Traitement antibiotique pour les partenaires sexuels des femmes présentant une vaginose bactérienne
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Setting: Italy, outpatient clinic. Trial design: multicentre randomized clinical trial, parallel, 2 arms. Funding sources: not mentioned. Ethical issues: ethical board and signed consent. | |
| Participants | Age of participants: from 18 to 45 years. Inclusion criteria:
Exclusion criteria: patients treated with systemic or topical Population Number of participants: 139 women, 139 men.
| |
| Interventions | Total number of intervention groups: Two groups. Intervention: clindamycin hydrochloride capsules, 150 mg by mouth 4 times daily for 7 consecutive days. | |
| Outcomes | The trial included evaluation at 1, 4 and 12 weeks after the start of treatment. The participants and their partners had a clinical examination, including the collection of samples of vaginal discharge to check for clue | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “…This was a double blind, randomised, controlled trial…” |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information to enable us to make a judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial authors did not adequately report the method implemented to blind study participants and personnel from knowledge of which intervention a participant received. "The clinicians were blind to the study treatment…” |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial authors did not adequately report the method implemented to blind outcome assessment from knowledge of which intervention a participant received. Comment: there was insufficient information to enable us to make a judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | For the outcomes clinical cure at 1, 4 and 12 weeks and gastrointestinal symptoms the risk of bias was low (e.g. no missing outcome data; missing outcome data was balanced across groups). However, for the outcome recurrence, the risk of bias was high according to the level of missing data (greater than 20%). |
| Selective reporting (reporting bias) | Unclear risk | The report did not have sufficient information to permit a judgment of “yes” or “no”. |
| Other bias | Low risk | This study appeared to be free of other sources of bias. |
| Methods | Setting: Finland, outpatient of primary care. Trial design: single randomized clinical trial, parallel, 2 arms. Funding sources: Orion pharmaceutical. Ethical issues: not mentioned. | |
| Participants | Age of participants: from 19 to 53 years. Inclusion criteria: Screening negative for Neisseria gonorrhoea, Chlamydia trachomatis, Candida albicans and Trichomonas vaginalis. Exclusion criteria: Population Number of participants: 90 women, 90 men.
| |
| Interventions | Total number of intervention groups:Two groups. | |
| Outcomes | The trial included evaluation at 1 and 3 months after the start of treatment. Women had a clinical examination, including the collection of PAP smear to check for clue Cure was defined as the absence of symptoms of BV or absence of clinically verified BV (Amsell criteria) Relapse was defined as patients who were asymptomatic after treatment and again developed symptoms of BV. Adverse events were not reported. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective randomised trial" Comment: there was insufficient information to enable us to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information to enable us to make a judgement. |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by a lack of blinding. |
| Blinding of outcome assessment (detection bias) | High risk | There was no blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by a lack of blinding. The outcomes symptomatic of improvement and relapse were subjectively assessed and the lack of blinding could have affected the results. The outcome of clinical cure was assessed objectively and the lack of blinding could not have affected the results. |
| Incomplete outcome data (attrition bias) | High risk | We judged the risk of bias as high for all outcomes according to the level of missing data (greater than 20%). For Group A, 7 participants were lost to follow‐up. In Group B 6 participants dropped out and in Group C 4 participants discontinued the trial. |
| Selective reporting (reporting bias) | Unclear risk | The report had insufficient information to permit judgment of “yes” or “no”. |
| Other bias | Low risk | Industry sponsored this trial. However, because the result were not positive, the study would be free of other sources of bias. |
| Methods | Setting: USA, outpatient of primary care. Trial design: multicentric randomized clinical trial, parallel, 2 arms. Funding sources: grants from the research committee, American Academy of Familiy Physicians; Family Health Foundation of America; Washington Academy of Family Physicians, and the Washington Familiy Health Foundation. Ethical issues: ethical board and signed consent. | |
| Participants | Age of participants: from 18 to 40 years. Inclusion criteria: Exclusion criteria: Population Number of participants: 161 women, 98 men
| |
| Interventions | Total number of intervention groups: Two groups. | |
| Outcomes | The trial included evaluations at 2 and 8 weeks after the onset of the treatment. Women had a clinical examination, including the collection of samples of vaginal discharge to check for clue The trial authors defined cure as the absence of at least 3 of the 4 Amsell criteria. They did not define recurrence. Participants reported adverse events as symptoms of BV. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished by blocks of varying sizes (4, 8 or 12) so that an equal number of women in each block entered each of the four treatment groups". Comment: this was probably done. |
| Allocation concealment (selection bias) | Low risk | "Randomization was accomplished by blocks of varying sizes (4, 8 or 12) so that an equal number of women in each block entered each of the four treatment groups" Comment: this was probably done. We considered that the authors implemented a valid randomized method which implies that the allocation concealment was probably through the use of consecutively numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | “placebo was used to ensure that both subjects and physicians did not know the subject's treatment” "metronidazole was identically coloured and shaped to placebo". |
| Blinding of outcome assessment (detection bias) | Low risk | “placebo was used to ensure that both subjects and physicians did not know the subject's treatment” "metronidazole was identically coloured and shaped to placebo". The outcomes of cure rate and side effect were objectively assessed. Comment: this was probably done. |
| Incomplete outcome data (attrition bias) | Low risk | 20% or fewer participants were excluded and also intention to treat analyses was reported. |
| Selective reporting (reporting bias) | Unclear risk | The report had insufficient information to permit a judgment of “yes” or “no”. |
| Other bias | High risk | This trial was supported by grants from the research committee, American Academy of Family Physicians; Family Health Foundation of America; Washington Academy of Family Physicians, and the Washington Family Health Foundation. |
| Methods | Setting: Sweden, Denmark, Finland and Norway. Outpatient clinic. Trial design: multicentric randomized clinical trial, parallel, 2 arms. Funding sources: grants from Rhone‐Paulenc Pharma Norden AS and Örebro County Council Research Committee. Ethical issues: ethical board and signed consent. | |
| Participants | Age of participants: from 17 to 56 years. Inclusion criteria: Exclusion criteria: Population Number of participants: 241 women: 241 men | |
| Interventions | Total number of intervention groups: Two groups. | |
| Outcomes | The triaI included evaluation at 1, 4, and 12 weeks after starting treatment. The trial asked participants about symptoms of BV and had a clinical examination including collection of samples of vaginal discharge to check for odour of the discharge, vaginal pH, KOH test and wetness of the vaginal discharge. The trial authors defined cure as the disappearance of at least 2 previous signs or symptoms reported. The trial authors defined recurrence as participants who were previously healthy after treatment but developed a new episode of BV (Amsell criteria). Adverse events were not reported. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomly allocated to one of two groups for a double blind trial”. Comment: there was insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information to make a judgement |
| Blinding of participants and personnel (performance bias) | Low risk | “They were randomly allocated to one of two groups for a double blind trial”, “Whose male consorts were given inert but identical placebo tablets”. Comment: this was probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “They were randomly allocated to one of two groups for a double blind trial”, “Whose male consorts were given inert but identical placebo tablets”. |
| Incomplete outcome data (attrition bias) | Unclear risk | For the outcomes of symptomatic improvement and clinical cure at first and fourth week the risk of bias was low (e.g. no missing outcome data; missing outcome data balanced across groups). For the same outcomes but assessed at 12 weeks, the risk of bias was high according to the level of missing data (greater than 20%). |
| Selective reporting (reporting bias) | Unclear risk | The report had insufficient information to permit judgment of “yes” or “no”. |
| Other bias | Low risk | The trial was sponsored by industry. However, because the results were not positives, the study would be free of other sources of bias. |
| Methods | Setting: USA, outpatient of family practice clinic. Trial design: single randomized clinical trial, parallel, 2 arms. Funding sources: Searle Pharmaceuticals and School of Human Medicine, University of Wyoming. Ethical issues: ethical board and signed consent. | |
| Participants | Age of participants: from 18 to 45 years. Inclusion criteria: Exclusion criteria: Population Number of participants: 82 women, 82 men.
Number of participants who received other(s) intervention(s) or placebo: No treatment was administred to 55 participants, 32 in yhe 2‐g single dose group and 23 in the seven day regimen group. Baseline characteristics: women were treated with metronidazole 500 mg twice a day for 7 days or 2 g single doses. The trial authors did not provide more information. | |
| Interventions | Total number of intervention groups: Two groups. | |
| Outcomes | The trial included evaluation at 7 to 10 and 21 days after starting treatment. All participants had a clinical examination, including the collection of samples of vaginal discharge to check for clue cells, KOH test, vaginal pH and cultures for gonorrhoea. At both visits, the participants were evaluated by a Likert‐type questionnaire for symptoms of vaginitis (vulvar itching, vulvar burning, odour and quantity of discharge). Cure was defined if G vaginalis was not isolated on culture and if symptoms had markedly improved or been assent (Likert‐type questionnaire). The participants reported adverse events as symptoms. | |
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random number list". Comments: this was probably done. |
| Allocation concealment (selection bias) | Low risk | "Regimen dispensed by the clinic pharmacist" Comment: this was probably done. Telephone or central randomization. |
| Blinding of participants and personnel (performance bias) | High risk | There was no blinding or incomplete blinding, and the outcome or outcome measurement is likely to have been influenced by a lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | "The clinical practitioner and the laboratory personnel were "blinded" in that they did not know to which treatment group the patient would be assigned". |
| Incomplete outcome data (attrition bias) | High risk | More than 20% were excluded: "18 did no return at 21 days after treatment, 14 were excluded because they failed to return at 7 – 10 day". |
| Selective reporting (reporting bias) | Unclear risk | The report had insufficient information to permit judgment of “yes” or “no”. |
| Other bias | Low risk | Searle Pharmaceuticals provided the medication, Other funds for this project were provided by the School of Human Medicine, University of Wyoming. |
| Methods | Setting: Denmark, outpatient clinic. Trial design: single randomized clinical trial, parallel, 2 arms. Funding sources: Rhone‐Poulenc Pharma Norden A/S. Ethical issues: ethical board and sign consent. | |
| Participants | Age of participants: from 17 to 50 years. Inclusion criteria: Exclusion criteria: Population Number of participants: 126 women, 126 men.
| |
| Interventions | Total number of intervention groups: Two groups. | |
| Outcomes | The trial included evaluation at 1 and 5 weeks after the start of treatment. The women had a clinical examination, including the collection of samples of vaginal discharge to check for clue The trial authors defined cure as the absence or symptoms of BV (increased discharge, malodour, burning sensation or itching) and without at least 3 of the 4 Amsell criteria. The trial authors defined recurrence as participants that were clinically cured after treatment and then developed a new episode of BV (Amsel criteria). Adverse events were not reported. | |
| Notes | None. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The partners were allocated to receive metronidazole or placebo by random allocation in blocks of four” Comment: this was probably done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information to make a judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial authors did not adequately report the method implemented to blind study participants and personnel from knowledge of which intervention a participant received. "The women were treated with metronidazole tablets, 2 g on days 1 and 3. The partner was given the same treatment or a placebo". |
| Blinding of outcome assessment (detection bias) | Low risk | The trial authors did not adequately report the method implemented to blind study participants and personnel from knowledge of which intervention a participant received. "The women were treated with metronidazole tablets, 2 g on days 1 and 3. The partner was given the same treatment or a placebo". |
| Incomplete outcome data (attrition bias) | Low risk | 20% or fewer participants were excluded and also intention to treat analyses was reported. |
| Selective reporting (reporting bias) | Unclear risk | The report did not have sufficient information to permit a judgment of “yes” or no”. |
| Other bias | Low risk | Rhone‐Poulenc Pharma Norden A/S supported the trial. |
| Methods | Setting: Thailand, outpatient clinic. Trial design: single randomized clinical trial, parallel, 2 arms. Funding sources: Faculty of Medicine, Chiang Mai University. Ethical issues: ethical board and signed consent. | |
| Participants | Age of participants: from 17 to 40 years. Inclusion criteria: Exclusion criteria: Population Number of participants: 250 women, 250 men.
| |
| Interventions | Total number of intervention groups: Two groups. | |
| Outcomes | The trial included evaluation at 1 and 4 weeks after the start of treatment. The women had a clinical examination, including the collection of samples of vaginal discharge to check for clue The trial authors defined clinical cure as the proportion of women who remained without at least 2 of the 4 criteria for BV (Amsell criteria). The trial authors defined symptomatic improvement as participants with an absence of symptoms of BV (abnormal vaginal discharge or pruritus vulvae, or both). The participants reported adverse events as symptoms. | |
| Notes | None. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomized into two groups using a table of random numbers”. Comment: this was probably done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information to make a judgement. |
| Blinding of participants and personnel (performance bias) | Low risk | “Either 2 g tinidazole or identical‐looking placebo packed similarly in packets of four tablets”. Comment: this was probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | “Either 2 g tinidazole or identical‐looking placebo packed similarly in packets of four tablet”. Comment: this was probably done. |
| Incomplete outcome data (attrition bias) | Low risk | 20% or fewer participants were excluded and also intention to treat analyses was reported. |
| Selective reporting (reporting bias) | Unclear risk | The report had insufficient information to permit a judgment of “yes” or “no”. |
| Other bias | Low risk | The study was supported by grant from the Faculty of Medicine Endowment Fund for Medical Research, Chiang Mai University. Comment: the industry probably did not influence the results. |
Abbreviations: BV: bacterial vaginosis. PAP: Papanicolaou ; IUCD:inrauterine divice, USA: United States of America, DMPA: Depot medroxyprogesterone acetate.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Randomized controlled trial (RCT), but all sexual partners were treated. | |
| RCT, but sexual partners did not received antibiotic treatment. | |
| RCT, but all sexual partners were treated. | |
| Not a RCT. | |
| Not a RCT. | |
| RCT, but all sexual partners were treated. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| Not a RCT. | |
| RCT, but all sexual partners were treated. |
Abbreviations: RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomized controlled trial of treatment of male partners of women With BV |
| Methods | Double‐blinded RCT |
| Participants | Male partners of women with recurrent BV |
| Interventions | Metronidazole 500 mg PO twice a day for 7 days versus placebo capsules PO twice a day for 7 days. |
| Outcomes | Recurrence of BV in the female, recurrence/persistence of BV, number of couples with concordance of biotypes/strains of Gardnerella vaginalis and time to recurrence. |
| Starting date | February 2015 |
| Contact information | Jane R Schwebke, MD; e‐mail: [email protected] |
| Notes | Study sponsor: University of Alabama at Birmingham |
Abbreviations: BV: bacterial vaginosis. RCT: randomized controlled trial. PO: per os.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV between the first and fourth week Show forest plot | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.43] |
| Analysis 1.1 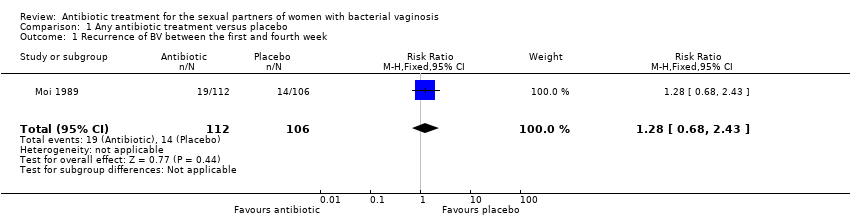 Comparison 1 Any antibiotic treatment versus placebo, Outcome 1 Recurrence of BV between the first and fourth week. | ||||
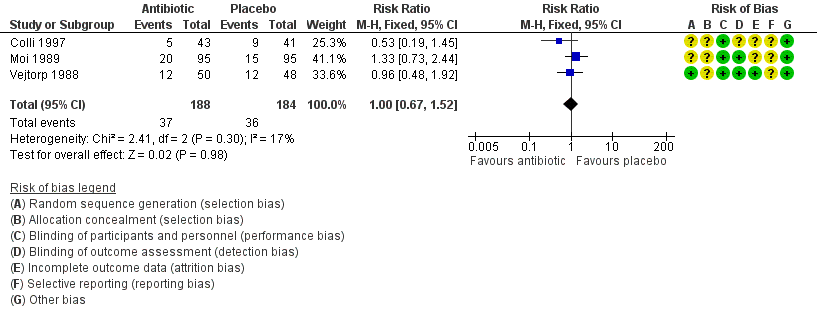
| 2 Recurrence of BV after the fourth week Show forest plot | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.52] |
| Analysis 1.2 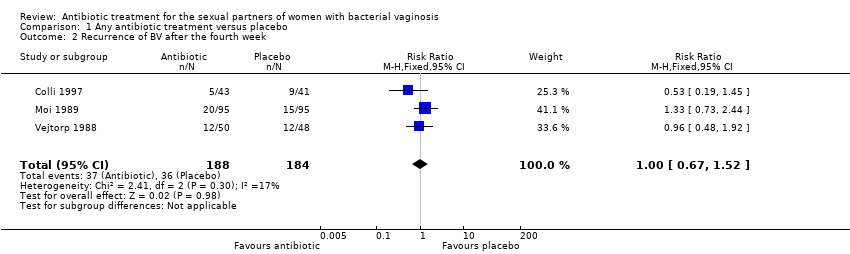 Comparison 1 Any antibiotic treatment versus placebo, Outcome 2 Recurrence of BV after the fourth week. | ||||
| 3 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| Analysis 1.3  Comparison 1 Any antibiotic treatment versus placebo, Outcome 3 Clinical improvement during the first week. | ||||
| 4 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| Analysis 1.4  Comparison 1 Any antibiotic treatment versus placebo, Outcome 4 Clinical improvement between the first and fourth week. | ||||
| 5 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| Analysis 1.5  Comparison 1 Any antibiotic treatment versus placebo, Outcome 5 Clinical improvement after the fourth week. | ||||
| 6 Symptomatic improvement during the first week Show forest plot | 3 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| Analysis 1.6 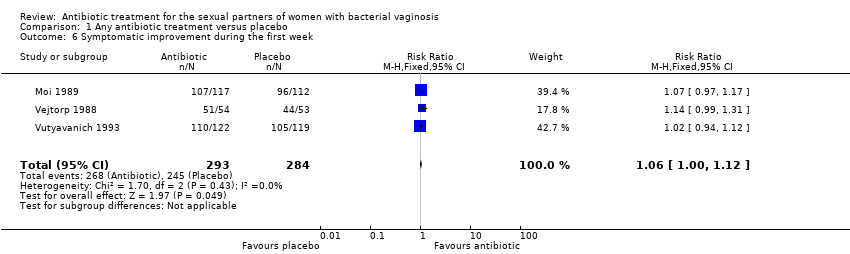 Comparison 1 Any antibiotic treatment versus placebo, Outcome 6 Symptomatic improvement during the first week. | ||||
| 7 Symptomatic improvement between the first and fourth week Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| Analysis 1.7  Comparison 1 Any antibiotic treatment versus placebo, Outcome 7 Symptomatic improvement between the first and fourth week. | ||||
| 8 Symptomatic improvement after the fourth week Show forest plot | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| Analysis 1.8  Comparison 1 Any antibiotic treatment versus placebo, Outcome 8 Symptomatic improvement after the fourth week. | ||||
| 9 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| Analysis 1.9 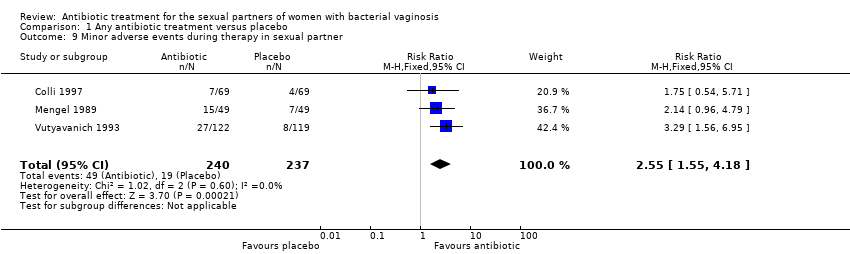 Comparison 1 Any antibiotic treatment versus placebo, Outcome 9 Minor adverse events during therapy in sexual partner. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV after the fourth week Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.65, 4.55] |
| Analysis 2.1  Comparison 2 Any antibiotic treatment versus no intervention, Outcome 1 Recurrence of BV after the fourth week. | ||||
| 2 Clinical improvement between the first and fourth week Show forest plot | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.25] |
| Analysis 2.2  Comparison 2 Any antibiotic treatment versus no intervention, Outcome 2 Clinical improvement between the first and fourth week. | ||||
| 3 Symptomatic improvement after the fourth week Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.11] |
| Analysis 2.3  Comparison 2 Any antibiotic treatment versus no intervention, Outcome 3 Symptomatic improvement after the fourth week. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV after the fourth week Show forest plot | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.52] |
| Analysis 3.1 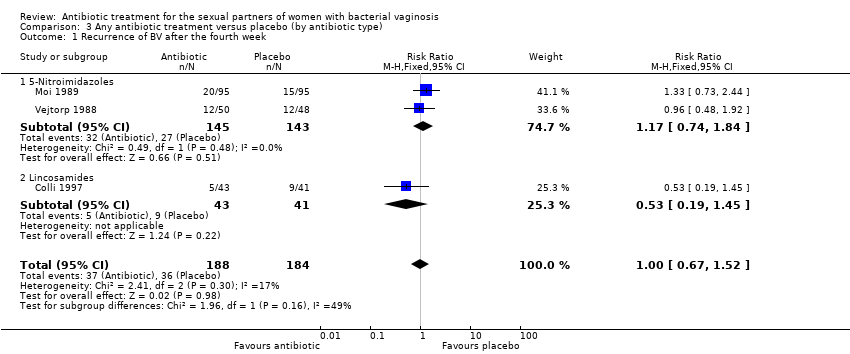 Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 1 Recurrence of BV after the fourth week. | ||||
| 1.1 5‐Nitroimidazoles | 2 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.74, 1.84] |
| 1.2 Lincosamides | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.45] |
| 2 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| Analysis 3.2  Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 2 Clinical improvement during the first week. | ||||
| 2.1 5‐Nitroimidazoles | 3 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.03] |
| 2.2 Lincosamides | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.10] |
| 3 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| Analysis 3.3 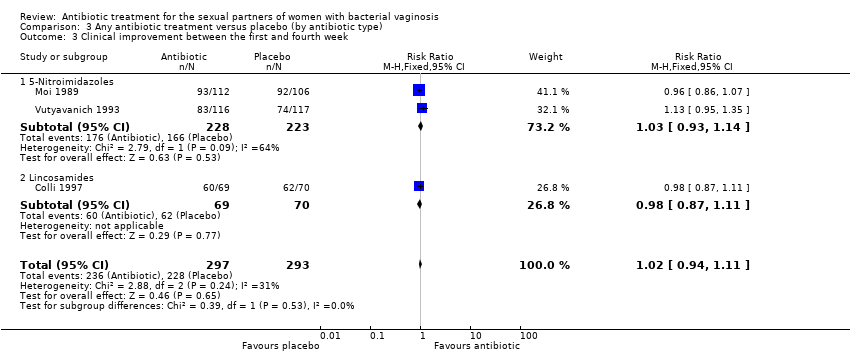 Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 3 Clinical improvement between the first and fourth week. | ||||
| 3.1 5‐Nitroimidazoles | 2 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 3.2 Lincosamides | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.11] |
| 4 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| Analysis 3.4 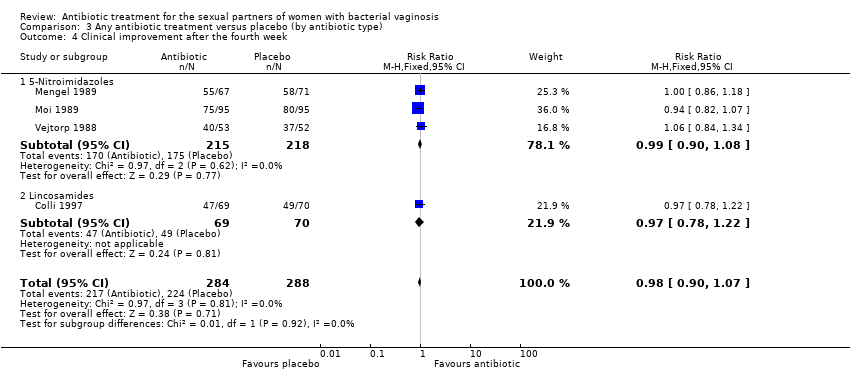 Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 4 Clinical improvement after the fourth week. | ||||
| 4.1 5‐Nitroimidazoles | 3 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.08] |
| 4.2 Lincosamides | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.22] |
| 5 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| Analysis 3.5  Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 5 Minor adverse events during therapy in sexual partner. | ||||
| 5.1 5‐Nitroimidazoles | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.60, 4.77] |
| 5.2 Lincosamides | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| Analysis 4.1 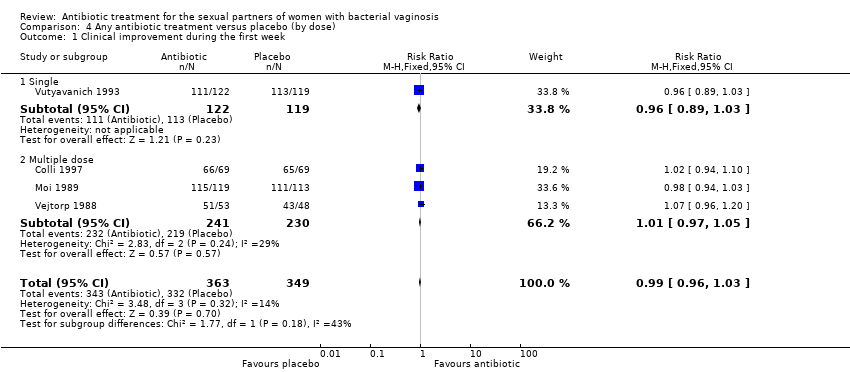 Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 1 Clinical improvement during the first week. | ||||
| 1.1 Single | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.03] |
| 1.2 Multiple dose | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.97, 1.05] |
| 2 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| Analysis 4.2 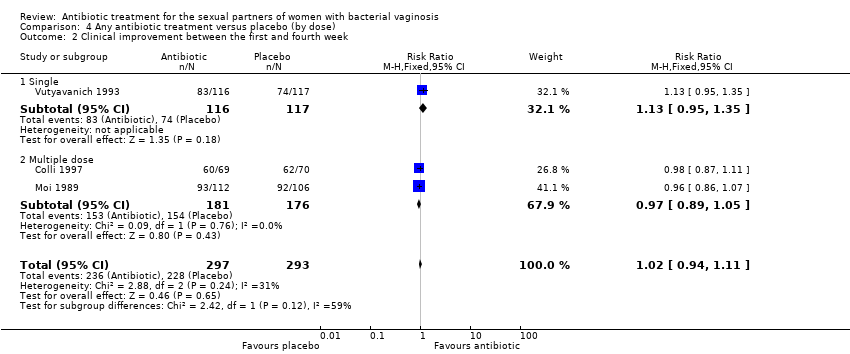 Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 2 Clinical improvement between the first and fourth week. | ||||
| 2.1 Single | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.95, 1.35] |
| 2.2 Multiple dose | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 3 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| Analysis 4.3  Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 3 Clinical improvement after the fourth week. | ||||
| 3.1 Single | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.18] |
| 3.2 Multiple dose | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.09] |
| 4 Symptomatic improvement during the first week Show forest plot | 3 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| Analysis 4.4  Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 4 Symptomatic improvement during the first week. | ||||
| 4.1 Single | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.12] |
| 4.2 Multiple dose | 2 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.01, 1.18] |
| 5 Symptomatic improvement between the first and fourth week Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| Analysis 4.5  Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 5 Symptomatic improvement between the first and fourth week. | ||||
| 5.1 Single | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.08] |
| 5.2 Multiple dose | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 6 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| Analysis 4.6  Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 6 Minor adverse events during therapy in sexual partner. | ||||
| 6.1 Single | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.60, 4.77] |
| 6.2 Multiple dose | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV after the fourth week Show forest plot | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.52] |
| Analysis 5.1  Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 1 Recurrence of BV after the fourth week. | ||||
| 1.1 Low risk | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.48, 1.92] |
| 1.2 Unclear risk | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.62, 1.71] |
| 2 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| Analysis 5.2  Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 2 Clinical improvement during the first week. | ||||
| 2.1 Low risk | 2 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 2.2 Unclear risk | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.03] |
| 3 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| Analysis 5.3  Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 3 Clinical improvement between the first and fourth week. | ||||
| 3.1 Low risk | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.95, 1.35] |
| 3.2 Unclear risk | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 4 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| Analysis 5.4 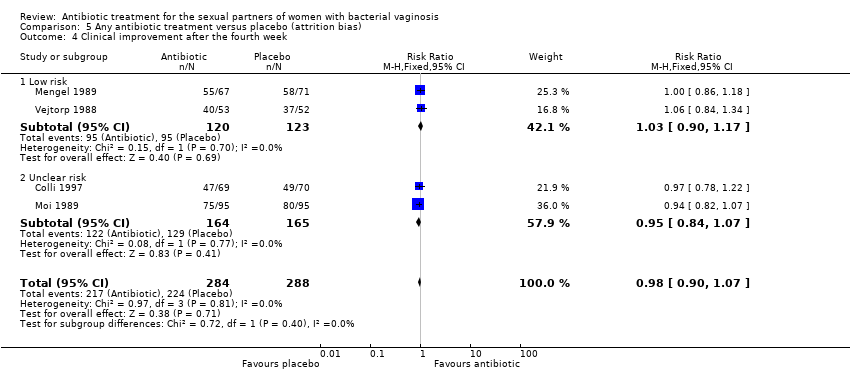 Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 4 Clinical improvement after the fourth week. | ||||
| 4.1 Low risk | 2 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 4.2 Unclear risk | 2 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| 5 Symptomatic improvement during the first week Show forest plot | 3 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| Analysis 5.5  Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 5 Symptomatic improvement during the first week. | ||||
| 5.1 Low risk | 2 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
| 5.2 Unclear risk | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.97, 1.17] |
| 6 Symptomatic improvement between the first and fourth week Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| Analysis 5.6  Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 6 Symptomatic improvement between the first and fourth week. | ||||
| 6.1 Low risk | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.08] |
| 6.2 Unclear risk | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 7 Symptomatic improvement after the fourth week Show forest plot | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| Analysis 5.7  Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 7 Symptomatic improvement after the fourth week. | ||||
| 7.1 Low risk | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.29] |
| 7.2 Unclear risk | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 8 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| Analysis 5.8  Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 8 Minor adverse events during therapy in sexual partner. | ||||
| 8.1 Low risk | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.60, 4.77] |
| 8.2 Unclear risk | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
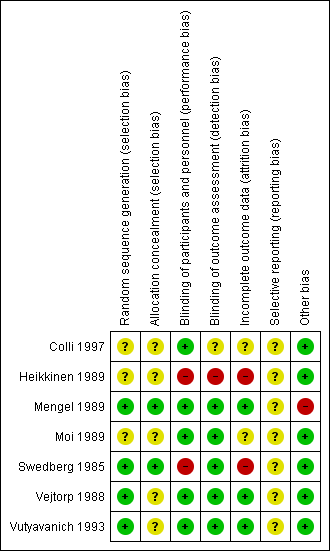
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Forest plot of comparison: 1 Any antibiotic treatment versus placebo, outcome: 1.2 Recurrence of BV after the fourth week.
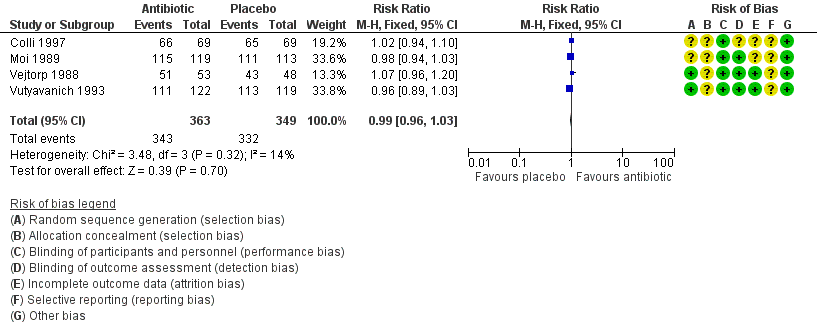
Forest plot of comparison: 1 Any antibiotic treatment versus placebo, outcome: 1.3 Clinical improvement during the first week.

Forest plot of comparison: 1 Any antibiotic treatment versus placebo, outcome: 1.4 Clinical improvement between the first and fourth week.

Forest plot of comparison: 1 Any antibiotic treatment versus placebo, outcome: 1.5 Clinical improvement after the fourth week.

Forest plot of comparison: 1 Any antibiotic treatment versus placebo, outcome: 1.6 Symptomatic improvement during the first week.

Forest plot of comparison: 1 Any antibiotic treatment versus placebo, outcome: 1.9 Minor adverse events during therapy in sexual partner.

Comparison 1 Any antibiotic treatment versus placebo, Outcome 1 Recurrence of BV between the first and fourth week.

Comparison 1 Any antibiotic treatment versus placebo, Outcome 2 Recurrence of BV after the fourth week.
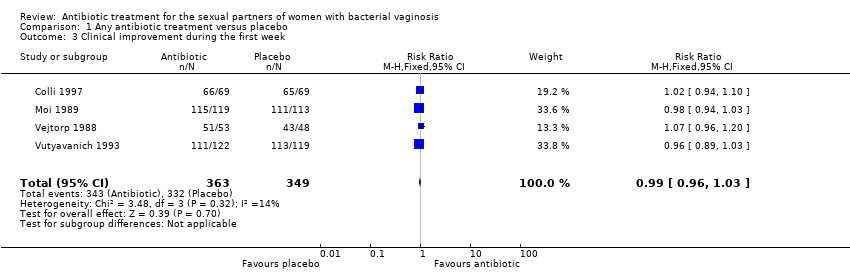
Comparison 1 Any antibiotic treatment versus placebo, Outcome 3 Clinical improvement during the first week.
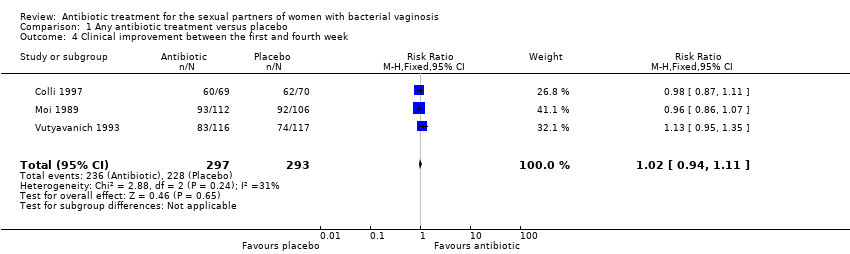
Comparison 1 Any antibiotic treatment versus placebo, Outcome 4 Clinical improvement between the first and fourth week.
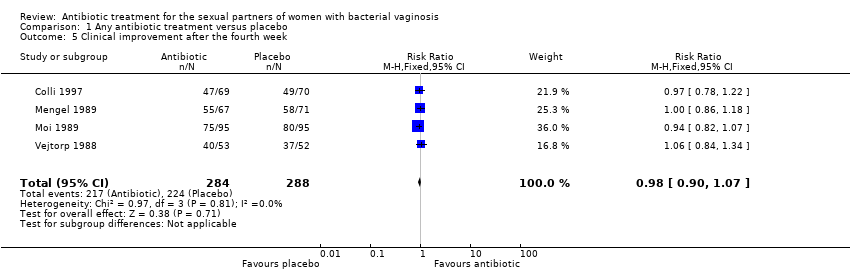
Comparison 1 Any antibiotic treatment versus placebo, Outcome 5 Clinical improvement after the fourth week.

Comparison 1 Any antibiotic treatment versus placebo, Outcome 6 Symptomatic improvement during the first week.
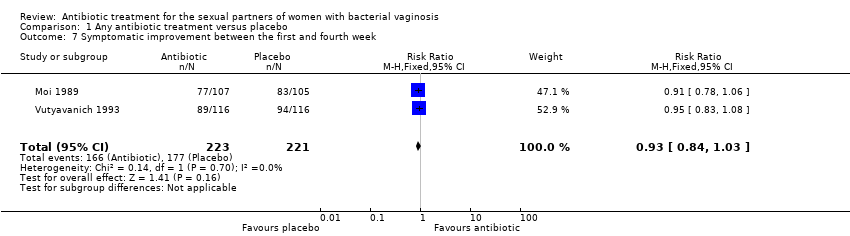
Comparison 1 Any antibiotic treatment versus placebo, Outcome 7 Symptomatic improvement between the first and fourth week.

Comparison 1 Any antibiotic treatment versus placebo, Outcome 8 Symptomatic improvement after the fourth week.

Comparison 1 Any antibiotic treatment versus placebo, Outcome 9 Minor adverse events during therapy in sexual partner.
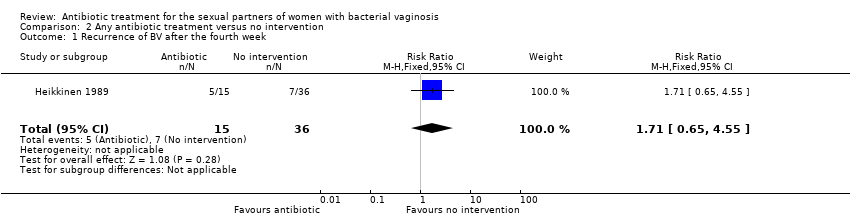
Comparison 2 Any antibiotic treatment versus no intervention, Outcome 1 Recurrence of BV after the fourth week.

Comparison 2 Any antibiotic treatment versus no intervention, Outcome 2 Clinical improvement between the first and fourth week.

Comparison 2 Any antibiotic treatment versus no intervention, Outcome 3 Symptomatic improvement after the fourth week.

Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 1 Recurrence of BV after the fourth week.

Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 2 Clinical improvement during the first week.

Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 3 Clinical improvement between the first and fourth week.

Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 4 Clinical improvement after the fourth week.
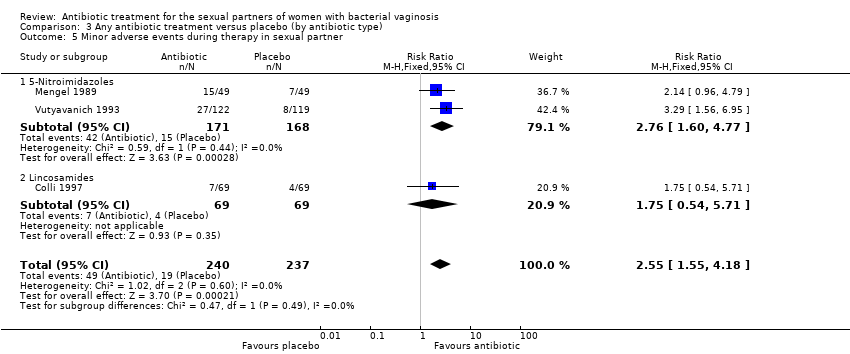
Comparison 3 Any antibiotic treatment versus placebo (by antibiotic type), Outcome 5 Minor adverse events during therapy in sexual partner.

Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 1 Clinical improvement during the first week.

Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 2 Clinical improvement between the first and fourth week.

Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 3 Clinical improvement after the fourth week.

Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 4 Symptomatic improvement during the first week.

Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 5 Symptomatic improvement between the first and fourth week.
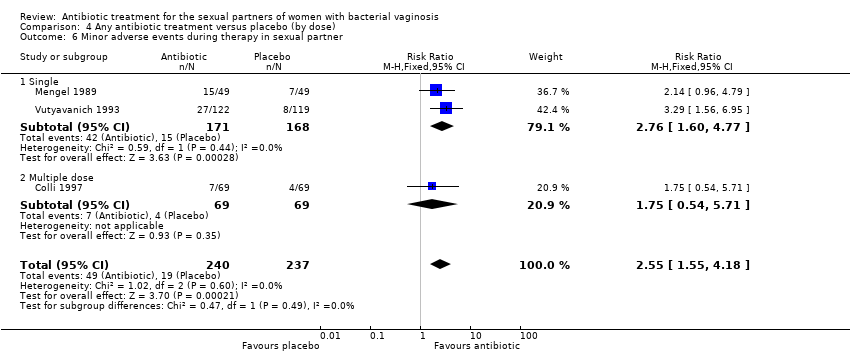
Comparison 4 Any antibiotic treatment versus placebo (by dose), Outcome 6 Minor adverse events during therapy in sexual partner.

Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 1 Recurrence of BV after the fourth week.
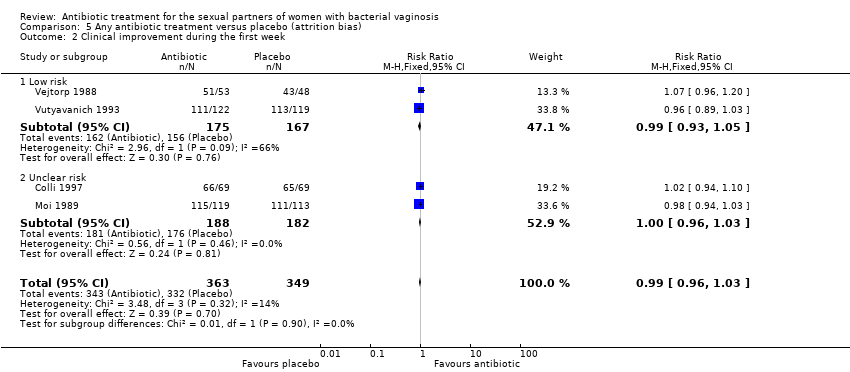
Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 2 Clinical improvement during the first week.

Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 3 Clinical improvement between the first and fourth week.

Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 4 Clinical improvement after the fourth week.
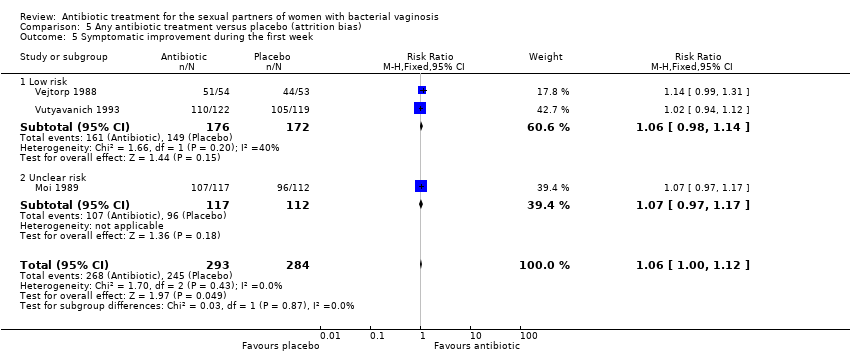
Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 5 Symptomatic improvement during the first week.
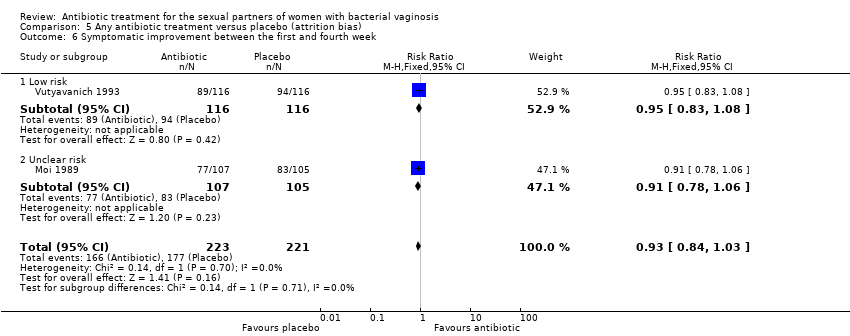
Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 6 Symptomatic improvement between the first and fourth week.

Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 7 Symptomatic improvement after the fourth week.

Comparison 5 Any antibiotic treatment versus placebo (attrition bias), Outcome 8 Minor adverse events during therapy in sexual partner.
| Any antibiotic treatment versus placebo for the sexual partners of woman with bacterial vaginosis | |||||
| Patient or population: sexual partners of women with bacterial vaginosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with placebo | Risk with any antibiotic treatment | ||||
| Recurrence follow‐up 4 to 12 weeks | Study population | RR 1.00 | 372 | ⊕⊝⊝⊝ | |
| 196 per 1000 | 196 per 1000 | ||||
| Clinical improvement follow‐up 1 to 4 weeks | Study population | RR 1.02 | 590 | ⊕⊕⊕⊕ | |
| 778 per 1000 | 794 per 1000 | ||||
| Clinical improvement follow‐up 4 to 12 weeks | Study population | RR 0.98 | 572 | ⊕⊕⊕⊕ | |
| 778 per 1000 | 762 per 1000 | ||||
| Symptomatic improvement during the first week | Study population | RR 1.06 | 577 | ⊕⊕⊕⊕ | |
| 863 per 1000 | 914 per 1000 | ||||
| Symptomatic improvement follow‐up 1 to 4 weeks | Study population | RR 0.93 | 444 | ⊕⊕⊕⊕ | |
| 801 per 1000 | 745 per 1000 | ||||
| Symptomatic improvement follow‐up 4 to 12 weeks | Study population | RR 1.03 | 296 | ⊕⊕⊕⊕ | |
| 743 per 1000 | 766 per 1000 | ||||
| Minor adverse events in sexual partner | Study population | RR 2.55 | 477 | ⊕⊕⊝⊝ | |
| 80 per 1000 | 204 per 1000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for significant imprecision as the 95% CI was below 0.75 and over 1.25. | |||||
| Any antibiotic treatment versus no intervention | |||||
| Patient or population: sexual partners of women with bacterial vaginosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with no intervention | Risk with any antibiotic treatment | ||||
| Recurrence follow‐up 4 to 12 weeks | Study population | RR 1.71 | 51 | ⊕⊝⊝⊝ | |
| 194 per 1000 | 333 per 1000 | ||||
| Clinical improvement follow‐up 1 to 4 weeks | Study population | RR 0.93 | 152 | ⊕⊝⊝⊝ | |
| 851 per 1000 | 792 per 1000 | ||||
| Symptomatic improvement follow‐up 4 to 12 weeks | Study population | RR 0.66 | 70 | ⊕⊝⊝⊝ | |
| 630 per 1000 | 416 per 1000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 2 levels due to imprecision as OIS was not achieved and the 95% CI crosses through 0.75 and 1.25. | |||||
| Any antibiotic treatment versus placebo | |||||
| Patient or population: sexual partners of women with bacterial vaginosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with placebo | Risk with any antibiotic treatment | ||||
| Recurrence | Study population | RR 1.28 | 218 | ⊕⊕⊝⊝ | |
| 132 per 1000 | 169 per 1000 | ||||
| Clinical improvement during the first week | Study population | RR 0.99 | 712 | ⊕⊕⊕⊕ | |
| 951 per 1000 | 942 per 1000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 2 levels for imprecision as the 95% CI crosses through 0.75 and 1.25 and OIS is not achieved. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV between the first and fourth week Show forest plot | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.43] |
| 2 Recurrence of BV after the fourth week Show forest plot | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.52] |
| 3 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| 4 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| 5 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| 6 Symptomatic improvement during the first week Show forest plot | 3 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| 7 Symptomatic improvement between the first and fourth week Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 8 Symptomatic improvement after the fourth week Show forest plot | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 9 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV after the fourth week Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.65, 4.55] |
| 2 Clinical improvement between the first and fourth week Show forest plot | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.25] |
| 3 Symptomatic improvement after the fourth week Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV after the fourth week Show forest plot | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.52] |
| 1.1 5‐Nitroimidazoles | 2 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.74, 1.84] |
| 1.2 Lincosamides | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.45] |
| 2 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| 2.1 5‐Nitroimidazoles | 3 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.03] |
| 2.2 Lincosamides | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.10] |
| 3 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| 3.1 5‐Nitroimidazoles | 2 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 3.2 Lincosamides | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.11] |
| 4 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| 4.1 5‐Nitroimidazoles | 3 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.08] |
| 4.2 Lincosamides | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.22] |
| 5 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| 5.1 5‐Nitroimidazoles | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.60, 4.77] |
| 5.2 Lincosamides | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| 1.1 Single | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.03] |
| 1.2 Multiple dose | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.97, 1.05] |
| 2 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| 2.1 Single | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.95, 1.35] |
| 2.2 Multiple dose | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 3 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| 3.1 Single | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.18] |
| 3.2 Multiple dose | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.09] |
| 4 Symptomatic improvement during the first week Show forest plot | 3 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| 4.1 Single | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.12] |
| 4.2 Multiple dose | 2 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.01, 1.18] |
| 5 Symptomatic improvement between the first and fourth week Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 5.1 Single | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.08] |
| 5.2 Multiple dose | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 6 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| 6.1 Single | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.60, 4.77] |
| 6.2 Multiple dose | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of BV after the fourth week Show forest plot | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.52] |
| 1.1 Low risk | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.48, 1.92] |
| 1.2 Unclear risk | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.62, 1.71] |
| 2 Clinical improvement during the first week Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.03] |
| 2.1 Low risk | 2 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 2.2 Unclear risk | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.03] |
| 3 Clinical improvement between the first and fourth week Show forest plot | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| 3.1 Low risk | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.95, 1.35] |
| 3.2 Unclear risk | 2 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 4 Clinical improvement after the fourth week Show forest plot | 4 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.07] |
| 4.1 Low risk | 2 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 4.2 Unclear risk | 2 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| 5 Symptomatic improvement during the first week Show forest plot | 3 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| 5.1 Low risk | 2 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
| 5.2 Unclear risk | 1 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.97, 1.17] |
| 6 Symptomatic improvement between the first and fourth week Show forest plot | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 6.1 Low risk | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.08] |
| 6.2 Unclear risk | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 7 Symptomatic improvement after the fourth week Show forest plot | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 7.1 Low risk | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.29] |
| 7.2 Unclear risk | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 8 Minor adverse events during therapy in sexual partner Show forest plot | 3 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.55, 4.18] |
| 8.1 Low risk | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.60, 4.77] |
| 8.2 Unclear risk | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |

