Antifibrinolitička terapija za prevenciju oralnog krvarenja kod pacijenata s hemofilijom ili Von Willebrandovom bolesti prilikom manjih oralnokirurških zahvata ili ekstrakcije zuba.
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Trial design: randomised double‐blind trial Follow up: 5 days | |
| Participants | Country: United Kingdom Setting: University Department of Medicine and Dental Unit, Glasgow Royal Infirmary Inclusion criteria: people with haemophilia undergoing dental extraction Exclusion criteria: ‐ history of haematuria in the previous 4 weeks ‐ presence of red cells in a fresh sample of urine Sample size: TXA group: ‐ total number of participants: n = 14 ‐ total number of episodes with dental extractions: n = 16 ‐ mean number (range) of roots extracted: n = 6.9 (2 ‐ 22) Placebo group: ‐ total number of participants: n = 14 ‐ total number of episodes with dental extractions: n = 16 ‐ mean number (range) of roots extracted: n=5.5 (2 ‐ 12) Numbers analysed/randomised: ‐ TXA group: 16/16 ‐ placebo group: 16/16 Participant characteristics: ‐ age 13 ‐ 65 years ‐ sex (M/F): not reported, probably all males ‐ TXA group: 11 haemophilia A, 3 haemophilia B | |
| Interventions | Both intervention and control group: FVIII or FIX equivalent of 1000 mL of human plasma intravenously 1 hour preoperatively. Intervention group: Start TXA tablets 2 hours preoperatively and continuation of TXA 1 g, 3 times a day for 5 days postoperatively. Control group: start placebo tablets 2 hours preoperatively and continuation of placebo treatment 3 times a day for 5 days postoperatively. | |
| Outcomes | 1. Amount of postoperative blood loss (mL) 2. Number of postoperative bleeding requiring intervention 3. Side effects or adverse events 4. Change in haemoglobin level from baseline 5. Need for and dose of clotting factor concentrates Outcome measurements: collection of oral and fecal secretions every 24 hours for five postoperative days. | |
| Notes | Bleeding definition: not clearly defined. Blood loss postoperatively, not further specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "By means of a double‐blind technique and random allocation to the trial the patients received either tranexamic acid ... or placebo tablets." (patients and methods page 322). No further details about the randomisation process are given. |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment is not reported. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up: the results of all participants are reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures are reported for all trial participants. |
| Other bias | Low risk | There is no indication that a contamination bias or other source of bias has occurred. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The physician" (and the participants) "did not know the results of the laboratory assays or which tablet the patient was receiving." The blinding procedure is not detailed in the paper. |
| Blinding of outcome assessment (detection bias) | Low risk | "The clinician did not know the results of the laboratory assays or which tablet the patient was receiving." |
| Methods | Trial design: double‐blind quasi‐randomised controlled trial in 2 centres Follow up: 7 ‐ 10 days | |
| Participants | Country: United Kingdom Setting: Oxford Haemophilia Centre and a centre at Cardiff Inclusion criteria: patients aged 12 or more, with factor‐VIII or IX levels of 15% average normal or less, admitted to the hospital for extraction of permanent teeth under general anaesthesia and antibiotic prophylaxis Exclusion criteria: ‐ People with factor‐VIII inhibitors ‐ People with active hematuria within the previous months ‐ People with renal failure (blood urea > 60 mg/100 ml) ‐ People in whom major surgical procedures other than tooth extraction were planned during the 10 day of follow up Sample size: Total 31 participants (Oxford n = 23, Cardiff n = 8) EACA group: ‐ number of participants: n = 15 (Oxford n = 11, Cardiff n = 4) ‐ average number of teeth removed: Oxford 7.7; Cardiff 7.5 Placebo group: ‐ number of participants: n = 16 (Oxford n = 12, Cardiff n = 4) ‐ average number of teeth removed: Oxford 6.7; Cardiff 9.0 Participant characteristics: ‐ mean age (years, age range not stated) Oxford: placebo 32.2; EACA 32.1 ‐ Sex (M/F): 31/0 ‐ Baseline: EACA group: 12 haemophilia A, 3 haemophilia B placebo group: 12 haemophilia A, 4 haemophilia B mean per cent average normal plasma factor Oxford placebo: 3.6 ; EACA: 2.4 Cardiff placebo: 3.5 ; EACA: 3.8 | |
| Interventions | Both intervention and control group: 1 initial dose of plasma replacement therapy within 1 hour preoperatively aimed to raise factor VIII of IX level to 50%. Intervention group: Plasma replacement therapy immediately followed by intravenous infusion of 6 g EACA in 250 ml isotonic saline and 6 g orally 4 times daily for 7 ‐ 10 days. Control group: Plasma replacement therapy immediately followed by intravenous infusion of placebo (isotonic saline alone) and placebo tablets orally 4 times daily for 7 ‐ 10 days. | |
| Outcomes | 1. Number of postoperative bleeding episodes requiring intervention 2. Side effects or adverse events 3. Change in haemoglobin level from baseline 4. Major bleeding, requiring transfusion of packed red blood cells 5. Need for and dose of clotting factor concentrates | |
| Notes | Bleeding definition: Intra‐oral bleeding, not further specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “… Thereafter, on the basis of the patients’ ages, results of factor‐VIII or factor‐IX assays and number and type of teeth to be extracted, the physician responsible for administration of the test agents assigned the patients to the treatment groups (EACA or placebo) on the basis of a pair‐matching technique.” |
| Allocation concealment (selection bias) | High risk | "...the physician responsible for administration of the test agents assigned the patients to the treatment groups (EACA or placebo) on the basis of a pair‐matching technique." |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow up. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures were reported. |
| Other bias | Low risk | There is no indication that a contamination bias or other source of bias has occurred. |
| Blinding of participants and personnel (performance bias) | Unclear risk | “..the physicians responsible for the care of the patient were kept ‘blind’ to all assigned treatments for the duration of the trial” The blinding procedure is not detailed in the paper. |
| Blinding of outcome assessment (detection bias) | Low risk | “In the event of intraoral or extraoral bleeding, the decision to administer therapeutic materials was made by physicians unaware of the assigned treatment.” |
EACA: epsilon aminocaproic acid
FIX: factor IX
FVII: factor VIII
TXA: tranexamic acid
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not on dental extraction or oral surgery. | |
| Not on dental extraction or oral surgery. | |
| Letter to editor, not a clinical trial. | |
| Editorial and case report, not a RCT. | |
| Editorial, not a clinical trial. | |
| Letter to the editor, not a RCT. | |
| Case series with historical controls, not a RCT. | |
| Case series, not a RCT. | |
| Letter to editor, not a clinical trial. | |
| Description of one of the included studies (Walsh 1971), not a RCT. | |
| Case series, not a RCT. | |
| Not on dental extraction or oral surgery. | |
| Not on dental extraction or oral surgery. | |
| Case series, not a RCT. | |
| Groups not comparable with respect to effect of tranexamic acid only. Dental scaling is not considered as oral surgery. | |
| Case series, not a RCT. | |
| Case report, not a RCT. | |
| Not a RCT. | |
| Case series, not a RCT. | |
| Not on dental extraction or oral surgery. | |
| Case report, not a RCT. | |
| No placebo or usual care as comparison. | |
| No placebo or usual care as comparison. | |
| Case series, not a RCT. | |
| No full text available. | |
| Not on TXA or EACA, not on dental extraction or oral surgery. | |
| All patients received TXA systemically. | |
| Case series, no RCT. |
EACA: epsilon aminocaproic acid
RCT: randomised controlled trial
TXA: tranexamic acid
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people with postoperative bleedings Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | ‐0.57 [‐0.76, ‐0.37] |
| Analysis 1.1  Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 1 Number of people with postoperative bleedings. | ||||
| 2 Number of side effects requiring withdrawal Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.13] |
| Analysis 1.2 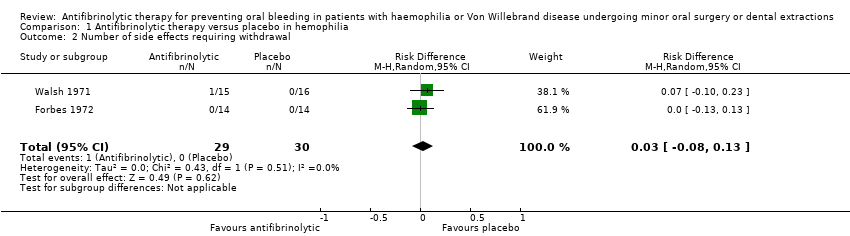 Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 2 Number of side effects requiring withdrawal. | ||||
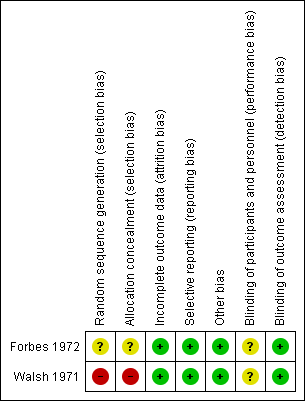
Risk of bias summary of the included studies
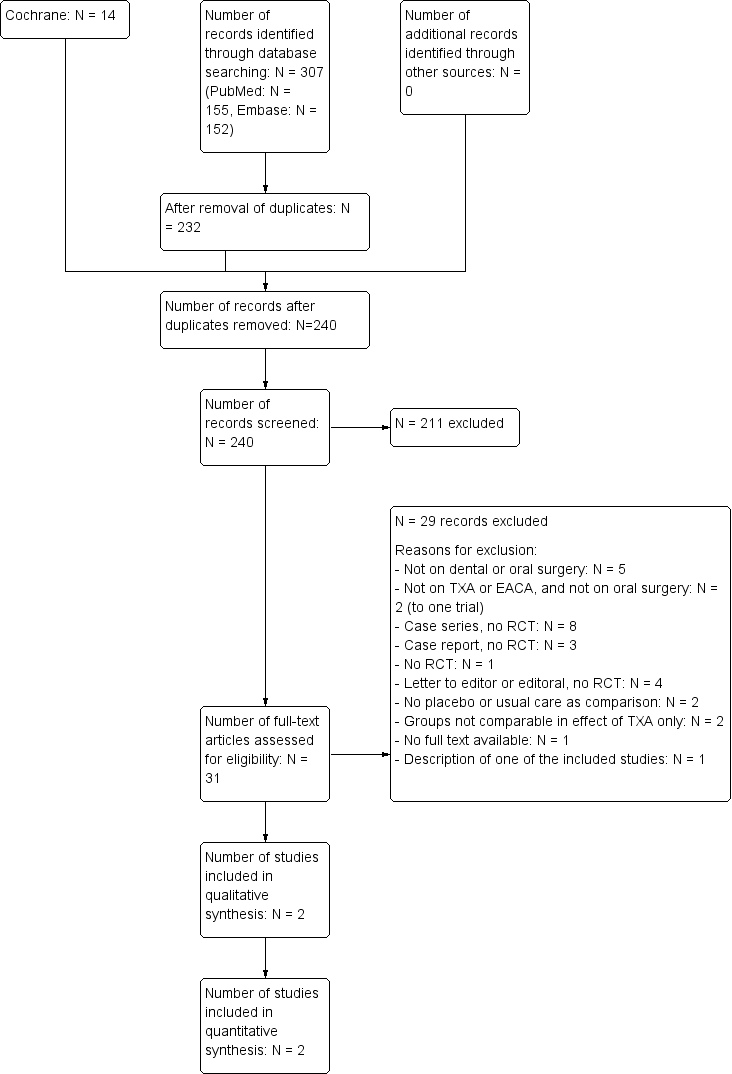
Trial flow diagram
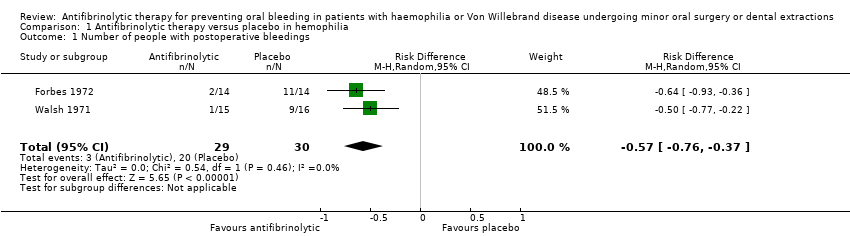
Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 1 Number of people with postoperative bleedings.

Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 2 Number of side effects requiring withdrawal.
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Outcomes | Anticipated absolute effects* | Relative effect | № of participants | Number needed to treat (NNT) | Quality of the evidence | Comments | |
| Risk with placebo or usual care | Risk with antifibrinolytic therapy | ||||||
| Postoperative bleedings requiring intervention | Study population | Risk difference: 57 | 59 | 1.8 | ⊕⊕⊕⊝ | ||
| 67 per 100 (20 events) | 10 per 100 (3 events) | ||||||
| Side effects or other adverse events | Study population | Risk difference: 3.4 | 59 | 0.3 | ⊕⊕⊝⊝ | ||
| 0 events | 1 event | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 The magnitude of effect was considered large in this study, therefore the quality of evidence was rated up one level. 2 Small sample sizes and lack of studies 3 Heterogeneity between the included trials regarding the proportion of severe people with haemophilia included, the concomitant standard therapy and fibrinolytic agent treatment regimens used | |||||||
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Primary outcomes | 1. Postoperative bleedings requiring intervention | 2. Side effects or other adverse events | |||||
| Absolute risks % (n/ntotal) | Risk difference (RD) | Number needed to treat (NNT) | Absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | |||
| Control | Intervention | Control | Intervention | ||||
| 79% (11/14) | 14% (2/14) | 65% (37 ‐ 93) | 1.5 | 0% (0/14) | 0% (0/14) | 0% | |
| 56% (9/16) | 7% (1/15) | 49% (21 ‐ 77) | 2.0 | 0% (0/16) | 7% (1/15) | 7% (‐0.06 ‐ 20) | |
| n: number; CI: confidence interval | |||||||
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | ||||||||
| 1. Number of people with postoperative bleeding | 2. Side effects or other adverse events | |||||||
| Combined absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | Number needed to treat (NNT) | Combined absolute risks % (n/ntotal) | Risk difference (RD)(% (95% CI) | Number needed to treat (NNT) | |||
| Control | Intervention | Control | Intervention | |||||
| 67% (20/30) | 10% (3/29) | 56% (36% ‐ 76%) | 1.8 | 0% (0/30) | 3.4 (1/29) | 3.4 (‐32 ‐ 38) | 0.3 | |
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||
| Patient or population: Patients with haemophilia or Von Willebrand disease Settings: Hospitals, Hemophilia centres Intervention: Tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: Placebo or no intervention or usual care with or without placebo | ||||||
| Secondary outcomes | 1. (Mean) fall in haemoglobin level from baseline | 2. Amount of postoperative blood loss: mean per patient mL (range) | 3. Need for and dose of clotting factor concentrates: Mean number of units IU (range) of replacement therapy per root extracted (A) / per patient (B) | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| 1.4 g/100mL | 0.3 g/100mL | 84.1 mL (4‐323) | 61.2 mL (1‐749) | 617 IU (0‐15800) in eleven participants | 30 IU and 65 IU in two participants | |
| Trial centre 1: 6.3% Trial centre 2: 6.3% | Trial centre 1: 3.9% Trial centre 2: 3.7% | NR | NR | Trial centre 1: 2331 IU Trial centre 2: 1881 IU | Trial centre 1: 145 IU Trial centre 2: 0 IU | |
| Abbreviations:NR: not reported | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people with postoperative bleedings Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | ‐0.57 [‐0.76, ‐0.37] |
| 2 Number of side effects requiring withdrawal Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.13] |

