Maßnahmen zur Vorbeugung oraler Mukositis bei Krebspatienten in Behandlung: orale Kryotherapie
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: parallel (2 arms) Location: Iran Number of centres: 1 Study duration: April to September 2013 | |
| Participants | Inclusion criteria: full consciousness; suffering Hodgkin or non‐Hodgkin lymphoma or multiple myeloma; good oral health; isolation in a separate room; undergoing similar basic chemotherapy; undergoing first course of chemotherapy; undergoing autologous BMT Exclusion criteria: patient dissatisfaction; loss of consciousness; susceptible to other diseases that could potentially disrupt treatment; use of analgesics continuously prior to start of study; receiving combined therapies such as radiotherapy; fever; neutropenia; mucositis prior to the treatment; respiratory diseases; oral infections; systemic diseases affecting oral health (especially periodontal tissues); more than 2 weeks interval between chemotherapy and transplantation; changes in treatment protocol during the study Cancer type: haematological (Hodgkin: Gp A: 31%; Gp B: 46%; non‐Hodgkin: Gp A: 13%; Gp B: 23%; multiple myeloma: Gp A: 56%; Gp B: 31%) Cancer treatment: melphalan for Hodgkin and non‐Hodgkin lymphoma; melphalan, cytarabine, etoposide, and lomustine for multiple myeloma ("There were no differences in terms of...treatment regimen") Any other potentially important prognostic factors: "There were no differences in terms of...educational status"; smokers: Gp A: 13%; Gp B: 31% Age at baseline (years): Gp A: 43 (range 19 to 66); Gp B: 39.8 (range 21 to 62) Gender: Gp A: 56% male; Gp B: 62% male Number randomised: 33 (Gp A: 17; Gp B: 16) Number evaluated: 29 (Gp A: 16; Gp B: 13) | |
| Interventions | Comparison: cryotherapy versus normal saline Gp A: prior to BMT, ice cubes held in mouth 5 min before start of chemotherapy, held for 30‐min periods with maximum 20‐min breaks between each period, until 5 min after completion of chemotherapy Gp B: prior to BMT, 30 to 50 cc of saline mouthwash used 30 min before start of chemotherapy, then again every half‐hour, until 6 hours after completion of chemotherapy Compliance: not reported Duration of treatment (intended): not reported but probably variable depending on chemotherapy regimen | |
| Outcomes |
| |
| Notes | Sample size calculation: based on detection of MD of 0.51, with 80% power at 5% significance level, and accounting for 40% attrition (14 per group required) Adverse effects: not reported Funding: "financial support of Tabriz University of Medical Sciences" Declarations/conflicts of interest: "Authors declare no conflict of interest in this study" Other information of note: the information on this study is obtained from a pre‐publication copy of the study report provided to us by the authors, and also from correspondence with the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
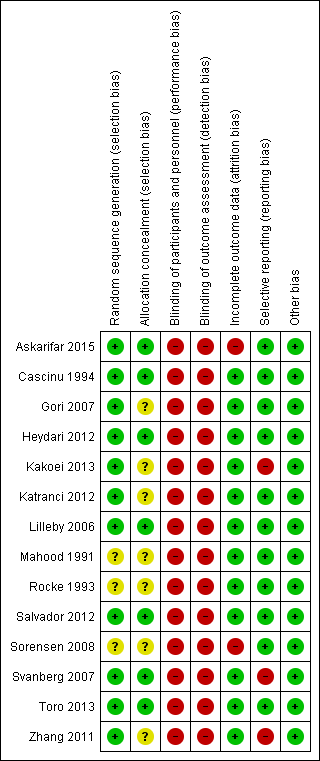
| Random sequence generation (selection bias) | Low risk | Quote: "The patients randomly were allocated into control and intervention groups using size‐2 random blocks and based on a 1:1 allocation ratio random numbers were generated by "Random software Allocation" software" Comment: computer generated randomisation so probably done adequately |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients randomly were allocated into control and intervention groups using size‐2 random blocks and based on a 1:1 allocation ratio random numbers were generated by "Random software Allocation" software" Comment: appears to be third‐party randomisation which should have ensured that the allocation sequence was not manipulated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | High risk | 12% of randomised participants were not included in the analyses (Gp A: 6%; Gp B: 19%). All drop‐outs were due to fever but this could be a risk of bias if the fever was linked to oral mucositis |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: Pesaro, Italy Number of centres: 1 Study duration: not reported | |
| Participants | Inclusion criteria: first ever course of chemotherapy Exclusion criteria: not reported Cancer type: Gp A: 98% gastrointestinal, 2% prostrate; Gp B: 98% gastrointestinal, 2% prostrate Cancer treatment: 5FU, different dosages and co‐treatments (LV, IFN, VP16) equally distributed between groups due to stratification Any other potentially important prognostic factors: performance status (EOCG): Gp A: 0 = 50%, 1 = 32%, 2 = 18%; Gp B: 0 = 50%, 1 = 35%, 2 = 15%; denture wearers equally distributed between groups due to stratification Age at baseline (years): Gp A: median 60 (range 38 to 73); Gp B: median 58 (range 44 to 72) Gender: Gp A: 68% male; Gp B: 70% male Number randomised: 84 (Gp A: 44; Gp B: 40) Number evaluated: 84 (Gp A: 44; Gp B: 40) | |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: ice chips placed in mouth 5 min before 5FU and continuously swished around, then replenished before the previous ice had completely melted, for total 30 min Gp B: 5FU only Compliance: all Gp A participants received cryotherapy in the first cycle but 2 participants "noted an 'ice cream' headache which caused them to refuse this technique after the second and third cycle of chemotherapy, respectively" Duration of treatment (intended): not reported (variable and dependent on number of cycles of cancer treatment) | |
| Outcomes |
| |
| Notes | Sample size calculation: not reported Adverse effects: 2 participants in the cryotherapy group "noted an 'ice cream' headache" Funding: not reported Declarations/conflicts of interest: not reported Other information of note: mean oral mucositis score reported by smoking status for each group for the first cycle only (smokers had higher mean oral mucositis score than non‐smokers in both groups) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised to a control arm or to receive cryotherapy" Comment: adequate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised to a control arm or to receive cryotherapy" Comment: third‐party randomisation and use of sealed envelopes should have ensured that the allocation sequence was not manipulated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately. Although only the oral mucositis outcome was mentioned in the methods, this is more likely to be related to reporting quality rather than bias as the study pre‐dates the CONSORT statement (CONSORT 2010) |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: various locations in Italy Number of centres: "multicentre" but unclear how many centres; co‐ordinated by the Institute of Hematology and Medical Oncology, University of Bologna Study duration: October 2004 to January 2006 | |
| Participants | Inclusion criteria: undergoing allogeneic HSCT and MTX‐containing GVHD prophylaxis; minimum age 8 years Exclusion criteria: clinical evidence of oral mucositis; participants not receiving at least 3 administrations of MTX following HSCT Cancer type: haematological (types were equally distributed between groups) Cancer treatment: pre‐transplant radio/chemotherapy generally comparable between groups; total body irradiation: Gp A: 30.6%; Gp B: 28.3% Any other potentially important prognostic factors: stem cell donor related: Gp A: 45.1%; Gp B: 58.3%; stem cell source: Gp A: marrow = 33.9%, peripheral blood = 66.1%; Gp B: marrow = 28.3%, peripheral blood = 71.7%; folinic acid rescue: Gp A: 43.5%; Gp B: 38.3% Age at baseline (years): Gp A: median 35.5 (range 9 to 59); Gp B: median 40 (range 8 to 66) Gender: Gp A: 51.6% male; Gp B: 50% male Number randomised: 130 (not reported by group) Number evaluated: 122 (Gp A: 62; Gp B: 60) | |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: after allogeneic HSCT, ice chips (mineral water) or popsicles placed in mouth for minimum 60 min starting from the time of low‐dose MTX administration (20 mg/m2 on day +1, 15 mg/m2 on days +3, +6 and +11) as an IV infusion lasting 5 min (± 2), and replenished when melted Gp B: after allogeneic HSCT, MTX as above Compliance: "Six patients enrolled in the cryotherapy arm did not actually complete cryotherapy as planned because of refusal or poor tolerance. However, the exclusion of these patients did not change the results" Duration of treatment (intended): 4 occasions (minimum of 60 min) on 4 separate days (days 1, 3, 6 and 11) | |
| Outcomes |
| |
| Notes | Sample size calculation: based on previous study, 90% power at 5% significance (unclear whether required sample size was achieved) Adverse effects: not reported, only refers to the 6 participants who did not complete cryotherapy due to "refusal or poor tolerance" Funding: "We thank the Italian HSCT Nurses Group (GITMO) for sponsoring the study" Declarations/conflicts of interest: not reported Data handling by review authors: data is maximum oral mucositis score across all cycles of MTX Other information of note: univariate and multivariate analyses showed severe (grade 3 to 4) oral mucositis was significantly associated with total body irradiation and lack of folinic acid rescue | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After giving their informed consent, patients were included in a preformed randomization list that was updated by the coordinating center. Randomization was performed at the ratio of 1 patient per arm with no further stratifications" Comment: adequate method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After giving their informed consent, patients were included in a preformed randomization list that was updated by the coordinating center. Randomization was performed at the ratio of 1 patient per arm with no further stratifications" Comment: co‐ordinating centre mentioned, but unclear whether or not they allocated participants remotely from this centre (central randomisation by a third party) |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | 6% of the participants were not included in the analyses but this attrition was not reported by group. However, the amount of attrition was low and reasons are fully reported |
| Selective reporting (reporting bias) | Low risk | Data for the primary outcome of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: Mashhad, Iran Number of centres: 2 Study duration: March 2007 to April 2008 | |
| Participants | Inclusion criteria: able to undergo one of the chemotherapy regimens described in the study at a standard dose; normal laboratory levels (including complete blood counts); normal kidney and hepatic function; participant or carer able to read and write Exclusion criteria: previous chemotherapy; not undergoing one of the combined courses of chemotherapy described in the study; treated with head and neck radiotherapy; diabetic Cancer type: Gp A: 55% colorectal, 45% breast; Gp B: 45% colorectal, 55% breast Cancer treatment:
Any other potentially important prognostic factors: no statistically significant differences between groups in the following factors: tooth status, smoking status, mouthwash use, brushing habit, BMI, educational status Age at baseline (years): Gp A: mean 59.5 (SD 12.35); Gp B: mean 63.25 (SD 15.06) Gender: 40% male overall and reports that there were no statistically significant differences between groups Number randomised: 80 (Gp A: 40; Gp B: 40) Number evaluated: 80 (Gp A: 40; Gp B: 40) | |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: ice chips placed in mouth 5 min before chemotherapy until 5 min after and continuously swished around, then replenished before the previous ice had completely melted Gp B: chemotherapy only Compliance: well tolerated, no discontinuation of therapy, and most participants kept their mouths constantly cool for most of the chemotherapy session Duration of treatment (intended): mean duration of cryotherapy was 20 to 45 min for a session; those receiving MAYO regimen (see above) had cryotherapy for each of the 5 days of treatment, whilst those receiving CAF/CMF regimen (see above) had cryotherapy on the single day of treatment | |
| Outcomes |
Obtained from correspondence:
| |
| Notes | Sample size calculation: not reported Adverse effects: 8 (20%) of participants in the cryotherapy group complained of chills Funding: "This work was supported by the department of research, Mashhad University of Medical Science" Declarations/conflicts of interest: not reported Other information of note: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by the use of a random numbers table" Comment: adequate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by the use of a random numbers table" Comment: third‐party randomisation should have ensured that the allocation sequence was not manipulated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: Kerman, Iran Number of centres: 1 Study duration: not reported | |
| Participants | Inclusion criteria: partial or complete exposure of head and neck to radiation; minimum radiation dose of 2500 to 3000 cGy (trial registry says Gy) per session; beginning radiotherapy at the start of the study and continuing constantly for the following 2 weeks Exclusion criteria: existing oral mucositis; systemic disease or medication affecting oral condition; less than 15 or more than 55 years of age Cancer type: head and neck (not reported by group) Cancer treatment: radiotherapy to the head and neck Any other potentially important prognostic factors: no statistically significant difference between groups in smoking status or education level Age at baseline (years): Gp A: mean 42.9 (SD 14.9); Gp B: mean 49.1 (SD 15.4) Gender: 57.5% male overall and reports that there were no statistically significant differences between groups Number randomised: 40 (Gp A: 20; Gp B: 20) Number evaluated: 40 (Gp A: 20; Gp B: 20) | |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: ice cubes placed in mouth and sucked for 5 min before radiotherapy and for a further 5 min after the session Gp B: standard oral care Compliance: only states "no lapse during the study" Duration of treatment (intended): 10 min per day for 2 weeks | |
| Outcomes |
| |
| Notes | Sample size calculation: 80% power at 5% significance level to detect a 40% difference in treatment effect (as there were no drop‐outs, it is assumed that this was achieved) Adverse effects: not reported but presumably none were expected due to the 5‐min periods of cryotherapy Funding: "This study was financially supported by the Office of Vice Chancellor for Research of Kerman University of Medical Sciences" Declarations/conflicts of interest: not reported Other information of note: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were divided into experimental and control groups using block randomization technique with the formula of AABB, ABAB, ABBA, BBAA, BABA, and BAAB" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The participants were divided into experimental and control groups using block randomization technique with the formula of AABB, ABAB, ABBA, BBAA, BABA, and BAAB" |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | High risk | Oral pain not mentioned in the trial registry record and not described in the methods of the published trial report. It is possible that this was reported because it showed favourable results for cryotherapy |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: Gaziantep, Turkey Number of centres: 1 Study duration: not reported | |
| Participants | Inclusion criteria: due to receive first course of chemotherapy; healthy oral mucosa; no dental problems Exclusion criteria: receiving more than 1 combination chemotherapy course or antineoplastic drug treatment with half‐life of 30 min or more; discomfort in the mouth; head‐neck cancer Cancer type: gastric 33.3%; colon 33.3%; rectal 16.9%; pancreatic 9.9%; unknown 6.6% (equal numbers per group); stage of disease equally distributed between groups Cancer treatment: 5FU and LV Any other potentially important prognostic factors: education level, denture wearers, toothbrushing habits, smoking status, nutrition, dry mouth, lack of appetite and systemic disease all equally distributed between groups Age at baseline (years): not reported Gender: 50% male in both groups Number randomised: 60 (Gp A: 30; Gp B: 30) Number evaluated: 60 (Gp A: 30; Gp B: 30) | |
| Interventions | Comparison: cryotherapy versus routine care Gp A: ice chips placed in mouth 5 min before 5FU + LV, during treatment and within 15 min after treatment, for total 30 min; continuously swished around, then replenished before the previous ice had completely melted; whole procedure repeated for 5 consecutive days Gp B: routine care Compliance: "Oral cryotherapy was tolerated well by the patients. The majority of the patients reported that they managed to keep the oral cavity constantly cooled most of the time that the chemotherapy treatment was administered. Patients who experienced discomfort during the cryotherapy application continued their treatment after a maximum 30‐60 s break" Duration of treatment (intended): 30 min per day for 5 days (first cycle only) | |
| Outcomes |
| |
| Notes | Sample size calculation: not reported Adverse effects: "The patients completed the procedure quite comfortably, without any problems during the application" Funding: not reported Declarations/conflicts of interest: "None declared" Other information of note: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by MedCalc software to give equal chance to assign each intervention group" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation was performed by MedCalc software to give equal chance to assign each intervention group" |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: Seattle, USA Number of centres: 1 Study duration: August 2003 to June 2005 | |
| Participants | Inclusion criteria: minimum age 18 years with multiple myeloma; scheduled to receive single‐agent high‐dose melphalan (200 mg/m2) followed by autologous PBSCT 2 days later Exclusion criteria: previous autologous PBSCT Cancer type: haematological (multiple myeloma) Cancer treatment: high‐dose melphalan (200 mg/m2) followed by autologous PBSCT Any other potentially important prognostic factors: not reported Age at baseline (years): Gp A: median 59 (range 51 to 71); Gp B: median 57 (range 33 to 72) Gender: Gp A: 76.2% male; Gp B: 63.2% male Number randomised: 41 (Gp A: 21; Gp B: 20) Number evaluated: 40 (Gp A: 21; Gp B: 19) (above figures for age and gender are for evaluated participants) | |
| Interventions | Comparison: cryotherapy versus saline rinse Gp A: 2 days before stem cell infusion, 1 ounce of ice chips held in mouth 30 min prior to beginning single‐agent high‐dose melphalan (200 mg/m2) infusion, replenished when melted, procedure continued for 6 hours after the end of the 30‐min melphalan infusion Gp B: 2 days before stem cell infusion, 1 ounce of room temperature normal saline swished around the mouth and spat out 30 min prior to beginning single‐agent high‐dose melphalan (200 mg/m2) infusion, procedure repeated every 30 min for 6 hours after the end of the 30‐min melphalan infusion Compliance: 14 participants had at least 5 hours of cryotherapy, and 2 had at least 2 hours, whilst 5 did not report the duration. Some participants stopped using the ice chips due to their coldness. Average frequency of use: cryotherapy: 1 cup/hour; saline: 1 to 4 rinses/hour Duration of treatment (intended): 7 hours | |
| Outcomes |
Patient‐reported events:
| |
| Notes | Sample size calculation: required sample size was achieved ("sample size of 40 was chosen to provide 91% power to observe a statistically significant difference (at the 2‐sided significance level of 0.05) in the probability of grades 3–4 mucositis under the assumption that the true probabilities of severe mucositis are 0.25 for patients receiving ice chips and 0.75 for patients receiving normal saline") Adverse effects: not reported, only refers to some participants that stopped using the ice chips due to their coldness Funding: "This work was supported by Friends of Jose Carreras International Leukemia Foundation Presidential Award, NCI P01 CA‐18029" Declarations/conflicts of interest: not reported Data handling by review authors: for patient‐reported oral pain, we used the mean, number of participants and P value to calculate a single SD to be used for both groups (we used the overall mean pain scores rather than the number of days of pain or the mean of the highest value); only medians and ranges presented for the outcomes days of TPN, IV narcotics and hospitalisation, so we present the results, as reported in the study report, in an additional table Other information of note: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients...were randomized to receive either ice chips or room temperature normal saline rinses" Comment: adequate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "patients...were randomized to receive either ice chips or room temperature normal saline rinses" Comment: central randomisation should have ensured that the allocation sequence was not manipulated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 randomised participant, from the control group, was not included in the analyses. The participant withdrew consent because he wanted to use ice chips |
| Selective reporting (reporting bias) | Low risk | Although the data for some outcomes of this review were not presented in a way amenable to meta‐analysis, this is unlikely to be due to bias |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: cross‐over (2 arms) Location: USA Number of centres: unclear (multicentre) Study duration: not reported | |
| Participants | Inclusion criteria: first ever course of chemotherapy Exclusion criteria: not reported Cancer type: not reported but must be solid due to chemotherapy regimen Cancer treatment: 5FU and LV Any other potentially important prognostic factors: not reported Age at baseline (years): not reported Gender: not reported Number randomised: 95 (Gp A: 50; Gp B: 45) Number evaluated: 93 (Gp A: 50; Gp B: 43) | |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: ice chips placed in mouth 5 min before receiving 5FU (425 mg/m2) and LV (20 mg/m2) by IV over a few minutes, and continuously swished around, then replenished before the previous ice had completely melted, for total 30 min, whole procedure repeated for 5 consecutive days Gp B: 5FU (425 mg/m2) and LV (20 mg/m2) only for 5 consecutive days Compliance: not clearly reported. Only states "well tolerated by most patients" Duration of treatment (intended): 30 min per day for 5 days (first cycle only) | |
| Outcomes |
| |
| Notes | Sample size calculation: not reported Adverse effects: "A few patients noted mild, temporary mouth numbness or an "ice cream" headache which rapidly resolved after cessation of cryotherapy. Also, some patients ascribed nausea to the oral ice chips (the nausea may have actually been from the 5FU)" Funding: "supported in part by Public Health Service grants...and Community Clinical Oncology Program grants" Declarations/conflicts of interest: not reported Data handling by review authors: we only used the data from the first treatment cycle (rather than cross‐over data); physician judgement of oral mucositis was preferred over participant judgement as we deemed that this may be more objective and less biased Other information of note: mean oral mucositis score reported by smoking status for each group for the first cycle only (smokers had statistically significantly lower mean oral mucositis score than non‐smokers, but data not available for all participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Prior to therapy, patients were stratified by age and whether or not they had dentures. They were then randomized to a control arm or to receive cryotherapy" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Prior to therapy, patients were stratified by age and whether or not they had dentures. They were then randomized to a control arm or to receive cryotherapy" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | Only 2 randomised participants, both from the control group, were not included in the physician‐judged oral mucositis analyses. We do not believe that this could pose a risk of bias significant enough to have led to a distortion of the true intervention effect |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: cross‐over (2 arms) Location: USA Number of centres: unclear (multicentre) Study duration: not reported | |
| Participants | Inclusion criteria: first course of chemotherapy Exclusion criteria: not reported Cancer type: not reported but must be solid due to chemotherapy regimen Cancer treatment: 5FU and LV, different dosages taken orally or by IV, equally distributed between groups due to stratification Any other potentially important prognostic factors: participants were stratified by smoking status, dentures and institution/centre; gum condition not used for stratification but Gp B (60 min cryotherapy) had worse gums at baseline (P = 0.02) Age at baseline (years): Gp A: median 65 (range 24 to 79); Gp B: median 65 (range 25 to 85) Gender: Gp A: 55% male; Gp B: 51% male Number randomised: 179 (Gp A: 90; Gp B: 89) Number evaluated: 178 (Gp A: 89; Gp B: 89) | |
| Interventions | Comparison: 30 min of cryotherapy versus 60 min of cryotherapy Gp A: ice chips placed in mouth 5 min before receiving 5FU and LV, and continuously swished around, then replenished before the previous ice had completely melted, for total 30 min, whole procedure repeated for 5 consecutive days Gp B: as above but for total 60 min Compliance: well tolerated with high degree of compliance. Only a few participants stopped cryotherapy early (due to nausea, headache or chills). Many participants in the 60‐min group were unhappy with the duration of cryotherapy, indicating that 30 min of cryotherapy is preferred Duration of treatment (intended): 30 or 60 min per day for 5 days (first cycle only) | |
| Outcomes |
| |
| Notes | Sample size calculation: not reported Adverse effects: only a few participants stopped cryotherapy early (due to nausea, headache or chills) Funding: "supported in part by Public Health Service grants...from the National Cancer Institute, Department of Health and Human Services" Declarations/conflicts of interest: not reported Data handling by review authors: we only used the data from the first treatment cycle (rather than cross‐over data); physician judgement of oral mucositis was preferred over participant judgement as we deemed that this may be more objective and less biased Other information of note: in exploratory analyses, age over 65 years was statistically significantly (P < 0.001) associated with severity of mucositis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to receive cryotherapy for either 30 or 60 minutes" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomized to receive cryotherapy for either 30 or 60 minutes" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 randomised participant, from the cryotherapy group, was not included in the analyses due to an unrelated medical problem |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: Toronto, Canada Number of centres: 1 Study duration: May to December 2007 | |
| Participants | Inclusion criteria: minimum 18 years of age; able to read and understand instructions on oral cryotherapy and basic oral care in English; diagnosis of multiple myeloma; due to receive high‐dose melphalan (200 mg/m2); due to receive growth factors as part of treatment protocol; no pre‐existing oral disease Exclusion criteria: previous radiotherapy to the head and neck region; amyloidosis (when abnormal proteins collect together and build up in tissues/organs) involving the heart, kidneys, or tongue; receiving investigational drugs during the trial period Cancer type: haematological (multiple myeloma) Cancer treatment: high‐dose melphalan (200 mg/m2) followed by autologous SCT Any other potentially important prognostic factors: college/university educated: Gp A: 43%; Gp B: 16%; smokers: Gp A: 17%; Gp B: 9%; drinks alcohol: Gp A: 35%; Gp B: 27% Age at baseline (years): Gp A: mean 56 (SD 8.9) (range 43 to 72); Gp B: mean 62 (SD 7.7) (range 43 to 72) Gender: Gp A: 61% male; Gp B: 55% male Number randomised: 46 (Gp A: 23; Gp B: 23) Number evaluated: 45 (Gp A: 23; Gp B: 22) | |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: on day ‐1 (the day before autologous SCT), ice chips held in mouth 5 min prior to receiving high‐dose melphalan (200 mg/m2), replenished before melted, procedure continued until 5 min after melphalan infusion, for total 60 min. Basic oral care also received (described below) from day ‐1 Gp B: on day ‐1, basic oral care began (described below) Compliance: all participants were able to perform the basic oral care procedures and all participants in the cryotherapy group complied with the intervention Duration of treatment (intended): basic oral care from day ‐1 to day 12 (14 days); oral cryotherapy for 60 min on day ‐1 | |
| Outcomes |
| |
| Notes | Sample size calculation: 17 participants per group needed to detect an effect size of 1 (presumably the authors mean a MD of 1 on the WHO scale) at 80% power and 5% significance Adverse effects: 4 participants (17.4%) in the cryotherapy group experienced side effects (teeth sensitivity and chills) Funding: "The authors acknowledge the financial support of the University Health Network Nursing Research and Canadian Nurses Foundation for the successful implementation of the project" Declarations/conflicts of interest: "The authors have nothing to disclose and declare no conflicts of interest" Other information of note: although the authors report that the MD of 0.71 in OM severity (at day 9) was statistically significant, they state that it is not clinically significant because most participants only had OM grades of 0 to 1; conversely, the authors report that the difference in days of hospital stay was not statistically significant, but that the difference of approximately 1 day is clinically significant; we note an error in Table 2 where the mean in the control group at day 6 should be 0.5 rather than 0.05 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were allocated based on a randomization process performed by the biostatistics staff at the study site, using consecutively numbered sealed envelopes containing a computer‐generated random number list patient assignment" Comment: adequate method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were allocated based on a randomization process performed by the biostatistics staff at the study site, using consecutively numbered sealed envelopes containing a computer‐generated random number list patient assignment" Comment: these methods should have ensured that the allocation sequence was not manipulated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | Although described as blinded, the subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 randomised participant, from the control group, was not included in the analyses due to an adverse event related to the melphalan on the day of its administration |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (3 arms) Location: Denmark Number of centres: unclear (appears to be multicentre) Study duration: 2001 to 2005 | |
| Participants | Inclusion criteria: gastric or colorectal cancers; due to receive first course of chemotherapy; healthy oral mucosa Exclusion criteria: history of head and neck radiotherapy; symptoms of any infections; history of dental or mouth discomfort when consuming hot/cold food and drinks Cancer type: approximately 95% colorectal cancer in each group, with the remainder having gastric cancer Cancer treatment: 5FU and LV Any other potentially important prognostic factors: participants were stratified by age, smoking status and dentures; performance status score was equally distributed between groups Age at baseline (years): Gp A: 40 or older = 97%, median 62 (range 36 to 84); Gp B: 40 or older = 95%, median 61 (range 30 to 81); Gp C: 40 or older = 92%, median 62 (range 28 to 82) Gender: Gp A: 60% male; Gp B: 53% male; Gp C: 53% male Number randomised: 225 (Gp A: 75; Gp B: 75; Gp C: 75) Number evaluated: reported in the study: 206 (Gp A: 67; Gp B: 66; Gp C: 73); data available for OM incidence: 197 (Gp A: 63; Gp B: 64; Gp C: 70) | |
| Interventions | Comparison: cryotherapy versus saline rinse (placebo version of chlorhexidine) versus chlorhexidine rinse Gp A: crushed ice placed in mouth 10 min before receiving 5FU (425 mg/m2) and LV (20 mg/m2) by IV, and for 35 min after the start of infusion (total 45 min), whole procedure repeated for 5 consecutive days every 4 weeks Gp B: same cancer treatment but saline mouthwash (with same taste additives as Gp C), 10 ml swished around the mouth for 1 min, 3 times per day for 21 days Gp C: same cancer treatment but chlorhexidine 0.1% mouthwash, same schedule as Gp B Compliance: (complete) Gp A: 87%; Gp B: 80%; Gp C: 75%; (partial) Gp A: 13%; Gp B: 20%; Gp C: 25% Duration of treatment (intended): 45 min per day for 5 days per chemotherapy cycle (cryotherapy); 3 min per day for 21 days per chemotherapy cycle (chlorhexidine and saline rinses) | |
| Outcomes |
| |
| Notes | Sample size calculation: considering a 15% decrease in incidence of grade 3 to 4 OM as being clinically meaningful, with 80% power and at the 5% significance level, 75 patients required per group. Therefore, considering drop‐outs, required sample size was not achieved Adverse effects: taste disturbance: Gp A: 24/67 (36%); Gp B: 25/66 (38%); Gp C: 35/73 (48%); headache: Gp A: 14/67 (21%); Gp B: 9/66 (14%); Gp C: 10/73 (14%) Funding: "Supported by a grant from the National University Hospitals Research Foundation in Denmark" Declarations/conflicts of interest: not reported Data handling by review authors: the study authors report that data were available on 206 participants, however, for OM incidence, some of this number of participants are not given a grade and are listed as 'NA' (abbreviation not explained). We subtracted the 'NA' participants from the total number of participants and have addressed this problem under 'Incomplete outcome data (attrition bias)' Other information of note: smoking status, performance status and being aged 40 or older (underpowered ‐ only 11 participants less than 40) were not statistically significantly associated with severity of OM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were stratified...and randomized after informed consent to 1 of 3 prophylactic regimens" Comment: insufficient information to determine method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were stratified...and randomized after informed consent to 1 of 3 prophylactic regimens" Comment: insufficient information to determine whether or not the random sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | High risk | For OM incidence, 12% of randomised participants were not included in the analysis (Gp A: 16%; Gp B: 15%; Gp C: 9%). Reasons are not reported but there is a potential risk of bias if this differential drop‐out is related to outcomes/prognosis |
| Selective reporting (reporting bias) | Low risk | Data for outcomes of this review were reported appropriately |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (2 arms) Location: Uppsala, Sweden Number of centres: 1 Study duration: January 2002 to August 2004 | |
| Participants | Inclusion criteria: more than 18 years of age; able to communicate in Swedish; about to receive BMT Exclusion criteria: not reported Cancer type: 1 participant per group had testicular cancer, the remainder had haematological cancers Cancer treatment: type of chemotherapy prior to BMT was fairly equally distributed between groups; participants were stratified by autologous BMT (both groups 79.5%) versus allogeneic URD BMT (both groups 20.5%) Any other potentially important prognostic factors: tobacco use equally distributed between groups (Gp A: 17.9%; Gp B: 15.4%) Age at baseline (years): Gp A: mean 49.8 (SD 14.4); Gp B: mean 54.3 (SD 11) Gender: Gp A: 66.7% male; Gp B: 48.7% male Number randomised: 78 (Gp A: 39; Gp B: 39) Number evaluated: 78 (Gp A: 39; Gp B: 39) | |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: prior to BMT (unclear how far in advance); ice chips placed in mouth or rinsing with ice‐cold water starting 5 min before receiving chemotherapy by IV, and replenished for the duration of the chemotherapy session Gp B: chemotherapy prior to BMT Compliance: 58% to 75% of the participants reported that they kept the mouth constantly cooled for the entire duration of the chemotherapy; 71% to 100% did so more than half the time Duration of treatment: not reported (variable and dependent on type of chemotherapy) | |
| Outcomes |
| |
| Notes | Sample size calculation: 36 participants per group needed for 80% power at 5% significance level (based on outcome 'duration of IV opioids') Adverse effects: 7 (18%) of participants found cryotherapy unpleasant, with 4 of those (10%) finding it very unpleasant due to shooting pain from the teeth Funding: "This study was in part supported by FoU funds, Uppsala läns landsting, Sweden" Declarations/conflicts of interest: not reported Data handling by review authors: we were unable to use the OMAS score but the authors provided full data for oral mucositis on the WHO scale; oral pain was not reported adequately so we were unable to report on this outcome Other information of note: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random assignment to EXP or CTR group was performed in blocks of six outside of the clinic by an independent researcher" Comment: if using block‐randomisation, it could be assumed that the independent researcher would have done this adequately |
| Allocation concealment (selection bias) | Low risk | Quote: "A random assignment to EXP or CTR group was performed in blocks of six outside of the clinic by an independent researcher" Comment: third‐party randomisation should have ensured that the allocation sequence was not manipulated |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | High risk | Oral mucositis using OMAS and oral pain inadequately reported. Data are presented in the text for mucositis scores on day 10 (autologous patients) and day 16 (allogeneic patients), and this appears to be based on statistical significance. The study authors have since provided full data on the WHO scale. Therefore, our rating for this domain is based on the fact that the text only states that there was no significant differences for oral pain |
| Other bias | Low risk | 45 staff members assessed mucositis using the OMAS instrument. Calibration is not mentioned. However, we were unable to use any data for this outcome so we can discount any potential bias |
| Methods | Trial design: parallel (3 arms) Location: San Antonio, Texas, USA Number of centres: 1 Study duration: first subject randomised August 2009; last subject randomised January 2013 | |
| Participants | Inclusion criteria: minimum 18 years of age; diagnosis of multiple myeloma and scheduled to receive high‐dose melphalan (70 to 100 mg/m2/day) for 2 days, as a single agent, for conditioning regimen prior to HSCT Exclusion criteria: received palifermin (Kepivance) in the past 30 days; received any investigational drug in the past 30 days; received radiation therapy in the past 30 days; oral mucositis at the time of randomisation; altered mental status precluding understanding of the informed consent process and/or completion of the necessary assessments Cancer type: haematological (multiple myeloma) Cancer treatment: high‐dose melphalan, prior to autologous HSCT Any other potentially important prognostic factors: no differences between groups in race/ethnicity, Karnofsky performance score, diabetes, denture wearing, smoking status Age at baseline (years): Gp A: median 62 (range 39 to 75); Gp B: median 61.5 (range 43 to 70) Gender: both groups 95% male Number randomised: 78 (Gp A: 40; Gp B: 38) (numbers are for the 2 groups of interest to this review) Number evaluated: 78 (Gp A: 40; Gp B: 38) | |
| Interventions | Comparison: cryotherapy plus saline rinse versus saline rinse Gp A: on day ‐2 and ‐1 (HSCT was on day 0), approximately 1 ounce of crushed ice held in the mouth 15 min prior to the initiation of melphalan infusion, replenished as soon as it had completely melted, this procedure continued during the melphalan infusion and for 90 min after the end of the infusion. After completion of cryotherapy, standard care was followed until the end of the study (see Gp B below) Gp B: standard care (0.9% sodium chloride irrigation solution): rinsing of mouth twice, with 1 ounce (30 ml) of room temperature 0.9% NaCl (normal saline), 4 times daily after admission and until end of study Compliance: 100% compliance with cryotherapy but 3 subjects from the saline group refused to follow protocol but were kept in study Duration of treatment (intended): 2 consecutive days (exact duration of cryotherapy sessions unclear) | |
| Outcomes |
| |
| Notes | Sample size calculation: "This study will achieve a power of 80% to detect a 0.30 reduction in the proportion of subjects experiencing mucositis relative to saline with 55 subjects per arm and relative to Caphosol with 43 subjects per arm, using 2‐sided pairwise Pearson chi‐square testing with the Bonferroni corrected significance level of 0.017 (PASS, Version 08.0.8, NCSS Kaysville, Utah 2008). Assuming no loss to follow‐up and complete data, the required sample size for this study is therefore 55 subjects per arm, giving a total required sample size of 165 subjects. The study was terminated early because of ethical concerns after an interim analysis" Adverse effects: no serious adverse events Funding: "There was no outside funding for this study" Declarations/conflicts of interest: "No conflict of interest for all authors" Other information of note: only abstracts available but the authors have provided extra information through email correspondence to allow inclusion in this review. Study report is currently being written up and secondary outcome data have not yet been analysed, so we will include these in the next update of the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomized to the above mentioned groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "were randomized to the above mentioned groups" |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scale used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | This domain is not yet applicable as the study report is currently being written up |
| Other bias | Low risk | No other sources of bias are apparent |
| Methods | Trial design: parallel (4 arms) Location: Beijing, China Number of centres: 1 Study duration: June 2009 to May 2010 | |
| Participants | Inclusion criteria: patients due to receive high‐dose MTX Exclusion criteria: not reported Cancer type: osteosarcoma (bone cancer) Cancer treatment: MTX plus vincristine plus LV Any other potentially important prognostic factors: not reported Age at baseline (years): not reported Gender: not reported Number randomised: 147 (Gp A: 52; Gp B: 66; Gp C: 29) (numbers are for the 3 groups of interest to this review) Number evaluated: 147 (Gp A: 52; Gp B: 66; Gp C: 29) | |
| Interventions | Comparison: cryotherapy versus LV versus high dose LV Gp A: from the start of MTX, 100 ml ice water to be used for gargling (amount per gargle and frequency of gargling not specified), for 3 consecutive days Gp B: from the start of MTX, 3 mg LV dissolved in 100 ml water to be used for gargling per day, for 3 consecutive days (amount per gargle not specified) Compliance: not reported Duration of treatment (intended): 3 days (actual length of time the ice water or LV was held in the mouth is not reported) | |
| Outcomes |
| |
| Notes | Sample size calculation: not reported Adverse effects: not reported Funding: not reported/not obtained from translation Declarations/conflicts of interest: not reported/not obtained from translation Other information of note: it is unclear why the authors report OM severity at day 4, after they state that symptoms usually occur 5 to 7 days after chemotherapy. No participants had severe OM (grades 3 or 4) on day 4. If any participants developed severe OM after day 4, then the study will not reflect this and the incidence of severe OM in this type of study will have been underestimated. We would recommend that authors report the maximum grade experienced by each participant in future publications, or at least report a range of appropriate time points | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated to 4 groups using a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated to 4 groups using a random number table" |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind participants and personnel to allocated groups |
| Blinding of outcome assessment (detection bias) | High risk | The subjective elements in the scales used to measure oral mucositis could have introduced bias into the assessments |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the analyses |
| Selective reporting (reporting bias) | High risk | Oral mucositis was measured on the day of chemotherapy and on day 10, but severity is reported on day 4 |
| Other bias | Low risk | No other sources of bias are apparent |
5FU = fluorouracil; autologous = patients' own cells; allogeneic = cells from donor; BMI = body mass index; BMT = bone marrow transplantation; CI = confidence interval; EOCG = Eastern Oncology Co‐operative Group; GVHD = graft‐versus‐host disease; HSCT = haematopoietic stem cell transplantation; IFN = interferon; IV = intravenous; LV = leucovorin; MD = mean difference; MTX = methotrexate; NCI‐CTC = National Cancer Institute common toxicity criteria; OM = oral mucositis; OMAS = oral mucositis assessment scale; PBSCT = peripheral blood stem cell transplantation; SCT = stem cell transplantation; SD = standard deviation; TPN = total parenteral nutrition; URD = unrelated donor; VAS = visual analogue scale; VP16 = vepesid/etoposide; WHO = World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not RCT (historical controls) | |
| Cross‐over study. First‐period data not presented | |
| Plain versus flavoured ice. Cross‐over study. First‐period data not presented | |
| Cryotherapy plus laser versus laser. We cannot exclude the possibility of interaction between interventions and therefore we would not be confident in stating that any improved/reduced effect is due to the cryotherapy | |
| Not RCT (participants were allocated by alternation) | |
| Not RCT (historical controls) | |
| Plain versus flavoured ice versus standard care. Cross‐over study. First‐period data not presented | |
| Translated from Japanese: case series of 5 cancer patients | |
| Not RCT (allocation based on date of birth) | |
| Unclear if RCT. Author was contacted during previous update of this Cochrane review but we received no response | |
| Not RCT (no mention of random allocation to groups) |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Trial design: parallel (2 arms) Location: Chandigarh, India Number of centres: 1 Study duration: 6 months (dates unclear from trials registry) |
| Participants | Inclusion criteria: aged from 18 to 80 years; males or females newly diagnosed with head and neck cancer; radical radiotherapy planned; normal mucosa Exclusion criteria: palliative radiotherapy planned; coming for booster dose of radiation Cancer type: head and neck Cancer treatment: radiotherapy to the head and neck Any other potentially important prognostic factors: unclear from trials registry Age at baseline (years): unclear from trials registry Gender: unclear from trials registry Number randomised: 60 (Gp A: 30; Gp B: 30) Number evaluated: unclear from trials registry |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: ice cubes held in mouth for 4 min before radiotherapy and for a further 4 min after the session Gp B: standard oral care Compliance: unclear from trials registry Duration of treatment: unclear from trials registry |
| Outcomes |
|
| Notes | Emailed study investigators 29/06/2015 for publication details or full unpublished study data "This was an M.Sc dissertation by a student from the institute of nursing and I was one of the guides. I shall try and contact this student for complete details and then get back to you. With regards, Sushmita Ghoshal" |
| Methods | Trial design: parallel (2 arms) Location: Suzhou, China Number of centres: 1 Study duration: unclear from abstract |
| Participants | Inclusion criteria: unclear from abstract Exclusion criteria: unclear from abstract Cancer type: unclear from abstract Cancer treatment: unclear from abstract (followed by HSCT) Any other potentially important prognostic factors: unclear from abstract Age at baseline (years): unclear from abstract Gender: unclear from abstract Number randomised: 37 (number per group unclear from abstract) Number evaluated: unclear from abstract |
| Interventions | Comparison: full cryotherapy versus partial cryotherapy Gp A: oral cryotherapy from the beginning of chemotherapy infusion until the end of infusion Gp B: oral cryotherapy starting half way through chemotherapy infusion until the end of infusion Compliance: unclear from abstract Duration of treatment: unclear from abstract |
| Outcomes |
|
| Notes | Unable to obtain email addresses for study investigators. Awaiting publication of full trial report |
| Methods | Trial design: parallel (2 arms) Location: Columbus, Ohio, USA Number of centres: 1 Study duration: April 2012 to March 2015 |
| Participants | Inclusion criteria: minimum 18 years of age; diagnosed with multiple myeloma and due to receive autologous STC Exclusion criteria: any other medical condition (including mental illness or substance abuse) which may interfere with ability to give informed consent, co‐operate, and participate in the study, or which may interfere with the interpretation of the results Cancer type: haematological (multiple myeloma) Cancer treatment: high‐dose melphalan followed by autologous SCT Any other potentially important prognostic factors: unclear from trials registry Age at baseline (years): unclear from trials registry Gender: unclear from trials registry Number randomised: 146 (Gp A: 73; Gp B: 73) Number evaluated: unclear from trials registry |
| Interventions | Comparison: 2 hours of cryotherapy versus 6 hours of cryotherapy Gp A: 1 ounce of shaved ice placed in mouth starting 15 min before receiving chemotherapy, and replenished for 2 hours Gp B: 1 ounce of shaved ice placed in mouth starting 15 min before receiving chemotherapy, and replenished for 6 hours Compliance: unclear from trials registry Duration of treatment: 2 hours or 6 hours |
| Outcomes |
|
| Notes | Emailed study investigators 24/09/2015 for publication details or full unpublished study data |
| Methods | Trial design: parallel (2 arms) Location: USA Number of centres: unclear from abstract Study duration: unclear from abstract |
| Participants | Inclusion criteria: unclear from abstract Exclusion criteria: unclear from abstract Cancer type: unclear from abstract Cancer treatment: high‐dose melphalan (> 140 mg/m2) either alone or as part of the BEAM regimen, prior to autologous HSCT Any other potentially important prognostic factors: unclear from abstract Age at baseline (years): unclear from abstract Gender: unclear from abstract Number randomised: 40 (number per group unclear from abstract) Number evaluated: unclear from abstract |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: oral cryotherapy 5 min before the start of chemotherapy infusion, continuing until 30 min after the completion of infusion Gp B: chemotherapy only Compliance: unclear from abstract Duration of treatment: unclear from abstract |
| Outcomes |
|
| Notes | Emailed study investigators 29/09/2015 for publication details or full unpublished study data |
| Methods | Trial design: parallel (2 arms) Location: unclear from abstract Number of centres: unclear from abstract Study duration: unclear from abstract |
| Participants | Inclusion criteria: minimum 18 years of age Exclusion criteria: unclear from abstract Cancer type: breast Cancer treatment: super intensive chemotherapy (various types) Any other potentially important prognostic factors: unclear from abstract Age at baseline (years): unclear from abstract Gender: females Number randomised: 150 (number per group unclear from abstract) Number evaluated: unclear from abstract |
| Interventions | Comparison: cryotherapy versus control (unclear from abstract) Gp A: details of regimen not described in abstract Gp B: details of regimen not described in abstract Compliance: unclear from abstract Duration of treatment (intended): unclear from abstract |
| Outcomes |
|
| Notes | Emailed study investigators 29/09/2015 for publication details or full unpublished study data |
autologous = patients' own cells; HSCT = haematopoietic stem cell transplantation; NCI‐CTC = National Cancer Institute common toxicity criteria; SCT = stem cell transplantation; WHO = World Health Organization
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomized controlled trial of cryotherapy for prevention and reduction of severity of oral mucositis in children undergoing hematopoietic stem cell transplantation |
| Methods | Trial design: parallel (2 arms) Location: unclear from trials registry Number of centres: unclear from trials registry Study duration: October 2012 to estimated September 2015 |
| Participants | Inclusion criteria: aged between 4 and 18 years; due to receive chemotherapy as conditioning treatment prior to HSCT in Sweden; sufficient knowledge of Swedish to understand the protocols Exclusion criteria: not reported Cancer type: haematological (multiple myeloma) Cancer treatment: chemotherapy prior to HSCT Any other potentially important prognostic factors: unclear from trials registry Age at baseline (years): 4 to 18 Gender: unclear from trials registry Number randomised: 50 (estimated enrolment ‐ not reported by group) Number evaluated: unclear from trials registry |
| Interventions | Comparison: cryotherapy versus standard oral care Gp A: ice chips/ice cream/ice water used during chemotherapy infusion, replenished continuously (children receiving a 24‐hour infusion instructed to use cryotherapy for 1 hour, 4 times per day) Gp B: standard oral care Compliance: unclear from trials registry Duration of treatment: unclear from trials registry |
| Outcomes |
|
| Starting date | October 2012 |
| Contact information | Gustaf Ljungman ([email protected]); Tove Kamsvåg Magnusson ([email protected]) |
| Notes |
| Trial name or title | Randomized controlled, open‐label study on the use of cryotherapy in the prevention of chemotherapy‐induced mucositis in stem cell transplant patients |
| Methods | Trial design: parallel (2 arms) Location: Florida, USA Number of centres: unclear from trials registry Study duration: March 2015 to estimated March 2019 |
| Participants | Inclusion criteria: minimum 18 years of age; due to receive etoposide chemotherapy (minimum dose of 30 mg/kg) as conditioning treatment prior to autologous SCT Exclusion criteria: prior radiation to head and neck; known oropharynx involvement in malignancy; history of non‐compliance or lack capacity to give informed consent Cancer type: unclear from trials registry Cancer treatment: etoposide (minimum dose of 30 mg/kg) prior to autologous SCT Any other potentially important prognostic factors: unclear from trials registry Age at baseline (years): unclear from trials registry Gender: unclear from trials registry Number randomised: 48 (estimated enrolment ‐ not reported by group) Number evaluated: unclear from trials registry |
| Interventions | Comparison: cryotherapy versus no treatment Gp A: ice chips/other very cold or frozen food used starting 15 min before starting etoposide infusion for a 30‐min period, then 3 saline rinses over 15‐min period. This alternating cycle is repeated until 30 min after completion of etoposide infusion (total 150 min) Gp B: at start of etoposide infusion 3 saline rinses over 15‐min period followed by 30‐rest period (no treatment). This alternating cycle is repeated until 30 min after completion of etoposide infusion (total 150 min) Compliance: unclear from trials registry Duration of treatment: 150 min |
| Outcomes |
|
| Starting date | March 2015 |
| Contact information | Christina Cline ([email protected]); Leslie Pettiford ([email protected]) |
| Notes |
autologous = patients' own cells; ChiMES = Children's International Mucositis Evaluation Scale; HSCT = haematopoietic stem cell transplantation; SCT = stem cell transplantation; TPN = total parenteral nutrition; WHO = World Health Organization
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 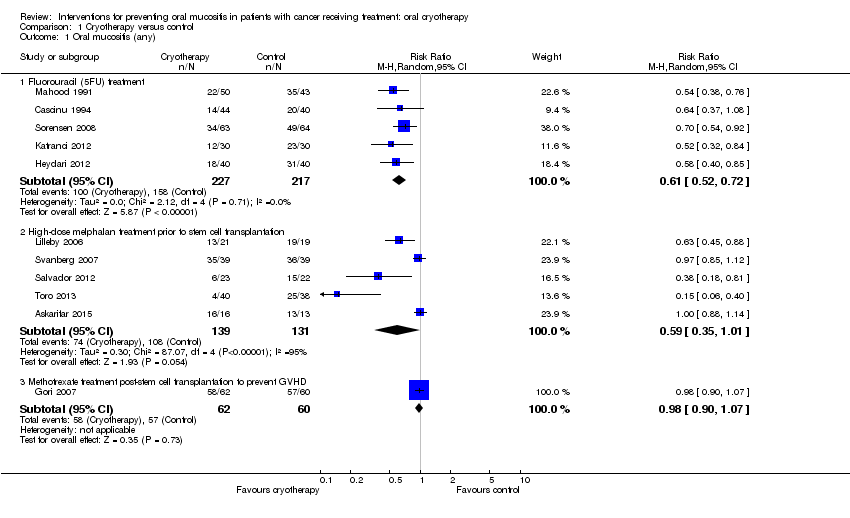 Comparison 1 Cryotherapy versus control, Outcome 1 Oral mucositis (any). | ||||
| 1.1 Fluorouracil (5FU) treatment | 5 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.52, 0.72] |
| 1.2 High‐dose melphalan treatment prior to stem cell transplantation | 5 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.01] |
| 1.3 Methotrexate treatment post‐stem cell transplantation to prevent GVHD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 2 Oral mucositis (moderate + severe) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Cryotherapy versus control, Outcome 2 Oral mucositis (moderate + severe). | ||||
| 2.1 Fluorouracil (5FU) treatment | 5 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.41, 0.65] |
| 2.2 High‐dose melphalan treatment prior to stem cell transplantation | 5 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| 2.3 Methotrexate treatment post‐stem cell transplantation to prevent GVHD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 3 Oral mucositis (severe) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Cryotherapy versus control, Outcome 3 Oral mucositis (severe). | ||||
| 3.1 Fluorouracil (5FU) treatment | 5 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.27, 0.61] |
| 3.2 High‐dose melphalan treatment prior to stem cell transplantation | 5 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.20, 0.72] |
| 3.3 Methotrexate treatment post‐stem cell transplantation to prevent GVHD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.25] |
| 4 Interruptions to cancer treatment Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.20, 0.95] |
| Analysis 1.4  Comparison 1 Cryotherapy versus control, Outcome 4 Interruptions to cancer treatment. | ||||
| 5 Interruptions to cancer treatment (days of interruption) Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐9.26, ‐6.74] |
| Analysis 1.5 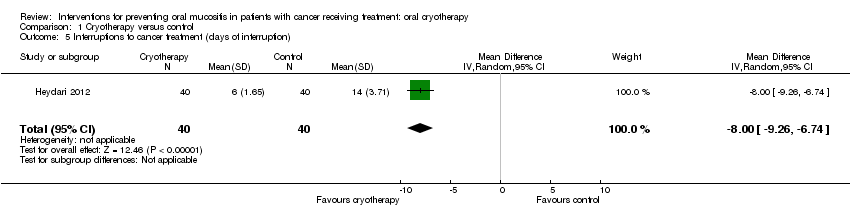 Comparison 1 Cryotherapy versus control, Outcome 5 Interruptions to cancer treatment (days of interruption). | ||||
| 6 Oral pain (0 to 10 scale) Show forest plot | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.11, ‐0.89] |
| Analysis 1.6 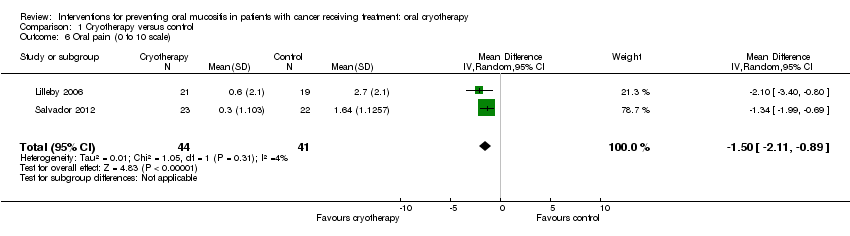 Comparison 1 Cryotherapy versus control, Outcome 6 Oral pain (0 to 10 scale). | ||||
| 7 Normalcy of diet (days of total parenteral nutrition) Show forest plot | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐2.18 [‐4.33, ‐0.03] |
| Analysis 1.7 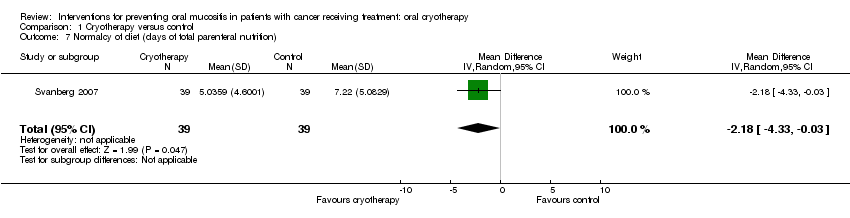 Comparison 1 Cryotherapy versus control, Outcome 7 Normalcy of diet (days of total parenteral nutrition). | ||||
| 8 Duration of hospitalisation (days) Show forest plot | 2 | 123 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.97, 0.19] |
| Analysis 1.8  Comparison 1 Cryotherapy versus control, Outcome 8 Duration of hospitalisation (days). | ||||
| 9 Duration of opioid use (days) Show forest plot | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐5.33, 0.77] |
| Analysis 1.9 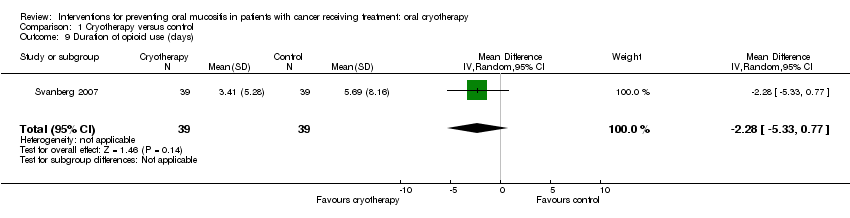 Comparison 1 Cryotherapy versus control, Outcome 9 Duration of opioid use (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.62, 1.29] |
| Analysis 2.1  Comparison 2 Different oral cryotherapy regimens, Outcome 1 Oral mucositis (any). | ||||
| 2 Oral mucositis (moderate + severe) Show forest plot | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.30] |
| Analysis 2.2  Comparison 2 Different oral cryotherapy regimens, Outcome 2 Oral mucositis (moderate + severe). | ||||
| 3 Oral mucositis (severe) Show forest plot | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.23, 1.58] |
| Analysis 2.3  Comparison 2 Different oral cryotherapy regimens, Outcome 3 Oral mucositis (severe). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.32] |
| Analysis 3.1  Comparison 3 Cryotherapy versus chlorhexidine, Outcome 1 Oral mucositis (any). | ||||
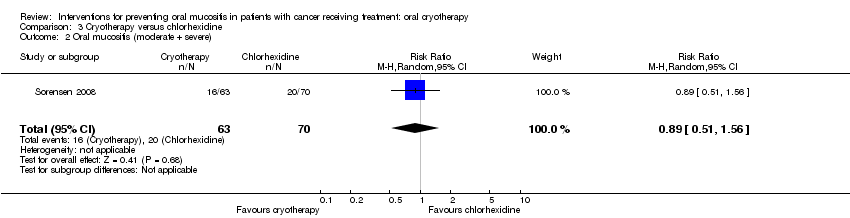
| 2 Oral mucositis (moderate + severe) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.51, 1.56] |
| Analysis 3.2 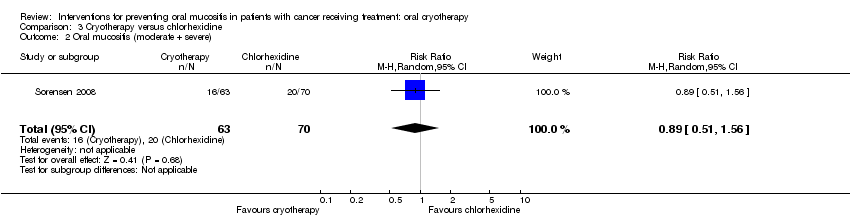 Comparison 3 Cryotherapy versus chlorhexidine, Outcome 2 Oral mucositis (moderate + severe). | ||||
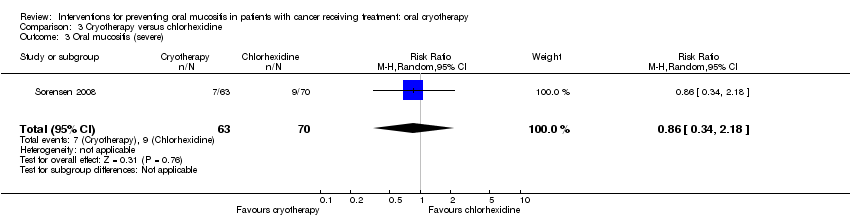
| 3 Oral mucositis (severe) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.18] |
| Analysis 3.3  Comparison 3 Cryotherapy versus chlorhexidine, Outcome 3 Oral mucositis (severe). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1 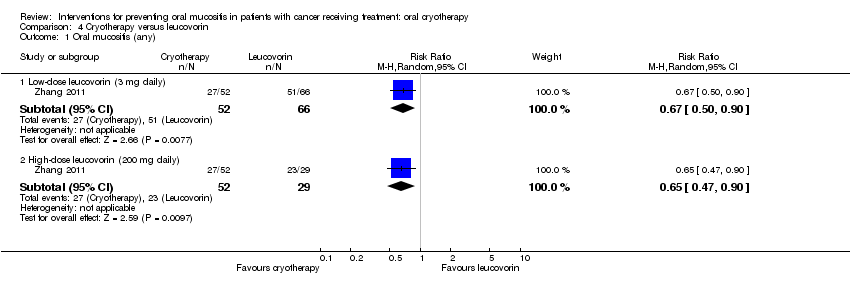 Comparison 4 Cryotherapy versus leucovorin, Outcome 1 Oral mucositis (any). | ||||
| 1.1 Low‐dose leucovorin (3 mg daily) | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.50, 0.90] |
| 1.2 High‐dose leucovorin (200 mg daily) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
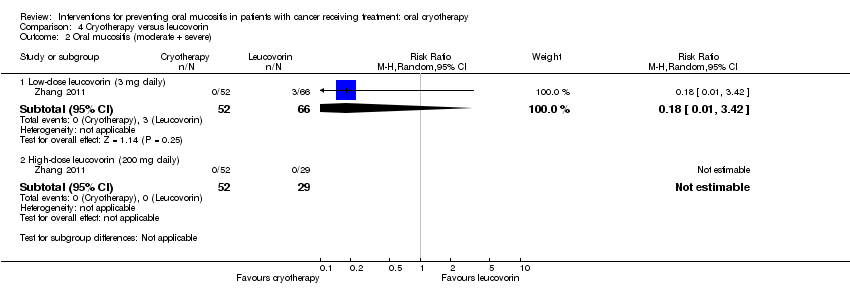
| 2 Oral mucositis (moderate + severe) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2 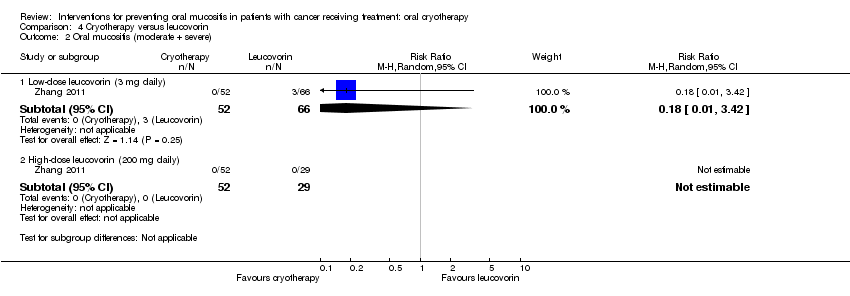 Comparison 4 Cryotherapy versus leucovorin, Outcome 2 Oral mucositis (moderate + severe). | ||||
| 2.1 Low‐dose leucovorin (3 mg daily) | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.42] |
| 2.2 High‐dose leucovorin (200 mg daily) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Oral mucositis (severe) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Cryotherapy versus leucovorin, Outcome 3 Oral mucositis (severe). | ||||
| 3.1 Low‐dose leucovorin (3 mg daily) | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 High‐dose leucovorin (200 mg daily) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
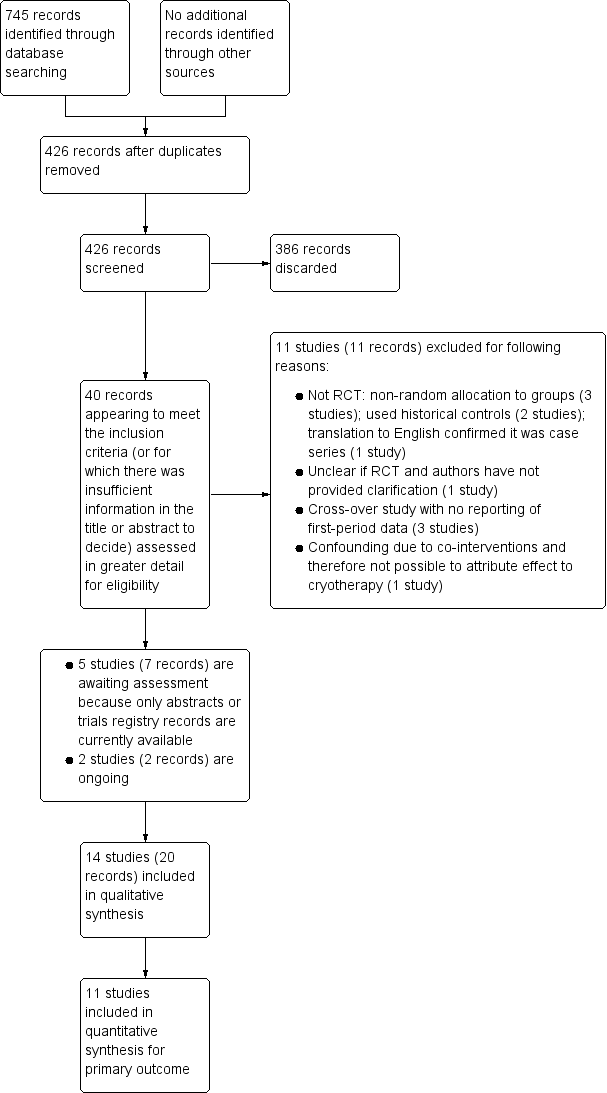
Study flow diagram

Comparison 1 Cryotherapy versus control, Outcome 1 Oral mucositis (any).
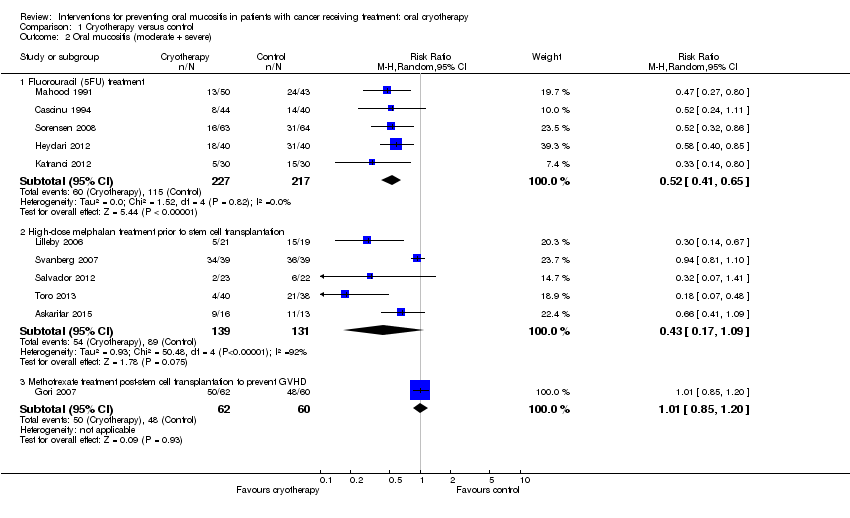
Comparison 1 Cryotherapy versus control, Outcome 2 Oral mucositis (moderate + severe).
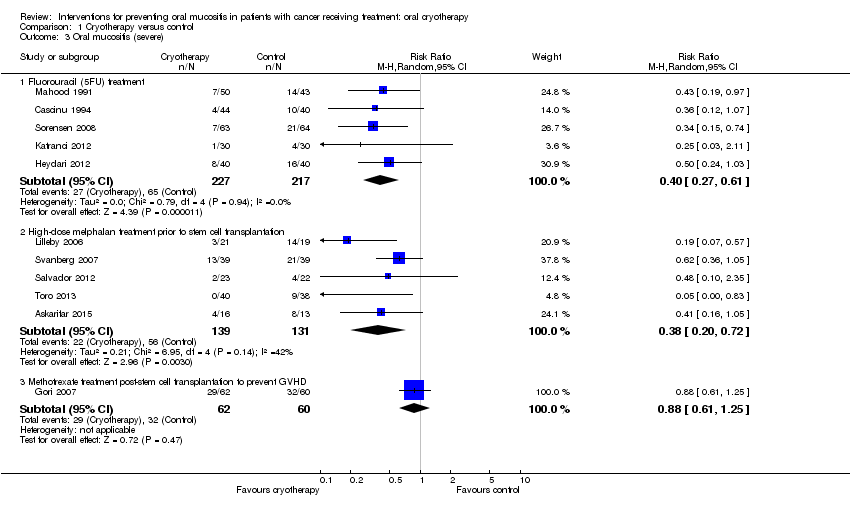
Comparison 1 Cryotherapy versus control, Outcome 3 Oral mucositis (severe).
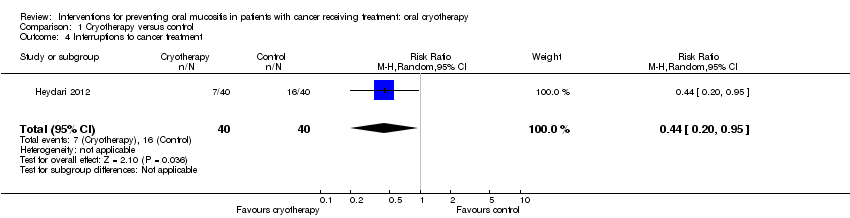
Comparison 1 Cryotherapy versus control, Outcome 4 Interruptions to cancer treatment.
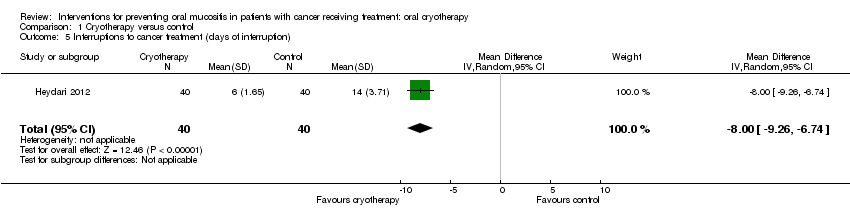
Comparison 1 Cryotherapy versus control, Outcome 5 Interruptions to cancer treatment (days of interruption).
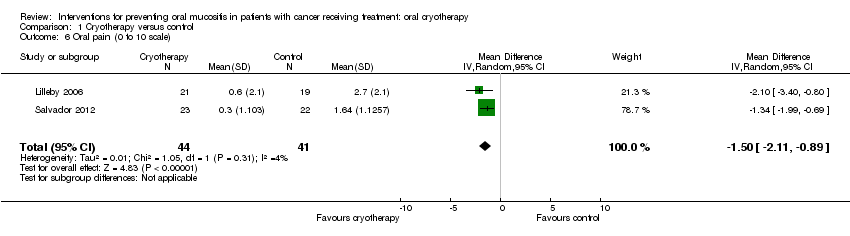
Comparison 1 Cryotherapy versus control, Outcome 6 Oral pain (0 to 10 scale).
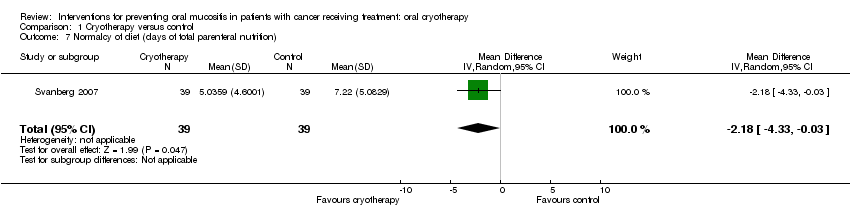
Comparison 1 Cryotherapy versus control, Outcome 7 Normalcy of diet (days of total parenteral nutrition).

Comparison 1 Cryotherapy versus control, Outcome 8 Duration of hospitalisation (days).
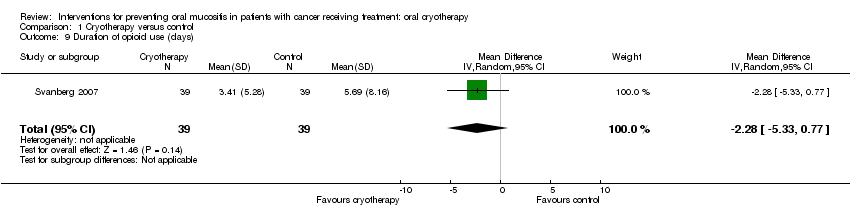
Comparison 1 Cryotherapy versus control, Outcome 9 Duration of opioid use (days).
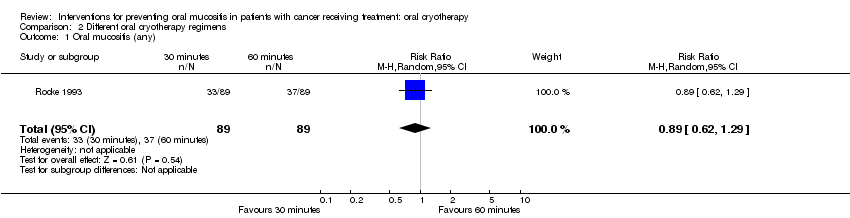
Comparison 2 Different oral cryotherapy regimens, Outcome 1 Oral mucositis (any).
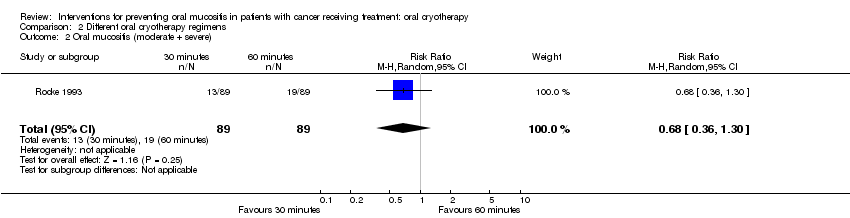
Comparison 2 Different oral cryotherapy regimens, Outcome 2 Oral mucositis (moderate + severe).

Comparison 2 Different oral cryotherapy regimens, Outcome 3 Oral mucositis (severe).
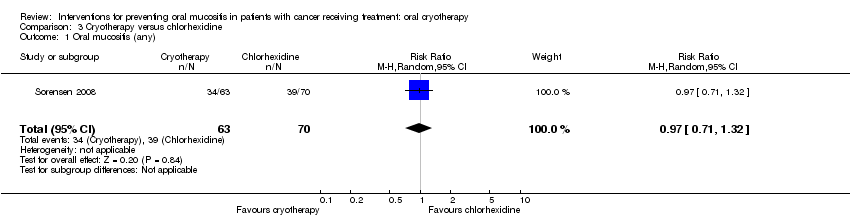
Comparison 3 Cryotherapy versus chlorhexidine, Outcome 1 Oral mucositis (any).
Comparison 3 Cryotherapy versus chlorhexidine, Outcome 2 Oral mucositis (moderate + severe).
Comparison 3 Cryotherapy versus chlorhexidine, Outcome 3 Oral mucositis (severe).

Comparison 4 Cryotherapy versus leucovorin, Outcome 1 Oral mucositis (any).
Comparison 4 Cryotherapy versus leucovorin, Outcome 2 Oral mucositis (moderate + severe).

Comparison 4 Cryotherapy versus leucovorin, Outcome 3 Oral mucositis (severe).
| Cryotherapy versus control for preventing oral mucositis in adults receiving fluorouracil‐based treatment for solid cancers | ||||||
| Patient or population: adults** with solid cancers receiving fluorouracil‐based cancer treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with cryotherapy | |||||
| Oral mucositis (any) | 728 per 1000 | 444 per 1000 | RR 0.61 | 444 | ⊕⊕⊕⊝ | Oral cryotherapy reduced the risk of developing oral mucositis by 39% (95% CI 28% to 48%). We would need to treat 4 people (95% CI 3 to 5 people) with oral cryotherapy to prevent 1 additional person from developing oral mucositis |
| Oral mucositis (moderate + severe) | 530 per 1000 | 276 per 1000 | RR 0.52 | 444 | ⊕⊕⊕⊝ | Oral cryotherapy reduced the risk of developing moderate to severe oral mucositis by 48% (95% CI 35% to 59%). We would need to treat 4 people (95% CI 4 to 6 people) with oral cryotherapy to prevent 1 additional person from developing moderate to severe oral mucositis |
| Oral mucositis (severe) | 300 per 1000 | 120 per 1000 | RR 0.40 | 444 | ⊕⊕⊕⊝ | Oral cryotherapy reduced the risk of severe oral mucositis by 60% (95% CI 39% to 73%). We would need to treat 6 people (95% CI 5 to 9 people) with oral cryotherapy to prevent 1 additional person from developing severe oral mucositis |
| Interruptions to cancer treatment | 400 per 1000 | 176 per 1000 | RR 0.44 | 80 | ⊕⊝⊝⊝ | Oral cryotherapy reduced the risk of treatment interruption by 56% (95% CI 5% to 80%). We would need to treat 5 people (95% CI 4 to 50 people) with oral cryotherapy to prevent 1 additional person from having a treatment interruption |
| Oral pain (1 to 5 scale: 1 = never, 2 = 1 day of week, 3 = 2 to 3 days of week, 4 = most of week, 5 = 7 days of week) | The mean oral pain (1 to 5 scale) was 3.64 | The mean oral pain (1 to 5 scale) in the cryotherapy group was 1.93 lower (2.37 to 1.49 lower) | ‐ | 80 | ⊕⊝⊝⊝ | The duration of oral pain experienced was less in the oral cryotherapy group (Additional Table 1) |
| Quality of life | No studies assessed this outcome | |||||
| Normalcy of diet (days of total parenteral nutrition) | No studies assessed this outcome | |||||
| Duration of hospitalisation (days) | No studies assessed this outcome | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All 5 studies at high risk of performance and detection bias 2 Single study at high risk of performance and detection bias 3 Low sample size and wide confidence interval 4 Indirect due to single study in 1 setting (in terms of country, healthcare system, and participants potentially differing from other countries) ** There were no studies conducted on children | ||||||
| Cryotherapy versus control for preventing oral mucositis in adults receiving high‐dose melphalan‐based treatment prior to haematopoietic stem cell transplantation for haematological cancers | ||||||
| Patient or population: adults** with haematological cancers receiving high‐dose melphalan‐based treatment prior to haematopoietic stem cell transplantation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with cryotherapy | |||||
| Oral mucositis (any) | 824 per 1000 | 486 per 1000 | RR 0.59 | 270 | ⊕⊕⊝⊝ | Oral cryotherapy appears to reduce the risk of developing oral mucositis. However, there is great uncertainty about our estimate, and there is an extremely small chance of a 1% increase in the risk of developing oral mucositis compared to control. The point estimate suggests a 41% reduction in the risk of developing oral mucositis, with the confidence interval ranging from a 65% reduction to a 1% increase in risk. We would need to treat 3 people with oral cryotherapy to prevent 1 additional person from developing oral mucositis, with the confidence interval ranging from 2 people (to prevent 1 additional oral mucositis case) to 111 people to cause 1 additional person to develop oral mucositis |
| Oral mucositis (moderate + severe) | 679 per 1000 | 292 per 1000 | RR 0.43 | 270 | ⊕⊕⊝⊝ | Oral cryotherapy appears to reduce the risk of developing moderate to severe oral mucositis. However, there is great uncertainty about our estimate, and there is a very small chance of a 9% increase in the risk of developing moderate to severe oral mucositis compared to control. The point estimate suggests a 57% reduction in the risk of developing moderate to severe oral mucositis, with the confidence interval ranging from an 83% reduction to a 9% increase in risk. We would need to treat 3 people with oral cryotherapy to prevent 1 additional person from developing moderate to severe oral mucositis, with the confidence interval ranging from 2 people (to prevent 1 additional moderate to severe oral mucositis case) to 17 people to cause 1 additional person to develop moderate to severe oral mucositis |
| Oral mucositis (severe) | 427 per 1000 | 162 per 1000 | RR 0.38 | 270 | ⊕⊕⊕⊝ | Oral cryotherapy reduced the risk of developing severe oral mucositis by 62% (95% CI 28% to 80%). We would need to treat 4 people (95% CI 3 to 9 people) with oral cryotherapy to prevent 1 additional person from developing severe oral mucositis |
| Interruptions to cancer treatment | No studies assessed this outcome | |||||
| Oral pain on a 0 (no pain) to 10 (maximum pain) scale | The weighted mean oral pain (0 to 10 scale) was 2.13 | The mean oral pain (0 to 10 scale) in the cryotherapy group was 1.5 lower (2.11 to 0.89 lower) | ‐ | 85 | ⊕⊕⊝⊝ | Oral cryotherapy reduced oral pain by 70% although the clinical importance of a 1.5 point‐reduction on a 0 to 10 scale is questionable |
| Quality of life | 1 study assessed this outcome but the data are currently unavailable as the study report and analysis have not yet been completed | |||||
| Normalcy of diet (days of total parenteral nutrition ‐ TPN) | The mean number of days of TPN was 7 | The mean number of days of TPN in the intervention group was 2.18 days fewer (4.33 to 0.03 fewer) | ‐ | 78 | ⊕⊝⊝⊝ | Oral cryotherapy reduced the duration of TPN by 2.18 days. There was some additional very low quality evidence, from a single small study at high risk of bias, reporting only median, range and P value, that oral cryotherapy reduced the number of days of TPN (Additional Table 1) |
| Duration of hospitalisation (days) | The mean duration of hospitalisation (days) was 0 | The mean duration of hospitalisation (days) in the intervention group was 1.39 undefined fewer (2.97 fewer to 0.19 more) | ‐ | 123 | ⊕⊕⊝⊝ | There is insufficient evidence to show that oral cryotherapy reduces the duration of hospitalisation. This is supported by additional very low quality evidence, from a single small study at high risk of bias, reporting only median, range and P value, that there is insufficient evidence to show a reduction in this outcome (Additional Table 1) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All 5 studies at risk of performance and detection bias, with 1 study at added risk of attrition bias 2 The I2 value indicates that a considerable amount (> 90%) of the variability in effect estimates is due to heterogeneity rather than chance 3 Both studies at high risk of performance and detection bias 4 Low sample size from 2 small studies 5 Single study at high risk of performance and detection bias 6 Low sample size and wide confidence interval 7 Indirect due to single study in 1 setting (in terms of country, healthcare system, and participants potentially differing from other countries) ** There were no studies conducted on children | ||||||
| Outcome | Study | Description of data | Cryotherapy | Control | Result |
| Oral mucositis | Kakoei 2013 | WHO 0 to 4 scale; physician‐judged rating used; day 14 data used due to highest control group mean (should be noted that on days 1 and 7 the cryotherapy group mean is higher but with no statistically significant difference) | n = 20 mean 0.95 (SD 0.58) | n = 20 mean 1.2 (SD 0.89) | MD ‐0.25 (95% CI ‐0.72 to 0.22); P = 0.29 (there is no evidence of a difference in mucositis severity) |
| Oral pain | Heydari 2012 | 1 to 5 scale: 1 = never, 2 = 1 day of week, 3 = 2 to 3 days of week, 4 = most of week, 5 = 7 days of week | n = 40 mean 1.71 (SD 0.74) | n = 40 mean 3.64 (SD 1.20) | MD ‐1.93 (95% CI ‐2.37 to ‐1.49); P < 0.00001 (cryotherapy statistically significantly reduced the duration of pain experience) |
| Normalcy of diet | Lilleby 2006 | Days of TPN (we use the P value quoted in the study report) | n = 21 median 2 (range 0 to 15) | n = 19 median 5.5 (range 0 to 21) | P = 0.04 (cryotherapy statistically significantly reduced the number of days of TPN) |
| Number of days in hospital | Lilleby 2006 | Days of hospitalisation (we use the P value quoted in the study report) | n = 21 median 9 (range 0 to 22) | n = 19 median 14 (range 0 to 30) | P = 0.11 (there is no evidence of a difference in the number of days of hospitalisation) |
| Number of days of treatment with opioid analgesics | Lilleby 2006 | Days of IV narcotics (we use the P value quoted in the study report) | n = 21 median 0 (range 0 to 10) | n = 19 median 5.5 (range 0 to 13) | P = 0.0003 (cryotherapy statistically significantly reduced the number of days of IV narcotics) |
| CI = confidence interval; IV = intravenous; MD = mean difference; n = number of participants analysed; N/A = not applicable; SD = standard deviation; TPN = total parenteral nutrition | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluorouracil (5FU) treatment | 5 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.52, 0.72] |
| 1.2 High‐dose melphalan treatment prior to stem cell transplantation | 5 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.01] |
| 1.3 Methotrexate treatment post‐stem cell transplantation to prevent GVHD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.07] |
| 2 Oral mucositis (moderate + severe) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluorouracil (5FU) treatment | 5 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.41, 0.65] |
| 2.2 High‐dose melphalan treatment prior to stem cell transplantation | 5 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.09] |
| 2.3 Methotrexate treatment post‐stem cell transplantation to prevent GVHD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 3 Oral mucositis (severe) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluorouracil (5FU) treatment | 5 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.27, 0.61] |
| 3.2 High‐dose melphalan treatment prior to stem cell transplantation | 5 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.20, 0.72] |
| 3.3 Methotrexate treatment post‐stem cell transplantation to prevent GVHD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.25] |
| 4 Interruptions to cancer treatment Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.20, 0.95] |
| 5 Interruptions to cancer treatment (days of interruption) Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐9.26, ‐6.74] |
| 6 Oral pain (0 to 10 scale) Show forest plot | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.11, ‐0.89] |
| 7 Normalcy of diet (days of total parenteral nutrition) Show forest plot | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐2.18 [‐4.33, ‐0.03] |
| 8 Duration of hospitalisation (days) Show forest plot | 2 | 123 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.97, 0.19] |
| 9 Duration of opioid use (days) Show forest plot | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐5.33, 0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.62, 1.29] |
| 2 Oral mucositis (moderate + severe) Show forest plot | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.30] |
| 3 Oral mucositis (severe) Show forest plot | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.23, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.32] |
| 2 Oral mucositis (moderate + severe) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.51, 1.56] |
| 3 Oral mucositis (severe) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral mucositis (any) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low‐dose leucovorin (3 mg daily) | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.50, 0.90] |
| 1.2 High‐dose leucovorin (200 mg daily) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.47, 0.90] |
| 2 Oral mucositis (moderate + severe) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low‐dose leucovorin (3 mg daily) | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.42] |
| 2.2 High‐dose leucovorin (200 mg daily) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Oral mucositis (severe) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Low‐dose leucovorin (3 mg daily) | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 High‐dose leucovorin (200 mg daily) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

