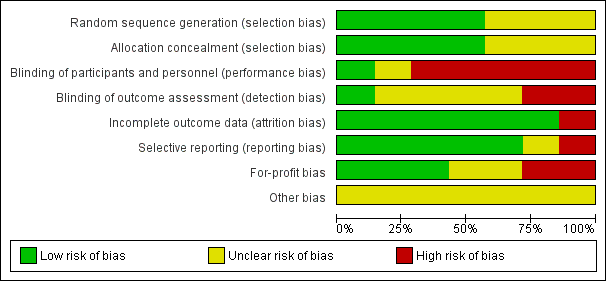
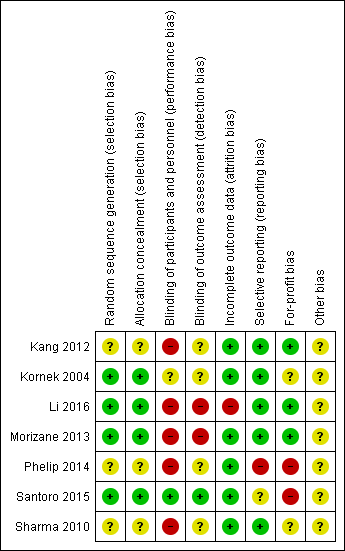
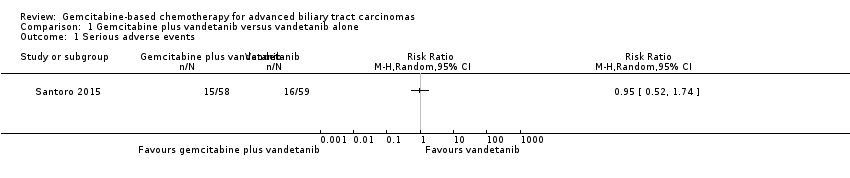
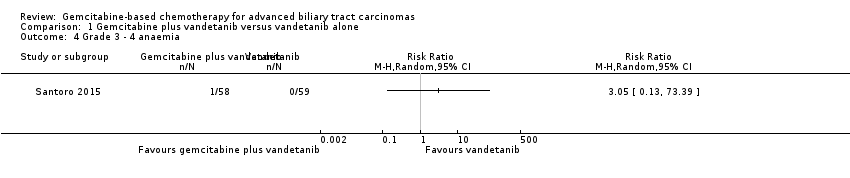
| Examples from table | Explanation |
| Outcomes | The tables provide the findings for the most important outcomes for someone making a decision. These include potential benefits and harms, whether the included trials provide data for these outcomes or not. Additional findings may be reported elsewhere in the review. |
| Assumed control group risk | Assumed control group risks can be based either on the control group risks reported in the included trials or on epidemiological data from elsewhere. When only one control group risk is provided, it is normally the median control group risk across the trials that provided data for that outcome. Risk is the probability of an outcome occurring. The control group risk is the risk of an outcome occurring in the comparison group (without the intervention). |
| Corresponding intervention group risk | Risk is the probability of an outcome occurring. The intervention group risk is the risk of an outcome occurring in the group receiving the intervention. |
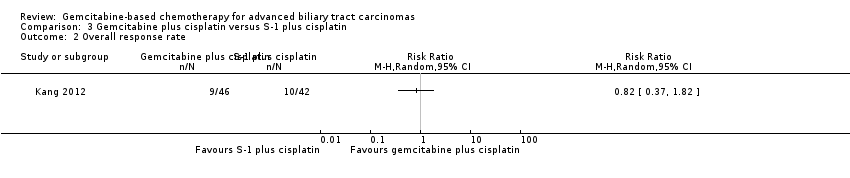
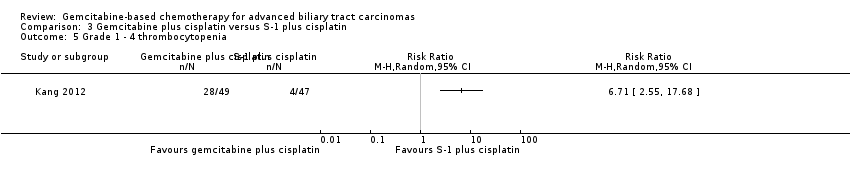
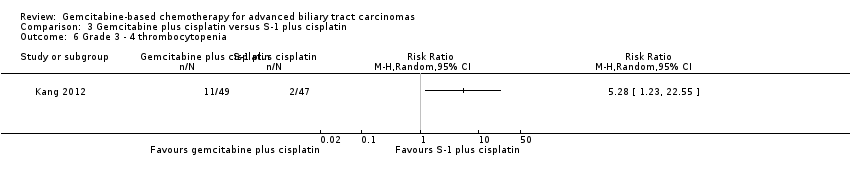
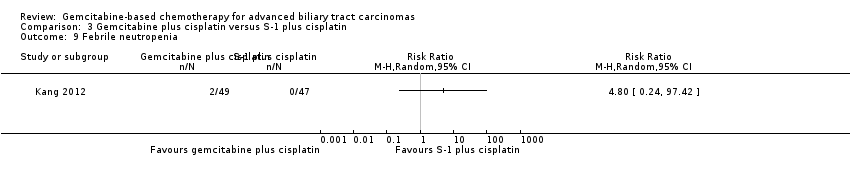
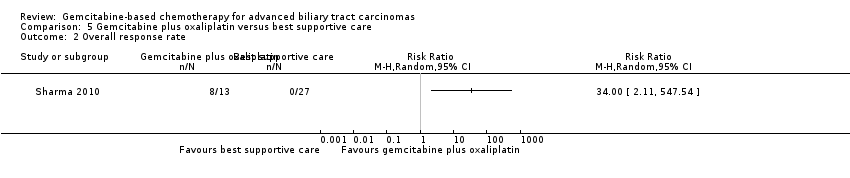
| Relative effect | Relative effect or RR (risk ratio) Relative effects are ratios. Here the relative effect is expressed as a risk ratio. Risk is the probability of an outcome occurring. An RR is the ratio between the risk in the intervention group and the risk in the control group. If the risk in the control group is 10% (100 per 1000) and the risk in the intervention group is 1% (10 per 1000), the RR is 10/100 or 0.10. If the RR is exactly 1.0, this means that there is no difference between the occurrence of the outcome in the intervention and the control group. It is unusual for the RR to be exactly 1.0, and understanding what it means if it is above or below this value depends on whether the outcome being counted is judged to be good or bad. If the RR is greater than 1.0, the intervention increases the risk of the outcome. If it is a good outcome (e.g. the birth of a healthy baby), an RR > 1.0 indicates a desirable effect for the intervention, whereas if the outcome is bad (e.g. death), an RR > 1.0 would indicate an undesirable effect. If the RR is less than 1.0, the intervention decreases the risk of the outcome. This indicates a desirable effect if it is a bad outcome (e.g. death) and an undesirable effect if it is a good outcome (e.g. birth of a healthy baby). |
| What is the difference between absolute and relative effects? The effect of an intervention can be described by comparing the risk of the intervention group with the risk of the control group. Such a comparison can be made in different ways. One way to compare two risks is to calculate the difference between the risks. This is the absolute effect. Consider the risk for blindness in a person with diabetes over a five‐year period. If the risk for blindness is found to be 20 in 1000 (2%) in a group of people treated conventionally and 10 in 1000 (1%) in people treated with a new drug, the absolute effect is derived by subtracting the intervention group risk from the control group risk: 2%/1% = 1%. Expressed in this way, it can be said that the new drug reduces the five‐year risk for blindness by 1% (absolute effect is 10 fewer per 1000). Another way to compare risks is to calculate the ratio of the two risks. Given the data above, the relative effect is derived by dividing the two risks, with the intervention risk being divided by the control risk: 1% ÷ 2% = ½ (0.50). Expressed in this way, as the 'relative effect', the five‐year risk for blindness with the new drug is 1/2 the risk with the conventional drug. Here the table presents risks as x per 1000 (or 100, etc.) instead of %, as this tends to be easier to understand. Whenever possible, the table presents the relative effect as the RR. Usually the absolute effect is different for groups that are at high and low risk, whereas the relative effect often is the same. Therefore, when it is relevant, we have reported indicative risks for groups at different levels of risk. Two or three indicative control group risks and the corresponding intervention group risks are presented when there are important differences across different populations. |
| Mean difference | The mean difference (MD) is the average difference between the intervention group and the control group across trials. Here a weighted MD is used, which means the results of some of the trials make a greater contribution to the average than others. Trials with more precise estimates for their results (narrower confidence intervals) are given more weight. This way of measuring effect is used when combining or comparing data for continuous outcomes, such as weight, blood pressure, or pain measured on a scale. When different scales are used to measure the same outcome, e.g. different pain scales, a standardised mean difference (SMD) may be provided. This is a weighted mean difference standardised across trials giving the average difference in standard deviations for the measures of that outcome. |
| Confidence interval | A confidence interval (CI) is a range around an estimate that conveys how precise the estimate is; in this example the result is the estimate of the intervention group risk. The CI is a guide to how sure we can be about the quantity we are interested in (here the true absolute effect). The narrower the range between the two numbers, the more confident we can be about what the true value is; the wider the range, the less sure we can be. The width of the CI reflects the extent to which chance may be responsible for the observed estimate (with a wider interval reflecting more chance). |
| 95% confidence interval | As explained above, the CI indicates the extent to which chance may be responsible for the observed numbers. In the simplest terms, a 95% CI means that we can be 95% confident that the true size of effect is between the lower and upper confidence limit (e.g. 0 and 3 in the blindness drugs example mentioned above). Conversely, there is a 5% chance that the true effect is outside of this range. |
| Not statistically significant | Statistically significant means that a result is unlikely to have occurred by chance. The usual threshold for this judgement is that the results, or more extreme results, would occur by chance with a probability of less than 0.05 if the null hypothesis (no effect) were true. When results are not statistically significant, as in this example, this is stated to alert users to the possibility that the results may have occurred by chance. |
| Number of participants (trials) | The table provides the total number of participants across trials and the number of trials that provided data for that outcome. This indicates how much evidence there is for the outcome. |
| Certainty of the evidence | The certainty of the evidence is a judgement about the extent to which we can be confident that the estimates of effect are correct. These judgements are made using the GRADE system, and are provided for each outcome. The judgements are based on the type of study design (randomised trials versus observational trials), the risk of bias, the consistency of the results across trials, and the precision of the overall estimate across trials. For each outcome, the certainty of the evidence is rated as high, moderate, low, or very low using the following definitions: -
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect -
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different -
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect -
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. |
| ‐ | A ‐ indicates that the information is not relevant. |