丙型肝炎病毒相关性混合性冷球蛋白血症的治疗
Abstract
研究背景
丙型肝炎病毒(HCV)相关性混合性冷球蛋白血症表现为中小血管炎,由IgM型活化的类风湿因子引起,这种类风湿因子由 B 细胞扩增产生。免疫复合物主要沉积在皮肤、关节、肾脏和周围神经纤维。目前的治疗方法旨在清除 HCV 感染,去除冷球蛋白和 B 细胞克隆增殖。最佳治疗尚未确定。
研究目的
本综述旨在研究目前治疗 HCV 相关性混合混合性冷球蛋白血症和活动性血管炎(皮肤或肾小球肾炎)治疗方案的获益和危害。
检索策略
我们通过联系信息专家,使用与本文相关的检索词,检索了截止2017年11月30日Cochrane肾脏与移植专业注册库(Cochrane Kidney and Transplant Specialised Register)。检索了如下数据库:CENTRAL、MEDLINE、EMBASE、会议论文集、国际试验注册库(International Clinical Trials Register (ICTRP) )、Search Portal和ClinicalTrials.gov。
标准/纳入排除标准
我们纳入的所有随机对照试验 (RCTs) 和准RCTs的干预措施,包括了HCV相关冷球蛋白血症血管炎的治疗 (免疫抑制剂和血浆置换治疗) 。
数据收集与分析
两位作者独立评估检索到文献的标题和摘要。联系纳入研究的作者,以获取缺失信息。统计分析使用随机效应模型,结果表示为风险比 (RR) 或均差 (MD) 与95%CI。方案中的主要结局为肾脏病、皮肤血管炎、肌肉骨骼症状、周围关节关节痛、周围神经病、肝脏受累、肺间质受累、广泛的血管炎和死亡。其他结局包括:治疗持续时间、实验室检查、不良反应、抗病毒治疗失败率、B 细胞淋巴瘤、内分泌紊乱和治疗费用。
主要结果
本综述纳入了十项研究 (394 名受试者)。它们都没有评估直接抗病毒药物。七项研究为单中心研究、三项为多中心。研究时间从6月到36月不等。偏倚风险总体为不清楚或很低。研究了三种不同的干预措施:使用利妥昔单抗(3 项研究,118 名受试者);干扰素(IFN,与其他方案比较(5 项研究,223 名受试者));IFN使用六月与一年相比(1 项研究,36 名受试者);免疫吸附与仅免疫抑制治疗比较(1 项研究,17 名受试者)。
使用利妥昔单抗可以轻度改善皮肤血管炎(2 项研究,78 名受试者:RR=0.57, 95%CI=0.28 ‐ 1.16,中等证据),对肾病疗效甚微或没有改善(中等证据)。实验室数据方面,利妥昔单抗对冷球蛋白 (MD=‐2.01%, 95%CI=‐10.29% ‐ 6.27%, 低等证据) 和 HCV 复制的影响不确定。与免疫抑制药物相比,利妥昔单抗可能略微增加输液反应 (3 项研究,118 名受试者:RR=4.33, 95%CI=0.76 ‐ 24.75, 中等证据) 。然而由于不良反应相似终止了治疗(3 研究, 118 名受试者:RR=0.97, 95%CI=0.22 ‐ 4.36, 中等证据)。
lFN 对临床症状的影响仅在定性结果部分进行了讨论。实验室参数的评估,六个月时IFN对丙氨酸转氨酶 (ALT)几乎或没有影响(2 项研究,39 名受试者:MD=‐5.89 UI/L,95%CI=‐55.77 ‐ 43.99);六个月时类风湿因子活性(1 项研究,13 名受试者:MD=97.00 UI/mL, 95%CI=‐187.37 ‐ 381.37),或18个月时 C4 水平(2 项研究,49 名受试者:MD=‐0.04 mg/dL,95%CI=‐2.74 ‐ 2.67)。另一方面,18个月干扰素可能会减少 ALT(2 项研究,39 名受试者:MD=‐28.28 UI/L, 95%CI=‐48.03 ‐ ‐8.54)和 Ig M (‐595.75 mg/dL, 95%CI=‐877.2 ‐ ‐314.3),但均是低等证据。一项研究报告发现,干扰素组输液反应可能比免疫抑制剂治疗组 (RR=27.82, 95%CI=1.72 ‐ 449.18) 高,干扰素因不良反应而停用者可能更多(4 项研究, 148 名受试者:RR=2.32, 95%CI=0.91 ‐ 5.90,低等证据)。干扰素疗法可能改善皮肤血管炎(3 项研究,95 名受试者:RR=0.60,95%CI=0.36 ‐ 1.00)和蛋白尿(2 项研究,49 名受试者:MD=‐1.98g/24h,95%CI=‐2.89 ‐ ‐1.07),18个月时血清肌酐无变化(2项研究,49 名受试者:MD=‐30.32μmol/L, 95%CI=‐80.59 ‐ 19.95)。
IFN治疗六月与一年相比,维持应答不同。六月组89%复发,仅11% 维持一年长期应答;而一年组仅78%复发、22%长期应答。一年治疗与六月相比,副作用更多(两例患者因严重副作用停止治疗)。
一项研究发现,免疫吸附对皮肤血管炎(RR=0.44, 95%CI =0.05 ‐ 4.02)、周围神经病(RR=2.70, 95%CI= 0.13 ‐ 58.24)、外周关节痛(RR=2.70, 95%CI=0.13 ‐ 58.24)、冷球蛋白(MD=0.01%, 95%CI=‐1.86 ‐ 1.88)在六个月时的疗效不确定,未报告输液反应。然而,评估临床积分后发现,与单独免疫抑制治疗相比,免疫吸附对严重临床并发症的缓解更高(80% vs 33%, P = 0.05)。
就死亡而言,由于大多数研究没有报告死亡,或者没有把死亡作为研究结局,所以不可能给出联合干预疗效的估计。
作者结论
治疗HCV相关性混合性冷球蛋白血症,抗病毒治疗清除HCV感染和利妥昔单抗终止免疫应答,可能是有益的。对于皮肤血管炎和一些实验室检查,抗病毒治疗联合美罗华阻碍B 细胞克隆增殖是合适的。本综述证据的适用性受到一定限制,因为没有任何直接抗病毒药物的研究,而这类药物迫切需要指导治疗。
PICO
Plain language summary
丙型肝炎病毒相关性冷球蛋白血症血管炎的治疗
问题是什么?
在新型直接抗病毒药物治疗出现之前,丙型肝炎病毒 (HCV)是 世界上第二个最常见的慢性感染性病毒 。HCV 可引起慢性肝病以及肝外症状,其中最常见的是混合性冷球蛋白血症。低温下沉淀的免疫球蛋白 (4ºC 以下) 加温至37ºC 后重新溶解,称为冷球蛋白 ("cyro" 来源于希腊语中的冷)。冷球蛋白通过两条主要途径引起器官损伤:高粘滞综合征和免疫介导机制。由此引起的冷球蛋白血症血管炎,是一种由致病蛋白所产生的中小血管炎症,这种致病蛋白称为活化的IgM型类风湿因子,由 B 细胞扩增产生。免疫复合物主要沉积在皮肤、关节、肾脏和周围神经纤维。我们想评估在HCV 感染的情况下,任何治疗方案对皮肤、关节、肾脏或周围神经纤维损伤的治疗效果。
我们做了什么?
我们纳入了10项随机对照试验 (RCT) ,涉及394名受试者, 符合纳入标准。本综述研究了三种可能控制疾病的策略,并研究了其对短期和长期预后的影响:干扰素或新型直接抗病毒药物清除HCV 感染;免疫吸附去除冷球蛋白;利妥昔单抗阻碍B 细胞克隆增殖。这些策略可以在成年人中使用, 通常联合使用抗病毒药物。
我们发现了什么?
本综述的局限是这些研究未涉及新型直接抗病毒药物,研究了利妥昔单抗、干扰素抗病毒或免疫吸附对比其他免疫抑制药物在皮肤、神经或肾脏疾病方面的一些获益。这三项干预措施没有头对头的比较。
结论
治疗HCV相关性混合性冷球蛋白血症,始终推荐使用抗病毒药物清除HCV感染(在本综述中,我们仅评估了干扰素治疗, 因为没有新型抗病毒药物治疗该病的RCT研究发表)。根据HCV相关性混合性冷球蛋白血症的扩展 ,推荐联合抗病毒治疗与利妥昔单抗阻碍B细胞克隆增殖,推荐免疫吸附去除冷球蛋白,因为它们能更快、更持久的改善皮肤、关节、肾脏或周围神经纤维损伤。为了指导治疗,迫切需要新型抗病毒药物的研究。
Authors' conclusions
Summary of findings
| Rituximab compared to no rituximab for hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Patient or population: hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no rituximab | Risk with rituximab | ||||
| Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months: number of affected patients | Study population | RR 0.57 | 78 (2) | ⊕⊕⊕⊝ | |
| 26 per 100 | 15 per 100 | ||||
| SCr at 18 months | The mean SCr at 18 months was 194.3 μmol/L | MD was 8.8 μmol/L lower | ‐ | 37 (1) | ⊕⊕⊕⊝ |
| Cryocrit at 12 months | The mean cryocrit at 12 months was 6.55% | MD was 2.01% lower | ‐ | 41 (2) | ⊕⊕⊝⊝ |
| Adverse effects ‐ infusion reactions: number of events | Study population | RR 4.33 | 118 (3) | ⊕⊕⊕⊝ | |
| 0 per 100 | 0 per 100 | ||||
| Activity outcomes | De Vita 2012 found a significant reduction in the BVAS at 2 months in the rituximab group (from mean ± SD: 11.9 ± 5.4 to 7.1 ± 5.7; P < 0.001), and this difference persisted at 6 months (6.9 ± 6.8; P < 0.001), 12 months (5.4 ± 6.2; P 0.0001), and 24 months (4.4 ± 4.6; P < 0.0001). Without differences in the control group. Sneller 2012 BVAS scores became significantly lower in the rituximab group at month 4 (from 10.2 ± 8.4, at 6 months: 0 ± 0; P < 0.02). Without differences in control group. | ‐ | 81 (2) | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 95% CI overlaps no effect, and the CI fails to exclude important benefit or important harm 2 Base on tests of heterogeneity which test the null hypothesis that all studies have the same underlying magnitude of effect, have a low P‐value (P = 0.00001), indicating to reject the null hypothesis I2 statistic, which quantifies the proportion of the variation in point estimates due to among‐study differences, is large (97%) 3 High risk of performance bias: non‐blinded participants and personnel | |||||
Background
Description of the condition
The second most frequent chronic viral infection in the world is hepatitis C virus (HCV) infection. Its estimated prevalence is 3% globally (around 170 million cases). HCV causes chronic liver disease as well as extra‐hepatic symptoms such as lymphoproliferative disorders, kidney disease, or the most common, mixed cryoglobulinaemia. HCV is characterised by two remarkable immunologic characteristics: escape of immune response in more than 80% of infected people and production of monoclonal/polyclonal rheumatoid factor in 20% to 40% (Dammacco 2000).
Immunoglobulins that precipitate at low temperature (below 4ºC) and re‐dissolve after warming to 37ºC are called cryoglobulins ("cryo" comes from the Greek word for cold). Cryoglobulins cause organ damage via two main routes: hyperviscosity syndrome and immune‐mediated mechanisms. Although plasma cryoglobulins are detected in approximately 50% of HCV‐infected patients, only 2% develop mixed cryoglobulinaemia systemic vasculitis (Charles 2009; Lauletta 2012; Saadoun 2008). This vasculitis is the manifestation of an inflammation of small and medium‐sized vessels produced by a pathogenic IgM with rheumatoid factor activity generated by an expansion of B‐cells (Dammacco 2010). The immune complexes formed precipitate mainly in the skin, joints, kidneys or peripheral nerve fibres. Disease manifestations are variable, ranging from moderate clinical symptoms (arthralgia, weakness that is nearly always present, lower limb palpable purpura, Raynaud's phenomenon, livedo reticularis, cutaneous ulcers, urticarial or oedema) or appearance of endocrine disorders (autoimmune thyroiditis, subclinical hypothyroidism, thyroid cancer or diabetes) to life threatening complications such as widespread vasculitis, glomerulonephritis (GN) or B‐cell lymphoma (Ferri 2004; Matignon 2009; Saadoun 2008; Sansonno 2005; Sansonno 2007; Dammacco 2013).
Mixed cryoglobulinaemia is diagnosed by cryoglobulin testing, laboratory tests for evaluating visceral involvement and histopathological data to demonstrate a particular organ affection. The diagnosis requires demonstration of the presence of cryoglobulins in serum and typical organ involvement (Ramos‐Casals 2012).
The demonstration of the role of HCV as the etiological factor in more than 90% of mixed cryoglobulinaemia patients has been critical to establish the treatment goals. Current therapeutic approaches are aimed at elimination of HCV infection, removal of cryoglobulins and also of the B‐cell clonal expansions (Lauletta 2012).
Description of the intervention
The main possible interventions to treat mixed cryoglobulinaemia are eradicating the HCV infection and to attenuate immune response which leads to cryoglobulinaemic vasculitis by the use of different immunosuppressive medications, or extracorporeal therapies (including plasma exchange therapy, cryofiltration or immunoadsorption apheresis) used individually or in combination (Schwartz 2013; Siami 1995; Stefanutti 2009). However, the best strategy to treat this disease is not well defined.
Antiviral therapy against HCV with the combination of interferon (IFN)‐alpha, pegylated (PEG)‐IFN‐alpha and ribavirin (a nucleoside antimetabolite agent) were the gold‐standard for the treatment of HCV infection, but nowadays direct‐acting antiviral agents (DAA) are becoming the mainstay to eradicating HCV infection (Desbois 2017; EASL 2011; Latt 2012; Martin 2017). The effectiveness of these strategies were demonstrated by a complete clinical response and sustained virological response (described as undetectable HCV RNA levels for at least 6 months after cessation of therapy) in 75% with DAA and 42.8% without DAA of HCV‐related mixed cryoglobulinaemia patients (Cacoub 2017,Liang 2013; Lok 2012; Maasoumy 2013; Myers 2012).
There are also different possibilities of immunosuppressive treatments (corticosteroids or cyclophosphamide); biological therapies whose usefulness is only anecdotal such as tumour necrosis factor blockade by infliximab or etanercept (Bartolucci 2002; Chandesris 2004; Josselin 2008); anti‐interleukin‐6 therapy (Cohen 2012); IFN therapy (Kotter 2010); or rituximab (a chimeric monoclonal antibody specifically directed to CD20 antigen) (De Vita 2012; Sneller 2012). These strategies are sometimes ineffective, contraindicated, poorly tolerated, or may be associated with serious side effects (De Vita 2012). Therapeutic apheresis may be a useful option and can have immediate beneficial effects for the treatment of severe vasculitis or for clinical manifestations that are resistant to other treatments (Cacoub 2014; Pietrogrande 2011; Ramunni 2008; Schwartz 2013; Stefanutti 2009). There have been other approaches proposed for mixed cryoglobulinaemia, such as: imatinib (a tyrosine kinase inhibitor), thalidomide (an anti‐angiogenic drug), bortezomib (a proteasome inhibitor) or proleukin (IL‐2 therapy).
How the intervention might work
The principal target of any disease treatment is to remove the etiologic factor of the disorder. As a result of this assumption, in the case of HCV‐related mixed cryoglobulinaemia, it seems that it is essential to eradicate the virus. However, the extrahepatic clinical manifestations of HCV infection can persist or relapse despite clearance of the HCV virus because they are mainly consequence of B‐cell clonal expansions that can become independent of HCV stimulation (Langhans 2017; Schiavinato 2017). Consequently, B‐cell clonal deletion with immunosuppression therapy may be a required step. The use of therapeutic apheresis is to remove a component of the blood which contributes to a disease state, in this case, cryoglobulins. Nevertheless, the concentration of cryoglobulin does not correlate with clinical severity or with response to therapy. Nowadays, the decision of immediate removal of cryoglobulins by plasma exchange is based on the severity of the disease manifestations, although it is unknown if it may have independent positive effects on survival.
Why it is important to do this review
Different therapeutic approaches in combination or alone have been assessed in several studies. Recent clinical research performed on the promising role of the biological agents or the therapeutic apheresis. Mixed cryoglobulinaemia involves important use of hospital resources, it entails morbidity and it also affects the quality of life of the HCV patients. The prognosis and natural history of mixed cryoglobulinaemia are variable. It principally depends on the extent of vasculitic lesions and kidney‐associated disease. In fact, the overall five‐year survival ranges from 90% to 50% when kidneys are involved (Terrier 2013). Currently, there is no evidence‐based strategy to manage mixed cryoglobulinaemia.
Objectives
This review aims to look at the benefits and harms of the currently available treatment options to treat the HCV‐associated mixed cryoglobulinaemia with active manifestations of vasculitis (cutaneous or GN).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at interventions directed at treatment of HCV‐associated mixed cryoglobulinaemia with active manifestations of vasculitis (immunosuppressive medications and plasma exchange therapy) have been included. There were no language restrictions.
Types of participants
Inclusion criteria
All patients with HCV‐associated mixed cryoglobulinaemia (male or female), of any age, with or without chronic kidney disease (CKD) with active manifestations of vasculitis (cutaneous, peripheral neuropathy or GN).
The diagnosis of active vasculitis in patients with HCV‐associated mixed cryoglobulinaemia could have been done following these criteria (Dammacco 2010): detection of serum cryoglobulins associated with purpura, arthralgia, and weakness; anti‐HCV antibodies positivity and polymerase chain reaction‐based assay to detect HCV RNA in serum; liver biopsy showing chronic hepatitis; negativity for hepatitis B surface antigen and human immunodeficiency virus. This review have not been limited by this criteria and accepted any definition described in the study reports.
Exclusion criteria
We excluded all studies focusing only on cryoglobulinaemic peripheral neuropathy.
Types of interventions
All possible comparisons between treatments have been eligible and have been analysed separately as well as in combination. These included the following.
-
Immunosuppressive medications (including rituximab, glucocorticoids, azathioprine, cyclophosphamide)
-
Different drug preparations
-
Different doses
-
Different frequency
-
Different duration
-
Different routes of administration
-
Monotherapy, dual therapy
-
-
Extracorporeal therapies
-
Plasma exchange therapy
-
Cryofiltration or cryoglobulin apheresis
-
Immunoadsorption apheresis
-
-
Treatment for HCV (including IFN, PEG‐IFN alpha‐2b, ribavirin, boceprevir, telaprevir and other direct‐acting antiviral agents).
Types of outcome measures
Primary outcomes
-
Death
-
Decreasing clinical manifestations (measured in different time points: 1, 6, 12 and 24 months)
-
Kidney disease (serum creatinine (SCr), glomerular filtration rate (GFR), proteinuria, active urinary sediment, need of dialysis, biopsy with GN)
-
Skin vasculitis (number and diameter of lesions, purpura, cutaneous ulcers or others)
-
Musculoskeletal symptoms (weakness (percentage of patients with complete response any time), peripheral joint arthralgia (percentage of patients with complete response any time) and peripheral neuropathies (percentage of patients with complete response any time))
-
Liver involvement (serum liver enzymes; echographic, histologic hepatitis or both; hepatocellular carcinoma)
-
Interstitial lung involvement characterized by subclinical alveolitis (presence of symptoms with radiologic, pulmonary function test alterations or both)
-
Widespread vasculitis involving medium‐small sized arteries, capillaries and venules with multiple organ involvement: skin, kidney, lungs, central nervous system, and gastrointestinal tract (pain simulating an acute abdomen)
-
Secondary outcomes
-
Duration of the therapy (months)
-
Laboratory findings (at different time points): rheumatoid factor activity (IU/mL); quantification of C3, C4 and C1q levels (mg/dL); serum levels of IgA, IgG and IgM (mg/dL); type I, II and III cryoglobulin levels (mg/L); cryocrit percentage (%); B cell clonal expansion (CD20, CD19, CD20/CD5) in circulation (%); anti‐HCV antibody titre (IU/L); serum HCV‐RNA (IU/mL); fast glycaemia, cholesterol (total, LDL, HDL); thyroid function (thyrotropin, free thyroxine and triiodothyronine)
-
Adverse effects of medication including: leukopenia (leukocytes/mL); thrombocytopenia (platelets/mL); aspartate aminotransferase (IU/L); total bilirubin (mg/dL); infusion reactions (number); discontinuations of treatment due to adverse drug reactions (number); infections (pneumonia, urosepsis); change in blood pressure; cardiovascular events (angina, myocardial infarction, heart failure); gastrointestinal bleeding; haemorrhagic alveolitis.
-
Antiviral therapy failure or not indicated
-
B‐cell lymphoma
-
Endocrine disorders (autoimmune thyroiditis, subclinical hypothyroidism, thyroid cancer or diabetes)
-
Economic costs of treatment (including hospitalisation rate).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 30 November 2017 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney and transplant journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of clinical practice guidelines, review articles and relevant studies.
-
Email seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that might include relevant data or information on studies had been retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by three authors using standard data extraction forms. It was planned to translate studies reported in non‐English language journals before assessment, but at the end that was not necessary. Where more than one publication of one study existed, reports had been grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data was used. Any discrepancy between published versions had been highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (death, adverse effects of medications, active urinary sediment, need of dialysis, antiviral therapy failure) results have been expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (SCr, GFR, proteinuria, economic cost of the treatment, laboratory findings, duration of the therapy), the mean difference (MD) have been used, or the standardised mean difference (SMD) if different scales have been used. In case it was necessary to deal with change scores (decreasing global disease activity, reduction of the number or diameter of skin lesions), the statistical approach have been to include the baseline outcome measurements as a covariate in a regression model or analysis of covariance (ANCOVA) and have been included in the meta‐analysis using the generic inverse‐variance method. When the study outcomes were expressed using the median, the minimum and maximum values, in order to combine results, the sample mean and standard deviation (SD) was estimated using a published validated method (Wan 2014).
We approached time to event data expressing the intervention effect as a hazard ratio using to analyse it the generic inverse variance method. For counts and rates the results of a study was expressed as a RR and the (natural) logarithms of the rate ratios have been combined across studies using the generic inverse‐variance method (Higgins 2011).
Unit of analysis issues
It was planned that cross‐over studies would not be included, but we found one study and we analysed only the first part of the trial before the interventions were crossed. It was planned that, although it would have been highly unlikely to find other studies with non‐standard designs, if a cluster‐RCT had been included, statistical advice would have been asked for determining the most appropriate method to use and if studies with multiple intervention groups were included, they have been analysed combining groups to create a single pair‐wise comparison.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population have been carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals have been investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) have been critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). A guide to the interpretation of I2 values is as follows.
-
0% to 40%: might not be important
-
30% to 60%: may represent moderate heterogeneity
-
50% to 90%: may represent substantial heterogeneity
-
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
Funnel plots were to be used to assess for the potential existence of small study bias (Higgins 2011). There were insufficient studies to do this.
Data synthesis
Data have been pooled using the random‐effects model but the fixed‐effect model was also be used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses have been used to explore possible sources of heterogeneity (participants, interventions and study quality). Heterogeneity among participants could be related to the HCV genotype (1 or 2) and efficacy of the antiviral agents therapy for HCV (IFN, ribavirin, boceprevir, telaprevir), age, liver involvement (considering all the possible criteria being used: severe versus non‐severe liver fibrosis, i.e. F3‐F4 versus F0‐F2 in the Metavir scoring system or following a histologic criteria) or presence of chronic kidney disease. Heterogeneity in treatments could be related to prior immunosuppressive agent(s) used and the agent, previous administration of immunosuppressive drugs, concurrent use of extracorporeal therapies, the route of administration (e.g. whether oral or intravenous), the doses of drug given (if single or multiple doses) and the duration of the treatment (e.g. 1, 2, 6, 12 months). Adverse effects have been tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used. Where possible, the risk difference with 95% CI has been calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size.
-
Repeating the analysis excluding unpublished studies
-
Repeating the analysis taking account of risk of bias, as specified above
-
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
-
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
This was not possible due to the limited number of studies.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the Summary of findings table 1 evaluating the intervention comparing rituximab versus other immunosuppressive drugs.
-
Clinical manifestations: skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months
-
Laboratory findings: SCr (μmol/L) 18 months
-
Laboratory findings: cryocrit (%) 12 months
-
Adverse effects of the medication: infusion reactions (number)
Results
Description of studies
Results of the search
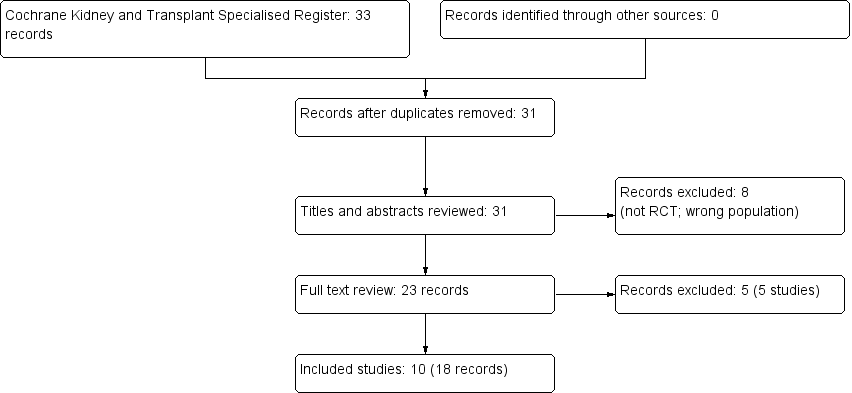
After searching the Register we identified 33 records. No relevant titles were found by handsearching at clinicaltrials.gov (to date 30 November 2017) or in major conferences lists from 2000 to 2015 (American Society of Nephrology, European Renal Association–European Dialysis and Transplant Association, and International Society of Nephrology). After duplicates were removed and titles and abstracts screened we retrieved 23 full‐text articles for further assessment. Of these, 10 studies (18 records) were included and 5 studies (5 records) were excluded (Figure 1).

Flow chart showing source and identification of studies for inclusion.
Included studies
See: Characteristics of included studies
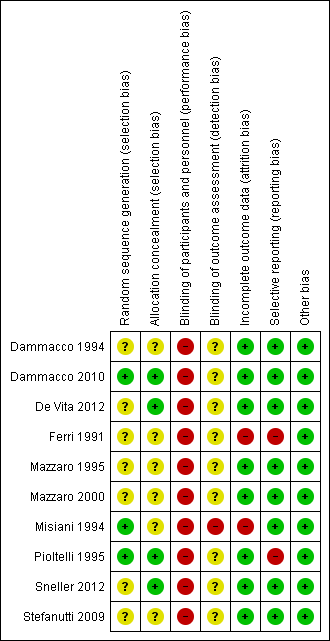
Ten studies (394 participants) were included (Dammacco 1994; Dammacco 2010; De Vita 2012; Ferri 1991; Mazzaro 1995; Mazzaro 2000; Misiani 1994; Pioltelli 1995; Sneller 2012; Stefanutti 2009). All studies were published in English.
One study author responded to queries about missing data stating he did not have the missing information (Dammacco 1994). No more responses were obtained.
Design
All the included studies were RCTS and were unblinded. The duration of the studies varied from six to 36 months. Seven studies were single‐centre (Dammacco 1994; Dammacco 2010; Ferri 1991; Mazzaro 2000; Misiani 1994; Sneller 2012; Stefanutti 2009) and three were multicentre (De Vita 2012; Mazzaro 1995; Pioltelli 1995). One study was conducted in the USA (Sneller 2012), and the rest were conducted in Italy (Dammacco 1994; Dammacco 2010; De Vita 2012; Ferri 1991; Mazzaro 1995; Mazzaro 2000; Misiani 1994; Pioltelli 1995; Stefanutti 2009).
All the participants had the diagnosis of HCV‐related mixed cryoglobulinaemia. In all studies potentially life‐threatening vasculitis was an exclusion criterion. The median age of participants ranged between 50 to 70 years old and they were predominantly women.
Intervention and comparators
Three different interventions were found and analysed. Some studies included a combination of more than one agent.
Rituximab versus no rituximab treatment
Three studies (118 participants) compared rituximab with non‐rituximab treatment (Dammacco 2010; De Vita 2012; Sneller 2012).
Dammacco 2010 included antiviral treatment in both groups, while in the other two studies patient inclusion implied that therapy with antiviral agents had failed, had been poorly tolerated, or was considered to be contraindicated (De Vita 2012; Sneller 2012).
De Vita 2012 prescribed doses of rituximab was 1 g at day 1 and 15 (with standard premedication that included 100 mg of methylprednisolone) and only if relapse or clinical disease reappeared another course of medication was given only if there had been previous response to the drug. In the other two studies 375 mg/m2 once a week for one month followed by two five‐monthly infusions with premedication with 20 mg of 6‐methyl‐prednisolone in Dammacco 2010, and without any steroids in Sneller 2012.
Antiviral treatment was given simultaneously in both intervention groups using Peg‐IFN‐alpha (180 µg or 1.5 µg/kg) weekly plus ribavirin (1000 or 1200 mg) daily for 48 weeks (Dammacco 2010). The comparator groups in the two studies without antiviral treatment were with the previous immunosuppressive agents they had been receiving at the time of enrolment and were allowed to increase or initiate new ones as needed to manage worsening disease activity (glucocorticoids, azathioprine, cyclophosphamide or methotrexate) or plasma exchange.
Interferon treatment
Six studies (259 participants) evaluated the use of IFN. Five studies (223 participants) compared IFN with no IFN (Dammacco 1994; Ferri 1991; Mazzaro 2000; Misiani 1994; Pioltelli 1995), and one study (36 participants) compared the use PEG‐IFN‐alpha‐2b for six months versus one year (Mazzaro 1995). Two studies compared the use of IFN versus maintaining previous immunosuppressive medications (Ferri 1991; Misiani 1994). In Mazzaro 2000, IFN was compared to oral prednisone at a dose of 0.2 mg/kg/d for six months, and Pioltelli 1995 compared IFN‐alpha‐2b (3 MU, 3 times/week) versus deflazacort (1 or 0.2 mg/kg/d depending on the severity of disease) for at least two months and, if no improvement was obtained, was continued for a maximum of four months.
Other interventions
One study (17 participants) compared the use of immunoadsorption apheresis with immunosuppressive therapy versus only immunosuppressive therapy (Stefanutti 2009). The therapy also included PEG‐IFN (3 MU, 3 times/week), cyclophosphamide (7.5 to 15 mg/kg, IV), CSA (2 to 5 mg/kg/d), ribavirin (10 to 15 mg/kg/d), or melphalan (0.1 to 0.2 mg/kg/d). The immunoadsorption apheresis was done using selesorb with a plasma volume exchange of 45 mL/Kg. The number of treatments for patients was between 24 and 35.
Outcomes
All studies were included in the meta‐analyses.
None of the included studies reported: GRF; kidney biopsy (Mazzaro 2000 only reported basal kidney biopsy data); echographic or histologic hepatitis (Dammacco 1994 and Mazzaro 2000 reported basal data of liver biopsy); hepatocellular carcinoma; interstitial lung involvement; widespread vasculitis (Stefanutti 2009 reported basal data); C1q levels; IgG; type I, II and III cryoglobulin levels; CD20/CD5; fast glycaemia; cholesterol; thyroid function (thyrotropin, free thyroxine and triiodothyronine); total bilirubin; economic costs of treatment (including hospitalisation rate).
All studies reported death at different time‐points.
Kidney involvement was reported differently in the studies: Dammacco 1994 referred to general improvement without specifying which was the improved parameter, and De Vita 2012 reported the presence of active urinary sediment. SCr, proteinuria and the need of dialysis was reported in the four studies (Dammacco 2010; De Vita 2012; Mazzaro 2000; Misiani 1994).
Six studies presented data on weakness, peripheral arthralgia, or peripheral neuropathies (Dammacco 1994; De Vita 2012; Ferri 1991; Mazzaro 1995; Misiani 1994; Stefanutti 2009), and eight studies reported data of skin vasculitis (Dammacco 1994; Dammacco 2010; De Vita 2012; Mazzaro 1995; Mazzaro 2000; Misiani 1994; Sneller 2012; Stefanutti 2009).
Some adverse events related to treatment were reported although this was not systematic. All reported withdrawal from study treatment, antiviral treatment failure and infusion reactions in rituximab group.
In De Vita 2012, the authors offered the patients in the non‐rituximab group in whom treatment failed, the opportunity to be switched to rituximab in an open‐label extension. At the end, 23/29 patients from the non‐rituximab group switched to rituximab. We decided to perform intention‐to‐treat analyses, and the events of those switched patients are presented with the non‐rituximab group.
Excluded studies
See: Characteristics of excluded studies.
Five studies did not meet our inclusion criteria and were excluded because of the following reasons: one study was a protocol, with no results identified (Anonymous 1989), and four studies did not include participants meeting our inclusion criteria for HCV‐associated mixed cryoglobulinaemia (Candela 1994; Ferri 1989; Lauta 1995; Vacca 1992).
Risk of bias in included studies
See Characteristics of included studies and Figure 2 and Figure 3.
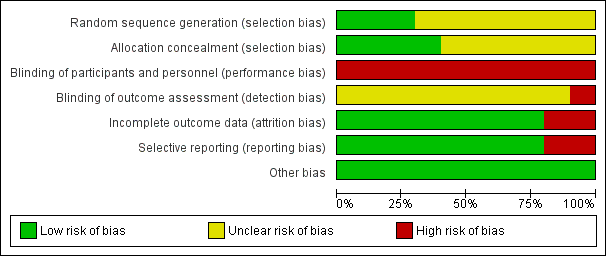
The risk of bias for many domains was generally unclear or low.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
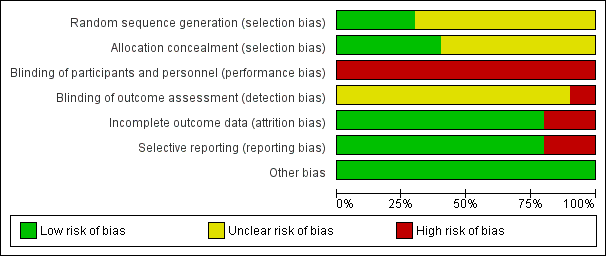
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Random sequence generation was judged to be at low risk of bias in three studies (Dammacco 2010; Misiani 1994; Pioltelli 1995) and unclear in seven studies (Dammacco 1994; De Vita 2012; Ferri 1991; Mazzaro 1995; Mazzaro 2000; Sneller 2012; Stefanutti 2009).
Allocation concealment
Allocation concealment was judged to be at low risk of bias in four studies (Dammacco 2010; De Vita 2012; Pioltelli 1995; Sneller 2012) unclear in six studies (Dammacco 1994; Ferri 1991; Mazzaro 1995; Mazzaro 2000; Misiani 1994; Stefanutti 2009).
Blinding
Performance bias (blinding of participants and investigators) was judged to be at high risk of bias for all 10 studies.
Misiani 1994 that refers to "the examiner searched for signs of cutaneous vasculitis", which introduced a high risk of detection bias. The risk of bias was unclear in the remaining nine studies.
Incomplete outcome data
Two studies were judged to be at high risk of attrition bias (Ferri 1991; Misiani 1994). Ferri 1991 reported five patients had drop‐out and the authors did not present data for them, and Misiani 1994 reported that missing outcome data was balanced in number across intervention groups, but the authors did not impute these using appropriate methods. All other studies were judged to be at low risk of bias.
Selective reporting
Two studies were judges to be at high risk of reporting bias (Ferri 1991; Pioltelli 1995). Ferri 1991 specified that "renal and neurological involvement" would be evaluated however they did not report these outcomes, and in Pioltelli 1995 there was no data at the end of the study of purpura, joint pain, weakness, kidney impairment or neurological impairment. All other studies were judged to be at low risk of bias.
Other potential sources of bias
All studies appear to be free of other sources of bias.
Effects of interventions
See: summary of findings Table for the main comparison for the main comparison IFN versus other immunosuppressive drug for patients affected by mixed cryoglobulinaemia
There are some outcomes that it was planned to analyse as it was published in the protocol, but that has not been possible because they were not reported in the available studies.
Rituximab versus no rituximab treatment
Three studies compared rituximab with non‐rituximab treatment (Dammacco 2010; De Vita 2012; Sneller 2012).
Death
Two studies reported 100% of survival (Dammacco 2010; Sneller 2012). De Vita 2012 found no differences in death between the groups at 24 months when intention‐to‐treat analysis was done: one in the non‐rituximab group, two in the rituximab group, and three in the rituximab‐switch group (Analysis 1.1.3 (3 studies, 107 participants): RR 0.52, 95% CI 0.1 to 2.61; I2 = 0%).
Kidney disease
Two studies reported kidney disease parameters, however they did not report the same outcomes (Dammacco 2010; De Vita 2012).
De Vita 2012 (46 participants) reported little or no difference between rituximab and control for active urinary sediment at 1 month (Analysis 1.2.1) or the need for dialysis at 24 months (Analysis 1.2.2). Dammacco 2010 (37 participants) reported little or no difference between rituximab and control for SCr (Analysis 1.3.1; Analysis 1.3.2) and proteinuria (Analysis 1.3.1; Analysis 1.3.4) at 18 and 36 months. The mean proteinuria given in Dammacco 2010 is a high value because authors only give results of those patients with a value of proteinuria > 0.25 g/24 h, and they were only three.
Skin vasculitis
Skin vasculitis was reported by two studies (Dammacco 2010; De Vita 2012).
At one month De Vita 2012 reported little or no difference between rituximab and control (Analysis 1.2.3). At 18 to 24 months rituximab may slightly improve skin vasculitis (Analysis 1.2.4 (2 studies, 78 participants): RR 0.57, 95% CI 0.28 to 1.16; I2 = 0%; moderate certainty evidence).
Rheumatoid factor activity
De Vita 2012 described rheumatoid factor levels decreased at the end of six months of rituximab treatment (mean ± SD: 286.14 ± 67.81 IU/mL at baseline versus 122.31 ± 43.03 IU/mL at 6 months; P = 0.0003), with no differences between the randomised rituximab group and the rituximab‐switch group (data is not presented in any analysis because the authors did not give any information of the control group).
Cryocrit
Two studies reported cryocrit (%) at 12 months (Dammacco 2010; Sneller 2012). The mean and standard deviation was estimated from the median and interquartile range using (Wan 2014). The heterogeneity of I2 of 97% when assessing the use of rituximab or not in terms of cryocrit variation (Analysis 1.3.5 (41 participants): MD ‐2.01%, 95% CI ‐10.29% to 6.27%; low certainty evidence) may indicate an error in the data because of inconsistent findings across studies.
HCV‐RNA
Sneller 2012 reported that rituximab treatment alone did not appear to affect HCV replication (numerical data is not given analysed because only mean values were reported). Dammacco 2010 reported the use of antiviral treatment with or without rituximab might make no difference to viral replication (Analysis 1.3.9 (17 participants): MD ‐435182.00 IU/mL, 95% CI ‐1051224.79 to 180860.79).
Adverse effects
Three studies reported adverse effects (Dammacco 2010; De Vita 2012; Sneller 2012) however only infusion reactions and discontinuation of treatment could be meta‐analysed.
Rituximab may slightly increase infusion reactions compared to other immunosuppressive medication (Analysis 1.4.1 (3 studies, 118 participants): RR 4.33, 95% CI 0.76 to 24.75; I2 = 0%; moderate certainty evidence), while there was little or no difference in discontinuation of treatment due to adverse reactions (Analysis 1.4.2 (3 studies, 118 participants): RR 0.97, 95% CI 0.22 to 4.36; I2 = 0%; moderate certainty evidence).
Sneller 2012 reported no differences in infection (Analysis 1.4.3), leukopenia (Analysis 1.4.8) or thrombocytopenia (Analysis 1.4.9) at six months. De Vita 2012 reported no differences for infections (Analysis 1.4.4) (the authors found only four infections during the 24 months of the study: one in the rituximab group, one in the non‐rituximab group, and two additional infection in rituximab switch group), cardiovascular events (Analysis 1.4.5) (one angina episode in rituximab group, one myocardial infarction and one heart failure episode in non‐rituximab group, and three heart failure episodes in rituximab switch group), gastrointestinal bleeding (Analysis 1.4.6), or haemorrhagic alveolitis (Analysis 1.4.7) at 24 months.
Antiviral therapy failure
As it was previously described, antiviral failure or not indicated was one inclusion criteria in De Vita 2012 and Sneller 2012 studies, when we analysed data of this 2 studies, the use of rituximab makes little of no difference to antiviral failure (Analysis 1.5 (3 studies, 118 participants): RR 1.21, 95% CI 0.77 to 1.9), but what was interesting is that in Dammacco 2010, the authors found that in rituximab group slightly increases the rate of antiviral failure compared to non‐rituximab group (RR 2.85, 95% CI 1.44 to 5.62).
Other outcomes
The duration of therapy was always shorter in the rituximab group than in the control group and did not differ between studies.
Patients were treated for six months in control group and one month in rituximab group in De Vita 2012. A second course of rituximab was administered to 15 patients who presented relapse. In Sneller 2012, rituximab was given during 22 days and control group received 12 months of treatment. Both groups were receiving concomitant antiviral treatment for 12 months in Dammacco 2010 and rituximab was given once a week for one month and then two, five‐monthly infusions in the treatment group.
Activity outcomes
Although it was not initially planned we decided to include activity outcomes as they were the main comparison in the majority of included studies. The activity was quantified using the Birmingham Vasculitis Activity Score (BVAS), which is the most recognized validated instrument for standardised assessment of disease activity in vasculitis clinical trials.
De Vita 2012 reported the global disease activity. They concluded that no difference in the BVAS was observed between the two treatment groups at baseline (mean ± SD: 9.6 ± 3.6 in the control group versus 11.9 ± 5.4 in the rituximab group; P = 0.06) or at two months (9.6 ± 4.5 in the control group versus 7.1 ± 5.7 in the rituximab group; P = 0.076). While no significant improvement in the non‐rituximab group was observed for the BVAS at baseline versus two months (P = 0.715), a significant reduction in the BVAS at two months was seen in the rituximab group (P < 0.001), and this difference persisted at 6 months (6.9 ± 6.8; P < 0.001), 12 months (5.4 ± 6.2; P < 0.0001), and 24 months (4.4 ± 4.6; P < 0.0001).
Sneller 2012 reported differences between groups. The authors explained that at baseline, BVAS were comparable between the two groups (P = 0.977), but became significantly lower in the rituximab group starting at four months with P values < 0.02.
Interferon versus control
Six studies evaluated the use of IFN (Dammacco 1994; Ferri 1991; Mazzaro 1995; Mazzaro 2000; Misiani 1994; Pioltelli 1995).
To include the four groups from Dammacco 1994, the approach used to overcome a unit‐of‐analysis error for a study that could contribute multiple, correlated, comparisons was to combine groups to create a single pair‐wise comparison (IFN combined with IFN plus prednisone group (32 participants) and prednisone alone plus control group (33 participants)). Data from the cross‐over trial of Ferri 1991 was analysed by including only data from the first period because we considered that in this particular case, carry‐over would be a problem. The numerical outcomes for Misiani 1994 were presented using medians and minimum and maximum values, and in order to combine results, the mean and SD was estimated using a published validated method (Wan 2014).
Death
Four studies (Dammacco 1994; Ferri 1991; Mazzaro 2000; Pioltelli 1995) reported no deaths at 12 and 24 months (Analysis 2.1; Analysis 2.2).
Kidney disease
Two studies reported kidney disease parameters at 18 months (Mazzaro 2000; Misiani 1994)
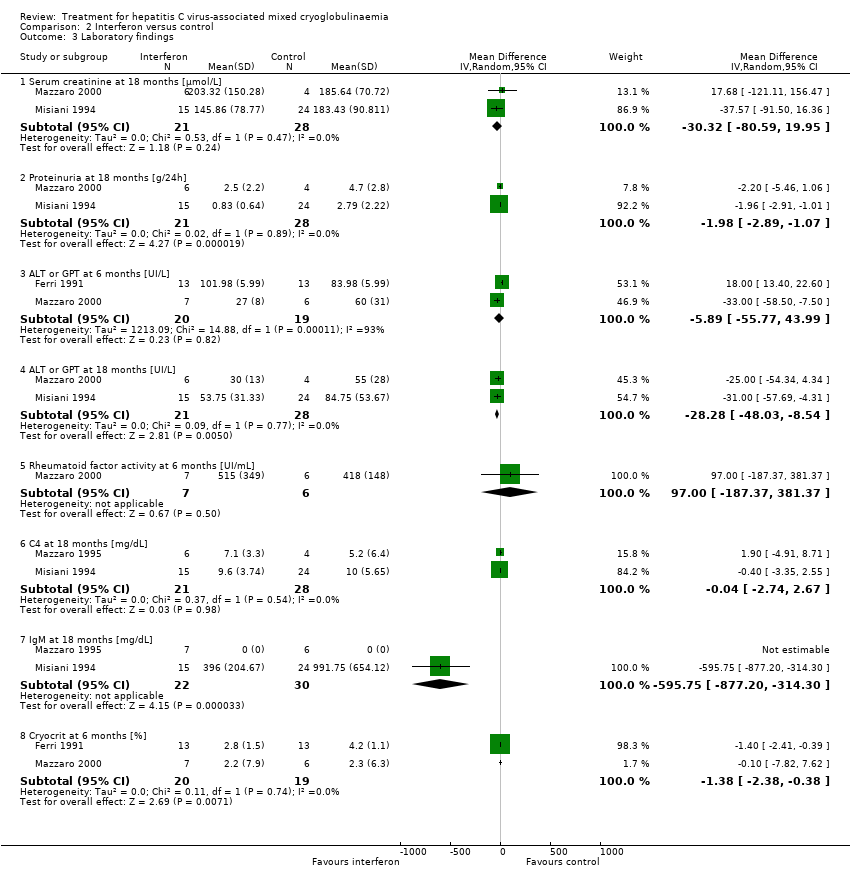
IFN made little or no difference to SCr at 18 months (Analysis 2.3.1 (2 studies, 49 participants): MD ‐30.32 μmol/L, 95% CI ‐80.59 to 19.95; I2 = 0%), however proteinuria was probably slightly reduced with IFN (Analysis 2.3.2 (2 studies, 49 participants): MD ‐1.98 g/24 h, 95% CI ‐2.89 to ‐1.07; I2 = 0%)
Vasculitis
Five studies reported vasculitis (Dammacco 1994; Ferri 1991; Mazzaro 2000; Misiani 1994; Pioltelli 1995), however only three could be meta‐analysed (Dammacco 1994; Ferri 1991; Misiani 1994).
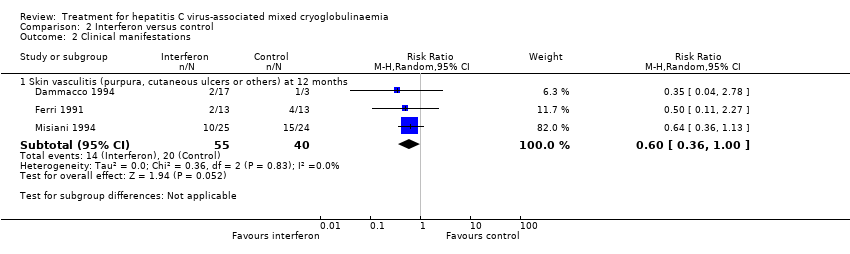
IFN therapy probably reduced the skin vasculitis lesions compared to other immunosuppressive treatment (Analysis 2.2 (3 studies, 95 participants): RR 0.60, 95% CI 0.36 to 1.00; I2 = 0%; moderate certainty evidence).
Dammacco 1994 described that lFN controlled the activity in more than a half of patients and when it was combined with prednisone, a more prompt response and delayed relapse was found, but relapse was common after three months of treatment withdrawal from all treatment modalities. Similar results were found by Mazzaro 2000, who described that both IFN (lymphoblastoid) and prednisone were associated with an improvement in clinical symptoms even in non‐responders; complete recovery was achieved in only one patient in the IFN group, suggesting that complete remission could only be obtained through virus eradication. Misiani 1994 concluded that the beneficial effect of IFN was limited to patients in whom HCV‐RNA disappeared from the serum, whereas it worsened in those without response or in the control group. Pioltelli 1995 did not find any difference between the effects of deflazacort versus IFN.
Liver involvement
IFN compared to control made little or no difference to ALT at six months (Analysis 2.3.3 (2 studies, 39 participants): MD ‐5.89 UI/L, 95% CI ‐55.77 to 43.99; I2 = 93%), however IFN probably decreased ALT at 18 months (Analysis 2.3.3 (2 studies, 39 participants): MD ‐28.28 UI/L, 95% CI ‐48.03 to ‐8.54; I2 = 0%).
Rheumatoid factor activity
Mazzaro 2000 reported little or no difference between IFN and control in rheumatoid factor activity at 6 months (Analysis 2.3.5 (13 participants): MD 97.00 UI/mL, 95% CI ‐187.37 to 381.37)
C4 levels
There was little no difference in C4 levels at 18 months (Analysis 2.3.6 (2 studies, 49 participants): MD ‐0.04 mg/dL, 95% CI ‐2.74 to 2.67).
IgM
IFN probably decreased IgM at 18 months (Analysis 2.3.7 (2 studies, 52 participants): MD ‐595.75 mg/dL, 95% CI ‐877.2 to ‐314.3; I2 = 0%)
Cryocrit
There was little or no difference in cryocrit at six months between IFN and control (Analysis 2.3.8 (2 studies, 39 participants): MD ‐1.38%, 95% CI ‐2.38 to ‐0.38; I2 = 0%).
HCV‐RNA
Three studies evaluated the effect of IFN on the serum HVC‐RNA but their results could not be meta‐analysed; Misiani 1994 did not express the numerical data (only specified if it was positive or negative after the treatment) and the other two studies gave the results at different time points (Mazzaro 2000 at 6 months and Dammacco 1994 at 12 months).
Adverse effects
Five studies reported adverse effects (Dammacco 1994; Ferri 1991; Mazzaro 2000; Misiani 1994; Pioltelli 1995).
Dammacco 1994 reported infusion reactions may be higher in IFN group compared to immunosuppressive therapy (Analysis 2.4.1 (65 participants): RR 27.82, 95%CI 1.72 to 449.18, low certainty evidence)
IFN may lead to higher discontinuations of treatment due to adverse reactions (Analysis 2.4.2 (4 studies, 148 participants): RR 2.32, 95% CI 0.91 to 5.90; I2 = 0%; low certainty evidence).
Antiviral failure
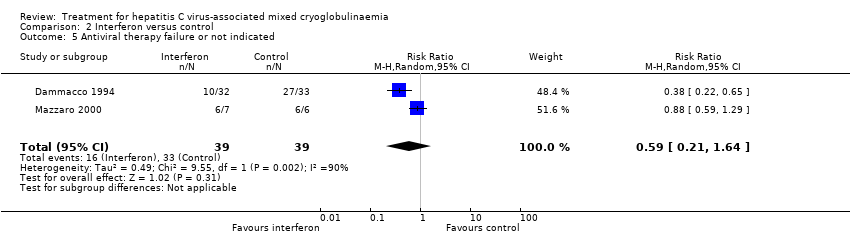
No difference was found in antiviral failure between groups (Analysis 2.5 (2 studies, 78 participants) RR 0.59, 95% CI 0.21 to 1.64).
Other outcomes
In narrative results, Mazzaro 2000 found minor side‐effects only in the IFN group (fever, fatigue and a flu‐like syndrome in most patients during the first two weeks) with one patient withdrawal (because of thrombocytopenia). Misiani 1994 reported IFN was well tolerated except for influenza‐like illness and two cases discontinued because of atrial fibrillation and depression. Pioltelli 1995 described a higher incidence of major toxic effects in IFN group (25% of patients: one ictus cerebri, one refractory headache, one refractory fever, one refractory diarrhoea, one an anaphylaxis episode, one impotentia coeundi, and one hypersomnia) versus 14% in deflazacort group (two femur fractures, one diabetes mellitus, one Cushing’s syndrome).
The duration of the therapy was always similar between the two groups and it was not different between studies.
Interferon: six months versus one year treatment
One study compared six months of IFN with 12 months (Mazzaro 1995).
Mazzaro 1995 reported no deaths at 24 months.
For the rest of available data, only narrative results can be presented.
-
Complete response in 28% for six months duration treatment compared 39% of one year treatment; partial response was 50% in both groups and minor response was of 22% for six months and 11% for one year. Differences were observed in terms of the maintenance of the response, 89% of patients in the six months group presented a relapse and only 11% maintained a long‐term response at one year, while in the one year group only 78% relapsed and long‐term response was observed in 22%.
-
The one‐year therapy was linked to a higher number of side‐effects (severe enough to cause the discontinuation of treatment in two cases) than the six‐month schedule.
Immunoadsorption apheresis versus immunosuppressive drug therapy
One study compared immunoadsorption apheresis with immunosuppressive drug therapy (Stefanutti 2009).
Stefanutti 2009 reported no deaths at 24 months. They also was also little or no difference between the treatments to skin vasculitis at six months (Analysis 4.2.1), peripheral neuropathies at six months (Analysis 4.2.2); peripheral joint arthralgia at six months (Analysis 4.2.3), cryocrit (Analysis 4.3), or to infusion reactions (Analysis 4.4).
Stefanutti 2009 reported clinical score changes were more favourable in combined therapy with immunoadsorption apheresis plus immunosuppressants compared to immunosuppressive treatment alone (no numerical data). Higher remission of severe clinical complications was found with combined therapy (80% versus 33%, P = 0.05). It is also reported that "no adverse reactions or complications were noted".
Discussion
Summary of main results
We have assessed a total of 10 studies involving 394 participants in this systematic review of treatment for hepatitis‐C virus‐associated mixed cryoglobulinaemia.
As discussed in other studies, the relevant clinical features to assess possible treatment strategies are kidney disease, skin vasculitis, musculoskeletal symptoms, and peripheral neuropathies. Moreover, the relevant biochemical parameters to assess immune response to the treatment are: C4 levels and the amount of cryoglobulins that can be estimated by quantifying the cryoglobulin levels, cryocrit, or rheumatoid factor. Three different interventions were examined: rituximab (three studies); IFN (six studies); and one study evaluated the use of immunoadsorption apheresis.
The majority of the studies under‐reported relevant data or gave compound results that could not have been disaggregated, especially related to clinical outcomes. Although we send emails seeking information about that data to the study authors, we did not obtain any positive responses. As there were few events and participants for some outcomes, we found wide CIs crossing the line of no effect. For all the interventions, the effect on death was uncertain. Rituximab probably improved skin vasculitis wounds. All interventions make little of no difference to peripheral neuropathies, peripheral joint arthralgia, and kidney disease although FN probably slightly reduced proteinuria as reported by Mazzaro 2000 and Misiani 1994. In narrative results, some authors reported that lFN improved clinical symptoms with a more prompt response and delayed relapse, however a study specified that the beneficial effect of IFN was limited to patients in whom HCV‐RNA disappeared from the serum. Also in narrative description, clinical score changes were more favourable in combined therapy with immunoadsorption plus immunosuppressive therapy compared to immunosuppressive treatment alone, with higher remission of severe clinical complications found with combined therapy. Moreover, the use of rituximab improved BVAS scores, which became significantly lower at six months in the rituximab group. Dammacco 2010 found that combining rituximab with antiviral treatment (IFN and ribavirin) significantly increased the rate of clinical response and sustained a higher long‐term remission rate compared to antiviral treatment alone. There was uncertain direction of effect for cryocrit variation and for HCV replication when rituximab or immunoadsorption were assessed, but narrative results of De Vita 2012 described that rheumatoid factor levels decreased at the end of month six after rituximab treatment. On the other hand, IFN may decrease ALT, IgM at 18 months and cryocrit at six months. Rituximab or immunoadsorption plus other immunosuppressive strategies made no difference to infusion reactions or discontinuations of the treatment due to adverse reactions. However, infusion reactions, discontinuation of the treatment and side effects were probably higher in IFN group compared to immunosuppressive therapy.
It seems logical that to treat HCV‐associated mixed cryoglobulinaemia, one of the main aspects might be to remove its disease causative agent with antiviral therapy. Our results show that it may be beneficial to eliminate HCV infection by using antiviral treatment to improve the vasculitis signs and symptoms. However, in this review we only have been able to evaluate IFN treatment because no RCT with new antivirals looking at this disease have been published, although cohort studies and case reports demonstrated an achievement of a sustained virological response and complete clinical and immunological remission (Cornella 2015; Gragnani 2016; Saadoun 2016; Sise 2016; Suwanthawornkul 2015). See characteristics of these cohort studies in Table 1. For skin vasculitis and for some laboratory findings, it may be appropriate to combine antiviral treatment with deletion of B‐cell clonal expansions by using of rituximab.
| Study ID | Study design | Patient profile | Objective | No. of patients | Treatment | Results | Adverse effects |
| Non‐randomised, non‐controlled prospective study | HCV RNA (+) + cryoglobulinaemic syndrome with organ damage and B‐cell lymphoproliferative syndrome | To assess the hepatovirological response, the clinical and immunological efficacy and the safety of using sofosbuvir‐based direct‐acting antiviral therapy in patients with HCV‐associated mixed cryoglobulinaemia(according to the latest guidelines) | 44 | 1) Sofosbuvir+ribavirin (18) 2) Sofosbuvir+simeprevir (12) ± ribavirin (6 of 12 patients) 3) Sofosbuvir+daclatasvir (4) ± ribavirin (1 patient) 4) Sofosbuvir+ledipasvir (10) ± ribavirin (3 patients) | Hepatovirological response ‐ Undetectable HCV RNA negative rate 100% at week 4; 12SVR and 24SVR remained 100% negative ‐ Decrease of ALT from 77.7 ± 10.3 IU/L at baseline to 27.3 ± 10.3 IU/L at 24SVR ‐ Decrease of AST from 55.2 ± 60.4 IU/L at baseline to 22.6 ± 8.3 IU/L at 24SVR (P < 0.001) Clinical efficacy ‐ Decrease of BVAS from 5.41 ± 3.53 at baseline to 1.27 ± 1.68 at 24SVR (P < 0.001) Immunological efficacy ‐ Decrease of cryocrit level from 7.2 ± 15.4% at baseline to 1.8 ± 5.1% at 24SVR (P < 0.001) | Total: 26/44 (59%) Withdrawals: 1 patient withdrew ribavirin while continuing sofosbuvir+simeprevir Death: none Relapse: none Most frequent AE: Anaemia (13, all receiving ribavirin); fatigue (15); nausea (7) | |
| Case series | Five patients with HVC RNA + with detectable cryoglobulins in plasma and symptomatic mixed cryoglobulinaemia Patient 1: bilateral foot neuropathy and purpura Patient 2: painful left foot drop and purpura Patient 3: purpura and MPGN Patient 4: MPGN Patient 5: MPGN + low grade lymphoma | Review of one centre's experience in treating patients with mixed cryoglobulinaemia with new oral antiviral agents and to assess common factors associated with persistence of mixed cryoglobulinaemia despite SVR | 5 | Patient 1: PEG‐IFN+ribavirin+boceprevir Patient 2: Firstly with rituximab (5 weeks); later with: PEG‐IFN+ribavirin+telaprevir for 4 weeks; PEG‐IFN+ribavirin for 12 weeks; and PEG‐IFN+ribavirin+sofosbuvir for 15 weeks (telaprevir discontinued for persistent viral load > 1000 IU/mL) Patient 3: PEG‐IFN+ribavirin+telaprevir for 47 weeks Patient 4: PEG‐IFN+ribavirin for 24 weeks; afterwards adding sofosbuvir until completing 12 weeks of triple therapy (total 36 weeks) Patient 5: 2 cycles of rituximab: weekly infusions 375mg/m2 during 4 weeks in 2010 then two extra doses of rituximab in 2013 ‐PEG‐IFN+ribavirin+sofosbuvir for 8 weeks in 2014 | Patient 1: complete clearance of virus at week 8; complete clearance of cryoglobulins at week 28. Persistence of neuropathy Patient 2: SVR and no detectable cryoglobulins at month 6 after last triple therapy. Persistence of neuropathy. Patient 3: clearance of HCV at week 4 after PEG‐IFN+ribavirin+telaprevir, with persistent cryoglobulins. Active MPGN and vasculitis after ending of previous treatment (responding to steroid therapy). Patient 4: SVR with persistence of cryoglobulinaemia. Kidney function remained stable. | ||
| Retrospective case series | HCV RNA > 1000 IU/mL + circulating purpura + cutaneous ulcers, Raynaud’s phenomenon, arthralgia, sicca syndrome, gastrointestinal vasculitis, neurologic involvement or renal involvement | Comparison of 2 historical cohorts: one treated with PEG‐IFN and ribavirin and the other sofosbuvir+simeprevir (8/12) or sofosbuvir+ribavirin (4/12). Evaluation of 12SVR, relapses, clinical, immunological (cryoglobulins) and biochemical (AST, Hb) response and adverse effects; without statistical comparison between them | 22 | 1) PEG‐IFN+ribavirin 2) IFN‐free regimens 2a) Sofosbuvir 400 mg/24 h + simeprevir 150 mg/24h 2b) Sofosbuvir 400 mg/24 h + ribavirin (adjusted to kidney function) | PEG‐IFN+ribavirin ‐ SVR12: 1/10 IFN‐free regimens ‐ SVR12: 10/12 (95%) ‐ ALT decreased from 42 U/L at baseline to 20 U/L after treatment ‐ Cryoglobulin levels decreased from 1.5% (0.5% to 4%) at baseline to 0.5% (0% to 2%) after treatment ‐ Decrease of proteinuria in all cases of kidney involvement (table IV) | Total PEG‐IFN+ribavirin: 10/10 IFN‐free regimens: 8/12 Withdrawals PEG‐IFN+ribavirin: 5/10 IFN‐free regimens: 1/12 (anxiety and insomnia) Deaths: none Relapses IFN‐free regimens: 2 (genotype 1 sofosbuvir+simeprevir; genotype 4 sofosbuvir+ribavirin) | |
| Open‐label, non‐controlled, prospective cohort study | Chronic active HCV infection with signs of mixed cryoglobulinaemia. All 23 patients had positive cryoglobulins in plasma at baseline or earlier | To analyse the safety and efficacy of Peg‐IFN‐alpha/ribavirin/protease inhibitor combination in HCV‐mixed cryoglobulinaemia | 23 | Peg‐IFN‐alpha+ribavirin a) + telaprevir (375 mg, 3 times/d for 12 weeks) for 48 weeks (15 patients) b) + boceprevir (800 mg, 3 times/d for 44 weeks) for 48 weeks (8 patients) | Complete clinical responders (improvement in all baseline clinical manifestations): 13 patients (56.5%) at week 24 Virological response (i.e., HCV RNA negative) was of 69.6% at week 24 (P = 0.005). Cryoglobulin level: decreased from 0.44 to 0.06 g/L(P = 0.0006) C4 level: increased from 0.09 to 0.15 g/L (P = 0.045) No significant difference was found between the two treatment regiments | Total: 105 Withdrawals: 8 patients (34.7%) ( virological non‐response (5); virological relapse (2); depression (1)) Death: none Relapse: 1 Most frequent AE: fatigue (87%); neutropenia (78.3%); thrombocytopenia (65.2%); infection (47.8%); pruritus (39.1%); depression (21.7%); nausea (21.7%) | |
| Open‐label, non‐controlled, prospective cohort study | Active HCV infection and active mixed cryoglobulinaemia. Excluded non‐active mixed cryoglobulinaemia, HIV or HBV active infection and current decompensated cirrhosis | To evaluate safety and efficacy of an oral IFN‐free regimen, sofosbuvir+ribavirin, in HCV‐mixed cryoglobulinaemia | 24 | Sofosbuvir (400 mg/d) + ribavirin (200 to 1400 mg/d) for 24 weeks “Rituximab was used in four cases, in addition to prednisone and plasmapheresis in two patients” | Complete response (improvement of ALL the affected organs involved at baseline) at week 24: 21 (87.5%) HCV RNA clearance at week 24: 22/24 (91.7%) SVR12: 74% Cryocrit: decrease from 0.35 g/L at baseline to 0 at 12 weeks after end‐of‐treatment. C4: increase from 0.1 g/L at baseline to 0.22 g/L at 12 week after end‐of treatment “No difference of outcome was found in patients who received immunosuppressive treatment or not” | Total: 14/24 (54%) Withdrawals: 2 (8%) (hallucination and irritability (1); grade 4 anaemia (1)) Death: 2 (severe pneumonia in the context of B cell lymphoma; pulmonary embolism in the context of hepatocellular carcinoma) Relapses: none Most frequent AE: (fatigue (25%); anaemia (25%); insomnia (21%); infection (17%); alopecia (8%)) |
12SVR ‐ 12 week SVR; 24SVR ‐ 24 week SVR; AE ‐ adverse event; ALT ‐ alanine aminotransferase; AST‐ aspartate aminotransferase; BVAS ‐ Birmingham Vasculitis Activity Score; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; IFN ‐ interferon; MPGN ‐ membranoproliferative glomerulonephritis; PEG ‐ pegylated; SVR ‐ sustained viral response
Overall completeness and applicability of evidence
The main problem of this review is that the treatment of HCV‐associated mixed cryoglobulinaemia has changed during the last months. Treatment with new direct‐acting antivirals has shown high cure rates (Cacoub 2017) but the absence of published or ongoing trials of these new antivirals in this population did not let us include them in our systematic review, although we know that this therapy is nowadays the mainstay of the treatment. Another problem has been that the data was incomplete in several areas despite our comprehensive search and direct contact with all of the authors of published studies; data found sometimes could not have been meta‐analysed.
Quality of the evidence
Due to the lack of studies on the topic that combined antiviral treatment with other interventions or RCTs that evaluate new antivirals that during last year has been demonstrated to be the gold‐standard to treat HCV infection, our conclusions must be considered cautiously because of several potential limitations in the available data.
We are aware that the width of the CIs could reflect a lack of statistical power which is directly related to the sample size of the included studies that evaluated the same outcomes that could be meta‐analysed.
Potential biases in the review process
This review has three main limitations. Firstly, there are currently no RCTs using new antiviral agents on this topic. Secondly, no study included patients in the worse scenario of mixed cryoglobulinaemia (severe renal disease or potential life threatening complications) which imply there is no evidence about the best treatment strategy for these cases. Lastly, the evaluation of other interventions (rituximab, deflazacort or immunoadsorption apheresis) was without being combined with antiviral treatment except in Dammacco 2010. Two out of the three included studies who assessed rituximab (De Vita 2012; Sneller 2012) enrolled patients in whom antiviral therapy had failed to induce remission, either because of a lack of sustained virologic response or regimen‐related toxicity. Limiting our study to only one study with combined therapy made it not possible to assess the efficacy and effect of rituximab on the underlying HCV infection with concomitant antiviral therapy.
Agreements and disagreements with other studies or reviews
No previous systematic reviews have been published on this topic but during the last two years, some narrative reviews assessed this subject (Cacoub 2014; Cacoub 2015; Dammacco 2016; Terrier 2013) and their results did not differ from the ones presented in this review.
Flow chart showing source and identification of studies for inclusion.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Rituximab versus no rituximab, Outcome 1 Death.

Comparison 1 Rituximab versus no rituximab, Outcome 2 Clinical manifestations.

Comparison 1 Rituximab versus no rituximab, Outcome 3 Laboratory findings.

Comparison 1 Rituximab versus no rituximab, Outcome 4 Adverse effects of the medication.

Comparison 1 Rituximab versus no rituximab, Outcome 5 Antiviral therapy failure or not indicated.

Comparison 2 Interferon versus control, Outcome 1 Death.
Comparison 2 Interferon versus control, Outcome 2 Clinical manifestations.
Comparison 2 Interferon versus control, Outcome 3 Laboratory findings.

Comparison 2 Interferon versus control, Outcome 4 Adverse effects of the medication.
Comparison 2 Interferon versus control, Outcome 5 Antiviral therapy failure or not indicated.

Comparison 3 Interferon for 6 months versus 1 year, Outcome 1 Death at 24 months.
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 1 Death at 24 months.
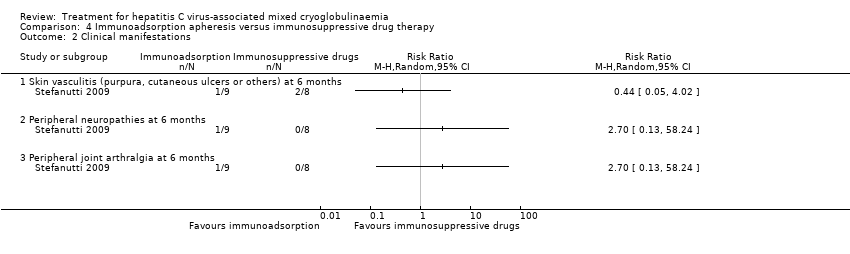
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 2 Clinical manifestations.
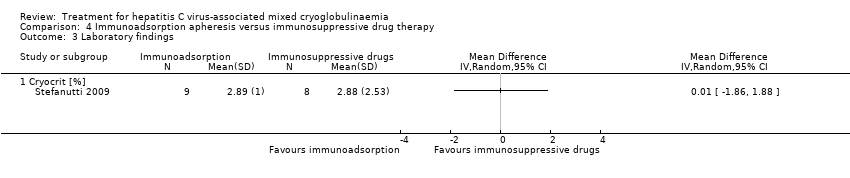
Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 3 Laboratory findings.

Comparison 4 Immunoadsorption apheresis versus immunosuppressive drug therapy, Outcome 4 Adverse effects of the medication.
| Rituximab compared to no rituximab for hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Patient or population: hepatitis C virus‐associated mixed cryoglobulinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no rituximab | Risk with rituximab | ||||
| Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months: number of affected patients | Study population | RR 0.57 | 78 (2) | ⊕⊕⊕⊝ | |
| 26 per 100 | 15 per 100 | ||||
| SCr at 18 months | The mean SCr at 18 months was 194.3 μmol/L | MD was 8.8 μmol/L lower | ‐ | 37 (1) | ⊕⊕⊕⊝ |
| Cryocrit at 12 months | The mean cryocrit at 12 months was 6.55% | MD was 2.01% lower | ‐ | 41 (2) | ⊕⊕⊝⊝ |
| Adverse effects ‐ infusion reactions: number of events | Study population | RR 4.33 | 118 (3) | ⊕⊕⊕⊝ | |
| 0 per 100 | 0 per 100 | ||||
| Activity outcomes | De Vita 2012 found a significant reduction in the BVAS at 2 months in the rituximab group (from mean ± SD: 11.9 ± 5.4 to 7.1 ± 5.7; P < 0.001), and this difference persisted at 6 months (6.9 ± 6.8; P < 0.001), 12 months (5.4 ± 6.2; P 0.0001), and 24 months (4.4 ± 4.6; P < 0.0001). Without differences in the control group. Sneller 2012 BVAS scores became significantly lower in the rituximab group at month 4 (from 10.2 ± 8.4, at 6 months: 0 ± 0; P < 0.02). Without differences in control group. | ‐ | 81 (2) | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 95% CI overlaps no effect, and the CI fails to exclude important benefit or important harm 2 Base on tests of heterogeneity which test the null hypothesis that all studies have the same underlying magnitude of effect, have a low P‐value (P = 0.00001), indicating to reject the null hypothesis I2 statistic, which quantifies the proportion of the variation in point estimates due to among‐study differences, is large (97%) 3 High risk of performance bias: non‐blinded participants and personnel | |||||
| Study ID | Study design | Patient profile | Objective | No. of patients | Treatment | Results | Adverse effects |
| Non‐randomised, non‐controlled prospective study | HCV RNA (+) + cryoglobulinaemic syndrome with organ damage and B‐cell lymphoproliferative syndrome | To assess the hepatovirological response, the clinical and immunological efficacy and the safety of using sofosbuvir‐based direct‐acting antiviral therapy in patients with HCV‐associated mixed cryoglobulinaemia(according to the latest guidelines) | 44 | 1) Sofosbuvir+ribavirin (18) 2) Sofosbuvir+simeprevir (12) ± ribavirin (6 of 12 patients) 3) Sofosbuvir+daclatasvir (4) ± ribavirin (1 patient) 4) Sofosbuvir+ledipasvir (10) ± ribavirin (3 patients) | Hepatovirological response ‐ Undetectable HCV RNA negative rate 100% at week 4; 12SVR and 24SVR remained 100% negative ‐ Decrease of ALT from 77.7 ± 10.3 IU/L at baseline to 27.3 ± 10.3 IU/L at 24SVR ‐ Decrease of AST from 55.2 ± 60.4 IU/L at baseline to 22.6 ± 8.3 IU/L at 24SVR (P < 0.001) Clinical efficacy ‐ Decrease of BVAS from 5.41 ± 3.53 at baseline to 1.27 ± 1.68 at 24SVR (P < 0.001) Immunological efficacy ‐ Decrease of cryocrit level from 7.2 ± 15.4% at baseline to 1.8 ± 5.1% at 24SVR (P < 0.001) | Total: 26/44 (59%) Withdrawals: 1 patient withdrew ribavirin while continuing sofosbuvir+simeprevir Death: none Relapse: none Most frequent AE: Anaemia (13, all receiving ribavirin); fatigue (15); nausea (7) | |
| Case series | Five patients with HVC RNA + with detectable cryoglobulins in plasma and symptomatic mixed cryoglobulinaemia Patient 1: bilateral foot neuropathy and purpura Patient 2: painful left foot drop and purpura Patient 3: purpura and MPGN Patient 4: MPGN Patient 5: MPGN + low grade lymphoma | Review of one centre's experience in treating patients with mixed cryoglobulinaemia with new oral antiviral agents and to assess common factors associated with persistence of mixed cryoglobulinaemia despite SVR | 5 | Patient 1: PEG‐IFN+ribavirin+boceprevir Patient 2: Firstly with rituximab (5 weeks); later with: PEG‐IFN+ribavirin+telaprevir for 4 weeks; PEG‐IFN+ribavirin for 12 weeks; and PEG‐IFN+ribavirin+sofosbuvir for 15 weeks (telaprevir discontinued for persistent viral load > 1000 IU/mL) Patient 3: PEG‐IFN+ribavirin+telaprevir for 47 weeks Patient 4: PEG‐IFN+ribavirin for 24 weeks; afterwards adding sofosbuvir until completing 12 weeks of triple therapy (total 36 weeks) Patient 5: 2 cycles of rituximab: weekly infusions 375mg/m2 during 4 weeks in 2010 then two extra doses of rituximab in 2013 ‐PEG‐IFN+ribavirin+sofosbuvir for 8 weeks in 2014 | Patient 1: complete clearance of virus at week 8; complete clearance of cryoglobulins at week 28. Persistence of neuropathy Patient 2: SVR and no detectable cryoglobulins at month 6 after last triple therapy. Persistence of neuropathy. Patient 3: clearance of HCV at week 4 after PEG‐IFN+ribavirin+telaprevir, with persistent cryoglobulins. Active MPGN and vasculitis after ending of previous treatment (responding to steroid therapy). Patient 4: SVR with persistence of cryoglobulinaemia. Kidney function remained stable. | ||
| Retrospective case series | HCV RNA > 1000 IU/mL + circulating purpura + cutaneous ulcers, Raynaud’s phenomenon, arthralgia, sicca syndrome, gastrointestinal vasculitis, neurologic involvement or renal involvement | Comparison of 2 historical cohorts: one treated with PEG‐IFN and ribavirin and the other sofosbuvir+simeprevir (8/12) or sofosbuvir+ribavirin (4/12). Evaluation of 12SVR, relapses, clinical, immunological (cryoglobulins) and biochemical (AST, Hb) response and adverse effects; without statistical comparison between them | 22 | 1) PEG‐IFN+ribavirin 2) IFN‐free regimens 2a) Sofosbuvir 400 mg/24 h + simeprevir 150 mg/24h 2b) Sofosbuvir 400 mg/24 h + ribavirin (adjusted to kidney function) | PEG‐IFN+ribavirin ‐ SVR12: 1/10 IFN‐free regimens ‐ SVR12: 10/12 (95%) ‐ ALT decreased from 42 U/L at baseline to 20 U/L after treatment ‐ Cryoglobulin levels decreased from 1.5% (0.5% to 4%) at baseline to 0.5% (0% to 2%) after treatment ‐ Decrease of proteinuria in all cases of kidney involvement (table IV) | Total PEG‐IFN+ribavirin: 10/10 IFN‐free regimens: 8/12 Withdrawals PEG‐IFN+ribavirin: 5/10 IFN‐free regimens: 1/12 (anxiety and insomnia) Deaths: none Relapses IFN‐free regimens: 2 (genotype 1 sofosbuvir+simeprevir; genotype 4 sofosbuvir+ribavirin) | |
| Open‐label, non‐controlled, prospective cohort study | Chronic active HCV infection with signs of mixed cryoglobulinaemia. All 23 patients had positive cryoglobulins in plasma at baseline or earlier | To analyse the safety and efficacy of Peg‐IFN‐alpha/ribavirin/protease inhibitor combination in HCV‐mixed cryoglobulinaemia | 23 | Peg‐IFN‐alpha+ribavirin a) + telaprevir (375 mg, 3 times/d for 12 weeks) for 48 weeks (15 patients) b) + boceprevir (800 mg, 3 times/d for 44 weeks) for 48 weeks (8 patients) | Complete clinical responders (improvement in all baseline clinical manifestations): 13 patients (56.5%) at week 24 Virological response (i.e., HCV RNA negative) was of 69.6% at week 24 (P = 0.005). Cryoglobulin level: decreased from 0.44 to 0.06 g/L(P = 0.0006) C4 level: increased from 0.09 to 0.15 g/L (P = 0.045) No significant difference was found between the two treatment regiments | Total: 105 Withdrawals: 8 patients (34.7%) ( virological non‐response (5); virological relapse (2); depression (1)) Death: none Relapse: 1 Most frequent AE: fatigue (87%); neutropenia (78.3%); thrombocytopenia (65.2%); infection (47.8%); pruritus (39.1%); depression (21.7%); nausea (21.7%) | |
| Open‐label, non‐controlled, prospective cohort study | Active HCV infection and active mixed cryoglobulinaemia. Excluded non‐active mixed cryoglobulinaemia, HIV or HBV active infection and current decompensated cirrhosis | To evaluate safety and efficacy of an oral IFN‐free regimen, sofosbuvir+ribavirin, in HCV‐mixed cryoglobulinaemia | 24 | Sofosbuvir (400 mg/d) + ribavirin (200 to 1400 mg/d) for 24 weeks “Rituximab was used in four cases, in addition to prednisone and plasmapheresis in two patients” | Complete response (improvement of ALL the affected organs involved at baseline) at week 24: 21 (87.5%) HCV RNA clearance at week 24: 22/24 (91.7%) SVR12: 74% Cryocrit: decrease from 0.35 g/L at baseline to 0 at 12 weeks after end‐of‐treatment. C4: increase from 0.1 g/L at baseline to 0.22 g/L at 12 week after end‐of treatment “No difference of outcome was found in patients who received immunosuppressive treatment or not” | Total: 14/24 (54%) Withdrawals: 2 (8%) (hallucination and irritability (1); grade 4 anaemia (1)) Death: 2 (severe pneumonia in the context of B cell lymphoma; pulmonary embolism in the context of hepatocellular carcinoma) Relapses: none Most frequent AE: (fatigue (25%); anaemia (25%); insomnia (21%); infection (17%); alopecia (8%)) | |
| 12SVR ‐ 12 week SVR; 24SVR ‐ 24 week SVR; AE ‐ adverse event; ALT ‐ alanine aminotransferase; AST‐ aspartate aminotransferase; BVAS ‐ Birmingham Vasculitis Activity Score; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; IFN ‐ interferon; MPGN ‐ membranoproliferative glomerulonephritis; PEG ‐ pegylated; SVR ‐ sustained viral response | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 One month | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Six months | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 24 months | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 2 Clinical manifestations Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Active urinary sediment at 1 month | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.65] |
| 2.2 Need for dialysis at 24 months | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Skin vasculitis (purpura, cutaneous ulcers or others) at 1 month | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.55, 12.27] |
| 2.4 Skin vasculitis (purpura, cutaneous ulcers or others) at 18 and 24 months | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.16] |
| 2.5 Skin vasculitis (purpura, cutaneous ulcers or others) at 36 months | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.08] |
| 3 Laboratory findings Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum creatinine at 18 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐8.80 [‐29.27, 11.67] |
| 3.2 Serum creatinine at 36 months [µmol/L] | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 17.60 [‐4.23, 39.43] |
| 3.3 Proteinuria at 18 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.42, 0.42] |
| 3.4 Proteinuria at 36 months [g/24 h] | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.84, 1.32] |
| 3.5 Cryocrit at 12 months [%] | 2 | 41 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐10.29, 6.27] |
| 3.6 Serum HCV‐RNA at 12 months [IU/mL] | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐435182.0 [‐1051224.79, 180860.79] |
| 4 Adverse effects of the medication Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [0.76, 24.75] |
| 4.2 Discontinuation of the treatment due to adverse drug reactions | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.22, 4.36] |
| 4.3 Infection (pneumonia, urosepsis) at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.4 Infection (pneumonia, urosepsis) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.12] |
| 4.5 Cardiovascular events (angina, myocardial infarction, heart failure) at 24 months | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.61] |
| 4.6 Gastrointestinal bleeding | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 73.12] |
| 4.7 Haemorrhagic alveolitis | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.12] |
| 4.8 Leukopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.81] |
| 4.9 Thrombocytopenia at 6 months | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.21] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 3 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.77, 1.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 12 months | 4 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 24 months | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical manifestations Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 12 months | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.00] |
| 3 Laboratory findings Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum creatinine at 18 months [µmol/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐30.32 [‐80.59, 19.95] |
| 3.2 Proteinuria at 18 months [g/24h] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐2.89, ‐1.07] |
| 3.3 ALT or GPT at 6 months [UI/L] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐5.89 [‐55.77, 43.99] |
| 3.4 ALT or GPT at 18 months [UI/L] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐28.28 [‐48.03, ‐8.54] |
| 3.5 Rheumatoid factor activity at 6 months [UI/mL] | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 97.0 [‐187.37, 381.37] |
| 3.6 C4 at 18 months [mg/dL] | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐2.74, 2.67] |
| 3.7 IgM at 18 months [mg/dL] | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐595.75 [‐877.20, ‐314.30] |
| 3.8 Cryocrit at 6 months [%] | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.38, ‐0.38] |
| 4 Adverse effects of the medication Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 27.82 [1.72, 449.18] |
| 4.2 Discontinuations of the treatment due to adverse drug reactions | 4 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.91, 5.90] |
| 5 Antiviral therapy failure or not indicated Show forest plot | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 24 months Show forest plot | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical manifestations Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Skin vasculitis (purpura, cutaneous ulcers or others) at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Peripheral neuropathies at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Peripheral joint arthralgia at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Laboratory findings Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Cryocrit [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects of the medication Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infusion reactions | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

