نقش دنرواسیون کلیه برای هیپرتانسیون مقاوم به درمان
چکیده
پیشینه
هیپرتانسیون مقاوم به درمان میان جمعیت عمومی هیپرتانسیو بسیار شایع است و مدیریت بالینی این وضعیت همچنان مشکلساز است. رویکردهای مختلف، از جمله درمان آنتیهیپرتانسیو قویتر، تغییر سبک زندگی یا هر دو، تا حد زیادی در بهبود پیامدهای بیماران و کاهش خطر ابتلا به بیماری قلبیعروقی و کلیوی ناموفق بودهاند. از آنجایی که کارکرد بیش از حد بالای کلیهها، از علل اصلی هیپرتانسیون مقاوم است، تخریب عصب سمپاتیک کلیه (دنرواسیون کلیه (renal denervation))، اخیرا به عنوان جایگزین احتمالی درمانی برای درمان این وضعیت پیشنهاد شده است.
اهداف
ما به دنبال ارزیابی تاثیرات کوتاهمدت و طولانیمدت دنرواسیون کلیه در افراد مبتلا به هیپرتانسیون مقاوم به درمان بر پیامدهای بالینی بودیم، از جمله رویدادهای قلبیعروقی کشنده و غیر‐کشنده، مورتالیتی به هر علتی، بستری شدن در بیمارستان، کیفیت زندگی، کنترل فشار خون، هیپرتروفی بطن چپ، پروفایل قلبیعروقی و متابولیک، و عملکرد کلیه و همچنین حوادث جانبی بالقوه مرتبط با این پروسیجر.
روشهای جستوجو
بانکهای اطلاعاتی زیر را با استفاده از کلمات جستوجوی مرتبط تا 17 فوریه 2016 جستوجو کردیم: پایگاه ثبت تخصصی گروه هیپرتانسیون در کاکرین، پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ MEDLINE؛ EMBASE و ClinicalTrials.gov.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شدهای (randomised controlled trials; RCTs) را که دنرواسیون کلیه را با درمان استاندارد یا پروسیجر ساختگی برای هیپرتانسیون مقاوم به درمان مقایسه کردند، بدون اعمال محدودیت زبانی، در نظر گرفتیم.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم دادهها را استخراج کرده و خطرات سوگیری (bias) مطالعه را بررسی کردند. اثرات درمان را بر پیامدهای بالینی موجود و حوادث جانبی را با استفاده از متاآنالیز اثرات‐تصادفی خلاصه کردیم. با استفاده از آمارههای Chi² و I²، ناهمگونی را در اثرات تخمین زده شده درمان ارزیابی کردیم. خلاصه تخمینهای درمان را با تفاوت میانگین (MD) یا تفاوت میانگین استاندارد شده (SMD) برای پیامدهای پیوسته و با خطر نسبی (RR) برای پیامدهای دو‐حالتی همراه با 95% فواصل اطمینان (CI) محاسبه کردیم.
نتایج اصلی
12 مطالعه واجد شرایط (1149 شرکتکننده) را یافتیم. در چهار مطالعه، دنرواسیون کلیه با پروسیجر ساختگی مقایسه شد؛ یک مطالعه، تخریب پروگزیمال را با دنرواسیون کامل کلیه مقایسه کرد؛ در مطالعات باقیمانده، دنرواسیون کلیه در مقابل درمان استاندارد یا آنتیهیپرتانسیو قویتر تست شد.
هیچ کدام از کارآزماییهای وارد شده به منظور دستیابی به نقاط پایانی بالینی سخت به عنوان پیامدهای اولیه طراحی نشده بودند.
در مقایسه با کنترل، شواهد با کیفیت پائین وجود داشت که نشان داد دنرواسیون کلیه، خطر ابتلا به انفارکتوس میوکارد (4 مطالعه، 742 شرکتکننده؛ RR: 1.31؛ 95% CI؛ 0.45 تا 3.84)، سکته مغزی ایسکمیک (4 مطالعه، 823 شرکتکننده؛ RR: 1.15؛ 95% CI؛ 0.36 تا 3.72) یا آنژین ناپایدار (2 مطالعه، 201 شرکتکننده؛ RR: 0.63؛ 95% CI؛ 0.08 تا 5.06) را کاهش نداد و شواهد با کیفیت متوسط نشان داد که دنرواسیون کلیه، تاثیری بر پایش فشار خون آمبولاتوری (ambulatory blood pressure monitoring; ABPM) فشار خون سیستولیک در 24 ساعت (5 مطالعه، 797 شرکتکننده؛ MD: 0.28 میلیمتر جیوه؛ 95% CI؛ 3.74‐ تا 4.29)، BP دیاستولیک (4 مطالعه، 756 شرکتکننده؛ MD: 0.93 میلیمتر جیوه؛ 95% CI؛ 4.50‐ تا 6.36)، BP سیستولیک (6 مطالعه، 886 شرکتکننده؛ MD: ‐4.08 میلیمتر جیوه ؛ 95% CI؛ 15.26‐ تا 7.11) یا BP دیاستولیک (5 مطالعه، 845 شرکتکننده؛ MD: ‐1.30 میلیمتر جیوه ؛ 95% CI؛ 7.30‐ تا 4.69) اندازهگیری شده در مطب ندارد. علاوه بر این، شواهد با کیفیت پائین نشان داد که این پروسیجر هیچ تاثیری بر سرم کراتینین (3 مطالعه، 736 شرکتکننده؛ MD: 0.01 میلیگرم/دسیلیتر؛ 95% CI؛ 0.12‐ تا 0.14)، تخمین نرخ فیلتراسیون گلومرولار (estimated glomerular filtration rate; eGFR) یا دفع کراتینین نداشت (4 مطالعه، 837 شرکتکننده، MD: ‐2.09 میلیلیتر/دقیقه؛ 95% CI؛ 8.12‐ تا 3.95). بر اساس شواهد با کیفیت پائین، دنرواسیون کلیوی به طور معنیداری اپیزودهای برادیکاردی را در مقایسه با کنترل افزایش داد (3 مطالعه، 220 شرکتکننده؛ RR: 6.63؛ 95% CI؛ 1.19 تا 36.84)، در حالی که خطر سایر حوادث جانبی قابل مقایسه یا قابل ارزیابی نبود.
دادههای مربوط به مورتالیتی به هر علتی، بستری شدن در بیمارستان، رویدادهای قلبیعروقی کشنده، کیفیت زندگی، اپیزودهای فیبریلاسیون دهلیزی، هیپرتروفی بطن چپ، شدت آپنه خواب، نیاز به درمان با پیوند کلیه و پروفایل متابولیک یا اندک بودند یا وجود نداشت.
شواهد مربوط به پیامدهای قلبیعروقی و حوادث جانبی، دارای کیفیت پائین و شواهد مربوط به عدم تاثیر بر فشار خون و عملکرد کلیه، دارای کیفیت متوسط بودند.
نتیجهگیریهای نویسندگان
شواهد با کیفیت پائین درباره بیماران مبتلا به هیپرتانسیون مقاوم به درمان وجود دارد که نشان میدهد دنرواسیون کلیه، رویدادهای عمده قلبیعروقی و عملکرد کلیه را تغییر نمیدهد. شواهد با کیفیت متوسط وجود داشت که نشان داد دنرواسیون کلیه، فشار خون را تغییر نمیدهد و شواهد با کیفیت پائین نشان داد که دنرواسیون کلیه باعث افزایش اپیزودهای برادیکاردی میشود. کارآزماییهای آینده بر اساس پیامدهای بیمار‐محور به جای پیامدهای جایگزین، با دورههای پیگیری طولانیمدت، حجم نمونه بزرگتر و روشهای پروسیجرال استانداردتر برای مشخص کردن کاربرد این پروسیجر در این جمعیت ضروری هستند.
PICO
خلاصه به زبان ساده
دنرواسیون کلیه برای بهبود پیامدها در افراد مبتلا به هیپرتانسیون مقاوم به درمان
سوال مطالعه مروری
مزایا و آسیبهای دنرواسیون کلیه (renal denervation) در افراد دارای هیپرتانسیون مقاوم به درمان بر پیامدهای مهم بالینی شامل موربیدیتیها و مورتالیتیهای قلبیعروقی، کنترل فشار خون، عملکرد کلیه و وقوع حوادث جانبی متعدد چه هستند.
پیشینه
هیپرتانسیون مقاوم به درمان، وضعیتی است که با سطوح بالای فشار خون علیرغم مصرف چندین داروی پائین آورنده فشار خون (آنتیهیپرتانسیو) با حداکثر دوز، تشخیص داده میشود. تخمین شیوع این بیماری بین 10% تا 20% از جمعیت عمومی هیپرتانسیو است. علیرغم رویکردهای درمانی و شیوه زندگی که پیشنهاد شده، مدیریت افراد مبتلا به هیپرتانسیون مقاوم به درمان با بروز بالای پیامدهای ضعیف و حوادث قلبیعروقی جانبی دشوار است. اخیرا، دنرواسیون عصب سمپاتیک کلیه، یک پروسیجر شامل تخریب اعصاب کلیه با استفاده از یک کاتتر رادیوفرکوئنسی که از طریق ایجاد حداقل یک شکاف با حداقل تهاجم وارد میشود، به عنوان یک درمان جایگزین احتمالی برای درمان این بیماری ظاهر شده است.
ویژگیهای مطالعه
دوازده مطالعه با کیفیت متغیر شناسایی شدند که در مجموع شامل 1149 شرکتکننده بودند. میان مطالعات از نظر طراحی، روشها و کورسازی محققین، ناهمگونی زیادی وجود داشت. بیشتر مطالعات، تاثیر دنرواسیون کلیه را بر پیامدهای جایگزین (به عنوان مثال کنترل فشار خون) به جای پیامدهای بیمار‐محور (به عنوان مثال مورتالیتی یا کیفیت زندگی) بررسی کردند.
نتایج کلیدی
به طور کلی، هیچ شواهدی مربوط به اثربخشی دنرواسیون کلیه نسبت به درمان استاندارد بر موربیدیتی و مورتالیتی قلبیعروقی وجود نداشت. به طور مشابه، دنرواسیون کلیه، تاثیرات ملموسی بر کنترل فشار خون و عملکرد کلیه نداشت. با این حال، با افزایش خطر اپیزودهای برادیکاردی (ضربان قلب بسیار پائین) مرتبط بود.
کیفیت شواهد
کیفیت شواهد برای موربیدیتی قلبیعروقی و حوادث جانبی در سطح پائین و برای عدم تاثیر بر فشار خون و عملکرد کلیه در سطح متوسط بود. شواهد تا 17 فوریه 2016 بهروز است.
نتیجهگیریها
شواهد موجود برای حمایت از استفاده از دنرواسیون کلیه برای بهبود خطر بیماری قلبیعروقی و کلیوی و کنترل فشار خون در بیماران مبتلا به هیپرتانسیون مقاوم به درمان نامشخص است. مطالعات آینده با هدف قرار دادن پیامدهای بیمار‐محور، با طولانیتر کردن دوره پیگیری و تعداد بیشتر شرکتکنندگان برای پی بردن به اینکه این پروسیجر میتواند برای این افراد مزیتی داشته باشد یا خیر، مورد نیاز هستند.
Authors' conclusions
Summary of findings
| Renal denervation versus sham denervation or standard treatment | |||||
| Patient or population: people with resistant hypertension | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect estimate | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Sham denervation/ Standard treatment | Renal denervation | ||||
| myocardial infarction | 14 per 1000 | 18 per 1000 (6 to 54) | RR 1.31 (0.45 to 3.84) | 742 (4 studies) | ⊕⊕⊝⊝ |
| ischaemic stroke | 12 per 1000 | 14 per 1000 (4 to 45) | RR 1.15 (0.36 to 3.72) | 823 (4 studies) | ⊕⊕⊝⊝ |
| unstable angina | 20 per 1000 | 12 per 1000 (2 to 101) | RR 0.63 (0.08 to 5.06) | 201 (2 studies) | ⊕⊕⊝⊝ |
| systolic 24‐hour ABPM (mmHg) | ‐ | ‐ | MD 0.28 (‐3.74 to 4.29) | 797 | ⊕⊕⊕⊝ |
| diastolic 24‐hour ABPM (mmHg) | ‐ | ‐ | MD 0.93 (‐4.50 to 6.36) | 756 | ⊕⊕⊕⊝ |
| systolic office BP (mmHg) | ‐ | ‐ | MD ‐4.08 (‐15.26 to 7.11) | 886 | ⊕⊕⊕⊝ |
| diastolic office BP (mmHg) | ‐ | ‐ | MD ‐1.30 (‐7.30 to 4.69) | 845 | ⊕⊕⊕⊝ |
| eGFR or creatinine clearance (mL/min/1.73m²) | ‐ | ‐ | MD ‐2.09 (‐8.12 to 3.95) | 837 | ⊕⊕⊕⊝ |
| *The assumed risk is the observed risk in the reference (control) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence Legend 1. Wide confidence intervals. 2. Only reported by less than half of the studies. | |||||
Background
Description of the condition
Resistant or refractory hypertension is characterised by blood pressure levels persistently above target, in spite of the concurrent use of three antihypertensive agents of different classes at best‐tolerated doses, including a diuretic (Calhoun 2008). Data from cross‐sectional and hypertension outcome studies suggest that this condition is not infrequent, with an estimated prevalence of 10% to 20% in the general hypertensive population (Myat 2012). Individuals with resistant hypertension are 50% more likely to experience poor outcomes and adverse cardiovascular events than those with controlled hypertension (Judd 2014). The lack of efficacy of multiple interventions in addition to pharmacological therapy, including dietary and lifestyle modifications, emphasises the importance of finding new effective and safe treatments for treating this condition.
Description of the intervention
Renal sympathetic denervation comprises the ablation of renal afferent and efferent nerves by a radiofrequency catheter through a minimally invasive, percutaneous intervention performed via femoral access. The thermal increase generated by the application of low‐dose radiofrequency energy is effective in disrupting large portions of nervous fibres located within the adventitia of the renal artery.
How the intervention might work
Sympathetic hyperactivity has long been acknowledged as a major player in the genesis of resistant hypertension (Huan 2013). In studies conducted in the eighties, surgical sympathectomy was effective in some individuals in lowering blood pressure and symptoms associated with severe hypertension. However, this procedure is no longer used because of considerable side effects (Leong 2014). As with sympathectomy, renal denervation might improve blood pressure control by reducing abnormal renal adrenergic nerve activity. Furthermore, since other conditions, such as congestive heart failure, atrial fibrillation, sleep breathing disorders, and diabetes mellitus are all associated with an overactive sympathetic drive, this procedure might result in pleiotropic benefits, including improvements in glycaemic levels, sleep apnoea, arrhythmias, and oxidative stress (Witkowski 2011). Of note, in spontaneously hypertensive rats, renal denervation was able to ameliorate metabolic control and to prevent hypertensive stroke and brain injury, in addition to controlling blood pressure (Nakagawa 2013a; Nakagawa 2013b).
Why it is important to do this review
As shown in a recent meta‐analysis, renal denervation reduced mean blood pressure at six months in individuals with persistent hypertension; intra‐procedural complications, including renal artery dissection and femoral pseudoaneurysms were rare (Davis 2013). Unfortunately, data were mostly derived from observational, uncontrolled studies with limited follow‐up, small sample sizes, and high heterogeneity in blood pressure measurement. Whether the benefits of renal denervation on blood pressure control are maintained in the long term, and particularly, whether this procedure might impact hard outcomes, such as mortality and cardiovascular events, remain unknown at this time. New evidence, based on larger, randomised controlled trials (RCTs), is now accruing, and long‐term data on the efficacy of renal denervation on surrogate and hard end points in the long term are becoming available. Therefore, an updated assessment of the efficacy and safety profile of this procedure is mandatory to define whether the benefits of implementing renal denervation in the clinical management of individuals with resistant hypertension outweigh the harms.
Objectives
To evaluate the short‐ and long‐term effects of renal sympathetic denervation in individuals with resistant hypertension on:
-
patient‐centred end points, including cardiovascular morbidity and mortality, all‐cause mortality, hospital admissions, and quality of life;
-
blood pressure control;
-
cardiovascular and metabolic profile;
-
kidney function;
-
adverse events, including but not limited to bradycardia, hypotension episodes, femoral artery pseudoaneurysm, and renal artery dissection.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth, or other predictable methods) of individuals with resistant hypertension undergoing renal sympathetic denervation procedures, without duration or language restrictions.
Types of participants
Adults (older than 18 years), with refractory or resistant hypertension, defined by the presence of a clinic blood pressure above target (higher than 140/90 mmHg, or higher than 130/80 mmHg in individuals with type 2 diabetes mellitus), despite the concomitant use of three or more antihypertensive drugs of different classes, including a diuretic.
Types of interventions
Any transcatheter renal sympathetic denervation procedures performed using contemporary percutaneous catheters and radiofrequency probes compared with standard medical therapy or sham intervention.
Types of outcome measures
Primary outcomes
-
Fatal and non‐fatal cardiovascular events, including but not limited to myocardial infarction, cerebrovascular accidents, and congestive heart failure
-
All‐cause mortality
-
Any hospitalisation and duration of hospital stay (if long‐term data are available)
-
Quality of life (assessed using validated scales or any other instrument as reported by authors, such as the Short‐Form Health Survey (SF‐36))
Secondary outcomes
-
Blood pressure control (change in office and clinic systolic, diastolic, and mean blood pressure)
-
Left ventricular hypertrophy
-
Atrial fibrillation episodes
-
Obstructive sleep apnoea severity (apnoea‐hypopnoea index)
-
Kidney function (change in serum creatinine, glomerular filtration rate (GFR), proteinuria or albuminuria, need for renal replacement therapy)
-
Metabolic profile (change in lipid and blood glucose levels and insulin resistance indices)
-
Withdrawal due to adverse effects, including but not limited to bradycardia and hypotensive episodes, femoral artery pseudoaneurysm, renal artery dissection, transient dizziness, pitting oedema, flank pain, and anaemia
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches in the following databases for randomised controlled trials without language, publication year or publication status restrictions:
-
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 16 February 2016);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2) via the Cochrane Register of Studies Online (CRSO) (searched 15 February 2016);
-
MEDLINE Ovid (from 1946 onwards), and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 15 February 2016);
-
PubMed (searched 16 February 2016);
-
Embase Ovid (searched 15 February 2016);
-
ClinicalTrials.gov (www.clinicaltrials.gov) searched 15 February 2016);
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) searched 15 February 2016).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases are provided in Appendix 1.
Searching other resources
-
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE and Epistemonikos for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
-
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
-
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
-
We did not perform a separate search for adverse effects of interventions used for the treatment of hypertension. We considered adverse effects described in included studies only.
-
We checked the reference lists of cardiology and nephrology textbooks for additional resources.
Data collection and analysis
Selection of studies
Two authors (AP and LR) independently screened titles and abstracts, and retained studies and reviews that might include relevant data or information on trials for review in detail; studies that were not applicable were excluded. The same authors (AP and LR) independently assessed retrieved abstracts, and if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Two authors (AP and LR) independently carried out data extraction using a standard electronic data extraction form. We arranged for translations of studies reported in non‐English language journals before assessment. If more than one publication of a study existed, we grouped the reports together and used the publication with the most complete data in the analyses. If relevant outcomes were published only in earlier versions of the study, we used such data.
Assessment of risk of bias in included studies
Two authors (AP and DB) independently assessed the following items using the 'Risk of bias' assessment tool (Higgins 2011).
-
Sequence generation (selection bias);
-
Allocation concealment (selection bias);
-
Blinding (detection bias)
-
Participants and personnel
-
Outcome assessors;
-
-
Completeness of outcome data (attrition bias);
-
Selective outcome reporting (reporting bias);
-
Other sources of bias:e.g. funding bias.
Measures of treatment effect
We expressed dichotomous outcome results as risk ratios (RRs) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, we reported results as mean differences (MD) or standardised mean differences (SMD) if different scales were reported, with 95% CI.
Unit of analysis issues
We appraised unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Dealing with missing data
We requested additional information from the corresponding author(s) by email. We carefully evaluated important data, such as numbers of screened and randomised participants, as well as numbers of intention‐to‐treat, as‐treated, and per‐protocol populations. We explored attrition in the study, such as drop‐outs, losses to follow‐up, and withdrawals. We appraised issues of missing data and imputation methods (such as last‐observation‐carried‐forward) according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We tested heterogeneity with a Chi² test on n ‐ 1 degrees of freedom, using an alpha of 0.05 for statistical significance, and used the I² statistic (Higgins 2003). We considered I² values of 25%, 50%, and 75% to correspond to low, medium, and high levels of heterogeneity.
Assessment of reporting biases
Where possible, we had planned to construct funnel plots to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
We analysed data for each outcome using Review Manager 5.3 (RevMan 2014) in an attempt to estimate the overall effect. We used the Mantel‐Haenszel method for the fixed‐effect model, except when statistical heterogeneity was observed, in which case we applied the random‐effects model, to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We had planned subgroup analyses to explore possible sources of heterogeneity (e.g. participants, treatments). Heterogeneity among participants could be related to age, the presence of comorbidities (e.g. diabetes, cardiovascular diseases), the presence or severity of renal function impairment, and the duration and severity of hypertension (e.g. number and dosage of antihypertensive drugs used). Heterogeneity in treatments could be related to the type and duration of the renal sympathetic denervation procedure and the type of catheter and radiofrequency probe used. We also planned an exploration of the effect of short‐ and long‐term follow‐up as a source of significant heterogeneity between studies.
Sensitivity analysis
If applicable, we had planned sensitivity analyses to explore the influence of the following factors on effect size:
-
repeating the analysis excluding any large studies, to establish how much they impact on the results;
-
repeating the analysis taking into account the risk of bias;
-
repeating the analysis excluding unpublished studies.
Summary of findings' table
We had planned to construct a summary table via the GRADEpro‐GDT(GRADEpro GDT 2015), reporting:
-
a summary of findings from all the primary outcomes
-
a summary of findings from some secondary outcomes, that have been pre‐selected according to their clinical importance. These include blood pressure outcomes (24 h‐ABPM and office blood pressure), renal function (serum creatinine and eGFR), bradycardia and hypotensive episodes.
-
the quality of the body of evidence supporting each of these outcomes
Results
Description of studies
The literature search was current to 17 February 2016.
Results of the search
The search identified 1057 records; we also identified two additional records from personal searches. Full‐text assessment of 157 records resulted in the inclusion of twelve eligible studies (58 articles), comprising a total of 1149 participants (DENER‐HTN 2015; Desch 2015; Franzen 2012; Oslo RDN 2014; Prague‐15 2016; RELIEF 2012; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Warchol 2014; Xiang 2014; HTN‐JAPAN 2015; ReSET 2015), and 26 ongoing trials (30 articles; INSPIRED; DEPART; RENO; RSD4CKD; ReSET‐2; RENSYMPIS; NCT01848275; RDNP‐2012‐01; ALLEGRO‐HTN; PaCE; EnligHTN IV; RAPID II; NCT01968785; SYMPLICITY HTN‐4; KPS; NCT02021019; DENERVHTA; ENSURE; NCT02346045; RSDforAF; SYMPATHY; NCT02444442; NCT02608632; NCT02667912; NCT01918111; NTR3444). We contacted the authors of some of the included studies for additional information about study methods, and unreported data; three investigators responded to our queries (DENER‐HTN 2015; Prague‐15 2016; SYMPLICITY HTN‐2 2010). Figure 1 depicts the flow of study selection.

Study flow diagram.
Included studies
All twelve included studies were parallel RCTs (Characteristics of included studies). All studies were conducted in adults. Study duration ranged from 3 to 12 months. All studies except DENER‐HTN 2015, Warchol 2014, and ReSET 2015 excluded patients with estimated glomerular filtration rate (eGFR) less than 45 mL/min/1.73 m². The renal sympathetic denervation procedure was performed with the electrode radiofrequency Symplicity catheter system in nine studies (DENER‐HTN 2015; Desch 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Warchol 2014; HTN‐JAPAN 2015; ReSET 2015). Ablation was performed with an off‐the‐shelf saline‐irrigated radiofrequency catheter in RELIEF 2012. In Xiang 2014, ablation was made with the IBI‐Therapy, St. Jude Medical radiofrequency catheter. In Franzen 2012, details of the denervation procedure were not provided. In seven studies, a series of four to six ablations per renal artery was performed (DENER‐HTN 2015; Desch 2015; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Xiang 2014; HTN‐JAPAN 2015). In Oslo RDN 2014, an average of eight (range 6 to11) radiofrequency ablations were applied per renal artery. The number of ablations was not reported in four studies (Franzen 2012; RELIEF 2012; Warchol 2014; ReSET 2015). In four studies, renal denervation was compared to sham procedure (Desch 2015; RELIEF 2012; SYMPLICITY HTN‐3 2014; ReSET 2015). Xiang 2014 compared a proximal ablation to a complete renal artery denervation. SYMPLICITY HTN‐2 2010, Warchol 2014, Franzen 2012, and HTN‐JAPAN 2015 compared renal denervation plus antihypertensive medications with antihypertensive medications alone. In three studies, the effects of renal denervation plus standard antihypertensive therapy were tested against an intensified pharmacological regimen (DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016). Outcomes available from studies were: incidence of myocardial infarction (DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐3 2014), ischaemic stroke (DENER‐HTN 2015; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014), unstable angina (Prague‐15 2016; SYMPLICITY HTN‐2 2010), all‐cause‐mortality and hospitalisations (SYMPLICITY HTN‐3 2014), 24‐hour ambulatory blood pressure monitoring (ABPM) or blood pressure (BP; DENER‐HTN 2015; Desch 2015; Oslo RDN 2014; Prague‐15 2016; RELIEF 2012; SYMPLICITY HTN‐3 2014; Warchol 2014; ReSET 2015; HTN‐JAPAN 2015), office ABPM or BP (DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; RELIEF 2012; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Warchol 2014; Xiang 2014; HTN‐JAPAN 2015), home BP (DENER‐HTN 2015; HTN‐JAPAN 2015), left ventricular hypertrophy (Prague‐15 2016), and kidney function (serum creatinine, eGFR; DENER‐HTN 2015; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; HTN‐JAPAN 2015). In addition, DENER‐HTN 2015; Desch 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Xiang 2014, and HTN‐JAPAN 2015 looked systematically at the incidence of adverse effects associated to the procedure.
Excluded studies
We excluded 698 records, 629 of which were excluded at title and abstract screening (Figure 1). Sixty‐nine records were excluded after full‐text evaluation. Reasons for exclusion were: inappropriate population, problem, or both (163 reports); inappropriate intervention, outcome, or both (328 reports); not an RCT (28 reports); editorial, comment or letter without reporting randomised trial data (179 reports). See Characteristics of excluded studies.
Risk of bias in included studies
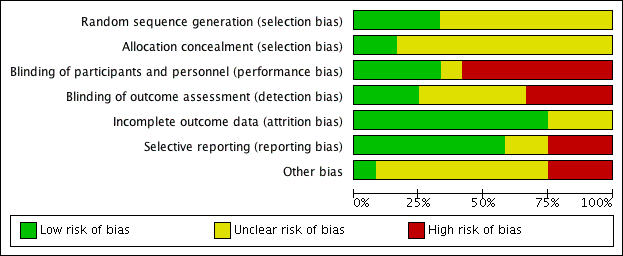
We have shown summaries of the risks of bias in the included studies in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The overall risk of selection bias was highly variable. Random sequence generation was detailed in four studies with a low risk of bias (DENER‐HTN 2015; Desch 2015; Oslo RDN 2014; Xiang 2014), while there were insufficient data to inform assessment in the remainder. Only two of the included studies adequately described the allocation concealment methodologies that were applied (Oslo RDN 2014; SYMPLICITY HTN‐2 2010); this information was not stated in the remainder.
Blinding
The risk of performance and detection bias was also variable. Six studies were fully open label, thus allowing a high risk of both biases (Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010; Warchol 2014; Xiang 2014; HTN‐JAPAN 2015). DENER‐HTN 2015 was an open‐label trial but outcome assessors were blinded to the procedure. ReSET 2015 was double‐blinded; participants and personnel were unaware of treatment arm, while blinding of outcome assessment was not stated. In Desch 2015 and SYMPLICITY HTN‐3 2014, participants and outcome assessors were blinded to the treatment. In RELIEF 2012, patients were blinded to renal denervation or sham procedure, while outcome assessor blinding was unclear. In Franzen 2012, no overall information on blinding was specified.
Incomplete outcome data
The overall drop‐out rate ranged from 3% to 37% with no differences among groups, with the exception of DENER‐HTN 2015 and SYMPLICITY HTN‐3 2014, in which drop‐outs were more prevalent in the treatment arm, and in Prague‐15 2016, in which 31 participants (62%) dropped out from the control group. Four studies reported no drop‐outs (Oslo RDN 2014; Warchol 2014; Xiang 2014; HTN‐JAPAN 2015). The information provided on attrition bias was insufficient to permit assessment in three studies (Franzen 2012; RELIEF 2012; ReSET 2015). Six studies were analysed on an intention‐to‐treat basis (DENER‐HTN 2015; Oslo RDN 2014; SYMPLICITY HTN‐3 2014; Warchol 2014; Xiang 2014; HTN‐JAPAN 2015). In SYMPLICITY HTN‐2 2010, analyses were performed on a per‐protocol basis. In Desch 2015 and Prague‐15 2016, results were analysed on both a per‐protocol and intention‐to‐treat basis.
Selective reporting
All the predefined outcomes were reported in seven studies (DENER‐HTN 2015; Desch 2015; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Xiang 2014; HTN‐JAPAN 2015). Some prespecified outcomes were not reported in RELIEF 2012 (office BP, serum creatinine) or in ReSET 2015 (daytime and night time BP, dipping status, diastolic and systolic ventricular function, left ventricular hypertrophy, renal sodium excretion, pulse wave velocity, a 25% or more decline in eGFR). Possible selective reporting was unclear in the remainder.
Other potential sources of bias
Five studies declared to be funded from industry (DENER‐HTN 2015; Oslo RDN 2014; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; HTN‐JAPAN 2015). In DENER‐HTN 2015, the authors stated that the sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. In Oslo RDN 2014, the involvement of industry was unclear. In SYMPLICITY HTN‐2 2010, SYMPLICITY HTN‐3 2014, and HTN‐JAPAN 2015 the authors declared that data were monitored, collected, and managed by the sponsor. No other sources of apparent bias were noticed in the other studies.
Effects of interventions
See: Summary of findings for the main comparison
The main effects of renal denervation on the primary outcomes and on the most important secondary outcomes are summarized in summary of findings Table for the main comparison.
Primary outcomes
Non‐fatal cardiovascular events
In a meta‐analysis of four studies (742 participants), renal denervation was not significantly associated with a lower risk of myocardial infarction than sham or standard treatment (RR 1.31, 95% CI 0.45 to 3.84; Analysis 1.1); there was no heterogeneity (Chi² = 0.79; P = 0.85; I² = 0%; DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐3 2014). In data pooled from four studies (823 participants), renal denervation was not significantly associated with a lower risk of ischaemic stroke than no treatment (RR 1.15, 95% CI 0.36 to 3.72; Analysis 1.2); there was no heterogeneity (Chi² = 1.27; P = 0.74; I² = 0%; DENER‐HTN 2015; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014). In a meta‐analysis of two studies (201 participants), renal denervation was not associated with a lower risk of unstable angina than standard therapy (RR 0.63, 95% CI 0.08 to 5.06; Analysis 1.3); there was no heterogeneity (Chi² = 0.29; P = 0.59; I² = 0%; Prague‐15 2016; SYMPLICITY HTN‐2 2010).
All‐cause mortality
Data on all‐cause mortality were provided by one study, in which two patients in the renal denervation group and one in the sham procedure group died (SYMPLICITY HTN‐3 2014).
Hospitalisation
Data on hospitalisation were only available in SYMPLICITY HTN‐3 2014. Five patients in the renal denervation and one patient in the sham group had hospital admissions for atrial fibrillation episodes; nine patients in the renal denervation group and three in the sham group required hospitalisation for a new‐onset of heart failure.
Secondary outcomes
24‐hour ambulatory blood pressure monitoring (ABPM)
Twenty‐four hour ABPM was measured in eight studies (DENER‐HTN 2015; Desch 2015; Oslo RDN 2014; Prague‐15 2016; RELIEF 2012; SYMPLICITY HTN‐3 2014; HTN‐JAPAN 2015; ReSET 2015). In a meta‐analysis of five studies (797 participants), renal denervation did not produce significant changes in systolic 24‐hour ABPM when compared with sham or standard therapy (MD 0.28 mmHg, 95% CI ‐3.74 to 4.29; Analysis 1.4); there was low heterogeneity (Chi² = 7.27; P = 0.12; I² = 45%; DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐3 2014; HTN‐JAPAN 2015). Similarly, renal denervation was not superior to sham or standard therapy in reducing diastolic 24‐hour ABPM (4 studies, 756 participants; MD 0.93 mmHg, 95% CI ‐4.50 to 6.36; Analysis 1.5). There was high heterogeneity in this latter analysis (Chi² = 22.50, P < 0.0001; I² = 87%) that could not be further explained due to the paucity of studies available (DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐3 2014).
In RELIEF 2012, the 24‐hour systolic/diastolic BP decreased by ‐17/‐12 mmHg (P = 0.006/P = 0.001) in the bilateral renal denervation group versus ‐5/‐5 mmHg (P = 0.22/P = 0.42) in the sham control group. In ReSET 2015, renal denervation (RD) and sham procedures showed a similar reduction in 24‐hour systolic ABPM after six‐month follow‐up (‐6.1 ± 18.9 (RD) versus ‐4.3 ± 15.1 mmHg (sham)). HTN‐JAPAN 2015 recorded no difference between groups in 24‐hour diastolic BP (‐3.8 mmHg, 95% CI ‐8.3 to 0.6; P = 0.091). In Desch 2015, the mean change for the 24‐hour systolic BP was −7.0 mmHg (95%CI −10.8 to −3.2) for patients undergoing renal denervation and −3.5 mmHg (95%CI −6.7 to −0.2) in the sham group (P = 0.15), as analysed on an intention‐to‐treat basis. In the per‐protocol population, the change in 24‐hour systolic BP at six months was −8.3 mmHg (95%CI −11.7 to −5.0) for patients undergoing renal denervation and −3.5 mmHg (95%CI −6.8 to −0.2) in the sham group (P = 0.042). No statistically significant changes in 24‐hour diastolic BP were recorded in either the intention‐to‐treat or per‐protocol analysis. All these single‐study data were directly retrieved from the correspondent papers.
Office BP
In separate meta‐analyses of six studies (886 participants) and five studies (845 participants), renal denervation had no conclusive effects on systolic or diastolic office BP when compared with sham procedure or standard therapy (systolic: MD ‐4.08 mmHg, 95% CI ‐15.26 to 7.11; Analysis 1.6; DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; HTN‐JAPAN 2015; diastolic: MD ‐1.30 mmHg, 95% CI ‐7.30 to 4.69; Analysis 1.7; DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014. There was high heterogeneity in these analyses (Chi² = 59.87; P < 0.00001; I² = 92% and Chi² = 27.44; P < 0.00001; I² = 85%, respectively) that could not be further explained due to the low number of studies included.
In Xiang 2014, at six‐month follow‐up, the average office systolic/diastolic BP decreased significantly from 191.2/98.3 at baseline to 136.3/80.2 mmHg in the group undergoing proximal ablation, and from 181.4/98.5 to 136.5/79.5 mmHg in the group undergoing the whole ablation. HTN‐JAPAN 2015 recorded a greater average diastolic office BP reduction in the renal denervation group than in the control group, with a change difference of ‐6.9 mmHg (95% CI ‐13.2 to 0.5; P = 0.036). These data were obtained from the correspondent study article.
Home BP
In HTN‐JAPAN 2015, no change difference in home systolic and diastolic BP was observed between the renal denervation and control groups (‐5.6 mmHg (95% CI ‐14.5 to 3.2; P = 0.205) and ‐4.8 mmHg (95% CI ‐9.8 to 0.3; P = 0.065), respectively). In DENER‐HTN 2015, the mean change in home systolic and diastolic BP was ‐15.4 mmHg (95% CI ‐20.4 to ‐10.4) and ‐8.7 mmHg (95% CI ‐12.1 to ‐5.4) in patients undergoing renal denervation and ‐11.8 mmHg (95% CI ‐16.5 to ‐7.1) and ‐6.7 mmHg (95% CI‐9.8 to ‐3.5) in the control group, with no differences between groups (P = 0.30 and P = 0.37) for systolic and diastolic BP, respectively.
Left ventricular mass (LVH)
Twelve‐month follow‐up data on left ventricular mass (LVM) and LVM indexed (LVMI) were provided by one study, which reported no significant difference in change between the renal denervation and control groups (10 (95% CI ‐13 to 33) and 2.3 (95% CI ‐2.7 to 7.4) for LVM and LVMI, respectively (Prague‐15 2016).
Kidney function
In a meta‐analysis of three studies (736 participants), renal denervation had no tangible effects over sham or standard treatment on serum creatinine levels (MD 0.01 mg/dL, 95% CI ‐0.12 to 0.14; Analysis 1.8), with high heterogeneity (Chi² = 12.75; P = 0.002; I² = 84%), which could not be further explored, as only three studies were included (Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014). Nevertheless, SYMPLICITY HTN‐3 2014 reported five cases in the renal denervation group and one case in the sham group, who had an increase in serum creatinine levels greater than 50% from baseline. One case of 50% increase in serum creatinine was also reported in the renal denervation group after six months of follow‐up in HTN‐JAPAN 2015.
In another meta‐analysis of four studies (837 participants), renal function, as estimated by eGFR or creatinine clearance, remained unaffected after renal denervation compared to control (MD ‐2.09 mL/min, 95% CI ‐8.12 to 3.95; Analysis 1.9), with moderate heterogeneity (Chi² = 7.09, P = 0.07; I² = 58%), which could not be further explored (DENER‐HTN 2015; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014).
Prague‐15 2016 recorded an unspecified decline in renal function in one patient undergoing the standard treatment.
Adverse events
Major adverse events were systematically collected by seven studies (DENER‐HTN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Xiang 2014; HTN‐JAPAN 2015). HTN‐JAPAN 2015 reported no periprocedural complications in either the renal denervation or control arms. No study provided information on the occurrence of transient dizziness or anaemia.
Bradycardia
In a meta‐analysis of three studies (220 participants), renal denervation was significantly associated with an almost seven‐fold higher risk of bradycardia symptoms than other treatments (RR 6.63, 95% CI 1.19 to 36.84; Analysis 1.10), with no heterogeneity (Chi² = 0.63; P = 0.73; I² = 0%; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010).
Femoral artery pseudoaneurysm
Pooled data from two studies (201 participants) showed that renal denervation was not statistically associated with a higher risk for femoral artery pseudoaneurysm than standard therapy (RR 3.96, 95% CI 0.44 to 35.22; Analysis 1.11), with no heterogeneity (Chi² = 0.04; P = 0.84; I² = 0%; Prague‐15 2016; SYMPLICITY HTN‐2 2010).
Renal artery dissection
In Prague‐15 2016, there was one case of renal artery dissection related to the procedure.
Renal artery vasospasm
Four cases of renal artery vasospasm in patients undergoing renal denervation were observed in Prague‐15 2016. Xiang 2014 reported two cases of renal artery vasospasm in the whole ablation group versus none in the proximal ablation group.
New renal‐artery stenosis
SYMPLICITY HTN‐3 2014 reported one case of re‐stenosis in the renal denervation group (documented as new renal artery stenosis of more than 70%) within the six‐month follow‐up.
Flank pain
In a meta‐analysis of two studies (199 participants), renal denervation was not significantly associated with a higher risk of flank pain than control (RR 4.30, 95% CI 0.48 to 38.28; Analysis 1.12), with no heterogeneity (Chi² = 0.08; P = 0.78; I² = 0%; DENER‐HTN 2015; SYMPLICITY HTN‐2 2010).
Pitting oedema
One case of oedema requiring hospital admission was provided by SYMPLICITY HTN‐2 2010.
Hypotensive episodes
In a meta‐analysis of two studies (119 participants), the renal denervation procedure was not associated with a higher risk of hypotensive episodes than no treatment (RR 0.67, 95% CI 0.07 to 6.64; Analysis 1.13), with low heterogeneity (Chi² = 1.61; P = 0.20; I² = 38%; Oslo RDN 2014; SYMPLICITY HTN‐2 2010).
Hypertensive crisis
In data pooled from three studies (722 participants), renal denervation was not significantly associated with a higher risk for hypertensive episodes than no treatment (RR 0.71, 95% CI 0.35 to 1.45; Analysis 1.14), with no heterogeneity (Chi² = 1.83; P = 0.40; I² = 0%; DENER‐HTN 2015; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014).
Hyperkalemia
In a meta‐analysis of two studies (200 participants), patients in the renal denervation group had no higher risk of hyperkalaemia than those in standard therapy (RR 0.48, 95% CI 0.01 to 21.33; Analysis 1.15). There was moderate heterogeneity in this analysis (Chi² = 3.17; P = 0.07; I² = 68%), which could not be further explored, as only two studies were included (Prague‐15 2016; DENER‐HTN 2015).
Syncope
In DENER‐HTN 2015, one patient in the control group experienced an episode of syncope.
Embolic event
In SYMPLICITY HTN‐3 2014, one case of embolic event resulting in end‐organ damage was reported in the renal denervation group.
Withdrawals
Nine studies provided information on withdrawals (DENER‐HTN 2015; Desch 2015; HTN‐JAPAN 2015; Oslo RDN 2014; Prague‐15 2016; SYMPLICITY HTN‐2 2010; SYMPLICITY HTN‐3 2014; Warchol 2014; Xiang 2014). SYMPLICITY HTN‐3 2014 recorded 14 (3.8%) withdrawals from the renal denervation group and two (1.2%) from the control arm. In SYMPLICITY HTN‐2 2010, there were three withdrawals from both the intervention and control arms. DENER‐HTN 2015 reported five (10%) withdrawals from the renal denervation group. In Desch 2015, six participants (17%) withdrew from the renal denervation and two (5.55%) from the sham group. Prague‐15 2016 recorded seven (13.7%) and 31 (62%) withdrawals from the renal denervation and control groups, respectively. Four studies reported no withdrawals (Oslo RDN 2014; Warchol 2014; Xiang 2014; HTN‐JAPAN 2015).
Outcomes not stated
No RCT provided data on the following outcomes: fatal cardiovascular events, quality of life, atrial fibrillation episodes, sleep apnoea severity, need for renal replacement therapy, proteinuria, albuminuria, or metabolic profile (blood glucose, insulin resistance).
Sensitivity analyses, investigation of heterogeneity, and publication bias
Although planned, such analyses were not performed due to the small number of studies retrieved and analysed.
Discussion
Summary of main results
In patients with resistant hypertension, a renal denervation procedure did not reduce the risk of major cardiovascular events, including myocardial infarction, ischaemic stroke, and unstable angina, compared with controls. Furthermore, this procedure had no definite effects on 24‐hour ABPM, office systolic or diastolic blood pressure, and no apparent benefits on renal function, while it increased the risk of bradycardia episodes. Conversely, renal denervation was not associated with a significantly higher risk of other adverse effects, such as femoral artery pseudo‐aneurysm, flank pain, hypotensive or hypertensive episodes, and long‐term hyperkalaemia. Data on mortality, hospitalisations, and other adverse effects were limited to single studies.
Overall completeness and applicability of evidence
The evidence on the benefits of this procedure remains inconclusive, and hence, poorly applicable in clinical practice. Many clinically relevant outcomes, such as fatal cardiovascular events, quality of life, sleep apnoea severity, need for renal replacement therapy, and metabolic profile, were not explored in any included RCT. Heterogeneity was high to very high in the majority of analyses carried out, hampering the overall reliability of findings. Although exploration of heterogeneity was not feasible due to the paucity of studies included in each analysis, it can be speculated that differences among individual study designs (e.g. use of sham procedure or standard therapy as control, presence or/ absence of blinding in outcome assessment) may represent one of the main causes underlying this phenomenon. In most trials, both study groups were simultaneously treated with optimal anti‐hypertensive therapy to decrease blood pressure to an established target. Administration of these drugs was variable and non‐reproducible. Procedural methods were also heterogeneous among studies, particularly in terms of type of catheter employed, number of applications, energy delivered and target portion of renal artery. Sakakura et al. recently observed that nervous fibres are mostly concentrated in the middle and proximal segments of the renal artery while their number decrease in the distal segment (Sakakura 2014). Recent data evidenced maximum procedural efficacy after ablation in the whole circumference of renal artery and a dose‐response dependency directly related to the amount of energy delivered (Kandzari 2015).The lack of standardized methods for renal denervation may hamper the reliability of comparisons among studies and, in some cases, even raise the question as to whether the procedure was truly successful (Esler 2015). For instance, in a corollary analysis of the SYMPLICITY HTN‐2 2010 trial the measurement of norepinephrine spillover seemed to indicate that in only 47% of patients renal denervation was truly achieved. Hence, technical bias should be considered in future trials as a potential cause of lack of response in many patients and reliable markers to confirm successful denervation are advocated. In addition, accumulating evidence indicated that the phenomena of re‐innervation of renal arteries after denervation may seriously hamper the achievement of long term benefits (Booth 2015).
Quality of the evidence
The GRADE quality of the evidence (Guyatt 2008) was low for cardiovascular morbidity outcomes and adverse effects and moderate for blood pressure and renal function outcomes. The quality of evidence was mostly influenced by the imprecision of results (wide confidence intervals) and/or the low number of studies providing quantitative data on the same outcome.
Potential biases in the review process
Points of strength of this review are represented by a peer‐reviewed protocol, a systematic search of electronic databases, and data extraction, analysis, and 'Risk of bias' assessment completed independently by two authors, according to current methodological standards. The main limitation is represented by the data obtainable from the included studies. Studies were mainly focused on small populations and short treatment periods. As a result, most trials were not adequately powered to capture exhaustive information on hard, patient‐centred outcomes, such as fatal or non‐fatal cardiovascular events. The limited evidence available also prevented more complex analyses, such as sensitivity analyses, interaction tests, and analysis for publication bias.
Agreements and disagreements with other studies or reviews
In a recent systematic review, renal denervation was apparently efficacious in reducing mean blood pressure at six months in individuals with resistant hypertension (Davis 2013). Unfortunately, this review was mostly based on data from observational, uncontrolled studies with limited follow‐up, small sample sizes, and high heterogeneity in blood pressure measurement. Findings from our review were in line with observations made by a more recent meta‐analysis of the European Network Coordinating Research On Renal Denervation (ENCOReD) Consortium (Fadl Elmula 2015). The authors confirmed the current lack of evidence supporting a widespread use of this procedure in clinical practice, advocating for future clinical trials with a longer observation time, which enrol hypertensive patients with fewer comorbidities.
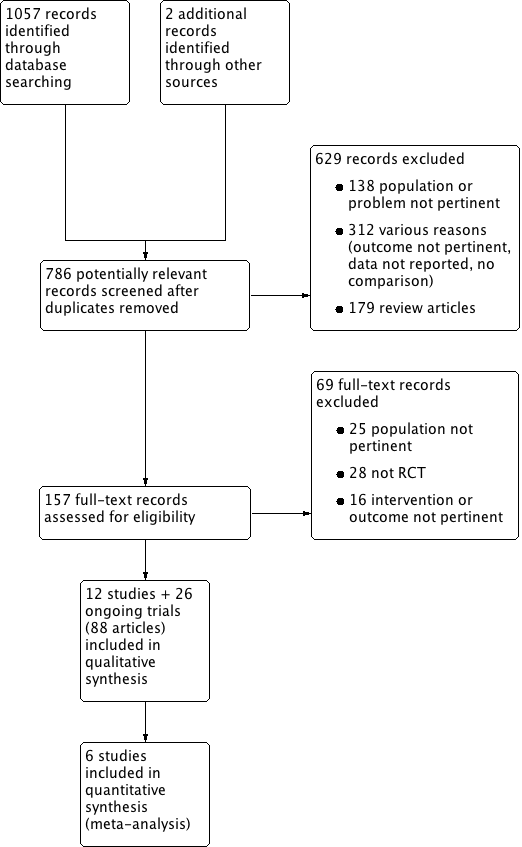
Study flow diagram.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 1 Myocardial infarction.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 2 ischaemic stroke.
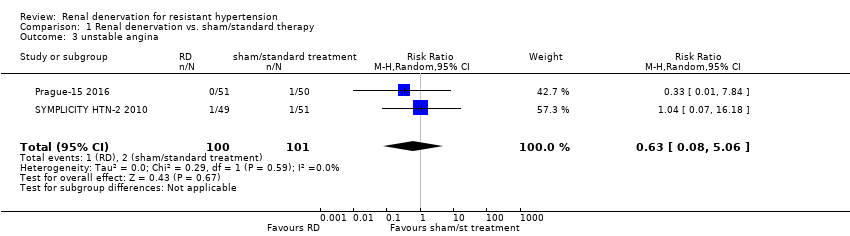
Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 3 unstable angina.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 4 systolic 24‐hour ABPM.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 5 diastolic 24‐hour ABPM.
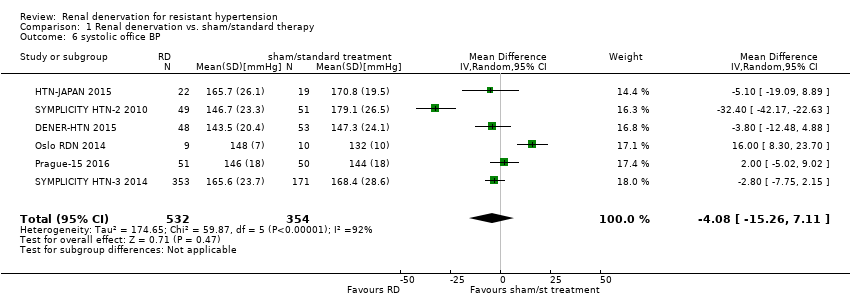
Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 6 systolic office BP.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 7 diastolic office BP.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 8 serum creatinine.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 9 eGFR/creatinine clearance.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 10 bradycardia.
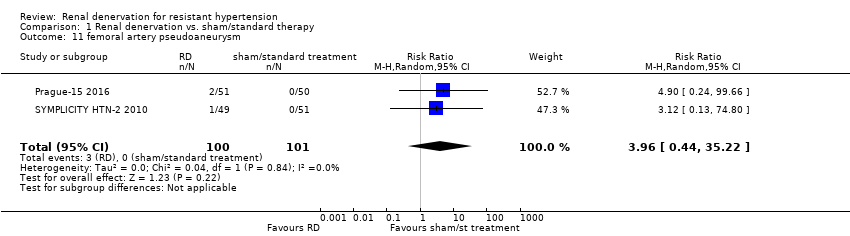
Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 11 femoral artery pseudoaneurysm.
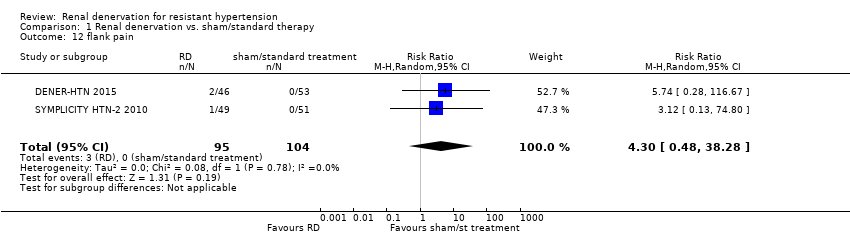
Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 12 flank pain.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 13 hypotensive episodes.
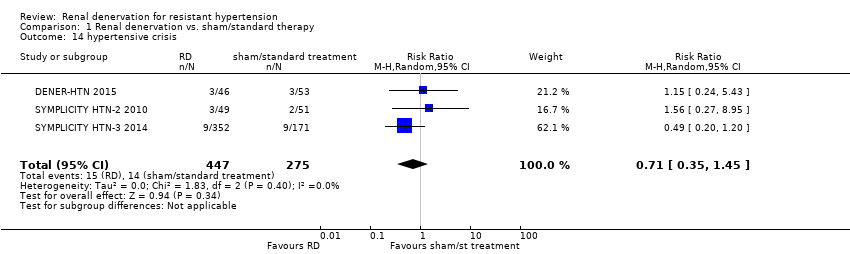
Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 14 hypertensive crisis.

Comparison 1 Renal denervation vs. sham/standard therapy, Outcome 15 hyperkalemia.
| Renal denervation versus sham denervation or standard treatment | |||||
| Patient or population: people with resistant hypertension | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect estimate | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Sham denervation/ Standard treatment | Renal denervation | ||||
| myocardial infarction | 14 per 1000 | 18 per 1000 (6 to 54) | RR 1.31 (0.45 to 3.84) | 742 (4 studies) | ⊕⊕⊝⊝ |
| ischaemic stroke | 12 per 1000 | 14 per 1000 (4 to 45) | RR 1.15 (0.36 to 3.72) | 823 (4 studies) | ⊕⊕⊝⊝ |
| unstable angina | 20 per 1000 | 12 per 1000 (2 to 101) | RR 0.63 (0.08 to 5.06) | 201 (2 studies) | ⊕⊕⊝⊝ |
| systolic 24‐hour ABPM (mmHg) | ‐ | ‐ | MD 0.28 (‐3.74 to 4.29) | 797 | ⊕⊕⊕⊝ |
| diastolic 24‐hour ABPM (mmHg) | ‐ | ‐ | MD 0.93 (‐4.50 to 6.36) | 756 | ⊕⊕⊕⊝ |
| systolic office BP (mmHg) | ‐ | ‐ | MD ‐4.08 (‐15.26 to 7.11) | 886 | ⊕⊕⊕⊝ |
| diastolic office BP (mmHg) | ‐ | ‐ | MD ‐1.30 (‐7.30 to 4.69) | 845 | ⊕⊕⊕⊝ |
| eGFR or creatinine clearance (mL/min/1.73m²) | ‐ | ‐ | MD ‐2.09 (‐8.12 to 3.95) | 837 | ⊕⊕⊕⊝ |
| *The assumed risk is the observed risk in the reference (control) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence Legend 1. Wide confidence intervals. 2. Only reported by less than half of the studies. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 4 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.45, 3.84] |
| 2 ischaemic stroke Show forest plot | 4 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.36, 3.72] |
| 3 unstable angina Show forest plot | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.06] |
| 4 systolic 24‐hour ABPM Show forest plot | 5 | 797 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐3.74, 4.29] |
| 5 diastolic 24‐hour ABPM Show forest plot | 4 | 756 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐4.50, 6.36] |
| 6 systolic office BP Show forest plot | 6 | 886 | Mean Difference (IV, Random, 95% CI) | ‐4.08 [‐15.26, 7.11] |
| 7 diastolic office BP Show forest plot | 5 | 845 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐7.30, 4.69] |
| 8 serum creatinine Show forest plot | 3 | 736 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 9 eGFR/creatinine clearance Show forest plot | 4 | 837 | Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐8.12, 3.95] |
| 10 bradycardia Show forest plot | 3 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [1.19, 36.84] |
| 11 femoral artery pseudoaneurysm Show forest plot | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.96 [0.44, 35.22] |
| 12 flank pain Show forest plot | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.48, 38.28] |
| 13 hypotensive episodes Show forest plot | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.07, 6.64] |
| 14 hypertensive crisis Show forest plot | 3 | 722 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
| 15 hyperkalemia Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.01, 21.33] |

