Nutzen und Risiko von früher versus später Ureterstententfernung nach Nierentransplantation
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Other information
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement "...were randomly assigned to two groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | No blinding but unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for: ‐ 3 patients forgot to return for stent removal at 6 weeks and it was removed at 12 weeks ‐ 4 patients removed from analysis (2 in each group) due to stent migration |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Very long length of stay 3 to 4 weeks, maybe associated with increased risk of nosocomial infection |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Other information
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer generated random numbers created by study coordinator |
| Allocation concealment (selection bias) | Low risk | Allocation kept in sealed opaque envelopes until opened on day 7 by ward nurses |
| Blinding of participants and personnel (performance bias) | Low risk | No blinding but unlikely to impact outcome |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, however CONSORT diagram not included |
| Selective reporting (reporting bias) | Low risk | No study protocol data but all reported outcomes are accounted for |
| Other bias | Low risk | The study appears to be free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Other information
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block of 4 |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding not possible but unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) | High risk | No evidence of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for but no CONSORT diagram |
| Selective reporting (reporting bias) | High risk | No information on what type of urological complications were encountered |
| Other bias | Low risk | The study appears to be free of other biases |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
Other information
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online computer generated randomisation process, block stratified with randomly varying block sizes |
| Allocation concealment (selection bias) | Low risk | Allocation revealed to clinicians at time of randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding not possible but unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) | High risk | Not possible |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram included detailing full follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | The study appears to be free of other biases |
BI ‐ bladder indwelling; CIT ‐ cold ischaemic time; DGF ‐ delayed graft function; DPTA ‐ diethylenetriaminepentaacetic acid; ESKD ‐ end‐stage kidney disease; IQR ‐ interquartile range; IV ‐ intravenous; M/F ‐ male/female; MSU ‐ midstream urine; mTORi ‐ mammalian target of rapamycin inhibitor; MUC ‐ major urological complications; PC ‐ percutaneous; PU ‐ per‐urethral; PP ‐ per protocol; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; USS ‐ urinary ultrasound; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not enough information included in abstract available regarding numbers of patients in intervention arms therefore unable to include in analysis. Authors did not respond to our contact for more information. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Pilot study: prospective randomised controlled trial of ureteric double J stenting with early vs standard stent removal to improve graft and patient outcome and reduce urological complications after renal transplantation |
| Methods | Prospective RCT comparing early removal of ureteric stent at day 4 (attached to catheter) compared to late removal 4‐6 weeks post‐op cystoscopically. |
| Participants | All patients > 16 years on the kidney transplant waiting list at a single centre Exclusion criteria: neurogenic bladder dysfunction or re‐transplant |
| Interventions | Double J stent sutured to urinary catheter and removed simultaneously on day 4 post‐transplant |
| Outcomes | Primary outcome (1): graft outcome assessed using histology from renal biopsy, SCr and eGFR Primary outcome (2): at 12 months post‐transplant patient mortality data will be recorded Secondary outcome: MUC |
| Starting date | 01/05/2010 |
| Contact information | Dr Adam Bartlett, [email protected] |
| Notes | No outcome data to be obtained regarding UTI |
| Trial name or title | Randomised controlled trial of early versus late ureteric stent removal post kidney transplant |
| Methods | Parallel RCT |
| Participants | Sample size set at 350 based on power calculations. To include all adults receiving at kidney either living or deceased donor |
| Interventions | Group A ‐ removal of ureteric stent on day 6‐8 post‐transplant Group B ‐ removal of ureteric stent during week 4‐6 post‐transplant |
| Outcomes | Primary outcome: composite incidence of UTI and ureteric complications Secondary outcome: incidence of UTI, urine leak, stenosis, patient death, graft loss, surgical complications, immunological complications, readmission and length of stay, medical complications Measure at 3 months post‐transplant |
| Starting date | 1/1/2014 |
| Contact information | Dept of Surgery Addenbrookes |
| Notes |
eGFR ‐ estimated glomerular filtration rate; MUC ‐ major urological complications; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; UTI ‐ urinary tract infection
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major urological complications Show forest plot | 5 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.61, 5.71] |
| Analysis 1.1  Comparison 1 Major urological complications, Outcome 1 Major urological complications. | ||||
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.52, 5.36] |
| 1.2 Per‐urethral stents | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.03, 74.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urinary tract infection Show forest plot | 5 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| Analysis 2.1  Comparison 2 Urinary tract infection, Outcome 1 Urinary tract infection. | ||||
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.29, 0.70] |
| 1.2 Per‐urethral stents | 2 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.03] |
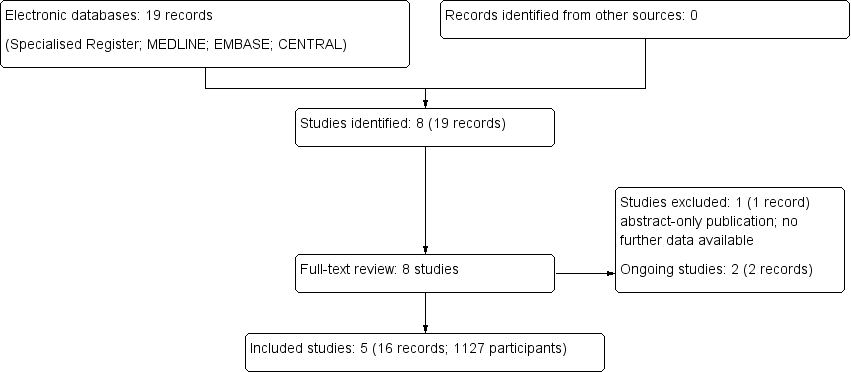
Flow chart of study selection

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
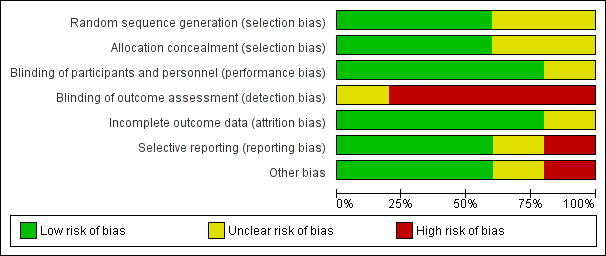
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Major urological complications, Outcome 1 Major urological complications.

Comparison 2 Urinary tract infection, Outcome 1 Urinary tract infection.
| Early versus late ureteric stent removal after kidney transplantation | |||||
| Patient or population: kidney transplant recipients | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with late removal | Risk with early removal | ||||
| Major urological complications: all stents | Study population | RR 1.87 | 1127 (5) | ⊕⊕⊕⊕ | |
| 12 per 1,000 | 23 per 1,000 | ||||
| Major urological complications: bladder indwelling stents | Study population | RR 1.67 | 539 (3) | ⊕⊕⊕⊕ | |
| 15 per 1,000 | 24 per 1,000 | ||||
| Major urological complications: per‐urethral stents | Study population | RR 1.51 | 588 (2) | ⊕⊕⊕⊕ | |
| 10 per 1,000 | 15 per 1,000 | ||||
| Urinary tract infection: all stents | Study population | RR 0.49 | 1126 (5) | ⊕⊕⊕⊝ | |
| 185 per 1,000 | 91 per 1,000 | ||||
| Urinary tract infection: bladder indwelling stents | Study population | RR 0.45 | 539 (3) | ⊕⊕⊕⊝ | |
| 209 per 1,000 | 94 per 1,000 | ||||
| Urinary tract infection: per‐urethral stents | Study population | RR 0.60 | 587 (2) | ⊕⊕⊝⊝ | |
| 164 per 1,000 | 98 per 1,000 | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 All studies were unblinded, however, this was unavoidable given the nature of the intervention. The majority of studies provided minimal information on processes of randomisation and allocation 2 Inconsistent definition and variable reporting of urinary tract infection across included studies | |||||
| Study ID | Adverse events |
| Two patients in the late group required re‐stenting due to ureteric stenosis | |
| Three patients in the late group had forgotten stents that were subsequently removed at 12 weeks | |
| Six patients in the early and 5 patients in the late group had acute rejection that required intervention | |
| No adverse events reported | |
| Sixteen patients did not receive their allocated treatment as there were technical difficulties attaching the stent to the catheter. In the early removal group, 1 patient's stent removal was delayed by 1 day because the urethral catheter balloon needed percutaneous needle puncture due to the stent suture There were 5 complications in patients who had early stent removal and these were all related to the percutaneous technique used in which the stent was tied to the catheter |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Major urological complications Show forest plot | 5 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.61, 5.71] |
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.52, 5.36] |
| 1.2 Per‐urethral stents | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.03, 74.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urinary tract infection Show forest plot | 5 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.29, 0.70] |
| 1.2 Per‐urethral stents | 2 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.03] |

