การใช้ยาเพื่อรักษาการติดเชื้อไวรัสตับอักเสบบีเฉียบพลัน
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised clinical trial. | |
| Participants | Country: US. Number randomised: 55. Postrandomisation dropouts: not stated. Revised sample size: 55. Mean age: not stated. Females: not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. | |
| Outcomes | Mortality. | |
| Notes | Follow‐up period: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Accession were stratified by a centralised computer program for stage of encephalopathy…" |
| Allocation concealment (selection bias) | Low risk | Quote: "Accession were stratified by a centralised computer program for stage of encephalopathy…" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: the placebo was indistinguishable from the HBIG treatment, but they did not say whether participants or clinicians (or both) were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: the placebo was indistinguishable from the HBIG treatment, but they did not say whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither adverse events nor progression to chronic HBV. |
| For‐profit bias | Low risk | Comment: study funded by the National Heart and Lung Institute. |
| Other bias | Low risk | Comment: no risk of other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Romania. Number randomised: 31. Postrandomisation dropouts: not stated. Revised sample size: 31. Mean age: not stated. Females: not stated. Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. | |
| Outcomes | Seroconversion. | |
| Notes | Follow‐up period: minimum 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although authors stated double blind, further details (e.g. whether placebo used) not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although authors stated double blind, further details (e.g. whether placebo used) not reported. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: mortality, adverse events, and progression to chronic HBV not reported. |
| For‐profit bias | Unclear risk | Comment: information not available. |
| Other bias | Low risk | Comment: no risk of other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: India. Number randomised: 71. Postrandomisation dropouts: 0 (0%). Revised sample size: 71. Mean age: 37 years. Females: 19 (26.8%). Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. | |
| Outcomes | Mortality, adverse events, progression to chronic HBV infection, and seroconversion. | |
| Notes | Follow‐up period: minimum 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done with a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: investigators and participants blinded to randomisation arm. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: investigators and participants blinded to randomisation arm. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality, adverse events, and progression to chronic HBV reported. |
| For‐profit bias | Unclear risk | Comment: source of funding not reported. |
| Other bias | Low risk | Comment: was no risk of other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Romania. Number randomised: 200. Postrandomisation dropouts: 0 (0%). Revised sample size: 200. Mean age: 36 years. Females: 107 (53.5%). Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 groups. Group 3: no intervention (n = 105). | |
| Outcomes | Mortality, progression to chronic HBV infection, and seroconversion. | |
| Notes | Follow‐up period: minimum 11 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated list of random numbers was used." |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was a prospective, open‐label study conducted in Romania… Given the different formulation and appearance of the administered drugs (lamivudine comes as oval‐shaped, white tablets and entecavir as triangular, blue tablets) and the individualised nature of the usual care administered in the control group, masking could not be performed and patients and physicians were aware of the allocated groups." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "This was a prospective, open‐label study conducted in Romania… Given the different formulation and appearance of the administered drugs (lamivudine comes as oval‐shaped, white tablets and entecavir as triangular, blue tablets) and the individualised nature of the usual care administered in the control group, masking could not be performed and patients and physicians were aware of the allocated groups." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: intention‐to‐treat analysis performed including all randomised participants. |
| Selective reporting (reporting bias) | High risk | Comment: mortality and adverse events reported; however, progression to chronic HBV not reported. |
| For‐profit bias | Low risk | Quote: "Financial support and sponsorship: nil." |
| Other bias | Low risk | Comment: no risk of other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Greece. Number randomised: 100. Postrandomisation dropouts: not stated. Revised sample size: 100. Mean age: 33 years. Females: 39 (39%). Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. | |
| Outcomes | Adverse events. | |
| Notes | Follow‐up period: minimum 5 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used in this double‐blind trial, it was not clear whether placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used in this double‐blind trial, it was not clear whether placebo was identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: information not available. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor progression to chronic HBV reported. |
| For‐profit bias | High risk | Comment: 1 of the coauthors was an employee of a pharmaceutical company. |
| Other bias | Low risk | Comment: no risk of other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Greece. Number randomised: 100. Postrandomisation dropouts: 0 (0%). Revised sample size: 100. Mean age: 32 years. Females: 39 (39%). Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. | |
| Outcomes | Adverse events, quality of life, seroconversion, and time to seroconversion. | |
| Notes | Follow‐up period: minimum 5 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to one of the three treatment options using the randomization sequences prepared by Schering‐Plough Corporation, the sponsor of the study." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly assigned to one of the three treatment options using the randomization sequences prepared by Schering‐Plough Corporation, the sponsor of the study." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "neither the patients nor the investigators knew which drug the patients received." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "neither the patients nor the investigators knew which drug the patients received." |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants were included for the analysis of most outcomes. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor progression to chronic HBV reported. |
| For‐profit bias | High risk | Comment: trial funded by a party with vested interest in the results (funded by Schering‐Plough). |
| Other bias | Low risk | Comment: no risk of other bias. |
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Number randomised: 40. Postrandomisation dropouts: 5 (12.5%). Revised sample size: 35. Mean age: 41 years. Females: 5 (14.3%). Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 groups. | |
| Outcomes | Adverse events, seroconversion, and time to seroconversion. | |
| Notes | Follow‐up period: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: information not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used in this double‐blind trial, it was not clear whether placebo was identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used in this double‐blind trial, it was not clear whether placebo was identical. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: neither mortality nor progression to chronic HBV reported. |
| For‐profit bias | Low risk | Quote: "the study was not supported by any manufacturer of lamivudine." |
| Other bias | Low risk | Comment: no risk of other bias. |
anti‐HBc: antibody to hepatitis B core antigen; HBIG: hepatitis B immunoglobulin; HBsAg: hepatitis B surface antigen; HBV: hepatitis B virus.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Hepatitis B virus infection not confirmed in all participants. | |
| Not participants with hepatitis B virus infection. | |
| Not a randomised clinical trial. | |
| Hepatitis B virus infection not confirmed in all participants. | |
| Quasi‐randomised study (allocation based on alphabet of participant's name). | |
| Not a randomised clinical trial. | |
| Quasi‐randomised study (allocation based on admission order). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Mortality Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 1 Mortality. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Hepatitis B immunoglobulin (HBIG) versus placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Lamivudine versus placebo or no intervention | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 Lamivudine versus entecavir | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 Entecavir versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Chronic hepatitis B virus infection Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2 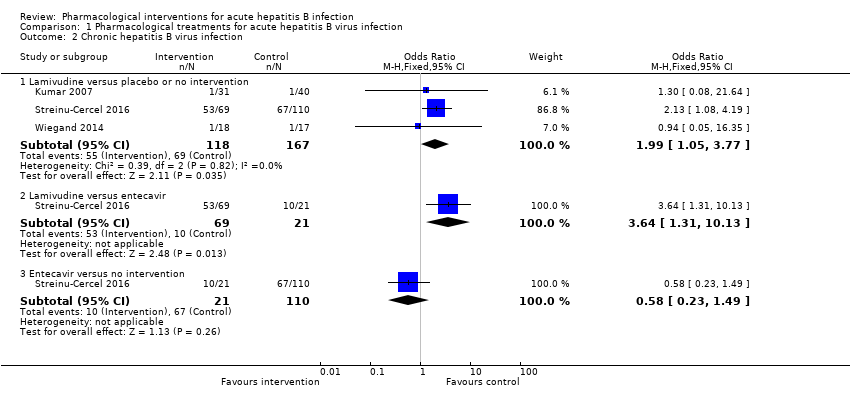 Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 2 Chronic hepatitis B virus infection. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 Lamivudine versus placebo or no intervention | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.05, 3.77] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 Lamivudine versus entecavir | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.31, 10.13] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.3 Entecavir versus no intervention | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.49] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Serious adverse events Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 3 Serious adverse events. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 Interferon versus placebo | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 Lamivudine versus placebo or no intervention | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 Lamivudine versus entecavir | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.4 Entecavir versus no intervention | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Adverse events proportion Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4  Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 4 Adverse events proportion. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Interferon versus placebo | 2 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 348.16 [45.39, 2670.26] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Lamivudine versus placebo or no intervention | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.34, 5.94] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Adverse events number Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5  Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 5 Adverse events number. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 Lamivudine versus placebo or no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Health‐related quality of life Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.6
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 6 Health‐related quality of life. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 Interferon versus placebo | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Seroconversion Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.7  Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 7 Seroconversion. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 Interferon versus placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.2 Lamivudine versus placebo or no intervention | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.3 Lamivudine versus entecavir | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.4 Entecavir versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Time to seroconversion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.8  Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 8 Time to seroconversion. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 Interferon versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Time to seroconversion [weeks] Show forest plot | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.9
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 9 Time to seroconversion [weeks]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 Lamivudine versus placebo or no intervention | Other data | No numeric data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
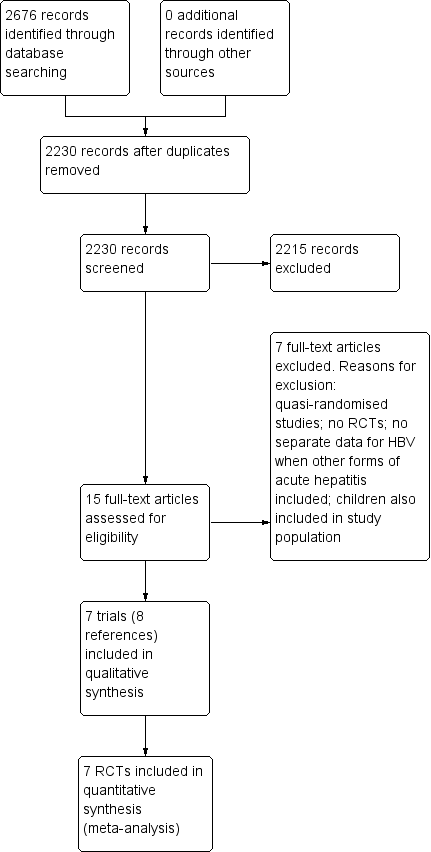
Study flow diagram. HBV: hepatitis B virus; RCT: randomised clinical trial.
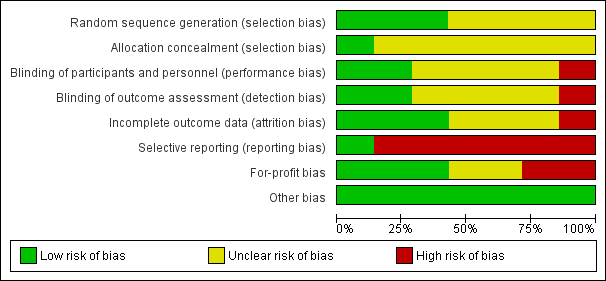
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial sequential analysis of progression to chronic hepatitis B virus infection (lamivudine versus placebo or no treatment) and adverse events (proportion) (interferon versus placebo): Using the control group proportion observed in the trials (Pc = 41.3% and 34.9% respectively), alpha error of 2%, beta error of 90%, relative risk reduction (RRR) of 20%, and diversity observed in the analysis (0%), the accrued sample sizes (285 and 200 respectively) were only small proportions of the diversity‐adjusted required information sizes (DARIS) (progression to chronic hepatitis B = 1783; adverse events (proportion) = 2303). While the Z‐curve (blue lines) crossed the conventional boundary of P = 0.05 (dotted green lines) favouring placebo or no treatment, it did not cross any of the trial sequential monitoring boundaries (dotted red lines). There was a high risk of random errors.

Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 1 Mortality.
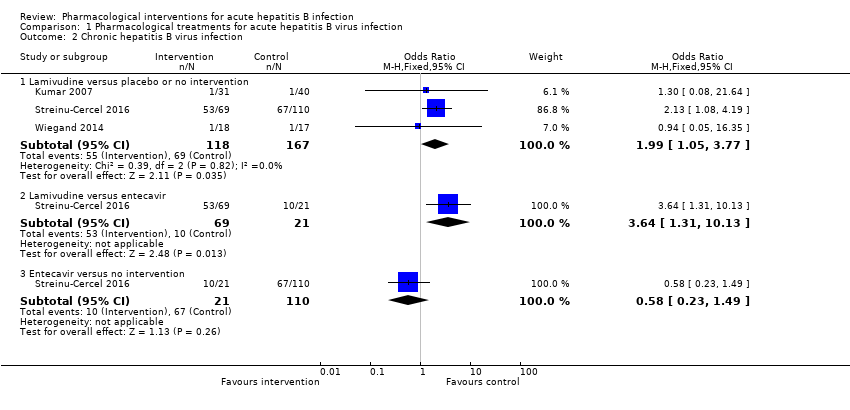
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 2 Chronic hepatitis B virus infection.
| Study | Number of events (intervention) | Number of participants (intervention) | Number of events (control) | Number of participants (control) |
| Interferon versus placebo | ||||
| Tassopoulos 1989 | 0 | 67 | 0 | 33 |
| Lamivudine versus placebo or no intervention | ||||
| Kumar 2007 | 0 | 31 | 0 | 40 |
| Streinu‐Cercel 2016 | 0 | 69 | 0 | 110 |
| Lamivudine versus entecavir | ||||
| Streinu‐Cercel 2016 | 0 | 69 | 0 | 21 |
| Entecavir versus no intervention | ||||
| Streinu‐Cercel 2016 | 0 | 21 | 0 | 110 |
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 3 Serious adverse events.
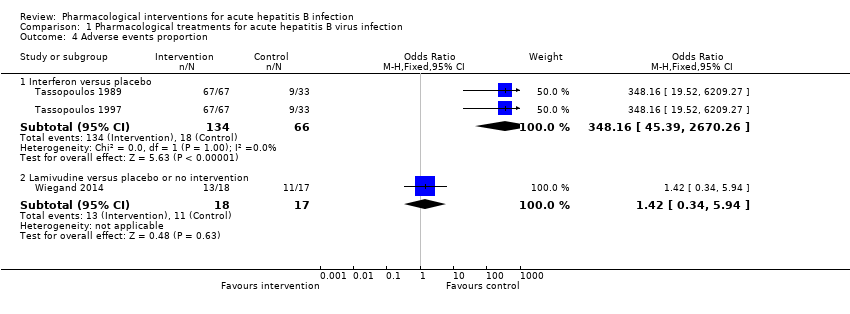
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 4 Adverse events proportion.
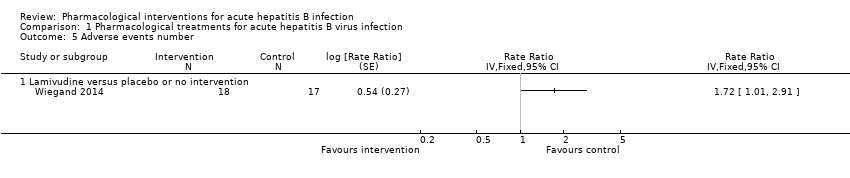
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 5 Adverse events number.
| Study | Mean (interferon) | Number of participants (interferon) | Mean (control) | Number of participants (control) | Mean difference | Statistical significance | Further details on scale used |
| Interferon versus placebo | |||||||
| Tassopoulos 1997 | 48.1 | 67 | 42.7 | 33 | 5.4 | Not stated | Scale not stated. Not reported whether higher score indicates better or worse |
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 6 Health‐related quality of life.
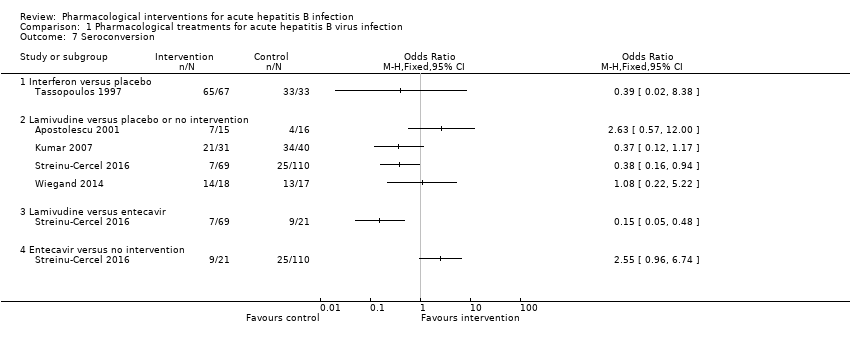
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 7 Seroconversion.

Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 8 Time to seroconversion.
| Study | Mean (lamivudine) | Number of participants (lamivudine) | Mean (control) | Number of participants (control) | Mean difference | Statistical significance |
| Lamivudine versus placebo or no intervention | ||||||
| Wiegand 2014 | 17 | 18 | 16 | 17 | 1 | P = 0.519 (not statistically significant) |
Comparison 1 Pharmacological treatments for acute hepatitis B virus infection, Outcome 9 Time to seroconversion [weeks].
| Lamivudine versus no intervention for acute hepatitis B virus infection | |||||
| Patient or population: people with acute HBV infection Settings: secondary or tertiary care Intervention: lamivudine Control: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Lamivudine | ||||
| Short‐term mortality (< 1 year) | 33 per 1000 | 43 per 1000 | OR 1.29 | 250 | ⊕⊝⊝⊝ |
| Progression to chronic HBV infection (6 to 12 months) | 413 per 1000 | 584 per 1000 | OR 1.99 | 285 | ⊕⊝⊝⊝ |
| Progression to fulminant HBV infection | None of the trials reported this information. | ||||
| Serious adverse events (6 to 12 months) | There were no serious adverse events in either group. | 250 (2 trials) | ⊕⊝⊝⊝ | ||
| Adverse events (proportion) (12 months) | 647 per 1000 | 722 per 1000 | OR 1.42 | 35 | ⊕⊝⊝⊝ |
| Adverse events (number of events) (12 months) | 1235 per 1000 | 2124 per 1000 | Rate ratio 1.72 | 35 | ⊕⊝⊝⊝ |
| Health‐related quality of life (1 week) | None of the trials reported this information. | ||||
| *The basis for the assumed risk is the mean control group risk in the control group across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HBV: hepatitis B virus; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The risk of bias in the trial(s) was high (downgraded by 1 level for risk of bias). | |||||
| Interferon versus no intervention for acute hepatitis B virus infection | |||||
| Patient or population: people with acute HBV infection Settings: secondary or tertiary care Intervention: interferon Control: no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Interferon | ||||
| Short‐term mortality | None of the trials reported this information. | ||||
| Progression to chronic HBV infection | None of the trials reported this information. | ||||
| Progression to fulminant HBV infection | None of the trials reported this information. | ||||
| Serious adverse events | There were no serious adverse events in either group. | 100 (1 trial) | ⊕⊝⊝⊝ | ||
| Adverse events (proportion) (4 to 6 months) | 273 per 1000 | 992 per 1000 | OR 348.16 | 200 | ⊕⊕⊝⊝ |
| Adverse events (number of events) | None of the trials reported this information. | ||||
| Health‐related quality of life (1 week) | The scale used to report the health‐related quality of life was not stated. Neither was information on whether higher score meant better or worse available. The mean score in the placebo group was 42.7 units. The mean score in the interferon group was 5.4 units higher. There was no information to calculate the 95% confidence intervals or P value. | 100 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk is the mean control group risk in the control group across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HBV: hepatitis B virus; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The risk of bias in the trial(s) was high (downgraded by 1 level for risk of bias). | |||||
| Study name | Intervention | Control | Follow‐up period (months) | Sequence generation | Allocation concealment | Blinding of participants and healthcare providers | Blinding of outcome assessors | Incomplete outcome data | Selective outcome reporting | Source of funding | Other bias | Overall risk of bias |
| Hepatitis B Immunoglobulin | Placebo | Not stated | Low | Low | Unclear | Unclear | Unclear | High | Low | Low | High | |
| Interferon | Placebo | Min 5 | Unclear | Unclear | Unclear | Unclear | Unclear | High | High | Low | High | |
| Interferon | Placebo | Min 5 | Unclear | Unclear | Low | Low | Low | High | High | Low | High | |
| Lamivudine | No intervention | Min 3 | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear | Low | High | |
| Lamivudine | Placebo | Min 12 | Low | Unclear | Low | Low | Low | Low | Unclear | Low | Unclear | |
| Lamivudine | Placebo | Not stated | Unclear | Unclear | Unclear | Unclear | High | High | Low | Low | High | |
| Lamivudine | Control 1: entecavir | Min 11 | Low | Unclear | High | High | Low | High | Low | Low | High | |
| Min: minimum. | ||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Hepatitis B immunoglobulin (HBIG) versus placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Lamivudine versus placebo or no intervention | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Lamivudine versus entecavir | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Entecavir versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Chronic hepatitis B virus infection Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Lamivudine versus placebo or no intervention | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.05, 3.77] |
| 2.2 Lamivudine versus entecavir | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.64 [1.31, 10.13] |
| 2.3 Entecavir versus no intervention | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.49] |
| 3 Serious adverse events Show forest plot | Other data | No numeric data | ||
| 3.1 Interferon versus placebo | Other data | No numeric data | ||
| 3.2 Lamivudine versus placebo or no intervention | Other data | No numeric data | ||
| 3.3 Lamivudine versus entecavir | Other data | No numeric data | ||
| 3.4 Entecavir versus no intervention | Other data | No numeric data | ||
| 4 Adverse events proportion Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Interferon versus placebo | 2 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 348.16 [45.39, 2670.26] |
| 4.2 Lamivudine versus placebo or no intervention | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.34, 5.94] |
| 5 Adverse events number Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Lamivudine versus placebo or no intervention | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Health‐related quality of life Show forest plot | Other data | No numeric data | ||
| 6.1 Interferon versus placebo | Other data | No numeric data | ||
| 7 Seroconversion Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Interferon versus placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Lamivudine versus placebo or no intervention | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Lamivudine versus entecavir | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Entecavir versus no intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Time to seroconversion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Interferon versus placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Time to seroconversion [weeks] Show forest plot | Other data | No numeric data | ||
| 9.1 Lamivudine versus placebo or no intervention | Other data | No numeric data | ||

