Intervenciones de autocuidado, incluidos los planes de acción para las exacerbaciones, versus la atención habitual en pacientes con enfermedad pulmonar obstructiva crónica
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: RCT Follow‐up: 24 months Control group: usual care | |
| Participants | Recruitment: general practice Assessed for eligibility: 748 Randomly assigned: Intervention (I): 55; Control (C): 55 Completed: I: 49; C: 44 Mean age: I: 65.5 ± 11.5 years; C: 63.5 ± 10.3 years Gender (% male): I: 67; C: 51 COPD diagnosis: GOLD, mild, moderate, severe airflow obstruction Inclusion of participants in the acute phase: not reported Major inclusion criteria: aged at least 35 years, post‐bronchodilator ratio of FEV₁/FVC < 0.70 Major exclusion criteria: post‐bronchodilator FEV₁ < 30% predicted, treatment by a respiratory physician, severe comorbid conditions with a reduced life expectancy, inability to communicate in the Dutch language, and objections to one or more of the modes of disease management used in the study | |
| Interventions | Mode: individual sessions at the general practice, paper modules "Living well with COPD", telephone calls Duration: 2 to 4 individual face‐to‐face sessions of one hour each scheduled over 4 to 6 consecutive weeks, 6 telephone calls to reinforce self‐management skills Professional: practice nurse of each participating practice Training of case managers: before the study, all nurses were trained in how to apply the self‐management programme Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD Self‐management topics: (home) exercise, (maintenance) medication, coping with breathlessness/breathing techniques, maintaining a healthy lifestyle, managing stress and anxiety. Exercise programme: no Smoking cessation programme: no Behavioural change techniques: 10 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents. Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support | |
| Outcomes | 1. change from baseline in health‐related quality of life (CRQ) 2. change in CRQ domain scores 3. exacerbation frequency and management 4. total and five domain scores for self‐efficacy (CSES) | |
| Notes | A third group of participants (N = 55) were assigned to routine monitoring through scheduled periodic monitoring visits as an adjunct to usual care. However, this group does not include an action plan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomised participants by using a computer generated two block randomisation procedure with stratification on severity of COPD (mild or moderate v severe airflow obstruction), smoking status (current v former smoker), and frequency of exacerbations in the previous 24 months (< 2 v ≥ 2 exacerbations)." p. 2 Comment: Random sequence generation was adequately performed |
| Allocation concealment (selection bias) | Unclear risk | "We randomly allocated patients to usual care, self management or routine monitoring." p. 2. "To ensure that the investigators were blinded to individual treatment allocation, practice nurses informed the patients of their allocation." p. 2 Comment: No information on who performed the allocation |
| Blinding of participants and personnel (performance bias) | High risk | "This was a 24 month, multicentre, investigator blinded, three arm, parallel group, randomised controlled trial." p. 2 Comment: No blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Low risk | "Investigator blinded study." p. 2 "Outcome assessment with standardised questionnaires and a telephonic exacerbation assessment system (TEXAS)." Comment: Outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Baseline characteristics did not differ between dropouts and participants who completed follow‐up (p. 3). The dropout rate was lowest in the self management group, which may suggest that participants in this group were more motivated to adhere to COPD treatment because they were more “involved” in the long term management of their disease. p. 4 Our primary analysis was based on intention to treat principle and included all available data for all participants. We did not impute any missing data. p. 3 Comment: Almost 16% of the participants dropped out during follow‐up (intervention 11%; usual care 20%). However, baseline characteristics did not differ between dropouts and participants who finished follow‐up. Exclusion is well described in flow chart. Intention‐to‐treat analyses were used |
| Selective reporting (reporting bias) | Low risk | "Data sharing: Technical appendix, statistical code, and dataset are available from the corresponding author." p. 5 Comment: Not all secondary outcome measures were assessed. However, no signs for selective outcome reporting |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 and 24 months Control group: usual care | |
| Participants | Recruitment: hospital (outpatient) Assessed for eligibility: not reported Randomly assigned: I: 96; C: 95 Completed: I: 86; C: 79 Mean age: I: 69.4 ± 6.5 years; C: 69.6 ± 7.4 years Gender (% male): I: 52; C: 59 COPD diagnosis: FEV₁ after the use of a bronchodilator between 25% and 70% of the predicted normal value and FEV₁–FVC ratio less than 70% Inclusion of participants in the acute phase: no Major inclusion criteria: hospitalised at least once in the preceding year for an exacerbation, stable COPD (respiratory symptoms and medication unchanged for at least 4 weeks before enrolment), at least 50 years of age, current or previous smoker (at least 10 pack‐years), FEV₁ after the use of a bronchodilator between 25% and 70% of the predicted normal value 14 and FEV₁–FVC ratio less than 70%, no previous diagnosis of asthma, left congestive heart failure, terminal disease, dementia, or uncontrolled psychiatric illness, no participation in a respiratory rehabilitation programme in the past year, and no long term‐care facility stays. Major exclusion criteria: participants with asthma as a primary diagnosis and those with major comorbidities (documented left ventricular failure and any terminal disease), dementia or uncontrolled psychiatric illness | |
| Interventions | Mode: individual sessions at the participant's home, "Living well with COPD" programme with patient workbook, telephone calls Duration: seven face‐to‐face individual sessions of one hour each scheduled in seven to eight consecutive weeks, 18 telephone calls (weekly calls for eight weeks educational period, after eight weeks monthly phone calls for 12 months) Professional: experienced health professionals (nurses, respiratory therapists, a physiotherapist) who acted as case managers with the supervision and collaboration of the treating physician Training of case managers: "The programme was supervised by experienced and trained health professionals..." p. 586 “Half‐day training sessions were dedicated to interactive lecturing sessions on each aspect of COPD given by different members of the multidisciplinary team. The rest of the training days included workshops oriented toward how to assess patient needs and the acquisition of motivational and teaching skills using group discussion, demonstration and practice of techniques, case scenarios, and role modeling." Bourbeau 2006, p. 1705 Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, coping with breathlessness/breathing techniques, other: energy conservation during day‐by‐day activities, relaxation exercises, adopting a healthy lifestyle, leisure activities and travelling, long‐term oxygen when appropriate Exercise programme: yes, home‐based exercise program. The exercise teaching began at about the 7th week, and the training program was initiated with a supervised session at home. The exercise program included warm‐up and stretching exercises, muscle exercises, and cardiovascular exercises (stationary bicycle, walking, or climbing stairs). Participants were encouraged to follow the exercise program at least 3 times per week for 30 to 45 minutes per session. Smoking cessation programme: no Behavioural change techniques: 10 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, other: symptom monitoring list for different situations (stress, environmental change, and respiratory tract infection) linked to appropriate therapeutic actions | |
| Outcomes | 1. hospital admissions 2. scheduled and unscheduled physician visits 3. emergency department visits 4. health‐related quality of life (SGRQ) 5. pulmonary function 6. functional exercise capacity 7. exacerbations | |
| Notes | Completed first year of follow‐up: N = 165 (based on hospital registry database) Completed second year of follow‐up: N = 175 (based on provincial health insurance and hospitalisation database records) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “… central computer generated list of random numbers. Randomisation was stratified per center and in blocks of 6, and patients were assigned to the self‐management programme (intervention group) or to usual care.” p. 586 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | “The blocking factor was not known by the investigators or their staff in each participating center." p. 586 Comment: Allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | "Since a double‐blind design was impossible..." p. 586 Comment: Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | "... an independent evaluator unaware of the patient assignment was responsible for the evaluation process in each center. The evaluator was cautioned not to ask about the workbook modules and types of contact.” p. 586 Comment: Outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | “At the end of the 2nd year of follow‐up, data were available for 75 patients in the standard‐care group (two subjects were lost to follow‐up, nine patients died in the 1st year and nine in the 2nd year) and 83 patients following the self‐management programme (five patients died in the 1st year and eight in the 2nd year).” Gadoury 2005, p. 855 Comment: Drop out in the usual care group was somewhat higher than in the self‐management group; however, an intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selective reporting, however no protocol available. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: hospital (inpatient) Assessed for eligibility: 1405 Randomly assigned: I: 232; C: 232 Completed: I: 211; C: 200 Mean age: I: 70.0 ± 9.3 years; C: 68.3 ± 9.2 years Gender (% male): I: 38; C: 35 COPD diagnosis: chronic irreversible airflow limitation with FEV₁ less than 70% predicted and a FEV₁ /FVC ratio of less than 70%. FVC is defined as the total amount of air that can be expelled from the chest by a forced expiratory manoeuvre Inclusion of participants in the acute phase: not reported Major inclusion criteria: admitted to hospital with an acute exacerbation of COPD Major exclusion criteria: a history of asthma or left ventricular failure, evidence of active malignant disease or any evidence of confusion/poor memory, assessed with the abbreviated mental test (scores of 9/10 or 10/10 required). | |
| Interventions | Mode: individual sessions at the participant's home, adapted "Living well with COPD" booklets, telephone calls Duration: four face‐to‐face individual sessions of 40 minutes each scheduled fortnightly over a two month period. There were also 828 phone calls to the intervention group participants (mean 4.6 phone calls per intervention patient). There were at least six subsequent home visits (but more frequently on request) thereafter for a total of 12 months Professional: study nurse Training of case managers: "Study nurses’ training was based on self regulation theory ." (p. 2). "Nurses were trained to deliver a structured self management programme in four fortnightly home visits (…). Nurses without previous respiratory training completed three half day training sessions." (p. 3) Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques Exercise programme: no Smoking cessation programme: no Behavioural change techniques: 12 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, comparison of outcomes, regulation, antecedents, self‐belief Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support | |
| Outcomes | 1. time to first acute hospital admission with a COPD exacerbation 2. death due to COPD within 12 months of randomisation 3. morbidity (change from baseline at six and 12 months in SGRQ) 4. likelihood of anxiety or depression (HADS) 5. sense of self efficacy (CSES) 6. quality of life (EuroQol 5D) | |
| Notes | Self management materials based on the Living Well with COPD programme and previously adapted for the UK population and healthcare setting by an iterative process, were used (p. 2). Extra information author: "We used adapted “Living with COPD” booklets and daily diary cards (Stockley et al. – originally developed for use in Bronchiecistasis, piloted these and adapted them for this study, to include a line for recording steroid and antibiotic usage." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “We used a minimisation technique to stratify randomisation of participants by demographic factors (deprivation category of area of residence,11 age and sex, FEV1 per cent predicted at the time of randomisation, smoking status, participation in pulmonary rehabilitation within two years, and number of previous admissions) to control for key aspects of disease severity and predictors of readmission. We constructed a computer generated sequence by using the method of randomised permuted blocks of length four, with allocations being made at random and two by minimisation.” p. 2 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | “Treatment group allocations were obtained by telephone, after baseline assessment had been made. This registered the participant on the system, and a researcher entered the characteristics necessary for the minimisation algorithm by using an interactive voice response system. The researcher did not know whether a participant was being allocated at random or by minimisation and could therefore not determine the next treatment allocation before enrolling each participant” p. 2 Comment: Allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: No blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Participants received monthly telephone calls from an independent researcher, blinded to the patients’ randomisation status, to collect information on health service usage and exacerbations.” p. 2 Comment: Outcome assessor partly blinded (researcher was blinded, participants were not blinded). |
| Incomplete outcome data (attrition bias) | High risk | “The number of questionnaires available for analysis varied between outcomes and time points owing to the number of questionnaires returned and the completeness of the returned questionnaires.” p. 4 “Completion rates for study questionnaires were also disappointing and were lower in the control arm of the study. Consequently, the apparent improvements in the intervention arm (impacts subscale of St George’s Respiratory Questionnaire, hospital anxiety and depression scale anxiety) could be biased, and these results cannot be taken as convincing evidence in favour of the intervention.” p. 5 Comment: A lot of missing data for study questionnaires. |
| Selective reporting (reporting bias) | High risk | “Participants received monthly telephone calls from an independent researcher, blinded to the patients’ randomisation status, to collect information on health service usage and exacerbations.” Comment: Healthcare usage and number of exacerbations during follow‐up were not reported. Difference in length of hospital stay (all causes and sub classified by principle diagnosis) not reported. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: outpatient clinic Assessed for eligibility: not reported Randomly assigned: I: 38; C: 12 Completed: I: 30; C: 11 Mean age: I: 63.8 ± 8.4 years; C: 64.6 ± 6.8 years Gender (% male): 63% of 41 participants who completed the study; the distribution of males per group is not reported COPD diagnosis: GOLD, COPD with obstruction confirmed by spirometry and FEV₁ / FVC < 70% Inclusion of participants in the acute phase: not reported Major inclusion criteria: diagnosis of COPD with obstruction proven by spirometry and a FEV1/FVC < 70% Major exclusion criteria: comorbidities which significantly influences symptoms, capacity or spirometry (symptomatic cardiopulmonary disease) | |
| Interventions | Mode: group sessions (six to eight participants) at the participant's home Duration: four face‐to‐face group sessions of two hours each with the final session scheduled six weeks later Professional: respiratory nurse under supervision of a respiratory specialist Training of case managers: nurses were trained for 10 hours Self‐management components: action plan COPD exacerbations, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, other: travelling, daily live (life style modification) Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, coping with breathlessness/breathing techniques Exercise programme: no Smoking cessation programme: yes, motivation and guidance by the smoking cessation program Behavioural change techniques: eight clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, comparison of behaviour, associations, comparison of outcomes, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, avoid situations in which viral infection might be prevalent, contact healthcare providers for support | |
| Outcomes | 1. mMRC 2. courses of antibiotics 3. FEV₁ (L) 4. hospital admissions 5. 6MWT | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The method used to generate the random sequence generation was not clearly reported. |
| Allocation concealment (selection bias) | Unclear risk | Information from the author: ‘Pick of envelope. Enrolment and selection were right before the start of the study – a selection bias cannot be fully excluded.’ Comment: This information is too concise to assess the risk of bias for allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Blinding of outcome assessment was not reported. |
| Incomplete outcome data (attrition bias) | High risk | Comment: Eight participants in the intervention group and one participant in the control group dropped out. Reasons for dropout were not clearly reported, and only participants who completed follow‐up were included in the baseline characteristics and analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs for selective outcome reporting, results were reported extensively; however, no protocol was available. |
| Other bias | Unclear risk | Comment: Per protocol analysis, baseline characteristics only assessed for the participants who completed the study. No differences reported for baseline characteristics between the withdrawals after randomisation (N = 9) and the participants who completed the study |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: hospital (inpatient) Assessed for eligibility: 850 Randomly assigned: I: 65; C: 90 Completed: I: 48; C: 72 Mean age: I: 70 ± 9 years; C: 72 ± 9 years Gender (% male): I: 77; C: 88 COPD diagnosis: 21 (14%) of participants had an FEV₁/FVC > 70%. However, these participants cannot be identified from the article. Inclusion of participants in the acute phase: yes, during hospitalisation Major inclusion criteria: admitted because of a previous episode of exacerbation requiring hospitalisation for > 48 hours Major exclusion criteria: not living in the healthcare area, severe comorbid conditions, logistical limitations due to extremely poor social conditions and being admitted to a nursing home | |
| Interventions | Mode: individual and group sessions at the hospital and the participant's home, telephone calls, ICT platform Duration: 3‐13 face‐to‐face individual sessions, one group session of 40 minutes and six phone calls; three individual sessions at the hospital of 40 minutes each and one to 10 (depending on the patient's needs) of 20 minutes each at the participant's home. Barcelona: one joint visit at home. Leuven: GP regularly visited participants at home. Weekly phone calls during the first month and phone calls after three and nine months Professional: respiratory nurse, GP, primary care team (physician, nurse, social worker) Training of case managers: GP's in Leuven were trained, also by the specialized respiratory nurse specifically trained for the study intervention Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, other: reinforcement of the logistics for treatment of comorbidities and social support was carried out accordingly Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: travelling, end‐of‐life decision making, interpretation of medical testing, irritant avoidance, anxiety and panic control Exercise programme: no Smoking cessation programme: no Behavioural change techniques: 10 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, other: reinforcement of the logistics for treatment of comorbidities and social support was carried out accordingly | |
| Outcomes | 1. all‐cause (re‐)hospitalisations 2. all‐cause mortality 3. use of healthcare resources | |
| Notes | This study was conducted in two cities, Barcelona (Spain) and Leuven (Belgium), with marked differences in the primary care settings. Consequently, the intervention required customisation to country specificities, particularly regarding the interactions between hospital and primary care teams. The subgroup from Barcelona (Spain) was also reported in Garcia‐Aymerich 2007. However, in the current study other outcome measures and different numbers of participants were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All 155 patients included in the study were blindly assigned (1:1 ratio) using computer generated random numbers to either IC or usual care (UC)." p. 124 Comment: Random sequence generation was adequately performed |
| Allocation concealment (selection bias) | Low risk | "Adequacy of the assignment process to either IC or UC was ensured by both the generation of the allocation sequence by a random process and preventing foreknowledge of the treatment assignments in the specialised team that implemented the allocation sequence." p. 128 Comment: Allocation was adequately concealed |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Early assessment of patients at study admission was identical for both groups. Assessment included a blind administration of a questionnaire, described in detail elsewhere. (…) Assessment of the use of healthcare resources by phone or personal interview was carried out at 1, 3, 6, 9 and 12 months in both arms of the study. Data regarding admissions during follow‐up were obtained from hospital records. Data regarding mortality were obtained from hospital records and direct family interviews.” p. 125 Comment: Only part of the baseline assessment was blinded; the other assessments were not blinded, and it is unclear who performed the phone, personal or family interviews. |
| Incomplete outcome data (attrition bias) | Low risk | “A strength of the present analysis was that there were no subjects lost to follow‐up, since all drop‐outs were due to appearance of exclusion criteria or death (fig. 1) and, in any case, valid information about re‐hospitalisations was available from the national health services.” p. 128 Comment: Data on healthcare utilization were presented for all included participants, leading to a low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selective reporting; however, no protocol available |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: guideline‐based usual care | |
| Participants | Recruitment: outpatient clinic Assessed for eligibility: 467 Randomly assigned: I: 209; C: 217 Completed: I: 201 continued, 101 completed baseline and 1‐year study visits; C: 207 continued, 108 completed baseline and 1‐year study visits Mean age: I: 66.2 ± 8.4 years; C: 65.8 ± 8.2 years Gender (% male): I: 97.6; C: 96.3 COPD diagnosis: GOLD, a post‐bronchodilator ratio of FEV₁/FVC < 0.70 with an FEV₁ < 80% predicted. At baseline and 1‐year study visits, post‐bronchodilator spirometry performed according to ATS criteria Inclusion of participants in the acute phase: no Major inclusion criteria: hospitalised for COPD in the 12 months before enrolment, post‐bronchodilator ratio of FEV₁ to FVC < 0.70 with an FEV₁ < 80% predicted, age older than 40 years, current or past history of cigarette smoking (> 10 pack‐years), at least 1 visit in the past year to either a primary care or pulmonary clinic at a Veterans Affairs medical centre, no COPD exacerbation in the past 4 weeks, ability to speak English, and access to a telephone. Major exclusion criteria: primary diagnosis of asthma or any medical conditions that would impair ability to participate in the study or to provide informed consent. | |
| Interventions | Mode: individual and group sessions at hospital outpatient clinics, telephone calls, educational booklet Duration: four face‐to‐face individual sessions of 90 minutes each scheduled weekly. The individual lessons were reinforced during a group session and by six phone calls, one per month for three months and every three months thereafter. Professional: case manager (various health‐related professionals) Training of case managers: before starting the study, all case managers received a three‐day training course with workshops covering detailed aspects of the self‐management programme, and all were supervised by the site investigator Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD Self‐management topics: smoking cessation, exercise, (maintenance) medication, coping with breathlessness/breathing techniques Exercise programme: no Smoking cessation programme: no Behavioural change techniques: nine clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support | |
| Outcomes | 1. time from randomisation to first COPD hospitalisation 2. all‐cause mortality 3. number of exacerbations 4. health‐related quality of life 5. patient satisfaction 6. medication adherence 7. COPD‐related knowledge, skill acquisition and self‐efficacy | |
| Notes | This multi site RCT of an educational and acute care management programme was stopped early when a safety monitoring board noted excess mortality in the intervention group. The mean follow‐up time was 250 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation lists were generated on the basis of random, permuted blocks of variable size to ensure approximate balance over time.” p. 674 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | “The CSP Coordinating Center in Boston, Massachusetts, randomly assigned eligible patients in equal numbers to 2 groups, stratifying patients per site to allow for possible regional differences in patient characteristics and clinical practice patterns.” p. 674 Comment: The allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | “The 2 groups differed on the basis of a complex behavioral intervention that made blinding impossible.” p. 674 Comment: No blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | “Telephone‐based ascertainment of study outcomes (COPD hospitalizations and exacerbations) was performed by centralized research staff blinded to assignment. All outcomes were collected by centralized staff blinded to study group, and all hospitalizations were adjudicated by a committee that was also blinded to study group.” p. 674 Comment: Outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | “This multi‐site, randomised, controlled trial of an educational and acute care management programme was stopped early when a safety monitoring board noted more deaths in the intervention group.” p. 674 Comment: There is incomplete outcome data due to early termination of the study. |
| Selective reporting (reporting bias) | Low risk | Comment: The primary and secondary outcomes were reported, only healthcare costs as (secondary objective) were not reported. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: hospital (outpatient) Assessed for eligibility: not reported Randomly assigned: I: 31; C: 31 Completed: I: 26; C: 27 Mean age: I: 57 ± 9 years; C: 58 ± 10 years Gender (% male): I: 48; C: 52 COPD diagnosis: FEV₁ equal to or higher than 40% and lower than 80% of predicted Inclusion of participants in the acute phase: not reported Major inclusion criteria: participants with COPD, <70 years of age, a FEV₁ equal to or higher than 40% and lower than 80% of predicted Major exclusion criteria: not suffering from any serious disease such as unstable coronary heart disease, heart failure, serious hypertension, diabetes mellitus, kidney or liver failure | |
| Interventions | Mode: individual and group sessions at an outpatient clinic Duration: one to two face‐to‐face individual sessions by a nurse and one to two face‐to‐face individual sessions by a physiotherapist of 40 minutes each. Two two‐hour group education sessions (five to eight persons) were scheduled on two separate days. Professional: nurse, physiotherapist, pharmacist, medical doctor Training of case managers: specially trained nurse Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, other: compliance, self‐care Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, coping with breathlessness/breathing techniques Exercise programme: no Smoking cessation programme: no Behavioural change techniques: nine clusters: goals and planning, social support, feedback and monitoring, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support | |
| Outcomes | 1. health‐related quality of life (SGRQ and four simple questions) 2. hospital admissions 3. days lost from work 4. GP consultation 5. FEV₁ % predicted | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients signed a written consent and were then randomly assigned using random number tables supplied by an external statistician in sealed envelopes" Gallefoss 2002, p. 425 Comment: Random sequence generation was adequately performed |
| Allocation concealment (selection bias) | Low risk | "The patients signed a written consent and were then randomly assigned using random number tables supplied by an external statistician in sealed envelopes" Gallefoss 2002, p. 425 Comment: Allocation was adequately concealed |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Blinding of outcome assessment was not reported; not clear who performed the measurements. |
| Incomplete outcome data (attrition bias) | Low risk | “In the intervention group, four patients failed to complete the educational programme (social problems (n = 1), unannounced emigration (n = 1), failure to meet at educational group sessions for unknown reasons (n = 1) and serious myocardial infarction (n = 1)). Another patient was withdrawn from the study during the follow‐up due to lymphoma (n = 1). This left us with 26 patients (81%) for a 1‐year follow‐up. The patients who were withdrawn from the intervention group did not, to our knowledge, have any serious deterioration in their obstructive lung disease, and none were hospitalised. In the control group four patients were withdrawn (lack of co‐operation (n = 2), diagnosis of rectal cancer (n = 1) and emigration (n = 1)). Two of the withdrawn control group patients were hospitalised for exacerbations of their COPD. This left us with 27 patients (84%) for the 1‐year follow‐up” Gallefoss 2002, p. 427 Comment: The number of dropouts was relatively low, and reasons for dropout were comparable over groups. |
| Selective reporting (reporting bias) | Low risk | Comment: No signs of selective outcome reporting; study extensively described in various articles. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: hospital (inpatient) Eligible: not reported Randomly assigned: I: 44; C: 69 Completed: I: 21; C: 41 Mean age: I (follow‐up): 72 ± 10 years, I (no follow‐up): 73 ± 6 years; C (follow‐up): 73 ± 9 years, C (no follow‐up): 74 ± 8 years Gender (% male): I: 75; C: 93 COPD diagnosis: some of the participants had an FEV₁/FVC > 70%. However, these participants cannot be identified from the article. Inclusion of participants in the acute phase: yes, during hospitalisation Major inclusion criteria: admitted because of a previous episode of exacerbation requiring hospitalisation for > 48 hours Major exclusion criteria: not living in the healthcare area or living in a nursing home, lung cancer or other advanced malignancies, logistical limitations due to extremely poor social conditions and extremely severe neurological or cardiovascular comorbidities | |
| Interventions | Mode: individual sessions at the hospital and the participant's home, telephone calls, ICT platform Duration: 3‐13 face‐to‐face individual sessions at the hospital of 40 minutes each and one to 10 (depending on the patient's needs) of 20 minutes each at the participant's home. six phone calls, weekly during the first month and phone calls after three and nine months. Professional: specialised respiratory nurse and primary care team (physician, nurse, social worker) Training of case managers: an educational session of approximately two hours duration on self‐management of the disease was administered at discharge, also by the specialized respiratory nurse specifically trained for the study Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, other: reinforcement of the logistics for treatment of comorbidities and social support was carried out accordingly Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: travelling, end‐of‐life decision making, interpretation of medical testing, irritant avoidance, anxiety and panic control Exercise programme: no Smoking cessation programme: no Behavioural change techniques: 10 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, other: reinforcement of the logistics for treatment of comorbidities and social support was carried out accordingly | |
| Outcomes | 1. health‐related quality of life (SGRQ and EQ‐5D) 2. FEV₁ (L) 3. FEV₁/FVC 4. clinical factors (comorbidities, MRC dyspnoea, BMI) 5. lifestyle (smoking, alcohol, physical activity) 6. self‐management (knowledge, identification and early treatment, adherence 7. satisfaction with health services | |
| Notes | The current study was conducted in Barcelona (Spain) only. This subgroup was also reported in Casas 2006. However, in the current study other outcome measures and different number of participants were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “and were blindly assigned (1:2 ratio) using computer generated random numbers either to integrated care (IC) or to usual care (UC).” p. 1463 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | “and were blindly assigned (1:2 ratio) using computer generated random numbers either to integrated care (IC) or to usual care (UC).” p. 1463 Comment: No information on allocation concealment. The allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | “Early assessment of patients at their admission to the study was identical for both groups. It included a blind administration of a questionnaire, described in detail elsewhere.” p. 1464 Comment: The administration of a questionnaire was blinded. |
| Incomplete outcome data (attrition bias) | High risk | “During follow‐up, a priori defined exclusion criteria, such as lung cancer, appeared in 9 subjects. Twente‐one subjects died, and 16 were lost to follow‐up. Only 57% of subjects finished the study at 12 months. (…) Since date about outcome variables was not available in the lost subjects (whether due to exclusion, loss to follow‐up or death), an intention‐to‐treat principle was not possible.” p. 1464 Comment: More than 40% of the data on functional status and HRQoL reported was missing, leading to a high risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Comment: VAS was reported, but the Euroqol (EQ‐5D) was not reported. No signs of selective reporting; however, no protocol available. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 (and 72 months passive follow‐up thereafter) Control group: usual care | |
| Participants | Recruitment: hospital (outpatient) Assessed for eligibility: 860 Randomly assigned: I: 71; C: 84 Completed: I: 54; C: 55 Mean age: I: 73 ± 8 years; C: 75 ± 9 years Gender (% male): I: 83; C: 86 COPD diagnosis: a person not involved in the study identified the cases with COPD (ICD9‐CM 491, 492, 493 or 496) as the primary diagnosis for admission. However, lung function testing was also assessed before randomisation Inclusion of participants in the acute phase: no Major inclusion criteria: clinically stable COPD participants with a history of at least two hospital admissions owing to severe respiratory exacerbations during two consecutive years, we considered a broad spectrum of COPD diagnostic terms that include chronic obstructive inflammatory diseases namely, emphysema, asthma, tuberculosis, chronic bronchitis and COPD, aged above 45 years and living at home within the healthcare area of the hospital (Barcelona‐Esquerra) Major exclusion criteria: nursing home or not living in the area, participants in another randomised controlled trial, exitus prior to contact | |
| Interventions | Mode: individual and group sessions at an outpatient clinic and at the participant's home Duration: at least one face‐to‐face individual session of 40 minutes at the patient's home within 72 hours after entry into the study by the primary care team (participants without mobility problems), four face‐to‐face individual sessions of 15 minutes education each at the patient's home by the primary care team (participants with mobility problems), one two‐hour individual or group educational programme of 40 minutes. Three group sessions for participants without mobility problems (two comprehensive assessments of 90 minutes each at the outpatient clinic and one two‐hour educational programme) and for participants with mobility problems, the programme was done at home. In all visits, the nurses dedicated 15 minutes for education. Professional: specialised respiratory nurse, primary care team (physician, nurse and social worker) Training of case managers: the community care teams received training: a two‐hour face‐to‐face educational training and one‐day stay at the hospital ward, aiming at enhancing home‐based management of frail COPD participants. Self‐management components: action plan COPD exacerbations, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, exercise or physical activity component, other: instructions on non‐pharmacological treatment Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: vaccination Exercise programme: yes, no extra information available Smoking cessation programme: yes, no extra information available Behavioural change techniques: eight clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, comparison of behaviour, associations, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, avoid situations in which viral infection might be prevalent, contact healthcare providers for support, self‐treatment of comorbidities | |
| Outcomes | 1. mental status 2. activities of daily living (Lawton index) 3. anxiety and depression (HADS) 4. health‐related quality of life (SGRQ) 5. sleepiness (Epworth sleepiness scale) 6. 6MWT 7. nocturnal pulse oximetry and body mass distribution 8. exacerbations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer‐generated list of random numbers with no restrictions and administered by personnel who were not involved in the study ensured blinded randomisation (1:1 ratio).” p. 2 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | “(…) and administered by personnel who were not involved in the study” p. 2 Comment: The allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) | Low risk | “A blind evaluation of the study group carried out before randomisation and after the 12‐month follow‐up consisted of a patient interview and analysis of medical records, self‐administered questionnaires and lung function testing.” p. 2 Comment: Outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | “The RCT was not included in the clinicaltrials.gov registry because at that time it was not compulsory.” p. 5 Comment: Not all outcome measures are reported (e.g., Epworth sleepiness scale, lung function, 6‐MWT). However, no protocol available. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care | |
| Participants | Recruitment: hospital (inpatient) Assessed for eligibility: 1225 Randomly assigned: I: 93; C: 79 Completed: I: 93; C: 79 Mean age: I: 64.88 ± 10.86 years; C: 64.43 ± 10.47 years Gender (% male): I: 43.4; C: 46.8 COPD diagnosis: based on spirometric testing in the prior year that demonstrated airflow obstruction (FEV₁/FVC , 70% and FEV₁ < 80%) based on GOLD criteria. If spirometric data were not available, a previously validated questionnaire was used in the diagnosis of COPD for purposes of study inclusion. The presence of airflow obstruction was then confirmed by spirometry prior to discharge. Inclusion of participants in the acute phase: yes, during hospitalisation Major inclusion criteria: diagnosis of COPD with the presence of an acute exacerbation, age > 40 years, and current or ex‐smoker with a history equivalent to at least 20 pack‐years. The diagnosis of AECOPD was made by the primary team but was confirmed by the research team prior to assessing eligibility for inclusion. AECOPD was defined as an acute event characterized by a worsening of the patient’s respiratory symptoms beyond normal day‐to‐day variations, leading to a change in medication. If there was a question about a true diagnosis of AECOPD, a pulmonologist on the research team evaluated the patient. Major exclusion criteria: a medical history of asthma, interstitial lung disease, bronchiectasis, presence of airway hardware (e.g., tracheal stents), lung cancer, any other cancer with an associated life expectancy of < 1 year, any cancer where the patient was receiving active chemotherapy or radiation treatment, active substance abuse, or neuromuscular disorders affecting the respiratory system, language barriers, residence in a nursing home, ICU stay during the current admission, and significant delirium or dementia. | |
| Interventions | Mode: individual sessions at a hospital and at the participant's home, telephone calls Duration: one face‐to‐face individual session of one hour at the hospital by a member of the research team 24 hours prior to the anticipated discharge day. 48 hours after discharge, participants were contacted by telephone to reinforce the items in the bundle. Professional: research team and research nurse Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, education regarding COPD, smoking cessation, other: the primary team was notified if a patient was identified as having anxiety or depressive symptoms, and referral to outpatient behavioral health services or pharmacologic treatment was deferred to the primary team Self‐management topics: smoking cessation, diet, correct device use, coping with breathlessness/breathing techniques, other: assess current behaviours to manage COPD Exercise programme: no Smoking cessation programme: yes, active smokers received smoking cessation counselling and, with patient agreement, were enrolled in the Henry Ford Health System Smoking Cessation Program Behavioural change techniques: eight clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, comparison of behaviour, associations, repetition and substitution, antecedents Action plan components: contact healthcare providers for support | |
| Outcomes | 1. 30‐day risk of readmission or ED visits for AECOPDs 2. 90‐day rate of COPD readmission | |
| Notes | The trial was stopped early after an interim analysis at 3 years did not demonstrate that further accrual could achieve the desired 10% difference in the primary composite end point of ED visit or rehospitalisation between the two groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer‐generated list was used to randomise patients in a 1:1 ratio, stratified by age and sex, to either the bundle or the control group.” Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: No information provided regarding blinding of participants and personnel. However, the participants and personnel could not be blinded as all participants assigned to the bundle group received a 60‐min visit by a member of the research team. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: No information provided regarding blinding of outcome assessment; however, objective outcome measures are used. |
| Incomplete outcome data (attrition bias) | Unclear risk | “The trial was stopped early after an interim analysis at 3 years did not demonstrate that further accrual could achieve the desired 10% difference in the primary composite end point of ED visit or rehospitalization between the two groups.” p. 1229 Comment: it seems that there were no dropouts after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | “The primary end point was the difference in the composite risk of hospitalizations or ED visits for AECOPDs between the two groups in the 30 days following discharge.” p. 1229 Comment: According to the protocol available in the Clinical Trials register the primary outcomes were the 30 day readmission rate and the time until readmission or ER visit, 30 days. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual hospital outpatient care | |
| Participants | Recruitment: hospital (outpatient clinic) Assessed for eligibility: not reported Randomly assigned: I: 86; C: 87 Completed: I: 71; C: 72 Mean age: I: 65.63 ± 10.1 years; C: 67.3 ± 9.2 years Gender (% male): I: 44.2; C: 43.7 COPD diagnosis: confirmed diagnosis of COPD (by the hospital consultant) for at least 1 year, having a FEV₁ of 30–80% of the predicted normal value Inclusion of participants in the acute phase: not reported Major inclusion criteria: confirmed diagnosis of COPD for at least 1 year, having a FEV₁ of 30–80% of the predicted normal value and >45 years old Major exclusion criteria: having congestive heart failure, moderate to severe learning difficulties (as judged by hospital consultant), attended a pulmonary rehabilitation programme in the last 6 months, and severe mobility problems or terminal illness | |
| Interventions | Mode: individual sessions at an outpatient clinic, telephone calls, booklet on techniques for expectoration Duration: one face‐to‐face individual session of 45 minutes (one hour for smokers) and two telephone calls at three and nine months Professional: clinical pharmacist, respiratory specialist, respiratory nurse Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques Exercise programme: no Smoking cessation programme: yes, advice, using the motivational interviewing technique, was provided to the participants who still smoked and referral to a special smoking cessation programme run within the hospital was made Behavioural change techniques: ten clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support | |
| Outcomes | 1. health‐related quality of life (SGRQ) 2. FEV₁ 3. hospital admissions for acute exacerbations 4. ED visits for acute exacerbations 5. GP visits, scheduled and unscheduled 6. knowledge of medication and disease management (COPD knowledge questionnaire) 7. adherence to prescribed medication | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Recruited patients were randomly assigned to one of two groups: the intervention group and the usual care (control group). Both groups were matched as closely as possible for the following parameters: severity of COPD (measured by FEV1), age, gender and other concomitant illness. The randomisation was carried out using the minimization method described by Gore.” p. 589 Comment: Random sequence generation was performed adequately. |
| Allocation concealment (selection bias) | Low risk | “Recruited patients were randomly assigned to one of two groups: the intervention group and the usual care (control group). Both groups were matched as closely as possible for the following parameters: severity of COPD (measured by FEV1), age, gender and other concomitant illness. The randomisation was carried out using the minimization method described by Gore.” p. 589 Comment: Allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Baseline measurements were performed by the research pharmacist (…) for operational reasons, the researcher could not be blinded to the group to which the patient belonged.” p. 590 Comment: Outcome assessment was not blinded; it was not clearly reported how the research pharmacist was related to the study. |
| Incomplete outcome data (attrition bias) | Low risk | “A per‐protocol analysis was used. (…) During the study period, three patients from the intervention group and five from the control group died and a total of 22 patients withdrew from the study; 12 patients from the intervention group and 10 from the control group.” p. 590 Comment: In both groups, 15 participants (17%) dropped out during the 12‐month follow‐up. Reasons for dropout were comparable across groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selective reporting; however, no protocol available. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care | |
| Participants | Recruitment: hospital (outpatient clinic) Assessed for eligibility: not reported Randomly assigned: I: 21; C: 21 Completed: I: 21; C: 21 Mean age: I: 56.6 ± 5.7 years; C: 56.2 ± 4.1 years Gender (% male): I: 61.9; C: 76.2 COPD diagnosis: diagnosed by a pulmonologist according to ATS criteria Inclusion of participants in the acute phase: not reported Major inclusion criteria: diagnosed by a pulmonologist according to ATS criteria, consent for participation in the study, being literate and having sufficient knowledge (at least to understand and fill out the questionnaires), having physical and mental ability to tolerate the interventions, absence of disease that limit the function and other medical conditions affecting the mortality Major exclusion criteria: primary diagnosis of asthma, hospitalisation during the intervention, main treatment with oxygen and occurrence of serious unexpected stresses during the study. | |
| Interventions | Mode: group sessions at a hospital (outpatient clinic), telephone calls Duration: eight face‐to‐face educational group sessions of 60‐90 minutes each (3‐4 member groups) with one week interval and during this 8‐week programme, participants of the intervention group were followed up by phone Professional: psychologist, trained psychiatric residents Training of case managers: the psychiatric residents are trained, but no further information is provided Self‐management components: action plan COPD exacerbations, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, coping with breathlessness/breathing techniques, other: healthy lifestyle, avoid places with air pollution, healthy sleep, sexual habits, stress management, free time activities, travelling, and behavioral interventions focusing on common issues like independence, decreased self‐esteem, feeling insecure, limited relation with family and friends Exercise programme: yes, simple regular exercise programme at home Smoking cessation programme: no Behavioural change techniques: six clusters: goals and planning, feedback and monitoring, shaping knowledge, natural consequences, associations, regulation Action plan components: avoid situations in which viral infection might be prevalent | |
| Outcomes | 1. severity of disease (CCQ questionnaire) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Blinding of outcome assessment was not reported. Not clear who performed the measurements. |
| Incomplete outcome data (attrition bias) | Low risk | “We also encouraged and followed up the patients by phone and even when someone was absent, we teached [sic] him/her over the phone. In this way, all patients accompanied us till the end of the course and no patient was excluded from the study." p. 28 Comment: All participants completed follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selective reporting, although only one outcome measure was reported. No protocol available. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: general practice Assessed for eligibility: not reported Randomly assigned: I: 44; C: 49 Completed: I: 35; C: 45 Mean age: I: 71.1 (95% CI 68.7‐73.5) years; C: 69.1 (95% CI 63.5‐74.7) years Gender (% male): I: 34.1; C: 65.3 COPD diagnosis: GOLD, a diagnosis of moderate or severe COPD Inclusion of participants in the acute phase: no (use of the plan was commenced at a time when each patient was in a stable condition) Major inclusion criteria: diagnosis of COPD, aged 55 years or over, at least one hospital admission or two acute exacerbations of COPD requiring GP care during the previous 12 months, a Mini Mental State Examination score > 22 Major exclusion criteria: terminally ill, coexisting lung cancer, admission to hospital with cardiac disease within previous 12 months, receiving home oxygen therapy. | |
| Interventions | Mode: individual sessions at a GP, hospital, ambulance service, emergency department or home‐based Duration: four face‐to‐face individual sessions, during the 12‐months period all participants were visited by a respiratory nurse at three, six and 12 months to provide routine support and further education regarding use of the plan Professional: respiratory physician, respiratory nurse, GP, ED consultant, medical staff hospital Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations Self‐management topics: (maintenance) medication Exercise programme: no Smoking cessation programme: no Behavioural change techniques: eight clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, comparison of behaviour, associations, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, self‐treatment of comorbidities, other: when/how to use oxygen therapy and when to use diuretics | |
| Outcomes | 1. health care utilisation (GP visits, hospital admissions, ambulance calls) 2. quality of life (SGRQ) 3. medication use (courses of oral steroids and antibiotics) | |
| Notes | Three participants subsequently withdrew for personal reasons. However, it was not reported in what group. A further 13 died during the follow‐up period (nine in the intervention group and four in the control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to the intervention (care plan) or control (usual care) groups." p. 192 Comment: The method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly assigned to the intervention (care plan) or control (usual care) groups." p. 192 Comment: The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Quality of life was measured by the St George’s Respiratory Questionnaire (SGRQ). The questionnaire was administered by the research nurse (DMcN) at each visit.” Comment: The blinding of outcome assessment was not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | “Three subsequently withdrew for personal reasons. A further 13 died during the follow‐up period (…) [nine in the intervention group and four in the control group (NS)]” p. 192 Comment: The number of withdrawals was higher in the intervention group compared to the control group. However, no information provided regarding the differences in dropout rates. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selective reporting, however no protocol available. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 6 months Control group: usual care | |
| Participants | Recruitment: general practice Assessed for eligibility: 326 Randomly assigned: I: 89; C: 95 Completed: I: 65; C: 79 Mean age: I: 69 ± 8 years; C: 69 ± 10.1 years Gender (% male): I: 60.7; C: 49.5 COPD diagnosis: a diagnosis of COPD confirmed by spirometry, with a FEV₁/FVC ratio < 0.7 Inclusion of participants in the acute phase: no Major inclusion criteria: have a diagnosis of COPD confirmed by spirometry, with a FEV₁/FVC ratio < 0.7, grade 2‐5 MRC dyspnoea scale, clinically stable for 4 weeks Major exclusion criteria: unable to undertake an exercise regime due to neurological, musculoskeletal or cognitive comorbidities, unable to read English to the reading age of an 8‐year‐old, completed pulmonary rehabilitation within the previous 12 months | |
| Interventions | Mode: individual sessions at a GP or home‐based, telephone calls, workbook Duration: one face‐to‐face individual session for 30‐45 minutes by a physiotherapist and two telephone calls at two and four weeks into the programme to reinforce skills and providing encouragement to progress Professional: physiotherapist, trainee health psychologist Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques Exercise programme: yes, home exercise programme consisting of a daily walking programme, and resistance training of the upper and lower limbs using free weights three times per week. Smoking cessation programme: no Behavioural change techniques: 11 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents, identity Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, avoid situations in which viral infection might be prevalent, contact healthcare providers for support, other: discussion with participants about self‐administration and requesting rescue medication from their primary care physician | |
| Outcomes | 1. health status (CRQ dyspnoea domain) 2. fatigue, emotion and mastery domains of the CRQ 3. disease knowledge (Bristol COPD Knowledge Questiionnaire) 4. anxiety and depression (HADS) 5. exercise capacity (ISWT, ESWT) 6. self‐efficacy (Pulmonary Rehabilitation Adapted Index of Self‐Efficacy) 7. healthcare utilisation (admissions, GP visits, ED visits, nurse home visits) 8. medication use (courses of antibiotics) 8. self‐reported smoking status | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician (J. Bankart).” p. 1539 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Unclear risk | “Randomisation was conducted by the trial investigator responsible for administering the intervention (K.E. Mitchell).” p. 1539 Comment: The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | “Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effects in the intervention arm. We cannot, therefore, rule out the possible impact of attention.” p. 1546 Comment: No blinding of participants. |
| Blinding of outcome assessment (detection bias) | Low risk | “The assessments at week 6 and 6 months were conducted by a member of the research team who was blind to randomisation allocation (V. Johnson‐Warrington).” p. 1540 Comment: The outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | “There were no significant differences in demographics or baseline variables between those who completed and those who did not complete the study. Analysis was carried out on an intention‐to‐treat basis. Missing data were imputed in Stata using multiple imputed chained equations. Analysis on imputed data sets were carried out using the micombine command in Stata, which analyses each dataset separately and combines the results.” p. 1540 Comment: No signs of incomplete outcome data. A bit more missing data in control group, maximum around 20%. |
| Selective reporting (reporting bias) | Low risk | Comment: No signs for selective outcome reporting. The primary outcome measure and most of the secondary outcome measures were reported. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: hospital (outpatient clinic) Assessed for eligibility: 615 Randomly assigned: I: 127; C: 121 Completed: I: 122; C: 114 Mean age: I: 65 ± 7 years; C: 65 ± 7 years Gender (% male): I: 85; C: 84 COPD diagnosis: clinical diagnosis of stable COPD, as defined by ATS criteria; FEV₁% predicted (pre): 25% to 80%; FEV₁/VC (pre): < 60% Inclusion of participants in the acute phase: no Major inclusion criteria: clinical diagnosis of stable COPD, no history of asthma, no exacerbation in the month prior to enrolment, current or former smoker, aged 40 to 75 years, baseline pre‐bronchodilator (FEV₁) 25–80% predicted, pre‐bronchodilator ratio FEV₁/VC < 60% Major exclusion criteria: maintenance treatment of oral steroids or antibiotics, medical condition with low survival or serious psychiatric morbidity, any other active lung disease | |
| Interventions | Mode: group sessions (approximately eight participants) at the outpatient clinic and community‐based, educational booklet Duration: five face‐to‐face group sessions for two hours each by a respiratory nurse (four sessions with a one‐week interval and the last (feedback) session was given three months after the fourth session) and one or two small group training sessions per week for 30‐45 minutes by a physiotherapist trained in COPD care Professional: respiratory nurse, respiratory physiotherapist Training of case managers: physiotherapists trained in COPD care Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: ergonomic posture and energy conservation during daily activities or work, communication and social relationships, coping with disease, recognising participants’ individual capacity, social interactions and behavioural changes Exercise programme: yes, one or two 1 h small group training sessions per week under guidance of a physiotherapist Smoking cessation programme: no Behavioural change techniques: ten clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support | |
| Outcomes | 1. health‐related quality of life (SGRQ) 2. self‐confidence 3. walking distance (6MWT) 4. exacerbations 5. COPD symptoms 6. healthcare utilisation (doctor consultations, hospital admissions) 7. healthcare costs (days lost from work) 8. preference‐based utilities (EuroQol, QALYs) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed in blocks of four, stratified by sex and smoking status, using sealed envelopes. ” p. 816 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | "Randomisation was performed in blocks of four, stratified by sex and smoking status, using sealed envelopes. ” p. 816 Comment: The allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: Outcome assessment was not blinded. However, measurements were performed by an assessor who was independent of the study. |
| Incomplete outcome data (attrition bias) | Low risk | "In the intervention group five patients (three deaths, two other) dropped out, as did seven patients (three deaths, two carcinoma, two other) in the control group." page 818 Comment: The number of withdrawals and reasons for withdrawal in both groups were comparable. Moreover, an intention‐to‐treat analysis was used and drop out was low. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selective reporting; however, no protocol available |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: hospital (outpatient, university‐based centre) Assessed for eligibility: 101 Randomly assigned: I: 23; C: 22 Completed: I: 20; C: 18 Mean age: I: 65 (range 59‐74) years; C: 61 (range 56‐65) years Gender (% male): I: 90; C: 77.8 COPD diagnosis: a FEV₁/FVC ratio of less than 0.70 Inclusion of participants in the acute phase: no Major inclusion criteria: stable COPD, 40 years of age or older, FEV₁/FVC ratio of less than 0.70, not previously been involved in pulmonary rehabilitation or had lived in a long‐term care facility, understood, read, and wrote French. Major exclusion criteria: previous diagnosis of asthma, oxygen dependence, unstable and/or uncontrolled cardiac disease, musculoskeletal problems precluding exercise training, a terminal disease, dementia or an uncontrolled psychiatric illness | |
| Interventions | Mode: individual and group sessions (four‐eight participants) at the hospital, telephone calls Duration: eight face‐to‐face group sessions (two per week) for two hours each by a health professional for four weeks, eight exercise sessions for 30‐45 min each under the supervision of a qualified exercise trainer, three telephone calls to encourage personalised endurance training and on reporting symptoms Professional: health professional and qualified exercise trainer Training of case managers: not reported Self‐management components: action plan COPD exacerbations, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques Exercise programme: yes, after each educational session (8 in total) within the same group, participants performed the usual exercise program used in our laboratory (i.e. cycling at the level of the ventilatory threshold for 30‐45 min under the supervision of a qualified exercise trainer). Smoking cessation programme: no Behavioural change techniques: nine clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, avoid situations in which viral infection might be prevalent | |
| Outcomes | 1. exercise training (change in 6MWD) 2. 6MWT 3. COPD‐specific health status (SGRQ) 4. perceived health status (Nottingham Health Profile) 5. maximal exercise capacity (peak work rate) 6. daily physical activity (Voorrips questionnaire) 7. healthcare utilisation (hospital admissions) 8. healthcare costs (cost of medication, hospitalisations) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were randomly assigned either to the self‐management programme or usual care group. The trial statistician, MCP, generated the random allocation sequence using the random procedure in SAS (SAS v.9.1 e SAS Institute, Cary NC), with a 1:1 allocation using block size of 5 (…)” p. 379 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | “(…) After the physician had obtained the patient’s consent, he sent by fax the randomisation form to the Clinical Research Unit (AJ) for allocation consignment re‐addressed by fax” p. 379 Comment: Alllocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | “Due to the nature of the intervention conditions, it is not possible to blind research participants or assessors. Several stratagems were adopted in an effort to ensure that objectivity was maintained as rigorously as possible. Participants were unaware of their group allocation until they had completed all of their pre‐intervention assessment” p. 379 Comment: participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | “(…) The individuals carrying out the assessment were not part of the intervention team. Research participants were asked not to divulge information regarding their group allocation in conversation during assessment at 12 month.” p. 379 Comment: Outcome assessment was not blinded; however, assessors were not part of the intervention team. |
| Incomplete outcome data (attrition bias) | Low risk | “One patient from the intervention group did not fulfil our adherence criteria to the 4‐week programme, and also did not complete the 1‐year evaluation. Six more patients were not available for follow‐up evaluation; four in the usual care group, and two in the intervention group. The withdrawals were due to miscellaneous medical conditions (n = 3), and COPD exacerbation (n = 3). Due to the missing data, we did not retain these patients in our 1‐year analyses” p. 380 “Baseline characteristics of the patients who withdrew from the study were similar to those of patients who completed the trial” p. 380 Comment: The number of withdrawal was relatively low and equally distributed over groups. Also, reasons for withdrawal in the two groups were comparable. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selectively reporting; however, no protocol available. |
| Other bias | Unclear risk | Comment: Per protocol analysis, baseline characteristics were not reported for all randomised participants. |
| Methods | Design: cluster‐RCT Follow‐up: 12 months Control group: conventional care | |
| Participants | Recruitment: general practice Assessed for eligibility: 700 Randomly assigned: I: 83; C: 52 Completed: I: 71; C: 46 Mean age: 68 (range 44‐84) years for total group Gender (% male): 41.5% for total group COPD diagnosis: diagnosis of COPD by ICD‐9‐CM codes and GP records for a clinical diagnosis of moderate to severe COPD Inclusion of participants in the acute phase: not reported Major inclusion criteria: clinical diagnosis of moderate to severe COPD Major exclusion criteria: chronic asthma, bronchiectasis, comorbidity more significant than COPD, unable to give informed consent; prognosis < 12 months, LTOT or too unwell, deceased, no longer enrolled with participating GP or moved out of area, unable to contact patient; insufficient practice nurse resource | |
| Interventions | Mode: individual sessions at the outpatient clinic, GP and at the participant's home Duration: at least 17 individual face‐to‐face sessions (monthly visits to practice nurse to review their progress (N = 12), at least three monthly visits to GP (n = 4), at least one home visit by the respiratory nurse specialist and one following hospital admissions) Professional: respiratory physician, respiratory nurse specialist, GP Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, other: annual influenza vaccination and attendance at a PR programme were recommended Self‐management topics: smoking cessation, exercise, (maintenance) medication, correct device use Exercise programme: no Smoking cessation programme: no Behavioural change techniques: eight clusters: goals and planning, feedback and monitoring, shaping knowledge, natural consequences, comparison of behaviour, associations, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support | |
| Outcomes | 1. quality of life (SF‐36) 2. CRQ 3. distance walked (Shuttle Walk Test) 4. hospital admissions 5. spirometry (FEV₁ (L), FEV₁ % predicted) 6. medication use (courses of oral steroids and antibiotics) | |
| Notes | Randomisation is done at the level of GP practice; 26 practices were randomised to intervention and 25 were randomised to usual care. Analysis is performed at the level of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Fifty‐one eligible practices with 116 GPs were randomised, using a set of computer‐generated random numbers (…)” p. 609 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | High risk | Comment: The study was cluster‐randomised. Therefore, there was no allocation concealment provided. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “For all patients, an initial assessment with the GP and practice nurse included clinical history and the Short Form (SF)‐36. Spirometry, the Shuttle Walk Test and the Chronic Respiratory Questionnaire (CRQ) were administered at the hospital outpatient clinic by a respiratory physician, respiratory nurses and experienced interviewers, respectively. At the completion of a 12‐month trial period, an identical reassessment was undertaken.” p. 609 Comment: Blinding of outcome assessment was not reported, measurements were predominantly performed by study personnel at the outpatient clinic. |
| Incomplete outcome data (attrition bias) | Low risk | “During the trial period, six patients died, six patients withdrew from the study, four patients developed cancer and two patients moved from the area. The 12 month follow‐up assessment was completed by 117 patients (71 INT, 46 CON), although hospital admission data were available for all 135 patients.” p. 609 Comment: 12 participants dropped out in the intervention group (14%), six in the control group (12%). Reasons were comparable. Intention‐to‐treat analysis was performed on the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs of selective reporting; however, no protocol available. |
| Other bias | Low risk | “GP practices were randomised rather than patients to try to avoid contamination of treatment groups within practices.” p. 609 “The characteristics of non‐participating and participating practices were similar, so a selection bias between INT and CON practices seems unlikely.” p. 613 Comment: We additionally assessed this study on bias specifically important in cluster‐randomised trials. In Rea’s study, the general practises were randomly assigned before the participants were included. For reasons unknown, the number of participants screened and included was lower in the control group than in the intervention group. The study authors state that baseline characteristics were not significantly different between groups. Therefore, the risk of recruitment bias is unclear, and risk of bias for baseline imbalance is low. The risk of bias due to loss of clusters is low because no clusters were lost after participant enrolment. Rea et al. did not correct for clustering in their analyses, so risk of bias due to incorrect analysis is high. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care | |
| Participants | Recruitment: hospital (Veterans Affairs medical centres) Assessed for eligibility: 1739 Randomly assigned: I: 372; C: 371 Completed: I: 336; C: 323 Mean age: I: 69.1 ± 9.4 years; C: 70.7 ± 9.7 years Gender (% male): I: 97.6%; C: 98.4% COPD diagnosis: clinical diagnosis of COPD with post‐bronchodilator spirometry showing an FEV₁ < 70% predicted and a FEV₁/FVC < 0.70 Inclusion of participants in the acute phase: not reported Major inclusion criteria: a diagnosis of COPD at high risk of hospitalisation as predicted by one or more of the following during the previous year: hospital admission or ED visit for COPD, chronic home oxygen use or course of systemic corticosteroids for COPD Major exclusion criteria: inability to have access to a home telephone line or sign a consent form, any condition that would preclude effective participation in the study or likely to reduce life expectancy to less than a year | |
| Interventions | Mode: group sessions at an outpatient clinic, one‐page handout summary and number for help line, telephone calls Duration: one group session of 1‐1.5 hours by a respiratory therapist case manager, 12 monthly phone calls of 10‐15 minutes each Professional: respiratory therapist case manager Training of case managers: "case managers were respiratory therapists who had completed a one‐day training session.” Appendix 1, p. 2 Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations; education regarding COPD, smoking cessation Self‐management topics: smoking cessation, exercise, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: oximetry, recommendation concerning influenza and pneumococcal vaccinations, instruction in hand hygiene Exercise programme: no Smoking cessation programme: yes (optional), smoking cessation counselling Behavioural change techniques: 10 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences; comparison of behaviour, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support | |
| Outcomes | 1. hospital admissions and ED visits for COPD 2. all‐cause hospitalisations and all‐cause ED visits 3. hospital and intensive care unit lengths of stay 4. respiratory medication use 5. change in respiratory quality of life (SGRQ) 6. all‐cause mortality | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “We assigned subjects in equal proportions to each of the two treatment arms by permuted‐block randomisation.” Appendix 1, p. 3 Comment: Information on the method of random sequence allocation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Information on the method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) | High risk | “We performed a randomised, adjudicator‐blinded, controlled, 1‐year trial (…).” p. 890 Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | “Blinded pulmonologists independently reviewed all discharge summaries and ED reports and assigned a primary cause for each.” p. 891 Comment: Outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | “All patients were followed for 12 months or until the time of death if it occurred before 12 months.” p. 981 “Fifty‐five percent of patients in the usual care group and 60% of patients in the disease management group returned a completed the Saint George’s Respiratory Questionnaire in response to a single mailing at the end of the study.” p. 982 Comment: Low response rates on SGRQ leading to a high risk of bias. However, data on healthcare utilisation seem complete with no risk of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: All primary and secondary outcome measures were reported; no signs of selective reporting. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 2 months Control group: usual care | |
| Participants | Recruitment: hospital (inpatient) Assessed for eligibility: 62 Randomly assigned: I: 20; C: 20 Completed: I: 17, C: 17 Mean age: I: 66.6 ± 7.12 years; C: 68.1 ± 6.46 years Gender (% male): I: 55.0, C: 75.0 COPD diagnosis: a diagnosis of moderate COPD, based on the GOLD staging system Inclusion of participantsin the acute phase: yes, during hospitalisation Major inclusion criteria: diagnosis of moderate COPD, based on the GOLD staging system, confirmed discharge date at the discretion of the responsible medical doctors, age 65‐75 years, independent mobility Major exclusion criteria: history of other lung diseases, any concomitant diseases that could interfere with the general condition, neuromuscular impairment that would interfere with the patient’s mobility | |
| Interventions | Mode: individual sessions at the hospital and at the outpatient clinic, telephone calls, written instruction Duration: three face‐to‐face individual sessions (two inpatient sessions for 90+45 minutes each on the day before discharge and on the day of discharge, one outpatient session for 90 minutes on the first follow‐up day which is usually planned one week after discharge) by two nurse interventionists, booster sessions were delivered through two phone calls with a two‐week interval Professional: nurse interventionists Training of case managers: "intervention sessions were delivered by two nurse interventionists selected on the basis of their previous experience in COPD care. They also received 6 hours of training sessions to ensure their consistency." p. 152 Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: exercise, (maintenance) medication, coping with breathlessness/breathing techniques, other: identifying barriers to self‐care adherence Exercise programme: yes, each face‐to‐face session consisted of the education accompanied by practicing exercise. Participants learned 10 sets of upper and lower extremities stretching with pursed lip breathing. They also performed a 10‐minute‐per‐toleration walk on a course 30‐m corridor in the unit. The written instruction, plus illustrations, was given Smoking cessation programme: no Behavioural change techniques: nine clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, associations, repetition and substitution, regulation, identity, self‐belief Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations | |
| Outcomes | 1. exercise capacity (PEFR and 6MWD) 2. health‐related quality of life (SGRQ) 3. self‐care adherence (medication and exercise compliance) | |
| Notes | Randomisation after matching for lung function, age and gender. Not all participants fulfilled the inclusion criterion 'a diagnosis of moderate COPD, based on the GOLD staging systems’, because the mean FEV1/FVC % predicted was > 0.70 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “After being matched for lung function, age, and gender, participants were randomly allocated to either an experimental or a control group.” p. 149 Comment: There is no method described that was used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | “After being matched for lung function, age, and gender, participants were randomly allocated to either an experimental or a control group.” p. 149 Comment: There is no method described that was used to conceal the allocation. |
| Blinding of participants and personnel (performance bias) | High risk | “This single‐blinded, randomised control group, pre‐/posttest study (…)” p. 148 Comment: No blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Most of the outcome measures were self‐reported. There is insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | “There were no significant differences in the aforementioned baseline characteristics between the final sample and those who withdrew.” p. 149 Comment: Whereas Table 3 states that data of 20 participants have been analysed, this cannot be the case, because t‐tests have been used and not all participants had complete data (15% non‐complete data in each group). |
| Selective reporting (reporting bias) | Unclear risk | Comment: No signs for selective outcome reporting, results were reported extensively; however, no protocol available. |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 9 months Control group: usual care | |
| Participants | Recruitment: hospital, primary care physiotherapy practices Assessed for eligibility: not reported (101 participants eligible) Randomly assigned: I: 15; C: 14 Completed: I: 10; C: 2 Mean age: I: 64.1 ± 9.0 years; C: 62.8 ± 7.4 years Gender (% male): I: 50.0; C: 50.0 COPD diagnosis: GOLD II‐IV, a clinical diagnosis of COPD according to the GOLD criteria Inclusion of participants in the acute phase: no Major inclusion criteria: fulfil COPE‐II study (effects of self‐treatment and an exercise programme within a self‐management programme in outpatients with COPD) criteria: no exacerbation in the month prior to enrolment, three or more exacerbations or one hospitalisation for respiratory problems in the 2 years preceding study entry, a computer with Internet access at home Major exclusion criteria: serious other disease with a low survival rate, other diseases influencing bronchial symptoms and/or lung function, severe psychiatric illness, uncontrolled diabetes mellitus or a hospitalisation for diabetes mellitus in the 2 years preceding the study, need for regular oxygen therapy, maintenance therapy with antibiotics, known a1‐ antitrypsin deficiency, disorders or progressive disease seriously influencing walking ability | |
| Interventions | Mode: individual and group sessions at the outpatient clinic, primary care physiotherapy practices and at the participant's home, web‐based teleconsultation module Duration: at least one face‐to‐face individual session by the primary care physiotherapist (no protocol for education, offered as blended care, depending on physiotherapist and patient) and a teleconsultation module. For research purposes there was one intake by a physiotherapist for baseline measure activity coach and explanations. Furthermore, there were additional meetings after one, three, six and nine months. Before the start of the programme, participants had to attend two group sessions of 90 minutes each by a nurse practitioner Professional: respiratory nurse practitioner, respiratory physiotherapist Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, coping with breathlessness/breathing techniques Exercise programme: yes, a web‐based exercise program on the web portal. For every individual patient, exercise schemes were created by the patient’s physiotherapist via the web portal. A scheme represents which exercises should be performed by the patient for which day, and which part of the day. Every exercise consists of a text description and movie. The patient is able to log in at home, follow the exercise scheme, execute the exercises, and provide feedback to the physiotherapist. There was no standardized exercise protocol: the physiotherapist could freely select the exercises for each patient for the online exercise program. This exercise program could be adapted during the intervention period following the progress of the patient at the discretion of the therapist. Both primary and secondary care professionals could supervise the patient at a distance by checking progress on the web portal. Smoking cessation programme: no Behavioural change techniques: 11 clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, regulation, antecedents, self‐belief Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support | |
| Outcomes | 1. use of application 2. adherence (online diary, exercise scheme) 3. satisfaction (Client Satisfaction Questionnaire) 4. hospitalisations (number and length of stay) 5. emergency department visits 6. exacerbations 7. level of activity (activity coach, accelerometer) 8. self‐perceived activity levels (Baecke Phsycial Activity Questionnaire) 9. exercise tolerance (6MWT) 10. fatigue (Multidimensional Fatigue Inventory 20) 11. health status (CCQ) 12. dyspnoea (MRC) 13. quality of life (EuroQol‐5D) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised using a computer‐generated randomisation list (Blocked Stratified Randomisation version 5; Steven Piantadosi), where randomisation was applied in blocks of two and four.” p. 936 Comment: Random sequence generation was adequately performed. |
| Allocation concealment (selection bias) | Low risk | “Participants were allocated by a data manager in order of inclusion following the randomisation list, placed in a sealed envelope.” p. 936 Comment: The allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “The decision‐support diary automatically identified exacerbations following previously described criteria for the intervention group, while the control group filled in a paper version of the diary” p. 938 Comment: Unclear whether outcome assessors were blinded. Questionnaires used are validated questionnaires. |
| Incomplete outcome data (attrition bias) | High risk | “A large number of patients were not able or willing to continue study participation: 33% in the intervention group and 86% in the control group.” p. 939 Comment: Most outcome measures are reported for 3 months follow‐up, whereas there was a total of 9 months follow‐up. There was a high number of withdrawals for the 9 months follow‐up (more dropouts in the control group). |
| Selective reporting (reporting bias) | High risk | Comment: Not all outcome measures were reported for the 9 month follow‐up. Exacerbations (duration) was not reported. Also, no information or results provided for the use of diaries in the control group. |
| Other bias | Unclear risk | Comment: Per protocol analysis, baseline characteristics only assessed for the participants who completed the study. No differences reported for baseline characteristics among the withdrawals after randomisation (N = 6) and the participants who completed the questionnaires at T0 (inclusion). |
| Methods | Design: RCT Follow‐up: 24 months Control group: usual care | |
| Participants | Recruitment: hospital (inpatient) Assessed for eligibility: 199 Randomly assigned: I: 91; C: 81 Completed: I: 51; C: 49 Mean age: I: 74.1 ± 9.26 years; C: 72.6 ± 9.33 years Gender (% male): I: 42.9; C: 43.2 COPD diagnosis: GOLD stage III or IV Inclusion of participants in the acute phase: yes, during hospitalisation Major inclusion criteria: admission due to AECOPD, COPD (GOLD stage III or IV, 2007), living in the Trondheim municipality, ability to communicate in Norwegian, ability to sign the informed consent form Major exclusion criteria: any serious diseases that might cause a very short lifespan (expected survival time less than six months) | |
| Interventions | Mode: individual sessions at the participant's home, telephone calls, e‐learning programme, "My COPD book" Duration: six face‐to‐face individual sessions (one at discharge, five joint visits at home at approximately three days, 14 days, six months, 12 months, and 24 months post‐discharge) by the specialist nurse, one interactive 15‐minute e‐learning programme, at least 24 telephone calls (routinely phone calls at least once a month and during COPD exacerbations) Professional: specialist nurse Training of case managers: "an education session for home‐care nurses: a three‐hour theoretical session covering several aspects of COPD and two days of practice at the DTM (Department of Thoracic Medicine)" p. 3 Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques Exercise programme: no Smoking cessation programme: no Behavioural change techniques: eight clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, associations, repetition and substitution, regulation, antecedents Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, avoid situations in which viral infection might be prevalent, contact healthcare providers for support | |
| Outcomes | 1. hospital utilisation (admissions caused by AECOPD, in‐hospital days due to AECOPD) 2. mortality 3. inhaled medication use (LAMA, LABA) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “They were randomly allocated to either integrated care (IC) or usual care (UC) based on their address of permanent residence. In order to create two pairs of districts with approximately equal numbers of citizens, a pair‐wise matching of districts was carried out. It was decided by lottery that participants from District Pair 1 were assigned to the UC group, and participants from District Pair 2 were assigned to the IC group.” p. 2 Comment: It was unclear whether random sequence allocation was performed on patient or health centre level. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided about the allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | “The study was a prospective, open, single‐centre intervention study.” p. 2 Comment: No blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | “Data concerning HA (hospital admissions) and HD (hospital days) were collected from the hospital registry database’s medical charts.” p. 3 Comment: Unclear who was the outcome assessor. |
| Incomplete outcome data (attrition bias) | High risk | “Data from patients who completed a minimum of two years of follow‐up were included in the analysis.” p. 3 Comment: A lot of missing data; after two years of follow‐up 58% of the included participants were available for evaluation. |
| Selective reporting (reporting bias) | High risk | “Information concerning the number and duration of the COPD exacerbations, as well as the time from onset of symptoms until the start of self‐initiated treatment is insufficient due to many incomplete registrations in “My COPD book” p. 9 Comment: No mortality reported; however, Figure 1 shows higher mortality for the IC group, N = 35 (38.4%), compared to the UC group, N = 21 (25.9%) |
| Other bias | Low risk | None noted. |
| Methods | Design: RCT Follow‐up: 3 to 5 months Control group: usual care | |
| Participants | Recruitment: nurse‐led primary healthcare clinic Assessed for eligibility: 110 Randomly assigned: I: 26, C: 26 Completed: I: 26, C: 26 Mean age: I: 66 ± 9.4 years; C: 67 ± 10.4 years Gender (% male): I: 50.0, C: 50.0 COPD diagnosis: mild, moderate, severe or very severe COPD based on spirometry, lung capacity after bronchodilator use, based on GOLD criteria Inclusion of participants in the acute phase: not reported Major inclusion criteria: diagnosed with mild, moderate, severe or very severe COPD based on spirometry, lung capacity after bronchodilator use, based on GOLD criteria Major exclusion criteria: diagnosed severe mental disorders such as schizophrenia, dementia or alcohol or drug abuse | |
| Interventions | Mode: individual sessions at the outpatient and nurse‐led primary healthcare clinic Duration: two face‐to‐face individual sessions for self‐care education during 3‐5 months for one hour each by the nurse Professional: COPD nurse, physician, if needed: dietician, medical social worker, physical therapist, occupational therapist Training of case managers: not reported Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: instructions on the coughing technique to prevent infections and exacerbations, measurement on oxygen saturation before and after exertion, psycho‐social counselling and support, counselling on infection prevention Exercise programme: yes (optional), dialogue on physical activity and exercise. When needed, a dietician, a medical social worker, a physical therapist and an occupational therapist were consulted. Smoking cessation programme: yes (optional), motivational dialogue on smoking cessation based on Behavioural change techniques: ten clusters: goals and planning, feedback and monitoring, social support, shaping knowledge, natural consequences, comparison of behaviour, associations, repetition and substitution, comparison of outcomes, reward and threat, regulation, antecedents, identity, scheduled consequences, self‐belief, covert learning. Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, contact healthcare providers for support | |
| Outcomes | 1. health‐related quality of life (SGRQ) 2. smoking 3. COPD knowledge | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was performed when two patients with the same variables agreed to participate in the study by assigning each individual an identity number. An independent person drew lots for allocation to either intervention or control group." p. 2‐3 Comment: The random sequence generation was performed adequately. |
| Allocation concealment (selection bias) | Low risk | "The randomisation was performed when two patients with the same variables agreed to participate in the study by assigning each individual an identity number. An independent person drew lots for allocation to either intervention or control group." p. 2‐3 Comment: Allocation was adequately concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Blinding of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) | High risk | “Each visit lasted for about 1 hour and the same nurse (Eva Österlund Efraimsson) was responsible for all consultations. At the first and last visits, all patients responded to the two questionnaires, which were completed by each participant in an undisturbed area. The nurse in charge was available to answer questions and to check that the patients responded to all the items.” p. 180 Comment: Outcome assessment was not blinded, and measurements were performed/supervised by the same person who provided the intervention (who was also the principal investigator) |
| Incomplete outcome data (attrition bias) | Low risk | “The drop‐out rate was 10 patients (five women and five men) (…) The severity of the illness was evenly distributed between men and women: three women and three men had moderate COPD, one woman and one man had severe COPD and one woman and one man had very severe COPD. The drop‐out group did not differ from the sample in any of these aspects.” p. 180 Comment: The dropout group did not differ from the sample. However, it was unclear when the participants did dropout and in which group |
| Selective reporting (reporting bias) | Unclear risk | Comment: All subscales of the two questionnaires used were reported; no signs of selective reporting were noted. However, no protocol available. |
| Other bias | Low risk | None noted. |
6MWD: 6 Minute Walking Distance; 6MWT: 6 Minute Walking Test; CCQ: Clinical COPD Questionnaire; COPD: Chronic Obstructive Pulmonary Disease; CRQ: Chronic Respiratory Questionnaire; CSES: COPD Self‐Efficacy Scale; ED: emergency department; ESWT: endurance shuttle walk test; EQ‐5D: EuroQol‐5 Dimensions; FEV₁: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; ICD‐9‐CM: International Statistical Classification of Diseases and Related Health Problems, Ninth Revision, Clinical Modification; ISWT: incremental shuttle walk test; LABA: long‐acting beta agonists; LAMA: long‐acting muscarinic antagonists; (m)MRC: modified Medical Research Council dyspnoea score; PEFR: peak expiratory flow rate; RCT: Randomised Controlled Trial; SF‐36: 36‐item Short Form quality of life; SGRQ: St. George's Respiratory Questionnaire; QALY: Quality‐Adjusted Life Year.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No verification COPD | |
| No RCT | |
| No usual care control group | |
| No self‐management | |
| No self‐management | |
| No self‐management | |
| No self‐management | |
| No written action plan AECOPD | |
| No self‐management | |
| No written action plan AECOPD | |
| No usual care control group | |
| No RCT | |
| No usual care control group | |
| No self‐management | |
| No RCT | |
| No COPD | |
| No self‐management | |
| No self‐management | |
| No written action plan AECOPD | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No usual care control group | |
| No usual care control group | |
| No stratification for COPD | |
| No self‐management | |
| No COPD | |
| No RCT | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No RCT | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No usual care control group | |
| No usual care control group | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No self‐management | |
| No self‐management | |
| No written action plan AECOPD | |
| No self‐management | |
| No usual care control group | |
| No RCT | |
| No peer‐reviewed full‐text available | |
| No usual care control group | |
| No self‐management | |
| No self‐management | |
| No stratification COPD | |
| No usual care control group | |
| No usual care control group | |
| No written action plan AECOPD | |
| No peer‐reviewed full‐text | |
| No usual care control group | |
| No self‐management | |
| No stratification for COPD | |
| No self‐management | |
| No written action plan AECOPD | |
| No COPD | |
| No self‐management | |
| No stratification for COPD | |
| No RCT | |
| No self‐management | |
| No written action plan AECOPD | |
| No stratification for COPD | |
| No stratification for COPD | |
| No written action plan AECOPD | |
| No self‐management | |
| No iterative process | |
| No verification COPD | |
| No peer‐reviewed full‐text available | |
| No self‐management | |
| No self‐management | |
| No self‐management | |
| No self‐management | |
| No peer‐reviewed full‐text | |
| No RCT | |
| No COPD | |
| No self‐management | |
| No self‐management | |
| No stratification for COPD | |
| No written action plan AECOPD | |
| No verification COPD | |
| No written action plan AECOPD | |
| No self‐management | |
| No self‐management | |
| No peer‐reviewed full‐text available | |
| No iterative process | |
| No stratification for COPD | |
| No written action plan AECOPD | |
| No verification COPD | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No self‐management | |
| No stratification for COPD | |
| No usual care control group | |
| No iterative process | |
| No RCT | |
| No written action plan AECOPD | |
| No self‐management | |
| No written action plan AECOPD | |
| No usual care control group | |
| No usual care control group | |
| No self‐management | |
| No verification COPD | |
| No verification COPD | |
| No self‐management | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No usual care control group | |
| No written action plan AECOPD | |
| No self‐management | |
| No self‐management | |
| No peer‐reviewed full‐text available | |
| No peer‐reviewed full‐text available | |
| No self‐management | |
| No self‐management | |
| No iterative process | |
| No usual care control group | |
| No self‐management | |
| No RCT | |
| No peer‐reviewed full‐text available | |
| No RCT | |
| No usual care control group | |
| No self‐management | |
| No RCT | |
| No self‐management | |
| No peer‐reviewed full‐text available | |
| No usual care control group | |
| No usual care control group | |
| No usual care control group | |
| No usual care control group | |
| No peer‐reviewed full‐text available | |
| No self‐management | |
| No iterative process | |
| No COPD | |
| No written action plan AECOPD | |
| No usual care control group | |
| No self‐management | |
| No written action plan AECOPD | |
| No self‐management | |
| No self‐management | |
| No usual care control group | |
| No self‐management | |
| No self‐management | |
| No peer‐reviewed full‐text available | |
| No peer‐reviewed full‐text available | |
| No peer‐reviewed full‐text available | |
| No iterative process | |
| No peer‐reviewed full‐text available | |
| No usual care control group | |
| No peer‐reviewed full‐text available | |
| No RCT | |
| No peer‐reviewed full‐text available | |
| No peer‐reviewed full‐text available | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No COPD | |
| No verification COPD | |
| No written action plan AECOPD | |
| No self‐management | |
| No self‐management | |
| No usual care control group | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No verification COPD | |
| No peer‐reviewed full‐text available | |
| No usual care control group | |
| No usual care control group | |
| No peer‐reviewed full‐text available | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No self‐management | |
| No written action plan AECOPD | |
| No self‐management | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No stratification for COPD | |
| No usual care control group | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No usual care control group | |
| No self‐management | |
| No COPD | |
| No iterative process | |
| No self‐management | |
| No written action plan AECOPD | |
| No self‐management | |
| No COPD | |
| No written action plan AECOPD | |
| No self‐management | |
| No self‐management | |
| No self‐management | |
| No iterative process | |
| No RCT | |
| No self‐management | |
| No RCT | |
| No RCT | |
| No written action plan AECOPD | |
| No self‐management | |
| No COPD | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No written action plan AECOPD | |
| No stratification for COPD | |
| No COPD |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants | Recruitment: hospital (inpatient) Randomly assigned: I: 108; C: 107 Mean age: I: 67.9 ± 9.8 years; C: 68.1 ± 9.2 years Gender (% male): I: 43; C: 48 COPD diagnosis: participants admitted for a COPD exacerbation Inclusion of participants in the acute phase: yes, during hospitalisation Major inclusion criteria: admitted for a COPD exacerbation, age older than 40 years, current or past cigarette smoking history of more than 10 pack‐years, ability to speak English, and access to a telephone Major exclusion criteria: any medical conditions that would impair their ability to participate in the study or to provide informed consent or if they were receiving hospice care |
| Interventions | Mode: individual sessions at the hospital and after discharge, telephone follow‐up calls Duration: one visit at the hospital of 2 hours, and at least one in person after discharge, with subsequent sessions conducted by telephone. Professional: the Mayo Clinic: registered nurse, Health Partners Regions Hospital: respiratory therapist Training of case managers: both interventionists (registered nurse and respiratory therapist) received the same training, which included 1) face‐to‐face training on theory and strategies associated with self‐management education and motivational interviewing; 2) reading materials that detailed skills and strategies associated with self‐management education and motivational interviewing; 3) role play‐based experiential learning of intervention strategies with patient vignettes; and 4) recorded intervention sessions were reviewed and interventionists were provided tailored training. Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: introduction of the concept of self‐management by providing copy of the book 'Living a Health Life with Chronic Conditions' Exercise programme: yes Smoking cessation programme: no Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support |
| Outcomes | 1. rate of COPD‐related rehospitalisations 2. disease‐specific quality of life (CRQ) 3. measured physical activity at 6 and 12 months (average number of steps and minutes per day spent in daily physical activities of at least moderate intensity) |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
| Methods | Design: RCT Follow‐up: 2 months Control group: usual care |
| Participants | Recruitment: not reported Assessed for eligibility: not reported Randomly assigned: I: 20; C: 20 Completed: not reported Mean age: I: 71.6 ± 7.1 years; C: 67.4 ± 9.3 years Gender (% male): I: 93.8; C: 87.5 COPD diagnosis: FEV₁/FVC < 70 % Inclusion of participants in the acute phase: not reported Major inclusion criteria: not reported Major exclusion criteria: not reported |
| Interventions | Mode: one‐on‐one interviews, telephone follow‐up, or home visits Duration: 2 months multidisciplinary self‐management education program Professional: not reported (multidisciplinary) Training of case managers: not reported Self‐management components: education regarding COPD Self‐management topics: not reported Exercise programme: not reported Smoking cessation programme: not reported Action plan components: no information was reported whether an action plan for COPD exacerbations was used |
| Outcomes | 1. self‐efficacy 2. exercise tolerance (6‐minute walking test) 3. quality of life (Taiwan Mandarin Chinese version of the SGRQ) |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
| Methods | Design: cluster‐RCT Follow‐up: 6 months Control group: usual care |
| Participants | Recruitment: pharmacies Assessed for eligibility: not reported Randomly assigned: not reported Completed: not reported Mean age: not reported Gender (% male): not reported COPD diagnosis: physician‐diagnosed COPD, the use of inhaled medication or a known diagnosis of COPD Inclusion of participants in the acute phase: no Major inclusion criteria: physician‐diagnosed COPD, age 40 years or older, the ability to answer questionnaires in English Major exclusion criteria: severe disease, defined as a known FEV₁/FVC < 30 %, a diagnosis of dementia or a prescription for cholinesterase inhibitors, a terminal illness, physician‐diagnosed asthma, participation in another clinical trial, no consent provided |
| Interventions | Mode: not reported Duration: not reported Professional: pharmacist, family physician Training of case managers: staff pharmacists working at all participating pharmacies from both arms of the study will be offered training on the design of the study, including how to administer questionnaires, proper patient recruitment, and consenting of participants Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, other: medication review, pulmonary rehabilitation Self‐management topics: smoking cessation, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques Exercise programme: no Smoking cessation programme: provision of, or referral to, smoking cessation counselling (where applicable) Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment. The form is divided into two sections, each section having three subcategories. These include: (1) “My Symptoms” (I feel well, I feel worse, I feel much worse) and (2) “My Actions” (stay well, take action, call for help). This action plan is easy to read and simple to follow. A copy of this will be provided to the patient and to the patient’s physician (faxed). When needed, a prescription suggestion for antibiotics and oral steroids will be provided to the physician for signature and fax back to the pharmacy, to facilitate the action plan |
| Outcomes | 1. medication adherence, difference in the change in the Medication Possession Ratio from baseline to 6 months between the intervention and control groups 2. quality of life (SGRQ) 3. medication inhalation technique (pharmacist‐scored scale) 4. healthcare resource utilisation (frequency of physician visits, hospitalisations, ED visits, and pharmacy visits) 5. antibiotic and orally administered corticosteroid use for acute exacerbations of COPD as reported by the patient at 6 months |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. There is patient referral to pulmonary rehabilitation in collaboration with their family physician. |
| Methods | Design: RCT Follow‐up: 6 months Control group: usual care |
| Participants | Recruitment: Assessed for eligibility: not reported Randomly assigned: I: 25; C: 25 Completed: not reported Mean age: total group: 59.3 ± 1.2 years Gender (% male): not reported COPD diagnosis: not reported Inclusion of participants in the acute phase: not reported Major inclusion criteria: COPD combined with essential hypertension Major exclusion criteria: not reported |
| Interventions | Mode: mobile telephone counselling Duration: monthly mobile telephone counselling for 6 months Professional: not reported Training of case managers: not reported Self‐management components: education regarding COPD Self‐management topics: not reported Exercise programme: not reported Smoking cessation programme: not reported Action plan components: no information was reported whether an action plan for COPD exacerbations was used |
| Outcomes | 1. Primary outcomes: self‐reported exacerbation and hospitalisation, 6‐minute walking distance test, CAT score, mMRC and SF‐36 quality of life |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
| Methods | Design: RCT Follow‐up: 3 months Control group: usual care |
| Participants | Recruitment: hospital (outpatient) Assessed for eligibility: not reported Randomly assigned: I: 20, C: 20 Completed: I: 19, C: 19 Mean age: I: 66.6 ± 9.1 years; C: 65.0 ± 8.2 years Gender (% male): I: 45, C: 50 COPD diagnosis: GOLD stage 3 or 4 COPD Inclusion of participants in the acute phase: Not reported Major inclusion criteria: GOLD stage 3 or 4 COPD, and a telephone land line Major exclusion criteria: active treatment for lung cancer, illiteracy, non‐English speaking, and inability to complete a 6‐min walk test |
| Interventions | Mode: individual face‐to‐face session, telecommunication device, home‐based Duration: one face‐to‐face session, 60 health buddy sessions (telecommunication) for 20 minutes each weekday morning during 3 months Professional: registered respiratory therapist Training of case managers: not reported Self‐management components: self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component. Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, coping with breathlessness/breathing techniques, other: oxygen therapy Exercise programme: yes Smoking cessation programme: no Action plan components: no information was provided whether an action plan for COPD exacerbations was used |
| Outcomes | 1. quality of life (SGRQ) 2. COPD hospitalisations 3. COPD ER visits 4. healthcare costs |
| Notes | We could not verify with the authors whether this study did meet our eligibility criteria. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants | Recruitment: health centre (primary care setting) Assessed for eligibility: 1553 Randomly assigned: I: 72; C: 74 Completed: I: 47; C: 57 Mean age: I: 69.6 (95% CI 67.7‐71.4) years; C: 68.6 (95% CI 66.4‐70.8) years Gender (% male): I: 91.7; C: 91.9 COPD diagnosis: confirmed COPD diagnosis registered in the patient's clinical record Inclusion of participants in the acute phase: no Major inclusion criteria: confirmed COPD diagnosis registered in the patient’s clinical record, clinical assistance at primary care centres in the Malaga area, prescription of scheduled inhalation therapy and written informed consent Major exclusion criteria: other respiratory conditions that are not included in the COPD definition (bronchiectasis, asthma or cystic fibrosis) or cognitive impairment problems registered in their clinical record (dementia, Alzheimer’s, Parkinson’s or cognitive decline) |
| Interventions | Mode: group session at the beginning of the study and individual sessions during the follow‐up visits Duration: one group session of 2 hours and three individual sessions Professional: two professionals with special training in motivational techniques and in the use of inhaler devices Training of case managers: two professionals with special training in motivational techniques and in the use of inhaler devices Self‐management components: education regarding COPD, other: treatment adherence, motivational and cognitive aspects Self‐management topics: (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: social and family support Exercise programme: no Smoking cessation programme: no Action plan components: no information was provided whether an action plan for COPD exacerbations was used |
| Outcomes | 1. adherence to a medication regimen 2. functional status (forces spirometry) 3. health‐related quality of life (SGRQ and EuroQoL‐5D) |
| Notes | We could not verify with the authors whether this study did meet our eligibility criteria. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants | Recruitment: four primary care practices Assessed for eligibility: not reported Randomly assigned: I: 77; C: 74 Completed: total group: 145 (80%) completed 12 months Mean age: I: total group: 67.7 ± 10.2 years Gender (% male): total group: 45.5 COPD diagnosis: not reported Inclusion of participants in the acute phase: not reported Major inclusion criteria: not reported Major exclusion criteria: not reported |
| Interventions | Mode: not reported Duration: case management and comprehensive education at baseline and 3 months, telephone contacts at 6 and 9 months Professional: respiratory educator, physician Training of case managers: certified respiratory educator and physician Self‐management components: education regarding COPD Self‐management topics: not reported Exercise programme: not reported Smoking cessation programme: not reported Action plan components: no information was reported whether an action plan for COPD exacerbations was used |
| Outcomes | 1. quality of life (COPD Assessment Test) at 12 months 2. health service utilisation (urgent/emergent visit for COPD, outpatient visit, emergency room visit, hospitalisation) |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
| Methods | Design: RCT Follow‐up: 48 months Control group: usual care |
| Participants | Recruitment: healthcare units/centres in rural areas Assessed for eligibility: 8,217 Randomly assigned: I: 4,197; C: 4,020 Completed: I: 3,418; C: 2,803 Mean age: I: 71.2 ± 7.4 years; C: 71.5 ± 7.8 years Gender (% male): I: 47.8; C: 47.9 COPD diagnosis: the subjects had to have a diagnosis of COPD according to the criteria proposed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Inclusion of participants in the acute phase: no Major inclusion criteria: at baseline, the subjects had to have a diagnosis of COPD according to the criteria proposed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Major exclusion criteria: presence of fever, active tuberculosis, changes in radiographic images or medication in the 4 weeks immediately preceding recruitment, primary diagnosis of asthma or obvious bronchiectasis, cystic fibrosis, interstitial lung disease, previous lung‐volume‐reduction surgery, lung transplantation, pneumonectomy, uncontrolled or serious conditions that could potentially affect spirometry tests, and refusal to fill out psychological questionnaires |
| Interventions | Mode: group and individual face‐to‐face sessions Duration: 104 group sessions of 40‐60 minutes lecture each every 2 weeks, 104 individual follow‐up sessions at least once every two weeks. Every 2 months, the professionals examined the subjects collectively at the health‐care units Professional: respiratory specialist, nurse psychologist, (respiratory) physiotherapist, peer led dietician, GPs, psychiatrists, rehabilitation specialists, other experts Training of case managers: GPs took 2 days of training for health management in the intervention Self‐management components: education regarding COPD, smoking cessation, exercise or physical activity component, other: psychological counselling, review and adjustment of outpatient COPD medication Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, other: recommendations concerning influenza and pneumococcal vaccinations, instruction on hand hygiene Exercise programme: yes Smoking cessation programme: yes Action plan components: no information was provided whether an action plan for COPD exacerbations was used |
| Outcomes | 1. health status (BODE index) 2. changes in COPD knowledge, awareness and risk factors (survey) 3. changes in anxiety and depression symptoms (HADS) 4. changes in hospital admissions and ED visits 5. changes in medication regimens |
| Notes | We could not verify with the authors whether this study did meet our eligibility criteria. |
| Methods | Design: RCT Follow‐up: 4 months Control group: usual care |
| Participants | Recruitment: not reported Assessed for eligibility: not reported Randomly assigned: total: 29 participants Completed: I: 14; C: 11 Mean age: total group: 70.3 ± 7.5 years Gender (% male): total group: 86.2 COPD diagnosis: moderate to very severe COPD Inclusion of participants in the acute phase: not reported Major inclusion criteria: not reported Major exclusion criteria: not reported |
| Interventions | Mode: 40 educational contents on iPad interactive app Duration: not reported Professional: not reported Training of case managers: not reported Self‐management components: action plan COPD exacerbations, education regarding COPD Self‐management topics: exercise, coping with breathlessness/breathing techniques Exercise programme: no (motion pictures of stretching training) Smoking cessation programme: no Action plan components: no information was reported whether an action plan for COPD exacerbations was used |
| Outcomes | 1. quality of life (SGRQ) 2. exercise capacity (6‐minute walking distance) 3. patient’s need for information about their disease (LINQ) |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
| Methods | Design: RCT Follow‐up: 6 months Control group: usual care |
| Participants | Recruitment: hospital (inpatient) Assessed for eligibility: 2,689 Randomly assigned: I: 214; C: 214 Completed: I: 211, C: 212 Mean age: I: 56 (50‐60.25) years; C: 56.5 (51‐61) years Gender (% male): I: 43.5, C: 49.5 COPD diagnosis: spirometry‐confirmed: an FEV₁/FVC 0.7 or an FEV₁ 80% predicted (pre‐bronchodilator) Inclusion of participants in the acute phase: yes Major inclusion criteria: > 18 and < 65 years of age, spirometry‐confirmed COPD, at high risk for hospitalisation or ED visits as predicted by a hospital admission or ED visit in the previous 12 months for a COPD exacerbation, chronic home use of oxygen, or treatment with a course of systemic corticosteroids in the preceding 12 months Major exclusion criteria: not being expected to survive the hospitalisation, the presence of metastatic cancer, bed‐bound individuals, non‐English speaking, and inability to provide informed consent |
| Interventions | Mode: in‐patient care coordinated by respiratory therapists, scheduled telephone calls Duration: 1‐h education in‐service Professional: respiratory therapist Training of case managers: respiratory therapist case managers conducting the education in‐services all took the COPD Educator Certification Preparation Course. Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, education regarding COPD, smoking cessation Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, other: recommendations concerning influenza and pneumococcal vaccinations, instruction in hand hygiene Exercise programme: no Smoking cessation programme: yes Action plan components: self‐treatment of exacerbations, contact healthcare providers for support |
| Outcomes | 1. combined non‐hospitalised emergency department visits and hospital readmissions for a COPD exacerbation during the 6‐month follow‐up 2. hospital readmissions 3. non‐hospitalised emergency department visits for causes other than COPD exacerbations 4. hospital and ICU lengths of stay for readmission 5. all‐cause mortality |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants | Recruitment: hospital (outpatient) Randomly assigned: I: 51; C: 45 Mean age: I: 68.2 ± 7.2 years; C: 67.1 ± 6.8 years Gender (% male): I: 88.9; C: 92.2 COPD diagnosis: post‐bronchodilator FEV₁/FVC ratio < 70% (GOLD 2007) Inclusion of participants in the acute phase: no Major inclusion criteria: clinical stability (at least in the 3 months prior to randomisation, with no change in medication or usual symptoms), active smoker or prior history of smoking of at least 10 pack‐years; post‐bronchodilator FEV₁/FVC ratio < 70%, normal cognitive status to read and understand written texts, and receive training in inhalation techniques or self‐care education sessions, physical status that allows for regular walking or exercise Major exclusion criteria: diagnoses of asthma, advanced heart failure, unstable ischaemic heart disease, terminal disease, dementia, or uncontrolled psychiatric disorders, no ability to read texts, participation in any pulmonary rehabilitation program in the previous year |
| Interventions | Mode: group education and individual training sessions Duration: group education session (6 to 8 participants) and individual training session at the start, two extra visits with a respiratory nurse at 1 and 3 months of follow‐up. Professional: nurses, physiotherapists, medical specialists in respiratory medicine Training of case managers: professionals previously trained in the intervention's features Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component Self‐management topics: exercise, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques Exercise programme: yes Smoking cessation programme: no Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, other: sheets with instructions on physical exercises for stable periods |
| Outcomes | 1. combined number of hospital admissions and accident and emergency visits for COPD 2. hospitalisations and ED visits for COPD exacerbations 3. lengths of hospital stay 4. use of antibiotics and corticosteroids 5. all‐cause mortality |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
| Methods | Design: cluster‐RCT Follow‐up: 12 months Control group: usual care |
| Participants | Recruitment: general practices in Sydney Randomly assigned: I: 144; C: 110 Mean age: not reported Gender (% male): not reported COPD diagnosis: COPD on post‐bronchodilator spirometry Inclusion of participants in the acute phase: no Major inclusion criteria: participants newly identified as having COPD on post‐bronchodilator spirometry Major exclusion criteria: not reported |
| Interventions | Mode: not reported Duration: not reported Professional: practice nurse, GP teams Training of case managers: Self‐management components: not reported Self‐management topics: not reported Exercise programme: not reported Smoking cessation programme: not reported Action plan components: no information was reported whether an action plan for COPD exacerbations was used |
| Outcomes | 1. quality of life (SGRQ) 2. other quality of life measures 3. lung function 4. disease knowledge 5. smoking and immunization status 6. inhaler technique 7. health service use |
| Notes | Awaiting assessment: further information is needed before inclusion when the review is next updated. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | An international randomised study of a home‐based self‐management program for severe COPD: the COMET |
| Methods | Design: RCT Follow‐up: 24 months Control group: usual care |
| Participants | Recruitment: hospital (outpatient) Randomly assigned: I: 172; C: 173 Mean age: not reported Gender (% male): not reported COPD diagnosis: COPD GOLD III or IV, post‐bronchodilator FEV₁ < 60% Inclusion of participants in the acute phase: no Major inclusion criteria: post‐bronchodilator FEV₁ < 60%, smoking history of ≥ 10 pack‐years, ≥ 1 moderate to severe exacerbation in the prior 12 months Major exclusion criteria: probability of survival < 6 months, be uninsured, or permanently living in a nursing home |
| Interventions | Mode: individual home sessions during the run‐in period, and group or telephone individual sessions during follow‐up Duration: 3‐ to 5‐week run‐in period with four individual home sessions, during follow‐up every 3 months seen by hospital physician, and monthly group or telephone individual sessions Professional: healthcare professionals (case‐managers) Training of case managers: case‐managers undergo a standardised initial 4‐day training with specific focus on motivational communication Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD Self‐management topics: smoking cessation, exercise, diet, (maintenance) medication, coping with breathlessness/breathing techniques, other: all participants on oxygen therapy are monitored with a wearable device that records time of oxygen use and respiration rate. Exercise programme: no Smoking cessation programme: no Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, other: self‐measurements of pulse oximetry, spirometry, and body temperature |
| Outcomes | 1. number of unscheduled all‐causes hospital days 2. hospital days due to COPD exacerbations 3. moderate‐to‐severe COPD exacerbations 4. all‐cause and COPD‐related healthcare use 5. anxiety and depression levels (HADS) 6. health status (SGRQ, 15D HRQoL questionnaire) 7. compliance to oxygen therapy 8. cost‐effectiveness |
| Starting date | 2010 |
| Contact information | Jean Bourbeau, Center for Innovative Medicine, McGill University Health Centre, e‐mail: [email protected] |
| Notes |
| Trial name or title | A self‐management approach using self‐initiated action plans for symptoms with ongoing nurse support in patients with chronic obstructive pulmonary disease (COPD) and comorbidities: the COPE‐III study |
| Methods | Design: RCT Follow‐up: 12 months Control group: usual care |
| Participants | Recruitment: hospital (outpatient) Randomly assigned: I: 102; C: 99 Mean age: I: 68.8 ± 9.0 years; C: 68.2 ± 8.9 years Gender (% male): I: 64.7; C: 63.6 COPD diagnosis: a clinical diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 criteria (GOLD II‐IV) Inclusion of participants in the acute phase: no Major inclusion criteria: aged at least 40 years, a clinical diagnosis of COPD according to the GOLD criteria (FEV₁ < 80% of the predicted value and FEV₁/FVC < 0.70), clinically stable at the time of inclusion, at least one clinically relevant comorbidity (ischaemic heart disease, chronic heart failure, diabetes mellitus, anxiety, depression), and at least three COPD exacerbations or one hospitalisation for respiratory problems in the two years preceding study entry. Major exclusion criteria: terminal cancer, end stage of COPD or another serious disease with low survival rate, other serious lung disease, participants with cognitive impairment |
| Interventions | Mode: group and individual sessions at the hospital, telephone follow‐up calls Duration: two or three face‐to‐face group sessions of 1.5 hours each and two individual sessions of one hour each scheduled in four consecutive weeks, three telephone calls to reinforce self‐management skills Professional: respiratory, cardiac, mental health and diabetes nurses Training of case managers: sessions were guided by a trained case‐manager (experienced respiratory nurses) and supported by cardiac, mental health and/or diabetes nurses Self‐management components: action plan COPD exacerbations, iterative process with feedback on actions, self‐recognition of COPD exacerbations, education regarding COPD Self‐management topics: exercise, diet, (maintenance) medication, correct device use, coping with breathlessness/breathing techniques, Exercise programme: no Smoking cessation programme: no Action plan components: self‐recognition of exacerbations, self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, self‐treatment of comorbidities |
| Outcomes |
|
| Starting date | 2012 |
| Contact information | Anke Lenferink, Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, the Netherlands. E‐mail: [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HRQoL: adjusted SGRQ total score Show forest plot | 10 | 1582 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] |
| Analysis 1.1 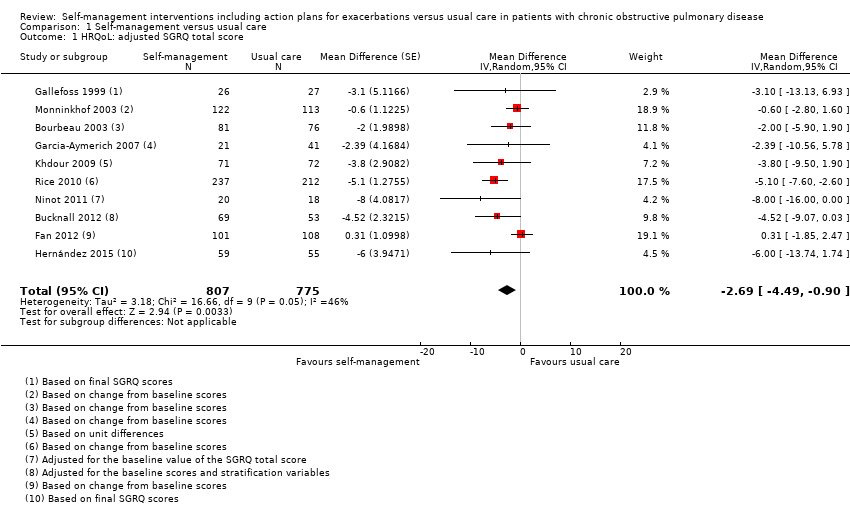 Comparison 1 Self‐management versus usual care, Outcome 1 HRQoL: adjusted SGRQ total score. | ||||
| 2 Healthcare utilisation: respiratory‐related hospital admissions (number of patients with at least one admission) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 1.2 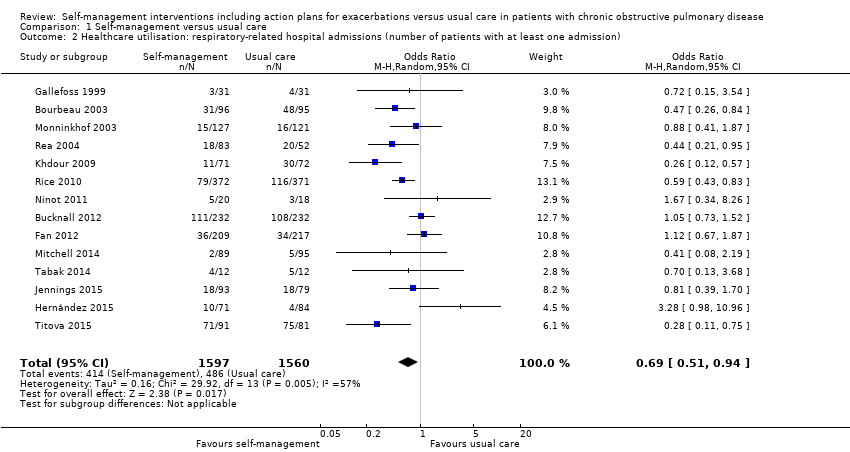 Comparison 1 Self‐management versus usual care, Outcome 2 Healthcare utilisation: respiratory‐related hospital admissions (number of patients with at least one admission). | ||||
| 3 Healthcare utilisation: respiratory‐related hospital admissions (mean number per patient) Show forest plot | 5 | 873 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.36, 0.05] |
| Analysis 1.3 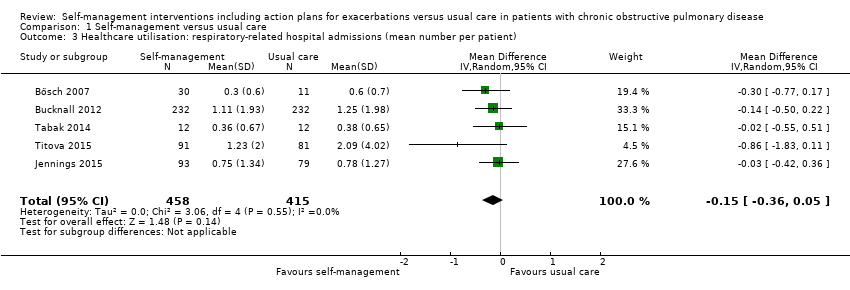 Comparison 1 Self‐management versus usual care, Outcome 3 Healthcare utilisation: respiratory‐related hospital admissions (mean number per patient). | ||||
| 4 Healthcare utilisation: all‐cause hospital admissions (number of patients with at least one admission) Show forest plot | 10 | 2467 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.54, 1.03] |
| Analysis 1.4  Comparison 1 Self‐management versus usual care, Outcome 4 Healthcare utilisation: all‐cause hospital admissions (number of patients with at least one admission). | ||||
| 5 Healthcare utilisation: all‐cause hospital admissions (mean number per patient) Show forest plot | 4 | 736 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.38, 0.29] |
| Analysis 1.5  Comparison 1 Self‐management versus usual care, Outcome 5 Healthcare utilisation: all‐cause hospital admissions (mean number per patient). | ||||
| 6 Healthcare utilisation: all‐cause hospitalisation days (per patient) Show forest plot | 7 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.01, 0.71] |
| Analysis 1.6  Comparison 1 Self‐management versus usual care, Outcome 6 Healthcare utilisation: all‐cause hospitalisation days (per patient). | ||||
| 7 Healthcare utilisation: emergency department visits (mean number per patient) Show forest plot | 3 | 827 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| Analysis 1.7 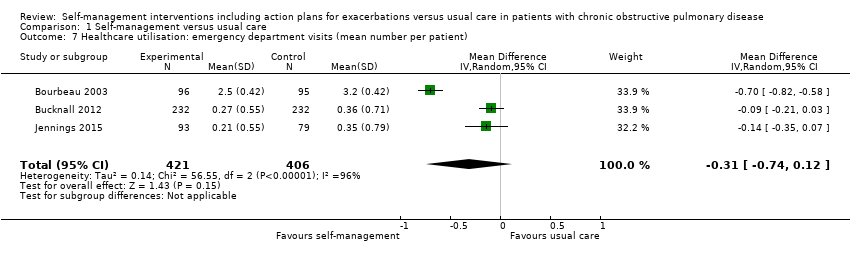 Comparison 1 Self‐management versus usual care, Outcome 7 Healthcare utilisation: emergency department visits (mean number per patient). | ||||
| 8 Healthcare utilisation: GP visits (mean number per patient) Show forest plot | 3 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐2.64, 1.93] |
| Analysis 1.8  Comparison 1 Self‐management versus usual care, Outcome 8 Healthcare utilisation: GP visits (mean number per patient). | ||||
| 9 Health status: (modified) Medical Research Council Dyspnoea Scale ((m)MRC) Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.44, 0.18] |
| Analysis 1.9 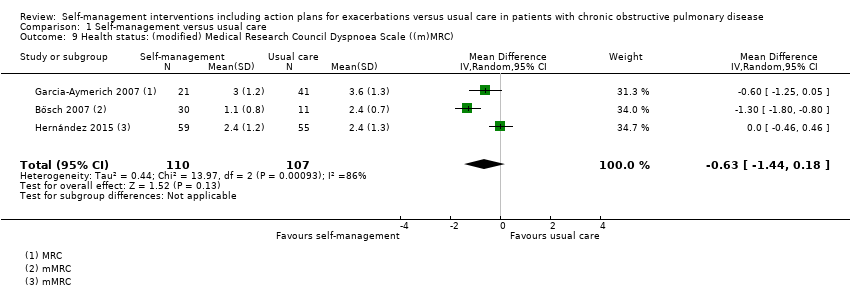 Comparison 1 Self‐management versus usual care, Outcome 9 Health status: (modified) Medical Research Council Dyspnoea Scale ((m)MRC). | ||||
| 10 COPD exacerbations (mean number per patient) Show forest plot | 4 | 740 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.28, 0.29] |
| Analysis 1.10 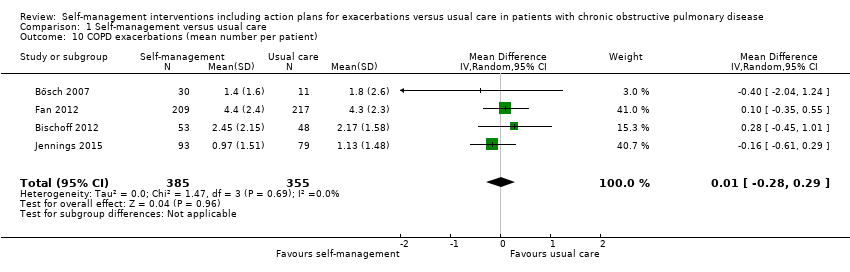 Comparison 1 Self‐management versus usual care, Outcome 10 COPD exacerbations (mean number per patient). | ||||
| 11 Courses of oral steroids (number of patients used at least one course) Show forest plot | 4 | 963 | Odds Ratio (M‐H, Random, 95% CI) | 4.38 [0.55, 34.91] |
| Analysis 1.11 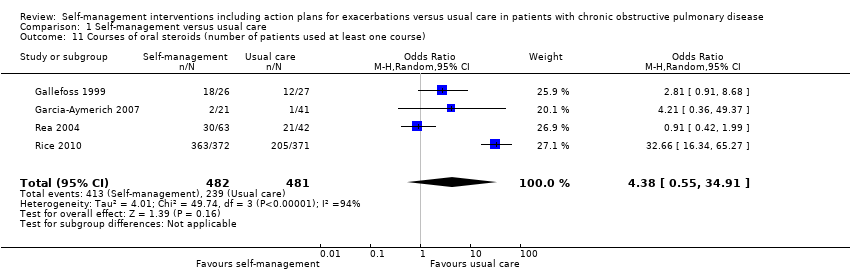 Comparison 1 Self‐management versus usual care, Outcome 11 Courses of oral steroids (number of patients used at least one course). | ||||
| 12 Mortality: all‐cause mortality Show forest plot | 16 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Self‐management versus usual care, Outcome 12 Mortality: all‐cause mortality. | ||||
| 12.1 All‐cause mortality | 16 | 3296 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 12.2 All‐cause 1‐year mortality | 12 | 2620 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 13 Mortality: respiratory‐related mortality Show forest plot | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.13 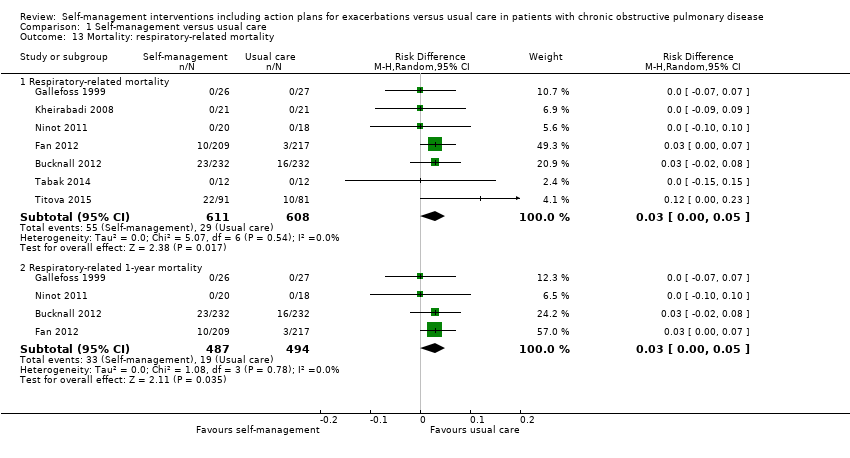 Comparison 1 Self‐management versus usual care, Outcome 13 Mortality: respiratory‐related mortality. | ||||
| 13.1 Respiratory‐related mortality | 7 | 1219 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.00, 0.05] |
| 13.2 Respiratory‐related 1‐year mortality | 4 | 981 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.00, 0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by follow‐up duration) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.1 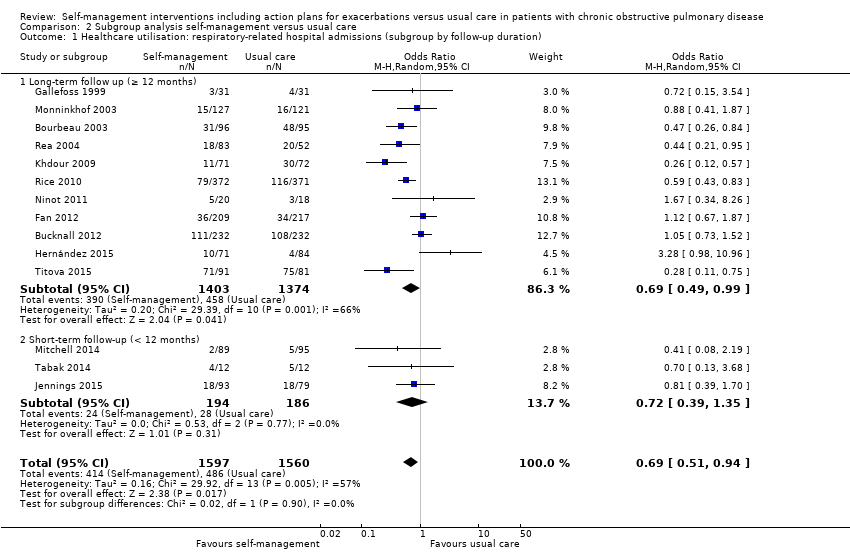 Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 1 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by follow‐up duration). | ||||
| 1.1 Long‐term follow up (≥ 12 months) | 11 | 2777 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.49, 0.99] |
| 1.2 Short‐term follow‐up (< 12 months) | 3 | 380 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.39, 1.35] |
| 2 HRQoL: adjusted SGRQ total score (subgroup by exercise programme) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| Analysis 2.2  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 2 HRQoL: adjusted SGRQ total score (subgroup by exercise programme). | ||||
| 2.1 Exercise programme | 4 | Mean Difference (Random, 95% CI) | ‐2.34 [‐5.09, 0.40] | |
| 2.2 No exercise programme | 6 | Mean Difference (Random, 95% CI) | ‐2.95 [‐5.63, ‐0.27] | |
| 3 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by exercise programme) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.3  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 3 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by exercise programme). | ||||
| 3.1 Exercise programme | 6 | 840 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.47, 1.65] |
| 3.2 No exercise programme | 8 | 2317 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 4 HRQoL: adjusted SGRQ total score (subgroup by smoking cessation programme) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| Analysis 2.4 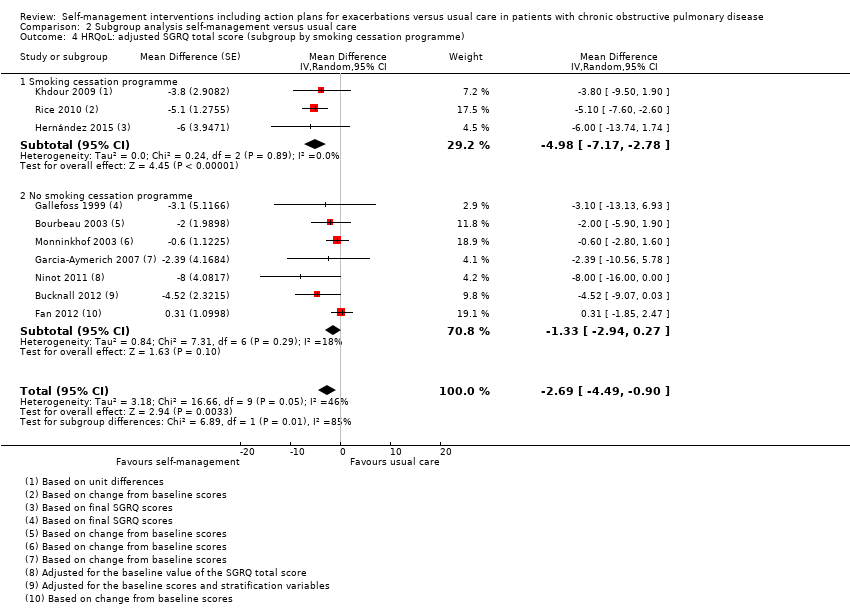 Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 4 HRQoL: adjusted SGRQ total score (subgroup by smoking cessation programme). | ||||
| 4.1 Smoking cessation programme | 3 | Mean Difference (Random, 95% CI) | ‐4.98 [‐7.17, ‐2.78] | |
| 4.2 No smoking cessation programme | 7 | Mean Difference (Random, 95% CI) | ‐1.33 [‐2.94, 0.27] | |
| 5 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by smoking cessation programme) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.5  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 5 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by smoking cessation programme). | ||||
| 5.1 Smoking cessation programme | 4 | 1213 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.45] |
| 5.2 No smoking cessation programme | 10 | 1944 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 1.00] |
| 6 HRQoL: adjusted SGRQ total score (subgroup by median number of BCT clusters) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| Analysis 2.6 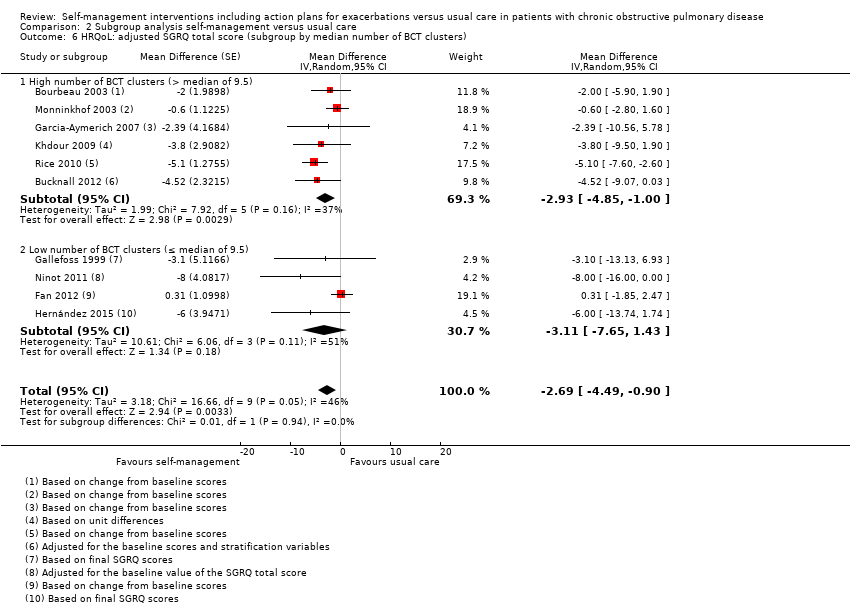 Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 6 HRQoL: adjusted SGRQ total score (subgroup by median number of BCT clusters). | ||||
| 6.1 High number of BCT clusters (> median of 9.5) | 6 | Mean Difference (Random, 95% CI) | ‐2.93 [‐4.85, ‐1.00] | |
| 6.2 Low number of BCT clusters (≤ median of 9.5) | 4 | Mean Difference (Random, 95% CI) | ‐3.11 [‐7.65, 1.43] | |
| 7 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by median number of BCT clusters) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.7 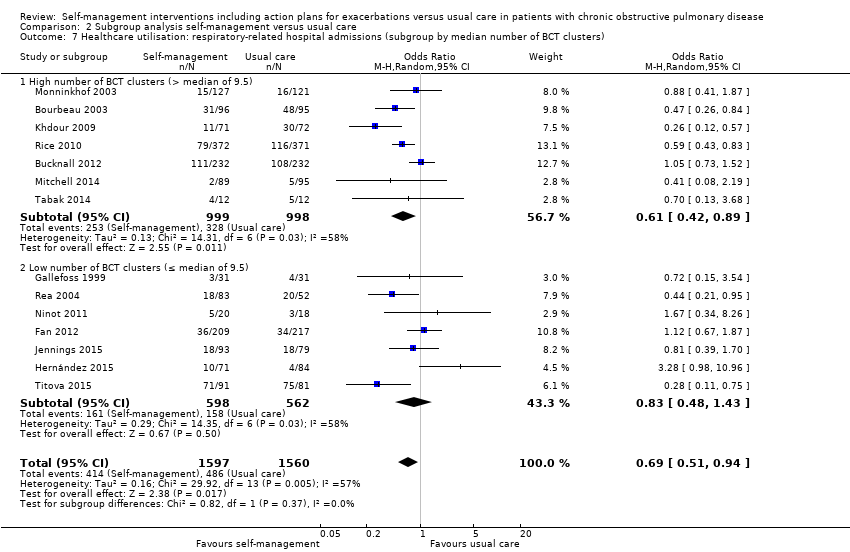 Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 7 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by median number of BCT clusters). | ||||
| 7.1 High number of BCT clusters (> median of 9.5) | 7 | 1997 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.89] |
| 7.2 Low number of BCT clusters (≤ median of 9.5) | 7 | 1160 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.43] |
| 8 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by number of BCT clusters) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.8 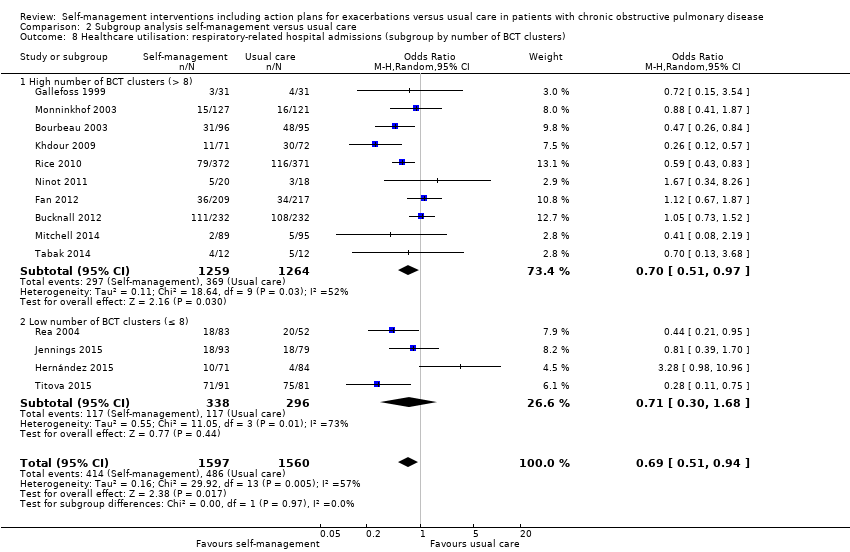 Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 8 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by number of BCT clusters). | ||||
| 8.1 High number of BCT clusters (> 8) | 10 | 2523 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.51, 0.97] |
| 8.2 Low number of BCT clusters (≤ 8) | 4 | 634 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.30, 1.68] |
| 9 HRQoL: adjusted SGRQ total score (subgroup by case manager support) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| Analysis 2.9 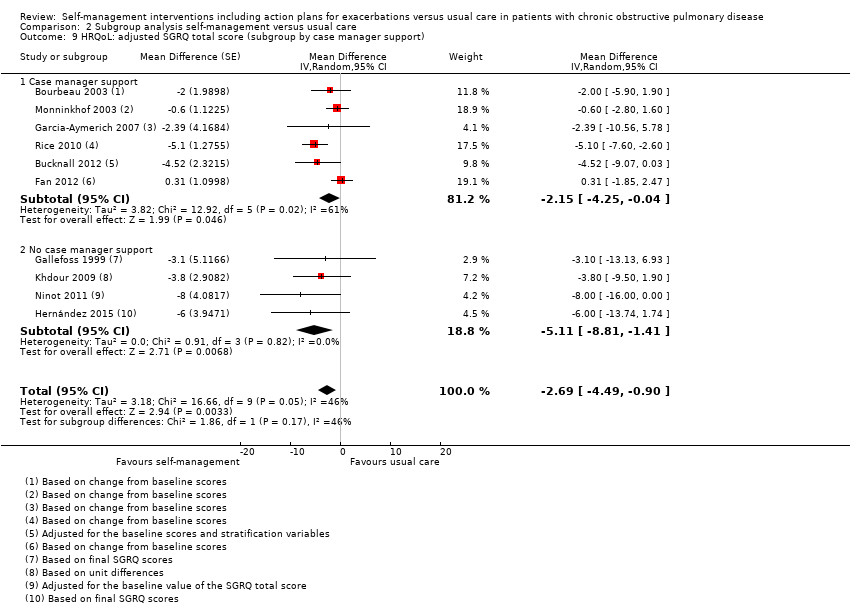 Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 9 HRQoL: adjusted SGRQ total score (subgroup by case manager support). | ||||
| 9.1 Case manager support | 6 | Mean Difference (Random, 95% CI) | ‐2.15 [‐4.25, ‐0.04] | |
| 9.2 No case manager support | 4 | Mean Difference (Random, 95% CI) | ‐5.11 [‐8.81, ‐1.41] | |
| 10 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by case manager support) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.10  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 10 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by case manager support). | ||||
| 10.1 Case manager support | 8 | 2403 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.49, 0.93] |
| 10.2 No case manager support | 6 | 754 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.36, 1.77] |
| 11 HRQoL: adjusted SGRQ total score (subgroup by intervention duration) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| Analysis 2.11  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 11 HRQoL: adjusted SGRQ total score (subgroup by intervention duration). | ||||
| 11.1 Intervention duration ≥ 6 months | 7 | Mean Difference (Random, 95% CI) | ‐2.96 [‐5.20, ‐0.72] | |
| 11.2 Intervention duration < 6 months | 3 | Mean Difference (Random, 95% CI) | ‐2.57 [‐6.96, 1.82] | |
| 12 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by intervention duration) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.12  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 12 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by intervention duration). | ||||
| 12.1 Intervention duration ≥ 6 months | 9 | 2453 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.96] |
| 12.2 Intervention duration < 6 months | 5 | 704 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.32] |
| 13 HRQoL: adjusted SGRQ total score (subgroup by action plan component 'adaptation of maintenance medication') Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| Analysis 2.13  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 13 HRQoL: adjusted SGRQ total score (subgroup by action plan component 'adaptation of maintenance medication'). | ||||
| 13.1 Action defined for adaptation of maintenance medication | 6 | Mean Difference (Random, 95% CI) | ‐3.75 [‐6.16, ‐1.33] | |
| 13.2 No action defined for adaptation of maintenance medication | 4 | Mean Difference (Random, 95% CI) | ‐2.02 [‐4.77, 0.72] | |
| 14 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'adaptation of maintenance medication' Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.14 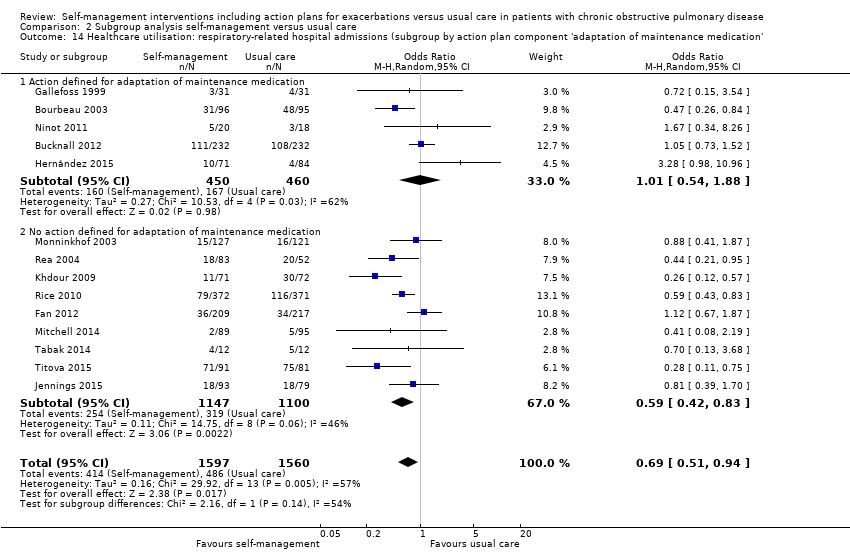 Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 14 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'adaptation of maintenance medication'. | ||||
| 14.1 Action defined for adaptation of maintenance medication | 5 | 910 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.88] |
| 14.2 No action defined for adaptation of maintenance medication | 9 | 2247 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.42, 0.83] |
| 15 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'when to avoid situations in which viral infections might be prevalent') Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| Analysis 2.15  Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 15 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'when to avoid situations in which viral infections might be prevalent'). | ||||
| 15.1 Action defined 'when to avoid situations in which viral infections might be prevalent' | 4 | 549 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.25, 3.13] |
| 15.2 No action defined 'when to avoid situations in which viral infections might be prevalent' | 10 | 2608 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.91] |

Study flow diagram
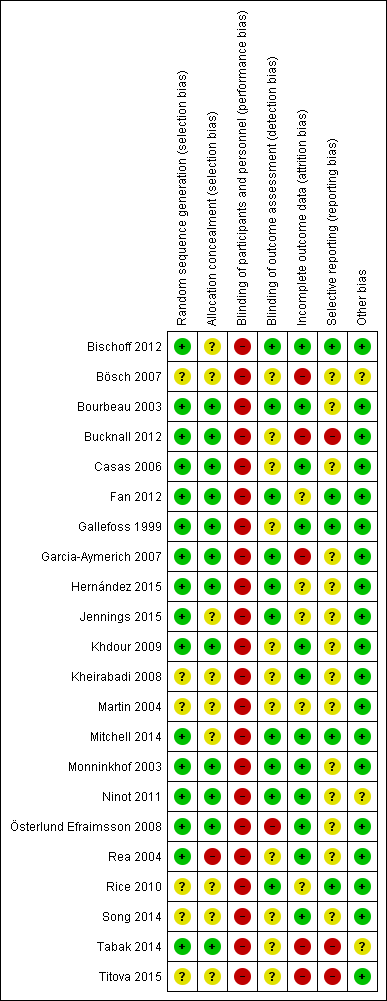
Risk of bias summary for each study according to authors' judgements
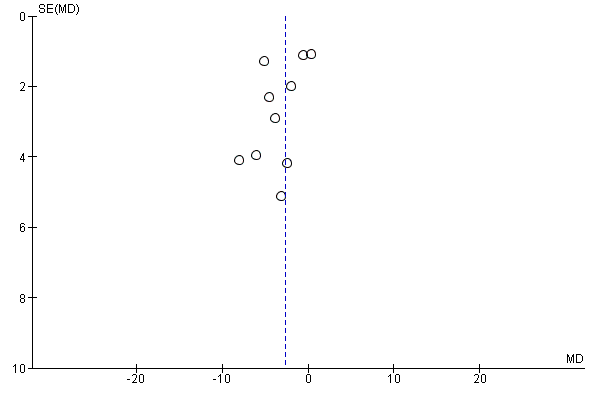
Funnel plot of comparison: Self‐management versus usual care, outcome: 1.1 HRQoL: adjusted SGRQ total score

Funnel plot of comparison: Self‐management versus usual care, outcome: 1.2 Healthcare utilisation: respiratory‐related hospital admissions (number of patients with at least one admission)
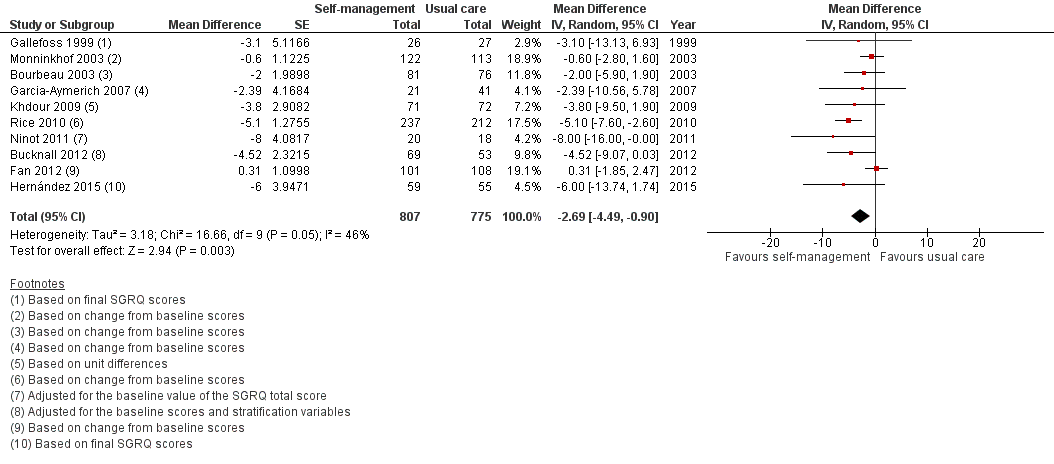
Forest plot of comparison: Self‐management versus usual care, outcome: 1.1 HRQoL: adjusted SGRQ total score after 12 months of follow‐up

Forest plot of comparison: Self‐management versus usual care, outcome: 1.2 Healthcare utilisation: respiratory‐related hospital admissions (number of patients with at least one admission)
Cates plot of COPD participants with high baseline risk of respiratory‐related hospital admissions in self‐management interventions including action plans for AECOPD compared to usual care. In the usual care group, 39 of 100 participants had at least one respiratory‐related hospital admission over 52 weeks, compared with 31 (95% CI 25 to 38) of 100 participants in the self‐management intervention group with the highest baseline risks for respiratory‐related hospital admissions
Cates plot of COPD participants with low baseline risk of respiratory‐related hospital admissions in self‐management interventions with action plans for AECOPD compared to usual care. In the usual care group, 23 of 100 participants had at least one respiratory‐related hospital admission over 52 weeks, compared with 17 (95% CI 13 to 22) of 100 participants in the self‐management intervention group

Forest plot of comparison: Self‐management versus usual care, outcome: 1.13 All‐cause mortality

Forest plot of comparison: Self‐management versus usual care, outcome: 1.14 Respiratory‐related mortality
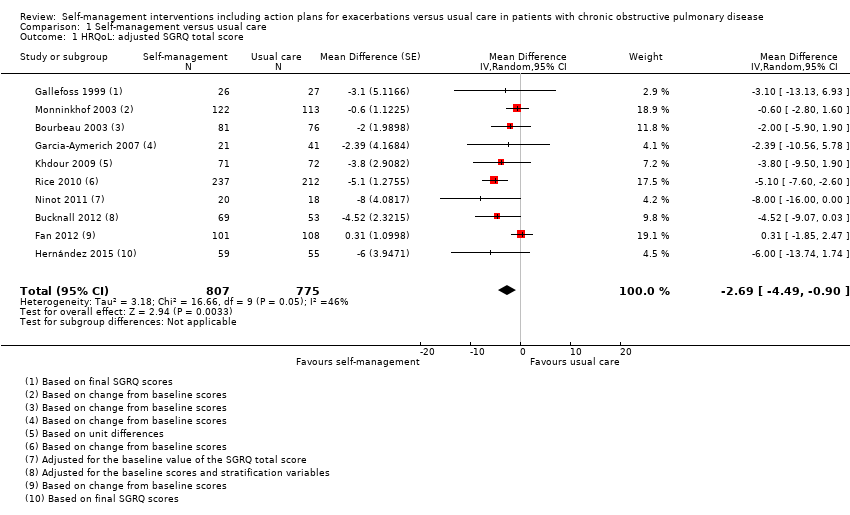
Comparison 1 Self‐management versus usual care, Outcome 1 HRQoL: adjusted SGRQ total score.

Comparison 1 Self‐management versus usual care, Outcome 2 Healthcare utilisation: respiratory‐related hospital admissions (number of patients with at least one admission).

Comparison 1 Self‐management versus usual care, Outcome 3 Healthcare utilisation: respiratory‐related hospital admissions (mean number per patient).
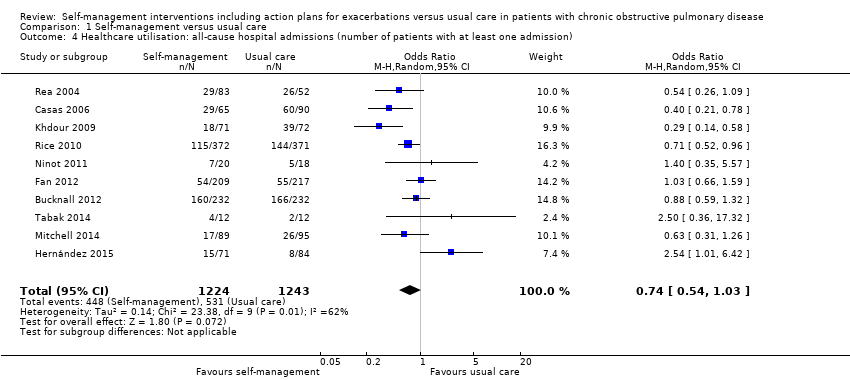
Comparison 1 Self‐management versus usual care, Outcome 4 Healthcare utilisation: all‐cause hospital admissions (number of patients with at least one admission).
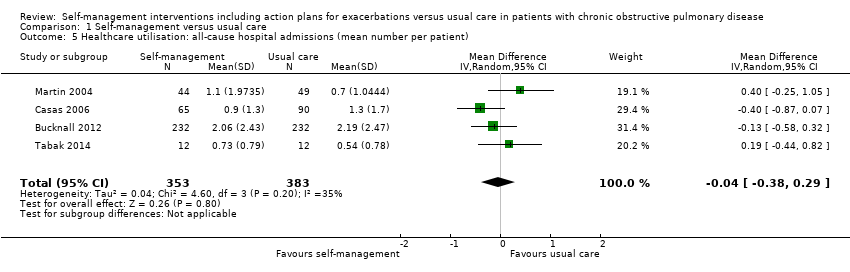
Comparison 1 Self‐management versus usual care, Outcome 5 Healthcare utilisation: all‐cause hospital admissions (mean number per patient).

Comparison 1 Self‐management versus usual care, Outcome 6 Healthcare utilisation: all‐cause hospitalisation days (per patient).
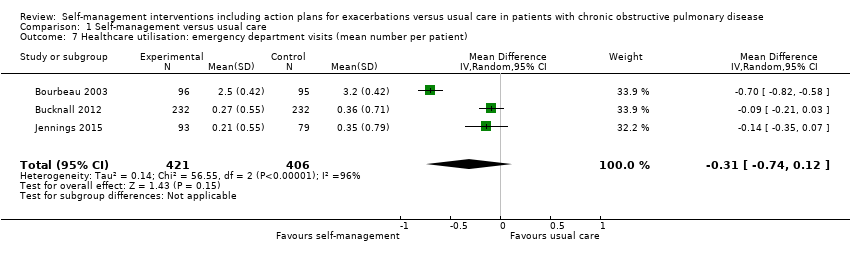
Comparison 1 Self‐management versus usual care, Outcome 7 Healthcare utilisation: emergency department visits (mean number per patient).
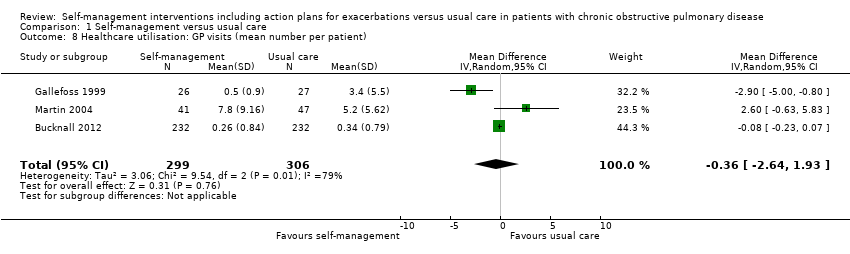
Comparison 1 Self‐management versus usual care, Outcome 8 Healthcare utilisation: GP visits (mean number per patient).
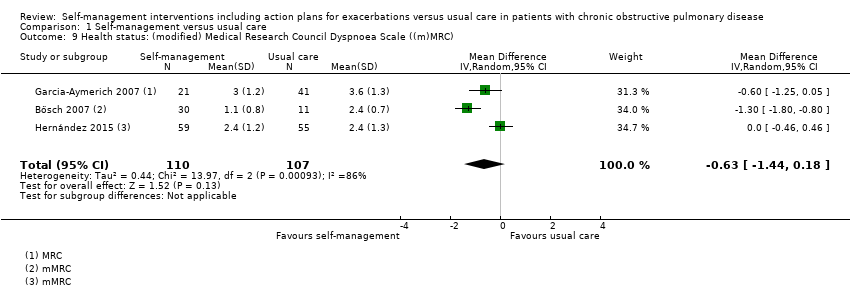
Comparison 1 Self‐management versus usual care, Outcome 9 Health status: (modified) Medical Research Council Dyspnoea Scale ((m)MRC).

Comparison 1 Self‐management versus usual care, Outcome 10 COPD exacerbations (mean number per patient).
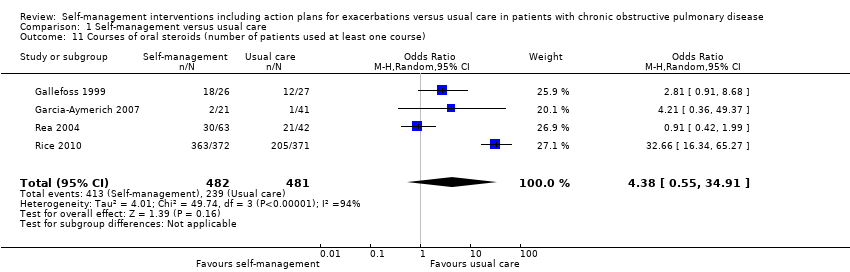
Comparison 1 Self‐management versus usual care, Outcome 11 Courses of oral steroids (number of patients used at least one course).
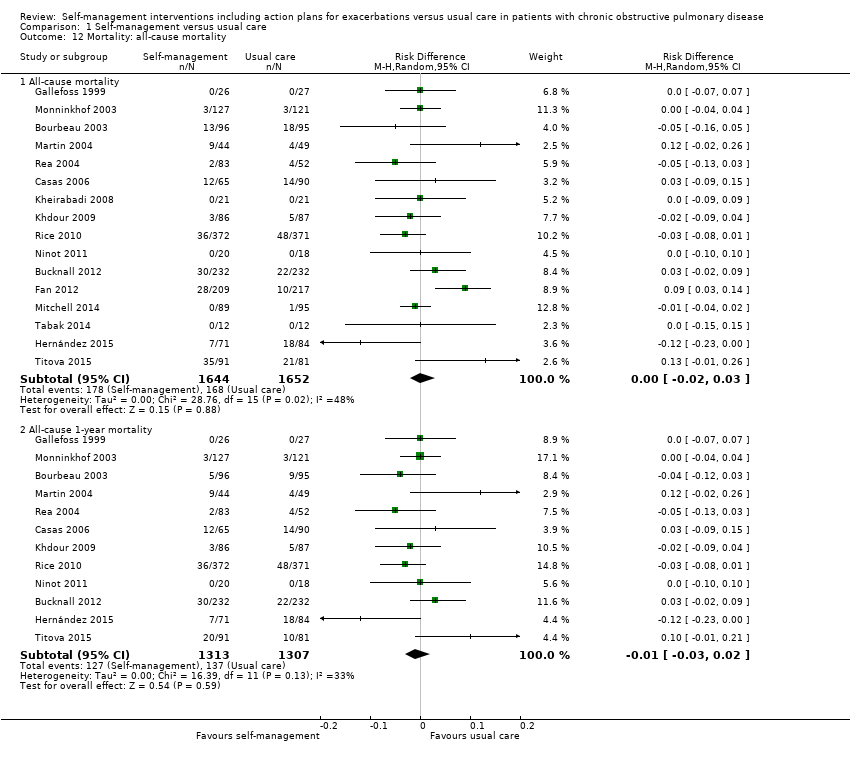
Comparison 1 Self‐management versus usual care, Outcome 12 Mortality: all‐cause mortality.

Comparison 1 Self‐management versus usual care, Outcome 13 Mortality: respiratory‐related mortality.

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 1 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by follow‐up duration).
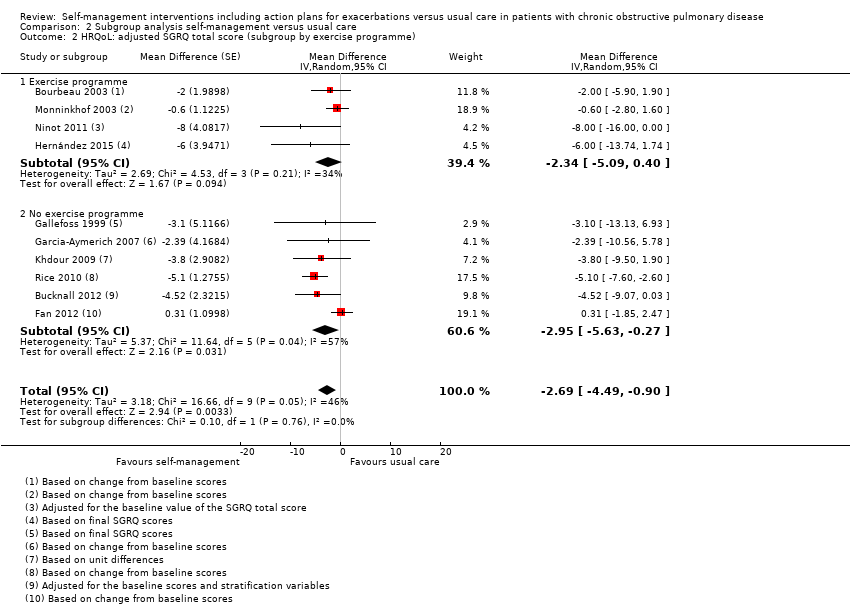
Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 2 HRQoL: adjusted SGRQ total score (subgroup by exercise programme).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 3 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by exercise programme).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 4 HRQoL: adjusted SGRQ total score (subgroup by smoking cessation programme).
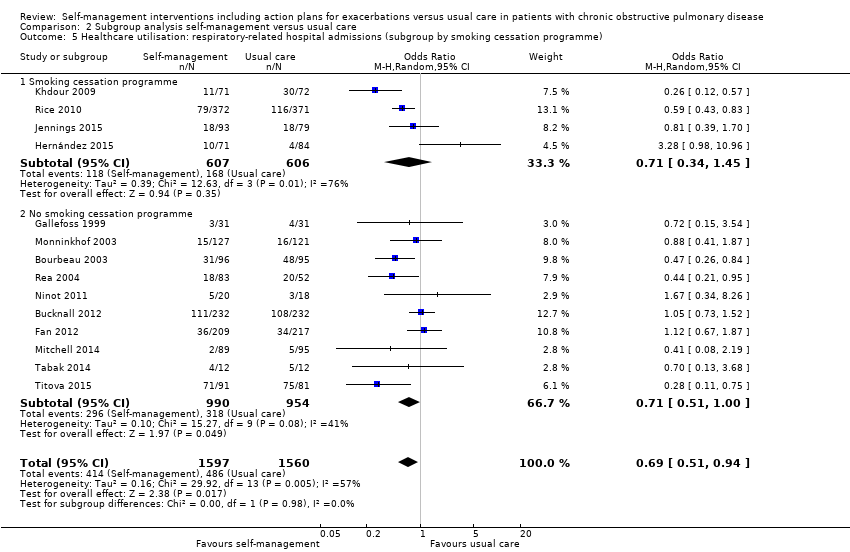
Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 5 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by smoking cessation programme).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 6 HRQoL: adjusted SGRQ total score (subgroup by median number of BCT clusters).
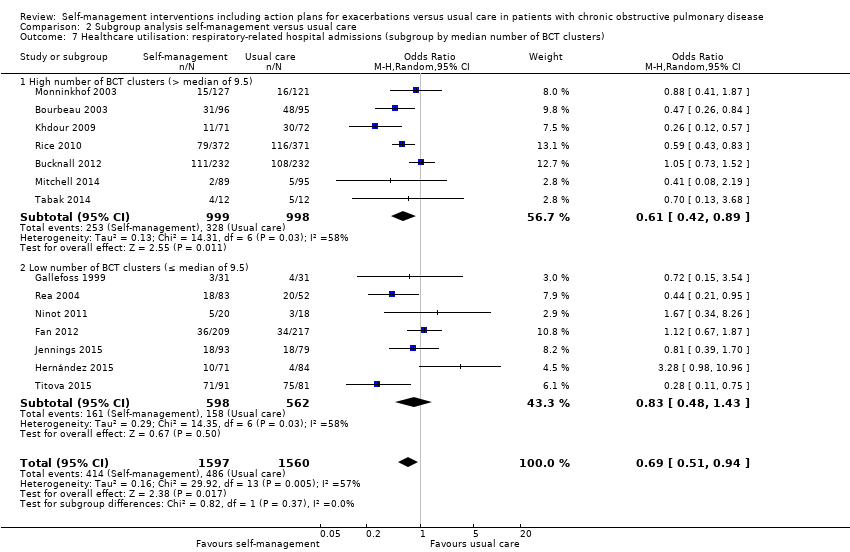
Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 7 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by median number of BCT clusters).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 8 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by number of BCT clusters).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 9 HRQoL: adjusted SGRQ total score (subgroup by case manager support).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 10 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by case manager support).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 11 HRQoL: adjusted SGRQ total score (subgroup by intervention duration).

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 12 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by intervention duration).
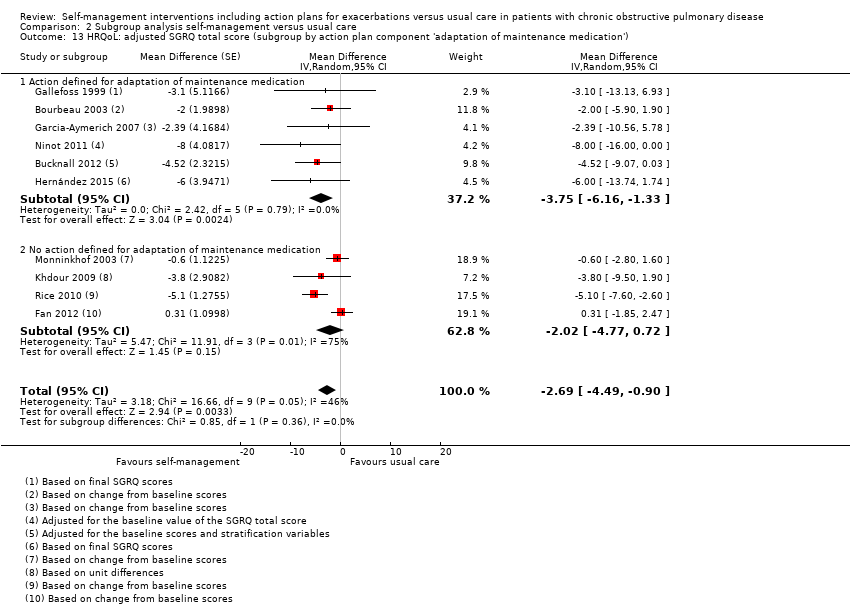
Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 13 HRQoL: adjusted SGRQ total score (subgroup by action plan component 'adaptation of maintenance medication').

Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 14 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'adaptation of maintenance medication'.
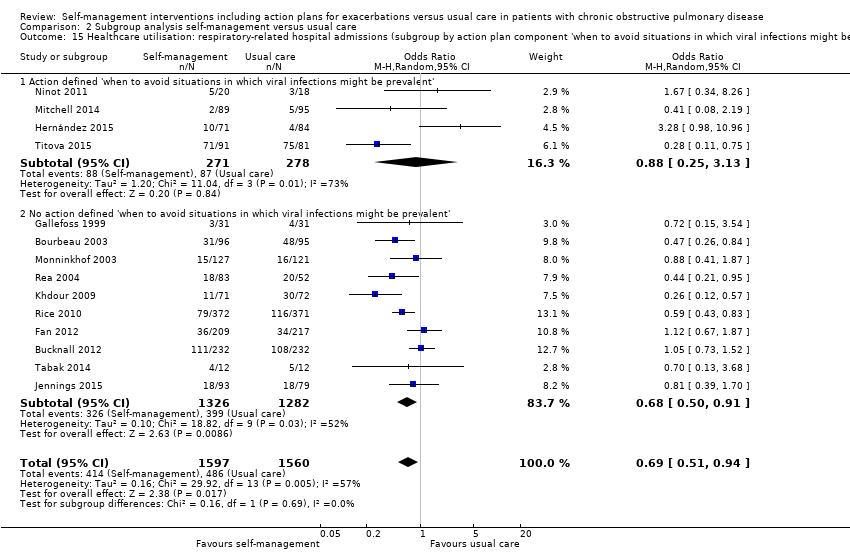
Comparison 2 Subgroup analysis self‐management versus usual care, Outcome 15 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'when to avoid situations in which viral infections might be prevalent').
| Self‐management interventions including action plans for exacerbations compared to usual care for patients with COPD | ||||||
| Patient or population: patients with chronic obstructive pulmonary disease (COPD) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with self‐management interventions including action plans for exacerbations | |||||
| Health‐related quality of life (HRQoL) | The mean HRQoL ranged from 37.7 to 70.4 points | MD 2.69 points lower | ‐ | 1582 | ⊕⊕⊕⊕ | Lower score indicates better health‐related quality of life. |
| Respiratory‐related hospital admissions | 312 per 1,000 | 238 per 1,000 | OR 0.69 | 3,157 | ⊕⊕⊕⊝ | |
| All‐cause hospital admissions | 427 per 1000 | 356 per 1,000 | OR 0.74 | 2,467 | ⊕⊕⊕⊝ | |
| All‐cause mortality | 102 per 1000 | 107 per 1,000 | OR 1.06 | 3,296 | ⊕⊕⊕⊝ | Pooled risk difference of 0.0019 (95% CI ‐0.0225 to 0.0263). |
| Respiratory‐related mortality | 48 per 1000 | 89 per 1,000 | OR 1.94 | 1,219 | ⊕⊝⊝⊝ | Pooled risk difference of 0.028 (95% CI 0.0049 to 0.0511). |
| Dyspnoea | The mean dyspnoea ranged from 2.4 to 2.6 | MD 0.63 lower | ‐ | 217 | ⊕⊕⊝⊝ | Lower score indicates improvement in dyspnoea. |
| COPD exacerbations | The mean COPD exacerbations ranged from 1.13 to 4.3 | MD 0.01 higher | ‐ | 740 | ⊕⊕⊕⊝ | |
| Courses of oral steroids | 497 per 1000 | 812 per 1000 | OR 4.38 | 963 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Heterogeneity was substantial (I² = 57%) (inconsistency ‐1). 2 Heterogeneity was substantial (I² = 62%) (inconsistency ‐1). 3 Imprecision of pooled effect size (imprecision ‐1). 4 Explorative meta‐analysis. Four studies (Gallefoss 1999; Kheirabadi 2008; Ninot 2011; Tabak 2014) with no events and a high risk of bias for three studies (Bucknall 2012; Tabak 2014; Titova 2015) for incomplete outcome data and selective reporting. Two studies (Bucknall 2012; Fan 2012) dominated the overall effect and heavily influenced the OR (risk of bias ‐1, inconsistency ‐1, imprecision ‐1). 5 Heterogeneity was high (I² =86%). Only three studies were included in this meta‐analysis (inconsistency ‐1, imprecision ‐1). 6 Only four studies were included in this meta‐analysis (imprecision ‐1). 7 COPD exacerbations were defined as worsening of respiratory symptoms beyond normal day‐to‐day variations that required treatment with bronchodilators, oral steroids and/or antibiotics 8 Heterogeneity was high (I² = 94%). Only four studies were included in this meta‐analysis (inconsistency ‐1, imprecision ‐1). | ||||||
| Study | Included participants (N) | Lost to follow‐up (%) | Age (years; mean (SD)) | Gender (% male) | FEV₁ (% predicted unless stated otherwise (SD)) | |||||
| Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | Self‐management | Usual care | |
| 55 | 55 | 10.9 | 20.0 | 65.5 (11.5) | 63.5 (10.3) | 67.0 | 51.0 | 66.3 (16.5) | 67.0 (18.0) | |
| 38 | 12 | 21.1 | 8.3 | 63.8 (8.4) | 64.6 (6.8) | 63.0% of completers | 45.9 (17.5) | 47.8 (16.9) | ||
| 96 | 95 | 10.4 | 16.8 | 69.4 (6.5) | 69.6 (7.4) | 52.0 | 59.0 | 1.0 L (0.33) | 0.98 (0.31) | |
| 232 | 232 | 9.1 | 13.8 | 70.0 (9.3) | 68.3 (9.2) | 38.0 | 35.0 | 41.2 (13.4) | 39.8 (13.8) | |
| 65 | 90 | 26.2 | 20.0 | 70 (9.0) | 72 (9.0) | 77.0 | 88.0 | 43 (20) | 41 (15) | |
| 44 | 69 | 52.3 | 40.6 | 72 (10.0) | 73 (9.0) | 75.0 | 93.0 | 1.2 L (IQR 0.8 to 1.4) | 1.0 L (IQR 0.8‐1.5) | |
| 209 | 217 | 3.8a; 51.7b | 4.6a; 50.2b | 66.2 (8.4) | 65.8 (8.2) | 97.6 | 96.3 | 38.2 (14.3) | 37.8 (14.5) | |
| 31 | 31 | 16.0 | 13.0 | 57 (9.0) | 58 (10.0) | 48.0 | 52.0 | 59 (9) | 56 (11) | |
| 71 | 84 | 23.9 | 34.5 | 73 (8.0) | 75 (9.0) | 83.0 | 86.0 | 41 (19) | 44 (20) | |
| 93 | 79 | 0 | 0 | 64.9 (10.9) | 64.4 (10.5) | 43.1 | 46.8 | 44.1 (23.1) | 48.3 (22.2) | |
| 86 | 87 | 17.4 | 17.2 | 65.6 (10.1) | 67.3 (9.2) | 44.2 | 43.7 | 52.0 (15.9) | 52 (17.8) | |
| 21 | 21 | 0 | 0 | 56.6 (5.7) | 56.2 (4.1) | 61.9 | 76.2 | N/A | N/A | |
| 44 | 49 | 20.5 | 8.2 | 71.1 (95% CI 68.7 to 73.5) | 69.1 (95% CI 63.5 to 74.7) | 34.1 | 65.3 | 35.4 (95% CI 31.6 to 39.2) | 34.3 (95% CI 31.2 to 37.4) | |
| 89 | 95 | 26.9 | 16.8 | 69 (8.0) | 69 (10.1) | 60.7 | 49.5 | 56.0 (16.8) | 59.6 (17.4) | |
| 127 | 121 | 3.9 | 5.8 | 65 (7.0) | 65 (7.0) | 85.0 | 84.0 | 56.1 (15.4) | 58.4 (14.5) | |
| 23 | 22 | 13.0 | 18.2 | 65 (range 59 to 74) | 61 (range 56 to 65) | 90.0 | 77.8 | 56 (range 42 to 67) | 54 (range 42 to 57) | |
| 26 | 26 | 0 | 0 | 66 (9.4) | 67 (10.4) | 50.0 | 50.0 | N/A | N/A | |
| 83 | 52 | 14.5 | 11.5 | 68 (range 44 to 84) for the total group | 41.5% for the total group | 51.8 (18.1) | 50.0 (20.3) | |||
| 372 | 371 | 9.7 | 12.9 | 69.1 (9.4) | 70.7 (9.7) | 97.6 | 94.8 | 36.1 (14.5) | 38.2 (14.4) | |
| 20 | 20 | 15.0 | 15.0 | 66.6 (7.1) | 68.1 (6.5) | 55.0 | 75.0 | 57.0 (10.0) | 60.4 (24.9) | |
| 15 | 14 | 33.3 | 85.7 | 64.1 (9.0) | 62.8 (7.4) | 50.0 | 50.0 | 50.0 (IQR 33.3 to 61.5) | 36.0 (IQR 26.0 to 53.5) | |
| 91 | 81 | 44.0 | 39.5 | 74.1 (9.3) | 72.6 (9.3) | 42.9 | 43.2 | 33.6 (9.9) | 33.0 (9.7) | |
| adiscontinued; bincomplete baseline and 1‐year study visits; CI: confidence interval; IQR: interquartile range; L: liters; N/A: not applicable. | ||||||||||
| Study | Follow‐up (months) | Setting; provision intervention | Duration intervention | Content intervention | Content action plan |
| 24 | General practice; trained practice nurse | 2 to 4 FTF individual sessions (60 min each) scheduled in 4‐6 consecutive weeks, 6 phone calls | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support | |
| 12 | Outpatient clinic; trained respiratory nurse under supervision of a respiratory specialist | 4 FTF group sessions (120 min each) and final session scheduled 6 weeks later | Self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, other: travelling, daily live | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, avoid situations in which viral infection might be prevalent, contact healthcare providers for support | |
| 24 | Hospital (outpatient); trained professionals (nurses, respiratory therapists, a physiotherapist) | 7 FTF individual sessions (60 min each) scheduled in 7‐8 consecutive weeks, 18 phone calls | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, other: symptom monitoring list linked to appropriate therapeutic actions | |
| 12 | Hospital (inpatient); trained study nurse | 4 FTF individual sessions (40 min each) in 2 months, at least 6 subsequent home visits, 828 phone calls intervention group | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support | |
| 12 | Hospital (inpatient); trained respiratory nurse and GP, physician, nurse, social worker | 3 to 13 FTF individual sessions, 1 x group (40 min), 6 phone calls; Barcelona: 1 joint visit at home. Leuven: GP regularly visited patients at home | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, other: reinforcement of the logistics for treatment of comorbidities and social support | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, other: reinforcement of the logistics for treatment of comorbidities | |
| 12 | Hospital (inpatient); trained specialised respiratory nurse and physician, nurse, social worker | 3 to 13 FTF individual sessions at the hospital (40 min each) or at home (20 min), 6 phone calls | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, other: reinforcement of the logistics for treatment of comorbidities and social support | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support, other: reinforcement of the logistics for treatment of comorbidities | |
| 12 | Outpatient clinic; trained case manager (various health‐related professionals) | 4 FTF individual sessions (90 min each) scheduled weekly, 1x group, 6 phone calls | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD | Self‐recognition and self‐treatment of exacerbations, contact healthcare providers for support | |
| 12 | Hospital (outpatient); trained nurse, physiotherapist, pharmacist, medical doctor | 1 to 2 FTF individual sessions by a nurse and 1 to 2 by physiotherapist (40 min each), 2 x group (120 min each) | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, other: compliance, self‐care | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, contact healthcare providers for support | |
| 12 | Hospital (outpatient); trained specialised respiratory nurse, physician, nurse, social worker | Participants with no mobility problems: 1 FTF individual session (40 min) at home by primary care team, 3 x group at outpatient clinic (2 x 90 min, 1x 120 min) Participants with mobility problems: 4 FTF individual sessions (15 min each), 1 x individual (120 min) or 1 x group (40 min), all at home by primary care team | Self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, exercise or physical activity component, other: instructions on non‐pharmacological treatment | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, avoid situations in which viral infection might be prevalent, contact healthcare providers for support, self‐treatment of comorbidities | |
| 3 | Hospital (inpatient); research team and research nurse | 1 FTF individual session (60 min) at the hospital by research team member 24 hours prior to discharge, phone call 48 hours after discharge | Iterative process, education regarding COPD, smoking cessation, other: primary team was notified if patient was identified as having anxiety or depressive symptoms | Contact healthcare providers for support | |
| 12 | Hospital (outpatient); clinical pharmacist, respiratory specialist, respiratory nurse | 1 FTF individual session of 45 min (60 min for smokers) and 2 phone calls | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation | Self‐recognition and self‐treatment of exacerbations, contact healthcare providers for support | |
| 3 | Hospital (outpatient); psychologist, trained psychiatric residents | 8 FTF group sessions (60 to 90 minutes each) with 1 week interval and follow‐up by phone | Self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component | Avoid situations in which viral infection might be prevalent | |
| 12 | General practice; respiratory physician and nurse, GP, ED consultant, medical staff hospital | 4 FTF individual sessions and respiratory nurse visits at 3, 6 and 12 months | Iterative process, self‐recognition of COPD exacerbations | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, self‐treatment of comorbidities, other: when to use oxygen therapy and diuretics | |
| 6 | General practice; physiotherapist, trainee health psychologist | 1 FTF individual session (30‐45 min) by a physiotherapist and 2 phone calls | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component | Self‐recognition and self‐treatment of exacerbations, avoid situations in which viral infection might be prevalent, contact healthcare providers for support, other: self‐administration, requesting rescue medication | |
| 12 | Hospital (outpatient); trained respiratory nurse, respiratory physiotherapist | 5 FTF group sessions (120 min each) by a respiratory nurse (4 x with a 1‐week interval and 3 months later) and 1 to 2 x groups (30 to 45 min) by a physiotherapist | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component | Self‐recognition and self‐treatment of exacerbations, contact healthcare providers for support | |
| 12 | Hospital (outpatient); health professional and qualified exercise trainer | 8 FTF group sessions (120 min each) by a health professional for 4 weeks, 8 exercise sessions (30 to 45 min each) by a qualified exercise trainer, 3 phone calls | Self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component | Self‐recognition and self‐treatment of exacerbations, use of maintenance treatment, avoid situations in which viral infection might be prevalent | |
| 3 to 5 | Primary healthcare clinic; COPD nurse, physician, if needed: dietician, medical social worker, physical and occupational therapist | 2 FTF individual sessions for self‐care education during 3 to 5 months (60 min each) by the nurse | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, smoking cessation, exercise or physical activity component | Self‐recognition and self‐treatment of exacerbations, contact healthcare providers for support | |
| 12 | General practice; respiratory physician, respiratory nurse specialist, GP | At least 17 individual FTF sessions (monthly visits to practice nurse (N = 12), 3‐monthly to GP (N = 4), 1 x home visit by the respiratory nurse specialist, 1 x after admission) | Iterative process, self‐recognition of COPD exacerbations, other: annual influenza vaccination and PR programme attendance | Self‐recognition and self‐treatment of exacerbations, contact healthcare providers for support | |
| 12 | Hospital (Veterans Affairs medical centres); trained respiratory therapist case manager | 1 group session (60 to 90 min) by a respiratory therapist case manager, 12 monthly phone calls (10 to 15 min each) | Iterative process, self‐recognition of COPD exacerbations; education regarding COPD, smoking cessation | Self‐recognition and self‐treatment of exacerbations, contact healthcare providers for support | |
| 2 | Hospital (inpatient); trained nurse interventionists | 3 FTF individual sessions (2 x inpatient (90 + 45 min each) on the day before and on the day of discharge, 1 x outpatient (90 min) on the first follow‐up day) by 2 nurse interventionists, 2 phone calls with a 2‐week interval | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component | Self‐recognition and self‐treatment of exacerbations | |
| 9 | Hospital (outpatient); primary care physiotherapy practices; respiratory nurse practitioner, respiratory physiotherapist | 2 group sessions (90 min each) by a nurse practitioner, 1 FTF individual session and 1 x intake by the physiotherapist, additional meetings after 1, 3, 6 and 9 months | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD, exercise or physical activity component | Self‐recognition and self‐treatment of exacerbations, contact healthcare providers for support | |
| 24 | Hospital (inpatient); trained specialist nurse | 6 FTF individual sessions (1 x at discharge, 5 x home visits at 3 and 14 days, and at 6, 12, 24 months) by the specialist nurse, 1 e‐learning programme (15 min), at least 24 phone calls | Iterative process, self‐recognition of COPD exacerbations, education regarding COPD | Self‐recognition and self‐treatment of exacerbations, avoid situations in which viral infection might be prevalent, contact healthcare providers for support | |
| COPD: Chronic Obstructive Pulmonary Disease; FTF: face‐to‐face; PR: pulmonary rehabilitation | |||||
| Outcome of interest | Number of studies |
| Primary outcomes | |
| Health‐related quality of life | 16 |
| Respiratory‐related hospital admissions | 16 |
| Secondary outcomes | |
| All‐cause hospital admissions | 11 |
| All‐cause hospitalisation days | 8 |
| Respiratory‐related hospitalisation days | 5 |
| Emergency department visits | 9 |
| General practitioner visits | 7 |
| Specialist visits | 4 |
| Rescue medication use | 2 |
| Health status | 3 |
| COPD exacerbations | 6 |
| Use of courses of oral corticosteroids or antibiotics | 9 |
| All‐cause mortality | 16 |
| Respiratory‐related mortality | 7 |
| Self‐efficacy | 2 |
| Days lost from work | 2 |
| COPD: Chronic Obstructive Pulmonary Disease | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HRQoL: adjusted SGRQ total score Show forest plot | 10 | 1582 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] |
| 2 Healthcare utilisation: respiratory‐related hospital admissions (number of patients with at least one admission) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 3 Healthcare utilisation: respiratory‐related hospital admissions (mean number per patient) Show forest plot | 5 | 873 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.36, 0.05] |
| 4 Healthcare utilisation: all‐cause hospital admissions (number of patients with at least one admission) Show forest plot | 10 | 2467 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.54, 1.03] |
| 5 Healthcare utilisation: all‐cause hospital admissions (mean number per patient) Show forest plot | 4 | 736 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.38, 0.29] |
| 6 Healthcare utilisation: all‐cause hospitalisation days (per patient) Show forest plot | 7 | 1982 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.01, 0.71] |
| 7 Healthcare utilisation: emergency department visits (mean number per patient) Show forest plot | 3 | 827 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.74, 0.12] |
| 8 Healthcare utilisation: GP visits (mean number per patient) Show forest plot | 3 | 605 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐2.64, 1.93] |
| 9 Health status: (modified) Medical Research Council Dyspnoea Scale ((m)MRC) Show forest plot | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.44, 0.18] |
| 10 COPD exacerbations (mean number per patient) Show forest plot | 4 | 740 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.28, 0.29] |
| 11 Courses of oral steroids (number of patients used at least one course) Show forest plot | 4 | 963 | Odds Ratio (M‐H, Random, 95% CI) | 4.38 [0.55, 34.91] |
| 12 Mortality: all‐cause mortality Show forest plot | 16 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 All‐cause mortality | 16 | 3296 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 12.2 All‐cause 1‐year mortality | 12 | 2620 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 13 Mortality: respiratory‐related mortality Show forest plot | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Respiratory‐related mortality | 7 | 1219 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.00, 0.05] |
| 13.2 Respiratory‐related 1‐year mortality | 4 | 981 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.00, 0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by follow‐up duration) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 1.1 Long‐term follow up (≥ 12 months) | 11 | 2777 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.49, 0.99] |
| 1.2 Short‐term follow‐up (< 12 months) | 3 | 380 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.39, 1.35] |
| 2 HRQoL: adjusted SGRQ total score (subgroup by exercise programme) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| 2.1 Exercise programme | 4 | Mean Difference (Random, 95% CI) | ‐2.34 [‐5.09, 0.40] | |
| 2.2 No exercise programme | 6 | Mean Difference (Random, 95% CI) | ‐2.95 [‐5.63, ‐0.27] | |
| 3 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by exercise programme) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 3.1 Exercise programme | 6 | 840 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.47, 1.65] |
| 3.2 No exercise programme | 8 | 2317 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 4 HRQoL: adjusted SGRQ total score (subgroup by smoking cessation programme) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| 4.1 Smoking cessation programme | 3 | Mean Difference (Random, 95% CI) | ‐4.98 [‐7.17, ‐2.78] | |
| 4.2 No smoking cessation programme | 7 | Mean Difference (Random, 95% CI) | ‐1.33 [‐2.94, 0.27] | |
| 5 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by smoking cessation programme) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 5.1 Smoking cessation programme | 4 | 1213 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.45] |
| 5.2 No smoking cessation programme | 10 | 1944 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 1.00] |
| 6 HRQoL: adjusted SGRQ total score (subgroup by median number of BCT clusters) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| 6.1 High number of BCT clusters (> median of 9.5) | 6 | Mean Difference (Random, 95% CI) | ‐2.93 [‐4.85, ‐1.00] | |
| 6.2 Low number of BCT clusters (≤ median of 9.5) | 4 | Mean Difference (Random, 95% CI) | ‐3.11 [‐7.65, 1.43] | |
| 7 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by median number of BCT clusters) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 7.1 High number of BCT clusters (> median of 9.5) | 7 | 1997 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.89] |
| 7.2 Low number of BCT clusters (≤ median of 9.5) | 7 | 1160 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.43] |
| 8 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by number of BCT clusters) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 8.1 High number of BCT clusters (> 8) | 10 | 2523 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.51, 0.97] |
| 8.2 Low number of BCT clusters (≤ 8) | 4 | 634 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.30, 1.68] |
| 9 HRQoL: adjusted SGRQ total score (subgroup by case manager support) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| 9.1 Case manager support | 6 | Mean Difference (Random, 95% CI) | ‐2.15 [‐4.25, ‐0.04] | |
| 9.2 No case manager support | 4 | Mean Difference (Random, 95% CI) | ‐5.11 [‐8.81, ‐1.41] | |
| 10 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by case manager support) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 10.1 Case manager support | 8 | 2403 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.49, 0.93] |
| 10.2 No case manager support | 6 | 754 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.36, 1.77] |
| 11 HRQoL: adjusted SGRQ total score (subgroup by intervention duration) Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| 11.1 Intervention duration ≥ 6 months | 7 | Mean Difference (Random, 95% CI) | ‐2.96 [‐5.20, ‐0.72] | |
| 11.2 Intervention duration < 6 months | 3 | Mean Difference (Random, 95% CI) | ‐2.57 [‐6.96, 1.82] | |
| 12 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by intervention duration) Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 12.1 Intervention duration ≥ 6 months | 9 | 2453 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.96] |
| 12.2 Intervention duration < 6 months | 5 | 704 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.32] |
| 13 HRQoL: adjusted SGRQ total score (subgroup by action plan component 'adaptation of maintenance medication') Show forest plot | 10 | Mean Difference (Random, 95% CI) | ‐2.69 [‐4.49, ‐0.90] | |
| 13.1 Action defined for adaptation of maintenance medication | 6 | Mean Difference (Random, 95% CI) | ‐3.75 [‐6.16, ‐1.33] | |
| 13.2 No action defined for adaptation of maintenance medication | 4 | Mean Difference (Random, 95% CI) | ‐2.02 [‐4.77, 0.72] | |
| 14 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'adaptation of maintenance medication' Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 14.1 Action defined for adaptation of maintenance medication | 5 | 910 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.88] |
| 14.2 No action defined for adaptation of maintenance medication | 9 | 2247 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.42, 0.83] |
| 15 Healthcare utilisation: respiratory‐related hospital admissions (subgroup by action plan component 'when to avoid situations in which viral infections might be prevalent') Show forest plot | 14 | 3157 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.94] |
| 15.1 Action defined 'when to avoid situations in which viral infections might be prevalent' | 4 | 549 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.25, 3.13] |
| 15.2 No action defined 'when to avoid situations in which viral infections might be prevalent' | 10 | 2608 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.91] |

