Антигипертензивная фармакотерапия для профилактики внезапной сердечной смерти у людей с артериальной гипертензией
Abstract
Background
High blood pressure is an important public health problem because of associated risks of stroke and cardiovascular events. Antihypertensive drugs are often used in the belief that lowering blood pressure will prevent cardiac events, including myocardial infarction and sudden death (death of unknown cause within one hour of the onset of acute symptoms or within 24 hours of observation of the patient as alive and symptom free).
Objectives
To assess the effects of antihypertensive pharmacotherapy in preventing sudden death, non‐fatal myocardial infarction and fatal myocardial infarction among hypertensive individuals.
Search methods
We searched the Cochrane Hypertension Specialised Register (all years to January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (2016, Issue 1), Ovid MEDLINE (1946 to January 2016), Ovid EMBASE (1980 to January 2016) and ClinicalTrials.gov (all years to January 2016).
Selection criteria
All randomised trials evaluating any antihypertensive drug treatment for hypertension, defined, when possible, as baseline resting systolic blood pressure of at least 140 mmHg and/or resting diastolic blood pressure of at least 90 mmHg. Comparisons included one or more antihypertensive drugs versus placebo, or versus no treatment.
Data collection and analysis
Review authors independently extracted data. Outcomes assessed were sudden death, fatal and non‐fatal myocardial infarction and change in blood pressure.
Main results
We included 15 trials (39,908 participants) that evaluated antihypertensive pharmacotherapy for a mean duration of follow‐up of 4.2 years. This review provides moderate‐quality evidence to show that antihypertensive drugs do not reduce sudden death (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.81 to 1.15) but do reduce both non‐fatal myocardial infarction (RR 0.85, 95% CI 0.74, 0.98; absolute risk reduction (ARR) 0.3% over 4.2 years) and fatal myocardial infarction (RR 0.75, 95% CI 0.62 to 0.90; ARR 0.3% over 4.2 years). Withdrawals due to adverse effects were increased in the drug treatment group to 12.8%, as compared with 6.2% in the no treatment group.
Authors' conclusions
Although antihypertensive drugs reduce the incidence of fatal and non‐fatal myocardial infarction, they do not appear to reduce the incidence of sudden death. This suggests that sudden cardiac death may not be caused primarily by acute myocardial infarction. Continued research is needed to determine the causes of sudden cardiac death.
PICOs
Резюме на простом языке
Лекарственные средства, используемые для снижения артериального давления, не сокращают число внезапных смертей
Высокое артериальное давление увеличивает риск инсульта и сердечного приступа/инфаркта. У людей с умеренно повышенным артериальным давлением лекарства, снижающие артериальное давление, сокращают частоту инсультов и сердечных приступов/инфарктов. Неизвестно, сокращают ли лекарства, снижающие артериальное давление, число внезапных смертей (смерть по неизвестной причине в течение одного часа после появления острых симптомов или в течение 24 часов наблюдения пациента без каких‐либо симптомов). Мы нашли 15 клинических испытаний с общим числом участников 39908 человек, в которых изучали, сокращают ли лекарства, снижающие артериальное давление, число внезапных смертей. Этот обзор представляет доказательства среднего качества, показывающие, что лекарства, снижающие артериальное давление, сокращают частоту сердечных приступов/инфарктов, но не снижают частоту внезапных сердечных смертей. Это предполагает то, что сердечный приступ/инфаркт может не являться первопричиной внезапной сердечной смерти. Для установления причин внезапной сердечной смерти необходимы дальнейшие исследования.
Authors' conclusions
Summary of findings
| Antihypertensive pharmacotherapy versus control for prevention of sudden cardiac death in hypertensive individuals | ||||||
| Patient or population: people with hypertension (defined as baseline systolic resting blood pressure ≥ 140 mmHg and/or resting diastolic blood pressure ≥ 90 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with antihypertensive pharmacotherapy | |||||
| Sudden cardiac death (mean follow‐up: 4.2 years) | Study population | RR 0.96 | 39908 | ⊕⊕⊕⊝ | ||
| 13 per 1000 | 12 per 1000 | |||||
| Non‐fatal myocardial infarction (mean follow‐up: 4.2 years) | Study population | RR 0.85 0.98) | 39908 | ⊕⊕⊕⊝ | ||
| 20 per 1000 | 17 per 1000 | |||||
| Fatal myocardial infarction (mean follow‐up: 4.2 years) | Study population | RR 0.75 0.90) | 39908 | ⊕⊕⊕⊝ | ||
| 12 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded 1 level for imprecision (wide confidence intervals) | ||||||
Background
Description of the condition
High blood pressure is an important public health problem because of associated risks of stroke and cardiovascular events. It is most often of unknown origin, is relatively easy to detect and can be lowered with antihypertensive drugs. Extensive epidemiological data support the well‐known relationship between blood pressure and risk of cardiovascular disease, particularly the importance of systolic blood pressure as a determinant of risk (Glynn 2010).
Major coronary heart disease manifests as and is captured in trials by three different outcomes: non‐fatal myocardial infarction, fatal myocardial infarction and sudden cardiac death. Antihypertensive therapy could have different effects on different outcomes, and some study authors suspect that it could increase the incidence of sudden cardiac death (Hoes 1994), which is defined as sudden unexpected death within one hour of the onset of acute symptoms or within 24 hours of observation of the patient as alive and symptom free (Chugh 2004).
Most reviews have focused on the effects of blood pressure‐lowering drugs on total fatal and non‐fatal myocardial infarction, but not specifically on sudden cardiac death. Instead, sudden cardiac death is included with total myocardial infarction because it is assumed that it is totally or most often caused by acute myocardial infarction. In fact, sudden cardiac death could have several causes not related to acute myocardial infarction (see below) (Zheng 2001).
Description of the intervention
The intervention of interest in this systematic review is any antihypertensive drug used alone or in combination with other antihypertensive drugs as part of stepped‐care therapy. The control in this review is placebo or no treatment.
How the intervention might work
Antihypertensive drugs lower blood pressure through a variety of mechanisms. Major classes of antihypertensive drugs include thiazide diuretics, beta‐blockers, drugs inhibiting the renin‐angiotensin system, calcium channel blockers, direct vasodilators, centrally active drugs and others. Several systematic reviews have shown the benefits of antihypertensive drug therapy in reducing cardiovascular morbidity and mortality in patients of all ages with moderate to severe hypertension (Gueyffier 1999; Musini 2009). At the present time, it is not known whether the benefits of antihypertensive therapy outweigh the harms in individuals with uncomplicated mild (grade 1) hypertension (Diao 2012; Sundström 2015).
It has been hypothesised, but not proven, that the benefits of antihypertensive drugs in reducing the incidence of stroke, myocardial infarction and heart failure are mediated through blood pressure reduction. If this is so, all drugs that lower blood pressure to the same degree should be similarly effective in reducing cardiovascular events. Some evidence suggests that this is not the case, and that different classes of antihypertensive drugs have differential effects on different outcomes (Chen 2010; Wiysonge 2012; Wright 2009; Xue 2015).
Effects of antihypertensive drugs on sudden cardiac death are potentially more complicated, as sudden cardiac death could be caused by acute myocardial infarction, but also could result from spontaneous fatal arrhythmia (ventricular fibrillation, torsade de pointes, asystole) or another sudden fatal event (e.g. ruptured aortic aneurysm, intracerebral haemorrhage). A particular class of antihypertensive drugs could be beneficial for one cause and deleterious for another. Other causes of sudden death cannot be ruled out because autopsies are not systematically done even in the event of sudden death in a randomised trial.
Why it is important to do this review
No published systematic review has compared the effects of antihypertensive drugs versus placebo or no treatment on the incidence of sudden cardiac death. If antihypertensive pharmacotherapy or a specific class of antihypertensive therapy increases sudden cardiac death, it is important to establish this, as it raises important questions: Is it appropriate to classify sudden cardiac death under total myocardial infarction? Are approaches available to prevent the increase in sudden cardiac death associated with use of antihypertensive drugs? Should patients with a particularly increased risk of sudden cardiac death be identified and treated differently?
Objectives
To assess the effects of antihypertensive pharmacotherapy in preventing sudden cardiac death, non‐fatal myocardial infarction and fatal myocardial infarction among hypertensive individuals.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials of antihypertensive drugs in predominantly hypertensive patients (> 50%) with duration of follow‐up of at least one year. Trials must include a comparative control group given placebo or no treatment.
We excluded trials that compared two specific antihypertensive therapies without placebo or with no treatment control, trials of blood pressure‐lowering devices and trials providing multi‐factorial interventions.
Trials had to provide data on the incidence of sudden cardiac death among participants in treated and control groups.
Types of participants
Most participants must have had high blood pressure (> 50%) at baseline. High blood pressure is defined as baseline resting systolic blood pressure (SBP) of at least 140 mmHg and/or resting diastolic blood pressure (DBP) of at least 90 mmHg.
Trials were not limited by any other factor nor by baseline risk. We excluded trials that recruited participants in the first month following an acute cardiovascular event.
Types of interventions
Treatment interventions
Antihypertensive drug classes, including angiotensin‐converting enzyme inhibitors, angiotensin receptor antagonists, renin inhibitors, beta‐adrenergic blockers, combined alpha‐ and beta‐blockers, calcium channel blockers, diuretics, alpha‐adrenergic blockers, central sympatholytics, direct vasodilators and antihypertensive drugs with unknown mechanisms of action.
Control interventions
Placebo and no treatment.
Types of outcome measures
Primary outcomes
-
Sudden cardiac death.
Secondary outcomes
-
Fatal myocardial infarction.
-
Non‐fatal myocardial infarction.
-
Withdrawals due to adverse effects.
Search methods for identification of studies
Electronic searches
We searched the Database of Abstracts of Reviews of Effectiveness (DARE) for related reviews.
This review falls within the scope of the search strategies developed for Wright 2009, for which we searched the following databases: the Hypertension Group Specialised Register (all years to January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (2016, Issue 1), Ovid MEDLINE (1946 to January 2016), Ovid EMBASE (1980 to January 2016), the WHO International Clinical Trials Registry Platform (all years to January 2016), and ClinicalTrials.gov (all years to January 2016).
The Hypertension Group Specialised Register includes controlled trials from searches of CAB Abstracts, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, Food Science and Technology Abstracts (FSTA), Global Health, the Latin American Caribbean Health Sciences Literature (LILACS), MEDLINE, ProQuest Dissertations & Theses, PsycINFO, Web of Science and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
We searched electronic databases using a strategy that combined the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (sensitivity‐maximising version (2008 revision)) with selected Medical Subject Heading (MeSH) terms and free text terms. We applied no language restrictions. We translated the MEDLINE search strategy (Appendix 1) for use with EMBASE, CENTRAL, the Hypertension Group Specialised Register and ClinicalTrials.gov, using the appropriate controlled vocabulary as applicable.
Searching other resources
We searched the reference lists of all relevant studies and systematic reviews for additional studies. We contacted authors of included studies to request information about any other relevant unpublished or ongoing studies.
We examined other sources to retrieve potential studies for the review:
-
Food and Drug Administration (FDA), to ask whether this group had related clinical trial information in its possession.
Data collection and analysis
Selection of studies
One review author (GT) screened citations/abstracts obtained as a result of the search strategies. We rejected articles on the initial screen if we determined from the title or from the abstract that the article was not a report of a randomised clinical trial. We also rejected articles that were irrelevant to the review, or that clearly did not meet the inclusion criteria. We obtained in full text trials that might be of relevance and citations of uncertain relevance. Two review authors (GT, YM) independently assessed full‐text articles for inclusion, using predetermined inclusion criteria. We recorded reasons for exclusion of trials. We provided a full accounting of search results in the form of a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) study flow diagram (Liberati 2009).
Data extraction and management
We extracted and managed data using the Cochrane Review Manager software, RevMan 5.3. Review authors (GT, YM, AL, LT and JMW) extracted data independently, using a piloted, standard form, then cross‐checked data. We resolved discrepancies by discussion and by consensus, or we sought adjudication by a third review author if we could not reach consensus.
Assessment of risk of bias in included studies
Review authors used the 'Risk of bias' tool to assess each trial according to guidelines provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Potential parameters of methodological quality listed in the 'Risk of bias' table include method used to randomise participants; whether randomisation was completed in an appropriate and blinded manner; whether participants, providers and/or outcome assessors were blinded to assigned therapy; whether the control group received placebo or no treatment; the proportion of participants who did not complete follow‐up; selective reporting of outcomes; and any other biases, including whether or not funding was provided by the drug industry.
Two review authors independently assessed risk of bias within each included study on the basis of these domains, assigning ratings of 'low risk of bias', 'high risk of bias' and 'unclear risk of bias' (uncertain risk of bias). Review authors resolved disagreements by discussion or by obtaining a third party opinion. When published articles did not include sufficient detail to permit full assessment, we contacted study authors to ask for clarification of methods used.
We presented the risk of bias assessment in a table for each study.
Measures of treatment effect
We measured most of our outcomes as the proportion of participants suffering an event, including sudden cardiac death, fatal myocardial infarction, non‐fatal myocardial infarction and withdrawal due to adverse effects. When performing a meta‐analysis, we used risk ratios.
Unit of analysis issues
For the subgroup analysis of the two‐arm Medical Research Council (MRC) trial (MRC‐TMH 1985), we halved the placebo group to prevent double counting.
Dealing with missing data
When information was missing from the included studies, we contacted investigators (using email, letter and/or fax) to request the missing information, and we explored the INDANA (Individual Data Analysis of Antihypertensive Drug Intervention Trials) database.
'Summary of findings' table
We presented data on the following outcomes: sudden cardiac death, non‐fatal myocardial infarction, fatal myocardial infarction and proportion of participants who experienced at least one withdrawal due to an adverse event. We presented dichotomous outcomes as risk ratios and 95% confidence intervals. We presented data regarding numbers of participants and studies for each outcome. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (Grade working group 2004) to determine the quality of the evidence and downgraded the quality if (1) most (> 50%) included studies had high risk of bias; (2) the outcome had significant statistical heterogeneity (I² > 50%); and (3) the outcome had wide 95% CIs, indicating that the intervention effect was highly variable.
Assessment of heterogeneity
We tested for heterogeneity of treatment effects between trials by using a standard Chi2 statistic for heterogeneity and an alpha of 0.05 for statistical significance, and we estimated heterogeneity using the I2 test. I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
We applied the fixed‐effect model to obtain summary statistics of pooled trials, unless significant between‐study heterogeneity was present, in which case we used the random‐effects model. If significant heterogeneity was present, and if I2 was greater than 50%, we explored reasons for heterogeneity (variation in trial methods, interventions and study population characteristics) in an attempt to find potential explanations for the observed variation.
Assessment of reporting biases
Positive results are consistently more likely to be published than negative results, leading to publication bias, which can result in overestimation of the effect size of studies. If we identified sufficient trials (≥ 10 studies), we visually inspected funnel plots for small‐study effects, and we considered the causes of funnel plot asymmetry, including publication bias. We considered use of additional statistical tests such as Egger's test when appropriate (Cochrane Handbook for Systematic Reviews of Interventions).
Data synthesis
We performed data synthesis and analyses using the most current version of the Cochrane Review Manager software, RevMan 5.3.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to look for variation in treatment effect among classes of first‐line antihypertensive drugs, namely, first‐line diuretics versus other first‐line antihypertensive drugs.
Sensitivity analysis
We performed sensitivity analyses to test for robustness of results on trial quality. We did this by restricting analyses to trials with placebo and those judged to have low risk of bias. We also performed sensitivity analyses to see whether recruiting only patients with diabetes affected trial results.
Results
Description of studies
Details for the included trials can be found in the Characteristics of included studies table. Reasons for exclusion of excluded trials can be found in the Characteristics of excluded studies table.
Results of the search
We identified 61 trials, included 15 trials (39,908 participants) and excluded 46 trials. We have summarised the study selection process in the PRISMA flow diagram shown in Figure 1.

PRISMA study flow diagram.
Included studies
We have provided details of included trials in the Characteristics of included studies table.
Studies recruited patients with high blood pressure, defined as baseline resting SBP of at least 140 mmHg and/or resting DBP of at least 90 mmHg. The average age of participants across all included trials was 63 years. Mean follow‐up of studies ranged from 1.5 to 8.3 years. Most participants were recruited from industrialised countries, including the USA (57%) and European multi‐sites (43%).
Most trials evaluated first‐line diuretics (EWPHBPE 1985; HSCGS 1974; MRC‐TMH 1985 Diuretic arm; OSLO 1986; SHEP 1991; SHEP‐Pilot 1989: USPHSHCSG 1977; VA‐I 1967; VA‐II 1970; VA‐NHLBI 1978), except for DIABHYCAR 2004 and UKPDS38 1998, both of which evaluated an angiotensin‐converting enzyme inhibitor, and SYST‐EUR 1997, which evaluated a first‐line calcium channel blocker. Five trials evaluated first‐line beta‐blockers (Coope 1986; MRC‐TMH 1985 Betablocker arm; OSLO 1986; STOP 1991; UKPDS38 1998).
Females represented 38% of the population studied. Four trials (OSLO 1986; VA‐I 1967; VA‐II 1970; VA‐NHLBI 1978) included only men (2320 participants). Eight trials reported ethnicity. African Americans accounted for the following percentages in these trials: HSCGS 1974 80%; SHEP 1991 13.8%; SHEP‐Pilot 1989 18%; USPHSHCSG 1977 28%; VA‐I 1967 53.8%; VA‐II 1970 42%; VA‐NHLBI 1978 25%; and UKPDS38 1998 8%. Seven trials did not report ethnicity (Coope 1986; DIABHYCAR 2004; EWPHBPE 1985; MRC‐TMH 1985; OSLO 1986; STOP 1991; SYST‐EUR 1997).
Twelve trials reported initial mean SBP of participants ranging from 145 mmHg to 196 mmHg and DBP ranging from 75 mmHg to 102 mmHg. The final mean SBP ranged from 127 mmHg to 180 mmHg and DBP from 44 mmHg to 92 mmHg. Two studies (VA‐I 1967; VA‐NHLBI 1978) reported initial and final mean DBP: respectively, VA‐I 1967 121 mmHg and 91 mmHg, and VA‐NHLBI 1978 93 mmHg and 83 mmHg. VA‐II 1970 reported only the initial mean SBP/DBP: 164/104 mmHg.
Ten trials reported baseline prevalence of diabetes as follows: Coope 1986 0%; DIABHYCAR 2004 100%; EWPHBPE 1985 0%; MRC‐TMH 1985 0%; SHEP 1991 10.1%; SYST‐EUR 1997 10.5%; UKPDS38 1998 100%; USPHSHCSG 1977 0%; VA‐I 1967 9.1%; and VA‐NHLBI 1978 0%.
Three trials (SHEP 1991; SHEP‐Pilot 1989; SYST‐EUR 1997) restricted recruitment to persons with systolic hypertension, defined as SBP 160 to 219 mmHg and DBP < 90 mmHg (SHEP 1991; SHEP‐Pilot 1989), DBP < 95 mmHg (SYST‐EUR 1997) or simply systolic > 140 mmHg (DIABHYCAR 2004). Five trials based entry on diastolic hypertension (USPHSHCSG 1977; VA‐I 1967; VA‐II 1970; VA‐NHLBI 1978; UKPDS38 1998); six trials based entry on either systolic or diastolic hypertension (Coope 1986; EWPHBPE 1985; HSCGS 1974; MRC‐TMH 1985; OSLO 1986; STOP 1991).
Excluded studies
We excluded 46 trials and have provided reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We assessed risk of bias from six domains for each of the included studies.
Allocation
We judged most of the trials as having low risk of bias for sequence generation and allocation concealment. For trials with insufficient reporting, we judged risk of bias as unclear.
Blinding
Twelve of the 15 trials blinded participants to therapy (DIABHYCAR 2004; EWPHBPE 1985; HSCGS 1974; MRC‐TMH 1985; SHEP 1991; SHEP‐Pilot 1989; STOP 1991; SYST‐EUR 1997; USPHSHCSG 1977; VA‐I 1967; VA‐II 1970; VA‐NHLBI 1978) and of these all but MRC‐TMH 1985 also blinded providers to therapy. Eight trials specifically reported blinding of outcome assessors (Coope 1986; EWPHBPE 1985; SHEP 1991; SHEP‐Pilot 1989; STOP 1991; SYST‐EUR 1997; UKPDS38 1998; VA‐II 1970).
Incomplete outcome data
Participants who were lost to follow‐up or who dropped out prematurely were usually included in the clinical outcome or safety analysis of each trial. Most trials used intention‐to‐treat analysis; we therefore judged them to have low risk of attrition bias.
Selective reporting
All studies reported sudden cardiac death and myocardial infarction numbers; therefore, we can consider them as having low risk of reporting bias.
Other potential sources of bias
We defined publication bias as selective publication of studies with positive results; this is another source of bias that may have skewed the results of this review. The most common way to investigate whether an effect estimate is subject to publication bias is to examine for funnel plot asymmetry. Examining the funnel plot for sudden cardiac death in this review revealed no asymmetry to suggest high risk of publication bias (Figure 2).

Funnel plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.1 Sudden cardiac death.
Thirteen of the 15 trials were funded by government agencies, not by industry, and thus were judged to have low risk of other bias.
We have summarised our judgements about each risk of bias item for each included study and about each risk of bias item presented as percentages across all included studies in Figure 3 and Figure 4, respectively.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See summary of findings Table for the main comparison. We performed analyses on the combined results of all 15 studies.
Sudden cardiac death
For the 39,908 participants included in this review, treatment with antihypertensive drugs as compared with no treatment or placebo did not affect sudden cardiac death (RR 0.96, 95% CI 0.81 to 1.1) (Analysis 1.1, Figure 5).

Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.1 Sudden cardiac death.
Non‐fatal myocardial infarction and fatal myocardial infarction
Antihypertensive pharmacotherapy resulted in a significant reduction in non‐fatal myocardial infarction (RR 0.85, 95% CI 0.74 to 0.98, ARR 0.3% over 4.2 years) (Analysis 1.2, Figure 6) and fatal myocardial infarction (RR 0.75, 95% CI 0.62 to 0.90, ARR 0.3% over 4.2 years) (Analysis 1.3, Figure 7). We found no evidence of heterogeneity among included trials.

Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.2 Non‐fatal myocardial infarction.
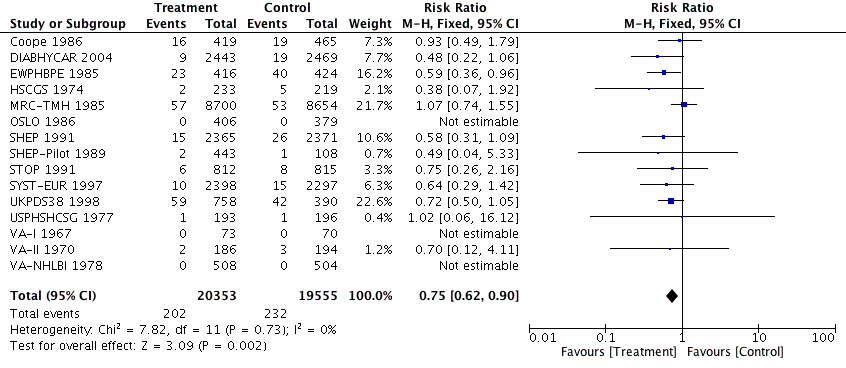
Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.3 Fatal myocardial infarction.
Subgroup analysis
To analyse whether a class effect of drugs was evident, we performed a subgroup analysis of first‐line diuretics versus other antihypertensive drugs. In clinical trials, medications given to treat hypertension are often added to achieve the goal of lowering blood pressure. For this reason, we analysed data related to first‐line treatment only.
The risk ratio for sudden cardiac death was 1.08 (95% CI 0.82 to 1.43) when diuretics were used as first‐line agents, and 0.94 (0.74 to 1.19) for other antihypertensive treatments (Analysis 2.1, Figure 8). We noted no significant heterogeneity between subgroups based on use of first‐line drugs.

Forest plot of comparison: 3 Subgroup analysis, first‐line diuretic versus others, outcome: 3.1 Sudden cardiac death.
Withdrawals due to adverse effects (WDAEs)
Ten of 15 trials reported the numbers of participants who dropped out of trials because of adverse drug effects. WDAEs occurred in 6.2% of the no treatment group, and this percentage increased to 12.8% in the drug treatment group (RR 2.06, 95% CI 1.11 to 3.83, absolute risk increase 6.6%). Heterogeneity was high for this outcome (I2 = 98%).
Discussion
Summary of main results
Fifteen trials evaluated antihypertensive pharmacotherapy of various drug classes in a total of 39,908 hypertensive individuals, with periods of follow‐up ranging from 1.5 to nine years (mean 4.2 years).
This systematic review demonstrates that antihypertensive pharmacotherapy used in the treatment of hypertensive patients has not been proven to significantly reduce sudden cardiac death (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.81 to 1.15). In contrast, in the same trials, antihypertensive therapy reduced both non‐fatal myocardial infarction (RR 0.85, 95% CI 0.74 to 0.98) and fatal myocardial infarction (RR 0.75, 95% CI 0.62 to 0.90).
These findings suggest that sudden cardiac death is not totally or mostly caused by acute myocardial infarction, as if this were the case, it would have been reduced by antihypertensive therapy to a similar degree (e.g. RR 0.72 to 0.82). Therefore, sudden cardiac death could most often result from a condition unaffected by antihypertensive therapy, or more likely is caused in part by acute myocardial infarction and is reduced by antihypertensive therapy and is caused in part as well by another mechanism (e.g. ventricular arrhythmias), which is increased by antihypertensive therapy, resulting in an overall null effect.
High blood pressure is a well‐known risk factor for cardiac hypertrophy. Increased left ventricular mass detected by echocardiography is associated with increased risk for sudden cardiac death after other known coronary disease risk factors were accounted for (Haider A). It is unclear, however, whether antihypertensive therapies that promote the regression of left ventricular hypertrophy would reduce the risk of sudden death (Messerli 1999).
These facts make the lack of risk reduction of sudden death difficult to explain, unless we assume that at least some antihypertensive drugs may increase the incidence of sudden death. Diuretics have long been suspected to potentially induce sudden death by causing hypokalaemia and subsequent ventricular arrhythmia (Hoes 1995). On the other hand, beta‐blockers are supposed to prevent sudden death due to arrhythmia (Hjalmarson 1997; Kaplan 1997), at least for secondary prevention of myocardial infarction.
In this review, we tested this possibility by performing a subgroup analysis of diuretics versus control for sudden cardiac death; we found that the pooled risk ratio of 1.08 (95% CI 0.82 to 1.43) did not affirm a differential effect for diuretics, and the pooled effect for other drugs was not different (RR 0.94, 95% CI 0.74 to 1.19). However, so few trials used other first‐line antihypertensive drugs that the subgroup analysis was not a good test of that possibility.
Sudden death represents 19.4% of total deaths and is more frequent than fatal myocardial infarction (13.4% of total deaths). It has been estimated that 40.7% of sudden deaths are due to coronary causes (Loire 1996), providing the rationale for including them within major coronary events. In North America and Europe, their annual incidence ranges from 50 to 100 per 100,000 in the general population (Byrne 2008; Chugh 2004; Vreede‐Swagemakers 1997; Vaillancourt 2004).
The fact that antihypertensive pharmacotherapy does not prevent this frequent cause of death is a matter of public health concern. One important improvement in coronary death prevention seen during the past two decades results from coronary angioplasty in acute coronary syndrome. These results, however, do not call into question the prescription of antihypertensive drugs, which remain associated with a significant reduction in coronary and cerebrovascular events. This shows that additional research is needed to determine the causes of sudden death. It also suggests that sudden cardiac death may be a misnomer, and that until we have attained a good estimate of its different causes, this might be better labelled 'sudden death'.
Overall completeness and applicability of evidence
This comprehensive review provides the most up‐to‐date evidence from available randomised controlled trials. We have done our best to contact study authors to collect data on sudden cardiac death from the more recent studies (HOPE 2000; HYVET 2008; HYVET‐Pilot 2003), which did not report it. We applied no language restrictions when selecting studies.
Quality of the evidence
Overall, we judged the risk of bias of studies included in this review as moderate to low. However, only six of the 15 trials (53%) described the method of concealment of allocation. Most trials with unclear concealment of allocation were described as 'randomised', and study authors provided no other details. Some study authors stated that these trials were double‐blind but offered no information about how this was achieved.
Potential biases in the review process
One potential bias that deserves attention is the fact that most trials used a combination of antihypertensive medications as stepped‐care therapy. As a result, the drugs used and the amount of blood pressure reduction achieved were heterogeneous.
For most long‐term and large‐scale studies, it is impossible to maintain single first‐line drug treatment because a single drug frequently does not lower blood pressure to a level considered low enough. In most cases, physicians in the included studies added other antihypertensive drugs (e.g. reserpine) to reach the target blood pressure.
We excluded many studies because they did not report the incidence of sudden cardiac death. This lack of completeness could have biased the analysis, but as most of these eligible studies were published before 1991, it is unlikely that their data will become available.
Agreements and disagreements with other studies or reviews
This is the first systematic review undertaken to specifically assess the effects of antihypertensive therapy on sudden cardiac death. Estimates of effects of risk reduction on fatal and non‐fatal myocardial infarction provided here are similar to those reported by other reviews (Musini 2009; Wright 2009).

PRISMA study flow diagram.

Funnel plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.1 Sudden cardiac death.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.1 Sudden cardiac death.

Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.2 Non‐fatal myocardial infarction.

Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.3 Fatal myocardial infarction.

Forest plot of comparison: 3 Subgroup analysis, first‐line diuretic versus others, outcome: 3.1 Sudden cardiac death.

Comparison 1 Antihypertensive drug therapy versus control, Outcome 1 Sudden cardiac death.

Comparison 1 Antihypertensive drug therapy versus control, Outcome 2 Non‐fatal myocardial infarction.

Comparison 1 Antihypertensive drug therapy versus control, Outcome 3 Fatal myocardial infarction.
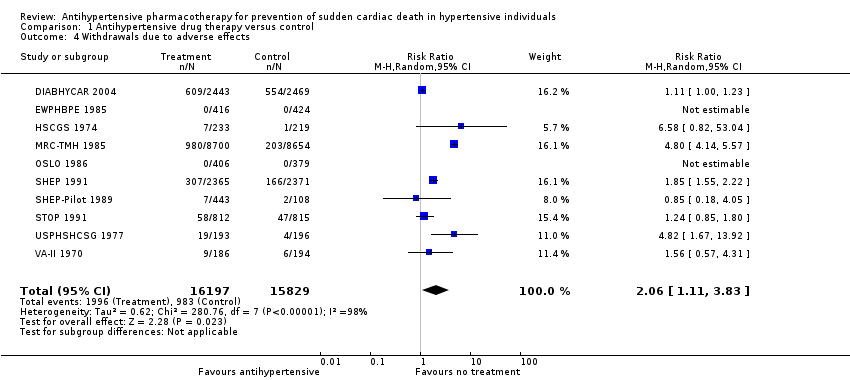
Comparison 1 Antihypertensive drug therapy versus control, Outcome 4 Withdrawals due to adverse effects.

Comparison 2 Subgroup analysis, first‐line diuretic versus others, Outcome 1 Sudden cardiac death.
| Antihypertensive pharmacotherapy versus control for prevention of sudden cardiac death in hypertensive individuals | ||||||
| Patient or population: people with hypertension (defined as baseline systolic resting blood pressure ≥ 140 mmHg and/or resting diastolic blood pressure ≥ 90 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with antihypertensive pharmacotherapy | |||||
| Sudden cardiac death (mean follow‐up: 4.2 years) | Study population | RR 0.96 | 39908 | ⊕⊕⊕⊝ | ||
| 13 per 1000 | 12 per 1000 | |||||
| Non‐fatal myocardial infarction (mean follow‐up: 4.2 years) | Study population | RR 0.85 0.98) | 39908 | ⊕⊕⊕⊝ | ||
| 20 per 1000 | 17 per 1000 | |||||
| Fatal myocardial infarction (mean follow‐up: 4.2 years) | Study population | RR 0.75 0.90) | 39908 | ⊕⊕⊕⊝ | ||
| 12 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded 1 level for imprecision (wide confidence intervals) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sudden cardiac death Show forest plot | 15 | 39908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.15] |
| 2 Non‐fatal myocardial infarction Show forest plot | 15 | 39908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 3 Fatal myocardial infarction Show forest plot | 15 | 39908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.62, 0.90] |
| 4 Withdrawals due to adverse effects Show forest plot | 10 | 32026 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.11, 3.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sudden cardiac death Show forest plot | 15 | 38281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.19] |
| 1.1 First‐line diuretic | 10 | 17912 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 1.2 First‐line other antihypertensive drugs | 5 | 20369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.19] |

