Etrolizumab za liječenje (indukciju remisije) aktivnog ulceroznog kolitisa
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, placebo‐controlled, double‐blind within‐cohort study comparing etrolizumab to placebo (N = 48) | |
| Participants | Male and female adults (18‐70 years) with a diagnosis of UC for > 12 weeks and a Mayo Clinic Score (MCS) of > 5 points at screening | |
| Interventions | SAD stage (n = 25): 5 cohorts of patients received etrolizumab or placebo Cohort A: IV etrolizumab 0.3 mg/kg (n = 4) or placebo (n = 1) Cohort B: IV etrolizumab 1.0 mg/kg (n = 4) or placebo (n = 1) Cohort C: IV etrolizumab 3.0 mg/kg (n = 4) or placebo (n = 1) Cohort D: IV etrolizumab 10.0 mg/kg (n = 4) or placebo (n = 1) Cohort E: SC etrolizumab 3.0 mg/kg (n = 4) or placebo (n = 1) MD stage (n = 23): 5 cohorts of patients received etrolizumab or placebo Cohort F: SC etrolizumab 0.5 mg/kg (n = 4) Cohort G: SC etrolizumab 1.5 mg/kg (n = 5) Cohort H: SC etrolizumab 3.0 mg/kg (n = 4) Cohort I: IV etrolizumab 4.0 mg/kg (n = 5) placebo: (n = 5) | |
| Outcomes | Primary outcomes: adverse events, serious adverse events, dose limiting toxicity, maximum tolerated dose pharmacodynamics evaluations (drug occupancy on target CD4+ lymphocytes; occupancy of etrolizumab; absolute number of T lymphocyte subsets) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Conducted by an interactive voice response system based on a process designed by a biostatistician |
| Allocation concealment (selection bias) | Low risk | Conducted by an interactive voice response system based on a process designed by a biostatistician |
| Blinding of participants and personnel (performance bias) | Unclear risk | double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals were similar across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
| Methods | Randomized, double‐blind, placebo‐controlled, phase 2 study comparing SC etrolizumab to matched placebo (N = 124) | |
| Participants | Adult patients (18‐75 years) with a diagnosis of UC for > 12 weeks and MCS > 5 points at screening (> 6 points at US sites) and a centrally read MCS > 2, a rectal bleeding subscore > 1, and disease extension > 25 cm from the anal verge | |
| Interventions | Etrolizumab 100 mg (n = 41): patients received 100 mg at weeks 0, 4 and 8, with placebo administered at week 2 Etrolizumab 300 mg (n = 40): patients received a 420 mg loading dose at week 0, followed by 300 mg at weeks 2, 4 and 8 | |
| Outcomes | Primary outcome: clinical remission at week 10 | |
| Notes | 124 patients were randomly assigned to placebo (n = 43), etrolizumab 100 mg (n = 41) or etrolizumab 300 mg (n = 40) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was conducted with an interactive voice and web response system |
| Allocation concealment (selection bias) | Low risk | Randomization was conducted with an interactive voice and web response system |
| Blinding of participants and personnel (performance bias) | Low risk | All patients, assessing physicians, the funder and its agents and study personnel were masked to treatment assignment, except for site pharmacists who prepared drugs but did not interact with patients |
| Blinding of outcome assessment (detection bias) | Low risk | All patients, assessing physicians, the funder and its agents and study personnel were masked to treatment assignment, except for site pharmacists who prepared drugs but did not interact with patients |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals were similar across groups |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A phase II open‐label extension study to evaluate the long‐term safety of rhuMAb beta7 in patients with moderate to severe ulcerative colitis |
| Methods | Patients will receive a repeating SC injection of etrolizumab; safety and efficacy will be assessed through 104 weeks |
| Participants | ˜ 116 patients Inclusion Criteria: Males and females between 18 to 75 years old with active ulcerative colitis Patients had failed to obtain a clinical response by week 10, or they obtained a clinical response by week 10 but they had a flare‐up between weeks 10 and 28 in a previous phase II study (ABS4986g) Patients in the Unitied States must discontinue concomitant immunosuppressive therapy before enrolment and completely taper off oral corticosteroids 24 weeks before study entry |
| Interventions | Group 1: SC injection of etrolizumab 150 mg/ml |
| Outcomes | Primary outcomes: adverse events, serious adverse events Secondary outcomes: clinically significant changes in vital signs and safety laboratory measures, discontinuation due to adverse events, incidence and nature of injection‐site reactions/hypersensitivity, incidence of infections complications, immunogenicity (incidence of anti‐therapeutic antibodies) |
| Starting date | November 2011 |
| Contact information | Genentech, Inc. |
| Notes | Study is active; enrolment is complete |
| Trial name or title | Phase III, double blind, placebo‐controlled, multicenter study of the efficacy and safety of etrolizumab during induction and maintenance in patients with moderate to severe active ulcerative colitis who are refractory to or intolerant of TNF inhibitors |
| Methods | Double‐blind, randomized, placebo‐controlled study; SC injection of placebo or etrolizumab 105 mg administered every 4 weeks |
| Participants | ˜800 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe active UC (determined by MCS score) who have experienced intolerance, loss of response or failure to respond to treatment with at least one TNF‐inhibitor in the past 5 years |
| Interventions | Group 1: Blinded (Cohort 2): etrolizumab induction (I) + maintenance (M) Group 2: Experimental: Blinded (Cohort 2): etrolizumab I + placebo M Group 3: Placebo Comparator: Blinded (Cohort 2): placebo I + M Group 4: Open‐label (Cohort 1): etrolizumab I + M Group 5: Open‐label (Cohort 1): etrolizumab I + placebo M |
| Outcomes | Primary outcomes: Clinical remission (determined by MCS) at week 14, maintenance of remission at week 66 |
| Starting date | May 2014 |
| Contact information | Reference Study ID Number: GA28950 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active; patients are being recruited |
| Trial name or title | An open label extension and safety monitoring study of moderate to severe ulcerative colitis patients previously enrolled in etrolizumab phase III studies |
| Methods | SC injection of placebo or etrolizumab 105 mg administered every 4 weeks for up to 7 years |
| Participants | ˜2600 patients Inclusion criteria: Part 1 (open‐label extension): patients are males and females over the age of 18 who were previously enrolled in a phase III study on etrolizumab who met the open‐label criteria outlined in the original study Part 2 (safety monitoring): patients are males and females over the age of 18 who previously enrolled in a phase III study on etrolizumab who were not eligible or chose not to participate in Part 1 |
| Interventions | Part 1: open‐label etrolizumab 105 mg Part 2: no intervention |
| Outcomes | Primary outcomes: long‐term efficacy as determined by partial Mayo Clinic Score (pMCS), incidence of adverse events |
| Starting date | September 2014 |
| Contact information | Reference Study ID Number: GA28951 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
| Trial name or title | Phase III, randomized, multicenter double‐blind, double dummy study to evaluate the efficacy and safety of etrolizumab compared with infliximab in patients with moderate to severe active ulcerative colitis who are naive to TNF inhibitors |
| Methods | SC injection of etrolizumab 105 mg administered every 4 weeks plus placebo IV infusions at weeks 0, 2 and 6, and then every 8 weeks, or, IV infusion of infliximab 5 mg/kg at weeks 0, 2 and 6, and then every 8 weeks plus SC placebo every 4 weeks |
| Participants | ˜720 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Patients had an inadequate response/intolerance to prior corticosteroid and/or immunosuppressant treatment |
| Interventions | Group 1 (experimental): etrolizumab + placebo Group 2 (active comparator): infliximab + placebo |
| Outcomes | Primary outcomes: proportion of patients in clinical remission (determined by MCS) Secondary outcomes: proportion of patients with clinical response (determined by MCS) at week 10, proportion of patients with sustained clinical response at weeks 10, 30 and 54 |
| Starting date | December 2014 |
| Contact information | Reference Study ID Number: GA29103 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
| Trial name or title | A phase III, randomized, double‐blind, double‐dummy, placebo‐controlled, multicenter study to evaluate the efficacy (induction of remission) and safety of etrolizumab compared with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients who are naive to TNF inhibitors (Study #1) |
| Methods | SC injection of etrolizumab 105 mg and adalimumab placebo administered at weeks 0, 2, 4, 6 and 8, or, SC injection of etrolizumab placebo and adalimumab 160 mg administered at week 0, 89 mg at week 2, and 40 mg at weeks 4, 6 and 8, or, etrolizumab placebo and adalimumab placebo administered at Weeks 0, 2, 4, 6 and 8 |
| Participants | ˜350 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Previous inadequate response to or intolerance of corticosteroids and/or immunosuppressant drugs |
| Interventions | Group 1: etrolizumab + adalimumab placebo Group 2: etrolizumab placebo + adalimumab Group 3: etrolizumab placebo + adalimumab placebo |
| Outcomes | Primary outcome: induction of remission compared with placebo (determined by MCS) |
| Starting date | November 2014 |
| Contact information | Contact: Reference Study ID Number: GA28948 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
| Trial name or title | Phase III, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy (maintenance of remission) and safety of etrolizumab compared with placebo in patients with moderate to severe active ulcerative colitis who are naive to TNF inhibitors |
| Methods | During the open‐label phase, patients will be given SC etrolizumab 105 mg every 4 weeks During the maintenance phase, patients will be given SC etrolizumab 105 mg or placebo every 4 weeks |
| Participants | ˜350 patients Inclusion criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Previous inadequate response to or intolerance of corticosteroids and/or immunosuppressant drugs |
| Interventions | Phase 1: open‐label SC etrolizumab 105 mg Phase 2: SC etrolizumab 105 mg or placebo |
| Outcomes | Primary outcome: maintenance of clinical remission among randomized patients in clinical remission at week 10 (determined by MCS) Secondary outcomes: maintenance of clinical remission among randomized patients in clinical remission at week 10 (determined by MCS) |
| Starting date | August 2014 |
| Contact information | Contact: Reference Study ID Number: GA28949 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
| Trial name or title | Phase III, randomized, double‐blind, double‐dummy, placebo‐controlled, multicenter study to evaluate the efficacy (induction and remission) and safety of etrolizumab compared with adalimumab and placebo in patients with moderate to severe ulcerative colitis in patients who are naive to TNF inhibitors (Study #2) |
| Methods | Patients were randomized to one of three treatment groups: experimental (etrolizumab and adalimumab placebo), active comparator (etrolizumab placebo and adalimumab) or placebo comparator (etrolizumab placebo and adalimumab placebo) for 8 weeks |
| Participants | ˜350 patients Inclusion Criteria: Males and females between 18 to 80 years of age with moderate to severe UC (determined by MCS) who are naive to anti‐TNF therapy Previous inadequate response to or intolerance of corticosteroids and/or immunosuppressant drugs |
| Interventions | Goup 1 (experimental): SC etrolizumab 105 mg every 4 weeks, plus SC adalimumab placebo at weeks 0, 2, 4, 6 and 8 Group 2 (active comparator): SC adalimumab 160 mg administered SC at Week 0; 80 mg administered SC at Week 2; 40 mg SC at Weeks 4, 6 and 8, plus SC etrolizumab placebo every 4 weeks Group 3: SC adalimumab placebo at weeks 0, 2, 4, 6 and 8, plus SC etrolizumab placebo every 4 weeks |
| Outcomes | Primary outcome: induction of remission compared with placebo (determined by the MCS) |
| Starting date | November 2014 |
| Contact information | Contact: Reference Study ID Number: GA28949 www.roche.com/about_roche/roche_worldwide.htm |
| Notes | Study is active: patients are being recruited |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to enter clinical remission at week 6 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| Analysis 1.1  Comparison 1 Etrolizumab versus placebo, Outcome 1 Failure to enter clinical remission at week 6. | ||||
| 1.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 1.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| 2 Failure to enter clinical remission at week 10 (Phase 1 study) Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| Analysis 1.2  Comparison 1 Etrolizumab versus placebo, Outcome 2 Failure to enter clinical remission at week 10 (Phase 1 study). | ||||
| 3 Failure to enter clinical remission at week 10 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.95] |
| Analysis 1.3  Comparison 1 Etrolizumab versus placebo, Outcome 3 Failure to enter clinical remission at week 10. | ||||
| 3.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.68, 0.96] |
| 3.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 4 Failure to respond at week 6 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| Analysis 1.4  Comparison 1 Etrolizumab versus placebo, Outcome 4 Failure to respond at week 6. | ||||
| 4.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.23] |
| 4.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] |
| 5 Failure to respond at week 10 (Phase 1 study) Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.26, 10.82] |
| Analysis 1.5  Comparison 1 Etrolizumab versus placebo, Outcome 5 Failure to respond at week 10 (Phase 1 study). | ||||
| 6 Failure to respond at week 10 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| Analysis 1.6  Comparison 1 Etrolizumab versus placebo, Outcome 6 Failure to respond at week 10. | ||||
| 6.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 6.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.36] |
| 7 Failure to enter endoscopic remission at week 6 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| Analysis 1.7 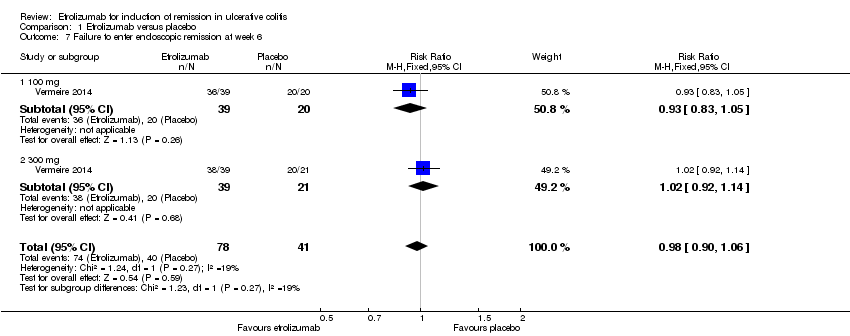 Comparison 1 Etrolizumab versus placebo, Outcome 7 Failure to enter endoscopic remission at week 6. | ||||
| 7.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 7.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.14] |
| 8 Failure to enter endoscopic remission at week 10 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| Analysis 1.8  Comparison 1 Etrolizumab versus placebo, Outcome 8 Failure to enter endoscopic remission at week 10. | ||||
| 8.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 8.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 9 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9 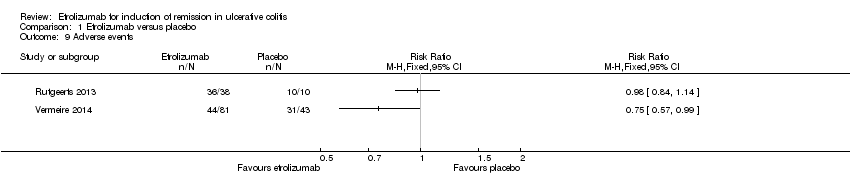 Comparison 1 Etrolizumab versus placebo, Outcome 9 Adverse events. | ||||
| 10 Serious adverse events Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.36, 2.34] |
| Analysis 1.10 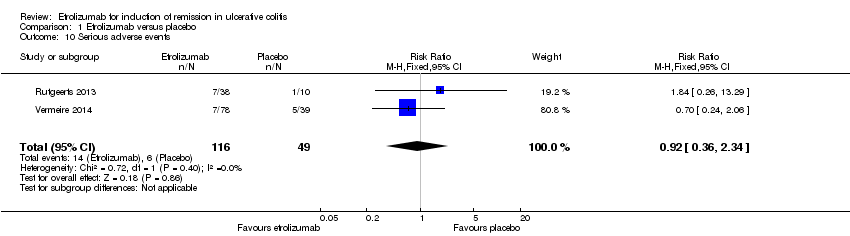 Comparison 1 Etrolizumab versus placebo, Outcome 10 Serious adverse events. | ||||
| 11 Withdrawal due to adverse events Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.26, 4.62] |
| Analysis 1.11  Comparison 1 Etrolizumab versus placebo, Outcome 11 Withdrawal due to adverse events. | ||||

Study flow diagram.
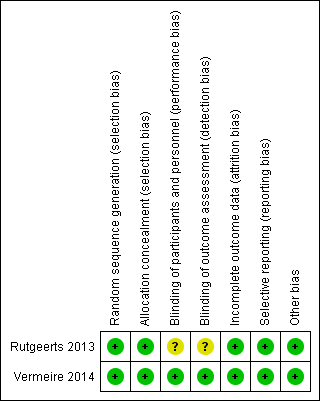
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Etrolizumab versus placebo, Outcome 1 Failure to enter clinical remission at week 6.
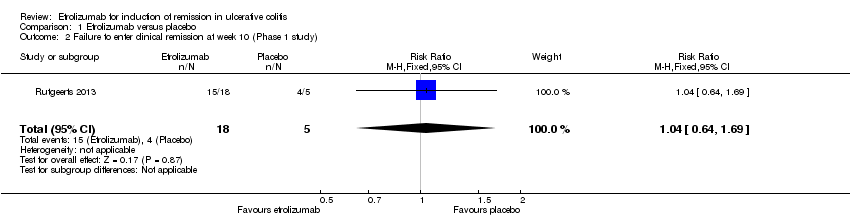
Comparison 1 Etrolizumab versus placebo, Outcome 2 Failure to enter clinical remission at week 10 (Phase 1 study).
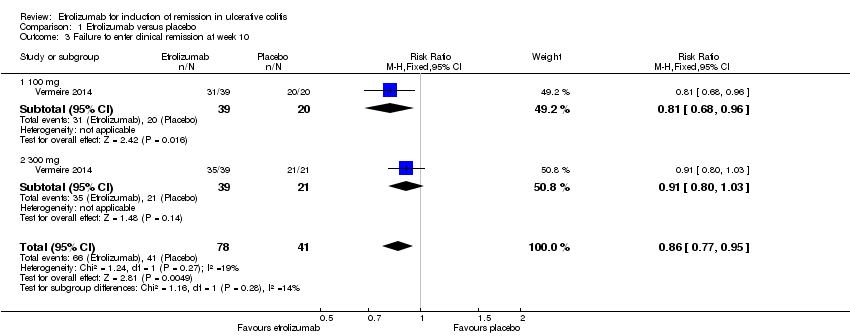
Comparison 1 Etrolizumab versus placebo, Outcome 3 Failure to enter clinical remission at week 10.
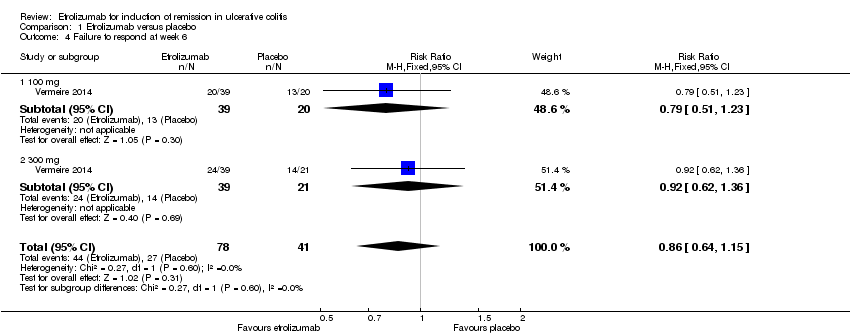
Comparison 1 Etrolizumab versus placebo, Outcome 4 Failure to respond at week 6.

Comparison 1 Etrolizumab versus placebo, Outcome 5 Failure to respond at week 10 (Phase 1 study).

Comparison 1 Etrolizumab versus placebo, Outcome 6 Failure to respond at week 10.
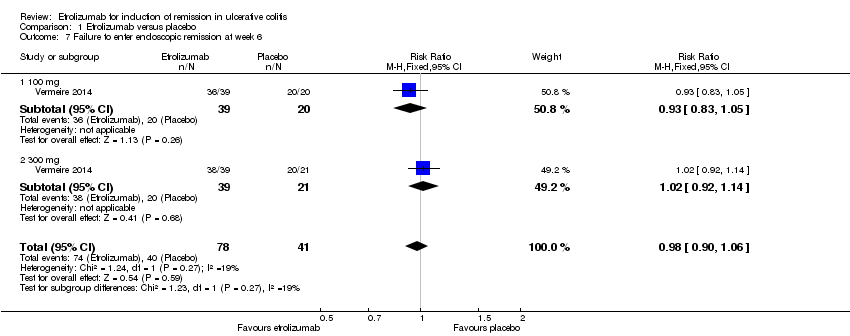
Comparison 1 Etrolizumab versus placebo, Outcome 7 Failure to enter endoscopic remission at week 6.
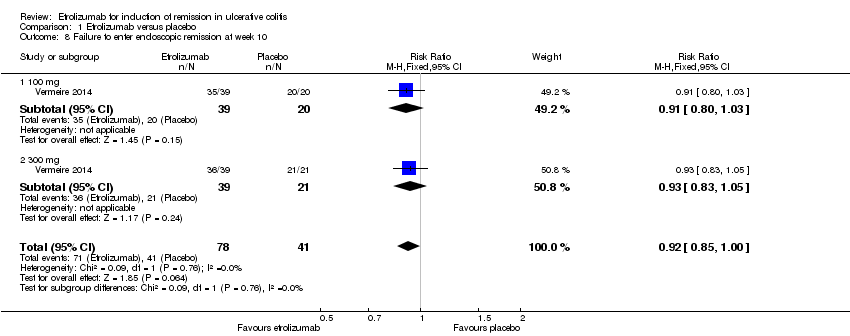
Comparison 1 Etrolizumab versus placebo, Outcome 8 Failure to enter endoscopic remission at week 10.

Comparison 1 Etrolizumab versus placebo, Outcome 9 Adverse events.
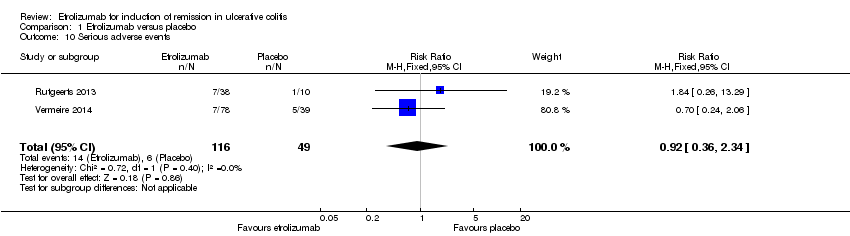
Comparison 1 Etrolizumab versus placebo, Outcome 10 Serious adverse events.

Comparison 1 Etrolizumab versus placebo, Outcome 11 Withdrawal due to adverse events.
| Etrolizumab versus placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Etrolizumab versus placebo | |||||
| Failure to enter clinical remission at week 10 | 1000 per 10001 | 860 per 1000 | RR 0.86 | 119 | ⊕⊕⊕⊝ | |
| Failure to enter clinical remission at week 10 ‐ 100 mg | 1000 per 10001 | 810 per 1000 | RR 0.81 | 59 | ⊕⊕⊕⊝ | |
| Failure to respond at week 10 | 707 per 10001 | 679 per 1000 | RR 0.96 | 119 | ⊕⊕⊕⊝ | |
| Failure to enter endoscopic remission at week 6 | 976 per 10001 | 956 per 1000 | RR 0.98 | 119 | ⊕⊕⊕⊝ | |
| Failure to enter endoscopic remission at week 10 | 1000 per 10001 | 920 per 1000 | RR 0.92 | 119 | ⊕⊕⊕⊝ | |
| Adverse events | 721 per 10001 | 541 per 1000 | RR 0.75 | 124 | ⊕⊕⊕⊝ | |
| Serious adverse events | 122 per 10001 | 113 per 1000 | RR 0.92 | 165 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (107 events). 3 Downgraded one level due to sparse data (51 events). 4 Downgraded one level due to sparse data (82 events). 5 Downgraded one level due to sparse data (114 events). 6 Downgraded one level due to sparse data (112 events). 7Downgraded one level due to sparse data (75 events). 8 Downgraded two levels due to very sparse data (20 events). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to enter clinical remission at week 6 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.06] |
| 1.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 1.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| 2 Failure to enter clinical remission at week 10 (Phase 1 study) Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| 3 Failure to enter clinical remission at week 10 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.95] |
| 3.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.68, 0.96] |
| 3.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 4 Failure to respond at week 6 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 4.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.23] |
| 4.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.36] |
| 5 Failure to respond at week 10 (Phase 1 study) Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.26, 10.82] |
| 6 Failure to respond at week 10 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.23] |
| 6.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 6.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.36] |
| 7 Failure to enter endoscopic remission at week 6 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| 7.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 7.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.14] |
| 8 Failure to enter endoscopic remission at week 10 Show forest plot | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 8.1 100 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 8.2 300 mg | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 9 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Serious adverse events Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.36, 2.34] |
| 11 Withdrawal due to adverse events Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.26, 4.62] |

