长期苯二氮卓类药物使用者停用苯二氮卓的药物干预
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Single‐centre | |
| Participants | Baseline characteristics Buspirone
Placebo
Inclusion criteria: above 18 years of age, continuous benzodiazepine therapy for a minimum of 6 months, wish to withdraw from benzodiazepines Exclusion criteria: use of other psychotropic medication, abuse of alcohol or drugs, major psychiatric or physical disease Pretreatment group differences: Mean benzodiazepine dosage at baseline was 15.5 mg in buspirone group and 7.5 mg in placebo group. | |
| Interventions | Benzodiazepine taper schedule: all participants switched to an equivalent dose of diazepam, stable dosage for 4 weeks, then taper with 25% each week for 4 weeks to 0, then 4 weeks without benzodiazepines.
| |
| Outcomes |
| |
| Identification | Sponsorship source: Bristol Myers CNS provided buspirone and placebo tablets and covered laboratory and administrative expenses. Country: UK Setting: Outpatients, participants referred from their GP, rapid benzodiazepine tapering regimen Declarations of interest: Not mentioned Author's name: Ashton CH Institution: Department of Pharmacological Sciences, University of Newcastle upon Tyne, NE2 4HH Email: Address: Department of Pharmacological Sciences, University of Newcastle upon Tyne, NE2 4HH | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned..." Comment: Not further described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: The study was carried out double‐blind using matching placebo tablets. Quote: "double‐blind...either buspirone or matching placebo tablets..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | High risk | Comment: No actions to adjust for high dropout (64%) in the intervention group compared with the placebo group (8%). |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available, but no reason to suspect selective outcome reporting |
| Other bias | Unclear risk | Comment: The role of Bristol Myers insufficiently described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 24 weeks Single‐centre | |
| Participants | Baseline characteristics Pronlonged‐release melatonin (PRM)
Placebo
Inclusion criteria: Age 18 years or above, an ICD‐10 diagnosis of schizophrenia (F20), schizoaffective disorder (F25), or bipolar disorder (F31). Bipolar patients were required to be euthymic a the time of inclusion. Treatment with antipsychotic drug(s) for at least 3 months before inclusion, treatment with 1 or more benzodiazepine derivatives or benzodiazepine‐related drugs for at least 3 months before inclusion, fertile women: negative pregnancy test at baseline and the use of safe contraceptives (intrauterine devices or hormonal contraception) throughout the trial period, written informed consent. Exclusion criteria: Known aggressive or violent behaviour, mental retardation, pervasive developmental disorder, or dementia, epilepsy, terminal illness, severe somatic comorbidity, or inability to understand Danish, allergy to compounds in the trial medication (melatonin, lactose, starch, gelatine, and talc), hepatic impairment, pregnancy or nursing, lack of informed consent. Pretreatment group differences: None | |
| Interventions | Benzodiazepine taper schedule: gradual reduction of usual benzodiazepine dosage (including benzodiazepine‐related drugs) at an approximate rate of 10% to 20% every second week.
| |
| Outcomes |
| |
| Identification | Sponsorship source: The Research Fund of the Mental Health Services of the Capital Region in Denmark financed the trial with a post doc grant and a grant for external randomisation and database management. Country: Denmark Setting: Mainly outpatients Declarations of interest: None Author's name: Baandrup L Institution: Centre for Neuropsychiatric Schizophrenia Research (CNSR) & Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), University of Copenhagen, Mental Health Centre Glostrup, Mental Health Services – Capital Region of Denmark, Glostrup Email: [email protected] Address: Mental Health Centre Glostrup, Mental Health Services – Capital Region of Denmark, DK‐2600 Glostrup | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central randomisation was performed by the Copenhagen Trial Unit (CTU) with computer‐generated, permuted randomisation allocation sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence and block sizes were kept unknown to the investigator. Allocation ratio was 1:1. The investigator contacted the CTU and provided a personal pin code, participant civil registration number, participant trial identification number, and the value of the stratification variable of benzodiazepine dosage (low (15 mg diazepam equivalents) or high (15 mg diazepam equivalents)) at baseline. Then the randomisation was announced as a trial medication container number and confirmation sent by e‐mail" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Thus, the placebo was matched to the study medication for taste, smell, colour, size and solubility. CTU held the randomisation code and the trial was not unblinded until all data were registered, primary analyses finished and conclusions drawn" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Trial participants, staff, and outcome assessors were blinded to the allocated treatment. We maintained blinding using matching placebo and an independent unit to perform the randomisation and do the packaging and labelling of the trial medication. Both PRM and placebo were encapsulated in lactose‐ containing gelatine capsules to optimise the blinding" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Data complete for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: Primary outcome, etc. reported in published trial protocol. |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks (4 weeks double‐blind followed by 2 weeks single‐blind placebo) Multicentre | |
| Participants | Baseline characteristics Alpidem
Placebo
Inclusion criteria: Outpatients with generalised anxiety disorder (GAD; DSM‐III‐R, item 300.02) or adjustment disorder with anxious mood (DSM‐III‐R, item 309.24). Consecutive patients of either sexes, aged between 18 and 60 years, taking non‐hypnotic benzodiazepines for anxiety as continuous course of therapy of at least 1 year duration, at a dose schedule corresponding to 30 mg or less of diazepam per day, were considered eligible. Exclusion criteria: Montgomery–Åsberg Depression Rating Scale was administered to exclude depressed patients (total score > 18). Pretreatment: No significant differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: all benzodiazepines abruptly discontinued at inclusion.
| |
| Outcomes |
| |
| Identification | Sponsorship source: No information Country: Italy Setting: Outpatients Declarations of interest: Not mentioned Author's name: Cassano GB Institution: Clinica Psichiatrica, University degli Studi di Pisa Email: Address: Clinica Psichiatrica, University degli Studi di Pisa, Ospedale Santa Chiara, Via Roma 67, 56100 Pisa | |
| Notes | The study lasted 6 weeks: a 4‐week comparative period (phase I) to prevent and treat benzodiazepine withdrawal symptoms (primary aim) was followed by a 2‐week single‐blind period with placebo (phase II) to monitor the occurrence of withdrawal symptoms after abrupt discontinuation of alpidem (secondary aim). 6 weeks was chosen as endpoint because alpidem is a Z‐drug. According to the review protocol, such studies are included if data are available on relevant outcomes AFTER withdrawal of the new benzodiazepine/Z‐drug, in this case after discontinuation of alpidem. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The Italian multicentre (15 centres), double‐blind, randomised (versus placebo), parallel group study" Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The diagnosis of withdrawal symptoms was made by a respected academic expert, in blind conditions, on the basis of the definition in the protocol" Comment: Done |
| Incomplete outcome data (attrition bias) | High risk | Comment: 10 (11.5%) discontinued in the alpidem group, 18 (21%) in the placebo group. |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol published but could not be retrieved. No reason to suspect selective outcome reporting |
| Other bias | Unclear risk | Comment: Source of financing not described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 1 month Single‐centre | |
| Participants | Baseline characteristics Homéogène
Sédatif PC
Placebo
Inclusion criteria: At least 18 years of age, at least 3 months use of benzodiazepines at low dosage (max 10 mg/day diazepam equivalents), clinically stable for at least 1 month Exclusion criteria: Severe insomnia, severe psychiatric disorders, alcohol or substance abuse disorder, previous seizures, current use of muscle relaxants, clonidine, or psychotropic drugs. Pretreatment: Higher scores on somatic symptoms in Homéogène group | |
| Interventions | Benzodiazepines substituted (no taper schedule) with study drug:
Both experimental drugs were homeopathic drugs. | |
| Outcomes |
| |
| Identification | Sponsorship source: Laboratoires Boiron, l'Agence Nationale de Valirisation de la Recherce Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Cialdella P Institution: Service de Pharmacologie Clinique, Faculté RTH Laënnec Email: Address: | |
| Notes | Homéogène and Sédatif groups were combined as 1 homeopathic drug group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Described as double‐blind, but lacks a description of what have been done to ensure blinding of participants and study personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described. Insufficient information to permit judgement of low or high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: 25% attrition. An ITT approach was used, but distribution of attrition between groups was not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: No obvious selective outcome reporting |
| Other bias | Unclear risk | Comment: Role of funding source not described, both industry and publicly funded. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 4 weeks Single‐centre | |
| Participants | Baseline characteristics Carbamazepine
Placebo
Inclusion criteria: > 60 years of age, GAD, benzodiazepine abuse, minimum duration of benzodiazepine treatment 6 months Exclusion criteria: None described. Pretreatment: No significant pretreatment differences | |
| Interventions | Benzodiazepine taper schedule: 25% benzodiazepine dose reduction every week
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not reported Country: Italy Setting: Outpatients Declarations of interest: Not mentioned Author's name: Di Constanzo E Institution: Servizio Psichiatrico Email: Address: Viale Spellanzon, 55 31015 Conegliano | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Described as double‐blind, but what has been done to ensure blinding of participants and study personnel is not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Insufficient information to permit judgement of low or high risk |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4 (26.6%) and 3 (21.4%) participants did not complete benzodiazepine cessation but participated in the study. |
| Selective reporting (reporting bias) | Low risk | Comment: No indication of selective outcome reporting |
| Other bias | Low risk | Comment: No apparent other source of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks double‐blind, 6 weeks single‐blind Single‐centre | |
| Participants | Baseline characteristics Controlled‐release melatonin
Placebo
Inclusion criteria: People with a daily use of benzodiazepines for more than 6 months, expressed willingness to discontinue the use, living independently Exclusion criteria: Cognitive impairment, liver or renal disorders Pretreatment: No significant differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: participants were encouraged to reduce their usual benzodiazepine therapy dosage 50% during week 2, 75% during weeks 3 and 4, and then to discontinue benzodiazepine therapy completely during weeks 5 and 6. Participants who did not succeed in stopping benzodiazepine therapy during period 1 were encouraged to further reduce benzodiazepine dosage 50%, 75%, and 100% during weeks 8, 9 and 10, 11 and 12, respectively.
| |
| Outcomes |
| |
| Identification | Sponsorship source: Neurim Pharmaceuticals sponsored study medication and study nurse; statistical evaluations performed independently. Country: Israel Setting: Outpatients, living independently Declarations of interest: Not mentioned Authors name: Doron Garfinkel Institution: Department of Neurobiochemistry, Tel Aviv University Email: [email protected] Address: Tel Aviv 69978, Israel | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects were randomised to receive either 2 mg of CRM therapy or a placebo that was identical in appearance" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Collection and entry of all data were completed before revealing the randomisation codes of the study" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All included participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No indications of selective reporting |
| Other bias | High risk | Comment: The trial was partly financed by a company with an interest in given result, the company's role in interpreting the data is not sufficiently described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group, stratifies for flunitrazepam/lormetazepam at baseline Blinding: Single Duration: 7 days Single‐centre | |
| Participants | Baseline characteristics Not reported Inclusion criteria: 18 to 40 years of age, flunitrazepam abuse at a dose of 10 to 12 mg/day (18 participants) or lormetazepam abuse at a dose of 8 to 10 mg/day (18 participants). All participants met the criteria of the DSM‐III‐R for benzodiazepine withdrawal syndrome. Abuse was defined as use for at least 9 months. Informed consent was obtained from all participants. Excluded criteria: Psychiatric patients were not included in the study. Daily urine samples were taken to rule out the abuse of morphine, methadone, cocaine, amphetamine, barbiturates, cannabis, and ethanol during the study. Pretreatment: Not reported | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: abrupt cessation
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not reported Country: Italy Setting: Inpatients Declarations of interest: Not mentioned Author's name: Gilberto Gerra Institution: University of Parma Email: Address: USL n. 4, Via Guasti S. Cecilia, 3, Parma 43100, Italy | |
| Notes | Results were reported separately for flunitrazepam and lormetazepam users. To avoid including several comparisons from the same study, we only included results for the lormetazepam users in this meta‐analysis (flunitrazepam is now very seldom used in clinical practice and in many countries is no longer registered for use). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Placebo groups B and D were only treated with saline solution for 7 days." Comment: Only participants were blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: Study only described as single‐blinded, therefore probably not done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No indication of selective outcome reporting |
| Other bias | Unclear risk | Comment: Insufficient information regarding sponsorship |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Single Duration: 8 days Single‐centre | |
| Participants | Baseline characteristics Flumazenil IV
Oxazepam tapering
Placebo
Inclusion criteria: History of benzodiazepine dependence according to DSM‐IV criteria. Exclusion criteria: Severe chronic liver or renal diseases or other chronic physical disorders, recent onset of significant weight loss or gain, endocrinopathies, neurological disorders, immunopathy, in particular HIV disease, a positive family history of cardiovascular disease and hypertension, current abuse of illicit drugs and alcohol Pretreatment: None in reported parameters | |
| Interventions | Intervention characteristics All participants received high doses of oxazepam (120 mg/day) during the last week before detoxification (pretreatment week).
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not mentioned Country: Italy Setting: Inpatients Declarations of interest: Not mentioned Author's name: Gilberto Gerra Institution: Addiction Research Center, Ser.T., AUSL, Parma, Italy Email: [email protected] Address: Gilberto Gerra, Centro Studi Farmacotossicodipendenze, Ser.T., A.U.S.L., Via Spalato 2,43100 Parma, Italy | |
| Notes | Only the comparison between flumazenil and placebo was considered relevant and included in the meta‐analysis, cf. Cochrane Handbook on multiple comparisons. Rate of relapse NOT reported for the placebo group because: (quote) Long‐term outcome of group C (placebo) patients was not evaluated in comparison with A and B patients because they received low‐dose benzodiazepine treatment for 2 weeks, immediately after the detoxification procedure, for ethical reasons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described Quote: "The study was single‐blind, randomised and placebo‐controlled." |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the trial was single‐blind, permitting direct clinical interventions in the case of dramatic withdrawal symptoms" |
| Blinding of outcome assessment (detection bias) | High risk | Comment: Not done |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Though not clearly described, judging from the text it appears that no participants withdrew during the 8‐day intervention trial. |
| Selective reporting (reporting bias) | Low risk | Comment: No reason to suspect selective outcome reporting |
| Other bias | Unclear risk | Comment: Funding not described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Multicentre | |
| Participants | Baseline characteristics Paroxetine
Placebo
Inclusion criteria: Participants were males or females aged > 18 years suffering from 1 or more of the following anxiety disorders of non‐severe degree in axis I: panic attack disorder (with or without agoraphobia), GAD, social anxiety/social phobia or mixed anxiety and depression disorder with significant anxiety; people continuously treated with benzodiazepines (any) for at least 6 consecutive months prior to the screening visit at doses between 2 and 8 mg/day of lorazepam or equivalent; a total score ≤ 16 on the HAM‐A and MADRS at screening and baseline. Exclusion criteria: People suffering (or diagnosed within the 6 months prior to screening) from 1 or more of the following conditions: major depressive episode; post‐traumatic stress disorder; obsessive‐compulsive disorder; eating behavioural disorders, people diagnosed with dysthymia or who had suffered from dysthymia in the 6 months prior to screening; people with a concomitant psychotic disorder, or history of psychotic disorder; people having a concomitant bipolar disorder or history of bipolar disorder, or having a cyclothymic disorder, or had suffered from it in the past; people who met DSM‐IV (protocol appendix O) criteria for substance (alcohol or drugs) abuse or dependence, except for benzodiazepine, within 6 months prior to screening; current suicidal or homicidal risk; and people who had electroconvulsive therapy in the 3 months prior to screening. Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 4‐week open‐label run‐in period during which participants were switched from their original benzodiazepine to an equivalent dosage of chlordemethyldiazepam (between 2 and 8 mg/d). The taper schedule during the treatment phase not described.
| |
| Outcomes |
| |
| Identification | Sponsorship source: GlaxoSmithKline Country: Italy Setting: Outpatients Declarations of interest: Not mentioned Comments: Unpublished phase III study Author's name: GlaxoSmithKline Institution: Email: Address: Clinical Study Register (www.gskclinicalstudyregister.com) 2002 | |
| Notes | Change scores extracted, final scores not available. Standard deviation calculated from CI using the following formula: SE = (upper limit – lower limit of CI)/3.92 Standard deviation σ = standard error x √n | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "12‐week double blind, multicentre, randomised, placebo‐controlled, parallel group" Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects were randomised to either paroxetine or placebo and entered the 12‐week double‐blind, randomised treatment phase. Dosage of paroxetine or matched placebo started with..." Comment: Double‐blind and using matched placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2 versus 8 participants withdrew, but ITT analysis data extracted for this meta‐analysis. |
| Selective reporting (reporting bias) | High risk | Comment: No protocol available, benzodiazepine dose at follow‐up not described. |
| Other bias | High risk | Comment: Study funded by the study drug manufacturer, no information available on involvement in design, data collection, etc. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks (6 weeks during tapering and 6 weeks post‐tapering) Multicentre | |
| Participants | Baseline characteristics Pregabalin
Placebo
Inclusion criteria: Adult outpatients aged 18 to 65 years were enrolled if they met DSM‐IV criteria for a primary lifetime diagnosis of GAD, and if they were receiving stable treatment with a benzodiazepine in daily doses of 1 to 4 mg/day (in alprazolam dose equivalents) for 8 to 52 weeks. A primary diagnosis of GAD was made, based on predominant clinical presentation, using the module P form of the Mini International Neuropsychiatric Interview (MINI)‐Plus version 5.0.0 (Sheehan et al, 1997). The current diagnosis of GAD could be sub‐threshold due to treatment. Exclusion criteria: (1) women who were pregnant, lactating, or of childbearing potential who were not using a medically approved form of contraception; (2) 17‐item HAM‐D total score > 15; (3) a history of anxiolytic non‐response to benzodiazepines or pregabalin, or hypersensitivity to either class of drug; (4) they met DSM‐IV criteria in the past 6 months of major depressive disorder, dysthymia, social phobia, post‐traumatic stress disorder, body dysmorphic disorder, or eating disorder; (5) met DSM‐IV criteria in the past 5 years of schizophrenia, psychotic disorder, bipolar affective disorder, obsessive–compulsive disorder, substance dependence (excluding nicotine), or in the past year for substance abuse; (6) currently receiving cognitive behavioural therapy for GAD or other anxiety disorder; (7) a history of seizure disorder, except febrile seizures of childhood; (8) a history of neuropathic pain or narrow angle glaucoma; (9) receiving treatment with fluoxetine (in past 5 weeks) or any psychotropic other than benzodiazepines (in past 2 weeks), or electroconvulsive therapy (in past 6 months); (10) positive urine drug screen for amphetamines, barbiturates, ethanol, narcotics, non‐benzodiazepine sedatives and hypnotics, cocaine, phencyclidine, cannabinoids or other illegal or illicit drugs; (11) considered by the investigator to be at risk for suicide or aggressive behaviour; (12) any serious or uncontrolled medical illness in the opinion of the investigator that would render the person unsuitable for the study; or (13) creatinine clearance 60 mL/min. Pretreatment: None | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: switch to equivalent dose alprazolam, 2‐week stabilisation phase before randomisation, 25% reduction per week, permitted up to 6 weeks to complete the alprazolam taper, after maintained 6 weeks on double‐blind treatment, then 1 week taper off study medication
| |
| Outcomes |
| |
| Identification | Sponsorship source: Funded by Pfizer Country: 20 investigational sites in Spain, Mexico, France, Italy, Costa Rica, the Czech Republic, and Guatemala Setting: Outpatients Declarations of interest: Dr Schweizer was at the time of the writing of the manuscript employee of Paladin Consulting Group Inc., which was a paid consultancy to Pfizer Inc. At the time the study was conducted and the paper was initially drafted, Dr Sallie J Hadley was an employee of Pfizer Inc. and owns stock in Pfizer. Dr Francine S Mandel was a full‐time employee of Pfizer Inc. Dr Edward Schweizer owns stock in Pfizer and has received payments for consulting and/or medical writing services from Alkermes, Bristol‐Myers Squibb, Sumitomo Dainippon Pharma, Eli Lilly, Memory Pharmaceuticals, Neurocrine Biosciences, and Pfizer Inc. Author's name: Sallie J Hadley Institution: Pfizer Inc., New York, NY, USA Email: [email protected] Address: Francine S Mandel, Pfizer Inc., 235 East 42nd Street, New York, NY, USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patients...were randomised on a one‐to‐one basis to 12 weeks of double‐blind treatment with either pregabalin or placebo" Comment: Described as "double‐blind" but what has been done to ensure blinding of participants and study personnel is not mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | High risk | Comment: High attrition rate in both the pregabalin group (46.4%) and the placebo group (62.0%) |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | High risk | Comment: Study funded by Pfizer, no indication of role of funding body in design, data collection, analysis, and interpretation of the data. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 3 months Multicentre | |
| Participants | Baseline characteristics Magnesium aspartate
Placebo
Inclusion criteria: Outpatients, 18 to 65 years of age, chronic users of lorazepam, alprazolam, or bromazepam (> 6 months, regular dose => 3 mg lorazepam equivalents), benzodiazepines prescribed due to an anxious disorder now in remission defined as score on Hamilton Anxiety < 14 and Raskin‐Depression < 6, no major psychiatric disorder, at least 1 trial of unsuccessful benzodiazepine withdrawal, a wish to discontinue benzodiazepine use Exclusion criteria: Severe hepatic or renal dysfunction, alcohol or substance use disorder, currently trying to discontinue use of tobacco, current psychotherapy, use of other psychotropics within 6 months, treatment with a magnesium salt or calcium within 1 month, regular use of magnesium aspartate during 1 month within the last 6 months Pretreatment: No significant pretreatment group differences | |
| Interventions | Benzodiazepine taper schedule: co‐administration of benzodiazepine and study drug for 1 month, gradual taper of benzodiazepine during the next month (50% of dosage for 2 weeks, 25% for 2 weeks, then stop), follow‐up during a third month after complete benzodiazepine discontinuation
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not reported Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Hantouche EG Institution: Département de Psychiatrie, Groupe Hospitaliers de la Pitrie‐Salpetriere Email: Address: 47, Boulevard de l'Hopital, 75013 Paris | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Described as double‐blind, what has been done to ensure blinding of participants and study personnel is not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Not described |
| Selective reporting (reporting bias) | Low risk | Comment: No indications of reporting bias |
| Other bias | Low risk | Comment: No apparent other sources of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 3 weeks Single‐centre | |
| Participants | Baseline characteristics Flumazenil IV challenge
Placebo
Inclusion criteria: People were recruited to the study if they had been taking benzodiazepines in usual therapeutic doses (< 30 mg per day of diazepam or equivalent) for 3 months or more, and if they had experienced withdrawal problems on discontinuing medication. Exclusion criteria: (i) regular intake of any other psychotropic medication, (ii) a diagnosis of schizophrenia, epilepsy, or cardiorespiratory disease Pretreatment: No significant pretreatment differences | |
| Interventions | Intervention characteristics
| |
| Outcomes |
| |
| Identification | Sponsorship source: Roche Products Ltd supplied unmarked ampoules of flumazenil and vehicle solution and a grant towards the cost of the project. Country: UK Setting: Outpatients (inpatients when receiving flumazenil challenge) Declarations of interest: Not mentioned Comments: The study was approved by the local ethics committee but, owing to the unexpectedly severe reactions shown in some participants, it was felt to be unethical to continue with the study after 10 participants had been tested using the original protocol. Author's name: Harrison‐Read PE Institution: Academic Unit of Psychiatry, St Charles Hospital, Exmoor Street, London W10 6DZ Email: Address: Academic Unit of Psychiatry, St Charles Hospital, Exmoor Street, London W10 6DZ | |
| Notes | Study discontinued due to unacceptable adverse effects (marked panic reaction in the 4 participants who received flumazenil), beginning within 30 seconds of the end of the injection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "High risk and low risk subjects were allocated separately at random to placebo or flumazenil challenge by an independent pharmacist." Comment: Description of how the sequence was generated was insufficient. |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was done by independent pharmacist. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "This ’challenge test’ was carried out double‐blind, with both subject and experimenter being unaware of the identify of the substance being injected" Comment: Described as double‐blind and using placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Immediately afterwards, the subject began filling in the BWSQ and the MRS, and then repeated these measures at 5, 15, 25, 35, 45 and 60 min post‐injection. Pulse and blood pressure were recorded as before" Comment: Description is insufficient to judge the risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All randomised participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No reason to suspect selective outcome reporting |
| Other bias | High risk | Comment: As the reaction to acute challenge with flumazenil proved to be unexpectedly severe, the study was stopped after only 10 participants had been recruited for the study: 4 were allocated to the flumazenil group and 6 to the placebo group. Despite separately randomising high‐ and low‐risk participants, the early cessation of the study led to unequal distribution between the 2 treatment groups: 1 out of the 4 participants in the flumazenil group and 3 out of the 6 in the placebo group were high‐risk participants. In addition, the study was supported by a company. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: Approximately 5 weeks (dependent on duration of taper phase) Single‐centre | |
| Participants | Baseline characteristics Carbamazepine: not available Placebo: not available Inclusion criteria: DSM‐III‐R diagnosis of panic disorder or generalised anxiety disorder Exclusion criteria: 1) Lifetime history of psychotic disorder, 2) Bipolar disorder, 3) Seizure disorder, 4) Severe head trauma, 5) Major depression, 6) Abuse of alcohol or other substances, 7) Obsessive‐compulsive disorder, 8) PTSD, 9) Pregnancy, 10) Active systemic illness with chronic medication Pretreatment: Reported to be non‐significant but not reported for the carbamazepine versus placebo group, only reported for the panic disorder versus the GAD group | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 25% every third day
| |
| Outcomes |
| |
| Identification | Sponsorship source: Supported by the Upjohn Company Country: Israel Setting: Outpatients Declarations of interest: Not mentioned Author's name: Ehud Klein Institution: Rambam Medical Center and University of Vermont Email: Address: Rambam Medical Center, Rapapport Faculty of Medicine, Technion‐IIT, Bat Galim, Haifa, Israel | |
| Notes | Baseline characteristics for the carbamazepine versus placebo group were not reported, only for panic disorder group versus generalised anxiety disorder group. Same problem when reporting the results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described in detail, but it is stated that randomisation was stratified by diagnosis and alprazolam daily dosage |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients entered the controlled portion of the study and were randomly assigned, in a double‐blind fashion, to receive either carbamazepine or placebo as adjunctive treatment...In order to maintain blindness of the study throughout the taper period, patients received a fixed number of capsules with a gradually increasing proportion of identical placebo capsules substituting for the alprazolam" Comment: The use of carbamazepine versus placebo (the primary interest for the current review) was double‐blinded with identical placebo. The alprazolam taper was single‐blind, but these data are not considered here. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) | High risk | Comment: Very high dropout rates (56% vs 71%), and no ITT analysis performed. |
| Selective reporting (reporting bias) | High risk | Comment: No reporting on benzodiazepine dosage or withdrawal symptoms |
| Other bias | High risk | Comment: Role of supporting company not described |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 28 days Single‐centre | |
| Participants | Baseline characteristics Carbamazepine
Tianeptine
Inclusion criteria: ICD‐10 criteria for benzodiazepine dependence, 18 to 65 years of age Exclusion criteria: Previously treated with 1 or both of the experimental drugs, psychotic symptoms, not treated with other psychotropic drugs until 2 weeks before inclusion, pregnant or nursing, substance abuse, severe somatic illness Pretreatment: No significant pretreatment differences | |
| Interventions | Benzodiazepines substituted with
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not mentioned Country: Poland Setting: Inpatients Declarations of interest: Not mentioned Comments: No data reported for the outcomes, only overall results from statistical analyses. Author's name: Kornowski J Institution: Psychiatric Hospital in Starogard Gdansk Email: [email protected] Address: 83‐200 Starogard Gdansk | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Blinding not described, not possible to judge whether participants and personnel were blinded, also it is not stated if the study was open‐label. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4 (17%) dropped out in each group because they ingested benzodiazepines during the trial(detected by urine screen), but all participants were included in the statistical analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent reporting bias |
| Other bias | Unclear risk | Comment: No apparent other sources of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks Single‐centre | |
| Participants | Baseline characteristics Only reported for the total sample: men: 41.7%; age: 39.1 years; years of benzodiazepine use: 8.4 years Inclusion criteria: More than 6 months of benzodiazepine use, physically dependent, no requirements of further benzodiazepine treatment as deemed by mental state assessment Exclusion criteria: Abuse of alcohol or other drugs Pretreatment: Not described | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 2 weeks on unchanged dosage, 2 weeks on halved benzodiazepine dosage, 2 weeks with no benzodiazepines (followed by 2 weeks with placebo in both groups and 2 weeks with no study medication)
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not mentioned Country: UK Setting: Outpatients Declarations of interest: Not mentioned Authors name: Malcolm Lader Institution: Institute of Psychiatry, University of London Email: Address: Institute of Psychiatry, University of London, De Crespigny Park, Denmark Hill, London, SE5 8AF England | |
| Notes | For reasons that are unclear, results are reported at week 3, i.e. after the first week of benzodiazepine reduction to half. That is, results are not available for week 6, when benzodiazepines have been tapered off. Figure 2 shows the temporal pattern for Hamilton Anxiety Scale (i.e. all time points available graphically) but only for the successful completers (5 buspirone, 6 placebo). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "During the first two weeks of withdrawal (3 and 4), buspirone or placebo was substituted for the benzodiazepine in an initial dosage of 5 mg (one capsule) twice daily, followed by 10 mg (two capsules) twice daily...The study was conducted double‐blind in that neither investigator nor patient knew whether placebo or buspirone was being administered during weeks 2 to 5" |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: It is stated that "investigators" were blinded. Judged as done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Successful completers: no attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Comment: No data on benzodiazepine dosage in the 2 groups, but the trial was designed to stop benzodiazepine use, and therefore dose reduction was not considered. However, the choice of using 3 weeks as primary time point does not seem justified. |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 8 weeks Single‐centre | |
| Participants | Baseline characteristics Alpidem (a Z‐drug)
Placebo
Inclusion criteria: Benzodiazepine use for more than 6 months, less than 30 mg/d diazepam equivalents, regarded as dependent (problems on previous attempts to lower the dosage), 18 to 65 years of age, within 20% of normal body weight Exclusion criteria: Major physical or psychiatric illness, drug abusers, women of child‐bearing age unless on adequate contraception Pretreatment: Not described | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 2 weeks on unchanged dosage, 2 weeks on halved benzodiazepine dosage, 2 weeks with no benzodiazepines (followed by 2 weeks with halved dosage study medication and 2 weeks with no study medication)
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not described Country: UK Setting: Outpatients Declarations of interest: Not mentioned Author's name: Lader M Institution: Institute of Psychiatry Email: Address: Institute of Psychiatry, De Crespigny Park, Denmark Hill, London SES 8AF, UK | |
| Notes | Anxiety and benzodiazepine withdrawal symptoms: the results are only shown in graphic as mean values, SDs not reported. SDs for HAM‐A were therefore imputed from Cassano 1996, which is a similar trial also using alpidem to facilitate benzodiazepine withdrawal. It was not possible to impute SDs for benzodiazepine withdrawal symptoms because withdrawal symptoms in Cassano 1996 were reported as a dichotomised variable, whereas they were reported as a continuous variable in Lader 1993. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was conducted double‐blind in that neither investigators nor patients knew whether placebo or alpidem was being administered during weeks 3‐8" Comment: Study described as double‐blinded and using placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: It is stated that "investigators" were blinded. Judged as done. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: High dropout, however only completion could be extracted from the study, and this is not biased by the high dropout rate. |
| Selective reporting (reporting bias) | Low risk | Comment: No indications of selective reporting |
| Other bias | Low risk | Comment: No other apparent bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 16 weeks Multicentre | |
| Participants | Baseline characteristics Lithium
Placebo
Inclusion criteria: Outpatients, 18 to 65 years old, receiving benzodiazepines for at least 6 months at a daily dose ranging from 10 to 40 mg diazepam or equivalent and wishing to withdraw benzodiazepine treatment Exclusion criteria: Anxiety disorder with a score of 15 or above on the HAM‐A, major depressive disorder, social phobia, alcohol or substance abuse according to Mini International Neuropsychiatric Interview, and/or other serious pathology. Tranquilisers including antihistamines, hypnotics, anxiolytics, and lithium salts were not allowed. Pretreatment: No significant pretreatment group differences | |
| Interventions | Benzodiazepine taper schedule: 4 weeks stable benzodiazepine and lithium versus placebo, 4 weeks benzodiazepine withdrawal ‐ reduction with 50% every week, 8 weeks lithium maintenance
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not described Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Lecrubier Institution: Inserm unité 302, service de psychiatrie AD Email: [email protected] Address: Hôpital Pitié‐Salpêtrière, 17, boulevard de l’hôpital, 75013 Paris, France | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blind, randomised study" Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Lithium gluconate and placebo were dispensed in vials and were indistinguishable in terms of appearance, taste and smell" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 244 participants were randomised: 146 to lithium and 98 to placebo. Only participants entering the benzodiazepine tapering phase were analysed (136 participants allocated to lithium and 94 to placebo), thus attrition rate of 7% and 4%, respectively. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Benzodiazepine dose at endpoint not reported, only participants who succeeded in discontinuing benzodiazepine usage. |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 4 weeks Multicentre | |
| Participants | Baseline characteristics Bromazepam
Cyamemazine
Inclusion criteria: Participants were aged 18 to 65 years, treated for anxiety for at least 3 months with benzodiazepines (bromazepam, lorazepam, alprazolam, or oxazepam) at a daily dose of 5 to 20 mg diazepam‐equivalent, and requiring a withdrawal. A < 18 score in the Hamilton Anxiety Rating Scale was required. Exclusion criteria: Female patients were excluded if they were pregnant or likely to become so or if they were breastfeeding. Individuals incapable of completing a questionnaire or of properly giving informed consent were also excluded. In addition, current treatment with any psychotropic drug or any other central nervous system active medication was forbidden. The presence of comorbid depression was also an exclusion criterion. Pretreatment: NS | |
| Interventions | Intervention characteristics
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not described. 1 of the authors affiliated with Sanofi‐Aventis. Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Patrick Lemoine Institution: Unite´ Clinique de Psychiatrie Biologique, Bron Email: [email protected] Address: 46bis rue Gallie´ni, 91360 Villemoisson‐sur‐Orge, France | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both drugs were administered in identical soft gelatin capsules" Comment: Sufficient blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: ITT analysis performed. |
| Selective reporting (reporting bias) | High risk | Comment: Protocol not available, unusual primary outcome (maximum amplitude of anxiety rebound). |
| Other bias | High risk | Comment: Role of Sanofi‐Aventis not described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 8 weeks Single‐centre | |
| Participants | Baseline characteristics Gabapentin
Placebo
Inclusion criteria: Meeting DSM‐IV criteria for current benzodiazepine abuse or dependence and opioid dependence, and being treated for opioid dependence with methadone, 18 to 65 years of age Exclusion criteria: (1) Any Axis I psychiatric disorder as defined by DSM‐IV‐TR that was unstable or would be disrupted by study medication or by an effort to discontinue benzodiazepines; (2) Acute physiological withdrawal or a history of seizures during alcohol or sedative‐hypnotic withdrawal; (3) Individuals with cocaine dependence as their primary substance use disorder diagnosis; (4) Individuals with unstable physical disorders or impaired kidney function; (5) Prescribed psychotropic medications other than methadone or medications prescribed for pain syndromes that would be disrupted by study medication or by an effort to discontinue benzodiazepines; (6) Anticonvulsants prescribed for pain syndromes; (7) Known sensitivity to gabapentin; (8) Individuals who had exhibited suicidal or homicidal behaviour within the past 2 years or had current active suicidal ideation; (9) Individuals physiologically dependent on any other drugs (excluding nicotine, caffeine, methadone); (10) Individuals currently prescribed gabapentin; and (11) Individuals requiring pharmacological detoxification from benzodiazepines in the past year and are unlikely to be able to tolerate taper off of benzodiazepines Pretreament differences: None reported. | |
| Interventions | Intervention characteristics
| |
| Outcomes | Benzodiazepine mean dose | |
| Identification | Sponsorship source: Funding for this work was provided by National Institute on Drug Abuse grants K23‐ DA021209 (Mariani), P50‐DA09236 (Kleber), K24‐ DA022412 (Nunes), and K24 029647 (Levin). Country: USA Setting: Methadone maintenance outpatients Declarations of interest: None Authors name: John J Mariani Institution: Division on Substance Abuse, New York State Psychiatric Institute, New York, NY, USA Email: [email protected] Address: New York State Psychiatric Institute, Division on Substance Abuse, 1051 Riverside Drive, Unit 66, New York, NY 10032, USA | |
| Notes | Data not reported sufficiently, not possible to extract results relevant to this review. The author has not responded to our queries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation method not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: All capsules were over‐capsulated with riboflavin to ensure compliance. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | High risk | Comment: Only 50% were retained in the study. |
| Selective reporting (reporting bias) | Low risk | Comment: Selective outcome reporting not evident. |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks (2 weeks taper off, 4 weeks assessment, ends day 45) Multicentre | |
| Participants | Baseline characteristics Captodiame
Placebo
Inclusion criteria: Participants aged 25 to 55 years who had been prescribed certain benzodiazepines (lorazepam, bromazepam, alprazolam, oxazepam, or clobazam) in the official recommended dose range for the treatment of an anxiety disorder for at least 6 months, stable benzodiazepine dosage over the 6‐month period. Since alertness was assessed with a driving simulation test, included participants were required to be in possession of a valid driving license for at least 5 years. Exclusion criteria: People with a history of alcohol dependence in the previous 5 years were excluded, as were those consuming excessive quantities of alcohol as defined in the CAGE questionnaire. Proven consumption (either openly declared or detected by urine testing) of illicit psychotropic drugs (opiates, cocaine, cannabis, amphetamines) or of any other sedatives also constituted grounds for exclusion. Additionally, people with severe, unstable, or uncontrolled hepatic, renal, or cardiac insufficiency, with glaucoma or prostate hypertrophy, or with any psychiatric disease other than generalised anxiety disorders were also excluded. Female individuals who were pregnant or breastfeeding were excluded. Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: half dose first week of experimental treatment, a quarter dose the second week, then discontinuation on day 14
| |
| Outcomes |
| |
| Identification | Sponsorship source: This study was funded by Laboratoires Bailly‐Creat, Paris, France, manufacturers of captodiame (Covatine), who financed the honoraria of the participating physicians and the statistical analysis. Country: France Setting: Outpatients Declarations of interest: Not mentioned Comments: Benzodiazepine dose during and after discontinuation not recorded/documented. Authors name: Merzecier‐Guyon C Institution: Centre d’Etudes et de Recherches en Médecine du Trafic, Annecy, France Email: [email protected] Address: Dr C Mercier‐Guyon, CERMT, BP 132, 74004 ANNECY Cedex, France | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "randomised, double‐blind, placebo‐controlled trial" Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All randomised participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "we have little information available on real benzodiazepine use during the discontinuation and follow‐up phases, which is the most relevant measure of successful benzodiazepine" Comment: No information due to study design, not interpreted as being left out intentionally |
| Other bias | High risk | Quote: "This study was funded by Laboratoires Bailly‐Creat, Paris, France, manufacturers of captodiamine (Covatine), who financed the honoraria of the participating physicians and the statistical analysis." Comment: No indication of sponsor's influence on study analysis, etc.; interpreted as high risk of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 16 weeks Single‐centre | |
| Participants | Baseline characteristics Not described Inclusion criteria: Referred to Benzodiazepine Withdrawal Clinic, benzodiazepines had been taken long term (> 6 months) at normal dose (< 30 mg/day of diazepam or equivalent), 18 to 70 years of age, body weight within normal limits Exclusion criteria: Major physical or psychiatric illnesses, drug abuse, women of childbearing age unless taking effective contraceptive measures Pretreatment: Not reported | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 4 weeks buspirone/placebo stabilisation, 6 weeks tapering to zero, 4 weeks of benzodiazepine abstinence, buspirone/placebo halved in dosage and then stopped 2 weeks later
| |
| Outcomes |
| |
| Identification | Sponsorship source: This study was supported by a grant from Bristol‐Myers Squibb UK to the Institute of Psychiatry. Country: UK Setting: Outpatients Declarations of interest: Not mentioned Author's name: Morton S Institution: Institute of Psychiatry, University of London Email: Address: Institute of Psychiatry, De Crespigny Park, London SE5 8AFUK | |
| Notes | Means only given in figures (HAM‐A and benzodiazepine withdrawal symptoms), no SDs reported, not possible to impute in a methodologically valid way. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Half the patients were given buspirone in flexible dosage according to the usual criteria of clinical need, at a minimum of 15 mg/day in divided doses. The others received matching placebo in equivalent flexible dosage, again according to apparent clinical need...The study was conducted double‐blind with reference to whether buspirone or placebo was being administered in weeks 2‐18" Comment: Done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No risk of bias regarding the main outcome (benzodiazepine cessation), but the secondary outcomes were only analysed for the participants who had discontinued treatment, i.e. half of the participants. |
| Selective reporting (reporting bias) | Low risk | Comment: No reason to suspect selective outcome reporting |
| Other bias | High risk | Comment: Financed by a grant from Bristol‐Myers Squibb, but further information on potential influence on design, etc. not provided. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel Blinding: None, open‐label Duration: 8 weeks Single‐centre | |
| Participants | Baseline characteristics: Not reported Inclusion criteria: The participant selection criteria were as follows: (i) those aged 20 to 70 years; (ii) those whose medical condition was stable and drug regimens unchanged for longer than 3 months; (iii) those who had been prescribed either alprazolam, bromazepam, etizolam, or lorazepam for at least 3 months prior to visiting the clinic; and (iv) those who were able to visit the clinic for an 8‐week intervention (or control) period. Exclusion criteria: DSM‐IV major depression Pretreatment: No baseline characteristics provided. | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 8‐week gradual benzodiazepine discontinuation
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not described Country: Japan Setting: Outpatients Declarations of interest: Not mentioned Author's name: Nakao M Institution: Division of Psychosomatic Medicine, Teikyo University Hospital Email: [email protected]‐u.ac.jp Address: Department of Hygiene and Public Health, Teikyo University School of Medicine, 2‐11‐1 Kaga, Itabashi‐ku, Tokyo, Japan | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: No placebo: open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | Comment: Not done |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All randomised participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol provided, but all relevant outcomes seem to be reported. |
| Other bias | Unclear risk | Comment: No other apparent biases, the funding of the study not described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 4 weeks Single‐centre | |
| Participants | Baseline characteristics Zopiclone
Flunitrazepam
Inclusion criteria: Long‐term usage of benzodiazepine hypnotics (range 6 months to 22 years), use of flunitrazepam for at least 3 months with stabilisation at a nightly dosage of 1 mg for at least 1 month before inclusion Exclusion criteria: Other benzodiazepine consumption, use of psychotropic medications Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: during the first part of the study, participants were either switched gradually to 1) zopiclone (3.75 mg and then 7.5 mg) over a 2‐week period (N = 7), or 2) continued their usual treatment of flunitrazepam 1 mg (N = 11). In the second part of the trial, the hypnotic (either zopiclone or flunitrazepam) was gradually withdrawn according to a 2‐step scheme over 2 weeks. | |
| Outcomes |
| |
| Identification | Sponsorship source: This study was supported by Rhone‐Poulenc Rorer Ltd, France, and the Technion Sleep Medicine Center, Israel Country: Israel Setting: Outpatients Declarations of interest: Not mentioned Authors name: Ruth Pat‐Horenczyk Institution: Technion Sleep Laboratory, Faculty of Medicine Email: Address: Technion Sleep Laboratory, Faculty of Medicine, Gutwirth Building, Technion‐Israel Institute of Technology, Haifa 32000, Israel | |
| Notes | Data from the benzodiazepine withdrawal questionnaires were not reported, thus only data on benzodiazepine relapse and insomnia could be extracted from this trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind randomised", "ZOP and FLU were encapsulated, and dummy placebo capsules were given to the patients who did not switch to ZOP, so that during the 5‐week period, all patients consumed", "two identical‐looking pills each night." Comment: Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) | High risk | Comment: 20/24 participants completed the 5‐week withdrawal programme, however analysis was performed on women only due to uneven gender distribution between groups and high dropout rate among men. All 5 male participants had been randomised to the zopiclone group, 3 of whom dropped out, and it was decided to perform the analyses on women only (n = 18). |
| Selective reporting (reporting bias) | Low risk | Comment: Unlikely |
| Other bias | High risk | Comment: Role of funding source not explicitly described. |
| Methods | Study design: Randomised controlled trial Study grouping: Cross‐over Blinding: Double Duration: 6 weeks Single‐centre | |
| Participants | Baseline characteristics Not reported for each group because of cross‐over design. Most (70%) of the 80 study participants were male. The mean age during study was 42.6 years, and the mean duration in MMT was 4.4 years. Almost half (48.8%) of the participants had other drug abuse in addition to benzodiazepines in the month prior to study entry. Specifically, 25 had positive urine for opiates, 12 for cocaine, 14 for cannabis, and 5 for amphetamines. With respect to lifetime psychiatric diagnosis, 9 participants (11.3%) had 1 of the psychotic disorders, 18 (22.5%) had an affective disorder, 8 (10%) had an adjustment disorder, 2 (2.5%) had an organic brain disorder, 38 (47.5%) had no DSM‐IV Axis I diagnosis (but all 38 had a DSM‐IV Axis II personality disorder), and 5 (6.3%) had no DSM‐IV Axis I or Axis II psychiatric diagnosis. Inclusion criteria: All patients who were admitted to the MMT clinic between July 1993 and July 2004 were eligible for inclusion in the study. This MMT clinic receives patients who meet DSM‐IV criteria for opioid dependence and report self administration of illicit heroin for 1 year or more. Exclusion criteria: None Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: run‐in phase: taper, until reaching 6 mg/day clonazepam or equivalent. Week 1 through 6: 0.5 mg/week dose reduction
| |
| Outcomes |
| |
| Identification | Sponsorship source: The study was supported (in part) by a grant from The Israel Anti Drug Authority. Country: Israel Setting: Outpatients in methadone maintenance treatment Declarations of interest: Not mentioned Author's name: Einat Peles Institution: Adelson Clinic, Tel Aviv Sourasky Medical Center Email: [email protected] Address: Adelson Clinic, Tel Aviv Sourasky Medical Center, 1 Henrietta Szold Street, Tel Aviv 64924, Israel | |
| Notes | Only data from the first period (first 6 weeks) of this cross‐over trial was included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutive container numbers" Comment: Done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "codes for melatonin first/placebo first were known only to the pharmacist" Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The codes for melatonin first/placebo first were known only to the pharmacist who prepared the sequence in a random manner and identified it to us only at the end of the study" Comment: Only pharmacist knew the code ‐ done. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "40 patients who started on melatonin and 40 patients who started on placebo. Sixty‐one patients (31 from the ‘melatonin‐first’ group and 30 from the ‘placebo‐first’ group) completed phase one (6 weeks). Forty‐four patients completed all 13 weeks of the study, with no differences between groups (60% of the 40 ‘melatonin‐first’ group and 50% of the 40 ‘placebo‐first’ group (P = 0.5)." Comment: Unclear how the high dropout after 6 weeks affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: The division of benzodiazepine continuers/discontinuers in the analysis seems to blur the effect of the study medication in itself. |
| Other bias | Low risk | Comment: No other apparent source of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 13 weeks Single‐centre | |
| Participants | Baseline characteristics No group difference. Only combined baseline characteristics reported:
Inclusion criteria: Age range of 21 to 70 years, had to have been on continuous daily treatment with diazepam, lorazepam, or alprazolam for a minimum of 1 year, and needed to be able to provide written informed consent Exclusion criteria: A screening medical history, physical examination, ECG, blood count, blood chemistry, urine analysis, and urine drug screens were performed to confirm each patient’s study eligibility. Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: gradual taper was initiated at approximately 25% reduction per week, with participants on lower therapeutic benzodiazepine doses possibly being tapered slightly faster, and participants on higher therapeutic doses being tapered slightly slower, but not longer than 6 weeks. After the taper was completed, participants were seen weekly for at least 5 weeks in order to determine their ability to stay off their benzodiazepine. During that time, participants continued to receive their double‐blind study medication. Study medication was discontinued at 5 weeks' post‐taper completion. From 5 to 12 weeks post‐taper, participants left the program and returned to their private physician for doctor’s choice management.
| |
| Outcomes |
| |
| Identification | Sponsorship source: This study was supported by USPHS Research Grant MHO8957. Country: USA Setting: Outpatients Declarations of interest: The study was supported by a US Public Health Service research grant Author's name: Rickels K Institution: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania Email: Address: University Science Center, 3600 Market Street, Suite 803, Philadelphia, PA 19104‐2649, USA | |
| Notes | We selected only the valproate vs placebo comparison for this meta‐analysis because we did not consider it relevant to combine the experimental intervention groups into a single group (cf. Cochrane Handbook 16.5.4 on how to include multiple groups from 1 study). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "randomly assigned under double blind conditions to study drug or placebo." Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) | High risk | Comment: 15 participants, 5 trazodone (12.2%), 5 valproate (26.3%), and 5 placebo (27.7%), dropped out during the pretreatment phase. The 15 dropouts were compared on a variety of demographic and illness variables with the 63 participants who entered taper, and no significant differences were present. |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | Unclear risk | Comment: Groups were not of equal size. No argument is provided. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 11 to 13 weeks Single‐centre | |
| Participants | Baseline characteristics No significant differences between the groups; only data for the combined participant group reported:
Inclusion criteria: Participants were required to have a diagnosis of generalised anxiety disorder according to DSM‐III‐R, to be at least 21 years old, and to have been taking diazepam, lorazepam, or alprazolam in therapeutic doses continuously for the past 12 months. Exclusion criteria: Panic disorder diagnosis Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 4‐week stabilisation phase, 4‐ to 6‐week taper phase: 25% reduction per week, 5‐week benzodiazepine‐free phase, the experimental drug continued for the first 3 weeks of the benzodiazepine‐free phase
| |
| Outcomes |
| |
| Identification | Sponsorship source: Supported by NIMH grant MH‐08957. Dr Greenblatt was supported by NIMH grant MH‐34223 and grant DA‐05258 from the National Institute on Drug Abuse. The medications used were provided by Bristol‐Myers Squibb CNS Group, Wallingford, CT. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Authors name: Karl Rickels Institution: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania, Philadelphia Email: [email protected] Address: University Science Center, 3600 Market St., Suite 803, Philadelphia, PA 19104 | |
| Notes | Adverse events not reported appropriately for a meta‐analysis. We selected only the imipramine versus placebo comparison for this meta‐analysis because we did not consider it relevant to combine the experimental intervention groups into a single group (cf. Cochrane Handbook 16.5.4 on how to include multiple groups from 1 study). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Medication was prepared double blind in identical capsules containing either 5 mg buspirone, 25 mg imipramine, or placebo" Comment: Sufficiently done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Not clearly described |
| Selective reporting (reporting bias) | Low risk | Comment: Probably not, relevant outcome measures |
| Other bias | Low risk | Comment: No other sources of bias evident. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks Single‐centre | |
| Participants | Baseline characteristics Ondansetron
Placebo
Inclusion criteria: DSM‐III‐R criteria for benzodiazepine dependence, a desire to discontinue use of benzodiazepines, daily use of alprazolam or lorazepam for > 3 months Exclusion criteria: Dosage of lorazepam > 8 mg/day or alprazolam > 5 mg/day, psychoactive substance use disorder (other than benzodiazepines), using other prescribed psychotropic medications (other benzodiazepine, antidepressants, antipsychotics, or anticonvulsants), psychosis, moderate to severe major depression, significant cognitive impairment, or suicidal ideation. Serious medical illness, pregnancy, liver enzymes elevated more than 3 times the upper limit, past history of head trauma Pretreatment: More anxiety patients in the placebo group | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: participants set their benzodiazepine tapering goals weekly with a study team member; the overall goal was benzodiazepine discontinuation within the treatment period (6 weeks).
| |
| Outcomes |
| |
| Identification | Sponsorship source: The study was supported in part by Glaxo Wellcome Canada. Country: Canada Setting: Outpatients Declarations of interest: Not mentioned Author's name: Myroslav Romach Institution: Departments of Pharmacology, Medicine, and Psychiatry and Faculty of Pharmacy, University of Toronto and Addiction Research Foundation, Toronto, Ontario, Canada Email: Address: Women's College Hospital, Department of Psychiatry, 76 Grenville St., Toronto, Ontario, Canada M5S 1B2 | |
| Notes | Not possible to extract results from this study due to poor reporting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: Described as double‐blind and identical‐appearing capsules |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not specifically described |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Out of 108 participants, only 11 (10%) participants dropped out. However, it is unclear if dropout was balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available, but no reason to suspect selective outcome reporting. |
| Other bias | High risk | Comment: Role of medicinal company as funding source not sufficiently described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 13 weeks Single‐centre | |
| Participants | Baseline characteristics No significant differences between groups; only data for the combined participant group reported:
Inclusion criteria: A diagnosis of panic disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition, at least 21 years old, and have been taking diazepam, lorazepam, or alprazolam in therapeutic doses continuously for at least the past 12 months (5 mg diazepam was considered equivalent to 1 mg lorazepam and 0.5 mg alprazolam). Individuals must also have expressed a desire to stop benzodiazepine intake. Exclusion criteria: Other psychotropic medication than benzodiazepines Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: after being kept on a stable benzodiazepine dose for 2 to 4 weeks within the therapeutic range during the screening period, participants were assigned to double‐blind treatment during which time their benzodiazepine intake was not altered. This pretreatment lasted 4 weeks, after which participants were tapered from their benzodiazepine dose over a 6‐week period. Benzodiazepine intake was reduced at the rate of 25% per week. Taper was followed by a 5‐week benzodiazepine‐free phase designed to prospectively assess the participant's clinical status in the initial period while being off benzodiazepines. Double‐blind study treatment was continued for the first 3 weeks of this phase. For the last 2 weeks, placebo was substituted for imipramine and buspirone.
| |
| Outcomes |
| |
| Identification | Sponsorship source: This research was supported by NIMH grant MH‐08957. Bristol‐Myers Squibb CNS Group (Wallingford, CT) provided all double‐blinded medications. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Authors name: Moira Rynn Institution: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania, Suite 670, 3535 Market Street, Philadelphia, PA 19104‐3309 Email: [email protected] Address: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania, Suite 670, 3535 Market Street, Philadelphia, PA 19104‐3309 | |
| Notes | Adverse events not appropriately described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Medication was prepared double blind in identical capsules" Comment: Done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Double‐blind identical capsules ‐ doneNot described |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of 52 patients randomised to 3 treatment conditions, 12 patients did not complete the pretreatment phase and 40 patients entered taper. The 2 patient groups did not differ in any baseline demographic or clinical measures with 1 exception. Dropouts had lower BZ doses at baseline, ex‐ pressed in diazepam equivalents, than patients entering taper (12.1 ± 7.7 versus 25.7 ± 19.5; F = 5.52; df = 1.50; P < 0.02). Dropouts also did not differ from taper patients in treatment assignment ( 2 = 0.69; df = 2; P = NS) or type of BZ at baseline ( 2 = 1.43; df = 2; P = NS). The main reason for dropping out of the program during the pre taper phase were adverse events (2 buspirone, 2 imipramine, and 1 placebo)." Comment: Acceptable and no difference between groups |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not available but no obvious selective outcome reporting |
| Other bias | Low risk | Comment: Role of BMS only to provide double‐blinded study medication |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 18 weeks Multicentre | |
| Participants | Baseline characteristics Atenolol
Placebo
Inclusion criteria: 18 to 60 years old, daily use of benzodiazepines for at least 8 weeks, not more than 15 mg of diazepam Exclusion criteria: Cerebrovascular or generalised vascular disease, heart block, thyrotoxicosis, premenstrual tension or other trigger of cyclical anxiety and depression, pregnancy, antihypertensive therapy or any drug likely to affect anxiety, and those for whom diazepam would be an unsuitable rescue Pretreatment: None reported. | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: follow‐up visits at 4‐week intervals, participants should have stopped taking benzodiazepines by their 4th visit
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not described Country: UK Setting: Outpatients Declarations of interest: Not mentioned Authors name: Saul PA Institution: General Practitioners, Stuart Clinical Research Group Email: Address: P. A. Saul, 555 Chorley Old Road, Bolton, Lancashire, BL2 6AF, UK | |
| Notes | None of the results were reported with mean and SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: Described as double‐blind and matching placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | High risk | Comment: High dropout rate: 59 out of 121 withdrew (48.7%) |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | Low risk | Comment: Apparently no other bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Blinding Duration: 8 to 10 weeks Single‐centre | |
| Participants | Baseline characteristics No significant differences between the intervention groups, therefore combined group reported:
Inclusion criteria: 18 years or older and receiving a daily dose of benzodiazepine continuously for at least the past year. Individuals were entered directly into the study if they were taking diazepam, alprazolam, or lorazepam in a dose of 40 mg or less diazepam equivalents (5 mg of diazepam = 0.5 mg of alprazolam = 1.0 mg of lorazepam). 6 individuals who were receiving a different benzodiazepine were switched to diazepam in an equivalent dose and stabilised for 3 weeks before entry into the study. Exclusion criteria: A history in the past year of alcohol or substance abuse or dependence, any acute or unstable medical condition, or not practicing adequate contraception Pretreatment: No significant group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 1 to 2 weeks pretreatment, benzodiazepine taper was initiated at a rate of 25% per week and completed over 4 weeks. Once the taper phase was completed and the participant had discontinued benzodiazepine intake, treatment with carbamazepine or placebo was continued for 2 to 4 weeks, then discontinued abruptly.
| |
| Outcomes |
| |
| Identification | Sponsorship source: This investigation was supported by Public Health Service research grant MH‐08957, Washington, DC. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Author's name: Edward Schweizer Institution: Psychopharmacology Research Unit, Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia Email: Address: 203 Piersol Bldg, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "random and double‐blind fashion", "Carbamazepine was provided in capsules that were identical to the placebo" Comment: Done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Carbamazepine levels were obtained in 12 of 19 patients, with the treating psychiatrist kept blind to the results." Comment: Judged as done since treating psychiatrist was kept blind |
| Incomplete outcome data (attrition bias) | High risk | Comment: Total dropout rate 15/55 (27%), 8 (30%) in the carbamazepine group and 7 (25%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Comment: No obvious selective outcome reporting |
| Other bias | Low risk | Comment: No other obvious sources of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 10 weeks Single‐centre | |
| Participants | Baseline characteristics Not reported Inclusion criteria: At least 18 years of age and taking diazepam, lorazepam, or alprazolam on a continuous daily basis for at least 1 year Exclusion criteria: Individuals were excluded from the study if they had a history of alcohol or substance abuse or dependence in the past year, or if they had any acute or unstable medical condition. Men could be of any age, while women had to be at least 2 years' postmenopause, or to have undergone an ovariectomy. Pretreatment: Unknown | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 2 to 3 weeks pretreatment with experimental drug, taper at the rate of 25% per week, after completion of taper experimental drug was continued for 4 weeks, then abruptly discontinued
| |
| Outcomes |
| |
| Identification | Sponsorship source: This study was supported by USPHS Research Grant MHO‐8957. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Author's name: Edward Schweizer Institution: University Science Center, Suite 803, 3600 Market Street, Philadelphia, PA 19104‐2649, USA Email: Address: University Science Center, Suite 803, 3600 Market Street, Philadelphia, PA 19104‐2649, USA | |
| Notes | Benzodiazepine withdrawal symptoms and anxiety not reported appropriately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "under random, double‐blind conditions", "either micronized oral progesterone in 300 mg capsules or matched placebo" Comment: Described as double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) | High risk | Comment: 8 (27%) participants in progesterone group versus 1 (8%) participant in placebo group dropped out during the pretreatment phase (due to sedation as side effect). |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | Low risk | Comment: No apparent other bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 2 weeks Multicentre | |
| Participants | Baseline characteristics Not reported Inclusion criteria: Monotherapy with diazepam or lorazepam, medication use for at least 4 months regularly, and were thought not to require continued prescription Exclusion criteria: Drugs other than benzodiazepines Pretreatment: Not reported | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: abrupt cessation
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not stated Country: UK Setting: General practice and outpatient psychiatric clinics Declarations of interest: Not mentioned Authors name: Peter Tyrer Institution: Mapperley Hospital, Nottingham, and Poisons Unit, New Cross Hospital, London Email: Address: Mapperley Hospital, Porchester Road, Nottingham NG3 6AA, UK | |
| Notes | Figure 1 reports withdrawal symptoms in each group, only mean score not SD, and no other measures from which the SD can be calculated. Since this was the only identified study using propranolol, and since the scale for withdrawal symptoms was not a validated scale used in other included studies, it was not possible to safely impute values for SD. Otherwise only dropout rate was reported for each group (55% in placebo group and 36% in propranolol group) = patients returning to same benzodiazepine treatment as previously. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "If patients agreed to enter this double‐blind study their benzodiazepines were stopped and replaced by propranolol or placebo tablets of identical appearance" Comment: Done |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: Daily self ratings, no third party involved in outcome assessment |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Of the 40 patients entering the study 18 (45%) dropped out during the two week period and took their benzodiazepine drugs again." Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: No obvious selective outcome reporting |
| Other bias | High risk | Quote: "I.C.I. Pharmaceuticals Division for providing the propranolol and placebo tablets." Comment: Role, if any, in the analyses not described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Single‐centre | |
| Participants | Baseline characteristics Dosulepin
Placebo
Inclusion criteria: Use of benzodiazepines for at least 6 months and had tried unsuccessfully to reduce or stop benzodiazepines due to apparent withdrawal symptoms, no other medication, written consent Exclusion criteria: Hypertension or the psychiatric diagnoses of major depressive disorder, a psychotic disorder, or melancholia Pretreatment: Not reported | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: reduction of the initial dosage by 20% every 2 weeks with the intention of stopping benzodiazepines entirely at week 8
| |
| Outcomes |
| |
| Identification | Sponsorship source: Research Department of Boots Drug Company funded the study. Country: UK Setting: Outpatients Declarations of interest: Not mentioned Authors name: Peter Tyrer Institution: St Charles Hospital, London Email: Address: St Charles Hospital, London WlO 6DZ | |
| Notes | For benzodiazepine withdrawal symptoms and anxiety, means were available from graphs only, but SDs were not reported. It was not possible to calculate SD from the presented data. Adverse events insufficiently reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation process not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "under cover of randomly allocated dothiepin or placebo tablets...administered using double‐blind procedure." Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) | High risk | Quote: "During the 14 weeks 45 patients (21 (51%) allocated to dothiepin and 24 (52%) to placebo) withdrew from the study." Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | High risk | Comment: Funded by a drug company. Role of funding source not described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 to 12 weeks depending on the alprazolam starting dose Multicentre | |
| Participants | Baseline characteristics Buspirone
Placebo
Inclusion criteria: 18 to 70 years of age, primary clinical anxiety, alprazolam pharmacotherapy for at least 3 months 0.75 to 3 mg daily, good physical health Exclusion criteria: Significant or uncontrolled organic disease, epilepsy or seizures, nursing/pregnant/not using contraceptive measures, substance use disorder, primary depression, panic disorder, psychosis, severe behaviour disorder, organic mental disorders, serious psychosomatic disorders, hypersensitivity to study drug, other drugs (psychotropics, beta‐blockers, carbamazepine, clonazepam) within 1 month before start of the study Pretreatment: No significant pretreatment group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: 2 weeks of concurrent treatment (study drug + stable benzodiazepine dosage), from the third week tapering of alprazolam 0.5 mg/day each week until the total daily dose was 1.5 mg, after which the rate of tapering was 0.25 mg/day each week; completion of the tapering process was to take from 2 to 8 weeks depending on the alprazolam starting dose. At completion, participants were to continue receiving the study drugs (buspirone or placebo) for an additional 2 weeks.
| |
| Outcomes |
| |
| Identification | Sponsorship source: Bristol‐Myers Squibb Country: USA Setting: Outpatients Declarations of interest: Not mentioned Authors name: Harold D Udelman Institution: Biomedical Stress Research Foundation, Phoenix, Arizona, USA Email: Not available Address: Biomedical Stress Research Foundation, 45 East Born Road, Phoenix, Arizona 85012 | |
| Notes | Means only given in figures (HAM‐A and benzodiazepine withdrawal symptoms), no SDs reported, not possible to impute in a methodologically valid way. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patients meeting the selection criteria were randomly assigned to one of two groups to receive either buspirone or placebo...Both buspirone and placebo (indistinguishable in physical appearance) were to be administered at a fixed dose..." Comment: Placebo was described as indistinguishable in physical appearance. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) | High risk | Comment: High attrition rate in buspirone group (42%) and in placebo group (53%) |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not available, but no indication of selective outcome reporting. |
| Other bias | High risk | Comment: Role of funding pharmaceutical company not described. |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 16 weeks (10 weeks to taper off and 6 weeks post‐taper) 9 general practices | |
| Participants | Baseline characteristics Melatonin
Placebo
Inclusion criteria: Adult patients who used benzodiazepines as a sleeping medication for more than 3 months (defined as long‐term use) at a minimum of 3 days per week Exclusion criteria: Use of more than 1 benzodiazepine at the same time, use of another type of sleep medication, use of stimulants and alcohol misuse (according to individual's GP), serious mental/somatic disease, or unfit to participate Pretreatment: No significant pretreatment differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: the benzodiazepine dose was converted to an equivalent dose of diazepam, which was stabilised for 2 weeks and then further converted every 2 weeks to 75%, 50%, 25%, 12.5%, and 0% of the original dose.
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not described Country: The Netherlands Setting: Outpatients in GP Declarations of interest: Not mentioned Authors name: Vissers FHJA Institution: Department of General Practice, Maastricht University Email: [email protected] Address: Department of General Practice, Maastricht University, P.O. Box 616, Maastricht 6200 MD, the Netherlands | |
| Notes | Insomnia: the Sleep Wake Experience List: mean and SD not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The patients, their GPs and the principal investigator were blinded for the study medication." Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Insufficient information to judge the risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All randomised participants were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Benzodiazepine withdrawal symptoms ambiguously reported. |
| Other bias | Low risk | Comment: No other apparent biases, funding not reported |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: None ‐ open‐label Duration: 3 weeks of inpatient treatment Single‐centre | |
| Participants | Baseline characteristics Valproate
Control
Inclusion criteria: DSM‐IV criteria for opioid dependence and benzodiazepine dependence Exclusion criteria: Pregnancy, active medical illnesses or severe mental disorders, history of convulsions, or unable to speak Finnish Pretreatment: At baseline, the median diazepam‐equivalent dose was 60 mg daily (range 20 to 160 mg) in the valproate group and 30 mg (range 8 to 75 mg) in the control group. | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: reduction with 10 mg diazepam equivalents daily until 40 mg per day, after which reductions were 5 mg daily
| |
| Outcomes |
| |
| Identification | Sponsorship source: The study was supported by Annual EVO Financing (special government subsidies) from the Department of Psychiatry, Helsinki University Central Hospital. No support was provided by any pharmaceutical company. Country: Finland Setting: Opioid maintenance treatment, inpatient setting, very rapid benzodiazepine‐tapering regimen Declarations of interest: None Author's name: Helena Vorma Institution: Helsinki University Central Hospital, Department of Psychiatry Email: [email protected] Address: Helsinki University Hospital, Department of Psychiatry, P.O. Box 590, FI‐00029 HUS, Finland | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "To prevent unequal treatment group sizes, we used block randomisation in blocks of six subjects. Sealed envelopes were used to keep the randomisation sequence unknown. The study was carried out as an open trial, with all outcome ratings assessed blindly to prevent detection bias." |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Not done, open‐label trial |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: All outcome assessments were done blindly. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Another 8 subjects discontinued participation in CIWA‐B ratings, but stayed in treatment." Comment: Not described why and from which group, accounted for by LOCF, but difficult to judge if this might give rise to any kind of bias |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol available; despite the rapid benzodiazepine taper regimen, it is remarkable that there is no indication of the benzodiazepine‐tapering success |
| Other bias | Unclear risk | Comment: Big difference in benzodiazepine dose between groups at baseline (valproate 60 mg/day, control group 30 mg/day) |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Unknown Duration: 3 months Single‐centre | |
| Participants | Baseline characteristics Trazodone
Placebo
Inclusion criteria: Benzodiazepine dependence syndrome (Criteria of Mental Disorders in China, Third Edition), insomnia Exclusion criteria: Abuse of alcohol or other psychoactive drugs, other mental disorders, serious somatic illness, allergic to study medication, suicidal risk, pregnancy, breastfeeding, lack of consent Pretreatment: No significant pretreatment group differences | |
| Interventions | Benzodiazepine taper schedule: benzodiazepine reduced to half dosage, when participant has had stable sleep for 5 days, then dosage is halved again and so forth.
| |
| Outcomes |
| |
| Identification | Sponsorship source: Not reported Country: China Setting: Outpatients Declarations of interest: Not mentioned Author's name: Zhang Hong‐Ju Institution: He'nan Provincial People's Hospital, Zhengzhou Email: [email protected] Address: Department of Neurology, He'nan Provincial People's Hospital, Zhengzhou 450003, He'nan, China | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Not described as blinded or open‐label |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Not described as blinded or open‐label |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Attrition: 2 of 38 (5%) participants |
| Selective reporting (reporting bias) | High risk | Comment: No data on use of benzodiazepine at follow‐up |
| Other bias | Low risk | Comment: No other apparent sources of bias |
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Multicentre | |
| Participants | Baseline characteristics Paroxetine
Placebo
Inclusion criteria: Benzodiazepine use for at least 3 months, a diagnosis of major depressive disorder (DSM‐III‐R), at least 18 years of age, written informed consent Exclusion criteria: Depression caused by organic factors, psychosis, schizophrenia, pregnancy, lactation, childbearing potential with a lack of adequate contraception, severe concomitant medical conditions, history of seizure disorders, use of other psychotropic medication during the 3 months prior to screening, clinically significant abnormalities in haematology or clinical chemistry, misuse of alcohol or illicit drugs, excessive use of benzodiazepines (more than 3 times the maximal dose), current suicidal risk Pretreatment: No significant pretreatment group differences | |
| Interventions | Intervention characteristics Benzodiazepine taper schedule: weeks 1 to 4: transfer to diazepamweeks 5 to 10: constant dose; weeks 11 to 12: 25% reduction per week; weeks 13 to 14: 12.5% reduction in 4 steps to 0
| |
| Outcomes |
| |
| Identification | Sponsorship source: One of the authors was employed by SmithKline Beecham, which also funded the study. Country: The Netherlands Setting: Outpatients Declarations of interest: Not mentioned Authors name: Frans Zitman Institution: Department of Psychiatry, Leiden and UMC Stat Radbond, Nijmegen Email: [email protected] Address: Department of Psychiatry, Leiden, BIP, P.O. Box 9600, 2300 RC Leiden | |
| Notes | Anxiety: Not sufficiently reported Withdrawal symptoms: Data not reported for placebo vs paroxetine, but for success vs no‐success groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation list (1‐330) in blocks of six was obtained by using the blocks of six was obtained by using the random number generator of SPSS/PC+ (SPSS, 1997)." Comment: Done |
| Allocation concealment (selection bias) | Low risk | Quote: "Based on this list, study medication (paroxetine, placebo) was blister packed and wrapped by Genfarma, The Netherlands. Blocks were sequentially distributed to GPs. Unused blocks were reallocated. The list was kept by the Medical Adviser on Safety of the medical department Adviser on Safety of the medical department of SmithKline Beecham, The Netherlands." Comment: Done |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patients were randomised to 20 mg of paroxetine or placebo in a 1:2 double‐blind fashion" Comment: What was done to ensure blinding of participants and personnel in terms of matching paroxetine/placebo is not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "After the database was closed and basic descriptive analyses were done, the actual codes were added to the database" Comment: Done |
| Incomplete outcome data (attrition bias) | High risk | Comment: 50% completed the programme in the paroxetine group and 43% in the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Some outcome data not reported for randomisation groups, but for success and no‐success group instead. |
| Other bias | High risk | Comment: Role of funding pharmaceutical company not described. |
AEs: adverse events
BWSQ: Benzodiazepine Withdrawal Symptom Questionnaire
CI: confidence interval
CIWA‐B: Clinical Institute Withdrawal Assessment Scale ‐ Benzodiazepines
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Revised 3rd Edition
DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision
ECG: electrocardiogram
GAD: generalised anxiety disorder
GP: general practitioner
HADS‐A Hospital Anxiety and Depression Scale ‐ Anxiety subscale
HAM‐A: Hamilton Anxiety Rating Scale
HAM‐D: Hamilton Depression Rating Scale
ICD‐10: International Statistical Classification of Diseases and Related Health Problems, 10th revision
ITT: intention‐to‐treat
IV: intravenous
LOCF: last observation carried forward
MADRS: Montgomery–Åsberg Depression Rating Scale
MMT: methadone maintenance treatment
NIMH: National Institute of Mental Health
NS: not specified
PSQI: Pittsburgh Sleep Quality Index
PTSD: post‐traumatic stress disorder
PWC: Physician Withdrawal Checklist
SAEs: serious adverse events
SD: standard deviation
SE: standard error
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong patient population: duration of benzodiazepine use above 1 month and no mention of dependence | |
| Wrong study design: not randomised | |
| Wrong study design: uncontrolled, observational study | |
| Wrong study design: uncontrolled, observational study | |
| Wrong study design: co‐intervention not delivered equally in both intervention groups | |
| Wrong study design: observational study | |
| Wrong intervention: switch to benzodiazepine‐like drug without discontinuation | |
| Wrong study design: not randomised | |
| Wrong study design: not randomised | |
| Wrong study design: co‐intervention not delivered equally in both intervention groups | |
| Wrong patient population: not chronic benzodiazepine users | |
| Wrong patient population: not chronic benzodiazepine users | |
| Wrong study design: co‐intervention not delivered equally in both intervention groups | |
| Wrong study design: not randomised | |
| Wrong study design: observational study | |
| Wrong study design: the study investigated gradual benzodiazepine withdrawal versus abrupt discontinuation | |
| Wrong study design: not randomised | |
| Wrong study design: observational study | |
| Wrong study design: study not designed to evaluate benzodiazepine discontinuation | |
| Wrong intervention: switch to benzodiazepine‐like drug without discontinuation | |
| Wrong study design: study not designed to evaluate benzodiazepine discontinuation | |
| Wrong study design: not randomised |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [1.08, 6.03] |
| Analysis 1.1  Comparison 1 Valproate versus placebo or no intervention, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Relapse to benzodiazepine use, end of intervention Show forest plot | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.90] |
| Analysis 1.2 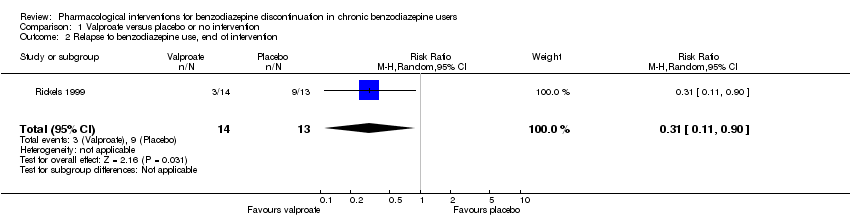 Comparison 1 Valproate versus placebo or no intervention, Outcome 2 Relapse to benzodiazepine use, end of intervention. | ||||
| 3 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.80, 3.09] |
| Analysis 1.3  Comparison 1 Valproate versus placebo or no intervention, Outcome 3 Benzodiazepine discontinuation, longest follow‐up. | ||||
| 4 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.39] |
| Analysis 1.4  Comparison 1 Valproate versus placebo or no intervention, Outcome 4 Relapse to benzodiazepine use, longest follow‐up. | ||||
| 5 Anxiety: HAM‐A (Hamilton Anxiety Rating Scale), end of intervention Show forest plot | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.47, 5.67] |
| Analysis 1.5  Comparison 1 Valproate versus placebo or no intervention, Outcome 5 Anxiety: HAM‐A (Hamilton Anxiety Rating Scale), end of intervention. | ||||
| 6 Benzodiazepine withdrawal symptoms, end of intervention Show forest plot | 2 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.68, 0.37] |
| Analysis 1.6 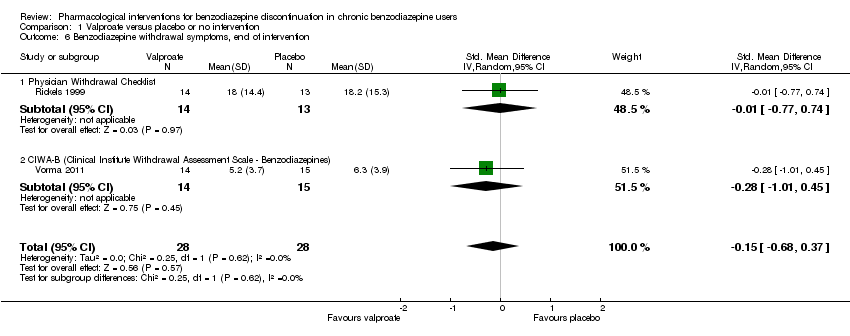 Comparison 1 Valproate versus placebo or no intervention, Outcome 6 Benzodiazepine withdrawal symptoms, end of intervention. | ||||
| 6.1 Physician Withdrawal Checklist | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.77, 0.74] |
| 6.2 CIWA‐B (Clinical Institute Withdrawal Assessment Scale ‐ Benzodiazepines) | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.01, 0.45] |
| 7 Discontinuation due to adverse events Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.7  Comparison 1 Valproate versus placebo or no intervention, Outcome 7 Discontinuation due to adverse events. | ||||
| 8 Serious adverse events Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.8  Comparison 1 Valproate versus placebo or no intervention, Outcome 8 Serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 3 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.99, 1.80] |
| Analysis 2.1  Comparison 2 Carbamazepine versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Benzodiazepine withdrawal symptoms Show forest plot | 2 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.43, 0.16] |
| Analysis 2.2 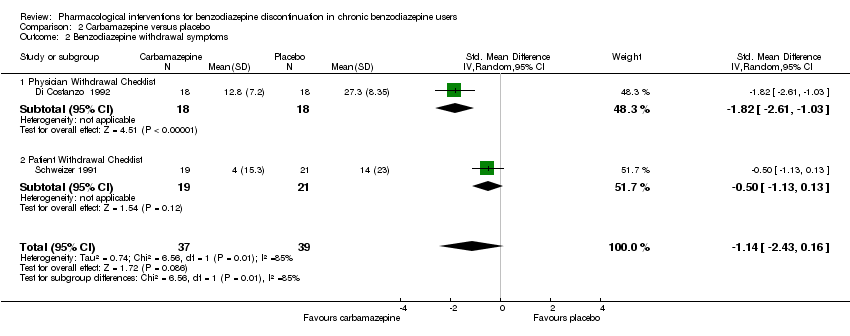 Comparison 2 Carbamazepine versus placebo, Outcome 2 Benzodiazepine withdrawal symptoms. | ||||
| 2.1 Physician Withdrawal Checklist | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐2.61, ‐1.03] |
| 2.2 Patient Withdrawal Checklist | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.13, 0.13] |
| 3 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.86, 2.29] |
| Analysis 2.3 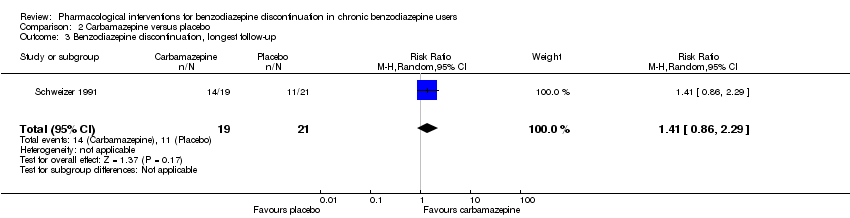 Comparison 2 Carbamazepine versus placebo, Outcome 3 Benzodiazepine discontinuation, longest follow‐up. | ||||
| 4 Relapse to benzodiazepine use Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.44] |
| Analysis 2.4 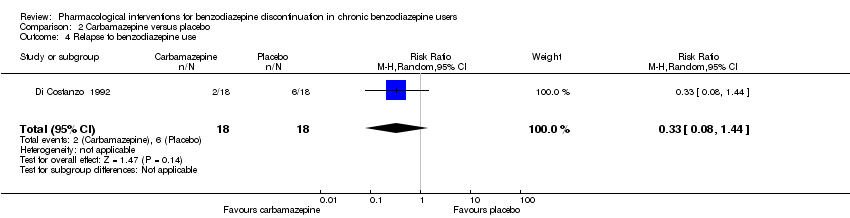 Comparison 2 Carbamazepine versus placebo, Outcome 4 Relapse to benzodiazepine use. | ||||
| 5 Serious adverse events Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.5  Comparison 2 Carbamazepine versus placebo, Outcome 5 Serious adverse events. | ||||
| 6 Non‐serious adverse events Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 126.48] |
| Analysis 2.6 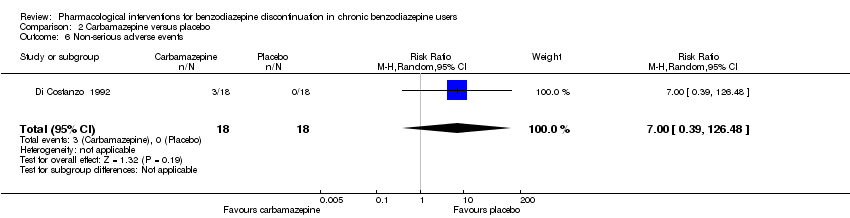 Comparison 2 Carbamazepine versus placebo, Outcome 6 Non‐serious adverse events. | ||||
| 7 Anxiety, HAM‐A Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐9.58, ‐2.42] |
| Analysis 2.7 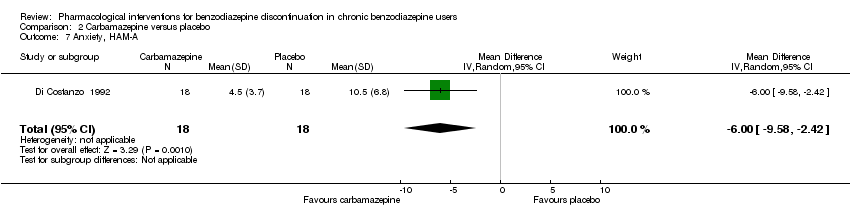 Comparison 2 Carbamazepine versus placebo, Outcome 7 Anxiety, HAM‐A. | ||||
| 8 Discontinuation due to adverse events Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.8  Comparison 2 Carbamazepine versus placebo, Outcome 8 Discontinuation due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.86, 1.28] |
| Analysis 3.1 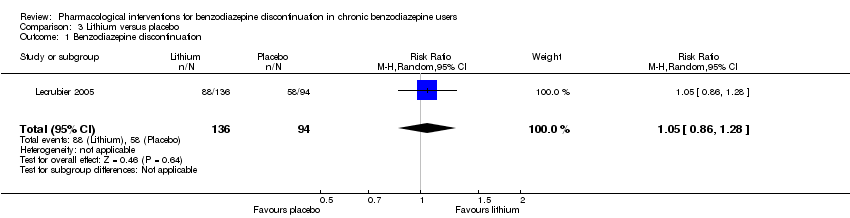 Comparison 3 Lithium versus placebo, Outcome 1 Benzodiazepine discontinuation. | ||||
| 2 Serious adverse events Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.2  Comparison 3 Lithium versus placebo, Outcome 2 Serious adverse events. | ||||
| 3 Non‐serious adverse events Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.49] |
| Analysis 3.3 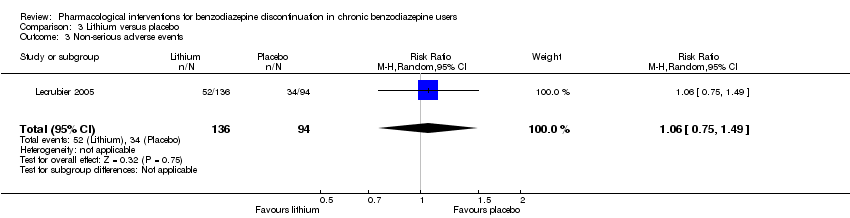 Comparison 3 Lithium versus placebo, Outcome 3 Non‐serious adverse events. | ||||
| 4 Discontinuation due to adverse events Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.13, 15.03] |
| Analysis 3.4 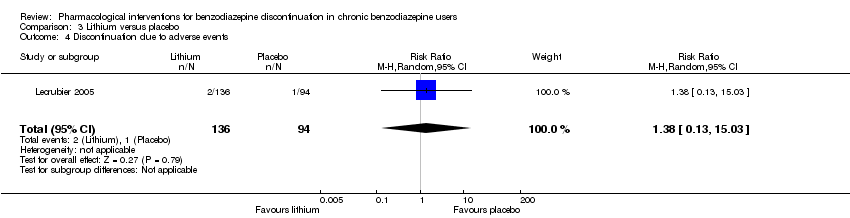 Comparison 3 Lithium versus placebo, Outcome 4 Discontinuation due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.92, 2.25] |
| Analysis 4.1  Comparison 4 Pregabalin versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention Show forest plot | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐3.1 [‐3.51, ‐2.69] |
| Analysis 4.2  Comparison 4 Pregabalin versus placebo, Outcome 2 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention. | ||||
| 3 Anxiety, HAM‐A, end of intervention Show forest plot | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐4.8 [‐5.28, ‐4.32] |
| Analysis 4.3  Comparison 4 Pregabalin versus placebo, Outcome 3 Anxiety, HAM‐A, end of intervention. | ||||
| 4 Serious adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.16, 2.85] |
| Analysis 4.4  Comparison 4 Pregabalin versus placebo, Outcome 4 Serious adverse events. | ||||
| 5 Non‐serious adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.40] |
| Analysis 4.5 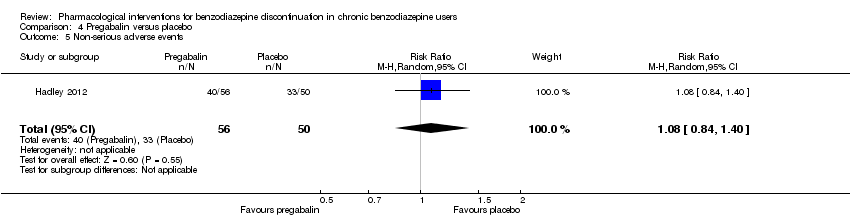 Comparison 4 Pregabalin versus placebo, Outcome 5 Non‐serious adverse events. | ||||
| 6 Discontinuation due to adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.59] |
| Analysis 4.6 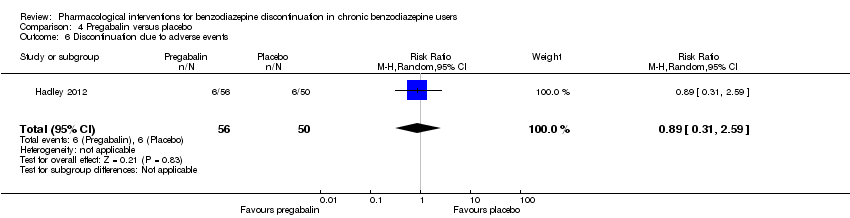 Comparison 4 Pregabalin versus placebo, Outcome 6 Discontinuation due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine withdrawal symptoms, BWSQ (Benzodiazepine Withdrawal Symptom Questionnaire), end of intervention Show forest plot | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.13, ‐0.87] |
| Analysis 5.1 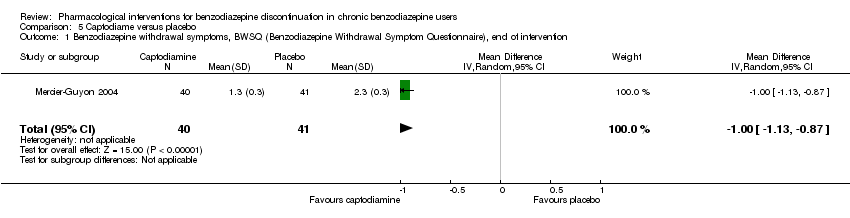 Comparison 5 Captodiame versus placebo, Outcome 1 Benzodiazepine withdrawal symptoms, BWSQ (Benzodiazepine Withdrawal Symptom Questionnaire), end of intervention. | ||||
| 2 Anxiety, HAM‐A, end of intervention Show forest plot | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐6.05, ‐5.35] |
| Analysis 5.2 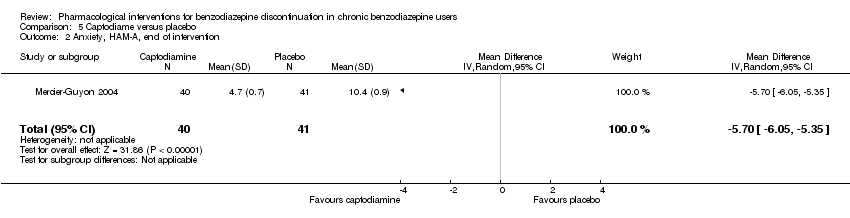 Comparison 5 Captodiame versus placebo, Outcome 2 Anxiety, HAM‐A, end of intervention. | ||||
| 3 Serious adverse events Show forest plot | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 5.3  Comparison 5 Captodiame versus placebo, Outcome 3 Serious adverse events. | ||||
| 4 Non‐serious adverse events Show forest plot | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 5.4 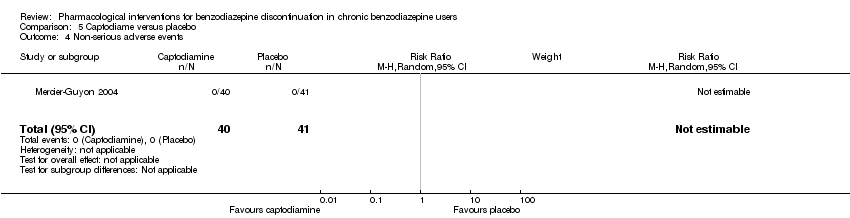 Comparison 5 Captodiame versus placebo, Outcome 4 Non‐serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.88, 2.39] |
| Analysis 6.1 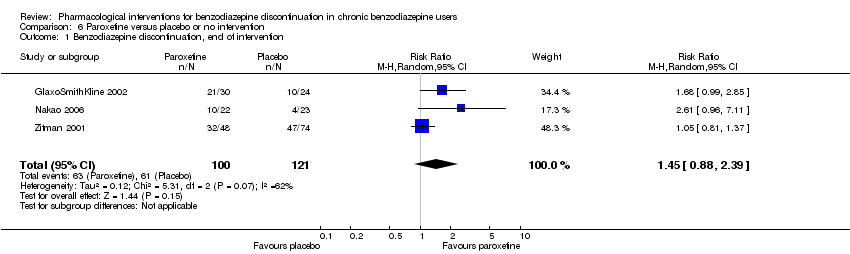 Comparison 6 Paroxetine versus placebo or no intervention, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Benzodiazepine withdrawal symptoms: BWSQ, end of intervention Show forest plot | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐5.34, ‐1.80] |
| Analysis 6.2 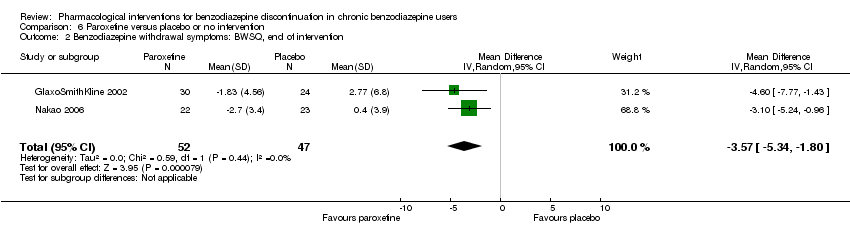 Comparison 6 Paroxetine versus placebo or no intervention, Outcome 2 Benzodiazepine withdrawal symptoms: BWSQ, end of intervention. | ||||
| 3 Anxiety: HAM‐A, end of intervention Show forest plot | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐9.64, ‐3.86] |
| Analysis 6.3 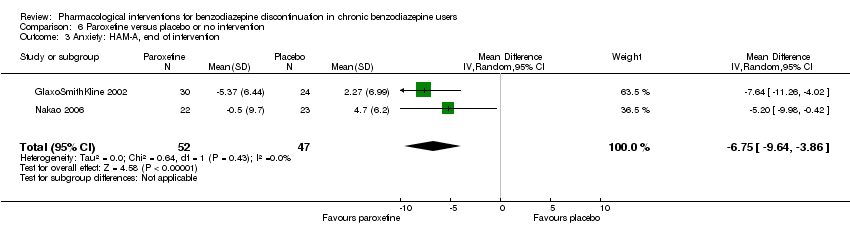 Comparison 6 Paroxetine versus placebo or no intervention, Outcome 3 Anxiety: HAM‐A, end of intervention. | ||||
| 4 Benzodiazepine withdrawal symptoms: BWSQ, longest follow‐up: 6 months Show forest plot | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐4.03, 3.77] |
| Analysis 6.4  Comparison 6 Paroxetine versus placebo or no intervention, Outcome 4 Benzodiazepine withdrawal symptoms: BWSQ, longest follow‐up: 6 months. | ||||
| 5 Serious adverse events Show forest plot | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.5  Comparison 6 Paroxetine versus placebo or no intervention, Outcome 5 Serious adverse events. | ||||
| 6 Non‐serious adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.35, 5.03] |
| Analysis 6.6  Comparison 6 Paroxetine versus placebo or no intervention, Outcome 6 Non‐serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.52, 1.28] |
| Analysis 7.1  Comparison 7 Tricyclic antidepressants versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Anxiety: HAM‐A (change from baseline), end of intervention Show forest plot | 2 | 66 | Mean Difference (IV, Random, 95% CI) | ‐10.38 [‐25.96, 5.20] |
| Analysis 7.2  Comparison 7 Tricyclic antidepressants versus placebo, Outcome 2 Anxiety: HAM‐A (change from baseline), end of intervention. | ||||
| 3 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.27, 3.82] |
| Analysis 7.3  Comparison 7 Tricyclic antidepressants versus placebo, Outcome 3 Benzodiazepine discontinuation, longest follow‐up. | ||||
| 4 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐19.78 [‐20.25, ‐19.31] |
| Analysis 7.4 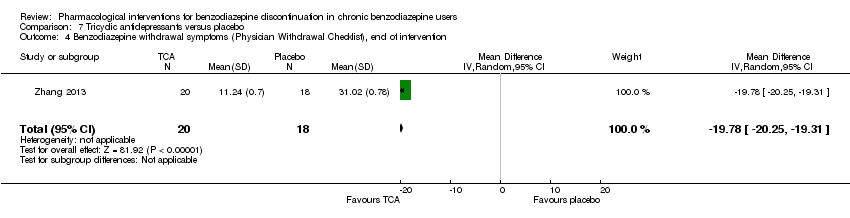 Comparison 7 Tricyclic antidepressants versus placebo, Outcome 4 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention. | ||||
| 5 Relapse to benzodiazepine use, end of intervention Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.73, 5.47] |
| Analysis 7.5 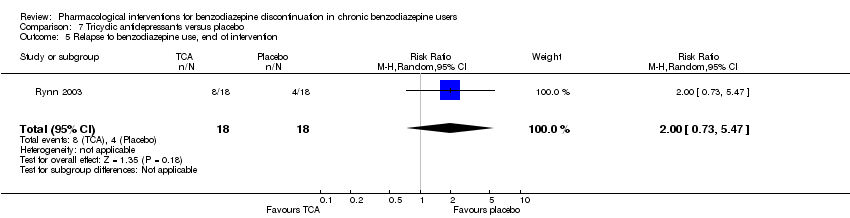 Comparison 7 Tricyclic antidepressants versus placebo, Outcome 5 Relapse to benzodiazepine use, end of intervention. | ||||
| 6 Discontinuation due to adverse events Show forest plot | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.42, 3.21] |
| Analysis 7.6  Comparison 7 Tricyclic antidepressants versus placebo, Outcome 6 Discontinuation due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.17, 0.99] |
| Analysis 8.1  Comparison 8 Alpidem versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Withdrawal syndrome (clinical diagnosis), end of intervention Show forest plot | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 4.86 [1.12, 21.14] |
| Analysis 8.2 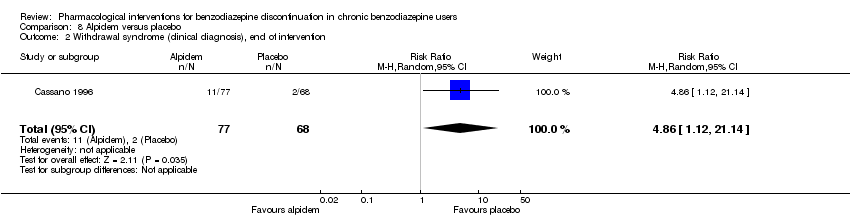 Comparison 8 Alpidem versus placebo, Outcome 2 Withdrawal syndrome (clinical diagnosis), end of intervention. | ||||
| 3 Anxiety, HAM‐A, end of intervention Show forest plot | 2 | 170 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.64, 1.45] |
| Analysis 8.3  Comparison 8 Alpidem versus placebo, Outcome 3 Anxiety, HAM‐A, end of intervention. | ||||
| 4 Relapse to benzodiazepine use, end of intervention Show forest plot | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.20] |
| Analysis 8.4  Comparison 8 Alpidem versus placebo, Outcome 4 Relapse to benzodiazepine use, end of intervention. | ||||
| 5 Serious adverse events Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 8.5  Comparison 8 Alpidem versus placebo, Outcome 5 Serious adverse events. | ||||
| 6 Discontinuation due to adverse events Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.05, 4.46] |
| Analysis 8.6  Comparison 8 Alpidem versus placebo, Outcome 6 Discontinuation due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 4 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.49, 1.37] |
| Analysis 9.1  Comparison 9 Buspirone versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Anxiety: HAM‐A/Hospital Anxiety Depression Scale, end of intervention Show forest plot | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.50, 0.86] |
| Analysis 9.2 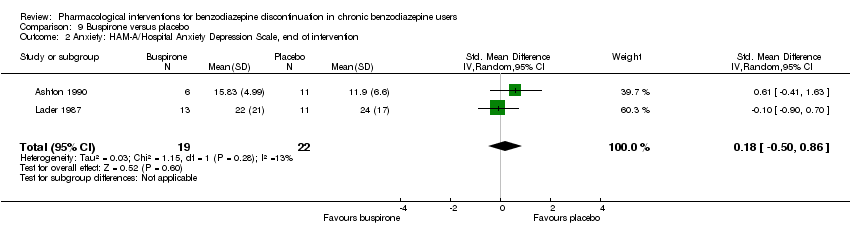 Comparison 9 Buspirone versus placebo, Outcome 2 Anxiety: HAM‐A/Hospital Anxiety Depression Scale, end of intervention. | ||||
| 3 Benzodiazepine withdrawal symptoms, end of intervention Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 4.69 [‐14.47, 23.85] |
| Analysis 9.3 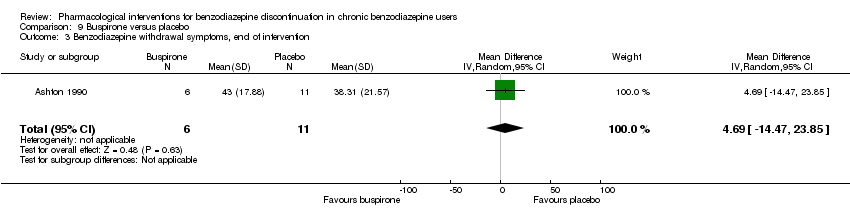 Comparison 9 Buspirone versus placebo, Outcome 3 Benzodiazepine withdrawal symptoms, end of intervention. | ||||
| 4 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.34, 1.05] |
| Analysis 9.4 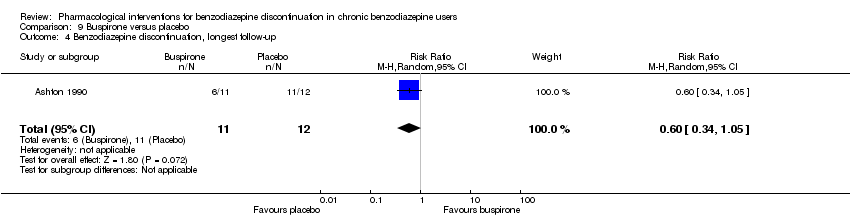 Comparison 9 Buspirone versus placebo, Outcome 4 Benzodiazepine discontinuation, longest follow‐up. | ||||
| 5 Benzodiazepine withdrawal symptoms, longest follow‐up Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐14.31, 11.63] |
| Analysis 9.5 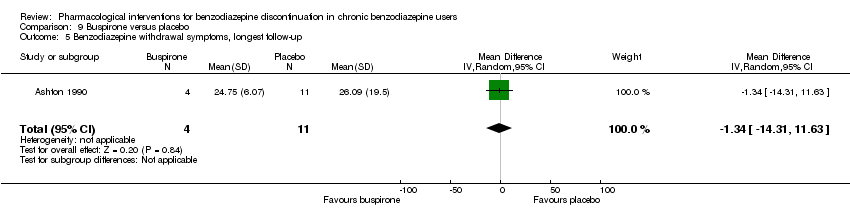 Comparison 9 Buspirone versus placebo, Outcome 5 Benzodiazepine withdrawal symptoms, longest follow‐up. | ||||
| 6 Anxiety, Hospital Anxiety Depression Scale, longest follow‐up Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 2.75 [‐2.83, 8.33] |
| Analysis 9.6 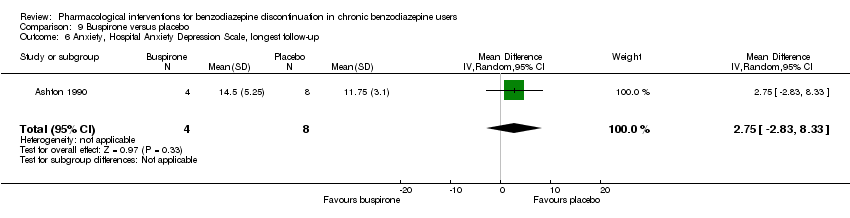 Comparison 9 Buspirone versus placebo, Outcome 6 Anxiety, Hospital Anxiety Depression Scale, longest follow‐up. | ||||
| 7 Discontinuation due to adverse events Show forest plot | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.92] |
| Analysis 9.7  Comparison 9 Buspirone versus placebo, Outcome 7 Discontinuation due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 4 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.73, 1.96] |
| Analysis 10.1  Comparison 10 Melatonin versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Insomnia Show forest plot | 3 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.70, 0.23] |
| Analysis 10.2  Comparison 10 Melatonin versus placebo, Outcome 2 Insomnia. | ||||
| 2.1 PSQI (Pittsburgh Sleep Quality Index) global score (higher = worse), end of intervention | 2 | 116 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.92, 0.31] |
| 2.2 Sleep quality (1 poorest, 10 excellent), end of intervention | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.34 [‐4.42, ‐2.26] |
| 3 Discontinuation due to adverse events Show forest plot | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.20, 22.26] |
| Analysis 10.3  Comparison 10 Melatonin versus placebo, Outcome 3 Discontinuation due to adverse events. | ||||
| 4 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.47, 2.27] |
| Analysis 10.4 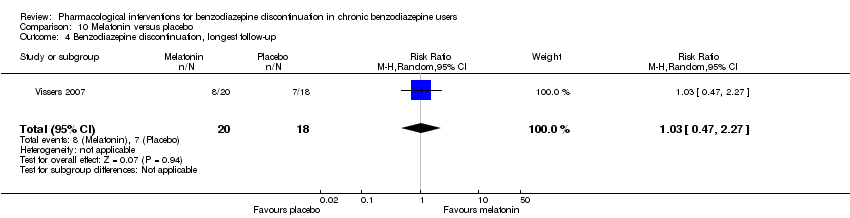 Comparison 10 Melatonin versus placebo, Outcome 4 Benzodiazepine discontinuation, longest follow‐up. | ||||
| 5 Adverse events Show forest plot | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.82] |
| Analysis 10.5  Comparison 10 Melatonin versus placebo, Outcome 5 Adverse events. | ||||
| 6 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.8 [0.37, 8.68] |
| Analysis 10.6  Comparison 10 Melatonin versus placebo, Outcome 6 Relapse to benzodiazepine use, longest follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine withdrawal symptoms, end of intervention Show forest plot | 3 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.71, ‐0.19] |
| Analysis 11.1  Comparison 11 Flumazenil versus placebo, Outcome 1 Benzodiazepine withdrawal symptoms, end of intervention. | ||||
| 2 Anxiety, HAM‐D (Hamilton Depression Rating Scale), end of intervention Show forest plot | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐1.3 [‐2.28, ‐0.32] |
| Analysis 11.2  Comparison 11 Flumazenil versus placebo, Outcome 2 Anxiety, HAM‐D (Hamilton Depression Rating Scale), end of intervention. | ||||
| 3 Benzodiazepine mean dose, end of intervention Show forest plot | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐22.06, 14.66] |
| Analysis 11.3  Comparison 11 Flumazenil versus placebo, Outcome 3 Benzodiazepine mean dose, end of intervention. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse to benzodiazepine use, end of intervention: 2 weeks Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.31, 1.30] |
| Analysis 12.1 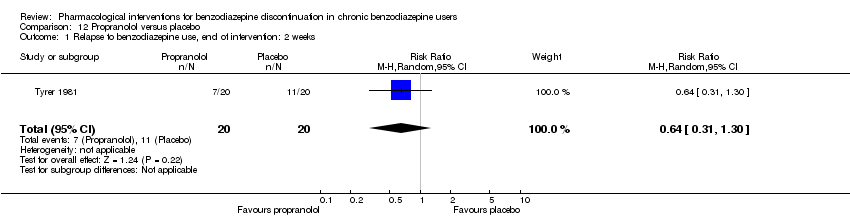 Comparison 12 Propranolol versus placebo, Outcome 1 Relapse to benzodiazepine use, end of intervention: 2 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.52, 2.54] |
| Analysis 13.1 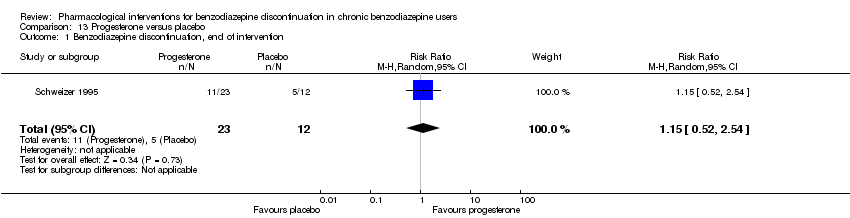 Comparison 13 Progesterone versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Non‐serious adverse events Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [1.15, 8.54] |
| Analysis 13.2 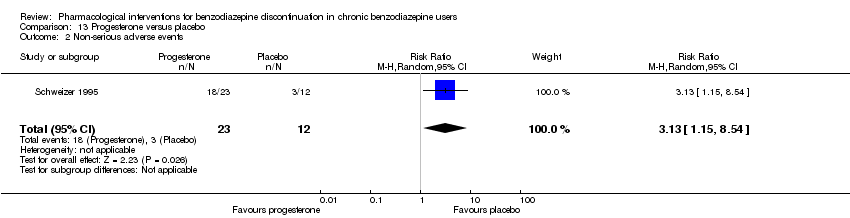 Comparison 13 Progesterone versus placebo, Outcome 2 Non‐serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.96] |
| Analysis 14.1 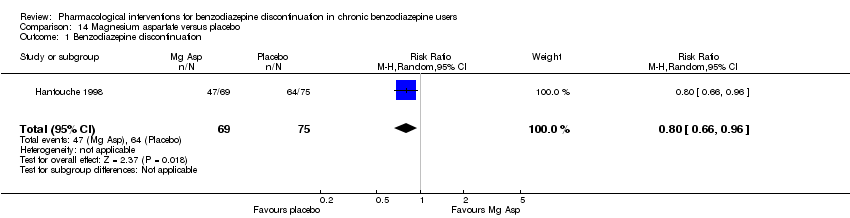 Comparison 14 Magnesium aspartate versus placebo, Outcome 1 Benzodiazepine discontinuation. | ||||
| 2 Anxiety Show forest plot | 1 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.73, 1.13] |
| Analysis 14.2 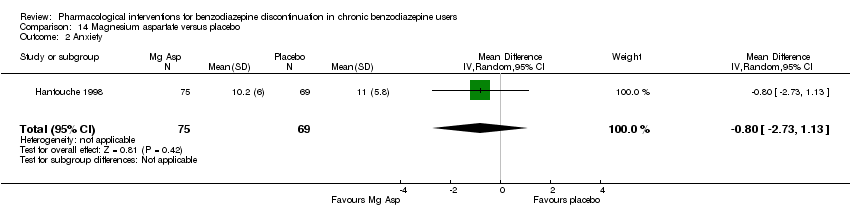 Comparison 14 Magnesium aspartate versus placebo, Outcome 2 Anxiety. | ||||
| 3 Relapse to benzodiazepine use Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.46, 1.87] |
| Analysis 14.3  Comparison 14 Magnesium aspartate versus placebo, Outcome 3 Relapse to benzodiazepine use. | ||||
| 4 Non‐serious adverse events Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.18, 1.35] |
| Analysis 14.4  Comparison 14 Magnesium aspartate versus placebo, Outcome 4 Non‐serious adverse events. | ||||
| 5 Discontinuation due to adverse events Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.13, 1.18] |
| Analysis 14.5 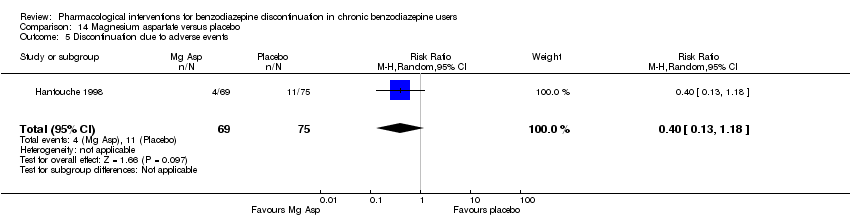 Comparison 14 Magnesium aspartate versus placebo, Outcome 5 Discontinuation due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation Show forest plot | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.36, 1.70] |
| Analysis 15.1  Comparison 15 Homéogène 46/Sedatif PC (homeopathic drugs) versus placebo, Outcome 1 Benzodiazepine discontinuation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.78, 1.29] |
| Analysis 16.1 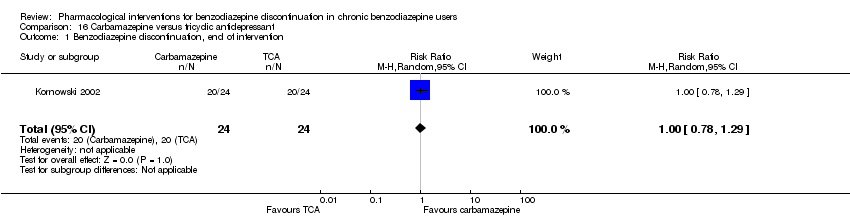 Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 1 Benzodiazepine discontinuation, end of intervention. | ||||
| 2 Relapse to benzodiazepine use Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.54] |
| Analysis 16.2  Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 2 Relapse to benzodiazepine use. | ||||
| 3 Serious adverse events Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 16.3 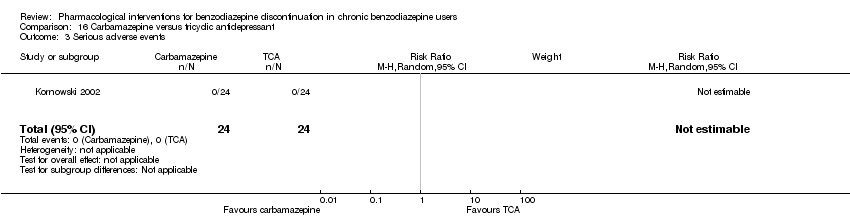 Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 3 Serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.14, 0.78] |
| Analysis 17.1  Comparison 17 Cyamemazine versus bromazepam, Outcome 1 Relapse to benzodiazepine use, longest follow‐up. | ||||
| 2 Anxiety: Maximum amplitude of rebound (HAM‐A), end of intervention Show forest plot | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐1.23, 2.23] |
| Analysis 17.2 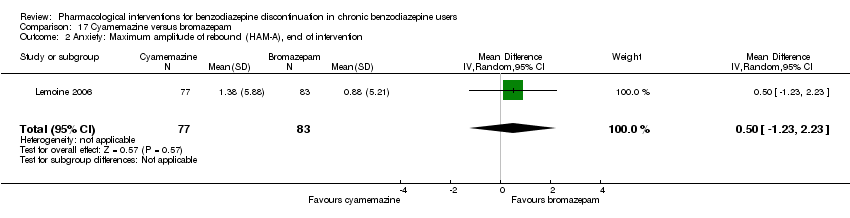 Comparison 17 Cyamemazine versus bromazepam, Outcome 2 Anxiety: Maximum amplitude of rebound (HAM‐A), end of intervention. | ||||
| 3 Discontinuation due to adverse events Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.79, 10.44] |
| Analysis 17.3 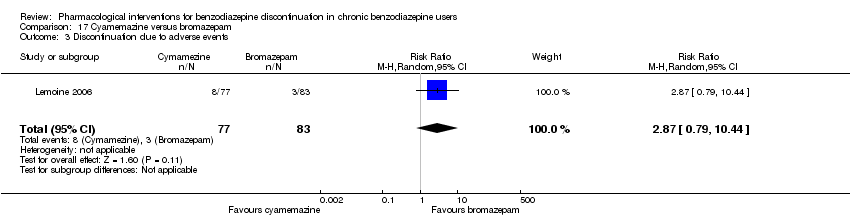 Comparison 17 Cyamemazine versus bromazepam, Outcome 3 Discontinuation due to adverse events. | ||||
| 4 Non‐serious adverse events Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.01, 2.78] |
| Analysis 17.4  Comparison 17 Cyamemazine versus bromazepam, Outcome 4 Non‐serious adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.78] |
| Analysis 18.1  Comparison 18 Zopiclone versus flunitrazepam, Outcome 1 Relapse to benzodiazepine use, longest follow‐up. | ||||
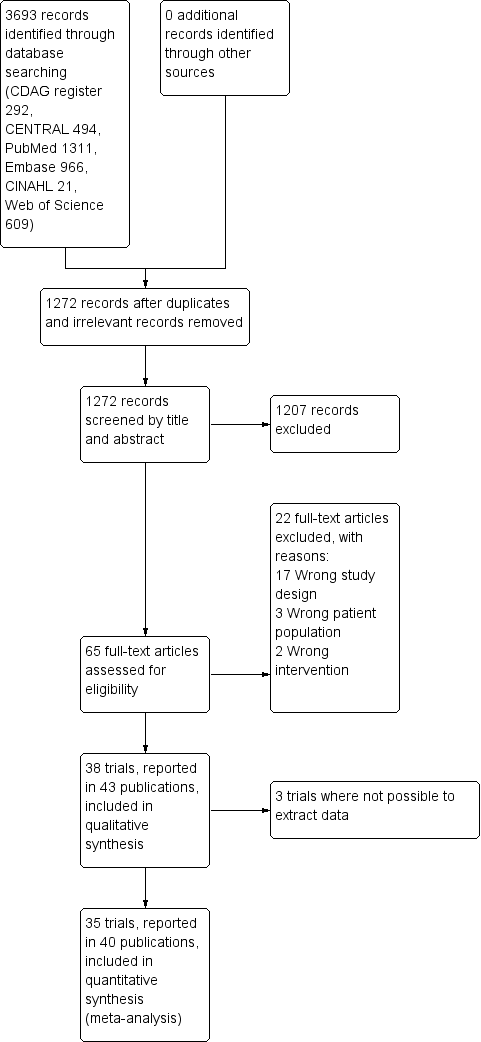
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
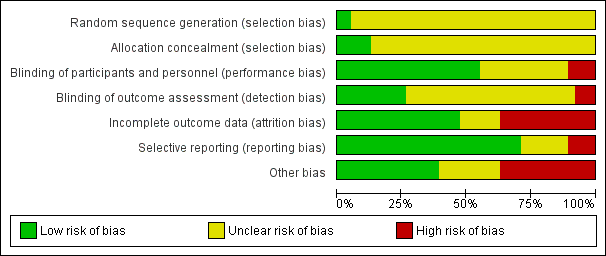
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Trial Sequential Analysis of comparison: 2 Carbamazepine versus placebo, outcome: 2.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in three trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction (RRR) of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 36% as observed in the trials. The diversity‐adjusted required information size (DARIS) was 2109 participants, and the Trial Sequential Analysis‐adjusted confidence interval is 0.24 to 2.38. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) touches the conventional statistical boundaries, but does not cross the trial sequential monitoring boundaries, and the diversity‐adjusted required information size is not met, showing that insufficient information has been accrued.

Trial Sequential Analysis of comparison: 6 Paroxetine versus placebo, outcome: 6.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in three trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 86% as observed in the trials. The diversity‐adjusted required information size was 9448 participants, and the Trial Sequential Analysis‐adjusted confidence interval could not be estimated due to lack of information. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries. The trial sequential monitoring boundaries and the diversity‐adjusted required information size are not shown as the accrued number of participants only amounted to 221/9448 (2.34%), showing that insufficient information has been accrued.

Trial Sequential Analysis of comparison: 6 Paroxetine versus placebo, outcome: 6.2 Benzodiazepine withdrawal symptoms Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ). Trial Sequential Analysis on benzodiazepine withdrawal symptoms assessed with BWSQ assessing a minimal relevant clinical difference (MIREDIF) of 2.25 points, and a variance of 20 points (empirical data), was performed based on a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0%. The diversity‐adjusted required information size (DARIS) was 229 participants, and the Trial Sequential Analysis‐adjusted confidence interval is ‐7.18 to 0.05. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 0.05. The red inward‐sloping lines represent the trial sequential monitoring boundaries. The cumulative Z‐curve touches the trial sequential monitoring boundaries, indicating that sufficient information was provided.

Trial Sequential Analysis of comparison: 6 Paroxetine versus placebo, outcome: 6.3 Anxiety, Hamilton Anxiety Rating Scale (HAM‐A). Trial Sequential Analysis on anxiety evaluated with HAM‐A assessing a minimal relevant clinical difference (MIREDIF) of 5 points, and a variance of 103 points, was performed based on a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0%. The diversity‐adjusted required information size (DARIS) was 236 participants, and the Trial Sequential Analysis‐adjusted confidence interval is ‐12.72 to ‐0.80. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 0.05. The red inward‐sloping lines represent the trial sequential monitoring boundaries. The cumulative Z‐curve crosses the trial sequential monitoring boundaries, indicating that sufficient information was provided.

Trial Sequential Analysis of comparison: 7 Tricyclic antidepressants versus placebo, outcome: 7.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in two trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction (RRR) of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 0% as observed in the trials. The diversity‐adjusted required information size (DARIS) was 1343 participants, and the Trial Sequential Analysis‐adjusted confidence interval is 0.20 to 7.55. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries or the trial sequential monitoring boundaries (red dotted lines), and the diversity‐adjusted required information size is not met, showing that insufficient information has been accrued.

Trial Sequential Analysis of comparison: 8 Alpidem versus placebo, outcome: 8.3 Anxiety, Hamilton Anxiety Rating Scale (HAM‐A). Trial Sequential Analysis on anxiety evaluated with HAM‐A assessing a minimal relevant clinical difference (MIREDIF) of 5 points, and a variance of 103 points (empirical data), was performed based on a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0%. The diversity‐adjusted required information size (DARIS) was 235 participants, and the Trial Sequential Analysis‐adjusted confidence interval is ‐6.28 to 3.08. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 0.05. The red inward‐sloping lines represent the trial sequential alpha‐spending monitoring boundaries, while the red outward‐sloping lines represent the beta‐spending (futility) boundaries. The cumulative Z‐curve crosses the beta‐spending (futility) boundaries, showing that an intervention effect, if any, is less than 5 points.

Trial Sequential Analysis of comparison: 9 Buspirone versus placebo, outcome: 9.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in four trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 60% as observed in the trials. The diversity‐adjusted required information size was 3381 participants, and the Trial Sequential Analysis‐adjusted confidence interval could not be estimated due to lack of information. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries. The trial sequential monitoring boundaries and the diversity‐adjusted required information size are not shown, as the accrued number of participants only amounted to 143/3381 (4.23%), showing that insufficient information has been accrued.
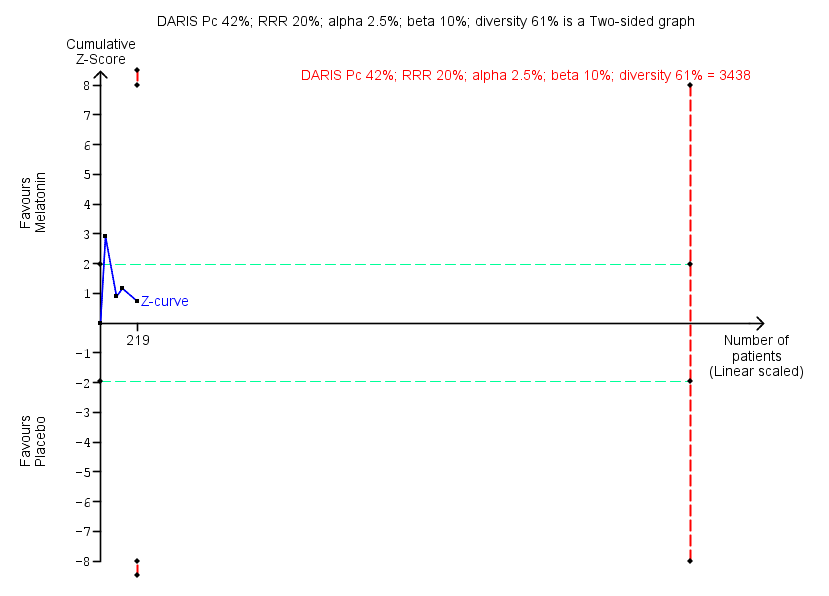
Trial Sequential Analysis of comparison: 10 Melatonin versus placebo, outcome: 10.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in four trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction (RRR) of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 61% as observed in the trials. The diversity‐adjusted required information size (DARIS) was 3438 participants, and the Trial Sequential Analysis‐adjusted confidence interval is 0.11 to 6.25. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries or the trial sequential monitoring boundaries (red dotted lines), and the diversity‐adjusted required information size is not met, showing that insufficient information has been accrued.

Comparison 1 Valproate versus placebo or no intervention, Outcome 1 Benzodiazepine discontinuation, end of intervention.
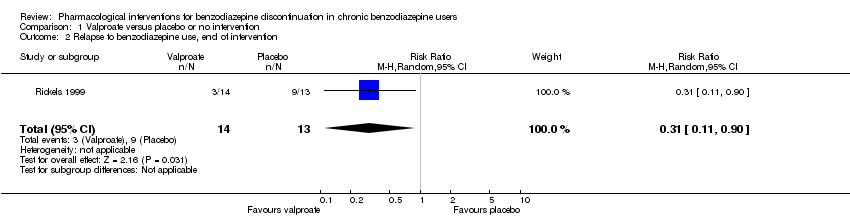
Comparison 1 Valproate versus placebo or no intervention, Outcome 2 Relapse to benzodiazepine use, end of intervention.
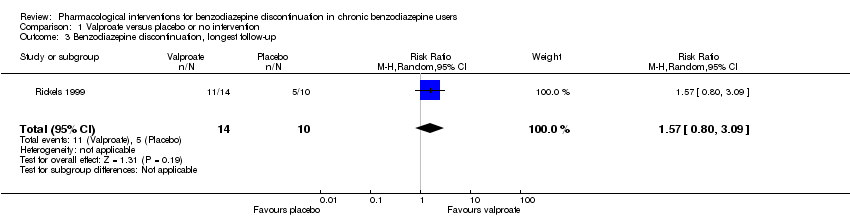
Comparison 1 Valproate versus placebo or no intervention, Outcome 3 Benzodiazepine discontinuation, longest follow‐up.

Comparison 1 Valproate versus placebo or no intervention, Outcome 4 Relapse to benzodiazepine use, longest follow‐up.
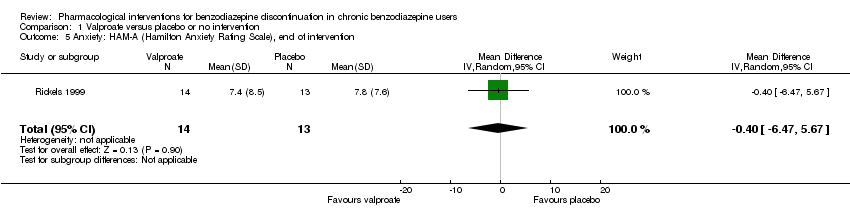
Comparison 1 Valproate versus placebo or no intervention, Outcome 5 Anxiety: HAM‐A (Hamilton Anxiety Rating Scale), end of intervention.

Comparison 1 Valproate versus placebo or no intervention, Outcome 6 Benzodiazepine withdrawal symptoms, end of intervention.
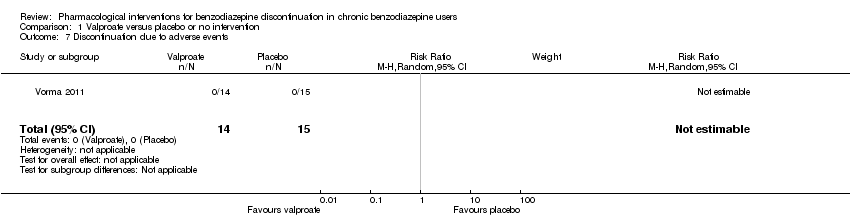
Comparison 1 Valproate versus placebo or no intervention, Outcome 7 Discontinuation due to adverse events.
Comparison 1 Valproate versus placebo or no intervention, Outcome 8 Serious adverse events.

Comparison 2 Carbamazepine versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.

Comparison 2 Carbamazepine versus placebo, Outcome 2 Benzodiazepine withdrawal symptoms.

Comparison 2 Carbamazepine versus placebo, Outcome 3 Benzodiazepine discontinuation, longest follow‐up.

Comparison 2 Carbamazepine versus placebo, Outcome 4 Relapse to benzodiazepine use.

Comparison 2 Carbamazepine versus placebo, Outcome 5 Serious adverse events.
Comparison 2 Carbamazepine versus placebo, Outcome 6 Non‐serious adverse events.

Comparison 2 Carbamazepine versus placebo, Outcome 7 Anxiety, HAM‐A.
Comparison 2 Carbamazepine versus placebo, Outcome 8 Discontinuation due to adverse events.

Comparison 3 Lithium versus placebo, Outcome 1 Benzodiazepine discontinuation.
Comparison 3 Lithium versus placebo, Outcome 2 Serious adverse events.
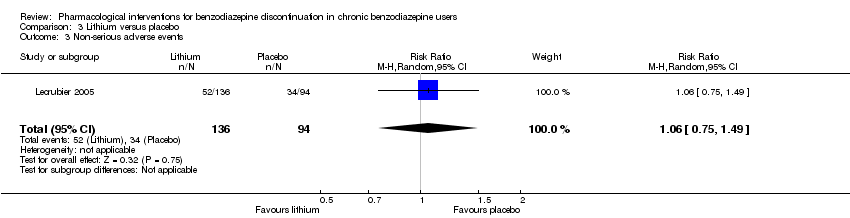
Comparison 3 Lithium versus placebo, Outcome 3 Non‐serious adverse events.
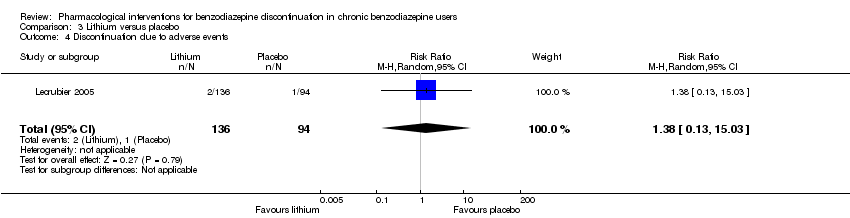
Comparison 3 Lithium versus placebo, Outcome 4 Discontinuation due to adverse events.

Comparison 4 Pregabalin versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
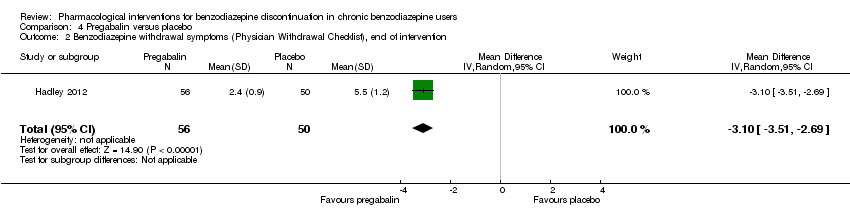
Comparison 4 Pregabalin versus placebo, Outcome 2 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention.
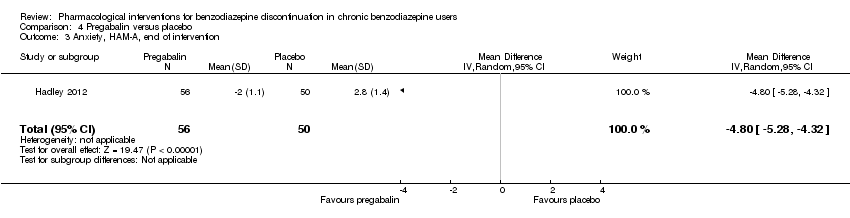
Comparison 4 Pregabalin versus placebo, Outcome 3 Anxiety, HAM‐A, end of intervention.

Comparison 4 Pregabalin versus placebo, Outcome 4 Serious adverse events.

Comparison 4 Pregabalin versus placebo, Outcome 5 Non‐serious adverse events.
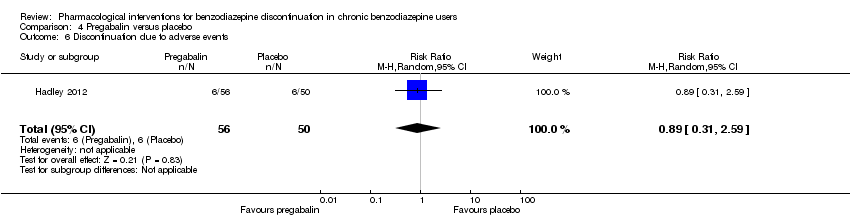
Comparison 4 Pregabalin versus placebo, Outcome 6 Discontinuation due to adverse events.
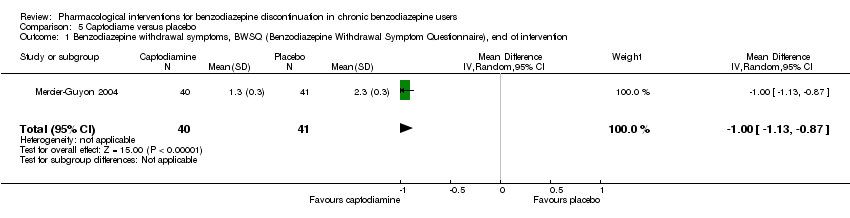
Comparison 5 Captodiame versus placebo, Outcome 1 Benzodiazepine withdrawal symptoms, BWSQ (Benzodiazepine Withdrawal Symptom Questionnaire), end of intervention.
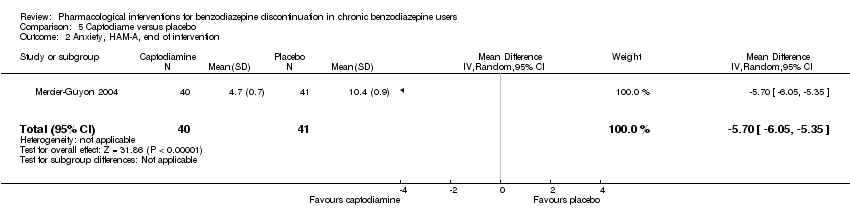
Comparison 5 Captodiame versus placebo, Outcome 2 Anxiety, HAM‐A, end of intervention.

Comparison 5 Captodiame versus placebo, Outcome 3 Serious adverse events.
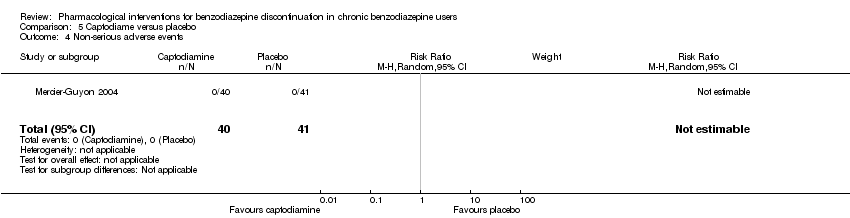
Comparison 5 Captodiame versus placebo, Outcome 4 Non‐serious adverse events.

Comparison 6 Paroxetine versus placebo or no intervention, Outcome 1 Benzodiazepine discontinuation, end of intervention.

Comparison 6 Paroxetine versus placebo or no intervention, Outcome 2 Benzodiazepine withdrawal symptoms: BWSQ, end of intervention.

Comparison 6 Paroxetine versus placebo or no intervention, Outcome 3 Anxiety: HAM‐A, end of intervention.
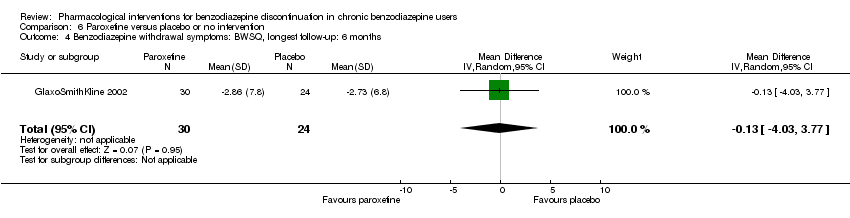
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 4 Benzodiazepine withdrawal symptoms: BWSQ, longest follow‐up: 6 months.

Comparison 6 Paroxetine versus placebo or no intervention, Outcome 5 Serious adverse events.
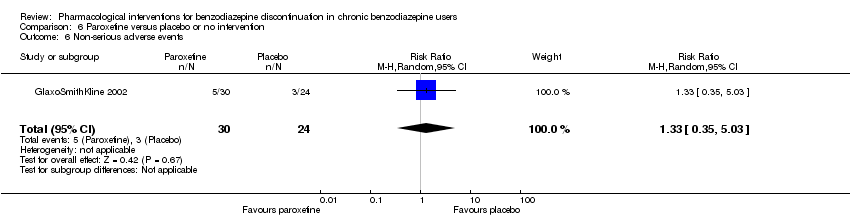
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 6 Non‐serious adverse events.

Comparison 7 Tricyclic antidepressants versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
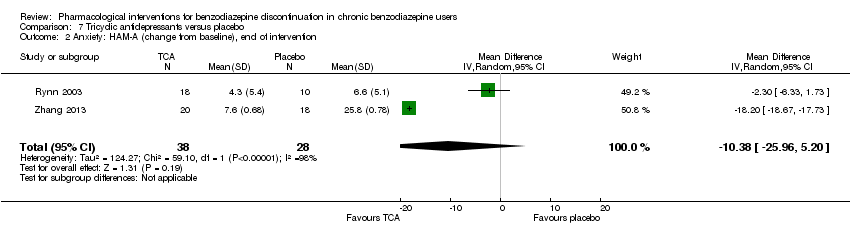
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 2 Anxiety: HAM‐A (change from baseline), end of intervention.

Comparison 7 Tricyclic antidepressants versus placebo, Outcome 3 Benzodiazepine discontinuation, longest follow‐up.
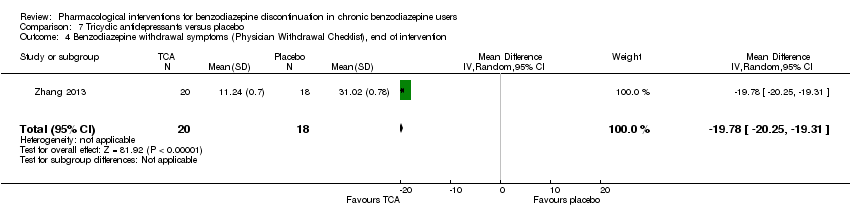
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 4 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention.
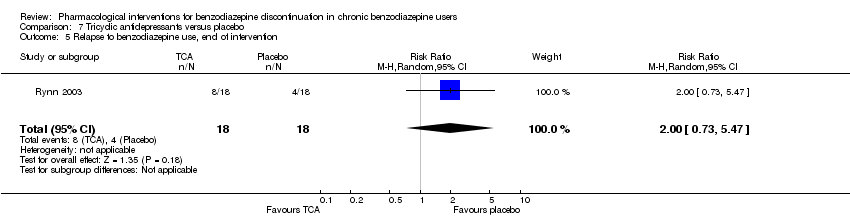
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 5 Relapse to benzodiazepine use, end of intervention.

Comparison 7 Tricyclic antidepressants versus placebo, Outcome 6 Discontinuation due to adverse events.
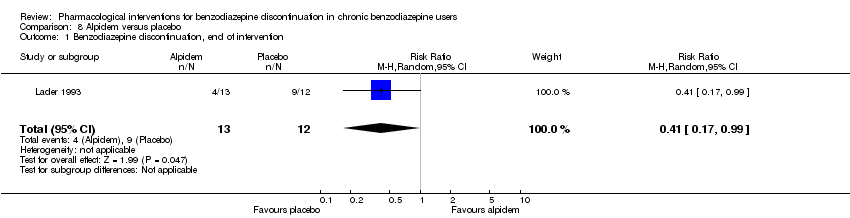
Comparison 8 Alpidem versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.

Comparison 8 Alpidem versus placebo, Outcome 2 Withdrawal syndrome (clinical diagnosis), end of intervention.

Comparison 8 Alpidem versus placebo, Outcome 3 Anxiety, HAM‐A, end of intervention.

Comparison 8 Alpidem versus placebo, Outcome 4 Relapse to benzodiazepine use, end of intervention.

Comparison 8 Alpidem versus placebo, Outcome 5 Serious adverse events.

Comparison 8 Alpidem versus placebo, Outcome 6 Discontinuation due to adverse events.

Comparison 9 Buspirone versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
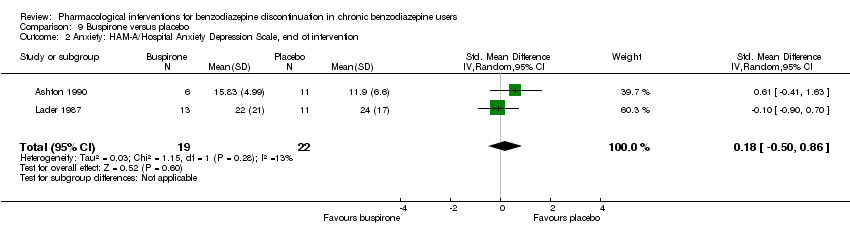
Comparison 9 Buspirone versus placebo, Outcome 2 Anxiety: HAM‐A/Hospital Anxiety Depression Scale, end of intervention.
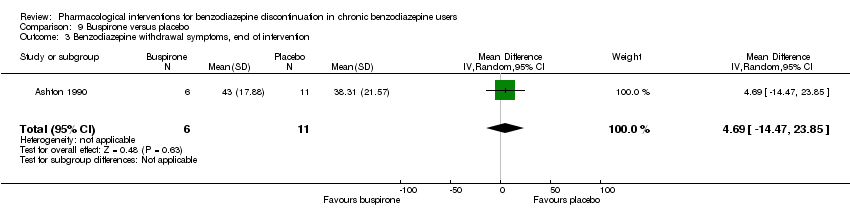
Comparison 9 Buspirone versus placebo, Outcome 3 Benzodiazepine withdrawal symptoms, end of intervention.

Comparison 9 Buspirone versus placebo, Outcome 4 Benzodiazepine discontinuation, longest follow‐up.
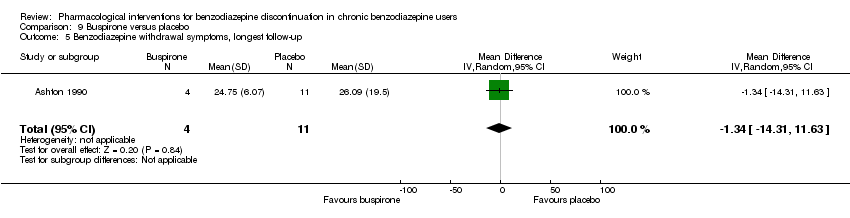
Comparison 9 Buspirone versus placebo, Outcome 5 Benzodiazepine withdrawal symptoms, longest follow‐up.
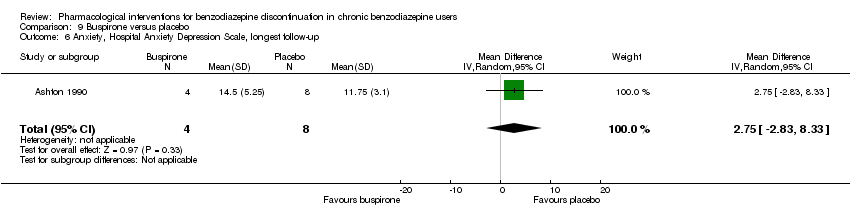
Comparison 9 Buspirone versus placebo, Outcome 6 Anxiety, Hospital Anxiety Depression Scale, longest follow‐up.

Comparison 9 Buspirone versus placebo, Outcome 7 Discontinuation due to adverse events.

Comparison 10 Melatonin versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.

Comparison 10 Melatonin versus placebo, Outcome 2 Insomnia.
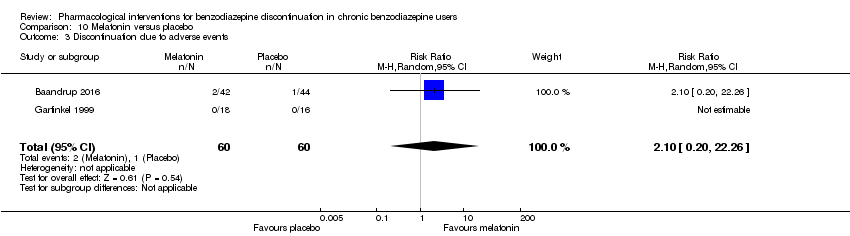
Comparison 10 Melatonin versus placebo, Outcome 3 Discontinuation due to adverse events.

Comparison 10 Melatonin versus placebo, Outcome 4 Benzodiazepine discontinuation, longest follow‐up.
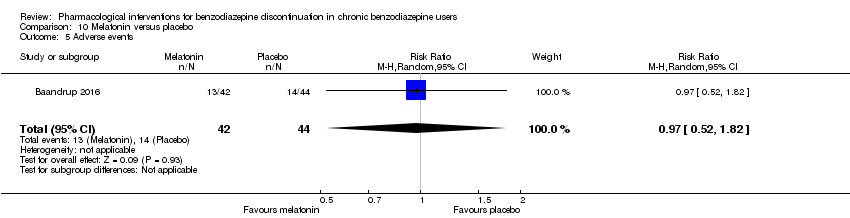
Comparison 10 Melatonin versus placebo, Outcome 5 Adverse events.
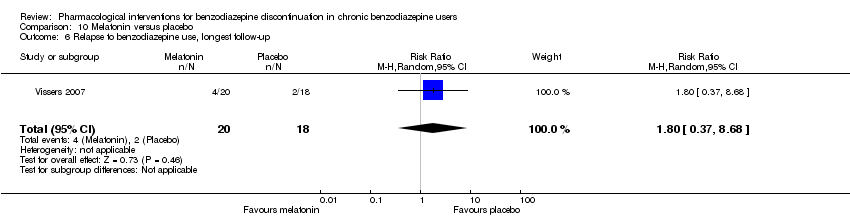
Comparison 10 Melatonin versus placebo, Outcome 6 Relapse to benzodiazepine use, longest follow‐up.
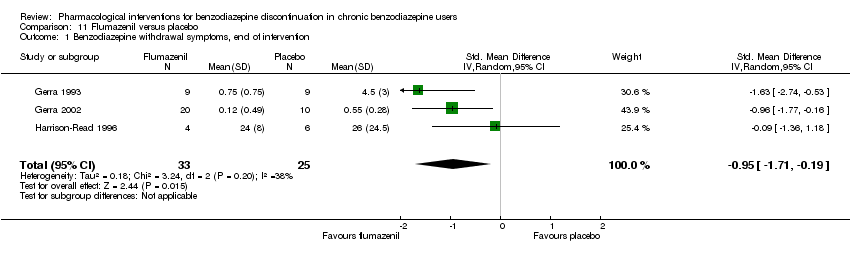
Comparison 11 Flumazenil versus placebo, Outcome 1 Benzodiazepine withdrawal symptoms, end of intervention.

Comparison 11 Flumazenil versus placebo, Outcome 2 Anxiety, HAM‐D (Hamilton Depression Rating Scale), end of intervention.

Comparison 11 Flumazenil versus placebo, Outcome 3 Benzodiazepine mean dose, end of intervention.

Comparison 12 Propranolol versus placebo, Outcome 1 Relapse to benzodiazepine use, end of intervention: 2 weeks.
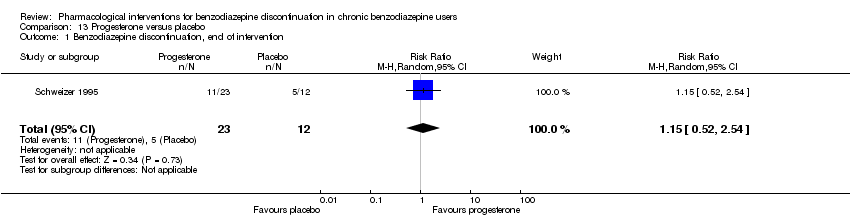
Comparison 13 Progesterone versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.

Comparison 13 Progesterone versus placebo, Outcome 2 Non‐serious adverse events.
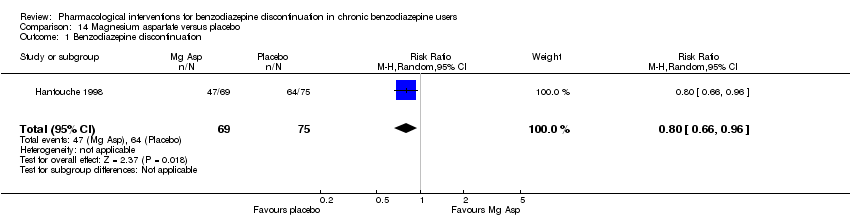
Comparison 14 Magnesium aspartate versus placebo, Outcome 1 Benzodiazepine discontinuation.

Comparison 14 Magnesium aspartate versus placebo, Outcome 2 Anxiety.

Comparison 14 Magnesium aspartate versus placebo, Outcome 3 Relapse to benzodiazepine use.
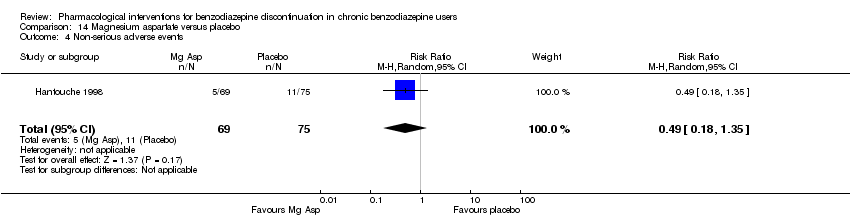
Comparison 14 Magnesium aspartate versus placebo, Outcome 4 Non‐serious adverse events.

Comparison 14 Magnesium aspartate versus placebo, Outcome 5 Discontinuation due to adverse events.
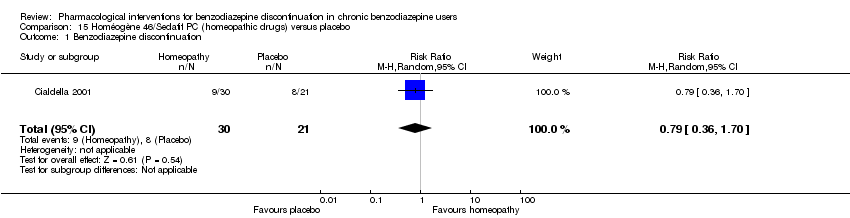
Comparison 15 Homéogène 46/Sedatif PC (homeopathic drugs) versus placebo, Outcome 1 Benzodiazepine discontinuation.
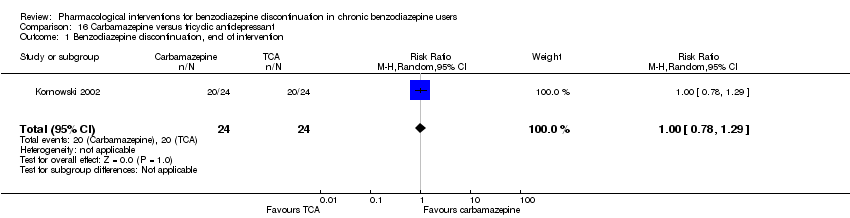
Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 1 Benzodiazepine discontinuation, end of intervention.
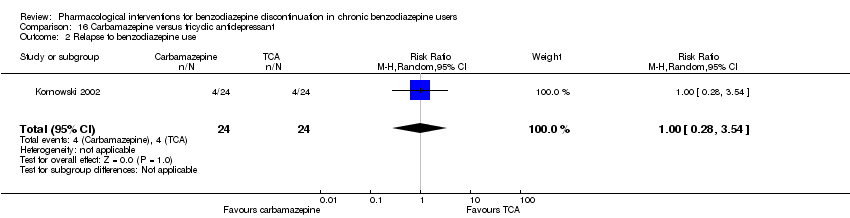
Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 2 Relapse to benzodiazepine use.
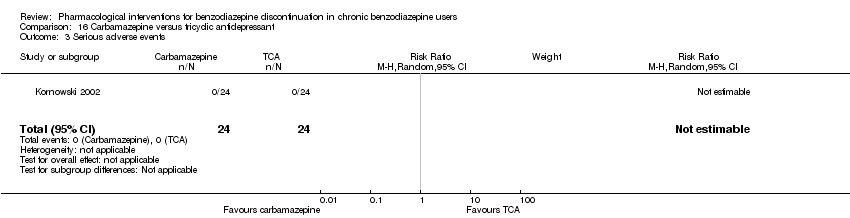
Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 3 Serious adverse events.
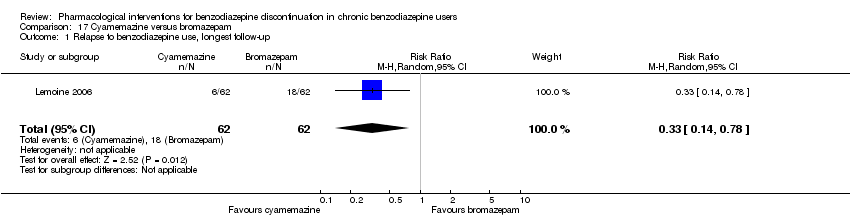
Comparison 17 Cyamemazine versus bromazepam, Outcome 1 Relapse to benzodiazepine use, longest follow‐up.

Comparison 17 Cyamemazine versus bromazepam, Outcome 2 Anxiety: Maximum amplitude of rebound (HAM‐A), end of intervention.

Comparison 17 Cyamemazine versus bromazepam, Outcome 3 Discontinuation due to adverse events.
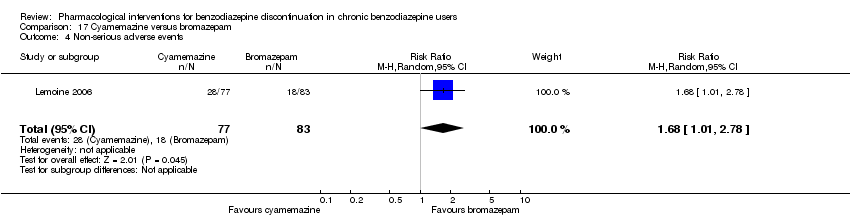
Comparison 17 Cyamemazine versus bromazepam, Outcome 4 Non‐serious adverse events.

Comparison 18 Zopiclone versus flunitrazepam, Outcome 1 Relapse to benzodiazepine use, longest follow‐up.
| Valproate compared with placebo or no intervention for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Valproate | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 2.55 | 27 | ⊕⊝⊝⊝ | The required information size of 1918 participants was not met. | |
| 679 per 1000 | 1000 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 1.57 | 24 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 785 per 1000 | |||||
| Benzodiazepine withdrawal symptoms, end of intervention | The mean benzodiazepine withdrawal symptoms in the intervention groups was | 56 | ⊕⊝⊝⊝ | SMD ‐0.15 (‐0.68 to 0.37). As a rule of thumb, 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. | ||
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | (0 study) | No included study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No details provided regarding random sequence generation, allocation concealment, and blinding, leading to unclear risk of selection bias, performance and detection bias (downgraded one level). | ||||||
| Carbamazepine compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Carbamazepine | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.33 | 147 | ⊕⊕⊝⊝ | Trial Sequential Analysis showed that only 7.0% of the required information size (2109) was reached, indicating that insufficient information has been obtained. | |
| 480 per 1000 | 638 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 1.41 | 40 | ⊕⊝⊝⊝ | ||
| 524 per 1000 | 739 per 1000 | |||||
| Benzodiazepine withdrawal symptoms, end of intervention | The mean benzodiazepine withdrawal symptoms in the intervention groups was | 76 | ⊕⊝⊝⊝ | SMD ‐1.14 (‐2.43 to 0.16). As a rule of thumb, 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. | ||
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | (0 study) | No included study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection bias. One study with high risk of attrition, reporting, and other bias (downgraded one level). | ||||||
| Lithium compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lithium | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.05 | 230 | ⊕⊕⊝⊝ | The required information size of 1918 participants was not met. | |
| 617 per 1000 | 648 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection, attrition, and reporting bias (downgraded one level). | ||||||
| Pregabalin compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pregabalin | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.44 | 106 | ⊕⊝⊝⊝ | The required information size of 1918 participants was not met. | |
| 360 per 1000 | 518 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, Physician Withdrawal Checklist (PWCL), end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, PWCL, end of intervention in the intervention group was | ‐ | 106 | ⊕⊝⊝⊝ | MD ‐3.10 (‐3.51 to ‐2.69) |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection bias and high risk of attrition and other bias (downgraded two levels). | ||||||
| Captodiame compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Captodiame | |||||
| Benzodiazepine discontinuation, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ), end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, BWSQ, end of intervention in the intervention group was | ‐ | 81 | ⊕⊝⊝⊝ | MD ‐1.00 (‐1.13 to ‐0.87) The required information size of 229 participants was not met. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection and reporting bias. High risk of other bias (downgraded one level). | ||||||
| Paroxetine compared with placebo or no intervention for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or Control | Paroxetine | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.45 | 221 | ⊕⊝⊝⊝ | Trial Sequential Analysis showed that only 2.34% of the required information size (9448) was reached, indicating that insufficient information has been obtained. | |
| 504 per 1000 | 731 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | (0 study) | ‐ | No included study measured this outcome. | |
| Benzodiazepine withdrawal symptoms, BWSQ, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, BWSQ, end of intervention in the intervention groups was | ‐ | 99 | ⊕⊝⊝⊝ | MD ‐3.57 (‐5.34 to ‐1.8). Trial Sequential Analysis showed that the required information size of 229 participants was not reached. However, the alpha‐spending boundaries for benefit were crossed, indicating that sufficient information was obtained, and the result was not due to random error. |
| Benzodiazepine withdrawal symptoms, BWSQ, longest follow‐up: 6 months | ‐ | The mean benzodiazepine withdrawal symptoms, BWSQ, longest follow‐up: 6 months in the intervention group was | ‐ | 54 | ⊕⊝⊝⊝ | MD ‐0.13 (‐4.03 to 3.77) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection and attrition bias. High risk of performance, detection, reporting, and other bias (downgraded two levels). | ||||||
| Tricyclic antidepressants compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tricyclic antidepressants | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.82 | 105 | ⊕⊝⊝⊝ | Trial Sequential Analysis showed that only 7.82% of the required information size (1343) was reached, indicating that insufficient information has been obtained. | |
| 451 per 1000 | 370 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 2.2 | 47 | ⊕⊕⊝⊝ | ||
| 375 per 1000 | 825 per 1000 | |||||
| Benzodiazepine withdrawal symptoms, Physician Withdrawal Checklist, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms in the intervention group was | ‐ | 38 (1 study) | ⊕⊝⊝⊝ | MD ‐19.78 (‐20.25 to ‐19.31) |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection bias and high risk of attrition and other bias (downgraded one level). | ||||||
| Alpidem compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Alpidem | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.41 | 25 | ⊕⊕⊝⊝ | The required information size of 1918 participants was not met. | |
| 750 per 1000 | 308 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Withdrawal syndrome (clinical diagnosis), end of intervention | Study population | RR 4.86 | 145 | ⊕⊝⊝⊝ | ||
| 29 per 1000 | 143 per 1000 | |||||
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Required information size not met (downgraded two levels due to imprecision). 3Unclear risk of selection and other bias, high risk of attrition bias (downgraded one level) | ||||||
| Buspirone compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Buspirone | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.82 | 143 | ⊕⊕⊝⊝ | Trial Sequential Analysis showed that only 4.23% of the required information size (3381) was reached, indicating that insufficient information has been obtained. | |
| 563 per 1000 | 462 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 0.60 | 23 | ⊕⊕⊝⊝ | ||
| 917 per 1000 | 550 per 1000 | |||||
| Benzodiazepine withdrawal symptoms, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, end of intervention in the intervention groups was | ‐ | 17 | ⊕⊝⊝⊝ | MD 4.69 (‐14.47 to 23.87) |
| Benzodiazepine withdrawal symptoms, longest follow‐up | ‐ | The mean benzodiazepine withdrawal symptoms, longest follow‐up in the intervention groups was | ‐ | 15 | ⊕⊝⊝⊝ | MD ‐1.34 (‐14.31 to 11.63) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection, performance, and reporting bias. High risk of attrition and other bias (downgraded one level). | ||||||
| Melatonin compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Melatonin | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.20 | 219 | ⊕⊝⊝⊝ | Trial Sequential Analysis showed that only 6.37% of the required information size (3438) was reached, indicating that insufficient information has been obtained. | |
| 417 per 1000 | 500 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 1.03 | 38 | ⊕⊝⊝⊝ | ||
| 389 per 1000 | 401 per 1000 | |||||
| Benzodiazpine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection, attrition, and reporting bias. High risk of other bias (downgraded one level). | ||||||
| Flumazenil compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Flumazenil | |||||
| Benzodiazepine discontinuation, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, end of intervention in the intervention groups was | ‐ | 58 | ⊕⊝⊝⊝ | SMD ‐0.95 (‐1.71 to ‐0.19) As a rule of thumb, 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection bias and high risk of performance, detection, and other bias (downgraded one level). | ||||||
| Progesterone compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Progesterone | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.15 | 35 | ⊕⊝⊝⊝ | The required information size of 1918 participants was not met. | |
| 417 per 1000 | 479 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection and attrition bias (downgraded one level). | ||||||
| Magnesium aspartate compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Magnesium aspartate | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.80 | 144 | ⊕⊝⊝⊝ | The required information size of 1918 participants was not met. | |
| 853 per 1000 | 683 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection, detection, and attrition bias (downgraded one level). | ||||||
| Homéogène 46/Sedatif PC (homeopathic drugs) compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Homéogène 46/Sedatif PC (homeopathic drugs) | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.79 | 51 | ⊕⊝⊝⊝ | The required information size was not met. | |
| 381 per 1000 | 301 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection, attrition, and other bias (downgraded one level). | ||||||
| Carbamazepine compared with tricyclic antidepressant for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tricyclic antidepressant | Carbamazepine | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.00 | 48 | ⊕⊕⊝⊝ | The required information size was not met. | |
| 833 per 1000 | 833 per 1000 | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear risk of selection, detection, and attrition bias (downgraded one level). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [1.08, 6.03] |
| 2 Relapse to benzodiazepine use, end of intervention Show forest plot | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.90] |
| 3 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.80, 3.09] |
| 4 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.39] |
| 5 Anxiety: HAM‐A (Hamilton Anxiety Rating Scale), end of intervention Show forest plot | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.47, 5.67] |
| 6 Benzodiazepine withdrawal symptoms, end of intervention Show forest plot | 2 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.68, 0.37] |
| 6.1 Physician Withdrawal Checklist | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.77, 0.74] |
| 6.2 CIWA‐B (Clinical Institute Withdrawal Assessment Scale ‐ Benzodiazepines) | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.01, 0.45] |
| 7 Discontinuation due to adverse events Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Serious adverse events Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 3 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.99, 1.80] |
| 2 Benzodiazepine withdrawal symptoms Show forest plot | 2 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.43, 0.16] |
| 2.1 Physician Withdrawal Checklist | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐2.61, ‐1.03] |
| 2.2 Patient Withdrawal Checklist | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.13, 0.13] |
| 3 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.86, 2.29] |
| 4 Relapse to benzodiazepine use Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.44] |
| 5 Serious adverse events Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Non‐serious adverse events Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 126.48] |
| 7 Anxiety, HAM‐A Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐9.58, ‐2.42] |
| 8 Discontinuation due to adverse events Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.86, 1.28] |
| 2 Serious adverse events Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐serious adverse events Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.49] |
| 4 Discontinuation due to adverse events Show forest plot | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.13, 15.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.92, 2.25] |
| 2 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention Show forest plot | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐3.1 [‐3.51, ‐2.69] |
| 3 Anxiety, HAM‐A, end of intervention Show forest plot | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐4.8 [‐5.28, ‐4.32] |
| 4 Serious adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.16, 2.85] |
| 5 Non‐serious adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.40] |
| 6 Discontinuation due to adverse events Show forest plot | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine withdrawal symptoms, BWSQ (Benzodiazepine Withdrawal Symptom Questionnaire), end of intervention Show forest plot | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.13, ‐0.87] |
| 2 Anxiety, HAM‐A, end of intervention Show forest plot | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐6.05, ‐5.35] |
| 3 Serious adverse events Show forest plot | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Non‐serious adverse events Show forest plot | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.88, 2.39] |
| 2 Benzodiazepine withdrawal symptoms: BWSQ, end of intervention Show forest plot | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐5.34, ‐1.80] |
| 3 Anxiety: HAM‐A, end of intervention Show forest plot | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐9.64, ‐3.86] |
| 4 Benzodiazepine withdrawal symptoms: BWSQ, longest follow‐up: 6 months Show forest plot | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐4.03, 3.77] |
| 5 Serious adverse events Show forest plot | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Non‐serious adverse events Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.35, 5.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.52, 1.28] |
| 2 Anxiety: HAM‐A (change from baseline), end of intervention Show forest plot | 2 | 66 | Mean Difference (IV, Random, 95% CI) | ‐10.38 [‐25.96, 5.20] |
| 3 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.27, 3.82] |
| 4 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐19.78 [‐20.25, ‐19.31] |
| 5 Relapse to benzodiazepine use, end of intervention Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.73, 5.47] |
| 6 Discontinuation due to adverse events Show forest plot | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.42, 3.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.17, 0.99] |
| 2 Withdrawal syndrome (clinical diagnosis), end of intervention Show forest plot | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 4.86 [1.12, 21.14] |
| 3 Anxiety, HAM‐A, end of intervention Show forest plot | 2 | 170 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.64, 1.45] |
| 4 Relapse to benzodiazepine use, end of intervention Show forest plot | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.20] |
| 5 Serious adverse events Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Discontinuation due to adverse events Show forest plot | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.05, 4.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 4 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.49, 1.37] |
| 2 Anxiety: HAM‐A/Hospital Anxiety Depression Scale, end of intervention Show forest plot | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.50, 0.86] |
| 3 Benzodiazepine withdrawal symptoms, end of intervention Show forest plot | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 4.69 [‐14.47, 23.85] |
| 4 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.34, 1.05] |
| 5 Benzodiazepine withdrawal symptoms, longest follow‐up Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐14.31, 11.63] |
| 6 Anxiety, Hospital Anxiety Depression Scale, longest follow‐up Show forest plot | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 2.75 [‐2.83, 8.33] |
| 7 Discontinuation due to adverse events Show forest plot | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 4 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.73, 1.96] |
| 2 Insomnia Show forest plot | 3 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.70, 0.23] |
| 2.1 PSQI (Pittsburgh Sleep Quality Index) global score (higher = worse), end of intervention | 2 | 116 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.92, 0.31] |
| 2.2 Sleep quality (1 poorest, 10 excellent), end of intervention | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.34 [‐4.42, ‐2.26] |
| 3 Discontinuation due to adverse events Show forest plot | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.20, 22.26] |
| 4 Benzodiazepine discontinuation, longest follow‐up Show forest plot | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.47, 2.27] |
| 5 Adverse events Show forest plot | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.82] |
| 6 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.8 [0.37, 8.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine withdrawal symptoms, end of intervention Show forest plot | 3 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.71, ‐0.19] |
| 2 Anxiety, HAM‐D (Hamilton Depression Rating Scale), end of intervention Show forest plot | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐1.3 [‐2.28, ‐0.32] |
| 3 Benzodiazepine mean dose, end of intervention Show forest plot | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐22.06, 14.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse to benzodiazepine use, end of intervention: 2 weeks Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.31, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.52, 2.54] |
| 2 Non‐serious adverse events Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [1.15, 8.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.96] |
| 2 Anxiety Show forest plot | 1 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.73, 1.13] |
| 3 Relapse to benzodiazepine use Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.46, 1.87] |
| 4 Non‐serious adverse events Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.18, 1.35] |
| 5 Discontinuation due to adverse events Show forest plot | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.13, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation Show forest plot | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.36, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepine discontinuation, end of intervention Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.78, 1.29] |
| 2 Relapse to benzodiazepine use Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.54] |
| 3 Serious adverse events Show forest plot | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.14, 0.78] |
| 2 Anxiety: Maximum amplitude of rebound (HAM‐A), end of intervention Show forest plot | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐1.23, 2.23] |
| 3 Discontinuation due to adverse events Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.79, 10.44] |
| 4 Non‐serious adverse events Show forest plot | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.01, 2.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse to benzodiazepine use, longest follow‐up Show forest plot | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.78] |

