遠距與面對面的氣喘檢查
Abstract
Background
Asthma remains a significant cause of avoidable morbidity and mortality. Regular check‐ups with a healthcare professional are essential to monitor symptoms and adjust medication.
Health services worldwide are considering telephone and internet technologies as a way to manage the rising number of people with asthma and other long‐term health conditions. This may serve to improve health and reduce the burden on emergency and inpatient services. Remote check‐ups may represent an unobtrusive and efficient way of maintaining contact with patients, but it is uncertain whether conducting check‐ups in this way is effective or whether it may have unexpected negative consequences.
Objectives
To assess the safety and efficacy of conducting asthma check‐ups remotely versus usual face‐to‐face consultations.
Search methods
We identified trials from the Cochrane Airways Review Group Specialised Register (CAGR) up to 24 November 2015. We also searched www.clinicaltrials.gov, the World Health Organization (WHO) trials portal, reference lists of other reviews and contacted trial authors for additional information.
Selection criteria
We included parallel randomised controlled trials (RCTs) of adults or children with asthma that compared remote check‐ups conducted using any form of technology versus standard face‐to‐face consultations. We excluded studies that used automated telehealth interventions that did not include personalised contact with a health professional. We included studies reported as full‐text articles, as abstracts only and unpublished data.
Data collection and analysis
Two review authors screened the literature search results and independently extracted risk of bias and numerical data. We resolved any disagreements by consensus, and we contacted study authors for missing information.
We analysed dichotomous data as odds ratios (ORs) using study participants as the unit of analysis, and continuous data as mean differences using the random‐effects models. We rated all outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Six studies including a total of 2100 participants met the inclusion criteria: we pooled four studies including 792 people in the main efficacy analyses, and presented the results of a cluster implementation study (n = 1213) and an oral steroid tapering study (n = 95) separately. Baseline characteristics relating to asthma severity were variable, but studies generally recruited people with asthma taking regular medications and excluded those with COPD or severe asthma. One study compared the two types of check‐up for oral steroid tapering in severe refractory asthma and we assessed it as a separate question. The studies could not be blinded and dropout was high in four of the six studies, which may have biased the results.
We could not say whether more people who had a remote check‐up needed oral corticosteroids for an asthma exacerbation than those who were seen face‐to‐face because the confidence intervals (CIs) were very wide (OR 1.74, 95% CI 0.41 to 7.44; 278 participants; one study; low quality evidence). In the face‐to‐face check‐up groups, 21 participants out of 1000 had exacerbations that required oral steroids over three months, compared to 36 (95% CI nine to 139) out of 1000 for the remote check‐up group. Exacerbations that needed treatment in the Emergency Department (ED), hospital admission or an unscheduled healthcare visit all happened too infrequently to detect whether remote check‐ups are a safe alternative to face‐to‐face consultations. Serious adverse events were not reported separately from the exacerbation outcomes.
There was no difference in asthma control measured by the Asthma Control Questionnaire (ACQ) or in quality of life measured on the Asthma Quality of Life Questionnaire (AQLQ) between remote and face‐to‐face check‐ups. We could rule out significant harm of remote check‐ups for these outcomes but we were less confident because these outcomes are more prone to bias from lack of blinding.
The larger implementation study that compared two general practice populations demonstrated that offering telephone check‐ups and proactively phoning participants increased the number of people with asthma who received a review. However, we do not know whether the additional participants who had a telephone check‐up subsequently benefited in asthma outcomes.
Authors' conclusions
Current randomised evidence does not demonstrate any important differences between face‐to‐face and remote asthma check‐ups in terms of exacerbations, asthma control or quality of life. There is insufficient information to rule out differences in efficacy, or to say whether or not remote asthma check‐ups are a safe alternative to being seen face‐to‐face.
PICO
淺顯易懂的口語結論
透過電話或網路檢查氣喘,是不是面對面諮詢的安全替代方案?
重點訊息
試圖回答這個問題的研究並未顯示兩種類型的檢查之間存在重要差異。然而,也沒有足夠的資訊顯示兩者之間存在風險和好處的差異。因此目前這個階段,我們不能說透過電話或是網路檢查氣喘,是否可以成為面對面諮詢的安全替代方案。
研究背景
定期與醫生或氣喘護理師聯繫,對於追蹤氣喘症狀和吸入劑的使用非常重要。對於需要管理越來越多的氣喘病患,和其他長期健康狀況而言,透過電話和網路科技或許是一個好辦法。這種方式被稱之為 “遠距審查” 或 “電子諮詢” ,而且可能是一種更容易讓病患和醫生之間保持聯繫的方式,但我們不知道這樣是否跟面對面諮詢的效果一樣好。
研究特點
我們總共發現 6 項研究,包括 2100 名受試者:其中的 4 項研究,共有 792 人可以合併作為主要結果,另外 2 項研究,因為研究設計差異非常大而被分開檢視(n = 1213 和 n = 95)。從這四項研究中匯整在一起的人,都有常規性的服用藥物,且我們排除了嚴重氣喘或其他肺部疾病的受試者。我們也分別檢視了另外兩項設計截然不同的研究:一項研究為比較氣喘患者可以自行選擇電話檢查,或是照常來診所面對面諮詢的做法、另一項研究則是減少病患服用口服類固醇的劑量,並利用科技來監測病患狀況。最後搜尋研究的時間為 2015 年 11 月 24 日。
主要結果
我們無法說明,透過電話或網路進行氣喘檢查的人,是否可能比面對面檢查的人,更需要或不需要口服類固醇來治療氣喘發作,並且由於多種原因我們不確定結果。研究中很少有人因為氣喘發作而需要在急診室或醫院接受治療,或者需要不定期去看醫生,進而判斷遠距檢查是否與面對面諮詢一樣好。研究無法顯示氣喘控制或生活品質方面的差異,但我們能夠排除某些可能性,像是遠端檢查在這些評量上,可能不如面對面諮詢來的好。所有證據的品質都被認為是低或中等。研究測試讓受試者選擇是否透過電話檢查,及可能帶來的好處。這增加了接受電話檢查的人數,但對於氣喘結果,顯示並無整體性的益處。
Authors' conclusions
Summary of findings
| Remote versus face‐to‐face check‐ups for asthma | ||||||
| Patient or population: adults or children with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with face‐to‐face check‐ups | Risk with remote check‐ups | |||||
| Exacerbations requiring oral corticosteroids | 21 per 1000 | 36 per 1000 | OR 1.74 | 278 | ⊕⊕⊝⊝ | Very imprecise. Data from the implementation study** were consistent. |
| Exacerbations requiring hospital admission 6 months | 5 per 1000 | 3 per 1000 | Peto OR 0.63 | 651 | ⊕⊕⊝⊝ | Very few events ‐ no conclusion could be drawn. The implementation study was more in favour of face‐to‐face check‐ups. |
| Asthma control (ACQ) Scale 0 to 6; lower is better | The mean ACQ score with face‐to‐face check‐ups improved by 0.11 | The mean ACQ score with remote check‐ups improved by 0.07 more (0.35 more to 0.21 less) | ― | 146 | ⊕⊕⊕⊝ | No difference and CIs ruled out significant harm of remote check‐ups (MCID for the ACQ is 0.5). The implementation study results were consistent. |
| Serious adverse events (including mortality) | ― | ― | ― | 0 RCTs | ― | No efficacy studies reported all‐cause SAEs. The implementation study recorded 12/554 and 8/659 in the remote and face‐to‐face groups respectively (OR 1.80, 95% CI 0.73 to 4.44) |
| Asthma‐related quality of life (AQLQ) Scale 1 to 7; higher is better 8 months | The mean AQLQ score with face‐to‐face check‐ups was 5.49 | The mean AQLQ score with remote check‐ups was 0.08 better (0.14 worse to 0.30 better) | ― | 544 | ⊕⊕⊕⊝ | No difference and CIs ruled out significant harm of remote check‐ups (MCID for the AQLQ is 0.5). The implementation study results were consistent. |
| Unscheduled healthcare visits 5 months | 120 per 1000 | 110 per 1000 | OR 0.91 | 531 | ⊕⊕⊝⊝ | Very few events ‐ we could not draw any conclusions. The implementation study was more precise and did not show a difference. |
| Lung function (trough FEV1) 6 months | The mean trough FEV1 with face‐to‐face check‐ups was 20 mL | The mean trough FEV1 with remote check‐ups was 166.76 mL better (78.03 more to 255.5 more) | ― | 253 | ⊕⊕⊕⊝ | People having remote check‐ups had better lung function than those seen face‐to‐face in the one study that measured it. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk with face‐to‐face check‐ups for continuous outcomes was calculated as a weighted mean of the face‐to‐face values. **The 'Implementation study', Pinnock 2007a, had a two‐cluster pragmatic design and was not pooled with the rest of the included studies (efficacy studies). Durations were calculated as a weighted mean duration of the studies contributing data to the analysis. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies were at high risk of bias for one or more blinding domains but it is unlikely that this had an effect on the objective outcomes (no downgrade). | ||||||
Background
Description of the condition
Asthma is a chronic disease of the airways, which causes reversible inflammation and narrowing of the airways and mucus production (GINA 2014). It commonly causes symptoms of wheezing, breathlessness, chest tightness and cough, although these vary between people and over time in their presence, frequency and severity (GINA 2014).
Despite the emergence and update of several national and international management guidelines which recommend a range of cost‐effective treatments based on frequency and severity of symptoms and exacerbations (e.g. BTS/SIGN 2014; GINA 2014), the disease remains a significant cause of avoidable morbidity and mortality worldwide (BTS/SIGN 2014; Global Asthma Report 2014; NRAD 2014). A national review of the 195 asthma deaths that occurred between February 2012 and January 2013 in the UK revealed that, in the year preceding their death, nearly one‐third had no record of seeing a general practitioner (GP) and nearly two‐thirds had not had an asthma check‐up in secondary care (NRAD 2014). The importance of self‐monitoring and regular check‐ups with a healthcare professional to monitor symptoms, and encourage adherence to preventer inhalers, is now well accepted (Gibson 2002; NRAD 2014), especially for people at high risk of severe asthma attacks.
Description of the intervention
Communication technologies, such as telephones and video conferencing, have been proposed as a way to conduct asthma check‐ups remotely. Conducting check‐ups in this way is a form of 'telehealth', otherwise referred to as 'telecare', 'digital health', 'telemedicine' or 'e‐health'. McLean 2013 described this field as "the use of information and communication technologies to deliver healthcare at a distance and to support patient self‐management through remote monitoring and personalised feedback". It may also be conceptualised as "an emerging field in the intersection of medical informatics, public health and business, referring to health services and information delivered or enhanced through the internet and related technologies (Eysenback 2001). Health services around the world are considering remote check‐ups as a way to manage the rising number of people with long‐term health conditions, to improve health outcomes and reduce the burden on emergency and inpatient services (Department of Health 2012; Steventon 2012).
The UK government outlined its aims for the widespread use of technology in health in their 2013 mandate, including wide availability of 'e‐consultations' by GPs, and significant progress towards home 'telemonitoring' of three million people with long‐term conditions by 2017 (Department of Health 2013). Researchers have studied the role of a range of technology‐based check‐ups and monitoring in asthma and other health conditions, including the use of telephone calls, email contact, text‐messaging and video‐conferencing (Laver 2013; McLean 2010; McLean 2011).
How the intervention might work
In the context of asthma, a condition that affects around 334 million people worldwide (Global Asthma Report 2014) and places a significant burden on healthcare systems, remote check‐ups may represent an unobtrusive and efficient way of maintaining contact with patients. Regular monitoring with communication technologies that does not disrupt a patient's life in the way that regular clinic visits might, may serve to enhance self management behaviours that have known benefits on morbidity and mortality, such as keeping personalised action plans up‐to‐date, and adherence to maintenance medications (NRAD 2014).
However, while governments and health services have highlighted the potential for cost savings and improved clinical outcomes of using remote check‐ups instead of face‐to‐face consultations, its use to monitor patients with potentially serious or life‐threatening conditions may not be without hazard. Focus groups have suggested that telehealth may be acceptable to patients and clinicians, but they have also raised concerns that it could actually discourage self management, or increase the likelihood of serious outcomes, by instilling a false sense of security (Pinnock 2007b).
The feasibility of using communication technologies in different situations and populations may be hampered by barriers, including insufficient healthcare infrastructure and funding (Lustig 2012). However, it may be a way to reduce inequality in health care related to socioeconomic status and rural living by improving access to services (Jannett 2003; Lustig 2012).
Why it is important to do this review
The release of the UK National Health Service (NHS) mandate in 2013 has seen a push to advance the use of telehealth for economic and clinical benefit. A recent overview of systematic reviews suggested that these benefits should not be assumed and that people at highest risk of serious health outcomes are likely to show the biggest gains (McLean 2013). For asthma, existing reviews have noted a large degree of variation in the way telehealth is defined and delivered in studies, to whom and to what it is compared (Jaana 2009; McLean 2010), and have been limited for this reason in the conclusions that could be drawn. This Cochrane review will focus on conducting asthma check‐ups remotely as a form of telehealth compared with usual face‐to‐face consultations in a hospital or clinic. A related Cochrane review will consider the evidence for remote monitoring of asthma control between visits with ongoing personalised feedback from a health professional (Kew 2015a).
Objectives
To assess the safety and efficacy of conducting asthma check‐ups remotely versus usual face‐to‐face consultations.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomised controlled trials (RCTs) of any duration. We included studies reported as full‐text articles, those published as an abstract only and unpublished data.
Types of participants
We included studies of adults or children with a diagnosis of asthma. We excluded studies that recruited participants with other long‐term health conditions, unless they presented data for people with asthma separately.
Types of interventions
We included trials that compared remote check‐ups conducted with any form of technology (e.g. telephone calls, video‐conferencing) versus standard face‐to‐face check‐ups. We included trials which compared the two types of check‐up on top of education or another co‐intervention. We excluded trials that used automated telehealth interventions and did not include personalised contact with a health professional.
Types of outcome measures
Primary outcomes
-
Exacerbations that required oral corticosteroidsa.
-
Asthma control (measured on a validated scale, e.g. the Asthma Control Questionnaire (ACQ)).
-
Serious adverse events (including mortality).
aIf trials reported exacerbations in a different way (e.g. required hospital emergency department (ED) visit), we analysed these separately.
Secondary outcomes
-
Asthma‐related quality of life (measured on a validated scale, e.g. the Asthma Quality of Life Questionnaire (AQLQ)).
-
Unscheduled healthcare visits.
-
Lung function (trough forced expiratory volume in one second (FEV1) preferred).
-
Adverse events/side effects.
Reporting of one or more of the outcomes listed here in the trial was not an inclusion criterion for this Cochrane review.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Review Group's Specialised Register (CAGR), which is maintained by the Information Specialist for the Cochrane Airways Review Group. The CAGR contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearches of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We used the search strategy in Appendix 2 to search for all records in the CAGR.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Portal (www.who.int/ictrp/en/) limited to interventional studies, using the condition term 'asthma', intervention terms 'remote OR telehealth OR telemedicine OR internet OR web', and title terms 'NOT education'. We searched all databases from their inception to 24 November 2015, and did not impose any restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references.
We searched for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed) on 20 July 2015.
Data collection and analysis
Selection of studies
Two review authors (KK and CJC) independently screened titles and abstracts for inclusion of all the potential studies identified from the literature searches and coded them as either 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication and two review authors (KK and CJC) independently screened the full‐text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we piloted on at least one study included in the review. One review author (KK) extracted the following study characteristics from included studies.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals and date of study.
-
Participants: number of participants, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: trial funding and notable conflicts of interest of the trial authors.
Two review authors (KK and CJC) independently extracted outcome data from the included studies. We noted in the 'Characteristics of included studies' table if the study authors did not report outcome data in a usable way. We resolved any disagreements by consensus. One review author (KK) transferred data into the Review Manager (RevMan) (RevMan 2014) file. We double‐checked that KK entered data correctly by comparing the data presented in the systematic review with the study reports. A second review author (CJC) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (KK and CJC) independently assessed the risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as either 'high', 'low' or 'unclear', and provided a quote from the study report together with a justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each domain listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When we considered treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of risk of bias in conducting the systematic review
We conducted the review according to the published Cochrane protocol, Kew 2015b, and reported any deviations from it in the 'Differences between protocol and review' section.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) and continuous data as either mean difference or standardised mean difference values. We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We narratively described skewed data reported as medians and interquartile ranges.
Where a single trial reported multiple trial arms, we only included the relevant trial arms. If the trial combined two comparisons (e.g. drug A versus placebo and drug B versus placebo) in the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
For dichotomous outcomes we used participants, rather than events, as the unit of analysis (i.e. number of adults admitted to hospital, rather than number of admissions per adult). However, if studies reported exacerbations as rate ratios, we analysed them on this basis. We did not anticipate the inclusion of cluster RCTs and hence presented a large two‐cluster implementation study, Pinnock 2007a, separately from the other studies. For the purposes of display in the analyses, we have referred to Pinnock 2007a as the 'cluster implementation study' and Chan 2007, Gruffydd‐Jones 2005, Pinnock 2003 and Rasmussen 2005 as the 'efficacy RCTs'. There were only two clusters so we included the data with participants as the unit of analysis. Although we presented the results of the cluster RCT on forest plots with the other studies, we did not pool the effects so weighting of the cluster trial within the analysis was not an issue. We presented Hashimoto 2011 in a separate comparison as the study focused on tapering OCS dose.
Dealing with missing data
We contacted the study authors or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when we identified a study as an abstract only). Where this was not possible, and we thought the missing data introduced serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity we reported it and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
If we were able to pool more than 10 trials, we created and examine a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model for all analyses, as we expected variation in effects due to differences in study populations and interventions. We performed sensitivity analyses with a fixed‐effect model when heterogeneity was statistically significant.
'Summary of findings' table
We created a 'Summary of findings' table using the seven outcomes listed above. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011, and used GRADEpro Guidelines Development Tool (GDT) software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of the evidence in footnotes, and we made comments to aid the reader's understanding of the Cochrane review, where necessary.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses for the primary outcomes, where there was a sufficient number of included studies.
-
Mean age (less than 16 years, 17 to 65 years, and greater than 65 years).
-
Type of technology (telephone calls, text‐messages, emails).
We used the formal test for subgroup interactions in Review Manager (RevMan) (RevMan 2014).
Sensitivity analysis
We carried out the following sensitivity analyses by exclusion of the following from the primary analyses.
-
Studies that recruited people with severe or life‐threatening asthma.
-
Unpublished data (obtained from trial authors or from conference abstracts).
-
Studies at high risk of selection biasa.
aInadequate selection procedures may result in unbalanced baseline characteristics between groups which could skew the data. Due to the nature of the studies, we anticipated that all or most included studies would be at high risk of performance or detection bias, so we discussed the possible effect of lack of blinding, in particular for subjective outcomes.
Results
Description of studies
Results of the search
We performed searches up to 24 November 2015. We identified 591 records from the Cochrane Airways Review Group's Specialised Register (CAGR). We also examined a total of 750 additional records, comprised of an older database search (n = 710), clinicaltrials.gov records (n = 29) and the World Health Organization (WHO) International Clinical Trials Registry Portal (ICTRP) (n = 11). After we removed duplicates, we screened the remaining 823 records and excluded 673 by looking at titles and abstracts alone. We retrieved full texts for the remaining 150 records and excluded 126 (collated into 95 studies). The other 25 records met all the inclusion criteria and we collated them as six included studies. We have presented a study flow diagram and the reasons for exclusion in Figure 1.

Study flow diagram
Included studies
Six studies including a total of 2100 participants met the inclusion criteria: we pooled four studies including 792 people in the main efficacy analyses, and presented the results of a cluster implementation study (n = 1213) and an oral steroid tapering study (n = 95) separately. We have presented a summary of study, participant and intervention characteristics in Table 1, and more details for each individual study are in the 'Characteristics of included studies' tables.
| Study ID | Total N | Country | Duration | Mean age | % male | % FEV1 | Intervention | Control |
| 120 | Hawaii, USA | 12 months | 9.6 | 62.5 | 100.5 | In‐home, website‐based case management and education. | In‐person education and case management. | |
| 194 | UK | 12 months | 50.2 | 45.4 | NR | 6‐monthly phone calls from trained asthma nurses. Formulation of individual AAP. | 6‐monthly usual face‐to‐face appointment with an asthma nurse. Symptoms, peak flow and inhaler technique checked, and participants were issued with an AAP. | |
| 95 | The Netherlands | 6 months | 50.1 | 45.3 | 73.9 | OCS dose adjustment guided by an internet‐based diary, decision support and monitoring with support from a study nurse. | OCS dose adjustment according to GINA by the specialist. | |
| 278 | UK | 3 months | 55.5 | 41.4 | NR | Telephone check‐up with the asthma nurse. | Face‐to‐face check‐ups in the surgery with the asthma nurse. | |
| 1728 | UK | 12 months | 42.6 | 44.6 | NR | Three invitations to book either a telephone or face‐to‐face check‐up. Non‐attenders were phoned and reviewed opportunistically. | Three invitations to book a face‐to‐face check‐up. Non‐attenders were not phoned opportunistically. | |
| 300 | Denmark | 6 months | 29 | 34.5 | 92.0 | Participants were given an AAP, online electronic diary and peak flow meter. Physicians gave participants instructions via e‐mail or telephone aided by computer decision support. | The specialists taught the participants how to adjust their medication on the basis of a peak flow meter and AAP. |
Total N: the total number of participants randomised in the study, included to groups not analysed in this Cochrane review
% FEV1: the baseline mean of the predicted normal values
Abbreviations: AAP = asthma action plan; NR = not reported; OCS = oral corticosteroids
Design, setting and duration
Three included studies were conducted in the UK (Gruffydd‐Jones 2005; Pinnock 2003; Pinnock 2007a), one in Hawaii, USA (Chan 2007), one in the Netherlands (Hashimoto 2011) and one in Denmark (Rasmussen 2005). Five studies were randomised controlled trials (RCTs) that lasted six or 12 months, and Pinnock 2007a was a before‐and‐after implementation study which randomised three practices rather than individual participants.
The design of Pinnock 2007a differed from the other studies in several respects. Firstly, it was cluster randomised which had an effect on the unit used for analysis and could not completely control for potential group differences between the practices. Secondly, it was a before‐and‐after design which meant the number and type of people on the practice lists was different at the two time points. Thirdly, the intervention offered a telephone check‐up as an option for asthma review so the study did not make a clean comparison between remote and face‐to‐face check‐ups, especially since most people in the telephone group did not choose that option. Fourthly, the primary aim of the study was to increase the number of people having a check‐up at all and so people in the intervention group were phoned opportunistically on top of being offered a phone check‐up, and this additional effort to contact participants may have been a confounding factor on the other study outcomes. We considered the study to be important so did not exclude it, and instead presented the study results separately from the main comparison for each outcome.
Population characteristics and inclusion criteria
The number of participants in each trial ranged from 95 in Hashimoto 2011 to 1728 in Pinnock 2007a; the median number of participants recruited was 236 and the total who received phone or face‐to‐face check‐ups was 2100. This was complicated by the design of Pinnock 2007a which assigned three GP practices rather than individual participants, although we will discuss the number of people for the descriptive purposes. Five studies recruited adults with a lower age limit of 17 or 18 and an upper age limits of between 45 (Rasmussen 2005) and 75 (Hashimoto 2011). Mean age of participants in the adult trials ranged from 29 to 55.5 years (median 50.1). One study recruited children between the ages of six and 17 years, and had a mean age of 9.6 years (Chan 2007). Studies included slightly more females than males (range 34.5% to 62.5% male, median 45%). Baseline characteristics related to the asthma severity were patchy and variable across studies. Hashimoto 2011 recruited people with more severe asthma than the other included studies; the mean percentage predicted FEV1 at baseline was 73.9%. Two other studies reported this measure of baseline severity at 92% (Rasmussen 2005) and 100.5% (Chan 2007).
In general the included studies did not describe the inclusion and exclusion criteria in great detail, and had varying requirements for the diagnosis and classification of asthma in their participants. With the exception of Hashimoto 2011, the studies recruited from the practice or clinic lists of their participating centres. Pinnock 2003 and Pinnock 2007a required participants to have received a prescription for asthma medications within the previous six and 12 months respectively, and both excluded participants with chronic obstructive disease. Pinnock 2003 further required participants to have been diagnosed with asthma for at least a year. Other inclusion criteria were related to computer or telephone access, and exclusion criteria were related to social, communication or medical difficulties that might preclude involvement in the intervention. Hashimoto 2011 only recruited participants on daily oral corticosteroids, and its inclusion criteria differed from the other studies. This study required participants to have a diagnosis of severe refractory asthma according to the major and minor criteria recommended by the American Thoracic Society (ATS 2000), and for their asthma to be uncontrolled despite intensive follow‐up by an asthma specialist for at least a year, chronic treatment with oral corticosteroids and high doses of inhaled steroids and long‐acting bronchodilators.
Interventions and comparisons
The interventions received in the active and comparison groups varied in several respects across studies, in particular regarding the length of observation, amount of professional contact and method of communication.
Four studies were designed to test remote check‐ups with standard face‐to‐face care. Chan 2007 was the only child study, and tested an in‐home website case management and education programme against an in‐person equivalent for 12 months. Participants in the active group of Gruffydd‐Jones 2005 received phone calls every six months from trained asthma nurses and those in the intervention group had equivalent face‐to‐face check‐ups with the asthma nurse. Both groups were given a personalised asthma action plan and discussed symptoms, peak flow and inhaler technique. Pinnock 2003 was described as a pragmatic RCT where participants were offered a telephone check‐up or a face‐to‐face consultation in the surgery, both with an asthma nurse. One of the study's main aims was the uptake of check‐ups with either method within three months of randomisation. Rasmussen 2005 was a six‐month study where physicians gave participants instructions via email or over the phone based on an agreed asthma action plan, an online diary and peak flow measurements uploaded by the participant. The comparison group received face‐to‐face instruction from an asthma specialist on how to adjust medication based on their asthma action plan and peak flow measurements.
As described above, Pinnock 2007a was a 12‐month implementation study where participants were given a structured recall with a choice of telephone or face‐to‐face for their asthma check‐up or a structured recall with no choice of a telephone check‐up (i.e. face‐to‐face only). We did not include a usual‐care group not subject to the methods to control for bias in the main comparison in this review. We chose to present the results of this study alongside the results of the four studies above, but in a separate subgroup so we did not pool the data with the main comparison. In addition to the choice of a telephone check‐up, people in the intervention group were called opportunistically if they did not respond or attend, which did not happen in the control group.
Hashimoto 2011 compared face‐to‐face check‐ups with internet‐delivered check‐ups for the specific purpose of tapering long‐term oral corticosteroid therapy (OCS), and for this reason we analysed the study as a separate comparison. The study was designed to test the effectiveness of a six‐month programme of OCS adjustment either via an internet diary and associated monitoring from an asthma nurse or via face‐to‐face check‐ups with a specialist according to Global Initiative for Asthma (GINA) guidelines.
Excluded studies
We excluded 125 records after viewing full texts, which we collated to represent 94 unique studies. The most common reason for exclusion was that the intervention did not meet the inclusion criteria (82 studies). Within this explanation, we explored the reasons and found that we often excluded studies because they used technology to facilitate self‐monitoring between usual face‐to‐face check‐ups (n = 22), and these studies meet the inclusion for another Cochrane review (Kew 2015a). Other excluded studies with interventions that did not meet the inclusion criteria used technology as education rather than for asthma check‐ups (n = 14), as automated monitoring systems without involvement from a health professional (n = 8), to validate an asthma questionnaire (n = 7), to improve or monitor adherence (n = 7), or to deliver a range of other interventions that did not match the remit of this review (including technology delivered counselling or behavioural interventions, parenting advice and monitoring interventions delivered without technology). We excluded eight studies as they were not RCTs (n = 8), and four because they made the wrong comparison (n = 4).
Risk of bias in included studies
We have shown a summary of the 'Risk of bias' judgements in Figure 2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
We rated all the included studies at low risk of bias for random sequence generation as they described the randomisation methods, such as centralised systems, random numbers tables or coin toss. We judged two studies at low risk of bias for allocation concealment after the study authors responded to our request to clarify the study methods. Also we considered one study at unclear risk of bias because it did not adequately describe the methods. Regarding Pinnock 2007a, the cluster implementation study, we rated it at high risk of bias for allocation concealment because of its two‐cluster design.
Blinding
Due to the nature of the interventions, none of the included studies were able to blind participants and personnel to group allocation (performance bias). For this reason, we chose to assess blinding of participants and personnel separately for the subjective and objective outcomes. In each study, we rated the subjective outcomes (Asthma Control Questionnaire (ACQ) and Asthma Quality of Life Questionnaire (AQLQ)) at high risk of bias and the objective outcomes (exacerbations, adverse events, FEV1) at low risk of bias.
While it was possible for studies to blind outcome assessors (detection bias), no included study described the procedures to do so. Gruffydd‐Jones 2005, Hashimoto 2011 (through personal communication) and Rasmussen 2005 explicitly stated that the outcomes assessors were not blinded to allocation so we rated them at high risk of bias, and the remaining studies as unclear.
Incomplete outcome data
We considered two included studies at low risk of bias (Pinnock 2003; Pinnock 2007a). The former had low and even dropout, and the latter was a real‐world implementation study where uptake rate was an integral part of the study. We rated four studies at high risk of bias due to incomplete outcome data, either because dropout was high or unbalanced between groups or missing data had not been sufficiently imputed to account for those not in the study at the end (or both) (Chan 2007; Gruffydd‐Jones 2005; Hashimoto 2011; Rasmussen 2005). Due to the nature of the question being posed in the studies, several were run in a real‐world context which made it more difficult to control for participants dropping out.
Selective reporting
There was no evidence of selective reporting in the six included studies so we rated them at low risk of bias. Where outcomes were unavailable in the published reports, the trial authors were able to provide the additional data or confirm they had not been measured.
Other potential sources of bias
We rated Pinnock 2007a at high risk of bias for several reasons related to its cluster before‐and‐after study design. It randomised two practices to the interventions which would not have controlled for baseline imbalances in the same way as individual randomisation, and this meant the participant population in each group was not static. The intervention was a telephone option and many in that practice opted for a usual face‐to‐face check‐up, which meant the study did not make a direct comparison of remote and face‐to‐face check‐ups. Additionally, people in the telephone option group were phoned opportunistically to increase uptake of check‐ups, which did not happen in the face‐to‐face group. These factors mean we cannot be certain that the mode of check‐up, and not the increased likelihood of being seen, was the variable measured and we considered this study as having a high risk of bias. For these reasons, we chose to present the study alongside the others but not to pool its results in the main analyses.
We did not identify any other sources of bias in the five other included studies, which we rated as having a low risk of bias.
Effects of interventions
See: Summary of findings for the main comparison Summary of findings table
We have presented data from studies that made a direct comparison between remote and face‐to‐face check‐ups as the main results (referred herein as the 'efficacy studies', and supplemented these by the results from the large two‐cluster implementation study, Pinnock 2007a. We have described data from the OCS tapering study, Hashimoto 2011, as a separate comparison below.
Primary outcomes
Exacerbations that required oral corticosteroids
One efficacy study reported the number of people who needed a course of oral corticosteroids for an exacerbation of asthma (Pinnock 2003). The confidence intervals (CIs) were very wide due to the small number of events in the analysis (odds ratio (OR) 1.74, 95% CI 0.41 to 7.44; 278 participants). In the face‐to‐face check‐up group, 21 people out of 1000 had exacerbations requiring oral steroids over three months, compared to 36 (95% CI nine to 139) out of 1000 in the remote group. We downgraded the evidence to low quality due to imprecision. The effect from the cluster implementation study was much more precise and favoured face‐to‐face check‐ups (OR 1.43, 95% CI 1.04 to 1.97); we have shown the two effects together in Analysis 1.1.
Exacerbations that required emergency department (ED) visit and hospital admission
The effect for exacerbations that required treatment in the ED also favoured face‐to‐face check‐ups over those conducted remotely, but this was uncertain due to the wide CIs from a small number of events (OR 2.60, 95% CI 0.63 to 10.64; 651 participants; three studies; Analysis 1.2). The result of the cluster implementation study was much smaller but also very imprecise (OR 1.19, 95% CI 0.38 to 3.71; 1212 participants; one study). Too few people in the efficacy studies had exacerbations that required a hospital admission to detect any difference between the two types of check‐up (Peto OR 0.63, 95% CI 0.06 to 6.32; 651 participants; three studies; Analysis 1.3), and the cluster implementation study showed a possible but not statistically significant benefit of face‐to‐face check‐up (Peto OR 2.18, 95% CI 0.83 to 5.69; 1213 participants; one study).
Asthma control
There was no difference in scores on the Asthma Control Questionnaire (ACQ) between participants in the remote and face‐to‐face groups (mean difference (MD) −0.07, 95% CI −0.35 to 0.21; 146 participants; one study; Analysis 1.4). Both CIs were within the minimal clinically important difference (MCID) for the scale (MCID = 0.5). We downgraded the quality of the evidence to moderate quality due to risk of bias because the outcome was a subjective rating scale that may have been affected by the inability to blind participants and personnel to group allocation. While the effect from the cluster implementation study was more in favour of remote check‐ups than the efficacy studies, the estimate and its CIs were still within the MCID for the scale so the difference was unimportant.
Serious adverse events (including mortality)
Only the cluster implementation study reported serious adverse events (SAEs) and showed a higher number in participants in the remote groups, although the CIs did not exclude the possibility that they were more common with face‐to‐face check‐ups (OR 1.80, 95% CI 0.73 to 4.44; 1213 participants; one study; Analysis 1.5). Given that SAEs are usually defined as those requiring hospital admission, it is likely that most of these events were the exacerbations requiring hospital admission described above.
Subgroup analyses
We were unable to conduct meaningful subgroup analyses on the basis of age and type of technology as planned, as there was an insufficient number of included studies to do this.
Sensitivity analyses
Severe or life‐threatening asthma
It was not necessary to conduct this sensitivity analysis because only Hashimoto 2011 specifically recruited people with severe or life‐threatening asthma and we analysed this study on its own due to the nature of the intervention.
Unpublished data
The trial author of Pinnock 2007a provided additional data for exacerbations requiring oral steroids, but we did not pool the study effect with the other study in the analysis so there was no basis for a sensitivity analysis.
High risk of selection bias
None of the included studies were at high risk of selection biases so we did not perform the planned sensitivity analysis.
Secondary outcomes
Asthma‐related quality of life
There was no difference between remote and face‐to‐face check‐ups on the Asthma Quality of Life Questionnaire (AQLQ) (MD 0.08, 95% CI −0.14 to 0.30; 544 participants; three studies; Analysis 1.6). While the effect was marginally in favour of remote check‐ups, both CIs were within the 0.5 minimal clinically important difference on the scale. As with the ACQ, we downgraded the evidence to moderate quality for risk of bias because the outcome was subjective and may have been affected by lack of blinding. The point estimate in the implementation study lay marginally in the opposite direction but the result was not inconsistent given the MCID (−0.02, 95% CI −0.23 to 0.19; 536 participants; one study).
Rasmussen 2005 also reported the AQLQ but the data were skewed and analysed non‐parametrically so we could not combine it with the other studies in the meta‐analysis (internet group median 6.42, range 4.11 to 7.00; specialist group median 6.17, range 3.98 to 7.00; GP group median 6.31, range 1.41 to 7.00). Chan 2007 also reported the parent version of the AQLQ with the following scores (remote group: mean 6.4, SD = 1, N = 60; face‐to‐face group: mean 6.2, SD = 0.8, N = 60). We considered the child version to be more similar to the way the scores were taken in the other studies which is why we included the child and not the parent scores in the meta‐analysis.
Unscheduled healthcare visits
The pooled estimate from two efficacy studies was based on very few events and was too imprecise to draw a conclusion (OR 0.91, 95% CI 0.45 to 1.85; 531 participants; two studies; Analysis 1.7). The direction and magnitude of the effect from the implementation study were consistent but the estimate was more precise (OR 0.95, 95% CI 0.75 to 1.21; 1213 participants; one study).
Lung function (trough FEV1 preferred)
Only one efficacy study reported trough FEV1 (mL), and showed a bigger improvement in the remote check‐up group (MD 166.76, 95% CI 78.03 to 255.50; 253 participants; one study; Analysis 1.8). We downgraded the evidence for imprecision to moderate quality because it was based on only 253 people, even though the confidence intervals excluded the possibility that face‐to‐face check‐ups were better. The implementation study was not designed to measure lung function.
Adverse events/side effects
Studies generally did not report adverse events separately from the asthma exacerbation and resource use outcomes. One study author confirmed that no participants in either group experienced adverse events, which did not allow us to display an effect estimate.
Remote versus face‐to‐face check‐up for oral corticosteroid tapering ‐ analysis of Hashimoto 2011
We chose to assess Hashimoto 2011 separately from the other studies as it was specifically aimed at assessing remote versus face‐to‐face check‐ups to guide dose reduction for people with asthma taking long‐term oral corticosteroids.
Mostly the CIs were too wide to tell whether one type of check‐up was better than the other at reducing harmful outcomes that might occur as a result of withdrawing oral steroids, or to say that they were equivalent. This was true for exacerbations requiring hospital admission (OR 0.88, 95% CI 0.25 to 3.13; Analysis 2.1), unscheduled healthcare visits (OR 2.31, 95% CI 0.23 to 23.14; Analysis 2.4) and adverse events (OR 1.51, 95% CI 0.13 to 17.29; Analysis 2.5). Scores on the ACQ (MD 0.14, 95% CI −0.15 to 0.43; Analysis 2.2) and AQLQ (MD −0.17, 95% CI −0.49 to 0.15; Analysis 2.3) were slightly better in the face‐to‐face group than those who were managed remotely, but the difference was below the minimal clinically important difference on both scales and the CIs did not exclude the possibility that remote check‐ups were better.
Discussion
Summary of main results
Six studies met the inclusion criteria (including a total of 2100 participants): we pooled four studies in the main efficacy analyses, which randomised 792 people to remote or face‐to‐face check‐up. In addition we also presented the results of a cluster implementation study (n = 1213) alongside the main results but did not pool them with the other included studies, and assessed one study that compared the two types of check‐up for oral steroid tapering in severe refractory asthma as a separate comparison (n = 95). Baseline characteristics relating to asthma severity were variable, but studies generally recruited people with asthma who took regular medications and excluded those with chronic obstructive pulmonary disease (COPD) or severe asthma. The studies could not be blinded and dropout was high in four of the six included studies, which may have biased the results.
We cannot say whether more people in the remote groups needed oral corticosteroids for an asthma exacerbation than those seen face‐to‐face because the confidence intervals (CIs) were very wide (OR 1.74, 95% CI 0.41 to 7.44; 278 participants; one study; low quality evidence). In the face‐to‐face check‐up groups, 21 people out of 1000 had exacerbations that required oral steroids over three months, compared to 36 (95% CI nine to 139) out of 1000 for the remote check‐up group. Exacerbations that needed treatment in the hospital emergency department (ED), hospital admission or an unscheduled healthcare visit happened too infrequently to detect whether remote check‐ups are a safe alternative to face‐to‐face consultations. Serious adverse events were not reported separately from the exacerbation outcomes.
There was no difference in asthma control measured by the Asthma Control Questionnaire (ACQ) or in quality of life measured on the Asthma Quality of Life Questionnaire (AQLQ) between remote and face‐to‐face check‐ups. We ruled out the significant harm of remote check‐ups for these outcomes but we were less confident because these outcomes are more prone to bias from lack of blinding.
The larger implementation study that compared two general practice populations demonstrated that offering telephone check‐ups and proactively phoning participants increased the number of people with asthma who received a review. However, we do not know whether the additional participants who had a telephone check‐up subsequently benefited in asthma outcomes.
Overall completeness and applicability of evidence
The design of this Cochrane review allowed us to focus on the specific question of whether regular asthma check‐ups can effectively be conducted remotely as opposed to face‐to‐face. By honing in on this form of telehealth, we were able to assess the evidence for a clearly defined application of technology‐based care that was more likely to lead to evidence that could be readily applied to real world settings. However, it did limit our assessments of possible effect moderators, such as age and type of technology, due to the small number of studies that met the inclusion criteria and reported the primary outcomes.
As described in the 'Included studies' section, Pinnock 2007a was a real‐word implementation study that differed from the other included studies in several respects. We chose to include this study because it addressed the feasibility of remote check‐ups in a real‐world setting, despite its design being dissimilar to the other included studies. By not pooling it with the other studies but presenting the results alongside them, we hoped to highlight the possible differences in a controlled comparison of remote and face‐to‐face check‐ups and what might actually happen in practice. While Pinnock 2007a's results do not lead to the cause and effect inferences that can be made from the efficacy RCTs, it does give an important insight into the relative merits of a possible incorporation of remote check‐ups into practice, and we consider the review to be more complete as a result. We did not set out to assess the possible benefit of improving access by offering remote check‐ups, especially for patients who are less likely to attend their annual check‐up. This is an important factor that can be addressed by implementation studies, such as Pinnock 2007a, better than classic randomised controlled trials (RCTs). Unfortunately, because there were only two clusters, the study results must be interpreted with caution and with the caveats both in this Cochrane review and in the study itself.
One efficacy study contributed data to the exacerbations requiring oral steroids analysis and this was provided by the study author (Pinnock 2003). It was not always possible to distinguish between courses of oral steroids for immediate treatment or future use (as part of an action plan). This highlights the importance of contacting study authors for additional data, as their own primary outcomes may differ from those of the review. This increased the completeness of the evidence base and our confidence in the results.
Quality of the evidence
We rated the evidence in this Cochrane review as low or moderate quality, mostly due to imprecision or risk of bias. We chose to assess blinding for the subjective outcomes separately from the objective outcomes. By differentiating the outcomes in this way, we were able to assess the effect lack of blinding was likely to have had on the confidence we have in the results. For the objective outcomes, we noted that the study designs did not allow for blinding of participants and personnel, but judged that this lack of blinding is unlikely to have made a difference to the number of people who had exacerbations, adverse events and lung function. There is a possibility that the lack of blinding may have still affected the way these outcomes were recorded but we did not consider this to sufficient to downgrade the evidence. For the subjective outcomes, it is more likely that the participant or investigators' knowledge of group allocation could have affected the way they responded to the asthma control and quality of life questionnaires, so we downgraded these outcomes for risk of bias.
We downgraded five outcomes for imprecision, including the primary outcome. We were fairly certain in the direction of the effect for the primary outcome, exacerbations requiring oral steroids, but there was only one efficacy study in the analysis and seven events, which reduced our confidence in the size and precision of the estimate significantly. Exacerbations that required an ED visit or a hospital admission, and unscheduled healthcare visits were all based on a very small number of events, which crucially meant we could not rule out significant harm of conducting remote check‐ups for people with asthma. Finally, we downgraded trough FEV1 for imprecision for a similar reason to the primary outcome: the effect was in favour of remote check‐ups, but the estimate was based on just one study of 253 people, so we weren't confident in the result. Visually, there was imprecision in the estimates for asthma control and quality of life, but the CIs for both were well with the established minimal clinically important differences for the scales (Juniper 1994; Juniper 1999).
We did not downgrade any of the outcomes for publication bias, inconsistency in the results or indirectness of the evidence. While not all studies reported the outcomes we were interested in, this was more likely to reflect the individual practices and designs of the studies (e.g. unable to measure lung function remotely) rather than selective reporting of outcome data. There was minimal statistical inconsistency in the analyses, and while there was a fair amount of variation in the aims and designs of the studies, we considered them all to match the PICO set out in the protocol for this review (Kew 2015b).
Potential biases in the review process
We made every effort to adhere to Cochrane methods during the review process. Both review authors extracted numerical data, performed 'Risk of bias' ratings in duplicate, and cross checked for accuracy and consistency. Throughout the process, we resolved any discrepancies through discussion. Neither review author has any conflicts of interest relating to this Cochrane review.
We performed broad literature searches that we independently screened in duplicate, and included studies regardless of language of publication or the existence of a full‐text paper. We also conducted comprehensive additional searches to identify unpublished studies that were not listed in the main electronic databases. It is unlikely that we missed studies during the study selection process, except for those not listed in non‐English language databases. We made every effort to contact study authors, although in some cases this was difficult due to when the studies were conducted and the effect this had both on the availability of contact details and the likelihood we could obtain data. We received detailed replies from two study authors which affected several analyses and the completeness of the 'Risk of bias' information.
Agreements and disagreements with other studies or reviews
Systematic reviews of the evidence for remote asthma check‐ups have generally had much broader inclusion criteria, assessing the use of any kind of telehealthcare for monitoring or educating people with asthma. As such, it is difficult to compare the results of this Cochrane review with their results because we designed this systematic review to assess specifically the use of technology systems to conduct asthma check‐ups remotely. For example, McLean 2010 included 21 studies that assessed a range of telehealth interventions against a range of control groups, and as such it was difficult to meta‐analyse the data in a way that led to conclusions with real‐world implications.
Systematic reviews that examined telehealth more broadly for asthma have generally approached the question from a different standpoint from our own. Other reviews have focused on possible benefits of remote healthcare over usual face‐to‐face care, whereas we were more concerned with highlighting the potential dangers of removing face‐to‐face contact with a healthcare professional. Conclusions made by McLean 2010 regarding the lack of benefit for relatively mild asthma and Zhao 2014 regarding the lack of improvements on asthma function scores do not disagree with our own, but are framed from the former standpoint. This is at least partly because these two reviews did not set out to compare remote or 'telehealthcare' against a consistent alternative form of monitoring. For most outcomes we looked at, we cannot conclude that remote check‐ups definitely lead to worse outcomes than face‐to‐face care, but neither can we conclude that remote check‐ups are a safe alternative to face‐to‐face care for either mild or severe asthma.
In their conclusions, Jaana 2009 focused on the attitudes and receptiveness of home telemonitoring for patients with respiratory illness, which we did not set out to assess, and commented on the "variations in study approaches and an absence of robust study designs and formal evaluations". We agree that the inherent differences in health systems and study designs continue to limit conclusions in this field, even when review questions are refined to one part of 'telehealthcare'.
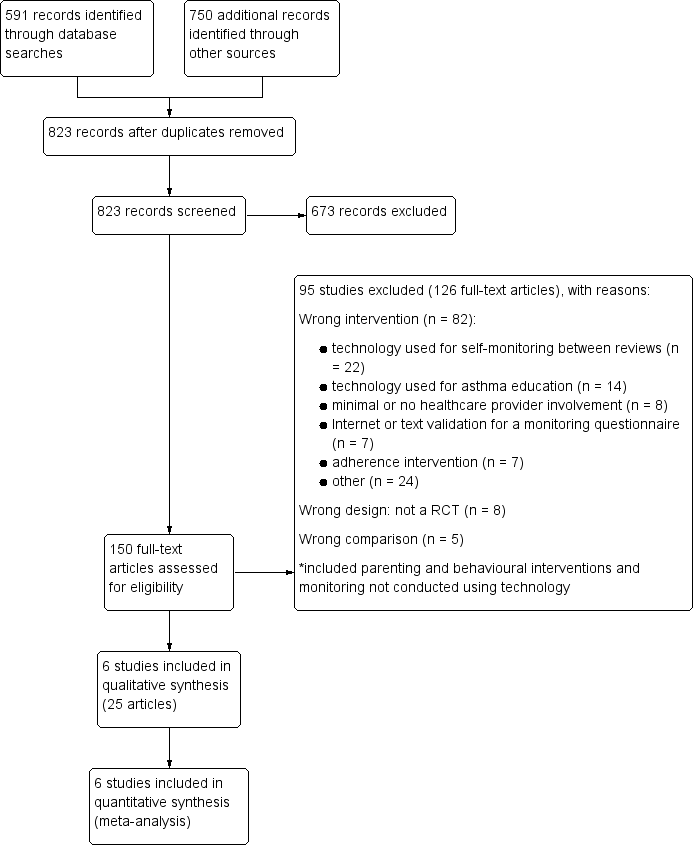
Study flow diagram

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
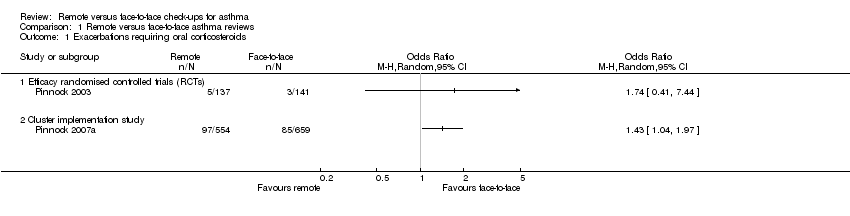
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 1 Exacerbations requiring oral corticosteroids.
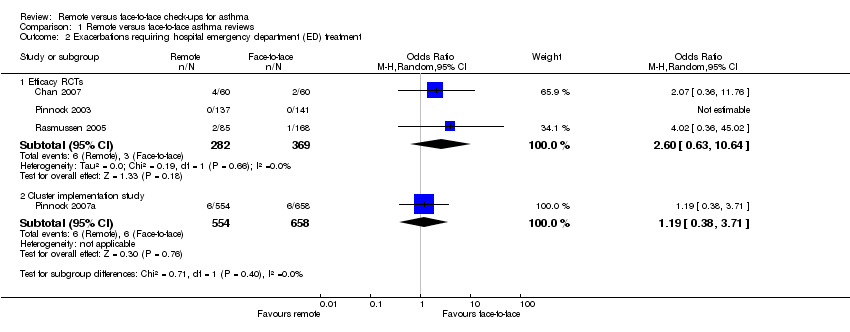
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 2 Exacerbations requiring hospital emergency department (ED) treatment.

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 3 Exacerbations requiring hospital admission.

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 4 Asthma control (Asthma Control Questionnaire (ACQ)).
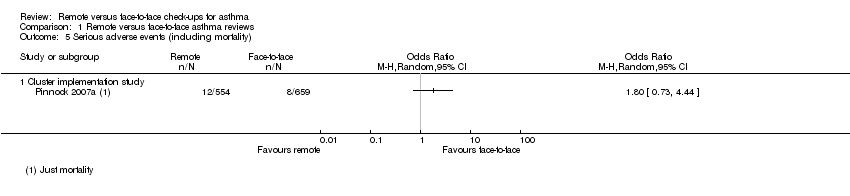
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 5 Serious adverse events (including mortality).

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 6 Asthma‐related quality of life (Asthma Quality of Life Questionnaire (AQLQ).
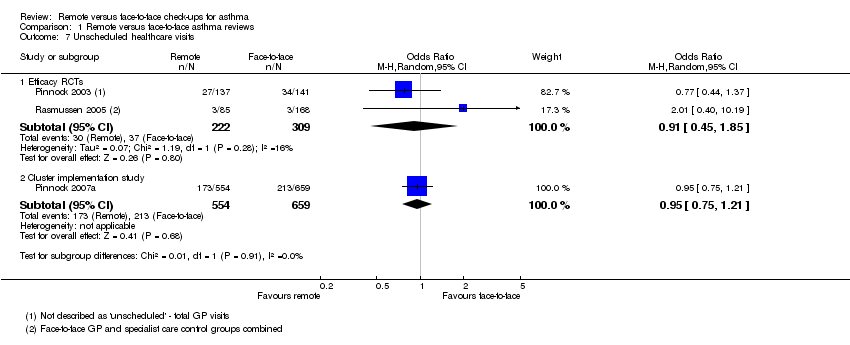
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 7 Unscheduled healthcare visits.

Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 8 Change in lung function (trough FEV1).
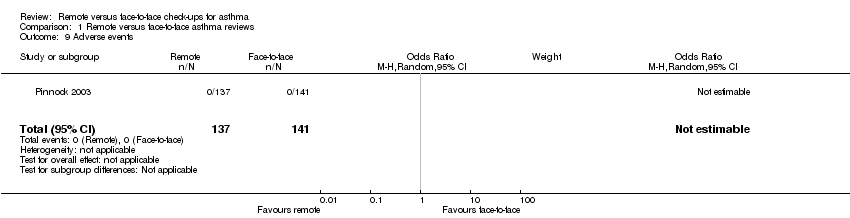
Comparison 1 Remote versus face‐to‐face asthma reviews, Outcome 9 Adverse events.

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 1 Exacerbations requiring hospital admission.

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 2 Asthma control (ACQ).

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 3 Asthma‐related quality of life (AQLQ).
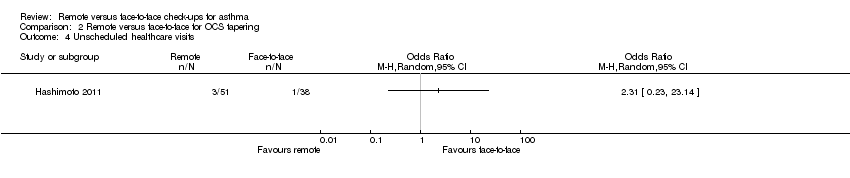
Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 4 Unscheduled healthcare visits.

Comparison 2 Remote versus face‐to‐face for OCS tapering, Outcome 5 Adverse events.
| Remote versus face‐to‐face check‐ups for asthma | ||||||
| Patient or population: adults or children with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with face‐to‐face check‐ups | Risk with remote check‐ups | |||||
| Exacerbations requiring oral corticosteroids | 21 per 1000 | 36 per 1000 | OR 1.74 | 278 | ⊕⊕⊝⊝ | Very imprecise. Data from the implementation study** were consistent. |
| Exacerbations requiring hospital admission 6 months | 5 per 1000 | 3 per 1000 | Peto OR 0.63 | 651 | ⊕⊕⊝⊝ | Very few events ‐ no conclusion could be drawn. The implementation study was more in favour of face‐to‐face check‐ups. |
| Asthma control (ACQ) Scale 0 to 6; lower is better | The mean ACQ score with face‐to‐face check‐ups improved by 0.11 | The mean ACQ score with remote check‐ups improved by 0.07 more (0.35 more to 0.21 less) | ― | 146 | ⊕⊕⊕⊝ | No difference and CIs ruled out significant harm of remote check‐ups (MCID for the ACQ is 0.5). The implementation study results were consistent. |
| Serious adverse events (including mortality) | ― | ― | ― | 0 RCTs | ― | No efficacy studies reported all‐cause SAEs. The implementation study recorded 12/554 and 8/659 in the remote and face‐to‐face groups respectively (OR 1.80, 95% CI 0.73 to 4.44) |
| Asthma‐related quality of life (AQLQ) Scale 1 to 7; higher is better 8 months | The mean AQLQ score with face‐to‐face check‐ups was 5.49 | The mean AQLQ score with remote check‐ups was 0.08 better (0.14 worse to 0.30 better) | ― | 544 | ⊕⊕⊕⊝ | No difference and CIs ruled out significant harm of remote check‐ups (MCID for the AQLQ is 0.5). The implementation study results were consistent. |
| Unscheduled healthcare visits 5 months | 120 per 1000 | 110 per 1000 | OR 0.91 | 531 | ⊕⊕⊝⊝ | Very few events ‐ we could not draw any conclusions. The implementation study was more precise and did not show a difference. |
| Lung function (trough FEV1) 6 months | The mean trough FEV1 with face‐to‐face check‐ups was 20 mL | The mean trough FEV1 with remote check‐ups was 166.76 mL better (78.03 more to 255.5 more) | ― | 253 | ⊕⊕⊕⊝ | People having remote check‐ups had better lung function than those seen face‐to‐face in the one study that measured it. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk with face‐to‐face check‐ups for continuous outcomes was calculated as a weighted mean of the face‐to‐face values. **The 'Implementation study', Pinnock 2007a, had a two‐cluster pragmatic design and was not pooled with the rest of the included studies (efficacy studies). Durations were calculated as a weighted mean duration of the studies contributing data to the analysis. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies were at high risk of bias for one or more blinding domains but it is unlikely that this had an effect on the objective outcomes (no downgrade). | ||||||
| Study ID | Total N | Country | Duration | Mean age | % male | % FEV1 | Intervention | Control |
| 120 | Hawaii, USA | 12 months | 9.6 | 62.5 | 100.5 | In‐home, website‐based case management and education. | In‐person education and case management. | |
| 194 | UK | 12 months | 50.2 | 45.4 | NR | 6‐monthly phone calls from trained asthma nurses. Formulation of individual AAP. | 6‐monthly usual face‐to‐face appointment with an asthma nurse. Symptoms, peak flow and inhaler technique checked, and participants were issued with an AAP. | |
| 95 | The Netherlands | 6 months | 50.1 | 45.3 | 73.9 | OCS dose adjustment guided by an internet‐based diary, decision support and monitoring with support from a study nurse. | OCS dose adjustment according to GINA by the specialist. | |
| 278 | UK | 3 months | 55.5 | 41.4 | NR | Telephone check‐up with the asthma nurse. | Face‐to‐face check‐ups in the surgery with the asthma nurse. | |
| 1728 | UK | 12 months | 42.6 | 44.6 | NR | Three invitations to book either a telephone or face‐to‐face check‐up. Non‐attenders were phoned and reviewed opportunistically. | Three invitations to book a face‐to‐face check‐up. Non‐attenders were not phoned opportunistically. | |
| 300 | Denmark | 6 months | 29 | 34.5 | 92.0 | Participants were given an AAP, online electronic diary and peak flow meter. Physicians gave participants instructions via e‐mail or telephone aided by computer decision support. | The specialists taught the participants how to adjust their medication on the basis of a peak flow meter and AAP. | |
| Total N: the total number of participants randomised in the study, included to groups not analysed in this Cochrane review | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations requiring oral corticosteroids Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Efficacy randomised controlled trials (RCTs) | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Cluster implementation study | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exacerbations requiring hospital emergency department (ED) treatment Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Efficacy RCTs | 3 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 2.60 [0.63, 10.64] |
| 2.2 Cluster implementation study | 1 | 1212 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.38, 3.71] |
| 3 Exacerbations requiring hospital admission Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Efficacy RCTs | 3 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.06, 6.32] |
| 3.2 Cluster implementation study | 1 | 1213 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [0.83, 5.69] |
| 4 Asthma control (Asthma Control Questionnaire (ACQ)) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Efficacy RCTs | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Cluster implementation study | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Serious adverse events (including mortality) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Cluster implementation study | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Asthma‐related quality of life (Asthma Quality of Life Questionnaire (AQLQ) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Efficacy RCTs | 3 | 544 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.14, 0.30] |
| 6.2 Cluster implementation study | 1 | 536 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 7 Unscheduled healthcare visits Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Efficacy RCTs | 2 | 531 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.85] |
| 7.2 Cluster implementation study | 1 | 1213 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.21] |
| 8 Change in lung function (trough FEV1) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Adverse events Show forest plot | 1 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations requiring hospital admission Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Asthma control (ACQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Asthma‐related quality of life (AQLQ) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Unscheduled healthcare visits Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |

