مداخلات دارویی در مدیریت درمانی کلانژیت صفراوی اولیه
Appendices
Appendix 1. Methods for network meta‐analysis if we find this is possible in future
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we will calculate the odds ratio with 95% credible interval (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. quality of life reported on the same scale), we will calculate the mean difference with 95% credible interval. We will use standardised mean difference values with 95% credible interval for quality of life if included trials use different scales. For count outcomes (e.g. number of adverse events and serious adverse events), we will calculate the rate ratio with 95% credible interval. For time‐to‐event data (e.g. mortality at maximal follow‐up), we will calculate hazard ratio with 95% credible interval.
Relative ranking
We will estimate the ranking probabilities for all treatments of being at each possible rank for each intervention. Then, we will obtain the surface under the cumulative ranking curve (SUCRA) (cumulative probability) and rankogram (Salanti 2011; Chaimani 2013).
Unit of analysis issues
We will collect data for all trial treatment groups that meet the inclusion criteria. The codes for analysis, that we will use, accounts for the correlation between the effect sizes from trials with more than two groups.
Assessment of heterogeneity
We will assess clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We will assess the presence of clinical heterogeneity by comparing effect estimates under different categories of potential effect modifiers. Different study designs and risk of bias may contribute to methodological heterogeneity.
We will assess the statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study standard deviation (tau2 and comparing this with values reported in the study of the distribution of between‐study heterogeneity (Turner 2012)), and by calculating the I2 statistic (using Stata/SE 14.2). If we identify substantial heterogeneity, clinical, methodological, or statistical, we will explore and address heterogeneity in a subgroup analysis (see 'Subgroup analysis and investigation of heterogeneity for network meta‐analysis' section).
Assessment of transitivity across treatment comparisons
We will evaluate the plausibility of transitivity assumption (the assumption that the participants included in the different studies with different immunosuppressive regimens can be considered to be a part of a multi‐arm randomised clinical trial and could potentially have been randomised to any of the treatments) (Salanti 2012). In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions. If there was any concern that the clinical safety and effectiveness are dependent upon the effect modifiers, we will not perform a network meta‐analysis on all participant subgroups.
Assessment of reporting biases
For the network meta‐analysis, we will judge the reporting bias by the completeness of the search (i.e. searching various databases and including conference abstracts), as we do not currently find any meaningful order to perform a comparison‐adjusted funnel plot as suggested by Chaimani 2012. However, if we find any meaningful order, for example, the control group used depended upon the year of conduct of the trial, we will use comparison‐adjusted funnel plot as suggested by Chaimani 2012.
Data synthesis
Methods for indirect and mixed comparisons
We will conduct network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We will obtain a network plot to ensure that the trials were connected by treatments using Stata/SE 14.2 (Chaimani 2013). We will exclude any trials that were not connected to the network. We will conduct a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3 as per the guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2014a). We will model the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and an arbitrarily selected reference group ('basic parameters') (Lu 2006) using appropriate likelihood functions and links. We will use binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link for time‐to‐event outcomes, and normal likelihood and identity link for continuous outcomes. We will perform a fixed‐effect model and random‐effects model for the network meta‐analysis. We will report both models for comparison with the reference group in a forest plot. For pairwise comparison, we will report the fixed‐effect model if the two models report similar results; otherwise, we will report the more conservative model.
We will use a hierarchical Bayesian model using three different initial values using codes provided by NICE DSU (Dias 2014a). We will use a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors). For the random‐effects model, we will use a prior distributed uniformly (limits: 0 to 5) for between‐trial standard deviation but assume similar between‐trial standard deviation across treatment comparisons (Dias 2014a). We will use a 'burn‐in' of 5000 simulations, check for convergence visually, and run the models for another 10,000 simulations to obtain effect estimates. If we do not obtain convergence, we will increase the number of simulations for 'burn‐in'. If we do not obtain convergence still, we will use alternate initial values and priors using methods suggested by Van Valkenhoef 2012. We will also estimate the probability that each intervention ranks at one of the possible positions using the NICE DSU codes (Dias 2014a).
Assessment of inconsistency
We will assess inconsistency (statistical evidence of the violation of transitivity assumption) by fitting both an inconsistency model and a consistency model. We will use the inconsistency models used in the NICE DSU manual, as we plan to use a common between‐study deviation for the comparisons (Dias 2014b). In addition, we will use the design‐by‐treatment full interaction model (Higgins 2012) and IF (inconsistency factor) plots (Chaimani 2013) to assess inconsistency. In the presence of inconsistency, we will assess whether the inconsistency is because of clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the ‘Subgroup analysis and investigation of heterogeneity for network meta‐analysis’ section.
If there was evidence of inconsistency, we will identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
Direct comparison
We will perform the direct comparisons using the same codes and the same technical details.
Sample size calculations
To control for the risk of random errors, we will interpret the information with caution when the accrued sample size in the network meta‐analysis (i.e. across all treatment comparisons) is less than the required sample size (required information size). For calculation of the required information size, see Appendix 3.
Subgroup analysis and investigation of heterogeneity for network meta‐analysis
We will assess the differences in the effect estimates between the subgroups listed in Subgroup analysis' and 'Investigation of heterogeneity' sections using meta‐regression with the help of the OpenBUGS code (Dias 2012a) if we include a sufficient number of trials. We will use the potential modifiers as study level co‐variates for meta‐regression. We will calculate a single common interaction term (Dias 2012a). If the 95% credible intervals of the interaction term do not overlap zero, we will consider this as evidence of difference in subgroups.
Presentation of results
We will present the effect estimates with 95% credible interval for each pairwise comparisons calculated from the direct comparisons and network meta‐analysis. We will also present the cumulative probability of the treatment ranks (i.e. the probability that the treatment is within the top two, the probability that the treatment is within the top three, etc.) in graphs (SUCRA) (Salanti 2011). We will also plot the probability that each treatment is best, second best, third best, etc. for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b).
We will present the 'Summary of findings' tables for mortality. In the 'Summary of findings', we will follow the approach suggested by Puhan and colleagues (Puhan 2014). First, we will calculate the direct and indirect effect estimates and 95% credible intervals using the node‐splitting approach (Dias 2010), that is calculate the direct estimate for each comparison by including only trials in which there was direct comparison of treatments and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of treatments. Then we will rate the quality of direct and indirect effect estimates using GRADE which takes into account the risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (Guyatt 2011). Then, we will present the estimates of the network meta‐analysis and rated the quality of network meta‐analysis effect estimates as the best quality of evidence between the direct and indirect estimates (Puhan 2014). In addition, in the same table, we will present illustrations and information on the number of trials and participants as per the standard 'Summary of findings' table.
Appendix 2. Search strategies
| Database | Time span | Search strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley) | Issue 2, 2017. | #1 MeSH descriptor: [Liver Cirrhosis, Biliary] explode all trees #2 (primary biliary cholangitis or PBC) #3 #1 or #2 |
| MEDLINE (OvidSP) | January 1947 to February 2017. | 1. exp Liver Cirrhosis, Biliary/ 2. (primary biliary cholangitis or PBC).ti,ab. 3. 1 or 2 4. randomized controlled trial.pt. 5. controlled clinical trial.pt. 6. randomized.ab. 7. placebo.ab. 8. drug therapy.fs. 9. randomly.ab. 10. trial.ab. 11. groups.ab. 12. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. exp animals/ not humans.sh. 14. 12 not 13 15. 3 and 14 |
| Embase (OvidSP) | January 1974 to February 2017. | 1. exp primary biliary cholangitis/ 2. (primary biliary cholangitis or PBC).ti,ab. 3. 1 or 2 4. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 5. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 6. 4 or 5 7. 3 and 6 |
| Science Citation Index Expanded (Web of Knowledge) | January 1945 to February 2017. | #1 TS=(primary biliary cholangitis or PBC) #2 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #3 #1 AND #2 |
| World Health Organization International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch/Default.aspx) | February 2017. | Condition: "primary biliary cholangitis" or PBC |
| February 2017. | Interventional Studies | "primary biliary cholangitis" OR PBC | Phase 2, 3, 4 |
Appendix 3. Sample size calculation
The five‐year mortality in people with primary biliary cholangitis is 20% (Kim 2000). The required information size based on a control group proportion of 20%, a relative risk reduction of 20% in the intervention group, type I error of 5%, and type II error of 20% is 2894 participants. Network analyses are more prone to the risk of random errors than direct comparisons (Del Re 2013). Accordingly, a greater sample size is required in indirect comparisons than direct comparisons (Thorlund 2012). The power and precision in indirect comparisons depends upon various factors, such as the number of participants included under each comparison and the heterogeneity between the trials (Thorlund 2012). If there is no heterogeneity across the trials, the sample size in indirect comparisons would be equivalent to the sample size in direct comparisons. The effective indirect sample size can be calculated using the number of participants included in each direct comparison (Thorlund 2012). For example, a sample size of 2500 participants in the direct comparison A versus C (nAC) and a sample size of 7500 participants in the direct comparison B versus C (nBC) results in an effective indirect sample size of 1876 participants. However, in the presence of heterogeneity within the comparisons, the sample size required is higher. In the above scenario, for an I2 statistic for each of the comparisons A versus C (IAC2) and B versus C (IBC2) of 25%, the effective indirect sample size is 1407 participants. For an I2 statistic for each of the comparisons A versus C and B versus C of 50%, the effective indirect sample size is 938 participants (Thorlund 2012). If there were only three groups and the sample size in the trials is more than the required information size, we planned to calculate the effective indirect sample size using the following generic formula (Thorlund 2012):
((nAC × (1 ‐ IAC2)) × (nBC × (1 ‐ IBC2))/((nAC × (1 ‐ IAC2)) + (nBC × (1 ‐ IBC2)).
There is currently no method to calculate the effective indirect sample size for a network analysis involving more than three intervention groups.
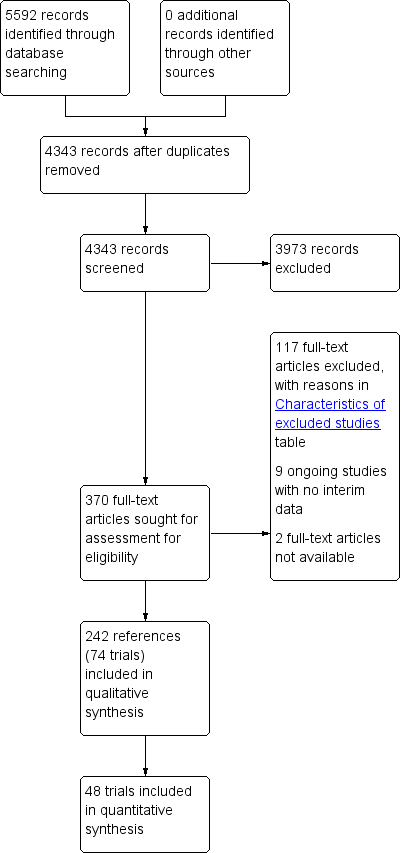
Study flow diagram.
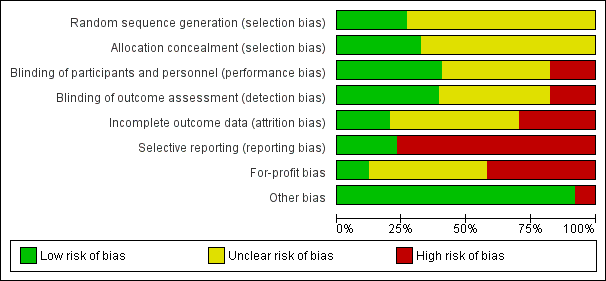
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
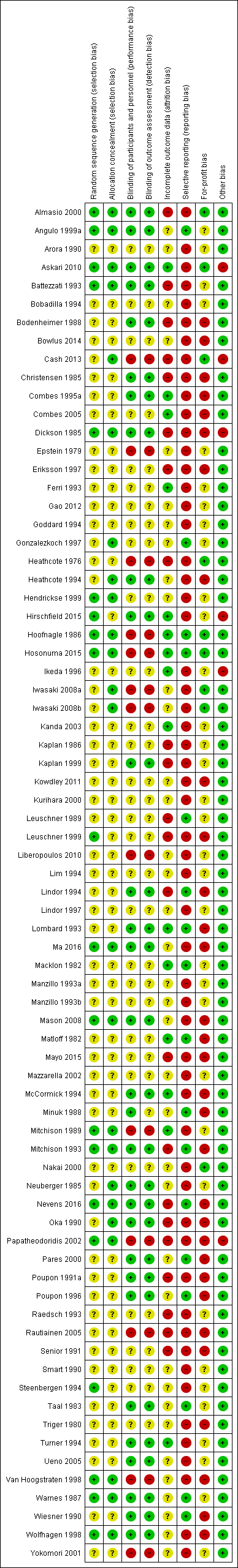
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
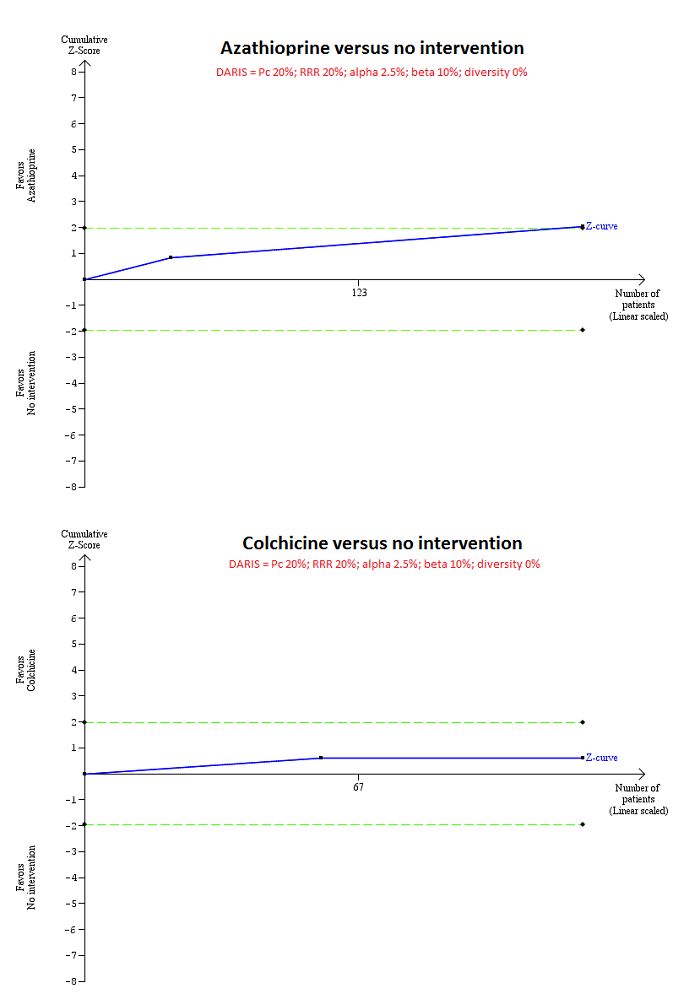
Trial Sequential Analysis of mortality at maximal follow‐up: azathioprine versus no intervention and colchicine versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 20%), and diversity observed in the analyses (0%), the accrued sample size (224 for azathioprine versus intervention and 122 for colchicine versus no intervention) was only a small fraction of the diversity adjusted required information size (DARIS) (4580 for both comparisons); therefore, the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) crossed the conventional boundaries (dotted green line) favouring azathioprine for azathioprine versus no intervention, but did not cross the conventional boundaries for colchicine versus no intervention. This indicates that there is a high risk of random errors in both these comparisons.
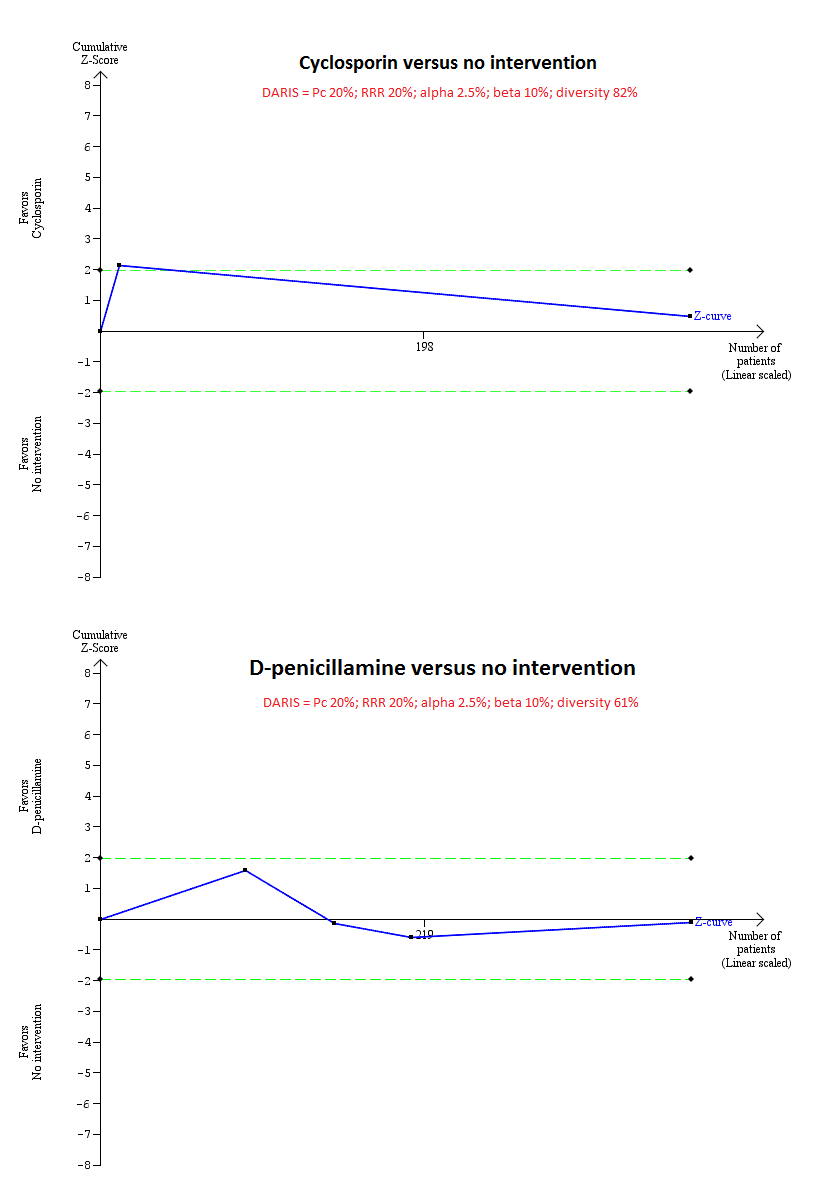
Trial Sequential Analysis of mortality at maximal follow‐up: ciclosporin versus no intervention and D‐penicillamine versus no intervention.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 20%), and diversity observed in the analyses (82% for ciclosporin versus no intervention and 61% for D‐penicillamine versus no intervention), the accrued sample size (394 for ciclosporin versus no intervention and 423 for D‐penicillamine versus no intervention) was only a small fraction of the diversity adjusted required information size (DARIS) (25,098 for ciclosporin versus no intervention and 11,623 for D‐penicillamine versus no intervention); therefore, the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) crossed the conventional boundaries (dotted green line) favouring ciclosporin for ciclosporin versus no intervention, but did not cross the conventional boundaries for D‐penicillamine versus no intervention. This indicates that there is a high risk of random errors in both these comparisons.
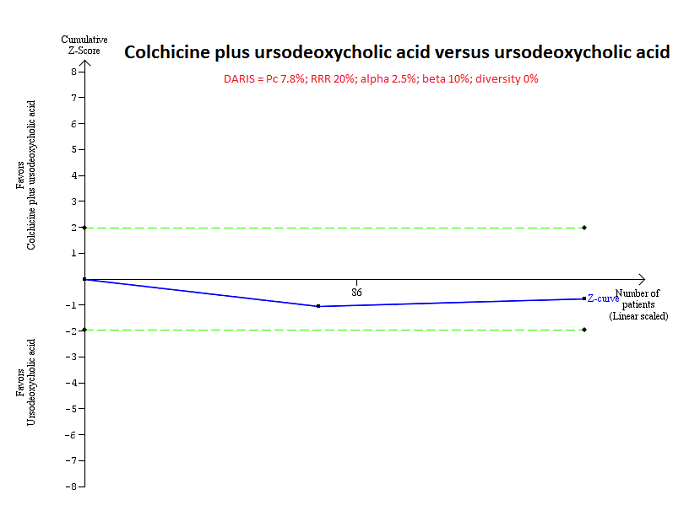
Trial Sequential Analysis of mortality at maximal follow‐up: colchicine plus UDCA versus UDCA.
Based on an alpha error of 2.5%, power of 90% (beta error of 10%), a relative risk reduction (RRR) of 20%, a control group proportion observed in the trials (Pc = 7.8%), and diversity observed in the analyses (0%), the accrued sample size (160 participants) was only a small fraction of the diversity adjusted required information size (DARIS) (13,316); therefore, the trial sequential monitoring boundaries were not drawn. The Z‐curve (blue line) did not cross the conventional boundaries (green dotted line). This indicates that there is a high risk of random errors in both this comparison.

Comparison 1 Main analysis, Outcome 1 Mortality at maximal follow‐up.
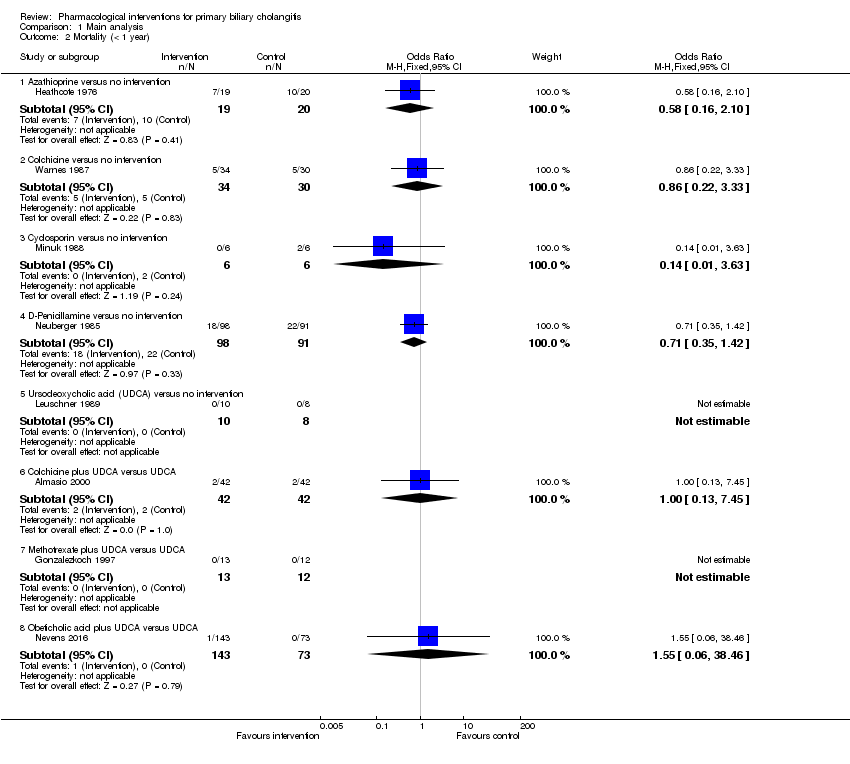
Comparison 1 Main analysis, Outcome 2 Mortality (< 1 year).

Comparison 1 Main analysis, Outcome 3 Mortality (1 to 5 years).
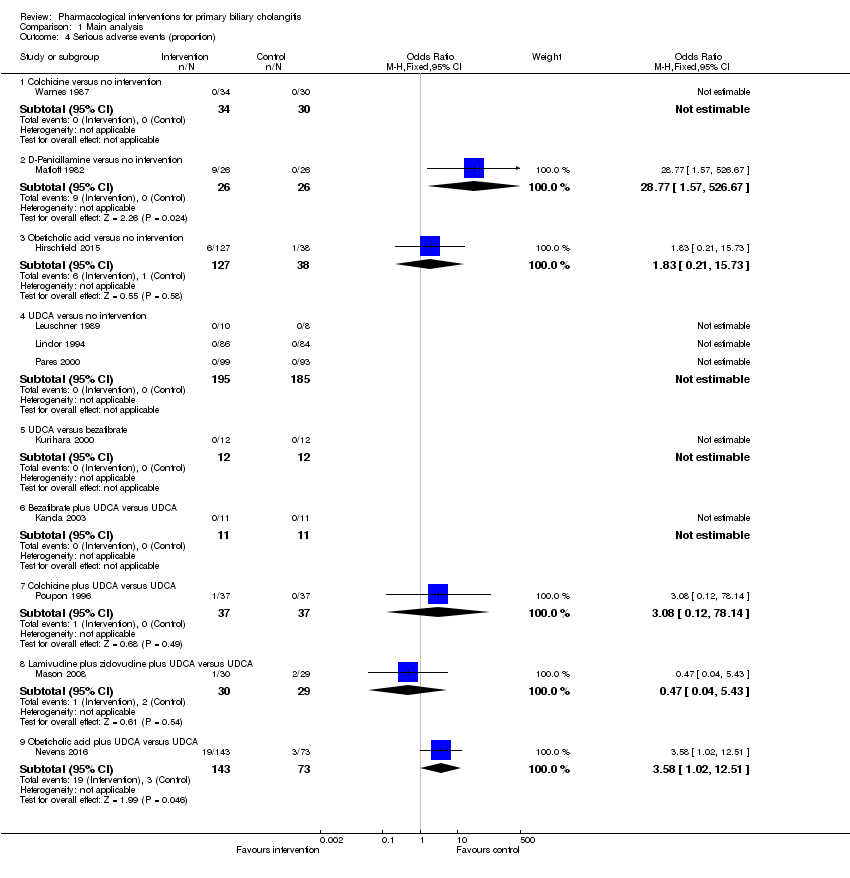
Comparison 1 Main analysis, Outcome 4 Serious adverse events (proportion).

Comparison 1 Main analysis, Outcome 5 Serious adverse events (number of events).

Comparison 1 Main analysis, Outcome 6 Adverse events (proportion).

Comparison 1 Main analysis, Outcome 7 Adverse events (number).
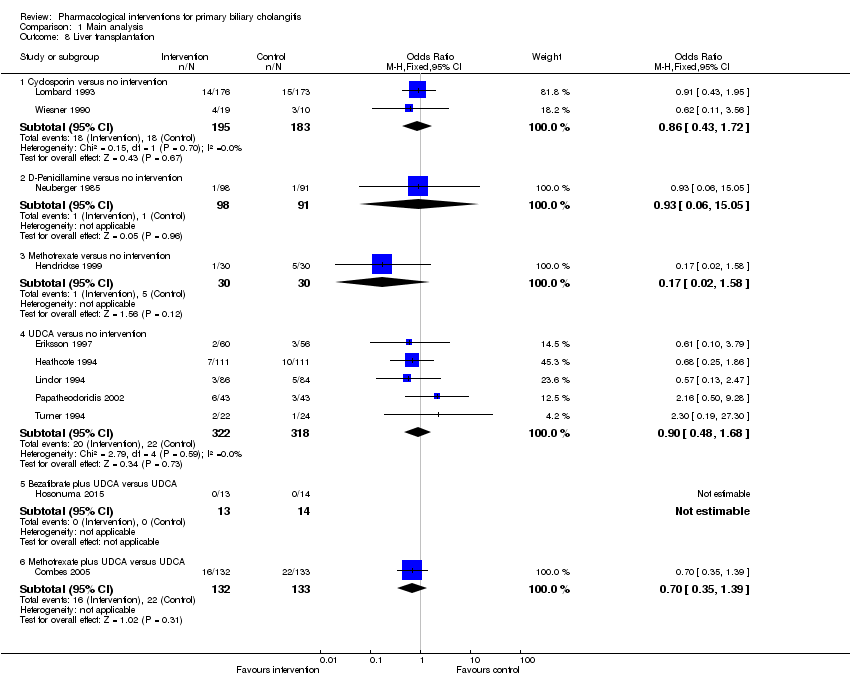
Comparison 1 Main analysis, Outcome 8 Liver transplantation.

Comparison 1 Main analysis, Outcome 9 Decompensated liver disease.
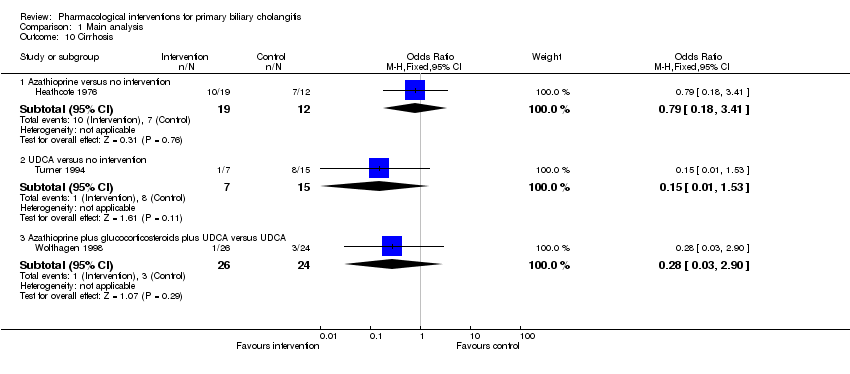
Comparison 1 Main analysis, Outcome 10 Cirrhosis.
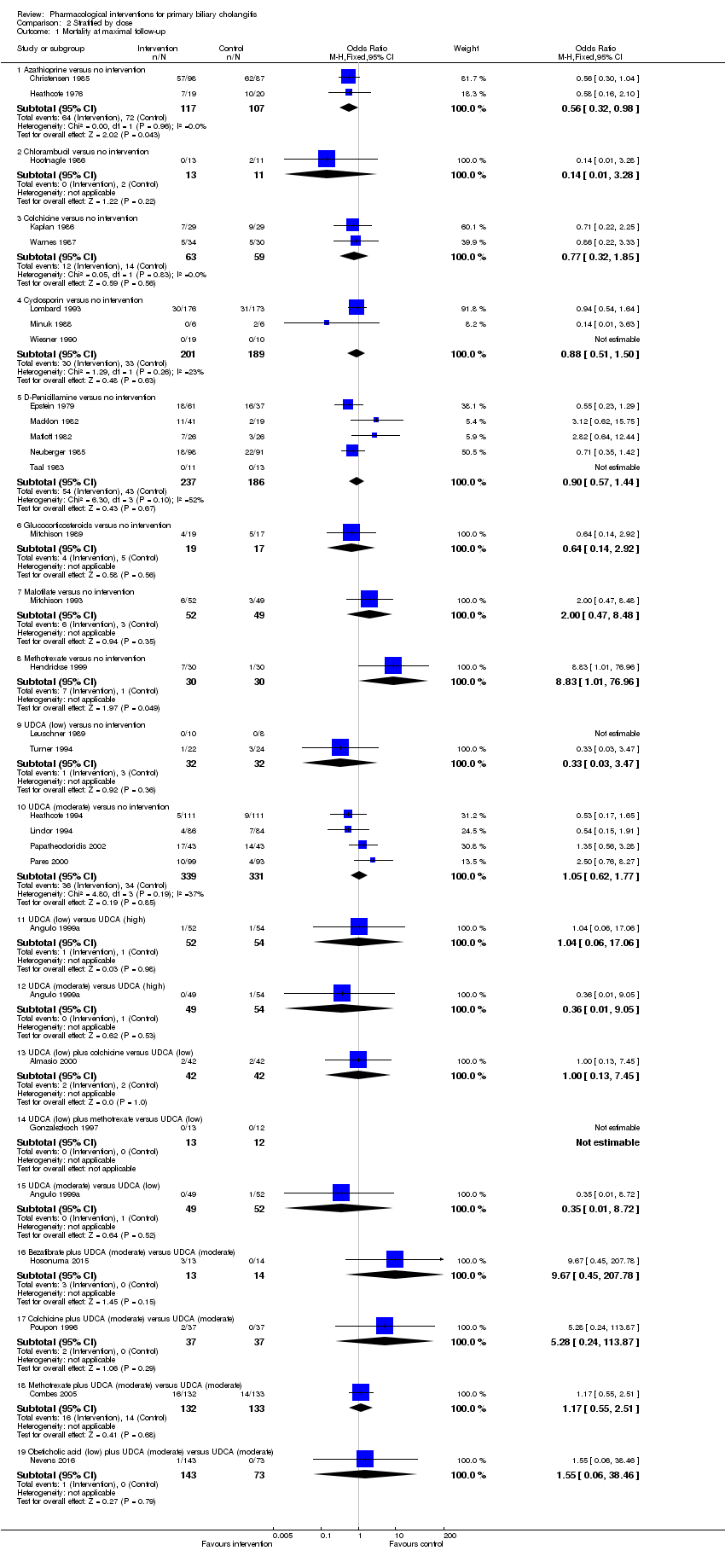
Comparison 2 Stratified by dose, Outcome 1 Mortality at maximal follow‐up.

Comparison 2 Stratified by dose, Outcome 2 Mortality (< 1 year).

Comparison 2 Stratified by dose, Outcome 3 Mortality (1 to 5 years).

Comparison 2 Stratified by dose, Outcome 4 Serious adverse events (proportion).
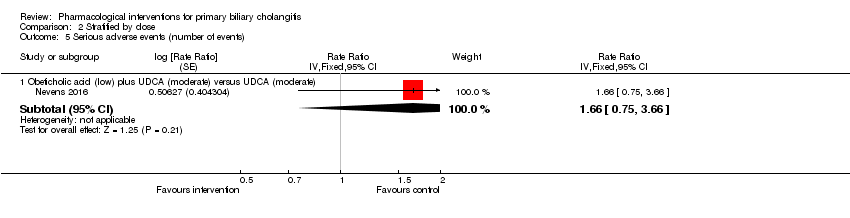
Comparison 2 Stratified by dose, Outcome 5 Serious adverse events (number of events).
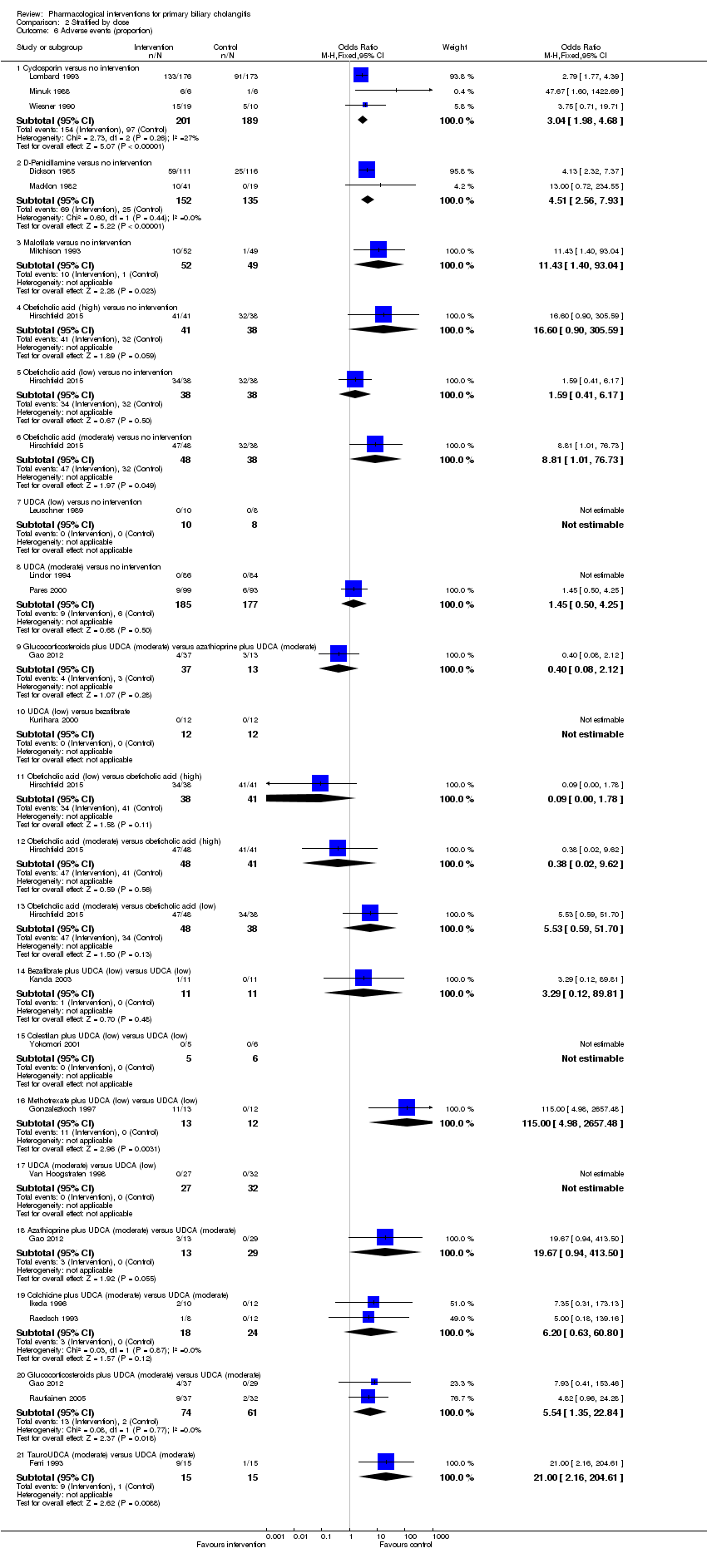
Comparison 2 Stratified by dose, Outcome 6 Adverse events (proportion).

Comparison 2 Stratified by dose, Outcome 7 Adverse events (number).

Comparison 2 Stratified by dose, Outcome 8 Liver transplantation.

Comparison 2 Stratified by dose, Outcome 9 Decompensated liver disease.

Comparison 2 Stratified by dose, Outcome 10 Cirrhosis.
| UDCA versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: UDCA Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | UDCA | ||||
| Mortality at maximal follow‐up Follow‐up: 12 to 89 months | 208 per 1000 | 206 per 1000 | OR 0.99 | 734 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) Follow‐up: 12 to 41 months | There were no events in either group | 380 | ⊕⊝⊝⊝ | ||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Azathioprine versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: azathioprine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Azathioprine | ||||
| Mortality at maximal follow‐up Follow‐up: 63 months in 1 trial and not stated in 1 trial | 208 per 1000 | 128 per 1000 | OR 0.56 | 224 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). | |||||
| Colchicine versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: colchicine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Colchicine | ||||
| Mortality at maximal follow‐up Follow‐up: 12 to 24 months | 208 per 1000 | 168 per 1000 | OR 0.77 | 122 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) Follow‐up: 12 months | There were no events in either group | 64 | ⊕⊝⊝⊝ | ||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Ciclosporin versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: ciclosporin Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Ciclosporin | ||||
| Mortality at maximal follow‐up Follow‐up: 31 to 35 months | 208 per 1000 | 188 per 1000 | OR 0.88 | 390 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). | |||||
| D‐Penicillamine versus no intervention for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: D‐penicillamine Comparison: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | D‐Penicillamine | ||||
| Mortality at maximal follow‐up (Follow‐up 24 to 66 months) | 208 per 1000 | 191 per 1000 | OR 0.90 | 423 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) (Follow‐up 24 months) | 4 per 1000 | 104 per 1000 | OR 28.77 | 52 | ⊕⊝⊝⊝ |
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Colchicine plus UDCA versus UDCA for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: colchicine + UDCA Comparison: UDCA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| UDCA | Colchicine + UDCA | ||||
| Mortality at maximal follow‐up Follow‐up: 24 months in 1 trial; not reported in 1 trial | 110 per 1000 | 185 per 1000 | OR 1.84 | 158 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) Follow‐up: not stated | 14 per 1000 | 42 per 1000 | OR 3.08 | 74 | ⊕⊝⊝⊝ |
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Methotrexate plus UDCA versus UDCA for primary biliary cholangitis | |||||
| Patient or population: people with primary biliary cholangitis Settings: secondary or tertiary care Intervention: methotrexate + UDCA Comparison: UDCA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| UDCA | Methotrexate + UDCA | ||||
| Mortality at maximal follow‐up Follow‐up: 11 to 91 months | 110 per 1000 | 126 per 1000 | OR 1.17 | 290 | ⊕⊝⊝⊝ |
| Serious adverse events (proportion) | None of the trials reported this outcome. | ||||
| Serious adverse events (number of events) | None of the trials reported this outcome. | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the mean control group proportion across all the trials. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Risk of bias in the trial(s) was high (downgraded by two levels). 3 There was moderate heterogeneity (downgraded by one level). | |||||
| Study name | No participants randomised | Post‐randomisation dropouts | No participants for whom outcome was reported | Intervention(s) | Control | Mean follow‐up period (months) |
| 20 | Not stated | 20 | Antioxidants | No intervention | Not stated | |
| 248 | 63 | 185 | Azathioprine | No intervention | 63 | |
| 45 | 6 | 39 | Azathioprine | No intervention | Not stated | |
| 24 | 0 | 24 | Chlorambucil | No intervention | 52 | |
| 57 | 10 | 47 | Colchicine | No intervention | 33 | |
| 60 | 3 | 57 | Colchicine | No intervention | 24 | |
| 64 | Not stated | 64* | Colchicine | No intervention | 19 (median) | |
| 40 | Not stated | 40 | Colchicine + UDCA | No intervention | 12 | |
| 349 | 0 | 349 | Ciclosporin | No intervention | 31 (median) | |
| 12 | 0 | 12 | Ciclosporin | No intervention | Not stated | |
| 40 | 11 | 29 | Ciclosporin | No intervention | 35 (median) | |
| 309 | 82 | 227 | D‐Penicillamine | No intervention | 60 (median) | |
| 98 | Not stated | 98 | D‐Penicillamine | No intervention | 66 | |
| 60 | 0 | 60 | D‐Penicillamine | No intervention | 37 | |
| 52 | 0 | 52 | D‐Penicillamine | No intervention | 24 | |
| 189 | Not stated | 189 | D‐Penicillamine | No intervention | Not stated | |
| 24 | Not stated | 24 | D‐Penicillamine | No intervention | 18 | |
| 35 | Not stated | 35 | D‐Penicillamine | No intervention | Not stated | |
| 36 | 0 | 36 | Glucocorticosteroids | No intervention | 36 | |
| 20 | Not stated | 20 | Lamivudine | No intervention | Not stated | |
| 104 | 3 | 101 | Malotilate | No intervention | 25 (median) | |
| 60 | Not stated | 60 | Methotrexate | No intervention | 68 | |
| 14 | Not stated | 14 | Methotrexate + UDCA | No intervention | 24 | |
| 45 | 3 | 42 | NGM282 | No intervention | Not stated | |
| 216 | Not stated | 216 | Obeticholic acid | No intervention | 12 | |
| 165 | 0 | 165 | Obeticholic acid | No intervention | 3 | |
| 59 | Not stated | 59 | Obeticholic acid | No intervention | Not stated | |
| 32 | Not stated | 32 | S‐Adenosyl methionine | No intervention | 1 | |
| 6 | Not stated | 6 | S‐Adenosyl methionine | No intervention | 2 | |
| 21 | 8 | 13 | Simvastatin | No intervention | 12 | |
| 28 | 0 | 28 | Tetrathiomolybdate | No intervention | Not stated | |
| 18 | 0 | 18 | Thalidomide | No intervention | Not stated | |
| 9 | Not stated | 9 | UDCA | No intervention | 5 | |
| 88 | 2 | 86 | UDCA | No intervention | 6 | |
| 151 | 0 | 151 | UDCA | No intervention | 24 | |
| 116 | 15 | 101 | UDCA | No intervention | 24 | |
| 222 | Not stated | 222 | UDCA | No intervention | 24 | |
| 20 | 0 | 18 | UDCA | No intervention | 12 | |
| 32 | Not stated | 32 | UDCA | No intervention | Not stated | |
| 180 | 10 | 170 | UDCA | No intervention | 24 | |
| 52 | 7 | 45 | UDCA | No intervention | Not stated | |
| 92 | 6 | 86 | UDCA | No intervention | 89 | |
| 192 | 0 | 192 | UDCA | No intervention | 41 (median) | |
| 149 | 3 | 146 | UDCA | No intervention | Not stated | |
| 20 | 1 | 19 | UDCA | No intervention | 18 | |
| 46 | 0 | 46 | UDCA | No intervention | 24 | |
| 57 | Not stated | 57 | Intervention 1: UDCA | No intervention | 15 | |
| 50 | Not stated | 50 | Azathioprine + glucocorticosteroids + UDCA | UDCA | 12 | |
| 45 | Not stated | 45 | Bezafibrate | UDCA | 12 | |
| 24 | Not stated | 24 | Bezafibrate | UDCA | Not stated | |
| 27 | 0 | 27 | Bezafibrate + UDCA | UDCA | 96 | |
| 22 | Not stated | 22 | Bezafibrate + UDCA | UDCA | 12 | |
| 22 | 0 | 22 | Bezafibrate + UDCA | UDCA | 7 | |
| 23 | Not stated | 23 | Bezafibrate + UDCA | UDCA | 12 | |
| 90 | 6 | 84 | Colchicine + UDCA | UDCA | Not stated | |
| 22 | 0 | 22 | Colchicine + UDCA | UDCA | 24 | |
| 74 | Not stated | 74 | Colchicine + UDCA | UDCA | 24 | |
| 28 | 8 | 20 | Colchicine + UDCA | UDCA | 24 | |
| 11 | Not stated | 11 | Colestilan + UDCA | UDCA | Not stated | |
| 10 | Not stated | 10 | Fenofibrate + UDCA | UDCA | Not stated | |
| 40 | 0 | 39 | Glucocorticosteroids + UDCA | UDCA | 24 | |
| 77 | 8 | 69 | Glucocorticosteroids + UDCA | UDCA | 36 | |
| 79 | Not stated | 79 | Intervention 1: glucocorticosteroids + UDCA | UDCA | Not stated | |
| 59 | 0 | 59 | Lamivudine + zidovudine + UDCA | UDCA | 6 | |
| 265 | 0 | 265 | Methotrexate + UDCA | UDCA | 91 (median) | |
| 25 | Not stated | 25 | Methotrexate + UDCA | UDCA | 11 | |
| 217 | Not stated | 216 | Obeticholic acid + UDCA | UDCA | 12 | |
| 30 | 0 | 30 | TUDCA | UDCA | 6 | |
| 199 | 8 | 191 | TUDCA | UDCA | 6 | |
| 87 | 2 | 85 | Colchicine | Methotrexate | 24 | |
| Comparison of doses | ||||||
| 150 | Not stated | 150 | Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | 12 | |
| 155 | Not stated | 155 | Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | 12 | |
| 61 | 2 | 59 | UDCA (moderate) | UDCA (low) | Not stated | |
| 42 | Not stated | 42 | UDCA (high) | UDCA (moderate) | 72 | |
| TUDCA: taurodeoxycholic acid; UDCA: ursodeoxycholic acid. | ||||||
| Name of studies | Intervention(s) | Control | Random sequence generation | Allocation concealment | Blinding of participants and health professionals | Blinding of outcome assessors | Missing outcome bias | Selective outcome reporting | For‐profit bias | Other bias |
| Antioxidants | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Azathioprine | No intervention | Unclear | Unclear | Low | Low | High | High | High | Low | |
| Azathioprine | No intervention | Unclear | Unclear | High | High | High | High | Low | Low | |
| Chlorambucil | No intervention | Low | Low | High | High | Low | Low | Low | Low | |
| Colchicine | No intervention | Unclear | Unclear | Low | Low | High | High | High | Low | |
| Colchicine | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | |
| Colchicine | No intervention | Low | Low | Low | Low | Unclear | Low | Unclear | Low | |
| Colchicine + UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Ciclosporin | No intervention | Unclear | Unclear | Low | Low | Low | Low | High | Low | |
| Ciclosporin | No intervention | Unclear | Unclear | Low | Unclear | Unclear | Low | High | Low | |
| Ciclosporin | No intervention | Unclear | Unclear | Low | Low | Unclear | Low | High | Low | |
| D‐Penicillamine | No intervention | Low | Low | Low | Low | High | High | High | High | |
| D‐Penicillamine | No intervention | Unclear | Unclear | High | High | Unclear | High | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Unclear | Unclear | Low | Low | High | Low | |
| D‐Penicillamine | No intervention | Unclear | Low | Low | Low | Unclear | High | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Low | Low | Unclear | Low | Unclear | Low | |
| D‐Penicillamine | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low | |
| Glucocorticosteroids | No intervention | Low | Low | High | High | Low | High | Unclear | Low | |
| Lamivudine | No intervention | Unclear | Unclear | Low | Low | Unclear | High | Unclear | Low | |
| Malotilate | No intervention | Low | Low | Low | Low | High | Low | High | Low | |
| Methotrexate | No intervention | Low | Low | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Methotrexate + UDCA | No intervention | Low | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| NGM282 | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | High | Low | |
| Obeticholic acid | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low | |
| Obeticholic acid | No intervention | Low | Unclear | Low | Low | Low | High | Unclear | High | |
| Obeticholic acid | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low | |
| S‐Adenosyl methionine | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| S‐Adenosyl methionine | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Simvastatin | No intervention | Unclear | Low | High | High | High | High | Low | High | |
| Tetrathiomolybdate | No intervention | Low | Low | Low | Low | Low | High | Low | High | |
| Thalidomide | No intervention | Unclear | Unclear | Low | Low | Low | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| UDCA | No intervention | Low | Low | Low | Low | High | High | Unclear | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | Low | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | High | Low | |
| UDCA | No intervention | Unclear | Low | Low | Low | Unclear | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | High | Low | Unclear | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | High | Low | High | Low | |
| UDCA | No intervention | Unclear | Low | Low | Low | High | High | High | Low | |
| UDCA | No intervention | Low | Low | High | High | High | High | High | High | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | Unclear | Low | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | High | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | High | High | High | Low | |
| UDCA | No intervention | Unclear | Unclear | Low | Low | Low | High | Unclear | Low | |
| Intervention 1: UDCA | No intervention | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Azathioprine + glucocorticosteroids + UDCA | UDCA | Low | Low | Low | Low | Unclear | High | High | Low | |
| Bezafibrate | UDCA | Unclear | Low | High | High | Unclear | High | Low | Low | |
| Bezafibrate | UDCA | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Bezafibrate + UDCA | UDCA | Low | Low | High | High | Low | Low | Low | Low | |
| Bezafibrate + UDCA | UDCA | Unclear | Low | High | High | Unclear | High | Low | Low | |
| Bezafibrate + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | |
| Bezafibrate + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | |
| Colchicine + UDCA | UDCA | Low | Low | Low | Low | High | High | Low | Low | |
| Colchicine + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | High | |
| Colchicine + UDCA | UDCA | Unclear | Unclear | Low | Low | Unclear | Low | High | Low | |
| Colchicine + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | High | High | Unclear | Low | |
| Colestilan + UDCA | UDCA | Unclear | Unclear | High | High | Unclear | High | Unclear | Low | |
| Fenofibrate + UDCA | UDCA | Unclear | Unclear | High | High | Unclear | High | Unclear | Low | |
| Glucocorticosteroids + UDCA | UDCA | Low | Unclear | Unclear | Unclear | High | High | High | Low | |
| Glucocorticosteroids + UDCA | UDCA | Unclear | Unclear | High | High | High | High | High | Low | |
| Glucocorticosteroids + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Lamivudine + zidovudine + UDCA | UDCA | Low | Low | Low | Low | Unclear | High | High | Low | |
| Methotrexate + UDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | High | Low | |
| Methotrexate + UDCA | UDCA | Unclear | Low | Unclear | Unclear | Unclear | Low | Unclear | Low | |
| Obeticholic acid + UDCA | UDCA | Low | Low | Low | Low | High | Low | High | Low | |
| TUDCA | UDCA | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Low | |
| TUDCA | UDCA | Low | Low | Low | Low | Unclear | High | High | Low | |
| Colchicine | Methotrexate | Unclear | Unclear | Low | Low | High | High | Unclear | Low | |
| Comparison of doses | ||||||||||
| Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| Intervention 1: UDCA (high) Intervention 2: UDCA (moderate) | UDCA (low) | Low | Low | Low | Low | Unclear | Low | Unclear | Low | |
| UDCA (moderate) | UDCA (low) | Low | Low | High | High | Unclear | High | High | Low | |
| UDCA (high) | UDCA (moderate) | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | |
| TUDCA: taurodeoxycholic acid; UDCA: ursodeoxycholic acid. | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximal follow‐up Show forest plot | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Azathioprine versus no intervention | 2 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 1.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 1.3 Colchicine versus no intervention | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.32, 1.85] |
| 1.4 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 1.5 D‐Penicillamine versus no intervention | 5 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.57, 1.44] |
| 1.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 1.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 1.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 1.9 UDCA versus no intervention | 6 | 734 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.64] |
| 1.10 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 1.11 Colchicine plus UDCA versus UDCA | 2 | 158 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.38, 8.91] |
| 1.12 Methotrexate plus UDCA versus UDCA | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 1.13 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2 Mortality (< 1 year) Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Azathioprine versus no intervention | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.10] |
| 2.2 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.22, 3.33] |
| 2.3 Cyclosporin versus no intervention | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.63] |
| 2.4 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 2.5 Ursodeoxycholic acid (UDCA) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Colchicine plus UDCA versus UDCA | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 2.7 Methotrexate plus UDCA versus UDCA | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 3 Mortality (1 to 5 years) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Azathioprine versus no intervention | 1 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 3.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 3.3 Colchicine versus no intervention | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.25] |
| 3.4 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.64] |
| 3.5 D‐Penicillamine versus no intervention | 4 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 3.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 3.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 3.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 3.9 UDCA versus no intervention | 5 | 716 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.64] |
| 3.10 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 3.11 Colchicine plus UDCA versus UDCA | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 3.12 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 4 Serious adverse events (proportion) Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 D‐Penicillamine versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.77 [1.57, 526.67] |
| 4.3 Obeticholic acid versus no intervention | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.21, 15.73] |
| 4.4 UDCA versus no intervention | 3 | 380 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 UDCA versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Bezafibrate plus UDCA versus UDCA | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Colchicine plus UDCA versus UDCA | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 78.14] |
| 4.8 Lamivudine plus zidovudine plus UDCA versus UDCA | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.43] |
| 4.9 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.02, 12.51] |
| 5 Serious adverse events (number of events) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Rate Ratio (Fixed, 95% CI) | 1.66 [0.75, 3.66] |
| 6 Adverse events (proportion) Show forest plot | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.98, 4.68] |
| 6.2 D‐Penicillamine versus no intervention | 2 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [2.56, 7.93] |
| 6.3 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.43 [1.40, 93.04] |
| 6.4 Obeticholic acid versus no intervention | 1 | 165 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.58 [1.31, 15.95] |
| 6.5 UDCA versus no intervention | 3 | 380 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.50, 4.25] |
| 6.6 Azathioprine plus UDCA versus UDCA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.67 [0.94, 413.50] |
| 6.7 Bezafibrate versus UDCA | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Bezafibrate plus UDCA versus UDCA | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.12, 89.81] |
| 6.9 Colchicine plus UDCA versus UDCA | 2 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.20 [0.63, 60.80] |
| 6.10 Colestilan plus UDCA versus UDCA | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Glucocorticosteroids plus UDCA versus UDCA | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.54 [1.35, 22.84] |
| 6.12 Methotrexate plus UDCA versus UDCA | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 115.0 [4.98, 2657.48] |
| 6.13 TauroUDCA versus UDCA | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [2.16, 204.61] |
| 6.14 Glucocorticosteroids plus UDCA versus azathioprine plus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.12] |
| 7 Adverse events (number) Show forest plot | 14 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 7.1 Chlorambucil versus no intervention | 1 | 24 | Rate Ratio (Random, 95% CI) | 3.67 [1.04, 12.87] |
| 7.2 Cyclosporin versus no intervention | 3 | 390 | Rate Ratio (Random, 95% CI) | 2.58 [1.26, 5.31] |
| 7.3 D‐Penicillamine versus no intervention | 3 | 303 | Rate Ratio (Random, 95% CI) | 2.99 [1.04, 8.63] |
| 7.4 Malotilate versus no intervention | 1 | 101 | Rate Ratio (Random, 95% CI) | 6.13 [1.38, 27.14] |
| 7.5 Obeticholic acid versus no intervention | 1 | 76 | Rate Ratio (Random, 95% CI) | 1.41 [1.13, 1.75] |
| 7.6 Azathioprine plus glucocorticosteroids plus UDCA versus UDCA | 1 | 50 | Rate Ratio (Random, 95% CI) | 1.32 [0.88, 1.97] |
| 7.7 Bezafibrate plus UDCA versus UDCA | 1 | 29 | Rate Ratio (Random, 95% CI) | 11.79 [0.65, 213.14] |
| 7.8 Colchicine plus UDCA versus UDCA | 1 | 24 | Rate Ratio (Random, 95% CI) | 5.91 [0.28, 123.08] |
| 7.9 Methotrexate plus UDCA versus UDCA | 1 | 27 | Rate Ratio (Random, 95% CI) | 30.64 [1.84, 510.76] |
| 7.10 TauroUDCA versus UDCA | 1 | 191 | Rate Ratio (Random, 95% CI) | 1.17 [0.81, 1.71] |
| 8 Liver transplantation Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.72] |
| 8.2 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 15.05] |
| 8.3 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.58] |
| 8.4 UDCA versus no intervention | 5 | 640 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.48, 1.68] |
| 8.5 Bezafibrate plus UDCA versus UDCA | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.39] |
| 9 Decompensated liver disease Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 D‐Penicillamine versus no active treatment | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 UDCA versus no intervention | 2 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.86, 2.98] |
| 9.3 Azathioprine plus UDCA versus UDCA | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.18] |
| 9.4 Colchicine plus UDCA versus UDCA | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.07] |
| 9.5 Glucocorticosteroids plus UDCA versus UDCA | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.69] |
| 9.6 Methotrexate plus UDCA versus UDCA | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.77, 2.33] |
| 9.7 Obeticholic acid plus UDCA versus UDCA | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 9.8 Glucocorticosteroids plus UDCA versus azathioprine plus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.10, 11.18] |
| 10 Cirrhosis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Azathioprine versus no intervention | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.41] |
| 10.2 UDCA versus no intervention | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 1.53] |
| 10.3 Azathioprine plus glucocorticosteroids plus UDCA versus UDCA | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at maximal follow‐up Show forest plot | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Azathioprine versus no intervention | 2 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.32, 0.98] |
| 1.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 1.3 Colchicine versus no intervention | 2 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.32, 1.85] |
| 1.4 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 1.5 D‐Penicillamine versus no intervention | 5 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.57, 1.44] |
| 1.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 1.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 1.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 1.9 UDCA (low) versus no intervention | 2 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.47] |
| 1.10 UDCA (moderate) versus no intervention | 4 | 670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.77] |
| 1.11 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.06] |
| 1.12 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.05] |
| 1.13 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 1.14 UDCA (low) plus methotrexate versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.15 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.72] |
| 1.16 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 1.17 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 1.18 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 1.19 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2 Mortality (< 1 year) Show forest plot | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Azathioprine versus no intervention | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.16, 2.10] |
| 2.2 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.22, 3.33] |
| 2.3 Cyclosporin versus no intervention | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.63] |
| 2.4 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.42] |
| 2.5 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.06] |
| 2.7 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.05] |
| 2.8 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 2.9 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.45] |
| 2.10 UDCA (low) plus methotrexate versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.72] |
| 3 Mortality (1 to 5 years) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Azathioprine versus no intervention | 1 | 185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 3.2 Chlorambucil versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 3.28] |
| 3.3 Colchicine versus no intervention | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.25] |
| 3.4 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.64] |
| 3.5 D‐Penicillamine versus no intervention | 4 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 3.6 Glucocorticosteroids versus no intervention | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.14, 2.92] |
| 3.7 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.47, 8.48] |
| 3.8 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.83 [1.01, 76.96] |
| 3.9 UDCA (low) versus no intervention | 1 | 46 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.47] |
| 3.10 UDCA (moderate) versus no intervention | 4 | 670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.77] |
| 3.11 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.67 [0.45, 207.78] |
| 3.12 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.24, 113.87] |
| 3.13 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.51] |
| 4 Serious adverse events (proportion) Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Colchicine versus no intervention | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 D‐Penicillamine versus no intervention | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.77 [1.57, 526.67] |
| 4.3 Obeticholic acid (high) versus no intervention | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.57, 46.17] |
| 4.4 Obeticholic acid (low) versus no intervention | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.22] |
| 4.5 Obeticholic acid (moderate) versus no intervention | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.05, 13.01] |
| 4.6 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 UDCA (moderate) versus no intervention | 2 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 UDCA (low) versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Obeticholic acid (low) versus obeticholic acid (high) | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.61] |
| 4.10 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.37] |
| 4.11 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.10, 61.39] |
| 4.12 Lamivudine plus zidovudine plus UDCA (moderate) versus UDCA (moderate) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.43] |
| 4.13 UDCA (moderate) versus obeticholic acid (low) plus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.98] |
| 4.14 Bezafibrate plus UDCA (low) versus UDCA (low) | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.15 UDCA (moderate) versus UDCA (low) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.16 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.12, 78.14] |
| 5 Serious adverse events (number of events) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.66 [0.75, 3.66] | |
| 6 Adverse events (proportion) Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cyclosporin versus no intervention | 3 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.98, 4.68] |
| 6.2 D‐Penicillamine versus no intervention | 2 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.51 [2.56, 7.93] |
| 6.3 Malotilate versus no intervention | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.43 [1.40, 93.04] |
| 6.4 Obeticholic acid (high) versus no intervention | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 16.6 [0.90, 305.59] |
| 6.5 Obeticholic acid (low) versus no intervention | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.41, 6.17] |
| 6.6 Obeticholic acid (moderate) versus no intervention | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.81 [1.01, 76.73] |
| 6.7 UDCA (low) versus no intervention | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 UDCA (moderate) versus no intervention | 2 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.50, 4.25] |
| 6.9 Glucocorticosteroids plus UDCA (moderate) versus azathioprine plus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.12] |
| 6.10 UDCA (low) versus bezafibrate | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Obeticholic acid (low) versus obeticholic acid (high) | 1 | 79 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.78] |
| 6.12 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | 89 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 9.62] |
| 6.13 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.53 [0.59, 51.70] |
| 6.14 Bezafibrate plus UDCA (low) versus UDCA (low) | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.29 [0.12, 89.81] |
| 6.15 Colestilan plus UDCA (low) versus UDCA (low) | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.16 Methotrexate plus UDCA (low) versus UDCA (low) | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 115.0 [4.98, 2657.48] |
| 6.17 UDCA (moderate) versus UDCA (low) | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.18 Azathioprine plus UDCA (moderate) versus UDCA (moderate) | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.67 [0.94, 413.50] |
| 6.19 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 2 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.20 [0.63, 60.80] |
| 6.20 Glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 2 | 135 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.54 [1.35, 22.84] |
| 6.21 TauroUDCA (moderate) versus UDCA (moderate) | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [2.16, 204.61] |
| 7 Adverse events (number) Show forest plot | 15 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 Chlorambucil versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 3.67 [1.04, 12.87] | |
| 7.2 Cyclosporin versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 1.87 [1.51, 2.32] | |
| 7.3 D‐Penicillamine versus no intervention | 3 | Rate Ratio (Fixed, 95% CI) | 2.64 [1.78, 3.91] | |
| 7.4 Malotilate versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 6.13 [1.38, 27.14] | |
| 7.5 Obeticholic acid (high) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.91 [1.50, 2.44] | |
| 7.6 Obeticholic acid (low) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.05 [0.80, 1.39] | |
| 7.7 Obeticholic acid (moderate) versus no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 1.25 [0.97, 1.62] | |
| 7.8 Obeticholic acid (low) versus obeticholic acid (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.55 [0.43, 0.70] | |
| 7.9 Obeticholic acid (moderate) versus obeticholic acid (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.66 [0.53, 0.81] | |
| 7.10 Obeticholic acid (moderate) versus obeticholic acid (low) | 1 | Rate Ratio (Fixed, 95% CI) | 1.19 [0.93, 1.53] | |
| 7.11 UDCA (low) versus UDCA (high) | 1 | Rate Ratio (Fixed, 95% CI) | 2.08 [0.78, 5.53] | |
| 7.12 UDCA (moderate) versus UDCA (high) | 1 | Rate Ratio (Fixed, 95% CI) | 0.73 [0.21, 2.60] | |
| 7.13 UDCA (low) plus methotrexate versus UDCA (low) | 1 | Rate Ratio (Fixed, 95% CI) | 30.64 [1.84, 510.76] | |
| 7.14 UDCA (moderate) versus UDCA (low) | 1 | Rate Ratio (Fixed, 95% CI) | 0.35 [0.11, 1.10] | |
| 7.15 Azathioprine plus glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.32 [0.88, 1.97] | |
| 7.16 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 11.79 [0.65, 213.14] | |
| 7.17 Colchicine plus UDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 5.91 [0.28, 123.08] | |
| 7.18 TauroUDCA (moderate) versus UDCA (moderate) | 1 | Rate Ratio (Fixed, 95% CI) | 1.17 [0.81, 1.71] | |
| 8 Liver transplantation Show forest plot | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Cyclosporin versus no intervention | 2 | 378 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.72] |
| 8.2 D‐Penicillamine versus no intervention | 1 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 15.05] |
| 8.3 Methotrexate versus no intervention | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.58] |
| 8.4 UDCA (low) versus no intervention | 2 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.24, 4.06] |
| 8.5 UDCA (moderate) versus no intervention | 3 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.76] |
| 8.6 UDCA (low) versus UDCA (high) | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.17 [0.13, 79.71] |
| 8.7 UDCA (moderate) versus UDCA (high) | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.37 [0.13, 84.70] |
| 8.8 UDCA (moderate) versus UDCA (low) | 1 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.06, 17.47] |
| 8.9 Bezafibrate plus UDCA (moderate) versus UDCA (moderate) | 1 | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.39] |
| 9 Decompensated liver disease Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 D‐Penicillamine versus no intervention | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 UDCA (moderate) versus no intervention | 2 | 351 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.84, 2.12] |
| 9.3 Obeticholic acid (low) plus UDCA (moderate) versus UDCA (moderate) | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 38.46] |
| 9.4 UDCA (low) plus colchicine versus UDCA (low) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.07] |
| 9.5 Azathioprine plus UDCA (moderate) versus UDCA (moderate) | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.18] |
| 9.6 Glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.69] |
| 9.7 Methotrexate plus UDCA (moderate) versus UDCA (moderate) | 1 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.79, 5.04] |
| 9.8 Glucocorticosteroids plus UDCA (moderate) versus azathioprine plus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.10, 11.18] |
| 10 Cirrhosis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Azathioprine versus no intervention | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.41] |
| 10.2 UDCA (low) versus no intervention | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 1.53] |
| 10.3 Azathioprine plus glucocorticosteroids plus UDCA (moderate) versus UDCA (moderate) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.90] |

