Monoterapia con fármacos antiepilépticos para la epilepsia: un metanálisis en red de datos individuales de participantes
Appendices
Appendix 1. Cochrane Register of Studies (CRS Web) search strategy
1. MeSH DESCRIPTOR Carbamazepine Explode All AND CENTRAL:TARGET
2. (Carbamazepin* OR Carbamazepen* OR Carbamezepin* OR CBZ OR SPD417 OR "Apo‐Carbamazepine" OR Atretol OR Biston OR Calepsin OR Carbagen OR Carbatrol OR Carbazepin* OR Carbelan OR Epitol OR Equetro OR Finlepsin OR Karbamazepin OR Lexin OR Neurotop OR "Novo‐Carbamaz" OR "Nu‐Carbamazepine" OR Sirtal OR Stazepin* OR "Taro‐Carbamazepine" OR Tegretal OR Tegretol OR Telesmin OR Teril OR Timonil):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
3. #1 OR #2 AND CENTRAL:TARGET
4. MeSH DESCRIPTOR Phenytoin Explode All AND CENTRAL:TARGET
5. (Aleviatin OR Antisacer OR Auranile OR Causoin OR Citrullamon OR Citrulliamon OR Comital OR Comitoina OR Convul OR Danten OR Dantinal OR Dantoin* OR Denyl OR "Di‐Hydan" OR "Di‐Lan" OR "Di‐Phetine" OR Didan OR Difenilhidantoin* OR Difenin OR Difetoin OR Difhydan OR Dihycon OR Dihydantoin OR Dilabid OR Dilantin* OR Dillantin OR Dintoin* OR Diphantoin OR Diphedal OR Diphedan OR Diphenat OR Diphenin* OR Diphentoin OR Diphentyn OR Diphenylan OR Diphenylhydantoin* OR Diphenylhydatanoin OR Ditoinate OR Ekko OR Elepsindon OR Enkelfel OR Epamin OR Epanutin OR Epasmir OR Epdantoin* OR Epelin OR Epifenyl OR Epihydan OR Epilan OR Epilantin OR Epinat OR Epised OR Eptal OR Eptoin OR Fenantoin OR Fenidantoin OR Fenitoin* OR Fentoin OR Fenylepsin OR Fenytoin* OR "Gerot‐epilan‐D" OR Hidan OR Hidant* OR Hindatal OR Hydant* OR Ictalis OR Idantoi* OR Iphenylhydantoin OR Kessodanten OR Labopal OR Lehydan OR Lepitoin OR Lepsin OR Mesantoin OR Minetoin OR "Neos‐Hidantoina" OR Neosidantoina OR Novantoina OR Novophenytoin OR "Om‐hidantoina" OR "Om‐Hydantoine" OR Oxylan OR Phanantin* OR Phenatine OR Phenatoine OR Phenhydan* OR Phenitoin OR Phentoin OR Phentytoin OR Phenytek OR Phenytex OR Phenytoin* OR PHT OR Ritmenal OR Saceril OR Sanepil OR Silantin OR Sinergina OR Sodanthon OR Sodanto* OR Solantin OR Solantoin OR Solantyl OR Sylantoic OR Tacosal OR Thilophenyl OR TOIN OR Zentronal OR Zentropil):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
6. #4 OR #5 AND CENTRAL:TARGET
7. MeSH DESCRIPTOR Valproic Acid Explode All AND CENTRAL:TARGET
8. (Avugane OR Baceca OR Convulex OR Delepsine OR Depacon OR Depakene OR Depakine OR Depakote OR Deproic OR DPA OR Encorate OR Epiject OR Epilex OR Epilim OR Episenta OR Epival OR Ergenyl OR Mylproin OR Orfiril OR Orlept OR Selenica OR Stavzor OR Valcote OR Valparin OR Valpro* OR VPA):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
9. #7 OR #8 AND CENTRAL:TARGET
10. MeSH DESCRIPTOR Phenobarbital Explode All AND CENTRAL:TARGET
11. (Adonal OR Aephenal OR Agrypnal OR Amylofene OR Aphenylbarbit OR Aphenyletten OR Barbenyl OR Barbinal OR Barbiphen* OR Barbipil OR Barbita OR Barbivis OR Barbonal OR Barbophen OR Bardorm OR Bartol OR Bialminal OR "Blu‐Phen" OR Cabronal OR Calmetten OR Calminal OR Cardenal OR Chinoin OR Codibarbita OR Coronaletta OR Cratecil OR Damoral OR Dezibarbitur OR Dormina OR Dormiral OR Dormital OR Doscalun OR Duneryl OR Ensobarb OR Ensodorm OR Epanal OR Epidorm OR Epilol OR Episedal OR Epsylone OR Eskabarb OR Etilfen OR Euneryl OR Fenbital OR Fenemal OR Fenobarbital OR Fenosed OR Fenylettae OR Gardenal OR Gardepanyl OR Glysoletten OR Haplopan OR Haplos OR Helional OR Hennoletten OR Henotal OR Hypnaletten OR Hypnette OR "Hypno‐Tablinetten" OR Hypnogen OR Hypnolone OR Hypnoltol OR Hysteps OR Lefebar OR Leonal OR Lephebar OR Lepinal OR Lepinaletten OR Linasen OR Liquital OR Lixophen OR Lubergal OR Lubrokal OR Lumen OR Lumesettes OR Lumesyn OR Luminal OR Lumofridetten OR Luphenil OR Luramin OR Molinal OR Neurobarb OR Nirvonal OR Noptil OR "Nova‐Pheno" OR Nunol OR Parkotal OR PB OR Pharmetten OR "Phen‐Bar" OR Phenaemal OR Phenemal* OR Phenobal OR Phenobarbit* OR Phenobarbyl OR Phenoluric OR Phenolurio OR Phenomet OR Phenonyl OR Phenoturic OR Phenylethylbarbit* OR Phenylethylmalonylurea OR Phenyletten OR Phenyral OR Phob OR Polcominal OR Prominal OR Promptonal OR "Seda‐Tablinen" OR Sedabar OR Sedicat OR Sedizorin OR Sedlyn OR Sedofen OR Sedonal OR Sedonettes OR Sevenal OR Sinoratox OR Solfoton OR "Solu‐Barb" OR Sombutol OR Somnolens OR Somnoletten OR Somnosan OR Somonal OR Spasepilin OR Starifen OR Starilettae OR Stental OR Talpheno OR Teolaxin OR Teoloxin OR Thenobarbital OR Theoloxin OR Triabarb OR Tridezibarbitur OR Triphenatol OR Versomnal OR Zadoletten OR Zadonal):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
12. #10 OR #11 AND CENTRAL:TARGET
13. MeSH DESCRIPTOR Oxcarbazepine Explode All AND CENTRAL:TARGET
14. (Oxcarbazepin* OR Actinium OR Barzepin OR Carbox OR Deprectal OR "GP 47680" OR Lonazet OR OCBZ OR Oxalepsy OR OXC OR Oxcarbamazepine OR Oxetol OR Oxpin OR Oxrate OR Oxtellar OR Oxypine OR Pharozepine OR Prolepsi OR Timox OR Trexapin OR Trileptal OR Trileptin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
15. #13 OR #14 AND CENTRAL:TARGET
16. MeSH DESCRIPTOR Lamotrigine Explode All AND CENTRAL:TARGET
17. (Lamotrigin* OR Elmendos OR Epilepax OR "GW 273293" OR Lamictal OR Lamictin OR Lamitor OR Lamitrin OR Lamogine OR Lamotrine OR LTG):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
18. #16 OR #17 AND CENTRAL:TARGET
19. MeSH DESCRIPTOR Gabapentin Explode All AND CENTRAL:TARGET
20. (Gabapentin* OR Aclonium OR Fanatrex OR Gabapetin OR Gabarone OR GBP OR Gralise OR Neogab OR Neurontin OR "Novo‐Gabapentin" OR Nupentin):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
21. #19 OR #20 AND CENTRAL:TARGET
22. MeSH DESCRIPTOR Topiramate Explode All AND CENTRAL:TARGET
23. (Topiramat* OR Qudexy OR Tipiramate OR Topamax OR "Topiramic acid" OR TPM):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
24. #22 OR #23 AND CENTRAL:TARGET
25. MeSH DESCRIPTOR Levetiracetam Explode All AND CENTRAL:TARGET
26. (Levetiracetam* OR Keppra OR LEV OR Levitiracetam):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
27. #25 OR #26 AND CENTRAL:TARGET
28. MeSH DESCRIPTOR Zonisamide Explode All AND CENTRAL:TARGET
29. (Zonisamid* OR Exceglan OR Excegram OR Excegran OR ZNS OR Zonegran):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
30. #28 OR #29 AND CENTRAL:TARGET
31. #3 OR #6 OR #9 OR #12 OR #15 OR #18 OR #21 OR #24 OR #27 OR #30 AND CENTRAL:TARGET
32. ((adjunct* or "add‐on" or "add on" or adjuvant* or combination* or polytherap*) not (monotherap* or alone or singl*)):TI AND CENTRAL:TARGET
33. #31 NOT #32 AND CENTRAL:TARGET
34. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
35. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
36. (epilep* OR seizure* OR convuls*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
37. #34 OR #35 OR #36 AND CENTRAL:TARGET
38. eclampsia:TI AND CENTRAL:TARGET
39. #37 NOT #38 AND CENTRAL:TARGET
40. #33 AND #39 AND CENTRAL:TARGET
41. >12/09/2019:CRSCREATED AND CENTRAL:TARGET
#40 AND #41 AND CENTRAL:TARGET
Appendix 2. MEDLINE search strategy
This strategy includes a modification of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2021).
1. exp Carbamazepine/
2. (Carbamazepin* or Carbamazepen* or Carbamezepin* or CBZ or SPD417 or "Apo‐Carbamazepine" or Atretol or Biston or Calepsin or Carbagen or Carbatrol or Carbazepin* or Carbelan or Epitol or Equetro or Finlepsin or Karbamazepin or Lexin or Neurotop or "Novo‐Carbamaz" or "Nu‐Carbamazepine" or Sirtal or Stazepin* or "Taro‐Carbamazepine" or Tegretal or Tegretol or Telesmin or Teril or Timonil).tw.
3. 1 or 2
4. exp Phenytoin/
5. (Aleviatin or Antisacer or Auranile or Causoin or Citrullamon or Citrulliamon or Comital or Comitoina or Convul or Danten or Dantinal or Dantoin* or Denyl or "Di‐Hydan" or "Di‐Lan" or "Di‐Phetine" or Didan or Difenilhidantoin* or Difenin or Difetoin or Difhydan or Dihycon or Dihydantoin or Dilabid or Dilantin* or Dillantin or Dintoin* or Diphantoin or Diphedal or Diphedan or Diphenat or Diphenin* or Diphentoin or Diphentyn or Diphenylan or Diphenylhydantoin* or Diphenylhydatanoin or Ditoinate or Ekko or Elepsindon or Enkelfel or Epamin or Epanutin or Epasmir or Epdantoin* or Epelin or Epifenyl or Epihydan or Epilan or Epilantin or Epinat or Epised or Eptal or Eptoin or Fenantoin or Fenidantoin or Fenitoin* or Fentoin or Fenylepsin or Fenytoin* or "Gerot‐epilan‐D" or Hidan or Hidant* or Hindatal or Hydant* or Ictalis or Idantoi* or Iphenylhydantoin or Kessodanten or Labopal or Lehydan or Lepitoin or Lepsin or Mesantoin or Minetoin or "Neos‐Hidantoina" or Neosidantoina or Novantoina or Novophenytoin or "Om‐hidantoina" or "Om‐Hydantoine" or Oxylan or Phanantin* or Phenatine or Phenatoine or Phenhydan* or Phenitoin or Phentoin or Phentytoin or Phenytek or Phenytex or Phenytoin* or PHT or Ritmenal or Saceril or Sanepil or Silantin or Sinergina or Sodanthon or Sodanto* or Solantin or Solantoin or Solantyl or Sylantoic or Tacosal or Thilophenyl or TOIN or Zentronal or Zentropil).tw.
6. 4 or 5
7. exp Valproic Acid/
8. (Avugane or Baceca or Convulex or Delepsine or Depacon or Depakene or Depakine or Depakote or Deproic or DPA or Encorate or Epiject or Epilex or Epilim or Episenta or Epival or Ergenyl or Mylproin or Orfiril or Orlept or Selenica or Stavzor or Valcote or Valparin or Valpro* or VPA).tw.
9. 7 or 8
10. exp Phenobarbital/
11. (Adonal or Aephenal or Agrypnal or Amylofene or Aphenylbarbit or Aphenyletten or Barbenyl or Barbinal or Barbiphen* or Barbipil or Barbita or Barbivis or Barbonal or Barbophen or Bardorm or Bartol or Bialminal or "Blu‐Phen" or Cabronal or Calmetten or Calminal or Cardenal or Chinoin or Codibarbita or Coronaletta or Cratecil or Damoral or Dezibarbitur or Dormina or Dormiral or Dormital or Doscalun or Duneryl or Ensobarb or Ensodorm or Epanal or Epidorm or Epilol or Episedal or Epsylone or Eskabarb or Etilfen or Euneryl or Fenbital or Fenemal or Fenobarbital or Fenosed or Fenylettae or Gardenal or Gardepanyl or Glysoletten or Haplopan or Haplos or Helional or Hennoletten or Henotal or Hypnaletten or Hypnette or "Hypno‐Tablinetten" or Hypnogen or Hypnolone or Hypnoltol or Hysteps or Lefebar or Leonal or Lephebar or Lepinal or Lepinaletten or Linasen or Liquital or Lixophen or Lubergal or Lubrokal or Lumen or Lumesettes or Lumesyn or Luminal or Lumofridetten or Luphenil or Luramin or Molinal or Neurobarb or Nirvonal or Noptil or "Nova‐Pheno" or Nunol or Parkotal or PB or Pharmetten or "Phen‐Bar" or Phenaemal or Phenemal* or Phenobal or Phenobarbit* or Phenobarbyl or Phenoluric or Phenolurio or Phenomet or Phenonyl or Phenoturic or Phenylethylbarbit* or Phenylethylmalonylurea or Phenyletten or Phenyral or Phob or Polcominal or Prominal or Promptonal or "Seda‐Tablinen" or Sedabar or Sedicat or Sedizorin or Sedlyn or Sedofen or Sedonal or Sedonettes or Sevenal or Sinoratox or Solfoton or "Solu‐Barb" or Sombutol or Somnolens or Somnoletten or Somnosan or Somonal or Spasepilin or Starifen or Starilettae or Stental or Talpheno or Teolaxin or Teoloxin or Thenobarbital or Theoloxin or Triabarb or Tridezibarbitur or Triphenatol or Versomnal or Zadoletten or Zadonal).tw.
12. 10 or 11
13. exp Oxcarbazepine/
14. (Oxcarbazepin* or Actinium or Barzepin or Carbox or Deprectal or "GP 47680" or Lonazet or OCBZ or Oxalepsy or OXC or Oxcarbamazepine or Oxetol or Oxpin or Oxrate or Oxtellar or Oxypine or Pharozepine or Prolepsi or Timox or Trexapin or Trileptal or Trileptin).tw.
15. 13 or 14
16. exp Lamotrigine/
17. (Lamotrigin* or Elmendos or Epilepax or "GW 273293" or Lamictal or Lamictin or Lamitor or Lamitrin or Lamogine or Lamotrine or LTG).tw.
18. 16 or 17
19. exp Gabapentin/
20. (Gabapentin* or Aclonium or Fanatrex or Gabapetin or Gabarone or GBP or Gralise or Neogab or Neurontin or "Novo‐Gabapentin" or Nupentin).tw.
21. 19 or 20
22. exp Topiramate/
23. (Topiramat* or Qudexy or Tipiramate or Topamax or "Topiramic acid" or TPM).tw.
24. 22 or 23
25. exp Levetiracetam/
26. (Levetiracetam* or Keppra or LEV or Levitiracetam).tw.
27. 25 or 26
28. exp Zonisamide/
29. (Zonisamid* or Exceglan or Excegram or Excegran or ZNS or Zonegran).tw.
30. 28 or 29
31. 3 or 6 or 9 or 12 or 15 or 18 or 21 or 24 or 27 or 30
32. ((adjunct$ or "add‐on" or "add on" or adjuvant$ or combination$ or polytherap$) not (monotherap$ or alone or singl$)).ti.
33. 31 not 32
34. exp Epilepsy/
35. exp Seizures/
36. (epilep$ or seizure$ or convuls$).tw.
37. 34 or 35 or 36
38. exp Pre‐Eclampsia/ or exp Eclampsia/
39. 37 not 38
40. exp controlled clinical trial/ or (randomi?ed or placebo or randomly).ab.
41. clinical trials as topic.sh.
42. trial.ti.
43. 40 or 41 or 42
44. exp animals/ not humans.sh.
45. 43 not 44
46. 33 and 39 and 45
47. limit 46 to ed=20190911‐20210412
48. 46 not (1$ or 2$).ed.
49. 48 and (2019$ or 2020$ or 2021$).dt.
50. 47 or 49
51. remove duplicates from 50
Appendix 3. SCOPUS search strategy
(((TITLE (carbamazepine OR carbamezepine OR cbz OR spd417 OR apo‐carbamazepine OR atretol OR biston OR calepsin OR carbagen OR carbamazepen OR carbatrol OR carbazepine OR carbelan OR epitol OR equetro OR finlepsin OR karbamazepin OR lexin OR neurotop OR novo‐carbamaz OR nu‐carbamazepine OR sirtal OR stazepin OR stazepine OR taro‐carbamazepine OR tegretal OR tegretol OR telesmin OR teril OR timonil OR phenytoin OR dihydantoin OR diphenylhydantoin OR diphenylhydantoine OR diphenylhydatanoin OR fenitoina OR phenytoine OR phenytoinum OR aleviatin OR antisacer OR auranile OR causoin OR citrullamon OR citrulliamon OR comital OR comitoina OR convul OR danten OR dantinal OR dantoinal OR dantoine OR denyl OR di‐hydan OR di‐lan OR di‐phetine OR didan OR difenilhidantoina OR difenin OR difetoin OR difhydan OR dihycon OR dilabid OR dilantin OR dilantine OR dillantin OR dintoin OR dintoina OR diphantoin OR diphedal OR diphedan OR diphenat OR diphenin OR diphenine OR dipheninum OR diphentoin OR diphentyn OR diphenylan OR ditoinate OR ekko OR elepsindon OR enkelfel OR epamin OR epanutin OR epasmir OR epdantoin OR epdantoine OR epelin OR epifenyl OR epihydan OR epilan OR epilantin OR epinat OR epised OR eptal OR eptoin OR fenantoin OR fenidantoin OR fentoin OR fenylepsin OR fenytoin OR fenytoine OR gerot‐epilan‐d OR hidan OR hidantal OR hidantilo OR hidantina OR hidantomin OR hindatal OR hydantal OR hydantin OR hydantoin OR hydantoinal OR hydantol OR ictalis OR idantoil OR idantoin OR iphenylhydantoin OR kessodanten OR labopal OR lehydan OR lepitoin OR lepsin OR mesantoin OR minetoin OR neos‐hidantoina OR neosidantoina OR novantoina OR novophenytoin OR om‐hidantoina OR om‐hydantoine OR oxylan OR phanantin OR phanatine OR phenatine OR phenatoine OR phenhydan OR phenhydanin OR phenitoin OR phentoin OR phentytoin OR phenytek OR phenytex OR ritmenal OR saceril OR sanepil OR silantin OR sinergina OR sodanthon OR sodantoin OR sodanton OR solantin OR solantoin OR solantyl OR sylantoic OR tacosal OR thilophenyl OR toin OR zentronal OR zentropil OR pht OR "Valproic Acid" OR avugane OR baceca OR convulex OR delepsine OR depacon OR depakene OR depakine OR depakote OR deproic OR epiject OR epilex OR epilim OR episenta OR epival OR ergenyl OR mylproin OR orfiril OR orlept OR selenica OR stavzor OR valcote OR valparin OR valpro OR valproate OR valproic OR vpa OR phenobarbital OR fenobarbital OR phenobarbitol OR phenobarbitone OR "Phenobarbituric Acid" OR phenylethylbarbiturate OR "Phenylethylbarbituric Acid" OR phenylethylmalonylurea OR adonal OR aephenal OR agrypnal OR amylofene OR aphenylbarbit OR aphenyletten OR barbenyl OR barbinal OR barbiphen OR barbiphenyl OR barbipil OR barbita OR barbivis OR barbonal OR barbophen OR bardorm OR bartol OR bialminal OR blu‐phen OR cabronal OR calmetten OR calminal OR cardenal OR chinoin OR codibarbita OR coronaletta OR cratecil OR damoral OR dezibarbitur OR dormina OR dormiral OR dormital OR doscalun OR duneryl OR ensobarb OR ensodorm OR epanal OR epidorm OR epilol OR episedal OR epsylone OR eskabarb OR etilfen OR euneryl OR fenbital OR fenemal OR fenosed OR fenylettae OR gardenal OR gardepanyl OR glysoletten OR haplopan OR haplos OR helional OR hennoletten OR henotal OR hypnaletten OR hypnette OR hypno‐tablinetten OR hypnogen OR hypnolone OR hypnoltol OR hysteps OR lefebar OR leonal OR lephebar OR lepinal OR lepinaletten OR linasen OR liquital OR lixophen OR lubergal OR lubrokal OR lumen OR lumesettes OR lumesyn OR luminal OR lumofridetten OR luphenil OR luramin OR molinal OR neurobarb OR nirvonal OR noptil OR nova‐pheno OR nunol OR parkotal OR pharmetten OR phen‐bar OR phenaemal OR phenemal OR phenemalum OR phenobal OR phenobarbyl OR phenoluric OR phenolurio OR phenomet OR phenonyl OR phenoturic OR phenyletten OR phenyral OR phob OR polcominal OR prominal OR promptonal OR seda‐tablinen OR sedabar OR sedicat OR sedizorin OR sedlyn OR sedofen OR sedonal OR sedonettes OR sevenal OR sinoratox OR solfoton OR solu‐barb OR sombutol OR somnolens OR somnoletten OR somnosan OR somonal OR spasepilin OR starifen OR starilettae OR stental OR talpheno OR teolaxin OR teoloxin OR thenobarbital OR theoloxin OR triabarb OR tridezibarbitur OR triphenatol OR versomnal OR zadoletten OR zadonal OR pb OR oxcarbazepine OR "GP 47680" OR ocbz OR oxcarbamazepine OR actinium OR barzepin OR carbox OR deprectal OR lonazet OR oxalepsy OR oxetol OR oxpin OR oxrate OR oxtellar OR oxypine OR pharozepine OR prolepsi OR timox OR trexapin OR trileptal OR trileptin OR oxc OR lamotrigine OR "GW 273293" OR lamotrigina OR lamotriginum OR lamictal OR lamotrine OR lamitrin OR lamictin OR lamogine OR lamitor OR ltg OR gabapentin OR gabapentine OR gabapentino OR gabapentinum OR gabapetin OR aclonium OR fanatrex OR gabarone OR neogab OR gralise OR neurontin OR novo‐gabapentin OR nupentin OR gbp OR topiramate OR tipiramate OR topiramatum OR "Topiramic acid" OR topamax OR tpm OR levetiracetam OR levetiracetamum OR levitiracetam OR keppra OR lev OR zonisamide OR zonisamida OR zonisamidum OR zonegran OR exceglan OR excegram OR excegran OR zns)) OR (ABS(carbamazepine OR carbamezepine OR cbz OR spd417 OR apo‐carbamazepine OR atretol OR biston OR calepsin OR carbagen OR carbamazepen OR carbatrol OR carbazepine OR carbelan OR epitol OR equetro OR finlepsin OR karbamazepin OR lexin OR neurotol OR novo‐carbamaz OR nu‐carbamazepine OR sirtal OR stazepin OR stazepine OR taro‐carbamazepine OR tegretal OR tegretol OR telesmin OR teril OR timonil OR phenytoin OR dihydantoin OR diphenylhydantoin OR diphenylhydantoine OR diphenylhydatanoin OR fenitoina OR phenytoine OR phenytoinum OR aleviatin OR antisacer OR auranile OR causoin OR citrullamon OR citrulliamon OR comital OR comitoina OR convul OR danten OR dantinal OR dantoinal OR dantoine OR denyl OR di‐hydan OR di‐lan OR di‐phetine OR didan OR difenilhidantoina OR difenin OR difetoin OR difhydan OR dihycon OR dilabid OR dilantin OR dilantine OR dillantin OR dintoin OR dintoina OR diphantoin OR diphedal OR diphedan OR diphenat OR diphenin OR diphenine OR dipheninum OR diphentoin OR diphentyn OR diphenylan OR ditoinate OR ekko OR elepsindon OR enkelfel OR epamin OR epanutin OR epasmir OR epdantoin OR epdantoine OR epelin OR epifenyl OR epihydan OR epilan OR epilantin OR epinat OR epised OR eptal OR eptoin OR fenantoin OR fenidantoin OR fentoin OR fenylepsin OR fenytoin OR fenytoine OR gerot‐epilan‐d OR hidan OR hidantal OR hidantilo OR hidantina OR hidantomin OR hindatal OR hydantal OR hydantin OR hydantoin OR hydantoinal OR hydantol OR ictalis OR idantoil OR idantoin OR iphenylhydantoin OR kessodanten OR labopal OR lehydan OR lepitoin OR lepsin OR mesantoin OR minetoin OR neos‐hidantoina OR neosidantoina OR novantoina OR novophenytoin OR om‐hidantoina OR om‐hydantoine OR oxylan OR phanantin OR phanatine OR phenatine OR phenatoine OR phenhydan OR phenhydanin OR phenitoin OR phentoin OR phentytoin OR phenytek OR phenytex OR ritmenal OR saceril OR sanepil OR silantin OR sinergina OR sodanthon OR sodantoin OR sodanton OR solantin OR solantoin OR solantyl OR sylantoic OR tacosal OR thilophenyl OR toin OR zentronal OR zentropil OR pht OR "Valproic Acid" OR avugane OR baceca OR convulex OR delepsine OR depacon OR depakene OR depakine OR depakote OR deproic OR epiject OR epilex OR epilim OR episenta OR epival OR ergenyl OR mylproin OR orfiril OR orlept OR selenica OR stavzor OR valcote OR valparin OR valpro OR valproate OR valproic OR vpa OR phenobarbital OR fenobarbital OR phenobarbitol OR phenobarbitone OR "Phenobarbituric Acid" OR phenylethylbarbiturate OR "Phenylethylbarbituric Acid" OR phenylethylmalonylurea OR adonal OR aephenal OR agrypnal OR amylofene OR aphenylbarbit OR aphenyletten OR barbenyl OR barbinal OR barbiphen OR barbiphenyl OR barbipil OR barbita OR barbivis OR barbonal OR barbophen OR bardorm OR bartol OR bialminal OR blu‐phen OR cabronal OR calmetten OR calminal OR cardenal OR chinoin OR codibarbita OR coronaletta OR cratecil OR damoral OR dezibarbitur OR dormina OR dormiral OR dormital OR doscalun OR duneryl OR ensobarb OR ensodorm OR epanal OR epidorm OR epilol OR episedal OR epsylone OR eskabarb OR etilfen OR euneryl OR fenbital OR fenemal OR fenosed OR fenylettae OR gardenal OR gardepanyl OR glysoletten OR haplopan OR haplos OR helional OR hennoletten OR henotal OR hypnaletten OR hypnette OR hypno‐tablinetten OR hypnogen OR hypnolone OR hypnoltol OR hysteps OR lefebar OR leonal OR lephebar OR lepinal OR lepinaletten OR linasen OR liquital OR lixophen OR lubergal OR lubrokal OR lumen OR lumesettes OR lumesyn OR luminal OR lumofridetten OR luphenil OR luramin OR molinal OR neurobarb OR nirvonal OR noptil OR nova‐pheno OR nunol OR parkotal OR pharmetten OR phen‐bar OR phenaemal OR phenemal OR phenemalum OR phenobal OR phenobarbyl OR phenoluric OR phenolurio OR phenomet OR phenonyl OR phenoturic OR phenyletten OR phenyral OR phob OR polcominal OR prominal OR promptonal OR seda‐tablinen OR sedabar OR sedicat OR sedizorin OR sedlyn OR sedofen OR sedonal OR sedonettes OR sevenal OR sinoratox OR solfoton OR solu‐barb OR sombutol OR somnolens OR somnoletten OR somnosan OR somonal OR spasepilin OR starifen OR starilettae OR stental OR talpheno OR teolaxin OR teoloxin OR thenobarbital OR theoloxin OR triabarb OR tridezibarbitur OR triphenatol OR versomnal OR zadoletten OR zadonal OR pb OR oxcarbazepine OR "GP 47680" OR ocbz OR oxcarbamazepine OR actinium OR barzepin OR carbox OR deprectal OR lonazet OR oxalepsy OR oxetol OR oxpin OR oxrate OR oxtellar OR oxypine OR pharozepine OR prolepsi OR timox OR trexapin OR trileptal OR trileptin OR oxc OR lamotrigine OR "GW 273293" OR lamotrigina OR lamotriginum OR lamictal OR lamotrine OR lamitrin OR lamictin OR lamogine OR lamitor OR ltg OR gabapentin OR gabapentine OR gabapentino OR gabapentinum OR gabapetin OR aclonium OR fanatrex OR gabarone OR neogab OR gralise OR neurontin OR novo‐gabapentin OR nupentin OR gbp OR topiramate OR tipiramate OR topiramatum OR "Topiramic acid" OR topamax OR tpm OR levetiracetam OR levetiracetamum OR levitiracetam OR keppra OR lev OR zonisamide OR zonisamida OR zonisamidum OR zonegran OR exceglan OR excegram OR excegran OR zns))) AND ((TITLE‐ABS‐KEY(epilep* OR "infantile spasm" OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency") OR (TITLE‐ABS‐KEY(syndrome) W/2 (aicardi OR angelman OR doose OR dravet OR janz OR jeavons OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR tassinari OR "unverricht lundborg" OR west)) OR TITLE(seizure OR convuls*) OR (TITLE‐ABS‐KEY(lafora*) W/4 (disease OR epilep*) AND NOT (TITLE(dog OR canine) OR INDEXTERMS(dog OR canine)))) AND NOT (TITLE(*eclampsia) OR INDEXTERMS(*eclampsia)) AND NOT INDEX(medl)) AND (TITLE(randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") OR ABS(randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study) AND NOT INDEX(medl))) AND NOT (TITLE((adjunct* OR "add‐on" OR "add on" OR adjuvant* OR combination* OR polytherap*) AND NOT (monotherap* OR alone OR singl*)))

Network plot of pairwise comparisons regardless of whether any outcome data (IPD or aggregate data) were available; all individuals included within the review, (total 22,040 participants), participants with focal seizures and participants with generalised tonic‐clonic seizures with or without other seizure types (shortened to 'generalised seizures' for brevity).
Out of a total of 22,040 participants, 15,148 participants were classified as experiencing focal onset seizures (69% of total), 5268 participants were classified as experiencing generalised onset seizures (24% of total) and 1624 had an unclassified or missing seizure type (7% of total).
Note that the size of the node indicates the number of studies the drug is included in and the thickness of the edges corresponds to the number of participants contributing to the comparison (i.e. larger node = more studies, thicker edge = more participants).
CBZ: carbamazepine; ESL: eslicarbazepine acetate; GBP: gabapentin; LCM: lacosamide; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures.

Network plot of pairwise comparisons in all included studies, studies providing individual participant data (IPD) and studies without IPD
Note that the size of the node indicates the number of studies the drug is included in and the thickness of the edges corresponds to the number of participants contributing to the comparison (i.e. larger node = more studies, thicker edge = more participants).
CBZ: carbamazepine; ESL: eslicarbazepine acetate; GBP: gabapentin; LCM: lacosamide; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHB: phenobarbitone; PHT: phenytoin; TPM: topiramate; VPS: sodium valproate; ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures.

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
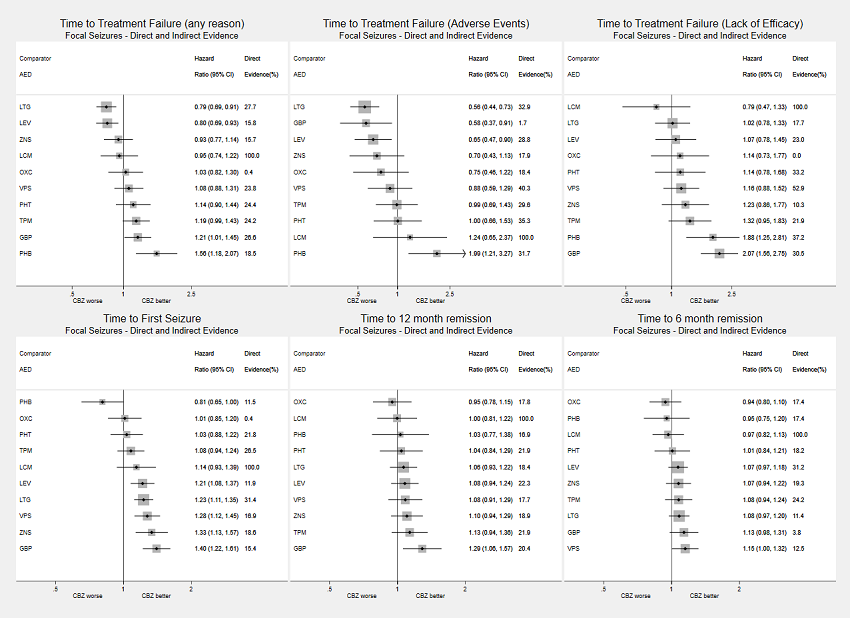
Network meta‐analysis results (direct and indirect evidence combined) for individuals with focal seizures, all drugs compared to carbamazepine (CBZ)
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures
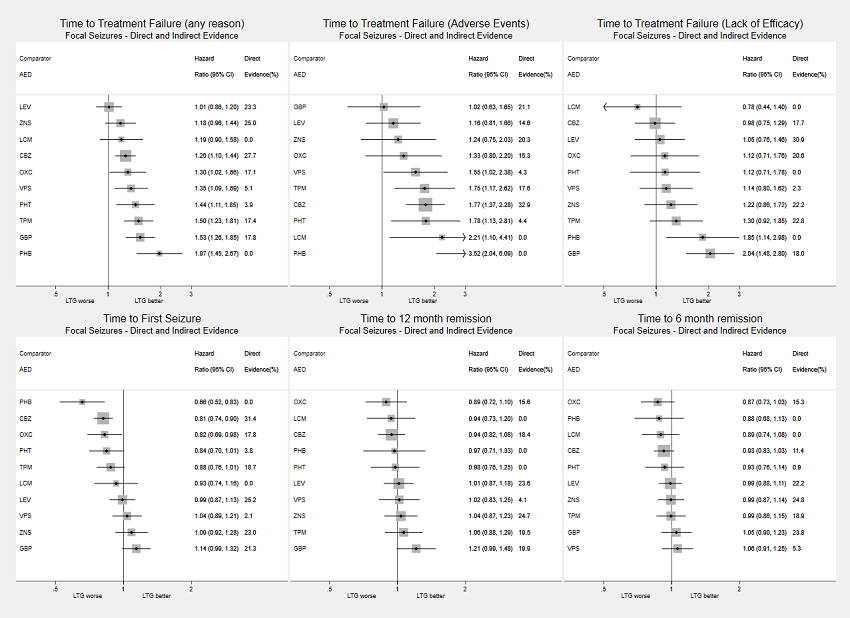
Network meta‐analysis results (direct and indirect evidence combined) for individuals with focal seizures, all drugs compared to lamotrigine (LTG)
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures
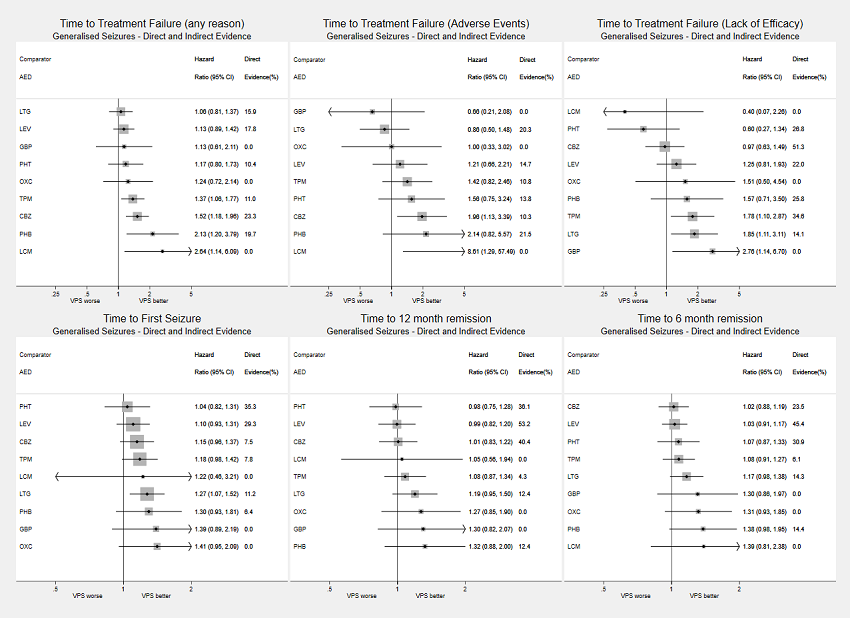
Network meta‐analysis results (direct and indirect evidence combined) for individuals with generalised seizures, all drugs compared to sodium valproate (VPS)
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Network meta‐analysis results (direct and indirect evidence combined) for individuals with focal seizures, all pairwise comparisons for time to treatment failure outcomes
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Network meta‐analysis results (direct and indirect evidence combined) for individuals with generalised seizures, all pairwise comparisons for time to treatment failure outcomes
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures
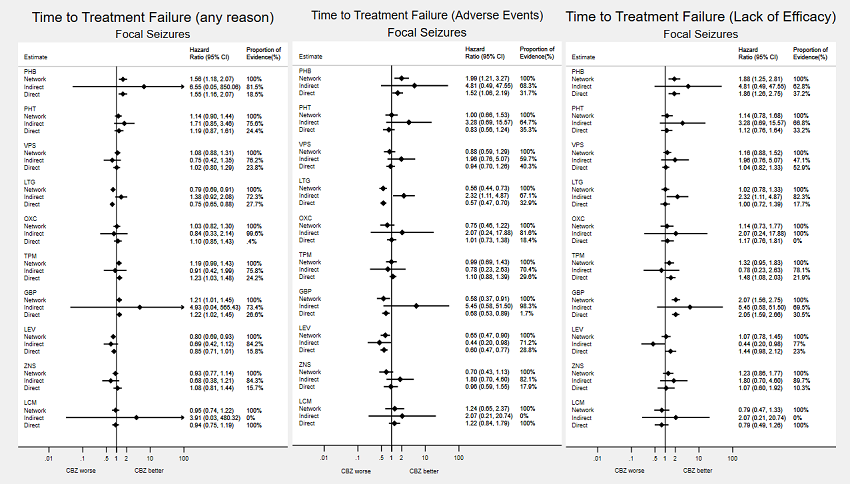
Consistency: direct, indirect and network estimates for individuals with focal seizures compared to carbamazepine (CBZ) for time to treatment failure outcomes. Numerical results from investigations of inconsistency for all pairwise comparisons are available from the corresponding author on request.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Consistency: direct, indirect and network estimates for individuals with focal seizures compared to lamotrigine (LTG) for time to treatment failure outcomes. Numerical results from investigations of inconsistency for all pairwise comparisons are available from the corresponding author on request.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Consistency: Direct, indirect and network estimates for individuals with generalised seizures compared to sodium valproate (VPS) for time to treatment failure outcomes. Numerical results from investigations of inconsistency for all pairwise comparisons are available from the corresponding author on request.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Network meta‐analysis results (direct and indirect evidence combined) for individuals with focal seizures, all pairwise comparisons for time to 12‐month remission, time to six‐month remission and time to first seizure
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Network meta‐analysis results (direct and indirect evidence combined) for individuals with generalised seizures, all pairwise comparisons for time to 12‐month remission, time to six‐month remission and time to first seizure
Note: direct evidence (%) is the proportion of the estimate contributed by direct evidence and the box size is proportional to the number of participants contributing direct evidence.
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Consistency: direct, indirect and network estimates for individuals with focal seizures compared to carbamazepine (CBZ) for time to 12‐month remission, time to six‐month remission and time to first seizure. Numerical results from investigations of inconsistency for all pairwise comparisons are available from the corresponding author on request.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Consistency: direct, indirect and network estimates for individuals with focal seizures compared to lamotrigine (LTG) for time to 12‐month remission, time to six‐month remission and time to first seizure. Numerical results from investigations of inconsistency for all pairwise comparisons are available from the corresponding author on request.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures

Consistency: direct, indirect and network estimates for individuals with generalised seizures compared to sodium valproate (VPS) for time to 12‐month remission, time to six‐month remission and time to first seizure. Numerical results from investigations of inconsistency for all pairwise comparisons are available from the corresponding author on request.
Note: direct evidence comes from studies that compared the drugs (head‐to‐head comparisons), indirect evidence comes from studies that did not compare the drugs (indirect comparisons) and network evidence comes from the whole network (head‐to‐head and indirect comparisons for all drugs).
Generalised tonic‐clonic seizures with or without other seizure types is shortened to 'Generalised seizures' for brevity.
AED: antiepileptic drug
CBZ: carbamazepine
CI: confidence interval
GBP: gabapentin
LCM: lacosamide
LEV: levetiracetam
LTG: lamotrigine
OXC: oxcarbazepine
PHB: phenobarbitone
PHT: phenytoin
TPM: topiramate
VPS: sodium valproate
ZNS: zonisamide
To see a magnified version of this figure, please see https://epilepsy.cochrane.org/network-meta-analysis-figures
| Antiepileptic drug monotherapy for epilepsy: time to treatment failure for participants with focal seizures (reference carbamazepine) | ||||||||
| Patient or population: adults and children with focal seizures Settings: outpatients globally, followed up in RCTs for up to 12 years Intervention: phenobarbitone, phenytoin, sodium valproate, lamotrigine, oxcarbazepine, topiramate, gabapentin, levetiracetam, zonisamide and lacosamide Comparison: carbamazepine | ||||||||
| Outcome | Intervention (experimental treatment)a | Comparison (reference treatment) | No of participants direct evidence | Relative effect sizes | Direct evidence (%)c | Certainty of the evidence | Interpretationg | |
|---|---|---|---|---|---|---|---|---|
| Direct evidence HR (95% CI)b; I2 (%) | Network meta‐analysis HR (95% CI)b | |||||||
| Any reason | Phenobarbone | Carbamazepine | 520 (4 studies) | 1.55 (1.16 to 2.07); I2 = 68% | 1.56 (1.18 to 2.07) | 18.5% | ⊕⊕⊕⊕ HIGH d,e,f | Carbamazepine better than phenobarbitone |
| Adverse events | 520 (4 studies) | 1.52 (1.06 to 2.19); I2 = 73% | 1.99 (1.21 to 3.27) | 31.7% | ⊕⊕⊕⊕ HIGH d,e,f | |||
| Lack of efficacy | 388 (3 studies) | 1.86 (1.26 to 2.75); I2 = 0% | 1.88 (1.25 to 2.81) | 37.2% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Phenytoin | Carbamazepine | 428 (3 studies) | 1.19 (0.87 to 1.61); I2 = 0% | 1.14 (0.90 to 1.44) | 24.4% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 428 (3 studies) | 0.83 (0.56 to 1.24); I2 = 0% | 1.00 (0.66 to 1.53) | 35.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 428 (3 studies) | 1.12 (0.76 to 1.64); I2 = 0% | 1.14 (0.78 to 1.68) | 33.2% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Sodium valproate | Carbamazepine | 814 (5 studies) | 1.02 (0.80 to 1.29); I2 = 0% | 1.08 (0.88 to 1.31) | 23.8% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 570 (3 studies) | 0.94 (0.70 to 1.26); I2 = 0% | 0.88 (0.59 to 1.29) | 40.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 814 (5 studies) | 1.04 (0.82 to 1.33); I2 = 0% | 1.16 (0.88 to 1.52) | 52.9% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Lamotrigine | Carbamazepine | 2203 (9 studies) | 0.75 (0.65 to 0.88); I2 = 0% | 0.79 (0.69 to 0.91) | 27.7% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine better than carbamazepine for treatment failures for any reason and due to adverse events |
| Adverse events | 2203 (9 studies) | 0.57 (0.47 to 0.70); I2 = 0% | 0.56 (0.44 to 0.73) | 32.9% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 2098 (8 studies) | 1.00 (0.72 to 1.39); I2 = 0% | 1.02 (0.78 to 1.33) | 17.7% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Oxcarbazepine | Carbamazepine | 599 (2 studies) | 1.10 (0.85 to 1.43); I2 = 66% | 1.03 (0.82 to 1.30) | 0.4% | ⊕⊕⊕⊕ HIGH d,e,f | No difference between drugs for any outcome |
| Adverse events | 599 (2 studies) | 1.01 (0.73 to 1.38); I2 = 0% | 0.75 (0.46 to 1.22) | 18.4% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 599 (2 studies) | 1.17 (0.76 to 1.81); I2 = 0% | 1.14 (0.73 to 1.77) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Topiramate | Carbamazepine | 976 (2 studies) | 1.23 (1.03 to 1.48); I2 = 0% | 1.19 (0.99 to 1.43) | 24.2% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 976 (2 studies) | 1.10 (0.88 to 1.39); I2 = 0% | 0.99 (0.69 to 1.43) | 29.6% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 976 (2 studies) | 1.48 (1.08 to 2.03); I2 = 0% | 1.32 (0.95 to 1.83) | 21.9% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Gabapentin | Carbamazepine | 681 (2 studies) | 1.22 (1.02 to 1.45); I2 = 0% | 1.21 (1.01 to 1.45) | 26.6% | ⊕⊕⊕⊕ HIGH d,e | Carbamazepine better for treatment failures for any reason and lack of efficacy Gabapentin better for treatment failures due to adverse events |
| Adverse events | 681 (2 studies) | 0.68 (0.53 to 0.89); I2 = 88% | 0.58 (0.37 to 0.91) | 1.7% | ⊕⊕⊕⊕ HIGH d,e,f | |||
| Lack of efficacy | 681 (2 studies) | 2.05 (1.59 to 2.66); I2 = 0% | 2.07 (1.56 to 2.75) | 30.5% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Levetiracetam | Carbamazepine | 1567 (3 studies) | 0.85 (0.71 to 1.01); I2 = 50% | 0.80 (0.69 to 0.93) | 15.8% | ⊕⊕⊕⊕ HIGH d,e,f | Leviracetam better than carbamazepine for treatment failures for any reason and due to adverse events |
| Adverse events | 1567 (3 studies) | 0.60 (0.47 to 0.77); I2 = 35% | 0.65 (0.47 to 0.90) | 28.8% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 1567 (3 studies) | 1.44 (0.98 to 2.12); I2 = 0% | 1.07 (0.78 to 1.45) | 23.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Zonisamide | Carbamazepine | 583 (1 study) | 1.08 (0.81 to 1.44); I2 = NA | 0.93 (0.77 to 1.14) | 15.7% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 583 (1 study) | 0.96 (0.59 to 1.55); I2 = NA | 0.70 (0.43 to 1.13) | 17.9% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 583 (1 study) | 1.07 (0.60 to 1.92); I2 = NA | 1.23 (0.86 to 1.77) | 10.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Lacosamide | Carbamazepine | 807 (1 study) | 0.94 (0.75 to 1.19); I2 = NA | 0.95 (0.74 to 1.22) | 100.0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 807 (1 study) | 1.22 (0.84 to 1.79); I2 = NA | 1.24 (0.65 to 2.37) | 100.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 807 (1 study) | 0.79 (0.49 to 1.26); I2 = NA | 0.79 (0.47 to 1.33) | 100.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Abbreviations: CI: confidence interval; HR: hazard ratio; NA: not applicable; RCT: randomised controlled trial | ||||||||
| GRADE Working Group grades of evidence | ||||||||
| aOrder of drugs in the table: drugs were ordered approximately by the date they were licensed as a monotherapy treatment (oldest first). gInterpretation of network meta‐analysis results taking into account direct evidence for the comparison and certainty of the evidence | ||||||||
| Antiepileptic drug monotherapy for epilepsy: time to treatment failure for participants with focal seizures (reference lamotrigine) | ||||||||
| Patient or population: adults and children with focal seizures Settings: outpatients globally, followed up in RCTs for up to 12 years Intervention: carbamazepine, phenobarbitone, phenytoin, sodium valproate, oxcarbazepine, topiramate, gabapentin, levetiracetam, zonisamide and lacosamide Comparison: lamotrigine | ||||||||
| Outcome | Intervention (experimental treatment)a | Comparison (reference treatment) | No of participants direct evidence | Relative effect sizes | Direct evidence (%)c | Certainty of the evidence | Interpretationg | |
|---|---|---|---|---|---|---|---|---|
| Direct evidence HR (95% CI)b; I2 (%) | Network meta‐analysis HR (95% CI)b | |||||||
| Any reason | Carbamazepine | Lamotrigine | 2203 (9 studies) | 1.33 (1.14 to 1.54); I2 = 0% | 1.26 (1.10 to 1.44) | 27.7% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine better than carbamazepine for treatment failures for any reason and due to adverse events |
| Adverse events | 2203 (9 studies) | 1.75 (1.43 to 2.14); I2 = 0% | 1.77 (1.37 to 2.28) | 32.9% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 2098 (8 studies) | 0.68 (0.49 to 0.94); I2 = 0% | 0.98 (0.75 to 1.29) | 17.7% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Phenobarbitone | Lamotrigine | No direct evidence | No direct evidence | 1.97 (1.45 to 2.67) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine probably better than phenobarbitone |
| Adverse events | No direct evidence | No direct evidence | 3.52 (2.04 to 6.09) | 0.0% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Lack of efficacy | No direct evidence | No direct evidence | 1.85 (1.14 to 2.98) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Phenytoin | Lamotrigine | 90 (1 study) | 0.82 (0.40 to 1.65); I2 = NA | 1.44 (1.11 to 1.85) | 3.9% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine better than phenytoin for treatment failures for any reason and due to adverse events |
| Adverse events | 90 (1 study) | 0.89 (0.33 to 2.37); I2 = NA | 1.78 (1.13 to 2.81) | 4.4% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | No direct evidence | No direct evidence | 1.12 (0.71 to 1.78) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Sodium valproate | Lamotrigine | 267 (3 studies) | 2.37 (1.32 to 4.27); I2 = 0% | 1.35 (1.09 to 1.69) | 5.1% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine better than sodium valproate for treatment failures for any reason and due to adverse events |
| Adverse events | 267 (3 studies) | 3.53 (1.28 to 9.71); I2 = 0% | 1.55 (1.02 to 2.38) | 4.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 267 (3 studies) | 1.77 (0.77 to 4.05); I2 = 0% | 1.14 (0.80 to 1.62) | 2.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Oxcarbazepine | Lamotrigine | 521 (1 study) | 1.37 (1.05 to 1.81); I2 = NA | 1.30 (1.02 to 1.66) | 17.1% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine better than oxcarbazepine for treatment failures for any reason |
| Adverse events | 521 (1 study) | 1.91 (1.33 to 2.73); I2 = NA | 1.33 (0.80 to 2.20) | 15.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 521 (1 study) | 1.21 (0.79 to 1.85); I2 = NA | 1.12 (0.71 to 1.76) | 20.6% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Topiramate | Lamotrigine | 699 (2 studies) | 1.62 (1.30 to 2.02); I2 = 0% | 1.50 (1.23 to 1.81) | 17.4% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine better than topiramate for treatment failures for any reason and due to adverse events |
| Adverse events | 699 (2 studies) | 2.20 (1.63 to 2.99); I2 = 0% | 1.75 (1.17 to 2.62) | 17.6% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 699 (2 studies) | 1.49 (1.07 to 2.08); I2 = 0% | 1.30 (0.92 to 1.85) | 22.8% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Gabapentin | Lamotrigine | 676 (1 study) | 1.64 (1.32 to 2.04); I2 = NA | 1.53 (1.26 to 1.85) | 17.8% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine better than gabapentin for treatment failures for any reason and due to lack of efficacy |
| Adverse events | 676 (1 study) | 1.50 (1.09 to 2.08); I2 = NA | 1.02 (0.63 to 1.65) | 21.1% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 676 (1 study) | 2.30 (1.70 to 3.11); I2 = NA | 2.04 (1.48 to 2.80) | 18.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Levetiracetam | Lamotrigine | 902 (2 studies) | 0.87 (0.71 to 1.07); I2 = 0% | 1.01 (0.86 to 1.20) | 23.3% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 902 (2 studies) | 0.84 (0.60 to 1.19); I2 = 32% | 1.16 (0.81 to 1.66) | 14.6% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 902 (2 studies) | 0.83 (0.57 to 1.21); I2 = 3% | 1.05 (0.76 to 1.46) | 30.9% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Zonisamide | Lamotrigine | 658 (1 study) | 1.01 (0.80 to 1.28); I2 = NA | 1.18 (0.96 to 1.44) | 25.0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 658 (1 study) | 0.90 (0.57 to 1.41); I2 = NA | 1.24 (0.75 to 2.03) | 20.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 658 (1 study) | 1.12 (0.78 to 1.59); I2 = NA | 1.22 (0.86 to 1.72) | 22.2% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Lacosamide | Lamotrigine | No direct evidence | No direct evidence | 1.19 (0.90 to 1.58) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | Lamotrigine probably better than lacosamide for treatment failures due to adverse events |
| Adverse events | No direct evidence | No direct evidence | 2.21 (1.10 to 4.41) | 0.0% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Lack of efficacy | No direct evidence | No direct evidence | 0.78 (0.44 to 1.40) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Abbreviations: CI: confidence interval; HR: hazard ratio; NA: not applicable; RCT: randomised controlled trial | ||||||||
| GRADE Working Group grades of evidence | ||||||||
| aOrder of drugs in the table: drugs were ordered approximately by the date they were licensed as a monotherapy treatment (oldest first). fWide confidence intervals around the NMA treatment‐effect estimate (downgraded once for imprecision) gInterpretation of network meta‐analysis results taking into account direct evidence for the comparison and certainty of the evidence | ||||||||
| Antiepileptic drug monotherapy for epilepsy: time to treatment failure for participants with generalised seizures (reference sodium valproate) | ||||||||
| Patient or population: adults and children with generalised tonic‐clonic seizures with or without other seizure types Settings: outpatients globally, followed up in RCTs for up to 12 years Intervention: carbamazepine, phenobarbitone, phenytoin, oxcarbazepine, lamotrigine, topiramate, gabapentin, levetiracetam and lacosamide Comparison: sodium valproate | ||||||||
| Outcome | Intervention (experimental treatment)a | Comparison (reference treatment) | No of participants direct evidence | Relative effect sizes | Direct evidence (%)c | Certainty of the evidence | Interpretationh | |
|---|---|---|---|---|---|---|---|---|
| Direct evidence HR (95% CI)b; I2 (%) | Network meta‐analysis HR (95% CI)b | |||||||
| Any reason | Carbamazepine | Sodium valproate | 405 (4 studies) | 1.26 (0.73 to 2.20); I2 = 7% | 1.52 (1.18 to 1.96) | 23.3% | ⊕⊕⊕⊕ HIGH d,e | Sodium valproate better than carbamazepine for treatment failures for any reason and due to adverse events |
| Adverse events | 117 (2 studies) | 0.74 (0.18 to 2.98); I2 = 0% | 1.96 (1.13 to 3.39) | 52.9% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 405 (4 studies) | 1.31 (0.71 to 2.42); I2 = 0% | 0.97 (0.63 to 1.49) | 51.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Phenobarbitone | Sodium valproate | 94 (2 studies) | 0.56 (0.20 to 1.54); I2 = 0% | 2.13 (1.20 to 3.79) | 19.7% | ⊕⊕⊕⊝ MODERATE d,e,f | Sodium valproate is probably better than phenobarbitone for treatment failures for any reason |
| Adverse events | 94 (2 studies) | 0.26 (0.06 to 1.05); I2 = 28% | 2.14 (0.82 to 5.57) | 4.1% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Lack of efficacy | 94 (2 studies) | 0.88 (0.25 to 3.07); I2 = 0% | 1.57 (0.71 to 3.50) | 25.8% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Any reason | Phenytoin | Sodium valproate | 326 (4 studies) | 0.65 (0.26 to 1.63); I2 = 22% | 1.17 (0.80 to 1.73) | 10.4% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 326 (4 studies) | 0.37 (0.06 to 2.13); I2 = 0% | 1.56 (0.75 to 3.24) | 13.8% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 326 (4 studies) | 0.49 (0.15 to 1.55); I2 = 0% | 0.60 (0.27 to 1.34) | 26.8% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Lamotrigine | Sodium valproate | 560 (3 studies) | 1.91 (0.93 to 3.90); I2 = 0% | 1.06 (0.81 to 1.37) | 15.9% | ⊕⊕⊕⊕ HIGH d,e | Sodium valproate better than lamotrigine for treatment failures due to lack of efficacy |
| Adverse events | 560 (3 studies) | 1.88 (0.68 to 5.21); I2 = 0% | 0.86 (0.50 to 1.48) | 20.3% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 560 (3 studies) | 1.98 (0.60 to 6.49); I2 = 0% | 1.85 (1.11 to 3.11) | 14.1% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Oxcarbazepine | Sodium valproate | No direct evidence | No direct evidence | 1.24 (0.72 to 2.14) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | Probably no difference between drugs for any outcome |
| Adverse events | No direct evidence | No direct evidence | 1.00 (0.33 to 3.02) | 0.0% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Lack of efficacy | No direct evidence | No direct evidence | 1.51 (0.50 to 4.54) | 0.0% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Any reason | Topiramate | Sodium valproate | 588 (2 studies) | 1.81 (0.91 to 3.60); I2 = 36% | 1.37 (1.06 to 1.77) | 11.0% | ⊕⊕⊕⊕ HIGH d,e | Sodium valproate better than topiramate for treatment failures for any reason and due to lack of efficacy |
| Adverse events | 588 (2 studies) | 1.53 (0.59 to 3.97); I2 = 54% | 1.42 (0.82 to 2.46) | 10.8% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 588 (2 studies) | 4.81 (1.14 to 20.3); I2 = 0% | 1.78 (1.10 to 2.87) | 34.6% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Gabapentin | Sodium Valproate | No direct evidence | No direct evidence | 1.13 (0.61 to 2.11) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | Sodium valproate better than gabapentin for treatment failures due to lack of efficacy |
| Adverse events | No direct evidence | No direct evidence | 0.66 (0.21 to 2.08) | 0.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | No direct evidence | No direct evidence | 2.76 (1.14 to 6.70) | 0.0% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Any reason | Levetiracetam | Sodium valproate | 1032 (2 studies) | 1.46 (0.63 to 3.38); I2 = 0% | 1.13 (0.89 to 1.42) | 17.8% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs for any outcome |
| Adverse events | 1032 (2 studies) | 0.79 (0.19 to 3.39); I2 = 0% | 1.21 (0.66 to 2.21) | 14.7% | ⊕⊕⊕⊕ HIGH d,e | |||
| Lack of efficacy | 1032 (2 studies) | 3.02 (0.43 to 21.1); I2 = 0% | 1.25 (0.81 to 1.93) | 22.0% | ⊕⊕⊕⊕ HIGH d,e | |||
| Any reason | Lacosamide | Sodium valproate | No direct evidence | No direct evidence | 2.64 (1.14 to 6.09) | 0.0% | ⊕⊕⊕⊝ MODERATE d,e,f | Sodium valproate probably better than lacosamide for treatment failures for any reason and may be better than lacosamide for treatment failures due to adverse events |
| Adverse events | No direct evidence | No direct evidence | 8.61 (1.29 to 57.5) | 0.0% | ⊕⊕⊝⊝ LOW d,e,g | |||
| Lack of efficacy | No direct evidence | No direct evidence | 0.40 (0.07 to 2.26) | 0.0% | ⊕⊕⊕⊝ MODERATE d,e,f | |||
| Abbreviations: CI: confidence interval; HR: hazard ratio; NA: not applicable; RCT: randomised controlled trial | ||||||||
| GRADE Working Group grades of evidence | ||||||||
| aOrder of drugs in the table: drugs were ordered approximately by the date they were licensed as a monotherapy treatment (oldest first). fWide confidence intervals around the NMA treatment‐effect estimate (downgraded once for imprecision) gVery wide confidence intervals around the NMA treatment‐effect estimate (downgraded twice for imprecision) hInterpretation of network meta‐analysis results taking into account direct evidence for the comparison and certainty of the evidence | ||||||||
| Antiepileptic drug monotherapy for epilepsy: time to 12‐month remission for individuals with focal seizures (reference carbamazepine) | |||||||
| Patient or population: adults and children with focal seizures Settings: outpatients globally, followed up in RCTs for up to 12 years Intervention: phenobarbitone, phenytoin, sodium valproate, lamotrigine, oxcarbazepine, topiramate, gabapentin, levetiracetam, zonisamide and lacosamide Comparison: carbamazepine | |||||||
| Intervention (experimental treatment)a | Comparison (reference treatment) | No of participants direct evidence | Relative effect sizes | Direct evidence (%)c | Certainty of the evidence | Interpretationf | |
|---|---|---|---|---|---|---|---|
| Direct evidence HR (95% CI)b; I2 (%) | Network meta‐analysis HR (95% CI) | ||||||
| Phenobarbitone | Carbamazepine | 525 (4 studies) | 1.00 (0.73 to 1.35); I2 = 42% | 1.03 (0.77 to 1.38) | 16.9% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Phenytoin | Carbamazepine | 430 (3 studies) | 1.03 (0.78 to 1.37); I2 = 0% | 1.04 (0.84 to 1.29) | 21.9% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Sodium valproate | Carbamazepine | 816 (5 studies) | 1.06 (0.86 to 1.30); I2 = 30% | 1.08 (0.91 to 1.29) | 17.7% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Lamotrigine | Carbamazepine | 907 (2 studies) | 1.08 (0.91 to 1.28); I2 = 0% | 1.06 (0.93 to 1.22) | 18.4% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Oxcarbazepine | Carbamazepine | 591 (2 studies) | 0.97 (0.78 to 1.20); I2 = 0% | 0.95 (0.78 to 1.15) | 17.8% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Topiramate | Carbamazepine | 962 (2 studies) | 1.20 (1.00 to 1.44); I2 = 0% | 1.13 (0.94 to 1.36) | 21.9% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Gabapentin | Carbamazepine | 666 (1 study) | 1.32 (1.09 to 1.60); I2 = NA | 1.29 (1.06 to 1.57) | 20.4% | ⊕⊕⊕⊕ HIGH d,e | Carbamazepine better than gabapentin |
| Levetiracetam | Carbamazepine | 1567 (3 studies) | 1.09 (0.92 to 1.29); I2 = 0% | 1.08 (0.94 to 1.24) | 22.3% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Zonisamide | Carbamazepine | 582 (1 study) | 1.05 (0.85 to 1.30); I2 = NA | 1.10 (0.94 to 1.29) | 18.9% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Lacosamide | Carbamazepine | 806 (1 study) | 1.00 (0.83 to 1.19); I2 = NA | 1.00 (0.81 to 1.22) | 100.0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Abbreviations: CI: confidence interval; HR: hazard ratio; NA: not applicable; RCT: randomised controlled trial | |||||||
| GRADE Working Group grades of evidence | |||||||
| aOrder of drugs in the table: drugs were ordered approximately by the date they were licensed as a monotherapy treatment (oldest first). fInterpretation of network meta‐analysis results taking into account direct evidence for the comparison and certainty of the evidence | |||||||
| Antiepileptic drug monotherapy for epilepsy: time to 12‐month remission for individuals with focal seizures (reference lamotrigine) | |||||||
| Patient or population: adults and children with focal seizures Settings: outpatients globally, followed up in RCTs for up to 12 years Intervention: carbamazepine, phenobarbital, phenytoin, sodium valproate, oxcarbazepine, topiramate, gabapentin, levetiracetam, zonisamide and lacosamide Comparison: lamotrigine | |||||||
| Intervention (experimental treatment)a | Comparison (reference treatment) | No of participants direct evidence | Relative effect sizes | Proportion of | Certainty of the evidence | Interpretationf | |
|---|---|---|---|---|---|---|---|
| Direct evidence HR (95% CI)b; I2 (%) | Network meta‐analysis HR (95% CI)b | ||||||
| Carbamazepine | Lamotrigine | 907 (2 studies) | 0.92 (0.78 to 1.09); I2 = 0% | 0.94 (0.82 to 1.08) | 18.4% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Phenobarbitone | Lamotrigine | No direct evidence | No direct evidence | 0.97 (0.71 to 1.33) | 0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Phenytoin | Lamotrigine | No direct evidence | No direct evidence | 0.98 (0.76 to 1.25) | 0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Sodium valproate | Lamotrigine | 267 (3 studies) | 1.35 (0.68 to 2.67); I2 = 0% | 1.02 (0.83 to 1.25) | 4.1% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Oxcarbazepine | Lamotrigine | 511 (1 study) | 0.87 (0.69 to 1.01); I2 = NA | 0.89 (0.72 to 1.10) | 15.6% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Topiramate | Lamotrigine | 683 (2 studies) | 1.12 (0.92 to 1.36); I2 = 0% | 1.06 (0.88 to 1.29) | 19.5% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Gabapentin | Lamotrigine | 660 (1 study) | 1.21 (1.00 to 1.47); I2 = NA | 1.21 (0.99 to 1.48) | 19.9% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Levetiracetam | Lamotrigine | 902 (2 studies) | 1.02 (0.86 to 1.20); I2 = 0% | 1.01 (0.87 to 1.18) | 23.6% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Zonisamide | Lamotrigine | 658 (1 study) | 1.07 (0.88 to 1.29); I2 = NA | 1.04 (0.87 to 1.23) | 24.7% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Lacosamide | Lamotrigine | No direct evidence | No direct evidence | 0.94 (0.73 to 1.20) | 0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Abbreviations: CI: confidence interval; HR: hazard ratio; NA: not applicable; RCT: randomised controlled trial | |||||||
| GRADE Working Group grades of evidence | |||||||
| aOrder of drugs in the table: drugs were ordered approximately by the date they were licensed as a monotherapy treatment (oldest first). fInterpretation of network meta‐analysis results taking into account direct evidence for the comparison and certainty of the evidence | |||||||
| Antiepileptic drug monotherapy for epilepsy: time to 12‐month remission for individuals with generalised seizures (reference sodium valproate) | |||||||
| Patient or population: adults and children with generalised seizures* Settings: outpatients globally, followed up in RCTs for up to 12 years Intervention: carbamazepine, phenobarbitone, phenytoin, lamotrigine, oxcarbazepine, topiramate, gabapentin, levetiracetam and lacosamide Comparison: sodium valproate | |||||||
| Intervention (experimental treatment)a | Comparison (reference treatment) | No of participants direct evidence | Relative effect sizes | Proportion of | Certainty of the evidence | Interpretationg | |
|---|---|---|---|---|---|---|---|
| Direct evidence HR (95% CI)b; I2 (%) | Network meta‐analysis HR (95% CI)b | ||||||
| Carbamazepine | Sodium valproate | 412 (4 studies) | 1.01 (0.72 to 1.43); I2 = 0% | 1.01 (0.83 to 1.22) | 40.4% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Phenobarbitone | Sodium valproate | 98 (2 studies) | 1.15 (0.53 to 2.49); I2 = 42% | 1.32 (0.88 to 2.00) | 12.4% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Phenytoin | Sodium valproate | 269 (4 studies) | 0.87 (0.55 to 1.40); I2 = 0% | 0.96 (0.75 to 1.28) | 36.1% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Lamotrigine | Sodium valproate | 555 (3 studies) | 1.27 (0.64 to 2.50); I2 = 0% | 1.19 (0.95 to 1.50) | 12.4% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Oxcarbazepine | Sodium valproate | No direct evidence | No direct evidence | 1.27 (0.85 to 1.90) | 0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Topiramate | Sodium valproate | 585 (2 studies) | 1.86 (0.94 to 3.71); I2 = 0% | 1.08 (0.87 to 1.34) | 4.3% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Gabapentin | Sodium valproate | No direct evidence | No direct evidence | 1.30 (0.82 to 2.07) | 0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Levetiracetam | Sodium valproate | 1032 (2 studies) | 1.10 (0.59 to 2.04); I2: 55% | 0.99 (0.82 to 1.20) | 53.2% | ⊕⊕⊕⊕ HIGH d,e,f | No difference between drugs |
| Lacosamide | Sodium valproate | No direct evidence | No direct evidence | 1.05 (0.56 to 1.94) | 0% | ⊕⊕⊕⊕ HIGH d,e | No difference between drugs |
| Abbreviations: CI: confidence interval; HR: hazard ratio; NA: not applicable; RCT: randomised controlled trial | |||||||
| GRADE Working Group grades of evidence | |||||||
| aOrder of drugs in the table: drugs were ordered approximately by the date they were licensed as a monotherapy treatment (oldest first). fLarge amount of heterogeneity present in pairwise meta‐analysis (direct evidence); heterogeneity likely due to difference in trial designs (e.g. age of participants). Despite heterogeneity, numerical results from direct evidence and from NMA were similar, therefore we judged that any heterogeneity present in pairwise meta‐analysis had not influenced the overall results (no downgrade of certainty of evidence). gInterpretation of network meta‐analysis results taking into account direct evidence for the comparison and certainty of the evidence | |||||||
| Trial\Drug | CBZ | PHB | PHT | VPS | LTG | OXC | LEV | TPM | GBP | ZNS | LCM | ESL | Total | Total randomiseda |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials providing individual participant data | ||||||||||||||
| 54 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 108 | 108 | |
| 301 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 282 | 0 | 0 | 583 | 583 | |
| 443 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 445 | 0 | 888 | 888 | |
| 0 | 0 | 144 | 0 | 0 | 143 | 0 | 0 | 0 | 0 | 0 | 0 | 287 | 287 | |
| 0 | 0 | 0 | 69 | 66 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 135 | 136 | |
| 66 | 0 | 0 | 0 | 70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 136 | 136 | |
| 63 | 0 | 0 | 0 | 61 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 124 | 124 | |
| 48 | 0 | 0 | 0 | 102 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 150 | 150 | |
| 291 | 0 | 0 | 0 | 0 | 0 | 288 | 0 | 0 | 0 | 0 | 0 | 579 | 579 | |
| 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 218 | 0 | 0 | 0 | 292 | 292 | |
| 0 | 0 | 81 | 85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 166 | 166 | |
| 54 | 10 | 54 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 167 | 173 | |
| 26 | 0 | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 52 | |
| 41 | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 84 | 84 | |
| 0 | 0 | 94 | 0 | 0 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 193 | 193 | |
| 61 | 58 | 63 | 61 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 243 | 243 | |
| 0 | 0 | 0 | 44 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 81 | 81 | |
| 53 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 110 | 110 | |
| 155 | 155 | 165 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 475 | 475 | |
| 236 | 0 | 0 | 244 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 480 | 480 | |
| 202 | 0 | 0 | 0 | 420 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 622 | 622 | |
| 19 | 18 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 55 | 55 | |
| 0 | 47 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 94 | 94 | |
| 95 | 97 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 192 | 192 | |
| Privitera 2003 (CBZ branch)b | 129 | 0 | 0 | 0 | 0 | 0 | 0 | 266 | 0 | 0 | 0 | 0 | 395 | 395 |
| Privitera 2003 (VPS branch)b | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 147 | 0 | 0 | 0 | 0 | 225 | 225 |
| 0 | 0 | 50 | 86 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 136 | 136 | |
| 0 | 0 | 128 | 0 | 0 | 0 | 0 | 133 | 0 | 0 | 0 | 0 | 261 | 261 | |
| 121 | 0 | 0 | 0 | 230 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 351 | 351 | |
| 151 | 0 | 0 | 149 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 300 | 300 | |
| 378 | 0 | 0 | 0 | 378 | 210 | 0 | 378 | 377 | 0 | 0 | 0 | 1721 | 1721 | |
| 0 | 0 | 0 | 0 | 330 | 0 | 330 | 0 | 0 | 330 | 0 | 0 | 990 | 990 | |
| 0 | 0 | 0 | 238 | 239 | 0 | 0 | 239 | 0 | 0 | 0 | 0 | 716 | 716 | |
| 0 | 0 | 0 | 260 | 0 | 0 | 260 | 0 | 0 | 0 | 0 | 0 | 520 | 520 | |
| 0 | 0 | 95 | 0 | 86 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 181 | 181 | |
| 0 | 0 | 0 | 109 | 117 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 226 | 227 | |
| Trinka 2013 (CBZ branch)b | 503 | 0 | 0 | 0 | 0 | 0 | 493 | 0 | 0 | 0 | 0 | 0 | 996 | 999 |
| Trinka 2013 (VPS branch)b | 0 | 0 | 0 | 353 | 0 | 0 | 350 | 0 | 0 | 0 | 0 | 0 | 703 | 703 |
| 0 | 0 | 70 | 70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 140 | 140 | |
| 130 | 0 | 0 | 130 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 260 | 260 | |
| 121 | 0 | 0 | 0 | 118 | 0 | 122 | 0 | 0 | 0 | 0 | 0 | 361 | 361 | |
| Total | 3815 | 439 | 1009 | 2025 | 2354 | 478 | 1843 | 1163 | 595 | 612 | 445 | 0 | 14,778 | 14,789 |
| Trials not providing individual participant data | ||||||||||||||
| Trial\Drug | CBZ | PHB | PHT | VPS | LTG | OXC | LEV | TPM | GBP | ZNS | LCM | ESL | Total | Total randomiseda |
| 0 | 0 | 18 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 37 | |
| 0 | 68 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 118 | 118 | |
| 36 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 71 | 71 | |
| 0 | 0 | 0 | 0 | 151 | 0 | 0 | 0 | 158 | 0 | 0 | 0 | 309 | 309 | |
| 59 | 0 | 58 | 64 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 181 | 181 | |
| 17 | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 35 | 35 | |
| 14 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 27 | 27 | |
| 26 | 25 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 76 | 76 | |
| 60 | 0 | 0 | 0 | 0 | 58 | 0 | 0 | 0 | 0 | 0 | 0 | 118 | 118 | |
| 15 | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 31 | 31 | |
| 0 | 0 | 0 | 121 | 0 | 128 | 0 | 0 | 0 | 0 | 0 | 0 | 249 | 249 | |
| 66 | 0 | 0 | 0 | 0 | 0 | 62 | 0 | 0 | 0 | 0 | 0 | 128 | 128 | |
| 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 12 | |
| 30 | 30 | 30 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 120 | 120 | |
| 100 | 0 | 0 | 0 | 0 | 94 | 0 | 0 | 0 | 0 | 0 | 0 | 194 | 194 | |
| 28 | 0 | 0 | 29 | 0 | 55 | 0 | 0 | 0 | 0 | 0 | 0 | 112 | 112 | |
| 152 | 150 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 302 | 302 | |
| 23 | 0 | 20 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 64 | 64 | |
| 0 | 0 | 0 | 0 | 21 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 48 | 48 | |
| 32 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 64 | 64 | |
| 0 | 0 | 0 | 30 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 60 | |
| 64 | 0 | 0 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 | 121 | 121 | |
| 70 | 0 | 0 | 0 | 73 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 143 | 143 | |
| 0 | 0 | 0 | 0 | 0 | 178 | 175 | 0 | 0 | 0 | 0 | 0 | 353 | 353 | |
| 6 | 0 | 0 | 3 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 15 | 15 | |
| 129 | 0 | 0 | 0 | 264 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 393 | 393 | |
| 82 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 73 | 0 | 0 | 155 | 155 | |
| 0 | 0 | 0 | 38 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 73 | 73 | |
| 30 | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 60 | |
| 15 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 33 | |
| 66 | 0 | 51 | 46 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 163 | 163 | |
| 0 | 0 | 0 | 0 | 50 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | |
| 215 | 0 | 0 | 0 | 0 | 0 | 218 | 0 | 0 | 0 | 0 | 0 | 433 | 433 | |
| 23 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43 | 43 | |
| 42 | 0 | 45 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 87 | 87 | |
| ? | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 37 | 37 | |
| 0 | 0 | 45 | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 94 | 94 | |
| 20 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 40 | |
| 42 | 0 | 0 | 0 | 0 | 0 | 0 | 46 | 0 | 0 | 0 | 0 | 88 | 88 | |
| 198 | 0 | 0 | 0 | 200 | 0 | 0 | 0 | 195 | 0 | 0 | 0 | 593 | 593 | |
| 92 | 0 | 0 | 0 | 93 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 185 | 185 | |
| 0 | 0 | 15 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 33 | |
| 0 | 0 | 0 | 34 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 66 | 66 | |
| 17 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 33 | |
| 30 | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 60 | 60 | |
| 88 | 0 | 0 | 30 | 121 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 239 | 239 | |
| 0 | 51 | 52 | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 151 | 151 | |
| 412 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 401 | 813 | 813 | |
| 0 | 0 | 0 | 0 | 11 | 16 | 11 | 0 | 0 | 0 | 0 | 0 | 38 | 38 | |
| 0 | 0 | 0 | 0 | 70 | 57 | 68 | 58 | 0 | 0 | 0 | 0 | 253 | 253 | |
| Totalc | 2305 | 383 | 374 | 602 | 1183 | 662 | 774 | 104 | 353 | 73 | 0 | 401 | 7251 | 7251 |
| Grand totalc | 6120 | 822 | 1383 | 2627 | 3537 | 1140 | 2617 | 1267 | 948 | 685 | 445 | 401 | 22,029 | 22,040 |
| Proportion of IPD | 62% | 53% | 73% | 77% | 67% | 42% | 70% | 92% | 63% | 89% | 100% | 0% | 67% | 67% |
| CBZ: carbamazepine aDrug allocated missing for 11 participants provided in the IPD | ||||||||||||||
| Trial | Gender | Epilepsy type | Epilepsy type reclassifiedc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Missing | Genb | Focal | Missing | Genb | Focal | Unclassifiedd | |
| 61 (56%) | 47 (44%) | 0 (0%) | 49 (45%) | 59 (55%) | 0 (0%) | 49 (45%) | 59 (55%) | 0 (0%) | |
| 347 (60%) | 236 (40%) | 0 (0%) | 0 (0%) | 583 (100%) | 0 (0%) | 0 (0%) | 583 (100%) | 0 (0%) | |
| 475 (53%) | 413 (47%) | 0 (0%) | 80 (9%) | 808 (91%) | 0 (0%) | 0 (0%) | 822 (93%) | 66 (7%) | |
| 174 (61%) | 113 (39%) | 0 (0%) | 105 (37%) | 182 (63%) | 0 (0%) | 78 (27%) | 182 (63%) | 27 (9%) | |
| 60 (44%) | 75 (55%) | 1 (1%) | 46 (34%) | 82 (60%) | 8 (6%) | 33 (24%) | 82 (60%) | 21 (15%) | |
| 56 (41%) | 80 (59%) | 0 (0%) | 54 (40%) | 82 (60%) | 0 (0%) | 35 (26%) | 82 (60%) | 19 (14%) | |
| 56 (45%) | 68 (55%) | 0 (0%) | 62 (50%) | 62 (50%) | 0 (0%) | 40 (32%) | 62 (50%) | 22 (18%) | |
| 83 (55%) | 67 (45%) | 0 (0%) | 45 (30%) | 105 (70%) | 0 (0%) | 0 (0%) | 105 (70%) | 45 (30%) | |
| 319 (55%) | 260 (45%) | 0 (0%) | 113 (20%) | 466 (80%) | 0 (0%) | 46 (8%) | 466 (80%) | 67 (12%) | |
| 157 (54%) | 135 (46%) | 0 (0%) | 0 (0%) | 292 (100%) | 0 (0%) | 0 (0%) | 292 (100%) | 0 (0%) | |
| 71 (43%) | 92 (55%) | 3 (2%) | 86 (52%) | 80 (48%) | 0 (0%) | 2 (1%) | 80 (48%) | 84 (51%) | |
| 86 (50%) | 81 (47%) | 6 (3%) | 84 (49%) | 89 (51%) | 0 (0%) | 84 (49%) | 89 (51%) | 0 (0%) | |
| 21 (40%) | 31 (60%) | 0 (0%) | 0 (0%) | 52 (100%) | 0 (0%) | 0 (0%) | 52 (100%) | 0 (0%) | |
| 48 (57%) | 36 (43%) | 0 (0%) | 0 (0%) | 84 (100%) | 0 (0%) | 0 (0%) | 84 (100%) | 0 (0%) | |
| 100 (52%) | 93 (48%) | 0 (0%) | 50 (26%) | 143 (74%) | 0 (0%) | 45 (23%) | 143 (74%) | 5 (3%) | |
| 117 (48%) | 126 (52%) | 0 (0%) | 141 (58%) | 102 (42%) | 0 (0%) | 97 (40%) | 102 (42%) | 44 (18%) | |
| 40 (49%) | 41 (51%) | 0 (0%) | 48 (59%) | 29 (36%) | 4 (5%) | 22 (27%) | 29 (36%) | 30 (37%) | |
| 57 (52%) | 53 (48%) | 0 (0%) | 15 (14%) | 95 (86%) | 0 (0%) | 6 (5%) | 95 (86%) | 9 (8%) | |
| 413 (87%) | 58 (12%) | 4 (1%) | 1 (0%) | 474 (100%) | 0 (0%) | 1 (0%) | 474 (100%) | 0 (0%) | |
| 445 (93%) | 35 (7%) | 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | |
| 329 (53%) | 293 (47%) | 0 (0%) | 3 (1%) | 619 (99%) | 0 (0%) | 1 (0%) | 619 (100%) | 2 (0%) | |
| 34 (62%) | 21 (38%) | 0 (0%) | 45 (82%) | 10 (18%) | 0 (0%) | 30 (55%) | 10 (18%) | 15 (27%) | |
| 47 (50%) | 45 (48%) | 2 (2%) | 34 (36%) | 60 (64%) | 0 (0%) | 34 (36%) | 60 (64%) | 0 (0%) | |
| 67 (35%) | 125 (65%) | 0 (0%) | 59 (31%) | 133 (69%) | 0 (0%) | 50 (26%) | 133 (69%) | 9 (5%) | |
| (CBZ branch)a | 215 (54%) | 180 (46%) | 0 (0%) | 88 (22%) | 285 (72%) | 22 (6%) | 50 (13%) | 285 (72%) | 60 (15%) |
| (VPS branch)a | 112 (50%) | 113 (50%) | 0 (0%) | 131 (58%) | 78 (35%) | 16 (7%) | 85 (38%) | 78 (35%) | 62 (27%) |
| 73 (54%) | 63 (46%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 111 (82%) | 0 (0%) | 25 (18%) | |
| 126 (48%) | 135 (52%) | 0 (0%) | 150 (57%) | 53 (20%) | 58 (22%) | 78 (30%) | 53 (20%) | 130 (50%) | |
| 188 (54%) | 163 (46%) | 0 (0%) | 114 (32%) | 237 (68%) | 0 (0%) | 76 (22%) | 237 (67%) | 38 (11%) | |
| 153 (51%) | 147 (49%) | 0 (0%) | 154 (51%) | 146 (49%) | 0 (0%) | 86 (29%) | 146 (49%) | 68 (22%) | |
| 944 (55%) | 777 (45%) | 0 (0%) | 190 (11%) | 1531 (89%) | 0 (0%) | 36 (2%) | 1531 (89%) | 154 (9%) | |
| 427 (60%) | 289 (40%) | 0 (0%) | 661 (92%) | 54 (8%) | 0 (0%) | 469 (65%) | 54 (8%) | 193 (27%) | |
| 561 (57%) | 429 (43%) | 0 (0%) | 0 (0%) | 990 (100%) | 0 (0%) | 0 (0%) | 984 (99%) | 9 (1%) | |
| 337 (65%) | 183 (35%) | 0 (0%) | 520 (100%) | 0 (0%) | 0 (0%) | 385 (74%) | 0 (0%) | 135 (26%) | |
| 101 (56%) | 80 (44%) | 0 (0%) | 91 (50%) | 90 (50%) | 0 (0%) | 57 (31%) | 90 (50%) | 34 (19%) | |
| 114 (50%) | 112 (49%) | 1 (0%) | 32 (14%) | 154 (68%) | 41 (18%) | 29 (13%) | 154 (68%) | 44 (19%) | |
| (CBZ branch)a | 551 (55%) | 448 (45%) | 0 (0%) | 141 (14%) | 858 (86%) | 0 (0%) | 46 (5%) | 858 (86%) | 95 (9%) |
| (VPS branch)a | 398 (57%) | 305 (43%) | 0 (0%) | 513 (73%) | 190 (27%) | 0 (0%) | 274 (39%) | 190 (27%) | 239 (34%) |
| 73 (52%) | 67 (48%) | 0 (0%) | 77 (55%) | 63 (45%) | 0 (0%) | 45 (32%) | 63 (45%) | 32 (23%) | |
| 122 (47%) | 138 (53%) | 0 (0%) | 152 (58%) | 108 (42%) | 0 (0%) | 152 (58%) | 108 (42%) | 0 (0%) | |
| 215 (60%) | 146 (40%) | 0 (0%) | 0 (0%) | 361 (100%) | 0 (0%) | 0 (0%) | 361 (100%) | 0 (0%) | |
| Total | 8373 (57%) | 6399 (43%) | 17 (< 1%) | 4270 (29%) | 10,369 (70%) | 150 (1%) | 2632 (18%) | 10,377 (70%) | 1780 (12%) |
| CBZ: carbamazepine aTrials designed in two strata based on whether recommended treatment would be CBZ or VPS. Within the two strata, participants were randomised to TPM in Privitera 2003/LEV in Trinka 2013 or CBZ/VPS depending on the strata. Data analysed according to the separate strata (CBZ branch or VPS branch) in this review | |||||||||
| Trial | Age (years) | Epilepsy duration (years) | Number of seizures in the last 6 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Missing | Median | Range | Missing | Median | Range | Missing | |
| 5.7 | 3.5 | 1 to 15 | 0 | 1.2 | 0 to 11.5 | 0 | 24 | 1 to 7200 | 5 | |
| 36.4 | 15.9 | 18 to 75 | 0 | 0.2 | 0 to 17.7 | 30 | 2 | 1 to 30 | 1 | |
| 41.9b | 17.5b | 16 to 73b | 2 | 0.8 | 0 to 40.3 | 2 | 4 | 1 to 1930 | 12 | |
| 26.8 | 10.7 | 15 to 91 | 1 | 0.4 | 0 to 25 | 0 | 3 | 0 to 252 | 0 | |
| 32.0 | 14.5 | 12 to 76 | 0 | 1 | 0 to 53 | 27 | 2 | 0 to 100 | 2 | |
| 34.0 | 15.8 | 13 to 70 | 0 | 1 | 0 to 17.9 | 0 | 4 | 1 to 960 | 0 | |
| 30.0 | 14.1 | 14 to 81 | 0 | 0.5 | 0 to 19.4 | 0 | 3 | 1 to 1020 | 0 | |
| 76.9 | 6.0 | 65 to 94 | 0 | NA | NA | 150 | 3 | 0 to 163 | 0 | |
| 39.0 | 16.2 | 15 to 82 | 0 | NA | NA | 579 | 3 | 1 to 1410 | 4 | |
| 35.7 | 16.6 | 12 to 86 | 0 | 0.3 | 0 to 7.7 | 5 | 4 | 1 to 146 | 6 | |
| 78.2 | 7.1 | 61 to 95 | 3 | NA | NA | 166 | 3 | 0 to 99 | 3 | |
| 9.9 | 3.6 | 2 to 15 | 6 | 0.5 | 0 to 13.7 | 6 | 3 | 1 to 900 | 6 | |
| 10.8 | 2.3 | 4 to 15 | 0 | NA | NA | 52 | 3 | 1 to 60 | 0 | |
| 8.8 | 2.1 | 5 to 13 | 0 | 0.4 | 0 to 4.5 | 0 | 3 | 2 to 11 | 0 | |
| 18.6 | 9.7 | 5 to 53 | 1 | 0.4 | 0 to 20 | 0 | 2 | 0 to 157 | 0 | |
| 32.3 | 14.8 | 13 to 77 | 3 | 1 | 0 to 39.8 | 4 | 2 | 1 to 579 | 3 | |
| 33.9 | 10.9 | 16 to 56 | 0 | NA | NA | 81 | 1 | 0 to 540 | 0 | |
| 35.8 | 12.2 | 16 to 60 | 0 | NA | NA | 110 | 2 | 0 to 200 | 0 | |
| 41.0 | 15.5 | 18 to 82 | 4 | 2 | 0.5 to 59 | 5 | 1 | 1 to 100 | 7 | |
| 47.1 | 16.1 | 18 to 83 | 0 | 3 | 1 to 68 | 19 | 12 | 1 to 2248 | 38 | |
| 27.2 | 21.4 | 2 to 83 | 1 | NA | NA | 622 | 3 | 1 to 9000 | 0 | |
| 27.5 | 8.5 | 14 to 55 | 0 | 7 | 3 to 11.5 | 18 | 12 | 6 to 42 | 0 | |
| 11.4 | 5.0 | 2 to 18 | 0 | 2.5 | 0.5 to 17 | 2 | NA | NA | 94 | |
| 29.0 | 17.6 | 2 to 68 | 0 | 5 | 0.5 to 44 | 0 | 2 | 0 to 100 | 0 | |
| (CBZ branch)a | 34.4 | 18.4 | 6 to 80 | 0 | NA | NA | 395 | 4 | 0 to 2400 | 0 |
| (VPS branch)a | 32.8 | 19.4 | 6 to 84 | 0 | NA | NA | 225 | 4 | 0 to 20000 | 0 |
| 20.9 | 14.2 | 3 to 64 | 0 | 0 | 0 to 3 | 15 | NA | NA | 136 | |
| 34.1 | 14.8 | 12 to 78 | 0 | NA | NA | 261 | 4 | 0 to 570 | 0 | |
| 32.1 | 14.2 | 12 to 72 | 2 | 0.7 | 0 to 26.8 | 3 | 3 | 1 to 145 | 1 | |
| 33.0 | 14.9 | 16 to 79 | 2 | NA | NA | 300 | 4 | 2 to 101 | 5 | |
| 38.3 | 18.2 | 5 to 86 | 0 | 1.4 | 0 to 68.6 | 1 | 4 | 0 to 1184 | 5 | |
| 22.5 | 14.0 | 5 to 77 | 0 | 1.3 | 0 to 60.8 | 0 | 3 | 0 to 2812 | 3 | |
| 38.8 | 21.2 | 5 to 91 | 0 | 1 | 0 to 59.3 | 7 | 6 | 1 to 99c | 1 | |
| 16.5 | 12.3 | 5 to 94 | 0 | 0.7 | 0 to 54.0 | 10 | 10 | 1 to 99c | 6 | |
| 34.1 | 16.7 | 13 to 74 | 1 | 1.3 | 0 to 28.5 | 1 | 3 | 1 to 600 | 0 | |
| 36.0 | 16.9 | 13 to 80 | 2 | NA | NA | 227 | 18 | 6 to 1080 | 37 | |
| (CBZ branch)a | 42.8 | 17.2 | 16 to 89 | 0 | NA | NA | 999 | NA | NA | 999 |
| (VPS branch)a | 36.5 | 17.8 | 16 to 85 | 1 | NA | NA | 703 | NA | NA | 703 |
| 35.2 | 16.1 | 14 to 70 | 0 | 0.8 | 0.1 to 30 | 0 | 2 | 0 to 60 | 0 | |
| 10.1 | 2.9 | 4 to 15 | 13 | 0.3 | 0 to 5.9 | 32 | 3 | 1 to 104 | 12 | |
| 71.5 | 7.2 | 60 to 95 | 0 | NA | NA | 361 | 2 | 1 to 96 | 7 | |
| Total (missing) | 42 (< 1%) | 7820 (47%) | 2096 (14%) | |||||||
| CBZ: carbamazepine aTrials designed in two strata based on whether recommended treatment would be CBZ or VPS. Within the two strata, participants were randomised to TPM in Privitera 2003/LEV in Trinka 2013 or CBZ/VPS depending on the strata. Data analysed according to the separate strata (CBZ branch or VPS branch) in this review bExact age not provided in IPD; age intervals only provided (largest age interval > 73 years). Mean age and SD calculated from aggregate data taken from Baulac 2017 journal article | ||||||||||
| Trial | EEG results | CT or MRI scan results | Neurological assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | Missing | Normal | Abnormal | Missing | Normal | Abnormal | Missing | |
| 49 (45%) | 54 (50%) | 5 (5%) | 21 (19%) | 5 (5%) | 82 (76%) | 0 (0%) | 0 (0%) | 108 (100%) | |
| 0 (0%) | 0 (0%) | 583 (100%) | 0 (0%) | 0 (0%) | 583 (100%) | 478 (82%) | 103 (18%) | 2 (0%) | |
| 262 (29%) | 624 (70%) | 2 (1%) | 508 (57%) | 379 (43%) | 1 (0%) | 452 (51%) | 436 (49%) | 0 (0%) | |
| 126 (44%) | 152 (53%) | 9 (3%) | 173 (60%) | 69 (24%) | 45 (16%) | 0 (0%) | 0 (0%) | 287 (100%) | |
| 0 (0%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 136 (100%) | 89 (65%) | 46 (34%) | 1 (1%) | |
| 62 (46%) | 72 (53%) | 2 (1%) | 82 (60%) | 12 (9%) | 42 (31%) | 123 (90%) | 13 (10%) | 0 (0%) | |
| 76 (61%) | 42 (34%) | 6 (5%) | 72 (58%) | 20 (16%) | 32 (26%) | 108 (87%) | 16 (13%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 150 (100%) | 62 (41%) | 87 (58%) | 1 (1%) | 90 (60%) | 60 (40%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 579 (100%) | 0 (0%) | 0 (0%) | 579 (100%) | 493 (85%) | 86 (15%) | 0 (0%) | |
| 107 (37%) | 179 (61%) | 6 (2%) | 0 (0%) | 0 (0%) | 292 (100%) | 0 (0%) | 0 (0%) | 292 (100%) | |
| 28 (17%) | 74 (45%) | 64 (39%) | 0 (0%) | 0 (0%) | 166 (100%) | 0 (0%) | 0 (0%) | 166 (100%) | |
| 0 (0%) | 0 (0%) | 173 (100%) | 0 (0%) | 0 (0%) | 173 (100%) | 152 (88%) | 15 (9%) | 6 (3%) | |
| 18 (35%) | 34 (65%) | 0 (0%) | 50 (96%) | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 52 (100%) | |
| 6 (7%) | 78 (93%) | 0 (0%) | 75 (89%) | 9 (11%) | 0 (0%) | 83 (99%) | 1 (1%) | 0 (0%) | |
| 92 (48%) | 99 (51%) | 2 (1%) | 126 (65%) | 12 (6%) | 55 (28%) | 0 (0%) | 0 (0%) | 193 (100%) | |
| 0 (0%) | 0 (0%) | 243 (100%) | 0 (0%) | 0 (0%) | 243 (100%) | 222 (91%) | 19 (8%) | 2 (1%) | |
| 0 (0%) | 0 (0%) | 81 (100%) | 0 (0%) | 0 (0%) | 81 (100%) | 0 (0%) | 0 (0%) | 81 (100%) | |
| 58 (53%) | 52 (47%) | 0 (0%) | 74 (67%) | 36 (33%) | 0 (0%) | 110 (100%) | 0 (0%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 475 (100%) | 0 (0%) | 0 (0%) | 475 (100%) | 0 (0%) | 0 (0%) | 475 (100%) | |
| 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | 0 (0%) | 480 (100%) | 0 (0%) | 0 (0%) | 480 (100%) | |
| 0 (0%) | 0 (0%) | 622 (100%) | 0 (0%) | 0 (0%) | 622 (100%) | 0 (0%) | 0 (0%) | 622 (100%) | |
| 0 (0%) | 0 (0%) | 55 (100%) | 37 (67%) | 0 (0%) | 18 (33%) | 55 (100%) | 0 (0%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 94 (100%) | 0 (0%) | 0 (0%) | 94 (100%) | 24 (26%) | 70 (74%) | 0 (0%) | |
| 180 (94%) | 12 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 192 (100%) | 0 (0%) | 0 (0%) | 192 (100%) | |
| (CBZ branch)a | 0 (0%) | 0 (0%) | 395 (100%) | 0 (0%) | 0 (0%) | 395 (100%) | 0 (0%) | 0 (0%) | 395 (100%) |
| (VPS branch)a | 0 (0%) | 0 (0%) | 225 (100%) | 0 (0%) | 0 (0%) | 225 (100%) | 0 (0%) | 0 (0%) | 225 (100%) |
| 0 (0%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 136 (100%) | 0 (0%) | 0 (0%) | 136 (100%) | |
| 0 (0%) | 0 (0%) | 261 (100%) | 0 (0%) | 0 (0%) | 261 (100%) | 0 (0%) | 0 (0%) | 261 (100%) | |
| 13 (4%) | 13 (4%) | 325 (93%) | 16 (5%) | 5 (1%) | 330 (94%) | 305 (87%) | 46 (13%) | 0 (0%) | |
| 0 (0%) | 0 (0%) | 300 (100%) | 0 (0%) | 0 (0%) | 300 (100%) | 0 (0%) | 0 (0%) | 300 (100%) | |
| 770 (45%) | 773 (45%) | 178 (10%) | 1000 (58%) | 437 (25%) | 287 (17%) | 1302 (76%) | 419 (24%) | 0 (0%) | |
| 187 (26%) | 494 (69%) | 35 (5%) | 322 (45%) | 44 (6%) | 350 (49%) | 605 (85%) | 111 (15%) | 0 (0%) | |
| 598 (60%) | 293 (30%) | 99 (10%) | 643 (65%) | 347 (35%) | 0 (0%) | 823 (83%) | 167 (17%) | 0 (0%) | |
| 210 (40%) | 44 (8%) | 266 (52%) | 153 (29%) | 367 (71%) | 0 (0%) | 485 (93%) | 35 (7%) | 0 (0%) | |
| 103 (57%) | 71 (39%) | 7 (4%) | 111 (61%) | 33 (18%) | 37 (20%) | 165 (91%) | 16 (9%) | 0 (0%) | |
| 51 (22%) | 121 (53%) | 55 (24%) | 0 (0%) | 0 (0%) | 227 (100%) | 0 (0%) | 0 (0%) | 227 (100%) | |
| (CBZ branch)1 | 0 (0%) | 0 (0%) | 999 (100%) | 0 (0%) | 0 (0%) | 999 (100%) | 0 (0%) | 0 (0%) | 999 (100%) |
| (VPS branch)1 | 0 (0%) | 0 (0%) | 703 (100%) | 0 (0%) | 0 (0%) | 703 (100%) | 0 (0%) | 0 (0%) | 703 (100%) |
| 70 (50%) | 70 (50%) | 0 (0%) | 17 (12%) | 10 (7%) | 113 (81%) | 0 (0%) | 0 (0%) | 140 (100%) | |
| 0 (0%) | 0 (0%) | 260 (100%) | 0 (0%) | 0 (0%) | 260 (100%) | 0 (0%) | 0 (0%) | 260 (100%) | |
| 117 (32%) | 242 (67%) | 2 (1%) | 78 (22%) | 282 (78%) | 1 (0%) | 0 (0%) | 0 (0%) | 361 (100%) | |
| Total | 3183 (22%) | 3593 (24%) | 8013 (54%) | 3620 (24%) | 2156 (15%) | 9013 (61%) | 6164 (42%) | 1659 (11%) | 6966 (47%) |
| CBZ: carbamazepine aTrials designed in two strata based on whether recommended treatment would be CBZ or VPS. Within the two strata, participants were randomised to TPM in Privitera 2003/LEV in Trinka 2013 or CBZ/VPS depending on the strata. Data analysed according to the separate strata (CBZ branch or VPS branch) in this review | |||||||||
| Trial | Outcomes | Summary of resultsb |
|---|---|---|
| Neuropsychological assessment and cognitive functioning at baseline, 6 months’ and 12 months’ follow‐up | MANOVA revealed no significant interaction effect of group and time in the assessments of neurological and psychological functioning. | |
| 1. Seizure remission of at least 50% | 1. 3 months: PHB = 25/68 (37%), LEV = 31/50 (62%); 6 months: PHB = 42/51 (82%), LEV = 40/45 (89%); 9 months: PHB = 49/51 (96%), LEV = 45/45 (100%); 12 months: PHB = 49/51 (96%), LEV = 45/45 (100%). | |
| 2. Psychological assessments | 2. "No significant influence" on psychological function in both group after 12 months | |
| 3. EEG abnormalities | 3. EEG abnormalities were reduced in both groups at 12 months. | |
| 4. Side effects | 4. Most common side effect of PHB was behavioural problems; most common side effect of LEV was irritability or sleep disturbance. | |
| 1. Proportion seizure‐free | 1. CBZ: 64%, PHB: 63% | |
| 2. Response rate and rate of side‐effects | 2. No statistically significant differences between groups | |
| 3. Seizure frequency | 3. Mean seizure frequency: CBZ: 0.66, PHB: 0.8 | |
| 4. Seizure duration | 4. Mean duration (seconds): CBZ: 12.63; PHB: 15 | |
| 1. Time to exit | 1. Median time to exit: GBP: 69 days, LTG: 48 days; HR: 1.043 (95% CI 0.602 to 1.809) | |
| 2. Percentage of completers | 2. Proportion of population completing the study – GBP: 71.6%, LTG: 67.1% | |
| 3. Time to withdrawal for any reason | 3. No difference between groups | |
| 4. Time to first seizure | 4. No difference between groups | |
| 5. Percentage who remained seizure‐free during the final 12 weeks of the 30‐week evaluation period | 5. GBP: 76.1%, LTG: 76.8% (ITT population) | |
| 6. Withdrawal rate due to adverse events | 6. Withdrawals during titration: GBP: 7, LTG: 10; Withdrawals after titration: GBP: 10, LTG: 13. | |
| 1. Seizure control: • excellent (complete freedom of seizures) • good (> 50% reduction in seizure frequency) • poor (< 50% reduction in seizure frequency or no response) | 1. Excellent: PHT: 67%; CBZ: 37%; VPS: 53% Good: PHT: 12%; CBZ: 37%; VPS: 25% Poor: PHT: 21%; CBZ: 25%; VPS: 22% | |
| 2. Side effects | 2. PHT: 10%; CBZ: 8%; VPS:11% | |
| 1. Seizure freedom | 1. CBZ: 76%, LEV: 76% | |
| 2. Proportion with adverse events | 2. CBZ: 65%, LEV: 50% | |
| 3. Discontinuations of the trial drug | 3. CBZ: 2 discontinuations due to failure to control seizures and interactions with other medications LEV: 3 discontinuations – 1 death from stroke and 2 due to AEs | |
| Event‐related potential recordings and neuropsychological assessments | No significant difference between groups | |
| 1. Cognitive/psychometric outcomes | 1. No significant difference between groups | |
| 2. Auditory event‐related potentials (neurophysiological outcome) | 2. No significant difference between groups | |
| 3.Incidence of allergic reactions | 3. 2 children from PHB group, 1 child from CBZ group and no children from VPS group withdrew from the study because of allergic reactions. | |
| 4. Seizure control | 4. No significant difference between groups | |
| 1. Response rate at 13 weeks and at 26 weeks | 1. 13 weeks: CBZ = 41/58 (71%), OXC = 45/60 (75%); 26 weeks: CBZ = 38/58 (66%), OXC = 43/60 (72%). | |
| 2. Seizure‐free rate at 13 weeks and at 26 weeks | 2. 13 weeks: CBZ = 29/58 (50%), OXC = 32/60 (53%); 26 weeks: CBZ = 25/58 (43%), OXC = 30/60 (50%). | |
| 3. Adverse events at 26 weeks | 3. CBZ = 23/58 (40%); OXC = 11/60 (18%) (P < 0.05) | |
| 1. Change in overnight sleep parameters from baseline after 4‐6 weeks of treatment | 1. Overall effect on sleep parameters was comparable between groups. LEV group PSG significant increase post‐treatment compared to baseline in sleep efficiency (P = 0.039) and in decrease of wake time after sleep onset (P = 0.047); no significant change in other sleep parameters. CBZ group post‐treatment compared to baseline: significant increases in the percentage of slow wave sleep (P = 0.038); no significant change in other sleep parameters | |
| 2. Change in sleep questionnaires and National Hospital Seizure Severity Scale (NHS3) from baseline after 4‐6 weeks of treatment | 2. No significant difference between baseline and post‐treatment between the 2 groups | |
| 1. The proportion of seizure‐free participants who had at least 1 seizure during the maintenance period | 1. OXC 56.6%; VPS 53.8% | |
| 2. Time to premature discontinuation due to adverse experiences | 2. No significant difference between groups | |
| 3. Rate of premature discontinuations for any reason | 3. OXC 40.6% ; VPS 33.9% | |
| 4. Overall assessments of efficacy and tolerability and therapeutic effect | 4. No significant difference between groups | |
| 5. Individual adverse experiences | 5. Proportion of participants experiencing at least 1 AE regardless of relationship to trial drug: OXC 89.8%; VPS 87.6% | |
| 6. Seizure frequency during maintenance | 6. Seizure frequency per week: OXC (n = 106) mean 0.17 median 0, VPS (n = 106) mean 0.40, median 0 | |
| 1. Frequency of seizures during the treatment period | 1. No significant difference between groups | |
| 2. Retention of treatment from the first intake | 2. Completed study: LEV 52/62, CBZ 54/66 Withdrawals: 8 poor compliance (LEV 4, CBZ 4); 7 severe adverse effects (LEV 3, CBZ 4); 7 unknown cause (LEV 3, CBZ 4) | |
| 3. Changes in cognitive measures and quality‐of‐life measures at the end of the treatment period | 3. Attention deficit on digital span end of follow up greater in CBZ group than LEV (P = 0.03) Stroop test worse in CBZ than LEV (P = 0.02) No significant difference between groups for other scales. Impairment of activities of daily living greater CBZ than LEV (P = 0.05) | |
| 4. Changes in EEG assessments at the end of the treatment period | 4. 4 participants (LEV 2, CBZ 2) had abnormal EEG at baseline, normal at end of treatment. Drug dose reduction (LEV 4, CBZ 2). Remaining participants unmodified versus baseline | |
| 5. Tolerability of treatment | 5. No significant difference between groups | |
| Changes in memory function from baseline after 3 weeks of treatment | Significant decrease in visual‐verbal memory for CBZ and acoustic memory for PHB. No significant differences for other tests | |
| 1. Proportion achieving 24‐month remission at 3 years | 1. PHB: 60%, PHT: 59%; CBZ: 62%; VPS: 64% | |
| 2. Exclusions after randomisation due to adverse effects or no efficacy | 2. PHB: 33%, PHT: 23%; CBZ: 30%; VPS: 23% | |
| 1.Changes in seizure frequency between baseline and the end of each maintenance period | 1. Baseline: OXC mean 2.9 (SD 7.0), median 1, range 0‐60; CBZ mean 5.8 (SD 14.7) median 1, range 0‐99 Maintenance phases: OXC mean 0.4 (SD 3.0) median 0, range 0‐27; CBZ mean 0.3 (SD 1.4) median 0, range 0‐12 | |
| 2. Side effects observed by participants and investigators at each visit | 2. Severe side effects: CBZ 25, OXC 13, statistically significant difference favouring OXC (P = 0.04) Participants without any side effects: CBZ 25, OXC 29 no significant difference between groups (P = 0.22) Nature of side effects same between groups, included tiredness, headache, dizziness, ataxia. Participants withdrawn due to severe side effects: CBZ 16, OXC 9 | |
| 3.Global evaluation of therapeutic efficacy and tolerability by the investigator at the end of each maintenance period | 3. Global efficacy: no significant difference between groups (P = 0.77); global tolerability (P = 0.11) Participants very good/good: CBZ 69 (73%), OXC 76 (84%) Participants poor/very poor: CBZ 26 (27%), OXC 15 (16%) | |
| 4. Changes in EEG tracings between baseline and the end of each maintenance period | 4. Clinically relevant changes observed in 2 participants only, both CBZ group, both stopped treatment | |
| 1. Cognitive testing | 1. No significant difference between treatment groups | |
| 2. Percentage of participants remaining seizure‐free throughout treatment | 2. OXC 58%; CBZ 46%; VPS 54% | |
| 3. Most common adverse events | 3. Most common (> 10% reported) side effects OXC fatigue and headache; CBZ fatigue and rash; VPS headache, increased appetite, alopecia | |
| 4. Treatment satisfaction on a 4‐point scale from poor to very good | 4. Good/very good: OXC investigators 84%, participants 82%, parents/carers 86%; Combined CBZ/VPS investigators 77%, participants 73%, parents/carers 80% | |
| 1. Adverse effects | 1. Minor adverse effects reported in PHB: 58 participants (39%) reported 86 AEs, CBZ: 46 participants (30%) reported 68 AEs | |
| 2. Withdrawals from allocated treatment | 2. All withdrawals: PHB: 18%, CBZ: 17%; withdrawals due to side‐effects: PHB: 5%, CBZ: 3% | |
| 3. Seizure frequency (during second 6 months of trial) | 3. Seizure‐free: PHB: 54%, CBZ: 52%; > 50% reduction of seizures: PHB: 23%, CBZ: 29% 50% reduction to 50% increase in seizures: PHB: 15%, CBZ: 13%; > 50% increase in seizures: PHB: 8%, CBZ: 6% | |
| 1. Cognitive assessments | 1. Significant difference favouring VPS test of speed of information processing No significant differences between treatment groups for any other cognitive tests | |
| 2. Withdrawals from randomised drug | 2. PHT: 30%; CBZ: 39%; VPS: 33% | |
| 1.Seizure reduction | 1. Seizure freedom: LTG: 38%, OXC: 44%; > 50% seizure reduction: LTG: 48%, OXC: 55% | |
| 2. Cognition, mood and health‐related quality of life | 2. Both groups showed improvement in verbal learning and in 1/4 measures of attention. In addition, participants under OXC improved in word fluency. Improved mood was reported with OXC only. | |
| 1.The appearance of a second seizure under treatment or by finishing the 12‐month follow‐up without seizures | 1. Number of participants experiencing early seizures as first event: LTG 2/32, CBZ 3/32 Number of participants remaining seizure‐free in the follow‐up period: LTG 23/32 (72%), CBZ 14/32 (44%), P = 0.05 | |
| 2. Tolerability: incidence of adverse events | 2. LTG 2/32 (6.25%), CBZ 12/32 (37.5%), P = 0.05 | |
| 3. Withdrawals due to adverse events | 3. LTG 1/32 (3%), CBZ 10/32 (31%), P = 0.02 | |
| 1. Seizure‐free | 1. 3 months: LTG = 8/30 (26.7%), VPS = 16/30 (53.3%); 6 months: LTG = 14/30 (46.7%), VPS = 19/30 (63.3%); 12 months: LTG = 17/30 (56.7%), VPS = 23/30 (76.7%). | |
| 2. At least 50% reduction in seizure frequency (not seizure‐free) | 2. 3 months: LTG = 2/30 (6.7%), VPS = 3/30 (10%); 6 months: LTG = 3/30 (10%), VPS = 3/30 (10%); 12 months: LTG= 3/30 (10%), VPS = 1/30 (3.3%). | |
| 3. Number of seizures per month (at 12 months) | 3. Mean (SD): LTG = 2.43 (1.87), VPS =1.70 (1.82) | |
| 4. Adverse events | 4. Adverse effects were recorded in 9 (30.0%) patients of VPS group and 17 (56.7%) in LTG group patients. Sedation, ataxia and tremor were recorded in patients taking VPS but these symptoms responded to a decrease in dosage. Skin rash developed within three months in 3 (10.00%) patients taking LTG; they withdrew from the study. | |
| 1. Neuropsychological outcomes | 1. No difference between groups in terms of social competence; school competence; internalising behaviour problems; externalising behaviour problems; total behaviour problems and anxiety. Significant decrease in depression in LEV group compared to CBZ group (P = 0.027) | |
| 2. Mean percentage change in seizure frequency from baseline | 2. LEV 95.7%, CBZ 97.1%, P = 0.686 | |
| 3. Seizure‐freedom rates | 3. LEV 66.7%, CBZ 57.8%, P = 0.317 | |
| 4. Incidence of adverse events | 4. LEV 33.3%, CBZ 46.9%. Number of AEs not significantly different between groups | |
| 1. Seizure freedom | 1.CBZ: 53% LTG: 56% | |
| 2. Cognitive assessments | 2. No significant difference between groups in overall cognitive score. In terms of individual assessments; only Stroop test B showed a statistically significant advantage for LTG. | |
| 1. Percentage of participants with a treatment failure after 50 weeks | 1. LEV = 19/173 (11.0%), OXC = 31/171 (18.1%) (full analysis set) | |
| 2. Time to treatment failure | 2. Hazard ratio (LEV vs OXC): 0.6 (0.3 to 1.0), log rank P = 0.0658 | |
| 3. Time to the first seizure defined as the time from the first dose of medication to the occurrence of the first seizure during the 48 weeks' treatment period | 3. Median months: LEV: 7.6, OXC: NA (fewer than 50% of participants in the OXC group had seizure recurrence) | |
| 4. Percentage of subjects who achieved seizure freedom for 24 consecutive weeks during the 48 weeks' treatment period at any time | 4. LEV = 93/173 (53.8%), OXC = 100/171 (58.5%) (full analysis set) | |
| 5. Percentage of subjects who achieved seizure freedom during the 48 weeks' treatment period | 5. LEV = 60/173 (34.7%), OXC = 70/171 (40.9%) (full analysis set) | |
| Cognitive performance and neuropsychological assessments | No significant difference between groups | |
| 1. Retention rate at study end | 1. LTG: 65%, CBZ: 70% | |
| 2. Terminal 24‐week seizure‐free rate and time interval from the end of dose titration phase to the first seizure | 2. Total seizure‐free rate LTG: 62%, CBZ: 63% Time to first seizure, mean (SD): weeks: LTG: 10 (5.09), CBZ: 10.82 (6.44) | |
| 1. Seizure remission rate at 24 weeks | 1. The 24‐week terminal remission rate was 51 out of 73 (69.9%) in ZNS group compared with 62 out of 82 (75.6%) in CBZ group (P = 0.9). | |
| 2. Time interval to first seizure recurrence (after the dose‐escalation period). | 2. The time interval to the first seizure recurrence was 40.9 ± 31.7 days in ZNS group (n = 13) and 47.8 ± 30.8 days in CBZ group (P = 0.75). | |
| 3. Incidence of adverse events | 3. The incidence of adverse events (AEs) was 67.1% in ZNS group and 53.7% in CBZ group (P = 0.088) (entire treatment period). | |
| 1. Seizure freedom | 1. LTG: 54%, VPS: 55%, no difference by seizure type | |
| 2. Retention on treatment | 2. LTG: 69%, VPS: 68 % | |
| 1. Serum S100B levels | 1. Decrease in serum S100B was significantly higher with CBZ group (0.004; 95% CI 0.001 to 0.006; P = 0.01) against OXC group | |
| 2. Quality of life (by the QOLIE‐31) | 2. An improvement in quality of life was seen in both groups for all domains. | |
| 3. Chalfont‐National Hospital seizure severity scale (NHS3) | 3. No difference in mean change of NHS3 between groups | |
| 4. Adverse events | 4. No adverse events reported on OXC. On CBZ, sedation and dizziness (n = 4) and vertigo (n = 3). All adverse drug reactions were mild, and the drug was not discontinued. | |
| Change in cognitive, intelligence (IQ), behavioural, and psychometric scores between baseline, 6 months, and 12 months | 1. No significant differences between treatment groups | |
| 2. Compliance, drug changes, and withdrawal rates | 2. Compliance: trend towards better compliance in CBZ group (not significant). Trend towards higher rate withdrawal from treatment in PHB group (not significant). More mild systemic side effects in CBZ group (significant). 3 children switched from CBZ to PHB and 1 from PHB to CB following adverse reactions. | |
| Seizure control at 6 and 12 months (excellent/good/fair/poor) | 3. 6 months: excellent/good: PHB = 15, CBZ = 13 12 months: excellent/good: PHB = 13, CBZ = 9 | |
| 1. Proportion of all randomised participants with seizure recurrence (by seizure type) | 1. Focal seizures ‐ PHT: 32%; CBZ: 40%; VPS: 41% Generalised seizures ‐ PHT: 35%; CBZ: 15%; VPS: 7% | |
| 2. Proportion of participants with optimum plasma levels with seizure recurrence (by seizure type) | 2. Focal seizures ‐ PHT: 24%; CBZ: 24%; VPS: 25% Generalised seizures ‐ PHT: 13%; CBZ: 0%; VPS: 0% | |
| 1. Seizure recurrence
| 1. Seizure recurrence at 2 weeks ‐ LTG: 43%, LEV: 35%, P = 0.42 Seizure recurrence at 4 weeks ‐ LTG: 39%, LEV: 33%, P = 0.53 Seizure recurrence at 8 weeks ‐ LTG: 35%, LEV: 28%, P = 0.50 Seizure recurrence at 12 weeks ‐ LTG: 33%, LEV: 24%, P = 0.35 Seizure recurrence at 20 weeks ‐ LTG: 31%, LEV: 13%, P = 0.03 | |
| 2. Abnormal laboratory values | 2. No significant difference between groups | |
| 3. Adverse events | 3. Proportion with AEs ‐ LTG: 53%, LEV: 67% | |
| 1. Proportion of subjects remaining seizure‐free during the 6‐month evaluation period | 1. LEV: 47.3%, CBZ: 68.4% | |
| 2. Proportion of subjects retained in the trial for the duration of the period covering the up‐titration period, stabilisation period, and evaluation period | 2. LEV: 48.4%, CBZ: 70.2% | |
| 3. Time to first seizure or discontinuation due to an adverse event /lack of efficacy during the evaluation period | 3. Number of events: LEV: 88, CBZ: 45 (times not reported) | |
| 4. Time to first seizure during the evaluation period | 4. Number of events: LEV: 87, CBZ: 39 (times not reported) | |
| 5. Time to first seizure during the period covering the up‐titration period, stabilisation period, and evaluation period from the first dose of trial drug | 5. Number of events: LEV: 97, CBZ: 57 (times not reported) | |
| 1. Cognitive assessments | 1. Compared to CBZ, participants on PHT became slower (motor speed of the hand) and their visual memory decreased. There was an equal decrease in negative mood (helplessness, irritability, depression) on PHT and CBZ. | |
| 2. Harmful side effects | 2. 3 participants taking PHT complained of tiredness, and 1 participant taking CBZ complained of facial skin problems, another tiredness and memory problems | |
| 1. Side effects (major and minor) | 1. Incidence of major side effects (proportion of analysed participants): PHT 23%; CBZ: 23% Minor side effects: cognitive impairment and sedation twice as likely on CBZ compared to PHT. Other minor side effects similar between groups | |
| 2. Treatment failure | 2. Treatment failures among analysed participants: PHT 4/35 (11%); CBZ: 5/35 (14%) | |
| 3. Seizure control | 3. Seizure control (among analysed participants with no major side effects): PHT: 86%; CBZ: 82% | |
| 4. Laboratory assessments | 4. Significantly lower mean LDH level at 24 weeks in CBZ participants than PHT participants. Other laboratory results similar across treatment groups | |
| 1. Discontinuations from the trial | 1. 8 discontinuations; due to generalised rash (n = 1), excessive tiredness (n = 1), withdrew consent (n = 2), renal transplant (n = 1), lost to follow‐up (n = 2), died (n = 1) | |
| 2. Treatment‐emergent side effects | 2. 6 participants reported treatment‐emergent side effects. | |
| 3. Seizure control | 3. No participants withdrew due to lack of seizure control. | |
| 1. Reduction in frequency of seizures: • excellent (100% reduction); • good (75%‐99% reduction); • fair (50%‐74% reduction); • poor (< 50% reduction) | 1. Excellent: PHT: 51%, VPS: 49% Good: PHT: 24%, VPS: 35% Fair: PHT: 18%, VPS: 10% Poor PHT: 2%, VPS: 6% | |
| 2. Adverse effects | 2. All reported AEs were minor and similar rates between groups. | |
| Cognitive measures before and after treatments | No significant differences between any tests of cognitive function taken before treatment and after 10‐12 weeks for both treatment groups | |
| 1. Seizure freedom and frequency of seizures during the trial | 1. Six months of seizure freedom: CBZ: 81%, TPM: 91% 50% reduction of seizures: CBZ: 84% TPM: 97% The average number of seizures was significantly less in the TPM group compared to the CBZ group at 6 and 9 months. | |
| 2. Adverse events during the trial | 2. AEs were mild and similar between groups. | |
| 3. Laboratory results | 3. No significant differences between groups | |
| 1. Retention in the trial for 12 months | 1. Significant difference between 3 treatment groups (P = 0.00022) CBZ more early terminators than GBP (P = 0.008) or LTG (P < 0.0001) | |
| 2. Seizure freedom at 12 months | 2. LTG 51.4%, GBP 47.4%, CBZ 64.3%, no significant difference between groups, P = 0.09 | |
| 3. Time to first, second, fifth and tenth seizure (time to seizures) | 3. No difference between groups for time to first, second, fifth and tenth seizure (P = 0.18, 0.13, 0.74, 0.95, respectively) | |
| 4. Drug toxicity (incidence of systemic and neurologic toxicities) | 4. More systemic toxicities on GBP than CBZ or LTG No significant differences in neurotoxicities between treatment groups over 12 months | |
| 5. Serum drug levels and compliance | 5. Mean serum levels: 6 weeks: GBP 8.67 ± 4.83; µg/mL, CBZ 6.79 ± 2.92 µg/mL and LTG 2.87 ± 1.60 µg/mL 52 weeks: GBP 8.54 ± 5.57 µg/mL, CBZ 6.48 ± 3.72 µg/mL and LTG 3.46 ± 1.68 µg/mL Overall medical compliance: 89% without significant group differences | |
| 6. Seizure‐free retention rates | 6. 3 months: LTG 49.7%, GBP 43.3%, CBZ 36.0%, significant difference between groups, P = 0.02 6 months: LTG 37.2%, GBP 33.0%, CBZ 28.9%, no significant difference between groups, P = 0.22 12 months: LTG 28.6%, GBP 23.2%, CBZ 22.8%, no significant difference between groups, P = 0.33 | |
| 1. Retention in the trial (time to treatment withdrawal for any cause) | 1. LTG 68 (73%), CBZ 61 (67%), no significant difference between groups | |
| 2. Seizure freedom after week 4 | 2. LTG 59 (63%), CBZ 69 (76%), no significant difference, P = 0.068 (ITT analysis) | |
| 3. Seizure freedom after week 20 | 3. LTG 71 (76%), CBZ 81 (89%), significant difference, P = 0.0234 (ITT analysis) | |
| 4. Time to first seizure | 4. Hazard ratio (LTG/CBZ) 1.50, 95% CI 0.94 to 2.40, P = 0.092 | |
| 5. Adverse event reports | 5. During treatment period, LTG 82 (88%) reported 378 AEs, CBZ 79 (86%) reported 310 AEs. No significant differences between groups for any AEs except for immune system Withdrew due to AE: LTG 13 (14%), CBZ 23 (25%), P = 0.078 | |
| 6. Tolerability according to the Liverpool Adverse Event profile (AEP) | 6. No difference between groups even when changes over time corrected for age, gender and baseline score | |
| 1. Seizures during treatment | 1. PHT: 33%; VPS: 39% | |
| 2. Adverse events | 2. All reported AEs were minor and similar rates between groups. | |
| 1. Anthropometric measures (weight, BMI) | 1. The mean weight and BMI were significantly higher in VPS group in LTG group at 12 months. | |
| 2. Clinical measures related to the menstrual cycle | 2. Menstrual disturbances were found in 11 women; among them nine (30%) received VPS and two (6%) received LTG | |
| 3. Reproductive hormone levels | 3. Mean testosterone level was significantly higher in the VPS group than in the LTG group at 6 and 12 months. There were no differences between groups in terms of other hormone levels. | |
| 4. Insulin resistance | 4. 11 (37%) of VPS group, and 2 (6%) of LTG group developed insulin resistance during the course of therapy. | |
| 1. Proportion of participants free of complex focal seizures during the maintenance period | 1. VPS 7/11 (64%), CBZ 9/14 (64%) | |
| 2. Proportion of participants reporting specific adverse events | 2. At least one AE reported VPS 15/16 (94%), CBZ 16/17 (94%) | |
| 1. Number of seizure‐free patients during trial weeks 17‐24
| 1. Focal: CBZ group 83/88 (94.3%), LTG group 78/88 (88.6%), no significant difference between groups Generalised: VPS group 25/30 (83.3%) LTG group 20/33 (60.6%), no significant difference between groups | |
| 2. “Leaving the study” (retention rates) | 2. Focal: CBZ group 81%, LTG group 91%, no significant difference between groups Generalised: VPS group 97%, LTG group 88%, not stated as significant or non‐significant difference | |
| 3. Adverse event rates | 3. At least 1 AE: Focal: CBZ 81 participants (91%), LTG 68 participants (77.3%) Generalised: VPS 25 participants (83.3%), LTG 24 participants (72.7%) Serious AEs: Focal: CBZ 8 participants (9%), LTG 6 participants (7%) Generalised: VPS 1 participant (3%), LTG 5 participants (15%) AEs considered related to study drug: Focal: CBZ 65 participants (74%), LTG 38 participants (43%) Generalised: VPS 16 participants (53%), LTG 15 participants (45.5%) | |
| 1. Quality of life by the QOLIE‐10 questionnaire before and after 26 weeks of therapy | 1. Mean quality of life score at baseline: CBZ group 31.14 ± 1.83, LEV group 29.76 ± 1.71 (P = 0.5861) Mean quality of life score after 26 weeks of treatment: CBZ group 58.41 ± 1.89, LEV 64.58 ± 2.02 (P = 0.0302) | |
| 2. Seizure freedom at 4 weeks, 12 weeks, 26 weeks and 6 months | 2. 4 weeks: CBZ group 85.72%, LEV group 85.72% (P = 1); 12 weeks: CBZ group 89.29%, LEV group 93.34% (P = 0.4595); 26 weeks: CBZ group 96.43%, LEV group 100% (P = 0.1212); 6 months: CBZ group 71.42% (20 participants), LEV group 78.57% (22 participants) (P = 0.2529) | |
| 3. Proportion of participants experiencing at least 1 adverse event) | 3. CBZ group 36.66%, LEV group 40% (P = 0.77) | |
| 1. Proportion with recurrence of seizures | 1. PHB: 31%, PHT: 27%, VPS: 21% | |
| 2. Adverse events | 2. PHB: 33%, PHT: 63%, VPS: 31% | |
| 1. Proportion of patients who were seizure‐free for the entire evaluation phase at the last evaluated dose level. | 1. Per protocol set: 71.1% of ESL group and 75.6% of the CBZ‐CR group Full analysis set: 70.8% of ESL group and 74.0% of CBZ group seizure‐free at the last evaluated dose level | |
| 2. Proportion of seizure‐free patients during 1‐year of treatment | 2. Per protocol set: 64.7% of ESL group and 70.3% of the CBZ‐CR group Full analysis set: 63.8% of ESL group and 68.7% of CBZ group seizure‐free at 1 year of treatment | |
| 3. Time to first seizure at the last evaluated dose (treatment failure time) | 3. Treatment failure (i.e. first seizure) was higher in the ESL group (12%) than in the CBZ‐CR group (6%). Treatment failure based on withdrawal from the study (post hoc) was also higher in the ESL group (7%) than in the CBZ‐CR group (3%). | |
| 4. Seizure characteristics of the first seizure during the evaluation period | 4. A similar proportion between treatment groups was found for seizure‐free patients during the evaluation period by most frequent baseline seizure type. | |
| 5. Dose level at which patients reached 26‐week seizure freedom | 5. The overall proportion of seizure‐free patients at each dose level was similar. | |
| 6. Treatment retention time (defined as the time to withdrawal due to adverse events [AEs] or lack of efficacy) | 6. The probability of patients withdrawing due to either AEs or lack of efficacy (ESL vs CBZ‐CR) was 26% vs 21% at 1 year. Retention time was similar across the drugs. | |
| 7. Changes in quality of life (Quality of Life in Epilepsy Inventory‐31 (QOLIE‐31) survey) | 7. Comparable improvements from baseline in the QOLIE‐31 scores were observed at the maintenance period visit and the final endpoint visit (between group differences not calculated). | |
| 8. Incidence of treatment‐emergent adverse events | 8. The percentage of patients who experienced at least 1 TEAE was similar (ESL: 76.3%, CBZ‐CR: 79.6%) | |
| 1. Semen quality | 1. OXC can improve the sperm quality in fast forward movement rate and survival rate. LEV and LTG have no effect on semen quality. | |
| 2. Sexual function and sex hormones | 2. OXC, LEV and LTG have no significant effect on sexual function or sex hormones. | |
| 1. Effective rate at 1 year | 1. OXC = 43/57 (75.4%), TPM = 49/58 (84.5%); LEV = 58/68 (85.5%), LTG = 61/70 (87.1%) | |
| 2. Retention rate at 1 year | 2. OXC = 35/57 (61.4%), TPM = 40/58 (68.9%); LEV = 50/68 (73.5%), LTG = 62/70 (88.6%) | |
| 3. Reasons for drug withdrawal | 3. The primary cause of drug withdrawal was seizure‐control ineffectiveness (28, 42.4%), adverse effects (13, 19.7%) and price (10, 15.2%). | |
| AE: adverse event | ||
| Trial | Time to treatment failurec | Time to first seizure | Time to 12‐month remissiond | Time to six‐month remissiond | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cens | Event | Total | Missing | Cens | Event | Total | Missing | Cens | Event | Total | Missing | Cens | Event | Total | Missing | |
| 0 | 0 | 0 | 108 | 38 | 66 | 104 | 4 | 0 | 0 | 0 | 108 | 0 | 0 | 0 | 108 | |
| 392 | 191 | 583 | 0 | 396 | 186 | 582 | 1 | 234 | 348 | 582 | 1 | 151 | 431 | 582 | 1 | |
| 562 | 325 | 887 | 1 | 481 | 403 | 884 | 4 | 343 | 541 | 884 | 4 | 224 | 660 | 884 | 4 | |
| 207 | 80 | 287 | 0 | 137 | 145 | 282 | 5 | 190 | 92 | 282 | 5 | 136 | 146 | 282 | 5 | |
| 99 | 36 | 135 | 1 | 64 | 71 | 135 | 1 | 0 | 0 | 0 | 136 | 90 | 45 | 135 | 1 | |
| 79 | 57 | 136 | 0 | 69 | 67 | 136 | 0 | 0 | 0 | 0 | 136 | 122 | 14 | 136 | 0 | |
| 79 | 45 | 124 | 0 | 52 | 72 | 124 | 0 | 0 | 0 | 0 | 124 | 96 | 28 | 124 | 0 | |
| 95 | 55 | 150 | 0 | 70 | 80 | 150 | 0 | 0 | 0 | 0 | 150 | 0 | 0 | 0 | 150 | |
| 323 | 256 | 579 | 0 | 350 | 229 | 579 | 0 | 260 | 319 | 579 | 0 | 177 | 402 | 579 | 0 | |
| 100 | 191 | 291 | 1 | 102 | 190 | 292 | 0 | 0 | 0 | 0 | 292 | 193 | 99 | 292 | 0 | |
| 0 | 0 | 0 | 166 | 66 | 81 | 147 | 19 | 117 | 30 | 147 | 19 | 58 | 89 | 147 | 19 | |
| 100 | 63 | 163 | 10 | 18 | 149 | 167 | 6 | 22 | 145 | 167 | 6 | 19 | 148 | 167 | 6 | |
| 44 | 8 | 52 | 0 | 40 | 12 | 52 | 0 | 11 | 41 | 52 | 0 | 8 | 44 | 52 | 0 | |
| 75 | 9 | 84 | 0 | 52 | 32 | 84 | 0 | 0 | 0 | 0 | 84 | 35 | 49 | 84 | 0 | |
| 153 | 40 | 193 | 0 | 106 | 84 | 190 | 3 | 112 | 78 | 190 | 3 | 84 | 106 | 190 | 3 | |
| 164 | 69 | 233 | 10 | 66 | 177 | 243 | 0 | 78 | 165 | 243 | 0 | 49 | 194 | 243 | 0 | |
| 58 | 23 | 81 | 0 | 38 | 29 | 67 | 14 | 68 | 13 | 81 | 0 | 30 | 50 | 80 | 1 | |
| 82 | 28 | 110 | 0 | 79 | 31 | 110 | 0 | 0 | 0 | 0 | 110 | 39 | 71 | 110 | 0 | |
| 266 | 208 | 474 | 1 | 226 | 238 | 464 | 11 | 325 | 149 | 474 | 1 | 281 | 193 | 474 | 1 | |
| 302 | 168 | 470 | 10 | 165 | 299 | 464 | 16 | 334 | 133 | 467 | 13 | 242 | 225 | 467 | 13 | |
| 510 | 111 | 621 | 1 | 309 | 312 | 621 | 1 | 0 | 0 | 0 | 622 | 0 | 0 | 0 | 622 | |
| 0 | 0 | 0 | 55 | 29 | 26 | 55 | 0 | 0 | 0 | 0 | 55 | 0 | 0 | 0 | 55 | |
| 0 | 0 | 0 | 94 | 41 | 49 | 90 | 4 | 82 | 8 | 90 | 4 | 63 | 27 | 90 | 4 | |
| 157 | 32 | 189 | 3 | 121 | 71 | 192 | 0 | 131 | 60 | 191 | 1 | 68 | 123 | 191 | 1 | |
| (CBZ branch)b | 221 | 174 | 395 | 0 | 208 | 187 | 395 | 0 | 316 | 79 | 395 | 0 | 194 | 201 | 395 | 0 |
| (VPS branch)b | 111 | 114 | 225 | 0 | 119 | 106 | 225 | 0 | 180 | 45 | 225 | 0 | 106 | 119 | 225 | 0 |
| 113 | 23 | 136 | 0 | 81 | 44 | 125 | 11 | 0 | 0 | 0 | 136 | 78 | 47 | 125 | 11 | |
| 230 | 31 | 261 | 0 | 197 | 64 | 261 | 0 | 0 | 0 | 0 | 261 | 0 | 0 | 0 | 261 | |
| 288 | 63 | 351 | 0 | 216 | 135 | 351 | 0 | 0 | 0 | 0 | 351 | 328 | 23 | 351 | 0 | |
| 210 | 76 | 286 | 14 | 91 | 198 | 289 | 11 | 92 | 198 | 290 | 10 | 77 | 213 | 290 | 10 | |
| 881 | 835 | 1716 | 5 | 389 | 1292 | 1681 | 40 | 596 | 1085 | 1681 | 40 | 355 | 1326 | 1681 | 40 | |
| 410 | 302 | 712 | 4 | 185 | 521 | 706 | 10 | 174 | 532 | 706 | 10 | 96 | 610 | 706 | 10 | |
| 567 | 423 | 990 | 0 | 300 | 690 | 990 | 0 | 341 | 649 | 990 | 0 | 203 | 787 | 990 | 0 | |
| 290 | 230 | 520 | 0 | 162 | 358 | 520 | 0 | 195 | 325 | 520 | 0 | 104 | 416 | 520 | 0 | |
| 108 | 73 | 181 | 0 | 100 | 81 | 181 | 0 | 0 | 0 | 0 | 181 | 157 | 24 | 181 | 0 | |
| 163 | 64 | 227 | 0 | 81 | 140 | 221 | 6 | 172 | 55 | 227 | 0 | 137 | 90 | 227 | 0 | |
| (CBZ branch)b | 760 | 239 | 999 | 0 | 572 | 427 | 999 | 0 | 780 | 219 | 999 | 0 | 336 | 663 | 999 | 0 |
| (VPS branch)b | 582 | 120 | 702 | 1 | 455 | 247 | 702 | 1 | 484 | 218 | 702 | 1 | 191 | 511 | 702 | 1 |
| 111 | 29 | 140 | 0 | 75 | 65 | 140 | 0 | 47 | 93 | 140 | 0 | 36 | 104 | 140 | 0 | |
| 187 | 59 | 246 | 14 | 59 | 187 | 246 | 14 | 84 | 162 | 246 | 14 | 19 | 227 | 246 | 14 | |
| 195 | 166 | 361 | 0 | 249 | 96 | 345 | 16 | 211 | 150 | 361 | 0 | 178 | 183 | 361 | 0 | |
| Total | 9274 | 5016 | 14290 | 499 | 6654 | 7937 | 14591 | 198 | 5979 | 5932 | 11911 | 2878 | 4760 | 8688 | 13,448 | 1341 |
| CBZ: carbamazepine aFor two studies, we could only calculate 'time to first seizure'; the study duration of Ogunrin 2005 was 12 weeks, and all randomised participants completed the study without withdrawing; and Banu 2007 did not record the dates of all seizures after randomisation and dates of withdrawal for allocated treatment for all participants. | ||||||||||||||||
| Reason for treatment failure/withdrawal | Classification for analysis | Randomised drugb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBZ | PHB | PHT | VPS | LTG | OXC | TPM | GBP | LEV | ZNS | LCM | Total | ||
| Adverse events | Event | 588 (45%) | 24 (19%) | 94 (38%) | 169 (29%) | 302 (41%) | 60 (38%) | 262 (50%) | 73 (21%) | 240 (40%) | 94 (39%) | 48 (29%) | 1954 (39%) |
| Inadequate response | Event | 258 (20%) | 20 (16%) | 28 (11%) | 157 (27%) | 192 (26%) | 39 (25%) | 123 (24%) | 205 (58%) | 159 (27%) | 58 (24%) | 47 (29%) | 1286 (26%) |
| Both adverse events and inadequate response | Event | 145 (11%) | 53 (42%) | 42 (17%) | 107 (18%) | 18 (2%) | 11 (7%) | 46 (9%) | 32 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 454 (9%) |
| Protocol violation/non compliance | Event | 94 (7%) | 6 (5%) | 57 (23%) | 20 (3%) | 68 (9%) | 40 (26%) | 39 (8%) | 17 (5%) | 21 (4%) | 3 (1%) | 11 (7%) | 376 (8%) |
| Withdrew consent | Event | 146 (11%) | 16 (13%) | 17 (7%) | 60 (10%) | 93 (13%) | 2 (1%) | 5 (1%) | 2 (1%) | 95 (16%) | 48 (20%) | 47 (29%) | 531 (11%) |
| Othera | Event | 66 (5%) | 6 (5%) | 9 (4%) | 66 (11%) | 60 (8%) | 4 (3%) | 45 (9%) | 23 (7%) | 81 (14%) | 35 (15%) | 11 (7%) | 406 (8%) |
| Total eventsb | 1297 (35%) | 125 (40%) | 247 (29%) | 579 (30%) | 733 (31%) | 156 (33%) | 520 (45%) | 352 (59%) | 596 (32%) | 238 (39%) | 164 (37%) | 5007 (35%) | |
| Illness or death | Censored | 31 (1%) | 14 (7%) | 16 (3%) | 12 (1%) | 19 (1%) | 2 (1%) | 16 (3%) | 10 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 120 (1%) |
| Remission of seizures | Censored | 49 (2%) | 4 (2%) | 37 (6%) | 91 (7%) | 65 (4%) | 12 (4%) | 44 (7%) | 21 (9%) | 52 (4%) | 23 (6%) | 0 (0%) | 398 (4%) |
| Lost to follow‐up | Censored | 103 (4%) | 31 (16%) | 58 (9%) | 69 (5%) | 47 (3%) | 28 (9%) | 18 (3%) | 0 (0%) | 41 (3%) | 0 (0%) | 15 (5%) | 410 (4%) |
| Otherc | Censored | 33 (1%) | 2 (1%) | 22 (4%) | 14 (1%) | 36 (2%) | 11 (3%) | 28 (4%) | 10 (4%) | 7 (1%) | 27 (7%) | 0 (0%) | 190 (2%) |
| Completed study | Censored | 2208 (91%) | 139 (73%) | 480 (78%) | 1146 (86%) | 1451 (90%) | 269 (84%) | 531 (83%) | 201 (83%) | 1149 (92%) | 322 (87%) | 265 (95%) | 8161 (88%) |
| Total censoredb | 2424 (65%) | 190 (60%) | 613 (71%) | 1332 (70%) | 1618 (69%) | 322 (67%) | 637 (55%) | 242 (41%) | 1249 (68%) | 372 (61%) | 280 (63%) | 9279 (65%) | |
| Totald | 3271 | 315 | 860 | 1911 | 2351 | 478 | 1157 | 594 | 1845 | 610 | 444 | 14,286 | |
| CBZ: carbamazepine aOther treatment‐related reasons included: physician's decision, drug‐related death, pregnancy or perceived remission, or nonspecific (drug‐related) reason. | |||||||||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 520 | 4 | 1.55 (1.16 to 2.07) | 68% | 18.5% | 1.56 (1.18 to 2.07) |
| CBZ vs PHT | 428 | 3 | 1.19 (0.87 to 1.61) | 0% | 24.4% | 1.14 (0.90 to 1.44) |
| CBZ vs VPS | 814 | 5 | 1.02 (0.80 to 1.29) | 0% | 23.8% | 1.08 (0.88 to 1.31) |
| CBZ vs LTG | 2203 | 9 | 0.75 (0.65 to 0.88) | 0% | 27.7% | 0.79 (0.69 to 0.91) |
| CBZ vs OXC | 599 | 2 | 1.10 (0.85 to 1.43) | 66% | 0.4% | 1.03 (0.82 to 1.30) |
| CBZ vs TPM | 976 | 2 | 1.23 (1.03 to 1.48) | 0% | 24.2% | 1.19 (0.99 to 1.43) |
| CBZ vs GBP | 681 | 2 | 1.22 (1.02 to 1.45) | 0% | 26.6% | 1.21 (1.01 to 1.45) |
| CBZ vs LEV | 1567 | 3 | 0.85 (0.71 to 1.01) | 50% | 15.8% | 0.80 (0.69 to 0.93) |
| CBZ vs ZNS | 583 | 1 | 1.08 (0.81 to 1.44) | NA | 15.7% | 0.93 (0.77 to 1.14) |
| CBZ vs LCM | 807 | 1 | 0.94 (0.75 to 1.19) | NA | 100.0% | 0.95 (0.74 to 1.22) |
| PHB vs PHT | 404 | 3 | 0.68 (0.51 to 0.91) | 56% | 22.4% | 0.73 (0.55 to 0.97) |
| PHB vs VPS | 75 | 2 | 0.36 (0.17 to 0.76) | 18% | 11.2% | 0.69 (0.50 to 0.95) |
| PHB vs LTG | No direct evidence | 0.0% | 0.51 (0.37 to 0.69) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.66 (0.47 to 0.93) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.76 (0.55 to 1.05) | |||
| PHB vs GBP | No direct evidence | 0.0% | 0.78 (0.56 to 1.08) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.51 (0.38 to 0.70) | |||
| PHB vs ZNS | No direct evidence | 0.0% | 0.60 (0.43 to 0.84) | |||
| PHB vs LCM | No direct evidence | 0.0% | 0.60 (0.42 to 0.88) | |||
| PHT vs VPS | 168 | 3 | 0.75 (0.49 to 1.15) | 0% | 13.2% | 0.94 (0.71 to 1.25) |
| PHT vs LTG | 90 | 1 | 1.22 (0.61 to 2.48) | NA | 3.9% | 0.70 (0.54 to 0.90) |
| PHT vs OXC | 325 | 2 | 0.80 (0.51 to 1.23) | 0% | 11.3% | 0.90 (0.68 to 1.20) |
| PHT vs TPM | 111 | 1 | 1.45 (0.27 to 7.92) | NA | 0.7% | 1.04 (0.79 to 1.38) |
| PHT vs GBP | No direct evidence | 0.0% | 1.06 (0.80 to 1.41) | |||
| PHT vs LEV | No direct evidence | 0.0% | 0.70 (0.54 to 0.92) | |||
| PHT vs ZNS | No direct evidence | 0.0% | 0.82 (0.61 to 1.10) | |||
| PHT vs LCM | No direct evidence | 0.0% | 0.83 (0.59 to 1.17) | |||
| VPS vs LTG | 267 | 3 | 0.48 (0.29 to 0.79) | 0% | 5.1% | 0.74 (0.59 to 0.92) |
| VPS vs OXC | No direct evidence | 0.0% | 0.96 (0.72 to 1.28) | |||
| VPS vs TPM | 129 | 2 | 0.79 (0.45 to 1.40) | 0% | 4.0% | 1.10 (0.86 to 1.42) |
| VPS vs GBP | No direct evidence | 0.0% | 1.13 (0.87 to 1.46) | |||
| VPS vs LEV | 190 | 2 | 1.08 (0.84 to 1.38) | 0% | 24.3% | 0.75 (0.59 to 0.95) |
| VPS vs ZNS | No direct evidence | 0.0% | 0.87 (0.66 to 1.14) | |||
| VPS vs LCM | No direct evidence | 0.0% | 0.88 (0.64 to 1.21) | |||
| LTG vs OXC | 521 | 1 | 1.37 (1.05 to 1.81) | NA | 17.1% | 1.30 (1.02 to 1.66) |
| LTG vs TPM | 699 | 2 | 1.62 (1.30 to 2.02) | 0% | 17.4% | 1.50 (1.23 to 1.81) |
| LTG vs GBP | 676 | 1 | 1.64 (1.32 to 2.04) | NA | 17.8% | 1.53 (1.26 to 1.85) |
| LTG vs LEV | 902 | 2 | 0.87 (0.71 to 1.07) | 0% | 23.3% | 1.01 (0.86 to 1.20) |
| LTG vs ZNS | 658 | 1 | 1.01 (0.80 to 1.28) | NA | 25.0% | 1.18 (0.96 to 1.44) |
| LTG vs LCM | No direct evidence | 0.0% | 1.19 (0.90 to 1.58) | |||
| OXC vs TPM | 509 | 1 | 1.18 (0.91 to 1.53) | NA | 20.9% | 1.15 (0.89 to 1.49) |
| OXC vs GBP | 521 | 1 | 1.19 (0.92 to 1.55) | NA | 21.2% | 1.18 (0.91 to 1.52) |
| OXC vs LEV | No direct evidence | 0.0% | 0.78 (0.60 to 1.02) | |||
| OXC vs ZNS | No direct evidence | 0.0% | 0.91 (0.67 to 1.22) | |||
| OXC vs LCM | No direct evidence | 0.0% | 0.92 (0.65 to 1.29) | |||
| TPM vs GBP | 664 | 1 | 1.02 (0.83 to 1.24) | NA | 25.7% | 1.02 (0.83 to 1.26) |
| TPM vs LEV | No direct evidence | 0.0% | 0.68 (0.54 to 0.85) | |||
| TPM vs ZNS | No direct evidence | 0.0% | 0.79 (0.61 to 1.02) | |||
| TPM vs LCM | No direct evidence | 0.0% | 0.80 (0.58 to 1.09) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.66 (0.53 to 0.83) | |||
| GBP vs ZNS | No direct evidence | 0.0% | 0.77 (0.59 to 1.00) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.78 (0.57 to 1.06) | |||
| LEV vs ZNS | 660 | 1 | 1.16 (0.91 to 1.48) | NA | 27.6% | 1.16 (0.94 to 1.43) |
| LEV vs LCM | No direct evidence | 0.0% | 1.18 (0.88 to 1.58) | |||
| ZNS vs LCM | No direct evidence | 0.0% | 1.01 (0.74 to 1.39) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 520 | 4 | 1.52 (1.06 to 2.19) | 73% | 31.7% | 1.99 (1.21 to 3.27) |
| CBZ vs PHT | 428 | 3 | 0.83 (0.56 to 1.24) | 0% | 35.3% | 1.00 (0.66 to 1.53) |
| CBZ vs VPS | 570 | 3 | 0.94 (0.70 to 1.26) | 0% | 40.3% | 0.88 (0.59 to 1.29) |
| CBZ vs LTG | 2203 | 9 | 0.57 (0.47 to 0.70) | 0% | 32.9% | 0.56 (0.44 to 0.73) |
| CBZ vs OXC | 599 | 2 | 1.01 (0.73 to 1.38) | 0% | 18.4% | 0.75 (0.46 to 1.22) |
| CBZ vs TPM | 976 | 2 | 1.10 (0.88 to 1.39) | 0% | 29.6% | 0.99 (0.69 to 1.43) |
| CBZ vs GBP | 681 | 2 | 0.68 (0.53 to 0.89) | 88% | 1.7% | 0.58 (0.37 to 0.91) |
| CBZ vs LEV | 1567 | 3 | 0.60 (0.47 to 0.77) | 35% | 28.8% | 0.65 (0.47 to 0.90) |
| CBZ vs ZNS | 583 | 1 | 0.96 (0.59 to 1.55) | NA | 17.9% | 0.70 (0.43 to 1.13) |
| CBZ vs LCM | 807 | 1 | 1.22 (0.84 to 1.79) | NA | 100.0% | 1.24 (0.65 to 2.37) |
| PHB vs PHT | 404 | 3 | 0.52 (0.36 to 0.77) | 80% | 15.1% | 0.50 (0.30 to 0.86) |
| PHB vs VPS | 75 | 2 | 0.15 (0.05 to 0.44) | 58% | 10.0% | 0.44 (0.24 to 0.80) |
| PHB vs LTG | No direct evidence | 0.0% | 0.28 (0.16 to 0.49) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.38 (0.19 to 0.75) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.50 (0.27 to 0.92) | |||
| PHB vs GBP | No direct evidence | 0.0% | 0.29 (0.15 to 0.58) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.33 (0.18 to 0.58) | |||
| PHB vs ZNS | No direct evidence | 0.0% | 0.35 (0.18 to 0.69) | |||
| PHB vs LCM | No direct evidence | 0.0% | 0.63 (0.28 to 1.41) | |||
| PHT vs VPS | 168 | 3 | 0.58 (0.30 to 1.10) | 0% | 14.2% | 0.87 (0.53 to 1.45) |
| PHT vs LTG | 90 | 1 | 1.12 (0.42 to 2.99) | NA | 4.4% | 0.56 (0.36 to 0.89) |
| PHT vs OXC | 325 | 2 | 0.35 (0.15 to 0.82) | 0% | 5.8% | 0.75 (0.43 to 1.31) |
| PHT vs TPM | 111 | 1 | 1.07 (0.18 to 6.40) | NA | 1.3% | 0.98 (0.59 to 1.65) |
| PHT vs GBP | No direct evidence | 0.0% | 0.58 (0.32 to 1.04) | |||
| PHT vs LEV | No direct evidence | 0.0% | 0.65 (0.39 to 1.09) | |||
| PHT vs ZNS | No direct evidence | 0.0% | 0.70 (0.37 to 1.30) | |||
| PHT vs LCM | No direct evidence | 0.0% | 1.24 (0.57 to 2.68) | |||
| VPS vs LTG | 267 | 3 | 0.33 (0.15 to 0.74) | 0% | 4.3% | 0.64 (0.42 to 0.98) |
| VPS vs OXC | No direct evidence | 0.0% | 0.85 (0.48 to 1.53) | |||
| VPS vs TPM | 129 | 2 | 0.94 (0.41 to 2.16) | 0% | 4.0% | 1.13 (0.70 to 1.82) |
| VPS vs GBP | No direct evidence | 0.0% | 0.66 (0.37 to 1.16) | |||
| VPS vs LEV | 190 | 2 | 1.93 (1.12 to 3.33) | 0% | 12.9% | 0.75 (0.46 to 1.21) |
| VPS vs ZNS | No direct evidence | 0.0% | 0.80 (0.44 to 1.45) | |||
| VPS vs LCM | No direct evidence | 0.0% | 1.42 (0.67 to 3.01) | |||
| LTG vs OXC | 521 | 1 | 1.91 (1.33 to 2.73) | NA | 15.3% | 1.33 (0.80 to 2.20) |
| LTG vs TPM | 699 | 2 | 2.20 (1.63 to 2.99) | 0% | 17.6% | 1.75 (1.17 to 2.62) |
| LTG vs GBP | 676 | 1 | 1.50 (1.09 to 2.08) | NA | 21.1% | 1.02 (0.63 to 1.65) |
| LTG vs LEV | 902 | 2 | 0.84 (0.60 to 1.19) | 32% | 14.6% | 1.16 (0.81 to 1.66) |
| LTG vs ZNS | 658 | 1 | 0.90 (0.57 to 1.41) | NA | 20.3% | 1.24 (0.75 to 2.03) |
| LTG vs LCM | No direct evidence | 0.0% | 2.21 (1.10 to 4.41) | |||
| OXC vs TPM | 509 | 1 | 1.16 (0.84 to 1.59) | NA | 21.6% | 1.32 (0.78 to 2.23) |
| OXC vs GBP | 521 | 1 | 0.79 (0.56 to 1.11) | NA | 22.7% | 0.77 (0.44 to 1.35) |
| OXC vs LEV | No direct evidence | 0.0% | 0.87 (0.49 to 1.56) | |||
| OXC vs ZNS | No direct evidence | 0.0% | 0.93 (0.48 to 1.82) | |||
| OXC vs LCM | No direct evidence | 0.0% | 1.66 (0.74 to 3.73) | |||
| TPM vs GBP | 664 | 1 | 0.68 (0.52 to 0.90) | NA | 29.8% | 0.58 (0.35 to 0.97) |
| TPM vs LEV | No direct evidence | 0.0% | 0.66 (0.41 to 1.07) | |||
| TPM vs ZNS | No direct evidence | 0.0% | 0.71 (0.39 to 1.28) | |||
| TPM vs LCM | No direct evidence | 0.0% | 1.26 (0.60 to 2.65) | |||
| GBP vs LEV | No direct evidence | 0.0% | 1.13 (0.65 to 1.97) | |||
| GBP vs ZNS | No direct evidence | 0.0% | 1.21 (0.63 to 2.32) | |||
| GBP vs LCM | No direct evidence | 0.0% | 2.16 (0.98 to 4.75) | |||
| LEV vs ZNS | 660 | 1 | 0.90 (0.57 to 1.42) | NA | 24.8% | 1.07 (0.64 to 1.78) |
| LEV vs LCM | No direct evidence | 0.0% | 1.90 (0.93 to 3.91) | |||
| ZNS vs LCM | No direct evidence | 0.0% | 1.78 (0.80 to 3.98) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 388 | 3 | 1.86 (1.26 to 2.75) | 0% | 37.2% | 1.88 (1.25 to 2.81) |
| CBZ vs PHT | 428 | 3 | 1.12 (0.76 to 1.64) | 0% | 33.2% | 1.14 (0.78 to 1.68) |
| CBZ vs VPS | 814 | 5 | 1.04 (0.82 to 1.33) | 0% | 52.9% | 1.16 (0.88 to 1.52) |
| CBZ vs LTG | 2098 | 8 | 1.00 (0.72 to 1.39) | 0% | 17.7% | 1.02 (0.78 to 1.33) |
| CBZ vs OXC | 599 | 2 | 1.17 (0.76 to 1.81) | 0% | 0.0% | 1.14 (0.73 to 1.77) |
| CBZ vs TPM | 976 | 2 | 1.48 (1.08 to 2.03) | 0% | 21.9% | 1.32 (0.95 to 1.83) |
| CBZ vs GBP | 681 | 2 | 2.05 (1.59 to 2.66) | 0% | 30.5% | 2.07 (1.56 to 2.75) |
| CBZ vs LEV | 1567 | 3 | 1.44 (0.98 to 2.12) | 0% | 23.0% | 1.07 (0.78 to 1.45) |
| CBZ vs ZNS | 583 | 1 | 1.07 (0.60 to 1.92) | NA | 10.3% | 1.23 (0.86 to 1.77) |
| CBZ vs LCM | 807 | 1 | 0.79 (0.49 to 1.26) | NA | 100.0% | 0.79 (0.47 to 1.33) |
| PHB vs PHT | 404 | 3 | 0.58 (0.40 to 0.85) | 0% | 43.7% | 0.61 (0.41 to 0.91) |
| PHB vs VPS | 75 | 2 | 0.74 (0.29 to 1.86) | 28% | 4.1% | 0.62 (0.39 to 0.97) |
| PHB vs LTG | No direct evidence | 0.0% | 0.54 (0.34 to 0.88) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.61 (0.33 to 1.10) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.71 (0.42 to 1.19) | |||
| PHB vs GBP | No direct evidence | 0.0% | 1.11 (0.67 to 1.81) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.57 (0.34 to 0.94) | |||
| PHB vs ZNS | No direct evidence | 0.0% | 0.66 (0.38 to 1.13) | |||
| PHB vs LCM | No direct evidence | 0.0% | 0.42 (0.22 to 0.82) | |||
| PHT vs VPS | 168 | 3 | 0.93 (0.52 to 1.68) | 0% | 13.8% | 1.02 (0.67 to 1.53) |
| PHT vs LTG | No direct evidence | 0.0% | 0.89 (0.56 to 1.41) | |||
| PHT vs OXC | 325 | 2 | 1.00 (0.06 to 16.0) | 0% | 0.6% | 1.00 (0.55 to 1.79) |
| PHT vs TPM | No direct evidence | 6.0% | 1.16 (0.69 to 1.94) | |||
| PHT vs GBP | No direct evidence | 0.0% | 1.82 (1.12 to 2.94) | |||
| PHT vs LEV | No direct evidence | 0.0% | 0.93 (0.57 to 1.52) | |||
| PHT vs ZNS | No direct evidence | 0.0% | 1.08 (0.64 to 1.82) | |||
| PHT vs LCM | No direct evidence | 0.0% | 0.70 (0.37 to 1.32) | |||
| VPS vs LTG | 267 | 3 | 0.65 (0.33 to 1.26) | 0% | 2.3% | 0.88 (0.62 to 1.24) |
| VPS vs OXC | No direct evidence | 0.0% | 0.98 (0.59 to 1.64) | |||
| VPS vs TPM | 129 | 2 | 0.33 (0.11 to 1.01) | 0% | 3.5% | 1.14 (0.74 to 1.75) |
| VPS vs GBP | No direct evidence | 0.0% | 1.79 (1.20 to 2.66) | |||
| VPS vs LEV | 190 | 2 | 1.09 (0.76 to 1.56) | 49% | 19.9% | 0.92 (0.62 to 1.36) |
| VPS vs ZNS | No direct evidence | 0.0% | 1.06 (0.69 to 1.64) | |||
| VPS vs LCM | No direct evidence | 0.0% | 0.68 (0.38 to 1.23) | |||
| LTG vs OXC | 521 | 1 | 1.21 (0.79 to 1.85) | NA | 20.6% | 1.12 (0.71 to 1.76) |
| LTG vs TPM | 699 | 2 | 1.49 (1.07 to 2.08) | 0% | 22.8% | 1.30 (0.92 to 1.85) |
| LTG vs GBP | 676 | 1 | 2.30 (1.70 to 3.11) | NA | 18.0% | 2.04 (1.48 to 2.80) |
| LTG vs LEV | 902 | 2 | 0.83 (0.57 to 1.21) | 3% | 30.9% | 1.05 (0.76 to 1.46) |
| LTG vs ZNS | 658 | 1 | 1.12 (0.78 to 1.59) | NA | 22.2% | 1.22 (0.86 to 1.72) |
| LTG vs LCM | No direct evidence | 0.0% | 0.78 (0.44 to 1.40) | |||
| OXC vs TPM | 509 | 1 | 1.24 (0.81 to 1.88) | NA | 24.0% | 1.16 (0.73 to 1.84) |
| OXC vs GBP | 521 | 1 | 1.91 (1.28 to 2.83) | NA | 28.7% | 1.82 (1.17 to 2.82) |
| OXC vs LEV | No direct evidence | 0.0% | 0.94 (0.56 to 1.57) | |||
| OXC vs ZNS | No direct evidence | 0.0% | 1.08 (0.63 to 1.86) | |||
| OXC vs LCM | No direct evidence | 0.0% | 0.70 (0.35 to 1.38) | |||
| TPM vs GBP | 664 | 1 | 1.54 (1.15 to 2.06) | NA | 32.8% | 1.57 (1.13 to 2.18) |
| TPM vs LEV | No direct evidence | 0.0% | 0.81 (0.52 to 1.24) | |||
| TPM vs ZNS | No direct evidence | 0.0% | 0.93 (0.59 to 1.47) | |||
| TPM vs LCM | No direct evidence | 0.0% | 0.60 (0.33 to 1.11) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.51 (0.35 to 0.77) | |||
| GBP vs ZNS | No direct evidence | 0.0% | 0.60 (0.39 to 0.91) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.38 (0.21 to 0.69) | |||
| LEV vs ZNS | 660 | 1 | 1.40 (0.96 to 2.05) | NA | 0.3% | 1.16 (0.80 to 1.67) |
| LEV vs LCM | No direct evidence | 0.0% | 0.74 (0.41 to 1.36) | |||
| ZNS vs LCM | No direct evidence | 0.0% | 0.64 (0.34 to 1.21) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 156 | 3 | 0.83 (0.35 to 1.95) | 12% | 23.5% | 1.40 (0.79 to 2.48) |
| CBZ vs PHT | 118 | 2 | 0.37 (0.13 to 1.05) | 0% | 10.9% | 0.77 (0.51 to 1.17) |
| CBZ vs VPS | 405 | 4 | 0.79 (0.46 to 1.38) | 7% | 23.3% | 0.66 (0.51 to 0.85) |
| CBZ vs LTG | 365 | 6 | 0.88 (0.57 to 1.38) | 0% | 31.9% | 0.70 (0.53 to 0.92) |
| CBZ vs OXC | 62 | 1 | 0.90 (0.39 to 2.13) | NA | 12.6% | 0.82 (0.47 to 1.42) |
| CBZ vs TPM | 193 | 2 | 0.84 (0.49 to 1.44) | 0% | 25.1% | 0.90 (0.67 to 1.22) |
| CBZ vs GBP | 73 | 1 | 0.71 (0.33 to 1.52) | NA | 16.5% | 0.75 (0.40 to 1.39) |
| CBZ vs LEV | 251 | 2 | 0.82 (0.49 to 1.35) | 0% | 55.9% | 0.74 (0.55 to 1.00) |
| CBZ vs LCM | 80 | 1 | 1.84 (0.82 to 4.13) | NA | 100.0% | 1.74 (0.78 to 3.86) |
| PHB vs PHT | 95 | 2 | 0.64 (0.20 to 2.04) | 0% | 15.9% | 0.55 (0.29 to 1.06) |
| PHB vs VPS | 94 | 2 | 1.80 (0.65 to 4.95) | 0% | 19.7% | 0.47 (0.26 to 0.84) |
| PHB vs LTG | No direct evidence | 0.0% | 0.50 (0.27 to 0.91) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.58 (0.27 to 1.25) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.65 (0.35 to 1.19) | |||
| PHB vs GBP | No direct evidence | 0.0% | 0.53 (0.23 to 1.21) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.53 (0.29 to 0.98) | |||
| PHB vs LCM | No direct evidence | 0.0% | 1.24 (0.47 to 3.31) | |||
| PHT vs VPS | 326 | 4 | 1.53 (0.61 to 3.83) | 22% | 10.4% | 0.85 (0.58 to 1.25) |
| PHT vs LTG | 91 | 1 | 0.90 (0.34 to 2.37) | NA | 12.8% | 0.90 (0.61 to 1.33) |
| PHT vs OXC | 155 | 2 | 1.38 (0.60 to 3.16) | 0% | 19.7% | 1.05 (0.62 to 1.78) |
| PHT vs TPM | 208 | 1 | 0.23 (0.03 to 1.78) | NA | 3.0% | 1.17 (0.78 to 1.76) |
| PHT vs GBP | No direct evidence | 0.0% | 0.96 (0.49 to 1.90) | |||
| PHT vs LEV | No direct evidence | 0.0% | 0.96 (0.62 to 1.49) | |||
| PHT vs LCM | No direct evidence | 0.0% | 2.25 (0.92 to 5.51) | |||
| VPS vs LTG | 560 | 3 | 1.91 (0.93 to 3.90) | 0% | 15.9% | 1.06 (0.81 to 1.37) |
| VPS vs OXC | No direct evidence | 0.0% | 1.24 (0.72 to 2.14) | |||
| VPS vs TPM | 588 | 2 | 1.81 (0.91 to 3.60) | 36% | 11.0% | 1.37 (1.06 to 1.77) |
| VPS vs GBP | No direct evidence | 0.0% | 1.13 (0.61 to 2.11) | |||
| VPS vs LEV | 1032 | 2 | 1.46 (0.63 to 3.38) | 0% | 17.8% | 1.13 (0.89 to 1.42) |
| VPS vs LCM | No direct evidence | 0.0% | 2.64 (1.14 to 6.09) | |||
| LTG vs OXC | 67 | 1 | 0.69 (0.03 to 1.60) | NA | 14.1% | 1.17 (0.68 to 2.02) |
| LTG vs TPM | 528 | 2 | 0.66 (0.33 to 1.31) | 0% | 17.1% | 1.30 (1.01 to 1.67) |
| LTG vs GBP | 78 | 1 | 0.55 (0.26 to 1.14) | NA | 18.4% | 1.07 (0.59 to 1.96) |
| LTG vs LEV | No direct evidence | 0.0% | 1.07 (0.77 to 1.48) | |||
| LTG vs LCM | No direct evidence | 0.0% | 2.50 (1.07 to 5.81) | |||
| OXC vs TPM | 75 | 1 | 0.95 (0.43 to 2.11) | NA | 16.8% | 1.11 (0.64 to 1.92) |
| OXC vs GBP | 65 | 1 | 0.79 (0.34 to 1.82) | NA | 18.1% | 0.92 (0.44 to 1.89) |
| OXC vs LEV | No direct evidence | 0.0% | 0.91 (0.51 to 1.63) | |||
| OXC vs LCM | No direct evidence | 0.0% | 2.13 (0.81 to 5.63) | |||
| TPM vs GBP | 86 | 1 | 0.83 (0.41 to 1.65) | NA | 22.7% | 0.83 (0.45 to 1.51) |
| TPM vs LEV | No direct evidence | NA | 0.0% | 0.82 (0.59 to 1.14) | ||
| TPM vs LCM | No direct evidence | NA | 0.0% | 1.92 (0.82 to 4.50) | ||
| GBP vs LEV | No direct evidence | NA | 0.0% | 1.00 (0.52 to 1.90) | ||
| GBP vs LCM | No direct evidence | NA | 0.0% | 2.33 (0.85 to 6.39) | ||
| LEV vs LCM | No direct evidence | NA | 0.0% | 2.34 (1.00 to 5.48) | ||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 156 | 3 | 0.33 (0.09 to 1.16) | 0% | 25.2% | 1.09 (0.42 to 2.87) |
| CBZ vs PHT | 118 | 2 | 0.62 (0.13 to 3.08) | 0% | 13.6% | 0.79 (0.37 to 1.73) |
| CBZ vs VPS | 117 | 2 | 1.36 (0.34 to 5.49) | 0% | 10.3% | 0.51 (0.29 to 0.88) |
| CBZ vs LTG | 368 | 7 | 1.03 (0.57 to 1.87) | 0% | 35.5% | 0.44 (0.26 to 0.73) |
| CBZ vs OXC | 62 | 1 | 0.59 (0.19 to 1.77) | NA | 17.8% | 0.51 (0.17 to 1.50) |
| CBZ vs TPM | 193 | 2 | 0.75 (0.37 to 1.49) | 0% | 30.1% | 0.72 (0.40 to 1.31) |
| CBZ vs GBP | 73 | 1 | 0.60 (0.21 to 1.68) | NA | 18.9% | 0.33 (0.11 to 1.02) |
| CBZ vs LEV | 251 | 2 | 0.62 (0.29 to 1.36) | 0% | 58.9% | 0.62 (0.32 to 1.19) |
| CBZ vs LCM | 80 | 1 | 3.51 (0.69 to 17.9) | NA | 100% | 4.39 (0.71 to 27.1) |
| PHB vs PHT | 95 | 2 | 1.82 (0.33 to 10.1) | 0% | 18.1% | 0.73 (0.25 to 2.15) |
| PHB vs VPS | 94 | 2 | 3.86 (0.95 to 15.7) | 0% | 21.5% | 0.47 (0.18 to 1.21) |
| PHB vs LTG | No direct evidence | 0.0% | 0.40 (0.14 to 1.11) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.47 (0.12 to 1.86) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.66 (0.23 to 1.86) | |||
| PHB vs GBP | No direct evidence | 0.0% | 0.31 (0.07 to 1.27) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.56 (0.19 to 1.66) | |||
| PHB vs LCM | No direct evidence | 0.0% | 4.01 (0.51 to 31.5) | |||
| PHT vs VPS | 326 | 4 | 2.71 (0.47 to 15.7) | 0% | 13.8% | 0.64 (0.31 to 1.34) |
| PHT vs LTG | 91 | 1 | 0.58 (0.14 to 2.50) | NA | 16.6% | 0.55 (0.26 to 1.17) |
| PHT vs OXC | 155 | 2 | 7.99 (0.51 to 124) | 0% | 0.0% | 0.64 (0.20 to 2.07) |
| PHT vs TPM | 208 | 1 | 0.10 (0.01 to 1.61) | NA | 4.5% | 0.91 (0.41 to 2.03) |
| PHT vs GBP | No direct evidence | 0.0% | 0.42 (0.12 to 1.49) | |||
| PHT vs LEV | No direct evidence | 0.0% | 0.78 (0.32 to 1.91) | |||
| PHT vs LCM | No direct evidence | 0.0% | 5.53 (0.77 to 39.9) | |||
| VPS vs LTG | 560 | 3 | 1.88 (0.68 to 5.21) | 0% | 20.3% | 0.86 (0.50 to 1.48) |
| VPS vs OXC | No direct evidence | 0.0% | 1.00 (0.33 to 3.02) | |||
| VPS vs TPM | 588 | 2 | 1.53 (0.59 to 3.97) | 54% | 10.8% | 1.42 (0.82 to 2.46) |
| VPS vs GBP | No direct evidence | 0.0% | 0.66 (0.21 to 2.08) | |||
| VPS vs LEV | 1032 | 2 | 0.79 (0.19 to 3.39) | 0% | 14.7% | 1.21 (0.66 to 2.21) |
| VPS vs LCM | No direct evidence | 0.0% | 8.61 (1.29 to 57.5) | |||
| LTG vs OXC | 67 | 1 | 0.48 (0.15 to 1.54) | NA | 16.1% | 1.17 (0.40 to 3.42) |
| LTG vs TPM | 528 | 2 | 0.55 (0.21 to 1.42) | 0% | 17.5% | 1.65 (0.92 to 2.95) |
| LTG vs GBP | 78 | 1 | 0.49 (0.16 to 1.47) | NA | 16.9% | 0.76 (0.25 to 2.34) |
| LTG vs LEV | No direct evidence | 0.0% | 1.41 (0.67 to 2.93) | |||
| LTG vs LCM | No direct evidence | 0.0% | 10.0 (1.51 to 66.4) | |||
| OXC vs TPM | 75 | 1 | 1.16 (0.39 to 3.45) | NA | 20.2% | 1.42 (0.49 to 4.12) |
| OXC vs GBP | 65 | 1 | 1.02 (0.30 to 3.50) | NA | 20.5% | 0.65 (0.17 to 2.49) |
| OXC vs LEV | No direct evidence | 0.0% | 1.21 (0.37 to 3.98) | |||
| OXC vs LCM | No direct evidence | 0.0% | 8.60 (1.04 to 71.2) | |||
| TPM vs GBP | 86 | 1 | 0.88 (0.32 to 2.43) | NA | 21.6% | 0.46 (0.15 to 1.39) |
| TPM vs LEV | No direct evidence | 0.0% | 0.85 (0.41 to 1.79) | |||
| TPM vs LCM | No direct evidence | 0.0% | 6.07 (0.90 to 41.2) | |||
| GBP vs LEV | No direct evidence | 0.0% | 1.84 (0.54 to 6.30) | |||
| GBP vs LCM | No direct evidence | 0.0% | 13.1 (1.55 to 111) | |||
| LEV vs LCM | No direct evidence | 0.0% | 7.13 (1.03 to 49.4) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 99 | 2 | 0.78 (0.21 to 2.95) | 0% | 22.7% | 1.63 (0.72 to 3.69) |
| CBZ vs PHT | 118 | 2 | 0.40 (0.13 to 1.30) | 0% | 26.5% | 0.62 (0.27 to 1.41) |
| CBZ vs VPS | 405 | 4 | 0.76 (0.41 to 1.40) | 0% | 51.3% | 1.04 (0.67 to 1.59) |
| CBZ vs LTG | 323 | 6 | 0.00 (0.00 to 0.02) | 98% | 0.1% | 1.92 (1.04 to 3.52) |
| CBZ vs OXC | 62 | 1 | 1.62 (0.34 to 7.70) | NA | 15.3% | 1.57 (0.51 to 4.81) |
| CBZ vs TPM | 193 | 2 | 1.16 (0.39 to 3.49) | 0% | 24.9% | 1.84 (1.03 to 3.28) |
| CBZ vs GBP | 73 | 1 | 1.35 (0.36 to 5.04) | NA | 19.0% | 2.86 (1.15 to 7.12) |
| CBZ vs LEV | 251 | 2 | 6.11 (0.75 to 50.0) | 0% | 28.2% | 1.29 (0.70 to 2.37) |
| CBZ vs LCM | 80 | 1 | 0.52 (0.09 to 2.86) | NA | 99.9% | 0.41 (0.08 to 2.22) |
| PHB vs PHT | 95 | 2 | 0.47 (0.11 to 1.95) | 0% | 27.0% | 0.38 (0.14 to 1.02) |
| PHB vs VPS | 94 | 2 | 1.14 (0.33 to 3.98) | 0% | 25.8% | 0.64 (0.29 to 1.42) |
| PHB vs LTG | No direct evidence | 0.0% | 1.18 (0.46 to 3.01) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.96 (0.26 to 3.63) | |||
| PHB vs TPM | No direct evidence | 0.0% | 1.13 (0.45 to 2.82) | |||
| PHB vs GBP | No direct evidence | 0.0% | 1.76 (0.55 to 5.65) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.79 (0.32 to 1.96) | |||
| PHB vs LCM | No direct evidence | 0.0% | 0.25 (0.04 to 1.65) | |||
| PHT vs VPS | 326 | 4 | 2.05 (0.64 to 6.54) | 0% | 26.8% | 1.66 (0.75 to 3.70) |
| PHT vs LTG | No direct evidence | 0.0% | 3.08 (1.22 to 7.76) | |||
| PHT vs OXC | No direct evidence | 0.0% | 2.52 (0.71 to 8.95) | |||
| PHT vs TPM | No direct evidence | 0.0% | 2.96 (1.20 to 7.29) | |||
| PHT vs GBP | No direct evidence | 0.0% | 4.60 (1.46 to 14.5) | |||
| PHT vs LEV | No direct evidence | 0.0% | 2.07 (0.83 to 5.15) | |||
| PHT vs LCM | No direct evidence | 0.0% | 0.66 (0.10 to 4.31) | |||
| VPS vs LTG | 560 | 3 | 1.98 (0.60 to 6.49) | 0% | 14.1% | 1.85 (1.11 to 3.11) |
| VPS vs OXC | No direct evidence | 0.0% | 1.51 (0.50 to 4.54) | |||
| VPS vs TPM | 588 | 2 | 4.81 (1.14 to 20.3) | 0% | 34.6% | 1.78 (1.10 to 2.87) |
| VPS vs GBP | No direct evidence | 0.0% | 2.76 (1.14 to 6.70) | |||
| VPS vs LEV | 1032 | 2 | 3.02 (0.43 to 21.1) | 0% | 22.0% | 1.25 (0.81 to 1.93) |
| VPS vs LCM | No direct evidence | 0.0% | 0.40 (0.07 to 2.26) | |||
| LTG vs OXC | 67 | 1 | 1.07 (0.27 to 4.26) | NA | 27.6% | 0.82 (0.28 to 2.42) |
| LTG vs TPM | 528 | 2 | 0.99 (0.31 to 3.09) | 0% | 30.8% | 0.96 (0.61 to 1.51) |
| LTG vs GBP | 78 | 1 | 0.89 (0.30 to 2.69) | NA | 21.5% | 1.49 (0.64 to 3.48) |
| LTG vs LEV | No direct evidence | 0.0% | 0.67 (0.33 to 1.35) | |||
| LTG vs LCM | No direct evidence | 0.0% | 0.22 (0.04 to 1.29) | |||
| OXC vs TPM | 75 | 1 | 0.92 (0.26 to 3.21) | NA | 25.5% | 1.17 (0.40 to 3.41) |
| OXC vs GBP | 65 | 1 | 0.84 (0.25 to 2.81) | NA | 31.6% | 1.83 (0.58 to 5.70) |
| OXC vs LEV | No direct evidence | 0.0% | 0.82 (0.25 to 2.70) | |||
| OXC vs LCM | No direct evidence | 0.0% | 0.26 (0.04 to 1.99) | |||
| TPM vs GBP | 86 | 1 | 0.91 (0.36 to 2.3) | NA | 0.0% | 1.55 (0.68 to 3.53) |
| TPM vs LEV | No direct evidence | 0.0% | 0.70 (0.36 to 1.35) | |||
| TPM vs LCM | No direct evidence | 0.0% | 0.22 (0.04 to 1.33) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.45 (0.17 to 1.22) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.14 (0.02 to 0.98) | |||
| LEV vs LCM | No direct evidence | 0.0% | 0.32 (0.05 to 1.91) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 525 | 4 | 1.00 (0.73 to 1.35) | 42% | 16.9% | 1.03 (0.77 to 1.38) |
| CBZ vs PHT | 430 | 3 | 1.03 (0.78 to 1.37) | 0% | 21.9% | 1.04 (0.84 to 1.29) |
| CBZ vs VPS | 816 | 5 | 1.06 (0.86 to 1.30) | 30% | 17.7% | 1.08 (0.91 to 1.29) |
| CBZ vs LTG | 907 | 2 | 1.08 (0.91 to 1.28) | 0% | 18.4% | 1.06 (0.93 to 1.22) |
| CBZ vs OXC | 591 | 1 | 0.97 (0.78 to 1.20) | 0% | 17.8% | 0.95 (0.78 to 1.15) |
| CBZ vs TPM | 962 | 2 | 1.20 (1.00 to 1.44) | 0% | 21.9% | 1.13 (0.94 to 1.36) |
| CBZ vs GBP | 666 | 1 | 1.32 (1.09 to 1.60) | NA | 20.4% | 1.29 (1.06 to 1.57) |
| CBZ vs LEV | 1567 | 3 | 1.09 (0.92 to 1.29) | 0% | 22.3% | 1.08 (0.94 to 1.24) |
| CBZ vs ZNS | 582 | 1 | 1.05 (0.85 to 1.30) | NA | 18.9% | 1.10 (0.94 to 1.29) |
| CBZ vs LCM | 806 | 1 | 1.00 (0.83 to 1.19) | NA | 100.0% | 1.00 (0.81 to 1.22) |
| PHB vs PHT | 465 | 4 | 0.91 (0.66 to 1.26) | 0% | 37.7% | 1.01 (0.75 to 1.36) |
| PHB vs VPS | 80 | 2 | 0.97 (0.54 to 1.73) | 0% | 8.7% | 1.05 (0.76 to 1.44) |
| PHB vs LTG | No direct evidence | 0.0% | 1.03 (0.75 to 1.42) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.92 (0.66 to 1.28) | |||
| PHB vs TPM | No direct evidence | 0.0% | 1.10 (0.78 to 1.54) | |||
| PHB vs GBP | No direct evidence | 0.0% | 1.25 (0.88 to 1.76) | |||
| PHB vs LEV | No direct evidence | 0.0% | 1.05 (0.76 to 1.44) | |||
| PHB vs ZNS | No direct evidence | 0.0% | 1.07 (0.77 to 1.48) | |||
| PHB vs LCM | No direct evidence | 0.0% | 0.97 (0.68 to 1.38) | |||
| PHT vs VPS | 245 | 4 | 0.92 (0.64 to 1.31) | 0% | 14.2% | 1.04 (0.81 to 1.33) |
| PHT vs LTG | 0.0% | 1.02 (0.80 to 1.31) | ||||
| PHT vs OXC | 318 | 2 | 0.94 (0.65 to 1.36) | 0% | 11.5% | 0.91 (0.71 to 1.17) |
| PHT vs TPM | No direct evidence | 0.0% | 1.09 (0.83 to 1.43) | |||
| PHT vs GBP | No direct evidence | 0.0% | 1.24 (0.93 to 1.64) | |||
| PHT vs LEV | No direct evidence | 0.0% | 1.04 (0.80 to 1.34) | |||
| PHT vs ZNS | No direct evidence | 0.0% | 1.06 (0.81 to 1.38) | |||
| PHT vs LCM | No direct evidence | 0.0% | 0.96 (0.71 to 1.29) | |||
| VPS vs LTG | 267 | 3 | 0.90 (0.54 to 1.49) | 0% | 4.1% | 0.98 (0.80 to 1.21) |
| VPS vs OXC | No direct evidence | 0.0% | 0.88 (0.68 to 1.12) | |||
| VPS vs TPM | 128 | 2 | 0.62 (0.33 to 1.18) | 59% | 0.9% | 1.05 (0.82 to 1.33) |
| VPS vs GBP | No direct evidence | 0.0% | 1.19 (0.92 to 1.53) | |||
| VPS vs LEV | 190 | 1 | 0.91 (0.74 to 1.11) | 0% | 29.3% | 1.00 (0.81 to 1.23) |
| VPS vs ZNS | No direct evidence | 0.0% | 1.02 (0.81 to 1.28) | |||
| VPS vs LCM | No direct evidence | 0.0% | 0.92 (0.70 to 1.20) | |||
| LTG vs OXC | 511 | 1 | 0.87 (0.69 to 1.01) | NA | 15.6% | 0.89 (0.72 to 1.10) |
| LTG vs TPM | 683 | 2 | 1.12 (0.92 to 1.36) | 0% | 19.5% | 1.06 (0.88 to 1.29) |
| LTG vs GBP | 660 | 1 | 1.21 (1.00 to 1.47) | NA | 19.9% | 1.21 (0.99 to 1.48) |
| LTG vs LEV | 902 | 2 | 1.02 (0.86 to 1.20) | 0% | 23.6% | 1.01 (0.87 to 1.18) |
| LTG vs ZNS | 658 | 1 | 1.07 (0.88 to 1.29) | NA | 24.7% | 1.04 (0.87 to 1.23) |
| LTG vs LCM | No direct evidence | 0.0% | 0.94 (0.73 to 1.20) | |||
| OXC vs TPM | 498 | 1 | 1.29 (1.02 to 1.63) | NA | 18.9% | 1.20 (0.95 to 1.51) |
| OXC vs GBP | 509 | 1 | 1.39 (1.10 to 1.75) | NA | 19.0% | 1.36 (1.07 to 1.72) |
| OXC vs LEV | No direct evidence | 0.0% | 1.14 (0.90 to 1.43) | |||
| OXC vs ZNS | No direct evidence | 0.0% | 1.16 (0.91 to 1.48) | |||
| OXC vs LCM | No direct evidence | 0.0% | 1.05 (0.79 to 1.40) | |||
| TPM vs GBP | 647 | 1 | 1.08 (0.88 to 1.32) | NA | 24.8% | 1.14 (0.91 to 1.41) |
| TPM vs LEV | No direct evidence | 0.0% | 0.95 (0.77 to 1.18) | |||
| TPM vs ZNS | No direct evidence | 0.0% | 0.97 (0.77 to 1.22) | |||
| TPM vs LCM | No direct evidence | 0.0% | 0.88 (0.67 to 1.16) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.84 (0.67 to 1.05) | |||
| GBP vs ZNS | No direct evidence | 0.0% | 0.86 (0.67 to 1.09) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.77 (0.58 to 1.03) | |||
| LEV vs ZNS | 660 | 1 | 1.05 (0.87 to 1.27) | NA | 28.0% | 1.02 (0.86 to 1.21) |
| LEV vs LCM | No direct evidence | 0.0% | 0.92 (0.72 to 1.18) | |||
| ZNS vs LCM | No direct evidence | 0.0% | 0.91 (0.70 to 1.17) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 158 | 3 | 1.90 (1.00 to 3.62) | 0% | 40.5% | 1.31 (0.87 to 1.98) |
| CBZ vs PHT | 121 | 2 | 0.90 (0.49 to 1.64) | 65% | 7.2% | 0.97 (0.73 to 1.31) |
| CBZ vs VPS | 412 | 4 | 0.99 (0.70 to 1.40) | 0% | 40.4% | 0.99 (0.82 to 1.21) |
| CBZ vs LTG | 74 | 1 | 0.92 (0.53 to 1.59) | NA | 17.8% | 1.19 (0.90 to 1.57) |
| CBZ vs OXC | 61 | 1 | 1.28 (0.67 to 2.43) | NA | 14.2% | 1.26 (0.83 to 1.91) |
| CBZ vs TPM | 191 | 2 | 0.84 (0.53 to 1.35) | 0% | 24.5% | 1.07 (0.83 to 1.39) |
| CBZ vs GBP | 72 | 1 | 0.90 (0.51 to 1.59) | NA | 19.1% | 1.29 (0.80 to 2.07) |
| CBZ vs LEV | 251 | 2 | 0.98 (0.63 to 1.54) | 77% | 15.3% | 0.99 (0.77 to 1.26) |
| CBZ vs LCM | 78 | 1 | 1.04 (0.58 to 1.88) | NA | 100.0% | 1.04 (0.58 to 1.87) |
| PHB vs PHT | 130 | 3 | 0.77 (0.36 to 1.65) | 6% | 9.9% | 0.74 (0.48 to 1.15) |
| PHB vs VPS | 98 | 2 | 0.87 (0.40 to 1.89) | 42% | 12.4% | 0.76 (0.50 to 1.14) |
| PHB vs LTG | No direct evidence | 0.0% | 0.90 (0.57 to 1.44) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.96 (0.56 to 1.66) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.82 (0.52 to 1.28) | |||
| PHB vs GBP | No direct evidence | 0.0% | 0.98 (0.54 to 1.80) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.75 (0.48 to 1.17) | |||
| PHB vs LCM | No direct evidence | 0.0% | 0.79 (0.39 to 1.62) | |||
| PHT vs VPS | 269 | 4 | 1.15 (0.72 to 1.83) | 0% | 36.1% | 1.02 (0.78 to 1.33) |
| PHT vs LTG | No direct evidence | 0.0% | 1.22 (0.87 to 1.71) | |||
| PHT vs OXC | 154 | 2 | 1.29 (0.68 to 2.46) | 0% | 19.2% | 1.29 (0.87 to 1.92) |
| PHT vs TPM | No direct evidence | 0.0% | 1.10 (0.79 to 1.52) | |||
| PHT vs GBP | No direct evidence | 0.0% | 1.32 (0.79 to 2.21) | |||
| PHT vs LEV | No direct evidence | 0.0% | 1.01 (0.73 to 1.40) | |||
| PHT vs LCM | No direct evidence | 0.0% | 1.07 (0.55 to 2.06) | |||
| VPS vs LTG | 555 | 3 | 1.27 (0.64 to 2.50) | 0% | 12.4% | 1.19 (0.95 to 1.50) |
| VPS vs OXC | No direct evidence | 0.0% | 1.27 (0.85 to 1.90) | |||
| VPS vs TPM | 585 | 2 | 1.86 (0.94 to 3.71) | 0% | 4.3% | 1.08 (0.87 to 1.34) |
| VPS vs GBP | No direct evidence | 0.0% | 1.30 (0.82 to 2.07) | |||
| VPS vs LEV | 1032 | 2 | 1.10 (0.59 to 2.04) | 55% | 53.2% | 0.99 (0.82 to 1.20) |
| VPS vs LCM | No direct evidence | 0.0% | 1.05 (0.56 to 1.94) | |||
| LTG vs OXC | 67 | 1 | 1.39 (0.75 to 2.59) | NA | 17.8% | 1.06 (0.69 to 1.63) |
| LTG vs TPM | 525 | 2 | 0.87 (0.52 to 1.45) | 0% | 23.9% | 0.90 (0.72 to 1.14) |
| LTG vs GBP | 78 | 1 | 0.98 (0.56 to 1.69) | NA | 22.7% | 1.09 (0.68 to 1.73) |
| LTG vs LEV | No direct evidence | 0.0% | 0.83 (0.62 to 1.12) | |||
| LTG vs LCM | No direct evidence | 0.0% | 0.88 (0.46 to 1.68) | |||
| OXC vs TPM | 74 | 1 | 0.62 (0.34 to 1.14) | NA | 18.3% | 0.85 (0.56 to 1.29) |
| OXC vs GBP | 65 | 1 | 0.70 (0.37 to 1.33) | NA | 17.7% | 1.02 (0.59 to 1.76) |
| OXC vs LEV | No direct evidence | 0.0% | 0.78 (0.50 to 1.22) | |||
| OXC vs LCM | No direct evidence | 0.0% | 0.82 (0.40 to 1.69) | |||
| TPM vs GBP | 85 | 1 | 1.13 (0.66 to 1.92) | NA | 24.7% | 1.20 (0.76 to 1.90) |
| TPM vs LEV | No direct evidence | 0.0% | 0.92 (0.70 to 1.22) | |||
| TPM vs LCM | No direct evidence | 0.0% | 0.97 (0.51 to 1.84) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.76 (0.47 to 1.26) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.80 (0.38 to 1.71) | |||
| LEV vs LCM | No direct evidence | 0.0% | 1.05 (0.56 to 1.99) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 525 | 4 | 0.90 (0.70 to 1.15) | 45% | 17.4% | 0.95 (0.75 to 1.20) |
| CBZ vs PHT | 430 | 3 | 0.97 (0.75 to 1.26) | 0% | 18.2% | 1.01 (0.84 to 1.21) |
| CBZ vs VPS | 816 | 5 | 1.15 (0.97 to 1.37) | 47% | 12.5% | 1.15 (1.00 to 1.32) |
| CBZ vs LTG | 1467 | 7 | 1.11 (0.96 to 1.27) | 20% | 11.4% | 1.08 (0.97 to 1.20) |
| CBZ vs OXC | 591 | 2 | 1.02 (0.84 to 1.24) | 0% | 17.4% | 0.94 (0.80 to 1.10) |
| CBZ vs TPM | 962 | 2 | 1.15 (0.98 to 1.34) | 0% | 24.2% | 1.08 (0.94 to 1.24) |
| CBZ vs GBP | 666 | 1 | 1.17 (1.00 to 1.38) | 70% | 3.8% | 1.13 (0.98 to 1.31) |
| CBZ vs LEV | 1567 | 3 | 1.10 (0.97 to 1.25) | 0% | 31.2% | 1.07 (0.97 to 1.18) |
| CBZ vs ZNS | 582 | 1 | 1.00 (0.82 to 1.20) | NA | 19.3% | 1.07 (0.94 to 1.22) |
| CBZ vs LCM | 806 | 1 | 0.97 (0.82 to 1.13) | NA | 100.0% | 0.97 (0.82 to 1.13) |
| PHB vs PHT | 465 | 4 | 0.90 (0.68 to 1.20) | 0% | 37.1% | 1.06 (0.83 to 1.35) |
| PHB vs VPS | 80 | 2 | 1.00 (0.58 to 1.72) | 0% | 7.5% | 1.21 (0.93 to 1.56) |
| PHB vs LTG | No direct evidence | 0.0% | 1.14 (0.88 to 1.46) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.99 (0.75 to 1.29) | |||
| PHB vs TPM | No direct evidence | 0.0% | 1.13 (0.86 to 1.48) | |||
| PHB vs GBP | No direct evidence | 0.0% | 1.19 (0.91 to 1.57) | |||
| PHB vs LEV | No direct evidence | 0.0% | 1.12 (0.87 to 1.44) | |||
| PHB vs ZNS | No direct evidence | 0.0% | 1.13 (0.87 to 1.47) | |||
| PHB vs LCM | No direct evidence | 0.0% | 1.02 (0.77 to 1.35) | |||
| PHT vs VPS | 245 | 4 | 0.91 (0.69 to 1.20) | 0% | 17.3% | 1.14 (0.93 to 1.39) |
| PHT vs LTG | 90 | 1 | 1.71 (0.57 to 5.12) | NA | 0.9% | 1.07 (0.88 to 1.31) |
| PHT vs OXC | 318 | 2 | 0.85 (0.63 to 1.16) | 0% | 12.6% | 0.93 (0.76 to 1.14) |
| PHT vs TPM | No direct evidence | 0.0% | 1.07 (0.86 to 1.33) | |||
| PHT vs GBP | No direct evidence | 0.0% | 1.13 (0.90 to 1.41) | |||
| PHT vs LEV | No direct evidence | 0.0% | 1.06 (0.87 to 1.30) | |||
| PHT vs ZNS | No direct evidence | 0.0% | 1.07 (0.86 to 1.32) | |||
| PHT vs LCM | No direct evidence | 0.0% | 0.96 (0.75 to 1.22) | |||
| VPS vs LTG | 266 | 3 | 0.83 (0.57 to 1.22) | 0% | 5.3% | 0.94 (0.80 to 1.10) |
| VPS vs OXC | No direct evidence | 0.0% | 0.82 (0.67 to 1.00) | |||
| VPS vs TPM | 128 | 2 | 0.76 (0.46 to 1.25) | 48% | 1.7% | 0.94 (0.78 to 1.12) |
| VPS vs GBP | No direct evidence | 0.0% | 0.99 (0.81 to 1.20) | |||
| VPS vs LEV | 190 | 2 | 0.95 (0.80 to 1.12) | 0% | 32.9% | 0.93 (0.80 to 1.09) |
| VPS vs ZNS | No direct evidence | 0.0% | 0.93 (0.78 to 1.12) | |||
| VPS vs LCM | No direct evidence | 0.0% | 0.84 (0.68 to 1.04) | |||
| LTG vs OXC | 511 | 1 | 0.87 (0.71 to 1.07) | NA | 15.3% | 0.87 (0.73 to 1.03) |
| LTG vs TPM | 683 | 2 | 1.01 (0.85 to 1.21) | 0% | 18.9% | 0.99 (0.86 to 1.15) |
| LTG vs GBP | 660 | 1 | 1.07 (0.90 to 1.27) | NA | 23.8% | 1.05 (0.90 to 1.23) |
| LTG vs LEV | 902 | 2 | 0.94 (0.81 to 1.10) | 0% | 22.2% | 0.99 (0.88 to 1.11) |
| LTG vs ZNS | 658 | 1 | 1.03 (0.87 to 1.23) | NA | 24.8% | 0.99 (0.87 to 1.14) |
| LTG vs LCM | No direct evidence | 0.0% | 0.89 (0.74 to 1.08) | |||
| OXC vs TPM | 498 | 1 | 1.16 (0.95 to 1.43) | NA | 19.0% | 1.14 (0.95 to 1.38) |
| OXC vs GBP | 509 | 1 | 1.23 (1.00 to 1.51) | NA | 20.4% | 1.21 (1.00 to 1.46) |
| OXC vs LEV | No direct evidence | 0.0% | 1.14 (0.95 to 1.37) | |||
| OXC vs ZNS | No direct evidence | 0.0% | 1.14 (0.94 to 1.39) | |||
| OXC vs LCM | No direct evidence | 0.0% | 1.03 (0.82 to 1.29) | |||
| TPM vs GBP | 647 | 1 | 1.06 (0.88 to 1.26) | NA | 26.5% | 1.06 (0.89 to 1.25) |
| TPM vs LEV | No direct evidence | 0.0% | 0.99 (0.85 to 1.17) | |||
| TPM vs ZNS | No direct evidence | 0.0% | 1.00 (0.83 to 1.19) | |||
| TPM vs LCM | No direct evidence | 0.0% | 0.90 (0.73 to 1.11) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.94 (0.80 to 1.12) | |||
| GBP vs ZNS | No direct evidence | 0.0% | 0.95 (0.79 to 1.14) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.85 (0.69 to 1.06) | |||
| LEV vs ZNS | 660 | 1 | 1.06 (0.89 to 1.26) | NA | 26.0% | 1.00 (0.88 to 1.15) |
| LEV vs LCM | No direct evidence | 0.0% | 0.90 (0.75 to 1.09) | |||
| ZNS vs LCM | No direct evidence | 0.0% | 0.90 (0.73 to 1.11) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 158 | 3 | 1.78 (1.05 to 3.02) | 13% | 25.5% | 1.35 (0.96 to 1.90) |
| CBZ vs PHT | 121 | 2 | 0.69 (0.39 to 1.23) | 31% | 9.9% | 1.05 (0.83 to 1.33) |
| CBZ vs VPS | 412 | 4 | 0.90 (0.65 to 1.23) | 30% | 23.5% | 0.98 (0.84 to 1.14) |
| CBZ vs LTG | 319 | 5 | 1.26 (0.80 to 2.00) | 0% | 19.1% | 1.14 (0.93 to 1.41) |
| CBZ vs OXC | 61 | 1 | 1.28 (0.71 to 2.29) | NA | 13.2% | 1.29 (0.90 to 1.83) |
| CBZ vs TPM | 191 | 2 | 0.89 (0.60 to 1.31) | 0% | 26.3% | 1.05 (0.86 to 1.29) |
| CBZ vs GBP | 72 | 1 | 1.03 (0.61 to 1.74) | NA | 18.0% | 1.28 (0.84 to 1.94) |
| CBZ vs LEV | 251 | 2 | 1.00 (0.73 to 1.38) | 60% | 23.8% | 1.01 (0.84 to 1.21) |
| CBZ vs LCM | 78 | 1 | 1.41 (0.82 to 2.42) | NA | 100.0% | 1.36 (0.81 to 2.28) |
| PHB vs PHT | 130 | 3 | 0.77 (0.39 to 1.49) | 0% | 21.2% | 0.78 (0.54 to 1.12) |
| PHB vs VPS | 98 | 2 | 0.67 (0.32 to 1.38) | 8% | 14.4% | 0.72 (0.51 to 1.02) |
| PHB vs LTG | No direct evidence | 0.0% | 0.85 (0.58 to 1.23) | |||
| PHB vs OXC | No direct evidence | 0.0% | 0.95 (0.60 to 1.51) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.78 (0.54 to 1.13) | |||
| PHB vs GBP | No direct evidence | 0.0% | 0.95 (0.56 to 1.60) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.75 (0.52 to 1.07) | |||
| PHB vs LCM | No direct evidence | 0.0% | 1.01 (0.54 to 1.87) | |||
| PHT vs VPS | 394 | 5 | 0.97 (0.65 to 1.47) | 0% | 30.9% | 0.93 (0.75 to 1.16) |
| PHT vs LTG | 91 | 1 | 0.51 (0.10 to 2.68) | NA | 1.9% | 1.09 (0.84 to 1.41) |
| PHT vs OXC | 154 | 2 | 1.41 (0.83 to 2.40) | 0% | 19.4% | 1.23 (0.88 to 1.71) |
| PHT vs TPM | No direct evidence | 0.0% | 1.00 (0.78 to 1.30) | |||
| PHT vs GBP | No direct evidence | 0.0% | 1.22 (0.78 to 1.90) | |||
| PHT vs LEV | No direct evidence | 0.0% | 0.96 (0.75 to 1.23) | |||
| PHT vs LCM | No direct evidence | 0.0% | 1.29 (0.73 to 2.29) | |||
| VPS vs LTG | 555 | 3 | 1.15 (0.66 to 2.00) | 0% | 14.3% | 1.17 (0.98 to 1.38) |
| VPS vs OXC | No direct evidence | 0.0% | 1.31 (0.93 to 1.85) | |||
| VPS vs TPM | 585 | 2 | 1.46 (0.83 to 2.56) | 55% | 6.1% | 1.08 (0.91 to 1.27) |
| VPS vs GBP | No direct evidence | 0.0% | 1.30 (0.86 to 1.97) | |||
| VPS vs LEV | 1032 | 2 | 1.13 (0.77 to 1.66) | 0% | 45.4% | 1.03 (0.91 to 1.17) |
| VPS vs LCM | No direct evidence | 0.0% | 1.39 (0.81 to 2.38) | |||
| LTG vs OXC | 67 | 1 | 1.18 (0.66 to 2.10) | NA | 16.0% | 1.13 (0.79 to 1.61) |
| LTG vs TPM | 525 | 2 | 0.75 (0.46 to 1.23) | 0% | 20.4% | 0.92 (0.77 to 1.10) |
| LTG vs GBP | 78 | 1 | 0.94 (0.56 to 1.59) | NA | 20.4% | 1.12 (0.74 to 1.69) |
| LTG vs LEV | No direct evidence | 0.0% | 0.88 (0.72 to 1.09) | |||
| LTG vs LCM | No direct evidence | 0.0% | 1.19 (0.68 to 2.08) | |||
| OXC vs TPM | 74 | 1 | 0.64 (0.37 to 1.11) | NA | 17.2% | 0.82 (0.57 to 1.17) |
| OXC vs GBP | 65 | 1 | 0.80 (0.45 to 1.44) | NA | 17.4% | 0.99 (0.61 to 1.61) |
| OXC vs LEV | No direct evidence | 0.0% | 0.78 (0.55 to 1.13) | |||
| OXC vs LCM | No direct evidence | 0.0% | 1.06 (0.56 to 1.97) | |||
| TPM vs GBP | 85 | 1 | 1.25 (0.76 to 2.06) | NA | 23.4% | 1.21 (0.81 to 1.82) |
| TPM vs LEV | No direct evidence | NA | 0.0% | 0.96 (0.78 to 1.18) | ||
| TPM vs LCM | No direct evidence | NA | 0.0% | 1.29 (0.74 to 2.25) | ||
| GBP vs LEV | No direct evidence | NA | 0.0% | 0.79 (0.52 to 1.21) | ||
| GBP vs LCM | No direct evidence | NA | 0.0% | 1.06 (0.55 to 2.07) | ||
| LEV vs LCM | No direct evidence | NA | 0.0% | 1.35 (0.78 to 2.33) | ||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 581 | 6 | 1.27 (0.93 to 1.73) | 46% | 11.5% | 0.81 (0.65 to 1.00) |
| CBZ vs PHT | 432 | 4 | 1.06 (0.77 to 1.45) | 0% | 21.8% | 1.03 (0.88 to 1.22) |
| CBZ vs VPS | 813 | 5 | 1.13 (0.89 to 1.43) | 31% | 16.9% | 1.28 (1.12 to 1.45) |
| CBZ vs LTG | 2184 | 9 | 0.88 (0.76 to 1.03) | 0% | 31.4% | 1.23 (1.11 to 1.35) |
| CBZ vs OXC | 591 | 2 | 1.08 (0.83 to 1.41) | 69% | 0.4% | 1.01 (0.85 to 1.20) |
| CBZ vs TPM | 962 | 2 | 1.24 (1.03 to 1.49) | 0% | 26.5% | 1.08 (0.94 to 1.24) |
| CBZ vs GBP | 666 | 2 | 1.43 (1.20 to 1.70) | 44% | 15.4% | 1.40 (1.22 to 1.61) |
| CBZ vs LEV | 1552 | 3 | 0.89 (0.75 to 1.06) | 68% | 11.9% | 1.21 (1.08 to 1.37) |
| CBZ vs ZNS | 582 | 1 | 1.16 (0.87 to 1.54) | NA | 18.6% | 1.33 (1.13 to 1.57) |
| CBZ vs LCM | 806 | 1 | 0.99 (0.79 to 1.25) | NA | 100.0% | 1.14 (0.93 to 1.39) |
| PHB vs PHT | 463 | 5 | 0.78 (0.58 to 1.05) | 0% | 49.9% | 1.28 (1.02 to 1.6) |
| PHB vs VPS | 80 | 2 | 0.67 (0.33 to 1.36) | 0% | 7.3% | 1.58 (1.24 to 2.01) |
| PHB vs LTG | No direct evidence | 0.0% | 1.52 (1.21 to 1.92) | |||
| PHB vs OXC | No direct evidence | 0.0% | 1.26 (0.97 to 1.63) | |||
| PHB vs TPM | No direct evidence | 0.0% | 1.34 (1.04 to 1.72) | |||
| PHB vs GBP | No direct evidence | 0.0% | 1.74 (1.35 to 2.24) | |||
| PHB vs LEV | No direct evidence | 0.0% | 1.51 (1.18 to 1.92) | |||
| PHB vs ZNS | No direct evidence | 0.0% | 1.65 (1.26 to 2.16) | |||
| PHB vs LCM | No direct evidence | 0.0% | 1.41 (1.05 to 1.90) | |||
| PHT vs VPS | 245 | 4 | 0.90 (0.58 to 1.39) | 0% | 13.4% | 1.23 (1.02 to 1.49) |
| PHT vs LTG | 90 | 1 | 1.12 (0.56 to 2.27) | NA | 3.8% | 1.19 (0.99 to 1.42) |
| PHT vs OXC | 318 | 2 | 0.78 (0.50 to 1.22) | 0% | 10.4% | 0.98 (0.80 to 1.20) |
| PHT vs TPM | 111 | 1 | 2.00 (0.36 to 11.2) | NA | 0.7% | 1.04 (0.85 to 1.28) |
| PHT vs GBP | No direct evidence | 0.0% | 1.36 (1.10 to 1.67) | |||
| PHT vs LEV | No direct evidence | 0.0% | 1.18 (0.96 to 1.43) | |||
| PHT vs ZNS | No direct evidence | 0.0% | 1.29 (1.03 to 1.62) | |||
| PHT vs LCM | No direct evidence | 0.0% | 1.10 (0.85 to 1.43) | |||
| VPS vs LTG | 257 | 3 | 0.44 (0.26 to 0.74) | 55% | 2.1% | 0.96 (0.83 to 1.12) |
| VPS vs OXC | No direct evidence | 0.0% | 0.79 (0.65 to 0.98) | |||
| VPS vs TPM | 128 | 2 | 0.65 (0.37 to 1.15) | 52% | 2.0% | 0.85 (0.71 to 1.01) |
| VPS vs GBP | No direct evidence | 0.0% | 1.10 (0.91 to 1.32) | |||
| VPS vs LEV | 190 | 2 | 1.00 (0.76 to 1.30) | 0% | 28.2% | 0.95 (0.81 to 1.13) |
| VPS vs ZNS | No direct evidence | 0.0% | 1.05 (0.85 to 1.28) | |||
| VPS vs LCM | No direct evidence | 0.0% | 0.89 (0.70 to 1.14) | |||
| LTG vs OXC | 511 | 1 | 1.07 (0.82 to 1.41) | NA | 17.8% | 0.82 (0.69 to 0.98) |
| LTG vs TPM | 683 | 1 | 1.27 (1.02 to 1.58) | NA | 18.7% | 0.88 (0.76 to 1.01) |
| LTG vs GBP | 660 | 1 | 1.58 (1.27 to 1.96) | NA | 21.3% | 1.14 (0.99 to 1.32) |
| LTG vs LEV | 891 | 2 | 0.86 (0.69 to 1.07) | 0% | 25.2% | 0.99 (0.87 to 1.13) |
| LTG vs ZNS | 658 | 1 | 1.04 (0.80 to 1.35) | NA | 23.0% | 1.09 (0.92 to 1.28) |
| LTG vs LCM | No direct evidence | 0.0% | 0.93 (0.74 to 1.16) | |||
| OXC vs TPM | 498 | 1 | 1.18 (0.91 to 1.53) | NA | 21.1% | 1.06 (0.88 to 1.29) |
| OXC vs GBP | 509 | 1 | 1.47 (1.14 to 1.90) | NA | 23.5% | 1.38 (1.14 to 1.67) |
| OXC vs LEV | No direct evidence | 0.0% | 1.20 (0.98 to 1.47) | |||
| OXC vs ZNS | No direct evidence | 0.0% | 1.32 (1.05 to 1.66) | |||
| OXC vs LCM | No direct evidence | 0.0% | 1.13 (0.87 to 1.46) | |||
| TPM vs GBP | 647 | 1 | 1.25 (1.02 to 1.53) | NA | 28.2% | 1.30 (1.11 to 1.53) |
| TPM vs LEV | No direct evidence | 0.0% | 1.13 (0.94 to 1.34) | |||
| TPM vs ZNS | No direct evidence | 0.0% | 1.24 (1.01 to 1.52) | |||
| TPM vs LCM | No direct evidence | 0.0% | 1.06 (0.83 to 1.35) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.87 (0.73 to 1.04) | |||
| GBP vs ZNS | No direct evidence | 0.0% | 0.95 (0.77 to 1.17) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.81 (0.64 to 1.04) | |||
| LEV vs ZNS | 660 | 1 | 1.13 (0.86 to 1.48) | NA | 27.6% | 1.10 (0.93 to 1.30) |
| LEV vs LCM | No direct evidence | 0.0% | 0.94 (0.74 to 1.19) | |||
| ZNS vs LCM | No direct evidence | 0.0% | 0.86 (0.66 to 1.11) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Comparisona | Direct Evidence | Network meta‐analysis | ||||
|---|---|---|---|---|---|---|
| Number of participants | Number of studies | HR (95% CI)b | I2 | Direct evidencec | HR (95% CI)b | |
| CBZ vs PHB | 237 | 5 | 1.59 (0.64 to 3.98) | 53% | 27.9% | 1.13 (0.82 to 1.56) |
| CBZ vs PHT | 150 | 3 | 0.50 (0.18 to 1.37) | 0% | 11.7% | 0.91 (0.71 to 1.16) |
| CBZ vs VPS | 411 | 4 | 0.74 (0.43 to 1.28) | 63% | 12.9% | 0.87 (0.73 to 1.04) |
| CBZ vs LTG | 367 | 7 | 0.74 (0.47 to 1.15) | 0% | 33.7% | 1.11 (0.90 to 1.37) |
| CBZ vs OXC | 61 | 1 | 0.93 (0.40 to 2.19) | NA | 13.0% | 1.23 (0.83 to 1.83) |
| CBZ vs TPM | 191 | 2 | 0.81 (0.47 to 1.38) | 0% | 27.1% | 1.03 (0.83 to 1.28) |
| CBZ vs GBP | 72 | 1 | 0.63 (0.30 to 1.34) | NA | 16.9% | 1.22 (0.77 to 1.91) |
| CBZ vs LEV | 251 | 2 | 0.95 (0.57 to 1.58) | 0% | 56.7% | 0.96 (0.77 to 1.20) |
| CBZ vs LCM | 78 | 1 | 1.77 (0.76 to 4.10) | NA | 100.0% | 1.06 (0.41 to 2.75) |
| PHB vs PHT | 161 | 4 | 0.36 (0.11 to 1.13) | 0% | 22.1% | 0.80 (0.57 to 1.14) |
| PHB vs VPS | 98 | 2 | 1.11 (0.42 to 2.91) | 70% | 7.5% | 0.77 (0.55 to 1.08) |
| PHB vs LTG | No direct evidence | 0.0% | 0.98 (0.69 to 1.41) | |||
| PHB vs OXC | No direct evidence | 0.0% | 1.09 (0.67 to 1.76) | |||
| PHB vs TPM | No direct evidence | 0.0% | 0.91 (0.63 to 1.31) | |||
| PHB vs GBP | No direct evidence | 0.0% | 1.08 (0.63 to 1.85) | |||
| PHB vs LEV | No direct evidence | 0.0% | 0.85 (0.59 to 1.23) | |||
| PHB vs LCM | No direct evidence | 0.0% | 0.94 (0.34 to 2.57) | |||
| PHT vs VPS | 394 | 4 | 1.38 (0.57 to 3.34) | 49% | 12.7% | 0.96 (0.76 to 1.21) |
| PHT vs LTG | 91 | 1 | 0.90 (0.34 to 2.36) | NA | 13.1% | 1.22 (0.94 to 1.59) |
| PHT vs OXC | 154 | 2 | 1.58 (0.68 to 3.67) | 0% | 19.5% | 1.36 (0.93 to 1.98) |
| PHT vs TPM | 208 | 1 | 0.18 (0.02 to 1.40) | NA | 3.0% | 1.14 (0.87 to 1.49) |
| PHT vs GBP | No direct evidence | 0.0% | 1.34 (0.83 to 2.17) | |||
| PHT vs LEV | No direct evidence | 0.0% | 1.06 (0.80 to 1.41) | |||
| PHT vs LCM | No direct evidence | 0.0% | 1.17 (0.44 to 3.14) | |||
| VPS vs LTG | 541 | 3 | 2.2 (1.02 to 4.73) | 0% | 18.5% | 1.27 (1.07 to 1.52) |
| VPS vs OXC | No direct evidence | 0.0% | 1.41 (0.95 to 2.09) | |||
| VPS vs TPM | 585 | 2 | 2.10 (1.06 to 4.17) | 68% | 6.9% | 1.18 (0.98 to 1.42) |
| VPS vs GBP | No direct evidence | 0.0% | 1.39 (0.89 to 2.19) | |||
| VPS vs LEV | 1032 | 1 | 1.51 (0.65 to 3.49) | 0% | 20.1% | 1.10 (0.93 to 1.31) |
| VPS vs LCM | No direct evidence | 0.0% | 1.22 (0.46 to 3.21) | |||
| LTG vs OXC | 67 | 1 | 0.94 (0.41 to 2.16) | NA | 14.3% | 1.11 (0.74 to 1.65) |
| LTG vs TPM | 525 | 1 | 0.80 (0.40 to 1.58) | NA | 17.7% | 0.93 (0.77 to 1.12) |
| LTG vs GBP | 78 | 1 | 0.63 (0.30 to 1.33) | NA | 18.6% | 1.10 (0.70 to 1.71) |
| LTG vs LEV | No direct evidence | 0.0% | 0.87 (0.68 to 1.10) | |||
| LTG vs LCM | No direct evidence | 0.0% | 0.96 (0.36 to 2.54) | |||
| OXC vs TPM | 74 | 1 | 0.85 (0.38 to 1.88) | NA | 17.6% | 0.84 (0.56 to 1.25) |
| OXC vs GBP | 65 | 1 | 0.68 (0.29 to 1.57) | NA | 18.2% | 0.99 (0.58 to 1.68) |
| OXC vs LEV | No direct evidence | 0.0% | 0.78 (0.51 to 1.19) | |||
| OXC vs LCM | No direct evidence | 0.0% | 0.87 (0.31 to 2.43) | |||
| TPM vs GBP | 85 | 1 | 0.80 (0.40 to 1.59) | NA | 23.7% | 1.18 (0.76 to 1.84) |
| TPM vs LEV | No direct evidence | 0.0% | 0.93 (0.73 to 1.19) | |||
| TPM vs LCM | No direct evidence | 0.0% | 1.03 (0.39 to 2.74) | |||
| GBP vs LEV | No direct evidence | 0.0% | 0.79 (0.49 to 1.27) | |||
| GBP vs LCM | No direct evidence | 0.0% | 0.87 (0.30 to 2.51) | |||
| LEV vs LCM | No direct evidence | 0.0% | 1.10 (0.42 to 2.94) | |||
| CBZ: carbamazepine a. Order of drugs in the table: most commonly used drug first (CBZ), then drugs were ordered approximately by the date they were licenced as a monotherapy treatment (oldest first). b. HRs and 95% CIs were calculated from fixed‐effects analyses. HR < 1 indicates an advantage to the second drug in the comparison; results in highlighted in bold are statistically significant. c. Proportion of the NMA estimate contributed by direct evidence | ||||||
| Drug | Number of studies for which AE data is available | Number of participants in studies with AE data available (% of total randomised) | Number of participants for which at least one AE is reported (% of total data available)a,b |
|---|---|---|---|
| CBZ | 46 (IPD for 18 studies) | 5748 (94%) | 3757 (65%) |
| PHB | 8 (IPD for 2 studies) | 640 (78%) | 275 (43%) |
| PHT | 15 (IPD for 3 studies) | 1057 (76%) | 614 (58%) |
| VPS | 21 (IPD for 7 studies) | 2250 (86%) | 1399 (62%) |
| LTG | 24 (IPD for 15 studies) | 3368 (95%) | 1733 (51%) |
| OXC | 10 (IPD for 2 studies) | 1021 (90%) | 634 (62%) |
| LEV | 14 (IPD for 6 studies) | 2503 (96%) | 1697 (68%) |
| TPM | 6 (IPD for 5 studies) | 1209 (95%) | 920 (76%) |
| GBP | 4 (IPD for 2 studies) | 948 (100%) | 506 (53%) |
| ZNS | 3 (IPD for 2 studies) | 685 (100%) | 377 (55%) |
| LCM | 1 (IPD provided) | 445 (100%) | 328 (74%) |
| ESL | 1 (No IPD available) | 401 (100%) | 306 (76%) |
| Total | 68 studies (IPD for 26 studies) | 20,275 (92%) | 12,546 (62%) |
| AE:adverse event aAdverse event data were provided as detailed individual participant data for 26 trials and we extracted summary adverse event information from 42 trial publications. No adverse event data were reported in 21 trial publications. bMost trial publications reported summaries only of the “most common” adverse events; the totals and frequencies are likely to be an underestimation of the true number of events and number of individuals experiencing events. Furthermore, detailed information was provided in the more recent trial publications and individual participant data requests of more recent trials, often involving newer antiepileptic drugs, such as LTG, LEV and TPM, which may indicate that these newer drugs are associated with more adverse events than older drugs such as PHB and PHT, for which less detailed information was available. It was also unclear whether all events reported were 'treatment‐emergent' or 'treatment‐related.' | |||
| Event (general description)a‐d | CBZ | PHB | PHT | VPS | LTG | OXC | TPM | GBP | LEV | ZNS | LCM | ESL | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drowsiness or fatigue | 1449 | 1 | 1271 | 449 | 573 | 236 | 628 | 326 | 549 | 96 | 61 | 41 | 5680 |
| Headache or migraine | 953 | 0 | 843 | 275 | 574 | 137 | 315 | 171 | 620 | 73 | 78 | 27 | 4066 |
| Dizziness or faintness | 751 | 0 | 617 | 174 | 363 | 140 | 269 | 160 | 416 | 44 | 88 | 38 | 3060 |
| Gastrointestinal disturbances | 762 | 20 | 703 | 259 | 403 | 33 | 236 | 142 | 305 | 77 | 64 | 0 | 3004 |
| Rash or skin disorder | 809 | 17 | 718 | 51 | 450 | 77 | 163 | 113 | 139 | 55 | 36 | 6 | 2634 |
| Mood or behavioural change | 294 | 45 | 320 | 158 | 186 | 27 | 415 | 121 | 272 | 80 | 18 | 3 | 1939 |
| Nausea or vomiting | 494 | 1 | 414 | 181 | 247 | 53 | 132 | 92 | 166 | 41 | 38 | 26 | 1885 |
| Cognitive disorder | 342 | 41 | 362 | 112 | 219 | 44 | 439 | 127 | 98 | 52 | 21 | 0 | 1857 |
| Fever or viral infection | 441 | 0 | 379 | 68 | 176 | 24 | 84 | 58 | 339 | 37 | 66 | 0 | 1672 |
| Pain | 405 | 1 | 346 | 65 | 255 | 6 | 154 | 48 | 252 | 31 | 70 | 0 | 1633 |
| Laboratory results abnormal | 651 | 0 | 367 | 106 | 117 | 8 | 47 | 19 | 91 | 32 | 119 | 35 | 1592 |
| Weight gain | 288 | 0 | 259 | 389 | 171 | 23 | 71 | 258 | 88 | 3 | 5 | 8 | 1563 |
| Anxiety/depression | 231 | 0 | 203 | 71 | 188 | 32 | 309 | 82 | 259 | 55 | 37 | 0 | 1467 |
| Respiratory disorder | 316 | 0 | 233 | 53 | 124 | 4 | 190 | 23 | 131 | 17 | 68 | 0 | 1159 |
| Anorexia or weight loss | 134 | 0 | 126 | 32 | 123 | 6 | 394 | 58 | 76 | 87 | 4 | 0 | 1040 |
| Tremor or twitch | 185 | 1 | 172 | 274 | 228 | 19 | 56 | 23 | 57 | 8 | 9 | 0 | 1032 |
| Paraesthesia or tingling | 66 | 0 | 56 | 22 | 36 | 2 | 708 | 34 | 29 | 9 | 11 | 0 | 973 |
| Sleep disorder or nightmares | 125 | 1 | 109 | 54 | 219 | 16 | 147 | 31 | 121 | 27 | 17 | 0 | 867 |
| Visual disturbance | 222 | 0 | 199 | 54 | 97 | 33 | 86 | 59 | 35 | 16 | 10 | 0 | 811 |
| Increased/worsened seizures | 174 | 0 | 151 | 31 | 164 | 6 | 58 | 48 | 142 | 6 | 22 | 1 | 803 |
| Renal/urinary disorder | 204 | 0 | 152 | 30 | 79 | 2 | 92 | 57 | 94 | 29 | 28 | 0 | 767 |
| Ataxia | 177 | 37 | 209 | 38 | 59 | 18 | 61 | 40 | 35 | 9 | 5 | 0 | 688 |
| Accidental injury | 149 | 0 | 100 | 28 | 110 | 5 | 95 | 36 | 58 | 8 | 62 | 0 | 651 |
| Infection | 149 | 0 | 121 | 19 | 90 | 4 | 56 | 27 | 63 | 5 | 26 | 0 | 560 |
| Dental problems | 102 | 0 | 93 | 28 | 71 | 5 | 61 | 24 | 74 | 10 | 15 | 0 | 483 |
| Menstrual problems | 114 | 0 | 110 | 28 | 31 | 1 | 22 | 18 | 39 | 4 | 5 | 0 | 372 |
| Hair loss | 48 | 0 | 47 | 137 | 23 | 15 | 39 | 8 | 20 | 6 | 2 | 0 | 345 |
| Impotence or loss of libido | 91 | 24 | 114 | 14 | 17 | 0 | 27 | 32 | 11 | 4 | 2 | 0 | 336 |
| Aphasia | 64 | 7 | 66 | 11 | 30 | 4 | 106 | 22 | 17 | 4 | 3 | 0 | 334 |
| Asthenia | 67 | 1 | 60 | 31 | 44 | 1 | 31 | 33 | 44 | 13 | 5 | 0 | 330 |
| CBZ: carbamazepine aVerbatim or reported terms extracted from publications or provided in individual participant data were grouped under the definitions by one review author (SJN) and any uncertainties in definition were discussed with the senior clinical author (AGM). bAdverse event data were provided as detailed individual participant data for 26 trials and we extracted summary adverse event information from 42 trial publications. No adverse event data were reported in 21 trial publications. c For each event, the number of events was extracted where reported; if only the number of participants experiencing the event was reported, it was assumed that each participant experienced the event once. Therefore, the frequency of some events may be underestimated. d Most trial publications reported summaries only of the “most common” adverse events; the totals and frequencies are likely to be an underestimation of the true number of events and number of individuals experiencing events. Furthermore, detailed information was provided in the more recent trial publications and individual participant data requests of more recent trials, often involving newer antiepileptic drugs, such as LTG, LEV and TPM. which may indicate that these newer drugs are associated with more adverse events than older drugs such as PHB and PHT, for which less detailed information was available. It was also unclear whether all events reported were 'treatment‐emergent' or 'treatment‐related.' | |||||||||||||

