Comparaison du suivi de l’asthme à distance et face à face
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: 12 month parallel RCT Setting: paediatric clinic at Tripler Army Medical Center, Hawaii Enrolment began in March 2003 and ended in December 2003. Participant data collection ended with the last participant’s final visit in February 2005 | |
| Participants | Population: 120 children were randomised to the virtual group (60) or the office‐based group (60) Baseline characteristics: mean age, years (SD): remote 10.2 (3.1); face‐to‐face 9.0 (3.0) % male: remote 61.7; face‐to‐face 63.3 % predicted FEV1 (SD): remote 104.1 (19.9); face‐to‐face 96.8 (13.0) Inclusion criteria: children aged 6 to 17 years with persistent asthma, dependent of active duty or retired USA military personnel, not moving from Oahu for 12 months after entry into the study, ability to receive cable modem connections in the home, willingness to complete questionnaires and monitoring Exclusion criteria: none stated | |
| Interventions | Intervention: virtual group participants received computers, internet connections and in‐home internet‐based case management and received education through the study website. Control: office‐based group patients received traditional in‐person education and case management. | |
| Outcomes | Control medication use, daily symptom diary, peak flow, patient and caregiver AQLQ, service utilisation, asthma knowledge retention Measured at 2 weeks, 6 weeks, 3 months, 6 months and 12 months | |
| Notes | Funding: grant from the US Army Medical Research Acquisition Activity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients underwent block randomisation with a table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide any details. |
| Blinding of participants and personnel | Low risk | It would not have been possible to blind participants and personnel to allocation due to the nature of the intervention. However, participants and personnel being aware of group allocation is unlikely to have affected the results for the objective outcomes (exacerbations and adverse events). |
| Blinding of participants and personnel | High risk | Participants and personnel being aware of group allocation could have affected their scores on subjective outcomes such as those measured on self‐report scales (Asthma Control Questionnaire (ACQ) and AQLQ). |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is possible to blind outcome assessment but the study did not provide any specific details of whether this was done. |
| Incomplete outcome data (attrition bias) | High risk | Dropout was much higher in the virtual group (23%) than the office group (8%). The study authors did not account for non‐adherent participants and other dropouts in the analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes were well reported. There was no protocol registration available to check all pre‐specified measures were included but there was no evidence of selective reporting. |
| Other bias | Low risk | We did not note any other possible sources of bias. |
| Methods | Study design: 12 month parallel RCT Setting: 1 practice in England, UK Participants were recruited between December 2002 and March 2003 | |
| Participants | Population: 194 people were randomised to the telephone group (97) or the clinic group (97) Baseline characteristics: mean age, years (standard deviation (SD)): remote 50.8 (15.4); face‐to‐face 49.6 (16.1) % male: remote 51.5; face‐to‐face 39.2 % predicted forced expiratory volume in one second (FEV1) (SD): not reported Inclusion criteria: adults with asthma aged 17 to 70 years and on the practice asthma list Exclusion criteria: housebound, did not possess a telephone or were unwilling to give informed consent | |
| Interventions | Intervention: participants were contacted by telephone at 6‐monthly intervals by 1 of 2 trained asthma nurses. The participant was then asked the RCPs ‘three questions’ plus two extra questions related to a high risk of asthma death. The nurse formulated an individualised asthma action plan with the participant, with advice on what to do if asthma control deteriorated. Control: participants received usual care by 6‐monthly check up via a dedicated asthma appointment with a diploma‐level asthma nurse. Symptom scores, inhaler technique and peak flow measurements were checked and all participants issued with an asthma action plan. | |
| Outcomes | ACQ, mini‐AQLQ, mild and severe exacerbations, healthcare costs, clinical time, inhaler use, unscheduled healthcare visits all given per patient year. Measured at baseline, 6 months and 12 months | |
| Notes | Funding: grant from Asthma UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study randomised participants using a random number tables on a 1 to 1 basis and stratified according to severity. |
| Allocation concealment (selection bias) | Unclear risk | The study did not provide any details. |
| Blinding of participants and personnel | Low risk | It would not have been possible to blind participants and personnel to allocation due to the nature of the intervention. However, participants and personnel being aware of group allocation is unlikely to have affected the results for the objective outcomes (exacerbations and adverse events). |
| Blinding of participants and personnel | High risk | Participants and personnel being aware of group allocation could have affected their scores on subjective outcomes such as those measured on self‐report scales (ACQ and AQLQ). |
| Blinding of outcome assessment (detection bias) | High risk | "assessors were not blinded to the interventions due to limited resources". |
| Incomplete outcome data (attrition bias) | High risk | "There were 20 withdrawals in the control group after the first visit, mainly due to non‐attendance and 6 in the telephone group, one of which was due to non‐attendance. As this trial is as real‐world as possible the fact that there was a high non‐attendance rate was taken account of in analysing the costs." |
| Selective reporting (reporting bias) | Low risk | Outcomes were well reported. There was no protocol registration available to check all pre‐specified measures were included but there was no evidence of selective reporting. |
| Other bias | Low risk | We did not note any other possible sources of bias. |
| Methods | Study design: pragmatic 6 month parallel RCT Setting: 2 academic tertiary care hospitals and 4 large community hospitals in The Netherlands. Participants were randomised between November 2007 and October 2008 | |
| Participants | Population: 95 people were randomised to the internet group (52) or the conventional face‐to‐face management group (38) Baseline characteristics: mean age, years (SD): remote 48.5 (12.4); face‐to‐face 52.4 (11.7) % male: remote 45; face‐to‐face 47 % predicted FEV1 (SD): remote 76.3 (24.7); face‐to‐face 71.3 (21.0) Inclusion criteria: adults (18 to 75 years) with a diagnosis of severe refractory asthma according to the major and minor criteria recommended by the American Thoracic Society. They had uncontrolled asthma despite intensive follow‐up by an asthma specialist for at least 1 year, chronic treatment with oral corticosteroids and high doses of ICS plus long‐acting bronchodilators. All were non‐smokers with a maximum smoking history of 15 pack‐years and had access to internet or mobile telephone Exclusion criteria: none stated | |
| Interventions | Intervention: dose adjustment of oral corticosteroids guided by an internet‐based management tool (internet group). Included electronic diary, decision support and monitoring support by a study nurse Control: dose adjustment of oral corticosteroids according to conventional asthma treatment by the pulmonologist, according to GINA (conventional management group) | |
| Outcomes | Cumulative sparing of oral corticosteroid therapy (OCS), ACQ, AQLQ, global satisfaction scale, FEV1, number of exacerbations and days of hospitalisation The authors defined an exacerbation as a decrease in morning FEV1 of at least 10% compared with the mean FEV1 from the week before, or a respiratory event requiring an increase in prednisone equivalent to at least 10 mg/day, or a course of antibiotics, with or without hospitalisation | |
| Notes | Funding: The Netherlands Organization for Health Research and Development (ZonMw) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study randomised participants by a computer random number generator and remained on the same allocation throughout the study. Communication: "The random codes were stratified for study center and initial dose of corticosteroid dose (lower or higher than 10 mg prednisone per day)". |
| Allocation concealment (selection bias) | Unclear risk | "unblinded after randomisation", implies it was concealed, but the study did not provide any further details. |
| Blinding of participants and personnel | Low risk | The treatment assignments were unblinded after randomisation to allow monthly corticosteroid dose adjustments according to conventional treatment by the physician or weekly adjustments according to the internet algorithm. While it was not possible to blind participants and personnel to allocation due to the nature of the intervention, participants and personnel being aware of group allocation is unlikely to have affected the results for the objective outcomes (exacerbations and adverse events). |
| Blinding of participants and personnel | High risk | Participants and personnel being aware of group allocation could have affected their scores on subjective outcomes such as those measured on self‐report scales (ACQ and AQLQ). |
| Blinding of outcome assessment (detection bias) | High risk | Communication: "This was a pragmatic study so the outcome assessors were not blind to the group allocation in order to allow monthly corticosteroid dose adjustments (according to conventional treatment by the physician) or weekly adjustments (according to the internet algorithm)". |
| Incomplete outcome data (attrition bias) | High risk | Five participants in the conventional management group withdrew consent before the study had started and the study excluded one participant because of poor adherence to the trial protocol. The study included 89 participants out of 95 in the intention‐to‐treat (ITT) analysis; 51 and 38. Dropout was higher in the conventional treatment group (16% versus 8%). |
| Selective reporting (reporting bias) | Low risk | The study was prospectively registered, and outcomes were well reported. |
| Other bias | Low risk | We did not note any other possible sources of bias. |
| Methods | Study design: pragmatic parallel RCT (duration of study participation varied across participants) Setting: 4 general practices in the UK | |
| Participants | Population: 278 people were randomised to remote telephone check‐up (137) or face‐to‐face check‐up (141) Baseline characteristics: mean age, years (SD): remote 54.6 (17.5); face‐to‐face 56.4 (17.5) % male: remote 41; face‐to‐face 42 % predicted FEV1 (SD): not reported Inclusion criteria: adults with asthma who had requested a prescription for a bronchodilator inhaler in the last 6 months Exclusion criteria: if diagnosis of asthma had been made within the previous year, if they had chronic obstructive pulmonary disease, if communication difficulties made a telephone check‐up impossible, or (at the general practitioner's (GP's) request) for major social or medical reasons. | |
| Interventions | Intervention: telephone check‐up with the asthma nurse. The nurse tried up to 4 times to contact the participants. Control: face‐to‐face check‐ups in the surgery also with the asthma nurse, one invitation was sent in the usual manner. Content of the check‐up was as the nurse deemed appropriate. | |
| Outcomes | Medical reviews, time taken to review participants in each arm, asthma morbidity on the short Q, asthma related quality of life on the mini AQLQ, participant satisfaction, costs | |
| Notes | Funding: originally developed at a General Practice Airways Group research meeting, which was organised by Mark Levy and funded by an educational grant from AstraZeneca. The trial was funded by British Lung Foundation (Grant No P00/9). Additionally, one study author was supported by an NHS R&D national primary care fellowship. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were centrally randomised in blocks of 10 to ensure that approximately equal numbers of participants were allocated to each study arm. |
| Allocation concealment (selection bias) | Low risk | "Centrally randomised" implies that allocation was undertaken independently and concealed. |
| Blinding of participants and personnel | Low risk | It would not have been possible to blind participants and personnel to allocation due to the nature of the intervention. However, participants and personnel being aware of group allocation is unlikely to have affected the results for the objective outcomes (exacerbations and adverse events). |
| Blinding of participants and personnel | High risk | Participants and personnel being aware of group allocation could have affected their scores on subjective outcomes such as those measured on self‐report scales (ACQ and AQLQ). |
| Blinding of outcome assessment (detection bias) | Unclear risk | “a researcher, blinded to allocation visited each of the practices and validated a random 20% sample of consultation data and data retrieved from records”. However, the participants and investigators could not be blinded to the interventions and, in most cases, the outcome assessors were not blinded to group allocation either. |
| Incomplete outcome data (attrition bias) | Low risk | The percentage of withdrawals was low and even between groups (5.1 and 4.3% in the remote and face‐to‐face groups respectively). |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not note any other possible sources of bias. |
| Methods | Study design: 12 month before‐and‐after implementation study Setting: 1 large English general practice on 3 sites | |
| Participants | Population: 3 practices were randomised to: 1. a choice of remote phone check‐ups or face‐to‐face check‐ups (554 on list), 2. face‐to‐face only check‐ups (659 on list), or 3. a usual care control group which was not included in this systematic review (515 on list) Baseline characteristics: mean age, years (SD): remote 43 (24.8); face‐to‐face 42.3 (24.4) % male: remote 44.2; face‐to‐face 44.9 % predicted FEV1 (SD): not reported Inclusion criteria: adults and adolescents with a diagnosis of asthma and prescribed asthma medication in the previous year Exclusion criteria: children under 12 years of age, diagnosis of COPD | |
| Interventions | Intervention: participants were identified from the practice computer database and sent 3 invitations over the study period. They could book either a telephone or face‐to‐face check‐up both at a pre‐arranged time. Participants who did not respond to the 3 invitations were phoned and reviewed opportunistically Control: participants were recalled to face‐to‐face only asthma check‐ups using invitations by post or as memos with repeat prescriptions. There was no option of telephone check‐ups and no systematic attempt was made to phone non‐attenders opportunistically. Group excluded: the usual‐care control group maintained their well established asthma clinic, and existing procedures (for example, invitations are issued in response to clinical need), but no systematic recall was undertaken | |
| Outcomes | Proportion reviewed, asthma morbidity and enablement on the mini AQLQ, ACQ, modified patient enablement instrument and Asthma Bother Profile, adverse events, costs | |
| Notes | Funding: Scientific Foundation Board of the Royal College of General Practitioners (SFB/2003/45) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study decided allocation to the telephone option by the public toss of a coin. |
| Allocation concealment (selection bias) | High risk | The study allocated individuals to treatment after the two clusters had been decided by the toss of a coin. |
| Blinding of participants and personnel | Low risk | It would not have been possible to blind participants and personnel to allocation due to the nature of the intervention. However, participants and personnel being aware of group allocation is unlikely to have affected the results for the objective outcomes (exacerbations and adverse events). |
| Blinding of participants and personnel | High risk | Participants and personnel being aware of group allocation could have affected their scores on subjective outcomes such as those measured on self‐report scales (ACQ and AQLQ). |
| Blinding of outcome assessment (detection bias) | Unclear risk | The nurses were aware of allocation but it was unclear whether it was the nurses measuring outcomes, or if it was someone independent from the study who could remain blind to allocation. The study did not describe this. The study stated that there were quality control checks blinded to allocation which confirmed accuracy of data transfer. |
| Incomplete outcome data (attrition bias) | Low risk | Real‐world implementation study, therefore the uptake rate by participants is part of the study, routine asthma check‐up was provided for 66.3% of participants in the telephone only group and 53.8% in the face‐to‐face only group. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | High risk | This study was a cluster implementation study with a before‐and‐after design. It randomised 2 practices to the interventions which would not have controlled for baseline imbalances in the same way as individual randomisation, and this meant the participant population in each group was not static. The intervention was a telephone option and many in that practice opted for a usual face‐to‐face check‐up, which meant the study was not making a direct comparison of remote and face‐to‐face check‐ups. Additionally, people in the telephone option group were phoned opportunistically to increase uptake of check‐ups which did not happen in the face‐to‐face group. These factors mean we cannot be certain that mode of check‐up, and not the increased likelihood of check‐up, was the variable being measured. |
| Methods | Study design: 6 month pragmatic parallel RCT Setting: general practices and specialist clinics in Copenhagen, Denmark | |
| Participants | Population: 300 people were randomised to remote check‐ups (100), face‐to‐face check‐ups with a specialist (100), and a usual care group not included in this review (100) Baseline characteristics: mean age, years (SD): remote 28 (NR); face‐to‐face 30 (NR) % male: remote 31.8; face‐to‐face 34.1 % predicted FEV1 (SD): remote 91 (NR); face‐to‐face 93 (NR) Inclusion criteria: 18 to 45 years with definite asthma, living in the catchment area of H:S Bispebjerg University Hospital of Copenhagen, Denmark Exclusion criteria: none stated | |
| Interventions | Intervention: participants were given a Peak Flow Meter and taught how to fill in a daily diary and respond to the computer’s advice. Physicians gave instructions via e‐mail or telephone to the participant. The intervention included an electronic diary, an asthma action plan and a decision support system for the physician. Control: the specialists taught the participants how to adjust their medication on the basis of a peak flow meter and written action plan Group not included: the GP group was asked to contact their GP and pass on a letter describing the study and giving the test results. GPs in Copenhagen had been sent a circular about asthma and GINA guidelines in the past. | |
| Outcomes | AQLQ, asthma self‐care, smoking, education, salary, sick leave, hospitalisations, medication compliance, adverse events, lung function Measured at baseline and 6 months | |
| Notes | Funding: Grants from H:S Corporation of University Hospital of Copenhagen, AstraZeneca, and private funds | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication: "The allocation sequences were computer‐generated by a senior respiratory physician. These sequences consisted of randomised blocks of 30 asthmatics". |
| Allocation concealment (selection bias) | Low risk | Communication: "The envelopes were packed by two medical students one month before the start of the study and the randomisation lists were stored in a separate, sealed envelope. The consecutively numbered and sealed envelopes contained the randomisation code. All envelopes were opened sequentially after the asthma diagnosis had been verified". |
| Blinding of participants and personnel | Low risk | It would not have been possible to blind participants and personnel to allocation due to the nature of the intervention. However, participants and personnel being aware of group allocation is unlikely to have affected the results for the objective outcomes (exacerbations and adverse events). |
| Blinding of participants and personnel | High risk | Participants and personnel being aware of group allocation could have affected their scores on subjective outcomes such as those measured on self‐report scales (ACQ and AQLQ). |
| Blinding of outcome assessment (detection bias) | High risk | Communication: "It was not possible to blind outcome assessors to group allocation". |
| Incomplete outcome data (attrition bias) | High risk | Of the 300 participants randomised, 253 participants completed both the screening and follow‐up visits. Dropout was unbalanced across groups (12%, 15% and 20%), and the study does not appear to have imputed data for missing values. |
| Selective reporting (reporting bias) | Low risk | The paper did not report all of the results from the questionnaires but the lead study author provided them on request. |
| Other bias | Low risk | We did not note any other possible sources of bias. |
Abbreviations: ACQ = Asthma Control Questionnaire; AQLQ = Asthma Quality of Life Questionnaire; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in one second; GINA = Global Initiative for Asthma; GP = general practitioner; ICS = inhaled corticosteroids; ITT = intention‐to‐treat analysis; NR = not reported; OCS = oral corticosteroids; RCP = respiratory care practitioner; RCT = randomised controlled trial; SD = standard deviation.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong intervention ‐ technology‐based self management between reviews. | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ minimal or no provider involvement | |
| Wrong design ‐ not a trial report | |
| Wrong comparison ‐ telemedicine portal used with or without home visits (both groups used the portal) | |
| Wrong design ‐ crossover RCT | |
| Wrong comparison ‐ phone calls for asthma education versus non‐asthma phone calls | |
| Wrong intervention ‐ asthma education intervention led by a pharmacist | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ study assessing validity of self‐reports | |
| Wrong intervention ‐ study assessing validity of self‐reports | |
| Wrong intervention ‐ minimal or no provider involvement | |
| Wrong intervention ‐ pharmacist led intervention about adherence | |
| Wrong intervention ‐ focus on asthma education, not monitoring with remote reviews | |
| Wrong intervention ‐ intervention to improve adherence to home PEF measurements | |
| Wrong intervention ‐ pharmacy led technology intervention to improve adherence | |
| Wrong intervention ‐ monitoring theophylline levels | |
| Wrong intervention ‐ phone calls to promote adherence, not remote reviews | |
| Wrong intervention ‐ asthma behavioural intervention using technology, not remote reviews | |
| Wrong intervention ‐ not remote reviews | |
| Wrong intervention ‐ counselling intervention not remote reviews | |
| Wrong intervention ‐ parenting intervention, not remote reviews | |
| Wrong design ‐ not a randomised controlled trial (RCT) | |
| Wrong design ‐ crossover RCT | |
| Wrong comparison ‐ comparing 2 types of electronic monitoring (FeNo versus symptoms) | |
| Wrong intervention ‐ asthma education and adherence monitoring by a pharmacist | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ coaching/education intervention using technology for multiple chronic conditions | |
| Wrong intervention ‐ not remote asthma reviews | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong design ‐ survey of RCT participants | |
| Wrong intervention ‐ adherence intervention | |
| Wrong intervention ‐ mostly automated home monitoring, not remote reviews | |
| Wrong intervention ‐ asthma coaching/education intervention over the phone, not remote reviews | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ self‐determination theory intervention, not remote reviews | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ support intervention, not remote reviews | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ management intervention for African American women, not remote reviews | |
| Wrong intervention ‐ mixed diagnosis study comparing models of delivering home care | |
| Wrong intervention ‐ minimal or no provider involvement | |
| Wrong intervention ‐ one phone call at discharge, not remote reviews | |
| Wrong intervention ‐ not technology‐based | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong design ‐ cross‐sectional analysis of an ongoing RCT, and mixed diagnosis | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ not about remote reviews | |
| Wrong intervention ‐ computer‐aided decision support during consultation | |
| Wrong intervention ‐ asthma education delivered via CD‐ROM and book versus book alone | |
| Wrong intervention ‐ remote monitoring of inhaler adherence | |
| Wrong intervention ‐ minimal or no provider involvement | |
| Wrong design ‐ comment on a RCT | |
| Wrong comparison ‐ remote monitoring using PEF or symptoms | |
| Wrong comparison ‐ phone monitoring plus asthma education versus phone education | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ focus on asthma education not remote reviews | |
| Wrong intervention ‐ focus on asthma education not remote reviews | |
| Wrong intervention ‐ focus on asthma education not remote reviews | |
| Wrong intervention ‐ counselling not remote reviews | |
| Wrong intervention ‐ computer‐aided decision support during consultation | |
| Wrong intervention ‐ post admission follow‐up | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ main focus of the study was to educate participants on how to use a peak flow meter | |
| Wrong intervention ‐ treatment awareness education delivered over the phone | |
| Wrong intervention ‐ minimal or no provider involvement | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ validating the Asthma Control Test for internet use | |
| Wrong design ‐ questionnaire not a RCT | |
| Wrong intervention ‐ study measuring validity of self‐report | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ minimal or no provider involvement | |
| Wrong intervention ‐ validation study | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong comparison ‐ phone calls on top of face‐to‐face review, not instead of | |
| Wrong intervention ‐ letter regarding validation of telephone delivery of the Asthma Control Questionnaire (ACQ) | |
| Wrong intervention ‐ minimal or no provider involvement | |
| Wrong intervention ‐ asthma education and motivational interviewing, not remote reviews | |
| Wrong intervention ‐ focus on asthma education not remote reviews | |
| Wrong intervention ‐ pharmacy‐led compliance intervention, not remote reviews | |
| Wrong intervention ‐ validating the Asthma Control Test via text messaging | |
| Wrong intervention ‐ general practitioner (GP) telephone access to paediatricians | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ multifaceted intervention, not just remote reviews | |
| Wrong intervention ‐ multifaceted intervention, not just remote reviews | |
| Wrong intervention ‐ minimal or no provider involvement. Medication reminder system | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ FeNO and Internet‐based monitoring | |
| Wrong intervention ‐ multi‐faceted intervention, not just about remote monitoring | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ technology‐based self management between reviews | |
| Wrong intervention ‐ asthma education via text, not remote reviews | |
| Wrong intervention ‐ computer works out best inhaler type for patient |
Abbreviations: RCT = randomised controlled trial; PEF = peak expiratory flow.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations requiring oral corticosteroids Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.1 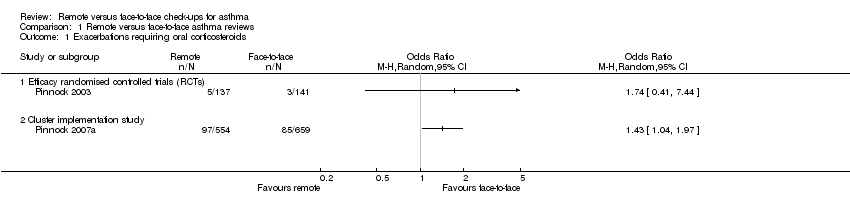 Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 1 Exacerbations requiring oral corticosteroids. | ||||
| 1.1 Efficacy randomised controlled trials (RCTs) | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Cluster implementation study | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
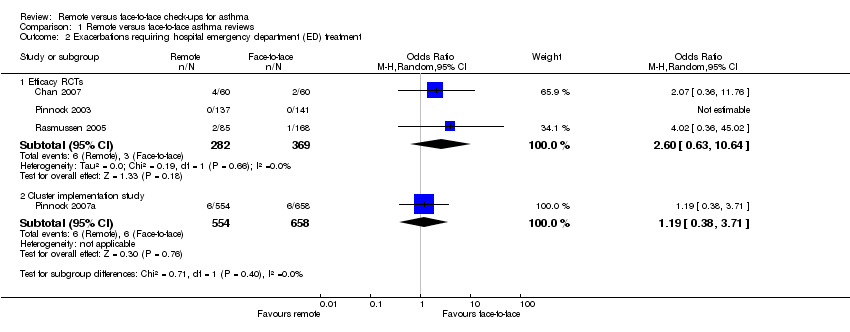
| 2 Exacerbations requiring hospital emergency department (ED) treatment Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2 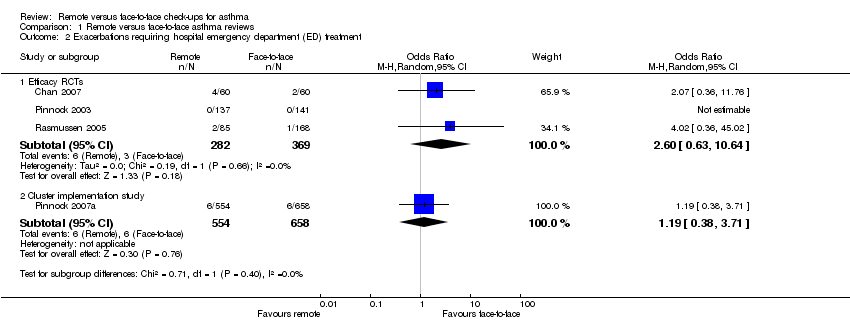 Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 2 Exacerbations requiring hospital emergency department (ED) treatment. | ||||
| 2.1 Efficacy RCTs | 3 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 2.60 [0.63, 10.64] |
| 2.2 Cluster implementation study | 1 | 1212 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.38, 3.71] |
| 3 Exacerbations requiring hospital admission Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 3 Exacerbations requiring hospital admission. | ||||
| 3.1 Efficacy RCTs | 3 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.06, 6.32] |
| 3.2 Cluster implementation study | 1 | 1213 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [0.83, 5.69] |
| 4 Asthma control (Asthma Control Questionnaire (ACQ)) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 4 Asthma control (Asthma Control Questionnaire (ACQ)). | ||||
| 4.1 Efficacy RCTs | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Cluster implementation study | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
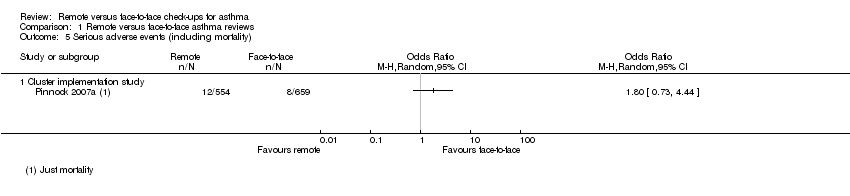
| 5 Serious adverse events (including mortality) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.5 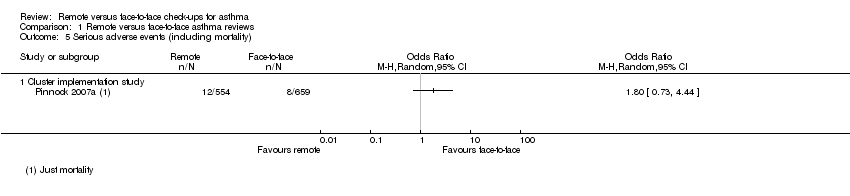 Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 5 Serious adverse events (including mortality). | ||||
| 5.1 Cluster implementation study | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Asthma‐related quality of life (Asthma Quality of Life Questionnaire (AQLQ) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 6 Asthma‐related quality of life (Asthma Quality of Life Questionnaire (AQLQ). | ||||
| 6.1 Efficacy RCTs | 3 | 544 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.14, 0.30] |
| 6.2 Cluster implementation study | 1 | 536 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.23, 0.19] |
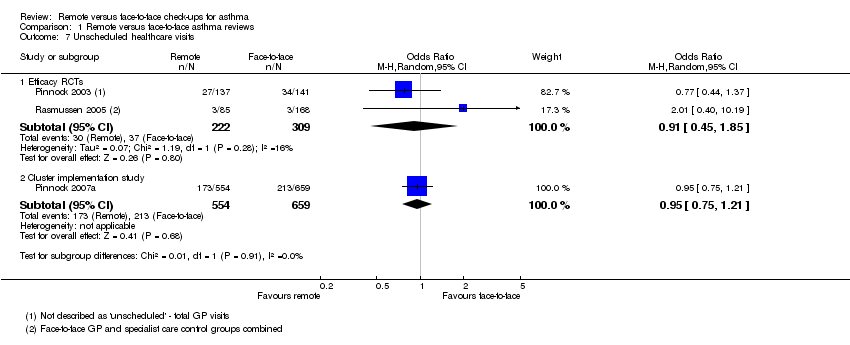
| 7 Unscheduled healthcare visits Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 7 Unscheduled healthcare visits. | ||||
| 7.1 Efficacy RCTs | 2 | 531 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.85] |
| 7.2 Cluster implementation study | 1 | 1213 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.21] |
| 8 Change in lung function (trough FEV1) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 8 Change in lung function (trough FEV1). | ||||
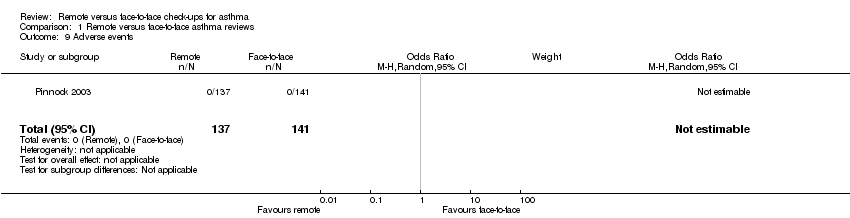
| 9 Adverse events Show forest plot | 1 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.9  Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 9 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations requiring hospital admission Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 1 Exacerbations requiring hospital admission. | ||||
| 2 Asthma control (ACQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 2 Asthma control (ACQ). | ||||
| 3 Asthma‐related quality of life (AQLQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.3 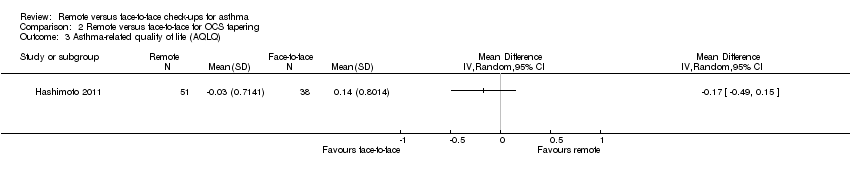 Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 3 Asthma‐related quality of life (AQLQ). | ||||
| 4 Unscheduled healthcare visits Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.4 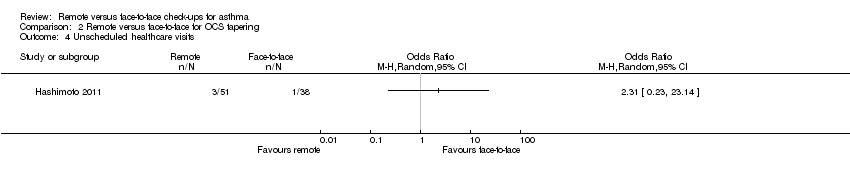 Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 4 Unscheduled healthcare visits. | ||||
| 5 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.5 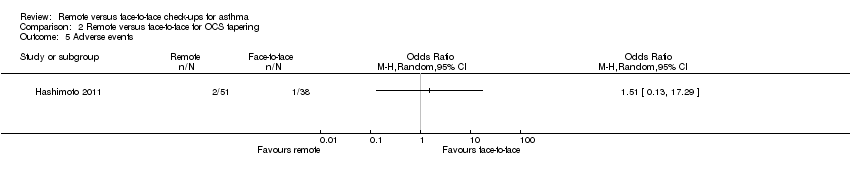 Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 5 Adverse events. | ||||
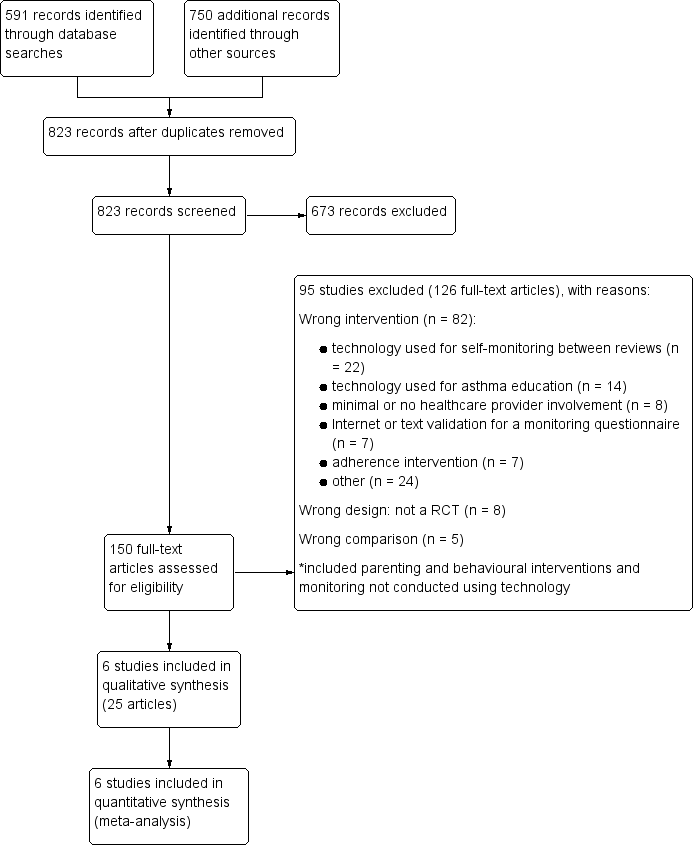
Study flow diagram

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
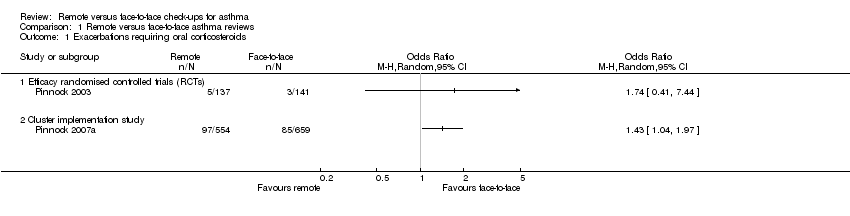
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 1 Exacerbations requiring oral corticosteroids.
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 2 Exacerbations requiring hospital emergency department (ED) treatment.

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 3 Exacerbations requiring hospital admission.

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 4 Asthma control (Asthma Control Questionnaire (ACQ)).
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 5 Serious adverse events (including mortality).

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 6 Asthma‐related quality of life (Asthma Quality of Life Questionnaire (AQLQ).
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 7 Unscheduled healthcare visits.

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 8 Change in lung function (trough FEV1).
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 9 Adverse events.

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 1 Exacerbations requiring hospital admission.

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 2 Asthma control (ACQ).

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 3 Asthma‐related quality of life (AQLQ).
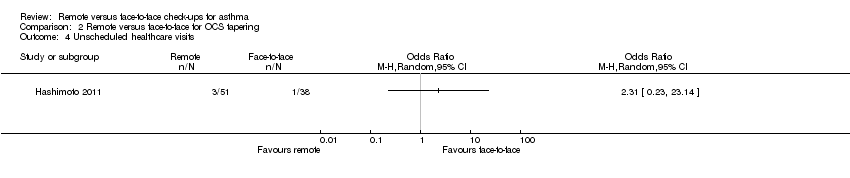
Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 4 Unscheduled healthcare visits.

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 5 Adverse events.
| Remote versus face‐to‐face check‐ups for asthma | ||||||
| Patient or population: adults or children with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with face‐to‐face check‐ups | Risk with remote check‐ups | |||||
| Exacerbations requiring oral corticosteroids | 21 per 1000 | 36 per 1000 | OR 1.74 | 278 | ⊕⊕⊝⊝ | Very imprecise. Data from the implementation study** were consistent. |
| Exacerbations requiring hospital admission 6 months | 5 per 1000 | 3 per 1000 | Peto OR 0.63 | 651 | ⊕⊕⊝⊝ | Very few events ‐ no conclusion could be drawn. The implementation study was more in favour of face‐to‐face check‐ups. |
| Asthma control (ACQ) Scale 0 to 6; lower is better | The mean ACQ score with face‐to‐face check‐ups improved by 0.11 | The mean ACQ score with remote check‐ups improved by 0.07 more (0.35 more to 0.21 less) | ― | 146 | ⊕⊕⊕⊝ | No difference and CIs ruled out significant harm of remote check‐ups (MCID for the ACQ is 0.5). The implementation study results were consistent. |
| Serious adverse events (including mortality) | ― | ― | ― | 0 RCTs | ― | No efficacy studies reported all‐cause SAEs. The implementation study recorded 12/554 and 8/659 in the remote and face‐to‐face groups respectively (OR 1.80, 95% CI 0.73 to 4.44) |
| Asthma‐related quality of life (AQLQ) Scale 1 to 7; higher is better 8 months | The mean AQLQ score with face‐to‐face check‐ups was 5.49 | The mean AQLQ score with remote check‐ups was 0.08 better (0.14 worse to 0.30 better) | ― | 544 | ⊕⊕⊕⊝ | No difference and CIs ruled out significant harm of remote check‐ups (MCID for the AQLQ is 0.5). The implementation study results were consistent. |
| Unscheduled healthcare visits 5 months | 120 per 1000 | 110 per 1000 | OR 0.91 | 531 | ⊕⊕⊝⊝ | Very few events ‐ we could not draw any conclusions. The implementation study was more precise and did not show a difference. |
| Lung function (trough FEV1) 6 months | The mean trough FEV1 with face‐to‐face check‐ups was 20 mL | The mean trough FEV1 with remote check‐ups was 166.76 mL better (78.03 more to 255.5 more) | ― | 253 | ⊕⊕⊕⊝ | People having remote check‐ups had better lung function than those seen face‐to‐face in the one study that measured it. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk with face‐to‐face check‐ups for continuous outcomes was calculated as a weighted mean of the face‐to‐face values. **The 'Implementation study', Pinnock 2007a, had a two‐cluster pragmatic design and was not pooled with the rest of the included studies (efficacy studies). Durations were calculated as a weighted mean duration of the studies contributing data to the analysis. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies were at high risk of bias for one or more blinding domains but it is unlikely that this had an effect on the objective outcomes (no downgrade). | ||||||
| Study ID | Total N | Country | Duration | Mean age | % male | % FEV1 | Intervention | Control |
| 120 | Hawaii, USA | 12 months | 9.6 | 62.5 | 100.5 | In‐home, website‐based case management and education. | In‐person education and case management. | |
| 194 | UK | 12 months | 50.2 | 45.4 | NR | 6‐monthly phone calls from trained asthma nurses. Formulation of individual AAP. | 6‐monthly usual face‐to‐face appointment with an asthma nurse. Symptoms, peak flow and inhaler technique checked, and participants were issued with an AAP. | |
| 95 | The Netherlands | 6 months | 50.1 | 45.3 | 73.9 | OCS dose adjustment guided by an internet‐based diary, decision support and monitoring with support from a study nurse. | OCS dose adjustment according to GINA by the specialist. | |
| 278 | UK | 3 months | 55.5 | 41.4 | NR | Telephone check‐up with the asthma nurse. | Face‐to‐face check‐ups in the surgery with the asthma nurse. | |
| 1728 | UK | 12 months | 42.6 | 44.6 | NR | Three invitations to book either a telephone or face‐to‐face check‐up. Non‐attenders were phoned and reviewed opportunistically. | Three invitations to book a face‐to‐face check‐up. Non‐attenders were not phoned opportunistically. | |
| 300 | Denmark | 6 months | 29 | 34.5 | 92.0 | Participants were given an AAP, online electronic diary and peak flow meter. Physicians gave participants instructions via e‐mail or telephone aided by computer decision support. | The specialists taught the participants how to adjust their medication on the basis of a peak flow meter and AAP. | |
| Total N: the total number of participants randomised in the study, included to groups not analysed in this Cochrane review | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations requiring oral corticosteroids Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Efficacy randomised controlled trials (RCTs) | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Cluster implementation study | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exacerbations requiring hospital emergency department (ED) treatment Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Efficacy RCTs | 3 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 2.60 [0.63, 10.64] |
| 2.2 Cluster implementation study | 1 | 1212 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.38, 3.71] |
| 3 Exacerbations requiring hospital admission Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Efficacy RCTs | 3 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.06, 6.32] |
| 3.2 Cluster implementation study | 1 | 1213 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [0.83, 5.69] |
| 4 Asthma control (Asthma Control Questionnaire (ACQ)) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Efficacy RCTs | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Cluster implementation study | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Serious adverse events (including mortality) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Cluster implementation study | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Asthma‐related quality of life (Asthma Quality of Life Questionnaire (AQLQ) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Efficacy RCTs | 3 | 544 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.14, 0.30] |
| 6.2 Cluster implementation study | 1 | 536 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 7 Unscheduled healthcare visits Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Efficacy RCTs | 2 | 531 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.85] |
| 7.2 Cluster implementation study | 1 | 1213 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.21] |
| 8 Change in lung function (trough FEV1) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Adverse events Show forest plot | 1 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations requiring hospital admission Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Asthma control (ACQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Asthma‐related quality of life (AQLQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Unscheduled healthcare visits Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |

