Toxina botulínica para la prevención de la migraña en adultos
Appendices
Appendix 1. Search strategies
CENTRAL (via CRSO)
#1 MESH DESCRIPTOR headache disorders EXPLODE ALL TREES
#2 MESH DESCRIPTOR headache EXPLODE ALL TREES
#3 ((headache* or migrain* or cephalgi* or cephalalgi* or hemicrani*)):TI,AB,KY
#4 #1 OR #2 OR #3
#5 MESH DESCRIPTOR botulinum toxins EXPLODE ALL TREES
#6 ((botulin* adj toxin*)):TI,AB,KY
#7 ((botulinum* or oculinu* or boto* or onabotulinum*)):TI,AB,KY
#8 MESH DESCRIPTOR Botulinum Toxins, Type A EXPLODE ALL TREES
#9 (clostridium botulinum):TI,AB,KY
#10 (clostridium botulin*):TI,AB,KY
#11 #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12 #4 AND #11
MEDLINE (via OVID)
#1 Exp headache disorders/
#2 headache/
#3 (headache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).mp.
#4 or/1‐3
#5 exp botulinum toxins/
#6 (botulin* adj toxin*).tw
#7 (botulinum* or oculinu* or boto* or onabotulinum*).tw.
#8 exp botulinum toxin type A/
#9 Exp clostridium botulinum/
#10 clostridium botulin*.tw.
#11 or/5‐10
12 randomized controlled trial.pt.
13 controlled clinical trial.pt.
14 randomized.ab.
15 placebo.ab.
16 drug therapy.fs.
17 randomly.ab.
18 trial.ab.
19 groups.ab.
20 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21 exp animals/ not humans.sh.
22 20 not 21
23 4 and 11 and 22
Embase (via OVID)
1 exp headache disorders/
2 headache/
3 (headache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).tw.
4 or/1‐3
5 exp botulinum toxins/
6 (botulin* adj toxin*).tw.
7 (botulinum* or oculinu* or boto* or onabotulinum*).tw.
8 exp botulinum toxin type A/
9 exp clostridium botulinum/
10 clostridium botulin*.tw.
11 or/5‐10
12 random$.tw.
13 factorial$.tw.
14 crossover$.tw.
15 cross over$.tw.
16 cross‐over$.tw.
17 placebo$.tw.
18 (doubl$ adj blind$).tw.
19 (singl$ adj blind$).tw.
20 assign$.tw.
21 allocat$.tw.
22 volunteer$.tw.
23 Crossover Procedure/
24 double‐blind procedure.tw.
25 Randomized Controlled Trial/
26 Single Blind Procedure/
27 or/12‐26
28 (animal/ or nonhuman/) not human/
29 27 not 28
30 4 and 11 and 29
Appendix 2. Botulinum toxin versus non‐established agents summary
Botulinum toxin versus other agent: histamine
One trial (Millán‐Guerrero 2009) with 100 participants compared a single round of injections of Botox (50 U) into the head and neck muscles with injections of histamine (1 to 10 µg) injected into upper arm twice a week for 12 weeks. The trial publication reports that there were no significant between‐group differences in number of migraine attacks per month (P = 0.52), duration of migraine (P = 0.21), headache intensity (P = 0.32), use of rescue medications (P = 0.12) and patient's global impression of disease (MIDAS score, P = 0.12) at the four week time point. Statistical analyses for between group comparisons are not reported for any outcome measures at the 12‐week time point. Number of migraine days, number of headache days, quality‐of‐life measures, adverse events data and cost‐effectiveness outcomes were not recorded for this trial.
Appendix 3. Dosing studies summary
Botulinum toxin versus clinically relevant different dose of botulinum toxin: Botox
Comparisons between different doses of Botox were studied in five trials and 1049 participants (Elkind I 2006; Elkind II 2006; Relja 2007; Saper 2007; Silberstein 2000). All but one (Elkind II 2006) also included a placebo arm and we discussed the results of the pooled treatment arms compared with placebo earlier. We attempted to organise data into four clinically relevant dose comparisons: ≥ 200 U and < 200 U; ≥ 150 U and < 150 U; ≥ 100 U and < 100 U; ≥ 50 U and < 50 U.
Primary outcome: number of migraine days per month
Two trials run in sequence (Elkind I 2006; Elkind II 2006) reported migraine days data for 353 participants comparing 50 U Botox versus 25 U Botox, we excluded the lower‐dose arm (7.5 U Botox) in Elkind I 2006 from this comparison to prevent double counting, as participants from that arm were re‐randomised into Elkind II 2006. No statistically significant between‐group difference (0.4 days, 95% CI ‐0.2 to 1.0, P = 0.18) resulted from aggregation of these data (Analysis 4.1). One additional trial (Relja 2007) recorded this outcome but did not report the results in a usable format.
Secondary outcomes
Efficacy outcomes
Two trials reported number of migraine attacks but only one of those provided meta‐analysable data (Relja 2007) for 377 participants. This trial's results did not show a statistically significant between‐group difference for arms treated with ≥ 200 U compared with pooled lower doses, or for treatment with ≥ 150 compared with < 150 U (P = 0.21 and P = 0.65 respectively). Silberstein 2000 randomised 82 participants into two dosing arms comparing treatment with 75 U of Botox and treatment with 25 U, they reported a reduction in favour of the group treated with 75 U Botox of 1.1 attacks (P ≤ 0.046). None of the trials included in this comparison reported meta‐analysable data for duration of migraine, proportion of responders or use of rescue medication. All trials recorded a measure of migraine severity, but none of the trials reported data for analysis or gave a clear description of the results for the dosing arms. All trials recorded a global impression scale, but no data were available for analysis. Two trials did not report their results in enough detail to draw comparisons between their dosing arms (Elkind I 2006; Relja 2007) and one trial failed to provide any statistical analysis comparing the two Botox‐treated arms. Elkind II 2006 reported only that no consistent statistically significant differences between groups were identified for the patient global assessment score.
Quality‐of‐life measures were recorded by three trials (Elkind I 2006; Elkind II 2006; Relja 2007). Two of these failed to provide enough detail to allow between‐group comparisons to be made for the dosing arms. Elkind II 2006 recorded data for the Migraine‐Specific Measure of Quality of Life questionnaire, the Migraine Impact Questionnaire, and the Headache Pain Specific Quality of Life questionnaire and reported that there were no consistent statistically significant differences between groups on any measure. No cost effectiveness analyses were identified for this comparison.
Safety outcomes
Doses administered in the two trials reporting data for treatment‐related adverse events were not comparable within our chosen categories. The ≥ 200 U versus < 200 U and ≥ 150 U versus < 150 U comparisons data from Relja 2007 did not show a statistically significant between‐group difference in risk of treatment‐related adverse events (P = 0.37 and P = 0.61 respectively). Elkind I 2006 compared two low dose arms (50 U versus 25 U) and found an increased risk of a treatment‐related adverse event for participants treated with the higher of these two doses, with a RR of 2.1 (95% CI 1.4 to 3.3).
There were no between‐group differences in the risk ratio (RR) of muscle weakness (200 U: RR 1.04, 95% CI 0.73 to 1.47, P = 0.85, N = 377; 150 U: RR 1.13, 95 % CI 0.78 to 1.64, P = 0.52, N = 377; Analysis 4.2), neck pain (200 U: RR 1.25, 95% CI 0.83 to 1.89, P = 0.28, N = 377; 150 U: RR 1.19, 95% CI 0.76 to 1.86, P = 0.45, N = 377; Analysis 4.4), or injection site pain (200 U: RR 1.23, 95% CI 0.13 to 11.97, P = 0.86, N = 500; 150 U: RR 1.45, 95% CI 0.48 to 4.41, P = 0.51, N = 377; 50 U: RR 1.19, 95% CI 0.34 to 4.12, P = 0.78, N = 82; Analysis 4.5) for any of the above and below dose comparisons analysed).
For the analysis of blepharoptosis‐related adverse events, we considered only doses administered into the frontalis and/or corrugator muscles, so the comparisons were: ≥ 50 U and < 50 U; ≥ 30 U and < 30 U; and ≥ 10 U and < 10 U of Botox (Analysis 4.3). The RR of blepharoptosis for higher doses of Botox was around 2 times that for lower doses in all cases but the result was statistically significant for the highest dose comparison only (50 U: RR 2.31, 95% CI 1.58 to 4.43, N = 377; 30 U: RR 2.36, 95% CI 0.58 to 9.65, P = 0.23, N = 459; 10 U: RR 2.42, 95% CI 0.99 to 5.94, P = 0.051, N = 406).
Botulinum toxin versus clinically relevant different dose of botulinum toxin: other botulinum toxin preparations
Two trials with 150 participants compared different dosing arms of Dysport (Chankrachang 2011; Petri 2009). Both included a placebo arm and were also involved in the relevant section earlier in this review. A further trial compared two different doses (33 U and 25 U), in 30 participants, of a further botulinum toxin agent, Prosigne, with a Botox arm (dose 25 U) and a placebo arm to attempt to establish dose equivalency (Lauretti 2014).
Primary outcome: number of migraine days per month
None of the trials reporting dose comparisons of other botulinum toxins recorded the primary outcome for this review.
Secondary outcomes
Efficacy outcomes
The number of headache days was recorded by Petri 2009 only and they did not report any data, but stated that the between‐group differences were not statistically significant. Two trials recorded number of migraine attacks (Chankrachang 2011; Petri 2009). Chankrachang 2011, N = 86, stated that there was no significant difference between the treated groups, for which the dose comparison was 240 U Dysport vs 120 U Dysport (P = 0.87). Petri 2009, N = 60, provided no statistical analysis comparing the groups treated with 210 U Dysport vs 80 U Dysport and no data to allow the analysis to be carried out by the review authors. Only Petri 2009 recorded the mean duration of migraine and frequency of use of rescue medications and again, the trial authors reported just that the difference between the groups was not statistically significant. None of the trials recorded proportion of responders. All trials recorded a measure of headache intensity. The trials studying Dysport both reported that there were no significant between‐group differences in migraine severity. The trial studying dose equivalency of Botox compared with Prosigne (Lauretti 2014) reported that there was no statistically significant between‐group difference for the 33 U Prosigne group. Chankrachang 2011 reported that there were no statistically significant between‐group differences in patient or investigator global assessment scores or MIDAS scores for the regular diary period of four weeks. No between‐group comparison was reported for patient global assessment scores by Petri 2009 and clinical global assessment scores were reported as showing no statistically significant between‐group difference. Only Chankrachang 2011 recorded a quality‐of‐life measure. They used the SF‐36 questionnaire and reported that there were no statistically significant between‐group differences. No cost‐effectiveness analyses were identified for this comparison.
Safety outcomes
The numbers of participants experiencing any adverse event were reported by both trials of Dysport. Meta‐analysis of these data, grouping arms treating with ≥ 150 U and < 150 U, showed no significant between group difference in the RR of experiencing an adverse event (RR 1.59, 95% CI 0.47 to 5.32, P = 0.45; Analysis 3.1). The specific adverse event types of interest for this review were not reported in the correct format for analysis, with the single exception that Petri 2009 stated that ptosis was experienced by one participant in each treated group (N = 32 per arm).

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison 1. Botulinum toxin type A versus placebo, outcome: 1.1 Number of migraine days. Mazza 2016 and Cady 2014 removed for sensitivity analysis of small trial effect. Data for Mazza 2016 is endpoint data.

Forest plot of comparison 1. Botulinum toxin type A versus placebo, outcome: 1.4 Severity of migraine (Visual Analogue Score 0‐10)

Forest plot of comparison 1. Botulinum toxin type A versus placebo, outcome: 1.6 Total adverse events

Comparison 1 Botulinum toxin type A versus placebo, Outcome 1 Number of migraine days.
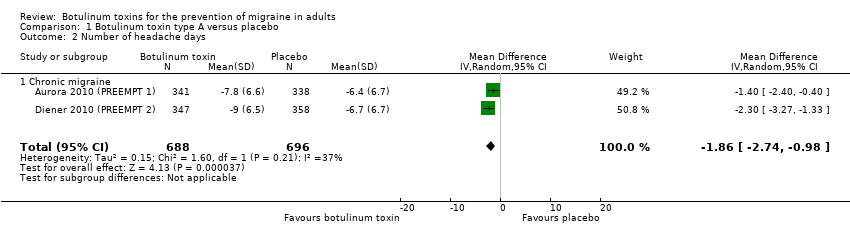
Comparison 1 Botulinum toxin type A versus placebo, Outcome 2 Number of headache days.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 3 Number of migraine attacks.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 4 Severity of migraine (Visual Analogue Score 0‐10).

Comparison 1 Botulinum toxin type A versus placebo, Outcome 5 Use of rescue medication.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 6 Total adverse events.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 7 Adverse event ‐ blepharoptosis.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 8 Adverse event ‐ muscle weakness.
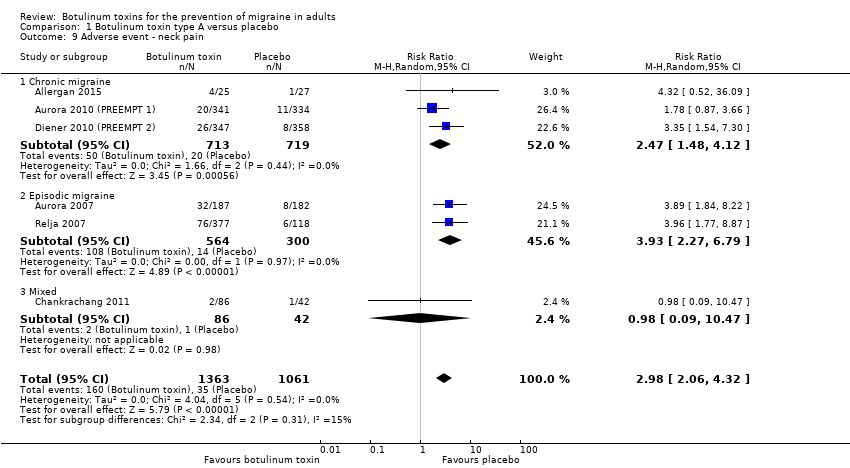
Comparison 1 Botulinum toxin type A versus placebo, Outcome 9 Adverse event ‐ neck pain.
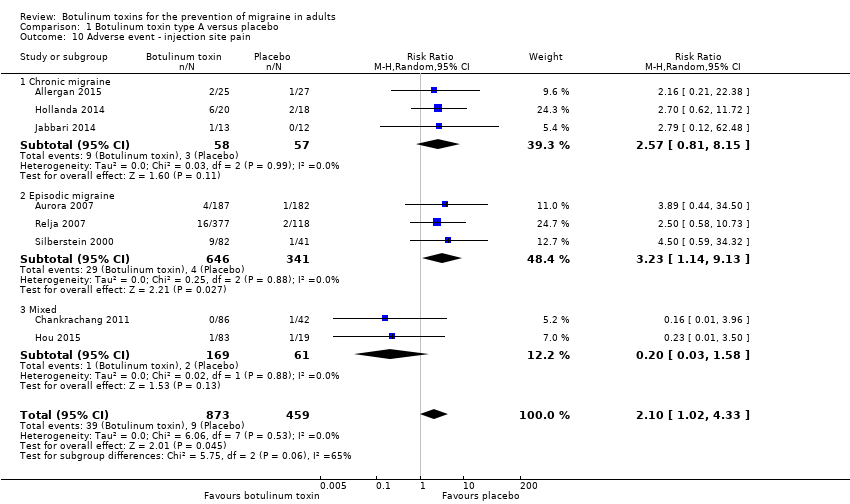
Comparison 1 Botulinum toxin type A versus placebo, Outcome 10 Adverse event ‐ injection site pain.

Comparison 1 Botulinum toxin type A versus placebo, Outcome 11 Total treatment related adverse events.
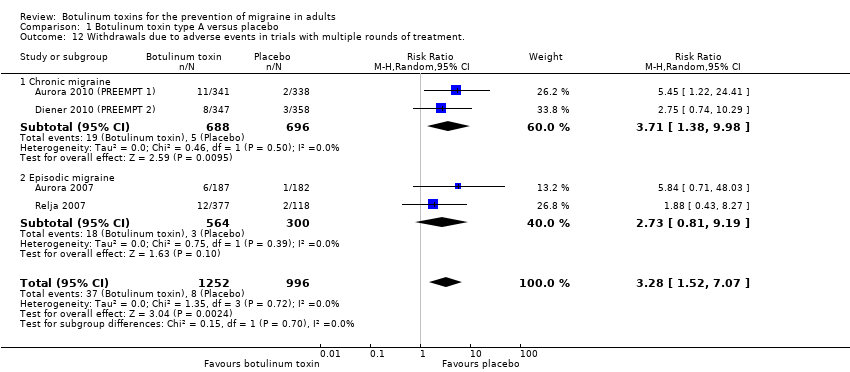
Comparison 1 Botulinum toxin type A versus placebo, Outcome 12 Withdrawals due to adverse events in trials with multiple rounds of treatment..
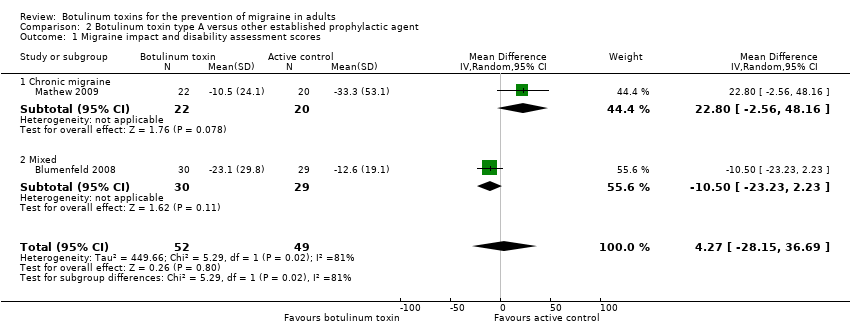
Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 1 Migraine impact and disability assessment scores.

Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 2 Total adverse events.

Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 3 Total treatment related adverse events.

Comparison 2 Botulinum toxin type A versus other established prophylactic agent, Outcome 4 Withdrawals due to adverse events in trials with multiple rounds of treatment..

Comparison 3 Dysport ≥ 150 U versus Dysport < 150 U, Outcome 1 Total adverse events.
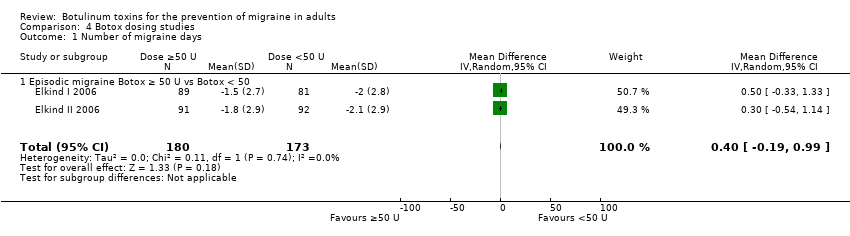
Comparison 4 Botox dosing studies, Outcome 1 Number of migraine days.

Comparison 4 Botox dosing studies, Outcome 2 Adverse event ‐ muscle weakness.

Comparison 4 Botox dosing studies, Outcome 3 Adverse event ‐ blepharoptosis.

Comparison 4 Botox dosing studies, Outcome 4 Adverse event ‐ neck pain.

Comparison 4 Botox dosing studies, Outcome 5 Adverse event ‐ injection site pain.
| Botulinum toxin type A compared to placebo for the prevention of migraine in adults | |||||
| Patient or population: adults with migraine | |||||
| Outcomes | Result with placebo | Result with botulinum toxin type A | Relative effect | № of participants | Quality of the evidence |
| Number of migraine days per month: chronic migraine only | The mean number of migraine days (chronic migraine only) ranged from 12 to 20 days | MD 3.1 days lower | ‐ | 1497 | ⊕⊕⊝⊝ |
| Number of migraine days per month | The mean number of migraine days ranged from 4 to 20 days | MD 2.4 days lower | ‐ | 1915 | ⊕⊝⊝⊝ |
| Number of headache days per month: chronic migraine only | The mean number of headache days (chronic migraine only) ranged from 13 to 13.4 days | MD 1.9 days lower | ‐ | 1384 | ⊕⊕⊕⊕ |
| Number of migraine attacks | The mean number of migraine attacks ranged from 1.9 to 7.8 attacks | MD 0.5 attacks lower | ‐ | 2004 | ⊕⊕⊝⊝ |
| Headache intensity measure (Visual Analogue Score 0‐10) | The mean severity of migraine (Visual Analogue Score 0‐10) ranged from 6.2 to 9.2 cm | MD 3.3 cm lower | ‐ | 209 | ⊕⊝⊝⊝ |
| Global impression scale | The mean global impression scale was 58.6 points | MD 1.6 points higher | ‐ | 45 | ⊕⊝⊝⊝ |
| Total number of participants experiencing an adverse event | Trial population | RR 1.28 | 3325 | ⊕⊕⊕⊝ | |
| 471 per 1000 | 603 per 1000 | ||||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded once due to inconsistency: statistical heterogeneity observed despite similarities in populations and doses. | |||||
| Botulinum toxin type A compared to other established prophylactic agent for the prevention of migraine in adults | |||||
| Patient or population: adults with migraine | |||||
| Outcomes | Result with other established prophylactic agent | Result with botulinum toxin type A | Relative effect | № of participants | Quality of the evidence |
| Number of migraine days per month: chronic migraine only | One trial using topiramate in its comparison arm reported narratively on this outcome stating that there was no significant difference between groups. | ‐ | 43 | ⊕⊝⊝⊝ | |
| Number of headache days per month | The mean number of headache days was 6.6 days | MD 1 day lower | ‐ | 59 | ⊕⊝⊝⊝ |
| Number of migraine attacks per month | ‐ | ‐ | ‐ | ‐ | ‐ |
| Headache intensity measure | The mean severity of migraine was 2.3 points | MD 0.4 points lower | ‐ | 46 | ⊕⊝⊝⊝ |
| Global impression of disease | The mean global impression of disease ranged from 9.8 to 16.5 points | MD 4.3 points higher | ‐ | 101 | ⊕⊝⊝⊝ |
| Total number of participants experiencing an adverse event | Trial population | RR 0.76 | 114 | ⊕⊝⊝⊝ | |
| 862 per 1000 | 724 per 1000 | ||||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded once due to risk of bias: unclear or high risk for selection, performance, detection and attrition bias. | |||||
| Term | Definition |
| Chronic migraine (IHS 1988) | Not defined |
| Chronic migraine (IHS 2004) | Description: migraine headache occurring on ≥ 15 days per month for > 3 months in the absence of medication overuse Diagnostic criteria |
| Chronic migraine (IHS 2013) | Description: headache occurring on ≥ 15 days per month for > 3 months, which has the features of migraine headache on at least 8 days per month Diagnostic criteria A. Headache (tension‐type‐like and/or migraine‐like on ≥ 15 days/month for > 3 months and fulfilling criteria B and C B. Occurring in a patient who has had at least 5 attacks fulfilling criteria B‐D for migraine without aura and/or criteria B and C for migraine with aura C. On 8 days per month for > 3 months, fulfilling any of the following:
D. Not better accounted for by another ICHD‐III diagnosis |
| Medication overuse headache (IHS 1988) | Not defined |
| Medication overuse headache (IHS 2004) | Diagnostic criteria |
| Medication overuse headache (IHS 2013) | Description: headache occurring on ≥ 15 days/month developing as a consequence of regular overuse of acute or symptomatic headache medication (on ≥ 10, or ≥ 15 days/month, depending on the medication) for > 3 months. It usually, but not invariably, resolves after the overuse is stopped. Diagnostic criteria |
| Migraine (IHS 2013) | Migraine has 2 major subtypes. Migraine without aura is a clinical syndrome characterised by headache with specific features and associated symptoms. Migraine with aura is primarily characterised by the transient focal neurological symptoms that usually precede or sometimes accompany the headache. |
| Migraine with aura (IHS 2013) | Description: recurrent attacks, lasting minutes, of unilateral fully reversible visual, sensory or other central nervous system symptoms that usually develop gradually and are usually followed by headache and associated migraine symptoms Diagnostic criteria C. At least 2 of the following 4 characteristics:
D. Not better accounted for by another ICHD‐3 diagnosis, and transient ischaemic attack has been excluded. |
| Migraine without aura (IHS 2013) | Description: recurrent headache disorder manifesting in attacks lasting 4‐72 h. Typical characteristics of the headache are unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity and association with nausea and/or photophobia and phonophobia. Diagnostic criteria A. At least 5 attacks1 fulfilling criteria B–D
D. During headache at least one of the following: 1. nausea and/or vomiting, 2. photophobia and phonophobia E. Not better accounted for by another ICHD‐III diagnosis. |
| SNARE complex (Goodsell 2013) | Soluble NSF‐attachment protein receptor (NSF: N‐ethylmaleimide‐sensitive factor) |
| SNAP‐25 (Goodsell 2013) | Synaptosomal‐associated protein‐25 |
| Trade name | Manufacturer | FDA‐issued name | Sero‐type |
| Botox | Allergan | OnabotulinumtoxinA | Botulinum toxin type A |
| Botox cosmetic | Allergan | OnabotulinumtoxinA | Botulinum toxin type A |
| Dysport | Ipsen | AbobotulinumtoxinA | Botulinum toxin type A |
| HengLi | Lanzhou Institute of biological products | Not issued | Botulinum toxin type A |
| Myobloc | Solstice | RimabotulinumtoxinB | Botulinum toxin type B |
| Prosigne | Lanzhou Institute of biological products | Not issued | Botulinum toxin type A |
| Xeomin | Merz | IncobotulinumtoxinA | Botulinum toxin type A |
| Allergan, Ipsen and Galderma all responded but were unable to provide additional eligible data. Merz, Solstice, and Lanzhou Institute of biological products were contacted without response. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of migraine days Show forest plot | 5 | 1915 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.02, ‐0.76] |
| 1.1 Chronic migraine | 4 | 1497 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.73, ‐1.41] |
| 1.2 Episodic migraine | 1 | 418 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.77, 0.37] |
| 2 Number of headache days Show forest plot | 2 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.74, ‐0.98] |
| 2.1 Chronic migraine | 2 | 1384 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.74, ‐0.98] |
| 3 Number of migraine attacks Show forest plot | 6 | 2004 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.34, 0.41] |
| 3.1 Chronic migraine | 1 | 679 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.71, 0.91] |
| 3.2 Episodic migraine | 3 | 1096 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.17, 0.43] |
| 3.3 Mixed | 2 | 229 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐6.78, 2.63] |
| 4 Severity of migraine (Visual Analogue Score 0‐10) Show forest plot | 4 | 209 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐4.16, ‐2.45] |
| 4.1 Chronic migraine | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐3.31, ‐2.09] |
| 4.2 Episodic migraine | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐4.9 [‐6.56, ‐3.24] |
| 4.3 Mixed | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐4.52, ‐2.48] |
| 5 Use of rescue medication Show forest plot | 2 | 717 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐3.09, 0.52] |
| 5.1 Chronic migraine | 2 | 717 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐3.09, 0.52] |
| 6 Total adverse events Show forest plot | 13 | 3325 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.47] |
| 6.1 Chronic migraine | 5 | 1494 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.40] |
| 6.2 Episodic migraine | 6 | 1673 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 6.3 Mixed | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.57, 3.76] |
| 7 Adverse event ‐ blepharoptosis Show forest plot | 7 | 1867 | Risk Ratio (M‐H, Random, 95% CI) | 7.29 [3.18, 16.73] |
| 7.1 Episodic migraine | 5 | 1637 | Risk Ratio (M‐H, Random, 95% CI) | 9.53 [3.87, 23.44] |
| 7.2 Mixed | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.18, 13.59] |
| 8 Adverse event ‐ muscle weakness Show forest plot | 6 | 2602 | Risk Ratio (M‐H, Random, 95% CI) | 13.67 [6.73, 27.75] |
| 8.1 Chronic migraine | 2 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 12.68 [3.49, 46.05] |
| 8.2 Episodic migraine | 4 | 1223 | Risk Ratio (M‐H, Random, 95% CI) | 14.12 [6.05, 32.94] |
| 9 Adverse event ‐ neck pain Show forest plot | 6 | 2424 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.06, 4.32] |
| 9.1 Chronic migraine | 3 | 1432 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.48, 4.12] |
| 9.2 Episodic migraine | 2 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 3.93 [2.27, 6.79] |
| 9.3 Mixed | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.09, 10.47] |
| 10 Adverse event ‐ injection site pain Show forest plot | 8 | 1332 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.02, 4.33] |
| 10.1 Chronic migraine | 3 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.81, 8.15] |
| 10.2 Episodic migraine | 3 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 3.23 [1.14, 9.13] |
| 10.3 Mixed | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.58] |
| 11 Total treatment related adverse events Show forest plot | 6 | 2893 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.73, 2.75] |
| 11.1 Chronic migraine | 2 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.85, 2.91] |
| 11.2 Episodic migraine | 4 | 1514 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.37, 3.08] |
| 12 Withdrawals due to adverse events in trials with multiple rounds of treatment. Show forest plot | 4 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 3.28 [1.52, 7.07] |
| 12.1 Chronic migraine | 2 | 1384 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [1.38, 9.98] |
| 12.2 Episodic migraine | 2 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [0.81, 9.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Migraine impact and disability assessment scores Show forest plot | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 4.27 [‐28.15, 36.69] |
| 1.1 Chronic migraine | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 22.8 [‐2.56, 48.16] |
| 1.2 Mixed | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐10.50 [‐23.23, 2.23] |
| 2 Total adverse events Show forest plot | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.88] |
| 2.1 Chronic migraine | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.14] |
| 2.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 1.00] |
| 3 Total treatment related adverse events Show forest plot | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
| 3.1 Chronic migraine | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.08] |
| 3.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.09] |
| 4 Withdrawals due to adverse events in trials with multiple rounds of treatment. Show forest plot | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.79] |
| 4.1 Chronic migraine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.11, 1.28] |
| 4.2 Mixed | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total adverse events Show forest plot | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.47, 5.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of migraine days Show forest plot | 2 | 353 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| 1.1 Episodic migraine Botox ≥ 50 U vs Botox < 50 | 2 | 353 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.19, 0.99] |
| 2 Adverse event ‐ muscle weakness Show forest plot | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.39] |
| 2.1 Botox ≥ 200 U versus Botox < 200 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.47] |
| 2.2 Botox ≥ 150 U versus Botox < 150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.78, 1.64] |
| 3 Adverse event ‐ blepharoptosis Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 ≥ 50 U versus < 50 U in frontalis and/or corrugator | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.20, 4.43] |
| 3.2 ≥ 30 U versus < 30 U in frontalis and/or corrugator | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [0.58, 9.65] |
| 3.3 ≥ 10 U versus < 10 U in frontalis and/or corrugator | 2 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.99, 5.94] |
| 4 Adverse event ‐ neck pain Show forest plot | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.91, 1.65] |
| 4.1 Botox ≥ 200 U versus Botox < 200 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.83, 1.89] |
| 4.2 Botox ≥ 150 U versus Botox < 150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.86] |
| 5 Adverse event ‐ injection site pain Show forest plot | 2 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.52, 2.51] |
| 5.1 Botox ≥ 200 U versus Botox < 200 U | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.13, 11.97] |
| 5.2 Botox ≥150 U versus Botox <150 U | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.48, 4.41] |
| 5.3 Botox ≥ 50 U versus Botox < 50 U | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.34, 4.12] |

