腕管减压手术后用于皮肤缝合的可吸收性缝线和非吸收性缝线
摘要
研究背景
腕管综合征是一种常见的问题,腕管减压手术是最有效的治疗方法。手术减压后,可以使用可吸收性缝线或者非吸收性缝线来缝合手掌皮肤。迄今为止,关于理想缝合材料的证据存在矛盾,这也是促成本系统综述的根本原因。
研究目的
评价成人选择性腕管减压手术后用于皮肤缝合的可吸收性缝线和非吸收性缝线在术后疼痛、手部功能、瘢痕满意度、伤口炎症和不良事件方面的影响。
检索策略
我们于2017年10月30日检索了以下数据库:Cochrane神经肌肉疾病组专业注册库(Cochrane Neuromuscular Specialised Register)、CENTRAL、MEDLINE和Embase。我们还在2017年10月30日检索了两个临床试验注册库。
纳入排除标准
我们纳入了所有比较了在任何形式的成人腕管减压手术后用于皮肤缝合的可吸收性缝线和非吸收性缝线的随机或半随机对照试验。
资料收集与分析
所分析的单位是手而不是人。我们对直接比较进行了meta分析,以生成关于疼痛评分(如伤口炎症)的具有95%置信区间(confidence intervals, CI)的标准化平均差(standardised mean differences, SMD)以及二分类结局具有95%CI的风险比(risk ratios, RR)。主要结局为术后疼痛。次要结局包括手部功能、瘢痕满意度、瘢痕炎症和不良事件(并发症)。我们使用GRADE评价主要结局的证据质量。
主要结果
我们纳入了五项随机对照试验(涉及255名受试者)。这些试验都是在欧洲进行的(英国、爱尔兰共和国、丹麦和荷兰)。引用的数据显示,受试者的平均年龄为48到53岁之间。试验评价了术后1到12周的结局。
根据开放式腕管减压手术(open carpal tunnel decompression, OCTD)10天后可吸收性缝线和非吸收性缝线的术后疼痛评分的meta分析,得出SMD为0.03(95%CI [‐0.43, 0.48]; 3项研究,受试者数量 (N)=137; I2=43%);SMD表明差异很小或者没有差异,但由于证据质量极低,其不确定性很高。在内窥镜辅助腕管减压术(endoscopic carpal tunnel decompression, ECTD)后10天,使用可吸收性缝线和非吸收性缝线的术后疼痛评分的SMD为‐0.81(95%CI [‐1.36, ‐0.25]; 1项研究; N=54);尽管SMD数据表明所产生的影响较大,极低质量证据意味着研究结果具有不确定性。只有OCTD的研究提供了受试者6周时的疼痛数据,此时SMD为0.06(95%CI [‐0.72, 0.84]; 4项研究; N=175; I2=84%),这表明几乎不存在或不存在证据表明差异,但是证据的不确定性也很高(极低质量证据)。OCTD后使用可吸收性缝线和非吸收性缝线在伤口炎症方面的RR为2.28(95%CI [0.24, 21.91]; N=95; I2=90%),在ECTD后其RR为0.93(95%CI [0.06, 14.09]; 1项研究; N=54)。由于证据质量极低,所以关于伤口炎症的影响的任何差异都具有不确定性。有一项研究报告了术后手部功能的情况,但并未发现缝合线类型在两周内存在差异的证据(均差MD=‐0.10, 95%CI [‐0.53, 0.33], N=36),在6周和12周的研究发现类似。只有ECTD试验报告了瘢痕满意度的数据,在可吸收性缝线组中,28人中有25人报告其使用结果“很好”,而使用非吸收性缝线的26人中只有18人报告瘢痕满意(RR=1.29, 95%CI [0.97, 1.72], N=54)。这些发现也非常不确定,因为我们认为其证据质量极低。所有的研究在大多数领域都存在高偏倚风险。没有试验报告不良事件。
作者结论
因为证据质量极低,在腕管减压手术后,尚不确定可吸收性缝线与非吸收性缝线相比,是否能带来更好、更差或同等的结局。使用可吸收性缝线无需拆线,这能够为患者和医疗保健提供者节省可观的费用。我们需要进行严格的、非劣效性的随机对照试验并进行经济分析,以了解缝合线类型的选择。
PICO
简语概要
腕管手术后用于皮肤缝合的可吸收性缝线与非吸收性缝线
本系统综述的目的是什么?
本Cochrane系统综述的目的为比较腕管手术后用于伤口缝合的可吸收性缝线和非吸收性缝线。我们收集并分析了关于这个问题的信息,发现了五项相关的研究。
关键信息
我们不知道在腕管综合征(carpal tunnel syndrome, CTS)手术后,是可吸收性缝线更适合伤口缝合,还是非吸收性缝线更适合。我们所发现的研究仅提供了极低质量证据,因此无法得出结论。
只有一项研究报告了术后手部功能和瘢痕满意度的数据,没有一项研究提供关于副作用的数据。我们没有足够高质量的证据来帮助选择CTS手术后所使用的缝合线然而,可吸收性缝线不需要拆除,因此可以节省时间和成本。
本系统综述研究了什么?
CTS是一种常见的疾病,可能会影响一只手或双手,常伴随比如拇指和手指的刺痛、麻木和无力的症状。这些症状通常是由于正中神经手从臂穿过手腕进入手掌时受到压力而引起的。神经在腕部穿过一个由腕骨和厚厚的组织组成的通道。如果该通道由于某种原因过小,则对神经的压力就会导致手部在使用时出现问题,以及CTS的其他症状。
CTS的治疗分为非手术治疗(用夹板固定和类固醇注射)以及手术治疗。CTS手术是最常见的非紧急手部手术。这种小手术通常在局部麻醉下进行。在开放式腕管手术中,切口足够大,以便外科医生能够直接看到腕管。在内窥镜辅助腕管手术中,外科医生在手腕上做两个小切口,一个用于手术器械的进入,另一个用于小型相机。通常,缝合皮肤需要用到缝合线,缝合线要么被身体自然吸收(可吸收性),要么必须拆除(非吸收性)。可吸收性缝线很方便,但是有些人认为它们可能会加重瘢痕和炎症。非吸收性缝线被认为会引起更少的炎症反应和更小的瘢痕,但是缝线的拆除会为患者和医疗保健系统带来更高的成本以及不便。
我们想评价证据质量以了解这两种缝合线在用于CTS手术时是否存在差异。
本系统综述的主要结果是什么?
通过彻底的检索,我们发现了五项比较这些缝合线的研究(总共涉及255名受试者)。所有的研究在设计或者实施方式上都存在一些问题。有四项研究的受试者接受了开放式腕管手术,一项研究的受试者接受了内窥镜辅助腕管手术。
由于有助于我们分析的证据质量极低,尚不确定可吸收性缝线和非吸收性缝线在术后10天或6周的疼痛情况、手部功能、瘢痕满意度或伤口炎症方面是否存在差异。这些研究并未报告副作用情况。
本系统综述的时效性如何?
本系统综述作者检索了截至2017年10月30日发表的研究。
Authors' conclusions
Summary of findings
| Absorbable sutures compared with non‐absorbable sutures for carpal tunnel decompression after open carpal tunnel decompression surgery | ||||||
| Patients: adults undergoing primary carpal tunnel decompression by open carpal tunnel decompression Intervention: absorbable sutures for wound closure Comparison: non‐absorbable sutures for wound closure Setting : secondary care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Non‐absorbable sutures | Absorbable sutures | |||||
| Postoperative pain: early pain (10 days postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative VAS pain after OCTD in the non‐absorbable suture groups was 0.03 SMD higher (0.43 lower to 0.48 higher) | 137 (3 RCTs) | ⊕⊝⊝⊝ | SMD 0.03 (95% CI ‐0.43 to 0.48) A SMD of 0.03 represents little or no difference between groups3 It is uncertain whether or not there is any difference in postoperative pain scores at 10 days because the quality of evidence is very low. | |
| Postoperative pain: late pain (6 weeks postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative pain (6 weeks) after open CTD in the non‐absorbable suture groups was | 175 (4 RCTs) | ⊕⊝⊝⊝ | SMD 0.06 (95% CI ‐0.72 to 0.84) A SMD of 0.06 represents little or no difference between groups. It is uncertain whether there is any difference in postoperative pain scores at 6 weeks because the quality of evidence is very low. | |
| Postoperative hand function (2 weeks postoperatively) Mean | The mean FSS score in the non‐absorbable suture group 2 weeks postoperatively was 1.6 | The mean FSS score 2 weeks postoperatively was 0.1 lower (0.53 lower to 0.33 higher) | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝ | MD ‐0.10, 95% CI ‐0.53 to 0.33) It is uncertain whether there is any difference between groups in postoperative hand function because the quality of evidence is very low. MD at 6 and 12 weeks follow‐up postoperatively were 0.00 (95% CI ‐0.39 to 0.39) and 0.00 (95% CI ‐0.37 to 0.37). |
| Wound Inflammation 6 to 12 weeks follow‐up | 370 per 1000 | 843 per 1000 (89 to 1000) | RR 2.28 (0.24 to 21.91) | 95 | ⊕⊝⊝⊝ | It is uncertain whether there is any difference between groups in the occurrence of postoperative wound inflammation because the quality of evidence is very low. |
| Postoperative scar satisfaction | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse outcomes including wound infection, scar breakdown or return to theatre | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| CI: confidence interval; OCTD: open carpal tunnel decompression; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale; CTS‐FSS: Carpal Tunnel Syndome Functional Status Scale | ||||||
| Quality of Evidence Grades | ||||||
| 1Downgraded twice based on study limitations (lack of allocation concealment, and lack of blinding of participants and assessors). 3Based on a rule‐of‐thumb guide to interpretation of SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988 in Higgins 2011). | ||||||
| Absorbable sutures compared with non‐absorbable sutures for endoscopic carpal tunnel decompression | ||||||
| Patients: adults undergoing primary carpal tunnel decompression by endoscopic carpal tunnel decompression Intervention: absorbable sutures for wound closure Comparison: non‐absorbable sutures for wound closure Setting: secondary care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Non‐absorbable sutures | Absorbable sutures | |||||
| Postoperative pain: early pain (10 days postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative VAS pain after ECTD in the non‐absorbable suture groups was 0.81 SMD lower (1.36 to 0.25 lower) | 54 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | SMD ‐0.81 (95% CI ‐1.36 to ‐0.25) A SMD of ‐0.81 represents a large difference between groups.3 It is uncertain whether or not there is any difference in postoperative pain scores at 10 days because the quality of evidence is very low. | |
| Postoperative pain: late pain (6 weeks postoperatively) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Postoperative hand function | ‐ | ‐ | ‐ | ‐ | Not measured | |
| Wound Inflammation 6 to 12 weeks follow‐up | 38 per 1000 | 36 per 1000 (2 to 542) | RR 0.93 (0.06 to 14.09) | 54 (1 RCT) | ⊕⊝⊝⊝ | It is uncertain whether there is any difference between groups in the occurrence of postoperative wound inflammation because the quality of evidence is very low. |
| Postoperative scar satisfaction | 692 per 1000 | 893 per 1000 (672 to 1000) | RR 1.29 (0.97 to 1.72) | ‐ | ⊕⊝⊝⊝ | |
| Adverse outcomes including wound infection, scar breakdown or return to theatre | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| CI: confidence interval; ECTD: endoscopic carpal tunnel decompression; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale | ||||||
| Quality of Evidence Grades | ||||||
| 1Downgraded twice for study limitations (lack of allocation concealment, and lack of blinding of participants and assessors). | ||||||
Background
Description of the condition
Carpal tunnel syndrome (CTS) is a constellation of symptoms resulting from compression of the median nerve within a fibro‐osseous tunnel between the carpal bones and palmar fascia, at the level of the wrist. The compression has various causes, which may or may not be reversible. Symptoms usually include numbness with 'pins and needles' in the hand, as well as pain at the wrist or in the arm, particularly at night. In advanced cases, sensation may be entirely lost and the thenar musculature (the muscles at the base of the thumb) may atrophy, resulting in severe functional impairment.
Most people with CTS will ultimately require surgery, as the most effective and definitive treatment for the condition is surgical decompression, which aims to relieve pressure on the nerve as it passes through the carpal tunnel (Verdugo 2008). Indeed, carpal tunnel decompression surgery is the most commonly performed elective procedure on the hand (Wildin 2006).
Description of the intervention
In order to decompress the median nerve, the overlying compressing tissue must be divided; this is traditionally performed via a longitudinal incision in the palm that extends distally, from the distal wrist crease for approximately 3 cm to 5 cm. The surgeon divides the skin, underlying fat, vessels and fascial layers in line with the wound until the tunnel is opened and the median nerve is fully exposed. Once the median nerve is decompressed, the skin alone is re‐approximated with sutures and the hand adequately dressed. Some surgeons prefer to decompress the carpal tunnel in a minimally invasive fashion through a smaller skin incision with or without telescopic instruments; these is known as minimally invasive or endoscopic carpal tunnel decompression (ECTD). Minimally invasive surgery means that most of the dissection cannot be done under direct vision, which poses a theoretical risk of inadvertent injury to the palmar cutaneous and recurrent motor branches of the median nerve, as well as inadequate decompression. Both techniques have advantages and limitations, though neither has been shown to be superior (Sanati 2007; Vasiliadis 2014). Therefore, most surgeons still adopt an open approach (open carpal tunnel decompression, OCTD) with a larger incision, as this operation is quicker and easier to perform (Beck 2007; Kohanzadeh 2012; Vasiliadis 2014).
Whilst the procedural steps of open and endoscopic carpal tunnel decompression surgery are largely consistent worldwide, there is conflicting evidence and ongoing debate regarding the ideal type of suture for closing the surgical incision in the palmar skin.
How the intervention might work
Some surgeons utilise absorbable sutures, which may be braided or monofilament and carry different half‐lives. Absorbable sutures rely on tissue hydrolysis to disintegrate, with the remnants being absorbed by phagocytes. The arguments for using absorbable sutures include that because a repeat visit is not required for suture removal and the dressings can remain undisturbed for longer, patients may even manage the dressings themselves at home and so satisfaction may be improved. There are also benefits for health professionals and the healthcare system, in terms of inherent reductions in clinical workload, resource utilisation and direct and indirect costs. However, these hypotheses have not been tested.
Conversely, some surgeons prefer to use non‐absorbable (usually monofilament) sutures, which are inert materials so theoretically cause less tissue trauma and foreign‐body reaction. This is thought to confer a more aesthetically pleasing scar. Monofilament sutures carry a hypothetically lower risk of wound infection, although there is no convincing evidence of this in relation to hand surgery.
Many randomised trials and observational studies have compared non‐absorbable and absorbable sutures for skin closure after carpal tunnel decompression surgery, but no consensus has yet been reached.
Why it is important to do this review
Carpal tunnel decompression surgery is the most commonly performed elective procedure on the hand, with approximately 72,000 operations performed annually in the UK (Wildin 2006). The use of a different suture to close the skin could affect the cost of the overall treatment and outcome. Firstly, if all surgeons in the UK used non‐absorbable sutures then the direct cost to the health care service for suture removal following carpal tunnel decompression would be in excess of GBP 3 million annually (PSSRU 2016), which only takes into account the cost of a single General Practice Nurse appointment for suture removal and dressing. This annual cost clearly does not account for other direct costs (e.g. instruments or dressings) or indirect costs to patients such as days off work, travel, etc. Therefore, if absorbable sutures are shown to be comparable or better than non‐absorbable sutures, then substantial cost savings could be made. Secondly, surgical‐site complications (e.g. pain, inflammation or infection) can adversely affect a person's day‐to‐day life and impair hand function, so if a simple alteration to the type of suture material used to close the skin could improve objective outcomes or reduce the rate of adverse events, then it is important to know.
In the early postoperative phase, healing can be complicated by wound dehiscence with or without infection or haematoma formation. Both situations can affect the underlying median nerve either by exposing it to the environment—which may result in desiccation and thus irreversible damage—or through compression by blood, pus, oedema, etc., similarly resulting in axonal loss. Wound dehiscence and exudative reactions may have additional adverse effects on the flexor tendons by inducing tenosynovitis and adhesions. Such adverse events can significantly impair hand function and adversely affect quality of life. These early adverse events usually warrant hospital admission and surgical reintervention. To avoid such undesirable outcomes, surgeons aim to use the most effective method of skin closure.
Late wound adverse events are usually related to either a symptomatic scar (which may be painful, tender, insensate or itchy) or recurrence of compressive symptoms. Recurrent CTS requires surgery and the wound is then prone to the same early complications as at initial operation. Late scar‐related adverse events can impact on quality of life and hand function, leading to a request for revision surgery. The type of wound closure may influence these late complications because absorbable sutures evoke an inflammatory reaction and may cause undesirable local tissue reactions. Conversely, non‐absorbable sutures stimulate a foreign body reaction from the immune system, which may also dysregulate wound healing.
For these reasons and the great uncertainty on the topic, a comprehensive evaluation of the ideal suture material for closing this standard incision is needed to reduce surgical‐site morbidity, improve outcomes and make the best use of resources.
Objectives
To assess the effects of absorbable versus non‐absorbable sutures for skin closure after elective carpal tunnel decompression surgery in adults on postoperative pain, hand function, scar satisfaction, wound inflammation and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs only. We included studies reported as full text, those published as abstract only and unpublished data. We placed no restrictions on language.
Types of participants
We included adults who had undergone primary open, minimally invasive or endoscopic elective carpal tunnel decompression surgery, as all such individuals require the skin incision to be closed using sutures.
We excluded participants with the following comorbidities and characteristics.
-
Children (those less than 18 years old). Elective carpal tunnel decompression is an operation which is more common in later life and the majority of cases will be in adults over the age of 18 years.
-
People who underwent other surgery simultaneously (e.g. Dupuytren's fasciectomy or trigger finger release at the same time as carpal tunnel decompression), as this may confound the outcomes.
-
People who underwent revision carpal tunnel decompression, as this could confound the results.
Types of interventions
We included trials comparing absorbable suture materials (e.g. Vicryl, Vicryl Rapide or Monocryl) with non‐absorbable suture materials (e.g. Prolene or Ethilon) available for commercial use. We excluded other methods of skin closure such as staples, tissue glue and suture strips (Steri‐Strips) because they are not comparable to sutures and moreover, not commonly used in this situation.
Types of outcome measures
Primary outcomes
Our primary outcome was postoperative pain, subcategorised into:
-
early pain: reported by participants at approximately 10 days postoperatively;
-
late pain: reported by participants at approximately 6 weeks postoperatively.
We have deviated from our protocol in the assessment of the primary outcome because we set out to compare patient‐reported pain in the short term (48 hours postoperatively), medium term (two to seven days postoperatively) and long term (more than seven days postoperatively). However, few studies reported pain scores comprehensively enough to permit assessment of three time points, and so we took a pragmatic decision to deviate from protocol to assess pain at 10 days and 6 weeks, which were common time points in the included trials (see also Differences between protocol and review).
We chose pain as our primary outcome because all early adverse events (arguably) have the common symptom of pain, which if substantial would stimulate people to seek medical consultation. However, quantitative measurement of postoperative pain following carpal tunnel syndrome (CTS) is difficult because there are many types of pain, which may coexist (including pillar pain, scar pain, tissue swelling and distension, hypersensitivity, etc.). Therefore, in order to generate appropriate data, we accepted pain measured on any validated pain scoring tool (including visual analogue scales (VAS) or Likert scales), and transformed the data for appropriate comparison in accordance with the established literature (Collins 1997).
Secondary outcomes
-
Postoperative hand function as measured by any validated hand tool, e.g. the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire (Hoang‐Kim 2011).
-
Scar satisfaction and wound inflammation as measured by a validated scale, e.g. the Vancouver Scar Scale (Baryza 1995).
-
Postoperative adverse events, e.g. wound infection, wound breakdown and return to theatre.
Search methods for identification of studies
Electronic searches
With an Information Specialist (AAG) within Cochrane Neuromuscular, we searched:
-
the Cochrane Neuromuscular Specialised Register (30 October 2017) Appendix 1;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (30 October 2017, in the Cochrane Register of Studies Web Online) Appendix 2;
-
MEDLINE (1966 to 30 October 2017) Appendix 3;
-
Embase (1980 to 30 October 2017) Appendix 4.
We also conducted a search of the US National Institutes for Health Clinical Trials Registry, ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Portal (ICTRP) (http://apps.who.int/trialsearch/) for ongoing incomplete trials or trials which were completed and unpublished, using the term 'carpal tunnel'. We searched all databases from their inception to the present (30 October 2017), and imposed no restriction on language of publication.
Searching other resources
We searched reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
Data collection and analysis
Selection of studies
Two review authors (RGW and JCRW) independently screened titles and abstracts of all the potential studies for inclusion and coded them as 'retrieve' (eligible or potentially eligible, or unclear) or 'do not retrieve'. We retrieved the full‐text study reports or publications and two review authors (RGW and JCRW) then independently screened the full text. Studies were labelled for inclusion or exclusion with explanations. We resolved any disagreements through discussion or, if required, by consulting a third review author (AF). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and 'Characteristics of excluded studies' table.
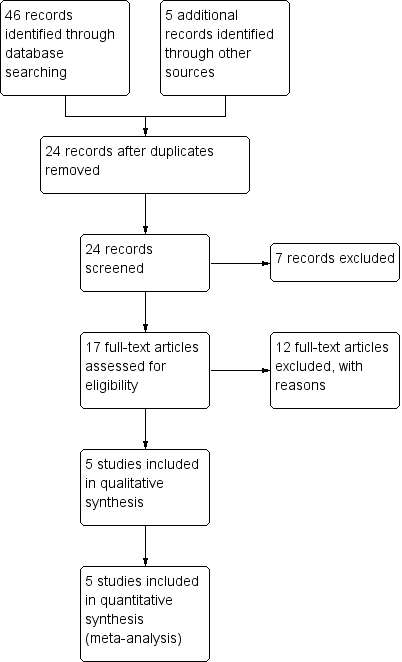
Flow diagram illustrating the study selection process.
Data extraction and management
We used a data extraction form for study characteristics and outcome data that had been piloted prior to conducting the review. Two review authors (RGW and JCRW) independently extracted data from included studies, including:
-
methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals and date of study;
-
participants: number of participants, number of hands randomised, number of bilateral cases, whether intervention randomised to hand or participant, mean age, age range, gender, severity of condition, diagnostic criteria, baseline characteristics, handedness, medical comorbidity, inclusion criteria and exclusion criteria;
-
interventions: intervention and comparison;
-
outcomes: primary and secondary outcomes specified and collected and time points reported;
-
notes: funding for trial and notable conflicts of interest of trial authors.
Both review authors (RGW and JCRW) then compared extracted data for accuracy. One dataset was then discarded and the remaining dataset used for the review. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (AF). One review author (JCRW) then transferred data into Review Manager (RevMan 2012) and a second review author (RGW) again independently checked all entered data against the original trial reports for accuracy. No reports required translation.
Assessment of risk of bias in included studies
Two review authors (JCRW and RGW) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author (AF). We assessed the risk of bias according to the following domains.
-
Random sequence generation
-
Allocation concealment
-
Blinding of participants and personnel
-
Blinding of outcome assessment
-
Incomplete outcome data
-
Selective outcome reporting
-
Other sources of bias, e.g. bias arising because of bilateral cases
We graded each potential source of bias as high, low or unclear, with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contribute to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratios and continuous data as standardised mean difference in order that outcomes that were conceptually the same (e.g. pain scores) but measured in different ways (e.g. a VAS in one study and a Likert scale) could be converted, standardised and pooled. For Erel 2001, we converted patient‐reported pain which was recorded on a 4‐point Likert scale to a continuous metric in order to permit pooling as above and applied the following conversions: no pain = 0, mild pain = 2.5, moderate pain = 5, and severe pain = 7.5; this method is in accordance with the literature (Collins 1997).
Unit of analysis issues
The unit of analysis was 'the hand' rather than 'the participant', as wound complications are a local phenomenon and we were interested to know if they were linked to the suture type used. Where studies considered simultaneous bilateral surgery, we included and analysed the data if the hand was randomised and individual data recorded for each side. Where an individual received different sutures on each hand and data per hand were not available or provided upon request, then we excluded the study.
Dealing with missing data
We contacted the corresponding authors of all eligible studies in order to attempt to obtain missing data and clarify unclear methodology. When we received no response, we sent a second email to the corresponding author. Thereafter, if we received no reply, we contacted all the co‐authors by email when their correspondence details were publicly available.
Assessment of heterogeneity
We used the I2 statistic to measure statistical heterogeneity among the trials in each analysis and interpreted this statistic in accordance with guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Assessment of reporting biases
Had more than 10 trials been available, we planned to create and examine a funnel plot to explore possibility of small‐study biases.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis with a fixed‐effect model. This decision was based on a preliminary review of the available literature, which suggested heterogeneity between trials.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes.
-
Postoperative pain:
-
early pain: reported by participants at approximately 10 days postoperatively;
-
late pain: reported by participants at approximately 6 weeks postoperatively.
-
-
Postoperative hand function.
-
Postoperative scar satisfaction.
-
Postoperative wound inflammation.
-
Postoperative adverse events, e.g. wound infection, wound breakdown and return to theatre.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence (studies that contribute data for the prespecified outcomes). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2003; Higgins 2011), using GRADEpro software.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses (Wade 2015).
-
Stratified by pre‐operative severity of carpal tunnel syndrome.
-
Stratified by medical comorbidity.
-
Stratified by unilateral versus bilateral carpal tunnel release (to determine effect of participant‐reported pain score).
We planned to use the following outcomes in subgroup analyses.
-
Postoperative pain.
-
Postoperative adverse events.
We would have used the formal test for subgroup interactions in Review Manager (RevMan 2012) however, none of the above variables were available to permit subgrouping.
Sensitivity analysis
We planned to carry out the following sensitivity analyses.
-
Repeat the analysis excluding unpublished studies (if there were any).
-
Repeat the analysis excluding studies at high risk of bias (non‐blinded trials, high risk of bias from the method of randomisation).
-
If there were one or more very large studies, repeat the analysis excluding them to look at how much they dominate the results.
-
Repeat the analysis excluding other types of studies, to explore effects of decisions made during the review process.
None of the above criteria set out in our protocol were met; however, during data collection and analysis we identified the operative method as an important source of heterogeneity. Therefore, we presented data from the ECTD trial separately (Hansen 2009). This was a deviation from protocol (Wade 2015) which is discussed below in Differences between protocol and review..
Results
Description of studies
Results of the search
From our searches, we identified 46 potential studies, of which 6 were from the Cochrane Neuromuscular Specialised Register, 8 were from CENTRAL,16 were from MEDLINE and 16 from Embase. After we removed duplicate records, 24 articles remained and we obtained them for assessment of eligibility. See Figure 1 for a flow chart illustrating the study selection process.
Included studies
See Characteristics of included studies.
We included five studies for meta‐analysis (Erel 2001; Hansen 2009; Kharwadkar 2005; Menovsky 2004; Theopold 2012).
The two‐group parallel randomised trial by Erel 2001 recruited 64 adults aged 18 to 80 years with carpal tunnel syndrome (CTS), undergoing open carpal tunnel decompression (OCTD). One group received a single deep subcutaneous absorbable 4‐0 Vicryl suture centrally and a completion continuous 4‐0 Vicryl intradermal suture for wound closure. The other group received interrupted 5‐0 Prolene sutures. The research took place in Hull, UK, over a six‐month period, although we do not know exactly when. The diagnostic criteria for CTS were not stated. The trial authors excluded those prone to poor wound healing (people taking corticosteroids, known to have "wound healing problems") and pregnant women. No hypothesis or primary outcome was stated. Outcomes were assessed at 10 days and 6 weeks postoperatively. The authors collected data on patient‐reported pain using a Likert scale (one to five) and the dichotomous outcomes of dehiscence, swelling, inflammation, infection and haematoma. No attrition was described. No funding source, conflicts of interest, or ethical review was described.
Hansen 2009 conducted a two‐group parallel, randomised trial of 58 hands (belonging to 50 participants) undergoing endoscopic carpal tunnel decompression (ECTD) for CTS confirmed by electrical studies. In one group the incision was closed with two interrupted 5‐0 non‐absorbable Novafil sutures and the other with a continuous intradermal 4‐0 monofilament Caprosyn suture. The study took place in Holstebro, Denmark, during 2006 to 2007. Participants with bilateral CTS having bilateral surgery underwent decompression on separate occasions and so were treated as independent cases. Inclusion criteria were not stated. The trialists appropriately excluded those with potential confounders for local pain and impaired healing (diabetes mellitus, inflammatory arthropathy and obesity), those ineligible for ECTD (due to prior wrist fracture or revisional surgery), and pregnant women. We assumed that the hand was the unit of randomisation but this is not defined. The power calculation lacked integral statistics (the desired alpha, beta and effect size) which precludes verification. Participants recorded their pain on a visual analogue scale (VAS) every day until follow‐up at days 10 to 14. Participants recorded scar satisfaction in private, three months postoperatively on a 5‐point ordinal scale ("very ugly", "ugly", "tolerable", "nice" or "very nice"). One participant withdrew from the trial on the day of surgery owing to symptom resolution, and two were excluded as the operation was converted to an open approach—we do not know if they were excluded pre‐ or post‐randomisation. One participant was lost to follow‐up and their data were excluded. The trial had ethical approval. The report does not state authors' conflicts of interest, predilection for suture material or any funding for the trial.
Kharwadkar 2005 was an ethically‐approved, two‐group, parallel randomised trial on 40 hands (belonging to 33 participants) with idiopathic CTS, treated with OCTD. In one group the incision was closed with 3‐0 Vicryl and the other with 3‐0 Prolene as subcuticular sutures. The trial took place in Scunthorpe, UK, in 2003. The diagnostic criteria for CTS were: 1) pain, paraesthesia or hypoaesthesia (or both) in the hand in the area innervated by the median nerve; and 2) electrophysiological confirmation of the diagnosis. The study included adults aged 18 to 75 years with CTS according to the above criteria who could also complete written questionnaires. The trialists excluded secondary CTS, those with a potential "double‐crush" (e.g. cervical radiculopathy or polyneuropathy) and those with "wound healing problems". The exclusion criteria were prior wrist surgery or trauma, secondary CTS or peripheral neuropathy, steroid use or "wound healing problems". No power calculation was provided. Participants completed validated questionnaires regarding their symptoms and function, the (Boston) Carpal Tunnel Syndrome Symptom Severity Scale (CTS‐SSS) and Functional Status Scale (CTS‐FSS) and independent assessments of outcomes were also undertaken at 2, 6 and 12 weeks postoperatively. There are many issues with Boston questionnaire, the foremost being that it is ostensibly not designed to measure the function of the hand; it was developed to quantify the symptoms of carpal tunnel syndrome (Levine 1993). Further: it is ambiguous with respect to which hand is being measured (or whether it measures both); it fails to adjust for unbalanced sided symptoms; and the difference between categories is subjective and may not be meaningful or externally valid. Presentation of these data as a quantification of hand function may be misleading. Further, as these outcome data are categorical, the authors should not present means (with standard deviations (SDs)). The trialists' methods section explains that rank‐based methods were used to analyse differences; we assume that the correct rank‐test was used for paired and unpaired data, although this is not stated. The direction of potential statistical bias cannot be confidently concluded. The trial report does not describe conflicts of interest, funding or pre‐existing suture preferences.
Menovsky 2004 conducted a three‐group parallel, randomised trial on 61 people with electromyographically proven idiopathic CTS undergoing unilateral OCTD. In one group the skin was closed with 4‐0 Ethilon, one with 4‐0 Vicryl and the other with 4‐0 stainless steel based cable sutures; all in interrupted format. No inclusion or exclusion criteria were described. They "randomised" participants to one of three groups The outcomes of pain (as measured on a 100‐point VAS) and complications were assessed at 10 days and six weeks postoperatively by the first author (TM) although the diagnostic criteria for "superficial wound infection" or "posttraumatic dystrophy" were not stated. Additionally, hands were photographed postoperatively for independent cosmetic analysis (comprising scar redness, granuloma formation and hypertrophy measured on a three‐point scale of "none", "mild" and "severe") but we do not know when the hands were photographed, the conditions under which the images were acquired or who assessed them. There was no description of a funding source, conflicts of interest, pre‐existing suture preferences or ethical review.
Theopold 2012 was a two‐group parallel, randomised trial of 47 patients with CTS seeking open decompression at Cork University Teaching Hospital, Ireland. In one group the skin was closed with 4‐0 Vircyl Rapide and the other with 4‐0 Novofil as interrupted sutures. We are not told when the trial took place. The inclusion criteria and diagnostic criteria for CTS are not described. The trial excluded patients undergoing revision surgery, those with an allergy to suture materials, those prone to hypertrophic or keloid scarring and people who were immunosuppressed. On the fourth postoperative day, participants were assessed for wound infection, with the criteria being "any patient that was prescribed antibiotics for their wound", but we do not know who reviewed the wound, made the diagnosis or if this was a post hoc assessment. On the 14th postoperative day, participants with non‐absorbable sutures had the material removed and the pain of this procedure was scored on a VAS (ranging from 0 to 10) but again, we do not know who recorded this score or the circumstances under which it was measured. Six weeks postoperatively, scars were assessed using a validated scale (Patient and Observer Scar Assessment Scale (POSAS)) by a surgeon blind to grouping, although again we do not know the identity of this assessor. The POSAS, available at www.posas.org, is an ordinal scale for various outcomes spanning 1 to 10. However, the authors report means and standard deviations, and compare these outcome measures with "Students [sic] t‐test" which is not entirely appropriate. Ten categories cannot be reliably approximated to the normal; a mean score is translatable back to the category and we assume that such data is skewed, so violating t‐distribution methods. Rank‐based methods would have been more appropriate, with the presentation of median differences and their spread. This raises concerns over the validity of the statistical methods used because the method may influence the effect estimate. A power calculation justified the trial authors' sample size. Nine participants (23.7%) were lost to follow‐up but we do not know the group‐specific rates. The trial does not describe funding, pre‐existing suture preferences, conflicts of interest or ethical review.
Excluded studies
See Characteristics of excluded studies. As literature on this topic is very limited, we listed all papers assessed for inclusion, rather than follow usual practice, which is to restrict Excluded studies to reports of potentially randomised trials.
We excluded Freshwater 2012 and Dellon 2001 because these were letters to the editor in response to two included trials (Theopold 2012 and Erel 2001, respectively). We also excluded the case reports by Acioly 2013, Hung 2008, Kokkalis 2015, Lu 2017 and Zingale 2003 as they were not applicable to our review. The prospective studies by Dosani 2013 and MacFarlane 2014 reported the costs, healing profiles and clinical outcomes of absorbable versus non‐absorbable sutures for skin closure following open carpal tunnel decompression; however, neither were randomised trials and so we excluded them. Although a potentially eligible study, we had to exclude Kundra 2010 because it included patients undergoing numerous different hand operations (trigger finger release, Dupuytren's excision and carpal tunnel surgery) which were randomised to compare the aesthetic outcomes of absorbable and non‐absorbable sutures only; they did not record any outcomes of relevance to this review. The investigators reported scar outcomes for the entire sample; therefore, we wrote to the authors requesting data specific to carpal tunnel decompression surgery and any other outcomes which may have been recorded (no protocol is provided to cross‐check) and although the first author replied, the original data were not available and so we were obliged to exclude the trial. We excluded the review articles by Scholten 2012 and Jerosch‐Herold 2006 because they contained no primary outcome data. We excluded the case series by Nassar 2014 and Kokkalis 2016 as they described the use of a synthetic biofilm after revision OCTD. Ramos‐Zúñiga 2017 reported the outcomes a series of patients undergoing ECTD and so was not applicable. We excluded Lattré 2016 as this described their experience with OCTD and the hypothenar fat pad flap. We excluded Magalhães 2017 as this systematic review had similar aims to our review; we cross‐checked their references and found that we had screened all the same citations except Bolster 2013, which we excluded as this trial compared different formats of suture (interrupted versus vertical mattress) after OTCD. The case series by Tosti 2017 was excluded as this considered catheter‐associated radial artery pseudoaneurysms.
Risk of bias in included studies
All studies were subject to risks of bias, and lacked important information about the study design and execution. We contacted the corresponding authors of all eligible studies in order to attempt to obtain missing data and clarify unclear methodology. The authors of Hansen 2009 replied and clarified our questions. We received no replies from the authors of other included studies (Erel 2001; Kharwadkar 2005; Menovsky 2004; Theopold 2012). See Figure 2 for a summary of 'Risk of bias' judgements.
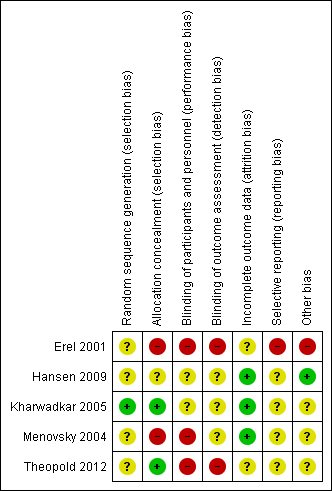
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both Kharwadkar 2005 and Theopold 2012 used sealed opaque envelopes to conceal allocation (presumably from participants, although not explicitly stated, because blinding the surgeon would not be possible), and so we judged these studies to have low risk of allocation bias. The risk of bias from the method of random sequence generation was low in Kharwadkar 2005, but unclear in Theopold 2012, as the report did not provide details.
We judged Hansen 2009 to have unclear risk of selection bias, as methods of randomisation and allocation concealment were inadequately described. We judged Erel 2001 and Menovsky 2004 as having unclear risk of bias from the method of randomisation and high risk of bias from unconcealed allocation.
Blinding
We assumed that blinding of participants and interviewers did not take place in Erel 2001, since these procedures were not described. Also Erel 2001 did not describe how outcomes were assessed, e.g. who determined the presence or absence of residual inflammation. This is particularly concerning given that "data were collected by telephone interview where possible". Also, the paper does not detail how the outcome of residual pain was measured, i.e. a mildly sore hand after substantial activity in the absence of analgesia is not the same as significant rest pain in the hand despite the use of opioid analgesia. We considered the trial at high risk of performance and detection bias.
Whilst Hansen 2009 did not qualify how they assessed for the presence of infection postoperatively, it is reasonable to deduce that an appropriately qualified healthcare professional with experience in hand surgery could reliably identify this adverse outcome. More importantly, we do not know if the same nurse removed the sutures and measured the outcome of infection. Similarly, this study states that the questionnaire regarding cosmesis was completed and placed into a closed envelope, but it is again unclear whether participants or researchers were blinded to the collected data and whether statistical analysis was undertaken with knowledge of the grouping. Therefore, we have judged the risk of performance bias as unclear (given the lack of information on participant blinding) and the risk of detection bias as unclear (for the same reason).
In Kharwadkar 2005, participants completed validated questionnaires regarding their hand function postoperatively and independent assessments of outcomes were also undertaken at 2, 6, and 12 weeks postoperatively. However, it is not clear whether the assessors were blinded to the intervention. The authors did not declare any conflicts of interest.
In the three‐group trial by Menovsky 2004, performance bias was likely as blinding was unclear and detection bias was possible for pain and numbness outcomes, given that the lead author and operating surgeon undertook the postoperative evaluation of these symptoms. Conversely, an independent group of four unnamed persons performed assessments of aesthetic outcomes based on photographs. This mixed picture led us to assign a judgement of 'unclear' risk of detection bias.
The data from Theopold 2012 are at high risk of performance and detection bias. Participants were not blinded and it is unclear who obtained the patient‐reported pain data on the 14th postoperative day; however, it is reasonable to assume that the methods were consistent and patient‐reported data from the 14th postoperative day is at high risk of bias. Data obtained six weeks postoperatively was via assessors blind to grouping. Overall we judged there to be a high risk of performance and detection bias given the majority of data (and those we have used) were obtained from unblinded participants and assessors.
Incomplete outcome data
Erel 2001 had no responses from six participants (9.4%) at 10 days for their primary outcome of pain, and had at least four dropouts (6.3%) at six weeks. We assessed the trial as having unclear risk of attrition bias. There was a high dropout rate (19%) in Theopold 2012 and so we assigned a judgement of 'unclear' risk of bias in this domain. Four people dropped out for reasons unrelated to the interventions in Hansen 2009. We considered this to represent a low risk of bias. Other studies reported complete data.
Selective reporting
The risk of selective outcome reporting by trial participants was high in Erel 2001 as numerical results were not reported for some outcomes, and unclear in Hansen 2009. We also assessed the other three trials as 'unclear' risk because there were no published protocols to which we could compare the published articles (Kharwadkar 2005; Menovsky 2004; Theopold 2012).
Other potential sources of bias
The contact author of Hansen 2009 confirmed in communication with review authors that the trialists had no conflicts of interest and that the trial was unfunded. He also declared a preference for absorbable sutures.
In Erel 2001 the Chi2 test was incorrectly used for some comparisons as they violated the assumptions that < 20% of expected counts are < 5. Ideally, they would have used exact methods (or resampling, also known as bootstrapping) as a wrongly used test of proportional difference is more likely to find a significant difference where none exists. We are unsure how the presumed continuous variable of 'weeks off work' was analysed but this is not an outcome in this review.
We assessed the risk of 'other bias' as unclear for Theopold 2012. The study reported no conflicts of interest and no specific funding, however, trialists did not provide information on pre‐existing suture preference.
Kharwadkar 2005 and Menovsky 2004 did not describe conflicts of interest or individual preferences for suture type and these omissions weaken the reliability of the results; we therefore assessed them as at unclear risk of bias. We feel that it is important for surgeons to state their prior biases, especially when outcome assessment is unblinded. This is because some surgeons may prefer one suture material over another and this could bias the assessment of outcomes.
Effects of interventions
See: Summary of findings for the main comparison Absorbable versus non‐absorbable sutures for skin closure after open carpal tunnel decompression surgery; Summary of findings 2 Absorbable versus non‐absorbable sutures for skin closure after endoscopic carpal tunnel decompression surgery
Absorbable versus non‐absorbable sutures after open carpal tunnel decompression (OCTD)
Primary outcome: postoperative pain
Postoperative pain at 10 days
This outcome was reported in Erel 2001, Kharwadkar 2005 and Menovsky 2004.
Ten days after OCTD, the standardised mean difference (SMD) in pain in absorbable versus non‐absorbable suture groups was 0.03 (95% confidence interval (CI) ‐0.43 to 0.48; 3 randomised controlled trials (RCTs), number of participants (N) = 137; I2 = 43%), as shown in Analysis 1.1 and Figure 3. As a rule of thumb, an SMD of less than 0.2 indicates no clear difference (Higgins 2011). The I2 suggests moderate heterogeneity, probably arising from variation in surgical techniques and outcome measures. Analyses using fixed‐effect instead of the random‐effects model had little effect on the findings. We judged the quality of the evidence to be very low according to GRADE criteria, as shown in summary of findings Table for the main comparison.
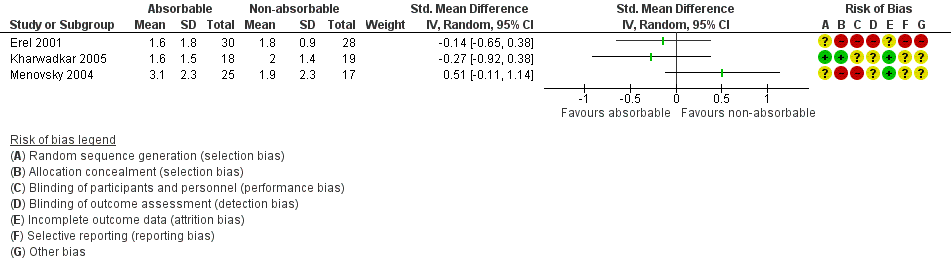
Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.1 Postoperative pain (10 days) after CTD.
Postoperative pain at 6 weeks
This outcome was reported in four trials of wound closure following OCTD (Erel 2001; Kharwadkar 2005; Menovsky 2004; Theopold 2012). The SMD in pain in absorbable versus non‐absorbable suture groups indicated little or no difference between suture types at six weeks (SMD 0.06, 95% CI ‐0.72 to 0.84; 4 trials, N = 175; I2 = 84%; Analysis 1.2, Figure 4). The I2 suggests substantial heterogeneity, probably arising from variation in surgical techniques and outcome measures. Analyses using fixed‐effect versus random‐effects models had little effect on the findings. The quality of evidence was very low (summary of findings Table for the main comparison).
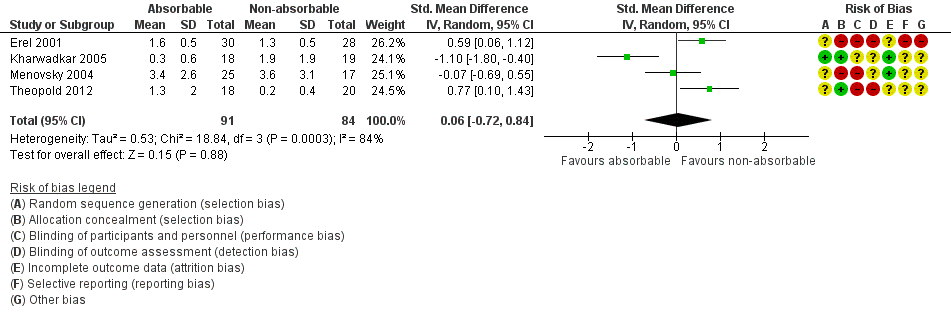
Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.2 Postoperative pain (6 weeks) after open CTD.
Secondary outcomes
Postoperative hand function
Kharwadkar 2005 (N = 36) was the only study to report hand function, which was measured using the Carpal Tunnel Syndome Functional Status Scale (CTS‐FSS). The report provided mean scores and standard deviations (SDs) for each group pre‐operatively, at 2 weeks (MD ‐0.10, 95% CI ‐0.53 to 0.33), 6 weeks (MD 0.00, 95% CI ‐0.39 to 0.39), and 12 weeks (0.00, 95% CI ‐0.37 to 0.37), suggesting no differences between the groups (Analysis 1.3). The evidence is very low quality (summary of findings Table for the main comparison).
Scar satisfaction and wound inflammation
Data for this outcome were provided in Kharwadkar 2005 and Erel 2001.
The risk ratio (RR) for wound inflammation with use of absorbable versus non‐absorbable sutures after OCTD was 2.28 (95% CI 0.24 to 21.91; 2 RCTs, N = 95; I2 = 90%), favouring non‐absorbable sutures (Analysis 1.4; Figure 5). The I2 suggests substantial heterogeneity, probably arising from variation in surgical techniques and outcome measures. The quality of evidence was very low (summary of findings Table for the main comparison).
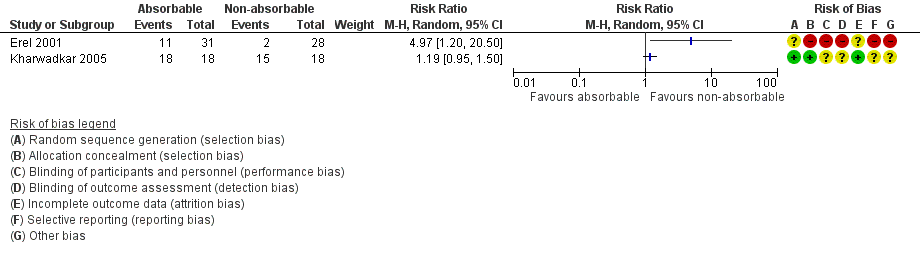
Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.3 Wound inflammation.
Scar satisfaction was not measured.
Postoperative adverse events
We were unable to perform a narrative review or pooled analysis of postoperative and adverse events as these outcomes were not reported in the included studies.
Absorbable versus non‐absorbable sutures after endoscopic carpal tunnel decompression
Primary outcome: postoperative pain
Postoperative pain at 10 days
Reported in Hansen 2009; the SMD for pain scores in the absorbable versus non‐absorbable suture groups 10 days postoperatively after ECTD was ‐0.81 (95% CI ‐1.36 to ‐0.25; 1 RCT, N = 54) (Analysis 2.1 and Figure 3). Using a rule of thumb for SMD interpretation, this is a large effect in favour of absorbable sutures. According to GRADE criteria, we judged the quality of this evidence to be very low, as shown in summary of findings Table 2.
Postoperative pain at 6 weeks
The ECTD study did not provide data for this outcome.
Secondary outcomes
Postoperative hand function
Not measured.
Scar satisfaction and wound inflammation
Reported in Hansen 2009; the RR for wound inflammation with use of absorbable versus non‐absorbable sutures after ECTD was 0.93 (95% CI 0.06 to 14.09; 1 RCT, N = 54) (Analysis 2.2 and Figure 5). We judged the quality of this evidence to be very low, as shown in summary of findings Table 2.
Participants in Hansen 2009 recorded scar satisfaction three months after the operation on a five‐point ordinal scale ("very ugly", "ugly", "tolerable", "nice" or "very nice"). In the absence of results from a validated scale we have reported the findings. The trial reported that 25 out of28 participants in the absorbable‐suture group judged the scar to have a nice appearance compared with 18 out of 26 in the non‐absorbable suture group (RR 1.29, 95% CI 0.97 to 1.72; 1 RCT, N = 54) (Analysis 2.3). One person in the group given non‐absorbable sutures reported that the scar was ugly or very ugly, compared with none in the group given absorbable sutures. We judged the quality of evidence very low, as shown in summary of findings Table 2.
Postoperative adverse events
We were unable to perform a narrative review or pooled analysis of postoperative adverse events as these outcomes were not reported in the included studies.
Discussion
Summary of main results
This review included five trials (255 randomised participants) comparing absorbable and non‐absorbable sutures, with various surgical techniques to decompress the carpal tunnel and then close the skin.
It is uncertain whether there is any difference in the outcomes following skin closure with absorbable versus non‐absorbable sutures after open or endoscopic carpal tunnel decompression surgery (OCTD and ECTD, respectively). We chose pain as the primary outcome as this is the most commonly reported outcome, all adverse events (are likely to) manifest with pain in the hand and anecdotally, surgical trainees are taught that absorbable sutures cause more inflammation and so, pain. However, some readers may question the utility of pain as an outcome in the study of skin closure after CTD and the effect of suture material. It remains unclear whether suture material has any meaningful effect on postoperative pain following OCTD or ECTD and whether this association is direct or acts via other factors (e.g. occult infection, foreign‐body reaction to retained suture material, or otherwise). We were seeking to add to the literature and help to clarify this uncertainty in this review, but as the quality of the evidence on this topic is very low, no reliable conclusions can be drawn with respect to the outcomes of postoperative pain.
By eliminating the need for suture removal, patients avoid future hospital or general practice appointments, which has the potential to save over GBP 3 million per year in the UK, which is based upon the direct cost of a nurse appointment for suture removal in a GP practice (PSSRU 2016). The cost saving would likely be much greater once other direct costs, of instruments, dressings, protective equipment, and infrastructure, are considered, as well as the speculative indirect cost savings for patients. Additionally, by giving the patient autonomy in their wound care they can be instructed to remove their own dressings at home (after a specified period of time), thoroughly wash their own hand(s) and redress as needed. Some readers may argue that the externalised knots of absorbable sutures require removal by a professional, which we accept; however, provided people are warned of this possibility, they can seek an appointment on their own terms, which again may confer fiscal savings.
Overall completeness and applicability of evidence
Despite an analysis of five randomised trials directly comparing absorbable and non‐absorbable sutures for carpal tunnel closure, we were unable to confidently reach any clinically useful conclusions on whether outcomes differ between these interventions. This is primarily due to the high risk of bias of each included trial. There is no robust evidence to support the use of one suture material over the other in terms of postoperative outcomes. A rigorous, well‐designed non‐inferiority randomised trial of absorbable versus non‐absorbable suture for carpal tunnel closure following decompression is indicated. The justification of a future trial should be based on the findings of our review and avoid the methodological flaws of the included studies. Scar satisfaction and postoperative hand function were each reported in one trial and no trials reported adverse events. Studies should report on all outcomes of relevance as described above.
Two trials used only interrupted sutures (Menovsky 2004; Theopold 2012), whilst the other three used a combination of subcuticular and interrupted sutures (Erel 2001; Hansen 2009; Kharwadkar 2005). At this point we should highlight that we assume "subcuticular", "intradermal" and "subcutaneous" sutures are all comparable, as each study uses different terminology. We take these terms to mean that the substance of the suture resides entirely within the dermis. Nonetheless, this is a potential confounding variable both for the individual trials and for our pooled analysis as each suture will manipulate the glabrous skin of the hand within the longitudinal crease of the ring ray differently so, for example, a mattress suture may evert the skin edges, an intradermal suture may align them neutrally, and a simple interrupted suture may achieve neutrality, inversion, or eversion of the skin edges depending on the surgeon's technique. Further, data from Kharwadkar 2005 is substantially different to the other trials, as shown in Figure 4; it is unclear why this study is an outlier but the only notable difference is that the quality of this trial was superior to all other studies and that they used subcuticular suturing, which is uncommon in hand surgery given that such a technique is more challenging on the palmar skin. Future studies should aim to compare the same format of suture (e.g. mattress only) and only differ in the suture material. Furthermore, most articles lack details about the suturing method, for example how sutures were secured (i.e. buried knots versus externalised knots versus loose ends with no knot), the type of needle used, etc., which prevents the application of the findings to clinical practice.
Our review did not consider a number of other outcomes from carpal tunnel surgery of interest to hand surgeons, patients and policy makers. Effective allocation of healthcare resources depends on cost‐effectiveness data. We were interested in the fiscal impacts of suture choice but, in the design phase of this review, agreed by consensus that the effectiveness and side‐effect profile of each suture material should be established first. Accordingly, cost outcomes did not feature as an outcome of interest in our protocol and, in fact, none of the included studies reported cost outcomes. Whilst our protocol was designed to capture serious adverse events, interventions in hand surgery are often designed to hasten return to usual activities of daily living or work. In common with the authors of the Cochrane Review of rehabilitation interventions following carpal tunnel release (Peters 2016), we consider an adequately powered return‐to‐work analysis unlikely given the high success rate of carpal tunnel surgery and the heterogeneity of trials, outcome definition and timing. Nevertheless, future versions will include return to work or usual activities as a secondary outcome, along with cost and quality of life, so that available data can be included. A further change in future versions will be specification of serious and rare adverse events, such as complex regional pain syndrome.
Quality of the evidence
Overall, the quality of evidence is very low; consequently the findings of our review are of limited transferability. To qualify this statement and descriptions in our summary of findings Table for the main comparison and summary of findings Table 2, we expand upon these reasons below.
-
Risk of bias—with the exception of Kharwadkar 2005, all studies were at high or unclear risk of numerous biases based on deficiencies in the published manuscripts, such as: failure to describe the details of the randomisation and allocation, lack of blinding of participants or outcome assessors, missing data due to attrition and the omission of statements of conflicts of interest and funding. All trials were conducted (and likely accepted for publication) prior to the genesis of the trial reporting guidance, therefore this information may exist but we were unable to clarify such details with authors and so accordingly, we downgraded the evidence twice.
-
Inconsistency—all studies yield widely different estimates of the effect of absorbable versus non‐absorbable sutures on pain after OCTD at 10 days, which could be a manifestation of heterogeneity of the intervention or of the outcome measurement; therefore, as a body of evidence, we have downgraded it once.
-
Indirectness—the pooled evidence comes from a variety of sources, using different sutures in unknown configurations and assessed using different outcome measures. We also question the statistical analysis of non‐linear measurement scales used for assessment of symptom and function. Therefore, we have concern over the generalisability of the data.
-
Imprecision—the sample sizes of all studies were small and the number of adverse events small too, so the confidence intervals (CIs) are all very wide. Conventional power calculations to detect a difference in means for the primary outcome of pain (according to the means in the included studies), with 90% power and a 5% level of significance, would warrant more than 1000 participants per study. Given that our review includes just 255 participants, we have downgraded the evidence twice for imprecision of the estimates.
-
Publication bias—we were unable to formally assess publication bias and so have not downgraded the evidence on this basis, but readers should be aware that the risk of such bias is possible.
Potential biases in the review process
We chose to use 'the hand' as our unit of analysis because all trials were based on unilateral surgery. Two trials included participants with bilateral carpal tunnel syndrome (Hansen 2009; Kharwadkar 2005), although each hand was decompressed on separate occasions and therefore, each hand recorded as an individual case. Previous reviews on topics related to CTS have highlighted the importance of correctly selecting the unit of analysis—'the hand' versus 'the patient' (Scholten 2007) and this statistical issue is familiar to hand surgeons (Sauerland 2003). This is particularly important when considering the outcomes of interest. For example, the unit of analysis should arguably be 'the patient' when satisfaction or return‐to‐work are the variables of interest, irrespective of whether the surgery is unilateral, staged bilateral, or simultaneous bilateral. Conversely, when one is considering a local phenomenon or complication (which is usually unilateral, such as infection) then we feel that the unit of analysis should be 'the hand'. More difficult outcomes are those of pain, inflammation, aesthetics, and function, as one side is not independent of the other, or the person in a holistic sense. We would argue that when the unit of analysis is in doubt (such as for local phenomena or holistic outcomes), that 'the hand' should be the unit of analysis in order to better capture sided differences. We accept that by using 'the hand' as the unit of measure, the sample size is artificially inflated, which will erroneously shrink the standard error of the effect size and narrow the confidence interval. Also, by using 'the hand' as the unit of measure, some statistical tests can no longer be used as the assumptions of independence are violated. Therefore, researchers should take great care when selecting their unit of analysis and seek professional statistical advice when designing their research.
Four trials concerned OCTD via a longitudinal skin incision (3 cm to 4 cm in length, as reported by two studies), whilst Hansen 2009 was based on ECTD through an incision of unknown size (although we assume that it is smaller than 3 cm). We chose to include data from the trial on endoscopic surgery because we feel that operatively, the procedure is sufficiently similar to OCTD (i.e. whether the incision is 1 cm (for endoscopic surgery) or 3 cm (for open surgery), both warrant closure with sutures and both are prone to the adverse outcomes we sought to investigate). Vasiliadis 2014 suggested that outcomes of ECTD and OCTD are different, as endoscopic surgery carried a lower risk of minor complications (RR 0.55, 95% CI 0.38 to 0.81, 18 studies) but a higher risk of transient nerve problems (i.e. neurapraxia, numbness, and paraesthesiae) and so they concluded that ECTR was safer when the total number of complications were assessed (RR 0.60, 95% CI 0.40 to 90, 20 studies). Therefore, some readers might disagree with our decision to include data on ECTD. To approach the problem in a balanced manner, we included data from Hansen 2009 but analysed the trial separately. Future trialists and reviewers may consider our opinions on this topic in the section Implications for research.
With hindsight, wound inflammation is a difficult outcome to define and moreover, it is debatable how important wound inflammation is in the context of pain, adverse outcomes or hand function. Wound inflammation is a normal part of the healing process and judging when this is pathologically is difficult for clinicians and more complex still for review authors. To handle the heterogeneity of outcome measures and time points at which wound inflammation was assessed, we elected to dichotomise the outcome, which certainly introduces difficulties in interpretation. We suggest that authors of future reviews consider whether wound inflammation is necessary to consider and if so, how best to handle these data.
The majority of studies reported pain at 10 days and at 6 weeks and we also reported these time points in this review (our protocol specified 48 hours, 48 hours to seven days and more than seven days). Although the review lacks data from the immediate postoperative period, this is of less relevance than long‐term healing.
As surgical trainees, RGW and JCRW have not formed their preferences for any particular suture type and use the suture preferred by the consultant in charge of care. AF, a consultant plastic surgeon and associate professor, uses absorbable 4‐0 Vicryl Rapide for his elective OCTD. However, he has no strong preference and this systematic review was encouraged and supervised in an unbiased fashion, in the pursuit of a high‐quality research synopsis.
Agreements and disagreements with other studies or reviews
We identified one other systematic review that considered studies of absorbable versus non‐absorbable sutures for closing the skin after OCTD. The findings differ from those of our review, in that the authors' conclusion was in favour of non‐absorbable sutures (Magalhães 2017). However, limitations in conduct and reporting in Magalhães 2017 account for the discrepancy between their findings and those of this review: the methods, search strategy, inclusion criteria and outcomes were not fully described and the authors did not assess the risk of bias or the quality of the evidence. There was a narrative summary of postoperative pain, infection, and scar and wound inflammation.but no meta‐analysis or measurement of heterogeneity.

Flow diagram illustrating the study selection process.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.1 Postoperative pain (10 days) after CTD.
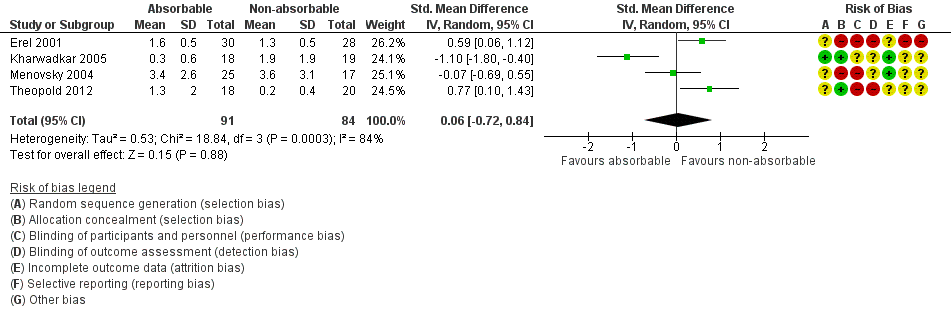
Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.2 Postoperative pain (6 weeks) after open CTD.

Forest plot of comparison: 1 Absorbable versus non‐absorbable sutures: open and endoscopic carpal tunnel decompression, outcome: 1.3 Wound inflammation.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 1 Postoperative pain (10 days) after CTD.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 2 Postoperative pain (6 weeks) after CTD.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 3 Postoperative hand function (BCQ‐FSS) after CTD.

Comparison 1 Absorbable versus non‐absorbable sutures: open endoscopic carpal tunnel decompression (CTD), Outcome 4 Wound inflammation.

Comparison 2 Absorbable versus non‐absorbable sutures: endoscopic carpal tunnel decompression (CTD), Outcome 1 Postoperative pain (10 days) after CTD.

Comparison 2 Absorbable versus non‐absorbable sutures: endoscopic carpal tunnel decompression (CTD), Outcome 2 Wound inflammation.

Comparison 2 Absorbable versus non‐absorbable sutures: endoscopic carpal tunnel decompression (CTD), Outcome 3 Scar satisfaction (scar assessed as 'nice') by participant after endoscopic CTD.
| Absorbable sutures compared with non‐absorbable sutures for carpal tunnel decompression after open carpal tunnel decompression surgery | ||||||
| Patients: adults undergoing primary carpal tunnel decompression by open carpal tunnel decompression Intervention: absorbable sutures for wound closure Comparison: non‐absorbable sutures for wound closure Setting : secondary care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Non‐absorbable sutures | Absorbable sutures | |||||
| Postoperative pain: early pain (10 days postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative VAS pain after OCTD in the non‐absorbable suture groups was 0.03 SMD higher (0.43 lower to 0.48 higher) | 137 (3 RCTs) | ⊕⊝⊝⊝ | SMD 0.03 (95% CI ‐0.43 to 0.48) A SMD of 0.03 represents little or no difference between groups3 It is uncertain whether or not there is any difference in postoperative pain scores at 10 days because the quality of evidence is very low. | |
| Postoperative pain: late pain (6 weeks postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative pain (6 weeks) after open CTD in the non‐absorbable suture groups was | 175 (4 RCTs) | ⊕⊝⊝⊝ | SMD 0.06 (95% CI ‐0.72 to 0.84) A SMD of 0.06 represents little or no difference between groups. It is uncertain whether there is any difference in postoperative pain scores at 6 weeks because the quality of evidence is very low. | |
| Postoperative hand function (2 weeks postoperatively) Mean | The mean FSS score in the non‐absorbable suture group 2 weeks postoperatively was 1.6 | The mean FSS score 2 weeks postoperatively was 0.1 lower (0.53 lower to 0.33 higher) | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝ | MD ‐0.10, 95% CI ‐0.53 to 0.33) It is uncertain whether there is any difference between groups in postoperative hand function because the quality of evidence is very low. MD at 6 and 12 weeks follow‐up postoperatively were 0.00 (95% CI ‐0.39 to 0.39) and 0.00 (95% CI ‐0.37 to 0.37). |
| Wound Inflammation 6 to 12 weeks follow‐up | 370 per 1000 | 843 per 1000 (89 to 1000) | RR 2.28 (0.24 to 21.91) | 95 | ⊕⊝⊝⊝ | It is uncertain whether there is any difference between groups in the occurrence of postoperative wound inflammation because the quality of evidence is very low. |
| Postoperative scar satisfaction | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse outcomes including wound infection, scar breakdown or return to theatre | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| CI: confidence interval; OCTD: open carpal tunnel decompression; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale; CTS‐FSS: Carpal Tunnel Syndome Functional Status Scale | ||||||
| Quality of Evidence Grades | ||||||
| 1Downgraded twice based on study limitations (lack of allocation concealment, and lack of blinding of participants and assessors). 3Based on a rule‐of‐thumb guide to interpretation of SMD: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988 in Higgins 2011). | ||||||
| Absorbable sutures compared with non‐absorbable sutures for endoscopic carpal tunnel decompression | ||||||
| Patients: adults undergoing primary carpal tunnel decompression by endoscopic carpal tunnel decompression Intervention: absorbable sutures for wound closure Comparison: non‐absorbable sutures for wound closure Setting: secondary care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Non‐absorbable sutures | Absorbable sutures | |||||
| Postoperative pain: early pain (10 days postoperatively) VAS 0 to 10 Verbal reporting scale No pain‐mild pain‐moderate pain‐severe pain (converted to numerical scale (0 to 3, 3 to 6, 6 to 10) | ‐ | The mean postoperative VAS pain after ECTD in the non‐absorbable suture groups was 0.81 SMD lower (1.36 to 0.25 lower) | 54 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | SMD ‐0.81 (95% CI ‐1.36 to ‐0.25) A SMD of ‐0.81 represents a large difference between groups.3 It is uncertain whether or not there is any difference in postoperative pain scores at 10 days because the quality of evidence is very low. | |
| Postoperative pain: late pain (6 weeks postoperatively) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Postoperative hand function | ‐ | ‐ | ‐ | ‐ | Not measured | |
| Wound Inflammation 6 to 12 weeks follow‐up | 38 per 1000 | 36 per 1000 (2 to 542) | RR 0.93 (0.06 to 14.09) | 54 (1 RCT) | ⊕⊝⊝⊝ | It is uncertain whether there is any difference between groups in the occurrence of postoperative wound inflammation because the quality of evidence is very low. |
| Postoperative scar satisfaction | 692 per 1000 | 893 per 1000 (672 to 1000) | RR 1.29 (0.97 to 1.72) | ‐ | ⊕⊝⊝⊝ | |
| Adverse outcomes including wound infection, scar breakdown or return to theatre | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| CI: confidence interval; ECTD: endoscopic carpal tunnel decompression; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale | ||||||
| Quality of Evidence Grades | ||||||
| 1Downgraded twice for study limitations (lack of allocation concealment, and lack of blinding of participants and assessors). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain (10 days) after CTD Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Postoperative pain (6 weeks) after CTD Show forest plot | 4 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.72, 0.84] |
| 3 Postoperative hand function (BCQ‐FSS) after CTD Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 2 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.53, 0.33] |
| 3.2 6 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.39, 0.39] |
| 3.3 12 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.37, 0.37] |
| 4 Wound inflammation Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative pain (10 days) after CTD Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Wound inflammation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Scar satisfaction (scar assessed as 'nice') by participant after endoscopic CTD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

