Deksametazon jako środek wspomagający przy wykonywaniu blokady nerwów obwodowych
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group RCT. | |
| Participants | In Canada, 75 ASA class I‐III participants aged 18‐80 years with BMI less than 35 m2 undergoing elective forearm or hand surgery with supraclavicular brachial plexus block were included. Those with cognitive or psychiatric history, pregnancy, diabetes mellitus, clavicular fracture, surgical procedure 180 minutes or longer, severe respiratory disease, chest or shoulder deformities on the operative side, preexisting chronic pain, preexisting neurological deficit or neuropathy in the upper extremities, allergy to any drugs used in the study or contraindication to peripheral nerve block such as local skin infection, coagulopathy or bleeding diathesis were excluded. | |
| Interventions | Block All participants underwent ultrasound‐guided supraclavicular block with bupivacaine 0.5 % 30 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: dexamethasone 8 mg intravenously, normal saline 2 ml perineurally. Placebo group: normal saline 2 ml perineurally and normal saline 2 ml intravenously. Intraperative anaesthesia/analgesia Intraoperative sedation with midazolam (1‐3 mg), fentanyl (1‐2 micrograms/kg) and/or propofol (25‐75 micrograms/kg/min) titrated to participant comfort. Postoperative anaesthesia/analgesia Fentanyl was administered every 5 minutes as needed up to 200 micrograms/hour to participants reporting moderate to severe pain (VAS 4 or greater) or at participant request. Participants requiring additional analgesics received acetaminophen 1g followed by oxycodone 5 mg as needed. | |
| Outcomes | Outcomes of interest for the review Duration of analgesia defined as time in hours to the first report of postoperative pain. Duration of motor block defined as time in hours to return to normal (or baseline) motor strength in the operative limb. Postoperative pain intensity (VAS) at 24 hours. Cummulative intraoperative opioid consumption converted to intravenous morphine equivalent. Cummulative postoperative opioid consumption converted to oral morphine equivalent at 24 hours. Incidence of postoperative nausea and vomiting at 24 hours after surgery. Participant satisfaction with pain relief (expressed as VAS) at 24 hours after surgery. Occurrence of any block‐related complications including new paraesthesia (numbness or tingling) or weakness in the operative limb at 2 weeks after surgery. Other outcomes Postoperative pain intensity at eight hours, and at seven days and 14 days. | |
| Notes | Funding: Drs. Faraj Abdallah and Richard Brull are supported by the Merit Award Program, Department of Anesthesia, University of Toronto. Conflicts of interest: Vincient Chan received equipment support from BK Medical, Philips Medical Systems, SonoSite and Ultrasonix. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was concealed by sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | The anaesthesiologist performing the block, the intraoperative anaesthesiologists, surgeons and nurses were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as states in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I or II participants aged 20‐69 years undergoing elective upper limb surgery (expected duration 60‐120 minutes) with ultrasound‐guided supraclavicular brachial plexus block. Participants with communication difficulties, hypersensitivity to local anaesthetics and dexamethasone, those on sedative medications and perioperative intravenous steroids were excluded from the study. | |
| Interventions | Block All participants received supraclavicular brachial plexus block with bupivacaine 0.5% 20 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally Placebo group: normal saline 2 ml intravenously. Intraoperative anaesthesia/analgesia Diazepam 0.15 mg orally the night before and on the morning of surgery. Postoperative anaesthesia/analgesia Diclofenac 1.5 mg intravenously for VAS > 30. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the onset of block and appearance of pain requiring analgesia. Duration of motor block defined as the time interval between complete motor paralysis to the compete return of motor power. Adverse events including nausea, vomiting, bradycardia, hypotension, convulsions, haematoma. Other outcomes Onset of block defined as the interval between injection of study drug to complete loss of cold perception and complete paralysis. Severity of pain at 90, 150, 210, 270, 330, 390 and 450 minutes after surgery. | |
| Notes | Funding: Gajira Raja Medical College, Gwalior, Madhya Pradesh, India. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Anesthesiologist performing the block was blinded, however, no indication whether other personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes were reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Canada and Thailand, 150 ASA class I‐II participants aged 18‐80 years undergoing forearm, wrist or hand surgery with ultrasound‐guided axillary block. Participants with sepsis, coagulopathy, allergy to local anaesthesia, hepatic or renal failure, pre‐existing upper limb neuropathy and who had prior surgery to the axilla were excluded. | |
| Interventions | Block All participants received ultrasound‐guided axillary nerve block with equal parts of lidocaine 2% and bupivacaine 0.5% with epinephrine 5 micrograms/ml 25 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg (0.8 mg) perineurally and normal saline 0.8 ml intravenously. Intravenous dexamethasone group: dexamethasone 8 mg (0.8 ml) intravenously and normal saline 0.8 ml perineurally. Intraperative anaesthesia/analgesia Intraoperative sedation with midazolam 0.015‐0.03 mg/kg and fentanyl 0.6 micrograms/kg intravenously. In the case of anxiety (as reported by the participant or determined by the blinded treating anaesthesiologist), propofol (25‐80 micrograms/kg/min) was administered. Postoperative anaesthesia/analgesia None reported. | |
| Outcomes | Outcomes of interest for the review Duration of motor block defined as time between block administration and time when participant regained movement of fingers. Duration of sensory block defined as time between block administration and time participant regained sensation of fingers. Duration of analgesia defined as time between block administration and time participant experienced pain in the operative site. Incidence of adverse events such as numbness, paraesthesia and motor deficit. Other outcomes Block performance time. Block onset time. Number of passes required to complete block. Block‐related pain as measured on 0‐10 pain scale. Incidence of vascular puncture. | |
| Notes | Funding: none. Conflict of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The number of participants with missing data was balanced between groups (8 in the IV dexamethasone group and 11 in the perineural dexamethasone group). |
| Selective reporting (reporting bias) | Low risk | Trial registered on clinicaltrials.gov NCT02629835. All outcomes reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 50 ASA class I‐II participants aged 15 to 54 years undergoing upper limb surgeries with supraclavicular block. Participants classified as ASA III to IV and those with infection at the block site, with comorbidities, coagulopathies and hypersensitivity to any of the study drugs were excluded. | |
| Interventions | Block All participants underwent supraclavicular block with ropivacaine 0.5% 30 ml using landmarks. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 1 mg was administered to all participants. Postoperative analgesia Diclofenac IM was administered when the participant reported pain. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as time interval between onset of sensory block to the time when participant first complains of pain at the site of surgery. Duration of motor block defined as interval between the time of loss of finger movements to the time the participant first regains finger movements. Intensity of pain assessed on a 5‐point VAS. Other outcomes Onset of sensory block defined as the time interval between administration of local anaesthetic to complete analgesia of forearm in relation to the distrubution of each major nerve as tested by pinprick over the forearm between elbow and wrist. Onset of motor block defined as time interval between administration of local anaesthetic to the time when finger movements are lost completely. | |
| Notes | Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Haemodynamic variables which could potentially be an indicator of adverse events were not reported as stated. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I‐II participants aged 20‐70 years undergoing elective surgery of the hand, forearm or elbow with supraclavicular brachial plexus block were included. Participants with uncontrolled diabetes mellitus, hypertension, peripheral neuropathy, hepatic or renal disease, pregnancy, acid peptic disease or hypersensitivity to local anaesthetics were excluded from the study. | |
| Interventions | Block All participants underwent nerve stimulator‐guided supraclavicular brachial plexus block with lidocaine 1.5% with adrenaline 1:200,000 (7 mg/kg) using nerve stimulator for guidance. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia None described. Postoperative analgesia Diclofenac IM 1.5 mg/kg was administered when the participant first complained of pain. Morphine IV 2 mg was administered every 10 minutes until VAS was less than 30. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time interval between brachial injection of local anaesthetic and the first postoperative pain. Duration of motor block defined as the time interval between brachial injection of local anaesthetic and complete recovery of motor function of all nerve distributions. Other outcomes Onset of sensory block defined as the time between the last brachial injection of local anaesthetic to the total abolition of pinprick response in all nerve distributions. Onset of motor block defined as the time between the last brachial injection of local anaesthetic to complete paralysis in all nerve distributions. | |
| Notes | Funding: none. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence table was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Anaesthesiologist who performed the block was blinded but no indication that other personnel (surgeon, nurses) were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant from each group was excluded. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Canada, 75 participants undergoing arthroscopic shoulder surgery with interscalene brachial plexus block. Exclusion criteria included participants with contraindications to interscalene block (coagulopathies, severe bronchopulmonary disease, contralateral diaphragmatic paralysis, prior contralateral pneumonectomy, preexisting neuropathy involving the surgical limb), preference for general anaesthesia, allergy or intolerance to one or more medications of the study protocol (dexamethasone, acetaminophen, morphine, or hydromorphone), chronic pain syndrome, chronic opioid use, chronic systemic corticosteroid use, weight\50 kg and pregnancy. | |
| Interventions | Block All participants received ultrasound and nerve stimulator‐guide interscalene brachial plexus block with ropivacaine 0.5% 20 ml. Dexamethasone/placebo Participants in the dexamethasone group received dexamethasone 10 mg diluted to a final volume with normal saline of 20 ml intravenously. Participants in the placebo group received 20 ml normal saline intravenously. Intraoperative anaesthesia/analgesia All participants received midazolam 1‐2 mg and/or fentanyl 25‐50 ug before block administration. Participants could receive an additional midazolam 1‐2 mg intravenously every 30 minutes and/or propofol 25‐100 ug/kg/min. Postoperative analgesia Acetaminopen 650 mg orally every six hours. Hydormorphone 1‐2 mg orally or morphine 5‐10 mg orally every 4 hours for pain score greater than or equal to 4. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time from the onset of block to the first analgesic request. Intensity of postoperative pain at 12, 24 and 48 hours. Cummulative opioid consumption at 12, 24 and 48 hours. Participant satisfaction. Adverse events including pruritus on administration of study drug and residual motor block at 24 and 48 hours postoperatively. Other outcomes Intensity of postoperative pain at 36 hours. Cummulative opioid consumption at 36 hours. Differences in variation of blood glucose concentration. | |
| Notes | This was a three‐arm study comparing dexamethasone 4 mg, dexamethasone 10 mg and placebo. In order to avoid unit of analysis issues we decided to include the dexamethasone 10 mg group and exclude the 4 mg group because high‐dose dexamethasone is used most often in clinical practice. Funding: funded by the Department of Anesthesiology, Hôpital Mainsonneve, Montréal, Quebec. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | One participant from the dexamethasone group and three participants from the placebo group were excluded. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as described in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Korea, 100 ASA class I‐II participants aged 20‐80 years undergoing elective arthroscopic shoulder surgery with interscalene brachial plexus block. Exclusion criteria included any neuropathy, coagulopathy, respiratory diseases, systemic steroid use or chronic opioid use, and uncontrolled diabetes mellitus. | |
| Interventions | Block All participants received ultrasound‐guided interscalene brachial plexus block with ropivacaine 0.75%, 60 mg. Dexamethasone/placebo Participants in the perineural dexamethasone group, participants received dexamethasone 5 mg perineurally + 3 ml 0.9% saline intravenously. Participants in the intravenous dexamethasone group received dexamethasone 5 mg intravenously + 4 ml 0.9% saline. Intraoperative anaesthesia/analgesia In all participants anaesthesia was induced with thiopental sodium 4 mg/kg, fentanyl 1‐2 ug/kg and rocuronium 0.6 mg/kg and maintained with sevoflurane. Postoperative analgesia Tramadol 50 mg intravenously for pain scores three or higher. Ketorolac 30 mg intravenously was given if tramadol was insufficient. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time from the completion of surgery to the first analgesic request. Severity of postoperative pain at 12, 24 and 48 hours. The incidence of adverse events including motor block, numbness and any other side effects in the first two days after surgery. Other outcomes Severity of postoperative pain at 6 hours. Number of participants requiring analgesic after surgery. | |
| Notes | Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how treatment allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant in the intravenous group was excluded from the study. |
| Selective reporting (reporting bias) | High risk | Protocol available from the Clinical Trials Registry of Korea. In the protocol, the primary outcome was stated as time to first analgesic request. In the study, the authors state the primary outcome was median analgesic time defined as the time to first analgesic request in > 50% of participants. |
| Other bias | Low risk | Appears to be free from any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In USA, 110 participants undergoing moderately to severely painful shoulder surgery with interscalene block were included. Participants with contraindication to interscalene block (severe lung disease, contralateral diaphragmatic paralysis and coagulopathy), pregnancy, pre‐existing neuropathy in the surgical limb, use of corticosteroids for two weeks or longer within six months of surgery or chronic opioid use were excluded from the study. | |
| Interventions | Block All participants underwent interscalene block with bupivacaine 0.5%, 30 ml, using nerve stimulator for guidance. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia General anaesthesia. No other details were provided. Postoperative analgesia Morphine IV 2 mg every 5 minutes for pain score > 2 was given in PACU. Acetominophen 325‐650 mg and oxycodone 5‐10 mg orally every 4 hours as needed for VAS > 4 after discharge from PACU. Morphine IV for pain unrelieved by oral analgesics (VRS persistently > 4). | |
| Outcomes | Outcomes of interest for the review Duration of sensory block. Postoperative pain intensity at rest and movement on postoperative day 1 and 2. Incidence of adverse events including numbness, paraesthesia, weakness in the operative limb, persistent hoarseness, respiratory difficulty, injection site infection or haematoma. Other outcomes Postoperative pain intensity on postoperative day 7. | |
| Notes | The effect of ropivacaine 0.5% was also examined; however to avoid unit of analysis errors, we chose to include only the bupivacaine arms since bupivacaine is more commonly used in clinical practice. Funding: support was solely from departmental sources. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed by sealed, sequentially numbered opaque envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol available on cllinicaltrials.gov. All outcomes reported as stated in the protocol. |
| Other bias | High risk | Study was stopped early for benefit according to predetermined stopping rule. |
| Methods | Parallel group RCT. | |
| Participants | In India, 80 ASA class I‐II participants aged 20‐50 years undergoing upper limb surgery with supraclavicular brachial plexus block were included. No exclusion criteria were stated. | |
| Interventions | Block All participants received supraclavicular brachial plexus block with ropivacaine 0.5% 30 ml using landmarks. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia None described. Postoperative analgesia Diclofenac IM 75 mg was administered when the VAS was greater than 4. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time interval between the end of local anaesthetic administration and complete resolution of sensory block (normal sensation). Duration of motor block defined as the time interval between the end of local anaesthetic administration and the recovery of full power in the relevant muscle group. Incidence of adverse events including hypotension (a 20% decrease in relation to baseline), bradycardia (heart rate less than 50 beats per minute), hypoxaemia (SpO2 < 90%) and nausea and vomiting. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic administration and complete sensory block. Onset of motor block defined as the time interval between the end of local anaesthetic administration and the time of no movement in the relevant group. Quality of intraoperative analgesia judged by the investigator on a 4‐point scale. | |
| Notes | Funding: none. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used to conceal treatment allocation. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as described in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Australia, 90 ASA class I‐III participants undergoing metatarsal osteotomy with ankle block were included. Participants classified as ASA greater than III, those less than 18 years old, those with coagulopathy, sepsis or infection at the operative site, allergy to ropivacaine, those taking regular opioids or glucocorticoids were excluded from the study. | |
| Interventions | Block All participants received ankle block with ropivacaine 0.75% 20 ml with ultrasound guidance. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: dexamethasone 8 mg intravenously and normal saline 2 ml mixed with the block solution. Placebo group: normal saline 2 ml mixed with the block solution and 2 ml intravenously. Intraperative anaesthesia/analgesia None reported. Postoperative analgesia Paracetamol 665 mg. Oxycodone 5 mg. Tamadol 50 mg. | |
| Outcomes | Outcomes of interest for the review Postoperative opioid consumption. Incidence of PONV. Other outcomes Pain score when block wore off, at seven days after surgery and maximum pain score during study period. Duration of block defined as the time when sensation and movement returned to normal. | |
| Notes | Funding: none. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Assume personnel were blinded since study drugs were prepared by a nurse not involved in the study and all study drugs were similar in appearance. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Belguim, 150 participants greater than 18 years undergoing arthroscopic shoulder surgery with interscalene block were included. Participants less than 18 years old, those with diabetes, brachial plexus neuropathies, severe bronchopulmonary disease, systemic glucocorticoid use, pregnancy, routine use of opioids or sensitivity to any of the study drugs were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided interscalene block with ropivacaine 0.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 10 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: normal saline 2 ml perineurally and dexamethasone 10 mg intravenously. Placebo group: normal saline 2 ml perineurally and normal saline 2 ml intravenously. Intraoperative anaesthesia/analgesia General anaesthesia was induced with target‐controlled propofol infusion 3‐5 micrograms/ml, remifentanil (loading dose 1 microgram/kg, continuous infusion 0.05‐0.3 microgram/kg/min) and cisatracurium 0.5 mg/kg. Postoperative analgesia Paracetamol was administered for VRS more than 2 on a 5‐point VRS. Diclofenac IV 50 mg was administered for inadequate analgesia with paracetamol. Piritramide IM 15‐20 mg was administered as needed. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time between performance of the block and the time to first analgesic request. Participant satisfaction measured on a 2‐point scale. Other outcomes Number of participants experiencing moderate to severe pain. Mean postoperative paracetamol consumption. Postoperative blood glucose concentrations. | |
| Notes | Funding: no information provided. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Assume operating room personnel were blinded since study drugs prepared by staff member not involved int he study and delivered in unidentifiable syringes, however, no indication whether other personnel were blinded (surgeon, recovery room and ward nurses) was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Only four participants were excluded from the placebo group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Belgim, 120 participants aged 18 years and older undergoing shoulder rotator cuff repair or subacromial decompression with interscalene brachial plexus block were included. Participants less than 18 years old, those with diabetes, brachial plexus neuropathies, severe bronchopulmonary disease, systemic glucocorticoid use, or pregnancy were excluded. | |
| Interventions | Block All participants received nerve‐stimulator/ultrasound‐guided interscalene block with 0.5% ropivacaine. Dexamethasone/placebo Dexamethasone group: dexamethasone 10 mg intravenously. Placebo group: normal saline intravenously. Intraoperative anaesthesia/analgesia Oral lorazepam 2.5 mg 1 hour before surgery + intravenous midazolam 2 mg and sufentanil 2‐5 micrograms before block placement. General anaesthesia was induced with target‐controlled propofol infusion 3‐5 micrograms/ml, remifentanil (loading dose 1 microgram/kg, continuous infusion 0.05‐0.3 microgram/kg/min) and cisatracurium 0.5 mg/kg. Postoperative analgesia Paracetamol for moderate or severe pain or on participant request to a maximum 4 grams/24 hours supplemented with diclofenac to a maximum of 100 mg/24 hours and intramuscular piritramide 15‐20 mg. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined by the interval between the time block was done and the time to first analgesia request. Arm weakness at 24 hours. Incidence of sleep disturbance, postoperative nausea and vomiting. Satisfaction. Other outcomes Number of participants with no/mild pain at 24 and 48 hours. | |
| Notes | This was a four‐arm study which included a placebo arm and three doses of dexamethasone: 1.25 mg. 2.5. mg and 10 mg. In order to avoid unit of analysis issues, we chose to include the dexamethasone 10 mg arm and placebo and exclude the other arms. Funding: supported through a grant from the Belgian Association for Regional Anesthesia. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Assume operating room personnel were blinded since study drugs prepared by staff member not involved the study and delivered in unidentifiable syringes, however, no indication whether other personnel were blinded (surgeon, recovery room and ward nurses) was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I‐II participants aged 18‐60 years undergoing elective and emergency upper limb surgery with supraclavicular block were included. Participants with uncontrolled diabetes or hypertension, peripheral neuropathy, hepatic or renal disease, pregnancy, acid peptic diease or allergy or hypersensitivity to local anaesthetics were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided supraclavicular block with bupivacaine 0.5% 15 ml + lidocaine 2% 15 ml + 5 micrograms 1:200,000 adrenaline. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Oral diazepam 0.15 mg/kg was administered the morning of surgery. Postoperative analgesia Diclofenac IM 1.5 mg/kg was administered when participant first complained of pain. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block as defined as the time from injection of local anaesthetic to the time rescue analgesia was given. Other outcomes Onset of sensory block as defined by the time from injection of local anaesthesia to patient report of dull sensation along any of the nerve distributions. Onset of motor block as defined by the time from injection of local anaesthesia to time patient felt heaviness on abduction of arm at shoulder. | |
| Notes | Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Only two participants were excluded from the study. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA class I‐II participants undergoing elective or emergency upper limb surgery with supraclavicular brachial plexus block were included. Participants with a history or uncontrolled diabetes, renal or liver disease, circulatory instability, pregnancy, peptic ulcer disease, allergy to local anaesthetics or receiving long‐term steroid therapy were excluded. | |
| Interventions | Block All participants underwent landmark‐guided supraclavicular block with lidocaine 2 % 15 ml + bupivacaine 0.5% 15 ml + adrenaline 1:200,000 Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 1 mg was administered after the block. Postoperative analgesia Diclofenac IM 1.5 mg/kg was administered when VAS was 5 or greater. | |
| Outcomes | Outcomes of interest for the review Intensity of postoperative pain measured on an 11‐point VAS every 3 hours after surgery. Duration of sensory block defined as the time from drug injection in brachial plexus to VAS = 5. Incidence of side effects in the intraoperative and postoperative period. Other outcomes Onset of sensory block defined as dull sensation along any nerve distrubution. Onset of motor block defined as the time when the patient felt heaviness on abduction of arm at the shoulder. | |
| Notes | Duration of block was reported as a range without any measure of central tendency. Pain scores were reported up to six hours in the placebo group and 15 hours in the dexamethasone group, therefore the data for this study could not be included in the meta‐analysis. Incidence of side effects was the only outcome that could be included in the analysis. Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how treatment allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | No protocol available. Pain scores were reported up to six hours in the placebo group and up to 15 hours in the dexamethasone group. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 112 ASA class I‐II participants aged 18‐70 years undergoing arthroscopic shoulder surgery with interscalene block were included. Participants with known hypersensitivity to study drugs or a contraindication to interscalene block were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided interscalene block with ropivacaine 0.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Alprazolam (by mouth) 0.5 mg was administered 2 hours before surgery. Midazolam IV 0.05 mg/kg was administered before block. Postoperative analgesia Diclofenac IM 1 mg/kg was administered when the VAS was greater than 3 or on participant request. Tramadol IV 1 mg/kg was administered if VAS was 3 or greater 45 minutes after diclofenac administration. | |
| Outcomes | Outcomes of interest for the review Duration of analgesia. Intensity of postoperative pain measured on an 11‐point VAS at 12 and 24 hours. Analgesic consumption at 24 hours. Incidence of block‐related complications. Other outcomes Intensity of postoperative pain at 1, 2, 3, 8, 16 and 20 hours. Onset of sensory block. Onset of motor block. | |
| Notes | Funding: none. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed in sealed, opaque envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Assume anaesthesiologist performing the block and operating room personnel were blinded since medication were prepared by an anaesthesiologist not involved in the study and delivered in similar syringes, however, no indication whether other personnel were blinded (surgeon, nurses). |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Twelve participants were excluded: six from the dexamethasone group and six from the placebo group. |
| Selective reporting (reporting bias) | High risk | No protocol available. All outcomes were reported as stated in the methods section, however, the SD was not reported for pain scores but was reported for other outcomes. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Japan, 39 participants aged 20 and 75 years undergoing arthroscopic shoulder surgery with interscalene block. Participants with coagulation disorder, skin infection at site of surgery, preexisting neuropathy involving upper limb, drug dependency, systemic opioid use within the previous six months, peptic ulcer disease, diabetes mellitus, renal or hepatic disease or pregnancy were excluded. | |
| Interventions | Block All participants underwent ultrasound‐guided interscalene block with ropivacaine 0.5% 20 ml after the surgical procedure. Dexamethasone/placebo Dexamethasone group: dexamethasone 4 mg perineurally. Placebo group: dexamethasone 4 mg intravenously. Intraoperative anaesthesia/analgesia Anaesthesia was induced and maintained by propofol 1mg/kg, remifentanil infusion 0.1 ‐0.3 microgram/kg/min, rocuronium 0.7 mg/kg and sevoflurane 1.0‐1.5 minimum alveolar concentration. Morphine 5 mg was administered after induction of anaesthesia. Postoperative analgesia Flurbiprofen IV was administered in the recovery room. Loxoprofen (by mouth) was administered after discharge from recovery room. Participants were instructed to request analgesia as soon as pain developed. | |
| Outcomes | Outcomes of interest for the review Intensity of postoperative pain measured on an 11‐point NRS the morning after surgery. Duration of sensory block defined as the interval between the time the block was performed and the first analgesic administration. Incidence of sleep disturbances measured on a 2‐point scale. Participant satisfaction measured on a 5‐point scale. Incidence of adverse events including nausea and vomiting, interscalene site infection, redness or neuropathy. | |
| Notes | Funding: no information provided. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was concealed by closed envelopes. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Three participants were excluded in the intravenous group, one in the placebo group and one in the perineural dexamethasone group. |
| Selective reporting (reporting bias) | High risk | There was an outlier in the intravenous dexamethasone group that was not included in the analysis. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RTC. | |
| Participants | In Korea, 40 ASA I‐II participants undergoing arthroscopic shoulder surgery with interscalene brachial plexus block were included. Participants with diabetes, pregnancy, coagulation disorders, sensitivity to local anaesthetic, severe chronic pulmonary disease, neurological deficiencies, neuropathy, infection at the surgical site, drug or alcohol dependency or history or chronic pain were excluded. | |
| Interventions | Block All participants received ultrasound‐guided interscalene block with levobupivacaine 0.5% 10 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 5 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 1‐3 mg and fentanyl IV 25‐50 micrograms was administered before block was performed. After block was performed, glycopyrrolate IV 0.2 mg, pentothal sodium IV 4 mg/kg, fentanyl 1‐2 micrograms/kg and rocuronium IV 0.6 mg/kg was administered. Postoperative analgesia Ketorolac IV or opioid IM was administered when the participant reported VAS more than 4 or on participant request. | |
| Outcomes | Intensity of postoperative pain measured on a 11‐point VAS assessed 12, 24 and 48 hours. Incidence of adverse events including nausea, vomiting, respiratory difficulties and neurological disabilities. | |
| Notes | This was a three‐arm study of 60 participants. In group III epinephrine 1:400 000 was given perineurally, however, the 20 participants of this arm are not included in this review as this is not an intervention of interest. Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how the treatment allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication of whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication of whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | The authors state analgesic consumption was not significantly different, however the results are not presented. In the abstract, the authors state they would assess sleep quality and satisfaction, however the results are not reported. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 80 ASA I‐II participants aged 16‐60 years undergoing elective upper limb surgery with supraclavicular brachial plexus block were included. Those with infection at the surgical site, local site anatomical abnormality, allergy to study drugs, use of corticosteroid for two weeks or longer, drug abuse, peripheral neuropathy, head injury, psychiatric disorder, severe pulmonary, cardiac, renal or endocrine disorder, peptic ulcer disease or pregnancy were excluded. | |
| Interventions | Block All participants underwent nerve‐stimulator‐guided supraclavicular block with ropivacaine 0.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: sterile water 2 ml perineurally. Intraoperative anaesthesia/analgesia None reported. Postoperative analgesia Diclofenac (by mouth) 50 mg was administered when participant reported VAS 3‐6. Diclofenac injection 75 mg was administered if participant reported VAS greater than 6. | |
| Outcomes | Outcomes of interest for the review Duration of analgesia as defined by the interval between the onset of sensory block and the initial use of rescue analgesia for surgical site pain. Duration of block. Intensity of postoperative pain measured on VAS. Postoperative analgesic consumption. Incidence of adverse events including nausea, vomiting, dysrhythmias, hypotension, convulsions, pneumothorax, pruritus, jerking movements and hypersensitivity reaction for the study drug. Other outcomes Onset of block. Peak effect of block. | |
| Notes | Authors did not specify the type of VAS. It was not possible to obtain pain scores from the figure in the manuscript. Authors were contacted to provide raw data, however it was unavailable; therefore not included in the analysis. Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in opaque, sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Korea, 34 ASA class I‐II participants aged 18 years and older undergoing elective forearm and hand surgery with ultrasound and nerve stimulator‐guided axillary brachial plexus block. Participants with hypertension, cardiac or hepatic disease, diabetes mellitus or coagulopathy were excluded from the study. | |
| Interventions | Block All participants received ultrasound and nerve stimulator‐guided axillary brachial plexus block with ropivacaine 0.5% 20 ml. Dexamethasone/placebo Perienural dexamethasone group: dexamethasone 10 mg perineurally Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Fentanyl 50 micrograms intravenously for pain score more than 4 on VAS. An additional 50 micrograms was given if pain persisted five minutes after first administration. Postoperative analgesia Not described. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block as defined as time between successful block and complete restoration of all the senses controlled by the radial, ulnar, median and musculocutaneous nerves. Incidence of adverse events including hypotension, bradycardia, hypoxaemia and nausea and vomiting. Other outcomes Onset of sensory block defined as time between the end of local anaesthetic injection and the loss of pinprick sensation. Quality of anaesthesia determined by the need for supplemental opioids during surgery. | |
| Notes | Funding: none reported. Conflicts of interest: none reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication of whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication of whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | A blinded observer recorded the onset of sensory block but unclear of whether the person who observed the primary outcome (duration of sensory block) was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes reported as described in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Canada and Thailand, 150 ASA class I‐III participants aged 18‐80 years undergoing forearm, wrist or hand surgery with ultrasound‐guided infraclavicular block. Participants with sepsis, coagulopathy, allergy to local anaesthesia, hepatic or renal failure, pre‐existing upper limb neuropathy and who had prior surgery in the infraclavicular fossa were excluded. | |
| Interventions | Block All participants received ultrasound‐guided Infraclavicular nerve block with equal parts of lidocaine 2% and bupivacaine 0.5% with epinephrine 5 micrograms/ml 35 ml. Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 5 mg (0.5 ml) perineurally and normal saline 0.5 ml intravenously. Intravenous dexamethasone group: dexamethasone 5 mg (0.5 ml) intravenously and normal saline 0.5 ml perineurally. Intraperative anaesthesia/analgesia Intraoperative sedation with midazolam 0.015‐0.03 mg/kg and fentanyl 0.6 micrograms/kg intravenously was administered as necessary. Postoperative anaesthesia/analgesia Not described. | |
| Outcomes | Outcomes of interest for the review Duration of motor block defined as time between block administration and time when participant regained movement of fingers. Duration of sensory block defined as time between block administration and time participant regained sensation of fingers. Duration of analgesia defined as time between block administration and time participant experienced pain in the operative site. Incidence of adverse events such as numbness, paraesthesia and motor deficit. Other outcomes Block performance time. Block onset time. Number of passes required to complete block. Block‐related pain as measured on 0‐10 pain scale. Incidence of vascular puncture. | |
| Notes | Funding: no information provided. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Number of participants and reasons for exclusion were balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Protocol published on clinicaltrials.in.th TCTR20150624001. All outcomes reported as per protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Iran, 60 ASA I‐II participants aged 20‐50 years undergoing elective hand and forearm surgery with axillary brachial plexus block were included. Participants with a history of peptic ulcer disease, diabetes, hepatic or renal failure, pregnancy and those receiving premedications including opioids, benzodiazepines and clonidine were excluded. | |
| Interventions | Block All participants received nerve stimulator‐guided axillary brachial plexus block with lidocaine 1.5% 34 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Not described. Postoperative analgesia Not described. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time interval between administration of local anaesthetic and the first postoperative pain. Duration of motor block defined as the time interval between administration of local anaesthetic and complete recovery of motor functions. Other outcomes Onset of sensory block defined as the time between the last injection and complete abolition of the pinprick response. Onset of motor block defined as the time between the last injection and complete paralysis in all nerve distributions. | |
| Notes | Funding: no information provided. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | The anaesthesiologists who evaluated the sensory and motor block were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Thirty participants were randomized to each group. Ten participants in the placebo group were excluded for failed block leaving 20 for analysis (33% excluded). In the dexamethasone group, six participants were excluded for failed block. The total of the remaining participants is reported to be 20. There are four participants that are not accounted for. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes were reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 90 ASA class I‐II participants aged 18‐65 years undergoing shoulder surgery with interscalene brachial plexus block were included. No exclusion criteria were stated. | |
| Interventions | Block All participants underwent nerve stimulator‐guided brachial plexus block with levobupivacaine 0.5% 35 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam 1 mg and fentanyl 30mg was administered before the block. Postoperative anaesthesia/analgesia Acetaminophen 325 mg. Participants were advised to take one or two tablets if the pain exceeded 3 on an 11‐point VAS. Ibuprophen 400 mg was administered if the pain persisted. | |
| Outcomes | Outcomes of interest for the review Duration of analgesia defined as the time in hours from the time of completion of surgery to the time participant felt pain from the incision at an intensity > 3 on numerical rating scale. Duration of motor block defined as the time of completion of nerve block to the time when patient was able to abduct the arm at least 2 inches away from the body. Other outcomes Total analgesic consumption defined as the number of analgesic used within the first 72 hours after surgery. | |
| Notes | This was a three‐arm study comparing dexamethasone 8 mg, dexamethasone 4 mg and placebo. In order to avoid unit of analysis errors, we chose to include the dexamethasone 8 mg arm and exclude the dexamethasone 4 mg arm since 8 mg is the dose most commonly used in clinical practice. Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Groups were allocated using a randomization table. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication of whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | The block was performed by a blinded anaesthesiologist, however there is no indication whether other personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Two participants were excluded from the placebo group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Canada, 45 ASA I‐III participants undergoing elective hand or forearm surgery with brachial plexus block were included. Participants scheduled for surgery less than 30 minutes or more than 120 minutes, hypersensitivity to local anaesthetics or dexamethasone, peripheral neuropathy, peptic ulcer, diabetes mellitus, coagulopathy or contraindication to supraclavicular brachial plexus block were excluded. | |
| Interventions | Block All participants underwent ultrasound‐guided brachial plexus block with mepivacaine 1.5% 30 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 0.03‐0.04 mg was administered before the block. Postoperative analgesia Fentanyl was administered in 25 microgram increments to participants with a pain score of 4 or greater on the VAS. Once oral intake was initiated, acetaminophen 300 mg/codeine 30 mg or acetaminophen 325 mg/oxycodone 5 mg was administered. | |
| Outcomes | Outcomes of interest for the review Intensity of postoperative pain measured on a 0‐100 mm VAS at 1 day, 7 days. Duration of sensory block defined as interval between the end of local anaesthetic injection and the patient's first report of postoperative pain at the surgical site. Postoperative analgesic consumption at 8 hours, 1 day, after surgery. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic injection and the loss of sensation to pinprick. Onset of motor block defined as the time interval between the end of local anaesthetic injection and paresis in the distributions of all 4 peripheral nerves. Intensity of pain measured on a 0‐100 mm VAS at 8 hours, 7 days and 14 days after surgery. Postoperative analgesic consumption at 0 hours, 8 hours, 7 days and 14 days after surgery. | |
| Notes | Funding: none reported. Conflicts of interest: Dr. Richard Brull is a consultant for B. Braun. Dr. Vincient Chan receives equipment support and honoraria from Philips Medcial Systems, SonoSite and GE Medical. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in opaque, sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Anaesthesiologist performing the block and the anaesthesiologist providing intraoperative care was blinded, however, there was no indication whether other personnel (surgeon, nurses) was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Number of participants with missing data balanced between groups (six in the dexamethasone group and seven in the placebo group). |
| Selective reporting (reporting bias) | Low risk | Protocol available on clinicaltrials.gov. All outcomes reported as stated. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In the USA, 80 participants aged 18 to 70 years undergoing elective ankle and foot surgery with sciatic nerve block were included. Participants with contraindication to regional anaesthesia, history of allergy to amide local anaesthetics, neurological deficit, coagulopathy, infection, type 1 or 2 diabetes mellitus, systemic use of corticosteroids within six months of surgery, chronic use of opioids, pregnancy and those undergoing midfoot and forefoot surgery were excluded. | |
| Interventions | Block All participants underwent ultrasound‐guided sciatic nerve block with bupivacaine 0.5% with epinephrine 1:300,000 (0.45 mg/kg) Dexamethasone/placebo Perineural dexamethasone group: dexamethasone 8 mg perineurally and normal saline 2 ml intravenously. Intravenous dexamethasone group: normal saline 2 ml perineurally and dexamethasone 8 mg intravenously. Placebo group: normal saline 2 ml perineurally and normal saline 2 ml intravenously. Intraoperative anaesthesia/analgesia Midazolam IV 2‐5 mg was administered to all participants and fentanyl IV 25‐50 micrograms was administered incrementally if necessary before the block. Propofol 25‐75 micrograms/kg/min was administered to provide sedation while maintaining responsiveness to tactile or verbal stimulation after the block. Postoperative analgesia Hydrocodone 10 mg + acetaminophen 325 mg every 4 hours as needed. | |
| Outcomes | Outcomes of interest for the review Intensity of postoperative pain measured on an 11‐point NRS on postoperative day one and day two. Postoperative opioid consumption on postoperative day one and two. Duration of sensory block defined as time to first pain not in saphenous distribution. Duration of motor block defined as time to first toe movement. Incidence of postoperative neurological sequale. Participant satisfaction measured on an 11‐point VAS. Other outcomes Quality of recovery measured by Quality of Recovery‐40 scale. Intensity of pain measured on an 11‐point NRS two weeks after surgery. Postoperative opioid consumption two weeks after surgery. | |
| Notes | Funding: Department of Anesthesiology, Northwestern University. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in opaque, sequentially numbered, sealed envelopes. |
| Blinding of participants (detection bias) | Low risk | No indication that participants were blinded in the paper, however the clincialtrials.gov document states that participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | No indication that participants were blinded in the paper, however the clincialtrials.gov document states that caregivers were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Number of participants with missing data balanced between groups; three in the intravenous dexamethasone group, one from the placebo group, and none from the perineural dexamethasone group. |
| Selective reporting (reporting bias) | Low risk | Protocol available on clinicaltrials.gov. All outcomes were reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In USA, 130 participants ASA I‐III participants undergoing shoulder surgery (arthroplasty, open and arthroscopic rotator cuff repair and acromisoplasty) with ultrasound‐guided brachial plexus block were included. Participants taking more than 60 mg oral morphine equivalents per day, those with diabetes mellitus, allergy to local anaesthetic or dexamethasone, coagulopathy, local infection or severe lung disease were excluded. | |
| Interventions | Block All participants received brachial plexus block with ropivacaine 0.5% 28 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally and normal saline 5 ml intravenously. Intravenous dexamethasone group: dexamethasone, 8 mg intravenously and normal saline 5 ml mixed with the block solution. Placebo group: normal saline 5 ml both intravenously and mixed with the block solution. Intraoperative anaesthesia/analgesia All participants received fentanyl IV up to 100 micrograms and midazolam up to 4 mg for sedation for block placement. All participants underwent general anaesthesia with propofol, fentanyl, rocuronium and/or succinylcholine, sevoflurane in air‐oxygen and ondansetron. Intraoperative fentanyl was limited to 250 micrograms and no long‐acting opioids were used. Neuromusclular block was reversed with neostigmine/glycopyrrolate. Postoperative analgesia For participants not discharged the day of surgery, ketorolac IV was given every six hours for the first 24 hours and intravenous morphine or hydromorphone and oral hydrocodone or oxycodone as needed. Participants who were discharged the day of surgery were prescribed ibuprofen 800 mg every eight hours and hydrocodone, oxycodone and non‐steroidal anti‐inflammatory medications as needed. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time until the patient detected complete resolution of sensory block in the shoulder region. 24‐hour postoperative opioid consumption. Pain scores at rest measured on 11‐point VAS 12 , 24 and 48 hours after surgery. Satisfaction with pain placebo. Incidence of adverse events. Other outcomes Pain scores at rest measured on 11‐point VAS 8 hours, 20 hours, and 1 week after surgery. | |
| Notes | Funding: none. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by a statistician. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation schedule was stored by the pharmacy and randomization occurred after informed consent was obtained and before any study drugs were prepared. |
| Blinding of participants (detection bias) | Low risk | The clinicaltrials.gov protocol states that participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | The clinicaltrials.gov document states personnel was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | The clinicaltrial.gov document states outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Only two participants were excluded form the perineural dexamethasone group, five from the intravenous group and three from the placebo group. |
| Selective reporting (reporting bias) | Low risk | Protocol was registered on clinicaltrial.gov. All outcomes were reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Brazil, 60 ASA class I‐II participants aged 18 years and older undergoing arthroscopic shoulder surgery with interscalene brachial plexus block. Exclusion criteria were: infection at the site, history of allergy to any of the study drugs, systemic use of corticosteroid for two weeks or longer, drug abuse, peripheral neuropathy, head injury, psychiatric disorder, coagulation disorder, severe pulmonary, cardiac, renal or endocrine disorder and pregnancy. | |
| Interventions | Block All participants received ultrasound and nerve stimulator‐guided interscalene brachial plexus block with ropivacaine 0.5% 20 ml. Dexamethasone/placebo Participants in the perineural dexamethasone group received dexamethasone 4 ml perineurally. Participants in the intravenous dexamethasone group received dexamethasone 4 mg intravenously + 1 ml normal saline perineurally. Participants in the control group received 1 ml of normal saline perineurally. Interoperative anaesthesia/analgesia All participants received fentanyl 50 micrograms intervenously. General anaesthesia was given by Total Anaesthesia Target Control Infusion induced with propofol 1% and remifentanil 50 micrograms then titrated to effect. Rocuronium 0.5 mg/kg was administered. Postoperative analgesia Parecoxib 40 mg was administered as soon as participant reported pain. Morphine 0.1 mg/kg was used as rescue medication. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as time between successful block and complete recovery of arm sensation. Duration of motor block defined as time interval between successful block and complete recovery of all movements in the arm. Severity of postoperative pain at 12 hours. Severity of postoperative pain at 24 hours. Postoperative opioid requirement. Incidence of postoperative nausea and vomiting. | |
| Notes | Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Protocol was published on www.ensaiosclinicos.gov.br. Adverse events not reported as per published protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Turkey, 30 ASA I‐II participants aged 18‐60 years undergoing elective hand and forearm surgery with brachial plexus block were included. Participants with severe hepatic, renal or cardiovascular disorders, electrolyte imbalance or pregnancy were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided brachial plexus block with prilocaine 2% 5 mg/kg. Dexamethasone/placebo Dexamethsone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Not described. Postoperative analgesia Diclofenac 1 mg/kg was administered to participants when they first complained of pain. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the first postoperative pain. Incidence of any side effects (nausea, vomiting, methaemoglobinaemia, cardiovascular issues). Other outcomes Onset of sensory block defined as the time between completion of local anaesthetic injection and no response to pinprick. Onset of motor block defined as the time between completion of local anaesthetic injection and paralysis. | |
| Notes | This was a three‐arm study in 75 participants. In group II, 15 participants received brachial plexus block with levobupivacaine however, this arm was not included in the review because there was no equivalent placebo or non‐active comparator group. Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | The anaesthesiologists who performed the block were blinded, however, there is no indication whether other personnel (surgeon, nurses) were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Pain scores, analgesic consumption, incidence of adverse events and vital signs not reported as stated. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In India, 53 ASA I‐II participants aged 18‐60 years undergoing upper limb surgery below mid‐humerus with infraclavicular brachial plexus block were included. Participants with head injury, psychiatric disorders, infection at surgical site, severe pulmonary, cardiac, renal, endocrine disorders, peptic ulcer disease, peripheral neuropathies, allergy to any of the study drugs were excluded. | |
| Interventions | Block All participants received nerve stimulator‐guided infraclavicular brachial plexus block with lignocaine 1.5% 0.6 ml/kg. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV was administered in incremental doses of 1 mg to a maximum of 3 mg and fentanyl was administered in 25 microgram incremental boluses to a maximum of 2 micrograms/kg before the block. Postoperative analgesia Patient controlled analgesia with morphine 1 mg/ml solution bolus 1 ml, lockout 5 min, 4 hour limit of 10 mg without background infusion. | |
| Outcomes | Outcomes of interest for the review Intensity of postoperative pain assessed by 11‐point NRS at 12 and 24 hours. Duration of sensory block defined as the time interval between the onset of sensory block and the first postoperative pain. Duration of motor block as defined as the time interval between the onset of motor block and complete recovery of motor functions. Patient satisfaction measured on a 4‐point scale. Other outcomes NRS assessed every 30 minutes until 6 hours and then at 6 hour intervals until 24 hours after surgery. | |
| Notes | This is a three‐arm study. In group C (19 participants), clonidine was given perineurally, however this was not an intervention of interest for our study therefore not included in the analysis. Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Authors state 53 participants were included in the study, however only 41 were included in the analysis. It is not clear how many participants were randomized to each group. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes reported as described in the methods section. |
| Other bias | High risk | No sample size was done a priori. An interim analysis showed significant difference with 13 participants in the placebo group and the study was stopped for benefit. |
| Methods | Parallel group RCT. | |
| Participants | In India, 60 ASA I‐II participants undergoing elective elbow, forearm and hand surgery with supraclavicular brachial plexus block were included. Participants classified as ASA III or more, those with history or peptic ulcer, diabetes mellitus, hepatic or renal failure, history of neurological, psychiatric, neuromuscular disease or hypersensitivity to any of the study drugs were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided supraclavicular brachial plexus block with bupivacaine 0.5% 38 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IV 0.03‐0.04 was administered before the block. Postoperative analgesia Diclofenac IM 75 mg was administered when participant reported VAS 30 or greater on a 100‐mm VAS. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time interval between the onset of sensory block and the first postoperative pain. Duration of motor block defined as the time interval between the onset of motor block and complete recovery of motor functions. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic injection and loss of sensation to pinprick in all nerve distrubution. Onset of motor block defined as the time interval between the end of local anaesthetic and paresis in all nerve distributions. Number of diclofenac injections required in the first 24 hours after surgery. | |
| Notes | Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Three participants per group were excluded. |
| Selective reporting (reporting bias) | High risk | Pain scores were not reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Bangledesh, 60 ASA I‐II participants ages 18 to 60 years undergoing elective upper limb surgery with supraclavicular brachial plexus block were included. Participants with coagulation disorder, skin infection at surgical site, pre‐existing upper limb neuropathy, drug dependency, systemic use of steroid within the past six months, peptic ulcer disease, diabetes mellitus, renal or hepatic disease or pregnancy were excluded. | |
| Interventions | Block All participants received a supraclavicular block with bupivacaine 0.5% 38 ml using paraesthesia technique. Dexamethasone/placebo Dexamethsaone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Not described. Postoperative analgesia Not described. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block. Intensity of postoperative pain measured on an 11‐point VAS at 12 and 24 hours. Incidence of sedation, nausea, vomiting, hypotension, arrhythmia and shivering. Other outcomes Onset of sensory block. Onset of motor block. Intensity of postoperative pain measured on an 11‐point VAS at 0.5 and 1 hour. | |
| Notes | Funding: none. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by card sampling method. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | Pain scores were assessed only up to 16 hours instead of up to 24 hours as stated in the methods section. The incidence of arrhythmias not reported as stated in the methods section. P values were reported for only statistically significant results. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In the USA, 78 participants aged 18 to 78 years undergoing elective arthroscopic shoulder surgery were included. Participants with coagulopathy, allergy to local anaesthetics, hypertension, peripheral neuropathy or chronic obstructive pulmonary disease were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided brachial plexus block with bupivacaine 0.5% with epinephrine 1:200,000 40 ml. Dexamethasone/placebo Dexamethsone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam 1‐2 mg and/or fentanyl 50‐100 micrograms was administered before the block. Anaesthesia was induced with propofol 2 mg/kg and maintained with sevoflurane 1.0‐1.5 MAC. Postoperative analgesia Acetaminophen 325 mg + hydrocodone 7.5 mg 1‐2 tablets was administered if pain score was greater than 3. If pain persisted, ibuprofen 400 mg was administered. | |
| Outcomes | Otcomes of interest for review Duration of sensory block defined as time of discharge to the time the patient experienced pain at or greater than 3. Duration of motor block defined as the time from discharge to the time when the patient was able to abduct the arm at least 2 inches away from the body. Participant satisfaction measured on a 5‐point scale. Other outcomes Number of acetaminophen 325 mg + hydrocodone 7.5 mg tablets taken in the first 72 hours after surgery. Incidence of adverse events. | |
| Notes | This was a three‐arm study comparing dexamethasone 8 mg, dexamethasone 4 mg and placebo. In order to avoid unit of analysis errors, we chose to include the dexamethasone 8 mg arm and exclude the dexamethasone 4 mg arm since 8 mg is the dose most commonly used in clinical practice. Funding: provided by Buffalo Anesthesiology Associates. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a randomization table. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Nurse who assessed the outcomes after discharge was blinded. Unclear whether the anaesthesiologist assessing the incidence of postoperative adverse events was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No participants were excluded from the dexamethasone group and two were excluded from the placebo group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In the USA, 120 ASA I‐III participants 18 years or older undergoing elective shoulder arthroscopy with interscalene brachial plexus block were included. Participants with a contraindication to bupivacaine, epinephrine, clonidine or dexamethasone as well as pregnant participants were excluded. | |
| Interventions | Block All participants underwent ultrasound‐guided supraclavicular block with bupivacaine 5 mg/ml + epinephrine 5 microgram/ml and clonidine 75 microgram/ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Anaesthesia was induced with propofol and maintained with sevoflurane, desflurane or propofol with nitrous oxide after the block. Postoperative anaesthesia/analgesia Participants were prescribed hydrocodone, oxycodone or hydromorphone. | |
| Outcomes | Outcomes of interest for the review Intensity of pain measured on an 11‐point VAS at 24 and 48 hours after surgery. Duration of sensory and motor block. Participants were given a diary to record the time at which they felt the sensory and motor block had resolved based on increase in pain, sensation and strength in the arm. Participant satisfaction with pain placebo measured on an 11‐point VAS Other outcomes Intensity of pain on admission to PACU, 1 and 2 hours after surgery and on discharge from PACU. | |
| Notes | Funding: departmental funding from the Department of Anesthesiology, Baystate Medical Center, Springfied, Massachuttes. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes reported as stated in the methods section. |
| Other bias | High risk | Sample size was 88; however, 120 participants were enrolled in order to obtain reliable data from 88 participants. |
| Methods | Parallel group RCT. | |
| Participants | In India, 50 participants ASA I‐II aged 20‐45 years undergoing upper extremity surgery with supraclavicular block were included. Participants classified as ASA III to IV, those with allergy to local anaesthetic or dexamethasone, coagulopathy, diabetes mellitus, local infection at block site, pre‐existing neuropathy of the surgical limb and systemic use of corticosteroids within six months of surgery were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided supraclavicular brachial plexus block with bupivacaine 0.5% 28 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 8 mg perineurally. Placebo group: normal saline 2 ml perineurally. Intraoperative anaesthesia/analgesia Midazolam IM 0.05 mg/kg was administered one hour before surgery. Postoperative analgesia Diclofenac IM 75 mg was administered as rescue analgesia. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time interval between the end for local anaesthetic administration to the time when the patient had VAS 4 or greater Incidence of nausea. Incidence of tingling/numbness. Other outcomes Onset of sensory block defined as the time interval between the end of local anaesthetic administration and loss of sensation to pin prick. Onset of motor block defined as the time between the end of local anaesthetic administration and the inability to move fingers. | |
| Notes | Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was achieved by simple random sampling. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | No indication whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | VAS scores not reported. |
| Other bias | Unclear risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Korea 36 ASA 1 to 2 participants aged 20 to 70 years undergoing arthroscopic shoulder surgery with interscalene brachial plexus block were included. Participants with coagulopathy, infection at block site, neurological deficit in the surgical limb, severe lung disease, contralateral diaphragmatic paralysis, systemic glucocorticoid use, chronic opioid use, peptic ulcer disease, uncontrolled diabetes mellitus or allergy to ropivacaine were excluded. | |
| Interventions | Block All participants underwent interscalene brachial plexus block with ropivacaine 0.75% using nerve stimulator guidance. Dexamethasone/placebo Dexamethsaone group: dexamethasone 7.5 mg perineurally. Placebo group: normal saline perineurally. Intraoperative anaesthesia/analgesia Thiopentone 4 mg/kg. Fentanyl one to two micrograms/kg. Rocuronium 0.6 mg/kg. Sevoflurane in 50% air/oxygen mixture 1.0 to 1.5 minimum alveolar concentration. Postoperative analgesia Tramadol 100 mg up to 3 times a day when pain was at least three on Numerical Rating Scale or patient request. Ketorolac 30 mg up to 90 mg a day for insufficient analgesia. | |
| Outcomes | Outcomes of interest for the review Duration of sensory block defined as the time the block was performed to the time of first analgesic request. Incidence of arm weakness and adverse events for the first 48 hours after surgery. Pain scores at 12, 24 and 48 hours after surgery. Other outcomes Number of participants not requiring analgesia. Analgesia consumption. Pain scores at 6 hours after surgery. | |
| Notes | This was a three‐arm study comparing three doses of dexamethasone (2.5 mg, 5 mg and 7.5 mg) and placebo. In order to avoid unit of analysis issues we chose to include the dexamethasone 7.5 mg arm since this is the dose used most often in practice and to exclude the other arms. Funding: none. Conflicts of interest: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication how group allocation was concealed. |
| Blinding of participants (detection bias) | Low risk | Participants were blinded. |
| Blinding of personnel (detection bias) | Low risk | Personnel was blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | OUtcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Protocol was registered with the Clinical Trial Registry of Korea. All outcomes reported as stated in the protocol. |
| Other bias | Low risk | Appears to be free of any other bias. |
| Methods | Parallel group RCT. | |
| Participants | In Nepal, 60 ASA I‐II participants undergoing forearm or hand surgery with brachial plexus block were included. Participants with uncontrolled hypertension, Ischaemic heart disease, acid peptic disease, neurological, psychiatric neuromuscular or respiratory disorder, drug addiction, pregnant or lactating women were excluded. | |
| Interventions | Block All participants underwent nerve stimulator‐guided brachial plexus block with lignocaine (1.5%) and adrenaline (1:200,000) 24 ml. Dexamethasone/placebo Dexamethasone group: dexamethasone 4 mg perineurally. Placebo group: nerve block only. Intraoperative anaesthesia/analgesia None described. Postoperative analgesia Diclofenac (by mouth) 50 mg was administered if VAS was 3‐5. Diclofenac IV 75 mg was administered if VAS was 6 or greater. | |
| Outcomes | Outcomes of interest for the review Intensity of postoperative pain on 11‐point VAS and 12 hours after surgery. Duration of analgesia defined as the time between onset of analgesia to first pain perception by the patient. Postoperative nausea and vomiting. Other outcomes Intensity of pain measured on VAS at 1 min, 15 minutes, 30 minutes, 1 hour, 2 hours, 3 hours, 6 hours after surgery. Postoperative analgesic consumption (non‐opioid). Surgeons satisfaction score measured on an 11‐point VAS. | |
| Notes | This is a three‐arm study of 90 participants. In group B (30 participants) neostigmine 0.5 mg was added to the block solution, however this is not an outcome of interest for this review and this group was not included in any of the analyses. Funding: no information provided. Conflicts of interest: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No indication of how group allocation was concealed. |
| Blinding of participants (detection bias) | Unclear risk | No indication whether participants were blinded. |
| Blinding of personnel (detection bias) | Unclear risk | Assume anaesthesiologist performing the block was blinded since study drugs were prepared by an anaesthesiologist not involved in the study, however no indication whether other personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No indication whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | No protocol available. The authors report mean and SD for all outcomes except pain scores. |
| Other bias | Low risk | Appears to be free of any other bias. |
ASA = Americal Anesthesiology Society; BMI = body mass index; IM = intramuscularly; IV = intravenously; kg = kilograms; MAC = maximum alveolar concentration; mg = milligrams; ml = millilitres; mm = millimetres; NRS = Numerical Rating Scale; PACU = Postanaesthesia care unit; PONV = postoperative nausea and vomiting; RCT = randomized controlled trial; SD = standard deviation; VAS = Visual Analalogue Scale; VRS = Verbal Rating Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| All participants received both perineural and intravenous dexamethasone. | |
| The majority of participants (81/89) received both perineural and intravenous dexamethasone. | |
| Data for all outcomes were reported as median and range (upper/lower), therefore could not be used in any meta‐analyses. | |
| No non‐active comparator. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Duration of analgesia after popliteal fossa nerve blockade: Effects of dexamethasone and buprenorphine |
| Methods | RCT |
| Participants | In the United States of America (New York, New York), participants undergoing ankle surgery. |
| Interventions | Drug: A. Control nerve block. IV dexamethasone (4 mg). |
| Outcomes | Time it takes for nerve block to wear off. |
| Starting date | October 2012. |
| Contact information | Hospital for special surgery, New York. |
| Notes | Completed study. Published results not available. |
| Trial name or title | The effect of systemic or perineural dexamethasone on the duration of interscalene nerve blocks with ropivacaine |
| Methods | RCT |
| Participants | In the United States of America (Clevland, Ohio) participants age 18 to 75 years undergoing shoulder surgery with interscalene brachial plexus block. |
| Interventions | Placebo comparator: ropivacaine with perineural dexamethasone Active comparator: ropivacaine with systemic steroid Other: ropivacaine plus dexamethasone anaesthetic. Other: ropivacaine plus saline plus dexamethasone anaesthetic. |
| Outcomes | The clinical duration of the interscalene nerve block, which will be measured by time from onset of sensory block until first administration of analgesic medication or requirement for initiation of the perineural catheter infusion. Maximum Verbal Response Score with rest (time frame: upon admission to PACU through postoperative day 2, postoperative day 14). Verbal VRS with movement (time frame: upon admission daily through postoperative day 2, postoperative day 14). Total opioid consumption (time frame: daily through postoperative day 2). |
| Starting date | September 2011. |
| Contact information | Principal Investigator: Kenneth Cummings, MD, The Cleveland Clinic. |
| Notes | Study terminated (accrual insufficient to complete study in a feasible time frame). No results available. |
| Trial name or title | Postoperative analgesia comparing subsartorial saphenous nerve block with and without dexamethasone in ACL reconstruction |
| Methods | RCT |
| Participants | In the United States of America (New York, New York), ASA I‐III participants age 16‐65 undergoing anterior cruciate ligament reconstruction. |
| Interventions | Bupivacaine 0.5% 13 ml + 1 mg dexamethasone perineurally. Bupivacaine 0.5% 13 ml + 4 mg dexamethasone perineurally. Bupivacaine 0.5% 13 ml perineurally. |
| Outcomes | Patient perceived duration of analgesia. Intensity of pain measured on NRS. Patient satisfaction measured on an 11‐point NRS. Postoperative morphine consumption. Incidence of opioid‐related side effects. |
| Starting date | July 2012. |
| Contact information | Hospital for Special Surgery, New York. |
| Notes | Completed study. No published results. |
| Trial name or title | Dexamethasone as an adjuvant to ropivacaine for femoral nerve blocks in children undergoing knee surgery |
| Methods | RCT |
| Participants | In the United States of America (Columbus, Ohio), ASA I‐II participants age 10‐19 years undergoing arthroscopic surgery of the knee. |
| Interventions | Ropivacaine 0.5% 2 mg/kg + 0.1 mg/kg dexamethasone given perineurally + normal saline intramuscularly. Ropivacaine 0.5% 2 mg/kg + 0.1 mg/kg dexamethasone given perineurally + dexamethasone 0.1 mg/kg intramuscularly. Ropivacaine 0.5% 2 mg/kg + normal saline given perineurally + normal saline intramuscularly. |
| Outcomes | Post‐PACU opioid consumption. |
| Starting date | July 2014. |
| Contact information | Giorgio Veneziano, Nationwide Children's Hospital. |
| Notes | Currently recruiting participants. |
| Trial name or title | Prolongation of pain free time by the use of dexamethasone in peripheral nerve blockade |
| Methods | RCT |
| Participants | In Austria, ASA I to II participants aged 18 years and older undergoing shoulder arthroscopy with interscalene block. |
| Interventions | Experimental: Ropivacaine and dexamethasone perineurally Active comparator: Ropivacaine + saline placebo. |
| Outcomes | Pain free time measured by the duration between block and the point to first analgesic request. VAS on movement and rest 10 hours after surgery. |
| Starting date | March 2014 |
| Contact information | Christoph Homann. |
| Notes | Recruitment status of this study is unknown because the information has not been verified recently. |
| Trial name or title | The effects of dexamethasone on low‐dose interscalene brachial plexus block |
| Methods | RCT |
| Participants | In Canada (Toronto, Ontario), ASA class I to II participants aged 18 to 80 years undergoing arthroscopic shoulder surgery with interscalene block. |
| Interventions | Active comparator: ropivacaine 0.5% + dexamethasone 4 mg given perineurally. Systemic dexamethasone: ropivacaine 0.5% given perineurally + dexamethasone 4 mg given intravenously. |
| Outcomes | Duration of sensory block defined as the time from completion of block to NRS > 0. Time to first opioid consumption. Duration of motor block. Postoperative oxygen saturation on room air. Pulse oximetry one hour after arrival in postoperative recovery room. Opioid consummation 12, 24 and 7 days after surgery. Incidence of nerve damage defined as persistent paraesthesia and sensory/motor block at 7 days. Postoperative nausea and vomiting assessed at 12 hours, 24 hours and 7 days after surgery. |
| Starting date | January 2015. |
| Contact information | Stephen Choi, Sunnybrook Health Science Centre. |
| Notes | Ongoing study July 2015. |
| Trial name or title | The effect of systemic or perineural dexamethasone on the duration of interscalene nerve blocks with ropivacaine |
| Methods | RCT |
| Participants | In the Unitied States of America (Cleveland, Ohio), participants undergoing shoulder surgery with interscalene block. |
| Interventions | Placebo comparator: ropivacaine 0.5% 30 ml + dexamethasone 8 mg given perineurally + normal saline 2 ml given intravenously. Active comparator: ropivacaine 0.5% 30 ml + normal saline 2 ml given perineurally + dexamethasone 8 mg given intravenously. |
| Outcomes | Duration of sensory block measured by the time of onset of sensory block until the first administration of analgesic or requirement for perineural catheter infusion. Maximum VAS at rest. Maximum VAS on movement. Postoperative opioid consumption. |
| Starting date | December 2011. |
| Contact information | Kenneth Cummings, The Cleveland Clinic. |
| Notes | This study is currently recruiting participants. |
| Trial name or title | Perineural steroids for peripheral nerve blocks |
| Methods | RCT |
| Participants | In the United States of America (Winston‐Salem, North Carolina), participants aged 18 to 90 years undergoing surgery with saphenous nerve block. |
| Interventions | Experimental: bupivacaine 0.25% 20 ml with epinephrine 1:400,000 + dexamethasone 4 mg. Placebo comparator: bupivacaine 0.25% 20 mg with epinephrine 1:400,000. |
| Outcomes | Duration of sensory block. Pain scores 24 and 36 hours after surgery. Incidence of postoperative nausea and vomiting. Incidence of neurologic complications. Opioid consumption 24‐36 hours after surgery. Time to first analgesic request. |
| Starting date | July 2015. |
| Contact information | Daryl Steven, Wake Forest Baptist Health. |
| Notes | This study is currently recruiting participants. |
| Trial name or title | Interscalene block with low‐dose IV vs. perineural dexamethasone for shoulder arthroscopy |
| Methods | RCT |
| Participants | In the United States of America (New York, New York), participants aged 18‐70 years undergoing arthroscopic shoulder surgery with ultrasound‐guided interscalene block. |
| Interventions | Active comparator: bupivacaine 0.5% 15 cc perineurally + dexamethasone 1 mg intravenously. Experinmental: bupivacaine 0.5% 15 cc perineurally + dexamethasone 1 mg perineurally. |
| Outcomes | Duration of sensory block. Pain scores for duration of stay in recovery room after surgery, postoperative day 2 and postoperative day 3. Opioid consumption on postoperative day 2 and 3. Adverse events. Opioid‐related symptom distress scale. Satisfaction with block. |
| Starting date | August 2015. |
| Contact information | Jennifer Cheng, Hosptial for Special Surgery, New York. |
| Notes | This study is currently recruiting participants. |
ASA = Amercian Society of Anesthesiologists; IV = intravenous; kg = kilograms; mg = milligrams; ml = millilitres; NRS = Numeric Rating Score; PACU = postanaesthesia care unit; RCT = randomized controlled trial; VAS = Visual Analogue Scale; VRS = Verbal Rating Scale.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of sensory block Show forest plot | 27 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| Analysis 1.1  Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 1 Duration of sensory block. | ||||
| 2 Duration of sensory block: long‐ versus medium‐acting local anaesthetic subgroups Show forest plot | 26 | 1572 | Mean Difference (IV, Random, 95% CI) | 6.78 [5.62, 7.94] |
| Analysis 1.2  Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 2 Duration of sensory block: long‐ versus medium‐acting local anaesthetic subgroups. | ||||
| 2.1 Long‐acting local anaesthetic | 20 | 1315 | Mean Difference (IV, Random, 95% CI) | 7.81 [6.40, 9.21] |
| 2.2 Medium‐acting local anaesthetic | 6 | 257 | Mean Difference (IV, Random, 95% CI) | 3.98 [1.76, 6.20] |
| 3 Duration of sensory block: additive versus no additive subgroups Show forest plot | 27 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| Analysis 1.3  Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 3 Duration of sensory block: additive versus no additive subgroups. | ||||
| 3.1 Additives | 6 | 336 | Mean Difference (IV, Random, 95% CI) | 7.29 [3.77, 10.81] |
| 3.2 No additives | 21 | 1289 | Mean Difference (IV, Random, 95% CI) | 6.60 [5.30, 7.89] |
| 4 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 27 | 1627 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.53, 7.86] |
| Analysis 1.4  Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 4 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 4.1 High‐dose dexamethasone | 23 | 1447 | Mean Difference (IV, Random, 95% CI) | 7.09 [5.81, 8.38] |
| 4.2 Low‐dose dexamethasone | 4 | 180 | Mean Difference (IV, Random, 95% CI) | 4.32 [1.80, 6.85] |
| 5 Duration of sensory block: high/unclear versus low risk of bias subgroups Show forest plot | 26 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| Analysis 1.5 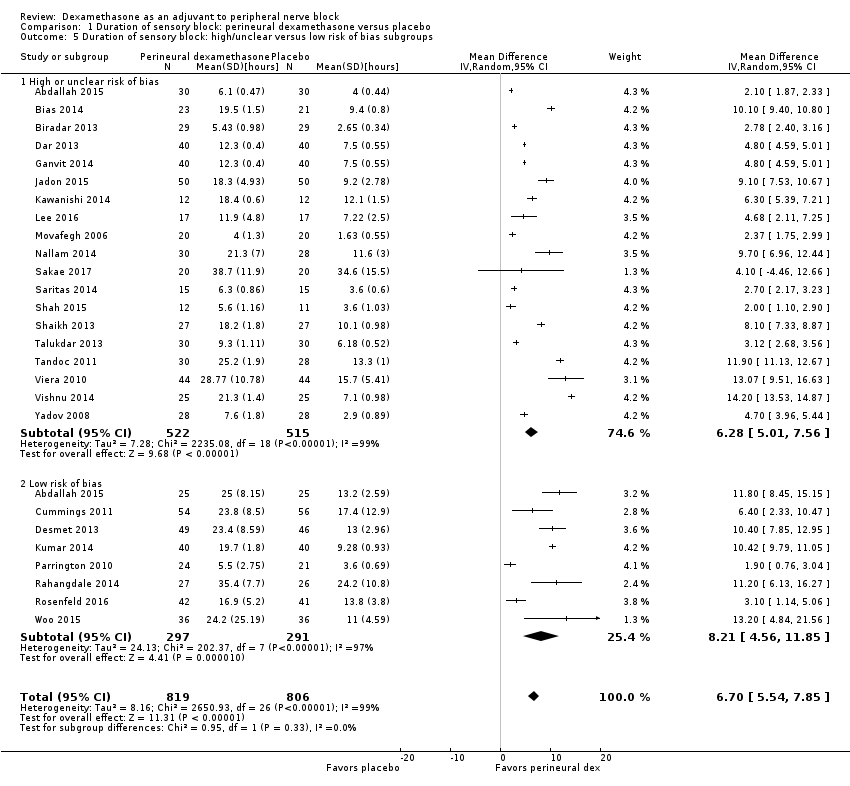 Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 5 Duration of sensory block: high/unclear versus low risk of bias subgroups. | ||||
| 5.1 High or unclear risk of bias | 19 | 1037 | Mean Difference (IV, Random, 95% CI) | 6.28 [5.01, 7.56] |
| 5.2 Low risk of bias | 8 | 588 | Mean Difference (IV, Random, 95% CI) | 8.21 [4.56, 11.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of motor block Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| Analysis 2.1 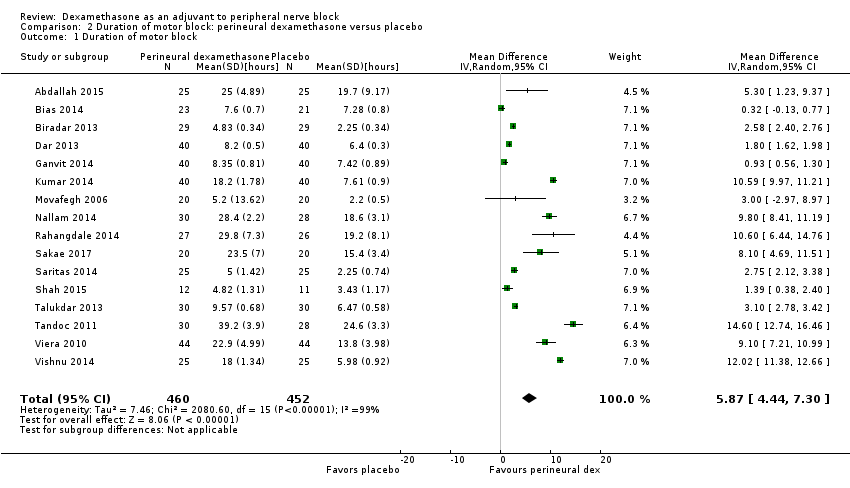 Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 1 Duration of motor block. | ||||
| 2 Duration of motor block: long‐ versus medium‐acting local anaesthetic subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| Analysis 2.2 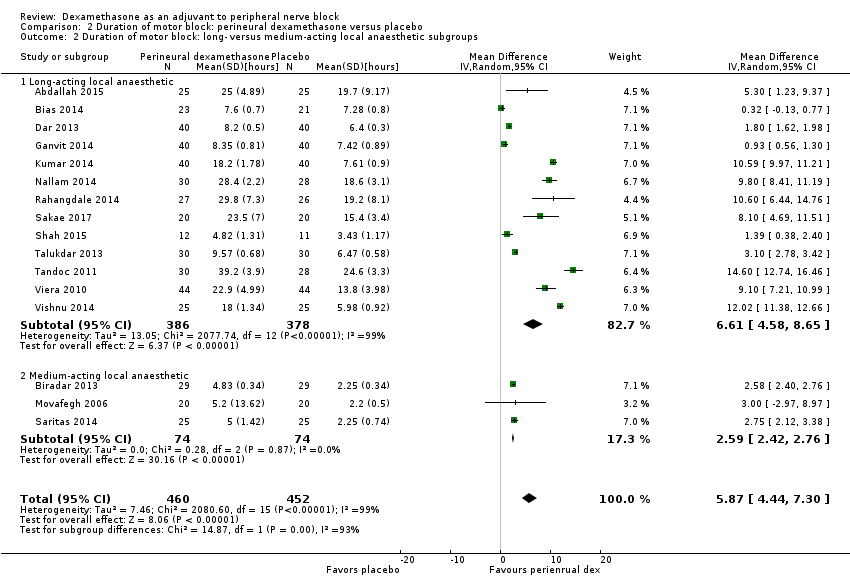 Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 2 Duration of motor block: long‐ versus medium‐acting local anaesthetic subgroups. | ||||
| 2.1 Long‐acting local anaesthetic | 13 | 764 | Mean Difference (IV, Random, 95% CI) | 6.61 [4.58, 8.65] |
| 2.2 Medium‐acting local anaesthetic | 3 | 148 | Mean Difference (IV, Random, 95% CI) | 2.59 [2.42, 2.76] |
| 3 Duration of motor block: additives verus no additives subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| Analysis 2.3 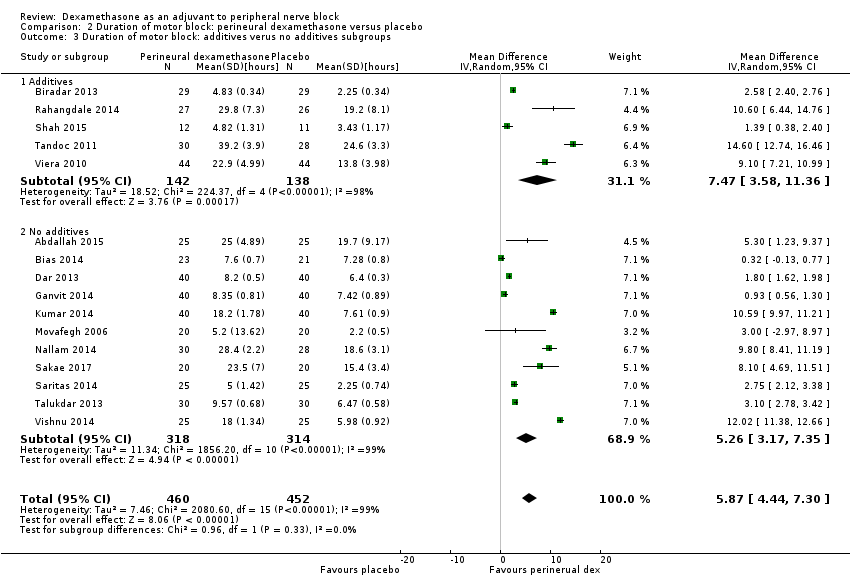 Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 3 Duration of motor block: additives verus no additives subgroups. | ||||
| 3.1 Additives | 5 | 280 | Mean Difference (IV, Random, 95% CI) | 7.47 [3.58, 11.36] |
| 3.2 No additives | 11 | 632 | Mean Difference (IV, Random, 95% CI) | 5.26 [3.17, 7.35] |
| 4 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| Analysis 2.4  Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 4 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 4.1 High‐dose dexamethasone | 15 | 872 | Mean Difference (IV, Random, 95% CI) | 5.75 [4.29, 7.22] |
| 4.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 8.1 [4.69, 11.51] |
| 5 Duration of motor block: high/unclear versus low risk of bias subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| Analysis 2.5  Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 5 Duration of motor block: high/unclear versus low risk of bias subgroups. | ||||
| 5.1 High/unclear risk of bias | 14 | 809 | Mean Difference (IV, Random, 95% CI) | 5.67 [4.18, 7.16] |
| 5.2 Low risk of bias | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 7.93 [2.74, 13.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall incidence of block‐related adverse events Show forest plot | 10 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.99, 1.39] |
| Analysis 3.1  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 1 Overall incidence of block‐related adverse events. | ||||
| 2 Numbness/tingling 14 days after surgery Show forest plot | 5 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.80, 3.89] |
| Analysis 3.2 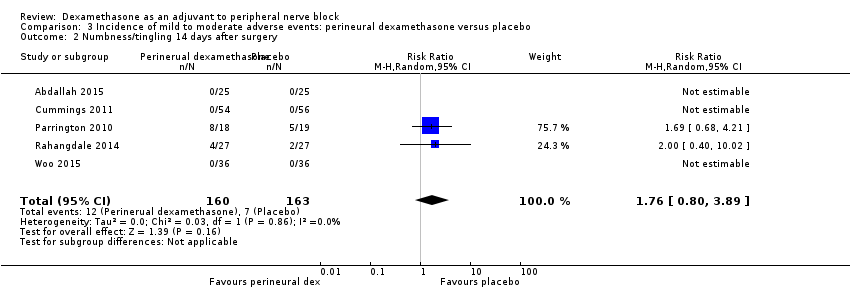 Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 2 Numbness/tingling 14 days after surgery. | ||||
| 3 Residual motor block/weakness 24 hours after surgery Show forest plot | 3 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.57, 38.68] |
| Analysis 3.3 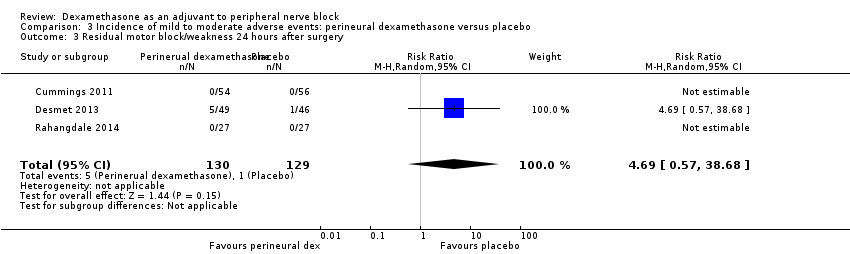 Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 3 Residual motor block/weakness 24 hours after surgery. | ||||
| 4 Horner Syndrome Show forest plot | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.36] |
| Analysis 3.4 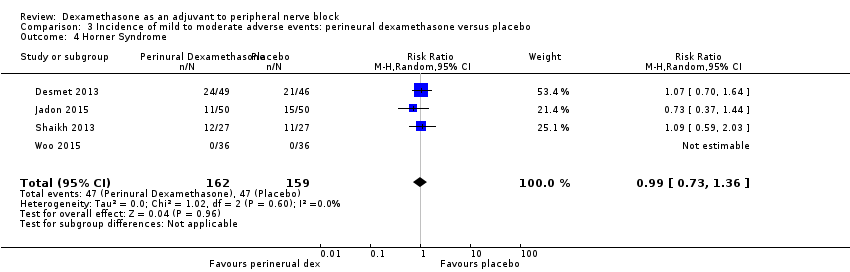 Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 4 Horner Syndrome. | ||||
| 5 Hoarseness Show forest plot | 4 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.65, 2.34] |
| Analysis 3.5 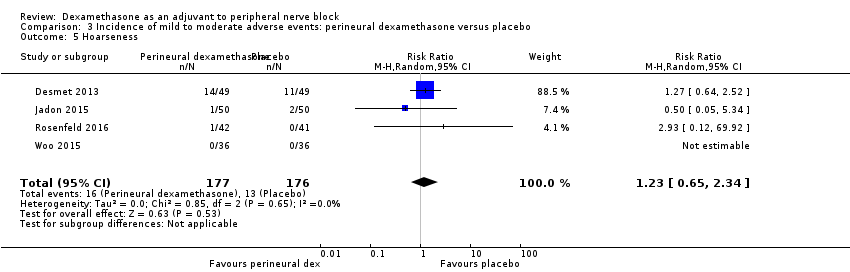 Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 5 Hoarseness. | ||||
| 6 Diaphragmatic paresis Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.66, 3.23] |
| Analysis 3.6 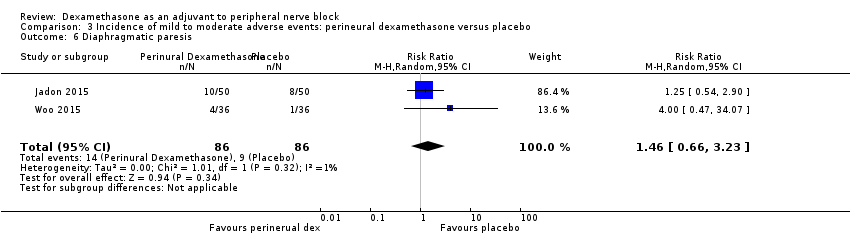 Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 6 Diaphragmatic paresis. | ||||
| 7 Dyspnoea Show forest plot | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.14] |
| Analysis 3.7  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 7 Dyspnoea. | ||||
| 8 Vascular injury Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.36] |
| Analysis 3.8  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 8 Vascular injury. | ||||
| 9 Cranial nerve 12 palsy Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.77] |
| Analysis 3.9 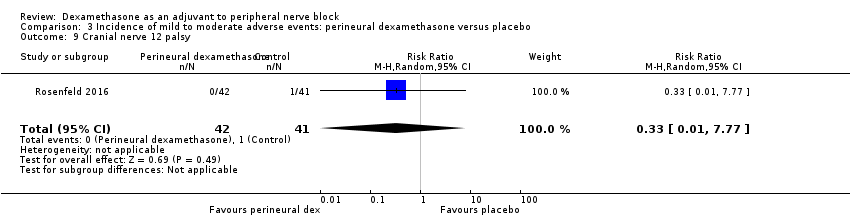 Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 9 Cranial nerve 12 palsy. | ||||
| 10 Bruising Show forest plot | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.07, 15.64] |
| Analysis 3.10  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 10 Bruising. | ||||
| 11 Overall non‐block‐related adverse events Show forest plot | 10 | 625 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.68] |
| Analysis 3.11  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 11 Overall non‐block‐related adverse events. | ||||
| 12 Postoperative nausea and vomiting Show forest plot | 10 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.26, 1.14] |
| Analysis 3.12 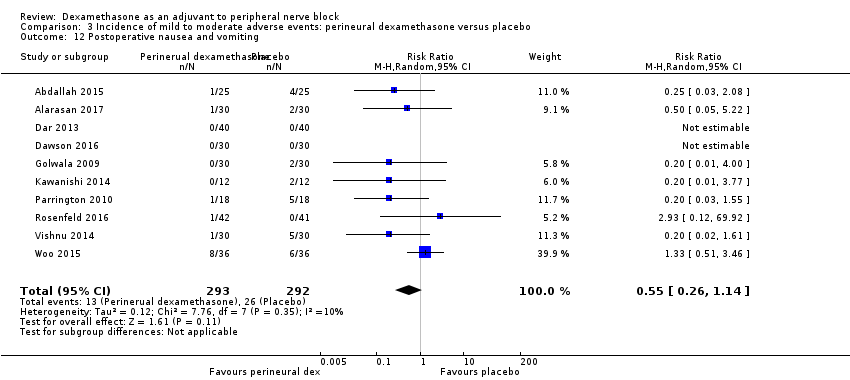 Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 12 Postoperative nausea and vomiting. | ||||
| 13 Deep sedation Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.38, 129.93] |
| Analysis 3.13  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 13 Deep sedation. | ||||
| 14 Dermatological symptoms (pruritus/rash) Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.32, 27.02] |
| Analysis 3.14  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 14 Dermatological symptoms (pruritus/rash). | ||||
| 15 Syncope/fainting Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.18, 20.71] |
| Analysis 3.15  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 15 Syncope/fainting. | ||||
| 16 Bradycardia Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.71] |
| Analysis 3.16  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 16 Bradycardia. | ||||
| 17 Hypotension Show forest plot | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.13] |
| Analysis 3.17  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 17 Hypotension. | ||||
| 18 Headache/10‐pound fluid gain/diarrhoea/frequent urination/muscle soreness Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.12, 69.92] |
| Analysis 3.18  Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 18 Headache/10‐pound fluid gain/diarrhoea/frequent urination/muscle soreness. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at12 hours Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| Analysis 4.1  Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at12 hours. | ||||
| 2 Postoperative pain intensity at 12 hours: medium‐ versus long‐acting local anaesthetic subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.53] |
| Analysis 4.2  Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 12 hours: medium‐ versus long‐acting local anaesthetic subgroups. | ||||
| 2.1 Long‐acting local anaesthesia | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐2.77, ‐1.66] |
| 2.2 Medium‐acting local anaesthesia | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.38, ‐0.06] |
| 3 Postoperative pain intensity at 12 hours: additive versus no additive subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| Analysis 4.3  Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 12 hours: additive versus no additive subgroups. | ||||
| 3.1 Additives | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.36, ‐0.04] |
| 3.2 No additives | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐2.77, ‐1.66] |
| 4 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| Analysis 4.4  Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 4.1 High‐dose dexamethasone | 3 | 177 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐3.29, ‐1.06] |
| 4.2 Low‐dose dexamethasone | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐2.75, ‐1.22] |
| 5 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| Analysis 4.5  Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 5 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups. | ||||
| 5.1 High/unclear versus low risk of bias | 3 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐2.53, ‐1.09] |
| 5.2 Low risk of bias | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐3.88, ‐1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 24 hours Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| Analysis 5.1  Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 24 hours. | ||||
| 2 Postoperative pain intensity at 24 hours: long‐ versus medium‐acting local anaesthetic subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| Analysis 5.2 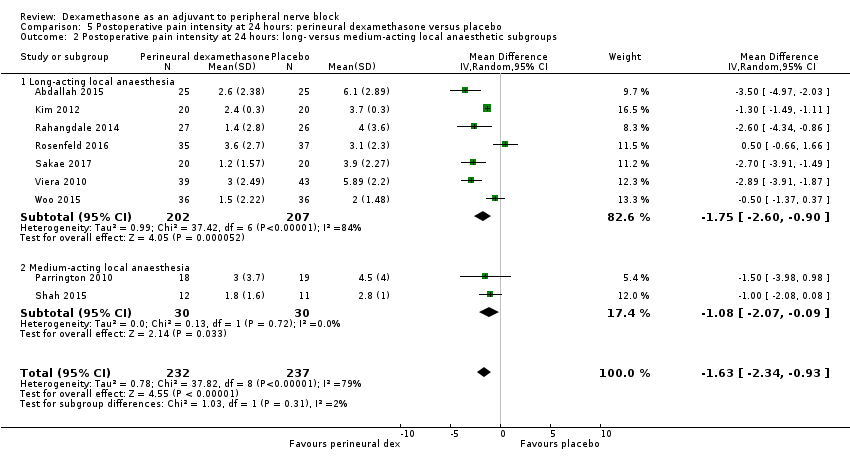 Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 24 hours: long‐ versus medium‐acting local anaesthetic subgroups. | ||||
| 2.1 Long‐acting local anaesthesia | 7 | 409 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐2.60, ‐0.90] |
| 2.2 Medium‐acting local anaesthesia | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.07, ‐0.09] |
| 3 Postoperative pain intensity at 24 hours: additive versus no additive subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| Analysis 5.3  Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 24 hours: additive versus no additive subgroups. | ||||
| 3.1 Additives | 3 | 158 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐3.43, ‐0.82] |
| 3.2 No additives | 6 | 311 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.31, ‐0.51] |
| 4 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| Analysis 5.4  Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 4.1 High‐dose dexamethasone | 7 | 389 | Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐2.71, ‐0.47] |
| 4.2 Low‐dose dexamethasone | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐3.21, ‐0.52] |
| 5 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| Analysis 5.5 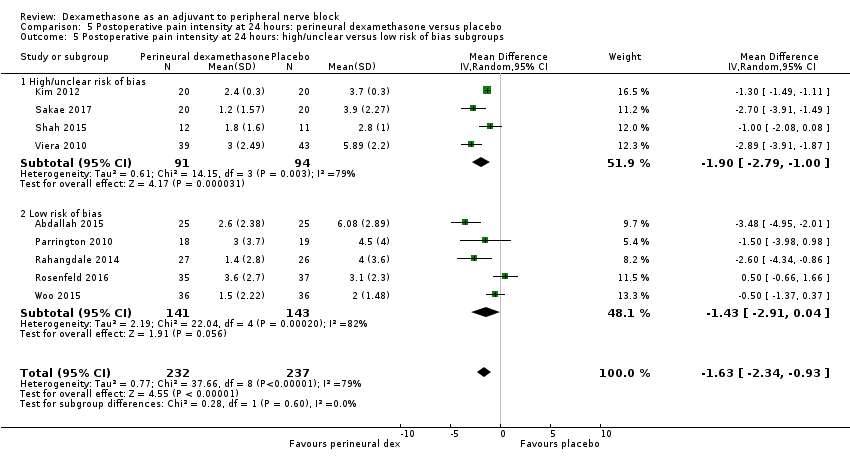 Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 5 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups. | ||||
| 5.1 High/unclear risk of bias | 4 | 185 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.79, ‐1.00] |
| 5.2 Low risk of bias | 5 | 284 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.91, 0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 48 hours Show forest plot | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| Analysis 6.1  Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 48 hours. | ||||
| 2 Postoperative pain intensity at 48 hours: additives versus no additives subgroups Show forest plot | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| Analysis 6.2  Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 48 hours: additives versus no additives subgroups. | ||||
| 2.1 No additives | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.36, ‐0.36] |
| 2.2 Additives | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.80, 1.34] |
| 3 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups Show forest plot | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| Analysis 6.3  Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups. | ||||
| 3.1 High/unclear risk of bias | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.24, 0.24] |
| 3.2 Low risk of bias | 3 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.30, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative opioid consumption at 24 hours Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| Analysis 7.1  Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative opioid consumption at 24 hours. | ||||
| 2 Opioid consumption at 24 hours medium‐ versus long‐acting local anaesthetic subgroups Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| Analysis 7.2  Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 2 Opioid consumption at 24 hours medium‐ versus long‐acting local anaesthetic subgroups. | ||||
| 2.1 Long‐acting local anaesthetic | 5 | 335 | Mean Difference (IV, Random, 95% CI) | ‐21.22 [‐35.20, ‐7.25] |
| 2.2 Medium‐acting local anaesthetic | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐33.91, 41.91] |
| 3 Opioid consumption at 24 hours: additive versus no additive subgroups Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| Analysis 7.3  Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 3 Opioid consumption at 24 hours: additive versus no additive subgroups. | ||||
| 3.1 Additives | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐30.17 [‐58.58, ‐1.76] |
| 3.2 No additives | 4 | 238 | Mean Difference (IV, Random, 95% CI) | ‐12.98 [‐26.28, 0.32] |
| 4 Opioid consumption at 24 hours: high/unclear versus low risk of bias subgroups Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| Analysis 7.4  Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 4 Opioid consumption at 24 hours: high/unclear versus low risk of bias subgroups. | ||||
| 4.1 High/unclear risk of bias | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐45.0 [‐57.58, ‐32.42] |
| 4.2 Low risk of bias | 5 | 292 | Mean Difference (IV, Random, 95% CI) | ‐13.55 [‐23.36, ‐3.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant satisfaction with pain control: perineural dexamethasone versus placebo Show forest plot | 4 | 224 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.05, 1.71] |
| Analysis 8.1 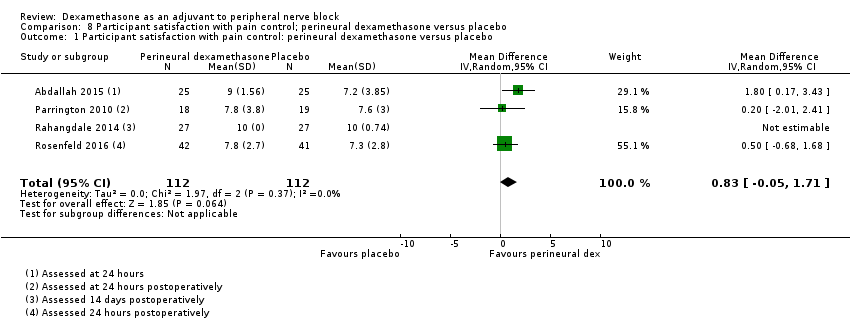 Comparison 8 Participant satisfaction with pain control; perineural dexamethasone versus placebo, Outcome 1 Participant satisfaction with pain control: perineural dexamethasone versus placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of sensory block Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| Analysis 9.1  Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 1 Duration of sensory block. | ||||
| 2 Duration sensory block: additive versus no additive subgroups Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| Analysis 9.2  Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 2 Duration sensory block: additive versus no additive subgroups. | ||||
| 2.1 Additives | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.20 [0.68, 11.72] |
| 2.2 No additives | 7 | 450 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.33, 9.08] |
| 3 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| Analysis 9.3  Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 3 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 3.1 High‐dose | 6 | 437 | Mean Difference (IV, Random, 95% CI) | 7.45 [5.55, 9.35] |
| 3.2 Low‐dose | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 2.25 [1.21, 3.30] |
| 4 Duration of sensory block: high/unclear versus low risk of bias subgroups Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| Analysis 9.4 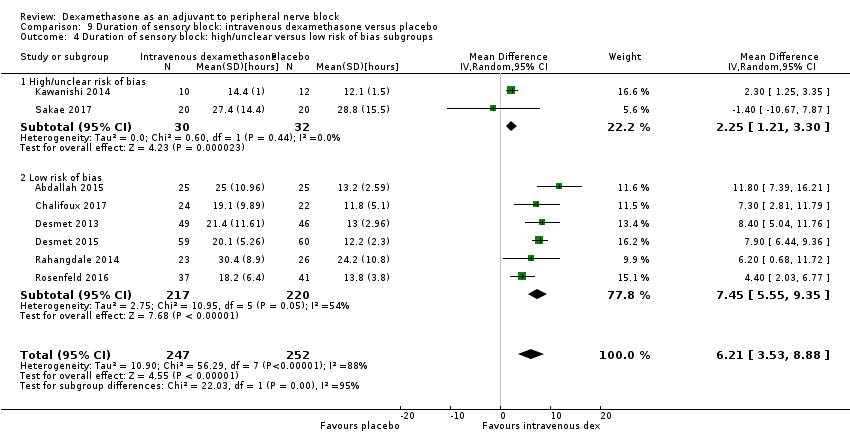 Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 4 Duration of sensory block: high/unclear versus low risk of bias subgroups. | ||||
| 4.1 High/unclear risk of bias | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 2.25 [1.21, 3.30] |
| 4.2 Low risk of bias | 6 | 437 | Mean Difference (IV, Random, 95% CI) | 7.45 [5.55, 9.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of motor block Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| Analysis 10.1  Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 1 Duration of motor block. | ||||
| 2 Duration of motor block: additive versus no additive subgroups Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.54 [3.11, 7.97] |
| Analysis 10.2  Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 2 Duration of motor block: additive versus no additive subgroups. | ||||
| 2.1 Additives | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.60 [2.32, 10.88] |
| 2.2 No additives | 2 | 90 | Mean Difference (IV, Random, 95% CI) | 3.67 [‐2.77, 10.11] |
| 3 Duration of motor block high‐ versus low‐dose dexamethasone subgroups Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| Analysis 10.3 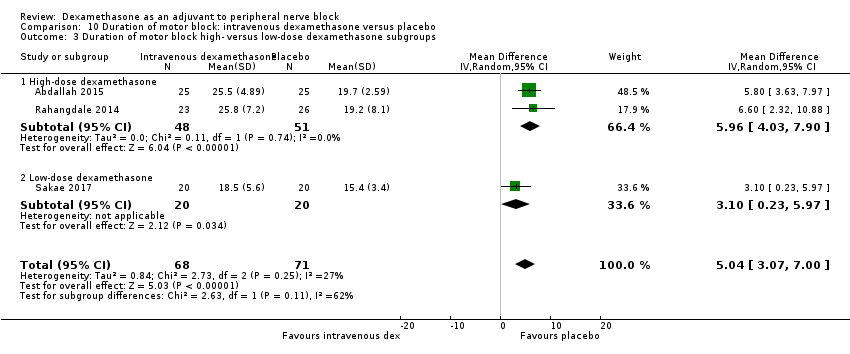 Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 3 Duration of motor block high‐ versus low‐dose dexamethasone subgroups. | ||||
| 3.1 High‐dose dexamethasone | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 5.96 [4.03, 7.90] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.23, 5.97] |
| 4 Duration of motor block: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| Analysis 10.4  Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 4 Duration of motor block: high/unclear versus low risk of bias subgroups. | ||||
| 4.1 High/unclear risk of bias | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 5.96 [4.03, 7.90] |
| 4.2 Low risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.23, 5.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall incidence of block‐related adverse events Show forest plot | 5 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.70] |
| Analysis 11.1  Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 1 Overall incidence of block‐related adverse events. | ||||
| 2 Numbness/tingling 14 days after surgery Show forest plot | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.31, 9.26] |
| Analysis 11.2  Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 2 Numbness/tingling 14 days after surgery. | ||||
| 3 Residual motor block/muscle weakness 24 hours after surgery Show forest plot | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [0.80, 8.90] |
| Analysis 11.3  Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 3 Residual motor block/muscle weakness 24 hours after surgery. | ||||
| 4 Horner syndrome Show forest plot | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.26] |
| Analysis 11.4  Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 4 Horner syndrome. | ||||
| 5 Hoarsenss Show forest plot | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.71] |
| Analysis 11.5 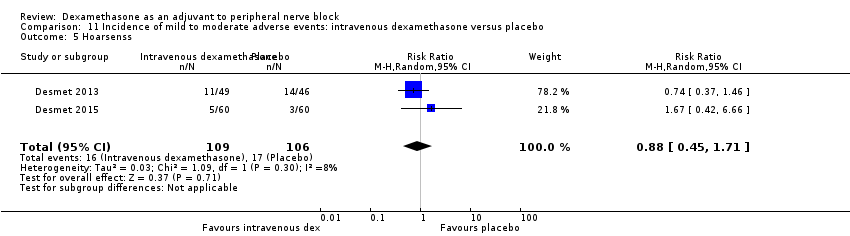 Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 5 Hoarsenss. | ||||
| 6 Dyspnoea Show forest plot | 3 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.11, 3.74] |
| Analysis 11.6 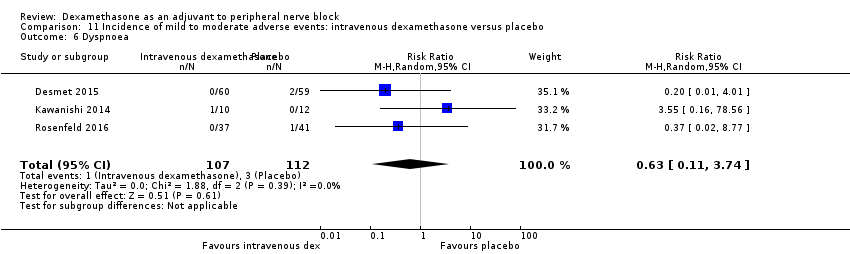 Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 6 Dyspnoea. | ||||
| 7 Cranial nerve 12 palsy Show forest plot | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.77] |
| Analysis 11.7  Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 7 Cranial nerve 12 palsy. | ||||
| 8 Overall non‐block‐related adverse events Show forest plot | 5 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.38, 3.97] |
| Analysis 11.8  Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 8 Overall non‐block‐related adverse events. | ||||
| 9 Postoperative nausea and vomiting Show forest plot | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.12, 3.78] |
| Analysis 11.9 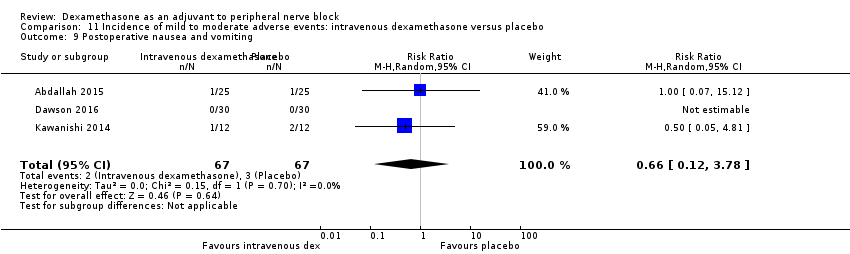 Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 9 Postoperative nausea and vomiting. | ||||
| 10 Dermatological symptoms Show forest plot | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.09, 40.62] |
| Analysis 11.10  Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 10 Dermatological symptoms. | ||||
| 11 Dizziness/wrist, hand or finger pain, constipation Show forest plot | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.77] |
| Analysis 11.11 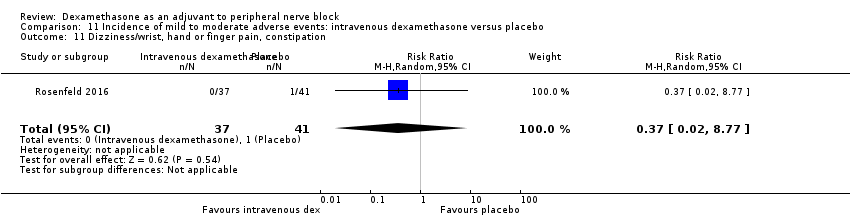 Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 11 Dizziness/wrist, hand or finger pain, constipation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 12 hours Show forest plot | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| Analysis 12.1 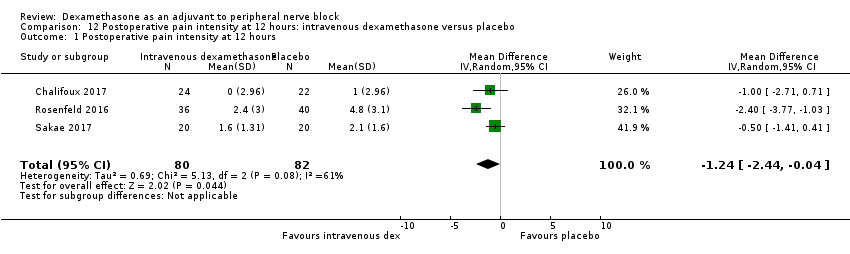 Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 12 hours. | ||||
| 2 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| Analysis 12.2 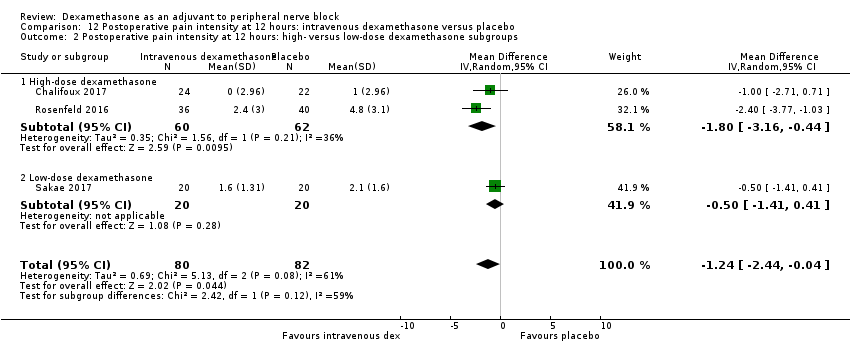 Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 2.1 High‐dose dexamethasone | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.16, ‐0.44] |
| 2.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.41, 0.41] |
| 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| Analysis 12.3  Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups. | ||||
| 3.1 High/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.41, 0.41] |
| 3.2 Low risk of bias | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.16, ‐0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 24 hours Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| Analysis 13.1  Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 24 hours. | ||||
| 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| Analysis 13.2  Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups. | ||||
| 2.1 Additive | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.75, 0.95] |
| 2.2 No additive | 4 | 208 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.48, ‐0.18] |
| 3 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| Analysis 13.3  Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 3.1 High‐dose dexamethasone | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.54, ‐0.07] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.49, 0.29] |
| 4 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| Analysis 13.4  Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups. | ||||
| 4.1 High/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.49, 0.29] |
| 4.2 Low risk of bias | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.54, ‐0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 48 hours Show forest plot | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| Analysis 14.1 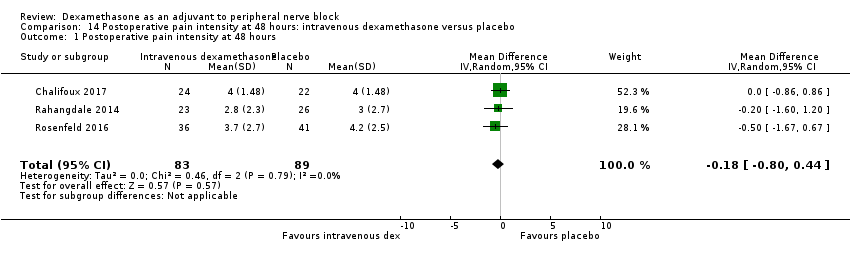 Comparison 14 Postoperative pain intensity at 48 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 48 hours. | ||||
| 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups Show forest plot | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| Analysis 14.2  Comparison 14 Postoperative pain intensity at 48 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups. | ||||
| 2.1 Additive | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.60, 1.20] |
| 2.2 No additive | 2 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.87, 0.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 24‐hour opioid consumption Show forest plot | 5 | 287 | Mean Difference (IV, Random, 95% CI) | ‐6.58 [‐10.56, ‐2.60] |
| Analysis 15.1 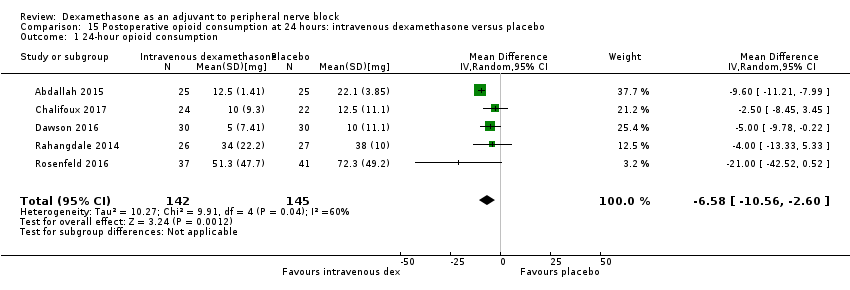 Comparison 15 Postoperative opioid consumption at 24 hours: intravenous dexamethasone versus placebo, Outcome 1 24‐hour opioid consumption. | ||||
| 2 24‐hour opioid consumption: additive verus no additive subgroups Show forest plot | 5 | 287 | Mean Difference (IV, Random, 95% CI) | ‐6.58 [‐10.56, ‐2.60] |
| Analysis 15.2  Comparison 15 Postoperative opioid consumption at 24 hours: intravenous dexamethasone versus placebo, Outcome 2 24‐hour opioid consumption: additive verus no additive subgroups. | ||||
| 2.1 Additive | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐13.33, 5.33] |
| 2.2 No additive | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐6.93 [‐11.41, ‐2.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative opioid consumption at 48 hours opioid consumption Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐22.5 [‐39.85, ‐5.15] |
| Analysis 16.1  Comparison 16 Postoperative opioid consumption at 48 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative opioid consumption at 48 hours opioid consumption. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant satisfaction with pain control Show forest plot | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐0.08, 2.22] |
| Analysis 17.1  Comparison 17 Participant satisfaction with pain control: intravenous dexamethasone versus placebo, Outcome 1 Participant satisfaction with pain control. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of sensory block Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| Analysis 18.1 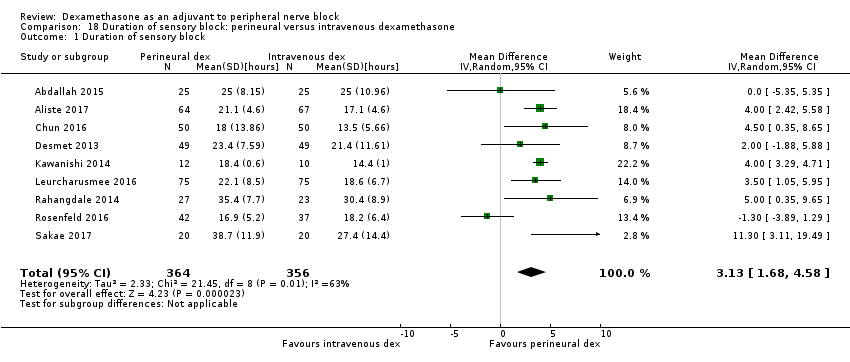 Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 1 Duration of sensory block. | ||||
| 2 Duration of sensory block additive versus no additive subgroups Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| Analysis 18.2 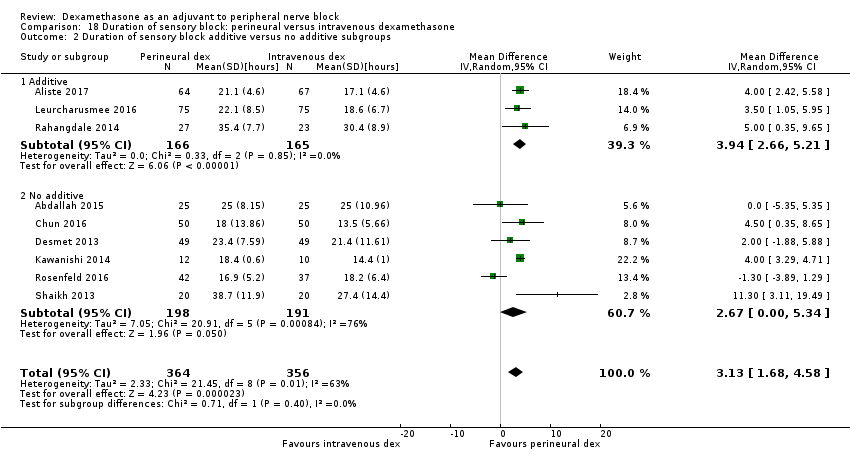 Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 2 Duration of sensory block additive versus no additive subgroups. | ||||
| 2.1 Additive | 3 | 331 | Mean Difference (IV, Random, 95% CI) | 3.94 [2.66, 5.21] |
| 2.2 No additive | 6 | 389 | Mean Difference (IV, Random, 95% CI) | 2.67 [0.00, 5.34] |
| 3 Duration sensory block high‐dose versus low‐dose dexamethasone subgroups Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| Analysis 18.3  Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 3 Duration sensory block high‐dose versus low‐dose dexamethasone subgroups. | ||||
| 3.1 High‐dose dexamethasone | 6 | 508 | Mean Difference (IV, Random, 95% CI) | 2.35 [0.04, 4.66] |
| 3.2 Low‐dose dexamethasone | 3 | 212 | Mean Difference (IV, Random, 95% CI) | 4.14 [2.48, 5.81] |
| 4 Duration sensory block high/unclear versus low risk of bias subgroups Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| Analysis 18.4  Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 4 Duration sensory block high/unclear versus low risk of bias subgroups. | ||||
| 4.1 High/unclear risk of bias | 3 | 162 | Mean Difference (IV, Random, 95% CI) | 4.67 [2.29, 7.04] |
| 4.2 Low risk of bias | 6 | 558 | Mean Difference (IV, Random, 95% CI) | 2.30 [0.23, 4.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of motor block Show forest plot | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| Analysis 19.1 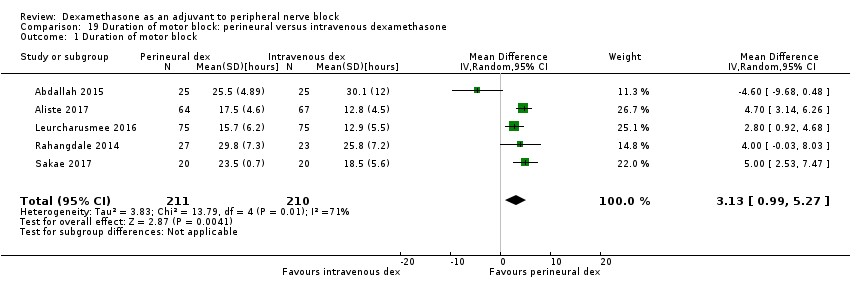 Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 1 Duration of motor block. | ||||
| 2 Duration of motor block: additive versus no additive subgroups Show forest plot | 5 | 340 | Mean Difference (IV, Random, 95% CI) | 2.75 [0.32, 5.19] |
| Analysis 19.2  Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 2 Duration of motor block: additive versus no additive subgroups. | ||||
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐0.03, 8.03] |
| 2.2 No additive | 4 | 290 | Mean Difference (IV, Random, 95% CI) | 2.39 [‐0.58, 5.37] |
| 3 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| Analysis 19.3 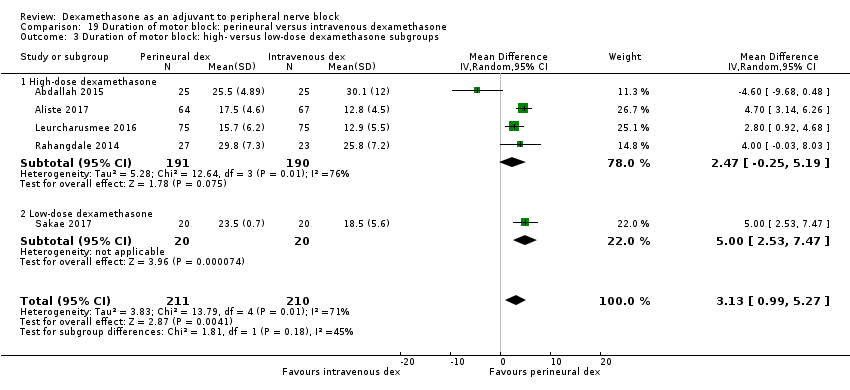 Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 3 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups. | ||||
| 3.1 High‐dose dexamethasone | 4 | 381 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐0.25, 5.19] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 5.0 [2.53, 7.47] |
| 4 Duration of motor block: high/unclear versus low risk of bias subgroups Show forest plot | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| Analysis 19.4  Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 4 Duration of motor block: high/unclear versus low risk of bias subgroups. | ||||
| 4.1 HIgh/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 5.0 [2.53, 7.47] |
| 4.2 Low risk of bias | 4 | 381 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐0.25, 5.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall incidence of block‐related adverse events Show forest plot | 5 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.93, 1.55] |
| Analysis 20.1 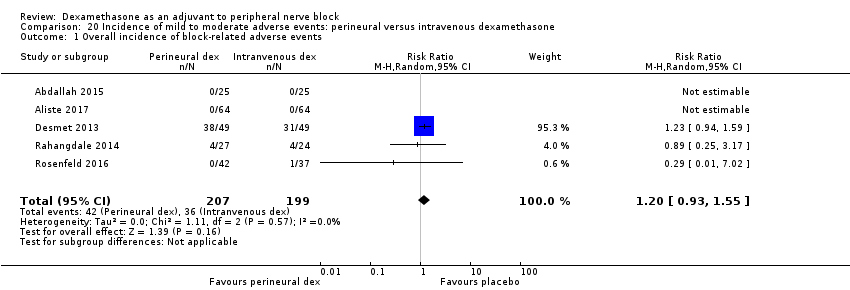 Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 1 Overall incidence of block‐related adverse events. | ||||
| 2 Numbness/tingling 14 days after surgery Show forest plot | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.27, 3.49] |
| Analysis 20.2 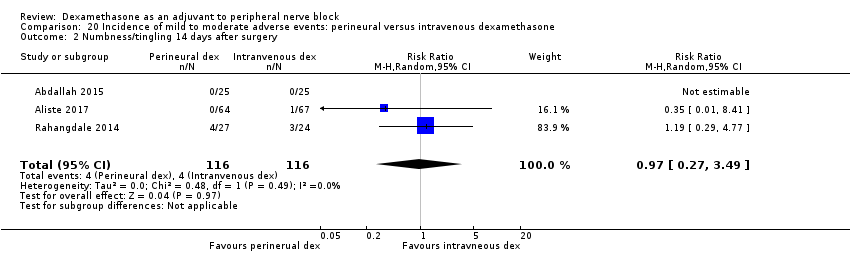 Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 2 Numbness/tingling 14 days after surgery. | ||||
| 3 Residual motor block/weakness at 24 hours Show forest plot | 3 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.37] |
| Analysis 20.3  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 3 Residual motor block/weakness at 24 hours. | ||||
| 4 Horner syndrome Show forest plot | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.77, 1.87] |
| Analysis 20.4  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 4 Horner syndrome. | ||||
| 5 Hoarsness Show forest plot | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.48, 2.09] |
| Analysis 20.5  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 5 Hoarsness. | ||||
| 6 Cranial nerve 12 motor palsy Show forest plot | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.39] |
| Analysis 20.6  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 6 Cranial nerve 12 motor palsy. | ||||
| 7 Overall incidence of non block‐related adverse events Show forest plot | 5 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.37, 4.78] |
| Analysis 20.7 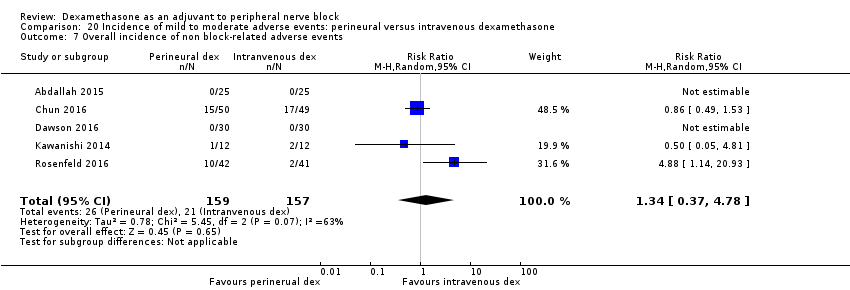 Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 7 Overall incidence of non block‐related adverse events. | ||||
| 8 Postoperative nausea and vomiting Show forest plot | 5 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.22, 1.80] |
| Analysis 20.8 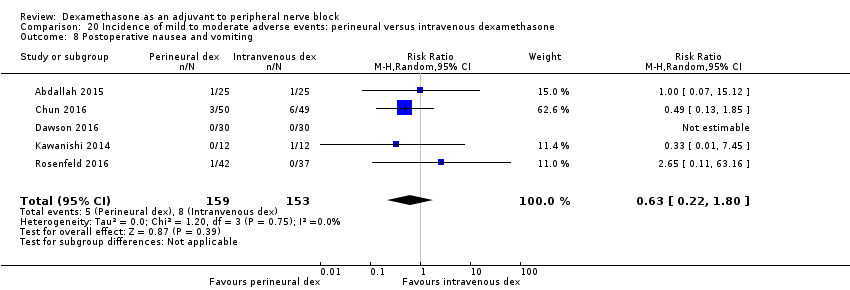 Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 8 Postoperative nausea and vomiting. | ||||
| 9 Dermatologicial symptoms (pruritus/rash) Show forest plot | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [0.22, 89.18] |
| Analysis 20.9  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 9 Dermatologicial symptoms (pruritus/rash). | ||||
| 10 Syncope/fainting Show forest plot | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [0.22, 89.18] |
| Analysis 20.10  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 10 Syncope/fainting. | ||||
| 11 Dizziness Show forest plot | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.06, 2.72] |
| Analysis 20.11  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 11 Dizziness. | ||||
| 12 Wrist, hand or finger pain Show forest plot | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 7.02] |
| Analysis 20.12 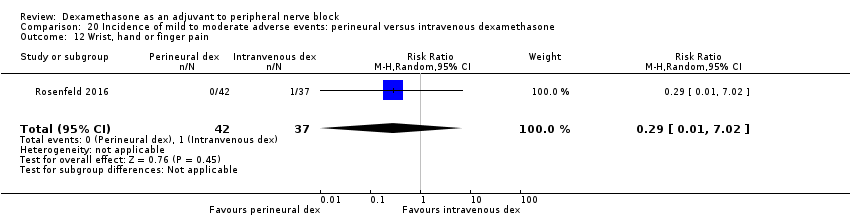 Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 12 Wrist, hand or finger pain. | ||||
| 13 Headache, 10‐pound fluid gain/diarrhoea/frequent urination/ muscle soreness Show forest plot | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.11, 63.16] |
| Analysis 20.13  Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 13 Headache, 10‐pound fluid gain/diarrhoea/frequent urination/ muscle soreness. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 12 hours Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| Analysis 21.1  Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 12 hours. | ||||
| 2 Postoperative pain intensity at 12 hours: low‐ versus high‐dose dexamethasone subgroups Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| Analysis 21.2  Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 12 hours: low‐ versus high‐dose dexamethasone subgroups. | ||||
| 2.1 Low‐dose dexamethasone | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.60, ‐0.47] |
| 2.2 High‐dose dexamethasone | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| Analysis 21.3 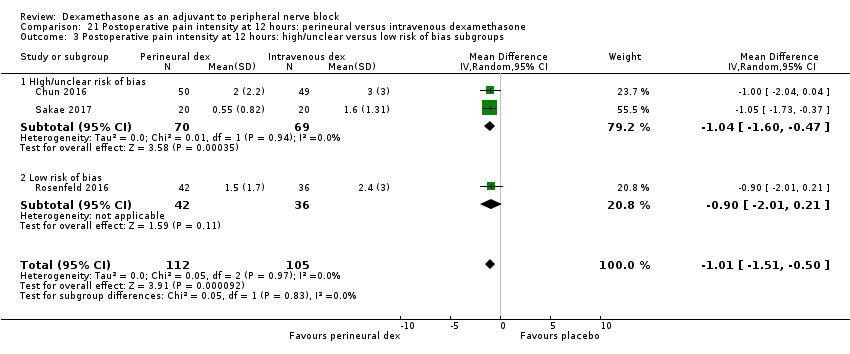 Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups. | ||||
| 3.1 HIgh/unclear risk of bias | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.60, ‐0.47] |
| 3.2 Low risk of bias | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 24 hours Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| Analysis 22.1 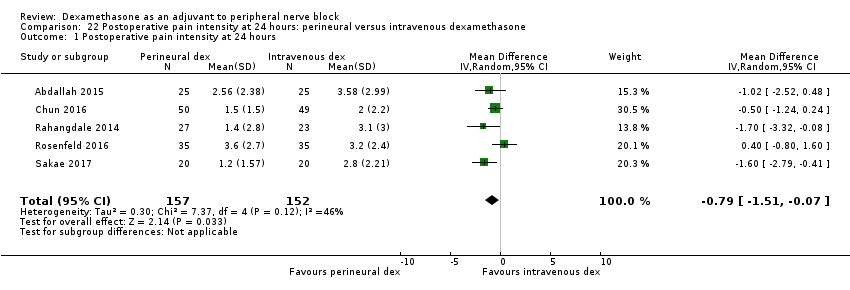 Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 24 hours. | ||||
| 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| Analysis 22.2  Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups. | ||||
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.32, ‐0.08] |
| 2.2 No additive | 4 | 259 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.41, 0.13] |
| 3 Postoperative pain intensity at 24 hours: low‐ versus high‐dose dexamethasonesubgroups Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| Analysis 22.3 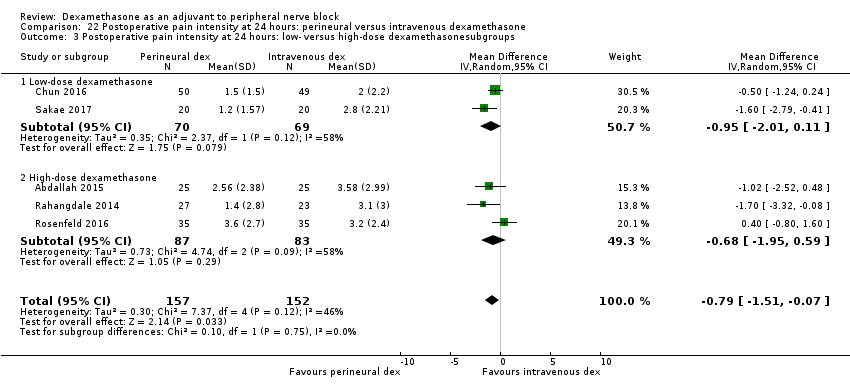 Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 24 hours: low‐ versus high‐dose dexamethasonesubgroups. | ||||
| 3.1 Low‐dose dexamethasone | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.01, 0.11] |
| 3.2 High‐dose dexamethasone | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.95, 0.59] |
| 4 Intensity of postoperative pain at 24 hours: high/unclear risk of bias versus low risk of bias subgroups Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| Analysis 22.4  Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 4 Intensity of postoperative pain at 24 hours: high/unclear risk of bias versus low risk of bias subgroups. | ||||
| 4.1 High/unclear risk of bias | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.01, 0.11] |
| 4.2 Low risk of bias | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.95, 0.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 48 hours Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| Analysis 23.1 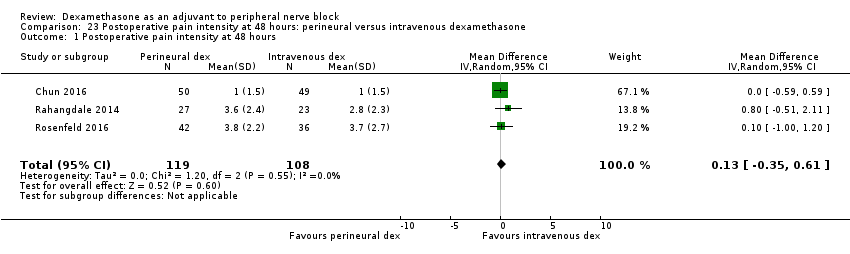 Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 48 hours. | ||||
| 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| Analysis 23.2 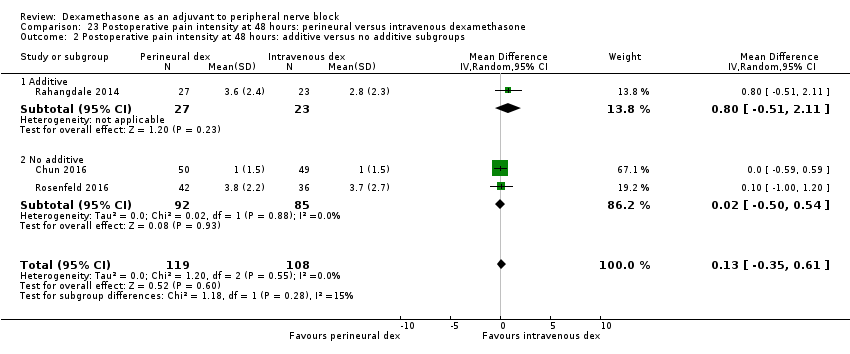 Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups. | ||||
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.51, 2.11] |
| 2.2 No additive | 2 | 177 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.50, 0.54] |
| 3 Postoperative pain intensity at 48 hours: low‐ versus high‐dose dexamethasone subgroups Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| Analysis 23.3  Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 48 hours: low‐ versus high‐dose dexamethasone subgroups. | ||||
| 3.1 Low‐dose dexamethasone | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 3.2 High‐dose dexamethasone | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.45, 1.24] |
| 4 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| Analysis 23.4  Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 4 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups. | ||||
| 4.1 High/unclear risk of bias | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 4.2 Low risk of bias | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.45, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Opioid consumption at 24 hours: perineural versus intravenous dexamethasone Show forest plot | 4 | 242 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐9.93, 2.19] |
| Analysis 24.1 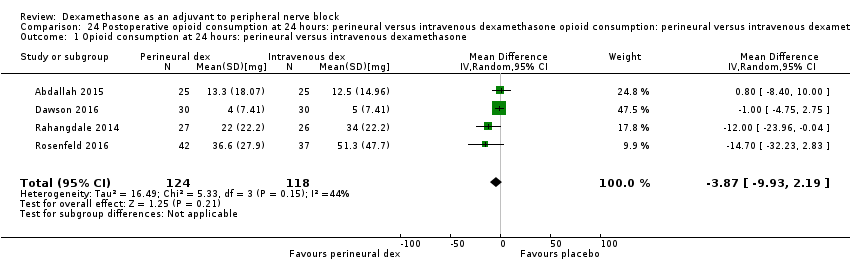 Comparison 24 Postoperative opioid consumption at 24 hours: perineural versus intravenous dexamethasone opioid consumption: perineural versus intravenous dexamethasone subgroups, Outcome 1 Opioid consumption at 24 hours: perineural versus intravenous dexamethasone. | ||||
| 2 24‐hour opioid consumption: additive versus no additive subgroups Show forest plot | 4 | 242 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐9.93, 2.19] |
| Analysis 24.2  Comparison 24 Postoperative opioid consumption at 24 hours: perineural versus intravenous dexamethasone opioid consumption: perineural versus intravenous dexamethasone subgroups, Outcome 2 24‐hour opioid consumption: additive versus no additive subgroups. | ||||
| 2.1 Additive | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐23.96, ‐0.04] |
| 2.2 No additive | 3 | 189 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐6.34, 3.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant satisfaction with pain control Show forest plot | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.33, 0.70] |
| Analysis 25.1  Comparison 25 Participant satisfaction with pain control: perineural versus intravenous dexamethasone, Outcome 1 Participant satisfaction with pain control. | ||||

Flow diagram of included studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
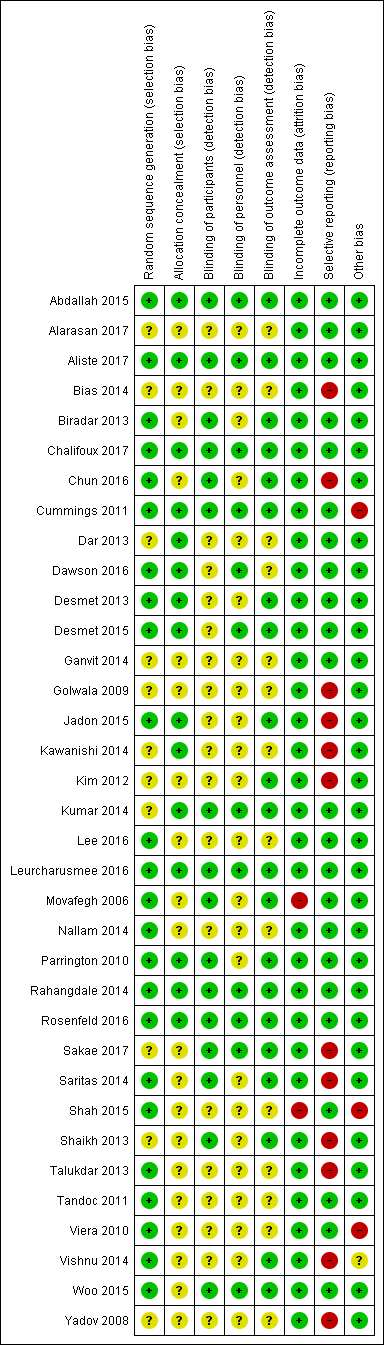
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
![Forest plot of comparison: 1 Duration of sensory block: perineural dexamethasone versus placebo, outcome: 1.1 Duration of sensory block [hours].](/es/cdsr/doi/10.1002/14651858.CD011770.pub2/media/CDSR/CD011770/image_n/nCD011770-AFig-FIG04.png)
Forest plot of comparison: 1 Duration of sensory block: perineural dexamethasone versus placebo, outcome: 1.1 Duration of sensory block [hours].

Forest plot of comparison: 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, outcome: 4.1 Postoperative pain intensity at12 hours.

Forest plot of comparison: 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, outcome: 5.1 Postoperative pain intensity at 24 hours.
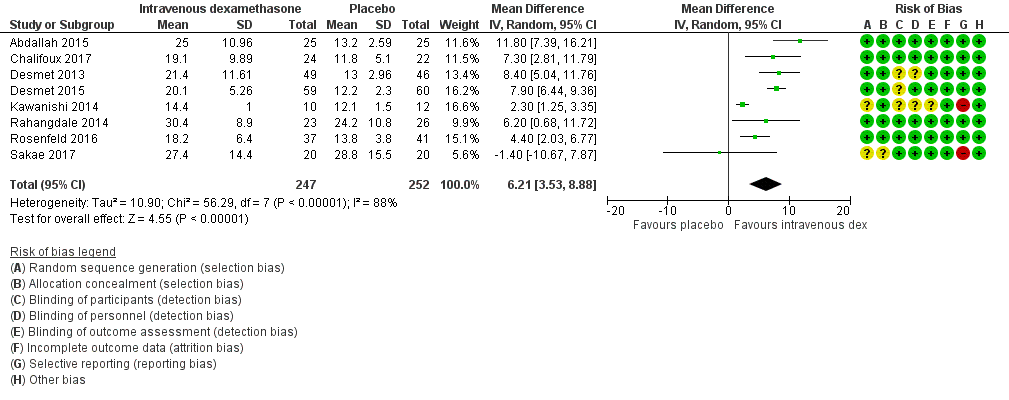
Forest plot of comparison: 9 Duration of sensory block: intravenous dexamethasone versus placebo , outcome: 9.1 Duration of sensory block.
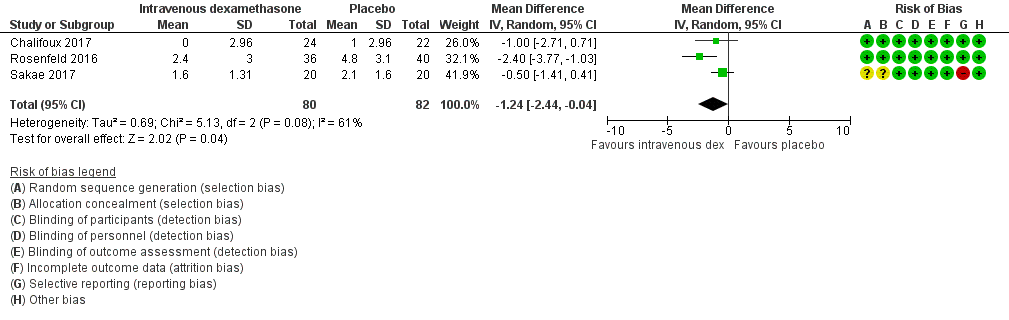
Forest plot of comparison: 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, outcome: 12.1 Postoperative pain intensity at 12 hours.

Forest plot of comparison: 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, outcome: 13.1 Postoperative pain intensity at 24 hours.

Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 1 Duration of sensory block.

Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 2 Duration of sensory block: long‐ versus medium‐acting local anaesthetic subgroups.
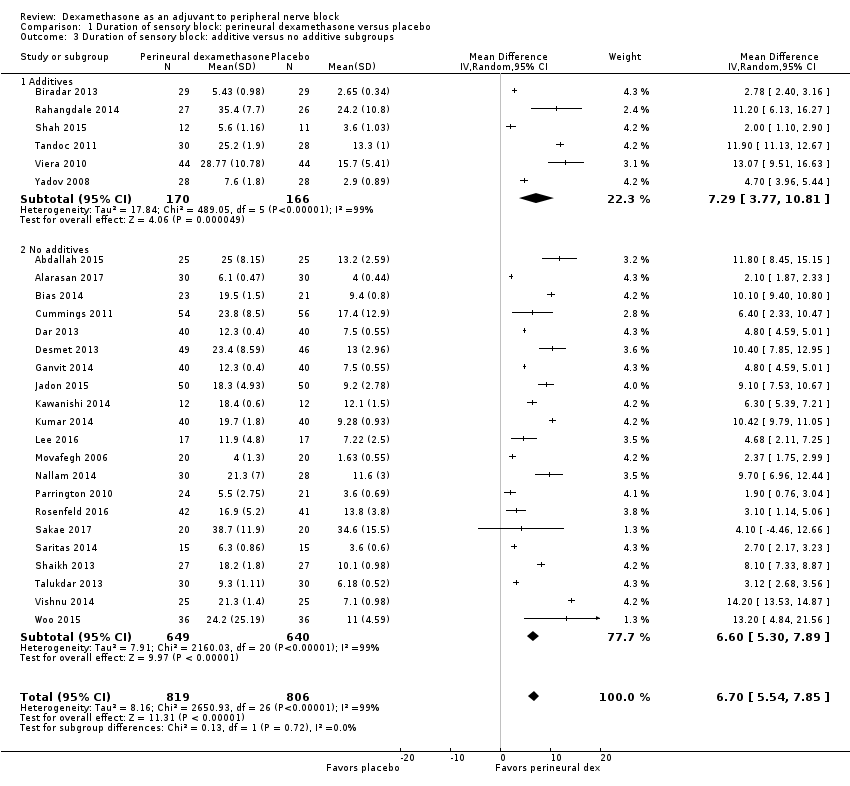
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 3 Duration of sensory block: additive versus no additive subgroups.
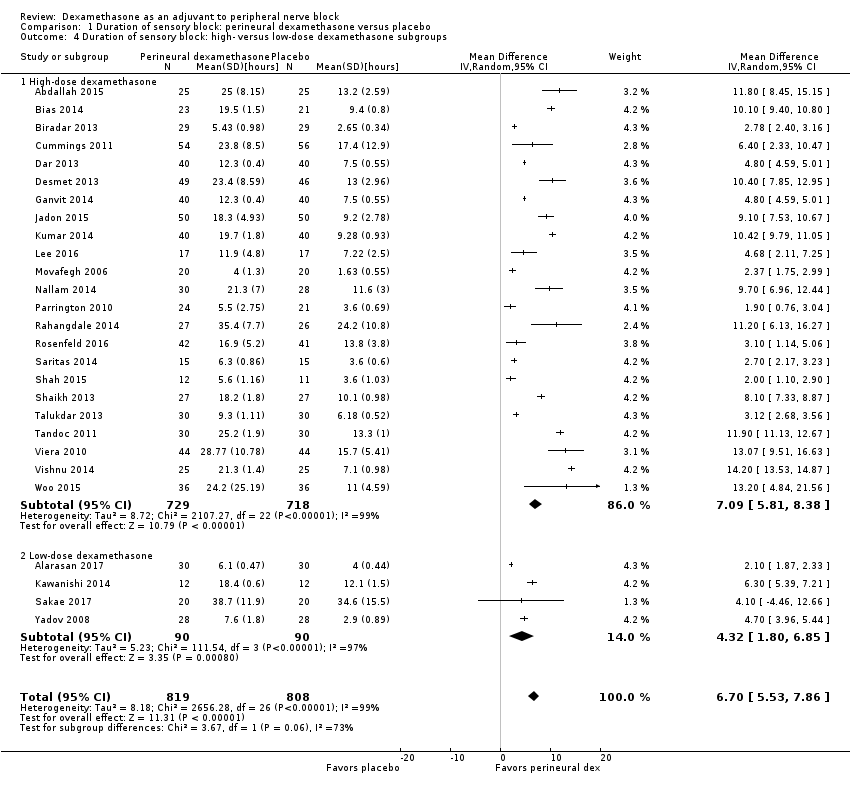
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 4 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups.
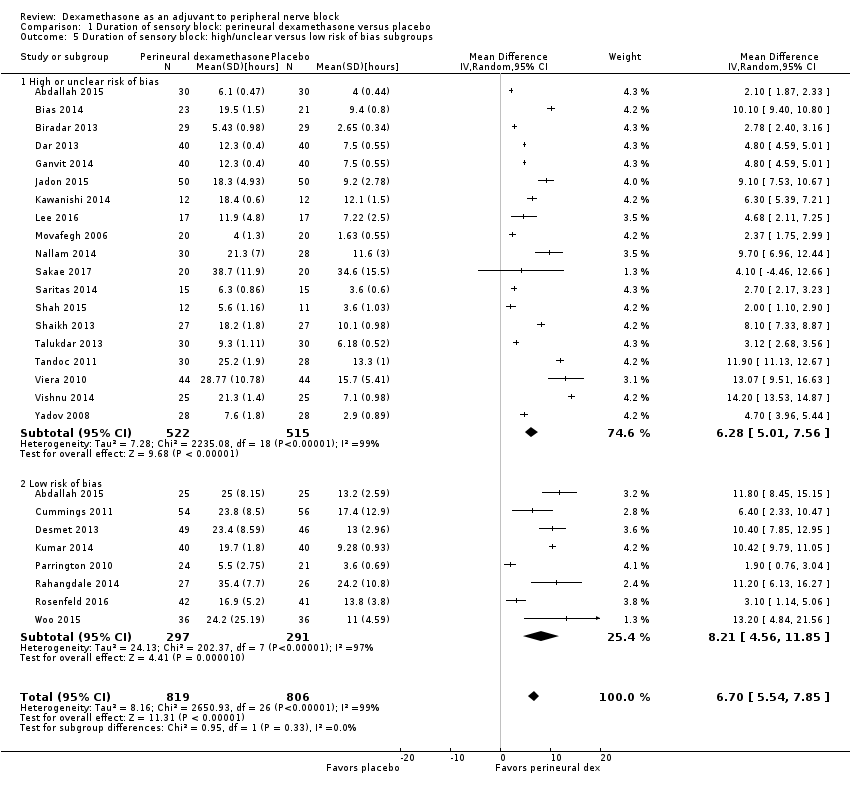
Comparison 1 Duration of sensory block: perineural dexamethasone versus placebo, Outcome 5 Duration of sensory block: high/unclear versus low risk of bias subgroups.
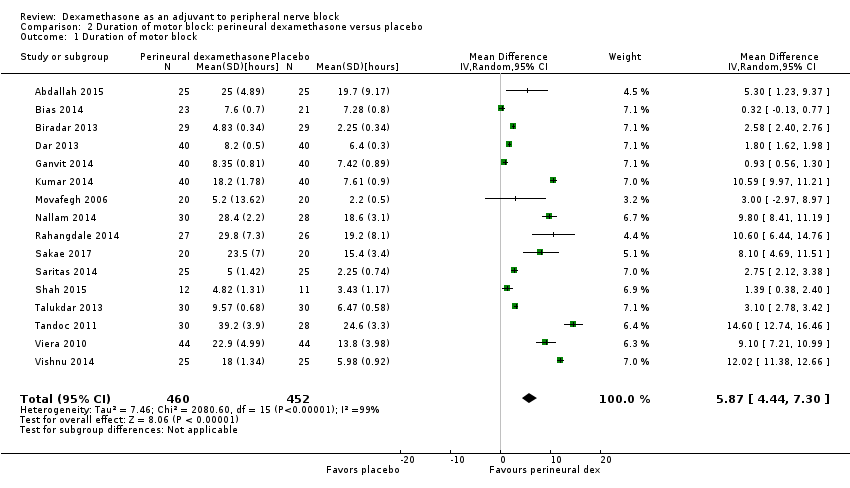
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 1 Duration of motor block.
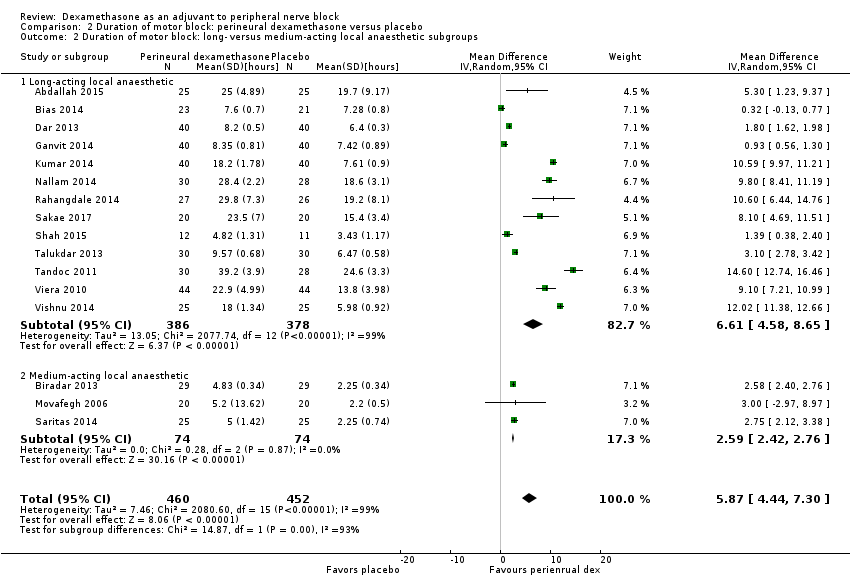
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 2 Duration of motor block: long‐ versus medium‐acting local anaesthetic subgroups.

Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 3 Duration of motor block: additives verus no additives subgroups.

Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 4 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups.
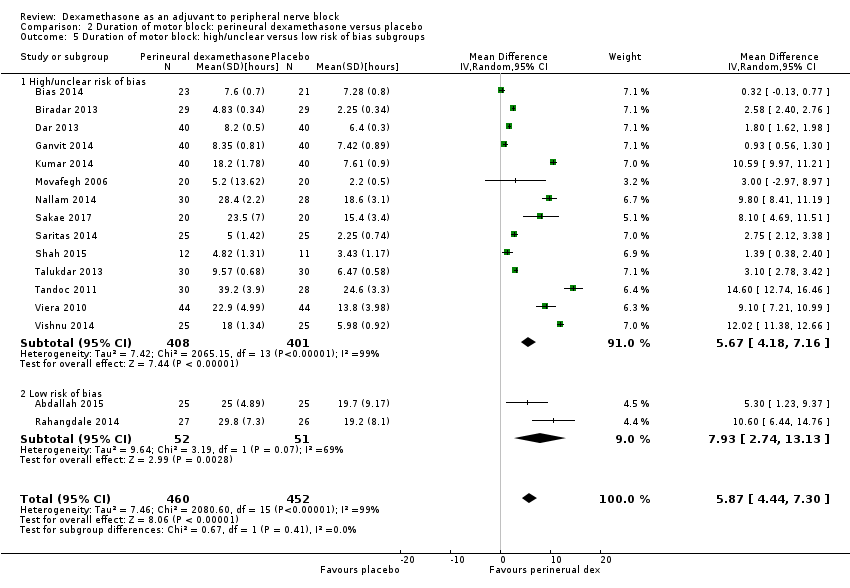
Comparison 2 Duration of motor block: perineural dexamethasone versus placebo, Outcome 5 Duration of motor block: high/unclear versus low risk of bias subgroups.
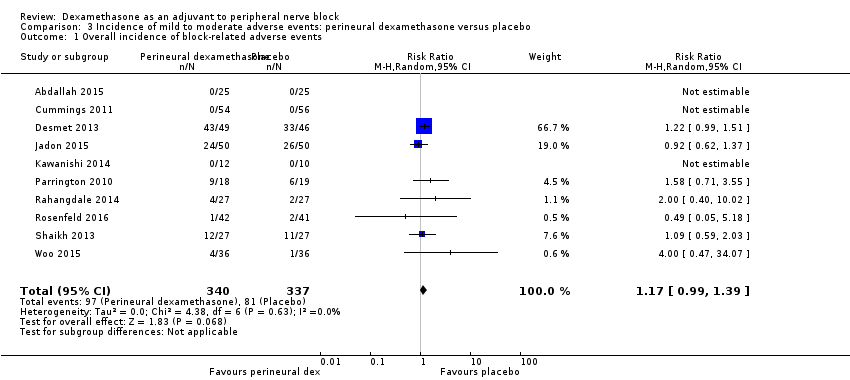
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 1 Overall incidence of block‐related adverse events.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 2 Numbness/tingling 14 days after surgery.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 3 Residual motor block/weakness 24 hours after surgery.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 4 Horner Syndrome.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 5 Hoarseness.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 6 Diaphragmatic paresis.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 7 Dyspnoea.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 8 Vascular injury.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 9 Cranial nerve 12 palsy.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 10 Bruising.
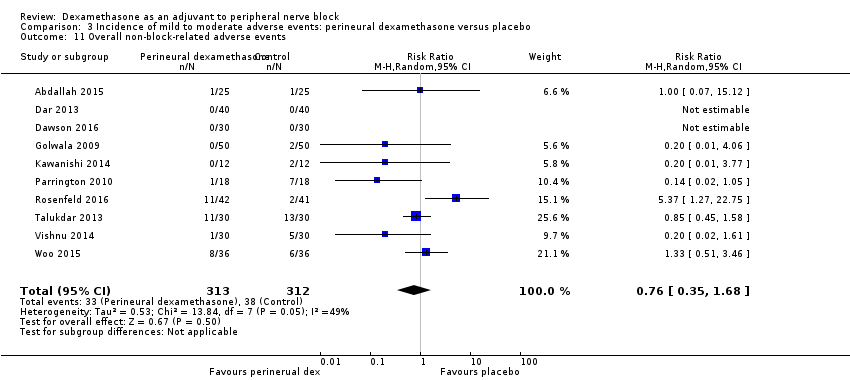
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 11 Overall non‐block‐related adverse events.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 12 Postoperative nausea and vomiting.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 13 Deep sedation.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 14 Dermatological symptoms (pruritus/rash).
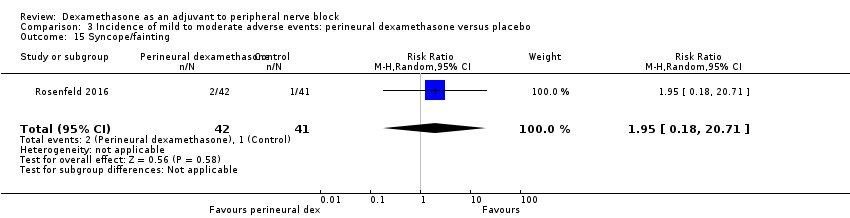
Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 15 Syncope/fainting.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 16 Bradycardia.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 17 Hypotension.

Comparison 3 Incidence of mild to moderate adverse events: perineural dexamethasone versus placebo, Outcome 18 Headache/10‐pound fluid gain/diarrhoea/frequent urination/muscle soreness.
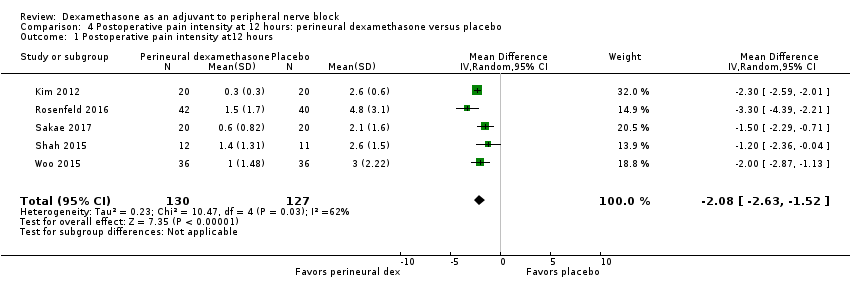
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at12 hours.
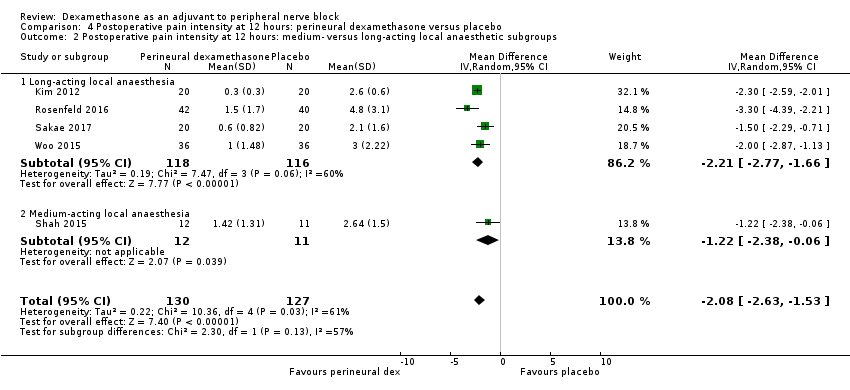
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 12 hours: medium‐ versus long‐acting local anaesthetic subgroups.
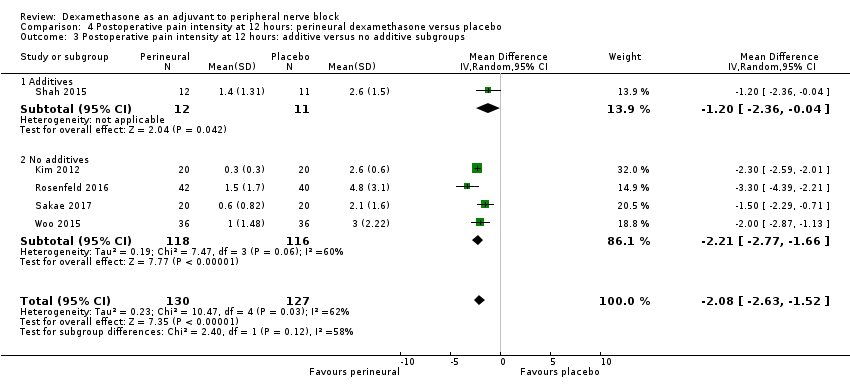
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 12 hours: additive versus no additive subgroups.

Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups.
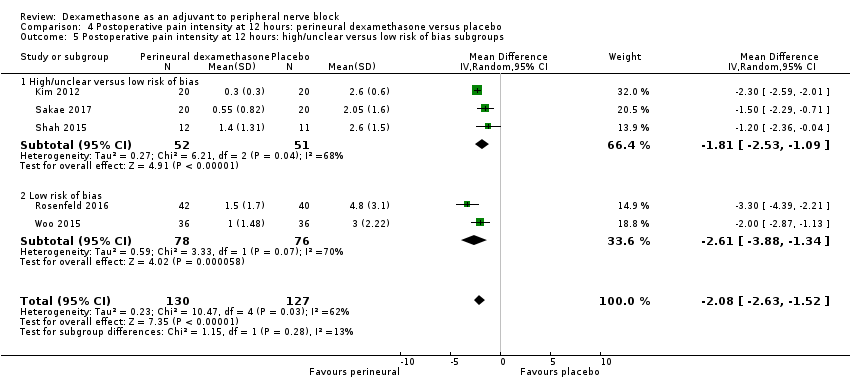
Comparison 4 Postoperative pain intensity at 12 hours: perineural dexamethasone versus placebo, Outcome 5 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups.

Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 24 hours.

Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 24 hours: long‐ versus medium‐acting local anaesthetic subgroups.
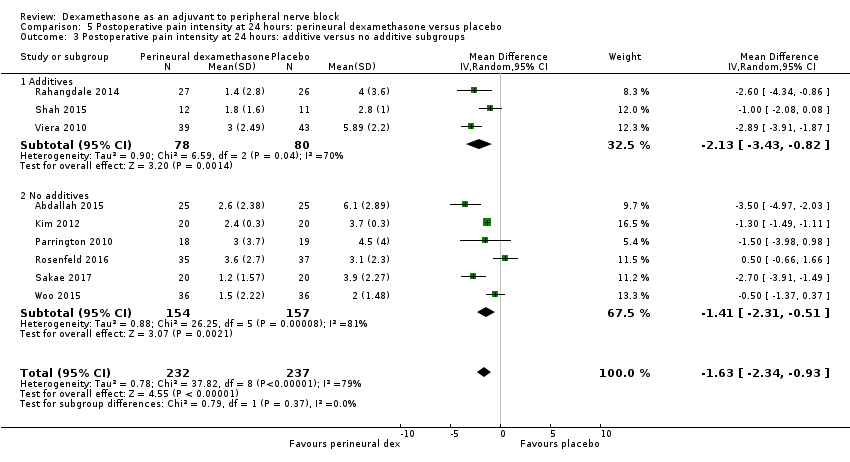
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 24 hours: additive versus no additive subgroups.
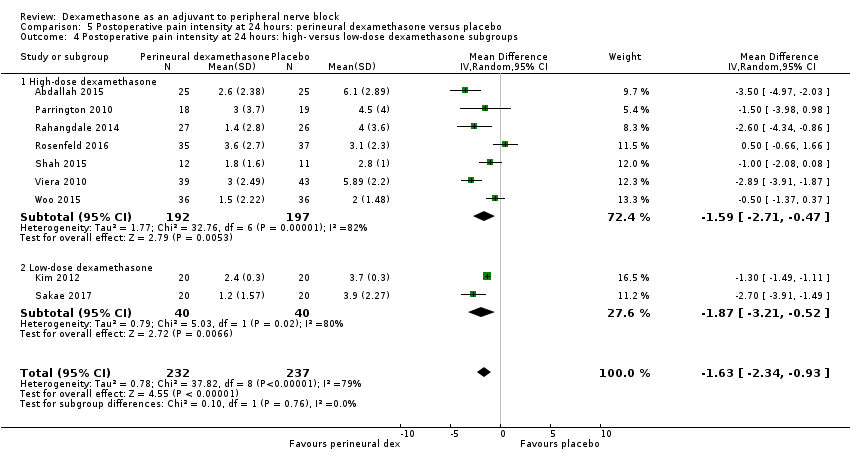
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups.
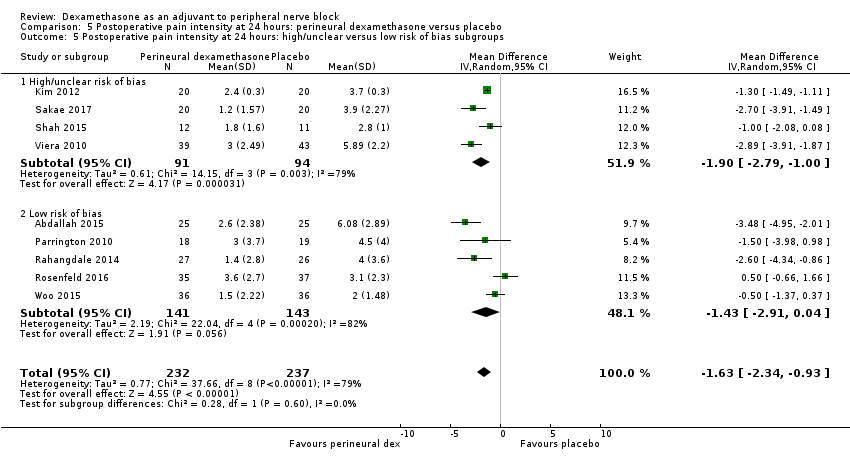
Comparison 5 Postoperative pain intensity at 24 hours: perineural dexamethasone versus placebo, Outcome 5 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups.

Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 48 hours.

Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 48 hours: additives versus no additives subgroups.
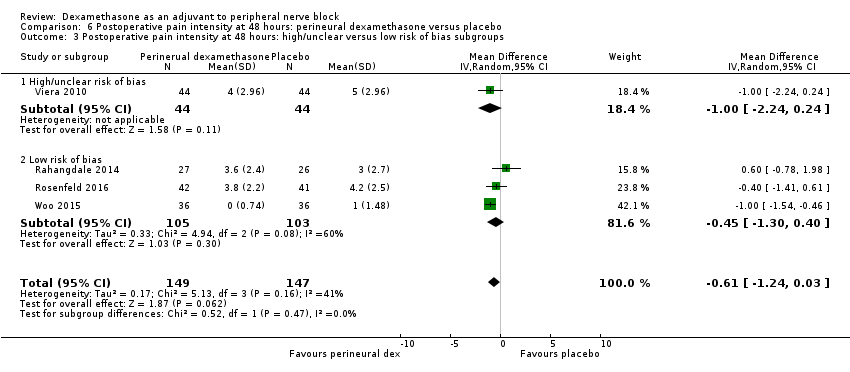
Comparison 6 Postoperative pain intensity at 48 hours: perineural dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups.
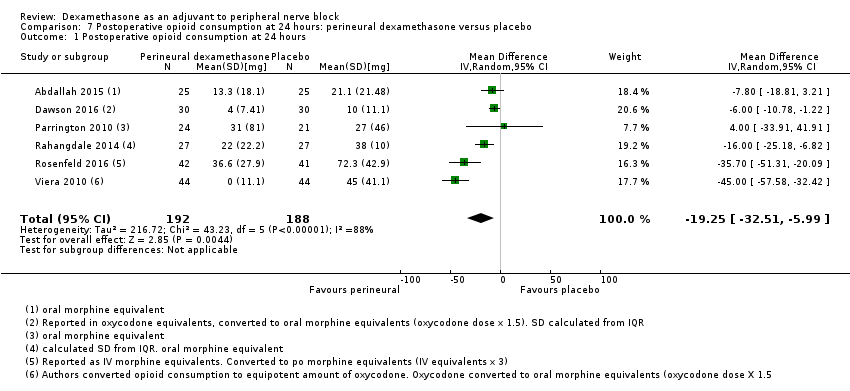
Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 1 Postoperative opioid consumption at 24 hours.

Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 2 Opioid consumption at 24 hours medium‐ versus long‐acting local anaesthetic subgroups.

Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 3 Opioid consumption at 24 hours: additive versus no additive subgroups.
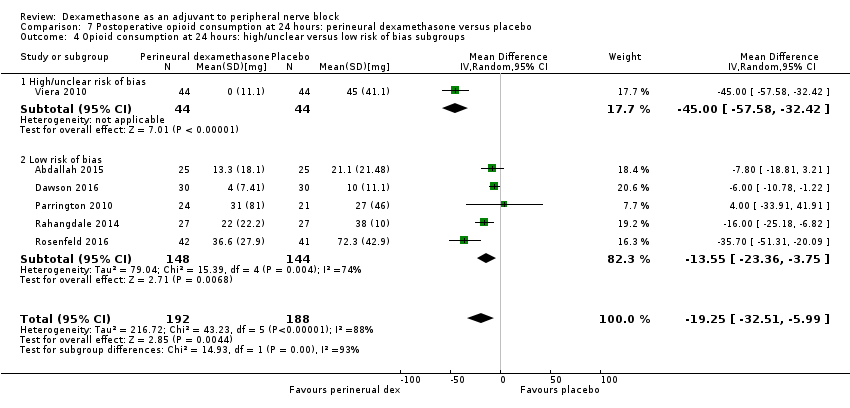
Comparison 7 Postoperative opioid consumption at 24 hours: perineural dexamethasone versus placebo, Outcome 4 Opioid consumption at 24 hours: high/unclear versus low risk of bias subgroups.
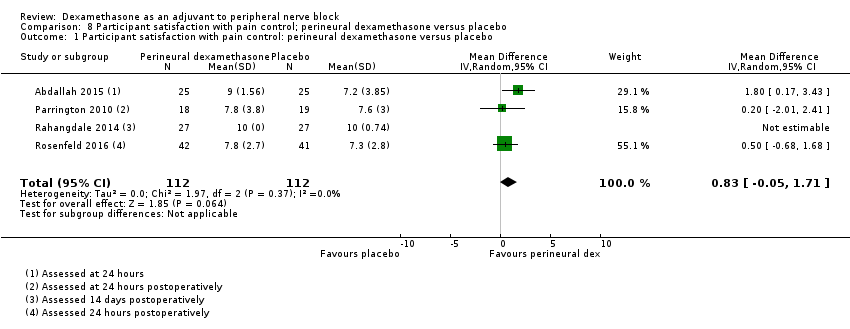
Comparison 8 Participant satisfaction with pain control; perineural dexamethasone versus placebo, Outcome 1 Participant satisfaction with pain control: perineural dexamethasone versus placebo.
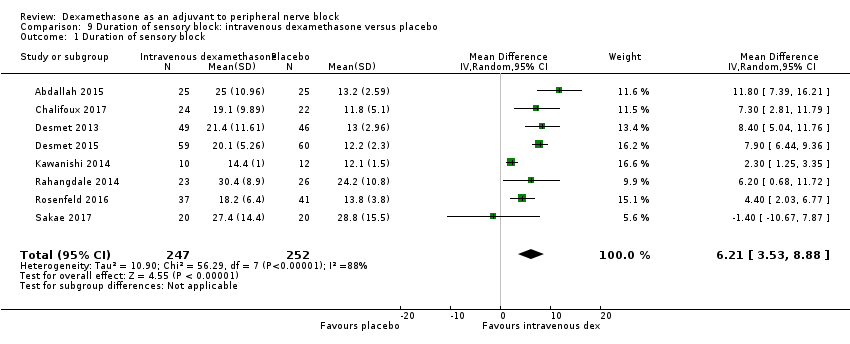
Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 1 Duration of sensory block.
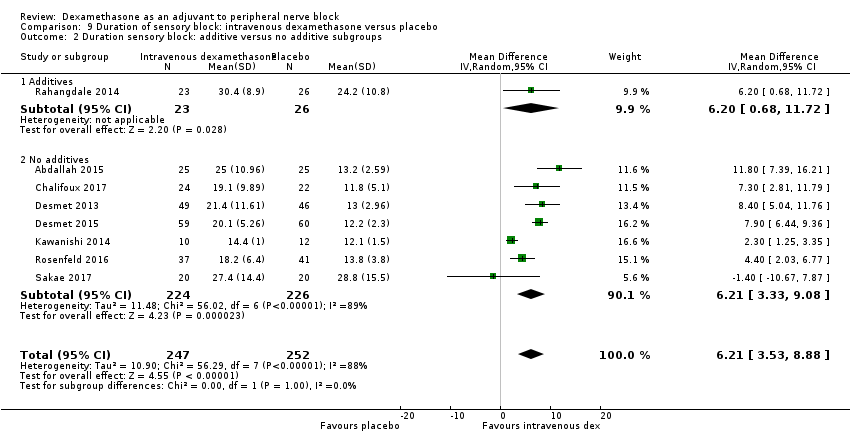
Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 2 Duration sensory block: additive versus no additive subgroups.

Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 3 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups.
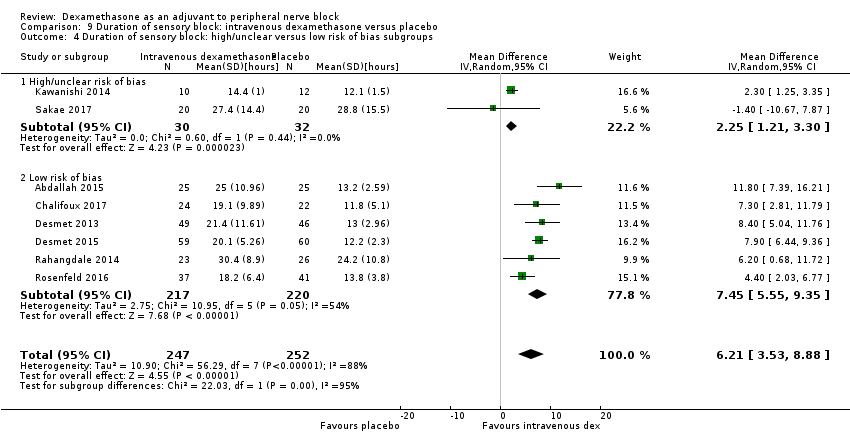
Comparison 9 Duration of sensory block: intravenous dexamethasone versus placebo, Outcome 4 Duration of sensory block: high/unclear versus low risk of bias subgroups.

Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 1 Duration of motor block.

Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 2 Duration of motor block: additive versus no additive subgroups.
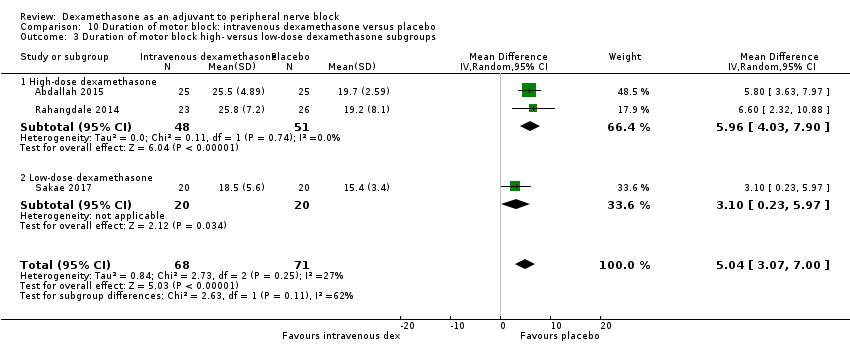
Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 3 Duration of motor block high‐ versus low‐dose dexamethasone subgroups.
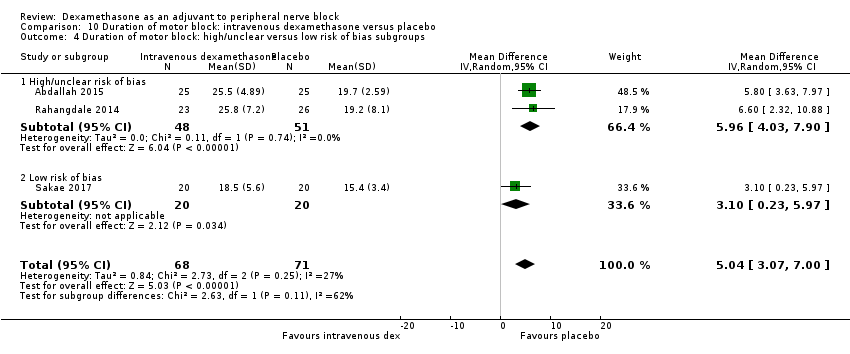
Comparison 10 Duration of motor block: intravenous dexamethasone versus placebo, Outcome 4 Duration of motor block: high/unclear versus low risk of bias subgroups.
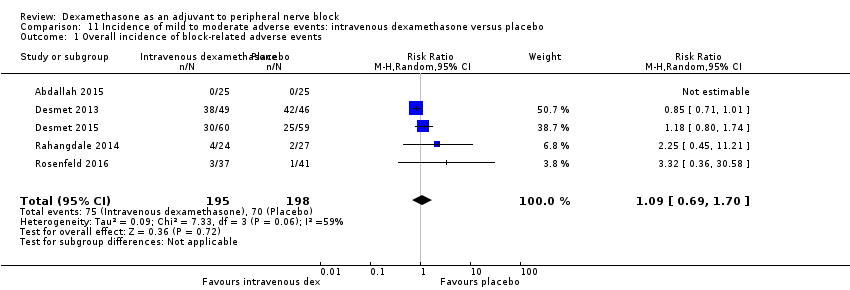
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 1 Overall incidence of block‐related adverse events.

Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 2 Numbness/tingling 14 days after surgery.

Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 3 Residual motor block/muscle weakness 24 hours after surgery.

Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 4 Horner syndrome.
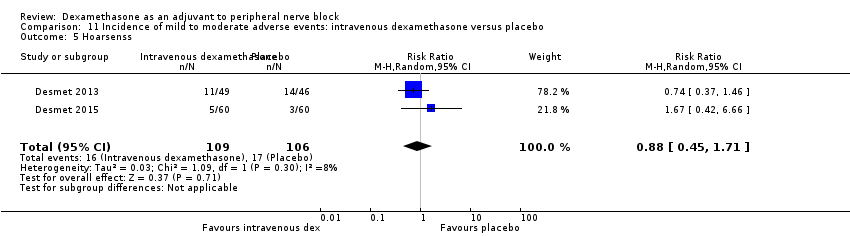
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 5 Hoarsenss.
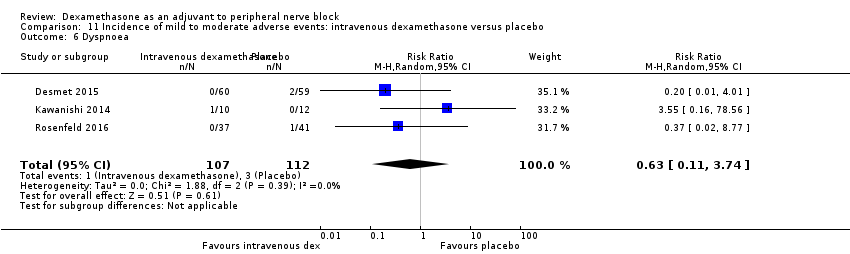
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 6 Dyspnoea.
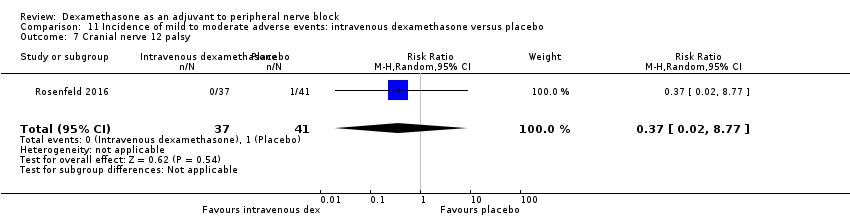
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 7 Cranial nerve 12 palsy.
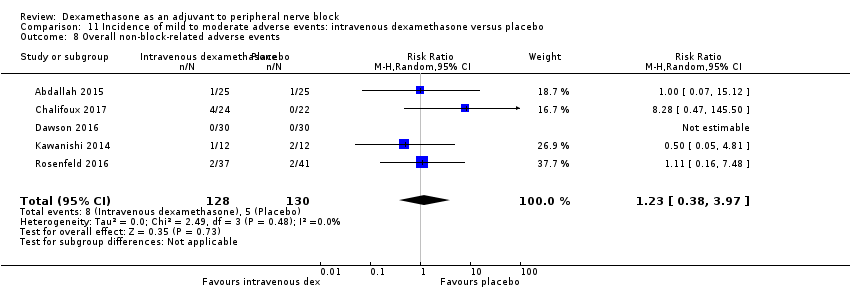
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 8 Overall non‐block‐related adverse events.
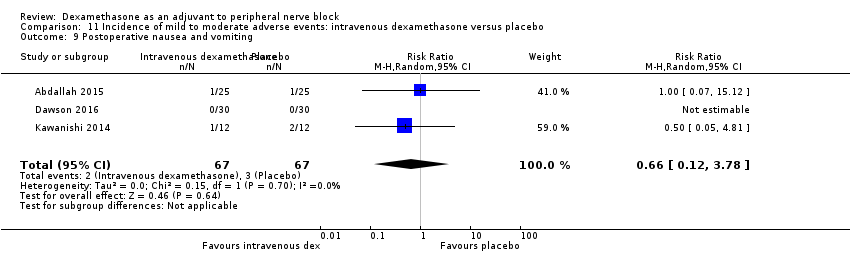
Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 9 Postoperative nausea and vomiting.

Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 10 Dermatological symptoms.

Comparison 11 Incidence of mild to moderate adverse events: intravenous dexamethasone versus placebo, Outcome 11 Dizziness/wrist, hand or finger pain, constipation.
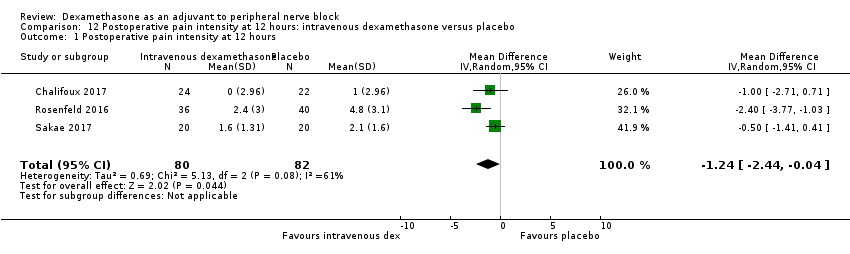
Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 12 hours.
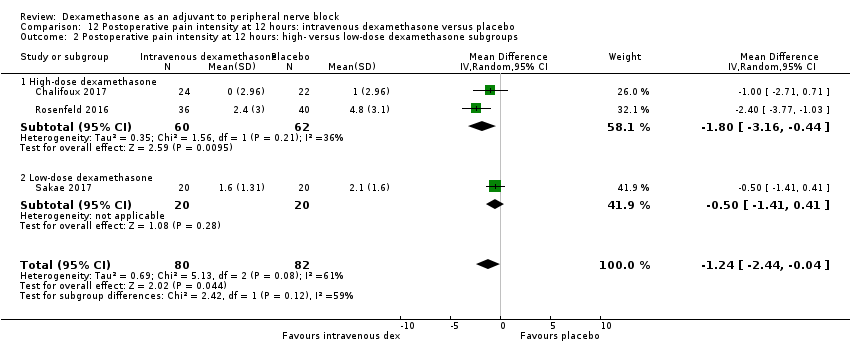
Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups.
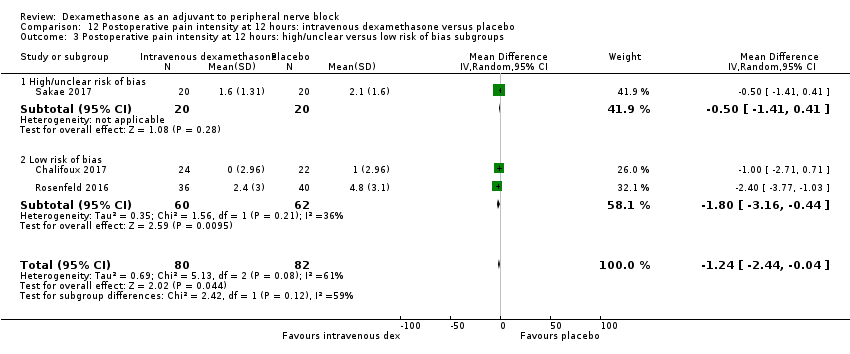
Comparison 12 Postoperative pain intensity at 12 hours: intravenous dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups.

Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 24 hours.

Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups.

Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 3 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups.
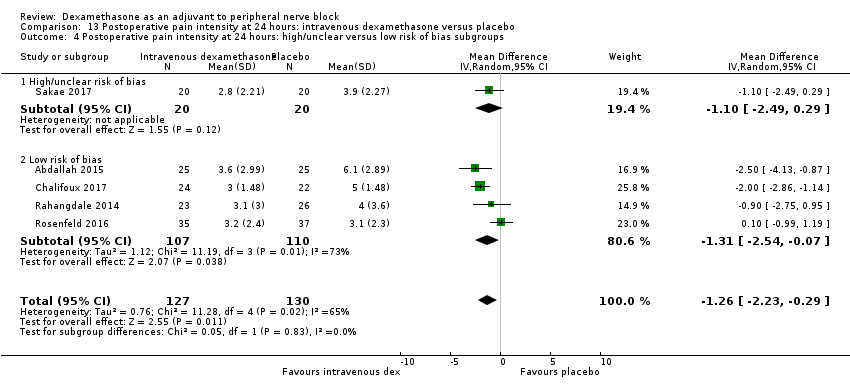
Comparison 13 Postoperative pain intensity at 24 hours: intravenous dexamethasone versus placebo, Outcome 4 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups.

Comparison 14 Postoperative pain intensity at 48 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative pain intensity at 48 hours.

Comparison 14 Postoperative pain intensity at 48 hours: intravenous dexamethasone versus placebo, Outcome 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups.

Comparison 15 Postoperative opioid consumption at 24 hours: intravenous dexamethasone versus placebo, Outcome 1 24‐hour opioid consumption.
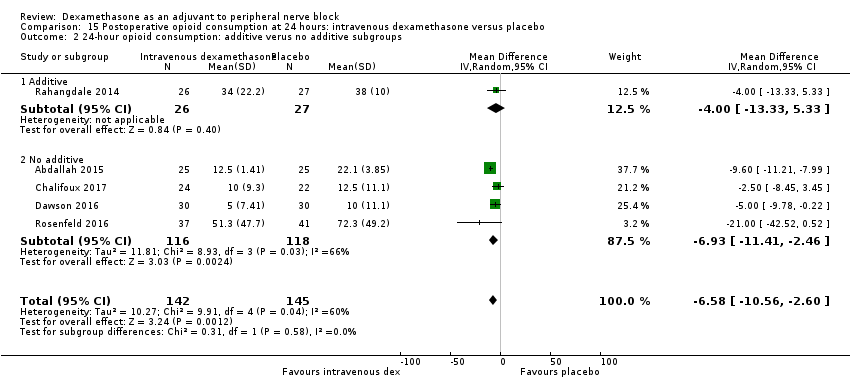
Comparison 15 Postoperative opioid consumption at 24 hours: intravenous dexamethasone versus placebo, Outcome 2 24‐hour opioid consumption: additive verus no additive subgroups.
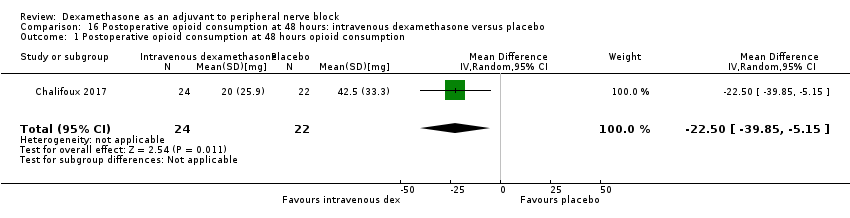
Comparison 16 Postoperative opioid consumption at 48 hours: intravenous dexamethasone versus placebo, Outcome 1 Postoperative opioid consumption at 48 hours opioid consumption.
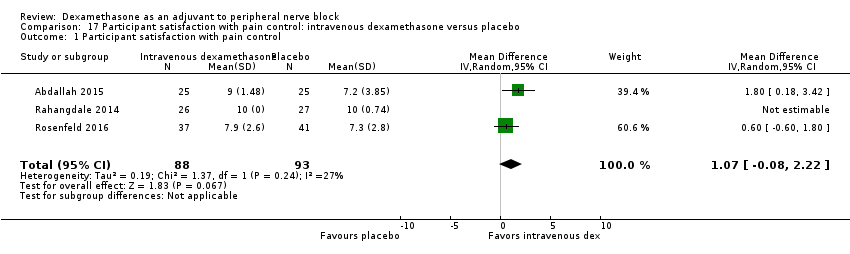
Comparison 17 Participant satisfaction with pain control: intravenous dexamethasone versus placebo, Outcome 1 Participant satisfaction with pain control.

Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 1 Duration of sensory block.
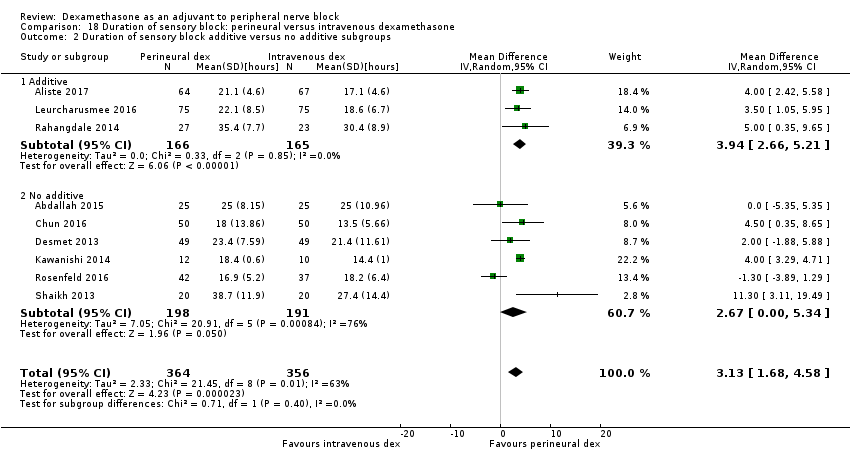
Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 2 Duration of sensory block additive versus no additive subgroups.

Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 3 Duration sensory block high‐dose versus low‐dose dexamethasone subgroups.

Comparison 18 Duration of sensory block: perineural versus intravenous dexamethasone, Outcome 4 Duration sensory block high/unclear versus low risk of bias subgroups.
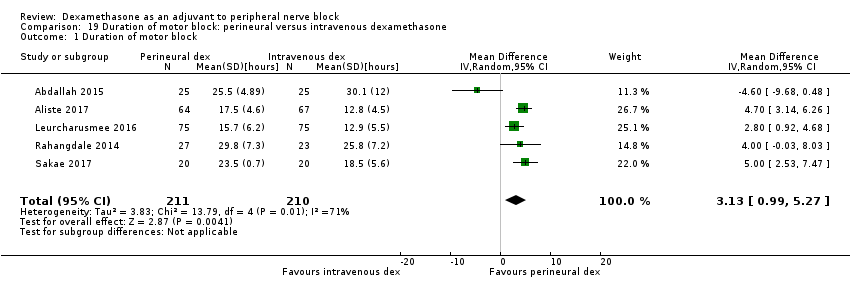
Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 1 Duration of motor block.

Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 2 Duration of motor block: additive versus no additive subgroups.

Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 3 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups.
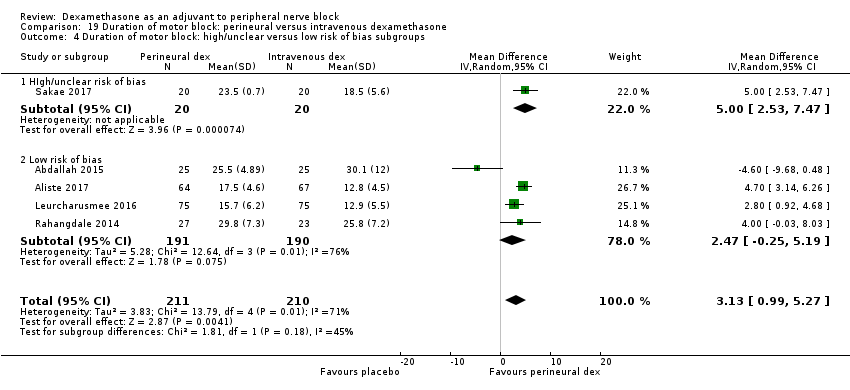
Comparison 19 Duration of motor block: perineural versus intravenous dexamethasone, Outcome 4 Duration of motor block: high/unclear versus low risk of bias subgroups.

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 1 Overall incidence of block‐related adverse events.
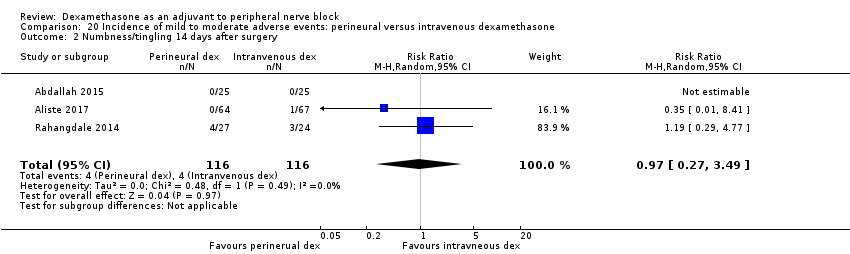
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 2 Numbness/tingling 14 days after surgery.

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 3 Residual motor block/weakness at 24 hours.
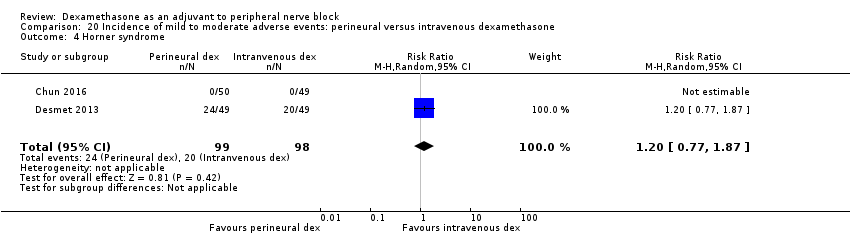
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 4 Horner syndrome.

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 5 Hoarsness.

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 6 Cranial nerve 12 motor palsy.
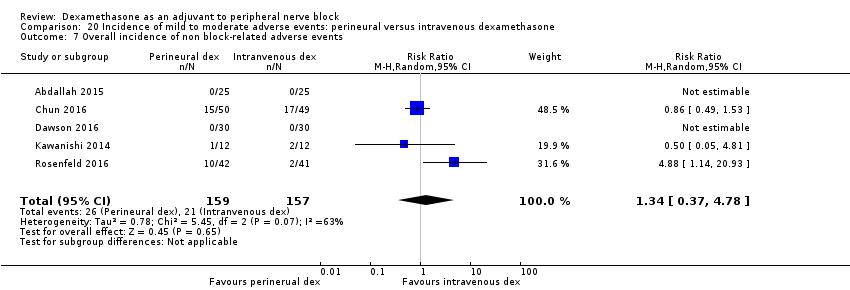
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 7 Overall incidence of non block‐related adverse events.
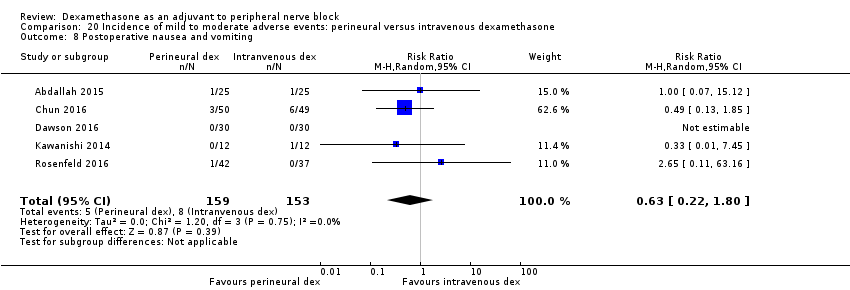
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 8 Postoperative nausea and vomiting.

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 9 Dermatologicial symptoms (pruritus/rash).

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 10 Syncope/fainting.

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 11 Dizziness.

Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 12 Wrist, hand or finger pain.
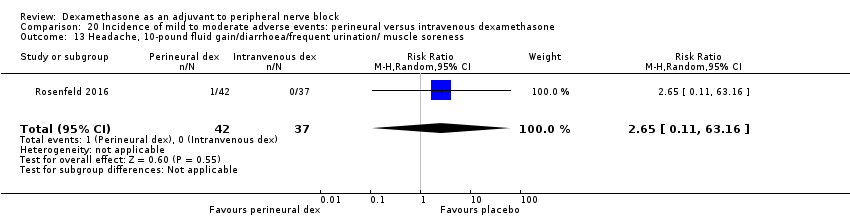
Comparison 20 Incidence of mild to moderate adverse events: perineural versus intravenous dexamethasone, Outcome 13 Headache, 10‐pound fluid gain/diarrhoea/frequent urination/ muscle soreness.
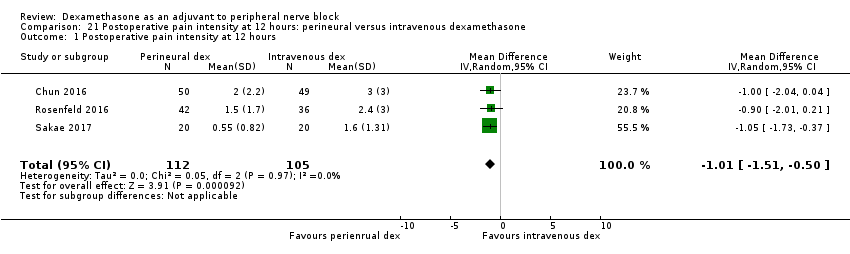
Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 12 hours.

Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 12 hours: low‐ versus high‐dose dexamethasone subgroups.
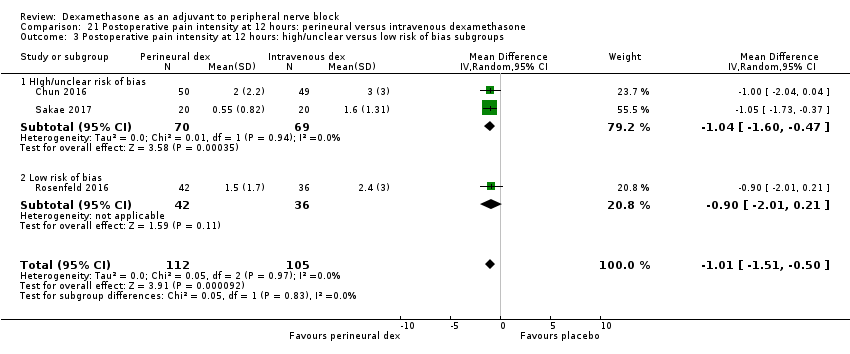
Comparison 21 Postoperative pain intensity at 12 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups.
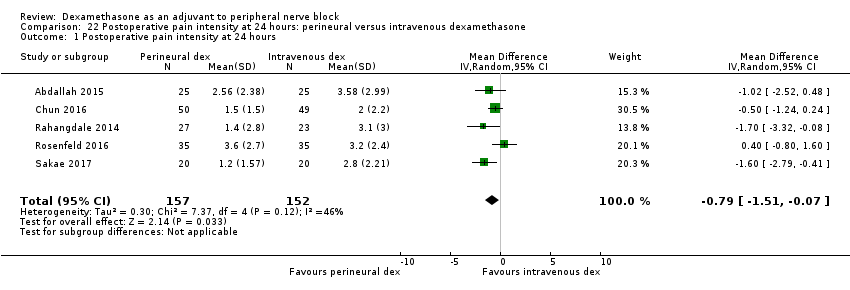
Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 24 hours.
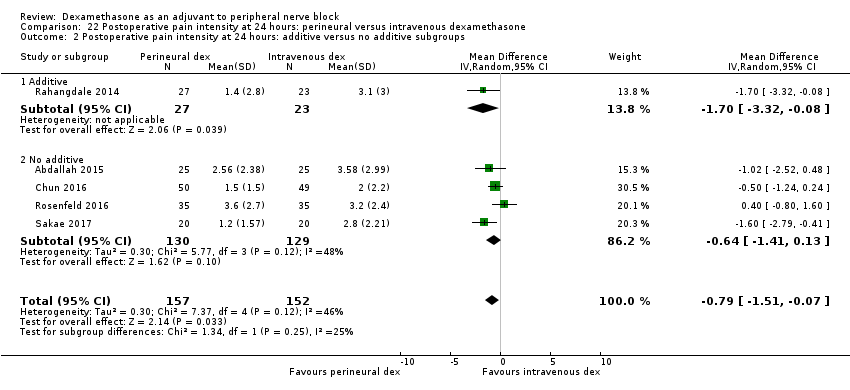
Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups.
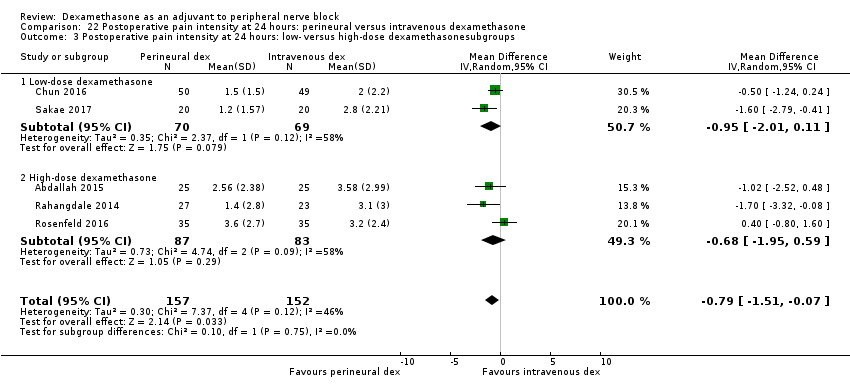
Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 24 hours: low‐ versus high‐dose dexamethasonesubgroups.

Comparison 22 Postoperative pain intensity at 24 hours: perineural versus intravenous dexamethasone, Outcome 4 Intensity of postoperative pain at 24 hours: high/unclear risk of bias versus low risk of bias subgroups.

Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 1 Postoperative pain intensity at 48 hours.
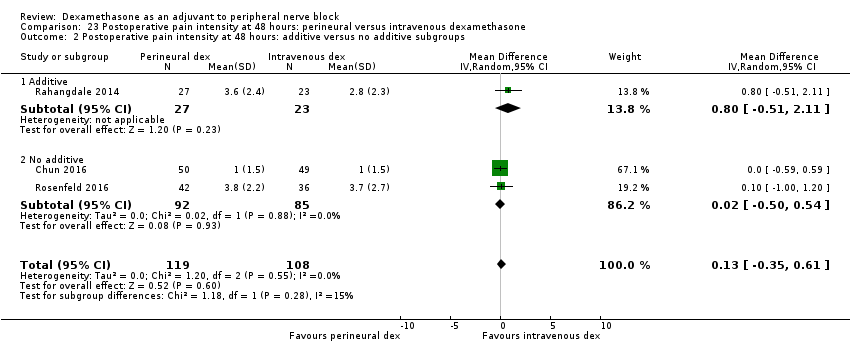
Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups.
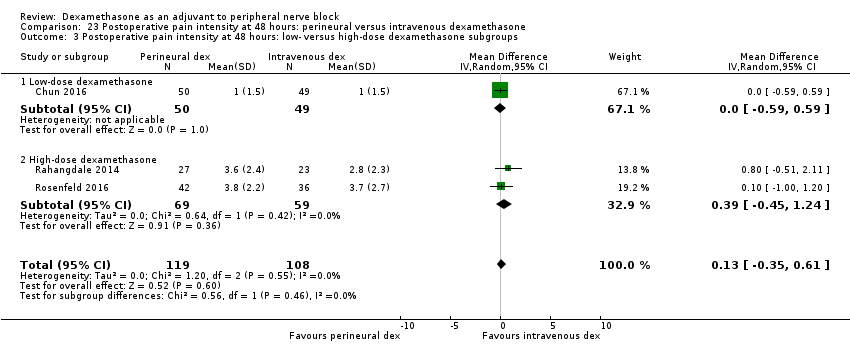
Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 3 Postoperative pain intensity at 48 hours: low‐ versus high‐dose dexamethasone subgroups.

Comparison 23 Postoperative pain intensity at 48 hours: perineural versus intravenous dexamethasone, Outcome 4 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups.
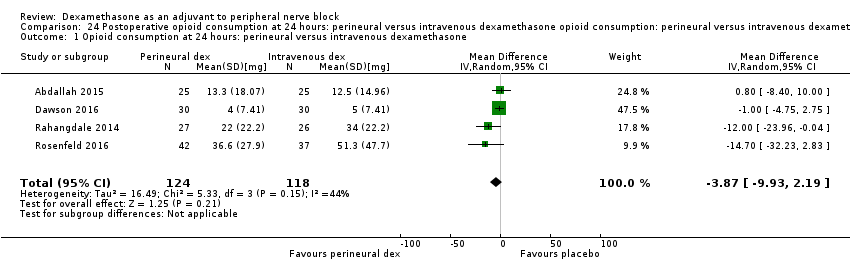
Comparison 24 Postoperative opioid consumption at 24 hours: perineural versus intravenous dexamethasone opioid consumption: perineural versus intravenous dexamethasone subgroups, Outcome 1 Opioid consumption at 24 hours: perineural versus intravenous dexamethasone.

Comparison 24 Postoperative opioid consumption at 24 hours: perineural versus intravenous dexamethasone opioid consumption: perineural versus intravenous dexamethasone subgroups, Outcome 2 24‐hour opioid consumption: additive versus no additive subgroups.

Comparison 25 Participant satisfaction with pain control: perineural versus intravenous dexamethasone, Outcome 1 Participant satisfaction with pain control.
| Patient or population: participants undergoing surgery with peripheral nerve block Intervention: perineural dexamethasone Comparison: placebo | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants | Quality of the evidence | |
| Risk with placebo | Risk with perineural dexamethasone | |||
| Duration of sensory block (we included all studies describing duration of sensory block, regardless of how it was described) | The mean duration of sensory block was 10.2 hours | The mean duration of sensory block in the perineural dexamethasone group was 6.70 hours longer (5.54 longer to 7.85 longer) | 1625 | ⨁⨁◯◯ |
| Incidence of serious adverse events (we used the NIH definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, disability or congenital anomaly) | In seven studies, authors reported that they assessed for serious adverse events. Five serious adverse events were reported in three studies: one block‐related adverse event (pneumothorax) occurred in one participant in a trial comparing perineural dexamethasone and placebo; however, group allocation was not reported. The remaining non‐block‐related events occurred in two trials comparing perineural dexamethasone, intravenous dexamethasone and placebo. Two participants in the placebo group required hospitalization within one week of surgery; one for a fall, and one for a bowel infection. One participant in the placebo group developed Complex Regional Pain Syndrome Type I and one in the intravenous dexamethasone group developed pneumonia. | 620 (7 RCTs) | ⨁◯◯◯ LOWb | |
| Postoperative pain intensity at 12 hours (assessed by pain scores on an 11‐point VAS) | The mean postoperative pain intensity at 12 hours was 3.0 | The mean postoperative pain score at 12 hours in the perineural dexamethasone group was 2.08 points lower (1.52 lower to 2.63 lower) | 257 | ⨁◯◯◯ LOWc |
| Postoperative pain intensity at 24 hours. (assessed by pain scores on an 11‐point VAS) | The mean postoperative pain intensity at 24 hours was 3.9 | The mean postoperative pain score at 24 hours in the perineural dexamethasone group was 1.63 points lower (0.93 lower to 2.34 lower) | 469 | ⨁⨁◯◯ |
| Postoperative pain intensity at 48 hours (assessed by pain scores on an 11‐point VAS) | The mean postoperative pain intensity at 48 hours was 3.3 | The mean postoperative pain score at 48 hours in the perineural dexamethasone group was 0.61 points lower (1.24 lower to 0.03 higher) | 296 | ⨁⨁◯◯ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded by one level for risk of bias as 19 out of 27 studies are at unclear risk of bias. Reasons include lack of reporting on random sequence generation, concealment allocation, blinding, and attrition bias. Downgraded by one level for inconsistency (I2 = 99%, P value for heterogeneity is < 0.00001) and heterogeneity is not explained by subgroup analyses; point estimates vary widely among studies, confidence intervals show minimal overlap). bDowngraded by one level for risk of bias as four out of the seven studies are at unclear risk of bias. Reasons include lack of reporting on random sequence generation, concealment allocation, blinding, and evidence of selective reporting bias. Downgraded by two levels for imprecision due to very low number of events. c Downgraded by one level for risk of bias. Three out of five studies are at unclear risk of bias. Reasons include lack of reporting on random sequence generation and allocation concealment and evidence of attrition bias, selective reporting bias, and stopping early for benefit. Downgraded by one level for inconsistency (I2 = 61%, P value for heterogeneity is 0.03) and heterogeneity is not explained by subgroup analyses; point estimates vary widely among studies, confidence intervals show minimal overlap dDowngraded by one level for inconsistency (I2 = 80%, P value for heterogeneity is < 0.00001) and heterogeneity is not explained by subgroup analyses; point estimates vary widely across studies. Downgraded by one level for imprecision (95% confidence interval includes no clinical effect and a clinical effect). By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS. e Downgraded by two levels for imprecision because of a sparse number of participants (n=296) and a very wide confidence interval demonstrating that the treatment effect is not statistically significant and of questionable clinical significance. | ||||
| Patient or population: participants undergoing surgery with peripheral nerve block Intervention: intravenous dexamethasone Comparison: placebo | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants | Quality of the evidence | |
| Risk with placebo | Risk with intravenous dexamethasone | |||
| Duration of sensory block (we included all studies describing duration of sensory block regardless of how it was described) | The mean duration of sensory block was 16.1 hours | The mean duration of sensory block in the intravenous dexamethasone group was 6.21 hours longer (3.53 longer to 8.88 longer) | 499 | ⨁⨁⨁◯ |
| Incidence of serious adverse events (we used the NIH definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, disability or congenital anomaly) | Please see incidence of serious adverse events in the perineural dexamethasone versus placebo 'Summary of findings' table. | |||
| Postoperative pain intensity at 12 hours (measured using pain scores on an 11‐point VAS) | The mean postoperative pain score at 12 hours was 2.6 | The mean postoperative pain score at 12 hours in the intravenous dexamethasone group was 1.24 points lower (2.44 lower to 0.04 lower) | 162 | ⨁⨁◯◯ |
| Postoperative pain intensity at 24 hours | The mean postoperative pain score at 24 hours was 4.4 | The mean postoperative pain score at 24 hours in the intravenous dexamethasone group was 1.26 points lower (2.23 lower to 0.29 lower) | 257 | ⨁⨁◯◯ |
| Postoperative pain intensity at 48 hours (measured using pain scores on an 11‐point VAS) | The mean postoperative pain score at 48 hours was 3.7 | The mean postoperative pain score at 48 hours in the intravenous dexamethasone group was 0.21 points lower (0.83 lower to 0.41 higher) | 172 | ⨁⨁◯◯ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded by one level for inconsistency (considerable heterogeneity (I2 = 88% and P value for heterogeneity <0.0001) and subgroup analyses did not explain observed heterogeneity. Downgraded by one level for imprecision because of a sparse number of participants (n=162). bDowngraded by one level for inconsistency (I2 = 61% and P value for heterogeneity 0.08) and subgroup analyses did not explain observed heterogeneity. Downgraded by one level for imprecision. Confidence interval includes both no clinical effect (minimally important difference 1.2 on VAS) and clinical effect (minimally important difference greater than 1.2 on VAS). cDowngraded by one level for inconsistency(I2 = 65% and P value for heterogeneity 0.02) and subgroup analyses did not explain observed heterogeneity. Point estimates vary widely across studies. Downgraded by one level for imprecision (95% confidence interval includes no clinical effect and a clinical effect). By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS. dDowngraded by two levels for precision (small sample size (n=172) and confidence interval crosses the line of null effect).. | ||||
| Patient or population: peripheral nerve block Setting: people undergoing upper or lower limb surgery with peripheral nerve block in hospitals in Australia, Belgium, Brazil, Canada and USA Intervention: perineural dexamethasone Comparison: intravenous dexamethasone | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants | Quality of the evidence | |
| Risk with intravenous dexamethasone | Risk with perineural dexamethasone | |||
| Duration of sensory block (we included all studies describing duration of sensory block regardless of how it was described) | The mean duration of sensory block was 20.6 hours | The mean duration of sensory block in the perineural dexamethasone group was 3.13 hours longer (1.68 longer to 4.58 longer) | 720 | ⨁⨁⨁◯ |
| Incidence of serious adverse events (we used the NIH definition of adverse events. A serious event includes death, a life‐threatening event that requires hospitalization or prolonged hospitalization, disability or congenital anomaly) | Please see incidence of serious adverse events in the perineural dexamethasone versus placebo 'Summary of findings' table. | |||
| Postoperative pain intensity at 12 hours (measured using pain scores on an 11‐point VAS) | The mean postoperative pain score at 12 hours was 2.3 | The mean postoperative pain score at 12 hours in the perineural dexamethasone group was 1.01 points lower (0.50 lower to 1.51 lower) | 217 | ⨁⨁◯◯ |
| Postoperative pain intensity at 24 hours (measured using pain scores on an 11‐point VAS) | The mean postoperative pain score at 24 hours was 2.9 | The mean postoperative pain score at 24 hours in the perineural dexamethasone group was 0.77 points lower (0.08 lower to 1.47 lower) | 309 | ⨁⨁⨁◯ |
| Postoperative pain intensity at 48 hours (measured using pain scores on an 11‐point VAS) | The mean postoperative pain score at 48 hours was 2.8 | The mean postoperative pain score at 48 hours in the perineural dexamethasone group was 0.13 points higher (0.35 lower to 0.61 higher) | 227 | ⨁⨁◯◯ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded by one level for inconsistency (I2 = 67% and P value for heterogeneity is 0.001). bDowngraded by one level for risk of bias. Two out of the three studies are at unclear risk of bias. Reasons include unclear random sequence generation, unclear allocation concealment, and selective outcome reporting. Downgraded by one level for imprecision because of a sparse number of participants (n=217) and because the 95% confidence interval includes no clinical effect and a clinical effect. By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS. cDowngraded by one level for imprecision (95% confidence interval includes no clinical effect and a clinical effect). By no clinical effect we mean the lower bound of the CI did not surpass our chosen MID threshold of 1.2 on VAS. dDowngraded by one level for risk of bias. The one study that is at unclear risk of bias contributes half the data for this outcome. Downgraded by one level for imprecision because of a small sample size (n=227). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of sensory block Show forest plot | 27 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| 2 Duration of sensory block: long‐ versus medium‐acting local anaesthetic subgroups Show forest plot | 26 | 1572 | Mean Difference (IV, Random, 95% CI) | 6.78 [5.62, 7.94] |
| 2.1 Long‐acting local anaesthetic | 20 | 1315 | Mean Difference (IV, Random, 95% CI) | 7.81 [6.40, 9.21] |
| 2.2 Medium‐acting local anaesthetic | 6 | 257 | Mean Difference (IV, Random, 95% CI) | 3.98 [1.76, 6.20] |
| 3 Duration of sensory block: additive versus no additive subgroups Show forest plot | 27 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| 3.1 Additives | 6 | 336 | Mean Difference (IV, Random, 95% CI) | 7.29 [3.77, 10.81] |
| 3.2 No additives | 21 | 1289 | Mean Difference (IV, Random, 95% CI) | 6.60 [5.30, 7.89] |
| 4 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 27 | 1627 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.53, 7.86] |
| 4.1 High‐dose dexamethasone | 23 | 1447 | Mean Difference (IV, Random, 95% CI) | 7.09 [5.81, 8.38] |
| 4.2 Low‐dose dexamethasone | 4 | 180 | Mean Difference (IV, Random, 95% CI) | 4.32 [1.80, 6.85] |
| 5 Duration of sensory block: high/unclear versus low risk of bias subgroups Show forest plot | 26 | 1625 | Mean Difference (IV, Random, 95% CI) | 6.70 [5.54, 7.85] |
| 5.1 High or unclear risk of bias | 19 | 1037 | Mean Difference (IV, Random, 95% CI) | 6.28 [5.01, 7.56] |
| 5.2 Low risk of bias | 8 | 588 | Mean Difference (IV, Random, 95% CI) | 8.21 [4.56, 11.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of motor block Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 2 Duration of motor block: long‐ versus medium‐acting local anaesthetic subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 2.1 Long‐acting local anaesthetic | 13 | 764 | Mean Difference (IV, Random, 95% CI) | 6.61 [4.58, 8.65] |
| 2.2 Medium‐acting local anaesthetic | 3 | 148 | Mean Difference (IV, Random, 95% CI) | 2.59 [2.42, 2.76] |
| 3 Duration of motor block: additives verus no additives subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 3.1 Additives | 5 | 280 | Mean Difference (IV, Random, 95% CI) | 7.47 [3.58, 11.36] |
| 3.2 No additives | 11 | 632 | Mean Difference (IV, Random, 95% CI) | 5.26 [3.17, 7.35] |
| 4 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 4.1 High‐dose dexamethasone | 15 | 872 | Mean Difference (IV, Random, 95% CI) | 5.75 [4.29, 7.22] |
| 4.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 8.1 [4.69, 11.51] |
| 5 Duration of motor block: high/unclear versus low risk of bias subgroups Show forest plot | 16 | 912 | Mean Difference (IV, Random, 95% CI) | 5.87 [4.44, 7.30] |
| 5.1 High/unclear risk of bias | 14 | 809 | Mean Difference (IV, Random, 95% CI) | 5.67 [4.18, 7.16] |
| 5.2 Low risk of bias | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 7.93 [2.74, 13.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall incidence of block‐related adverse events Show forest plot | 10 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.99, 1.39] |
| 2 Numbness/tingling 14 days after surgery Show forest plot | 5 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.80, 3.89] |
| 3 Residual motor block/weakness 24 hours after surgery Show forest plot | 3 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.57, 38.68] |
| 4 Horner Syndrome Show forest plot | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.36] |
| 5 Hoarseness Show forest plot | 4 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.65, 2.34] |
| 6 Diaphragmatic paresis Show forest plot | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.66, 3.23] |
| 7 Dyspnoea Show forest plot | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.14] |
| 8 Vascular injury Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.36] |
| 9 Cranial nerve 12 palsy Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.77] |
| 10 Bruising Show forest plot | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.07, 15.64] |
| 11 Overall non‐block‐related adverse events Show forest plot | 10 | 625 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.68] |
| 12 Postoperative nausea and vomiting Show forest plot | 10 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.26, 1.14] |
| 13 Deep sedation Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.38, 129.93] |
| 14 Dermatological symptoms (pruritus/rash) Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.32, 27.02] |
| 15 Syncope/fainting Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.18, 20.71] |
| 16 Bradycardia Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.71] |
| 17 Hypotension Show forest plot | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.13] |
| 18 Headache/10‐pound fluid gain/diarrhoea/frequent urination/muscle soreness Show forest plot | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.12, 69.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at12 hours Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 2 Postoperative pain intensity at 12 hours: medium‐ versus long‐acting local anaesthetic subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.53] |
| 2.1 Long‐acting local anaesthesia | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐2.77, ‐1.66] |
| 2.2 Medium‐acting local anaesthesia | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.38, ‐0.06] |
| 3 Postoperative pain intensity at 12 hours: additive versus no additive subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 3.1 Additives | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.36, ‐0.04] |
| 3.2 No additives | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐2.77, ‐1.66] |
| 4 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 4.1 High‐dose dexamethasone | 3 | 177 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐3.29, ‐1.06] |
| 4.2 Low‐dose dexamethasone | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐2.75, ‐1.22] |
| 5 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐2.63, ‐1.52] |
| 5.1 High/unclear versus low risk of bias | 3 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐2.53, ‐1.09] |
| 5.2 Low risk of bias | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐3.88, ‐1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 24 hours Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 2 Postoperative pain intensity at 24 hours: long‐ versus medium‐acting local anaesthetic subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 2.1 Long‐acting local anaesthesia | 7 | 409 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐2.60, ‐0.90] |
| 2.2 Medium‐acting local anaesthesia | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.07, ‐0.09] |
| 3 Postoperative pain intensity at 24 hours: additive versus no additive subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 3.1 Additives | 3 | 158 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐3.43, ‐0.82] |
| 3.2 No additives | 6 | 311 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.31, ‐0.51] |
| 4 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 4.1 High‐dose dexamethasone | 7 | 389 | Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐2.71, ‐0.47] |
| 4.2 Low‐dose dexamethasone | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐3.21, ‐0.52] |
| 5 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups Show forest plot | 9 | 469 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.34, ‐0.93] |
| 5.1 High/unclear risk of bias | 4 | 185 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.79, ‐1.00] |
| 5.2 Low risk of bias | 5 | 284 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.91, 0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 48 hours Show forest plot | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| 2 Postoperative pain intensity at 48 hours: additives versus no additives subgroups Show forest plot | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| 2.1 No additives | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.36, ‐0.36] |
| 2.2 Additives | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.80, 1.34] |
| 3 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups Show forest plot | 4 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.24, 0.03] |
| 3.1 High/unclear risk of bias | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.24, 0.24] |
| 3.2 Low risk of bias | 3 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.30, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative opioid consumption at 24 hours Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 2 Opioid consumption at 24 hours medium‐ versus long‐acting local anaesthetic subgroups Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 2.1 Long‐acting local anaesthetic | 5 | 335 | Mean Difference (IV, Random, 95% CI) | ‐21.22 [‐35.20, ‐7.25] |
| 2.2 Medium‐acting local anaesthetic | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐33.91, 41.91] |
| 3 Opioid consumption at 24 hours: additive versus no additive subgroups Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 3.1 Additives | 2 | 142 | Mean Difference (IV, Random, 95% CI) | ‐30.17 [‐58.58, ‐1.76] |
| 3.2 No additives | 4 | 238 | Mean Difference (IV, Random, 95% CI) | ‐12.98 [‐26.28, 0.32] |
| 4 Opioid consumption at 24 hours: high/unclear versus low risk of bias subgroups Show forest plot | 6 | 380 | Mean Difference (IV, Random, 95% CI) | ‐19.25 [‐32.51, ‐5.99] |
| 4.1 High/unclear risk of bias | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐45.0 [‐57.58, ‐32.42] |
| 4.2 Low risk of bias | 5 | 292 | Mean Difference (IV, Random, 95% CI) | ‐13.55 [‐23.36, ‐3.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant satisfaction with pain control: perineural dexamethasone versus placebo Show forest plot | 4 | 224 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.05, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of sensory block Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 2 Duration sensory block: additive versus no additive subgroups Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 2.1 Additives | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.20 [0.68, 11.72] |
| 2.2 No additives | 7 | 450 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.33, 9.08] |
| 3 Duration of sensory block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 3.1 High‐dose | 6 | 437 | Mean Difference (IV, Random, 95% CI) | 7.45 [5.55, 9.35] |
| 3.2 Low‐dose | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 2.25 [1.21, 3.30] |
| 4 Duration of sensory block: high/unclear versus low risk of bias subgroups Show forest plot | 8 | 499 | Mean Difference (IV, Random, 95% CI) | 6.21 [3.53, 8.88] |
| 4.1 High/unclear risk of bias | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 2.25 [1.21, 3.30] |
| 4.2 Low risk of bias | 6 | 437 | Mean Difference (IV, Random, 95% CI) | 7.45 [5.55, 9.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of motor block Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| 2 Duration of motor block: additive versus no additive subgroups Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.54 [3.11, 7.97] |
| 2.1 Additives | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 6.60 [2.32, 10.88] |
| 2.2 No additives | 2 | 90 | Mean Difference (IV, Random, 95% CI) | 3.67 [‐2.77, 10.11] |
| 3 Duration of motor block high‐ versus low‐dose dexamethasone subgroups Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| 3.1 High‐dose dexamethasone | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 5.96 [4.03, 7.90] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.23, 5.97] |
| 4 Duration of motor block: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 139 | Mean Difference (IV, Random, 95% CI) | 5.04 [3.07, 7.00] |
| 4.1 High/unclear risk of bias | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 5.96 [4.03, 7.90] |
| 4.2 Low risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.23, 5.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall incidence of block‐related adverse events Show forest plot | 5 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.70] |
| 2 Numbness/tingling 14 days after surgery Show forest plot | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.31, 9.26] |
| 3 Residual motor block/muscle weakness 24 hours after surgery Show forest plot | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [0.80, 8.90] |
| 4 Horner syndrome Show forest plot | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.26] |
| 5 Hoarsenss Show forest plot | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.71] |
| 6 Dyspnoea Show forest plot | 3 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.11, 3.74] |
| 7 Cranial nerve 12 palsy Show forest plot | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.77] |
| 8 Overall non‐block‐related adverse events Show forest plot | 5 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.38, 3.97] |
| 9 Postoperative nausea and vomiting Show forest plot | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.12, 3.78] |
| 10 Dermatological symptoms Show forest plot | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.09, 40.62] |
| 11 Dizziness/wrist, hand or finger pain, constipation Show forest plot | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 12 hours Show forest plot | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| 2 Postoperative pain intensity at 12 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| 2.1 High‐dose dexamethasone | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.16, ‐0.44] |
| 2.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.41, 0.41] |
| 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.44, ‐0.04] |
| 3.1 High/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.41, 0.41] |
| 3.2 Low risk of bias | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.16, ‐0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 24 hours Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 2.1 Additive | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.75, 0.95] |
| 2.2 No additive | 4 | 208 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.48, ‐0.18] |
| 3 Postoperative pain intensity at 24 hours: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 3.1 High‐dose dexamethasone | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.54, ‐0.07] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.49, 0.29] |
| 4 Postoperative pain intensity at 24 hours: high/unclear versus low risk of bias subgroups Show forest plot | 5 | 257 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.23, ‐0.29] |
| 4.1 High/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐2.49, 0.29] |
| 4.2 Low risk of bias | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.54, ‐0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 48 hours Show forest plot | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups Show forest plot | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| 2.1 Additive | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.60, 1.20] |
| 2.2 No additive | 2 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.87, 0.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 24‐hour opioid consumption Show forest plot | 5 | 287 | Mean Difference (IV, Random, 95% CI) | ‐6.58 [‐10.56, ‐2.60] |
| 2 24‐hour opioid consumption: additive verus no additive subgroups Show forest plot | 5 | 287 | Mean Difference (IV, Random, 95% CI) | ‐6.58 [‐10.56, ‐2.60] |
| 2.1 Additive | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐13.33, 5.33] |
| 2.2 No additive | 4 | 234 | Mean Difference (IV, Random, 95% CI) | ‐6.93 [‐11.41, ‐2.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative opioid consumption at 48 hours opioid consumption Show forest plot | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐22.5 [‐39.85, ‐5.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant satisfaction with pain control Show forest plot | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐0.08, 2.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of sensory block Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 2 Duration of sensory block additive versus no additive subgroups Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 2.1 Additive | 3 | 331 | Mean Difference (IV, Random, 95% CI) | 3.94 [2.66, 5.21] |
| 2.2 No additive | 6 | 389 | Mean Difference (IV, Random, 95% CI) | 2.67 [0.00, 5.34] |
| 3 Duration sensory block high‐dose versus low‐dose dexamethasone subgroups Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 3.1 High‐dose dexamethasone | 6 | 508 | Mean Difference (IV, Random, 95% CI) | 2.35 [0.04, 4.66] |
| 3.2 Low‐dose dexamethasone | 3 | 212 | Mean Difference (IV, Random, 95% CI) | 4.14 [2.48, 5.81] |
| 4 Duration sensory block high/unclear versus low risk of bias subgroups Show forest plot | 9 | 720 | Mean Difference (IV, Random, 95% CI) | 3.13 [1.68, 4.58] |
| 4.1 High/unclear risk of bias | 3 | 162 | Mean Difference (IV, Random, 95% CI) | 4.67 [2.29, 7.04] |
| 4.2 Low risk of bias | 6 | 558 | Mean Difference (IV, Random, 95% CI) | 2.30 [0.23, 4.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of motor block Show forest plot | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| 2 Duration of motor block: additive versus no additive subgroups Show forest plot | 5 | 340 | Mean Difference (IV, Random, 95% CI) | 2.75 [0.32, 5.19] |
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐0.03, 8.03] |
| 2.2 No additive | 4 | 290 | Mean Difference (IV, Random, 95% CI) | 2.39 [‐0.58, 5.37] |
| 3 Duration of motor block: high‐ versus low‐dose dexamethasone subgroups Show forest plot | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| 3.1 High‐dose dexamethasone | 4 | 381 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐0.25, 5.19] |
| 3.2 Low‐dose dexamethasone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 5.0 [2.53, 7.47] |
| 4 Duration of motor block: high/unclear versus low risk of bias subgroups Show forest plot | 5 | 421 | Mean Difference (IV, Random, 95% CI) | 3.13 [0.99, 5.27] |
| 4.1 HIgh/unclear risk of bias | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 5.0 [2.53, 7.47] |
| 4.2 Low risk of bias | 4 | 381 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐0.25, 5.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall incidence of block‐related adverse events Show forest plot | 5 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.93, 1.55] |
| 2 Numbness/tingling 14 days after surgery Show forest plot | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.27, 3.49] |
| 3 Residual motor block/weakness at 24 hours Show forest plot | 3 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.37] |
| 4 Horner syndrome Show forest plot | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.77, 1.87] |
| 5 Hoarsness Show forest plot | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.48, 2.09] |
| 6 Cranial nerve 12 motor palsy Show forest plot | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.39] |
| 7 Overall incidence of non block‐related adverse events Show forest plot | 5 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.37, 4.78] |
| 8 Postoperative nausea and vomiting Show forest plot | 5 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.22, 1.80] |
| 9 Dermatologicial symptoms (pruritus/rash) Show forest plot | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [0.22, 89.18] |
| 10 Syncope/fainting Show forest plot | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [0.22, 89.18] |
| 11 Dizziness Show forest plot | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.06, 2.72] |
| 12 Wrist, hand or finger pain Show forest plot | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 7.02] |
| 13 Headache, 10‐pound fluid gain/diarrhoea/frequent urination/ muscle soreness Show forest plot | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.11, 63.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 12 hours Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| 2 Postoperative pain intensity at 12 hours: low‐ versus high‐dose dexamethasone subgroups Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| 2.1 Low‐dose dexamethasone | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.60, ‐0.47] |
| 2.2 High‐dose dexamethasone | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| 3 Postoperative pain intensity at 12 hours: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.51, ‐0.50] |
| 3.1 HIgh/unclear risk of bias | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.60, ‐0.47] |
| 3.2 Low risk of bias | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 24 hours Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 2 Postoperative pain intensity at 24 hours: additive versus no additive subgroups Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.32, ‐0.08] |
| 2.2 No additive | 4 | 259 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.41, 0.13] |
| 3 Postoperative pain intensity at 24 hours: low‐ versus high‐dose dexamethasonesubgroups Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 3.1 Low‐dose dexamethasone | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.01, 0.11] |
| 3.2 High‐dose dexamethasone | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.95, 0.59] |
| 4 Intensity of postoperative pain at 24 hours: high/unclear risk of bias versus low risk of bias subgroups Show forest plot | 5 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.51, ‐0.07] |
| 4.1 High/unclear risk of bias | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.01, 0.11] |
| 4.2 Low risk of bias | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.95, 0.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain intensity at 48 hours Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 2 Postoperative pain intensity at 48 hours: additive versus no additive subgroups Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 2.1 Additive | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.51, 2.11] |
| 2.2 No additive | 2 | 177 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.50, 0.54] |
| 3 Postoperative pain intensity at 48 hours: low‐ versus high‐dose dexamethasone subgroups Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 3.1 Low‐dose dexamethasone | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 3.2 High‐dose dexamethasone | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.45, 1.24] |
| 4 Postoperative pain intensity at 48 hours: high/unclear versus low risk of bias subgroups Show forest plot | 3 | 227 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.61] |
| 4.1 High/unclear risk of bias | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 4.2 Low risk of bias | 2 | 128 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.45, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Opioid consumption at 24 hours: perineural versus intravenous dexamethasone Show forest plot | 4 | 242 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐9.93, 2.19] |
| 2 24‐hour opioid consumption: additive versus no additive subgroups Show forest plot | 4 | 242 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐9.93, 2.19] |
| 2.1 Additive | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐23.96, ‐0.04] |
| 2.2 No additive | 3 | 189 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐6.34, 3.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant satisfaction with pain control Show forest plot | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.33, 0.70] |

