Tratamiento con hierro para la anemia preoperatoria
Appendices
Appendix 1. Search strategies for 30 July 2018 electronic searches
Cochrane Injuries Group Specialised Register & Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library) (all years to Issue 10, 2018)
#1 MESH DESCRIPTOR iron EXPLODE ALL TREES
#2 MeSH descriptor: [Iron Compounds] explode all trees
#3 iron:TI,AB,KY
#4 (((ferric OR ferrous):TI,AB,KY
#5 MeSH descriptor: [Hematinics] explode all trees
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MESH DESCRIPTOR preoperative period EXPLODE ALL TREES
#8 MESH DESCRIPTOR preoperative care
#9 (((prior OR before) adj3 (surg* OR operat*))):TI,AB,KY
#10 (preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg* or ((pre or peri) next (operat* or procedur* or surgi* or surgu*))):ti,ab,kw
#11 (#7 OR #8 OR #9 OR #10)
#12 (#6 AND #11)
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (all years to 30 July 2018)
1. exp Iron/
2. exp Iron Compounds/
3. iron.ab,ti,kf.
4. (ferric or ferrous) .ab,ti,kf.
5. exp Hematinics/
6. or/1‐5
7. exp Anemia/
8. Iron/df [defciency]
9. (anaemi* or anemi*).ti,ab,kf.
10. exp Blood Transfusion/
11. transfusion.ab.
12. or/7‐11
13. (preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg* or ((pre or peri) adj (operat* or procedur* or surgi* or surgu*))).ti,ab,kf.
14. ((prior or before) adj3 (surg* or operat*)).ab,ti,kf.
15. exp Preoperative Period/
16. Preoperative Care/
17 or/13‐16
18 (6 and 12 and 17)
19. (randomi#ed or randomi#ation).ab,ti.
20. randomized controlled trial.pt.
21. controlled clinical trial.pt.
22. placebo.ab.
23. clinical trials as topic.sh.
24. randomly.ab.
25. trial.ti.
26. Comparative Study/
27. or/19‐26
28. (animals not (humans and animals)).sh.
29. 27 not 28
30. (18 and 29)
Ovid EMBASE 1974 to 30 July 2018
1. Iron/
2. Iron Derivative/
3. iron.ab,ti,kf.
4. (ferric or ferrous).ti,ab,kw.
5. exp antianemic agent/
6. or/1‐5
7. exp Anemia/
8. (anaemi* or anemi*).ti,ab,kw.
9. exp Blood Transfusion/
10. transfusion.ab.
11. or/7‐10
12. (preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg* or ((pre or peri) adj (operat* or procedur* or surgi* or surgu*))).ti,ab,kw.
13. ((prior or before) adj3 (surg* or operat*)).ab,ti,kw.
14. exp Preoperative Period/
15. or/12‐14
16. (randomi#ed or randomi#ation).ab,ti.
17. randomized controlled trial/
18. (RCT or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion* or number* or place* or recruit* or subsitut* or treat*))).ab,kw.
19. placebo/
20. placebo.ab.
21. randomly.mp. or "at random".ab.
22. trial.ti.
23. or/16‐22
24. exp animal/ not (exp human/ and exp animal/)
25. 23 not 24
26. 6 and 11 and 15 and 25
PubMed (to 30 July 2018)
(((((((("Comparative Study"[Publication Type]) OR "Randomized Controlled Trial"[Publication Type]) OR "Controlled Clinical Trial"[Publication Type])) OR (((((((randomized[Title/Abstract]) OR randomised[Title/Abstract]) OR placebo[Title/Abstract]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract]) OR group[Title/Abstract]))) NOT (("Animals"[Mesh]) NOT ("Animals"[Mesh] AND "Humans"[Mesh])))) AND (((((((("preoperative surgery"[Title/Abstract]) OR "before surgery"[Title/Abstract]) OR "before surgical intervention"[Title/Abstract]) OR "before operation"[Title/Abstract])) OR (("Preoperative Period"[Mesh]) OR "Preoperative Care"[Mesh:noexp]))) AND (((((iron[Title/Abstract]) OR Ferrous compound*[Title/Abstract]) OR ferric compound*[Title/Abstract])) OR ((("Iron"[Mesh]) OR "Ferric Compounds"[Mesh]) OR "Ferrous Compounds"[Mesh])))
Web of Science Indexes
SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI (all years to 3 November 2016)
Topic search
#1 (iron or ferric or ferrous)
#2 (preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg*)
#3 (pre‐operat* or peri‐operati* or pre‐procedur* or peri‐procedur* or pre‐surg* or peri‐surg*)
#4 (anemi* or anaemi* or transfus*)
#5 (#1 and (#2 or #3) and #4)
#6 (RCT or random* or placebo)
#7 (((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)))
#8 (trial)
#9 (#6 or #7 or #8)
#10 (#4 and #9)
ClinicalTrials.gov 30 July 2018
Basic Search: IRON AND (PREOPERATIVE OR PERIOPERATIVE OR PERIPROCEDURAL OR PRE‐OPERATIVE OR PERI‐OPERATIVE OR PERI‐PROCEDURAL)
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal 30 July 2018
Basic Search: ANEMIA AND IRON AND PREOPERATIVE OR ANEMIA AND IRON AND PERIOPERATIVE OR ANEMIA AND IRON AND PERIPROCEDURAL OR ANEMIA AND IRON AND PRE‐OPERATIVE OR ANEMIA AND IRON AND PERI‐OPERATIVE OR ANEMIA AND IRON AND PERI‐PROCEDURAL OR ANAEMIA AND IRON AND PREOPERATIVE OR ANAEMIA AND IRON AND PERIOPERATIVE OR ANAEMIA AND IRON AND PERIPROCEDURAL OR ANAEMIA AND IRON AND PRE‐OPERATIVE OR ANAEMIA AND IRON AND PERI‐OPERATIVE OR ANAEMIA AND IRON AND PERI‐PROCEDURAL
Appendix 2. Search strategies for 28 November 2019 electronic searches
Cochrane Injuries Group Specialised Register & Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library) (all years to 28 November 2019)
#1 MeSH descriptor: [Iron] in all MeSH products
#2 MeSH descriptor: [Iron Compounds] explode all trees
#3 iron:TI,AB,KW
#4 (ferric OR ferrous):TI,AB,KW
#5 MeSH descriptor: [Hematinics] in all MeSH products
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MeSH descriptor: [Preoperative Period] explode all trees
#8 MeSH descriptor: [Preoperative Care] explode all trees
#9 ((prior OR before) near/3 (surg* OR operat*)):TI,AB,KW
#10 (preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg* or ((pre or peri) next (operat* or procedur* or surgi* or surgu*))):ti,ab,kw
#11 (#7 OR #8 OR #9 OR #10)
#12 (#6 AND #11)
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (all years to 28 November 2019)
1. exp Iron/
2. exp Iron Compounds/
3. iron.ab,ti,kf.
4. (ferric or ferrous).ab,ti,kf.
5. exp Hematinics/
6. or/1‐5
7. exp Anemia/
8. Iron/df [deficiency]
9. (anaemi* or anemi*).ti,ab,kf.
10. exp Blood Transfusion/
11. transfusion.ab.
12. or/7‐11
13. (preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg* or ((pre or peri) adj (operat* or procedur* or surgi* or surgu*))).ti,ab,kf.
14. ((prior or before) adj3 (surg* or operat*)).ab,ti,kf.
15. exp Preoperative Period/
16. Preoperative Care/
17. or/13‐16
18. 6 and 12 and 17
19. (randomi#ed or randomi#ation).ab,ti.
20. randomized controlled trial.pt.
21. controlled clinical trial
22. placebo.ab.
23. clinical trials as topic.sh.
24. randomly.ab.
25. trial.ti.
26. Comparative Study/
27. or/19‐26
28. (animals not (humans and animals)).sh.
29. 27 not 28
30. 18 and 29
Ovid EMBASE 1974 to 28 November 2019
1. Iron/
2. Iron Derivative/
3. iron.ab,ti,kw.
4. (ferric or ferrous).ti,ab,kw.
5. exp antianemic agent/
6. or/1‐5
7. exp Anemia/
8. (anaemi* or anemi*).ti,ab,kw.
9. exp Blood Transfusion/
10. transfusion.ab.
11. or/7‐10
12.(preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg* or ((pre or peri) adj (operat* or procedur* or surgi* or surgu*))).ti,ab,kw.
13. ((prior or before) adj3 (surg* or operat*)).ab,ti,kw.
14. exp Preoperative Period/
15. or/12‐14
16. (randomi#ed or randomi#ation).ab,ti.
17. randomized controlled trial/
18. (RCT or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion* or number* or place* or recruit* or subsitut* or treat*))).ab,kw.
19. placebo/
20. placebo.ab.
21. randomly.mp. or "at random".ab.
22. trial.ti.
23. or/16‐22
24. exp animal/ not (exp human/ and exp animal/)
25. 23 not 24
26. 6 and 11 and 15 and 25
PubMed (to 28 November 2019)
(((((((("Comparative Study"[Publication Type]) OR "Randomized Controlled Trial"[Publication Type]) OR "Controlled Clinical Trial"[Publication Type])) OR (((((((randomized[Title/Abstract]) OR randomised[Title/Abstract]) OR placebo[Title/Abstract]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract]) OR group[Title/Abstract]))) NOT (("Animals"[Mesh]) NOT ("Animals"[Mesh] AND "Humans"[Mesh])))) AND (((((((("preoperative surgery"[Title/Abstract]) OR "before surgery"[Title/Abstract]) OR "before surgical intervention"[Title/Abstract]) OR "before operation"[Title/Abstract])) OR (("Preoperative Period"[Mesh]) OR "Preoperative Care"[Mesh:noexp]))) AND (((((iron[Title/Abstract]) OR Ferrous compound*[Title/Abstract]) OR ferric compound*[Title/Abstract])) OR ((("Iron"[Mesh]) OR "Ferric Compounds"[Mesh]) OR "Ferrous Compounds"[Mesh])))
Web of Science Indexes
SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI (all years to 28 November 2019)
Topic search
#1 (iron or ferric or ferrous)
#2 (preoperat* or perioperati* or preprocedur* or periprocedur* or presurg* or perisurg*)
#3 (pre‐operat* or peri‐operati* or pre‐procedur* or peri‐procedur* or pre‐surg* or peri‐surg*)
#4 (anemi* or anaemi* or transfus*)
#5 (#3 OR #2)
#6 (#4 AND #1)
#7 #6 and #5
#8 (RCT or random* or placebo)
#9 (((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)))
#10 (trial)
#11 (#10 or #9 or #8)
ClinicalTrials.gov 28 November 2019
Basic search: IRON AND (PREOPERATIVE OR PERIOPERATIVE OR PERIPROCEDURAL OR PRE‐OPERATIVE OR PERI‐OPERATIVE OR PERI‐PROCEDURAL)
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal 28 November 2019
Basic search: ANEMIA AND IRON AND PREOPERATIVE OR ANEMIA AND IRON AND PERIOPERATIVE OR ANEMIA AND IRON AND PERIPROCEDURAL OR ANEMIA AND IRON AND PRE‐OPERATIVE OR ANEMIA AND IRON AND PERI‐OPERATIVE OR ANEMIA AND IRON AND PERI‐PROCEDURAL OR ANAEMIA AND IRON AND PREOPERATIVE OR ANAEMIA AND IRON AND PERIOPERATIVE OR ANAEMIA AND IRON AND PERIPROCEDURAL OR ANAEMIA AND IRON AND PRE‐OPERATIVE OR ANAEMIA AND IRON AND PERI‐OPERATIVE OR ANAEMIA AND IRON AND PERI‐PROCEDURAL

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Six studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram for combined searches to 30 July 2018 (fully incorporated into review)
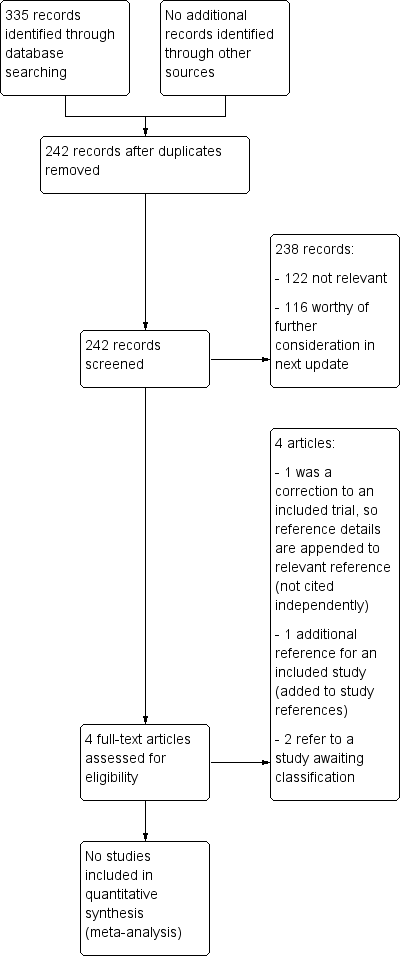
Study flow diagram for 28 November 2019 top‐up searches (not completely incorporated into review)

Comparison 1 Iron therapy versus placebo, no treatment or standard care, Outcome 1 Proportion of participants who received a blood transfusion.

Comparison 1 Iron therapy versus placebo, no treatment or standard care, Outcome 2 Quality of life (SF‐36) 4 weeks postoperatively.
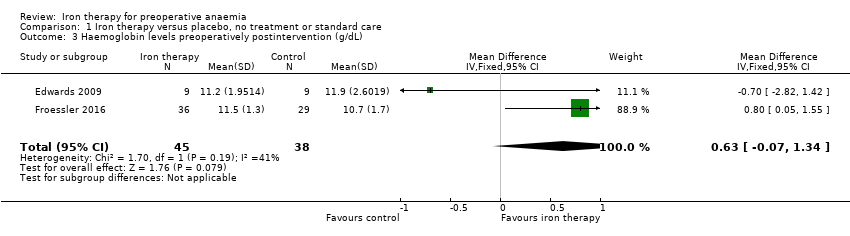
Comparison 1 Iron therapy versus placebo, no treatment or standard care, Outcome 3 Haemoglobin levels preoperatively postintervention (g/dL).

Comparison 1 Iron therapy versus placebo, no treatment or standard care, Outcome 4 Haemoglobin levels postintervention postoperatively (g/dL).

Comparison 1 Iron therapy versus placebo, no treatment or standard care, Outcome 5 Ferritin level post‐treatment (ng/mL).

Comparison 2 Intravenous versus oral iron therapy, Outcome 1 Number of participants who received a blood transfusion.
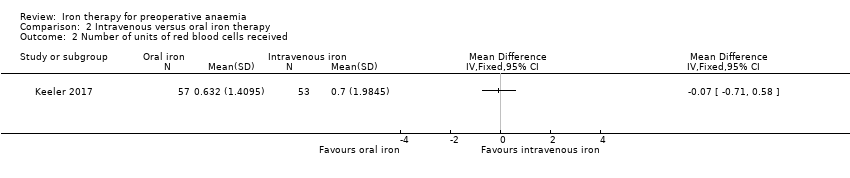
Comparison 2 Intravenous versus oral iron therapy, Outcome 2 Number of units of red blood cells received.
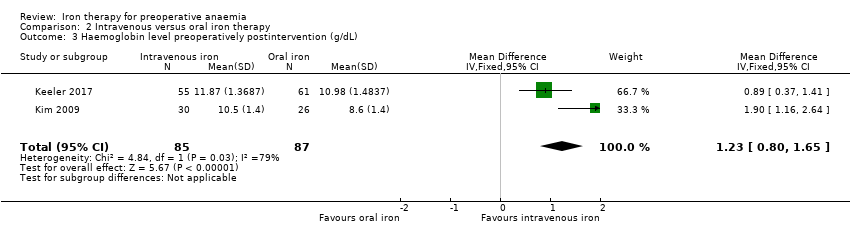
Comparison 2 Intravenous versus oral iron therapy, Outcome 3 Haemoglobin level preoperatively postintervention (g/dL).
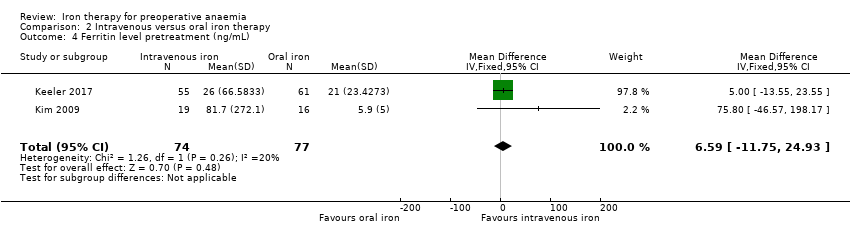
Comparison 2 Intravenous versus oral iron therapy, Outcome 4 Ferritin level pretreatment (ng/mL).

Comparison 2 Intravenous versus oral iron therapy, Outcome 5 Ferritin level preoperatively postintervention (ng/mL).
| Iron therapy compared to placebo, no treatment or standard care for preoperative anaemia | ||||||
| Patient or population: people with preoperative anaemia awaiting major surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo, no treatment or standard care | Iron therapy | |||||
| Proportion of participants who received a blood transfusion | 438 per 1000 | 386 per 1000 | RR 1.21 | 200 | ⊕⊕⊝⊝ | |
| Any validated measure of quality of life (measured by SF36) | 6 ± 17 | 8 ± 18 | ‐ | 72 (1 study) | ⊕⊝⊝⊝ Very lowb | |
| Haemoglobin levels at end of preoperative treatment (g/dL) | The mean haemoglobin level in the control groups was | The mean haemoglobin levels in the intervention groups was | MD 0.63 (‐0.07 to 1.34) | 83 | ⊕⊕⊝⊝ | |
| Haemoglobin levels post‐treatment and surgery (g/dL) | The mean haemoglobin level in the control groups was | The mean haemoglobin levels in the intervention groups was | MD 0.17 (‐0.29 to 0.63) | 86 | ⊕⊕⊝⊝ | |
| Ferritin at the end of preoperative treatment (ng/mL) | The mean ferritin level in the control group was 99 ng/mL | The mean ferritin level in the intervention groups was 149 ng/mL higher (26 higher to 272 higher) | MD 149.00 (25.68 to 272.32) | 76 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded 2 levels for imprecision with only four randomised control trials including subsets of anaemic participants, resulting in a small number of participants. | ||||||
| Intravenous iron therapy compared to oral iron therapy for preoperative anaemia | ||||||
| Patient or population: people with preoperative anaemia awaiting major surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron therapy | Intravenous iron therapy | |||||
| Proportion of participants who received a blood transfusion | No data available | No data available | ‐ | ‐ | ‐ | |
| Any validated measure of quality of life | No data available | No data available | ‐ | ‐ | ‐ | |
| Haemoglobin levels at end of preoperative treatment (g/dL) | The mean haemoglobin level in the oral iron groups was | The mean haemoglobin level in the IV iron groups was | MD 1.23 (0.80 to 1.65) | 172 | ⊕⊕⊝⊝ | |
| Haemoglobin levels post‐treatment and surgery (g/dL) | No data available | No data available | ‐ | ‐ | ‐ | |
| Ferritin preoperatively postintervention (ng/L) | The mean ferritin level in the oral iron groups was 23 ng/mL | The mean ferritin level in the IV iron groups was 395 ng/mL higher (228 higher to 562 higher) | MD 395.03 (227.72 to 562.35) | 151 (2 studies) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded twice overall: 1 level due to risk of bias (attrition bias), as the Kim 2009 study excluded participants with less than 80% compliance with therapy (compliance was lower in the oral group); and 1 level for imprecision as only two studies with a small number of participants contributed to the results. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who received a blood transfusion Show forest plot | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.87, 1.70] |
| 2 Quality of life (SF‐36) 4 weeks postoperatively Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Haemoglobin levels preoperatively postintervention (g/dL) Show forest plot | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.07, 1.34] |
| 4 Haemoglobin levels postintervention postoperatively (g/dL) Show forest plot | 2 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.29, 0.63] |
| 5 Ferritin level post‐treatment (ng/mL) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who received a blood transfusion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of units of red blood cells received Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Haemoglobin level preoperatively postintervention (g/dL) Show forest plot | 2 | 172 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [0.80, 1.65] |
| 4 Ferritin level pretreatment (ng/mL) Show forest plot | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | 6.59 [‐11.75, 24.93] |
| 5 Ferritin level preoperatively postintervention (ng/mL) Show forest plot | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | 395.03 [227.72, 562.35] |

