Prueba de detección cognitiva breve (Mini‐Cog) para el diagnóstico de la demencia de la enfermedad de Alzheimer y otras demencias en ámbitos de atención primaria
Appendices
Appendix 1. Appendix 1: Electronic database search strategy
| Source | Search strategy | Hits retrieved |
| ALOIS DTA (Cochrane Dementia and Cognitive Improvement Specialized Register) (see below for detailed explanation of what is contained within the ALOIS register) | Mini‐cog | September 2012: 19 January 2013: 0 |
| 1. MEDLINE In‐Process and other non‐indexed citations and MEDLINE 1950 to present (January 2013) (Ovid SP) | 1. "mini‐Cog".ti,ab. 2. minicog.ti,ab. 3. (MCE and (cognit* OR dement* OR screen* OR Alzheimer*)).ti,ab. 3. or/1‐3 | September 2012: 91 January 2013: 12 |
| 2. Embase 1974‐2013 January 02 (OvidSP) | 1. "mini‐cog*".mp. 2. minicog*.mp. 3. 1 or 2 | September 2012: 96 January 2013: 37 |
| 3. PsycINFO 1806 to January week 1 2013 (OvidSP) | 1. minicog*.mp. 2. "mini‐cog*".mp. 3. 1 or 2 | September 2012: 69 January 2013: 28 |
| 4.Biosis previews 1926 to present (January 2013) (ISI Web of Knowledge) | Topic=("mini‐cog*" OR "minicog*") Timespan=All Years. Databases=BIOSIS Previews. Lemmatization=On | September 2012: 33 January 2013: 7 |
| 5.Web of Science and conference proceedings (1945 to January 2013) | Topic=("mini‐cog*" OR "minicog*") Timespan=All Years. Databases=BIOSIS Previews. Lemmatization=On | September 2012: 93 January 2013: 20 |
| 6. LILACS (BIREME) (January 2013) | "mini‐cog" OR minicog [Words] | September 2012: 2 January 2013: 2 |
| Total before deduplication | September 2012: 403 January 2013: 106 | |
| Total after deduplication and first assessment | September 2012: 108 January 2013: 41 | |
In addition to the above single concept search based on the Index test, Cochrane Dementia and Cognitive Improvement ran a more complex, multi‐concept search each month primarily for the identification of diagnostic test accuracy studies of neuropsychological tests. Where possible they obtained the full texts of the studies identified. This approach is expected to help identify those papers where the index test of interest (in this case Mini‐Cog) is used and the paper contains usable data but where Mini‐Cog was not alluded to in the report's citation.
The MEDLINE strategy used is below. Similar strategies are also run in Embase and PsycINFO.
The Mini‐Cog search utilized only one search concept: the index test (Mini‐Cog):
1. "mini‐Cog".ti,ab.
2. minicog.ti,ab.
3. (MCE and (cognit* OR dement* OR screen* OR Alzheimer*)).ti,ab.
4. or/1‐3
The MEDLINE generic search run for the Cochrane Dementia and Cognitive Improvement DTA register:
1. "word recall".ti,ab.
2. ("7‐minute screen" OR “seven‐minute screen”).ti,ab.
3. ("6 item cognitive impairment test" OR “six‐item cognitive impairment test”).ti,ab.
4. "6 CIT".ti,ab.
5. "AB cognitive screen".ti,ab.
6. "abbreviated mental test".ti,ab.
7. "ADAS‐cog".ti,ab.
8. AD8.ti,ab.
9. "inform* interview".ti,ab.
10. "animal fluency test".ti,ab.
11. "brief alzheimer* screen".ti,ab.
12. "brief cognitive scale".ti,ab.
13. "clinical dementia rating scale".ti,ab.
14. "clinical dementia test".ti,ab.
15. "community screening interview for dementia".ti,ab.
16. "cognitive abilities screening instrument".ti,ab.
17. "cognitive assessment screening test".ti,ab.
18. "cognitive capacity screening examination".ti,ab.
19. "clock drawing test".ti,ab.
20. "deterioration cognitive observee".ti,ab.
21. ("Dem Tect" OR DemTect).ti,ab.
22. "object memory evaluation".ti,ab.
23. "IQCODE".ti,ab.
24. "mattis dementia rating scale".ti,ab.
25. "memory impairment screen".ti,ab.
26. "minnesota cognitive acuity screen".ti,ab.
27. "mini‐cog".ti,ab.
28. "mini‐mental state exam*".ti,ab.
29. "mmse".ti,ab.
30. "modified mini‐mental state exam".ti,ab.
31. "3MS".ti,ab.
32. “neurobehavio?ral cognitive status exam*”.ti,ab.
33. "cognistat".ti,ab.
34. "quick cognitive screening test".ti,ab.
35. "QCST".ti,ab.
36. "rapid dementia screening test".ti,ab.
37. "RDST".ti,ab.
38. "repeatable battery for the assessment of neuropsychological status".ti,ab.
39. "RBANS".ti,ab.
40. "rowland universal dementia assessment scale".ti,ab.
41. "rudas".ti,ab.
42. "self‐administered gerocognitive exam*".ti,ab.
43. ("self‐administered" and "SAGE").ti,ab.
44. "self‐administered computerized screening test for dementia".ti,ab.
45. "short and sweet screening instrument".ti,ab.
46. "sassi".ti,ab.
47. "short cognitive performance test".ti,ab.
48. "syndrome kurztest".ti,ab.
49. ("six item screener" OR “6‐item screener”).ti,ab.
50. "short memory questionnaire".ti,ab.
51. ("short memory questionnaire" and "SMQ").ti,ab.
52. "short orientation memory concentration test".ti,ab.
53. "s‐omc".ti,ab.
54. "short blessed test".ti,ab.
55. "short portable mental status questionnaire".ti,ab.
56. "spmsq".ti,ab.
57. "short test of mental status".ti,ab.
58. "telephone interview of cognitive status modified".ti,ab.
59. "tics‐m".ti,ab.
60. "trail making test".ti,ab.
61. "verbal fluency categories".ti,ab.
62. "WORLD test".ti,ab.
63. "general practitioner assessment of cognition".ti,ab.
64. "GPCOG".ti,ab.
65. "Hopkins verbal learning test".ti,ab.
66. "HVLT".ti,ab.
67. "time and change test".ti,ab.
68. "modified world test".ti,ab.
69. "symptoms of dementia screener".ti,ab.
70. "dementia questionnaire".ti,ab.
71. "7MS".ti,ab.
72. ("concord informant dementia scale" or CIDS).ti,ab.
73. (SAPH or "dementia screening and perceived harm*").ti,ab.
74. or/1‐73
75. exp Dementia/
76. Delirium, Dementia, Amnestic, Cognitive Disorders/
77. dement*.ti,ab.
78. alzheimer*.ti,ab.
79. AD.ti,ab.
80. ("lewy bod*" or DLB or LBD or FTD or FTLD or “frontotemporal lobar degeneration” or “frontaltemporal dement*).ti,ab.
81. "cognit* impair*".ti,ab.
82. (cognit* adj4 (disorder* or declin* or fail* or function* or degenerat* or deteriorat*)).ti,ab.
83. (memory adj3 (complain* or declin* or function* or disorder*)).ti,ab.
84. or/75‐83
85. exp "sensitivity and specificity"/
86. "reproducibility of results"/
87. (predict* adj3 (dement* or AD or alzheimer*)).ti,ab.
88. (identif* adj3 (dement* or AD or alzheimer*)).ti,ab.
89. (discriminat* adj3 (dement* or AD or alzheimer*)).ti,ab.
90. (distinguish* adj3 (dement* or AD or alzheimer*)).ti,ab.
91. (differenti* adj3 (dement* or AD or alzheimer*)).ti,ab.
92. diagnos*.ti.
93. di.fs.
94. sensitivit*.ab.
95. specificit*.ab.
96. (ROC or "receiver operat*").ab.
97. Area under curve/
98. ("Area under curve" or AUC).ab.
99. (detect* adj3 (dement* or AD or alzheimer*)).ti,ab.
100. sROC.ab.
101. accura*.ti,ab.
102. (likelihood adj3 (ratio* or function*)).ab.
103. (conver* adj3 (dement* or AD or alzheimer*)).ti,ab.
104. ((true or false) adj3 (positive* or negative*)).ab.
105. ((positive* or negative* or false or true) adj3 rate*).ti,ab.
106. or/85‐105
107. exp dementia/di
108. Cognition Disorders/di [Diagnosis]
109. Memory Disorders/di
110. or/107‐109
111. *Neuropsychological Tests/
112. *Questionnaires/
113. Geriatric Assessment/mt
114. *Geriatric Assessment/
115. Neuropsychological Tests/mt, st
116. "neuropsychological test*".ti,ab.
117. (neuropsychological adj (assess* or evaluat* or test*)).ti,ab.
118. (neuropsychological adj (assess* or evaluat* or test* or exam* or battery)).ti,ab.
119. Self report/
120. self‐assessment/ or diagnostic self evaluation/
121. Mass Screening/
122. early diagnosis/
123. or/111‐122
124. 74 or 123
125. 110 and 124
126. 74 or 123
127. 84 and 106 and 126
128. 74 and 106
129. 125 or 127 or 128
130. exp Animals/ not Humans.sh.
131. 129 not 130
Appendix 2. Appendix 2: QUADAS‐2
| Domain | Patient selection | Index test | Reference standard | Flow and timing |
| Description | Describe methods of patient selection: describe included patients (prior testing, presentation, intended use of index test and setting) | Describe the index test and how it was conducted and interpreted | Describe the reference standard and how it was conducted and interpreted | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 x 2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard |
| Signalling questions (yes, no, unclear) | Was a consecutive or random sample of patients enrolled? Was a case‐control design avoided? Did the study avoid inappropriate exclusions? | Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it pre‐specified? | Is the reference standard likely to correctly classify the target condition? Were the reference standard results interpreted without knowledge of the results of the index test? | Was there an appropriate interval between index test(s) and reference standard? Did all patients receive the same reference standard? Were all patients included in the analysis? |
| Risk of bias: (high, low, unclear) | Could the selection of patients have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the patient flow have introduced bias? |
| Concerns regarding applicability: (high, low, unclear) | Are there concerns that the included patients do not match the review question? | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? | — |
Anchoring statements to assist with assessment of risk of bias
Domain 1: patient selection
Risk of bias: could the selection of patients have introduced bias? (high, low, unclear)
Was a consecutive or random sample of patients enrolled?
Where sampling is used, the methods least likely to cause bias are consecutive sampling or random sampling, which should be stated and/or described. Non‐random sampling or sampling based on volunteers is more likely to be at high risk of bias.
Weighting: high risk of bias
Was a case‐control design avoided?
Case‐control study designs have a high risk of bias, but sometimes they are the only studies available especially if the index test is expensive and/or invasive. Nested case‐control designs (systematically selected from a defined population cohort) are less prone to bias but they will still narrow the spectrum of patients that receive the index test. Study designs (both cohort and case‐control) that may also increase bias are those designs where the study team deliberately increase or decrease the proportion of participants with the target condition, for example a population study may be enriched with extra dementia participants from a secondary care setting.
Weighting: high risk of bias
Did the study avoid inappropriate exclusions?
We will automatically grade the study as unclear if exclusions are not detailed (pending contact with study authors). Where exclusions are detailed, we will grade the study as 'low risk' if exclusions are felt to be appropriate by the review authors. Certain exclusions common to many studies of dementia are: medical instability; terminal disease; alcohol/substance misuse; concomitant psychiatric diagnosis; other neurodegenerative condition. However if 'difficult to diagnose' groups are excluded this may introduce bias, so exclusion criteria must be justified. For a community sample we would expect relatively few exclusions. We will label post hoc exclusions 'high risk' of bias.
Weighting: high risk of bias
Applicability: are there concerns that the included patients do not match the review question? (high, low, unclear)
The included patients should match the intended population as described in the review question. If not already specified in the review inclusion criteria, setting will be particularly important – the review authors should consider population in terms of symptoms; pre‐testing; potential disease prevalence. We will classify studies that use very selected participants or subgroups as having low applicability, unless they are intended to represent a defined target population, for example, people with memory problems referred to a specialist and investigated by lumbar puncture.
Domain 2: index test
Risk of bias: could the conduct or interpretation of the index test have introduced bias? (high, low, unclear)
Were the index test results interpreted without knowledge of the reference standard?
Terms such as 'blinded' or 'independently and without knowledge of' are sufficient and full details of the blinding procedure are not required. This item may be scored as 'low risk' if explicitly described or if there is a clear temporal pattern to the order of testing that precludes the need for formal blinding, i.e. all (neuropsychological test) assessments were performed before the dementia assessment. As most neuropsychological tests are administered by a third party, knowledge of dementia diagnosis may influence their ratings; tests that are self administered, for example using a computerized version, may have less risk of bias.
Weighting: high risk of bias
Were the index test thresholds pre‐specified?
For neuropsychological scales there is usually a threshold above which participants are classified as 'test positive'; this may be referred to as threshold, clinical cut‐off or dichotomiation point. Different thresholds are used in different populations. A study is classified as at higher risk of bias if the authors define the optimal cut‐off post hoc based on their own study data. Certain papers may use an alternative methodology for analysis that does not use thresholds and these papers should be classified as not applicable.
Weighting: low risk of bias
Were sufficient data on (neuropsychological test) application given for the test to be repeated in an independent study?
Particular points of interest include method of administration (for example self completed questionnaire versus direct questioning interview); nature of informant; language of assessment. If a novel form of the index test is used, for example a translated questionnaire, details of the scale should be included and a reference given to an appropriate descriptive text, and there should be evidence of validation.
Weighting: low risk of bias
Applicability: are there concerns that the index test, its conduct, or interpretation differ from the review question? (high, low, unclear)
Variations in the length, structure, language, and/or administration of the index test may all affect applicability if they vary from those specified in the review question.
Domain 3: reference standard
Risk of bias: could the reference standard, its conduct, or its interpretation have introduced bias? (high, low, unclear)
Is the reference standard likely to correctly classify the target condition?
Commonly used international criteria to assist with clinical diagnosis of dementia include those detailed in DSM‐IV and ICD‐10. Criteria specific to dementia subtypes include but are not limited to NINCDS‐ADRDA criteria for Alzheimer's dementia; McKeith criteria for Lewy Body dementia; Lund criteria for frontotemporal dementias; and the NINDS‐AIREN criteria for vascular dementia. Where the criteria used for assessment are not familiar to the review authors and Cochrane Dementia and Cognitive Improvement, this item should be classified as 'high risk of bias'.
Weighting: high risk of bias
Were the reference standard results interpreted without knowledge of the results of the index test?
Terms such as 'blinded' or 'independent' are sufficient and full details of the blinding procedure are not required. This may be scored as 'low risk' if explicitly described or if there is a clear temporal pattern to order of testing, i.e. all dementia assessments performed before (neuropsychological test) testing.
Informant rating scales and direct cognitive tests present certain problems. It is accepted that informant interview and cognitive testing is a usual component of clinical assessment for dementia, however specific use of the scale under review in the clinical dementia assessment should be scored as high risk of bias.
Weighting: high risk of bias
Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study?
Particular points of interest for dementia assessment include the training/expertise of the assessor, whether additional information was available to inform the diagnosis (for example neuroimaging, other neuropsychological test results), and whether this was available for all participants.
Weighting: variable risk, but high risk if method of dementia assessment not described
Applicability: are there concerns that the target condition as defined by the reference standard does not match the review question? (high, low, unclear)
There is the possibility that some methods of dementia assessment, although valid, may diagnose a far smaller or larger proportion of participants with disease than in usual clinical practice. For example, currently the reference standard for vascular dementia may under‐diagnose compared to usual clinical practice. In this instance the item should be rated as having poor applicability.
Domain 4: patient flow and timing
Risk of bias: could the patient flow have introduced bias? (high, low, unclear)
Was there an appropriate interval between the index test and reference standard?
For a cross‐sectional study design, there is potential for the subject to change between assessments, however dementia is a slowly progressive disease, which is not reversible. The ideal scenario would be a same‐day assessment, but longer periods of time (for example, several weeks or months) are unlikely to lead to a high risk of bias. For delayed‐verification studies the index and reference tests are necessarily separated in time given the nature of the condition.
Weighting: low risk of bias
Did all participants receive the same reference standard?
There may be scenarios where participants who score 'test positive' on the index test have a more detailed assessment for the target condition. Where dementia assessment (or reference standard) differs between participants this should be classified as high risk of bias.
Weighting: high risk of bias
Were all participants included in the final analysis?
Attrition will vary with study design. Delayed verification studies will have higher attrition than cross‐sectional studies due to mortality, and it is likely to be greater in participants with the target condition. Dropouts (and missing data) should be accounted for. Attrition that is higher than expected (compared to other similar studies) should be treated as a high risk of bias. We have defined a cut‐off of greater than 20% attrition as being high risk but this will be highly dependent on the length of follow‐up in individual studies.
Weighting: high risk of bias

Study flow diagram
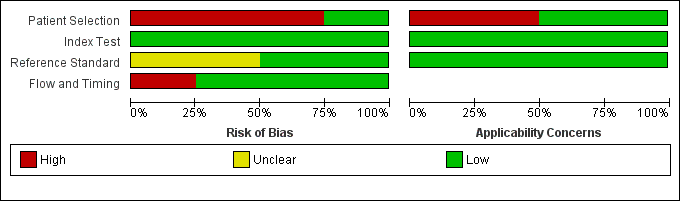
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
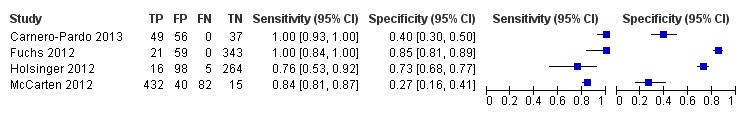
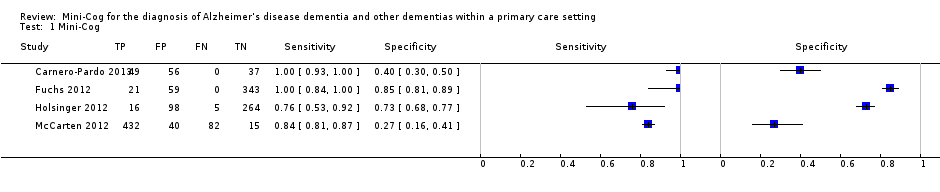
Forest plot of Analysis 1 Mini‐Cog
| Mini‐Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a primary care setting | ||||
| Population | The study populations were sampled from participants identified in primary care settings. | |||
| Setting | The primary care setting was identified as representing a sample that would be presenting to primary care settings where the Mini‐Cog might be used as a screening test to identify individuals who may benefit from additional evaluation. Studies that identified individuals in primary care where they received both the index test and a reference standard were used. | |||
| Indext test | The Mini‐Cog performed in insolation or scored based on results on the clock drawing test or three‐word recall were included. | |||
| Reference Standard | Clinical diagnosis of dementia was made using recognized standard diagnostic criteria. | |||
| Studies | Cross‐sectional studies were included, case control studies were excluded | |||
| Study | Accuracy (95% CI) | Number of participants | Dementia prevalence | Implications |
| Sensitivity: 1.00 (0.93 to 1.00) Specificity: 0.40 (0.30 to 0.50) | 142 | 34.5% | Participants were sampled including individuals who did have a pre‐existing history of dementia or cognitive impairment prior to assessment with the Mini‐Cog and reference standard but all participants had to have cognitive complaints suggestive of possible undiagnosed dementia or cognitive impairment. | |
| Sensitivity: 1.00 (0.84 to 1.00) Specificity: 0.85 (0.81 to 0.89) | 423 | 5.0% | The study excluded individuals with dementia at baseline, and those included in the study received a 36 month follow up assessment. Thus participants in the sample who were diagnosed with dementia were in the early stages of the disease. | |
| Sensitivity: 0.76 (0.53 to 0.92) Specificity: 0.73 (0.68 to 0.77) | 383 | 5.5% | Study involved evaluation of individuals in primary care settings without a documented history of dementia recorded at baseline. | |
| Sensitivity: 0.84 (0.81 to 0.87) Specificity: 0.27 (0.16 to 0.41) | 569 | 90.3% | Individuals with documented cognitive impairment were excluded from screening. Sampling involved screening of all participants in primary care and then offering further evaluation to individuals who either screened positive or negative on initial screening and who also agreed to have further evaluation. | |
| CI: confidence interval | ||||
| Study ID | Country | Participants (N) | Setting | Mini‐Cog scoring | Reference standard for dementia diagnosis | Dementia prevalence | Notes |
| Spain | 142 | 1 primary care location in Madrid and 3 primary care locations in Granada, only data from the Granada site was included | Standard scoring | DSM IV TR | 34.5% | The clock drawing test was incorporated into the reference standard at the Madrid site, data are presented for the Granada sites only. Screening was administered by professionals (no further specification) except for the clock drawing test component in Madrid, which was performed by a neurologist. | |
| Germany | 423 | Participants were randomly selected from 138 study centres in 6 metropolitan areas in Germany although study reports information from 29 sites recruited from Dusseldorf region | Standard scoring | DSM IV | 5.0% | Individuals with known dementia were excluded from the study. Study evaluated accuracy of the Mini‐Cog in detecting incident dementia at 36 months' follow‐up from enrolment. Screening tests were administered by a trained physician or psychologist. | |
| USA | 383 | Primary care locations affiliated with the Veterans Affairs near Durham, North Carolina | Standard scoring | DSM IV and NINCDS‐ADRDA | 5.5% | Excluded individuals with a known prior history of dementia based on diagnoses recorded in charts. The Mini‐Cog was administered by a research assistant. | |
| USA | 569 | 7 primary care settings affiliated with Veterans Affairs in Minneapolis, Minnesota | Standard scoring | DSM IV | 90.3% | Participants were first screened for possible dementia by trained advanced practice registered nurses based on interview during routine visit with those who initially screened positive being offered additional evaluation with the index and reference standards. Some individuals who did not screen positive at the initial interview requested and received additional evaluation. | |
| DSM IV: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; DSM IV TR: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (text revision); NINCDS‐ADRDA: Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association | |||||||
| Test | No. of studies | No. of participants |
| 1 Mini‐Cog Show forest plot | 4 | 1517 |