparator group (Gunn 1997) and considered the two inhaler types to be equivalent for a given dose.
Outcomes
Outcomes reported were inconsistent across included studies. All studies reported data on asthma control, although several studies used scales that were not validated and thus did not contribute data to the meta‐analysis. Most studies reported exacerbations requiring oral corticosteroids (OCS) (Godard 2008; Hawkins 2003; Knox 2007; Magnussen 2000), all‐cause serious adverse events (SAEs) (Godard 2008; Gunn 1997; Hawkins 2003; Knox 2007) and lung function (Godard 2008; Gunn 1997; Juniper 1991; Knox 2007; Magnussen 2000), although reported measures of lung function varied across studies. Two studies reported quality of life (QoL): One study used both the Juniper Asthma QoL Questionnaire and the Dupuy Psychological General Well Being Index (Gunn 1997), and the second study used the EuroQoL questionnaire and the St George's Respiratory Questionnaire (Hawkins 2003). Steroid related AEs and exacerbations requiring hospitalisation were each reported by two studies (Knox 2007 and Magnussen 2000; Godard 2008 and Hawkins 2003, respectively). Mortality and exacerbations requiring an emergency department visit were each reported by one study (Godard 2008 and Hawkins 2003, respectively).
Excluded studies
We excluded 53 references (related to 41 studies) following assessment of full‐text articles (Characteristics of excluded studies). We excluded 27 studies as they used an intervention that was not relevant to this review (e.g. a dose reduction of ICS was not used, or a concomitant reduction in ICS and LABA was used). Eight studies were excluded because they used a study design not appropriate for this review (e.g. cross‐over or non‐randomised design). We excluded six studies because they used a comparator not relevant to this review (n = 3; e.g. a dose reduction in the control group) or a route of administration not relevant to this review was used (n = 2; e.g. the intervention was OCS, not ICS) or because the patient population studied was not relevant (n = 1; e.g. participants were children).
Risk of bias in included studies
Please refer to the Characteristics of included studies tables for details on risk of bias and for supporting evidence for each study. Figure 2 provides a summary of risk of bias judgements, presented by study and domain (sequence generation, allocation concealment, blinding, incomplete data, selective reporting and 'other'). Figure 3 depicts the risk of bias for each domain, presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
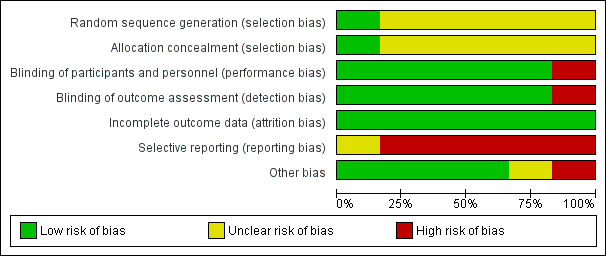
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Most studies (five of six) provided insufficient information regarding methods of random sequence generation and concealment of treatment allocation to allow a judgement on risk of bias (Godard 2008; Gunn 1997; Juniper 1991; Knox 2007; Magnussen 2000); therefore, the risk of bias for these studies was unclear. One study (Hawkins 2003) used a computer‐generated randomisation sequence and concealed allocation method, and was considered to be at low risk for selection bias.
Blinding
We considered five of six studies (Godard 2008; Hawkins 2003; Juniper 1991; Knox 2007; Magnussen 2000) to have low risk of performance and detection bias, as participants, personnel and outcome assessors were blinded to treatment allocation through adequate methods. One study (Gunn 1997) used an open‐label design, in which participants, personnel and outcome assessors were not blinded to treatment allocation; we considered this study to be at high risk of both performance and detection bias.
Incomplete outcome data
We considered all studies to be at low risk of attrition bias on the basis of low and balanced rates of participant withdrawal, which were adequately documented in the trial report.
Selective reporting
We judged five studies (Godard 2008; Gunn 1997; Hawkins 2003; Juniper 1991; Magnussen 2000) to be at high risk of reporting bias because no study protocol was available and there appeared to be either non‐standard presentation of the data or selective reporting of data that were likely recorded. One study (Knox 2007) appeared to report a fairly comprehensive set of outcomes (i.e. exacerbations, steroid‐related AEs, all‐cause SAEs, lung function and asthma control); however, a protocol was not available, so we judged the risk of bias as unclear.
Other potential sources of bias
We judged four studies to be at low risk of other bias, as no other concerns were identified (Hawkins 2003; Juniper 1991; Knox 2007; Magnussen 2000). We considered Godard 2008 to be at high risk of other bias because, contrary to the methods described, investigators randomised a relatively high proportion of participants who had asthma that was not well controlled and included them in the full analysis set. We judged Gunn 1997 to be at unclear risk of bias because there appeared to be some changes in the inhaler used to deliver the ICS at the same time as changes in dose, although we noted that the two inhaler types were considered equivalent for a given dose.
Effects of interventions
See: Summary of findings for the main comparison ICS dose reduction compared with no change in ICS dose (no concomitant LABA) for adults with asthma; Summary of findings 2 ICS dose reduction compared with no change in ICS dose (concomitant LABA) for adults with asthma
Structure of the analysis
As per the protocol, we chose to analyse participants who were receiving a concomitant LABA separately from those who were not receiving a concomitant LABA.
Structure of the meta‐analysis
We created two main comparison headings within the analysis tree. For each comparison, we elected to perform a meta‐analysis only when interventions and outcomes were sufficiently similar for pooling of the data.
Participants not taking concomitant a LABA: ICS reduction versus no change in ICS dose
This comparison comprised all studies that compared a reduction in the dose of ICS versus no change in ICS dose among participantsnot taking a concomitant LABA (Gunn 1997; Knox 2007; Magnussen 2000).
Participants taking a concomitant LABA: ICS reduction versus no change in ICS dose
This comparison comprised all studies that compared a reduction in the dose of ICS versus no change in ICS dose among participants taking a concomitant LABA (Godard 2008; Hawkins 2003; Juniper 1991)
Structure of the narrative synthesis
In the following sections, we present a narrative summary of study results according to the prespecified outcomes. We present primary outcomes (exacerbations requiring OCS, asthma control, all‐cause SAEs, steroid‐related AEs) followed by secondary outcomes (health‐related QoL, lung function, exacerbations requiring hospitalisation, exacerbations requiring an emergency department visit, mortality). For each outcome, we describe the effect of the intervention among participants not taking a concomitant LABA followed by the effect of the intervention among participants taking a LABA.
Primary outcomes
Exacerbations requiring oral corticosteroids
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring treatment with oral steroids (odds ratio (OR) 1.86, 95% confidence interval (CI) 0.16 to 21.09; n = 261 participants, two studies; I2 = 0%; Analysis 1.1; Figure 4). For people who stepped down their dose of ICS, we estimated that six more people per 1000 would have an exacerbation requiring oral steroids, but the confidence intervals ranged from seven fewer to 132 more people per 1000. We rated the quality of the evidence as very low after downgrading twice for imprecision (no events were reported by one of the contributing studies, and confidence intervals include the null effect (risk ratio (RR) 1.0) and appreciable benefit (RR 0.75) or harm (RR 1.25)) and once for indirectness, as the evidence was based on studies operating out of specialist centres.
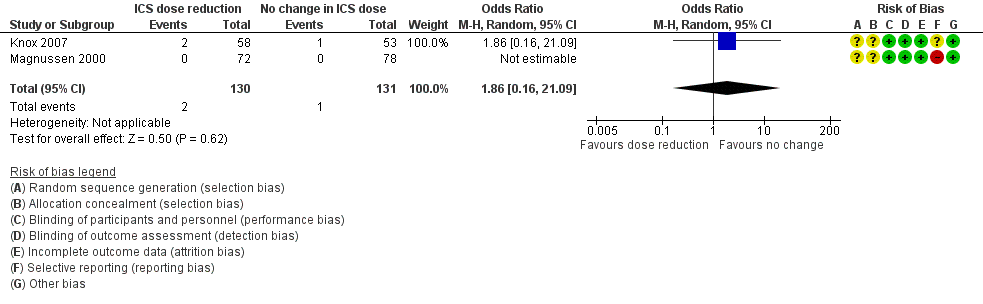
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.1 Exacerbation requiring OCS.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring treatment with oral steroids (OR 1.31, 95% CI 0.82 to 2.08; n = 569 participants; two studies; I2 = 0%; Analysis 2.1; Figure 5). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 38 more people per 1000 would have an exacerbation requiring oral steroids, but the confidence intervals ranged from 23 fewer to 118 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.1 Exacerbation requiring OCS.
Asthma control
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to asthma control (mean difference (MD) ‐0.22, 95% CI ‐1.05 to 0.61; n = 150 participants; one study; Analysis 1.2). We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for indirectness (single study representative of a single setting and drug regimen).
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to asthma control as measured by the short asthma morbidity score (change from baseline: MD 0.16, 95% CI ‐0.34 to 0.66; n = 242 participants; one study; scale 0 (perfect control) to 8 (very poor control); Analysis 2.2). We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for indirectness (single study representative of a single setting and drug regimen). Nor did stepping down the dose of ICS result in clear benefit or harm with respect to asthma control as measured by the Asthma Severity Questionnaire (MD 1.13, 95% CI ‐0.24 to 2.49; scale 0 (best control) to 6 (worst control); Analysis 2.3). We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (single study representative of a single setting and drug regimen) and once for imprecision (confidence intervals include the null effect (MD 0) and appreciable harm (MD 1.5)).
All‐cause SAEs
ICS stepdown, no concomitant LABA
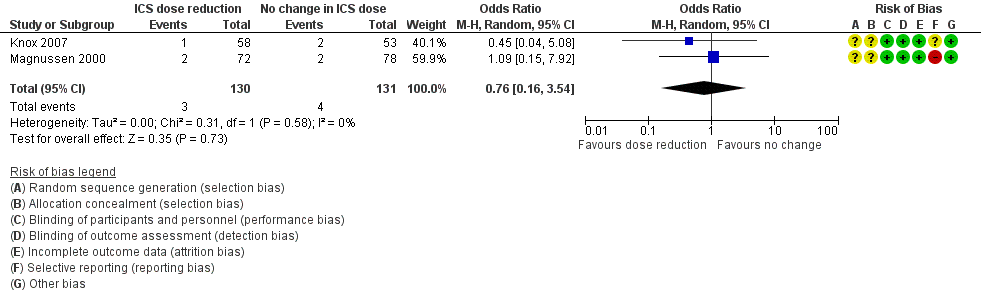
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to SAEs (OR 1.24, 95% CI 0.25 to 6.25; n = 742 participants; two studies; I2 = 5%; Analysis 1.3; Figure 6). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 1 more person per 1000 would have an SAE, but confidence intervals ranged from six fewer to 37 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include the null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.3 All‐cause SAEs.
ICS stepdown, concomitant LABA
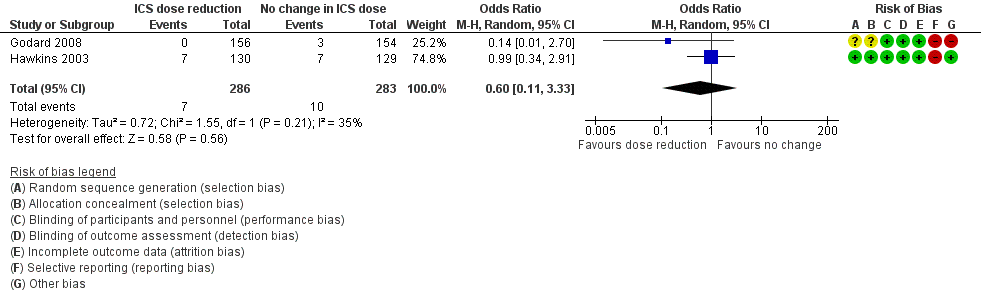
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to SAEs (OR 0.60, 95% CI 0.11 to 3.33; n = 569 participants; two studies; I2 = 35%; Analysis 2.4; Figure 7). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 13 fewer people per 1000 would have an SAE, but the confidence intervals ranged from 31 fewer to 74 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.4 All‐cause SAEs.
Steroid‐related AEs
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to steroid‐related AEs (OR 0.76, 95% CI 0.16 to 3.54; n = 261 participants; two studies; I2 = 0%; Analysis 1.4; Figure 8). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that eight fewer people per 1000 would have a steroid‐related AE, but confidence intervals ranged from 26 fewer to 69 more people per 1000. We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (representative of specialist centres) and once for imprecision (confidence intervals include the null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.4 Steroid‐related AEs.
ICS stepdown, concomitant LABA
No included studies reported data for steroid‐related AEs.
Secondary outcomes
Health‐related quality of life
ICS stepdown, no concomitant LABA
There was a statistically significant difference in health‐related quality of life (change from baseline) between groups as measured by the Asthma Quality of Life Questionnaire (change from baseline: MD ‐0.21, 95% CI ‐0.33 to ‐0.09; n = 554 participants, one study; scale 0 (worst) to 7 (best); Analysis 1.5). However, the mean difference and 95% confidence limits were below the minimal clinically important difference (MCID) of 0.5, indicating no clinically relevant difference between groups. We rated the quality of the evidence as very low after downgrading twice for risk of bias (selective reporting and lack of blinding for a subjective outcome measure) and once for indirectness (single study representative of a single setting and drug regimen).
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no benefit or harm with respect to health‐related quality of life as measured by St George's Respiratory Scale (change from baseline: MD 0.13, 95% CI ‐2.80 to 3.06; n = 229 participants, one study; scale 0 to 100 (greatest impact of chest disease on life); Analysis 2.6) or the EuroQoL (change from baseline: MD 2.32, 95% CI ‐1.64 to 6.28; n = 219 participants, one study; scale 0 to 100 (best imaginable health state); Analysis 2.5). With regards to the St George's Respiratory Scale, the mean difference and 95% confidence limits were below the MCID of 4 units, indicating no clinically relevant difference between groups. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for indirectness (single study representative of a single setting and drug regimen).
Lung function
ICS stepdown, no concomitant LABA
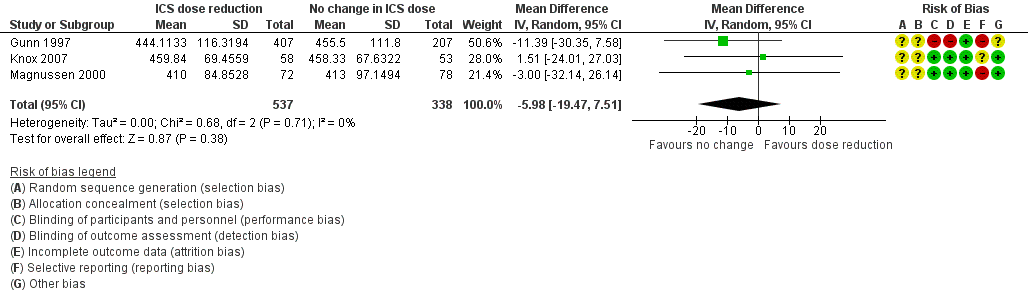
Stepping down the dose of ICS resulted in no benefit or harm with respect to lung function. There was no statistically significant change in percent predicted FEV1 (MD ‐0.02, 95% CI ‐0.12 to 0.08; n = 261 participants, two studies; I2 = 0%; Analysis 1.7; Figure 9) nor in morning PEFR (MD ‐5.98 L/min, 95% CI ‐19.47 to 7.51; n = 875 participants, three studies; I2 = 0%; Analysis 1.6; Figure 10). We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include the null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.7 Lung function, FEV1 (L).
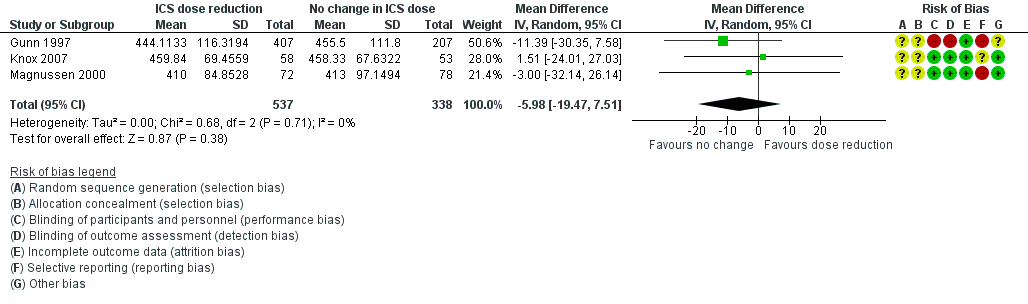
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.6 Lung function, PEFR morning (L/min).
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no benefit or harm with respect to lung function. There was no statistically significant between‐group differences for change in percent predicted FEV1 from baseline (MD ‐2.45, 95% CI ‐8.88 to 3.98; n = 14 participants, one study; Analysis 2.8) nor for change from baseline in morning PEFR (MD ‐4.54, 95% CI ‐12.08 to 3.00; n = 310 participants, one study; Analysis 2.7). We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (single study representative of a single setting and drug regimen) and once for imprecision (wide CI).
Exacerbations requiring hospitalisation
ICS stepdown, no concomitant LABA
No studies reported the number of participants requiring hospitalisation.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring hospitalisation (OR 4.06, 95% CI 0.45 to 36.86; n = 569 participants, two studies; I2 = 0%; Analysis 2.9; Figure 11). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 10 more people per 1000 would have an exacerbation requiring hospitalisation, but the confidence intervals ranged from 2 fewer to 112 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (wide CI).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.9 Exacerbation requiring hospitalisation.
Exacerbations requiring an emergency department visit
ICS stepdown, no concomitant LABA
No studies reported the number of participants requiring an emergency department visit.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring an emergency department visit (OR 2.00, 95% CI 0.18 to 22.33; n = 259 participants, one study; Analysis 2.10). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 7 more people per 1000 would have an exacerbation requiring an emergency department visit, but confidence intervals ranged from 7 fewer to 141 more people per 1000. We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for imprecision (wide CI) and once for indirectness (single study representative of a single setting and drug regimen).
Mortality
ICS stepdown, no concomitant LABA
No studies reported mortality data.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm in terms of mortality; the single study reporting data (N = 310 participants; Analysis 2.11) reported no deaths in either group. We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (single study representative of a single setting and drug regimen) and once for imprecision (no events reported).
Subgroup analyses
Magnitude of dose reduction
Review authors did not perform this prespecified subgroup analysis for either comparison because all of the included studies represented a 50% to 60% reduction in ICS dose.
Separate ICS/LABA inhalers versus combination ICS/LABA inhaler
This was relevant only to the second comparison (participants who were permitted to receive a concomitant LABA).
For exacerbations requiring oral corticosteroids, the use of individual ICS and LABA inhalers (Hawkins 2003) versus a combined inhaler (Godard 2008) did not appear to influence the overall OR (1.31, 95% CI 0.82 to 2.08) because results of the two contributing studies were comparable (OR 1.29, 95% CI 0.75 to 2.23 (Hawkins 2003) and OR 1.34, 95% CI 0.55 to 3.28 (Godard 2008), respectively).
We could not perform a subgroup analysis for asthma control as only one study contributed data to each measure of asthma control (short asthma morbidity score (Hawkins 2003) and asthma severity questionnaire (Juniper 1991)).
Sensitivity analyses
It was not possible for review authors to conduct the planned sensitivity analyses because of the paucity of included studies contributing to each outcome examined.
Discussion
Summary of main results
We included six studies, which randomised a total of 1654 participants (inhaled corticosteroid (ICS) dose reduction, no concomitant long‐acting beta agonist (LABA): n = 892 participants, three randomised controlled trials (RCTs); ICS dose reduction, concomitant LABA: n = 762, three RCTs). All included studies were RCTs with a parallel design that compared a fixed dose of ICS with a 50% to 60% reduction in the dose of ICS among adult participants with well‐controlled asthma. The duration of treatment ranged from 12 to 52 weeks (mean duration 21 weeks; median duration 14 weeks). Two studies were performed in the setting of primary care, two were performed in the secondary care setting and two provided no information on setting.
Meta‐analysis was hampered by the small number of studies that contributed to each comparison, combined with differences among outcomes reported in the included studies. However, a low level of heterogeneity was observed in the meta‐analyses that were performed. We found no statistically significant differences between groups (step‐down of ICS vs no change in ICS) with respect to any of the primary or secondary outcomes considered in this review and thus were unable to determine whether stepping down the dose of ICS in adults with asthma (compared with maintaining the previous dose of ICS) confers overall benefit. On one hand, we did not identify a statistically significant between‐group difference for measures of effectiveness such as asthma control, lung function or the number of participants experiencing exacerbations, which would support the guideline‐recommended use of an ICS dose reduction for patients with well‐controlled asthma. However, we noted a numerical trend towards a greater number of participants experiencing exacerbations, and we observed no benefit in terms of other safety outcomes. Moreover, we rated the quality of the evidence as generally low or very low, which means that we cannot be confident in the effect estimates (see below). Finally, whether concomitant treatment with a LABA influences the benefit/harm ratio for stepping down ICS remains unclear.
Overall completeness and applicability of evidence
Two of the included studies were performed in the primary care setting, two in the secondary care setting and in two cases the setting was not reported. Each comparison (± LABA) included one study in each of these three categories (primary care, secondary care, not stated); however, owing to the small number of studies contributing to each outcome, some outcomes may have been more representative of a particular setting. When this was the case, we accounted for this factor by downgrading the quality of the evidence for indirectness. Furthermore, single studies (representative of a single regimen or treatment duration) contributed to several of the outcomes, and most of the meta‐analyses comprised only two studies. Therefore, our results may be relevant to the particular treatment regimens represented in the individual studies. Finally, our results are relevant only to adult patients. It is possible that potential harms due to systemic effects associated with long‐term ICS use might be more relevant to children. To examine this, we would need to consider including paediatric studies in future iterations of this review, or in a separate review.
One of the concerns associated with stepping down the dose of ICS in patients with well‐controlled asthma is possible slow deterioration in asthma control over time as bronchial hyper‐responsiveness slowly returns. Moreover, long‐term exposure to steroids may result in the development of systemic side effects such as loss of bone density in adults and growth retardation in children (Colice 2006; Lipworth 1999; SIGN/BTS 2016). The mean duration of the included studies was 21 weeks (median duration 14 weeks), which is potentially insufficient for detecting long‐term deterioration in asthma control/lung function or for adequately assessing long‐term safety outcomes.
Quality of the evidence
Few relevant studies met the prespecified criteria for inclusion; this fact, combined with the use of varied outcome measures across included studies, limited the number of meta‐analyses that we could perform. In terms of risk of bias, the included studies were generally of moderate quality, although selective reporting introduced risk of bias in five out of six included studies. Furthermore, it was not clear whether adequate methods of randomisation sequence generation or concealment of allocation were used in all but one study.
We assessed the quality of evidence in this review using GRADE (Higgins 2011) and GRADEpro software; our findings in the 'Summary of findings tables'. summary of findings Table for the main comparison presents our findings for the first comparison (stepping down ICS vs no change in ICS, in patients not receiving a concomitant LABA), and summary of findings Table 2 presents our findings for the second comparison (stepping down ICS vs no change in ICS, in patients receiving a concomitant LABA). In summary, for both comparisons, we assessed the quality of the synthesised evidence as low or very low for most outcomes because of risk of bias (principally, selective reporting), imprecision (few events in a small number of studies, or wide confidence intervals) and indirectness (single studies representative of a single setting or drug regimen). Based on the quality of the evidence, we cannot be confident about the effect estimates presented in this review.
Potential biases in the review process
We followed standard procedures as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to minimise bias in the review process. With regard to the search process, the Cochrane Airways Group Information Specialist designed and conducted the main electronic search, two review authors independently sifted the search results and two review authors (one with expert clinical knowledge) reviewed the full‐text results. Consistent with Cochrane methods, we excluded no trials on the basis of language, publication status or outcomes reported, so we are confident that we identified all potentially relevant evidence from RCTs. In terms of our findings and conclusions, two review authors independently performed all steps in the review process for which a subjective decision was required (e.g. selection of studies, extraction of data, assessment of risk of bias, assessment of the overall quality of evidence using GRADE), and, if necessary, a third review author assisted in resolving disagreements. Finally, this review has undergone editorial and peer review such that the opinion of independent external experts has been considered. Together, these factors should ensure that our conclusions fairly represent the results synthesised during the review process.
Agreements and disagreements with other studies or reviews
Our findings agree well with those of a systematic review (Gionfriddo 2015) that examined the evidence for stepping down the dose of ICS from a scheduled regimen to an as‐needed basis. Those review authors found insufficient evidence to associate stepping down ICS dose with an effect on the number of asthma exacerbations. In contrast, the authors found some evidence for fewer symptom‐free days in patients who used an ICS on an as‐needed basis. In another systematic review, Hagan and colleagues similarly found that asthma exacerbations were statistically no more likely among individuals who reduced ICS than among those who maintained their ICS dose (Hagan 2014). The Hagan review included studies of both adults and children and permitted step‐down to ICS on an as‐needed basis. Nevertheless, their findings are consistent with those of our review.
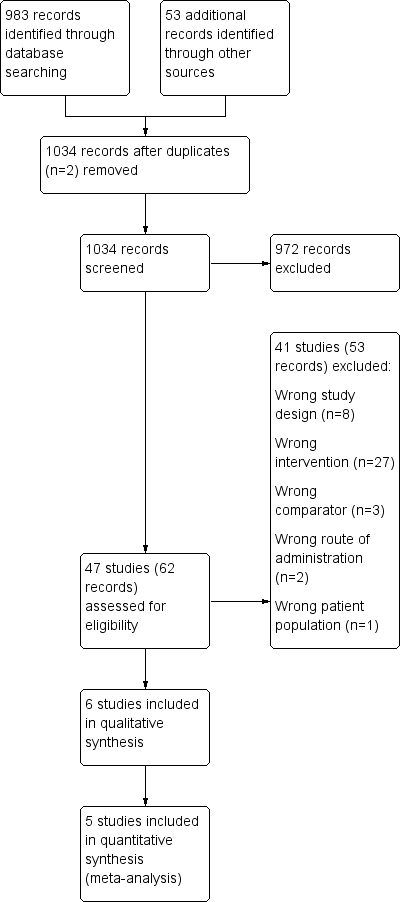
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
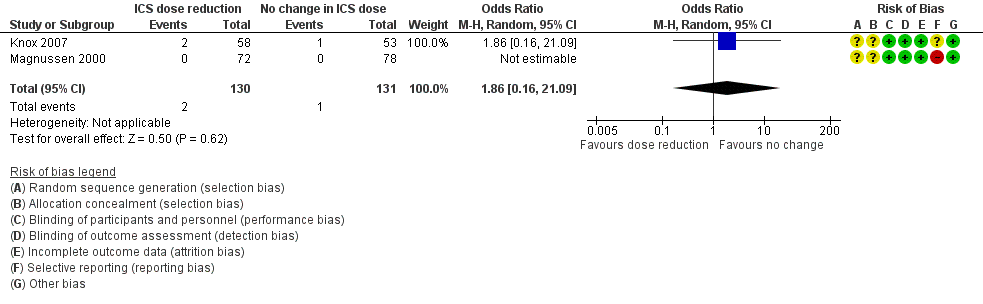
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.1 Exacerbation requiring OCS.
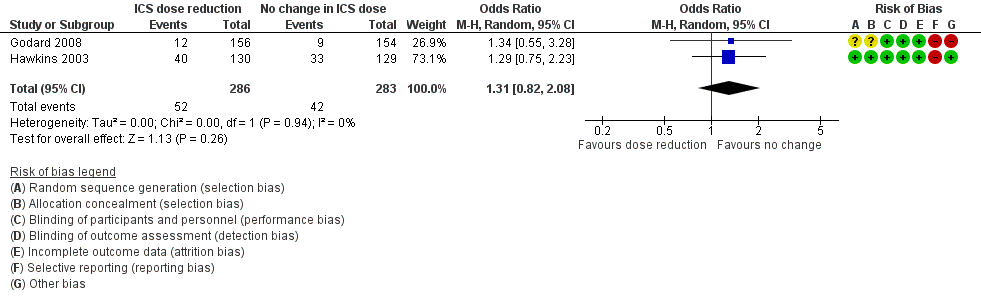
Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.1 Exacerbation requiring OCS.
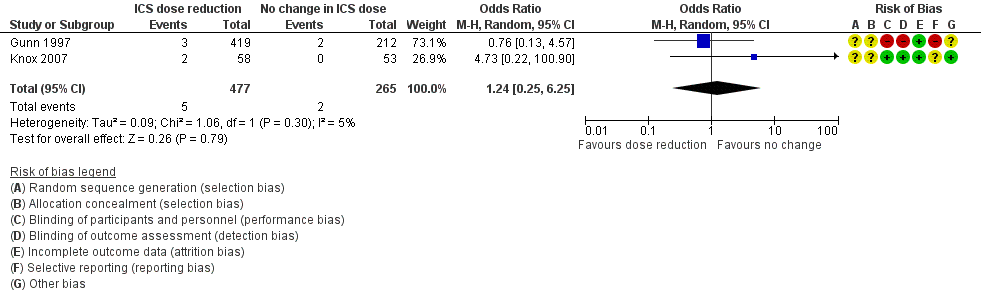
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.3 All‐cause SAEs.
Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.4 All‐cause SAEs.
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.4 Steroid‐related AEs.

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.7 Lung function, FEV1 (L).
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.6 Lung function, PEFR morning (L/min).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.9 Exacerbation requiring hospitalisation.

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 1 Exacerbation requiring OCS.
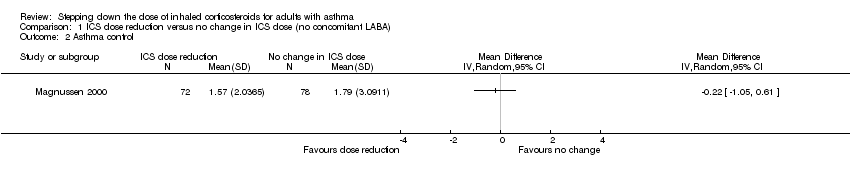
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 2 Asthma control.
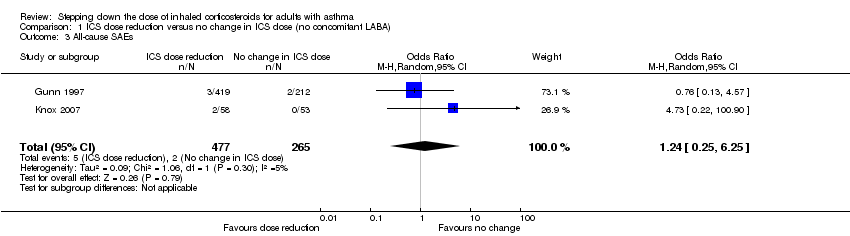
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 3 All‐cause SAEs.
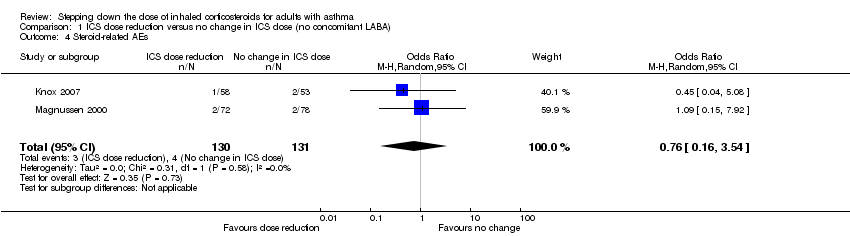
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 4 Steroid‐related AEs.
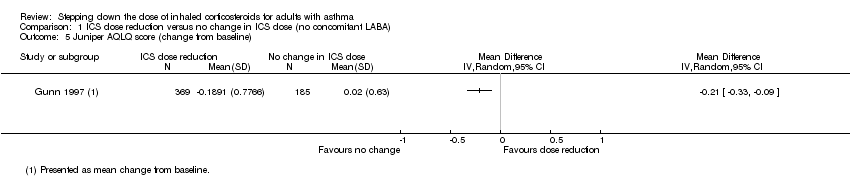
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 5 Juniper AQLQ score (change from baseline).

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 6 Lung function, PEFR morning (L/min).

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 7 Lung function, FEV1 (L).
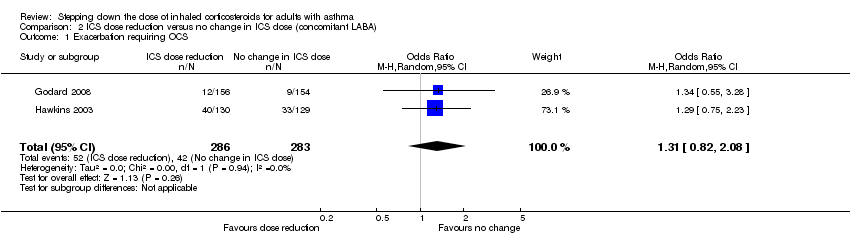
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 1 Exacerbation requiring OCS.
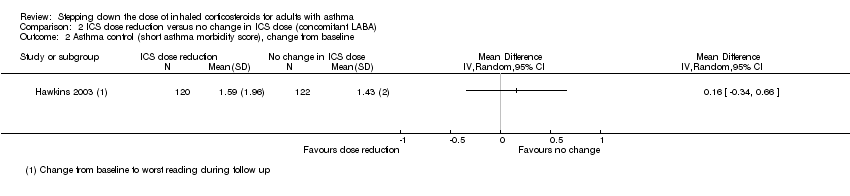
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 2 Asthma control (short asthma morbidity score), change from baseline.
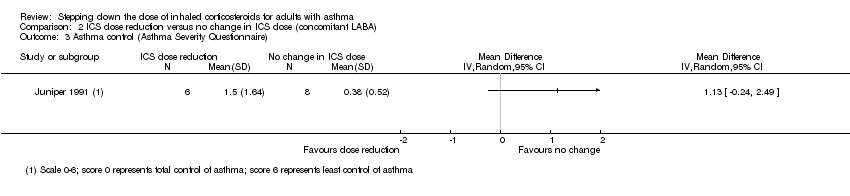
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 3 Asthma control (Asthma Severity Questionnaire).
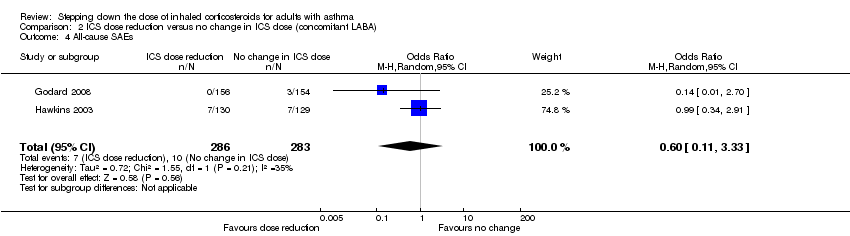
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 4 All‐cause SAEs.
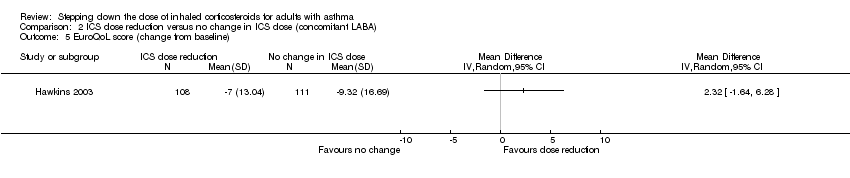
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 5 EuroQoL score (change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 6 St. George's Respiratory Scale score (change from baseline).
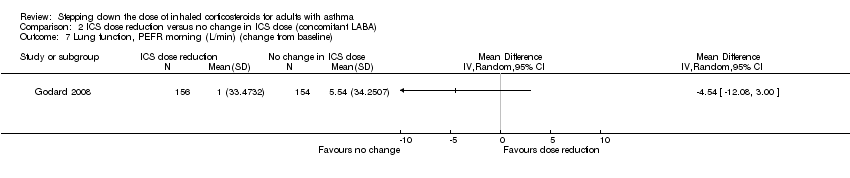
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 7 Lung function, PEFR morning (L/min) (change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 8 Lung function, reduction in FEV1 (% predicted, change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 9 Exacerbation requiring hospitalisation.
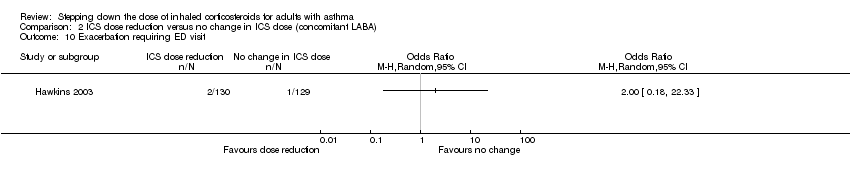
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 10 Exacerbation requiring ED visit.

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 11 Mortality.
| ICS dose reduction compared with no change in ICS dose (no concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (no concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 8 per 1000 | 14 per 1000 | OR 1.86 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Asthma control | Mean asthma control score in the no change in ICS dose group was 1.79. | MD 0.22 lower | ‐ | 150 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| All‐cause SAEs | 8 per 1000 | 9 per 1000 | OR 1.24 | 742 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Steroid‐related AEs | 31 per 1000 | 23 per 1000 | OR 0.76 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Health‐related quality of life (change from baseline) | Mean change from baseline in health‐related quality of life for the no change in ICS dose group was 0.02. | MD 0.21 lower | ‐ | 554 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence); MCID is 0.5 for AQLQ |
| Lung function, FEV1 (L) | Mean FEV1 in the no change in ICS dose group was 3.15 litres. | MD 0.02 litres lower | ‐ | 261 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Exacerbations requiring hospitalisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported by included studies |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). dThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (representative of specialist centres) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded twice for risk of bias (selective reporting and lack of blinding (subjective outcome)) and once for indirectness (single study representative of one setting and drug regimen). fThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ICS dose reduction compared with no change in ICS dose (concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 148 per 1000 | 186 per 1000 | OR 1.31 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring OCS (low‐quality evidence) |
| Asthma control (short asthma morbidity score) | Mean asthma control score was 1.43. | MD 0.16 higher | ‐ | 242 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to asthma control (low‐quality evidence) |
| All‐cause SAEs | 35 per 1000 | 22 per 1000 | OR 0.60 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to all‐cause SAEs (low‐quality evidence) |
| Steroid‐related AEs ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| St. George's Respiratory Scale score (change from baseline) Score 0‐100. 100 = greatest impact of chest disease on life; MCID is 4 units. | Mean change from baseline in HRQoL score was 7.4.c | MD 0.13 higher | ‐ | 229 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to HRQoL (low‐quality evidence) |
| Exacerbation requiring hospitalisation | 4 per 1000 | 14 per 1000 | OR 4.06 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring hospitalisation (low‐quality evidence) |
| Lung function, reduction in FEV1 (% predicted, change from baseline) | Mean change from baseline in % predicted FEV1 was ‐0.75%. | MD 2.45 lower | ‐ | 14 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to lung function (very low‐quality evidence) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cNote that study authors reported the change to the lowest SGRQ score during follow‐up. dThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (single study representative of one setting or drug regimen) and once for imprecision (wide CI). AE, adverse event; CI, confidence interval; FEV1, forced expiratory volume in one second; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; HRQoL, health‐related quality of life; ICS, inhaled corticosteroid; LABA, long‐acting beta agonist; MCID, minimum clinically important difference; MD, mean difference; OCS, oral corticosteroid; OR, odds ratio; RCT, randomised controlled trial; RR, risk ratio; SAE, serious adverse event. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.16, 21.09] |
| 2 Asthma control Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 All‐cause SAEs Show forest plot | 2 | 742 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.25, 6.25] |
| 4 Steroid‐related AEs Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.54] |
| 5 Juniper AQLQ score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Lung function, PEFR morning (L/min) Show forest plot | 3 | 875 | Mean Difference (IV, Random, 95% CI) | ‐5.98 [‐19.47, 7.51] |
| 7 Lung function, FEV1 (L) Show forest plot | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.08] |
| 2 Asthma control (short asthma morbidity score), change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Asthma control (Asthma Severity Questionnaire) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 All‐cause SAEs Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.33] |
| 5 EuroQoL score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 St. George's Respiratory Scale score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Lung function, PEFR morning (L/min) (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Lung function, reduction in FEV1 (% predicted, change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Exacerbation requiring hospitalisation Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 4.06 [0.45, 36.86] |
| 10 Exacerbation requiring ED visit Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Mortality Show forest plot | 1 | 310 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

