Лапароскопическая по сравнению с открытой гастрэктомией при раке желудка
Abstract
Background
Gastric cancer is the third most common cause of cancer‐related mortality in the world. Currently there are two surgical options for potentially curable patients (i.e. people with non‐metastatic gastric cancer), laparoscopic and open gastrectomy. However, it is not clear whether one of these options is superior.
Objectives
To assess the benefits and harms of laparoscopic gastrectomy or laparoscopy‐assisted gastrectomy versus open gastrectomy for people with gastric cancer. In particular, we planned to investigate the effects by patient groups, such as cancer stage, anaesthetic risk, and body mass index (BMI), and by intervention methods, such as method of anastomosis, type of gastrectomy and laparoscopic or laparoscopically‐assisted gastrectomy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index, ClinicalTrials.gov and the WHO ICTRP (World Health Organization International Clinical Trials Registry Platform) until September 2015. We also screened reference lists from included trials.
Selection criteria
Two review authors independently selected references for further assessment by going through all titles and abstracts. Further selection was based on review of full text articles for selected references.
Data collection and analysis
Two review authors independently extracted study data. We calculated the risk ratio (RR) with 95% confidence interval (CI) for binary outcomes, the mean difference (MD) or the standardised mean difference (SMD) with 95% CI for continuous outcomes and the hazard ratio (HR) for time‐to‐event outcomes. We performed meta‐analyses where it was meaningful.
Main results
In total, 2794 participants were randomised in 13 trials included in this review. All the trials were at unclear or high risk of bias. One trial (which included 53 participants) did not contribute any data to this review. A total of 213 participants were excluded in the remaining trials after randomisation, leaving a total of 2528 randomised participants for analysis, with 1288 undergoing laparoscopic gastrectomy and 1240 undergoing open gastrectomy. All the participants were suitable for major surgery.
There was no difference in the proportion of participants who died within thirty days of treatment between laparoscopic gastrectomy (7/1188: adjusted proportion = 0.6% (based on meta‐analysis)) and open gastrectomy (4/1447: 0.3%) (RR 1.60, 95% CI 0.50 to 5.10; risk difference 0.00, 95% CI ‐0.01 to 0.01; participants = 2335; studies = 11; I2 = 0%; low quality evidence). There were no events in either group for short‐term recurrence (participants = 103; studies = 3), proportion requiring blood transfusion (participants = 66; studies = 2), and proportion with positive margins at histopathology (participants = 28; studies = 1). None of the trials reported health‐related quality of life, time to return to normal activity or time to return to work. The differences in long‐term mortality (HR 0.94, 95% CI 0.70 to 1.25; participants = 195; studies = 3; I2 = 0%; very low quality evidence), serious adverse events within three months (laparoscopic gastrectomy (7/216: adjusted proportion = 3.6%) versus open gastrectomy (13/216: 6%) (RR 0.60, 95% CI 0.27 to 1.34; participants = 432; studies = 8; I2 = 0%; very low quality evidence), long‐term recurrence (HR 0.95, 95% CI 0.70 to 1.30; participants = 162; studies = 4; very low quality evidence), adverse events within three months (laparoscopic gastrectomy (204/268: adjusted proportion = 16.1%) versus open gastrectomy (253/1222: 20.7%) (RR 0.78, 95% CI 0.60 to 1.01; participants = 2490; studies = 11; I2 = 38%; very low quality evidence), quantity of perioperative blood transfused (SMD 0.05, 95% CI ‐0.27 to 0.38; participants = 143; studies = 2; I2 = 0%; very low quality evidence), length of hospital stay (MD ‐1.82 days, 95% CI ‐3.72 to 0.07; participants = 319; studies = 6; I2 = 83%; very low quality evidence), and number of lymph nodes harvested (MD ‐0.63, 95% CI ‐1.51 to 0.25; participants = 472; studies = 9; I2 = 40%; very low quality evidence) were imprecise. There was no alteration in the interpretation of the results in any of the subgroups.
Authors' conclusions
Based on low quality evidence, there is no difference in short‐term mortality between laparoscopic and open gastrectomy. Based on very low quality evidence, there is no evidence for any differences in short‐term or long‐term outcomes between laparoscopic and open gastrectomy. However, the data are sparse, and the confidence intervals were wide, suggesting that significant benefits or harms of laparoscopic gastrectomy cannot be ruled out. Several trials are currently being conducted and interim results of these trials have been included in this review. These trials need to perform intention‐to‐treat analysis to ensure that the results are reliable and report the results according to the CONSORT Statement.
PICO
Резюме на простом языке
Лапароскопическая (через "замочную скважину") операция против открытой операции при лечении людей с раком желудка
Вопрос обзора
Является ли лапароскопическое вмешательство ("хирургия замочной скважины") эквивалентным открытому хирургическому вмешательству при лечении пациентов с раком желудка?
Актуальность
Рак желудка занимает третье место в мире среди причин смерти от онкологических заболеваний. Если рак не распространился в другие области организма, и если человек может выдержать большую операцию, в зависимости от того, какая часть желудка поражена раком, удаление части желудка или всего желудка (гастрэктомия) является единственным способом лечения, направленным на долгосрочное излечение от рака. Гастрэктомия может быть выполнена как лапароскопическая операция (через отверстие ‐ "замочную скважину"), а также как открытая операция, подразумевающая большой разрез. Хотя при лапароскопическом способе разрез меньше, не ясно, является ли "хирургия замочной скважины" такой же безопасной, как открытая операция, и представляет ли она какие‐либо преимущества в отношении сокращения сроков выздоровления у людей, перенесших гастрэктомию. Мы стремились решить этот вопрос с помощью поиска медицинской литературы на предмет исследований, опубликованных до сентября 2015 года, в которых сравнивали лапароскопическую и открытую гастрэктомию у людей с раком желудка.
Характеристика исследований
Мы обнаружили 13 подходящих исследований (2794 участника) для этого обзора. В одном клиническом испытании не была представлена необходимая для нас информация. Информацию по 213 участникам не сообщали по разным причинам, в основном – по причине того, что пациенты не получили запланированного лечения. Всего 2528 участников перенесли либо лапароскопическую гастрэктомию (1288 участников), или открытую гастрэктомию (1240 участников). Решение, какую гастрэктомию будут проводить участнику ‐ лапароскопическую или открытую, было принято с использованием методов, схожих с подбрасыванием монеты. Этот процесс обеспечивает схожесть участников в двух группах. Все участники подходили для проведения большой операции.
Основные результаты
Не было различий между лапароскопической и открытой гастрэктомией в отношении краткосрочной смертности (лапароскопическая гастрэктомия: 6 смертей на 1000 операций; открытая гастрэктомия: 3 смерти на 1000 операций). Есть некоторая неопределенность в возможности прогнозирования числа смертей или исходов на основании информации из этих клинических испытаний. В связи с этой неопределенностью, мы смогли сделать вывод, что различий в краткосрочной (ранней) смертности между группами не было, хотя число смертей при проведении лапароскопической гастрэктомии было в 2 раза больше, чем при открытой. Ни в одном из клинических испытаний не сообщали о качестве жизни, связанном со здоровьем, времени возвращения к нормальной активности и работе. Различия в долгосрочной (поздней) смертности, серьезных осложнениях в течение трех месяцев (лапароскопическая гастрэктомия: 36 осложнений на 1000 операций; открытая гастрэктомия: 60 осложнений на 1000 операций), во всех осложнениях в течение трех месяцев (лапароскопическая гастрэктомия: 161 осложнение на 1000 операций; открытая гастрэктомия: 253 осложнения на 1000 операций); в ранних и поздних рецидивах рака; в числе людей, которым потребовалось переливание крови; в числе проведенных переливаний крови в течение первой недели после операции, а также в сроках госпитализации были неопределенными. Таким образом, нельзя исключить существенную пользу или вред лапароскопической гастрэктомии по сравнению с открытой гастрэктомией. Для сравнения пользы и вреда лапароскопической и открытой гастрэктомии необходимы дальнейшие хорошо спланированные клинические испытания.
Качество доказательств
В отношении всех исходов качество доказательств было очень низким. Качество доказательств в отношении краткосрочной (ранней) смертности было низким. Таким образом, есть большая неопределенность в отношении результатов.
Authors' conclusions
Summary of findings
| Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (primary outcomes) | |||||
| Patient or population: patients with gastric cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Open gastrectomy | Laparoscopic gastrectomy | ||||
| Short‐term mortality | 3 per 1000 | 6 per 1000 | RR 1.60 | 2335 | ⊕⊕⊝⊝ |
| Long‐term mortality (maximal follow‐up) | 448 per 1000 | 428 per 1000 | HR 0.94 | 195 | ⊕⊝⊝⊝ |
| Proportion with a serious adverse event (< 3 months) | 60 per 1000 | 36 per 1000 | RR 0.60 | 432 | ⊕⊝⊝⊝ |
| Health‐related quality of life during short‐term (four weeks to three months) or medium‐term (more than three months to one year) was not reported. | |||||
| *The basis for the assumed risk was the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 There was unclear or high risk bias within the trials (downgraded by two levels). | |||||
| Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (secondary outcomes) | ||||||
| Patient or population: patients with gastric cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open gastrectomy | Laparoscopic gastrectomy | |||||
| Long‐term recurrence (maximal follow‐up) | 450 per 1000 | 433 per 1000 | HR 0.95 | 162 | ⊕⊝⊝⊝ | |
| Proportion with an adverse event (< 3 months) | 207 per 1000 | 161 per 1000 | RR 0.78 | 2490 | ⊕⊝⊝⊝ | |
| Quantity of perioperative blood transfused | The mean quantity of perioperative blood transfused in the control groups was | The mean quantity of perioperative blood transfused in the intervention groups was | 143 | ⊕⊝⊝⊝ | SMD 0.05 (‐0.27 to 0.38) | |
| Length of hospital stay | The mean length of hospital stay in the intervention groups was | 319 | ⊕⊝⊝⊝ | |||
| Number of lymph nodes harvested | The mean number of lymph nodes harvested in the control groups was | The mean number of lymph nodes harvested in the intervention groups was | 472 | ⊕⊝⊝⊝ | ||
| There were no events in either group for short‐term recurrence (103 participants (3 studies)), proportion requiring blood transfusion (66 participants (2 studies)), proportion with positive resection margin (incomplete cancer resection) (14 participants (1 study)). | ||||||
| None of the trials reported on measures of earlier postoperative recovery such as time to return to normal activity or time to return to work. | ||||||
| *The basis for the assumed risk was the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was unclear or high risk of bias within the trials (downgraded by two levels). Please see Figure 1 and Figure 2 which show this. | ||||||

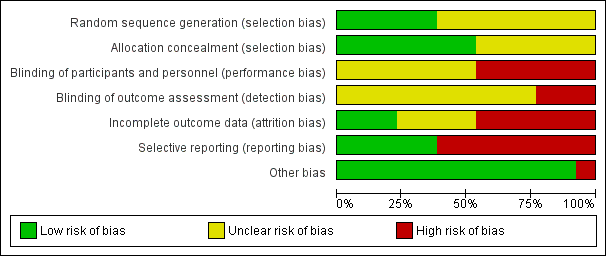
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
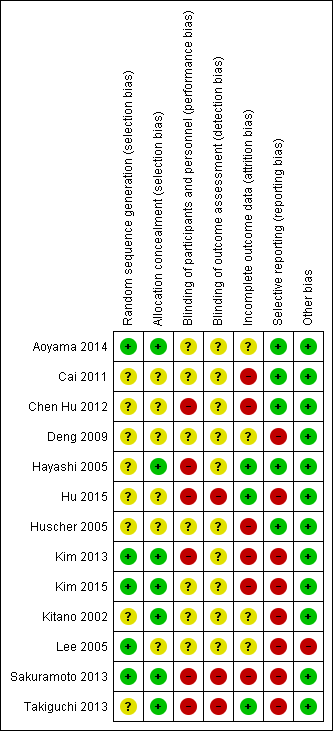
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Background
Description of the condition
Adenocarcinoma of the stomach (or stomach cancer) is the fifth most common cancer and the third most common cause of cancer‐related mortality in the world (IARC 2014). In 2012, there were about 950,000 newly diagnosed cases of gastric cancer and 725,000 deaths due to gastric cancer globally (IARC 2014). There is global variation in the incidence of gastric cancers with an age‐standardised annual incidence rate of 30 to 42 per 100,000 population in East Asian countries such as Japan, Mongolia, and Korea compared with an age‐standardised annual incidence rate of 1 to 5 per 100,000 population in Africa, Australia, the USA, and the UK (IARC 2014). The trend in mortality is different. For example, the age‐standardised annual mortality rate in Mongolia is 25 per 100,000 population compared with 13 per 100,000 population in Korea and Japan despite a higher age‐standardised annual incidence in Korea than Mongolia (IARC 2014).
There is a decreasing trend in the overall incidence of gastric cancers, possibly due to lifestyle changes, such as decreased consumption of salted and preserved foods, increased consumption of fruits and vegetables, decreased smoking and reduction ofHelicobacter pylori (H. pylori) (Cancer Research UK 2014; Jemal 2010).
The treatment of gastric cancer depends upon the stage of cancer. One of the common systems for staging cancer currently is the American Joint Committee on Cancer (AJCC) gastric cancer staging system ‐ AJCC 7th edition (AJCC 2010; Washington 2010). This system is based on the involvement of the different layers of the stomach by the tumour (T), nodal involvement (N), and the presence of metastases (M) (TNM classification). Early gastric cancer is cancer that is confined to the submucosa (T1) with or without nodal involvement, although this definition of early gastric cancer has been challenged since nodal status is an important prognostic factor in survival (Inoue 1991; Kim 1995). If the cancer has penetrated beyond the submucosa, it is called advanced gastric cancer. Metastatic gastric cancer corresponds to Stage IV of the AJCC gastric cancer staging system. The survival after diagnosis of gastric cancer depends upon the stage with five‐year survival ranging from 70% in Stage Ia cancer to 5% in Stage IV cancer (AJCC 2010; Washington 2010). The treatment of gastric cancer depends upon the stage of the disease. Potentially curative treatment is possible for stages I to III (Japanese Gastric Cancer Association 2011; Waddell 2013). Apart from T1aN0M0 stage, where endoscopic treatment may be performed, and stage IV, where palliative treatment is recommended, the remaining stages are treated by resection of the stomach (gastrectomy) (Bennett 2009; Japanese Gastric Cancer Association 2011; Waddell 2013).
Description of the intervention
In open gastrectomy, the surgical access to the abdominal cavity (and hence the stomach) is by upper midline incision, a bilateral subcostal incision (roof‐top or Chevron incision), or a transverse abdominal incision (Inaba 2004; Stuart 1997). In laparoscopy‐assisted gastrectomy, the surgical access to the abdominal cavity (and hence the stomach) is by a small abdominal incision (about 5 cm) and additional five or six small ports (holes) of about 0.5 cm to 1 cm each through which laparoscopic instruments can be inserted after the abdomen is distended using carbon dioxide pneumoperitoneum. Part of the surgery, usually the anastomosis restoring the continuity of the gastrointestinal tract, is performed outside the body (extracorporeal) (Lee 2013). The resected stomach is removed through the small abdominal incision. In totally laparoscopic gastrectomy, the surgical access to the abdominal cavity (and hence the stomach) is only by five or six small ports of about 0.5 to 1 cm each through which laparoscopic instruments can be inserted after the abdomen is distended using carbon dioxide pneumoperitoneum. The entire surgery is performed laparoscopically (Zhang 2015).
The standard operations are total gastrectomy and subtotal gastrectomy, and are recommended in the presence of nodal involvement or T2 to T4a tumours. Subtotal gastrectomy can be performed when a minimum of 2 to 5 cm proximal cancer‐free margin can be achieved, depending upon the depth of infiltration and the growth pattern of the cancer (Japanese Gastric Cancer Association 2011). Proximal gastrectomy can be performed for T1N0 proximal gastric cancers when more than half of the distal stomach can be preserved; and a pylorus‐preserving gastrectomy can be performed for T1N0 cancers of the middle third of the stomach when the distal margin of the tumour is at least 4 cm from the pylorus (Japanese Gastric Cancer Association 2011). The extent of lymph node excision, and the method of restoration of continuity of the gastrointestinal tract, are controversial (Japanese Gastric Cancer Association 2011; Memon 2011; Waddell 2013; Xiong 2013). Postoperative chemotherapy is recommended after gastrectomy for resectable gastric cancer (Diaz‐Nieto 2013; Waddell 2013).
How the intervention might work
For many surgical procedures, laparoscopic surgery is currently preferred over open surgery. This includes surgical procedures such as cholecystectomy (removal of gallbladder), colon cancer, and hysterectomy (Bijen 2009; Keus 2006; Reza 2006; Talseth 2014; Walsh 2009). The reason for this preference of laparoscopic surgery over open surgery is because of decreased pain, decreased blood loss, shorter hospital stay, earlier postoperative recovery, better cosmesis (physical appearance), and decreased costs (Bijen 2009; Keus 2006; Reza 2006; Talseth 2014; Walsh 2009).
Why it is important to do this review
While the smaller incision and earlier postoperative recovery appear to be potential advantages of laparoscopic gastrectomy or laparoscopy‐assisted gastrectomy, the safety of the laparoscopic approach (for a procedure that has a high complication rate) and cancer clearance after laparoscopic and laparoscopy‐assisted gastrectomy has to be ensured before the method can be widely recommended. There are concerns about cancer clearance, since port site metastases (recurrence of cancer at the laparoscopic port site) have been reported after many cancers (Kais 2014; Palomba 2014; Song 2014). Animal research has shown that the increased intra‐abdominal pressure during laparoscopy (pneumoperitoneum) may drive the malignant cells into ports, resulting in seeding of the port site and port site metastases (Hopkins 1999). Another reason is that the malignant cells may be adherent to the laparoscopic instruments that are introduced and removed through the ports, resulting in seeding of the port site and port site metastases (Hopkins 1999). Another issue is the adequacy of cancer clearance in terms of resection margins and the extent of lymph nodes removed with laparoscopy. Therefore, oncological safety (cancer clearance) is an important issue with laparoscopic and laparoscopic‐assisted gastrectomy. There is no Cochrane review on this topic.
Objectives
To assess the benefits and harms of laparoscopic gastrectomy or laparoscopy‐assisted gastrectomy versus open gastrectomy for people with gastric cancer. In particular, we planned to investigate the effects by patient groups, such as cancer stage, anaesthetic risk, and body mass index (BMI), and by intervention methods, such as method of anastomosis, type of gastrectomy and laparoscopic or laparoscopically‐assisted gastrectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported as full text, studies published as abstract only, and unpublished data.
Types of participants
We included adults undergoing gastrectomy for gastric adenocarcinoma (cancer). We included trials in which separate outcome data for people undergoing gastrectomy for gastric adenocarcinoma were available, even if some of the participants underwent gastrectomy for other causes, including lymphomas.
Types of interventions
We included trials comparing laparoscopic gastrectomy or laparoscopy‐assisted gastrectomy with open gastrectomy, provided that the only difference between the randomised groups was the use of laparoscopic (or laparoscopy‐assisted) or open method of access to the stomach. We excluded trials comparing totally laparoscopic gastrectomy with laparoscopy‐assisted gastrectomy or different methods of open or laparoscopic gastrectomy. We also excluded any trials comparing robot‐assisted gastrectomy with laparoscopic or open gastrectomy.
Types of outcome measures
Primary outcomes
-
Mortality
-
Short‐term mortality (in‐hospital mortality or mortality within three months)
-
Long‐term mortality (at maximal follow‐up)
-
-
Serious adverse events (within three months). We accepted the following definitions of serious adverse events.
-
Clavien‐Dindo classification (Clavien 2009; Dindo 2004): Grade III or more.
-
International Conference on Harmonisation ‐ Good Clinical Practice guideline (ICH‐GCP; ICH‐GCP 1996): serious adverse events defined as any untoward medical occurrence that results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, and results in persistent or significant disability/incapacity.
-
Individual complications that could clearly be classified as Grade III or more with the Clavien‐Dindo classification (Clavien 2009; Dindo 2004), or as a serious adverse event with the ICH‐GCP classification.
-
-
Health‐related quality of life (using any validated scale)
-
Short‐term (four weeks to three months)
-
Medium‐term (more than three months to one year)
-
Secondary outcomes
-
Recurrence (local recurrence, surgical wound recurrence (also called port site metastases in the laparoscopic group) or distal metastases)
-
Short‐term recurrence (within six months)
-
Long‐term recurrence (at maximal follow‐up)
-
-
Adverse events (within three months). We accepted all adverse events reported by the study author irrespective of the severity of the adverse event.
-
Perioperative blood transfusion requirements (during surgery or within one week after surgery) (whole blood or red cell transfusion).
-
Proportion of people requiring blood transfusion
-
Quantity of blood transfusion
-
-
Measures of earlier postoperative recovery
-
Length of hospital stay (including the index admission for gastrectomy and any surgical complication‐related re‐admission)
-
Time to return to normal activity (return to preoperative mobility without any additional carer support)
-
Time to return to work (in people who were employed previously)
-
-
Positive resection margins (presence of macroscopic or microscopic cancer tissue at the plane of resection) at histopathological examination after surgery.
-
Number of lymph nodes harvested during surgery.
We based the choice of the above clinical outcomes on the necessity to assess whether laparoscopic surgery results in adequate cancer clearance, is safe, and is beneficial in terms of decreased blood transfusion requirements; earlier postoperative recovery allowing earlier discharge from hospital, return to normal activity, and return to work; and improvement in health‐related quality of life. We highlight that the positive resection margins at histopathological examination after surgery, and the number of harvested lymph nodes during surgery, are surrogate outcomes, and we have included these in order to explore whether these are responsible for any differences in survival or mortality.
We included studies which met the inclusion criteria, irrespective of whether they reported the secondary outcomes.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished RCTs. The literature search identified potential studies in all languages. We translated the non‐English language papers and assessed them fully for potential inclusion in the review as necessary.
We searched the following electronic databases for identifying potential studies.
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 9) (Appendix 1).
-
MEDLINE (1966 to September 2015) (Appendix 2).
-
EMBASE (1988 to September 2015) (Appendix 3).
-
Science Citation Index (1982 to September 2015) (Appendix 4).
We also conducted a search of ClinicalTrials.gov (ClinicalTrials.gov; Appendix 5) and the World Health Organization ‐ International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/; Appendix 6). We performed all the searches on 5 September 2015.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies.
We searched for errata or retractions from eligible trials on PubMed on 7 October 2015 (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (LB and KG) independently screened titles and abstracts for inclusion all the potential studies that we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve' (ineligible). We retrieved the full text study reports for coded as 'retrieve' and two review authors (LB and KG) independently screened the full text, identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and Characteristics of excluded studies table.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. Two review authors (LB and KG) extracted study characteristics from included studies and detailed them in a Characteristics of included studies table. We extracted the following study characteristics.
-
Methods: study design, total duration of the study and run in, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: number, mean age, gender, tumour stage, tumour location, American Society of Anesthesiologists (ASA) status (ASA 2014), inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, and concomitant interventions.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (LB and KG) independently extracted outcome data from included studies. If outcomes were reported multiple times for the same time frame (for example, short‐term health‐related quality of life was reported at six weeks and three months), we chose the later time point (i.e. three months) for data extraction. For time‐to‐event outcomes where data is censored, we extracted data to calculate the natural logarithm of the hazard ratio (HR) and its standard error using the methods suggested in Parmar 1998.
We included all randomised participants for medium‐term and long‐term outcomes (e.g. mortality or quality of life), and this was not conditional upon the short‐term outcomes (e.g. being alive at three months or having a low or high quality of life index at three months).
We noted in the Characteristics of included studies table if outcome data are reported in an unusable way. We resolved disagreements by consensus. One review author (LB) copied across the data from the data collection form into Review Manager 5 (RevMan 2014). We double‐checked that the data were entered correctly by comparing the study reports with how the data are presented in the systematic review.
Assessment of risk of bias in included studies
Two review authors (LB and KG) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low, or unclear risk of bias and provided a quotation from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
We considered trials were at low risk of bias in all domains to be at overall low risk of bias. Other trials were considered to be at unclear or high risk of bias. When considering treatment effects, we took into account the risk of bias for the studies that contribute to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RRs) with 95% CIs and continuous data as mean differences (MDs) with 95% CIs when the outcome was reported or converted to the same units in all the trials (e.g. hospital stay, time to return to work) or standardised mean differences (SMDs) with 95% CIs when different scales were used for measuring the outcome (e.g. quality of life). We ensured that higher scores for continuous outcomes had the same meaning for the particular outcome, explained the direction to the reader, and reported where the directions were reversed, if this was necessary. We calculated the rate ratio (RaR) with 95% CIs for outcomes such as adverse events and serious adverse events, where it is possible for the same person to develop more than one adverse event (or serious adverse event). We did not identify any studies that reported the RaR of adverse events (or serious adverse events) in the intervention versus control based on Poisson regression. We calculated the HR for time‐to‐event outcomes such as long‐term mortality, long‐term recurrence, and time‐to‐first adverse event (or serious adverse event).
We undertook meta‐analyses only where this was meaningful (i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
A common way that trial authors indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we noted that the data were skewed by following the rough guide for identifying skewed distribution available in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and considered the implication of this.
Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. laparoscopy‐assisted gastrectomy versus open gastrectomy and totally laparoscopic gastrectomy versus open gastrectomy) had to be entered into the same meta‐analysis, we halved the control group to avoid double‐counting. The alternative way of including such trials with multiple arms is to pool the results of the laparoscopy‐assisted gastrectomy and totally laparoscopic gastrectomy and compare it with open gastrectomy. We performed a sensitivity analysis to determine if the results of the two methods of dealing with multi‐arm trials led to different conclusions.
Unit of analysis issues
The unit of analysis was individual participants undergoing gastrectomy. We did not encounter any cluster‐randomised trials for this review, and therefore did not require any specific methodology for this trial type.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as abstract only). If we were unable to obtain the information from the investigators or study sponsors, we imputed mean from median (i.e. consider median as the mean) and standard deviation from standard error, interquartile range, or P values according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), but assessed the impact of including such studies in a sensitivity analysis. If we were unable to calculate the standard deviation from the standard error, interquartile range, or P values, we imputed the standard deviation as the highest standard deviation in the remaining trials included in the outcome, fully aware that this method of imputation will decrease the weight of the studies in the meta‐analysis of MD and shift the effect towards no effect for SMD.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity as per the Cochrane Handbook for Systematic Reviews of Interventions (i.e. greater than 50% to 60%; Higgins 2011), we explored it by pre‐specified subgroup analysis.
Assessment of reporting biases
We attempted to contact study authors, asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results using a sensitivity analysis.
If we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible publication biases. We used Egger's test to determine the statistical significance of the reporting bias (Egger 1997). We considered a P value less than 0.05 statistically significant for reporting bias.
Data synthesis
We performed analyses using Review Manager 5 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Totally laparoscopic and laparoscopy‐assisted gastrectomy.
-
Different cancer stages (early gastric cancer and advanced gastric cancer; node‐positive and node‐negative gastric cancer). For this, we defined early gastric cancer as tumours confined to mucosa and submucosa, irrespective of lymph node metastasis (Japanese Gastric Cancer Association 2011).
-
Different types of gastrectomy (proximal, pylorus‐preserving, subtotal, total gastrectomy).
-
Different methods of anastomoses (stapler versus hand‐sewn anastomoses).
-
People with different anaesthetic risk (ASA I (a healthy person) or ASA II (a person with mild systemic disease) versus ASA III or more (a person with severe systemic disease or worse).
-
Different body mass index (BMI) (healthy weight (BMI 18.5 to 25) versus overweight or obese (BMI 25 or greater).
We used all primary outcomes in subgroup analyses.
We used the formal Chi2 test for subgroup differences to test for subgroup interactions.
Sensitivity analysis
We performed sensitivity analyses defined a priori, to assess the robustness of our conclusions. These involved:
-
excluding trials at unclear or high risk of bias (one of more of the risk of bias domains (other than blinding of surgeon) classified as unclear or high);
-
excluding trials in which either mean or standard deviation, or both are imputed;
-
excluding cluster‐RCTs in which the adjusted effect estimates are not reported; and
-
different methods of dealing with multi‐arm trials (see Measures of treatment effect).
'Summary of findings' table
We created two 'Summary of findings' tables. summary of findings Table for the main comparison includes all of the pre‐specified primary outcomes that have been reported in the studies (short‐term mortality, long‐term mortality, and serious adverse events); summary of findings Table 2 includes all of the pre‐specified secondary outcomes that have been reported in the studies (long‐term recurrence, adverse events, perioperative blood transfused, length of hospital stay, and number of lymph nodes harvested). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for the pre‐specified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and using GRADEproGDT software (GRADEproGDT 2015). We justified all decisions to downgrade or upgrade the quality of studies using footnotes and made comments to aid reader's understanding of the review, where necessary. We considered whether there was any additional outcome information that we were unable to incorporate into the meta‐analyses, noted this in the comments, and stated if it supported or contradicted the information from the meta‐analyses.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice, and our Implications for research will give the reader a clear sense of where the focus of any future research in the area should be, and what the remaining uncertainties are.
Results
Description of studies
Results of the search
We identified 18,369 references through electronic searches of the Cochrane Central Register of Controled trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index, ClinicalTrials.gov and WHO ICTRP (World Health Organization International Clinical Trials Registry Platform). After completion of manually removing duplicate references there were 11,953 references. We excluded 11,914 clearly irrelevant references through reading the abstracts. We sought 42 references in full text for further assessment. We did not identify any additional references to trials by searching the trial registry. We excluded 13 references (11 studies or comments) because of the reasons mentioned in the Characteristics of excluded studies tables and Excluded studies. We excluded five references (three trials) which were protocols of ongoing trials with no interim results available. Thirteen trials (24 references) met the inclusion criteria and were included in this review (Aoyama 2014; Cai 2011; Chen Hu 2012; Deng 2009; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013). The reference flow diagram is shown in Figure 3.
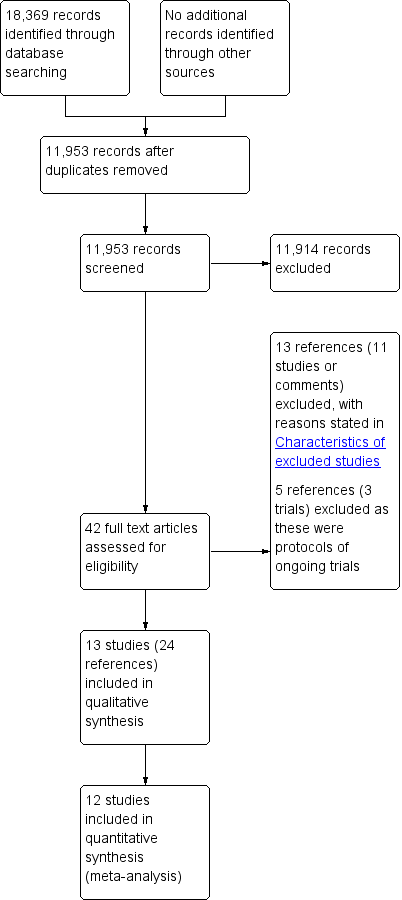
Study flow diagram.
Included studies
The thirteen trials compared laparoscopic with open gastrectomy (Aoyama 2014; Cai 2011; Chen Hu 2012; Deng 2009; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013). Twelve of the trials were two‐armed RCTs (Aoyama 2014; Cai 2011; Deng 2009; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013). The thirteenth was a four‐armed trial (Chen Hu 2012). Two of the arms involved laparoscopic surgery (fast‐track laparoscopic gastrectomy versus standard procedure laparoscopic gastrectomy) and two arms involved open gastrectomy (fast‐track laparoscopic gastrectomy versus standard procedure laparoscopic gastrectomy). The exact tumour stages included for each trial are reported in the Characteristics of included studies tables. Broadly, five trials included patients with early stage gastric cancer (Hayashi 2005; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013), three trials included patients with only advanced gastric cancer (Cai 2011; Kim 2013; Hu 2015), four trials included patients with early or advanced gastric cancer, a wide range of cancer staging (Aoyama 2014; Chen Hu 2012; Huscher 2005; Kim 2015), and one trial did not specify the cancer staging of included patients (Deng 2009).
Four of the trials included patients with an ASA risk score of III (Cai 2011; Hu 2015; Huscher 2005; Kim 2015), one trial did not include patients with ASA risk score III (Sakuramoto 2013), and the remaining eight trials did not specify their inclusion or exclusion (Aoyama 2014; Chen Hu 2012; Deng 2009; Hayashi 2005; Kim 2013; Kitano 2002; Lee 2005; Takiguchi 2013). None of the 13 trials specifically stated the inclusion or exclusion of patients with a BMI greater than 30.
Ten of the studies used laparoscopy‐assisted gastrectomy (Aoyama 2014; Cai 2011; Chen Hu 2012; Hayashi 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013), with three potentially using the totally laparoscopic method (Deng 2009; Hu 2015; Huscher 2005). There was no mention of an incision to remove the specimen in these trials, and the procedure was termed laparoscopic gastrectomy in these trials. However, it should be noted that some trials which were really laparoscopic‐assisted gastrectomy (based on the description of the procedure) reported the procedure as laparoscopic gastrectomy. So, it is not clear whether any of the trials used totally laparoscopic gastrectomy.
Twelve of the trials involved patients in whom subtotal (distal) gastrectomy was performed (Aoyama 2014; Chen Hu 2012; Deng 2009; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013); in Cai 2011, proximal, distal or total gastrectomy was performed. D1 nodal dissection was performed in both groups in one trial (Hayashi 2005). D2 nodal dissection was performed in both groups in four trials (Cai 2011; Deng 2009; Hu 2015; Kim 2013). D1 or more nodal dissections were performed in both groups in three trials (Aoyama 2014; Huscher 2005; Kim 2015). In two trials, selected groups of lymph nodes were dissected in both groups (Sakuramoto 2013; Takiguchi 2013). In one trial, selected nodes were dissected in the laparoscopic group, while D2 nodal dissection was performed in the open group (Lee 2005). Information on nodal dissection was not available in two trials (Chen Hu 2012; Kitano 2002). Seven of the trials used the Billroth I method of anastomosis alone (Aoyama 2014; Deng 2009; Hayashi 2005; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013), four of the trials used either the Billroth I or II anastomosis, Roux en Y anastomosis, oesophagogastrostomy or oesophageal jejunostomy (Cai 2011; Chen Hu 2012; Huscher 2005; Kim 2015), and two did not state the method of anastomosis used (Hu 2015; Kim 2013). Five trials used staples as the anastomotic method (Aoyama 2014; Hayashi 2005; Lee 2005; Sakuramoto 2013; Takiguchi 2013). In the remaining trials, either a combination of stapler and hand‐sewn anastomosis were used (Huscher 2005), or the information on stapler versus hand‐sewn anastomosis was not available (Cai 2011; Chen Hu 2012; Deng 2009; Hu 2015; Kim 2013; Kim 2015; Kitano 2002). Drains were routinely used in both groups in one trial (Sakuramoto 2013), and no routine drains were used in either group in one trial (Aoyama 2014). In one 2 x 2 factorial trial in which the safety and effectiveness of laparoscopic versus open gastrectomy and fast‐track surgery versus conventional surgery, drains were used in participants who underwent fast‐track surgery in both the laparoscopic and open groups (Chen Hu 2012). Two trials reported drains being used in the laparoscopic gastrectomy group, but did not report whether drains were used routinely in the open gastrectomy group (Lee 2005; Takiguchi 2013). The information on drain use was not available in the remaining trials (Cai 2011; Deng 2009; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002).
The follow‐up period was not available for one trial (Hu 2015). The follow‐up period in the remaining trials were as follows.
-
Until discharge (Deng 2009; Sakuramoto 2013)
-
30 days (Aoyama 2014; Chen Hu 2012; Kim 2013; Kim 2015).
-
14 months (Lee 2005); 22 months (Cai 2011); 26 months (Kitano 2002).
-
42 months (Hayashi 2005); 52 months (Huscher 2005); 60 months (Takiguchi 2013).
In total, 2794 participants were randomised in the 13 trials included in this review. One trial which included 53 participants did not contribute any data for this review, because none of the outcomes included in the review were reported (Deng 2009). Two hundred and thirteen participants were excluded in the remaining 12 trials that contributed data, leaving a total of 2528 participants for whom data were available. Of these 2528 participants, 1288 were randomised to laparoscopic gastrectomy and 1240 to open gastrectomy (Aoyama 2014; Cai 2011; Chen Hu 2012; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013).
Excluded studies
We excluded a total of 13 references (11 studies or comments). We excluded three references because they were reports of a 'quasi‐randomised' control trial in which block allocation equivalent to alternate assignment was used (Kim 2008). We excluded two references because they were comments on Kim 2008 (Kim 2009; Liakakos 2009), and we excluded one reference because it was an editorial (Kanellos 2009). We excluded seven references because they were not RCTs (Han 2014; Kawamura 2008; Lee 2008; Lee 2009; Li 2014; Lin 2014; Sakuramoto 2009).
We excluded five other references because they were protocols for three trials which have not yet reported the results (Haverkamp 2015; Straatman 2015; Yoshikawa 2012). A summary of these trials is reported in Characteristics of ongoing studies.
Risk of bias in included studies
All the trials were at unclear or high risk of bias as shown in Figure 1 and Figure 2.
Allocation
Four trials were free from selection bias (Aoyama 2014; Kim 2013; Kim 2015; Sakuramoto 2013). These trials had a low risk of bias in random sequence generation and allocation concealment. The remaining trials had unclear risk of bias in at least one of the aspects of random sequence generation or allocation concealment.
Blinding
Seven of the trials were at unclear risk of performance bias because of a lack of reported information (Aoyama 2014; Cai 2011; Deng 2009; Huscher 2005; Kim 2015; Kitano 2002; Lee 2005), with the remaining six being at high risk of performance bias, with patients and healthcare providers not being blinded (Chen Hu 2012; Hayashi 2005; Hu 2015; Kim 2013; Sakuramoto 2013; Takiguchi 2013).
Ten of the trials were at unclear risk of detection bias because of a lack of reported information (Aoyama 2014; Cai 2011; Chen Hu 2012; Deng 2009; Hayashi 2005; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005), with the remaining three being at high risk of detection bias, with outcome assessors not being blinded (Hu 2015; Sakuramoto 2013; Takiguchi 2013).
Incomplete outcome data
We classified three trials at low risk of attrition bias as they described no post‐randomisation drop‐outs (Hayashi 2005; Hu 2015; Takiguchi 2013). Four trials were at unclear risk of attrition bias as the reports did not describe the participant flow clearly (Aoyama 2014; Deng 2009; Kitano 2002; Lee 2005). Six trials were at high risk of attrition bias as they had post‐randomisation drop‐outs which were likely to affect the effect estimates (Cai 2011; Chen Hu 2012; Huscher 2005; Kim 2013; Kim 2015; Sakuramoto 2013).
Selective reporting
We classified six of the trials at low risk of reporting bias, with both postoperative mortality and morbidity reported (Aoyama 2014; Cai 2011; Chen Hu 2012; Hayashi 2005; Huscher 2005; Sakuramoto 2013). We classified seven of the trials at high risk of reporting bias, as one or both of these were not reported (Deng 2009; Hu 2015; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Takiguchi 2013).
Other potential sources of bias
In one trial, a more extensive procedure (subtotal gastrectomy) was performed in open gastrectomy group compared to laparoscopic group (distal gastrectomy) (Lee 2005). This could potentially favour the laparoscopic group in terms of decreased complications, but favour the open group in terms of decreased long‐term recurrence and mortality. We did not detect any other sources of bias in the remaining trials.
Effects of interventions
See: Summary of findings for the main comparison Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (primary outcomes); Summary of findings 2 Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (secondary outcomes)
The outcomes reported in these trials were: short‐term mortality, long‐term mortality, serious adverse events within three months, short‐term recurrence, long‐term recurrence, adverse events within three months, blood transfusion during or within a week of surgery, quantity of perioperative blood transfused, length of hospital stay, positive resection margins on histopathology, and number of lymph nodes harvested. The remaining outcomes of interest in the review, i.e. short‐ and medium‐term health‐related quality of life, time to return to normal activity, and time to return to work were not reported in any of the trials. The results are partially summarised in summary of findings Table for the main comparison and summary of findings Table 2.
Short‐term mortality
Eleven trials reported short‐term mortality which is defined as mortality in hospital or within thirty days of treatment (Aoyama 2014; Cai 2011; Chen Hu 2012; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013). We pooled the trials using a fixed‐effect model. There was no significant difference in the proportion of participants who died within 30 days of treatment between laparoscopic gastrectomy (7/1188: adjusted proportion (based on meta‐analysis) = 0.6%) and open gastrectomy (4/1447: 0.3%) (RR 1.60, 95% CI 0.50 to 5.10; participants = 2335; studies = 11; I2 = 0%; low quality evidence) (Analysis 1.1; summary of findings Table for the main comparison). There was no change to the conclusions when we used a random‐effects model or when we calculated the risk difference (RD 0.00, 95% CI ‐0.01 to 0.01; participants = 2335; studies = 11; I2 = 0%).
Long‐term mortality
Three trials reported long‐term mortality (Cai 2011; Huscher 2005; Takiguchi 2013). In one of these trials, all the participants were alive after a follow‐up period of 60 months (Takiguchi 2013). We pooled the trials using a fixed‐effect model. There was no significant difference in long‐term mortality between the groups (HR 0.94, 95% CI 0.70 to 1.25; participants = 195; studies = 3; I2 = 0%; very low quality evidence) (Analysis 1.2; summary of findings Table for the main comparison). There was no change to the conclusions when we used a random‐effects model. Approximately 55% to 60% of participants were alive after about 52 months (Huscher 2005).
Serious adverse events within three months
Eight trials reported serious adverse events within three months of treatment (Aoyama 2014; Cai 2011; Chen Hu 2012; Hayashi 2005; Huscher 2005; Kitano 2002; Lee 2005; Sakuramoto 2013). The serious adverse events in the trials included anastomotic leakage, anastomotic stenosis requiring balloon dilatation, pancreatic fistula, pancreatic injury, small bowel volvulus requiring adhesiolysis, bleeding requiring reoperation, abdominal abscess, myocardial infarction, acute respiratory distress syndrome, pleural effusion requiring puncture, and pneumonia. The type of serious adverse events were similar in nature between the groups. We pooled the trials using a fixed‐effect model. There was no significant difference in the proportion of participants who suffered a serious adverse event between laparoscopic gastrectomy (7/216: adjusted proportion = 3.6%) and open gastrectomy (13/216: 6%) within three months of treatment (RR 0.60, 95% CI 0.27 to 1.34; participants = 432; studies = 8; I2 = 0%; very low quality evidence) (Analysis 1.3; summary of findings Table for the main comparison). There was no change to the conclusions when we used a random‐effects model or when we calculated risk difference.
Health‐related quality of life
Short‐ and medium‐term health‐related quality of life were not reported in any of the trials.
Short‐term recurrence
Three trials reported short‐term recurrence, which is defined as local recurrence, surgical wound recurrence or distal metastases within six months (Hayashi 2005; Kitano 2002; Lee 2005). No events were reported for either laparoscopic (52 participants) or open gastrectomy (51 participants). Therefore, we could not calculate an effect estimate (participants = 103; studies = 3) (Analysis 1.4).
Long‐term recurrence
Four trials reported long‐term recurrence (> six months) (Hayashi 2005; Huscher 2005; Kitano 2002; Lee 2005). Three of these three trials did not report any recurrence in either group (Hayashi 2005; Kitano 2002; Lee 2005). There was no significant difference in the hazard ratio for recurrence more than six months after treatment between the two groups (HR 0.95, 95% CI 0.70 to 1.30; participants = 160; studies = 4; very low quality evidence) (Analysis 1.5; summary of findings Table 2). Since only one trial contributed to the analysis, the issue of fixed‐effect versus random‐effects meta‐analysis and assessment of heterogeneity did not arise.
Adverse events within three months
Eleven trials reported adverse events within three months of treatment (Aoyama 2014; Cai 2011; Chen Hu 2012; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013). We pooled the trials using a random‐effects model. There were significantly fewer adverse events following laparoscopic gastrectomy (204/268: adjusted proportion = 16.1%) versus open gastrectomy (253/1222: 20.7%) (RR 0.78, 95% CI 0.60 to 1.01; participants = 2490; studies = 11; I2 = 38%; very low quality evidence) (Analysis 1.6: summary of findings Table 2). However, on using the fixed‐effect model, there was no statistically significant difference between the groups (RR 0.78, 95% CI 0.66 to 0.92; participants = 2490; studies = 11; I2 = 38%). Two large trials had narrow confidence intervals and had results in opposite directions, and this may account for the differences between the fixed‐effect and random‐effects models. In addition, we performed a sensitivity analysis excluding Lee 2005, in which the laparoscopic group underwent a less invasive procedure than the open group. There was no change in the results by excluding this trial.
Blood transfusion during or within a week of surgery
Two trials reported the proportion of patients requiring blood transfusion during or within a week of surgery (Aoyama 2014; Takiguchi 2013). None of the participants in either group in either of the trials required a blood transfusion. Therefore, we could not estimate an effect estimate (participants = 66; studies = 2) (Analysis 1.7). Since both trials reported the mean and standard deviation, we did not perform any sensitivity analysis excluding studies in which standard deviation was imputed.
Quantity of perioperative blood transfused
Two trials reported quantity of perioperative blood transfused (Cai 2011; Lee 2005): Lee 2005 reported the number of units of blood transfused; and Cai 2011 reported the amount of blood transfused in SI units. We pooled the trials using a fixed‐effect model. There was no significant difference in the amount of blood transfused between the laparoscopic and open gastrectomy groups (SMD 0.05, 95% CI ‐0.27 to 0.38; participants = 143; studies = 2; I2 = 0%; very low quality evidence) (Analysis 1.8; summary of findings Table 2). There was no change to the conclusions when we used a random‐effects model.
Length of hospital stay
Eight trials reported length of hospital stay (Cai 2011; Chen Hu 2012; Hayashi 2005; Huscher 2005; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013). The length of hospital stay was significantly shorter in the laparoscopic group than the open group (MD ‐1.38 days, 95% CI ‐2.57 to ‐0.19; participants = 444; studies = 8; I2 = 76%; very low quality evidence) using the random‐effects model (Analysis 1.9; summary of findings Table 2). There was substantial heterogeneity as noted by I2 of 76% and Chi2 test for heterogeneity P value of 0.0001. Using the fixed‐effect model did not alter the results (MD ‐0.86 days, 95% CI ‐1.27 to ‐0.44; participants = 444; studies = 8; I2 = 76%). In two trials, median hospital stay rather than mean hospital stay was reported (Chen Hu 2012; Takiguchi 2013). In Takiguchi 2013, the standard deviation was calculated from the P value, while in Chen Hu 2012, the standard deviation was imputed as the highest standard deviation from the remaining trials. Excluding these two trials, there was no statistically significant difference between the two groups (MD ‐1.82 days, 95% CI ‐3.72 to 0.07; participants = 319; studies = 6; I2 = 83%) using the random‐effects model, although there was still a statistically significant difference between the groups using the fixed‐effect model (MD ‐0.68 days, 95% CI ‐1.31 to ‐0.06; participants = 319; studies = 6; I2 = 83%). The exclusion of Lee 2005, in which the laparoscopic group underwent a less invasive procedure did not alter the results.
Time to return to normal activity
This outcome was not reported in any of the trials.
Time to return to work
This outcome was not reported in any of the trials.
Positive resection margins at histopathological examination
One trial reported the number of patients with positive resection margins at histopathological examination (Kitano 2002). No events were reported for either laparoscopic or open gastrectomy. Therefore, we could not calculate an effect estimate (participants = 28; study = 1) (Analysis 1.10).
Number of lymph nodes harvested
Nine trials reported the number of lymph nodes harvested (Aoyama 2014; Cai 2011; Chen Hu 2012; Hayashi 2005; Huscher 2005; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013). We analysed the trials using a fixed‐effect model. There was no significant difference in the number of lymph nodes harvested between the two groups (MD ‐0.63, 95% CI ‐1.51 to 0.25; participants = 472; studies = 9; I2 = 40%; very low quality evidence) (Analysis 1.11; summary of findings Table 2). There was no change to the conclusions when we used a random‐effects model. Since higher numbers of lymph nodes harvested is a better surgical marker, we switched the direction of the X‐axis in Analysis 1.11, with trials to the left of the equivalence line favouring open gastrectomy. The mean or standard deviation or both were calculated from other information such as median, standard error, or P value or imputed from the maximum standard deviation in the included studies in four trials (Aoyama 2014; Chen Hu 2012; Huscher 2005; Takiguchi 2013). Excluding these trials did not alter the conclusions (MD ‐0.62, 95% CI ‐1.55 to 0.31; participants = 262; studies = 5; I2 = 70%).
Subgroup analysis
Of the planned subgroup analyses for the primary outcomes, only two were possible.
-
Different cancer stages (early gastric cancer and advanced gastric cancer). There was no short‐term mortality or long‐term mortality in laparoscopic or open gastrectomy groups in the early gastric cancer subgroup. So, we could not perform tests for subgroup differences (Analysis 2.1; Analysis 2.2). The test for subgroup differences was not statistically significant for subgroup analysis of serious adverse events, and there was a good overlap of confidence interval between the effect estimates of the different subgroups (Analysis 2.3).
-
Different types of gastrectomy (subtotal versus total gastrectomy). Of the 13 trials, 12 trials reported the use of subtotal gastrectomy (Aoyama 2014; Chen Hu 2012; Deng 2009; Hayashi 2005; Hu 2015; Huscher 2005; Kim 2013; Kim 2015; Kitano 2002; Lee 2005; Sakuramoto 2013; Takiguchi 2013). In Cai 2011, a mixture of different types of gastrectomies was performed. No separate data were available for the different types of gastrectomies. Excluding this trial did not alter the results (Analysis 2.4; Analysis 2.5; Analysis 2.6).
The remaining subgroup analyses were not possible for the following reasons.
-
Totally laparoscopic and laparoscopy‐assisted gastrectomy: it was not clear whether any of the trials used totally laparoscopic gastrectomy.
-
Different cancer stages (node‐positive and node‐negative gastric cancer): none of the trials reported the results separately for different nodal status.
-
Different methods of anastomoses (stapler versus hand‐sewn anastomoses): five trials used staples as the anastomotic method (Aoyama 2014; Hayashi 2005; Lee 2005; Sakuramoto 2013; Takiguchi 2013). In the remaining trials, either a combination of stapler and hand‐sewn anastomosis were used (Huscher 2005), or the information on stapler versus hand‐sewn anastomosis was not available (Cai 2011; Chen Hu 2012; Deng 2009; Hu 2015; Kim 2013; Kim 2015; Kitano 2002).
-
People with different anaesthetic risk (ASA I (a healthy person) or ASA II (a person with mild systemic disease) versus ASA III or more (a person with severe systemic disease or worse): none of the trials that included ASA III participants reported the results separately for ASA III participants.
-
Different BMI (healthy weight (BMI 18.5 to 25) versus overweight or obese (BMI 25 or greater)): none of the trials reported results separately for people with different BMI ranges.
Sensitivity analysis
The sensitivity analysis excluding trials in which either mean or standard deviation or both were imputed have been presented in the individual outcomes. Another sensitivity analysis excluding the trial in which the laparoscopic group underwent less invasive distal gastrectomy, and the open group underwent more invasive subtotal gastrectomy, did not alter the results. We performed the remaining sensitivity analyses for the following reasons.
-
All trials were at unclear or high risk of bias.
-
No cluster‐RCTs were included.
-
The only multi‐armed trial included was investigating fast‐track surgery. Since this variable was not of interest to this review, we considered the trial a two‐arm trial.
Reporting bias
Only two outcomes had 10 or more trials, namely, short‐term mortality and adverse events. We did not assess reporting bias using a funnel plot in the remaining comparisons. In the outcome, short‐term mortality, only three trials had one or more events in at least one of the groups (Hu 2015; Huscher 2005; Kim 2015). So, we did not assess reporting bias using a funnel plot for short‐term mortality either. Visual inspection revealed that studies with large variance were more prevalent in the laparoscopic group than the open group, suggesting potential reporting bias. However, the Egger's test was not statistically significant (P = 0.3144).
Discussion
Summary of main results
In this review, we compared laparoscopic versus open gastrectomy for people with non‐metastatic gastric cancer. There were no statistically significant differences in short‐term mortality, long‐term mortality, proportion of people with serious adverse events within three months of surgery, proportion of people with recurrence within six months, proportion of people with recurrence after six months, proportion of people requiring blood transfusion during or within a week of surgery, proportion of people with any adverse event within three months of surgery, quantity of perioperative blood transfused, proportion of people with positive resection margins at histopathological examination, or in the number of lymph nodes harvested by each technique. None of the trials reported patient oriented outcomes such as health‐related quality of life, time to return to normal activity, or time to return to work.
Short‐term mortality was reported in 2335 participants. Based on the number of participants included and the confidence intervals obtained by calculating the risk difference, it appears that there is no difference between laparoscopic and open gastrectomy in terms of mortality (i.e. this is lack of effect rather than lack of evidence of effect), although the risk of bias in the trials, mainly due to exclusion of participants who did not receive the planned treatment, introduces some doubt on this issue. Differences in serious adverse events within three months, length of hospital stay, long‐term recurrence, and long‐term mortality (which are the other major outcomes of interest for patients and healthcare funders) cannot be ruled out since the confidence intervals were wide.
Overall completeness and applicability of evidence
This review included participants undergoing either laparoscopic or open gastrectomy for gastric cancer. Although the American Society of Anesthesiologists (ASA) status was not reported in many trials, all the participants must have been fit for major surgery since both arms involve major surgeries. Thus, the results of this review are applicable only to patients with gastric cancer, with a variety of stages from early to advanced cancer, and are not applicable to patients who are not suitable for surgery either because of their anaesthetic risk or because of the location or presence of metastatic disease. It should also be noted that the results apply only to laparoscopy‐assisted distal gastrectomy since it was not clear whether three trials that did not report a small laparotomy incision performed totally laparoscopic gastrectomy, and because most trials in this review included participants undergoing distal gastrectomy.
Quality of the evidence
All the trials were at unclear or high risk of bias. Selection bias and funding bias were at unclear or low risk of bias in all trials. Those trials with unclear risk of bias were generally due to a lack of clear information. We graded both performance and detection bias as high risk of bias in a significant number of trials, with issues in blinding of the healthcare providers responsible for these. There was significant bias due to missing outcomes in the trials, with six of the studies at high risk of bias because of post‐randomisation drop‐outs. This can be easily avoided by using an intention‐to‐treat analysis, which involves reporting the outcomes for all randomised patients even if they do not receive the relevant treatment. There was significant selective reporting bias in the trials, with seven of the studies at high risk of bias generally because morbidity was not reported adequately. Severity of the outcomes is more important than stating whether an adverse event occurred.
There was significant heterogeneity in length of hospital stay. Because of the few trials included in each subgroup, the subgroup analysis may not be reliable and multiple subgroup analyses can lead to spurious results. A potential reason for this heterogeneity may be different criteria for discharging patients who had undergone gastrectomy in different trials. The trials did not report this sufficiently to determine if this was the reason for the differences in effect estimates in trials.
There was imprecision in many outcomes despite the inclusion of more than 2500 participants in the various trials included in this review. This was because of selective outcome reporting with trials not reporting even important outcomes such as serious adverse events.
Despite the shortcomings in the studies included in this review, these studies constitute the best level of evidence that is currently available. Overall, the evidence from this systematic review is more trustworthy than observational studies and expert opinions. This is because observational studies contain inherent bias. It is quite possible that people with lower tumour burden will be selected to undergo laparoscopic gastrectomy while those with greater tumour burden will undergo open gastrectomy. This will lead to bias due to confounding in observational studies.
Potential biases in the review process
We followed the Cochrane Handbook for Systematic Reviews of Interventions for this review (Higgins 2011). There were no language, publication status, or sample size restrictions. Thus, we minimised the bias due to selection of trials. There was suspicion of reporting bias for adverse events by visual inspection of funnel plots, although this was not substantiated by Egger's test. Since there was no restriction on the publication date, we included trials from the pre‐mandatory trial registration era. There is a possibility that some of the trials were not reported because of the direction of results. However, we have to be pragmatic and accept that it will be difficult to obtain useful data from these trials after such a long period of time. So, we have to arrive at conclusions based on the trials which have been published or reported in conferences. We calculated the hazard ratio for long‐term mortality and long‐term recurrence using methods suggested in Parmar 1998. This assumes constant proportional hazards. From the Kaplan‐Meier curves in the studies, the proportional hazards appeared constant for both long‐term mortality and long‐term recurrence.
Agreements and disagreements with other studies or reviews
This is the first Cochrane review to assess laparoscopic versus open gastrectomy for gastric cancer. We identified three previous systematic reviews of RCTs on this topic (Jiang 2013; Liang 2011; Sun 2012). The authors of these systematic reviews appear to suggest that laparoscopic surgery is better than open surgery for one or more short‐term outcomes, in particular, length of hospital stay. However, we are more cautious in concluding that laparoscopic surgery is better than open surgery because of the risk of bias in the studies included and the heterogeneity in the length of hospital stay.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 1 Short‐term mortality.
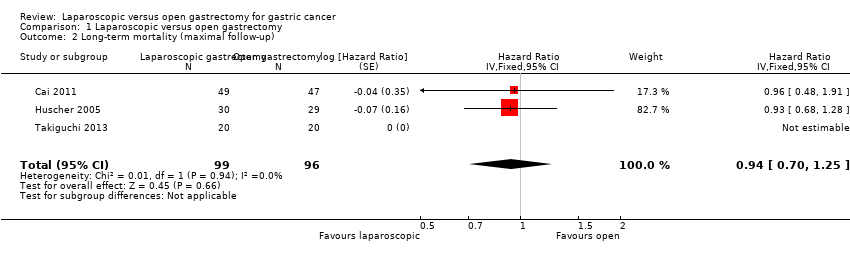
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 2 Long‐term mortality (maximal follow‐up).

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 3 Proportion with a serious adverse event (< 3 months).
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 4 Short‐term recurrence.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 5 Long‐term recurrence (maximal follow‐up).
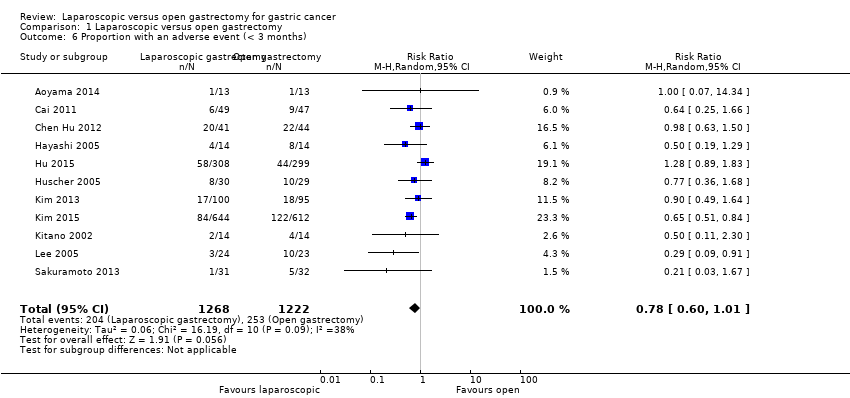
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 6 Proportion with an adverse event (< 3 months).

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 7 Proportion requiring blood transfusion during or within a week of surgery.
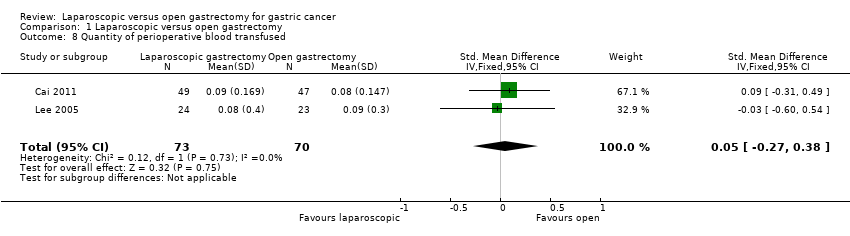
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 8 Quantity of perioperative blood transfused.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 9 Length of hospital stay.
Comparison 1 Laparoscopic versus open gastrectomy, Outcome 10 Proportion with positive resection margins at histopathological examination.

Comparison 1 Laparoscopic versus open gastrectomy, Outcome 11 Number of lymph nodes harvested.

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 1 Short‐term mortality (stratified by early versus advanced cancer).
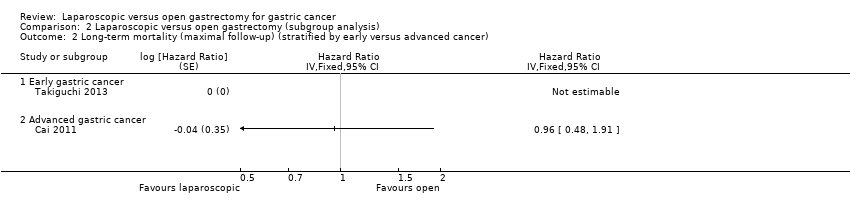
Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 2 Long‐term mortality (maximal follow‐up) (stratified by early versus advanced cancer).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 3 Proportion with a serious adverse event (< 3 months) (stratified by early versus advanced cancer).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 4 Short‐term mortality (stratified by type of gastrectomy).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 5 Long‐term mortality (maximal follow‐up) (stratified by type of gastrectomy).

Comparison 2 Laparoscopic versus open gastrectomy (subgroup analysis), Outcome 6 Proportion with a serious adverse event (< 3 months) (stratified by type of gastrectomy).
| Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (primary outcomes) | |||||
| Patient or population: patients with gastric cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Open gastrectomy | Laparoscopic gastrectomy | ||||
| Short‐term mortality | 3 per 1000 | 6 per 1000 | RR 1.60 | 2335 | ⊕⊕⊝⊝ |
| Long‐term mortality (maximal follow‐up) | 448 per 1000 | 428 per 1000 | HR 0.94 | 195 | ⊕⊝⊝⊝ |
| Proportion with a serious adverse event (< 3 months) | 60 per 1000 | 36 per 1000 | RR 0.60 | 432 | ⊕⊝⊝⊝ |
| Health‐related quality of life during short‐term (four weeks to three months) or medium‐term (more than three months to one year) was not reported. | |||||
| *The basis for the assumed risk was the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 There was unclear or high risk bias within the trials (downgraded by two levels). | |||||
| Laparoscopic gastrectomy compared to open gastrectomy for gastric cancer (secondary outcomes) | ||||||
| Patient or population: patients with gastric cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open gastrectomy | Laparoscopic gastrectomy | |||||
| Long‐term recurrence (maximal follow‐up) | 450 per 1000 | 433 per 1000 | HR 0.95 | 162 | ⊕⊝⊝⊝ | |
| Proportion with an adverse event (< 3 months) | 207 per 1000 | 161 per 1000 | RR 0.78 | 2490 | ⊕⊝⊝⊝ | |
| Quantity of perioperative blood transfused | The mean quantity of perioperative blood transfused in the control groups was | The mean quantity of perioperative blood transfused in the intervention groups was | 143 | ⊕⊝⊝⊝ | SMD 0.05 (‐0.27 to 0.38) | |
| Length of hospital stay | The mean length of hospital stay in the intervention groups was | 319 | ⊕⊝⊝⊝ | |||
| Number of lymph nodes harvested | The mean number of lymph nodes harvested in the control groups was | The mean number of lymph nodes harvested in the intervention groups was | 472 | ⊕⊝⊝⊝ | ||
| There were no events in either group for short‐term recurrence (103 participants (3 studies)), proportion requiring blood transfusion (66 participants (2 studies)), proportion with positive resection margin (incomplete cancer resection) (14 participants (1 study)). | ||||||
| None of the trials reported on measures of earlier postoperative recovery such as time to return to normal activity or time to return to work. | ||||||
| *The basis for the assumed risk was the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was unclear or high risk of bias within the trials (downgraded by two levels). Please see Figure 1 and Figure 2 which show this. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 11 | 2335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.50, 5.10] |
| 2 Long‐term mortality (maximal follow‐up) Show forest plot | 3 | 195 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.70, 1.25] |
| 3 Proportion with a serious adverse event (< 3 months) Show forest plot | 8 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.27, 1.34] |
| 4 Short‐term recurrence Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Long‐term recurrence (maximal follow‐up) Show forest plot | 4 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 6 Proportion with an adverse event (< 3 months) Show forest plot | 11 | 2490 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.01] |
| 7 Proportion requiring blood transfusion during or within a week of surgery Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Quantity of perioperative blood transfused Show forest plot | 2 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.27, 0.38] |
| 9 Length of hospital stay Show forest plot | 8 | 444 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.57, ‐0.19] |
| 10 Proportion with positive resection margins at histopathological examination Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Number of lymph nodes harvested Show forest plot | 9 | 472 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.51, 0.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality (stratified by early versus advanced cancer) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Early gastric cancer | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Advanced gastric cancer | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Long‐term mortality (maximal follow‐up) (stratified by early versus advanced cancer) Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Early gastric cancer | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Advanced gastric cancer | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Proportion with a serious adverse event (< 3 months) (stratified by early versus advanced cancer) Show forest plot | 5 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.60] |
| 3.1 Early gastric cancer | 4 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.39] |
| 3.2 Advanced gastric cancer | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 68.98] |
| 4 Short‐term mortality (stratified by type of gastrectomy) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Subtotal gastrectomy | 10 | 2239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.50, 5.10] |
| 5 Long‐term mortality (maximal follow‐up) (stratified by type of gastrectomy) Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Subtotal gastrectomy | 2 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Proportion with a serious adverse event (< 3 months) (stratified by type of gastrectomy) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Subtotal gastrectomy | 7 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.22, 1.22] |

