Dose unique de dipyrone (métamizole) contre la douleur postopératoire aiguë chez l’adulte
Appendices
Appendix 1. Search strategy for CENTRAL (via CRSO)
-
MESH DESCRIPTOR dipyrone EXPLODE ALL TREES (173)
-
(dipyrone OR metamizol*):TI,AB,KY (558)
-
(adolkin OR afebrin OR aminopyrine sulphonate OR anador OR analgin OR analginum OR ascorfebrina OR baralgin OR dolemicin OR dolo buscopan OR huberdor OR inalgon OR lasain OR lisalgil OR metamizol OR metamizole OR metamizole sodium OR methampyrone OR minalgin OR natrium novaminsulfonicum OR neo meubrina OR neu novalgin OR neu novalgine OR neuro‐brachont OR neuro‐formatin S OR nolotil OR noramidaophenum OR noraminophenazonum OR norgesic OR novalgina OR novalgine OR novamidazofen OR optalgin OR pirenil OR sulpyrinepyrethane OR trisalgina):TI,AB,KY (267)
-
1 OR 2 OR 3 (574)
-
MESH DESCRIPTOR Pain, Postoperative EXPLODE ALL TREES (9224)
-
((postoperative adj4 pain*) OR (post‐operative adj4 pain*) OR post‐operative‐pain* OR (post* adj4 pain*) OR (postoperative adj4 analgesi*) OR (post‐operative adj4 analgesi*) OR ("post‐operative analgesi*")):TI,AB,KY (16726)
-
((post‐surgical adj4 pain*) OR ("post surgical" adj4 pain*) OR (post‐surgery adj4 pain*)):TI,AB,KY (106)
-
(("pain‐relief after surg*") OR ("pain following surg*") OR ("pain control after")):TI,AB,KY (364)
-
(("post surg*" OR post‐surg*) AND (pain* OR discomfort)):TI,AB,KY (381)
-
((pain* adj4 "after surg*") OR (pain* adj4 "after operat*") OR (pain* adj4 "follow* operat*") OR (pain* adj4 "follow* surg*")):TI,AB,KY (762)
-
((analgesi* adj4 "after surg*") OR (analgesi* adj4 "after operat*") OR (analgesi* adj4 "follow* operat*") OR (analgesi* adj4 "follow* surg*")):TI,AB,KY (303)
-
5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 (19610)
-
3 and 12 (315)
Appendix 2. Search strategy for MEDLINE (via Ovid)
-
Dipyrone/ (1257)
-
(dipyrone or metamizol*).mp. (1752)
-
(adolkin or afebrin or aminopyrine sulphonate or anador or analgin or analginum or ascorfebrina or baralgin or dolemicin or dolo buscopan or huberdor or inalgon or lasain or lisalgil or metamizol or metamizole or metamizole sodium or methampyrone or minalgin or natrium novaminsulfonicum or neo meubrina or neu novalgin or neu novalgine or neuro‐brachont or neuro‐formatin S or nolotil or noramidaophenum or noraminophenazonum or norgesic or novalgina or novalgine or novamidazofen or optalgin or pirenil or sulpyrinepyrethane or trisalgina).mp. (902)
-
OR/1‐3 (1882)
-
Pain, postoperative/ (29234)
-
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* adj4 pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or ("post‐operative analgesi*")).mp. (47413)
-
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp. (334)
-
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")).mp. (598)
-
(("post surg*" or post‐surg*) AND (pain* or discomfort)).mp. (1228)
-
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp. (2753)
-
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp. (577)
-
OR/5‐11 (49482)
-
randomized controlled trial.pt. (406912)
-
controlled clinical trial.pt. (91324)
-
randomized.ab. (299530)
-
placebo.ab. (156120)
-
drug therapy.fs. (1820254)
-
randomly.ab. (212212)
-
trial.ab. (311214)
-
groups.ab. (1340408)
-
OR/13‐20 (3429681)
-
4 AND 12 AND 21 (231)
Appendix 3. Search strategy for EMBASE (via Ovid)
-
Dipyrone/ (6920)
-
(dipyrone or metamizol*).mp (7234)
-
(adolkin or afebrin or aminopyrine sulphonate or anador or analgin or analginum or ascorfebrina or baralgin or dolemicin or dolo buscopan or huberdor or inalgon or lasain or lisalgil or metamizol or metamizole or metamizole sodium or methampyrone or minalgin or natrium novaminsulfonicum or neo meubrina or neu novalgin or neu novalgine or neuro‐brachont or neuro‐formatin S or nolotil or noramidaophenum or noraminophenazonum or norgesic or novalgina or novalgine or novamidazofen or optalgin or pirenil or sulpyrinepyrethane or trisalgina).ti,ab (2122)
-
OR/1‐3(7518)
-
Pain, postoperative/ (40435)
-
((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* NEAR pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or ("post‐operative analgesi*")).mp. (78385)
-
((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp. (849)
-
(("pain‐relief after surg*") or ("pain following surg*") or ("pain control after")).mp. (923)
-
(("post surg*" or post‐surg*) AND (pain* or discomfort)).mp. (3234)
-
((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp. (4252)
-
((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp. (826)
-
OR/5‐11 (82424)
-
(random* or factorial* or crossover or "cross over" or cross‐over).tw. (1065325)
-
(placebo* or (doubl* adj blind*) or (singl* adj blind*)).tw. (291579)
-
(allocat* or allocat*).tw. (96761)
-
crossover Procedure/ (43946)
-
double‐blind procedure/ (125003)
-
Randomized Controlled Trial/ (382097)
-
OR/13‐18 (1297615)
-
4 AND 12 AND 19 (442)
Appendix 4. Search strategy for LILACS
dipyrone OR metamizole OR adolkin OR afebrin OR aminopyrine sulphonate OR anador OR analgin OR analginum OR ascorfebrina OR baralgin OR dolemicin OR dolo buscopan OR huberdor OR inalgon OR lasain OR lisalgil OR metamizol OR metamizole OR metamizole sodium OR methampyrone OR minalgin OR natrium novaminsulfonicum OR neo meubrina OR neu novalgin OR neu novalgine OR neuro‐brachont OR neuro‐formatin S OR nolotil OR noramidaophenum OR noraminophenazonum OR norgesic OR novalgina OR novalgine OR novamidazofen OR optalgin OR pirenil OR sulpyrinepyrethane OR trisalgina [Words] and (postoperative AND pain$) OR (post‐operative AND pain$) OR (post‐surgical AND pain$) OR (post AND surgical AND pain$) OR (post‐surgery AND pain$) OR (pain‐relief AND after AND surg$) OR (pain AND following AND surg$) or (pain AND control AND after) OR (pain$ AND after AND surg$) OR (pain$ AND after AND operat$) OR (pain$ AND follow$ AND operat$) OR (pain$ AND follow$ AND surg$) OR
(analgesi$ AND after AND surg$) OR (analgesi$ AND after AND operat$) OR (analgesi$ AND follow$ AND operat$) OR (analgesi$ AND follow$ AND surg$) OR ((post AND surg$ OR post‐surg$) AND (pain$ or discomfort)) [Words] and (( ensaio$ OR ensayo$ OR trial$) AND ( azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego)) OR (( simple$ OR single OR duplo$ OR doble$ OR double$) AND ( cego OR ciego OR blind OR mask )) [Words]
Appendix 5. Glossary
Categorical rating scale
The most common scale used is the five category scale (none, slight, moderate, good or lots, and complete). For analysis, numbers are given to the verbal categories (for pain intensity, none = 0, mild = 1, moderate = 2, and severe = 3, and for relief none = 0, slight = 1, moderate = 2, good or lots = 3, and complete = 4). Data from different participants are then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. Good correlation was found, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
Visual analogue scale (VAS)
For pain intensity, lines with left end labelled 'no pain' and right end labelled 'worst pain imaginable', and for pain relief lines with left end labelled 'no relief of pain' and right end labelled 'complete relief of pain', seem to overcome the limitation of forcing patient descriptors into particular categories. Participants mark the line at the point that corresponds to their pain or pain relief. The scores are obtained by measuring the distance between the no relief end and the person's mark, usually in millimetres. The main advantages of the VAS is that it is simple and quick to score, avoids imprecise descriptive terms, and provides many points from which to choose. More concentration and co‐ordination are needed, which can be difficult postoperatively or with neurological disorders.
Total pain relief (TOTPAR)
TOTPAR is calculated as the sum of pain relief scores over a period of time. If a patient had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the composite trapezoidal rule. This is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
Summed pain intensity difference (SPID)
SPID is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See 'Measuring pain' in Bandolier's Little Book of Pain, Oxford University Press, Oxford. 2003; pp 7‐13 (Moore 2003).
Appendix 6. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning grade of evidence (GRADEpro GDT 2016).
-
High = further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low = any estimate of effect is very uncertain.
We decrease grade if we find:
-
a serious (‐1) or very serious (‐2) limitation to study quality;
-
important inconsistency (‐1);
-
some (‐1) or major (‐2) uncertainty about directness;
-
imprecise or sparse data (‐1);
-
a high probability of reporting bias (‐1).
We increase grade if we find:
-
strong evidence of association ‐ significant relative risk of > 2 (< 0.5) based on consistent evidence from two or more; observational studies, with no plausible confounders (+1);
-
very strong evidence of association ‐ significant relative risk of > 5 (< 0.2) based on direct evidence with no major threats to validity (+2);
-
evidence of a dose response gradient (+1);
-
that all plausible confounders would have reduced the effect (+1).
Appendix 7. Summary of efficacy outcomes in individual studies
| Study ID | Treatment | Analgesia | Rescue medication | ||
| PI or PR | Number with 50% PR | Median time to use (h) | Number using | ||
| (1) dipyrone 500 mg, n = 20 (2) ibuprofen 400 mg, n = 20 (3) paracetamol 600 mg, n = 20 (4) aspirin 600 mg, n = 20 (5) placebo, n = 20 | TOTPAR 6: (1) 13.0 (2) 14.2 (3) 8.1 (4) 6.7 (5) 7.4 | (1) 12/20 (2) 13/20 (3) 7/20 (4) 5/20 (5) 6/20 | Not reported | Not reported | |
| (1) dipyrone 500 mg, n = 39 (2) aspirin 650 mg, n = 41 (3) flurbiprofen 50 mg, n = 40 (4) placebo, n = 39 | ‐ | (1) 28/39 (2) 25/41 (3) 25/40 (4) 16/39 | Not reported | at 6 h: (1) 6/39 (2) 1/41 (3) 4/40 (4) 20/39 | |
| (1) dipyrone 2000 mg, n = 35 (2) ibuprofen arginine 400 mg, n = 36 (3) placebo, n = 35 | VAS SPID 5: (1) 196.8 (2) 187.0 (3) 119.8 | (1) 26/35 (2) 25/36 (3) 16/35 | Not reported | Not reported | |
| (1) dipyrone 500 mg, n = 27 (2) ketoprofen 25 mg, n = 28 (3) ketoprofen 50 mg, n = 26 (4) placebo, n = 27 | TOTPAR 6: (1) 9.6 (2) 9.1 (3) 9.3 (4) 2.8 | (1) 19/27 (2) 19/28 (3) 18/26 (4) 5/27 | (1) > 6 (2) > 6 (3) > 6 (4) 1.3 | at 6 h: (1) 14/67 (2) 20/67 (3) 25/66 (4) 31/39 | |
| (1) dipyrone 500 mg, n = 27 (2) paracetamol 500 mg, n = 29 (3) placebo, n = 29 | TOTPAR 4: (1) 10.7 (2) 11.4 (3) 5.6 | (1) 21/27 (2) 24/29 (3) 10/27 | Not reported | at 4 h: (1) 2/27 (2) 0/29 (3) 8/29 | |
| (1) dipyrone 500 mg, n = 30 (2) paracetamol 500 mg, n = 30 (3) placebo, n = 30 | TOTPAR 4: (1) 11.9 (2) 10.1 (3) 4.7 | (1) 26/30 (2) 22/30 (3) 8/30 | Not reported | at 4 h: (1) 0/30 (2) 2/30 (3) 6/30 | |
| (1) dipyrone 1000 mg, n = 29 (2) paracetamol 1000 mg, n = 30 (3) placebo, n = 27 | TOTPAR 4: (1) 8.7 (2) 8.4 (3) 2.8 | (1) 18/29 (2) 17/30 (3) 3/27 | Not reported | Not reported | |
| (1) dipyrone 1000 mg, n = 28 (2) paracetamol 1000 mg, n = 28 (3) placebo, n = 29 | SPID 4: (1) 6.2 (2) 6.4 (3) 2.1 | (1) 22/28 (2) 21/28 (3) 7/29 | Not reported | at 4 h: (1) 1/28 (2) 0/28 (3) 11/29 | |
| h: hour; n: number of participants in treatment arm; PI: pain intensity; PR: pain relief; SPID: summed pain intensity difference; TOTPAR: total pain relief; VAS: visual analogue scale. | |||||
Appendix 8. Summary of adverse events and withdrawals
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other |
| (1) dipyrone 500 mg, n = 20 (2) ibuprofen 400 mg, n = 20 (3) paracetamol 600 mg, n = 20 (4) aspirin 600 mg, n = 20 (5) placebo, n = 20 | None | None | None | Not reported | |
| (1) dipyrone 500 mg, n = 39 (2) aspirin 650 mg, n = 41 (3) flurbiprofen 50 mg, n = 40 (4) placebo, n = 39 | (1) 4/39 (somnolence, dizziness, nausea, headache) (2) 6/41 (3) 7/40 (4) 8/39 (somnolence, dizziness, nausea, warm feeling) | Not reported | None reported | Not reported | |
| (1) dipyrone 2000 mg, n = 35 (2) ibuprofen arginine 400 mg, n = 36 (3) placebo, n = 35 | (1) 0/35 (2) 1/36 (3) 1/35 (headache) | None | None | None | |
| (1) dipyrone 500 mg, n = 27 (2) ketoprofen 25 mg, n = 28 (3) ketoprofen 50 mg, n = 26 (4) placebo, n = 27 | "No adverse events reported during this study" | None | None | None | |
| (1) dipyrone 500 mg, n = 27 (2) paracetamol 500 mg, n = 29 (3) placebo, n = 29 | (1) 1/27 (arterial hypertension) | None | None | Not reported | |
| (1) dipyrone 500 mg, n = 30 (2) paracetamol 500 mg, n = 30 (3) placebo, n = 30 | (2) 1/30 (vomiting) | None | None | None | |
| (1) dipyrone 1000 mg, n = 29 (2) paracetamol 1000 mg, n = 30 (3) placebo, n = 27 | Not reported | Not reported | Not reported | Not reported | |
| (1) dipyrone 1000 mg, n = 28 (2) paracetamol 1000 mg, n = 28 (3) placebo, n = 29 | "well tolerated" | Not reported | Not reported | Not reported | |
| n: number of participants in treatment arm. | |||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
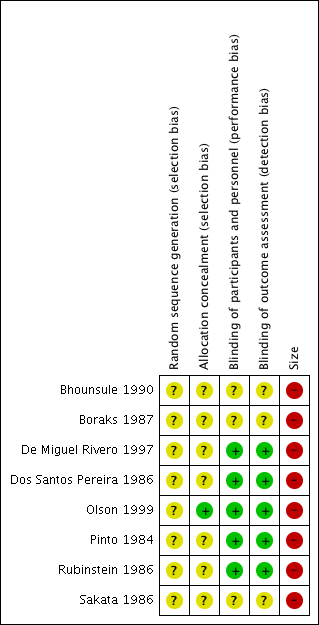
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
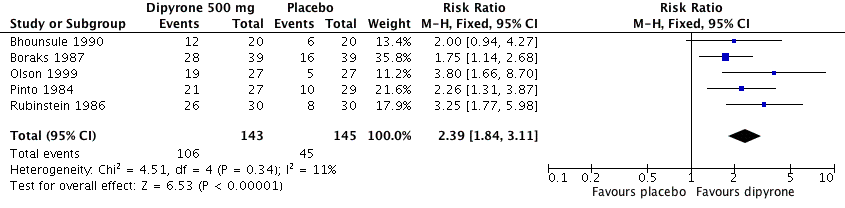
Forest plot of comparison: 1 Oral dipyrone 500 mg versus placebo, outcome: 1.1 Participants with ≥ 50% pain relief over 4 to 6 hours.

Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4 to 6 hours.

Comparison 1 Oral dipyrone 500 mg versus placebo, Outcome 2 Participants using rescue medication over 4 to 6 hours.
| Oral dipyrone 500 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with acute postoperative pain Settings: clinic Intervention: oral dipyrone 500 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Relative effect and NNT or NNTp | Number of studies, participants, events | Quality of the evidence | Comments | |
| intervention | comparator | |||||
| At least 50% of maximum pain relief over 4 to 6 h | 730 in 1000 | 320 in 1000 | RR 2.4 (95% CI 1.8 to 3.1) NNT 2.4 (1.9 to 3.1) | 5 studies, 288 participants, 151 events | Moderate | Small studies, few events |
| Participants remedicating within 4 to 6 h | 70 in 1000 | 340 in 1000 | RR 0.21 (0.11 to 0.40) NNTp 3.6 (2.7 to 5.4) | 4 studies, 248 participants, 51 events | Low | Small studies, very few events |
| Participants with at least one adverse event | Insufficient data for analysis | ‐ | ‐ | ‐ | ‐ | |
| Participants with a serious adverse event | None reported | None reported | ‐ | 5 studies, 288 participants, no events | Very low | Small studies, no events |
| CI: confidence interval; h: hour; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4 to 6 hours Show forest plot | 5 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.84, 3.11] |
| 2 Participants using rescue medication over 4 to 6 hours Show forest plot | 4 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.40] |

