Peritonealdialyse bei akutem Nierenversagen
Abstract
Background
Peritoneal dialysis (PD) has been suggested as an effective and safe dialysis modality in patients with acute kidney injury (AKI). However, whether PD is superior to extracorporeal therapy (e.g. haemodialysis) in terms of improving survival, recovery of kidney function, metabolic and clinical outcomes is still inconclusive.
Objectives
The aim of this review was to evaluate the benefits and harms of PD for patients with AKI compared with extracorporeal therapy or different PD modalities.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies to 29 May 2017 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials.gov. We also searched the China Biological Medicine Database.
Selection criteria
We included patients with AKI who were randomised to receive PD, extracorporeal therapy, or different PD modalities regardless of their age, sex, primary disease and clinical course.
Data collection and analysis
Screening, selection, data extraction and quality assessments for each retrieved article were carried out by two authors using standardised forms. Authors contacted when published data were incomplete. Statistical analyses were performed using the random effects model and results expressed as risk ratio (RR) with 95% confidence intervals (CI). Heterogeneity among studies was explored using the Cochran Q statistic and the I2 test. Outcomes of interest included all‐cause mortality, recovery of kidney function, weekly delivered Kt/V, correction of acidosis, fluid removal, duration of dialysis, and infectious complications. Confidence in the evidence was assessing using GRADE.
Main results
Six studies (484 participants) met our inclusion criteria. Five studies compared high volume PD with daily haemodialysis, extended daily haemodialysis, or continuous renal replacement therapy. One study focused on the intensity of PD. The overall risk of bias was low to unclear. Compared to extracorporeal therapy, PD probably made little or no difference to all‐cause mortality (4 studies, 383 participants: RR 1.12, 95% CI 0.81 to 1.55; I2 = 69%; moderate certainty evidence), or kidney function recovery (3 studies, 333 participants: RR 0.95, 95% CI 0.68 to 1.35; I2 = 0%; moderate certainty evidence). PD probably slightly reduces the amount of fluid removal compared to extracorporeal therapy (3 studies, 313 participants: MD ‐0.59 L/d, 95% CI ‐1.19 to 0.01; I2 = 89%; low certainty evidence), and probably made little or no difference to infectious complications (2 studies, 263 participants: RR 1.03, 95% CI 0.60 to 1.78; I2 = 0%; low certainty evidence). It is uncertain whether PD compared to extracorporeal therapy has any effects on weekly delivered Kt/V (2 studies, 263 participants: MD ‐2.47, 95% CI ‐5.17 to 0.22; I2 = 99%; very low certainty evidence), correction of acidosis (2 studies, 89 participants: RR 1.32, 95% CI 0.13 to 13.60; I2 = 96%; very low certainty evidence), or duration of dialysis (2 studies, 170 participants: MD ‐1.01 hours, 95% CI ‐91.49 to 89.47; I2 = 98%; very low certainty evidence). Heterogeneity was high and this may be due to the different extracorporeal therapies used.
One study (61 participants) reported little or no difference to all‐cause mortality, kidney function recovery, or infection between low and high and intensity PD. Weekly delivered Kt/V and fluid removal was lower with low compared to high intensity PD.
Authors' conclusions
Based on moderate (mortality, recovery of kidney function), low (infectious complications), or very low certainty evidence (correction of acidosis) there is probably little or no difference between PD and extracorporeal therapy for treating AKI. Fluid removal (low certainty) and weekly delivered Kt/V (very low certainty) may be higher with extracorporeal therapy.
PICO
Laienverständliche Zusammenfassung
Peritonealdialyse bei akutem Nierenversagen
Worum geht es?
Beim akuten Nierenversagen (ANV) handelt es sich um einen plötzlichen und in der Regel umkehrbaren Rückgang der glomerulären Filtrationsrate. Bisher konnte für Patienten mit ANV bei keiner bestimmten Form von Nierenersatztherapie (einer Behandlung, die auf den Ersatz der normalen Blutfilterfunktion der Niere abzielt) eindeutig eine Überlegenheit nachgewiesen werden. Die Wahl der Nierenersatztherapie hängt von einer Vielzahl von Faktoren ab, darunter der Verfügbarkeit der Behandlung, der Fachkompetenz des behandelnden Arztes, der hämodynamischen Stabilität usw.
Was haben wir getan?
In diesem Review sollten Nutzen und Schaden der Peritonealdialyse (PD) für Patienten mit ANV im Vergleich zur extrakorporalen Therapie (z. B. Hämodialyse) oder anderen Arten der PD bewertet werden. Die Recherche erfolgte im Cochrane Kidney and Transplant Register of Studies.
Was fanden wir heraus?
Sechs randomisierte kontrollierte Studien (mit 484 Patienten) erfüllten unsere Einschlusskriterien. In fünf Studien wurde die Hochvolumen‐Peritonealdialyse mit täglicher Hämodialyse, erweiterter täglicher Hämodialyse oder kontinuierlicher Nierenersatztherapie verglichen. In einer Studie wurden PD‐Therapien mit unterschiedlicher Intensität bei ANV‐Patienten verglichen. Im Vergleich zur extrakorporalen Therapie bestand bei der PD wahrscheinlich nur ein geringer oder gar kein Unterschied in Hinblick auf Todesfälle gleich welcher Ursache bzw. auf eine Wiederherstellung der Nierenfunktion. Der Flüssigkeitsentzug war bei der PD im Vergleich zur extrakorporalen Therapie wahrscheinlich leicht reduziert, der Unterschied in Hinblick auf infektiöse Komplikationen war gering oder gar nicht vorhanden. Es ist unklar, ob PD im Vergleich zur extrakorporalen Therapie Wirkungen auf die wöchentliche Dialyseeffektivität (Kt/V), die Korrektur der Übersäuerung oder die Dauer der Dialyse hat.
In einer Studie (mit 61 Teilnehmern) wurde im Vergleich zwischen PD‐Therapien mit niedriger oder, hoher Intensität nur ein geringer oder gar kein Unterschied in Hinblick auf Todesfälle gleich welcher Ursache, Wiederherstellung der Nierenfunktion oder Infektionen berichtet. Die wöchentliche Dialyseeffektivität (Kt/V) und der Flüssigkeitsentzug fielen bei der PD mit niedriger Intensität im Vergleich zur hochintensiven PD geringer aus.
Schlussfolgerungen
Zurzeit liegt keine ausreichende Evidenz vor, um zu bestimmen, ob es in Hinblick auf Todesfälle gleich welcher Ursache bzw. auf Wiederherstellung der Nierenfunktion zwischen PD, extrakorporaler Therapie oder unterschiedlich intensiver PD‐Therapie signifikante Unterschiede gibt.
Authors' conclusions
Summary of findings
| Peritoneal dialysis versus extracorporeal therapy for acute kidney injury | ||||||
| Patient or population: patients with acute kidney injury Settings: inpatient Intervention: peritoneal dialysis Comparison: extracorporeal therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Extracorporeal therapy | Peritoneal dialysis | |||||
| All‐cause mortality | 542 per 1000 | 607 per 1000 (439 to 841) | RR 1.12 (0.81 to 1.55) | 4 (383) | ⊕⊕⊕⊝ | Downgraded for study limitations |
| Recovery of kidney function | 284 per 1000 | 270 per 1000 (193 to 384) | RR 1.42 (0.74 to 2.75) | 3 (333) | ⊕⊕⊕⊝ | Downgraded for study limitations |
| Weekly delivered Kt/V | The mean delivered Kt/V was 2.47 lower (5.17 lower to 0.22 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 2 (263) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data | ||
| Correction of acidosis | 577 per 1000 | 762 per 1000 (70 to 1,000) | RR 1.32 (0.1 to 13.60) | 2 (120) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data |
| Fluid removal (L/d) | The mean fluid removal was 0.59 L/d lower (1.19 lower to 0.01 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 3 (313) | ⊕⊕⊝⊝ | Downgraded for study limitations and imprecision | ||
| Duration of dialysis (hours) | The mean duration of dialysis was 1.01 hours less (91.49 lower to 92.54 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 2 (170) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data | ||
| Infectious complications | 169 per 1000 | 174 per 1000 (101 to 301) | RR 1.03 (0.60 to 1.78) | 2 (263) | ⊕⊕⊝⊝ | Downgraded for study limitations and insufficient data |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Three studies did not report details about random sequence generation or allocation concealment or both 2Small numbers with wide CI 3Few studies (no more than 2) reported the relevant data | ||||||
Background
Description of the condition
Acute kidney injury (AKI) is generally characterised by an abrupt deterioration in kidney function, with accumulation of creatinine, urea, metabolic acids, electrolytes, and decreased urine output. AKI is increasingly prevalent globally and associated with significant morbidity and mortality (Hoste 2006; Hsu 2013; Piccinni 2011). A systematic review, which included 49 million patients and 312 cohort studies, found that AKI occurred in 20% adults and 33% of children who are hospitalised with acute illness. Mortality remains unacceptably high, unadjusted mortality associated with an episode of AKI has been estimated at 23.9% in adults and 13.8% in children (Susantitaphong 2013).
Description of the intervention
Extracorporeal therapy, including continuous renal replacement therapy (CRRT) and intermittent HD (IHD), is the most common therapies for the treatment of people with AKI. However, the effect of peritoneal dialysis (PD) for AKI remains unclear despite the use of PD preceding extracorporeal therapy for the treatment of AKI (Gabriel 2006). PD is a preferred dialysis option for most children with AKI, patients with vascular access failure, and those with unstable haemodynamics or who are at risk of bleeding (Ponce 2012). PD use has progressively declined in favour of extracorporeal therapy in many settings, particularly pump‐assisted modes of dialysis (Bunchman 1994; Ellis 1997).
Although extracorporeal therapy is now the treatment of choice for most people with AKI, PD is still widely used in some settings (Gabriel 2007a; Ponce 2011). There is no randomised controlled trial (RCT) evidence to indicate superiority in terms of improved survival, recovery of kidney function, metabolic and clinical outcomes among modes of dialysis for people with AKI (Gabriel 2007b; Gabriel 2008; George 2011; Ponce 2011).
Dialysis types vary significantly and their use may be influenced by the treatment setting. Although both extracorporeal therapy and PD are provided for people with AKI, PD is seldom used in developed countries because of efficacy concerns (Hyman 2002). However, in resource‐limited settings PD is favoured as a low cost and widely available dialysis option (Mohandas 2004; Sharma 2003). Moreover, PD has been shown to be effective in patients in hypercatabolic state (Gabriel 2008) and those with acidosis (Dell'Aquila 2006).
How the intervention might work
PD, which uses the peritoneum as a dialysis membrane by means of dispersion and ultrafiltration, is aimed to remove urinary toxins, excess fluid and correct electrolyte and acid‐base balance disorders. Improvements in PD have increased fluid removal efficacy and metabolic control in people with AKI (Chitalia 2002; Gabriel 2006; Gabriel 2007b).
Why it is important to do this review
Recent studies have shown that PD may be a cost‐effective alternative to extracorporeal therapy in resource‐limited healthcare settings. Furthermore, similar rates of mortality and recovery of kidney function in AKI patients have been reported between PD and extracorporeal therapy (Gabriel 2008; George 2011). Thus, we will seek to systematically review the current literature and to analyse all studies comparing PD with extracorporeal therapy or different PD modalities for the treatment of AKI.
Objectives
The aim of this review was to evaluate the benefits and harms of PD for patients with AKI compared with extracorporeal therapy or different PD modalities.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment will be obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the benefits and harms of PD comparing with extracorporeal therapy or different PD modalities for AKI were considered eligible for inclusion, whether or not mortality rate and kidney function recovery were set as the primary outcome. Cross‐over studies were excluded.
Types of participants
Inclusion criteria
We included patients with AKI who were randomised to receive PD or extracorporeal therapy, regardless of their age, sex, primary disease and clinical course.
Exclusion criteria
-
Patients who had previously received any mode of dialysis during the current illness, and who had pre‐renal AKI, urinary tract obstruction, rapidly progressive glomerulonephritis, a history of chronic kidney insufficiency, kidney transplant.
-
Patients receiving both PD and extracorporeal therapy.
Types of interventions
-
PD (including all different modalities) plus supportive treatment versus extracorporeal therapy (including all different modalities of IHD or CRRT) plus supportive treatment.
-
PD (including all different modalities) versus extracorporeal therapy (including all different modalities of IHD or CRRT)
-
Comparison of different modalities of PD.
Supportive treatment might include approaches to treat underlying kidney or other diseases and to improve disorders that were linked to AKI, such as sepsis, malaria, and acute tubular necrosis. Supportive treatment should be comparable between PD and extracorporeal therapy.
Types of outcome measures
All the outcomes were assessed at fixed time points (e.g. 30 days, 90 days, the end of follow‐up, at ICU discharge or hospital discharge).
Primary outcomes
All‐cause mortality (death from any cause at the end of follow‐up).
Secondary outcomes
-
Recovery of kidney function (kidney function recovery is defined as no need of dialysis with improvement of urine output and a progressive fall in serum creatinine (SCr))
-
Laboratory index (e.g. SCr, Kt/V, weekly endogenous creatinine clearance (CrCl), serum potassium, serum phosphorus)
-
Economic cost (total cost of dialysis and hospitalisation)
-
Metabolic and acid‐base control (pH and bicarbonate)
-
Fluid removal
-
Length of in‐hospital and ICU stays
-
Duration of dialysis (days)
-
Adverse events (including bleeding, peritonitis, respiratory Insufficiency, hypoalbuminaemia, infection)
-
Blood pressure during dialysis (mm Hg)
-
Vasopressor support
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 29 May 2017 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL).
-
Weekly searches of MEDLINE OVID SP.
-
Handsearching of kidney and transplant‐related journals and the proceedings of major kidney and transplant conferences.
-
Searching of the current year of EMBASE OVID SP.
-
Weekly current awareness alerts for selected kidney and transplant journals.
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the "Specialised Register" section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
-
The China Biological Medicine Database (CBM‐disc from 1979), a database of Chinese biomedical research literature.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that might be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that might include relevant data or information on studies will be retained initially. Two authors will independently assess retrieved abstracts, and if necessary the full text of these studies, to determine which studies satisfy the inclusion criteria.
Data extraction and management
Data extraction will be carried out independently by two authors using standard data extraction forms. Studies reported in non‐English and non‐Chinese language journals were translated before assessment. Where more than one publication of one study exists, reports were grouped together and the report with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancy between published versions was to be highlighted. Disagreements were resolved in consultation among all authors.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. all‐cause mortality, patient survival, recovery of kidney function) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. laboratory index, cost, RRT time, vascular booster dose, quality of life), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales have been used.
Unit of analysis issues
Special issues in the analysis of studies with non‐standard designs, such as cluster‐RCTs, were to be described.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing to corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). A guide to the interpretation of I2 values is as follows.
-
0% to 40%: might not be important
-
30% to 60%: may represent moderate heterogeneity
-
50% to 90%: may represent substantial heterogeneity
-
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
Funnel plots were to be used to assess for the potential existence of small study bias (Higgins 2011). There were insufficient studies to do this.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was be used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis to identify possible sources of heterogeneity. Differences in participant sources (ethnicity, age and underlying kidney diseases) and disparities related to intervention (different dialytic modalities, PD and HD dose and mode) might be attributed to heterogeneity. The following subgroup analyses were planned to investigate any observed heterogeneity.
-
Primary diseases
-
Disease severity
-
Extracorporeal therapy modes
-
Timing of treatment commencement.
Adverse effects were tabulated and assessed with descriptive techniques because they were likely to be different for the various agents used. Where possible, the risk difference (RD) with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
Where possible, we performed sensitivity analyses to evaluate the effect on the overall result of removing studies with low methodological quality. Studies with inadequate allocation concealment; achieving inadequate follow‐up and unblinded outcome assessment, or blinding of outcome assessment uncertain, were considered as being of low methodological quality. We performed sensitivity analyses to explore the influence of the following factors on effect size.
-
Repeating the analysis excluding unpublished studies.
-
Repeating the analysis taking account of risk of bias.
-
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results.
-
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
-
All‐cause mortality
-
Recovery of kidney function
-
Duration of dialysis (hours)
-
Delivered Kt/V
-
Correction of acidosis
-
Fluid removal (L/d)
-
Infectious complications
Results
Description of studies
Results of the search
After searching the Register of Studies and the China Biological Medicine Database we identified 968 records. After duplicates were removed and titles and abstracts screened we retrieved 21 full‐text articles for further assessment. Of these, six studies (15 records) were included and five studies (six records) were excluded (Figure 1).

Flow chart of the article selection process
Included studies
Six studies (Alhwiesh 2014; Gabriel 2008; George 2011; Phu 2002; Ponce 2011; Ponce 2013) enrolling 484 participants were included. Three studies focused on AKI patients with critical illness (Alhwiesh 2014; Phu 2002; George 2011), two studies on general AKI patients (Gabriel 2008; Ponce 2011), and one study enrolled any patient with AKI (Ponce 2013). Three studies were from Brazil (Gabriel 2008; Ponce 2011; Ponce 2013), one from India (George 2011), one from Saudi Arabia (Alhwiesh 2014), and one from Vietnam (Phu 2002). The characteristics of studies fulfilling the inclusion criteria are listed in Characteristics of included studies. In total, 233 patients underwent PD and 251 patients underwent extracorporeal therapy.
High volume PD (HVPD) was performed in all included studies as the modality of PD. One study (Ponce 2011) focused on high versus low intensity of PD, and the other studies compared PD with daily haemodialysis (DHD) (Gabriel 2008); extended daily haemodialysis (EDD) (Ponce 2013), or CRRT (Alhwiesh 2014; George 2011; Phu 2002).
Five studies reported all‐cause mortality (Gabriel 2008; George 2011; Phu 2002; Ponce 2011; Ponce 2013), four reported recovery of kidney function (Gabriel 2008; Phu 2002; Ponce 2011; Ponce 2013), two reported duration of dialysis (hours) (Gabriel 2008; George 2011), three reported delivered weekly Kt/V (Gabriel 2008; Ponce 2011; Ponce 2013), two reported the correction of acidosis (George 2011; Phu 2002), four reported fluid removal (L/d) (Gabriel 2008; George 2011; Ponce 2011; Ponce 2013); and three reported infection (Gabriel 2008; Ponce 2011; Ponce 2013).
Excluded studies
We excluded five studies. Three studies enrolled both AKI and CKD patients and data could not be separated (Arogundade 2005; Kalra 1989; Nand 1996), one study compared PD + verapamil to HD, however verapamil was not used in the HD group (Nand 1997a), and one study was a cross‐over study (Chitalia 2002).
Risk of bias in included studies
Details of the assessment of risk of bias of included studies are presented in Figure 2 and Figure 3. One study, available only as an abstract, reported limited information and we were unable to complete a risk of bias assessment (Alhwiesh 2014).
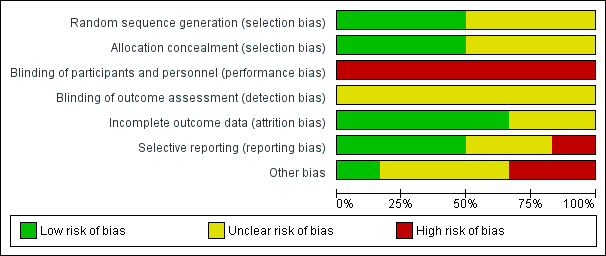
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
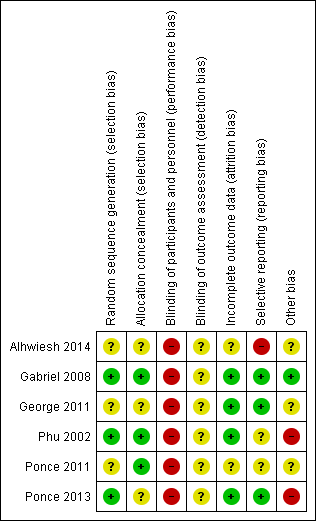
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Random sequence generation was judged to be at low risk of bias in three studies (Gabriel 2008; Phu 2002; Ponce 2013) and unclear in three studies (Alhwiesh 2014; George 2011; Ponce 2011).
Allocation concealment
Allocation concealment was judged to be at low risk of bias in three studies (Gabriel 2008; Phu 2002; Ponce 2011) and unclear in three studies (Alhwiesh 2014; George 2011; Ponce 2013).
Blinding
Performance bias was high in all studies and detection bias was unclear.
Incomplete outcome data
Attrition bias was judged to be low in four studies (Gabriel 2008; George 2011; Phu 2002; Ponce 2013) and unclear in two studies (Alhwiesh 2014; Ponce 2011).
Selective reporting
Reporting bias was judged to be high in one study (Alhwiesh 2014), low in three studies (Gabriel 2008; George 2011; Ponce 2013), and unclear in two studies (Phu 2002; Ponce 2011).
Other potential sources of bias
One study was stopped in advance, as only 50 patients were recruited over 3 years (George 2011). One study was stopped because of obvious differences in mortality (Phu 2002). In Ponce 2013, despite randomisation, there were significant differences between PD and extracorporeal therapy in some baseline characteristics, including pre‐dialysis BUN, and creatinine levels.
Effects of interventions
All‐cause mortality
PD compared to extracorporeal therapy probably makes little or no difference to all‐cause mortality (Analysis 1.1 (4 studies, 383 participants): RR 1.12, 95% CI 0.81 to 1.55; I2 = 69%; moderate certainty evidence). In Phu 2002, a total of 108 patients with AKI requiring dialysis were planned to be recruited. When 70 patients were enrolled, it showed an unexpected higher mortality rate in the PD group, so the study was stopped. AKI was mainly caused by falciparum malaria in this study, while in the other three studies AKI was mainly caused by sepsis or haemodynamic disturbances. When this study was removed from the analysis I2 was 0% with no change to the significance of the result (RR 1.00, 95% CI 0.85 to 1.17).
Ponce 2011 reported high versus low intensity PD probably makes little or no difference to all‐cause mortality (Analysis 2.1 (1 study, 61 participants): RR 1.03, 95% CI 0.65 to 1.63).
Recovery of kidney function
PD compared to extracorporeal therapy probably makes little or no difference to kidney function recovery (Analysis 1.2 (3 studies, 333 participants): RR 0.95, 95% CI 0.68 to 1.35; I2 = 0%; moderate certainty evidence).
Ponce 2011 reported high versus low intensity PD probably makes little or no difference to kidney function recovery (Analysis 2.2 (1 study, 61 participants): RR 1.00, 95% CI 0.74 to 1.35).
Weekly delivered Kt/V
It is uncertain whether the weekly delivered Kt/V is lower with PD compared to extracorporeal therapy (Analysis 1.3 (2 studies, 263 participants): MD ‐2.47, 95% CI ‐5.17 to 0.22; I2 = 99%; very low certainty evidence). Heterogeneity was high and this may be due to the different extracorporeal therapies used in the two studies (Gabriel 2008; Ponce 2013).
Ponce 2011 reported lower weekly delivered Kt/V with low intensity PD compared to high intensity PD (Analysis 2.3 (1 study, 61 participants): MD 1.13, 95% CI 0.91 to 1.35).
Correction of acidosis
It is uncertain whether PD compared to extracorporeal therapy corrected acidosis (Analysis 1.4 (2 studies, 89 participants): RR 1.32, 95% CI 0.13 to 13.60; I2 = 96%; very low certainty evidence). Heterogeneity was high and this may be due to the different extracorporeal therapies used in the two studies (George 2011; Phu 2002).
Correction of acidosis was not reported for high versus low intensity PD.
Fluid removal
PD probably slightly reduces the amount of fluid removal compared to extracorporeal therapy (Analysis 1.5 (3 studies, 313 participants): MD ‐0.59 L/d, 95% CI ‐1.19 to 0.01; I2 = 89%; low certainty evidence). Heterogeneity was high and this may be due to the different extracorporeal therapies used in the three studies (Gabriel 2008; George 2011; Ponce 2013).
Ponce 2011 reported less fluid removal with low intensity PD (Analysis 2.4 (1 study, 61 participants): MD 0.30 L/d, 95% CI ‐0.03 to 0.63).
Duration of dialysis
It is uncertain whether the duration of dialysis is reduced with PD compared to extracorporeal therapy (Analysis 1.6 (2 studies, 170 participants): MD ‐1.01 hours, 95% CI ‐91.49 to 89.47; I2 = 98%; very low certainty evidence). Heterogeneity was high and this may be due to the different extracorporeal therapies used in the two studies (Gabriel 2008; George 2011).
Duration of dialysis was not reported for high versus low intensity PD.
Adverse effects ‐ infection
PD probably made little or no difference to infectious complications compared to extracorporeal therapy (Analysis 1.7 (2 studies, 263 participants): RR 1.03, 95% CI 0.60 to 1.78; I2 = 0%; low certainty evidence).
Ponce 2011 reported little or no difference to the risk of infection between high and low intensity PD (Analysis 2.5 (1 study, 61 participants): RR 0.97, 95% CI 0.27 to 3.52).
Other outcomes
Gabriel 2008 reported that the HVPD and DHD were similar in metabolic control. SCr was stabilized after the same number of dialysis sessions. Mean SCr levels after four sessions of HVPD and DHD were 4.6 ± 1.0 and 5.5 ± 1.3 mg/100 mL respectively.
Phu 2002 and George 2011 compared the economic cost of dialysis. Phu 2002 observed the mean cost of the hospital stay (from the diagnosis of AKI to discharge) for patients assigned to PD was $1,580 (95% CI $1,170 to $2,000), as compared with $1,150 (95% CI $960 to $1,330) for patients assigned to CRRT. The mean costs per survivor were $3,000 (95% CI $2,210 to $3,790) for PD and $1,340 (95% CI $1,130 to $1,560) for haemofiltration. However, George 2011 observed that cost of disposables was higher in continuous venovenous haemodiafiltration (CVVHDF,) than in HVPD (INR 7184 ± 1436 versus INR 3009 ± 1643, P < 0.001).
Length of in‐hospital and ICU stays, blood pressure during dialysis (mm Hg), and vasopressor support were not reported in any of the included studies.
Discussion
Summary of main results
A total of six studies (484 participants) where included in our review. Five studies compared HVPD with DHD (Gabriel 2008), extended DHD (Ponce 2013), or CRRT (Alhwiesh 2014; George 2011; Phu 2002). One study focused on the intensity of PD (Ponce 2011). Overall, this systematic review represents a total of 484 patients. All of the studies came from low to middle income countries; there were no data available from high income countries. Overall there was little or no difference between PD and extracorporeal therapy on all‐cause mortality, recovery of kidney function recovery, duration of dialysis, correction of acidosis, and infectious complications. Fluid removal (L/d) was probably lower with PD and weekly delivered Kt/V might be higher with extracorporeal therapy. There was little or no difference between high and low intensity of PD for mortality or kidney function recovery.
Overall completeness and applicability of evidence
Although the effect of PD on AKI in high income countries may be unclear (Hyman 2002), PD is often used in low‐resource countries because of low cost, availability, and ease of administration (Phu 2002; Mohandas 2004; Sharma 2003). This finding may be considered surprising, because PD has been considered as a better dialysis modality that preserving renal haemodynamics and residual kidney function, as no extracorporeal circulation is required, which could theoretically result in better outcomes. The finding is in agreement with our included studies which all come from low‐resource countries, such as Brazil, Vietnam, and India. The reason for choice of PD is likely multifactorial; one reason is personal tendency of nephrologist, another important reason is different aetiology (Cerda 2008). In high income countries, patients with AKI often accompany with multiorgan failure and multiple comorbidities in elderly patients particular particularly in ICU. However, in low‐resource countries, AKI happens more often in young and previously healthy individuals, most of the patients have a single disease or condition, such as infection or toxins. These factors may affect the choice of dialysis modality.
Quality of the evidence
Only five studies provided data that could be potentially meta‐analysed, and there were several limitations of the quantity of evidence. First, it must be noted that half studies were small (fewer than 100 patients). Second, not all included studies reported all primary outcomes. For instance, 75% reported recovery of kidney function and fluid removal, 50% reported duration of dialysis, delivered weekly Kt/V, correction of acidosis and infection rate. Thus, there might be outcome reporting bias.
The high mortality rate among patients with AKI especially in intensive care units remains an unsolved problem in spite of the technological advances in RRT (Himmelfarb 2007; Ricci 2006; Uchino 2005). It has been reported that the mortality of patients with AKI has been estimated at 23.9% in adults and 13.8% in children (Susantitaphong 2013). There is no consensus in the literature on the best dialysis modality in patients with AKI; both PD and extracorporeal therapy are the possible options for the treatment of AKI. Phu 2002 reported that mortality with PD was threefold higher than mortality with HF; however, most of other studies didn't see a similar effect. The authors used intermittent PD with rigid catheters, an open system, manual exchanges, and a too‐short dwell time, which may contribute to the poor outcome. In addition, severe acidosis secondary to the combination of severe sepsis or malaria was a major contributor to the high mortality. Our review demonstrated that pooled mortality of dialysis patients was 58.2% for PD and 56.2% for extracorporeal therapy. Meta‐analysis suggested that all‐cause mortality was similar in both groups. This finding is consistent with the review of Chionh 2013, which suggested that no significant differences was observe in mortality between PD and extracorporeal therapy in patients with AKI, however most data was from the cohort studies. Our mortality data appeared equivalent with other centres in India, with a mortality of 60% observed in septic patients (Chatterjee 2009). It is speculated that the main reason influencing mortality was the severity of disease and the need for ventilator support, but not the modality of dialysis (George 2011). However, because of limited number of patients and RCTs, this finding should be interpreted with caution until more studies are available.
Three studies assessed the difference in recovery of kidney function between PD and extracorporeal therapy. Only one study identified that the recovery of kidney function of haemofiltration was superior to PD. Technical problems, severe primary infections and acidosis caused by infection may contribute to the poor outcome (Phu 2002). Meta‐analysis suggested that recovery of kidney function was similar in both groups. However, there were differences in the speed of kidney function recovery. One study suggested that the recovery of kidney function in patients treated with HF required for a significantly shorter period compared with PD (Phu 2002). However, Gabriel 2008 and Mehta 2007 both reported that PD was associated with a significantly faster recovery of kidney function.
In terms of other secondary outcomes, no differences were identified in duration of dialysis (hours), correction of acidosis, and fluid removal (L/d) between PD and extracorporeal therapy. Whereas, delivered Kt/V was higher in HD group. Most of the published studies had conflicting results. There are several factors that impact the outcome of PD, such as dialysate dose, dwell times, membrane permeability and area (Ronco 2006). Longer dwell times which may be conducive to fluid removal may reduce the urea and creatinine clearance (Jeloka 2006). A recent study had suggested that dialysis dose may be a major factor influencing the outcome of AKI treatment (Schiffl 2002). The reporting of these factors was either absent or different among the four included studies, making it difficult to draw a specific relationship between these factors and clinical outcomes. Further clinical subgroup analyses were not performed.
The most common complications related to PD and extracorporeal therapy are peritonitis, catheter infection, catheter leakage, catheter obstruction and migration. Two studies reported peritonitis related to PD and catheter infection related to extracorporeal therapy; infectious complications related to dialysis method were similar in both studies (Gabriel 2008; Ponce 2013). Peritonitis incidence was similar to previous studies in the literature (Gabriel 2006). The included studies didn't report length of in‐hospital or ICU stays, blood pressure during dialysis (mm Hg), or vasopressor support which might influence outcomes in AKI, leading to the lack of further analysis according to design in advance.
Potential biases in the review process
Both low quantity and quality of available evidence has meant that any differences between the two groups remain extremely uncertain. Some limitations of our study should be pointed out. First, only four studies were included in our meta‐analysis, eligible studies comparing specific outcomes were extremely limited. The limited sample size made it difficult to observe a clinically significant difference in outcomes. We therefore did not perform further subgroup analyses based on indicators such as modality of PD and extracorporeal therapy, differences of dialysis dose, primary diseases, disease severity, and timing of treatment commencement, which may have added to heterogeneity. Second, although we extracted data at the end of studies, the duration of each study varied. Some studies were terminated in advance, which possibly have added to generation of bias. Third, all studies in our review were performed in low to middle income countries. Four, unreported outcomes of interest in some studies possibly lead to reporting bias. Last, there was no enough data to evaluate the length of stays in the ICU, intradialytic BP and vasopressor requirements in our review, which might have significant impact in AKI outcomes. Regardless of these limitations, we have decreased bias throughout the methods of research identification, data selection, and statistical analysis. These steps should enhance accuracy of our review.
Agreements and disagreements with other studies or reviews
Chionh 2013 included 11 studies to evaluate the effect of PD on AKI patients in comparison with extracorporeal therapy, and reported no difference in mortality. In subgroup analyses, mortality was similar in observational studies (OR 0.96, 95% CI, 0.53 to 1.71) and RCTs (OR 1.50, 95% CI, 0.46 to 4.86). However, only three of our included studies (Gabriel 2008; George 2011; Phu 2002) were pooled in the meta‐analysis of mortality. We included four RCTs (Gabriel 2008; George 2011; Phu 2002; Ponce 2013), and also found no difference in mortality. There was no RCT focusing on PD for paediatric AKI patients, but a recent observational study (Basu 2016) showed that continuous PD was associated with lower mortality compared with daily HD in paediatric AKI patients. The recent International Society for Peritoneal Dialysis guidelines (Cullis 2014) on PD for AKI also suggested that PD should be considered as a suitable method of CRRT in patients with AKI both in high and low resource countries, and PD was also a safe and effective method of blood purification and fluid removal for paediatric AKI patients.

Flow chart of the article selection process

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
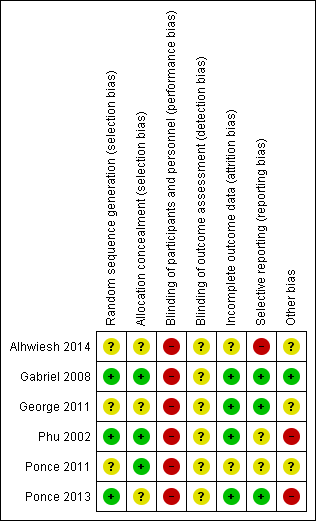
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
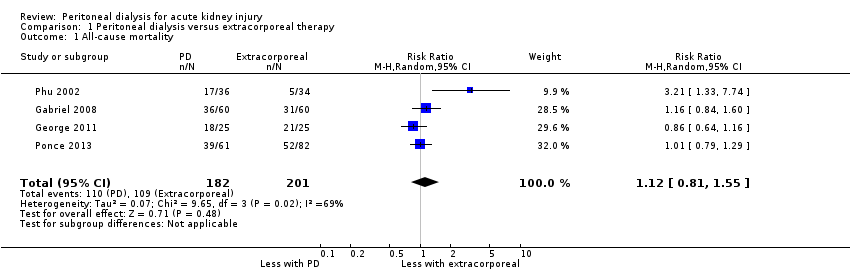
Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 1 All‐cause mortality.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 2 Recovery of kidney function.
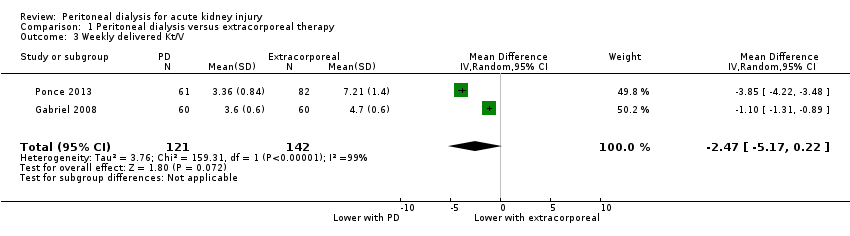
Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 3 Weekly delivered Kt/V.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 4 Correction of acidosis.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 5 Fluid removal.
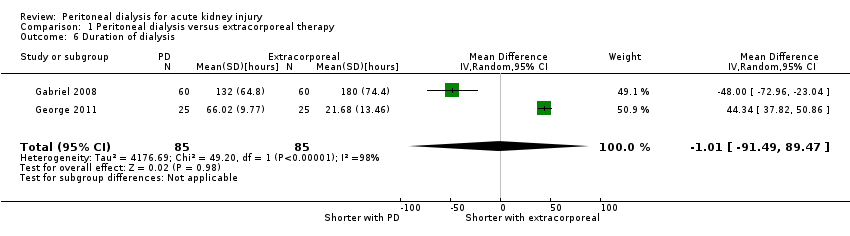
Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 6 Duration of dialysis.

Comparison 1 Peritoneal dialysis versus extracorporeal therapy, Outcome 7 Infectious complications (catheter infection or peritonitis).
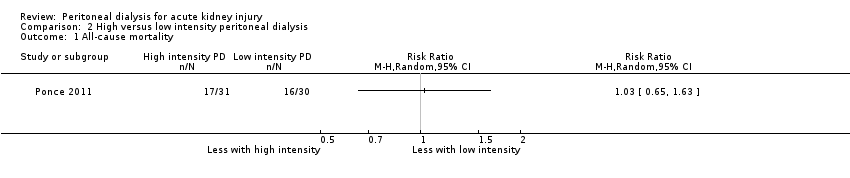
Comparison 2 High versus low intensity peritoneal dialysis, Outcome 1 All‐cause mortality.

Comparison 2 High versus low intensity peritoneal dialysis, Outcome 2 Recovery of kidney function.
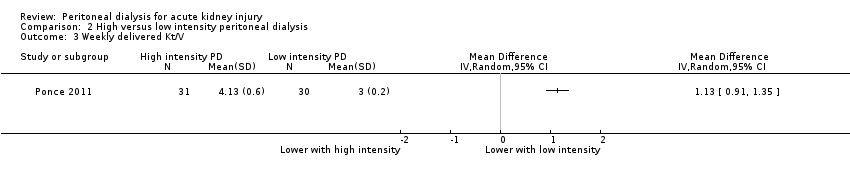
Comparison 2 High versus low intensity peritoneal dialysis, Outcome 3 Weekly delivered Kt/V.
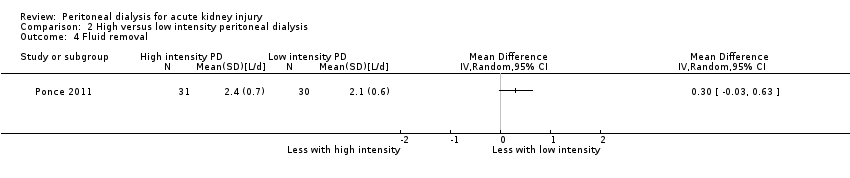
Comparison 2 High versus low intensity peritoneal dialysis, Outcome 4 Fluid removal.

Comparison 2 High versus low intensity peritoneal dialysis, Outcome 5 Infectious complications (catheter infection or peritonitis).
| Peritoneal dialysis versus extracorporeal therapy for acute kidney injury | ||||||
| Patient or population: patients with acute kidney injury Settings: inpatient Intervention: peritoneal dialysis Comparison: extracorporeal therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Extracorporeal therapy | Peritoneal dialysis | |||||
| All‐cause mortality | 542 per 1000 | 607 per 1000 (439 to 841) | RR 1.12 (0.81 to 1.55) | 4 (383) | ⊕⊕⊕⊝ | Downgraded for study limitations |
| Recovery of kidney function | 284 per 1000 | 270 per 1000 (193 to 384) | RR 1.42 (0.74 to 2.75) | 3 (333) | ⊕⊕⊕⊝ | Downgraded for study limitations |
| Weekly delivered Kt/V | The mean delivered Kt/V was 2.47 lower (5.17 lower to 0.22 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 2 (263) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data | ||
| Correction of acidosis | 577 per 1000 | 762 per 1000 (70 to 1,000) | RR 1.32 (0.1 to 13.60) | 2 (120) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data |
| Fluid removal (L/d) | The mean fluid removal was 0.59 L/d lower (1.19 lower to 0.01 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 3 (313) | ⊕⊕⊝⊝ | Downgraded for study limitations and imprecision | ||
| Duration of dialysis (hours) | The mean duration of dialysis was 1.01 hours less (91.49 lower to 92.54 higher) in the peritoneal dialysis group compared to the extracorporeal therapy group | 2 (170) | ⊕⊝⊝⊝ | Downgraded for study limitations, imprecision and insufficient data | ||
| Infectious complications | 169 per 1000 | 174 per 1000 (101 to 301) | RR 1.03 (0.60 to 1.78) | 2 (263) | ⊕⊕⊝⊝ | Downgraded for study limitations and insufficient data |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Three studies did not report details about random sequence generation or allocation concealment or both 2Small numbers with wide CI 3Few studies (no more than 2) reported the relevant data | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 4 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.55] |
| 2 Recovery of kidney function Show forest plot | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.35] |
| 3 Weekly delivered Kt/V Show forest plot | 2 | 263 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐5.17, 0.22] |
| 4 Correction of acidosis Show forest plot | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.13, 13.60] |
| 5 Fluid removal Show forest plot | 3 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.19, 0.01] |
| 6 Duration of dialysis Show forest plot | 2 | 170 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐91.49, 89.47] |
| 7 Infectious complications (catheter infection or peritonitis) Show forest plot | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.60, 1.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Recovery of kidney function Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Weekly delivered Kt/V Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Fluid removal Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Infectious complications (catheter infection or peritonitis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

