Sustavni antibiotici u terapiji malignih rana
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, cross‐over trial that explored the use of metronidazole for controlling the smell from fungating wounds in patients with breast cancer, using a placebo for comparison. Care setting and location: not clearly stated; however, the authors worked in hospitals in London, UK. | |
| Participants | People with breast cancer, and a fungating wound which had a troublesome smell and was unlikely to respond to irradiation or chemotherapy. Six of the nine who were recruited to the trial participated and completed both arms of the study. Mean age and sex were not reported. | |
| Interventions | Intervention: metronidazole 200 milligram tablets given orally three times daily (TDS) for 14 days; and placebo tablets TDS given orally for 14 days. Participants acting as their own controls. | |
| Outcomes | Smell scores and anaerobe culture results were reported. Smell was graded 0 to 3 with 0 indicating the smell was 'absent', 1 being 'not offensive', 2 being 'offensive but tolerable' and 3 being 'offensive and intolerable'. Anaerobe culture results were reported as '‐', '+', '++' and '+++'. | |
| Notes | Funding source was not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that "a randomised, double‐blind, cross‐over design" was used (p1232) but detail on how the random sequence was generated was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No detail about sequence generation or allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was described as double blind whereby both participants and personnel were blind to the intervention and control through the use of "identical tablets". |
| Blinding of outcome assessment (detection bias) | Low risk | It was stated that the participant, nurse and doctor independently assessed smell at each visit; "double‐blind" implied that participant and personnel were unaware of which treatment is being dispensed at any particular time. |
| Incomplete outcome data (attrition bias) | High risk | Original sample size was 9 but 3 participants were withdrawn; results are presented for 6 (2/3rds of the original sample). This is a small sample size that is likely to have impacted on the results. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration was not available, and because the outcomes of interest were not clearly specified, only inferred, we assessed the risk of bias for selective reporting as unclear. |
| Other bias | Unclear risk | Limited information on participant characteristics (e.g. age) and study setting. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This study was not a trial (RCT or CCT) but was a case report on 4 participants with fungating gynaecological tumours treated with metronidazole. | |
| This study was not a trial (RCT or CCT) but was a systematic review of the use of metronidazole (topical application) for odour control in malignant fungating wounds. | |
| This study was a double‐blind trial of 8 participants who received tinidazole (8 g over 7 days) pre‐ and post‐operatively for prevention of infection in people with oropharyngeal tumours and not for treatment of malignant wounds. | |
| This study was not a trial (RCT or CCT) but was an observational study of 9 participants with fungating breast carcinoma and treated with metronidazole 400 milligrams three times daily. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Malodour (Smell Score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Metronidazole versus Placebo, Outcome 1 Malodour (Smell Score). | ||||
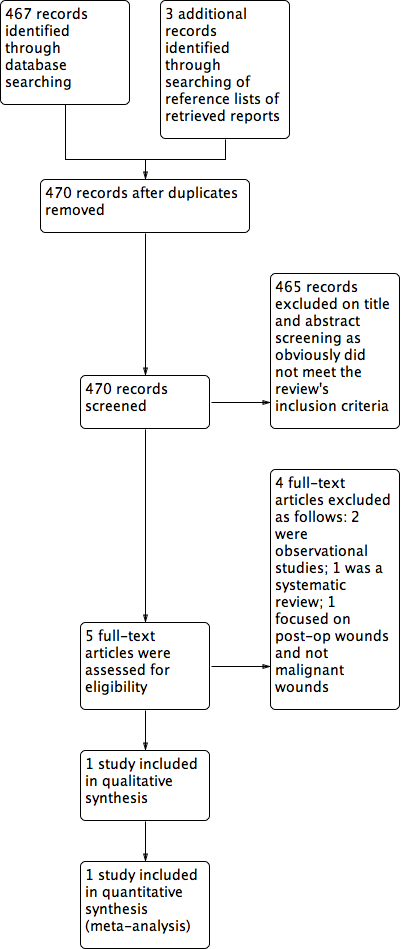
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
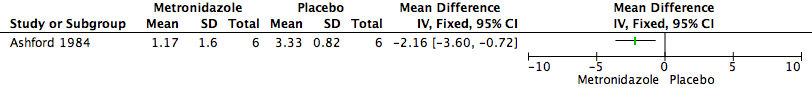
Forest plot of comparison: 1 Metronidazole versus Placebo, outcome: 1.1 Malodour (Smell Score).
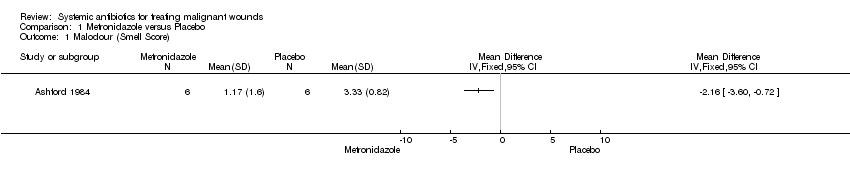
Comparison 1 Metronidazole versus Placebo, Outcome 1 Malodour (Smell Score).
| Metronidazole compared to Placebo for treating malignant wounds | ||||||
| Patient or population: treating malignant wounds | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Placebo | Risk with Metronidazole | |||||
| Malodour (smell score measured on a scale of 0 to 3 with higher scores indicating a more offensive smell) | The mean malodour (smell score) was 3.33 (range 2.0 to 4.0) | MD 2.16 lower | ‐ | 6 | ⊕⊝⊝⊝ | It is uncertain whether metronidazole leads to a reduction in malodour because the quality of the evidence is very low |
| Adverse effects | Study population | not estimable | 6 | NA | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded 3 levels: serious limitation — insufficient details provided to make clear judgements on random sequence generation and allocation concealment. There was also a 33% loss to follow‐up; very serious imprecision; a small sample size of 6 participants. 2 Smell was independently assessed at each visit by the patient, doctor, and nurse, who graded the smell as "absent" (0), "not offensive" (1), "offensive but tolerable" (2), or "offensive and intolerable" (3), and an amalgamated score calculated. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Malodour (Smell Score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

