Lesión del endometrio para el embarazo después del coito o la inseminación intrauterina
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial, 3 groups, set in an infertility clinic, United Arab Emirates March 2010 to March 2012 Number of participants randomised: 150 Number of participants analysed: 150 | |
| Participants | Inclusion criteria: diagnosed as primary or secondary unexplained infertility; semen count was ≥ 15 million/mL, motility grade a + b, ≥ 40 % before wash; age 22 to 35 years; having a good response as demonstrated by the presence of 1 to 3 follicles; undergoing intrauterine insemination (IUI) with stimulation protocol Exclusion criteria: endometriosis or intrauterine organic pathology (myoma, polyps and adhesions) by diagnostic laparoscopy, and diagnostic hysteroscopy performed 2 to 3 months before the IUI; known pelvic inflammatory disease; uni‐ or bilateral tubal block Cause subfertility: (primary/secondary) unexplained, mild male factor, ovulatory factor | |
| Interventions |
All groups: stimulation protocol consisted of Letrozol and follitropin alpha (Gonal‐F). Egg trigger was performed by recombinant human chorionic gonadotropin. Luteal phase support was performed using Dydrogesterone (Duphaston) Degree of endometrial injury: Tao brush Timing of endometrial injury: follicular phase days 8 to 9: either in the previous cycle (group A) or in the same cycle, as IUI (group B) Study length: 1 cycle Type of conception: IUI | |
| Outcomes | Reported in paper: clinical pregnancy; defined by human chorionic gonadotropin doubling and ultrasound confirmation Multiple pregnancy | |
| Notes | Funding source: no funding required as per author correspondence Conflict of interest: "none" Study was not registered as per author correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Reported as "Sealed envelopes". However, the study used sequentially numbered, opaque sealed envelopes, as we determined after author correspondence |
| Blinding of participants (performance bias) | High risk | The study did not report blinding of participants and it was unlikely; and we anticipate that lack of participant blinding introduced performance bias |
| Blinding of personnel (performance bias) | High risk | The study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The study did not report blinding of outcome assessors and it was unlikely; however, outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | The study did not report any missing outcome data and the study authors confirmed there were no drop‐outs after correspondence |
| Selective reporting (reporting bias) | Low risk | There was no protocol available and the trial was not registered (confirmed by author correspondence). However, the study reported all expected outcomes. The study authors confirmed live birth and pain data were not collected |
| Other bias | Low risk | We did not identify any other potential sources of bias |
| Methods | Randomised controlled trial, 2 groups, set in an infertility clinic, Cairo, Egypt, Ain Shams University Maternity Hospital Number of participants randomised: 80 Number of participants analysed: 73 | |
| Participants | Inclusion criteria: 20 to 35 years of age; undergoing intrauterine insemination (IUI), patent (functioning) fallopian tubes, body mass index between 20 ‐ 35 kg/m² Exclusion criteria: indication for in vitro fertilisation, pelvic inflammatory disease, poor responder to ovarian stimulation, bilateral tubal disease, severe male factor, intrauterine pathology (submucosal fibroid, polyp, adhesion), cervical or acute vaginal infection Cause subfertility: (primary/secondary) unexplained, mild male factor | |
| Interventions |
Both groups: controlled ovarian hyperstimulation (clomiphene and/or gonadotrophins) + IUI. All participants asked to remain abstinent or use barrier contraception in the preceding cycle. Degree of endometrial injury: pipelle Timing of endometrial injury: luteal phase (day 21 of cycle preceding IUI cycle) Study length: 1 cycle Type of conception: IUI | |
| Outcomes | Reported in paper
| |
| Notes | Only a thesis is available, which was published as part of a Master's degree in Obstetrics and Gynaecology at Baghdad University. The study does not appear to have been published external to the university library. Funding source: not reported Conflict of interest: not stated Study does not appear to be registered Author correspondence was not possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | The thesis did not describe how randomisation was carried out |
| Blinding of participants (performance bias) | High risk | The thesis did not report blinding of participants and it was unlikely; and we anticipate the lack of participant blinding to have introduced performance bias |
| Blinding of personnel (performance bias) | High risk | The thesis did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The thesis did not report blinding of outcome assessors and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | The study recruited 80 women, and 7 dropped out (2 in control group and 5 in intervention group). The thesis author did not provide reasons. |
| Selective reporting (reporting bias) | Unclear risk | The study does not appear to have been registered. The thesis only reported biochemical pregnancy and clinical pregnancy; however it is unclear whether there was any intention to follow‐up women to the stage of ongoing pregnancy or live birth. |
| Other bias | Unclear risk | Insufficient information was available to assess this bias. |
| Methods | Methods: Randomised controlled trial, 2 groups, set in Kasr Al‐Aini Teaching Hospital at Cairo University and a Middle East IVF Centre, Egypt February 2012 to October 2014 Number of participants randomised: 332 Number of participants analysed: 332 | |
| Participants | Inclusion criteria: women with unexplained infertility or couples with mild male factor infertility. Female partner aged less than 39 years; regular menstrual cycles; a body mass index of < 32 kg/m², a normal uterine cavity with normal thin endometrium measuring < 5 mm on day 4; bilateral tubal patency (demonstrated by laparoscopy or hysterosalpingography); normal hormonal profile Exclusion criteria: women diagnosed with infertility due to other causes; significant cardiovascular, pulmonary, renal, neurological or hepatic problems; presence of ovarian cyst > 2 cm before stimulation; abnormal endometrial cavity due to submucous myoma; endometrial polyp; intrauterine synechia; septate or bicornate uterus Cause subfertility: unexplained infertility or mild male factor | |
| Interventions |
Both groups: ovulation induction consisted of clomiphene citrate 100 mg/day from day 3 to 7, human menopausal gonadotrophin 75 IU/day from day 6 to 8. Transvaginal ultrasound was done on day 9, and when 2 to 3 follicles > 18 mm diameter were present a human chorionic gonadotrophin trigger of 10,000 IU was administered. Intrauterine insemination (IUI) was performed 36 hours after the trigger. Degree of endometrial injury: grasping forceps with teeth Timing of endometrial injury: follicular phase (day 4 to 7) of the preceding cycle Study length: 1 cycle Type of conception: IUI | |
| Outcomes | Reported in paper
| |
| Notes | Funding source: none NCT01544426 Author correspondence undertaken | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random number tables" |
| Allocation concealment (selection bias) | Low risk | "opaque sealed envelopes containing the participants' group allocation". The random allocation was put into envelopes every "24 hours at a location different from the study site and sent to an assigned nurse who opened each envelope just before the office hysteroscopy". The study authors confirmed via correspondence that envelopes were sequentially numbered and revealed that this was a mechanism to help to ensure no violation of allocation concealment |
| Blinding of participants (performance bias) | Low risk | The paper stated that "the patients were blinded to group allocation". Participants were undergoing either hysteroscopy or hysteroscopy and endometrial injury. Although no anaesthesia or analgesia was used and participant blinding was not formally tested, the control procedure is likely to simulate the intervention and therefore likely to have blinded participants to their allocation |
| Blinding of personnel (performance bias) | High risk | The study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The study did not report blinding of outcome assessors and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Four participants were lost to follow‐up from the intervention group and 2 from the control group, and no discontinued interventions. The study reported the number of participants missing and it was similar between groups. The study authors performed intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | Protocol/registration was available, and the study reported all prespecified outcomes. Confirmed pain was not recorded |
| Other bias | Low risk | We did not identify any other potential sources of bias |
| Methods | Randomised controlled trial, 2 groups, set in Mansoura University Hospital and a private practice, Egypt July 2009 to December 2010 Number of participants randomised: 105 Number of participants analysed: 105 | |
| Participants | Inclusion criteria: women aged between 20 and 39 years; ≥ 1 year of infertility; regular menstruation with the length of the cycle between 22 and 34 days; ovulation confirmed by appropriately timed mid‐luteal progesterone, fertile semen variables (according to World Health Organization criteria 1999); bilateral tubal patency (demonstrated by laparoscopy or hysterosalpingography) Exclusion criteria: not reported Cause subfertility: unexplained infertility | |
| Interventions |
Both: all women received pain medicine and doxycycline after procedure. Non‐hormonal contraception was advised to the participants in both groups in that cycle. Degree of endometrial injury: pipelle Timing of endometrial injury: luteal phase (days 21 to 26 of a spontaneous cycle, participants advised to use non‐hormonal contraception during the intervention cycle) Study length: 6 cycles Type of conception: intercourse at the participants' convenience The control group was administered a mock procedure which was not intended to cause injury but is likely to have done, and so this may be considered an inappropriate control intervention (uterine sound). | |
| Outcomes | Reported in paper
Obtained from author correspondence
| |
| Notes | Funding source: no external funding source other than salaries paid by Mansoura University (author correspondence) NCT01412606 Conflict of interests unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization carried out through a computer‐generated allocation sequence” |
| Allocation concealment (selection bias) | Low risk | “Allocation was through a nurse picking up a sealed opaque consecutively numbered envelope” |
| Blinding of participants (performance bias) | Low risk | Placebo procedure employing uterine sound, likely to simulate the intervention and therefore likely to have blinded participants to their allocation (although this was not formally tested). |
| Blinding of personnel (performance bias) | High risk | The study did not report blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The study did not report blinding of outcome assessors and was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Seven participants failed to undergo their allocated procedure, and a further 8 were lost to follow‐up. The study report the number of participants and reasons for missing outcome data, which were similar between groups. The study authors performed intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | The protocol was available. The study reported all prespecified outcomes. The authors confirmed by author correspondence that live birth and pain were not recorded |
| Other bias | Low risk | We did not identify any other potential sources of bias |
| Methods | Randomised controlled trial, 2 groups, set in Department of Obstetrics and Gynaecology, Faculty of Medicine at Benha University Hospital, and private centres for infertility, Egypt January 2010 to January 2015 Number of participants randomised: 154 Number of participants analysed: 154 | |
| Participants | Inclusion criteria: women with unexplained infertility assigned for intrauterine insemination (IUI) (requiring normal semen analysis); must have at least 1 patent (functioning) tube and no significant intrauterine or pelvic abnormalities (demonstrated on ultrasound, hysteroscopy or laparoscopy); normal serum follicular stimulating hormone levels of ≤ 12 mIU/mL Exclusion criteria: female partner > 40 years; ovarian cyst; uterine lesions; previous diagnosis of moderate‐severe endometriosis; body mas index ≥ 35 kg/m²; polycystic ovary syndrome or anovulatory; signs of hyperandrogaenemia Cause subfertility: unexplained infertility | |
| Interventions |
Both groups: participants given 100 mg clomiphene citrate on days 3 to 7 of spontaneous menstrual cycle, followed by daily 150 IU of human menopausal gonadotrophin. When 2 dominant follicles of 17 mm diameter or a luteinising hormone surge occurs participants are given 5000 IU of human chorionic gonadotrophin. 24 to 36 hours later IUI was performed. Degree of endometrial injury: no. 8 neonatal feeding tube Timing of endometrial injury: on the day of trigger Study length: 3 cycles (scratching performed only in the first cycle) Type of conception: IUI | |
| Outcomes | Reported in paper
| |
| Notes | Funding source: the authors received no financial support Conflics of interest: the author(s) declared no potential conflicts of interest NCT02349750 Author correspondence undertaken | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as "randomly" in the text. Author correspondence confirmed the sequence was computer generated. "Allocation list was generated by a computer" |
| Allocation concealment (selection bias) | High risk | "using sealed envelope". Author correspondence elaborated: "codes were inserted into envelopes by a third party (secretary). The participants and the physicians were blinded to the identity of each envelope until it is opened and paper unfolded by a nurse." However, the envelopes were not numbered |
| Blinding of participants (performance bias) | High risk | The study did not report blinding of participants and it was unlikely; and lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | The study did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The study did not report blinding of outcome assessors and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses to follow‐up/drops outs/discontinuation of treatment |
| Selective reporting (reporting bias) | Low risk | The study was retrospectively registered. The study authors confirmed that they did not record live birth and pain |
| Other bias | Low risk | We did not identify any other sources of bias |
| Methods | Randomised controlled trial, 2 groups, set in Outpatient Department, Department of Obstetrics and Gynaecology at All India Institute of Medical Sciences, India March 2014 to March 2015 Number of participants randomised: 92 (recruitment ongoing) Number of participants analysed: 86 (interim analysis) | |
| Participants | Inclusion criteria: women aged between 21 and 37 years with unexplained infertility; no or only mild male factor infertility; bilateral free spill on hysterosalpingography; normal hormone profile, no adnexal mass; body mass index 18.5 ‐ 29.9 kg/m²; euthyroid state with normal thyroid function tests; no associated medical problems like diabetes mellitus, hypertension, heart disease or drug allergies Exclusion criteria: severe male factor infertility; stage III or IV endometriosis; tubal factor infertility; baseline follicle stimulating hormone > 12 IU/L; less than 4 antral follicles per ovary; fibroid uterus; systemic diseases Cause subfertility: unexplained infertility and mild male factor | |
| Interventions |
Both groups: when follicle present with diameter 18 mm given 10,000 IU human chorionic gonadotrophin, then intrauterine insemination (IUI) after 36 to 38 hours. Degree of endometrial injury: Karman’s No. 4 cannula Timing of endometrial injury: day 8 of IUI cycle (scratching was performed in each of 3 IUI cycles) Study length: 3 cycles Type of conception: IUI | |
| Outcomes | Reported in paper
Obtained by author correspondence: an updated interim analysis was provided for the outcomes
| |
| Notes | Funding source: none Conflicts of interest: not reported CTRI/2015/12/006419 (retrospectively registered) Author correspondence undertaken Data included here is from an interim analysis as recruitment is ongoing (86 participants currently in analysis). An earlier interim analysis was reported in the abstract and multiple interim analyses, however this is not a risk of bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized into two groups by computer generated random table" |
| Allocation concealment (selection bias) | High risk | From author correspondence "The doctor has the list of allocations and patients were randomised according to the list". It therefore appears that allocations were not concealed |
| Blinding of participants (performance bias) | High risk | The study authors did not report blinding of participants and was unlikely; and lack of participant blinding is anticipated to introduce performance bias |
| Blinding of personnel (performance bias) | High risk | The study authors did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The study authors did not report any blinding of outcome assessors and it was unlikely; however most outcomes were unlikely to be influenced by lack of blinding. The outcome of self‐reported pain during the procedure may be influenced by knowledge of the intervention, but this outcome was only recorded in the intervention group as no sham procedure was employed |
| Incomplete outcome data (attrition bias) | Low risk | There was 1 participant lost to follow‐up from the intervention group and none from the control group. The study authors recorded all patient outcomes and performed intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | The study authors reported all expected outcomes. As data are from an interim analysis, the live birth information is not yet available. Pain data provided. |
| Other bias | Low risk | We did not identify any other sources of bias |
| Methods | Randomised controlled trial, 2 groups, set in Shiraz University Infertility Clinic, Iran January 2010 to March 2012 Number of participants randomised: 234 Number of participants analysed: 217 | |
| Participants | Inclusion criteria: unexplained infertility: normal ovulatory function, normal uterine cavity, bilateral tubal patency via hysterosalpingography and/or hysterolaparascopy if indicated. Women: between 23 to 35 years of age; infertility duration of 2 to 5 years; body mass index 18 ‐ 25 kg/m²; Anti‐mullerian hormone > 1 µg/L; follicle stimulating hormone < 10 mlU/mL on the 3rd day of the cycle; ≥ 10 to 12 follicles in antral follicle count; only received clomiphene citrate for their infertility during the 3 past months and no previous treatment with gonadotropins or any other interventions for treatment of their infertility. Men: normal semen analysis parameters (as defined by World Health Organisation criteria) Exclusion criteria: other known infertility etiologies such as hormonal disorders, infections, genetic anomalies, immunological problems and abnormal anatomic structures; painters, factory workers; smoking; alcohol abuse Cause of subfertility: unexplained infertility | |
| Interventions | Intervention group: mild endometrial local injury in the posterior wall of the uterus by standard pipelle endometrial sampling during the preovulatory days (the days of detecting urinary luteinising hormone surge) Control group: gynaecological examination using a mock pipelle biopsy without any endometrial manipulation (no entry of pipelle into internal os of cervix) Both groups: optimal superovulation by clomiphene‐citrate and regular timed intercourse (from luteinising hormone positive days until 8 days later every other day) Degree of endometrial injury: pipelle Timing of endometrial injury: follicular phase (days of detecting luteinising hormone surge, of a potential conception cycle) Study length: unclear in the paper. "About 3 menstrual cycles" according to author correspondence Type of conception: regularly timed intercourse The control group was administered a mock procedure which was not intended to cause injury but is likely to have done, and so this may be considered an inappropriate control procedure (pipelle inserted through external but not internal os). | |
| Outcomes | Reported in paper
Obtained by author correspondence
| |
| Notes | Funding source: Infertility Research Center of Shiraz University Conflict of interests: the authors reported none Trial registration number: IRCT2012082510657N1 (retrospective registration). Author correspondence undertaken but incomplete Though the authors report Parsanezhad 2013 and Dadras 2012 to be distinct studies, it is unclear how both were conducted at the same centre, in overlapping time periods and reported by overlapping authors. For this and other reasons, we excluded Dadras 2012 from the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author correspondence: "Allocation proceeded by randomly selecting one of the orderings and assigning the next block of participants to study groups according to the specified sequence". It is unclear how these sequences were generated and whether this was truly random. From author correspondence it appears that data from some participants enrolled at the beginning of the study period may have been removed from analysis to reduce any inter‐investigator discrepancies at the changeover of the study gynaecologists |
| Allocation concealment (selection bias) | High risk | Not reported in the paper. Author correspondence "Since we chose each block size of 2, there were 2 possible ways to equally assign participants to a block (AB or BA)". A block size of 2 means every second allocation is known, therefore this is a high‐risk method |
| Blinding of participants (performance bias) | Unclear risk | Use of a sham procedure (mock pipelle biopsy, insertion of pipelle into external but not internal os) reported in the paper and confirmed in author correspondence. However, there is no mention of a placebo procedure in the trial register and there was no assessment of whether participants were truly blinded by the placebo procedure |
| Blinding of personnel (performance bias) | High risk | The study authors did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The study authors did not report blinding of outcome assessors and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Number of missing outcome data: 17 (3 in the intervention group and 14 in the control group). The reasons for missing outcome data were reported. The proportion of missing outcomes compared with observed event risk was not enough to have a significant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Retrospective registration on Iranian registry of clinical trials. IRCT2012082510657N1. Methods in the registered trial do not entirely match the methods in the full report. However, all expected outcomes are reported. The study authors provided live birth rates and stated that pain was not recorded |
| Other bias | Low risk | We did not identify any other sources of bias |
| Methods | Randomised controlled trial, 3 groups, set in the Department of Obstetrics and Gynaecology at a tertiary care centre, India August 2012 to March 2014 Number of participants randomised: 225 (26 not randomised), total of 251 Number of participants analysed: 251 | |
| Participants | Inclusion criteria: women aged between 18 and 38 years with primary or secondary infertility who were attending the clinic planning stimulated intrauterine insemination (IUI), with either both or 1 patent (functioning) fallopian tube (demonstrated by laprohysteroscopy or hysterosalpingography). Exclusion criteria: known pelvic inflammatory disease with bilateral tubal blockage, severe male factor infertility with intrauterine pathology (submucosal fibroid, endometrial polyp, adhesions), acute vaginal or cervical infection. Cause subfertility: unexplained, mild male factor, tubal factor (unilateral) | |
| Interventions |
All groups: each participant underwent single IUI 36 hours after human chorionic gonadotrophin trigger, or 24 hours later if luteinising hormone surge was positive. Degree of endometrial injury: endometrial aspiration cannula Timing of endometrial injury: in group A injury was during the luteal phase between day 19 to 24 of the preceding spontaneous menstrual cycle, in group B injury was during the follicular phase before day 6 of the same spontaneous menstrual cycle. Endometrial scratching performed in the 1st cycle only. Study length: 1 cycle (the paper reports the pregnancy rates over 3 cycles, but as the number of participants attending for the 2nd and 3rd cycles are unbalanced the study authors provided the data for the first cycle only) Type of conception: IUI | |
| Outcomes | Reported in paper
| |
| Notes | Funding source: no financial support or sponsorship Conflicts of interest: none declared CTRI/2012/12/004356 (retrospectively) Author correspondence undertaken | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random allocation was generated using a random number table". From author correspondence it was discovered that 11 participants in the group A and 15 in B were not randomised, but were allocated to the intervention group to replace participants who dropped out. Therefore 26 participants were not randomly allocated. However the study authors provided the data for the randomised participants only |
| Allocation concealment (selection bias) | High risk | "sealed envelope system was used...allocation was done by the doctor posted in infertility outpatient department". The study authors confirmed the envelopes were not numbered |
| Blinding of participants (performance bias) | High risk | "This study was not blinded." 11 participants from group A, 15 participants from group B, and 0 participants from Group C failed to commence their allocated procedure. Although it was intended for all participants to complete 3 IUI cycles (unless they fell pregnant), only 93 cycles took place in group A, 156 in Group B, 113 Group C (number of cycles in Group C provided by author correspondence). Additionally this gave the Group B more opportunities to conceive and it is possible that this could account for the higher pregnancy rate in Group B. However, the study authors provided the data for the 1st cycle only |
| Blinding of personnel (performance bias) | High risk | The study authors did not report blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessors not reported and unlikely; however outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Intention to treat analysis performed and data available for those who did not attend for IUI. |
| Selective reporting (reporting bias) | Low risk | India No.CTRI/2012/12/004356 Retrospectively registered. The study authors confirmed that they did not record any live birth or pain. |
| Other bias | Low risk | Group B and C were not advised abstinence prior to their IUI cycle, but no pregnancies were reported during this period. |
| Methods | Randomised controlled trial, 2 groups, set in Shiraz University of Medical Sciences Infertility Clinic, Iran January 2011 to May 2012 Number of participants randomised: 146 Number of participants analysed: 144 | |
| Participants | Inclusion criteria: 18 to 40 years old participants who suffered from unexplained infertility, mild male factor and mild endometriosis; all women had normal plasma concentrations of day 3 luteinising hormone and follicle stimulating hormone (FSH); normal tests of renal and hepatic function; normal complete blood counts; normal hysterosalpingogram, laparoscopy and hysteroscopy and negative pregnancy tests. When the endometriosis was diagnosed, the stage was determined according to the revised American society for reproductive medicine classification and the score was recorded. Only those with mild endometriosis were included in the study while those with moderate to severe endometriosis were excluded from the study. Exclusion criteria: hirsutism, autoimmune disorders, endocrinopathies, and ovarian hyperstimulation syndrome, smoked cigarettes, alcohol abuse (either partner) Cause subfertility: unexplained, mild male factor, mild endometriosis | |
| Interventions |
Both groups: Received 100 mg/day of clomiphene citrate between day 5 to 9 of the menstrual cycle, and then 100 U/day of FSH from day 8. When at least one < 18 mm dominant follicle was seen on ultrasonography, 10,000 units of human chorionic gonadotrophin was given intramuscularly if estradiol levels were < 1500 pg/mL. IUI was performed 36 hours after the trigger. Degree of endometrial injury: Novak curette biopsy catheter (considered to be higher degree of injury than pipelle) Timing of endometrial injury: early follicular phase (days 6 to 8 of the menstrual cycle prior to IUI) Study length: 3 cycles of IUI Type of conception: IUI | |
| Outcomes | Reported in paper
| |
| Notes | Funding source: Infertility Research Center of Shiraz University of Medical Sciences, Shiraz, Iran Conflicts of interest: "none" Study registered retrospectively IRCT2012070810210N1 Author correspondence attempted but no useful response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Block randomisation', not further explained |
| Allocation concealment (selection bias) | Unclear risk | 'Block randomisation', not further explained. The same researchers have previously used blocks of 2 for randomisation, which is considered high‐risk as every 2nd allocation would be known in advance and therefore not concealed |
| Blinding of participants (performance bias) | High risk | Not blinded, and lack of participant blinding anticipated to introduce performance bias. Although it was intended for all 146 participants to complete 3 IUI cycles (unless they fell pregnant), only 126 cycles took place in the intervention group and 105 in the control group. Additionally this gave the intervention group more opportunities to conceive and it is possible that this could account for the higher pregnancy rate in this group |
| Blinding of personnel (performance bias) | High risk | The study authors did not report any blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | The study authors did not report blinding of outcome assessors and it was unlikely; however outcomes were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Two participants removed from intervention group due to ovarian hyperstimulation syndrome (OHSS). None lost from control group. |
| Selective reporting (reporting bias) | Unclear risk | It was unclear whether the study authors collected live birth and pain data as author correspondence was not possible. Retrospective registration on Iranian registry of clinical trials IRCT2012070810210N1. |
| Other bias | Low risk | We did not identify any other sources of bias. |
Abbreviations: IUI: intrauterine insemination, FSH: follicle stimulating hormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial | |
| This trial is available as an abstract only and appears to be associated with extensive bias as detailed below, therefore we excluded it.
We contacted the study authors but they did not satisfactorily address the above issues | |
| Author correspondence: the trial was discontinued after only 15 participants were recruited | |
| This study reported biochemical pregnancy rate only, and did not report or record any of the review outcomes. The trial authors confirmed this by correspondence | |
| Intervention is microhysteroscopy, not intentional injury |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria.
|
| Interventions | Intervention group: endometrial injury using a pipelle biopsy catheter on day (5, 6 or 7) of the stimulation cycle combined with the intrauterine insemination (IUI) Control group: stimulation cycle combined with the IUI only |
| Outcomes | Clinical pregnancy rate |
| Notes | NCT02542280 This study is registered as "ongoing, but not recruiting participants" and is therefore awaiting classification. We have been unable to contact the authors. |
| Methods | Unclear if the study is a randomised controlled trial as is described as "Randomized case control study" |
| Participants | "excluding anovulatory and polycystic ovarian syndrome subjects with previous one or more failed intrauterine insemination (IUI) cycles" |
| Interventions | "The study group patients were subjected to endometrial scratch by ‘‘Pipelle’’ on post ovulatory day 6‐8 in the preceding cycle. Both the groups underwent controlled ovarian stimulation with gonadotropins and IUI" |
| Outcomes | "Pregnancy" not further specified, and no actual results reported in the abstract. |
| Notes | Poster presentation from American Society Reproductive Medicine 2015. Author correspondence confirmed the results are soon to be submitted for publication, but they did not supply results. This study is awaiting classification as it is unclear if it was a true randomised controlled trial and no results are available. |
Abbreviations: IUI: intrauterine insemination.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Pipelle for pregnancy (PIP) in couples with subfertility related to unexplained infertility |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: a single endometrial pipelle biopsy performed between days 1 and 12 of a menstrual cycle Control group: a single placebo procedure performed between days 1 and 12 of a menstrual cycle |
| Outcomes | Live birth, miscarriage, ongoing pregnancy, clinical pregnancy, multiple pregnancy, pain during the procedure, bleeding following the procedure |
| Starting date | June 2014 |
| Contact information | Sarah Lensen; [email protected] |
| Notes | ACTRN12614000656639 Confirmed ongoing by author correspondence in December 2015 |
| Trial name or title | Pipelle for pregnancy (PIP) in couples with subfertility related to polycystic ovarian syndrome |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: a single endometrial pipelle biopsy performed between days 1 and 12 of a stimulated cycle (clomiphene, letrozole or metformin) Control group: a single placebo procedure performed between days 1 and 12 of a stimulated cycle (clomiphene, letrozole or metformin) |
| Outcomes | Live birth, miscarriage, ongoing pregnancy, clinical pregnancy, multiple pregnancy, pain during the procedure, bleeding following the procedure |
| Starting date | June 2014 |
| Contact information | Sarah Lensen; [email protected] |
| Notes | ACTRN12614000657628 Confirmed ongoing by author correspondence in December 2015 |
| Trial name or title | Endometrial Scratching During Laproscopic Ovarian Drilling in Subfertile PCOS Women (ESLOD) |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: endometrial Currettage (endometrial scraping) will be performed at the time of laparoscopic ovarian drilling Control group: no endometrial curettage at time of laparoscopic ovarian drilling |
| Outcomes | Live birth, pregnancy and miscarriage |
| Starting date | April 2014 |
| Contact information | Ahmed Gibreel, 00201004045733, [email protected] |
| Notes | Confirmed ongoing by author correspondence in December 2015 |
| Trial name or title | Effect of Local Endometrial Injury on Pregnancy Outcomes During Ovulation Induction Cycles |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: couples who will undergo endometrial sampling in the luteal phase of the cycle preceding ovulation induction (OI) by clomiphene citrate. Timing: from day 15 to day 24 of the cycle preceding OI cycle Group 2: couples who will undergo OI with clomiphene citrate. |
| Outcomes | Clinical pregnancy, miscarriage and multiple pregnancy |
| Starting date | February 2014 |
| Contact information | Mohamed Maher, +966558198655, [email protected] |
| Notes | NCT02345837 Confirmed ongoing by author correspondence in December 2015 |
| Trial name or title | Endometrial Injury versus Luteal Phase Support in Intrauterine Insemination Cycles |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: endometrial biopsy is performed on days 21 to 24 of the spontaneous menstrual cycle preceding the IUI treatment cycle. Two small biopsies are obtained from anterior and posterior walls of the uterus. Group 2: vaginal progesterone gel is administered for luteal phase support from the second day after insemination until pregnancy testing and is continued in the presence of pregnancy until the 12 weeks of pregnancy. Goup 3: participants undergo IUI cycles stimulated with gonadotropin without any intervention. |
| Outcomes | Pregnancy at 12 weeks |
| Starting date | June 2015 |
| Contact information | Selcuk Selcuk, 905321630488, [email protected] |
| Notes | NCT02492451 Confirmed ongoing by author correspondence in December 2015 |
Abbreviations, OI: ovulation induction, HSG: hysterosalpingogram
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 6 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.56, 3.15] |
| Analysis 1.1  Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 1 Live birth or ongoing pregnancy. | ||||
| 1.1 Live birth | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.12, 5.49] |
| 1.2 Ongoing pregnancy | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.46, 3.19] |
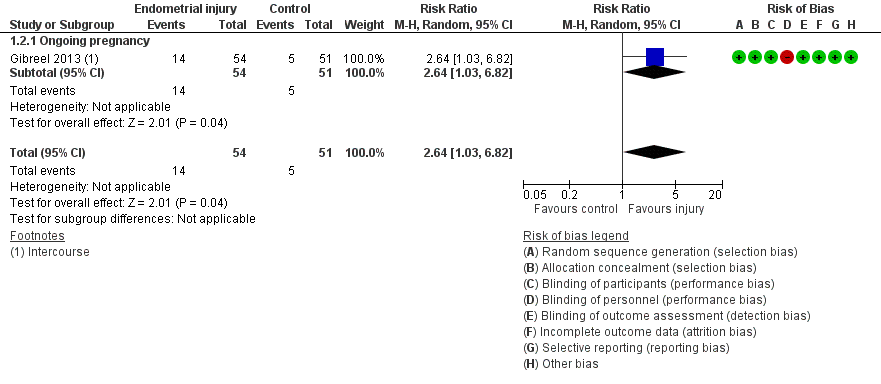
| 2 Live birth or ongoing pregnancy: sensitivity analysis Show forest plot | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| Analysis 1.2 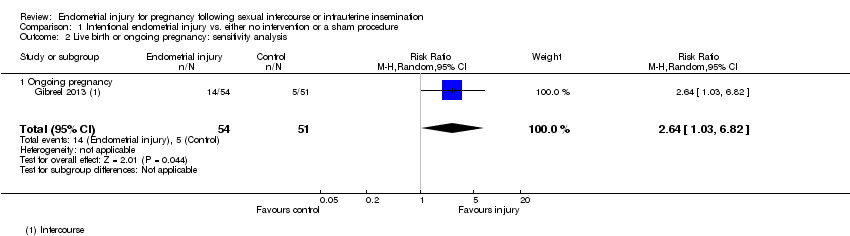 Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 2 Live birth or ongoing pregnancy: sensitivity analysis. | ||||
| 2.1 Ongoing pregnancy | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
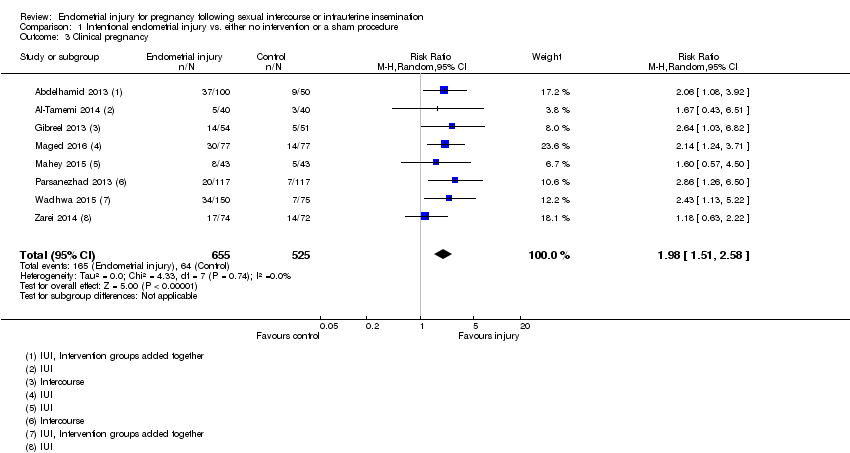
| 3 Clinical pregnancy Show forest plot | 8 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.51, 2.58] |
| Analysis 1.3 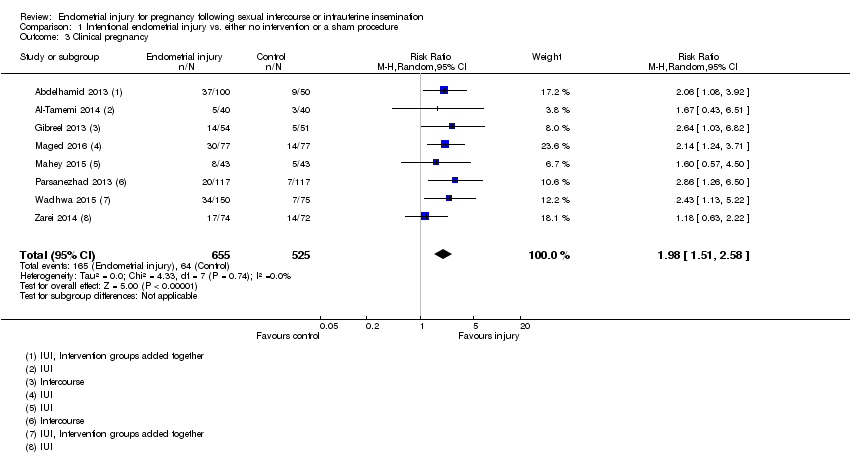 Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 3 Clinical pregnancy. | ||||
| 4 Miscarriage per clinical pregnancy Show forest plot | 6 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.39] |
| Analysis 1.4  Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 4 Miscarriage per clinical pregnancy. | ||||
| 5 Multiple pregnancy per clinical pregnancy Show forest plot | 6 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.31, 2.78] |
| Analysis 1.5  Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 5 Multiple pregnancy per clinical pregnancy. | ||||
| 6 Ectopic pregnancy per clinical pregnancy Show forest plot | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.46] |
| Analysis 1.6  Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 6 Ectopic pregnancy per clinical pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.71, 2.35] |
| Analysis 2.1  Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy. | ||||
| 2 Clinical pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.66, 2.01] |
| Analysis 2.2 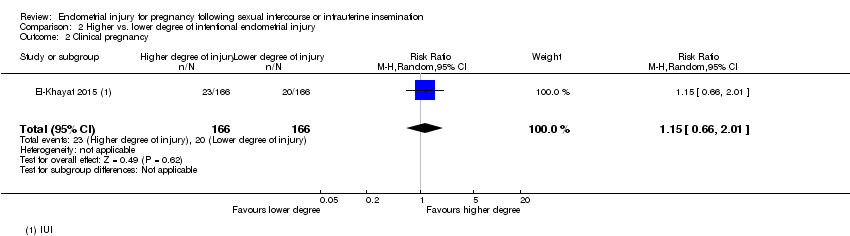 Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 2 Clinical pregnancy. | ||||
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.57] |
| Analysis 2.3 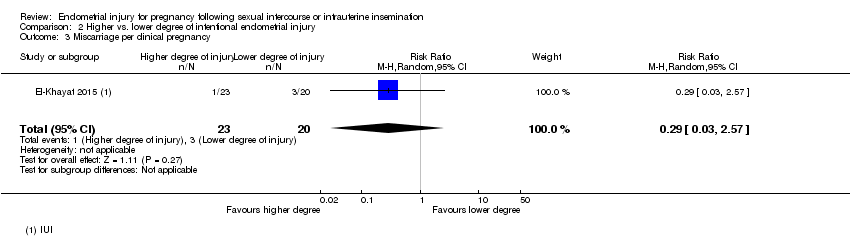 Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy. | ||||
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.20, 3.83] |
| Analysis 2.4 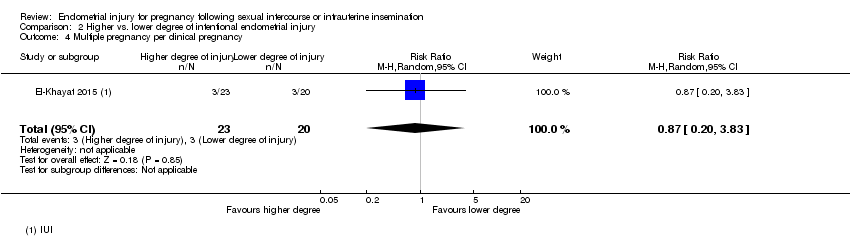 Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.16] |
| Analysis 3.1  Comparison 3 Timing of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy. | ||||
| 2 Clinical pregnancy Show forest plot | 2 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.36] |
| Analysis 3.2 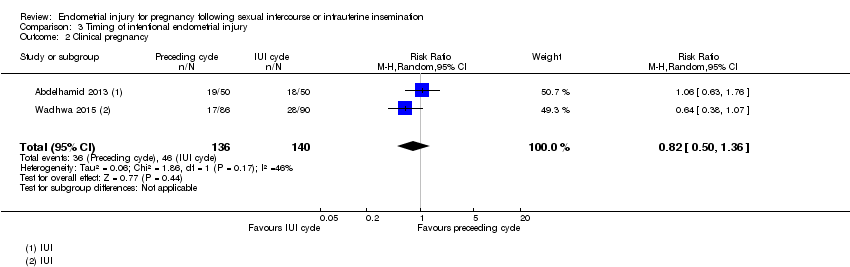 Comparison 3 Timing of intentional endometrial injury, Outcome 2 Clinical pregnancy. | ||||
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.03] |
| Analysis 3.3  Comparison 3 Timing of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy. | ||||
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.04] |
| Analysis 3.4  Comparison 3 Timing of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy. | ||||

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' category for each included study.

Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.1 Live birth or ongoing pregnancy: sensitivity analysis excluding studies at high or unclear risk of allocation concealment.
Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.2 Live birth or ongoing pregnancy: sensitivity analysis.

Forest plot of comparison: 2 Higher vs. lower degree of intentional endometrial injury, outcome: 2.1 Live birth or ongoing pregnancy.

Forest plot of comparison: 3 Timing of intentional endometrial injury, outcome: 3.1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 2 Live birth or ongoing pregnancy: sensitivity analysis.
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 3 Clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 4 Miscarriage per clinical pregnancy.
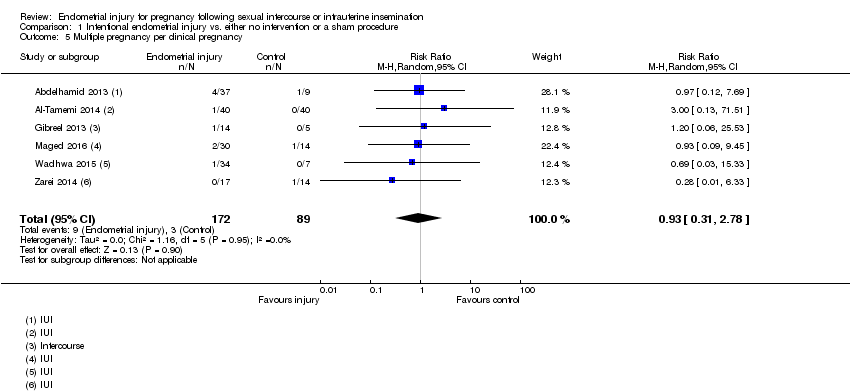
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 5 Multiple pregnancy per clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 6 Ectopic pregnancy per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 2 Clinical pregnancy.
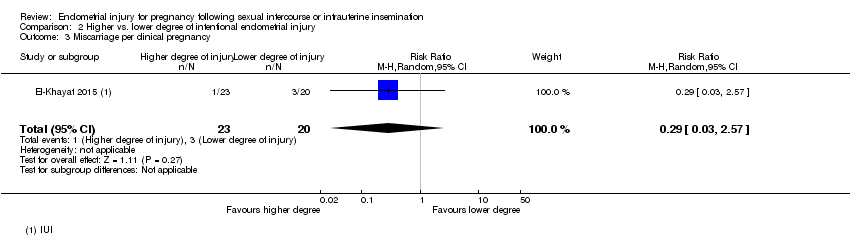
Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.
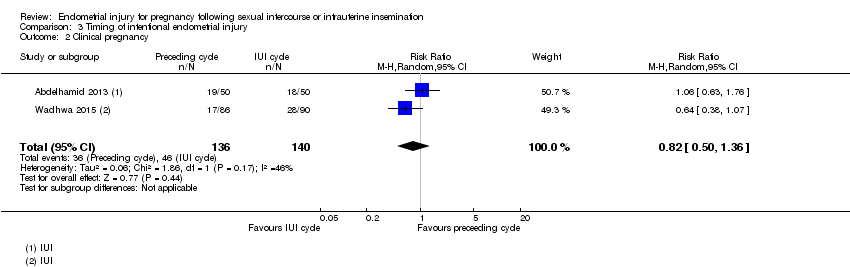
Comparison 3 Timing of intentional endometrial injury, Outcome 2 Clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.
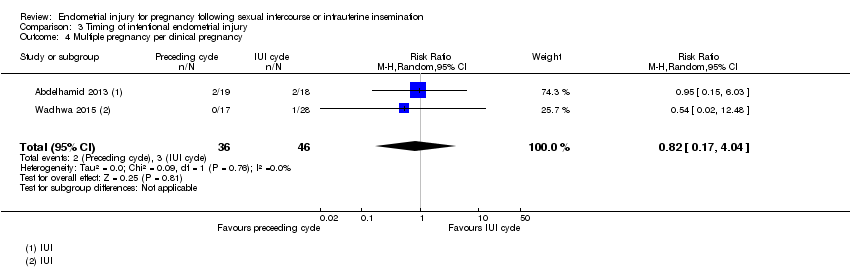
Comparison 3 Timing of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with either: no intervention, or a sham procedure | Risk with Intentional endometrial injury | ||||
| Live birth or ongoing pregnancy | 87 per 1000 | 194 per 1000 | RR 2.22 | 950 | ⊕⊝⊝⊝ |
| Live birth or ongoing pregnancy ‐ sensitivity | 98 per 1000 | 259 per 1000 | RR 2.64 | 105 | ⊕⊝⊝⊝ |
| Pain during the procedure | Pain was not recorded in the control group | Pain was only recorded in the intervention group with an average of 6/10, standard deviation (SD) = 1.5 | — | (1 RCT) | — |
| Clinical pregnancy | 122 per 1000 | 241 per 1,000 | RR 1.98 | 1180 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 2 levels for risk of bias as many of the included studies are associated with a high risk of bias. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with lower degree of intentional endometrial injury | Risk with Higher | ||||
| Live birth or ongoing pregnancy | 102 per 1000 | 132 per 1000 | RR 1.29 | 332 | ⊕⊕⊝⊝ |
| Pain during the procedure | — | — | — | (0 study) | — |
| Clinical pregnancy | 120 per 1000 | 139 per 1000 | RR 1.15 | 332 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for indirectness as there was only 1 included study. Therefore the result was applicable only to cases of hysteroscopy plus injury vs hysteroscopy alone, and not other cases of higher vs. lower injury. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with injury in preceding cycle | Risk with injury in IUI cycle | ||||
| Live birth or ongoing pregnancy | 267 per 1000 | 173 per 1000 | RR 0.65 | 176 | ⊕⊝⊝⊝ |
| Pain during the procedure | — | — | — | (0 RCTs) | — |
| Clinical pregnancy | 329 per 1000 | 269 per 1000 | RR 0.82 | 276 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for risk of bias as both studies were at high risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 6 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.56, 3.15] |
| 1.1 Live birth | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.12, 5.49] |
| 1.2 Ongoing pregnancy | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.46, 3.19] |
| 2 Live birth or ongoing pregnancy: sensitivity analysis Show forest plot | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 2.1 Ongoing pregnancy | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 3 Clinical pregnancy Show forest plot | 8 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.51, 2.58] |
| 4 Miscarriage per clinical pregnancy Show forest plot | 6 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.39] |
| 5 Multiple pregnancy per clinical pregnancy Show forest plot | 6 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.31, 2.78] |
| 6 Ectopic pregnancy per clinical pregnancy Show forest plot | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.71, 2.35] |
| 2 Clinical pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.66, 2.01] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.57] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.20, 3.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.16] |
| 2 Clinical pregnancy Show forest plot | 2 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.36] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.03] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.04] |

