دیالیز پریتونئال برای درمان نارسایی حاد کلیه
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Abstract‐only publication; data presented cannot be meta‐analysed |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using random‐number tables |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Part funded by Baxter Asia |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number tables |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed, opaque, double‐wrapped envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement as study was stopped early |
| Other bias | High risk | The study was stopped due to the difference in mortality rates |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | 4 patients lost to follow‐up; all analyses were performed according to the intention‐to‐treat principle, with no imputation for missing values. Data from patients lost to follow‐up were not analysed |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported |
| Other bias | Unclear risk | Study appears to be free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Intervention assignment was generated by a computerised random number generated with separate lists at each centre |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | High risk | There were significant differences between PD and extracorporeal therapy in some baseline characteristics, including pre‐dialysis BUN, and creatinine levels |
AKI ‐ acute kidney injury; APACHE ‐ Acute Physiology and Chronic Health Evaluation; AKNC ‐ Acute Kidney Network Criteria; ATN ‐ acute tubular necrosis; BUN ‐ blood urea nitrogen; CKD ‐ chronic kidney disease; CPD ‐ continuous peritoneal dialysis; CVVHDF ‐ continuous venovenous haemodiafiltration; EHD ‐ extended daily haemodialysis; HF ‐ haemofiltration; DHD ‐ daily haemodialysis; HVPD ‐ high volume peritoneal dialysis; ICU ‐ intensive care unit; M/F ‐ male/female; PD ‐ peritoneal dialysis; RCT ‐ randomised controlled trial; RRT ‐ renal replacement therapy; SCr ‐ serum creatinine; SD ‐ standard deviation; TPD ‐ tidal peritoneal dialysis
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong population: mix of AKI and CKD patients | |
| Cross‐over RCT; not appropriate study design for this review | |
| Wrong population: mix of AKI and CKD patients | |
| Wrong population: mix of AKI and CKD patients | |
| Wrong intervention: PD + verapamil versus HD; verapamil not used in the HD group |
AKi ‐ acute kidney injury; CKD ‐ chronic kidney disease; HD ‐ haemodialysis; PD ‐ peritoneal dialysis; RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
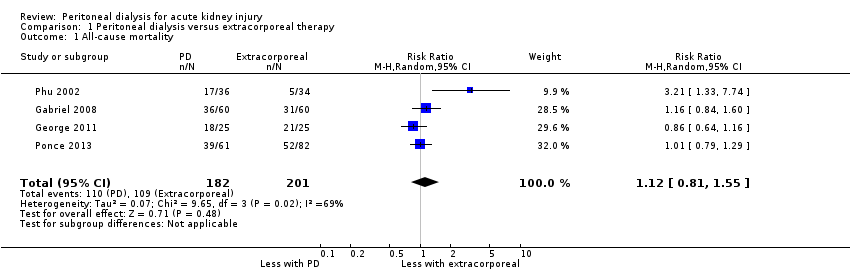
| 1 All‐cause mortality Show forest plot | 4 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.55] |
| Analysis 1.1  Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 1 All‐cause mortality. | ||||
| 2 Recovery of kidney function Show forest plot | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.35] |
| Analysis 1.2 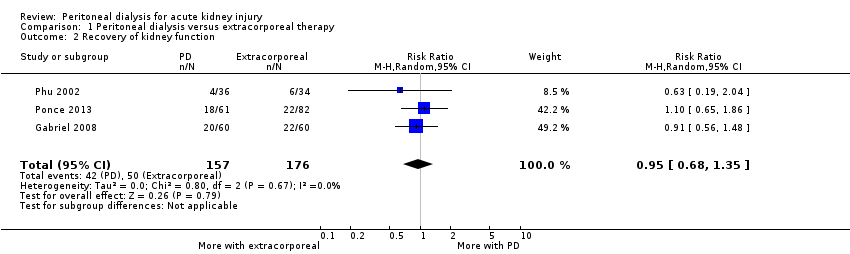 Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 2 Recovery of kidney function. | ||||
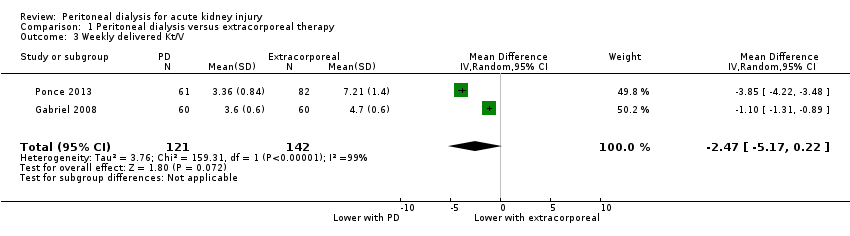
| 3 Weekly delivered Kt/V Show forest plot | 2 | 263 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐5.17, 0.22] |
| Analysis 1.3 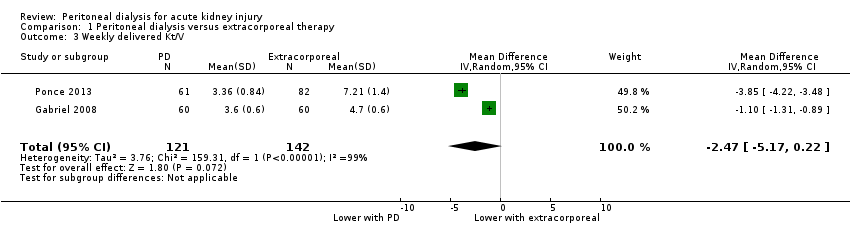 Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 3 Weekly delivered Kt/V. | ||||
| 4 Correction of acidosis Show forest plot | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.13, 13.60] |
| Analysis 1.4  Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 4 Correction of acidosis. | ||||
| 5 Fluid removal Show forest plot | 3 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.19, 0.01] |
| Analysis 1.5  Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 5 Fluid removal. | ||||
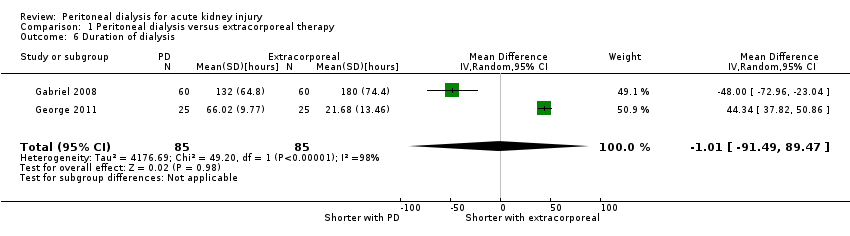
| 6 Duration of dialysis Show forest plot | 2 | 170 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐91.49, 89.47] |
| Analysis 1.6  Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 6 Duration of dialysis. | ||||
| 7 Infectious complications (catheter infection or peritonitis) Show forest plot | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.60, 1.78] |
| Analysis 1.7 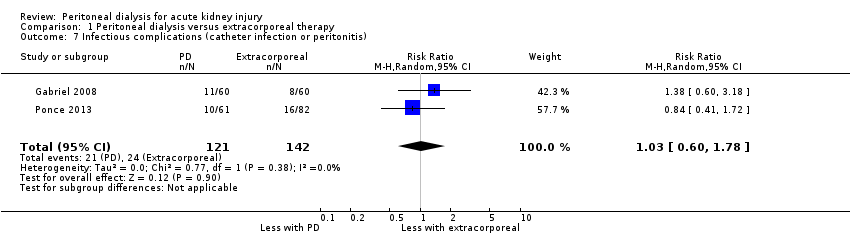 Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 7 Infectious complications (catheter infection or peritonitis). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 High versus low intensity peritoneal dialysis, Outcome 1 All‐cause mortality. | ||||
| 2 Recovery of kidney function Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 High versus low intensity peritoneal dialysis, Outcome 2 Recovery of kidney function. | ||||
| 3 Weekly delivered Kt/V Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 High versus low intensity peritoneal dialysis, Outcome 3 Weekly delivered Kt/V. | ||||
| 4 Fluid removal Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.4 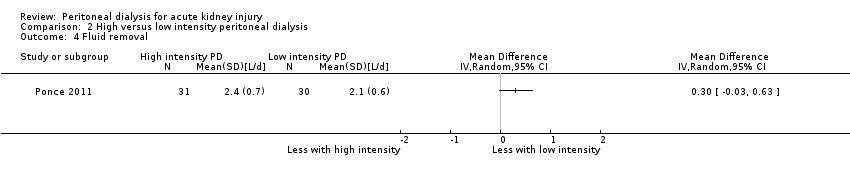 Comparison 2 High versus low intensity peritoneal dialysis, Outcome 4 Fluid removal. | ||||
| 5 Infectious complications (catheter infection or peritonitis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 High versus low intensity peritoneal dialysis, Outcome 5 Infectious complications (catheter infection or peritonitis). | ||||

Flow chart of the article selection process

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
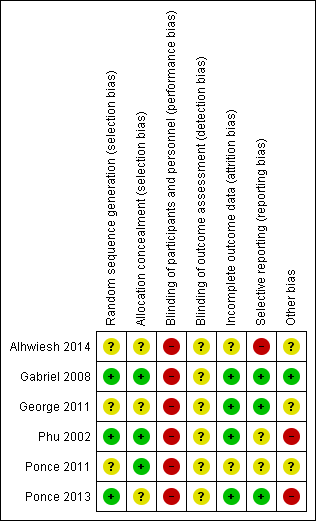
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 1 All‐cause mortality.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 2 Recovery of kidney function.
Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 3 Weekly delivered Kt/V.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 4 Correction of acidosis.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 5 Fluid removal.
Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 6 Duration of dialysis.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 7 Infectious complications (catheter infection or peritonitis).
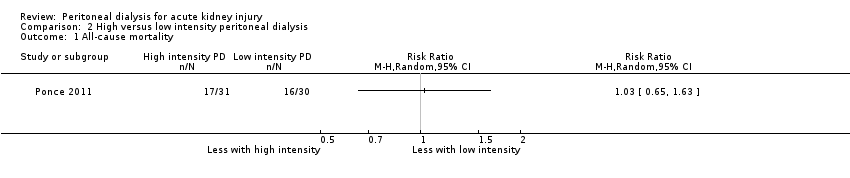
Comparison 2 High versus low intensity peritoneal dialysis, Outcome 1 All‐cause mortality.

Comparison 2 High versus low intensity peritoneal dialysis, Outcome 2 Recovery of kidney function.
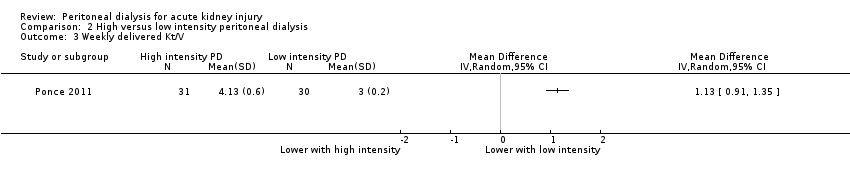
Comparison 2 High versus low intensity peritoneal dialysis, Outcome 3 Weekly delivered Kt/V.
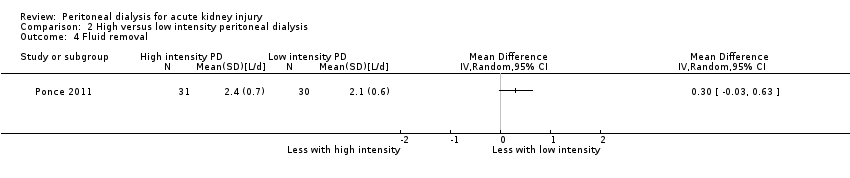
Comparison 2 High versus low intensity peritoneal dialysis, Outcome 4 Fluid removal.

Comparison 2 High versus low intensity peritoneal dialysis, Outcome 5 Infectious complications (catheter infection or peritonitis).
| Peritoneal dialysis versus extracorporeal therapy for acute kidney injury | ||||||
| Patient or population: patients with acute kidney injury Settings: inpatient Intervention: peritoneal dialysis Comparison: extracorporeal therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Extracorporeal therapy | Peritoneal dialysis | |||||
| All‐cause mortality | 542 per 1000 | 607 per 1000 (439 to 841) | RR 1.12 (0.81 to 1.55) | 4 (383) | ⊕⊕⊕⊝ | Downgraded for study limitations |
| Recovery of kidney function | 284 per 1000 | 270 per 1000 (193 to 384) | RR 1.42 (0.74 to 2.75) | 3 (333) | ⊕⊕⊕⊝ | Downgraded for study limitations |
| Weekly delivered Kt/V | The mean delivered Kt/V was 2.47 lower (5.17 lower to 0.22 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 2 (263) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data | ||
| Correction of acidosis | 577 per 1000 | 762 per 1000 (70 to 1,000) | RR 1.32 (0.1 to 13.60) | 2 (120) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data |
| Fluid removal (L/d) | The mean fluid removal was 0.59 L/d lower (1.19 lower to 0.01 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 3 (313) | ⊕⊕⊝⊝ | Downgraded for study limitations and imprecision | ||
| Duration of dialysis (hours) | The mean duration of dialysis was 1.01 hours less (91.49 lower to 92.54 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 2 (170) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data | ||
| Infectious complications | 169 per 1000 | 174 per 1000 (101 to 301) | RR 1.03 (0.60 to 1.78) | 2 (263) | ⊕⊕⊝⊝ | Downgraded for study limitations and insufficient data |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Three studies did not report details about random sequence generation or allocation concealment or both 2Small numbers with wide CI 3Few studies (no more than 2) reported the relevant data | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 4 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.55] |
| 2 Recovery of kidney function Show forest plot | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.35] |
| 3 Weekly delivered Kt/V Show forest plot | 2 | 263 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐5.17, 0.22] |
| 4 Correction of acidosis Show forest plot | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.13, 13.60] |
| 5 Fluid removal Show forest plot | 3 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.19, 0.01] |
| 6 Duration of dialysis Show forest plot | 2 | 170 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐91.49, 89.47] |
| 7 Infectious complications (catheter infection or peritonitis) Show forest plot | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.60, 1.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Recovery of kidney function Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Weekly delivered Kt/V Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Fluid removal Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Infectious complications (catheter infection or peritonitis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

