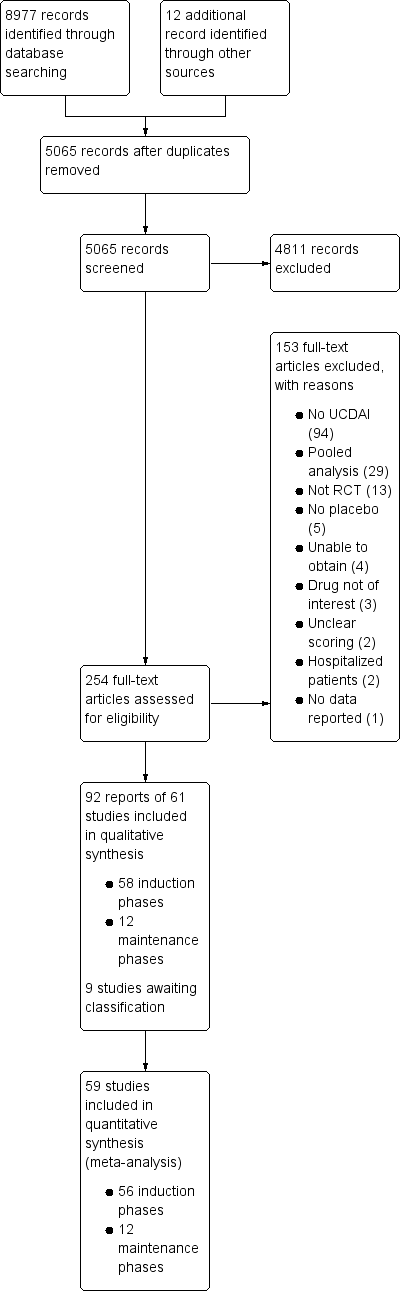
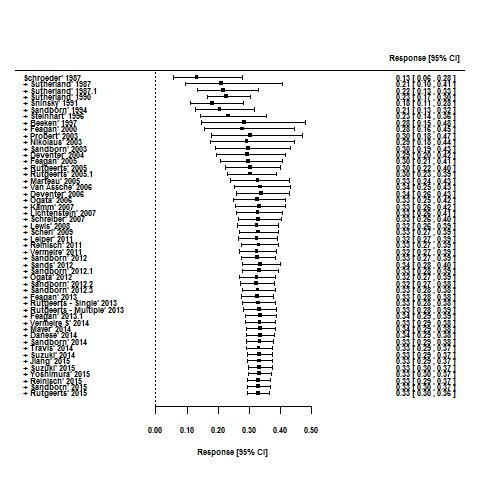
| Trial | Phase | Setting (number of centres) | Comparator | Placebo patients | Mean age | Follow‐up (weeks) | Mean entry UCDAI score | Response definition | Remission definition |
| 1 | Aoyama 2015 | induction (1) | Multicenter, single country (NS) | Budesonide foam | NS | NS | 6 | NS | NS | RBS = 0, endoscopic sub score < 1 and stool frequency sub score = 0 or decrease > 1 |
| 2 | Beeken 1997 | induction (2) | Multicenter, multinational (4) | Aminosalicylate | 13 | 48 | 6 | 7.8 | Mean/median score improvement | NS |
| 3 | Carbonnel 2016 | induction (3) | Multicenter, multinational (26) | Methotrexate | 51 | NS | 24 | NS | NS | Mayo Clinic subscore < 2 with no item >1 |
| 4 | Danese 2014 | induction (4) | Multicenter, multinational (30) | Tralokinumab | 55 | 41 | 24 | 8.3 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point |
| 5 | Deventer 2006 | induction (5) | Multicenter, multinational (30) | Alicaforsen | 22 | 50 | 6 | 6.5 | Decrease in RBS of 0‐1or more from baseline | NS |
| 6 | Deventer 2004 | induction (6) | Multicenter (NS) | Alicaforsen | 8 | | 4 | 7.5 | Percent reduction in DAI | NS |
| 7 | Feagan 2000 | induction (7) | Multicenter, single country (NS) | Vedolizumab | 8 | NS | 4 | 8 | Improvement in Baron ≥ 2 points | Mayo 0; Modified Baron 0 |
| 8 | Feagan 2005 | induction (8) | Multicenter, single country (20) | Vedolizumab | 63 | 38.9 | 6 | 6.7 | Improvement in UCCS ≥ 3 points | UCCS ≤ 1 and a modified Baron ≤ 1 |
| 9 | Feagan 2013a | induction (9) | Multicenter, multinational (26) | Mesalamine | 141 | 40.4 | 10 | NS | UCDAI decrease by ≥3 points | UCDAI, SFS and RBS scores of 0, and no fecal urgency |
| 10 | Feagan 2013b | induction (10) | Multicenter, multinational (211) | Vedolizumab | 149 | 41.2 | 6 | 8.6 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤ 2 points; no individual sub score > 1 point |
| | maintenance (1) | Multicenter, multinational (211) | Vedolizumab | 126 | 40.3 | 52 | 8.4 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤ 2 points; no individual sub score > 1 point |
| 11 | Hanauer 2000 | maintenance (2) | Multicenter (9) | Mesalamine | 34 | 37.3 | 96 | NS | NS | UCDAI score = 0 was the definition of clinical and endoscopic remission Relapse defined as symptoms of rectal bleeding or increase in stool frequency for > 1 wk and endoscopic evidence of inflammation |
| 12 | Jiang 2015 | induction (11) | Single centre | Infliximab | 41 | 34.5 | 8 | NS | Decrease in total MCS > 3 points or > 30% from baseline, with a decrease in RBS > 1 point or an absolute RBS of 0 or 1 | Total Mayo score = 2 points with no individual sub score > 1 point |
| | maintenance (3) | Single centre | Infliximab | 41 | 34.5 | 30 | NS | Decrease in total MCS > 3 points or > 30% from baseline, with a decrease in RBS > 1 point or an absolute RBS of 0 or 1 | Total Mayo score of < 2 points with no individual sub score > 1 point |
| 13 | Kamm 2007 | induction (12) | Multicenter. multinational (49) | MMX mesalamine | 86 | 43.2 | 8 | NS | UCDAI decrease by ≥3 points | UCDAI ≤1+ RBS=0 + SFS=0 ; and ≥1 point reduction in sigmoidoscopy score |
| 14 | Leiper 2011 | induction (13) | Single country (1) | Rituximab | 8 | 50 | 24 | 7.6 | Decrease in Mayo ≥ 3 points | Decrease in Mayo to ≤ 2 |
| 15 | Lewis 2008 | induction (14) | Multicenter, single country (15) | Rosiglitazone | 53 | | 12 | NS | Decrease in Mayo ≥2 points | Mayo score ≤ 2 |
| 16 | Lichtenstein 2007 | induction (15) | Multicenter, multinational (52) | MMX mesalamine | 93 | 42.6 | 8 | NS | UCDAI decrease by ≥3 points | UCDAI ≤1+ RBS=0 + SFS=0 ; and ≥1 point reduction in sigmoidoscopy score |
| 17 | Lichtenstein 2010 | maintenance (4) | Multicenter, multinational (48) | Mesalamine | 96 | 46 | 24 | NS | NS | Relapse free at 6 months |
| 18 | Marteau 2005 | induction (16) | Multicenter, multinational (43) | Mesalazine enema | 56 | NS | 8 | NS | UCDAI decrease by ≥2 points | UCDAI ≤1 |
| 19 | Mayer 2014 | induction (17) | Multicenter, multinational (54) | BMS‐936557 | 54 | 41.8 | 8 | 7.9 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point |
| 20 | Nikolaus 2003 | induction (18) | Multicenter, multinational (6) | rIFN‐β‐1a | 7 | | 6 | NS | Reduction of ≥3 points in the UCSS symptoms score and PGA | All clinical UCSS sub scores equal to 0, with a proctosigmoidoscopy score of 0 or 1 |
| 21 | Ogata 2006 | induction (19) | Multicenter, single country (17) | Tacrolimus | 21 | 30 | 2 | 9.4 | Reduction in DAI of more than 4 points with improvement of all categories | Complete resolution of all symptoms (all assessment scores were zero) |
| 22 | Ogata 2012 | induction (20) | Multicenter, single country (NS) | Tacrolimus | 30 | NS | 2 | 9.1 | Reduction in DAI of more than 4 points with improvement of all categories | Total DAI score 2 with all individual sub scores of 0 or 1 |
| 23 | Oren 1996 | induction (21) | Multicenter, single country (12) | Methotrexate | 37 | 38.9 | 36 | 6.8 | NS | MCS (including the endoscopic sub score) of < 3 with no steroid use, and without a score of < 2 without sigmoidoscopy results |
| | maintenance (5) | Multicenter, single country (12) | Methotrexate | 37 | 38.9 | 36 | 6.8 | NS | Relapse was an increase in the MCS of > 3 (not including sigmoidoscopy) and/or reintroduction of steroids at a dose of > 300 mg/month |
| 25 | Probert 2003 | induction (22) | Multicenter, multinational (4) | Infliximab | 20 | NS | 6 | 8.5 | Decrease in Baron of ≥ 1 | UCCS ≤ 2 AND/OR Baron score = 0 |
| 25 | Reinisch 2011 | induction (23) | Multicenter, multinational (94) | Adalimumab | 130 | NS | 8 | 8.7 | Decrease in Mayo > 3 points and decrease in the RBS >1/absolute RBS of 0 or 1 | Mayo score < 2 with no individual sub score > 1 |
| 26 | Reinisch 2015 | induction (24) | Multicenter, multinational (38) | Anrukinzumab | 21 | 36.6 | 32 | 6.6 | Decrease from baseline of ≥3 points in total Mayo score, with at least a 30% change, accompanied by ≥1 point decrease or absolute score of 0 or 1 in RBS | Defined as proportion of subjects with a total Mayo score ≤ 2, with no individual sub score > 1 |
| 27 | Rubin 2015 | induction (25) | NS | Budesonide MMX® | NS | NS | | | NS | rectal bleeding and stool frequency sub scores = 0 |
| 28 | Rutgeerts 2005a | induction (26) | Multicenter, multinational (62) | Infliximab | 121 | 41.4 | 8 | 8.4 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point |
| | maintenance (6) | | | | | | | | |
| 29 | Rutgeerts 2005b | induction (27) | Multicenter, multinational (55) | Infliximab | 123 | 39.3 | 8 | 8.5 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point |
| | maintenance (7) | | | | | | | | |
| 30 | Rutgeerts 2013a | induction (28) | Multicenter, multinational (15) | Etrolizumab | 5 | 30.2 | 4 | 9 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point |
| 31 | Rutgeerts 2013b | induction (29) | Multicenter, multinational (15) | Etrolizumab | 5 | 39 | 5 | 10 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point |
| 32 | Rutgeerts 2015 | induction (30) | | | | | | | | |
| 33 | Sandborn 1994 | induction (31) | Single centre | Cyclosporin | 20 | | 4 | NS | Reduction of ≥3 points in DAI | UCDAI=0 |
| 34 | Sandborn 2003 | induction (32) | Multicenter, single country (15) | Repifermin | 28 | NS | 6 | | Decrease in Mayo ≥3 points compared with baseline at week 4 | A score of zero on the sigmoidoscopy all sub scores = 0 (SFS, PGA, RBS) |
| 35 | Sandborn 2012a | induction (33) | Multicenter, multinational (103) | Adalimumab | 260 | 41.3 | 8 | 8.9 | Decrease in Mayo ≥ 3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual sub score >1 point |
| | maintenance (8) | | | | | | | | |
| 36 | Sandborn 2012b | induction (34) | Multicenter, multinational (108) | Budesonide MMX | 128 | | 8 | NS | ≥3‐point decrease in UCDAI, and ≥1‐point reduction in the endoscopy sub score | UCDAI ≤1+ RBS=0 + SFS=0; no mucosal on colonoscopy ; and ≥1 point reduction in sigmoidoscopy score |
| 37 | Sandborn 2012c | induction (35) | Multicenter, multinational (51) | Tofacitinib | 48 | 42.5 | 8 | 8.2 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | MCS = 2 with no individual sub score> 1 |
| 38 | Sandborn 2012d | induction (36) | Multicenter, multinational (142) | Abatacept | 140 | 40.9 | 12 | 8.8 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | MCS = 2 with no individual sub score> 1 |
| | maintenance (9) | Multicenter, multinational (142) | Abatacept | 66 | NS | 52 | NS | NS | NS |
| 39 | Sandborn 2013a (BUCF3001) | induction (37) | Multicenter, multinational (NS) | Budesonide Foam | NS | NS | | 7.9 | NS | Endoscopy score ≤ 1, RBS = 0 and improvement or no change from baseline in stool frequency subscales of MMDAI** |
| 40 | Sandborn 2013b (BUCF3002) | induction (38) | Multicenter, multinational (NS) | Budesonide Foam | NS | NS | NS | 8 | NS | Endoscopy score ≤ 1, RBS = 0 and improvement or no change from baseline in stool frequency subscales of MMDAI |
| 41 | Sandborn 2014a | maintenance (10) | Multicenter, multinational (217) | Golimumab | 331 | 39 | 8 | 8.3 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point |
| 42 | Sandborn 2014b | induction (39) | Multicenter, multinational (251) | Golimumab | 156 | 40.2 | 54 | 8.3 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point |
| 43 | Sandborn 2015 | induction (40) | Multicenter, multinational (75) | Eldelumab | 83 | 42.7 | 11 | 8.6 | Mayo score < 2 points
with no individual subscore > 1 point | Reduction
from baseline ≥ 3 points and ≥ 30% in Mayo score, reduction
≥ 1 in RBS, or absolute RBS
≤ 1 |
| 44 | Sands 2012 | induction (41) | Multicenter, multinational (46) | Basiliximab | 51 | 38 | 8 | NS | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point |
| 45 | Scherl 2009 | induction (42) | Multicenter, single country (55) | Balsalazide | 83 | 45.4 | 8 | 8 | ≥3 point improvement in modified Mayo, ≥1 point improvement in RBS | 0 for RBS and combined score of ≤2 for SFS and PGA using the Modified Mayo subscales |
| 46 | Schreiber 2007 | induction (43) | Multicenter, single country (35) | Tetomilast | 62 | 45.5 | 8 | 7.5 | Reduction of ≥3 points in DAI | UCDAI ≤1 |
| 47 | Schroeder 1987 | induction (44) | Single center | Mesalamine | 38 | 42.7 | 6 | NS | 'substantial' improvement in scores | Complete resolution of symptoms (total score 0) |
| 48 | Sninsky 1991 | induction (45) | Multicenter, single country (9) | Mesalamine | 52 | 39.2 | 6 | NS | Reduction in the PGA score and in at least one other component score | Complete resolution of all symptoms with all assessment scores 0 |
| 49 | Steinhart 1996 | induction (46) | Multicenter, single country (2) | Butyrate | 19 | 38.6 | 6 | 7.8 | Reduction of ≥2 points in UCDAI | UCDAI ≤1 |
| 50 | Sutherland 1987a | induction (47) | Multicenter, multinational (8) | Aminosalicylate | 77 | 36 | 6 | NS | PGA, % drop in DAI from baseline (total and subscores) | NS§ |
| 51 | Sutherland 1987b | induction (48) | Multicenter, single country (2) | Aminosalicylate | 30 | 36 | 6 | NS | PGA, mean DAI | NS |
| 52 | Sutherland 1990 | induction (49) | Multicenter, multinational (7) | Aminosalicylate | 44 | 37.8 | 6 | 8.2 | PGA, mean DAI | NS |
| 53 | Suzuki 2014 | maintenance (11) | Multicenter, single country (65) | Adalimumab | 96 | 41.3 | 52 | 8.5 | Decrease of > 3 points and > 30 % from
baseline plus a decrease in the RBS > 1 or an absolute score of < 1 | Full Mayo
score < 2 with no individual subscore > 1 |
| 54 | Suzuki 2015 | induction (51) | Multicenter, single country (NS) | Infliximab | 104 | NS | 8 | NS | NS | NS |
| | maintenance (12) | Multicenter, single country (NS) | Infliximab | 104 | NS | 30 | NS | NS | NS |
| 55 | Travis 2014 | induction (52) | Multicenter, multinational (69) | Budesonide MMX | 128 | 39.9 | 8 | 6.2 | ≥3‐point decrease in UCDAI, and ≥1‐point reduction in the endoscopy subscore | UCDAI ≤1+ RBS=0 + SFS=0; no mucosal on colonoscopy; and ≥1 point reduction in sigmoidoscopy score |
| 56 | Van Assche 2006 | induction (53) | Multicenter, multinational (40) | Daclizumab | 56 | 40.7 | 20 | 8 | Decrease in Mayo ≥ 3 points | Mayo 0 for endoscopy and RBS; Mayo 0/1 for SFS† and PGA‡ |
| 57 | Vermeire 2011 | induction (54) | Multicenter, multinational (17) | PF‐00547,659 | 20 | 47.9 | 4 | 7.5 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point |
| 58 | Vermeire 2014 | induction (55) | Multicenter. Multinational (40) | Etrolizumab | 43 | 37.5 | 10 | 9.1 | Decrease in Mayo ≥3 points and ≥30%; plus decrease in RBS of ≥1 point or absolute RBS 0 /1 | Mayo score ≤2 points; no individual subscore >1 point |
| 59 | Watanabe 2013 | induction (56) | Multicenter, single country (45) | Aminosalicylate | 64 | 41.3 | 4 | 5.5 | NS | Rectal mucosal score of 0 or 1 |
| 60 | Williams 1987 | induction (57) | Multicenter, single country (2) | NS | 13 | 42.7 | 6 | 7.4 | NS | DAI score of 0 |
| 61 | Yoshimura 2015 | induction (58) | Multicenter, single country (42) | AJM300 | 51 | 42.6 | 8 | 7.7 | Decrease in MCS of at least 3 points and
a decrease of at least 30% from the baseline score, with a
decrease of at least 1 point on the RBS or
an absolute RBS of 0 or 1 | MCS of 2 or lower and no subscore higher than 1 |