مقیاس ارزیابی رفتاری نوزادان (NBAS) و سیستم مشاهدات رفتاری نوزاد تازه متولد شده (NBO) در حمایت از مراقبان و بهبود پیامدها در مراقبان و نوزادان آنها
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Systematic Reviews (DARE); all in the Cochrane Library
#1 MeSH descriptor: [Parent‐Child Relations] explode all trees
#2 MeSH descriptor: [Object Attachment] this term only
#3 MeSH descriptor: [Early Intervention (Education)] this term only
#4 ((parent* or mother* or father*) near/3 (demonstrat* or teach* or train*)):ti,ab,kw (Word variations have been searched)
#5 Neonatal Behavio?r* Assessment Scale:ti,ab,kw (Word variations have been searched)
#6 Newborn Behavio?r* Assessment Scale:ti,ab,kw (Word variations have been searched)
#7 NBAS or BNAS or BNBAS:ti,ab,kw (Word variations have been searched)
#8 (Brazelton* or Brazleton*):ti,ab,kw (Word variations have been searched)
#9 Newborn Behavio?ral Observation* System:ti,ab,kw (Word variations have been searched)
#10 NBO:ti,ab,kw (Word variations have been searched)
#11 (newborn* or neonat* or baby or babies or infant*):ti,ab,kw (Word variations have been searched)
#12 MeSH descriptor: [Infant] explode all trees
#13 #11 or #12
#14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#15 #13 and #14
MEDLINE Ovid
1. Parent Child relations/
2. Mother Child Relations/
3. Father Child Relations/
4. Object attachment/
5. Early Intervention/
6. ((parent$ or mother$ or father$) adj3 (demonstrat$ or teach$ or train*)).tw.
7. Neonatal Behavio?r$ Assessment Scale.mp.
8. Newborn Behavio?r$ Assessment Scale.mp.
9. NBAS.tw.
10. BNAS.tw.
11. BNBAS.tw.
12. (Brazelton$ or Brazleton$).mp.
13. Newborn Behavio?ral Observation$ System.mp.
14. NBO.tw.
15. (newborn$ or neonat$ or baby or babies or infant$).tw.
16. exp Infant/
17. 15 or 16
18. or/1‐14
19. randomised controlled trial.pt.
20. controlled clinical trial.pt.
21. randomized.ab.
22. placebo.ab.
23. drug therapy.fs.
24. randomly.ab.
25. trial.ab.
26. groups.ab.
27. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28. exp animals/ not humans.sh.
29. 27 not 28
30. 17 and 18 and 29
Embase Ovid
1. Parent Child relations/
2. Mother Child Relations/
3. Father Child Relations/
4. Object attachment/
5. Early Intervention/
6. ((parent$ or mother$ or father$) adj3 (demonstrat$ or teach$ or train*)).tw.
7. Neonatal Behavio?r$ Assessment Scale.mp.
8. Newborn Behavio?r$ Assessment Scale.mp.
9. NBAS.tw.
10. BNAS.tw.
11. BNBAS.tw.
12. (Brazelton$ or Brazleton$).mp.
13. Newborn Behavio?ral Observation$ System.mp.
14. NBO.tw.
15. (newborn$ or neonat$ or baby or babies or infant$).tw.
16. exp Infant/
17. 15 or 16
18. or/1‐14
19. random$.tw.
20. factorial$.tw.
21. crossover$.tw.
22. cross over$.tw.
23. cross‐over$.tw.
24. placebo$.tw.
25. (doubl$ adj blind$).tw.
26. (singl$ adj blind$).tw.
27. assign$.tw.
28. allocat$.tw.
29. volunteer$.tw.
30. Crossover Procedure/
31. double‐blind procedure.tw.
32. Randomized Controlled Trial/
33. Single Blind Procedure/
34. or/19‐33
35. (animal/ or nonhuman/) not human/
36. 34 not 35
37. 17 and 18 and 36
PsycINFO Ovid
1. Parent Child relations/
2. Mother Child Relations/
3. Father Child Relations/
4. Attachment Behavior/
5. Early Intervention/
6. Parent training/
7. ((parent$ or mother$ or father$) adj3 (demonstrat$ or teach$ or train*)).tw.
8. Neonatal Behavio?r$ Assessment Scale.mp.
9. Newborn Behavio?r$ Assessment Scale.mp.
10. NBAS.tw.
11. BNAS.tw.
12. BNBAS.tw.
13. (Brazelton$ or Brazleton$).mp.
14. Newborn Behavio?ral Observation$ System.mp.
15. NBO.tw.
16. or/1‐15
17. (neonatal birth 1 mo or infancy 2 23 mo).ag.
18. (newborn$ or neonat$ or baby or babies or infant$).tw.
19. or/17‐18
20. clinical trials/
21. random$.tw.
22. (allocat$ or assign$).tw.
23. ((clinic$ or control$) adj trial$).tw.
24. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
25. (crossover$ or "cross over$").tw.
26. random sampling/
27. Experiment Controls/
28. exp experimental methods/
29. Placebo/
30. placebo$.tw.
31. exp program evaluation/
32. treatment effectiveness evaluation/
33. ((effectiv$ or evaluat$) adj3 (intervention$ or stud$ or research$)).tw.
34. or/20‐33
35. 16 and 19 and 34
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S26 S13 AND S16 AND S25
S25 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24
S24 (allocat* random*)
S23 (MH "Quantitative Studies")
S22 (MH "Placebos")
S21 placebo*
S20 (random* allocat*)
S19 (MH "Random Assignment")
S18 (Randomi?ed control* trial*)
S17 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S16 S14 OR S15
S15 (MH "Infant+")
S14 (newborn* or neonat* or baby or babies or infant*)
S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12
S12 NBO
S11 Newborn Behavio?ral Observation* System
S10 Newborn Behavio?ral Observation* System
S9 (Brazelton* or Brazleton*)
S8 NBAS or BNAS or BNBAS
S7 Newborn Behavio?r* Assessment Scale
S6 Newborn Behavio?r* Assessment Scale
S5 Neonatal Behavio?r* Assessment Scale
S4 ((parent* or mother* or father*) N3 (demonstrat* or teach* or train*))
S3 (MH "Early Intervention")
S2 (MH "Attachment Behavior")
S1 (MH "Parent‐Child Relations+")
Science Citation Index (SCI), Social Sciences Citation Index (SSCI), Conference Proceedings Citation Index ‐ Science (CPCI‐S); Conference Proceedings Citation Index ‐ Social Science & Humanities (CPCI‐SS&H), BIOSIS; all Web of Science
#1 TOPIC: ((((parent* or mother* or father*) near/3 (demonstrat* or teach* or train*))))
#2 TOPIC: ((Neonatal Behavio?r* Assessment Scale))
#3 TOPIC: ((Newborn Behavio?r* Assessment Scale))
#4 TOPIC: ((NBAS or BNAS or BNBAS))
#5 TOPIC: (((Brazelton* or Brazleton*)))
#6 TOPIC: ((Newborn Behavio?ral Observation* System))
#7 TOPIC: ((NBO))
#8 TOPIC: (((newborn* or neonat* or baby or babies or infant*)))
#9 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#10 #9 AND #8
#11 TS=Randomized clinical trial* OR TI=Randomized clinical
#12 TI=randomi* OR TS=randomi*
#13 TI=clin* OR TS=clin*
#14 TS=trial* OR TI=trial*
#15 #14 AND #13
#16 TS=(singl* OR Doubl* OR Tripl* OR Trebl*) OR TI=(singl* OR Doubl* OR Tripl* OR Trebl*)
#17 TS=(mask* OR blind*) OR TI=(mask* OR blind*)
#18 TS=crossover* OR TI=crossover*
#19 TS=(allocate* OR assign*) OR TI=(allocate* OR assign*)
#20 TS=random* OR TI=random*
#21 #20 AND #19
#22 #21 OR #18 OR #17 OR #16 OR #15 OR #12 OR #11
#23 #22 AND #10
ERIC EBSCOhost (Education Resources Information Center)
S19 S17 AND S18
S18 TI ( trial* OR random* OR crossover OR blind*) ) OR AB ( trial* OR random* OR crossover OR blind*) )
S17 S13 AND S16
S16 S14 OR S15
S15 (newborn* or neonat* or baby or babies or infant*)
S14 DE "Infants" OR DE "Neonates" OR DE "Premature Infants"
S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12
S12 DE "Early Intervention"
S11 DE "Attachment Behavior"
S10 DE "Parent Child Relationship"
S9 NBO
S8 Newborn Behavio?ral Observation* System
S7 Newborn Behavio?ral Observation* System
S6 (Brazelton* or Brazleton*)
S5 NBAS or BNAS or BNBAS
S4 Newborn Behavio?r* Assessment Scale
S3 Newborn Behavio?r* Assessment Scale
S2 Neonatal Behavio?r* Assessment Scale
S1((parent* or mother* or father*) N3 (demonstrat* or teach* or train*))
SCOPUS Elsevier
TITLE‐ABS‐KEY ( ( nbas OR bnas OR bnbas OR brazelton* OR brazleton* OR nbo OR ( parent* train* ) OR ( parent* teach* ) OR ( parent* demonstrat* ) OR ( neonatal behavior* assessment scale ) OR ( neonatal behaviour* assessment scale ) ) OR ( newborn behavior* assessment scale ) OR ( newborn behaviour* assessment scale ) AND ( newborn* OR neonat* OR baby OR babies OR infant* ) AND ( trial* OR random* OR crossover OR blind* ) )
Sociological Abstracts ProQuest
(ab(trial* OR random* OR crossover OR blind*) OR ti(trial* OR random* OR crossover OR blind*)) AND ((SU.EXACT("Infants") OR ((newborn* OR neonate* OR baby OR babies OR infant*) OR SU.EXACT("Infants"))) AND (((parent* OR mother* OR father*) NEAR/3 (demonstrate* OR teach* OR train*)) OR (neonateal Behavio?r* Assessment Scale) OR (Newborn Behavio?r* Assessment Scale) OR (NBAS OR BNAS OR BNBAS) OR (bromelton* OR Brazleton*) OR (Newborn Behavio?ral Observation* System) OR NBO OR SU.EXACT("Parent Child Relations")))
LILACS (Lating American and Caribbean Health Science Information database)
NBAS or BNAS or BNBAS or Brazelton$ or Brazleton$ or NBO or (parent$ train$) or (parent$ teach$) or (parent$ demonstrat$) or (Neonatal Behavior$ Assessment Scale) or (Neonatal Behaviour$ Assessment Scale) or (Newborn Behavior$ Assessment Scale) or (Newborn Behaviour$ Assessment Scale) [Words] and (newborn$ or neonat$ or baby or babies or infant$) [Words] and ((PT:"randomised controlled trial" OR PT:"controlled clinical trial" OR PT:"multicenter study" OR MH:"randomised controlled trials as topic" OR MH:"controlled clinical trials as topic" OR MH:"multicenter studies as topic" OR MH:"random allocation" OR MH:"double‐blind method" OR MH:"single‐blind method") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animals OR MH:rabbits OR MH:rats OR MH:primates OR MH:dogs OR MH:cats OR MH:swine OR PT:"in vitro") [Words]
ClinicalTrials.gov
Brazelton OR NBAS OR NBO
ISTRN
Brazelton OR NBAS OR NBO
UK Clinical Research Network Study Portfolio
Brazelton OR NBAS OR NBO
World Health Organisation (WHO) International Clinical Trials Registry Platform (ICTRP)
Brazelton OR NBAS OR NBO
Appendix 2. 'Risk of bias' domains and criteria for assigning ratings of low, high and unclear risk of bias
Sequence generation (selection bias)
Did the method used to generate the allocation sequence produce comparable groups?
-
Low risk of bias: used a truly random method of sequence generation such as a random number generator on a computer.
-
High risk of bias: used a quasi‐ or non‐random method of sequence generation such as assigning participants to groups based on case number or birth date.
-
Unclear risk of bias: the method of generation was not sufficiently described such as stating that 'participants were randomly assigned to treatment and control groups' without specifying the randomisation method.
Allocation concealment (selection bias)
Was the method used to conceal allocation sequence adequate, or could the allocation schedules have been foreseen in advance of, or during, recruitment? Could the schedules have been changed after assignment?
-
Low risk of bias: used adequate methods for allocation concealment such as central randomisation and sequentially numbered and sealed opaque envelopes.
-
High risk of bias: used methods that were inadequate for allocation concealment such as alternation, assignment by date of birth, and unsealed or non‐opaque envelopes.
-
Unclear risk of bias: the method of allocation concealment was not sufficiently described to allow assessment of whether it was adequate.
Blinding of participants and personnel (performance bias)
Were adequate steps taken to blind participants, intervention personnel, and investigators as to which treatment arm a given participant might have received?
-
Low risk of bias: used an adequate method of blinding or where lack of blinding was unlikely to affect results.
-
High risk of bias: used inadequate method of blinding or where no blinding method was used.
-
Unclear risk of bias: in cases where the method of blinding was not sufficiently described to allow assessment of whether it was adequate.
Blinding of outcome assessment (detection bias)
Were steps taken to blind outcome evaluators and data assessors as to which treatment a given participant might have received?
-
Low risk of bias: used adequate method of blinding or where lack of blinding was unlikely to affect results.
-
High risk of bias: used inadequate method of blinding or where no blinding method was used.
-
Unclear risk of bias: the method of blinding was not sufficiently described to allow assessment of whether it was adequate.
Incomplete outcome data (attrition bias)
Were incomplete data dealt with adequately by the study investigators? How were data on attrition and exclusions reported?
-
Low risk of bias: no outcome data were missing, or outcome data were missing for acceptable reasons such as attrition of healthy persons over a long‐term study, or missing outcome data were balanced across groups, or intention‐to‐treat analyses were used.
-
High risk of bias: a significant amount of outcome data were missing, or outcome data were missing for unacceptable reasons such as exclusion due to 'failure to improve', or missing outcome data were imbalanced across groups, or as‐treated analyses were used.
-
Unclear risk of bias: the reasons for missing outcome data were not sufficiently described to allow assessment of whether missing data were likely to affect the results.
Selective outcome reporting (reporting bias)
Was any attempt made to reduce the possibility of selective outcome reporting?
-
Low risk of bias: all of the expected outcomes were reported.
-
High risk of bias: not all the expected or pre‐specified outcomes were reported, or one or more reported primary outcomes were not pre‐specified, or outcomes were reported incompletely and could not be used.
-
Unclear risk of bias: it was unclear whether there was a risk of selective outcome reporting.
Other sources of bias
Was each study apparently free of other problems (e.g. baseline imbalance; contamination; fraudulence) that could put it at a high risk of bias? We examined, for example, baseline or pre‐treatment means, if available, to determine if any imbalance existed in those participant characteristics that were strongly related to outcome measures, as imbalance can cause bias in intervention effect estimates (Higgins 2017, section 8.14.1.2).
-
Low risk of bias: the study was apparently free of other sources of bias.
-
High risk of bias: the study was at risk of at least one other sources of bias.
-
Unclear risk of bias: there was insufficient information to assess if the study was at risk for other sources of bias.
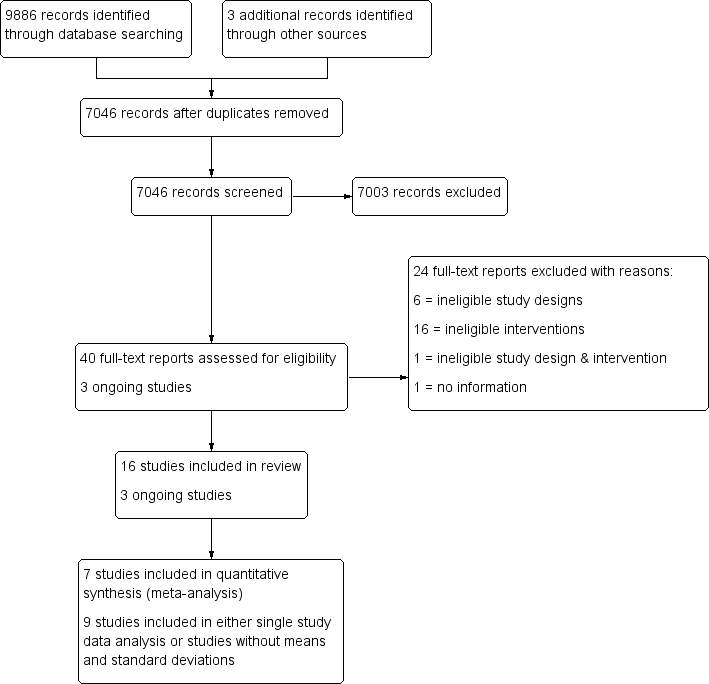
Study flow diagram.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Comparison 1 NBAS or NBO versus control, Outcome 1 Quality of caregiver‐infant interaction: postintervention.

Comparison 1 NBAS or NBO versus control, Outcome 2 Caregiver mental health (maternal depression): EPDS postintervention score.
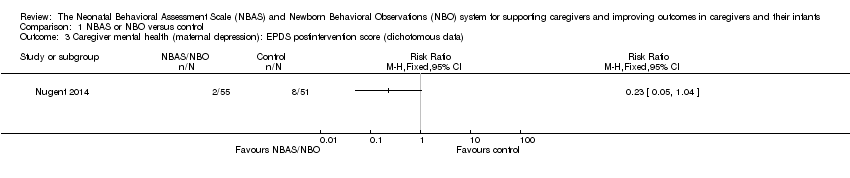
Comparison 1 NBAS or NBO versus control, Outcome 3 Caregiver mental health (maternal depression): EPDS postintervention score (dichotomous data).

Comparison 1 NBAS or NBO versus control, Outcome 4 Infant social, emotional, cognitive and motor development.

Comparison 1 NBAS or NBO versus control, Outcome 5 Caregiver perception of infant.
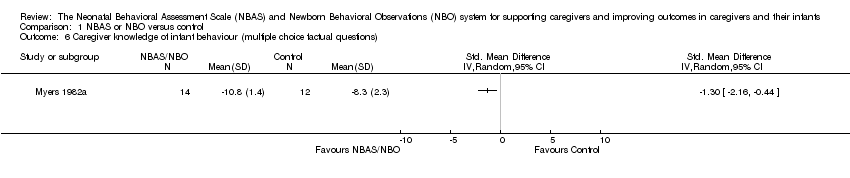
Comparison 1 NBAS or NBO versus control, Outcome 6 Caregiver knowledge of infant behaviour (multiple choice factual questions).

Comparison 1 NBAS or NBO versus control, Outcome 7 Caregiver stress.

Comparison 2 NBAS or NBO versus control: subgroup analysis, Outcome 1 Quality of caregiver‐infant interaction.
| NBAS or NBO versus control for caregiver‐infant interaction, caregiver mental health, and caregiver functioning | |||||
| Patient or population: caregiver‐infant dyads Settings: hospitals, clinics, home Intervention: NBAS or NBO Comparison: NBAS or NBO administered with no interaction | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control group | NBAS or NBO | ||||
| Quality of caregiver‐infant interaction (parental sensitivity, attunement, etc.); continuous data Assessed at postintervention using validated assessment scales for caregiver‐infant interaction: higher scores indicate better outcome | The mean score for quality of caregiver‐infant interaction ranged across control groups from 3.72 to 51.90 | The mean caregiver‐infant interaction score in the intervention groups was 0.53 lower (0.90 lower to 0.17 lower) | — | 304 | ⊕⊕⊝⊝ |
| Caregiver mental health (depression), dichotomous data Assessed at postintervention using the EPDS; lower scores indicate less depression | Low‐risk population | RR 0.23 (0.05 to 1.04) | 106 (1 study) | ⊕⊕⊝⊝ | |
| 157 per 1000 | 36 per 1000 (8 to 163) | ||||
| Medium‐risk population | |||||
| NA | NA | ||||
| High‐risk population | |||||
| NA | NA | ||||
| Infant social, emotional, cognitive and motor development Assessed when infant aged 4 months, using the BSID; higher scores indicate better development | The mean score for infant mental development in the control group was 107.83 | The mean score for infant mental development in the intervention groups was0.13 lower (0.48 lower to 0.22 higher) | — | 125 (1 study) | ⊕⊕⊝⊝ |
| Caregiver perception of infant (parents' perception of the degree of difficult temperament of the infant) Assessed at postintervention, 8 weeks after delivery; higher score indicates better outcome | The mean score for caregiver perception of infant in the control group was 18.90 | The mean score for caregiver perception of the infant in the intervention group was 0.36 lower (0.95 lower to 0.24 higher) | — | 44 (1 study) | ⊕⊕⊝⊝ |
| Caregiver stress (maternal perceptions of her adjustment to the parenting role) Assessed when infant aged 4 months, using the PSI | The mean score for parent‐related caregiver stress in the control group was 2.19 | The mean score for parent‐related caregiver stress in the intervention groups was0.00 (0.35 lower to 0.35 higher) | — | 125 (1 study) | ⊕⊕⊝⊝ |
| Caregiver knowledge (related to infants' physical capacities, including reflexes and senses) Assessed at postintervention, using multiple choice factual questions; higher scores indicate better outcome | The mean score for caregiver knowledge in the control groups was8.30 | The mean score for caregiver knowledge in the intervention groups was 1.30 higher (0.44 to 2.16 higher) | — | 26 (1 study) | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded due to risk of bias due to poor quality research (e.g. limitations in design, including inadequate randomisation or allocation procedures, together with attrition, which ranged from 4% to 20% in seven studies – none of which conducted an intention‐to‐treat analysis), inconsistency for the main outcome due to high levels of heterogeneity for the NBAS; and indirectness in terms of the low levels of generalisability to wider risk groups within the population. 2Downgraded due to risk of bias due to poor quality research (e.g. limitations in design, including inadequate randomisation or allocation procedures, together with attrition, which ranged from 4% to 20% in seven studies – none of which conducted an intention‐to‐treat analysis), and indirectness in terms of the low levels of generalisability to wider risk groups within the population. | |||||
| Unit of analysis issues | Cluster‐randomised trials The randomisation of clusters can result in an overestimate of the precision of the results (with a higher risk of a type I error) when their use has not been compensated for in the analysis. Had we included a cluster‐RCT, we planned to explore whether the authors had adequately controlled for the effects of clustering in the study. When they had, and when there was little difference between the study designs, and when there was unlikely to be an interaction between the effect of the intervention and the choice of randomisation method, we planned to combine the data from the cluster‐RCT with data from individual RCTs. When the effects of clustering had not been controlled for properly, we planned to derive an estimate of the intracluster correlation coefficient (ICC) from the study or that of a similar population, and to report whether an ICC had been used and conduct sensitivity analyses to determine the effect of using an ICC. We also planned to assess the impact of including data from a cluster‐RCT on the inclusion of the study in the meta‐analyses using a sensitivity analysis to explore the effects of the randomisation method. However, no cluster RCTs were identified or included. |
| Trials with multiple treatments groups In the event that we had identified a multi‐arm study in which the NBAS and NBO had been compared with an alternative treatment and a control group, we planned to only extract data from two arms (e.g. NBAS and control group). In the event that we had identified a multi‐arm study in which the NBAS had been compared with the NBO and involved only one control group, we planned to combine the data from the NBAS and NBO arms for primary analyses and to conduct secondary, subgroup analyses and split the control group data. However, we identified no multiple treatment groups. | |
| Cross‐over trials Cross‐over trials are not possible with this type of intervention, and none were identified. | |
| Assessment of reporting bias | We planned to draw funnel plots (estimated differences in treatment effects against their standard error) if there was a sufficient number of included studies (e.g. more than 10), to identify asymmetry due to publication bias and other small study effects. We also planned to assess whether there had been selective reporting of outcomes and to assess the impact of this using a sensitivity analysis. However, there were insufficient studies to undertake this analysis. |
| Subgroup analysis and investigation of heterogeneity | We planned to explore possible reasons for heterogeneity by undertaking the following, additional subgroup analyses, scrutinising studies to determine the extent of between‐trial differences.
However, it was only possible to undertake subgroup analysis for NBAS versus NBO, due to the small number of studies. |
| Sensitivity analysis | We planned to conduct sensitivity analyses on the basis of method of sequence generation only, to assess the robustness of the results, but this was not possible due to the small number of studies. |
| NBAS: Neonatal Behavioral Assessment Scale; NBO: Newborn Behavioral Observations system; RCT: randomised controlled trial. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of caregiver‐infant interaction: postintervention Show forest plot | 7 | 304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.90, ‐0.17] |
| 2 Caregiver mental health (maternal depression): EPDS postintervention score Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Caregiver mental health (maternal depression): EPDS postintervention score (dichotomous data) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Infant social, emotional, cognitive and motor development Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 BSID: mental development | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 BSID: psychomotor development | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Caregiver perception of infant Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Caregiver knowledge of infant behaviour (multiple choice factual questions) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Caregiver stress Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 PSI: parent‐related sources of stress | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 PSI: child‐related sources of stress | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of caregiver‐infant interaction Show forest plot | 7 | 304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.90, ‐0.17] |
| 1.1 NBAS | 5 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.99, ‐0.00] |
| 1.2 NBO | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.18, ‐0.20] |

