Использование наружных дренажей и отказ от дренирования у пациентов с хронической субдуральной гематомой через трепанационное отверстие
Abstract
Background
Chronic subdural haematoma (CSDH) is one of the most common types of intracranial haematoma, and often occurs in older people. Burr‐hole craniostomy, which is an evacuation through one or two burr holes drilled over the site of the haematoma, has been widely accepted as the most effective way to manage CSDH. Recurrences are a major problem and need reoperation, sometimes repeatedly.
Objectives
To assess the effects and safety of the use of external drains versus no drains after burr‐hole evacuation for the treatment of CSDH in adults.
Search methods
We ran our first search on 27 November 2014. We searched the Cochrane Injuries Group's Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library), MEDLINE (OvidSP), Embase Classic+Embase (OvidSP), PubMed, ISI WOS (SCI‐EXPANDED, SSCI, CPCI‐S and CPSI‐SSH), Chinese databases, and clinical trials registers, and screened reference lists. In compliance with the MECIR conduct standard 37, the Cochrane Injuries Group Information Specialist ran an update search within 12 months of publication (25 April 2016). We have screened these results but not incorporated the findings into the current review; as a result of the update search, one trial is awaiting classification.
Selection criteria
We included randomized controlled trials (RCTs) that compared external subdural drains with no drains after burr‐hole evacuation for the treatment of CSDH in adults.
Data collection and analysis
Two review authors identified potential articles from the literature search, extracted data independently using a data extraction form and assessed risk of bias using the Cochrane ‘Risk of bias' tool. For dichotomous data, where statistical heterogeneity was low, we calculated summary risk ratios with 95% confidence intervals using a fixed‐effect model.
Main results
Nine RCTs, including a total of 968 participants, reported outcomes specified by this review. Only one RCT reported the use of an adequate method of allocation concealment; this trial was a large, single‐centre, high quality study and was adequately reported. All included trials reported a reduced recurrence of CSDH with external subdural drains. We found a significant reduction in the risk of recurrence with subdural drains (RR 0.45, 95% CI 0.32 to 0.61, I2 = 38%; 9 studies, 968 participants; moderate‐quality evidence). There was no strong evidence of any increase in complications (RR 1.15; 95% CI 0.77 to 1.72, I2 = 0%; 7 studies, 710 participants; low‐quality evidence), mortality (RR 0.78, 95% CI 0.45 to 1.33, I2 = 22%; 5 studies, 539 participants; low‐quality evidence), or poor functional outcome (which included deaths) (RR 0.68, 95% CI 0.44 to 1.05, I2 = 31%; 5 studies, 490 participants; low‐quality evidence).
Authors' conclusions
There is some evidence that postoperative drainage is effective in reducing the symptomatic recurrence of CSDH. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Due to the low quality of the evidence for the secondary outcomes, the effect of drainage on the occurrence of surgical complications, mortality and poor functional outcome is uncertain. This uncertainty can be clarified with data from high‐quality studies which may be conducted in the future. There is no strong evidence of any increase in complications when drains are used.
PICOs
Резюме на простом языке
Установка дренажей у взрослых после хирургического вмешательства по поводу хронической субдуральной гематомы
Что такое хроническая субдуральная гематома (ХСГ)?
Хроническая субдуральная гематома (ХСГ) представляет собой скопление крови между мозгом и оболочками, окружающими его. ХСГ ‐ это распространенный тип (внутричерепной) гематомы, которая чаще всего встречается у пожилых людей. До 75% ХСГ возникают вследствие травмы головы, при этом травма может быть незначительной, не сопровождаясь потерей сознания, рвотой, судорогами, или какими‐либо другими посттравматическими симптомами. Симптомы ХСГ зависят от размера гематомы и участков мозга, на которые она оказывает давление. Она может сопровождаться спутанностью сознания или комой; проблемами с памятью; трудностями речи, глотания или ходьбы; сонливостью; головными болями; судорогами; а также слабостью или онемением конечностей и лица.
Как лечится ХСГ?
Наиболее эффективным способом лечения ХСГ является хирургическая операция, при которой эвакуация гематомы осуществляется через трепанационное отверстие. При выполнении этой процедуры одно или два отверстия (от 5 до 30 мм) просверливают в черепе над гематомой. Скопившиеся кровяные сгустки удаляют через сформированные отверстия. Иногда, в конце операции, хирурги устанавливают мягкий силиконовый дренаж через трепанационное отверстие для продолжения дренирования полости, которая была заполнена кровью (субдуральный дренаж). Эти дренажи эвакуируют жидкость, накапливающуюся в наружных собирательных сосудах, и оставляются на 24 до 48 часов после операции, до того как будут удалены. Альтернативный вариант, в конце операции, раны закрываются хирургически без установки наружных дренажей.
Почему дренажи могут быть полезны?
Рецидив (то есть формирование другой ХСГ в том же самом месте) является главной проблемой у больных с ХСГ, и пациентам может понадобиться дополнительная, повторная хирургическая процедура для ее удаления. Дренаж может уменьшить вероятность рецидива, но не используется рутинно. Авторы этого Кокрейновского обзора хотели выяснить, действительно ли внешнее дренирование после операции с трепанационным отверстием по поводу ХСГ снижает вероятность рецидива.
Характеристика исследований и основные результаты
Авторы обзора провели обширный поиск медицинской литературы по ноябрь 2014 года на предмет рандомизированных контролируемых испытаний (РКИ), соответствующих критериям включения, которые обеспечивают наиболее надежные доказательства. Они нашли девять РКИ, включающих 968 пациентов, в которых сравнивалось использование наружных дренажей у некоторых пациентов и отказ от дренирования у других, после операций с созданием трепанационного отверстия у больных ХСГ. Исследования были проведены в Индии, Турции, Иране, Германии, Великобритании и Японии. Все участники были взрослые люди, в основном старше 60 лет. Во всех исследованиях выполнялись схожие операции. Шесть исследований проводились в течение шести месяцев, остальные в течение трех, одного месяца и трёх недель (по одному для каждого).
Авторы исследований смогли статистически объединить результаты испытаний, которые показали, что использование дренажей сокращает риск рецидива ХСГ после создания трепанационных отверстий примерно на 50% по сравнению с группой пациентов, которым не были установлены дренажи (контрольная группа). Однако, не было обнаружено четкой разницы по сравнению дренирования и не дренирования в группах пациентов по числу послеоперационных осложнений (таких как: инфекционные осложнения, судороги или спонтанные кровотечения), летальных исходов или функциональных результатов (например, восстановление функций, нарушенных за счет ХСГ).
Качество доказательств
Результаты этого обзора могут быть изменены в будущем, когда появятся данные дополнительных исследований. В проведенных исследованиях или слишком мало участников или вмешательств, чтобы показать достоверные результаты, даже при их статистической обработке. В некоторых исследованиях не описан процесс рандомизации в деталях, поэтому они считаются не достаточно достоверными.
Будущие исследования также помогут установить:
‐ влияние установки наружных дренажей на возникновение послеоперационных осложнений, летальность и функциональные результаты;
‐ лучше устанавливать один или два дренажа при выполнении хирургического вмешательства;
‐ лучший способ расположения дренажных трубок в головном мозге;
‐ длительность оставления дренажа.
Authors' conclusions
Summary of findings
| Drains compared to no drains for burr‐hole evacuation of CSDH in adults | ||||||
| Patient or population: adults with burr‐hole evacuation of CSDH Settings: hospital settings in India,Turkey, Iran, Germany, UK and Japan | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No drain | Drain | |||||
| Overall recurrence | Study population | RR 0.45 | 968 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 97 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 104 per 1000 | |||||
| Recurrence with 2 burr holes (subgroup) | Study population | RR 0.46 | 306 | ⊕⊕⊝⊝ | ||
| 216 per 1000 | 99 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 106 per 1000 | |||||
| Recurrence with 1 burr hole (subgroup) | Study population | RR 0.17 | 156 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 36 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 36 per 1000 | |||||
| Recurrence with 1 or 2 burr holes (subgroup) | Study population | RR 0.52 | 506 | ⊕⊕⊕⊝ | ||
| 218 per 1000 | 113 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 111 per 1000 | |||||
| Complications | Study population | RR 1.15 | 710 | ⊕⊕⊝⊝ | ||
| 110 per 1000 | 127 per 1000 | |||||
| Moderate | ||||||
| 100 per 1000 | 115 per 1000 | |||||
| Mortality | Study population | RR 0.78 | 539 | ⊕⊕⊝⊝ | ||
| 100 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 44 per 1000 | |||||
| Poor functional outcome (includes death) | Study population | RR 0.68 | 490 | ⊕⊕⊝⊝ | ||
| 169 per 1000 | 115 per 1000 | |||||
| Moderate | ||||||
| 131 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one trial used adequate allocation concealment, and one trial used alternation as a randomisation method (Wakai 1990). | ||||||
Background
Description of the condition
Chronic subdural haematoma (CSDH; a collection of blood between the brain and its outer membrane that forms slowly) is one of the most common clinical entities in daily neurosurgical practice. It is generally a disease of older people, but also occurs during infancy.
The incidence of CSDH increases greatly with age and ranges from approximately 5 per 100,000 per year in the general population (Santarius 2004), to 8 to 58 per 100,000 per year in those over 65 years of age (Cousseau 2001). The proportion of people aged 65 years and older in the population is expected to double worldwide between 2000 and 2030 (Kinsella 2001), so interest in the best treatment for CSDH will increase.
Older people are at particular risk of CSDH because the brain atrophies with age (due to brain cell death). Pathogenesis of CSDH in older people is considered to be linked to gradual, age‐related atrophying of the brain that leads to tension on the dural border cell layer and on the points where veins traverse the dura. Even minor trauma may cause one of these veins to tear. Slow bleeding from the low‐pressure venous system can enable large haematomas to form before clinical signs appear (Haines 1993).
Most CSDHs are probably caused by head injury. A history of trauma has been obtained in 56% to 75% of patients (Fogelholm 1975a; Richter 1984; Ramachandran 2007), although the trauma is frequently trivial (i.e. involving no loss of consciousness, no vomiting, seizures or any post‐traumatic sequelae). Acute subdural haematoma (sudden formation of a subdural haematoma), with or without surgical intervention, can also lead to CSDHs. Other predisposing factors include chronic alcoholism, coagulopathy (problems with coagulation), use of anticoagulants, seizure disorders, and cerebrospinal fluid (CSF) shunts (Fogelholm 1975b; Meagher 2013).
CSDH is not uncommon in infants, but is relatively rare after childhood. In infants, unlike adults, the original blood clot is rarely encapsulated by a fibrous membrane forming a 'subdural hygroma' of similar shape and location to the original haematoma. Infant CSDH more commonly features a widespread fluid accumulation (Squier 2009).
Development of such chronic subdural fluid collections is not well understood. Suggested mechanisms include: osmotic accumulation of fluid and repeated haemorrhage into the healing granulating membrane, or continued exudation, or inflammation of the dural border cell layers (Markwalder 1981). Leakage of CSF through the subarachnoid membrane is considered to play a significant role in chronic subdural fluid collections (Stroobandt 1978).
Description of the intervention
In general, treatment for this disorder is surgical evacuation (removal) and three techniques are most often used: twist‐drill craniostomy, which produces a hole with a diameter less than 5 mm; burr‐hole craniostomy, which produces a hole with a diameter of 5 mm to 30 mm; and craniotomy in which a portion of the skull is removed to gain access to the haematoma (Mohamed 2003; Muzii 2005; Horn 2006). A meta‐analysis showed that all three techniques have about the same mortality rate (2% to 4%) (Weigel 2003). Craniotomy is associated with a much higher morbidity than craniostomy (12.3% versus 3% to 4%); while recurrence of CSDH is much higher with twist‐drill craniostomy than with burr‐hole craniostomy (33% versus 12.1%), or with craniotomy (33% versus 10.8%) (Santarius 2008). Burr‐hole craniostomy, an evacuation via one or two burr holes drilled over the site of the haematoma, with or without drainage, is the most popular surgical technique worldwide. After the holes have been drilled, the subdural collection of blood is washed out with warm saline. Then the holes are sealed surgically, or a drain is inserted for 24 h to 48 h.
How the intervention might work
To prevent recurrences, some neurosurgeons insert a subdural drain, which can be temporary (subdural‐to‐external) (Gelabert‐González 2005), or permanent (subdural‐to‐peritoneal) (Santarius 2010). Evidence from the last 20 years suggests that use of postoperative drains after burr‐hole evacuation of a primary CSDH is associated with a lower recurrence risk (Wakai 1990; Lind 2003; Ramachandran 2007). Surgical techniques, haematoma irrigation and drainage, and insufficient intraoperative and postoperative drainage have been suggested as potential contributors to increased risk of recurrence after surgery.
Why it is important to do this review
Although most CSDHs resolve following a single neurosurgical intervention, approximately 5% to 30% of haematomas recur and require re‐rinsing of the subdural fluid space, sometimes repeatedly (Weigel 2003). Drains may reduce recurrence, but are not used routinely. There is little data available in the literature to confirm the impact of using drainage after evacuation and irrigation of the cavity containing the CSDH on the outcome of the patients. It is time to provide clinicians with the best available evidence for the efficacy of external drainage after burr‐hole evacuation for the treatment of CSDH, particularly as a large rise in CSDH incidence is expected within the next twenty years (Cousseau 2001).
Objectives
To assess the effects and safety of the use of external drains versus no drains after burr‐hole evacuation for the treatment of CSDH in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing external subdural drains with no drains after burr‐hole evacuation for the treatment of CSDH.
Types of participants
People over 18 years of age, in any healthcare setting, who have been clinically diagnosed with CSDH and who have had a burr‐hole evacuation.
Types of interventions
Intervention: external subdural drainage after burr‐hole evacuation.
Comparison: no drainage.
Types of outcome measures
Primary outcomes
The primary outcome is risk of symptomatic recurrence, defined as reappearance of symptoms and signs attributable to an ipsilateral haematoma identified on a computed tomography (CT) scan within six months of the original drainage procedure.
Secondary outcomes
-
Mortality: all‐cause mortality at six months after operation.
-
Postoperative complications: all medical and surgical complications, such as subdural empyema (collection of pus), infection, cardiopulmonary complications.
-
Poor functional outcomes: defined as death or significant dependence in daily activities at six months (4 to 5 on modified Rankin scale (Van Swieten 1988); or 1 to 3 on the Glasgow Outcome Scale Extended (Teasdale 1974); or less than 15 on the Glasgow Coma Scale (Jennett 1975)).
Sample size calculation
Assuming a recurrence risk of 20% for high‐risk patients which reduces to 10% with external subdural drainage after burr‐hole evacuation, using standard alpha (0.05) and beta (0.10) values, the required sample size is 438 patients in total.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date, or publication status.
Electronic searches
The Cochrane Injuries Group Information Specialist searched the following sources:
-
Cochrane Injuries Group Specialised Register (27 November 2014);
-
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library; 2014 Issue 10);
-
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to 27 November 2014);
-
Embase Classic + Embase (OvidSP) (1947 to 27 November 2014);
-
PubMed (27 November 2014);
-
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to November 2014);
-
ISI Web of Science: Conference Proceedings Citation Index‐ Science (CPCI‐S) (1990 to November 2014);
-
Clinicaltrials.gov (clinicaltrials.gov) (accessed 27 November 2014);
-
WHO International Clinical Trials Registry Platform (who.int/ictrp/en/) (accessed 27 November 2014).
In compliance with the MECIR conduct standard 37, the Cochrane Information Specialist ran an update search within 12 months of publication (25 April 2016). We have screened these results, but not incorporated the findings into the current review.
The authors searched the following:
-
The Chinese Biomedical Database (CBM) (1 August 2016);
-
The Chinese National Knowledge Infrastructure (CNKI) (1 August 2016);
-
China Online Journals (1 August 2016).
Search strategies are reported in (Appendix 1 and Appendix 2). We adapted the MEDLINE search strategy as necessary for the other databases. We added the Cochrane Highly Sensitive Search Strategy for identifying randomized trials to the MEDLINE search strategy, and added the search strategy study design terms as used by the UK Cochrane Centre to the Embase strategy (Lefebvre 2011).
Changes to search methods are noted in Differences between protocol and review.
Searching other resources
We screened the reference lists of relevant published papers and all included studies.
Data collection and analysis
Selection of studies
The Cochrane Injuries Group Information Specialist collated the results of the electronic database searches and removed duplicates.
Two review authors (Zhu Y and Peng D) independently performed a preliminary screening of titles and abstracts considering the type of trial, participants, and intervention. In the event of disagreement, we would have discussed any differences of opinion. If disagreement persisted, we would have selected trials for inclusion on the basis of the full‐text article. We assessed the full‐text articles of the selected trials to examine eligibility and had no disagreements about inclusion. As stated in our protocol, if we had had any disagreements, we would have consulted our colleague, Ruxin Xing, who is an experienced author.
Data extraction and management
Using a predesigned form for this review, the two review authors (Zhu Y and Peng D) extracted information independently on aspects of trial design and risk of bias (including method of randomisation and allocation concealment, blinding of participants, blinding of outcome assessment, incomplete follow‐up, selective reporting, and other sources of bias), study characteristics (types of participants, interventions, and outcomes), as well as outcome data, and compared their results.
Assessment of risk of bias in included studies
We evaluated each included trial for risk of bias in six domains (i.e. sequence generation, allocation concealment, blinding, incomplete outcome data, potential for selective reporting and other sources of bias); we rated each domain as being at low, high or unclear risk of bias. Two review authors (Zhu Y and Peng D) made assessments for each domain independently using the 'Risk of bias' assessment tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
For dichotomous data, we calculated the risk ratio (RR) and 95% CI. We calculated the mean difference (MD) and 95% CI for continuous outcomes that were measured on the same scale; for continuous outcomes measured on different scales we planned to calculate the standardised mean difference.
Unit of analysis issues
We included trials where the unit of allocation was the individual participant; we did not exclude any trials on this basis, as all trials randomized individual participants.
Dealing with missing data
For included studies, we noted levels of attrition. We defined ‘high levels’ of missing data as more than 20% of participants lost to follow‐up. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis, which showed our result was robust. For all outcomes, we carried out analyses based on available data.
Assessment of heterogeneity
We explored both clinical and statistical heterogeneity. We examined trial characteristics in terms of participants, interventions, and outcomes for evidence of clinical heterogeneity. We planned to assess statistical heterogeneity using the Chi2 test, which examines the presence of heterogeneity, and the I2 statistic, which assesses the extent of inconsistency between trials (Higgins 2003). We considered trials to be statistically heterogeneous if the I2 statistic value was over 50% and the P value was less than 0.10 in the Chi2 test (Deeks 2011). We explored possible sources of heterogeneity through sensitivity analysis.
Assessment of reporting biases
When a sufficient number of trials (10 or more) becomes available, we plan to assess publication bias by using funnel plots (Sterne 2011).
Data synthesis
We performed statistical analysis using Review Manager 2014 (RevMan) and conducted meta‐analysis where the included trials were clinically homogeneous and there was no evidence of substantial statistical heterogeneity (I2 statistic value less than 75%, P value greater than 0.10) (Deeks 2011). For dichotomous outcomes, we planned to use the Mantel‐Haenszel method and to report the results as RR with 95% CI. We planned to provide a narrative summary of study results if it was not possible to pool the data (I2 statistic value was over 75% and the P value was less than 0.10 in the Chi2 test).
Subgroup analysis and investigation of heterogeneity
If significant heterogeneity had been present, and the number of included studies had been sufficient, we planned to explore the effects of different study characteristics to determine possible causes of heterogeneity.
Had there been enough studies, we planned to perform subgroup analyses of:
-
different age groups;
-
severity of brain injury (severe = GCS 8 or less, moderate = GCS 9 to 12, mild = GCS 13 or over) (Teasdale 1974);
-
number of burr holes (one or two);
-
type of drain (inserted frontal‐ward or occipital‐ward, passive or active drainage);
-
anticoagulation treatment.
Sensitivity analysis
We planned to test the robustness of the evidence by performing sensitivity analyses of pooled data in which we repeated the analysis excluding studies:
-
at high or unknown risk of selection bias (due to inadequate methods of allocation concealment);
-
with missing data; and
-
comparing the results of the fixed‐effect model to the random‐effects model (robust evidence should not be reversed by changing the model).
Summarising and interpreting results
We used the GRADE approach to assess the quality of evidence for each of the key outcomes. We used the GRADE profiler to import data from RevMan and create 'Summary of findings' tables (GRADEpro GDT 2014; Review Manager 2014).
In our assessments of the overall quality of evidence for each outcome that included pooled data, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
The search was performed by the Cochrane Injuries Group's Information Specilaist (27 November 2014). The search identified 4610 records from the databases outlined in Electronic searches. There were 4562 records after duplicates were removed. We screened all titles and abstracts for inclusion in the review. We excluded 4406 obviously ineligible studies at this point, and assessed 156 full‐text articles for eligibility. Following this, we excluded 138 texts. One record was identified through the reference lists of included trials (Ahmed 2011). We included nine trials in the qualitative and quantitative syntheses (see Figure 1 and Characteristics of included studies). We did not identify any potentially eligible trials from other sources.
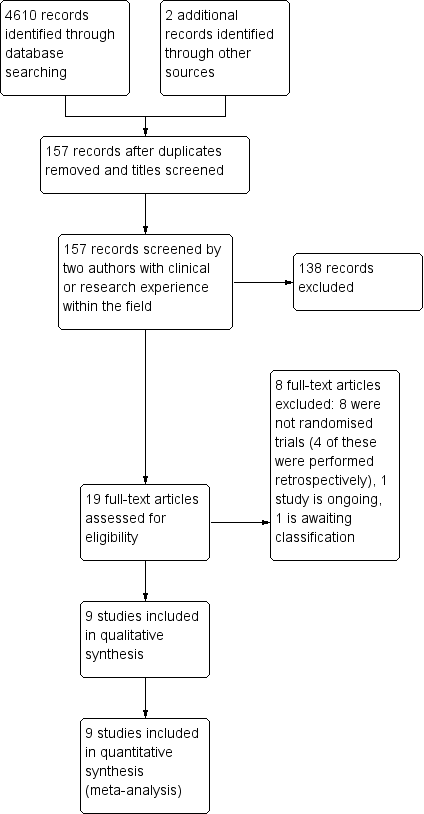
Study flow diagram
In total, we included nine trials (Laumer 1989; Wakai 1990; Tsutsumi 1997; Erol 2005; Santarius 2009; Ahmed 2011; Javadi 2011; Kutty 2014; Singh 2014) and excluded eight (Hennig 1999; Williams 2001; Okada 2002; Lind 2003; Gurelik 2007; Kiymaz 2007; Sindou 2010; Sarnvivad 2011). We identified one ongoing trial (NCT01785797).
From the updated search (25 April 2016) we screened 496 new references and found one new study, which we have placed in Studies awaiting classification. This study will be incorporated into the next version of the review, if appropriate, once we have obtained details of the randomisation process.
Included studies
See the Characteristics of included studies for details of the nine included studies.
Design
Six trials described the method of randomisation. Erol 2005, Laumer 1989 and Javadi 2011 did not describe any details of randomisation. All trials had two groups (one drain group and one control group) except Laumer 1989, which had three groups, one of which concerned internal drains, and so was not included in our analysis. Only Santarius 2009 reported the method of allocation concealment. All nine trials were conducted in single centres.
Sample sizes
Ahmed 2011 recruited 51 participants. Erol 2005 recruited 70 participants. Javadi 2011 recruited 40 participants. Kutty 2014 recruited 140 participants. Laumer 1989 recruited 144 participants. Santarius 2009 recruited 215 participants. Singh 2014 recruited 200 participants. Tsutsumi 1997 recruited 118 participants. Wakai 1990 recruited 38 participants.
Setting
Ahmed 2011, Singh 2014 and Kutty 2014 were conducted in India. Erol 2005 was conducted in Turkey. Javadi 2011 was conducted in Iran. Laumer 1989 was conducted in Gemany. Santarius 2009 was conducted in UK. Tsutsumi 1997 and Wakai 1990 were conducted in Japan.
Participants
All trials recruited adults with symptomatic CSDH proven by computed tomography (CT) or magnetic resonance imaging (MRI) scan. Five trials excluded people with ipsilateral haematomas who had undergone CSF diversion, or in whom surgery other than burr‐hole evacuation was indicated, or who did not need surgical treatment because of the size of CSDH or their clinical status, or had been operated on once or more for previous CSDH, or had calcified or ossified CSDHs (Tsutsumi 1997; Santarius 2009; Ahmed 2011; Javadi 2011; Singh 2014). Kutty 2014 and Wakai 1990 excluded people with bleeding diathesis or using oral anticoagulants. Two trials did not report the details of exclusion criteria (Laumer 1989; Erol 2005). There were more men than women in all the trials. The age and sex distribution of the participants was clear in all studies except for Laumer 1989 and Singh 2014.
Interventions
All interventions were very similar, with the biggest difference from a surgical viewpoint being the number of burr holes used. Single (Wakai 1990; Tsutsumi 1997), or double (Santarius 2009; Ahmed 2011; Javadi 2011), burr holes were drilled over the maximum width of the haematoma. In three trials the number of burr holes to be made was left to the discretion of the operating surgeon (Laumer 1989; Erol 2005; Singh 2014), but in Kutty 2014 the surgeon drilled one hole for participants in the drains group, and two holes for participants in the no‐drains group. Surgery was done under local or general anaesthesia. The dura mater was opened with a cruciate incision, and coagulated with bipolar diathermy. The subdural collection of blood was washed out with warm Ringer's lactate saline or normal saline. When a participant was assigned to the no‐drain group, the subdural space was filled with Ringer's lactate saline or normal saline and the scalp closed in two layers. Those assigned to the drain group had a soft silicone drain inserted into the subdural space through the burr hole, and tunnelled for a minimum of 5 cm away from the scalp incision. The subdural space was filled with Ringer's lactate saline or normal saline and the scalp was closed in two layers. The drain was connected to a soft collection bag that was kept for 24 h to 48 h and then removed. Bilateral CSDHs were treated as one case, and both sides received the same treatment.
Outcomes
In all nine trials the primary outcome measure was the risk of recurrence, defined as the risk of reoperation to treat recurrent chronic subdural haematoma in a participant. However, two studies performed CT scanning in all participants, with and without symptoms, within a month of surgery, and defined recurrent CSDH as either worsening neurological symptoms or haematoma increase on CT (Laumer 1989; Erol 2005). The other seven trials used the presence of both worsening neurological symptoms and haematoma increase on CT as an indication for reoperation.
The secondary outcomes included surgical complications (subdural empyema, acute haematoma, meningitis, seizure, fever, cardiopulmonary complications, etc.); mortality and clinical outcomes measured by the Glasgow Outcome Score (GOS), and Modified Rankin Score (MRS); and neurological deficits (i.e. limb weakness or dysphasia). Tsutsumi 1997 and Kutty 2014 did not report complications, but we analyzed complication risk in the other seven studies. Three studies did not report mortality and clinical outcome (Laumer 1989; Tsutsumi 1997; Kutty 2014).
Follow‐up in the Laumer 1989 study was only three weeks in duration. Erol 2005 used a follow‐up of one month, Kutty 2014 used a follow‐up of three months; follow‐up in the other six trials was six months.
Excluded studies
We excluded eight studies for reasons detailed in Characteristics of excluded studies. None of the excluded trials were RCTs, and some were done retrospectively (Okada 2002; Lind 2003; Kiymaz 2007; Sarnvivad 2011).
Risk of bias in included studies
See Figure 2 and Figure 3 for summaries of the results of the ‘Risk of bias' assessment.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.
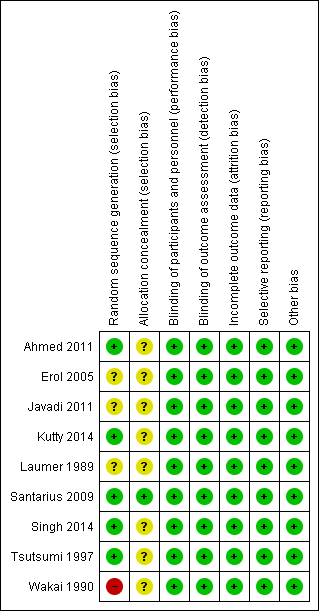
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged that five of the nine included trials used adequate methods of randomisation with regard to sequence generation (Tsutsumi 1997; Santarius 2009; Ahmed 2011; Kutty 2014; Singh 2014). Another three trials were described as randomized (Erol 2005; Javadi 2011; Laumer 1989), but the methods of random sequence generation used were not clear from their trial reports. One trial used an alternate method of randomisation by assigning participants sequentially to drains or no‐drains (Wakai 1990), this method of allocation has a high risk of bias. Only one trial described the use of sealed envelopes labelled with sequential study numbers (Santarius 2009), and so was rated as being at low risk of bias for this domain; we rated the other eight trials as being at unclear risk of bias.
Blinding
The nature of this intervention did not allow for masking of treatment allocation, so performance bias was inevitable in these nine trials. Outcome assessment for recurrence (which was defined as both worsening of neurological symptoms and haematoma increase on CT scan), mortality and complications of operation was objective, so, with the exception of Santarius 2009 and Javadi 2011, no studies used blinding. We judged that the performance bias and detection bias had little effect on the outcome assessment, and thus each trial was at low risk of bias for this domain.
Incomplete outcome data
All the included trials reported our primary outcome with no missing data, and only the Santarius 2009 trial had data missing for secondary outcomes. In the trial report the authors said that 50 participants did not respond to the postal questionnaire and one no longer wished to take part. Since the missing data were due to failure to respond to the postal questionnaire, we did not write to the trialists, as they would not have had the data. We performed analyses on both an intention‐to‐treat (ITT) basis and per protocol basis (PP) for missing data for poor functional outcome. When we performed a sensitivity analysis by removing the Santarius 2009 trial, the result was still robust (Analysis 1.5).
Selective reporting
We were able to check only one registered protocol (Santarius 2009), and found no reporting bias. Although we could not check the protocols, a further six trials reported important data (such as recurrence, complications, outcome, and mortality), therefore we judged the potential for reporting bias to be low for these seven trials. Two trials, Tsutsumi 1997 and Kutty 2014, reported our primary outcome only, but we found no selective reporting (although important outcomes, such as complications and mortality, were not reported).
Other potential sources of bias
We judged all nine trials to be at low risk of bias for this domain.
Effects of interventions
Risk of recurrence
The nine trials included a total of 968 participants. There were 47 cases of recurrent CSDH among 492 participants (9.6%) with a drainage system in place compared with 103 cases of recurrent CSDH in the 476 participants without a drainage system in place (21.6%). A fixed‐effect meta‐analysis showed a pooled RR of 0.45 (95% CI 0.32 to 0.61; P < 0.00001; I2 = 38%; Analysis 1.1). There was no indication of substantial statistical heterogeneity between the results of the different trials (Chi2 = 12.89; P = 0.12).
Sensitivity analysis
We performed a sensitivity analysis by removing the two short‐term studies (Laumer 1989; Erol 2005), and found a pooled RR of 0.33 (95% CI 0.22 to 0.50; P < 0.00001; I2 = 0%; Analysis 1.5). There was no indication of statistical heterogeneity between the results of the different trials (Chi2 = 4.28; P = 0.64). Since only one trial used adequate allocation concealment (Santarius 2009), we did not perform a sensitivity analysis in which we excluded studies with a high risk of bias for allocation concealment. We rated the quality of the evidence for this outcome as moderate because of risk of bias.
Subgroup analyses
We performed additional subgroup analysis according to the numbers of holes used during surgery. Three trials used double holes over the maximum width of the haematoma (Santarius 2009; Ahmed 2011; Javadi 2011), while two used single holes (Wakai 1990; Tsutsumi 1997), and the remaining four trials used a mixture of one or two holes. The three subgroups all showed a lower risk of recurrence in the drains treatment group. A fixed‐effect meta‐analysis of double holes showed a pooled RR of 0.46 (95% CI 0.26 to 0.80; P = 0.006; I2 = 0%). There was no indication of statistical heterogeneity between the results of the different trials (Chi2 = 1.11; P = 0.57; Analysis 1.1). We rated the quality of the evidence for this outcome as low for risk of bias and imprecision (estimate based on few events and wide CIs). A fixed‐effect meta‐analysis of single holes showed a pooled RR of 0.17 (95% CI 0.05 to 0.56; P = 0.004; I2 = 0%). There was no indication of statistical heterogeneity between the results of the different trials (Chi2 = 0.02; P = 0.88; Analysis 1.1). We rated the quality of the evidence for this outcome as low for risk of bias and imprecision (estimate based on few events and wide CIs). A fixed‐effect meta‐analysis of single or double holes showed a pooled RR of 0.52 (95% CI 0.34 to 0.79; P = 0.002; I2 = 63%; Analysis 1.1). We rated the quality of the evidence for this outcome as moderate for risk of bias. The sensitivity analysis showed that the two short‐term studies, Laumer 1989 and Erol 2005 were the source of the heterogeneity (Analysis 1.5).
We did not perform other subgroup analyses because the trials did not offer sufficiently detailed data.
Secondary outcomes
Complications
Two trials did not report complications (Tsutsumi 1997; Kutty 2014). We analyzed the remaining seven studies for risk of complications. These seven trials included 710 participants. There were 45 complications amongst 357 participants with drains (12.6%) compared with 39 complications among 353 participants without drains (10.8%). A fixed‐effect meta‐analysis showed a pooled RR of 1.15 (95% CI 0.77 to 1.72; P = 0.50; I2 = 0%; Analysis 1.2). No indication of statistical heterogeneity between the results of the different trials was present (Chi2 = 5.63; P = 0.47). We downgraded the quality of the evidence for this outcome from high to low quality because of risk of bias and imprecision (estimate based on few events and wide CIs).
Mortality
Five studies with a total of 539 participants could be analyzed for mortality at six months. There were 21 deaths among 270 participants with drains (7.8%), compared with 27 deaths among 269 participants without drains (10%). A fixed‐effect meta‐analysis showed a pooled RR of 0.78 (95% CI 0.45 to 1.33; P = 0.35; I2 = 22%; Analysis 1.3). No indication of statistical heterogeneity between the results of the different trials was present (Chi2 = 5.11; P = 0.28). There were few missing values for the outcome measures (less than 2.5%), so incomplete data for a response variable will not bias the results. We downgraded the quality of the evidence for this outcome from high to low quality because of risk of bias and imprecision (estimate based on few events and wide CIs).
Poor functional outcome
We defined poor functional outcomes as death or significant dependence in daily activities at six months (see Types of outcome measures for details). The figures presented here are for the combined outcome of death plus disability/dependence.
Five studies could be analyzed for poor functional outcome at six months. There were 27 poor outcomes among 241 participants with drains (11.2%), compared with 42 poor outcomes among 249 participants without drains (16.9%). A fixed‐effect meta‐analysis showed a pooled RR of 0.68 (95% CI 0.44 to 1.05; P = 0.08; I2 = 31%; Analysis 1.4). No indication of statistical heterogeneity between the results of the different trials was present (Chi2 = 5.77; P = 0.22). We downgraded the outcome from high quality to low quality for risk of bias and imprecision (estimate based on few events and wide CIs).
Discussion
This systematic review summarises the evidence from randomized controlled trials (RCTs) of external drains versus no drains after burr‐hole evacuation for the treatment of chronic subdural haematoma in adults.
Summary of main results
Nine RCTs with a total of 968 participants met the inclusion criteria for this review. These trials were homogeneous in their objectives, participant characteristics, operative method, and outcome measures. Only one trial reported the use of adequate allocation concealment methods (Santarius 2009); this was a large, single‐centre, high‐quality and adequately reported RCT. The effect of external subdural drains on the recurrence risk for chronic subdural haematomas (CSDH) was reported in all the included trials. We found a statistically significant reduction in the risk ratio (RR) for recurrence when a subdural drain was fitted (RR 0.45, 95% CI 0.32 to 0.61). Heterogeneity between the results of the different trials was acceptable (Chi2 = 12.89; P = 0.12; I2 = 38%). The result was robust even when we used the random‐effects model. We did not perform a sensitivity analysis based on the quality of allocation concealment status, because only one trial used adequate allocation concealment.
The sensitivity analysis we performed by removing the two short‐term studies found that I2 was 0% and the RR was also smaller (Laumer 1989; Erol 2005). With a shorter follow‐up time we would have expected the recurrence risk to be lower, as fewer people would present with symptoms of recurrence within the shorter time frame. However, our analysis showed that these two studies with shorter follow‐up times had higher risks of recurrence. We reviewed the studies and noted that clinical heterogeneity may contribute to this result, as there were differences in the definition of recurrence and in follow‐up times between trials. In the other trials, imaging was done if participants developed a recurrence of their original symptoms, or new symptoms suggestive of raised intracranial pressure, and routine postoperative imaging was not employed. These seven studies defined recurrence as the reappearance of clinical symptoms after initial surgery with computed tomography (CT) scans providing evidence of CSDH on the same site. However, the two short‐term studies, Laumer 1989 and Erol 2005, performed CT scanning on all participants within the first month after surgery, whether they had symptoms of recurrence, or not. The high level of recurrence in the participants in these two studies is a result of the detection of radiological recurrence of CSDH in the absence of clinical symptoms or in participants suffering from minimal complaints only. With a modified follow‐up regimen, these cases that were detected early might be resolved without reoperation.
Data on complications were not available for two trials (Tsutsumi 1997; Kutty 2014). Mortality and poor functional outcome were not reported in three trials (Laumer 1989; Tsutsumi 1997; Kutty 2014). The pooled risk ratio for surgical complications within six months was 1.15 (95% CI 0.77 to 1.72; P = 0.50), and the pooled risk ratio for mortality at six months was 0.78 (95% CI 0.45 to 1.33; P = 0.35). The results show that there is no clear difference in mortality and surgical complications between participants with a drain and those without a drain. The pooled risk ratio for poor functional outcome (i.e. death and disability) was 0.68 (95% CI 0.44 to 1.05; P = 0.08). These results were robust even when we changed the fixed‐effect model to the random‐effects model or used intention‐to‐treat analysis.
The prespecified subgroup analysis of the number of burr holes used by the surgeons shows that insertion of a postoperative drain reduced the recurrence risk for CSDH regardless of whether a single hole or double holes were used. It seems to be that drainage via a single burr hole results in a lower recurrence risk (RR 0.17) than drainage with double holes (RR 0.46) compared with no drainage.
Overall completeness and applicability of evidence
This review suggests that the existing evidence on effectiveness of external subdural drainage after burr‐hole evacuation of CSDH is reasonable. We identified nine relevant RCTs with a total of 968 participants. Althrough individual studies actually had small numbers of participants, the trials were homogeneous with respect to the characteristics of participants (for age and sex distribution), treatment methods (burr hole at the maximum width of the haematoma, insertion of a subdural tube after irrigation), duration of treatment (drain in place for one to two days after surgery), and outcome measures (seven out of nine trials used the same definition for the primary outcome of recurrence of CSDH). A different definition was used in two studies, which also had short follow‐up times, and this introduced clinical heterogeneity, although, overall, the level of statistical heterogeneity was acceptable. The outcomes, especially the primary outcome, could be combined in meta‐analysis and a robust conclusion could be drawn.
The insertion of an external subdural drain after burr‐hole evacuation treatment for CSDH reduces the risk of recurrence. We did not identify any evidence that showed that external subdural drains increased the risk of surgical complications, poor function outcome or mortality. As the insertion of an external subdural tube is inexpensive and easy to perform, and the participants studied in the these trials were located in different healthcare settings worldwide, this procedure could readily be added to the normal medical and surgical management of chronic subdural haematoma patients in hospitals around the world.
Quality of the evidence
We judged only one of the trials to be at a low risk for the method of allocation concealment and so the risk of bias in the remaining trials was high. We assessed the quality of the evidence using GRADE, and judged the evidence for recurrence risk as being of moderate quality, as it was downgraded one level due to a high risk of bias for allocation concealment. We judged the evidence to be of low quality for complications, mortality and poor functional outcome; these were downgraded two levels due to a high risk of bias for allocation concealment and wide confidence intervals.
The complete GRADE evidence profile is presented in summary of findings Table for the main comparison.
Potential biases in the review process
The process of searching for studies was thorough. We followed the review protocol strictly in the process of study selection, data extraction, and analysis. Despite the robustness of the pooled analysis, there were some limitations in the review process that may have resulted in potential biases. Firstly, we did not try to obtain missing information from trial investigators. Missing data exceeded 20% for a secondary outcome in Santarius 2009. One of the included trials used alternation as the means of randomisation, which is a method at high risk of bias. Three of the studies did not report on random sequence generation and only one trial reported a low‐risk method for allocation concealment.
Agreements and disagreements with other studies or reviews
Two other systematic reviews and meta‐analyses, Liu 2014 and Gabriel 2014, included six and seven RCTs respectively. They did not include two of the trials we included (Kutty 2014; Singh 2014), because they had not been published. As with our findings, these reviews saw little clinical and statistical heterogeneity in the included studies. According to Gabriel 2014, Liu 2014 and our pooled analyses of included RCTs, the risk of overall symptomatic recurrence was significantly reduced with the use of external subdural drains, and there were no clear differences between the two intervention groups for medical or surgical complications, or poor functional outcome and mortality. While the Gabriel 2014 pooled analysis showed that subdural drains were associated with a better functional recovery ‐ which our analysis did not demonstrate ‐ one of the trials included in Gabriel 2014 did not include deaths within the poor functional outcome category (Wakai 1990), and this may account for the difference in results.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Nine studies are included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Comparison of drains versus no drains, Outcome 1 Recurrence.
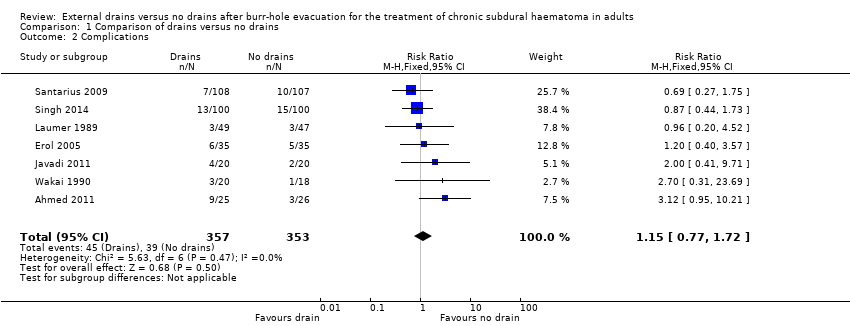
Comparison 1 Comparison of drains versus no drains, Outcome 2 Complications.
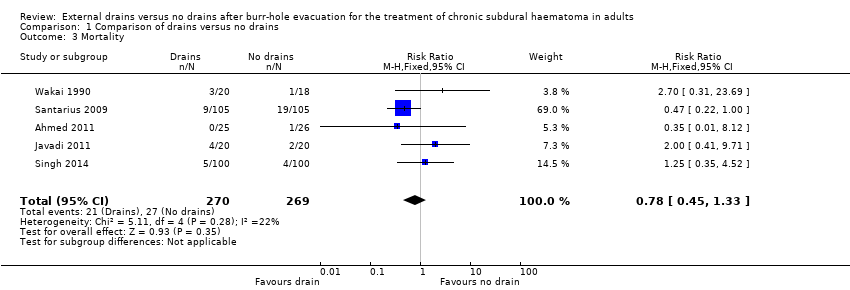
Comparison 1 Comparison of drains versus no drains, Outcome 3 Mortality.
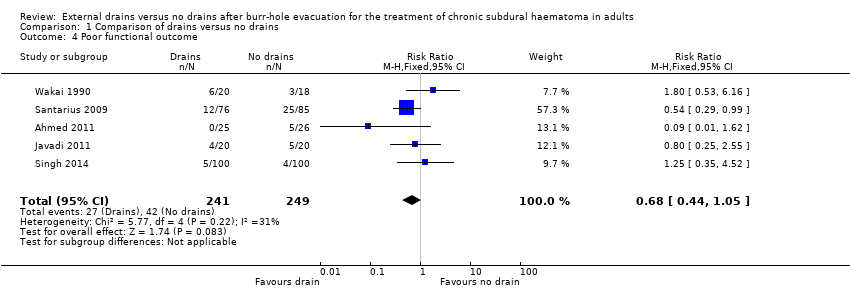
Comparison 1 Comparison of drains versus no drains, Outcome 4 Poor functional outcome.

Comparison 1 Comparison of drains versus no drains, Outcome 5 Sensitivity analysis of recurrence.
| Drains compared to no drains for burr‐hole evacuation of CSDH in adults | ||||||
| Patient or population: adults with burr‐hole evacuation of CSDH Settings: hospital settings in India,Turkey, Iran, Germany, UK and Japan | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No drain | Drain | |||||
| Overall recurrence | Study population | RR 0.45 | 968 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 97 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 104 per 1000 | |||||
| Recurrence with 2 burr holes (subgroup) | Study population | RR 0.46 | 306 | ⊕⊕⊝⊝ | ||
| 216 per 1000 | 99 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 106 per 1000 | |||||
| Recurrence with 1 burr hole (subgroup) | Study population | RR 0.17 | 156 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 36 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 36 per 1000 | |||||
| Recurrence with 1 or 2 burr holes (subgroup) | Study population | RR 0.52 | 506 | ⊕⊕⊕⊝ | ||
| 218 per 1000 | 113 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 111 per 1000 | |||||
| Complications | Study population | RR 1.15 | 710 | ⊕⊕⊝⊝ | ||
| 110 per 1000 | 127 per 1000 | |||||
| Moderate | ||||||
| 100 per 1000 | 115 per 1000 | |||||
| Mortality | Study population | RR 0.78 | 539 | ⊕⊕⊝⊝ | ||
| 100 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 56 per 1000 | 44 per 1000 | |||||
| Poor functional outcome (includes death) | Study population | RR 0.68 | 490 | ⊕⊕⊝⊝ | ||
| 169 per 1000 | 115 per 1000 | |||||
| Moderate | ||||||
| 131 per 1000 | 89 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one trial used adequate allocation concealment, and one trial used alternation as a randomisation method (Wakai 1990). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence Show forest plot | 9 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.61] |
| 1.1 Two holes | 3 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.80] |
| 1.2 One hole | 2 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.56] |
| 1.3 One or two holes | 4 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.79] |
| 2 Complications Show forest plot | 7 | 710 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.72] |
| 3 Mortality Show forest plot | 5 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.33] |
| 4 Poor functional outcome Show forest plot | 5 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 5 Sensitivity analysis of recurrence Show forest plot | 7 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.22, 0.50] |

