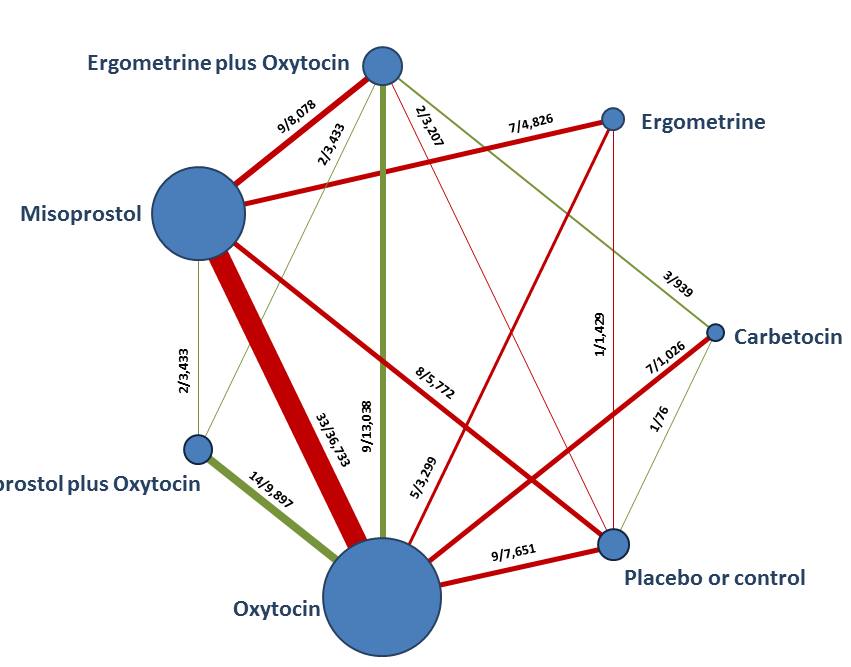
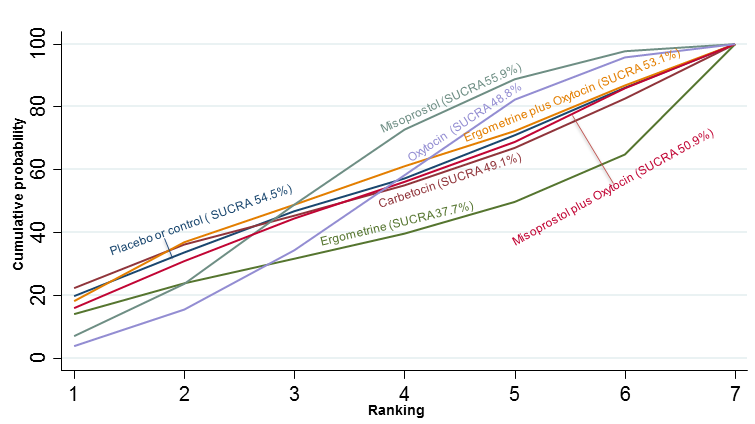
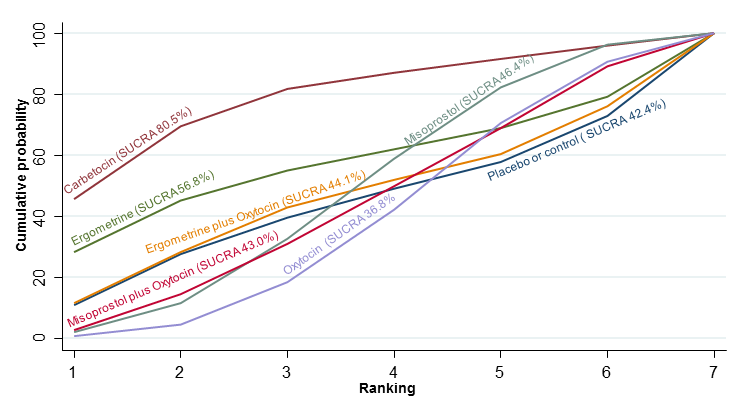
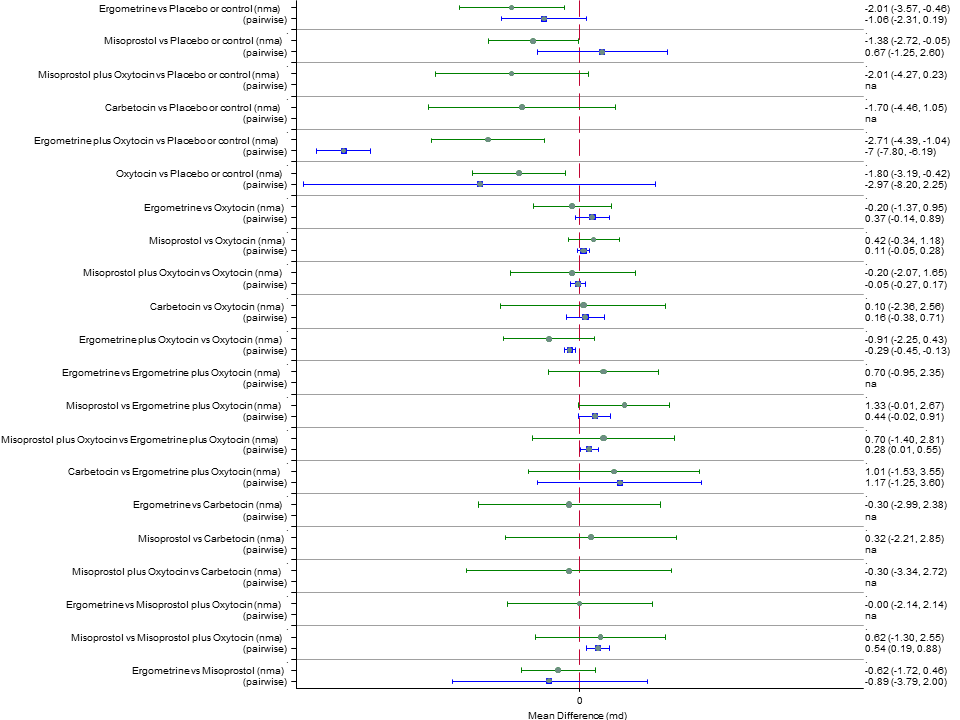
| Effects of uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis |
| Patient or population: Women giving birth and at the third stage of labour Settings: Hospital setting Intervention: Ergometrine plus oxytocin, Carbetocin, Misoprostol plus oxytocin Comparison: Oxytocin |
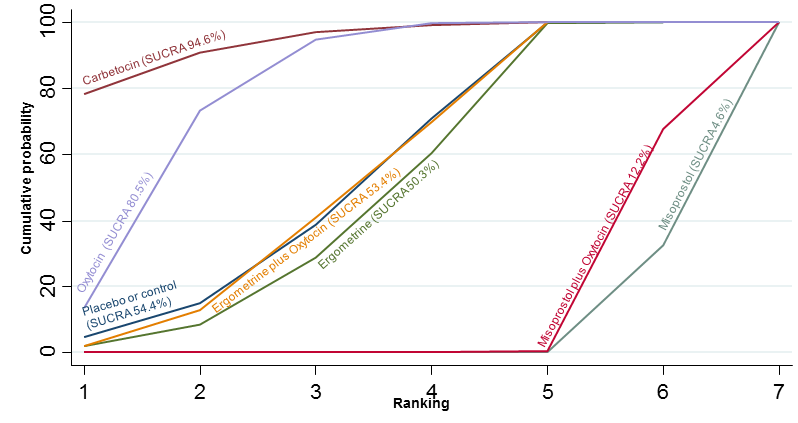
| PPH ≥500 mL | 7.2% (6 to 8.7) for vaginal births 51.7% (42.7 to 62.2) for caesareans | 7.6% (5.5 to 10.5) for vaginal births 53.9% (38.9 to 74.9) for caesareans | 7.7% (6.3 to 9.5) for vaginal births 54.7% (44.9 to 67.4) for caesareans | 10.5% (9.8 to 11.3) for vaginal births 74.9% (65.7 to 85.4) for caesareans | There was evidence of global inconsistency in this analysis ( P = 0.046). However, the comparisons in this table were consistent except for the comparison of ergometrine versus no treatment not included in this table‐based on a single study. |
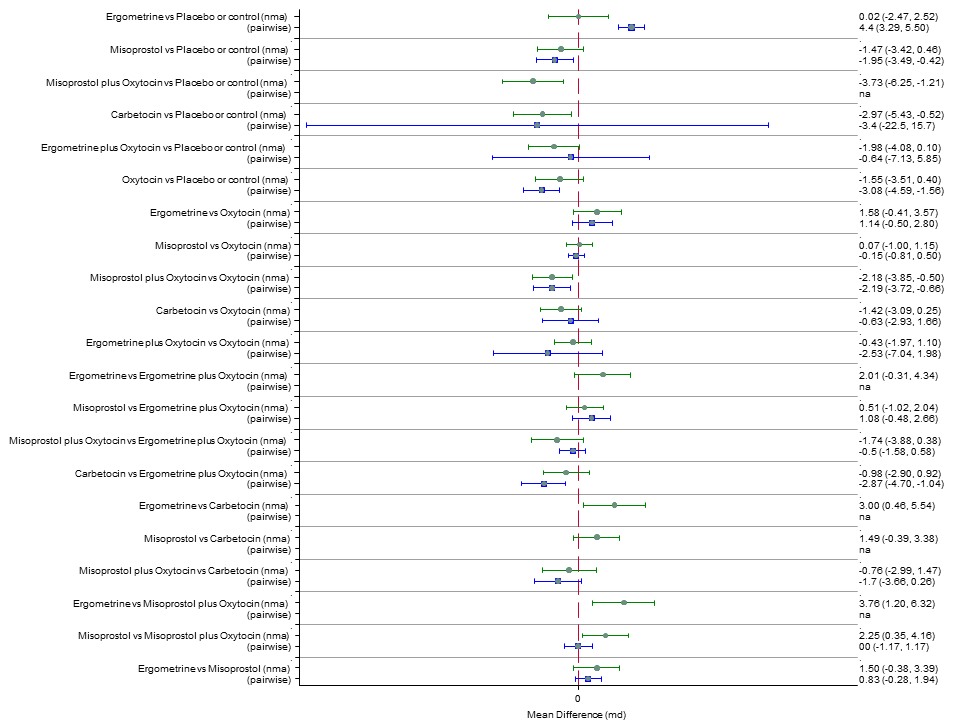
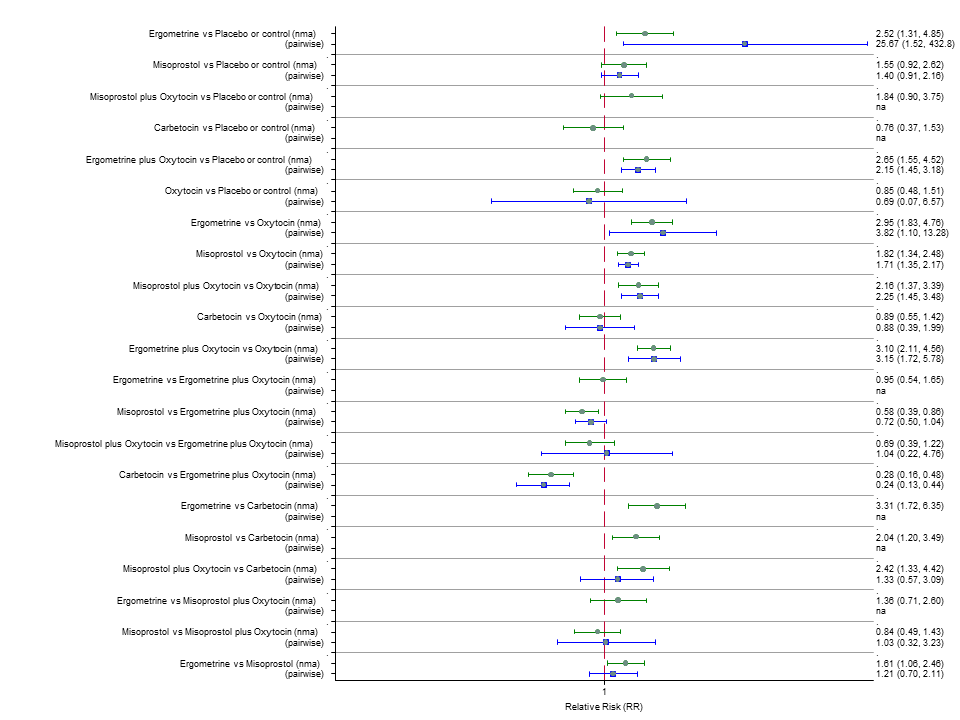
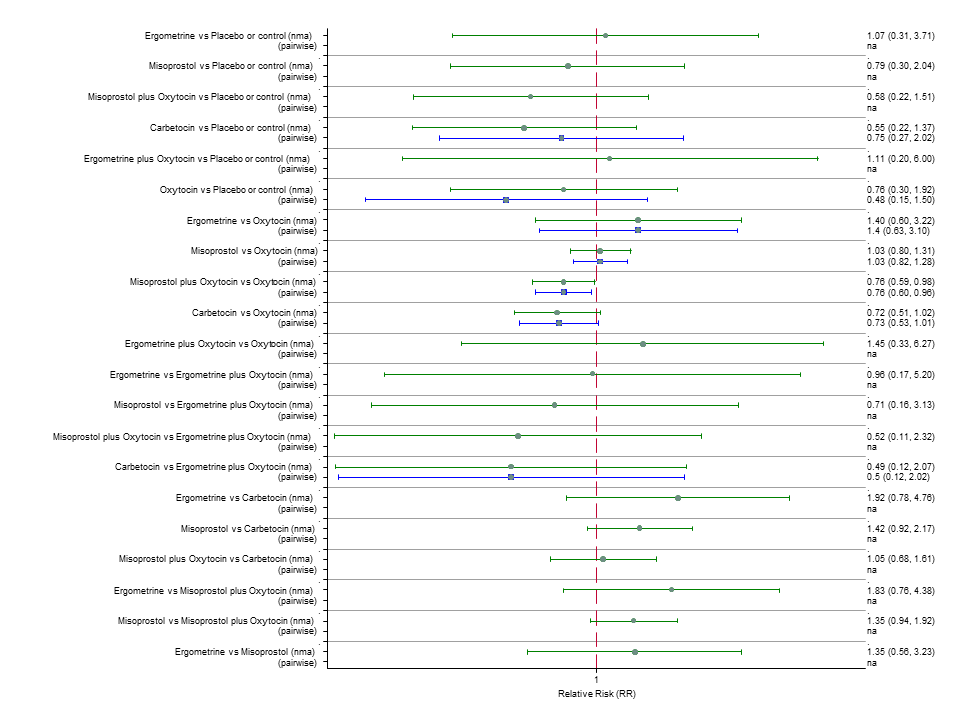
| RR 0.69 (0.57 to 0.83) (NMA) RR 0.72 (0.56 to 0.92) (Pairwise) | RR 0.72 (0.52 to 1.00) (NMA) RR 0.69 (0.45 to 1.07) (Pairwise) | RR 0.73 (0.60 to 0.90) (NMA) RR 0.74 (0.62 to 0.88) (Pairwise) | 1 |
| ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 10 studies (13,138 women, I2=57.4%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, imprecision and inconsistency based on 8 studies (917 women, I2 = 49.9%) | ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 12 studies (9651 women, I2 = 60.5%) | |
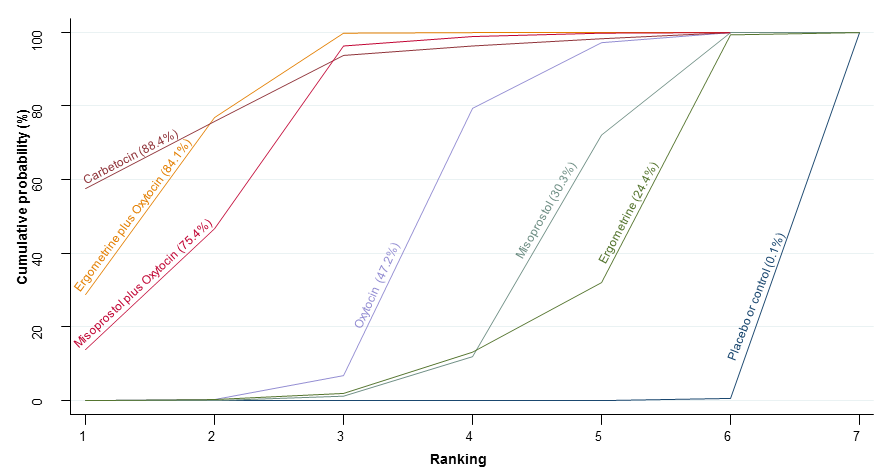
| PPH ≥1000 mL | 2.8% (2.2 to 3.4) for vaginal births 10.7% (8.5 to 13.2) for caesareans | 2.5% (1.4 to 4.6) for vaginal births 9.7% (5.3 to 17.8) for caesareans | 3.2% (2.6 to 4.1) for vaginal births 12.5% (10 to 15.8) for caesareans | 3.6% (3.4 to 3.9) for vaginal births 13.9% (11.7 to 16.6) for caesareans | There was no evidence of global inconsistency (P = 0.345) in this analysis. |
| RR 0.77 (0.61 to 0.95) (NMA) RR 0.73 (0.57 to 0.93) (Pairwise) | RR 0.70 (0.38 to 1.28) (NMA) RR 0.71 (0.38 to 1.35) (Pairwise) | RR 0.90 (0.72 to 1.14) (NMA) RR 0.89 (0.71 to 1.12) (Pairwise) | 1 |
| ⊕⊕⊕⊕ high confidence in estimate based on 9 studies (13,038 women, I2 = 0%) | ⊕⊕⊝⊝ low confidence in estimate due to risk of bias and imprecision based on 7 studies (1026 women, I2 = 0%) | ⊕⊕⊕⊝ moderate confidence in estimate due to imprecision based on 14 studies (9897 women, I2 = 0%) | |
| Vomiting | 1.9% (1.3 to 2.7) for vaginal births 16.1% (11 to 23.7) for caesareans | 0.5% (0.3 to 0.9) for vaginal births 4.6% (2.9 to 7.4) for caesareans | 1.3% (0.8 to 2) for vaginal births 11.2% (7.1 to 17.6) for caesareans | 0.6% (0.5 to 0.6) for vaginal births 5.2% (4.9 to 5.5) for caesareans | There was no evidence of global inconsistency (P = 0.06) in this analysis. |
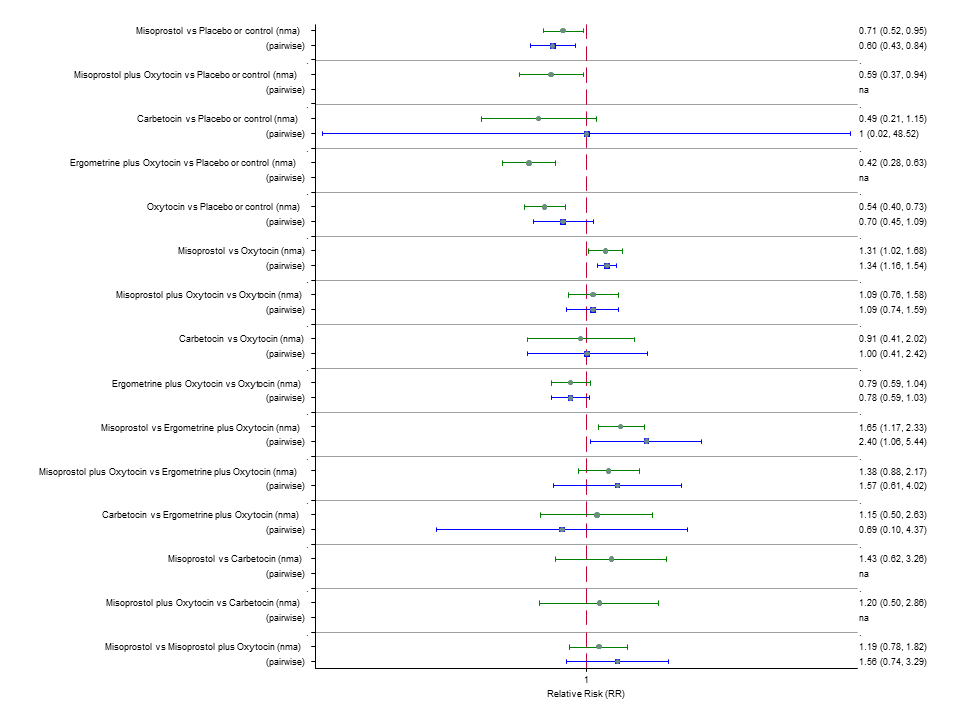
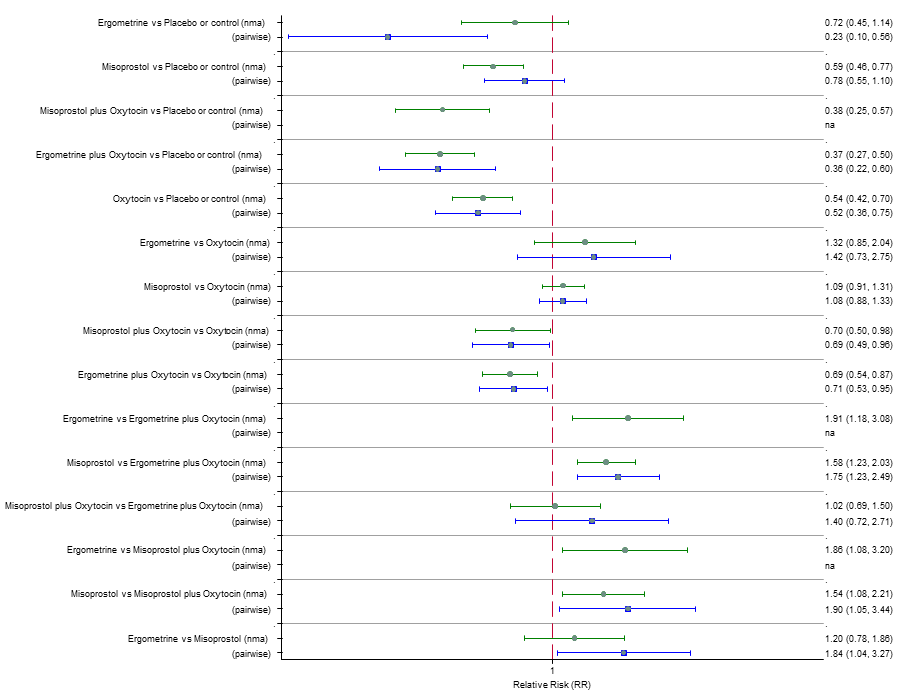
| RR 3.10 (2.11 to 4.56) (NMA) RR 3.15 (1.72 to 5.78) (Pairwise) | RR 0.89 (0.55 to 1.42) (NMA) RR 0.88 (0.39 to 1.99) (Pairwise) | RR 2.16 (1.37 to 3.39) (NMA) RR 2.25 (1.45 to 3.48) (Pairwise) | 1 |
| ⊕⊕⊕⊕ high confidence in estimate based on 8 studies (9811 women, I2 = 48.1%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, inconsistency and imprecision based on 10 studies (1939 women, I2 = 59.2%) | ⊕⊕⊕⊕ high confidence in estimate due to imprecision based on 9 studies (5015 women, I2 = 30.1%) | |
| Hypertension | 1.2% (0.4 to 4) for vaginal births 29.6% ( to ) for caesareans | 0.6% (0.1 to 3.3) for vaginal births 14.2% (2.5 to 79.7) for caesareans | Risks not available as no studies report this outcome | 0.7% (0.7 to 0.8) for vaginal births 16.7% (11.2 to 24.9) for caesareans | There was no evidence of global inconsistency (P = 0.481) in this analysis. |
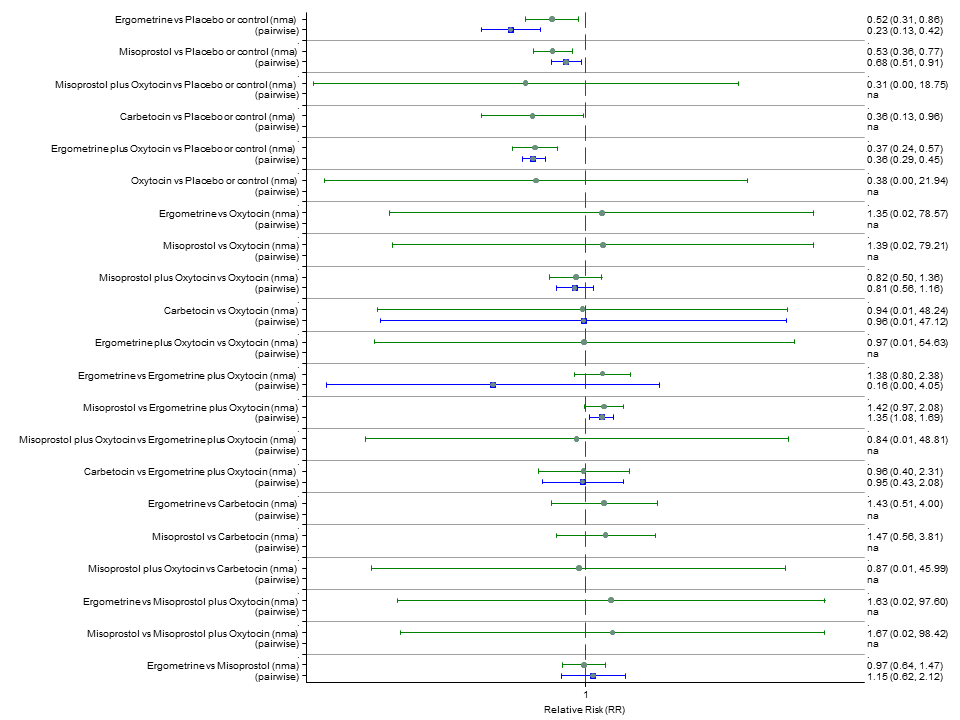
| RR 1.77 (0.55 to 5.66) (NMA) RR 0.95 (0.10 to 8.38) (Pairwise) | RR 0.85 (0.15 to 4.77) (NMA) | RR not available as no studies reported this outcome | 1 |
| ⊕⊕⊝⊝ low confidence in estimate due to inconsistency and imprecision based on 2 studies (1039 women, I2 = 73.2%) | ⊕⊕⊝⊝ low confidence in estimate due to imprecision and based only on indirect evidence | Quality of the evidence cannot be assessed as no studies report this outcome | |
| Fever | 3% (1.5 to 6) for vaginal births 11.7% (6.5 to 23.2) for caesareans | 3.1% (0.8 to 12.1) for vaginal births 12% (3.1 to 46.6) for caesareans | 11.4% (8 to 16.4) for vaginal births 44.2% (30.9 to 63.2) for caesareans | 3.6% (3.4 to 3.9) for vaginal births 13.9% (11.7 to 16.6) for caesareans | There was no evidence of global inconsistency (P = 0.352) in this analysis. |
| RR 0.84 (0.42 to 1.67) (NMA) RR 1.07 (0.47 to 2.43) (Pairwise) | RR 0.86 (0.22 to 3.35) (NMA) RR 2.11 (0.18 to 24.40) (Pairwise) | RR 3.18 (2.22 to 4.55) (NMA) RR 2.96 (1.95 to 4.51) (Pairwise) | 1 |
| ⊕⊕⊕⊝ moderate confidence in estimate due to imprecision based on 2 studies (1591 women, I2 = 0%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, inconsistency and imprecision based on 3 studies (292 women, I2 = 40.9%) | ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 15 studies (8209 women, I2 = 77.8%) | |
| *The risks in the ergometrine plus oxytocin, carbetocin, misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effects of the interventions (and its 95% CI). **The risk in the oxytocin group (and its 95% confidence interval) is based on a meta‐analysis of proportions from the studies included in this review for this group.
RR: Risk ratio |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |