Prueba de detección cognitiva breve (Mini‐Cog) para el diagnóstico de la demencia de la enfermedad de Alzheimer y otras demencias en ámbitos de atención primaria
Resumen
Antecedentes
La enfermedad de Alzheimer y otras formas de demencia se están volviendo cada vez más prevalentes con el envejecimiento de muchas poblaciones. La mayoría de los pacientes con demencia primero acudirán a ámbitos de atención primaria en busca de atención y evaluación. Se necesitan instrumentos breves de cribado de la demencia que puedan diagnosticar con exactitud la demencia en ámbitos de atención primaria. La Mini‐Cog es una prueba de detección cognitiva breve que se usa con frecuencia para evaluar la cognición en pacientes adultos mayores de diversos ámbitos.
Objetivos
Determinar la exactitud diagnóstica de la Mini‐Cog para diagnosticar la demencia de la enfermedad de Alzheimer y las demencias relacionadas en ámbitos de atención primaria.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane de Estudios de Precisión de Pruebas Diagnósticas para la Demencia y la Trastornos Cognitivos (Cochrane Dementia and Cognitive Improvement Register of Diagnostic Test Accuracy Studies), MEDLINE, Embase y otras cuatro bases de datos, inicialmente hasta septiembre de 2012. Desde entonces, se realizaron cuatro actualizaciones de la búsqueda utilizando los mismos métodos de búsqueda, y la más reciente fue en enero de 2017. Se utilizó el seguimiento de las citas (mediante la característica de la base de datos "artículos relacionados", cuando estuvo disponible) como un método adicional de búsqueda y se estableció contacto con los autores de los estudios elegibles para obtener datos no publicados.
Criterios de selección
Solo se incluyeron los estudios que evaluaron la Mini‐Cog como una prueba índice para el diagnóstico de la demencia de la enfermedad de Alzheimer o las formas relacionadas de demencia en comparación con un estándar de referencia que utilizara criterios validados para la demencia. Solo se incluyeron los estudios que se realizaron en poblaciones de atención primaria.
Obtención y análisis de los datos
Se extrajo y se describió la información sobre las características de los participantes de los estudios y el ámbito de los estudios. Mediante los criterios Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) se evaluó la calidad de los estudios y el riesgo de sesgo, así como la aplicabilidad de cada estudio para cada dominio de QUADAS‐2. Dos autores de la revisión extrajeron de forma independiente la información sobre los verdaderos positivos, los verdaderos negativos, los falsos positivos y los falsos negativos, e introdujeron los datos en Review Manager 5 (RevMan 5). Luego se utilizó RevMan 5 para determinar la sensibilidad, la especificidad y los intervalos de confianza del 95%. La sensibilidad y la especificidad de la Mini‐Cog en los estudios individuales se resumió en diagramas de bosque y también se graficaron en un gráfico de las características operativas del receptor. También se creó una tabla de "Riesgo de sesgo" y un gráfico de problemas de aplicabilidad para resumir la información relacionada con la calidad de los estudios incluidos.
Resultados principales
Hubo un total de cuatro estudios que cumplieron con los criterios de inclusión, con un total de 1517 participantes. La sensibilidad de la Mini‐Cog varió entre 0,76 y 1,00 en los estudios, mientras que la especificidad varió entre 0,27 y 0,85. Los estudios incluidos mostraron heterogeneidad significativa en cuanto a las metodologías y las poblaciones clínicas, lo que no permitió completar un metanálisis. Sólo un estudio (Holsinger 2012) se encontró que tenía un bajo riesgo de sesgo en todos los dominios metodológicos. Los resultados de este estudio informaron que la sensibilidad de la Mini‐Cog fue 0,76 y la especificidad fue 0,73. Se encontró que la calidad de los otros estudios incluidos fue baja debido al alto riesgo de sesgo, con limitaciones metodológicas principalmente en la selección de los participantes.
Conclusiones de los autores
Hay un número limitado de estudios que evalúan la exactitud de la Mini‐Cog para el diagnóstico de la demencia en ámbitos de atención primaria. Debido al escaso número de estudios, la amplia variedad en las estimaciones de la exactitud de la Mini‐Cog y las limitaciones metodológicas identificadas en la mayoría de los estudios, en este momento no hay evidencia suficiente para recomendar el uso de la Mini‐Cog como una prueba de detección de la demencia en la atención primaria. Se necesitan estudios adicionales para determinar la exactitud de Mini‐Cog en la atención primaria y si esta herramienta tiene suficiente exactitud como prueba diagnóstica para ser útil como prueba de detección en este ámbito.
Resumen en términos sencillos
¿Cuán exacta es la prueba Mini‐Cog cuando se utiliza para evaluar la demencia en la práctica general?
Antecedentes y justificación de la revisión
En la mayor parte del mundo hay un número cada vez mayor de personas de edad avanzada y, como resultado, los problemas relacionados con la memoria y las afecciones como la enfermedad de Alzheimer y otras formas de demencia se hacen cada vez más frecuentes. La mayoría de las personas con dificultades de memoria en primer lugar buscarán atención o se identificarán en el sistema de asistencia sanitaria a través de profesionales de la atención primaria como los médicos de familia o el personal de enfermería. Por lo tanto, se necesitan herramientas que puedan identificar a los pacientes que pueden presentar demencia o problemas de memoria significativos. Estas herramientas también deben poder descartar la demencia en las personas con afecciones relacionadas con la memoria que no presentan demencia ni problemas de memoria significativos. Dichas herramientas en la atención primaria deben ser relativamente fáciles de utilizar, de administración rápida y exactas para que se puedan utilizar en la atención primaria, y al mismo tiempo, que no sobrediagnostiquen ni subdiagnostiquen la demencia. La Mini‐Cog, una herramienta de cribado cognitivo breve, se ha indicado como una posible prueba de detección para la demencia en la atención primaria porque se ha informado que es exacto y relativamente fácil de administrar en ámbitos de atención primaria. La Mini‐Cog consiste en una tarea de memoria que incluye el recuerdo de tres palabras y una evaluación de una tarea de dibujo de relojes.
Características de los estudios
Se efectuaron búsquedas en las bases de datos electrónicas de artículos que evaluaron la Mini‐Cog y esta evidencia se actualizó hasta enero de 2017. La finalidad de la presente revisión fue comparar la exactitud de la Mini‐Cog para diagnosticar la demencia de cualquier tipo en ámbitos de atención primaria en comparación con la evaluación en profundidad realizada por especialistas en demencia. Se incluyeron los estudios que evaluaron personas con cualquier posible gravedad de demencia, independientemente de si se había completado una prueba cognitiva previa antes de la Mini‐Cog. En general, la revisión identificó cuatro estudios realizados en ámbitos de atención primaria que compararon la exactitud de la Mini‐Cog para la evaluación detallada de la demencia por especialistas en demencia.
Calidad de la evidencia
De los cuatro estudios incluidos en la revisión, todos excepto uno tuvieron limitaciones en cómo se evaluó la Mini‐Cog, lo que puede haber dado lugar a una sobrestimación de la exactitud de la prueba en los estudios restantes. En particular, el aspecto más problemático relacionado con la calidad de los estudios fue la manera en la que se seleccionaron los participantes para participar en los estudios de investigación, lo que puede haber contribuido de manera adicional a una sobrestimación de la exactitud de la Mini‐Cog en la mayoría de los estudios incluidos en la revisión.
Hallazgos clave
Los resultados del estudio de mayor calidad Holsinger 2012 encontró que la Mini‐Cog tuvo una sensibilidad del 76%, lo que indica que la Mini‐Cog no pudo detectar hasta el 24% de los pacientes con demencia (p.ej. los falsos negativos). En este mismo estudio, la especificidad de la Mini‐Cog fue del 73%, lo que indica que hasta el 27% de las personas se pueden identificar de manera incorrecta como con demencia en la Mini‐Cog, cuando estas personas en realidad no presentan una demencia subyacente (p.ej. falsos positivos). Se concluye que en este momento no hay evidencia suficiente para apoyar el uso sistemático de la Mini‐Cog como prueba de detección de la demencia en la atención primaria, y se necesitan estudios adicionales antes de concluir que la Mini‐Cog es útil en este ámbito.
Authors' conclusions
Summary of findings
| Mini‐Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a primary care setting | ||||
| Population | The study populations were sampled from participants identified in primary care settings. | |||
| Setting | The primary care setting was identified as representing a sample that would be presenting to primary care settings where the Mini‐Cog might be used as a screening test to identify individuals who may benefit from additional evaluation. Studies that identified individuals in primary care where they received both the index test and a reference standard were used. | |||
| Indext test | The Mini‐Cog performed in insolation or scored based on results on the clock drawing test or three‐word recall were included. | |||
| Reference Standard | Clinical diagnosis of dementia was made using recognized standard diagnostic criteria. | |||
| Studies | Cross‐sectional studies were included, case control studies were excluded | |||
| Study | Accuracy (95% CI) | Number of participants | Dementia prevalence | Implications |
| Sensitivity: 1.00 (0.93 to 1.00) Specificity: 0.40 (0.30 to 0.50) | 142 | 34.5% | Participants were sampled including individuals who did have a pre‐existing history of dementia or cognitive impairment prior to assessment with the Mini‐Cog and reference standard but all participants had to have cognitive complaints suggestive of possible undiagnosed dementia or cognitive impairment. | |
| Sensitivity: 1.00 (0.84 to 1.00) Specificity: 0.85 (0.81 to 0.89) | 423 | 5.0% | The study excluded individuals with dementia at baseline, and those included in the study received a 36 month follow up assessment. Thus participants in the sample who were diagnosed with dementia were in the early stages of the disease. | |
| Sensitivity: 0.76 (0.53 to 0.92) Specificity: 0.73 (0.68 to 0.77) | 383 | 5.5% | Study involved evaluation of individuals in primary care settings without a documented history of dementia recorded at baseline. | |
| Sensitivity: 0.84 (0.81 to 0.87) Specificity: 0.27 (0.16 to 0.41) | 569 | 90.3% | Individuals with documented cognitive impairment were excluded from screening. Sampling involved screening of all participants in primary care and then offering further evaluation to individuals who either screened positive or negative on initial screening and who also agreed to have further evaluation. | |
| CI: confidence interval | ||||
Background
Target condition being diagnosed
Alzheimer's disease and related forms of dementia are common among older adults with a prevalence of 8% in individuals aged over 65 years and increasing to a prevalence of approximately 43% in adults aged 85 years and older (Thies 2012). Given the increasing number of older adults in most developing countries, the prevalence of dementia is expected to increase considerably in the coming years (Ferri 2005). Alzheimer's disease and related forms of dementia are currently incurable and result in considerable direct and indirect costs, both in terms of formal health care and lost productivity from both the affected individuals and their caregivers (Thies 2012). There is a debate as to the value of arriving at a diagnosis of dementia earlier in the disease process. Diagnosing Alzheimer's disease in the pre‐clinical state using biomarker or neuroimaging modalities without the availability of effective treatments or interventions to alter the disease course may be harmful in some situations (Le Couteur 2013). However, qualitative research has demonstrated that many individuals with clinically diagnosed dementia and their caregivers would prefer to know a diagnosis of dementia early in the disease process, as knowledge of the diagnosis of dementia can help to facilitate a better understanding of observed cognitive and functional changes and facilitate more timely access to supports and services (Prorok 2013; Prorok 2016). A diagnosis of dementia is necessary to access certain services and supports for individuals and their caregivers; and pharmacological treatments such as cholinesterase inhibitors (Birks 2006; Rolinski 2012) or memantine (McShane 2009; Wilkinson 2012) have only been shown to be effective in providing temporary symptomatic improvement in cognitive function for individuals diagnosed with mild to moderate Alzheimer's disease.
The diagnosis of Alzheimer's disease is clinical and based on a history of progressive decline in cognition affecting memory and at least one other area of cognitive functioning (e.g. apraxia, agnosia, or executive dysfunction). There must be a decline from a previous level of functioning resulting in significant social or occupational impairment (APA 2000; APA 2013; McKhann 2001). A definitive diagnosis of Alzheimer's disease can only be achieved at autopsy but a clinical diagnosis using standardized criteria is associated with a sensitivity of 81% and a specificity of 70% when compared to autopsy‐proven cases (Knopman 2001; Nagy 1998).
Approximately 50% to 80% of all individuals with dementia are ultimately classified as having Alzheimer's disease (Blennow 2006; Brunnstrom 2009; Canadian Study of Health and Aging 1994). Vascular dementias may occur more abruptly or present with a step‐wise decline in cognition over time and account for approximately 15% to 20% of dementias (Brunnstrom 2009; Canadian Study of Health and Aging 1994; Feldman 2003; Lobo 2000). Dementia with a mixed Alzheimer's disease and vascular pathology is present in 10% to 30% of cases (Brunnstrom 2009; Crystal 2000; Feldman 2003). Less frequent causes of dementia include dementia with Lewy bodies (Brunnstrom 2009) or Parkinson's disease dementia (Aarsland 2005). People experiencing frontotemporal dementia account for a smaller proportion of dementias (4% to 8%) and often present with problems in executive function and changes in behaviour, while memory is relatively preserved (Brunnstrom 2009; Grecicus 2002).
Index test(s)
The Mini‐Cog is a brief cognitive screening test consisting of two components, a delayed, three‐word recall task and the clock drawing test (Borson 2000). The Mini‐Cog was initially examined in community settings and was designed to provide a relatively brief cognitive screening test that was free of educational and cultural biases. Different scoring algorithms were tested to determine which combination had the optimal balance of sensitivity and specificity (McCarten 2011; Scanlan 2001). The Mini‐Cog takes approximately three to five minutes to complete in routine practice (Borson 2000; Holsinger 2007; Scanlan 2001). The Mini‐Cog has been reported to have little potential for bias as a result of education or language (Borson 2000; Borson 2005).
Clinical pathway
Dementia typically begins with subtle cognitive changes and progresses gradually over the course of several years. Most older adults with memory complaints will first present to their general practitioner or other primary care healthcare provider (for example nurses or a nurse practitioner). There is a presumed period when people are asymptomatic, although the disease pathology may be progressing. Individuals or their relatives may first notice subtle impairments of short‐term memory or other areas of cognitive functioning. Gradually, additional cognitive deficits become apparent resulting in difficulty completing complex activities of daily living such as the management of finances and medications, or operating motor vehicles (Njegovan 2001). The attribution of cognitive symptoms to normal aging may cause delays in the diagnosis and treatment of Alzheimer's disease or other types of dementia (Prorok 2013). Therefore, there is a need for accurate brief dementia screening tests to help distinguish between the cognitive changes associated with normal aging and changes that might indicate dementia. Individuals with dementia often first present to primary care health care providers with cognitive complaints or functional changes that might indicate the possibility of a dementia (Feldman 2008). Even though most individuals with dementia are first evaluated in primary care, the absence of systematic dementia screening programs for dementia in many primary care settings may result in an underdiagnosis of individuals with dementia (Connolly 2011).
Prior test(s)
As the Mini‐Cog is recommended to be used as an initial screening test for dementia in primary care (Brodaty 2006; Ismail 2010; Milne 2008; Tsoi 2015) it is unlikely that individuals will have had any testing completed prior to the administration of the Mini‐Cog.
Role of index test(s)
Primary healthcare providers may administer brief cognitive screening tests and, depending on the results of the initial tests, an individual may then have additional investigations or cognitive tests to confirm if a diagnosis of dementia is present. In some settings, a positive result on a brief cognitive screening test may result in a referral to a dementia specialist, such as a neurologist, geriatrician, or geriatric psychiatrist. Some countries have recently recommended that brief cognitive screening tests be administered to all older adults in order to identify asymptomatic individuals who may have underlying undiagnosed cognitive impairment (Cordell 2013), although the utility of routine screening of asymptomatic individuals for dementia in primary care settings is controversial (UK National Screening Committee 2015). The Mini‐Cog would most often be used in most clinical settings as an initial screening test for dementia and not to arrive at a definitive diagnosis of dementia on its own. However, in the current review we evaluated the diagnostic test accuracy of the Mini‐Cog when compared to a reference standard diagnosis of dementia to determine the accuracy of the Mini‐Cog in keeping with previous Cochrane Reviews of diagnostic test accuracy of dementia cognitive tests.
Alternative test(s)
We will not be including alternative tests in this review because there are currently no standard tests available for the diagnosis of dementia. The diagnostic test accuracy of other cognitive tests is the subject of separate reviews (Creavin 2016; Davis 2015; Harrison 2014; Hendry 2014).
Cochrane Dementia and Cognitive Improvement (CDCI) is in the process of conducting a series of diagnostic test accuracy reviews of biomarkers and scales. CDCI is conducting reviews on individual tests compared to a reference standard and they plan to compare the results of different tests in an overview.
Rationale
Most individuals with dementia are first assessed and diagnosed in primary care settings (Prorok 2013). Individuals with dementia or cognitive disorders may present to primary care providers with cognitive symptoms although primary care providers may not identify older adults with cognitive symptoms in routine brief clinical encounters (Bradford 2009; Connolly 2011). Some studies have found that in primary care the majority of older adults with dementia are undiagnosed (Boustani 2005; Connolly 2011; Sternberg 2000) and mild dementia is particularly under‐diagnosed (Van den Dungen 2011). Accurate diagnosis of dementia is important in order to initiate dementia therapeutics including both non‐pharmacological treatments and pharmacological treatments such as cholinesterase inhibitors (Birks 2006; Rolinski 2012) and memantine (McShane 2009). Early diagnosis and treatment of dementia may also have long‐term clinical benefits for the patient and his or her caregivers during the course of disease progression (Bennett 2003; Prorok 2013; Thies 2012). Routine screening of all older adults for dementia in primary care using cognitive screening tests appears to improve dementia case detection rates when compared to usual care without routine screening of older adults (Eichler 2015). Comprehensive evaluation conducted by psychologists or dementia physician specialists such as general psychiatrists, geriatric psychiatrists, geriatricians, or neurologists using standardized diagnostic criteria is considered the reference standard for diagnosing dementia in older adults. However, access to these specialized resources is scarce and expensive and as such they are not practical to be used routinely in the evaluation of cognitive complaints (Pimlott 2009; Yaffe 2008). While there are some cognitive tests that can be performed by healthcare providers who are not dementia specialists, many of these tests are time consuming and may not be practical to use routinely in primary care settings (Brodaty 2006; Pimlott 2009). As such, brief but relatively accurate cognitive screening tests are required for healthcare providers in primary care settings as an initial test to identify individuals who may require more in‐depth evaluation of cognition either in primary care or in specialist settings.
The sensitivity and specificity of such brief screening tests are likely to vary depending upon the setting in which they are used (Holsinger 2007). If the Mini‐Cog was used in primary care settings, it could allow healthcare professionals or lay people to initially assess older adults for the possible presence of dementia. Individuals that screen positive for cognitive impairment on the Mini‐Cog would then be further investigated for the presence of dementia using additional cognitive tests or other investigations. Given that the Mini‐Cog is brief, widely available, easy to administer, and has been reported to have reasonable test accuracy properties (Brodaty 2006; Ismail 2010; Lin 2013; Lorentz 2002; Milne 2008) it may be well suited for use as an initial cognitive screening test in primary care, and has already been recommended as a suitable test for primary care dementia screening programmes in some countries (Cordell 2013). Other cognitive tests that may also be suitable for use in primary care settings include the Mini‐Mental State Examination (MMSE) (Holsinger 2007), the General Practitioner Assessment of Cognition (GPCOG), or the Memory Impairment Screen (Brodaty 2006), however each of these take longer to administer, and may be biased by language, culture, and education level (Matallana 2011) in contrast to the Mini‐Cog. The current review will examine the diagnostic accuracy of the Mini‐Cog in primary care settings. Separate DTA reviews are being undertaken for the Mini‐Cog in community (Fage 2015) and secondary care settings (Chan 2014).
Objectives
To determine the diagnostic accuracy of the Mini‐Cog for diagnosing Alzheimer's disease dementia and related dementias in a primary care setting.
Secondary objectives
To investigate the heterogeneity of test accuracy in the included studies and potential sources of heterogeneity. These potential sources of heterogeneity will include the baseline prevalence of dementia in study samples, thresholds used to determine positive test results, the type of dementia (Alzheimer's disease dementia or all causes of dementia), and aspects of study design related to study quality.
To identify gaps in the evidence where further research is required.
Methods
Criteria for considering studies for this review
Types of studies
We included all cross‐sectional studies from primary care settings with well‐defined populations that used the Mini‐Cog as an index cognitive test compared to a reference standard for the diagnosis of dementia. Case‐control studies were not included in this review. Studies had to use a reference standard to determine whether or not dementia was present. Studies used the Mini‐Cog as an initial cognitive test for dementia and not for the confirmation of a diagnosis of dementia. When possible, studies administered the index and reference tests to individuals where their diagnosis was not already known, although some studies may have used the test on people with a previously known diagnosis of Alzheimer's disease or a related dementia.
Participants
Study participants presented in a primary care setting and may or may not have been ultimately diagnosed with Alzheimer's disease or all‐cause dementia following additional evaluation. Participants may have had cognitive complaints or dementia at baseline although their cognitive status was not known to the individual administering the Mini‐Cog or the reference standard. Studies on participants with a developmental disability, which prevented them from completing the Mini‐Cog, were excluded.
Index tests
Mini‐Cog test
The Mini‐Cog consists of two components: a three‐word recall task that assesses memory and the clock drawing test that assesses cognitive domains such as cognitive function, language, visual‐motor skills and executive function. The standard scoring system involves assigning a score of 0 to 3 points on the word recall task for the correct recall of 0, 1, 2, or 3 words, respectively. The clock drawing test is scored as being either 'normal' or 'abnormal'. A positive test on the Mini‐Cog (i.e. indicating a possible diagnosis of dementia) is assigned if the delayed word recall score is 0 out of 3, or if their delayed recall score is either 1 or 2 and their clock drawing test is abnormal. A score of 3 on the delayed word recall or 1 to 2 on the word recall with a normal clock drawing is considered a negative test (i.e. no dementia is present) (Borson 2000).
Studies must have included the results of the Mini‐Cog. We planned to examine the potential effects of multiple scoring algorithms through subgroup analyses, although there were an insufficient number of studies identified to complete this analysis in our review.
Target conditions
The primary target condition of interest for this review was any stage of Alzheimer's disease or all‐cause dementia, which in primary care settings would most commonly be caused by Alzheimer's disease, vascular dementia, or some combination of these two pathologies.
Reference standards
While a definitive diagnosis can only be made post‐mortem at autopsy, there are clinical reference standard criteria for the diagnosis of the different forms of dementia. All dementia diagnostic criteria require that an individual has impairment in multiple areas of cognition that results in difficulties in daily functioning which is not directly caused by either the effects of a substance or general medical condition. We have included several potential reference standards for the diagnosis of all‐cause dementia or specific types of dementia. All‐cause dementia is commonly diagnosed using the Diagnostic and Statistical Manual of Mental Disorders IV (DSM‐IV) (APA 2000), DSM‐5 criteria for major neurocognitive disorder (APA 2013), or the International Classification of Diseases (ICD) diagnosis of dementia (WHO 2010). The standard clinical diagnostic criteria commonly used for Alzheimer's disease dementia include the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) for probable or possible Alzheimer's disease dementia (McKhann 1984; McKhann 2011). Diagnostic criteria for other types of dementia include the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINCDS‐AIREN) criteria for vascular dementia (Roman 1993), standard criteria for dementia with Lewy bodies (McKeith 2005) and for frontotemporal dementia (McKhann 2001).
Search methods for identification of studies
Electronic searches
We initially searched up to September 2012. Four subsequent updates to the initial search was performed using the same search methods: January 2013, February 2015, January 2016, and January 2017. We searched the Cochrane Dementia and Cognitive Improvement Register of Diagnostic Test Accuracy Studies that is currently under development, MEDLINE (OvidSP) (1950 to January 2017), Embase (OvidSP) (1974 to 31 January 2017), BIOSIS Previews (Thomson Reuters Web of Science) (1926 to January 2017), Science Citation Index (Thomson Reuters Web of Science) (1945 to January 2017), PsycINFO (OvidSP) (1806 to January week 4 2017), and LILACS (BIREME) (January 2017) (for results of the database search, see Figure 1). See Appendix 1 for details of the sources searched, the search strategies used and the number of citations retrieved, and to view the 'generic' search that is used regularly for Cochrane Dementia and Cognitive Improvement's Register. Similarly, we designed structured search strategies using search terms appropriate for each database. Controlled vocabulary such as MeSH terms and EMTREE were used where appropriate. We made no attempt to restrict studies based on the sampling frame or setting in the searches that we developed. This was meant to maximize the sensitivity and allow inclusion to be assessed on the basis of population‐based sampling at testing (see ‘Selection of studies’, below). We did not use search filters (collections of terms aimed at reducing the number of studies that need to be screened) as an overall limiter because those published have not proved sensitive enough (Whiting 2011). We did not apply any language restriction to the electronic searches.
Study flow diagram
A single review author with extensive experience in systematic reviews performed the initial searches. Two review authors independently screened abstracts and titles.
Searching other resources
We searched the reference lists of all relevant studies for additional relevant studies, since this has been reported to be a useful method to minimize overlooking potentially relevant studies for complex reviews (Greenhalgh 2005; Horsely 2011). We also used these studies to search electronic databases to identify additional studies through the use of the related article feature. We asked research groups authoring studies that were used in the analysis for unpublished data.
Data collection and analysis
Selection of studies
Studies had to address the following.
-
Make use of the Mini‐Cog as a cognitive test in a primary care setting.
-
Include patients from a primary care setting who may or may not have dementia or cognitive complaints.
-
Clearly explain how a diagnosis of dementia was confirmed according to a reference standard such as the DSM IV‐TR, DSM‐5, or NINCDS‐ADRDA at the same time or within the same four‐week time period that the Mini‐Cog was administered. Formal neuropsychological evaluation or neuroimaging was required for a diagnosis of dementia.
We first selected articles based on the abstract and title. Two review authors independently located the selected articles and assessed them for inclusion. A third review author resolved disagreements.
Data extraction and management
Two review authors extracted the following data from all included studies.
-
Author, journal, and year of publication.
-
Scoring algorithm for the Mini‐Cog including cut‐points used to define a positive screen; method of Mini‐Cog administration, including who administered and interpreted the test and their training.
-
Reference criteria and method used to confirm diagnosis of Alzheimer's disease or all‐cause dementia.
-
Baseline demographic characteristics of the study population including age, gender, ethnicity, spectrum of presenting symptoms, comorbidity, educational achievement, language, baseline prevalence of dementia, country, ApoE status, methods of participant recruitment and sampling procedures.
-
Length of time between administration of index test (Mini‐Cog) and the reference standard.
-
The sensitivity and specificity, and positive and negative likelihood ratios, of the index test in defining dementia.
-
Version of translation (if applicable).
-
Prevalence of dementia in the study population.
Assessment of methodological quality
To assess data quality we used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) criteria (Whiting 2011). The QUADAS‐2 criteria contain assessment domains for patient selection, the index test, reference test, and flow and timing. Each domain has suggested signalling questions to assist with the assessment of risk of bias for each domain. The potential risk of bias associated with each domain is rated as being at high, low, or uncertain risk of bias. In addition, using the guide provided in QUADAS‐2, we determined the applicability of the study to the review question for each domain. We used a standardized 'Risk of bias' template to extract data on the risk of bias for each study using the form provided by the UK Support Unit Cochrane Diagnostic Test Accuracy group. See Appendix 2 for details. We summarized quality assessment results using the methodological quality summary table and methodological summary graph in Review Manager 5 (RevMan 5) (RevMan 2014).
Statistical analysis and data synthesis
We performed the statistical analysis as per the Cochrane guidelines for diagnostic test accuracy reviews (Macaskill 2010). We planned to construct two‐by‐two tables for the Mini‐Cog results for both all‐cause dementia and Alzheimer's disease dementia where this information was available.
We entered data from individual studies including the true positives (TP), true negatives (TN), false positives (FP), and false negatives (FN) into RevMan 5. We determined these values by comparing the rates of TP, TN, FP, and FN for individuals with all‐cause dementia when compared to individuals without any form of dementia. For the primary analysis, we compared the diagnosis of all‐cause dementia to no dementia. We also calculated the sensitivity, specificity, positive and negative likelihood ratios, as well as measures of statistical uncertainty (e.g. 95% confidence intervals) from the raw data for the primary analysis of dementia when compared to no dementia in RevMan 5. We presented the data from each study graphically by plotting sensitivities and specificities on a coupled forest plot. If multiple thresholds were reported for the Mini‐Cog, we planned to use the hierarchical summary receiver operating characteristic (HSROC) method of Rutter and Gastconis for the meta‐analysis (Rutter 2001). We had initially planned a meta‐analysis for this review although, due to a limited number of studies and methodological limitations present in the included studies, we did not undertake a meta‐analysis as a part of the final review.
Investigations of heterogeneity
The potential sources of heterogeneity that we intended to examine included the baseline prevalence of cognitive impairment in the target population, the cut‐points used to determine a positive test result, the reference standard used to diagnose dementia, the type of dementia (Alzheimer's disease dementia or all‐cause dementia), the severity of dementia in the study sample (using dementia severity assessment scales such as the Clinical Dementia Rating (Morris 1993) scale or the Global Deterioration Scale (Reisberg 1982)), and aspects related to study quality as assessed with the QUADAS‐2.
To investigate the effects of the sources of heterogeneity, we planned to complete subgroup analyses. These involved visual examination of the forest plot of sensitivity and specificity and the receiver operating characteristic (ROC) plot within each subgroup (for example baseline prevalence, type of dementia, etc). Additionally, we planned a formal analysis using the HSROC model. This model can be extended to include covariates in order to assess whether threshold, accuracy, or the shape of the summary ROC (SROC) curve varies with participant or study characteristics. However, given the small number of studies included in our review and methodological limitations of studies we were unable to complete these planned subgroup analyses.
Sensitivity analyses
We planned to perform a sensitivity analysis in order to investigate the influence of study quality on the overall diagnostic accuracy of the Mini‐Cog test. We did not perform the sensitivity analysis as we did not undertake a meta‐analysis of the results.
Assessment of reporting bias
We had not planned to assess reporting bias because of current uncertainty about how it operates in test accuracy studies and the interpretation of existing analytical tools such as funnel plots.
Results
Results of the search
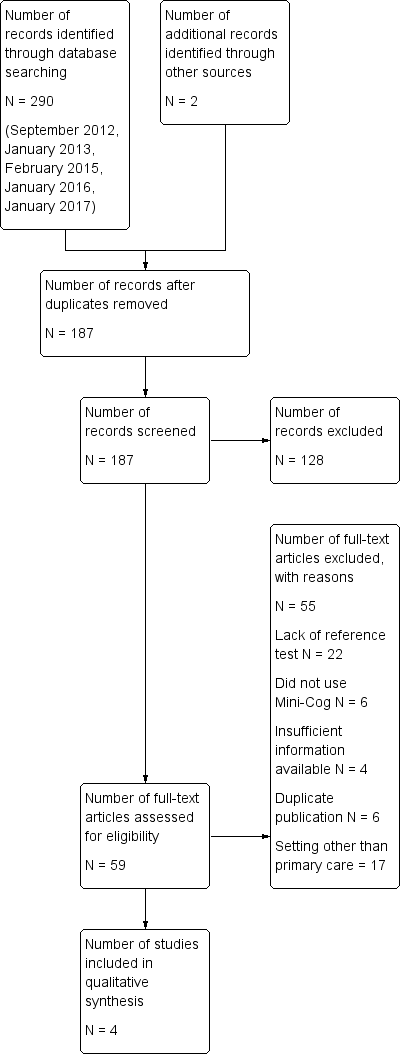
The results of the literature search are outlined below in Figure 1. A review of the electronic databases on four occasions between 2012 and 2017 identified a total of 292 articles. The same search strategy was employed for this review that was used in separate reviews of the Mini‐Cog in the community setting (Fage 2015) and secondary care setting (Chan 2014). After removal of duplications, two review authors independently reviewed a total of 187 abstracts and citations for inclusion criteria and suitability for inclusion in the final review.
We reviewed a total of 59 full‐text articles for eligibility to be included in the final review. Of these 59 articles, we excluded 55 due to a lack of a reference standard (N = 22), failure to include the Mini‐Cog as an index text (N = 6), duplicate publications (N = 6), incorrect setting (N = 17), or lack of sufficient data to be included in the review (N = 4).
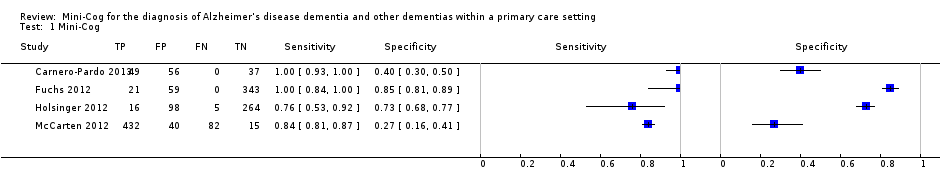
The search identified four independent studies from four different study reports (Carnero‐Pardo 2013; Fuchs 2012; Holsinger 2012; McCarten 2012). The characteristics of the studies are outlined in the summary of findings Table. These four studies included a total of 1517 participants and there was heterogeneity in the baseline prevalence of dementia across the studies, which ranged from 5% to 90%. Additional details regarding the design, setting, population, target condition and reference standard of each included study can be found in the Characteristics of included studies section.
Methodological quality of included studies
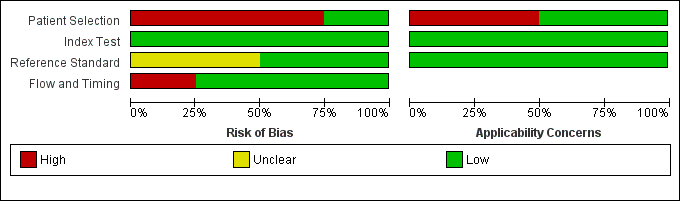
The results of the QUADAS‐2 assessment for the four studies are summarized in Figure 2 and the details of the risk of bias assessment for each of the included studies are presented in Figure 3. We judged three of the four studies as being at a high risk of bias in the patient selection domain (Carnero‐Pardo 2013; Fuchs 2012; McCarten 2012) as they did not enrol a consecutive or random sample of patients. For Fuchs 2012, it was unclear whether or not a case‐control design was avoided and the study failed to avoid inappropriate exclusions, thus introducing a high risk of patient selection bias. While all the included studies used the Mini‐Cog as the index test, McCarten 2012 adjusted the threshold of a positive screen in order to increase the sensitivity of the test by considering a positive screen on the Mini‐Cog for possible dementia being 3 or fewer points compared to the usual scoring of 2 or fewer points. We rated the risk of bias for the assessment of the reference standard as unclear for both Fuchs 2012 and McCarten 2012, as it was unclear whether the reference standard assessment results had been interpreted without knowledge of the Mini‐Cog results. We rated only one study as being at low risk of bias on all the QUADAS‐2 domains (Holsinger 2012).
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Findings
There were four study reports on four unique study populations that were selected for the final review (Carnero‐Pardo 2013; Fuchs 2012; Holsinger 2012; McCarten 2012). The Characteristics of included studies section of this review and Table 1 include a summary of the included studies. Additional features of these studies are also summarized in summary of findings Table. The baseline prevalence of dementia in the overall study samples varied from 5.0% (Fuchs 2012) to 90.3% (McCarten 2012). Two studies randomly recruited participants from Veteran Affairs Medical Centres either from routinely scheduled primary care appointments (McCarten 2012) or electronic medical records (Holsinger 2012), one study evaluated a random sample of medical records from primary practices in a defined geographic area (Fuchs 2012), and another study recruited from four primary care sites in two cities (Carnero‐Pardo 2013). The McCarten 2012 recruited individuals from primary care sites who either tested positive for possible dementia on the Mini‐Cog as part of a dementia screening programme or those individuals who tested negative on the Mini‐Cog but who requested additional evaluation of their cognition. The process for selection of participants in the McCarten 2012 study likely contributed to the high prevalence of dementia in this study, which was reported as 90.3%. Given that the McCarten 2012 study included individuals who initially tested negative and positive on the Mini‐Cog test, we decided to include it in the final review. Two studies excluded individuals with known cognitive impairment (Carnero‐Pardo 2013; McCarten 2012) and another two excluded individuals with a history of dementia at baseline (Fuchs 2012; Holsinger 2012). All studies used the DSM‐IV‐TR as the reference standard for the diagnosis of dementia and two studies based dementia diagnosis on additional reference standards, NINCDS‐ADRDA and NINCDS‐AIREN, as well (Fuchs 2012, Holsinger 2012). The diagnosis of dementia was agreed upon by consensus between two or more clinicians or researchers in all four studies. All studies used the original scoring system for the Mini‐Cog as proposed by Borson 2000 except for McCarten 2012, which used an adjusted scoring with a cut‐off of 3 or lower to indicate a positive test for dementia to increase the sensitivity of the Mini‐Cog compared to the usual cut‐off of 2 or lower. Three studies reported on the gender distribution of participants, with two studies reporting a majority of participants being female (Carnero‐Pardo 2013; Fuchs 2012) while one study reported a very low prevalence of female participants (Holsinger 2012).
| Study ID | Country | Participants (N) | Setting | Mini‐Cog scoring | Reference standard for dementia diagnosis | Dementia prevalence | Notes |
| Spain | 142 | 1 primary care location in Madrid and 3 primary care locations in Granada, only data from the Granada site was included | Standard scoring | DSM IV TR | 34.5% | The clock drawing test was incorporated into the reference standard at the Madrid site, data are presented for the Granada sites only. Screening was administered by professionals (no further specification) except for the clock drawing test component in Madrid, which was performed by a neurologist. | |
| Germany | 423 | Participants were randomly selected from 138 study centres in 6 metropolitan areas in Germany although study reports information from 29 sites recruited from Dusseldorf region | Standard scoring | DSM IV | 5.0% | Individuals with known dementia were excluded from the study. Study evaluated accuracy of the Mini‐Cog in detecting incident dementia at 36 months' follow‐up from enrolment. Screening tests were administered by a trained physician or psychologist. | |
| USA | 383 | Primary care locations affiliated with the Veterans Affairs near Durham, North Carolina | Standard scoring | DSM IV and NINCDS‐ADRDA | 5.5% | Excluded individuals with a known prior history of dementia based on diagnoses recorded in charts. The Mini‐Cog was administered by a research assistant. | |
| USA | 569 | 7 primary care settings affiliated with Veterans Affairs in Minneapolis, Minnesota | Standard scoring | DSM IV | 90.3% | Participants were first screened for possible dementia by trained advanced practice registered nurses based on interview during routine visit with those who initially screened positive being offered additional evaluation with the index and reference standards. Some individuals who did not screen positive at the initial interview requested and received additional evaluation. |
DSM IV: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; DSM IV TR: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (text revision); NINCDS‐ADRDA: Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association
The extracted data for each study, including sensitivity and specificity, are summarized in summary of findings Table and in the forest plot presented in Figure 4. The sensitivities of the Mini‐Cog in the individual studies were reported as 1.00 (Carnero‐Pardo 2013), 1.00 (Fuchs 2012), 0.76 (Holsinger 2012) and 0.84 (McCarten 2012). The specificity of the Mini‐Cog varied in the individual studies and was 0.40 (Carnero‐Pardo 2013), 0.85 (Fuchs 2012), 0.73 (Holsinger 2012) and 0.27 (McCarten 2012). The values for the positive and negative predictive values are summarized in summary of findings Table. Meta‐analysis of the diagnostic test accuracy of the Mini‐Cog was initially planned in this review, although due to the small number of studies and methodological limitations of included studies, we did not perform a meta‐analysis.
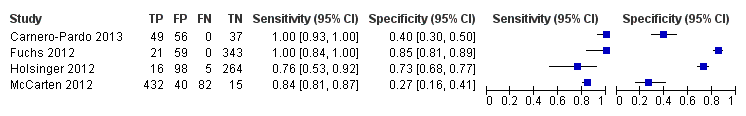
Forest plot of Analysis 1 Mini‐Cog
The small number of studies, significant heterogeneity between the studies, and overall poor quality of most of the included studies precluded the use of meta‐analysis to arrive at pooled estimates for the diagnostic test accuracy. The planned evaluations of heterogeneity and subgroup analyses were also not undertaken for these same reasons.
Discussion
Summary of main results
Overall we found a small number of studies that evaluated the test accuracy of the Mini‐Cog in primary care settings. The reported sensitivities and specificities of the Mini‐Cog varied significantly between studies, likely due to underlying differences in study populations and research methods utilized across the different studies. Of the included studies, only one study was of high quality with the remaining studies having methodological limitations that may have contributed to an overestimation of the accuracy of the Mini‐Cog in primary care. The heterogeneity of the study samples and methodological limitations present in the majority of the studies precluded formal meta‐analyses of study results and further analysis of some of the factors related to study design that may have affected the accuracy of the Mini‐Cog.
The one study in our review that was of high quality demonstrated that the accuracy of the Mini‐Cog has a sensitivity of 0.76 and specificity of 0.73 (Holsinger 2012). There is no agreed value for the sensitivity and specificity of cognitive screening tests in primary care settings. In primary care, it would be anticipated that the Mini‐Cog may be used initially as a screening test to identify individuals who would benefit from additional cognitive evaluation for dementia. In this situation, a brief test that has high sensitivity may be desirable. The sensitivity of the Mini‐Cog reported in the one high‐quality study identified in this review may not be high enough for the Mini‐Cog to be useful in this setting. One potential way that the sensitivity of the Mini‐Cog could be improved would be to modify the cut‐point on Mini‐Cog to increase its sensitivity, such as in McCarten 2011. Changing the cut‐point on the Mini‐Cog to improve its sensitivity would also likely reduce the specificity of Mini‐Cog, which would also need to be considered when using the Mini‐Cog in clinical settings. Although Holsinger 2012 reported that there was no statistical difference in the sensitivity of the Mini‐Cog when compared to the Modified Mini‐Mental State (3MS), the sensitivity of the 3MS was reported to be 0.86, which may be interpreted as a clinically significant difference in accuracy when compared to the sensitivity of the Mini‐Cog from the same study. The remaining studies of the Mini‐Cog in our review demonstrated higher sensitivities although these results must be interpreted with caution, as the remaining studies had methodological limitations that may have resulted in biased estimates of the sensitivity compared to Holsinger 2012. The low specificity of the Mini‐Cog reported in most studies would also make it unsuitable as a confirmatory test for dementia.
Multiple reviews of cognitive screening tests in primary care settings have identified the Mini‐Cog as a potentially appropriate cognitive test for primary care settings (Brodaty 2006; Ismail 2010; Lorentz 2002; Milne 2008; Tsoi 2015). These previous reviews have identified that the Mini‐Cog has some potentially attractive features as a cognitive test for primary care, such as being relatively brief and easy to measure and, in some studies, the sensitivity and specificity of the Mini‐Cog may appear to be adequate for use in this setting. However, one important limitation of these previous reviews is that the quality of the individual studies evaluating the Mini‐Cog was not taken into consideration when evaluating its accuracy. Based on the small number of included studies and the quality of these studies in this review, there is limited information regarding the accuracy of the Mini‐Cog for the diagnosis of Alzheimer's disease and other forms of dementia in primary care. Although the Mini‐Cog has been recommended as a test in primary care dementia screening programmes (Cordell 2013), at this time the existing evidence to support the routine use of the Mini‐Cog as a screening test in primary care is insufficient.
In addition, one feature common to all the included studies in this review may have also introduced a potential source of bias. All studies used a version of the Mini‐Cog that obtained the three‐word recall component of the Mini‐Cog as part of a larger neuropsychological test (i.e. the three‐word recall from the MMSE). The accuracy of the Mini‐Cog to diagnose dementia may have differed depending on whether the component tests were administered by themselves or if the results of the Mini‐Cog were derived from the results of more comprehensive testing. The three‐word recall component of the Mini‐Cog may be more sensitive and less specific when incorporated into a longer test battery. There may be a greater delay between registration of the three words and the recall task when this is incorporated into a longer test battery, compared to having the word recall task administered in isolation.
Strengths and weaknesses of the review
Strengths of our review include our use of a standardized search of electronic databases to identify both published and potentially unpublished studies evaluating the Mini‐Cog. We also used consistent data extraction processes throughout the review process. Importantly, we included an assessment of study quality, which identified that the majority of included studies had major methodological limitations and only one study was assessed at low risk of bias on all quality domains. In comparing the sensitivity reported in each study with the quality of studies, the three studies that were assessed as lower quality reported higher sensitivities than the one study that was found to be of higher quality study. Therefore, the results of the accuracy of the Mini‐Cog in each study should be interpreted with caution and the accuracy of the Mini‐Cog in some studies is potentially overestimated due to these methodological limitations. An additional limitation of this review was that we were unable to assess the accuracy of the Mini‐Cog in different types of dementia as initially planned. The Mini‐Cog may be more accurate in some forms of dementia, such as Alzheimer's disease, where memory is affected to greater extent early in the dementia process as compared to other types of dementia.
Applicability of findings to the review question
The Mini‐Cog would most commonly be used in primary care settings as a screening test to identify individuals who may or may not have identified cognitive complaints or dementia. Individuals testing positive on the Mini‐Cog would then likely be evaluated with additional cognitive tests in primary care or referred to specialists for further evaluation. Given the intended use of the Mini‐Cog in the diagnostic process as a screening tool, only two studies evaluated the Mini‐Cog as intended for use in most primary care settings to screen asymptomatic individuals for undetected dementia (Fuchs 2012; Holsinger 2012). Therefore, the results of some of the studies included in this review may not apply readily to the intended use of the Mini‐Cog in primary care settings. Additionally, the use of the Mini‐Cog as a diagnostic tool was the focus of separate reviews in the community setting (Fage 2015) and secondary care setting (Chan 2014).

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study

Forest plot of Analysis 1 Mini‐Cog
| Mini‐Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a primary care setting | ||||
| Population | The study populations were sampled from participants identified in primary care settings. | |||
| Setting | The primary care setting was identified as representing a sample that would be presenting to primary care settings where the Mini‐Cog might be used as a screening test to identify individuals who may benefit from additional evaluation. Studies that identified individuals in primary care where they received both the index test and a reference standard were used. | |||
| Indext test | The Mini‐Cog performed in insolation or scored based on results on the clock drawing test or three‐word recall were included. | |||
| Reference Standard | Clinical diagnosis of dementia was made using recognized standard diagnostic criteria. | |||
| Studies | Cross‐sectional studies were included, case control studies were excluded | |||
| Study | Accuracy (95% CI) | Number of participants | Dementia prevalence | Implications |
| Sensitivity: 1.00 (0.93 to 1.00) Specificity: 0.40 (0.30 to 0.50) | 142 | 34.5% | Participants were sampled including individuals who did have a pre‐existing history of dementia or cognitive impairment prior to assessment with the Mini‐Cog and reference standard but all participants had to have cognitive complaints suggestive of possible undiagnosed dementia or cognitive impairment. | |
| Sensitivity: 1.00 (0.84 to 1.00) Specificity: 0.85 (0.81 to 0.89) | 423 | 5.0% | The study excluded individuals with dementia at baseline, and those included in the study received a 36 month follow up assessment. Thus participants in the sample who were diagnosed with dementia were in the early stages of the disease. | |
| Sensitivity: 0.76 (0.53 to 0.92) Specificity: 0.73 (0.68 to 0.77) | 383 | 5.5% | Study involved evaluation of individuals in primary care settings without a documented history of dementia recorded at baseline. | |
| Sensitivity: 0.84 (0.81 to 0.87) Specificity: 0.27 (0.16 to 0.41) | 569 | 90.3% | Individuals with documented cognitive impairment were excluded from screening. Sampling involved screening of all participants in primary care and then offering further evaluation to individuals who either screened positive or negative on initial screening and who also agreed to have further evaluation. | |
| CI: confidence interval | ||||
| Study ID | Country | Participants (N) | Setting | Mini‐Cog scoring | Reference standard for dementia diagnosis | Dementia prevalence | Notes |
| Spain | 142 | 1 primary care location in Madrid and 3 primary care locations in Granada, only data from the Granada site was included | Standard scoring | DSM IV TR | 34.5% | The clock drawing test was incorporated into the reference standard at the Madrid site, data are presented for the Granada sites only. Screening was administered by professionals (no further specification) except for the clock drawing test component in Madrid, which was performed by a neurologist. | |
| Germany | 423 | Participants were randomly selected from 138 study centres in 6 metropolitan areas in Germany although study reports information from 29 sites recruited from Dusseldorf region | Standard scoring | DSM IV | 5.0% | Individuals with known dementia were excluded from the study. Study evaluated accuracy of the Mini‐Cog in detecting incident dementia at 36 months' follow‐up from enrolment. Screening tests were administered by a trained physician or psychologist. | |
| USA | 383 | Primary care locations affiliated with the Veterans Affairs near Durham, North Carolina | Standard scoring | DSM IV and NINCDS‐ADRDA | 5.5% | Excluded individuals with a known prior history of dementia based on diagnoses recorded in charts. The Mini‐Cog was administered by a research assistant. | |
| USA | 569 | 7 primary care settings affiliated with Veterans Affairs in Minneapolis, Minnesota | Standard scoring | DSM IV | 90.3% | Participants were first screened for possible dementia by trained advanced practice registered nurses based on interview during routine visit with those who initially screened positive being offered additional evaluation with the index and reference standards. Some individuals who did not screen positive at the initial interview requested and received additional evaluation. | |
| DSM IV: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition; DSM IV TR: Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (text revision); NINCDS‐ADRDA: Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association | |||||||
| Test | No. of studies | No. of participants |
| 1 Mini‐Cog Show forest plot | 4 | 1517 |