مقایسه ترلیپرسین در برابر سایر داروهای وازواکتیو برای سندرم هپاتورنال
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy | No of hits |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | November 2016. | (terlipressin* OR glypressin* OR vasoconstric*) AND hepatorenal syndrom* | 31 |
| The Cochrane Central Register of Controlled Trials (CENTRAL) | 2016, Issue 11. | #1 MeSH descriptor: [Vasoconstrictor Agents] explode all trees #2 terlipressin* or glypressin* or vasoconstric* #3 #1 or #2 #4 MeSH descriptor: [Hepatorenal Syndrome] explode all trees #5 hepatorenal syndrom* #6 #4 or #5 #7 #3 and #6 | 35 |
| MEDLINE Ovid | 1946 to November 2016. | 1. exp Vasoconstrictor Agents/ 2. (terlipressin* or glypressin* or vasoconstric*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 3. 1 or 2 4. exp Hepatorenal Syndrome/ 5. hepatorenal syndrom*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 6. 4 or 5 7. 6 and 3 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 9. 8 and 7 | 59 |
| Embase Ovid | 1974 to November 2016. | 1. exp Terlipressin/ 2. exp Vasoconstrictor Agent/ 3. (terlipressin* or glypressin* or vasoconstric*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 4. 1 or 3 or 2 5. exp Hepatorenal Syndrome/ 6. hepatorenal syndrom*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. 6 or 5 8. 4 and 7 9. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 10. 8 and 9 | 204 |
| Science Citation Index Expanded (Web of Science) | 1900 to November 2016. | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analysis) #3 #1 AND #2 #2 TS=(hepatorenal syndrom*) #1 TS=(terlipressin* or glypressin* or vasoconstric*) | 118 |
Appendix 2. Diagnostic criteria of hepatorenal syndrome (Arroyo 1996)
Diagnostic criteria described in Arroyo 1996.
Major criteria
-
Chronic or acute liver disease with advanced hepatic failure and portal hypertension.
-
Low glomerular filtration rate, as indicated by serum creatinine of greater than 133 mmol/L (1.5 mg/dL) or 24‐hour creatinine clearance less than 40 mL/minute.
-
Absence of shock, ongoing bacterial infection, and current or recent treatment with nephrotoxic drugs. Absence of gastrointestinal fluid losses (repeated vomiting or intense diarrhoea) or renal fluid losses (weight loss greater than 500 g/day for several days in people with ascites without peripheral oedema or 1000 g/day in people with peripheral oedema).
-
No sustained improvement in renal function (decrease in serum creatinine to 1.5 mg/dL or less or increase in creatinine clearance to 40 mL/minute or more) following diuretic withdrawal and expansion of plasma volume with 1.5 L of isotonic saline.
-
Proteinuria less than 500 mg/dL and no ultrasonographic evidence of obstructive uropathy or parenchymal renal disease.
Additional criteria
-
Urine volume less than 500 mL/day.
-
Urine sodium less than 10 mEq/L.
-
Urine osmolality greater than plasma osmolality.
-
Urine red blood cells less than 50 per high power field.
-
Serum sodium concentration less than 130 mEq/L.
Appendix 3. Diagnostic criteria of hepatorenal syndrome (Salerno 2007)
Diagnostic criteria described in Salerno 2007.
-
Cirrhosis with ascites.
-
Serum creatinine greater than 133 mmol/L (1.5 mg/dL).
-
No improvement of serum creatinine (decrease to a level of 133 mmol/L or less after at least two days with diuretic withdrawal and volume expansion with albumin. The recommended dose of albumin 1 g/kg of bodyweight per day up to a maximum of 100 g/day.
-
Absence of shock.
-
No current or recent treatment with nephrotoxic drugs.
-
Absence of parenchymal kidney disease as indicated by proteinuria greater than 500 mg/day, microhaematuria (more than 50 red blood cells per high power field) and abnormal renal ultrasonography.
Appendix 4. Diagnostic criteria of hepatorenal syndrome type of acute kidney injury in people with cirrhosis
Diagnostic criteria described in Angeli 2015.
-
Diagnosis of cirrhosis and ascites.
-
Diagnosis of acute kidney injury according to the International Ascites Club‐Acute Kidney Injury criteria (see below).
-
No response after two consecutive days of diuretic withdrawal and plasma volume expansion with albumin 1 g/kg of bodyweight.
-
Absence of shock.
-
No current or recent use of nephrotoxic drugs (non‐steroidal anti‐inflammatory drugs, aminoglycosides, iodinated contrast media, etc.).
-
No macroscopic signs of structural kidney injury, defined as: absence of proteinuria (greater than 500 mg/day), absence of microhaematuria (more than 50 red blood cells per high power field), normal findings on renal ultrasonography.
Definition of acute kidney injury according to the International Ascites Club‐Acute Kidney Injury criteria
Increase in serum creatinine of 0.3 mg/dL or greater (26.5 μmol/L or greater) within 48 hours; or a percentage increase serum creatinine of 50% or greater from baseline which is known, or presumed, to have occurred within the prior seven days.
| Staging of acute kidney injury | Stage 1 | Stage 2 | Stage 3 |
| ‐ | Increase in serum creatinine ≥ 0.3 mg/dL (≥ 26.5 μmol/L) or an increase in serum creatinine ≥ 1.5‐fold to 2‐fold from baseline. | Increase in serum creatinine > 2‐fold to 3‐fold from baseline. | Increase of serum creatinine > 3‐fold from baseline or serum creatinine ≥ 4.0 mg/dL (≥ 353.6 μmol/L) with an acute increase ≥ 0.3 mg/dL (≥ 26.5 μmol/L) or initiation of renal replacement therapy. |
| Progression of acute kidney injury | Progression | ‐ | Regression |
| ‐ | Progression of acute kidney injury to a higher stage or need for renal replacement therapy, or both. | ‐ | Regression of acute kidney injury to a lower stage. |
| Response to treatment | No response | Partial response | Full response |
| ‐ | No regression of acute kidney injury. | Regression of acute kidney injury stage with a reduction of serum creatinine to ≥ 0.3 mg/dL (≥ 26.5 μmol/L) above baseline value. | Return of serum creatinine to within 0.3 mg/dL (26.5 μmol/L) of baseline value. |
Baseline serum creatinine: a value of serum creatinine obtained in the previous three months, when available, can be used as baseline serum creatinine. In people with more than one value within the previous three months, the value closest to the admission time to the hospital should be used In people without a previous serum creatinine value, the serum creatinine on admission should be used as baseline.

Study flow diagram.
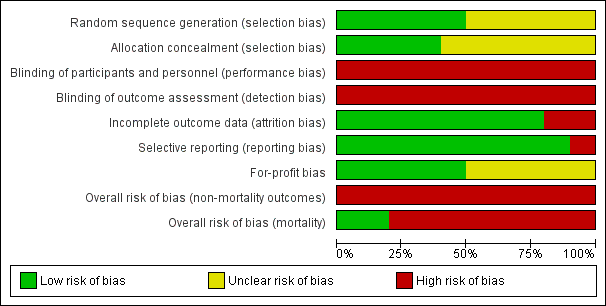
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

"Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
'+' = low risk of bias;
'‐' = high risk of bias;
'?' = unclear risk of bias.

Trial Sequential Analysis of 10 randomised clinical trials (474 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on mortality. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 20%, a control group risk (CGR) of mortality of 52%, and a model variance ‐ based heterogeneity correction of 30%. The risk ratio was 0.96 (97% confidence interval 0.79 to 1.18). The cumulative Z‐curve (blue line) did not cross the diversity‐adjusted trial monitoring boundary for benefit.
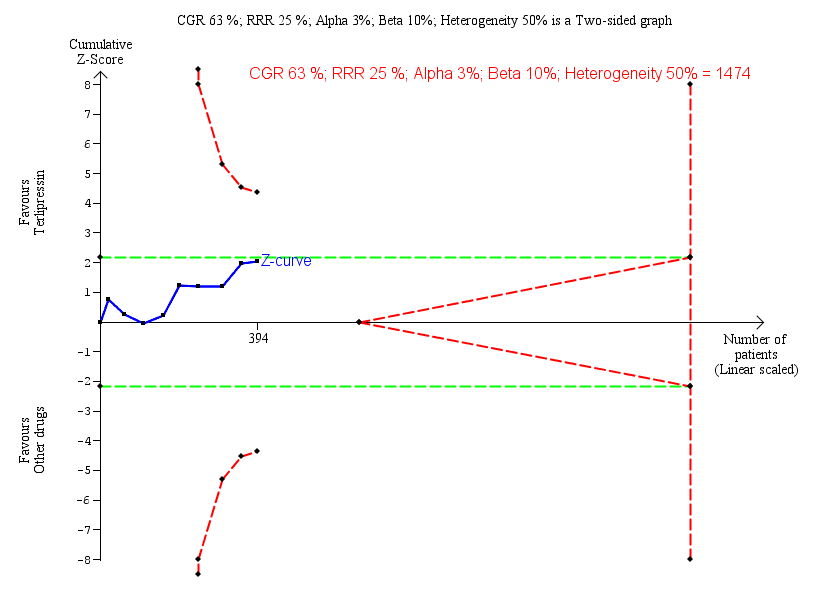
Trial Sequential Analysis of nine randomised clinical trials (394 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on lack of reversal of hepatorenal syndrome. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of lack of reversal of hepatorenal syndrome of 63%, and a heterogeneity correction of 50%. The risk ratio was 0.79 (97% confidence interval 0.48 to 1.31). The cumulative Z‐curve (blue line) does not cross the diversity‐adjusted trial monitoring boundary for benefit.

Trial Sequential Analysis of two randomised clinical trials (88 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on cardiovascular adverse events. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The diversity‐adjusted trial monitoring boundary for harm was not included in the figure due to insufficient information. The estimated required information size was 4831 participants. Accordingly, with an accrued number of participants of 88, the required number of participants was not achieved.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 1 Mortality: bias control.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 2 Mortality: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 3 Mortality: type of hepatorenal syndrome.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 4 Mortality: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 5 Hepatorenal syndrome: type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 6 Hepatorenal syndrome: type hepatorenal syndrome.
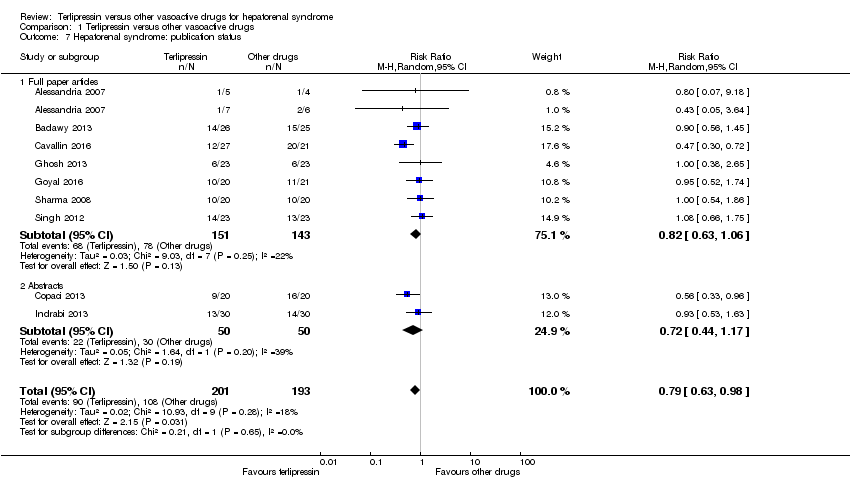
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 7 Hepatorenal syndrome: publication status.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 8 Serious adverse events, type of vasoactive drug.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 9 Serious adverse events, type of event.
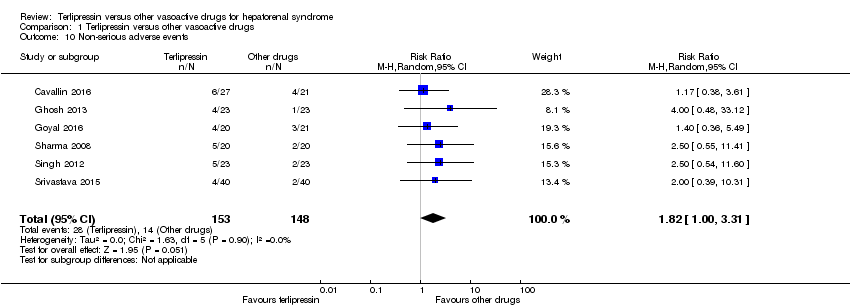
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 10 Non‐serious adverse events.

Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 11 Non‐serious adverse event: types.
| Terlipressin compared to other vasoactive drugs for hepatorenal syndrome | ||||||
| Patient or population: people with cirrhosis and hepatorenal syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with other vasoactive drugs | Risk with terlipressin | |||||
| Mortality (All‐cause) | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, 8/10 randomised clinical trials were at high risk of bias and, the results of Trial Sequential Analysis. | |
| 601 per 1000 | 577 per 1000 | |||||
| Hepatorenal syndrome (Number of participants who did not achieve reversal of hepatorenal syndrome) | Study population | RR 0.79 | 394 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 560 per 1000 | 442 per 1000 | |||||
| Serious adverse events | Study population | RR 0.96 | 474 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 609 per 1000 | 585 per 1000 | |||||
| Non‐serious adverse events: diarrhoea or abdominal pain, or both | Study population | RR 3.50 | 221 | ⊕⊝⊝⊝ | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of the Trial Sequential Analysis. | |
| 19 per 1000 | 65 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn the assessment of mortality, we classified two randomised clinical trials at low risk of bias and eight at high risk of bias. bThe randomised clinical trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that sample size did not reach the required information size for equivalence/inferiority meta‐analysis. cClinical heterogeneity. dWe classified all randomised clinical trials at high risk of bias in all non‐mortality outcomes. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality: bias control Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 1.1 Low risk of bias | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 1.2 High risk of bias | 8 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 2 Mortality: type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 2.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.40, 1.28] |
| 2.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 2.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 3 Mortality: type of hepatorenal syndrome Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 3.1 Type 1 hepatorenal syndrome | 9 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3.2 Type 2 hepatorenal syndrome | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 4 Mortality: publication status Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Full paper | 8 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 4.2 Abstract | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 5 Hepatorenal syndrome: type of vasoactive drug Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| 5.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.21] |
| 5.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.72] |
| 5.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 6 Hepatorenal syndrome: type hepatorenal syndrome Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 6.1 Type 1 hepatorenal syndrome | 8 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 6.2 Type 2 hepatorenal syndrome | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.36, 2.10] |
| 7 Hepatorenal syndrome: publication status Show forest plot | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 7.1 Full paper articles | 7 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 7.2 Abstracts | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Serious adverse events, type of vasoactive drug Show forest plot | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 8.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.23] |
| 8.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 8.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 9 Serious adverse events, type of event Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Death | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 9.2 Major cardiovascular events | 7 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.13, 5.98] |
| 10 Non‐serious adverse events Show forest plot | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.00, 3.31] |
| 11 Non‐serious adverse event: types Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Diarrhoea or abdominal pain, or both | 5 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.19, 10.27] |
| 11.2 Peripheral cyanosis | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 27.83] |
| 11.3 Minor cardiovascular events | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.93] |

