Lesión del endometrio para el embarazo después del coito o la inseminación intrauterina
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011424.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 junio 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Ginecología y fertilidad
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
SL conceived and developed the protocol with input and final approval from all authors.
SL and MM developed the search strategy, searched for trials and selected the included studies.
SL, MM and GT extracted data from the included studies.
SL and GT entered data into RevMan (RevMan 2014), and performed the analysis with guidance from WP and CN.
SL drafted the review in close collaboration with MM and GT.
All review authors helped to interpret the analyses. All review authors read and commented on the draft versions of the review, and approved the final version.
Sources of support
Internal sources
-
University of Auckland, New Zealand.
PhD Scholarship awarded to Sarah Lensen
-
University of Auckland Summer Research Scholarship, New Zealand.
Gabriella Templer was funded by The University of Auckland Summer Research Scholarships programme (Kate Edger Educational Charitable Trust) to enable her contribution to this review.
External sources
-
None, Other.
Declarations of interest
AG is an author of one of the included studies (Gibreel 2013) and has no other known conflicts of interest.
SL and CF are authors of two ongoing studies (ACTRN12614000657628; ACTRN12614000656639). SL has no other known conflicts of interest.
CF is a director/shareholder of a fertility/gynaecology clinic and undertakes private practice within those premises.
WPM has no known conflicts of interest.
CON has no known conflicts of interest.
GT has no known conflicts of interest.
MM has no known conflicts of interest.
AG has no known conflicts of interest.
When a review author was also the author of an included study, they were not involved in the process of appraising the study for inclusion, performing 'Risk of bias' assessments or data extraction.
Acknowledgements
We thank the Cochrane Gynaecology and Fertility Group. In particular, we are grateful to Marian Showell (Information Specialist) for her assistance in developing the search strategies and Vanessa Jordan (New Zealand Cochrane Fellow) for her assistance with methodological aspects of the protocol and review.
We thank the authors of included studies for corresponding with us regarding questions pertaining to this review.
We acknowledge Waleed El‐Khayat for sourcing a copy of one of the included studies from a university library local to him (Al‐Tamemi 2014).
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Oct 24 | Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination | Review | Bich Ngoc Bui, Sarah F Lensen, Ahmed Gibreel, Wellington P Martins, Helen Torrance, Frank J Broekmans | |
| 2021 Mar 18 | Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination | Review | Bich Ngoc Bui, Sarah F Lensen, Ahmed Gibreel, Wellington P Martins, Helen Torrance, Frank J Broekmans | |
| 2016 Jun 14 | Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination | Review | Sarah F Lensen, Marlies Manders, Carolina O Nastri, Ahmed Gibreel, Wellington P Martins, Gabriella E Templer, Cindy Farquhar | |
| 2014 Dec 10 | Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination | Protocol | Sarah F Lensen, Marlies Manders, Carolina O Nastri, Ahmed Gibreel, Wellington P Martins, Cindy Farquhar | |
Differences between protocol and review
We divided the domain of performance bias to more clearly convey the different risks by evaluating blinding of participants and of personnel separately.
Due to the high risk of bias associated with most of the included studies and subsequent low or very low quality of evidence, we conducted a sensitivity analysis excluding studies at high or unclear risk of bias for allocation concealment. We highlighted this analysis in the review to stress the concern around the low or very low quality of evidence, and we also included the analysis in the 'Summary of findings' table.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Abortion, Spontaneous [epidemiology];
- Bias;
- *Coitus;
- Endometrium [*injuries];
- *Fertilization in Vitro;
- Infertility [*therapy];
- Live Birth [*epidemiology];
- Pain [diagnosis, etiology];
- Pain, Procedural [diagnosis, etiology];
- *Pregnancy Rate;
- Randomized Controlled Trials as Topic;
- Reproductive Techniques, Assisted;
Medical Subject Headings Check Words
Adult; Female; Humans; Pregnancy;
PICO

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' category for each included study.

Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.1 Live birth or ongoing pregnancy: sensitivity analysis excluding studies at high or unclear risk of allocation concealment.
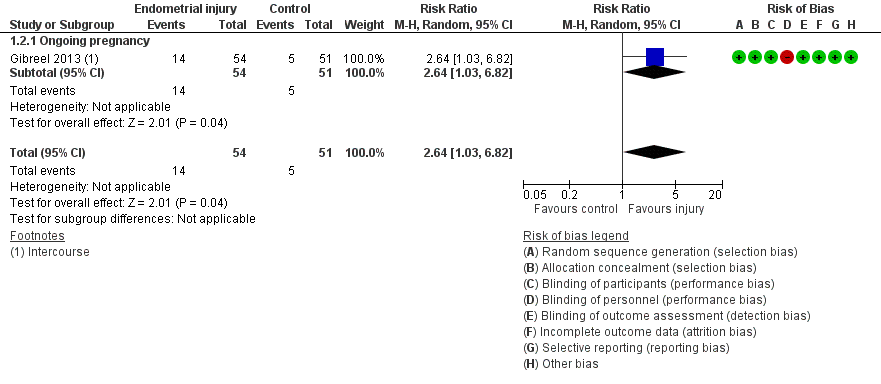
Forest plot of comparison: 1. Intentional endometrial injury vs. either no intervention or a sham procedure, outcome: 1.2 Live birth or ongoing pregnancy: sensitivity analysis.

Forest plot of comparison: 2 Higher vs. lower degree of intentional endometrial injury, outcome: 2.1 Live birth or ongoing pregnancy.

Forest plot of comparison: 3 Timing of intentional endometrial injury, outcome: 3.1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 1 Live birth or ongoing pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 2 Live birth or ongoing pregnancy: sensitivity analysis.
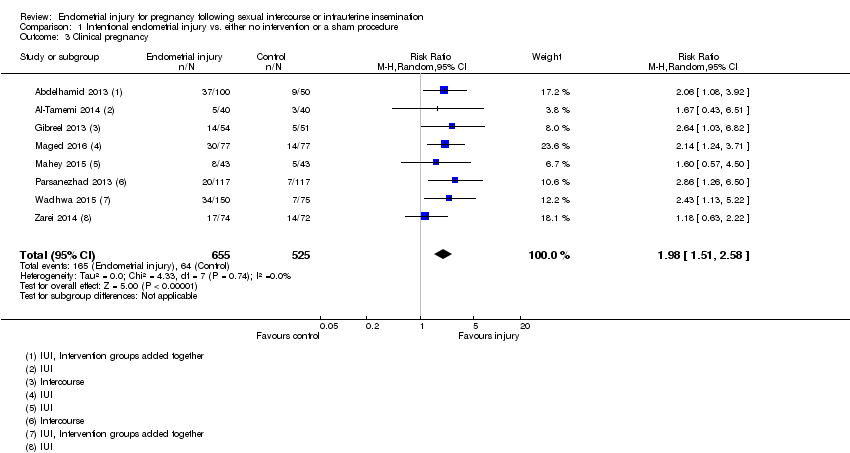
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 3 Clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 4 Miscarriage per clinical pregnancy.
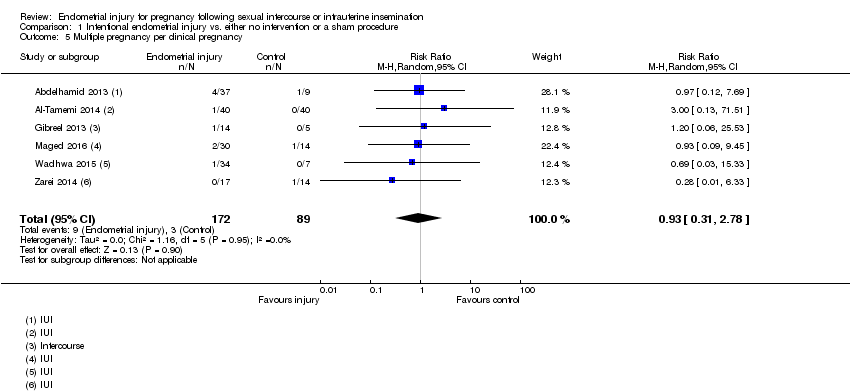
Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 5 Multiple pregnancy per clinical pregnancy.

Comparison 1 Intentional endometrial injury vs. either no intervention or a sham procedure, Outcome 6 Ectopic pregnancy per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 2 Clinical pregnancy.
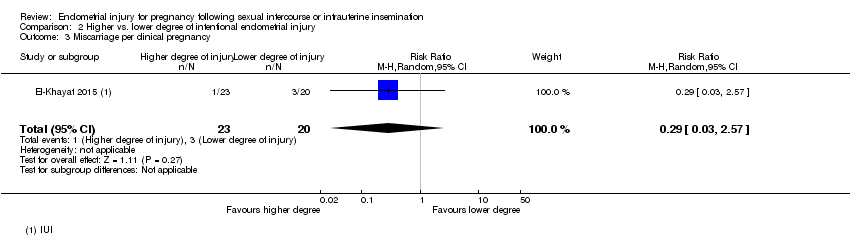
Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.

Comparison 2 Higher vs. lower degree of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 1 Live birth or ongoing pregnancy.
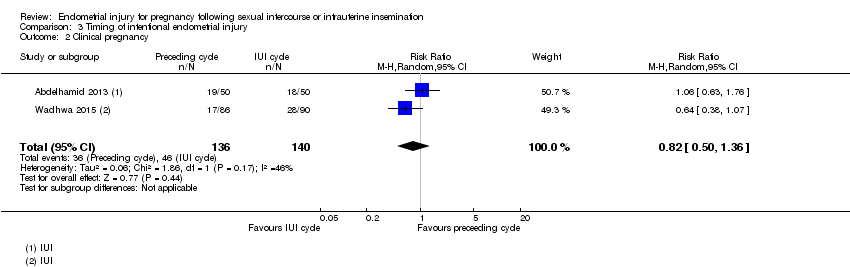
Comparison 3 Timing of intentional endometrial injury, Outcome 2 Clinical pregnancy.

Comparison 3 Timing of intentional endometrial injury, Outcome 3 Miscarriage per clinical pregnancy.
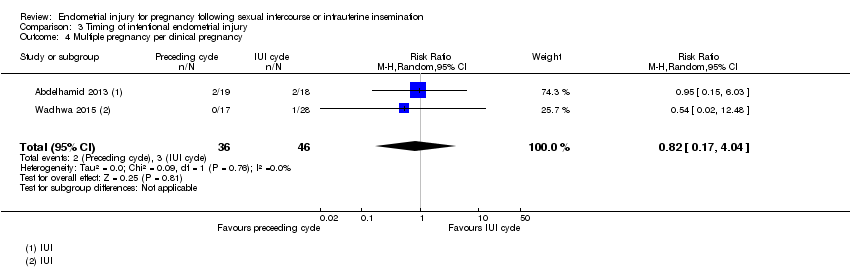
Comparison 3 Timing of intentional endometrial injury, Outcome 4 Multiple pregnancy per clinical pregnancy.
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with either: no intervention, or a sham procedure | Risk with Intentional endometrial injury | ||||
| Live birth or ongoing pregnancy | 87 per 1000 | 194 per 1000 | RR 2.22 | 950 | ⊕⊝⊝⊝ |
| Live birth or ongoing pregnancy ‐ sensitivity | 98 per 1000 | 259 per 1000 | RR 2.64 | 105 | ⊕⊝⊝⊝ |
| Pain during the procedure | Pain was not recorded in the control group | Pain was only recorded in the intervention group with an average of 6/10, standard deviation (SD) = 1.5 | — | (1 RCT) | — |
| Clinical pregnancy | 122 per 1000 | 241 per 1,000 | RR 1.98 | 1180 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 2 levels for risk of bias as many of the included studies are associated with a high risk of bias. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with lower degree of intentional endometrial injury | Risk with Higher | ||||
| Live birth or ongoing pregnancy | 102 per 1000 | 132 per 1000 | RR 1.29 | 332 | ⊕⊕⊝⊝ |
| Pain during the procedure | — | — | — | (0 study) | — |
| Clinical pregnancy | 120 per 1000 | 139 per 1000 | RR 1.15 | 332 | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for indirectness as there was only 1 included study. Therefore the result was applicable only to cases of hysteroscopy plus injury vs hysteroscopy alone, and not other cases of higher vs. lower injury. | |||||
| Patient or population: women trying to get pregnant from intercourse or intrauterine insemination (IUI) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | |
| Risk with injury in preceding cycle | Risk with injury in IUI cycle | ||||
| Live birth or ongoing pregnancy | 267 per 1000 | 173 per 1000 | RR 0.65 | 176 | ⊕⊝⊝⊝ |
| Pain during the procedure | — | — | — | (0 RCTs) | — |
| Clinical pregnancy | 329 per 1000 | 269 per 1000 | RR 0.82 | 276 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% CI) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 level for risk of bias as both studies were at high risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 6 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.56, 3.15] |
| 1.1 Live birth | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.12, 5.49] |
| 1.2 Ongoing pregnancy | 4 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.46, 3.19] |
| 2 Live birth or ongoing pregnancy: sensitivity analysis Show forest plot | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 2.1 Ongoing pregnancy | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.03, 6.82] |
| 3 Clinical pregnancy Show forest plot | 8 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.51, 2.58] |
| 4 Miscarriage per clinical pregnancy Show forest plot | 6 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.39] |
| 5 Multiple pregnancy per clinical pregnancy Show forest plot | 6 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.31, 2.78] |
| 6 Ectopic pregnancy per clinical pregnancy Show forest plot | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.71, 2.35] |
| 2 Clinical pregnancy Show forest plot | 1 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.66, 2.01] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.57] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.20, 3.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth or ongoing pregnancy Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.16] |
| 2 Clinical pregnancy Show forest plot | 2 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.36] |
| 3 Miscarriage per clinical pregnancy Show forest plot | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.03] |
| 4 Multiple pregnancy per clinical pregnancy Show forest plot | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.04] |

