Jedna doza dipirona (metamizola) za akutnu postoperativnu bol u odraslih
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, placebo controlled, single oral dose. Study duration 6 h Self assessment at 0, 0.5, 1.5, and 2 h, then hourly up to 6 h | |
| Participants | Post‐episiotomy N = 100 All F Age: not reported Baseline PI = severe | |
| Interventions | Dipyrone 500 mg, n = 20 Ibuprofen 400 mg, n = 20 Paracetamol 600 mg, n = 20 Aspirin 600 mg, n = 20 Placebo, n = 20 | |
| Outcomes | PI: standard 4‐point scale (0 to 3) PR: non‐standard 4‐point (1 to 4), standard wording, but non‐standard numbering Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method not described |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo controlled, single oral dose. Study duration 6 h Self assessment at 0, 0.5, and 1 h, then hourly up to 6 h | |
| Participants | Dental extraction N = 149 M 84, F 75 Mean age: 27 years Baseline PI = moderate or severe | |
| Interventions | Dipyrone 500 mg, n = 39 Aspirin 650 mg, n = 31 Flurbiprofen 50 mg, n = 40 Placebo, n = 39 | |
| Outcomes | PI: standard 4‐point scale (0 to 3) PR: 5‐point scale (1 to 5) standard wording, but non‐standard numbering PGE: standard 5‐point scale Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2/5 Rescue medication allowed after 1 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method not described |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo controlled, single and multiple dose phases. IM route of administration for dipyrone and oral route for ibuprofen arginine, with double‐dummy placebo. Duration of single dose phase 5 h Self assessment at 0, 0.25, 0.5, and 1 h, then hourly up to 5 h | |
| Participants | Orthopaedic surgery ‐ total hip replacement N = 106 M 48, F 58 Mean age 62 years Baseline PI ≥ 50/100 mm | |
| Interventions | Dipyrone 2000 mg IM, n = 35 Ibuprofen arginine 400 mg oral, n = 36 Placebo, n = 35 | |
| Outcomes | PI: 100 mm VAS (no pain to unbearable pain) PGE: standard 5‐point scale Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Rescue medication allowed after 1 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "Double dummy" design |
| Blinding of outcome assessment (detection bias) | Low risk | "Double dummy" design |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo controlled, single oral dose. Study duration 4 h Self assessment at 0, 0.15, and 1 h, then hourly up to 4 h | |
| Participants | Orthopaedic surgery N = 85 M 57, F 28 Mean age 39 years Baseline PI = moderate or severe | |
| Interventions | Dipyrone 1000 mg, n = 28 Paracetamol 1000 mg, n = 28 Placebo, n = 29 | |
| Outcomes | PI: standard 4‐point scale (0 to 3) PR: standard 5‐point scale (0 to 4) PGE: non‐standard 4‐point scale Use of rescue medication | |
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 Rescue medication allowed after 2 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Capsules of identical appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Capsules of identical appearance |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo controlled, single oral dose. Study duration 6 h Self assessment at 0, 0.25, 0.5, 1.0, and 1.5 h and then hourly up to 6 h | |
| Participants | Post‐episiotomy or 2nd degree vaginal tear N = 108 All F Mean age: 24 years Baseline PI = severe | |
| Interventions | Dipyrone 500 mg, n = 27 Ketoprofen 25 mg, n = 28 Ketoprofen 50 mg, n = 26 Placebo, n = 27 | |
| Outcomes | PI: standard 4‐point scale (0 to 3) PR: standard 5‐point scale (0 to 4) PGE: non‐standard 4‐point scale Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Remedication was allowed after 1 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "individual randomisation envelope for each patient entering the study" |
| Blinding of participants and personnel (performance bias) | Low risk | Observation by different person to one giving treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Observation by different person to one giving treatment |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo controlled, single oral dose. Study duration 4 h Self assessment at 0, 0.5, and 1 h, and then hourly up to 4 h | |
| Participants | Post‐tonsillectomy N = 85 M 33, F 52 Mean age: 23 years Baseline PI = moderate or severe | |
| Interventions | Dipyrone 500 mg, n = 27 Paracetamol 500 mg, n = 29 Placebo, n = 29 | |
| Outcomes | PI: standard 4‐point scale (0 to 3) PR: standard 5‐point scale (0 to 4) PGE: non‐standard 4‐point scale Use of rescue medication Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 Rescue medication allowed after 2 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Tablets identical in appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Tablets identical in appearance |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo controlled, single oral dose. Study duration 4 h Self assessment at 0, 0.5, and 1 h, and then hourly up to 4 h | |
| Participants | Urological surgery N = 90 M 60, F 30 Mean age 49 years Baseline PI = moderate and severe | |
| Interventions | Dipyrone 500 mg, n = 30 Paracetamol 500 mg, n = 30 Placebo, n = 30 | |
| Outcomes | PI: standard 4‐point scale (0 to 3) PR: standard 5‐point scale (0 to 4) PGE: non‐standard 4‐point scale Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Rescue medication allowed after 2 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Capsules of identical appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Capsules of identical appearance |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, placebo controlled, single oral dose. Study duration 4 h Self assessment at 0, 0.5, and 1 h, and then hourly up to 4 h | |
| Participants | Mainly orthopaedic surgery N = 86 M 49, F 37 Mean age: 32 years Baseline PI = moderate and severe | |
| Interventions | Dipyrone 1000 mg, n = 29 Paracetamol 1000 mg, n = 30 Placebo group, n = 27 | |
| Outcomes | PI: standard 4‐point scale (0 to 3) PR: standard 5‐point (0 to 4) PGE: non‐standard 4‐point scale | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method not described |
| Size | High risk | < 50 participants per treatment arm |
DB: double‐blind; F: female; h: hour; IM: intramuscular; M: male; N: number of participants in study; n: number of participants in treatment arm; PGE: Patient Global Evaluation; PI: pain intensity; PR: pain relief; R: randomised; VAS: visual analogue scale; W: withdrawals.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not randomised | |
| No placebo | |
| Included children | |
| Dipyrone given intra‐operatively | |
| Short study duration (3 h) | |
| Administered preoperatively | |
| Number of participants with moderate or severe pain not stated; included mild pain; non‐standard PR scale used | |
| Baseline pain not measured. Non‐standard PI scale. Small size (N = 7) | |
| Multiple doses; no single‐dose data | |
| Non‐standard PI scale | |
| No placebo | |
| Not randomised or blind | |
| Not blind | |
| Non‐standard PI and PR scales | |
| No placebo | |
| Short study duration (30 minutes). Non‐standard PI and PR scales | |
| Short study duration (1 h) | |
| Not randomised or blinded | |
| No placebo | |
| Non‐standard scales | |
| First dose administered preoperatively | |
| No placebo | |
| Did not present hourly pain outcome data | |
| No single dose data | |
| No placebo | |
| Baseline pain not measured. No single dose data | |
| Intraoperative administration, not established pain | |
| No placebo |
h: hour; N: number of participants in study; PI: pain intensity; PR: pain relief.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4 to 6 hours Show forest plot | 5 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.84, 3.11] |
| Analysis 1.1  Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4 to 6 hours. | ||||
| 2 Participants using rescue medication over 4 to 6 hours Show forest plot | 4 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.40] |
| Analysis 1.2 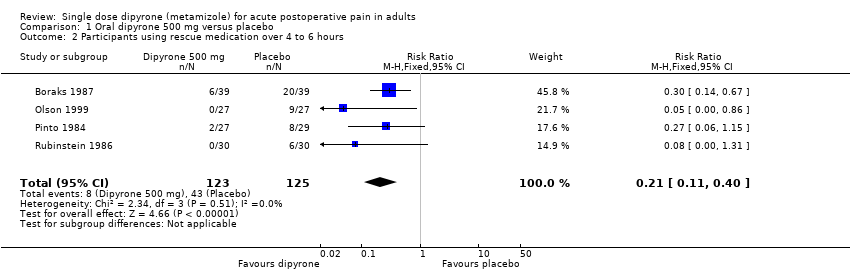 Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 2 Participants using rescue medication over 4 to 6 hours. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
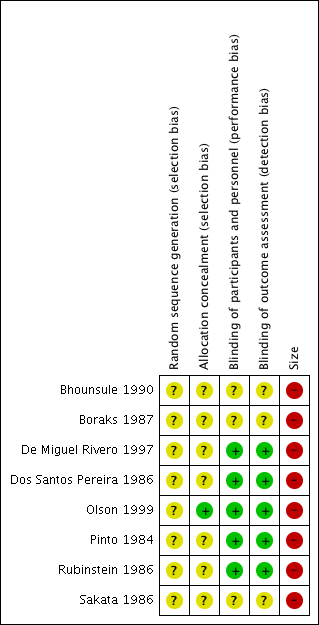
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
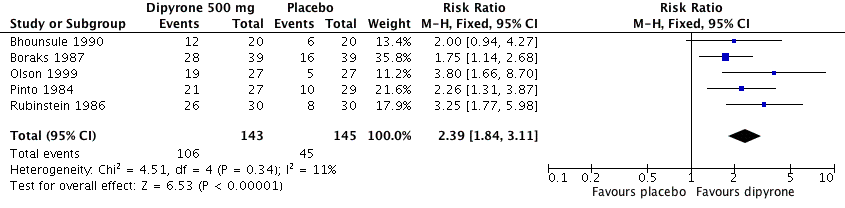
Forest plot of comparison: 1 Oral dipyrone 500 mg versus placebo, outcome: 1.1 Participants with ≥ 50% pain relief over 4 to 6 hours.

Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4 to 6 hours.

Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 2 Participants using rescue medication over 4 to 6 hours.
| Oral dipyrone 500 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with acute postoperative pain Settings: clinic Intervention: oral dipyrone 500 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Relative effect and NNT or NNTp | Number of studies, participants, events | Quality of the evidence | Comments | |
| intervention | comparator | |||||
| At least 50% of maximum pain relief over 4 to 6 h | 730 in 1000 | 320 in 1000 | RR 2.4 (95% CI 1.8 to 3.1) NNT 2.4 (1.9 to 3.1) | 5 studies, 288 participants, 151 events | Moderate | Small studies, few events |
| Participants remedicating within 4 to 6 h | 70 in 1000 | 340 in 1000 | RR 0.21 (0.11 to 0.40) NNTp 3.6 (2.7 to 5.4) | 4 studies, 248 participants, 51 events | Low | Small studies, very few events |
| Participants with at least one adverse event | Insufficient data for analysis | ‐ | ‐ | ‐ | ‐ | |
| Participants with a serious adverse event | None reported | None reported | ‐ | 5 studies, 288 participants, no events | Very low | Small studies, no events |
| CI: confidence interval; h: hour; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4 to 6 hours Show forest plot | 5 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.84, 3.11] |
| 2 Participants using rescue medication over 4 to 6 hours Show forest plot | 4 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.40] |

