Laparoskopowa czy otwarta gastrektomia w leczeniu raka żołądka.
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Method of Anastamosis: Billroth‐I; stapler Inclusion criteria | |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D1 or more nodal dissection; no routine drain | |
| Outcomes | The outcomes reported were short‐term mortality, complications, and lymph nodes harvested | |
| Notes | Conversion to open gastrectomy: 0/13 (0%) Follow‐up period: 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients are randomized to either the ODG arm or the LADG arm by minimization method balancing the arms with institution and clinical stage (IA/IB)" |
| Allocation concealment (selection bias) | Low risk | Quote: "After the confirmation of the eligibility criteria, registration is made by telephone, fax or web‐based system to the JCOG Data Center. Patients are randomized to either the ODG arm or the LADG arm by minimization method balancing the arms with institution and clinical stage (IA/IB)" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: Postoperative mortality and morbidity were reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: China Inclusion criteria Patients requiring gastrectomy for gastric cancer Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D2 nodal dissection; drain use not stated | |
| Outcomes | The outcomes reported were short‐term mortality, complications, lymph nodes harvested, length of hospital stay, and long‐term mortality | |
| Notes | Conversion to open gastrectomy: 2/61 (3.3%) Follow‐up period: 22 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: Postoperative mortality and morbidity were reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: China Method of Anastamosis: Billroth‐I or Billroth‐II; hand‐sewn or stapler anastomosis not stated Inclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: Nodal dissection not stated; routine drains were used in the part of group who underwent fast‐track surgery | |
| Outcomes | The outcomes reported were short‐term mortality, complications, lymph nodes harvested, and hospital stay | |
| Notes | Conversion to open gastrectomy: not reported Follow‐up period: 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Blinding of the surgeons and nurses was not feasible. Therefore, two specially trained doctors blinded to patients' allocated treatment group were in charge for assessing postoperative outcomes and follow‐up" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Blinding of the surgeons and nurses was not feasible. Therefore, two specially trained doctors blinded to patients' allocated treatment group were in charge for assessing postoperative outcomes and follow‐up" |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: Postoperative mortality and morbidity were reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: China Method of Anastamosis: Billroth‐I; hand‐sewn or stapler anastomosis not stated Inclusion criteria Patients undergoing distal gastrectomy for gastric cancer Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D2 nodal dissection; drain use not stated | |
| Outcomes | None of the outcomes of interest were reported | |
| Notes | Conversion to open gastrectomy: not reported Follow‐up period: until discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | High risk | Comment: Postoperative mortality and morbidity were not reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Method of Anastamosis: Billroth‐I; stapler Inclusion criteria Patients undergoing distal gastrectomy for early gastric cancer Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D1 nodal dissection; drain use not stated | |
| Outcomes | The outcomes reported were short‐term mortality, complications, lymph nodes harvested, length of hospital stay, and long‐term mortality | |
| Notes | Conversion to open gastrectomy: 0/14 (0%) Follow‐up period: 42 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization of the patients into two groups (LADG or ODG) was performed by the blind envelope method on the day before surgery, and the patients were informed of the results the same day" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Randomization of the patients into two groups (LADG or ODG) was performed by the blind envelope method on the day before surgery, and the patients were informed of the results the same day" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | Low risk | Comment: There were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: Postoperative mortality and morbidity were reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: China Method of Anastamosis: no information on type of anastomosis; hand‐sewn or stapler anastomosis not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D2 nodal dissection; drain use not stated | |
| Outcomes | The outcomes reported were short‐term mortality and complications | |
| Notes | Conversion to open gastrectomy: 14/308 (4.5%) Follow‐up period: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Masking: Open Label" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Masking: Open Label" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: There were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: The severity of postoperative complications was not reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: Italy Method of Anastamosis: Billroth‐I or Billroth‐II; some anastomoses by stapler and others by hand‐sewn anastomoses Inclusion criteria | |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D1 or D2 nodal dissection; drain use not stated | |
| Outcomes | The outcomes reported were short‐term mortality, complications, lymph nodes harvested, length of hospital stay, long‐term mortality, and long‐term recurrence | |
| Notes | Conversion to open gastrectomy: not reported Follow‐up period: 52 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: Postoperative mortality and morbidity were reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: South Korea Method of Anastamosis: no information on type of anastomosis; hand‐sewn or stapler anastomosis not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D2 nodal dissection; drain use not stated | |
| Outcomes | The outcomes reported were complications | |
| Notes | Conversion to open gastrectomy: not reported Follow‐up period: 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization is performed as block randomization in fixed block sizes in a 1:1 allocation ratio using a centralized web‐based randomization system (eVelos [http://eresearch.ncc. re.kr/eres/jsp/ereslogin.jsp])" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization is performed as block randomization in fixed block sizes in a 1:1 allocation ratio using a centralized web‐based randomization system (eVelos [http://eresearch.ncc. re.kr/eres/jsp/ereslogin.jsp])" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Blinding procedures are not possible in this trial due to the nature of the intervention" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "However blinded assessment of the primary & secondary outcomes were provided by blinded observers" (author replies) Comment: It is unclear how the decision on hospital discharge and serious adverse events were assessed (for example, by a second surgical team) |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: Mortality and the severity of postoperative complications were not reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: South Korea Method of Anastamosis: Billroth‐I, Billroth‐II, or Roux‐en‐Y anastomosis; hand‐sewn or stapler anastomosis not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: D1 or more nodal dissection; drain use not stated | |
| Outcomes | The outcomes reported were short‐term mortality and complications | |
| Notes | Conversion to open gastrectomy: not reported Follow‐up period: 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After confirming the patients met the inclusion/exclusion criteria by telephoning the data center, the patients were registered into the trial and then randomized to one of two groups (LADG or ODG) on the basis of a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was coordinated centrally by the independent data center and aimed to balance the arms according to each institution" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: The severity of postoperative complications was not reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Method of Anastamosis: Billroth‐I; hand‐sewn or stapler anastomosis not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: Nodal dissection not stated; drain use not stated | |
| Outcomes | The outcomes reported were short‐term mortality, complications, and long‐term recurrence | |
| Notes | Conversion to open gastrectomy: 0/14 (0%) Follow‐up period: 26 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "After providing written informed consent, the patients were randomly assigned to either LADG group (n = 10) and an ODG group (n = 10) with Billroth‐I reconstruction on the day before operation by use of numbered, sealed envelopes that were stratified by the surgeon" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | High risk | Comment: The severity of postoperative complications was not reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: South Korea Method of Anastamosis: Billroth‐I; stapler Inclusion criteria Patients with early gastric cancer undergoing distal gastrectomy Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: Selected nodes in laparoscopic group and D2 nodal dissection in open group; drain used routinely in laparoscopic group; information on drain use in open group was not available | |
| Outcomes | The outcomes reported were short‐term mortality, complications, lymph nodes harvested, length of hospital stay, and long‐term recurrence | |
| Notes | Conversion to open gastrectomy: 0/24 (0%) Follow‐up period: 14 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random number table, 23 patients were assigned to the open surgery group (group O) and 24 patients were assigned to the LADG group (group L)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: This information was not available |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: The severity of postoperative complications was not reported |
| Other bias | High risk | Comment: A more extensive procedure was performed in open gastrectomy group compared to laparoscopic group. This could potentially favour laparoscopic group in terms of decreased complications but favour open group in terms of decreased long‐term recurrence and mortality |
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Method of Anastamosis: Billroth‐I; stapler Inclusion criteria Over 20 and under 75 years of age with gastric cancer in the middle or lower part of the stomach for which distal gastrectomy was indicated Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: Selected group of nodes in the two groups; drains used routinely in both groups | |
| Outcomes | The outcomes reported were short‐term mortality, complications, and length of hospital stay | |
| Notes | Conversion to open gastrectomy: not reported Follow‐up period: until discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computer‐generated, nonstratified, blocked randomization scheme was managed centrally and concealed at the moment of inclusion" |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer‐generated, nonstratified, blocked randomization scheme was managed centrally and concealed at the moment of inclusion" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Due to the pragmatic nature of the trial, surgeons, care providers, and patients could not be blinded to the type of treatment that was performed". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Due to the pragmatic nature of the trial, surgeons, care providers, and patients could not be blinded to the type of treatment that was performed" |
| Incomplete outcome data (attrition bias) | High risk | Comment: There were post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: Postoperative mortality and morbidity were reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Method of Anastamosis: Billroth‐I; stapler Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to two groups Nodes dissected and drain use: Selected group of nodes in the two groups; drains used in laparoscopic group, no details in open group | |
| Outcomes | The outcomes reported were short‐term mortality, blood transfusion, length of hospital stay, lymph node harvest, and long‐term mortality | |
| Notes | Conversion to open gastrectomy: 0/20 (0%) Follow‐up period: 60 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization of the patients into two groups was performed by the blind envelop method on the day before operation, but the patients were not informed of the results at that time" |
| Blinding of participants and personnel (performance bias) | High risk | Comment: This was a single‐blinded study in which only patients were blinded |
| Blinding of outcome assessment (detection bias) | High risk | Comment: This was a single‐blinded study in which only patients were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: There were no post‐randomisation drop‐outs |
| Selective reporting (reporting bias) | High risk | Comment: The complications in the laparoscopic gastrectomy group and the severity of postoperative complications in the open gastrectomy group were not reported |
| Other bias | Low risk | Comment: No other source of bias was identified |
ASA: American Society of Anesthesiologist; EMR: Endoscopic mucosal resection; FEV1: forced expiratory volume in first second; JCOG: Japan Clinical Oncology Group; LADG: laparoscopy‐assisted distal gastrectomy; LAG: laparoscopy‐assisted gastrectomy; ODG: open distal gastrectomy
T: Tumour stage of TNM classification
N: Nodal stage of TNM classification
Example: T1‐2N0‐1: indicates T‐stage 1 or 2 and N‐stage 0 or 1
Early gastric cancer: clinical stage: T1Nany
Advaced gastric cancer: T>1Nany
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial | |
| Editorial | |
| Not a randomised controlled trial | |
| Quasi‐randomised study | |
| Comment on an excluded study (Kim 2008) | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Comment on an excluded study (Kim 2008) | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | LOGICA |
| Methods | Randomised controlled trial |
| Participants | Early or advanced staged gastric adenocarcinoma |
| Interventions | Laparosopy‐assisted versus open distal or total gastrectomy |
| Outcomes | Mortality, adverse events, health‐related quality of life, length of hospital stay, clear resection margins, number of lymph nodes dissected, and long‐term mortality |
| Starting date | December 2014 |
| Contact information | |
| Notes |
| Trial name or title | STOMACH |
| Methods | Randomised controlled trial |
| Participants | People with early or advanced gastric cancer receiving neoadjuvant chemotherapy |
| Interventions | Laparosopy‐assisted versus open total gastrectomy |
| Outcomes | Mortality, health‐related quality of life, length of hospital stay, number of lymph nodes dissected, and long‐term mortality |
| Starting date | Not stated |
| Contact information | |
| Notes |
| Trial name or title | LANDSCOPE |
| Methods | Randomised controlled trial |
| Participants | People with advanced gastric cancer receiving neoadjuvant chemotherapy |
| Interventions | Laparosopy‐assisted versus open distal gastrectomy |
| Outcomes | Mortality, adverse events, and long‐term recurrence |
| Starting date | December 2014 |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 11 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.50, 5.10] |
| Analysis 1.1  Comparison 1 Laparoscopic versus open gastrectomy, Outcome 1 Short‐term mortality. | ||||
| 2 Long‐term mortality (maximal follow‐up) Show forest plot | 3 | 195 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.70, 1.25] |
| Analysis 1.2 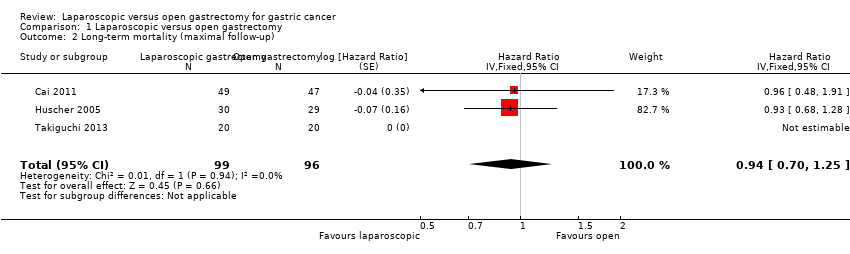 Comparison 1 Laparoscopic versus open gastrectomy, Outcome 2 Long‐term mortality (maximal follow‐up). | ||||
| 3 Proportion with a serious adverse event (< 3 months) Show forest plot | 8 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.27, 1.34] |
| Analysis 1.3  Comparison 1 Laparoscopic versus open gastrectomy, Outcome 3 Proportion with a serious adverse event (< 3 months). | ||||
| 4 Short‐term recurrence Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 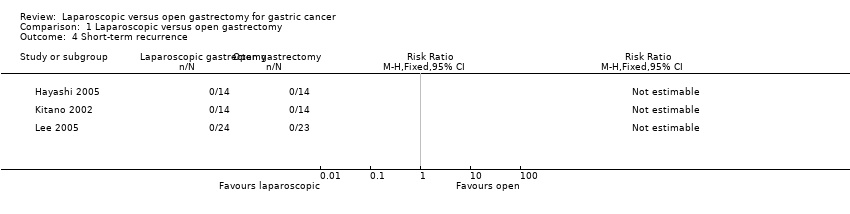 Comparison 1 Laparoscopic versus open gastrectomy, Outcome 4 Short‐term recurrence. | ||||
| 5 Long‐term recurrence (maximal follow‐up) Show forest plot | 4 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Laparoscopic versus open gastrectomy, Outcome 5 Long‐term recurrence (maximal follow‐up). | ||||
| 6 Proportion with an adverse event (< 3 months) Show forest plot | 11 | 2490 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.01] |
| Analysis 1.6  Comparison 1 Laparoscopic versus open gastrectomy, Outcome 6 Proportion with an adverse event (< 3 months). | ||||
| 7 Proportion requiring blood transfusion during or within a week of surgery Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Laparoscopic versus open gastrectomy, Outcome 7 Proportion requiring blood transfusion during or within a week of surgery. | ||||
| 8 Quantity of perioperative blood transfused Show forest plot | 2 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.27, 0.38] |
| Analysis 1.8  Comparison 1 Laparoscopic versus open gastrectomy, Outcome 8 Quantity of perioperative blood transfused. | ||||
| 9 Length of hospital stay Show forest plot | 8 | 444 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.57, ‐0.19] |
| Analysis 1.9 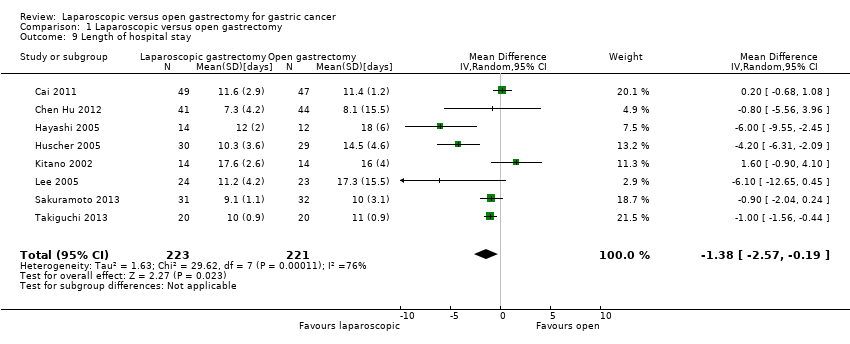 Comparison 1 Laparoscopic versus open gastrectomy, Outcome 9 Length of hospital stay. | ||||
| 10 Proportion with positive resection margins at histopathological examination Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10 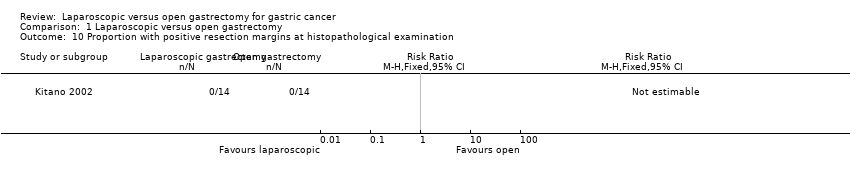 Comparison 1 Laparoscopic versus open gastrectomy, Outcome 10 Proportion with positive resection margins at histopathological examination. | ||||
| 11 Number of lymph nodes harvested Show forest plot | 9 | 472 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.51, 0.25] |
| Analysis 1.11 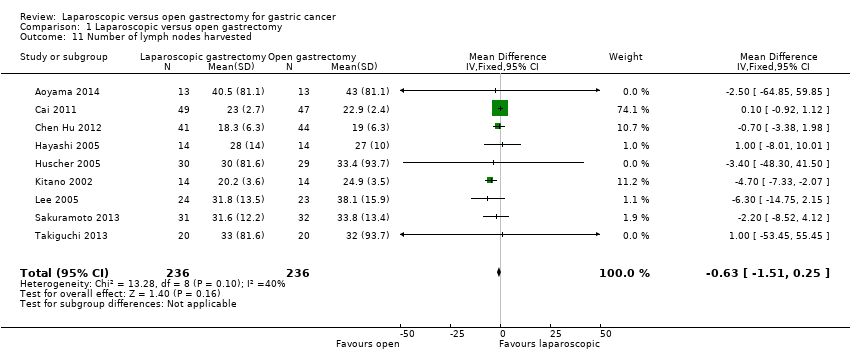 Comparison 1 Laparoscopic versus open gastrectomy, Outcome 11 Number of lymph nodes harvested. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality (stratified by early versus advanced cancer) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 1 Short‐term mortality (stratified by early versus advanced cancer). | ||||
| 1.1 Early gastric cancer | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Advanced gastric cancer | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Long‐term mortality (maximal follow‐up) (stratified by early versus advanced cancer) Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 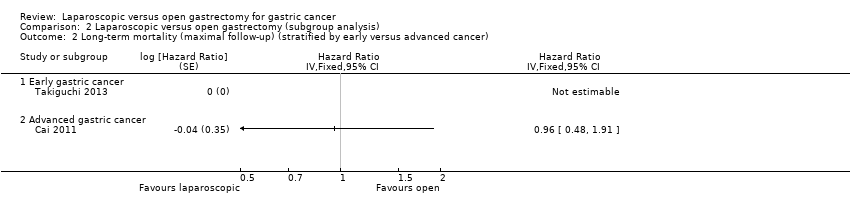 Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 2 Long‐term mortality (maximal follow‐up) (stratified by early versus advanced cancer). | ||||
| 2.1 Early gastric cancer | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Advanced gastric cancer | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Proportion with a serious adverse event (< 3 months) (stratified by early versus advanced cancer) Show forest plot | 5 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.60] |
| Analysis 2.3 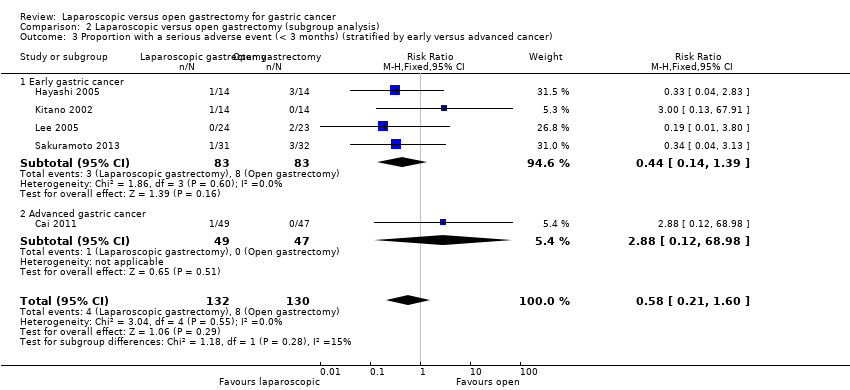 Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 3 Proportion with a serious adverse event (< 3 months) (stratified by early versus advanced cancer). | ||||
| 3.1 Early gastric cancer | 4 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.39] |
| 3.2 Advanced gastric cancer | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 68.98] |
| 4 Short‐term mortality (stratified by type of gastrectomy) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 4 Short‐term mortality (stratified by type of gastrectomy). | ||||
| 4.1 Subtotal gastrectomy | 10 | 2239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.50, 5.10] |
| 5 Long‐term mortality (maximal follow‐up) (stratified by type of gastrectomy) Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 5 Long‐term mortality (maximal follow‐up) (stratified by type of gastrectomy). | ||||
| 5.1 Subtotal gastrectomy | 2 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Proportion with a serious adverse event (< 3 months) (stratified by type of gastrectomy) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 6 Proportion with a serious adverse event (< 3 months) (stratified by type of gastrectomy). | ||||
| 6.1 Subtotal gastrectomy | 7 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.22, 1.22] |
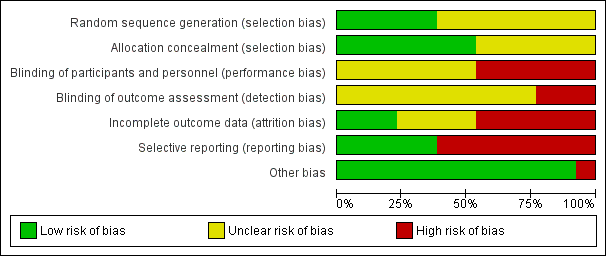
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 1 Short‐term mortality.
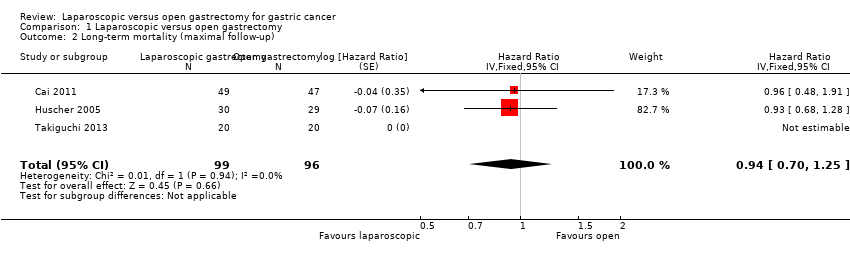
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 2 Long‐term mortality (maximal follow‐up).

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 3 Proportion with a serious adverse event (< 3 months).
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 4 Short‐term recurrence.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 5 Long‐term recurrence (maximal follow‐up).
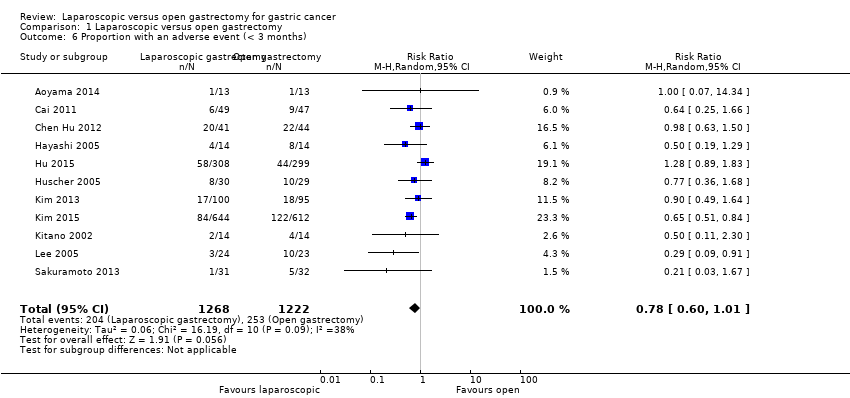
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 6 Proportion with an adverse event (< 3 months).

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 7 Proportion requiring blood transfusion during or within a week of surgery.
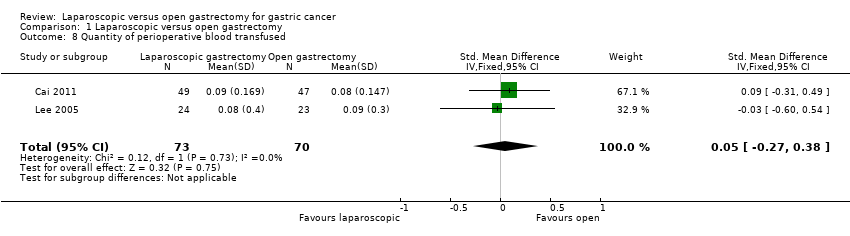
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 8 Quantity of perioperative blood transfused.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 9 Length of hospital stay.
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 10 Proportion with positive resection margins at histopathological examination.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 11 Number of lymph nodes harvested.

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 1 Short‐term mortality (stratified by early versus advanced cancer).
Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 2 Long‐term mortality (maximal follow‐up) (stratified by early versus advanced cancer).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 3 Proportion with a serious adverse event (< 3 months) (stratified by early versus advanced cancer).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 4 Short‐term mortality (stratified by type of gastrectomy).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 5 Long‐term mortality (maximal follow‐up) (stratified by type of gastrectomy).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 6 Proportion with a serious adverse event (< 3 months) (stratified by type of gastrectomy).
| Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (primary outcomes) | |||||
| Patient or population: patients with gastric cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Open gastrectomy | Laparoscopic gastrectomy | ||||
| Short‐term mortality | 3 per 1000 | 6 per 1000 | RR 1.60 | 2335 | ⊕⊕⊝⊝ |
| Long‐term mortality (maximal follow‐up) | 448 per 1000 | 428 per 1000 | HR 0.94 | 195 | ⊕⊝⊝⊝ |
| Proportion with a serious adverse event (< 3 months) | 60 per 1000 | 36 per 1000 | RR 0.60 | 432 | ⊕⊝⊝⊝ |
| Health‐related quality of life during short‐term (four weeks to three months) or medium‐term (more than three months to one year) was not reported. | |||||
| *The basis for the assumed risk was the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 There was unclear or high risk bias within the trials (downgraded by two levels). | |||||
| Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (secondary outcomes) | ||||||
| Patient or population: patients with gastric cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open gastrectomy | Laparoscopic gastrectomy | |||||
| Long‐term recurrence (maximal follow‐up) | 450 per 1000 | 433 per 1000 | HR 0.95 | 162 | ⊕⊝⊝⊝ | |
| Proportion with an adverse event (< 3 months) | 207 per 1000 | 161 per 1000 | RR 0.78 | 2490 | ⊕⊝⊝⊝ | |
| Quantity of perioperative blood transfused | The mean quantity of perioperative blood transfused in the control groups was | The mean quantity of perioperative blood transfused in the intervention groups was | 143 | ⊕⊝⊝⊝ | SMD 0.05 (‐0.27 to 0.38) | |
| Length of hospital stay | The mean length of hospital stay in the intervention groups was | 319 | ⊕⊝⊝⊝ | |||
| Number of lymph nodes harvested | The mean number of lymph nodes harvested in the control groups was | The mean number of lymph nodes harvested in the intervention groups was | 472 | ⊕⊝⊝⊝ | ||
| There were no events in either group for short‐term recurrence (103 participants (3 studies)), proportion requiring blood transfusion (66 participants (2 studies)), proportion with positive resection margin (incomplete cancer resection) (14 participants (1 study)). | ||||||
| None of the trials reported on measures of earlier postoperative recovery such as time to return to normal activity or time to return to work. | ||||||
| *The basis for the assumed risk was the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was unclear or high risk of bias within the trials (downgraded by two levels). Please see Figure 1 and Figure 2 which show this. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 11 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.50, 5.10] |
| 2 Long‐term mortality (maximal follow‐up) Show forest plot | 3 | 195 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.70, 1.25] |
| 3 Proportion with a serious adverse event (< 3 months) Show forest plot | 8 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.27, 1.34] |
| 4 Short‐term recurrence Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Long‐term recurrence (maximal follow‐up) Show forest plot | 4 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 6 Proportion with an adverse event (< 3 months) Show forest plot | 11 | 2490 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.01] |
| 7 Proportion requiring blood transfusion during or within a week of surgery Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Quantity of perioperative blood transfused Show forest plot | 2 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.27, 0.38] |
| 9 Length of hospital stay Show forest plot | 8 | 444 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.57, ‐0.19] |
| 10 Proportion with positive resection margins at histopathological examination Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Number of lymph nodes harvested Show forest plot | 9 | 472 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.51, 0.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality (stratified by early versus advanced cancer) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Early gastric cancer | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Advanced gastric cancer | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Long‐term mortality (maximal follow‐up) (stratified by early versus advanced cancer) Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Early gastric cancer | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Advanced gastric cancer | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Proportion with a serious adverse event (< 3 months) (stratified by early versus advanced cancer) Show forest plot | 5 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.60] |
| 3.1 Early gastric cancer | 4 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.39] |
| 3.2 Advanced gastric cancer | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 68.98] |
| 4 Short‐term mortality (stratified by type of gastrectomy) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Subtotal gastrectomy | 10 | 2239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.50, 5.10] |
| 5 Long‐term mortality (maximal follow‐up) (stratified by type of gastrectomy) Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Subtotal gastrectomy | 2 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Proportion with a serious adverse event (< 3 months) (stratified by type of gastrectomy) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Subtotal gastrectomy | 7 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.22, 1.22] |

