Intervenciones farmacológicas para la pancreatitis aguda
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised clinical trial | |
| Participants | Country: India Number randomised: 135 Postrandomisation dropouts: 6 (4.4%) Revised sample size: 129 Average age: 39 years Women: 13 (10.1%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 62 (48.1%) Moderate pancreatitis: not stated Severe pancreatitis: 67 (51.9%) Persistent organ failure: not stated Infected pancreatitis: 0 Inclusion criteria
| |
| Interventions | Group 1: ulinastatin (n = 30), 200,000 IU twice daily for 5 days Group 2: placebo (n = 32) | |
| Outcomes | Mortality, adverse events, organ failure, hospital stay Follow‐up: until discharge or maximum of 22 days | |
| Notes | Reasons for postrandomisation dropouts: withdrew consent, screening error, died | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled, multi‐centre trial across 15 centres in India". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled, multi‐centre trial across 15 centres in India". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Sweden | |
| Interventions | Group 1: aprotinin (n = 26), 500,000 KIU in lavage fluid every 2 h for an average of 2.7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, sepsis, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Comment: supported by grants from the ….Bayer AG…. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: India Number randomised: 44 Postrandomisation dropouts: 5 (11.4%) Revised sample size: 39 Average age: 39 years Women: 9 (23.1%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis within 96 h of onset of symptoms Exclusion criteria
| |
| Interventions | Group 1: antioxidants (n = 19): vitamin A, C, E ‐ initially parenterally and then orally when the participant could consume orally for a total of 14 days | |
| Outcomes | Mortality, serious adverse events, adverse events, organ failure, hospital stay Follow‐up: until discharge | |
| Notes | Reasons for postrandomisation dropouts: lost to follow‐up, withdrew consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]his was a single‐center, prospective randomized, open‐label with blinded endpoint assessment study of antioxidant therapy". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[t]his was a single‐center, prospective randomized, open‐label with blinded endpoint assessment study of antioxidant therapy". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Low risk | Quote: "[s]ource of support: Nil". |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial | |
| Participants | Country: Peru Number randomised: 80 Postrandomisation dropouts: 22 (27.5%) Revised sample size: 58 Average age: 50 years Women: 24 (41.4%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 58 (100%) Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with necrotising pancreatitis Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 24): imipenem 500 mg 4 times daily for 14 days | |
| Outcomes | Mortality, serious adverse events, adverse events, infected pancreatic necrosis, requirement for additional intervention, length of hospital stay Follow‐up: 2 months | |
| Notes | Reasons for postrandomisation dropouts: protocol violations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: multicentric, international Number randomised: 48 Postrandomisation dropouts: not stated Revised sample size: 48 Average age: 56 years Women: 17 (35.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 48 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: participants with acute severe pancreatitis with circulatory insufficiency or peritonitis Exclusion criteria
| |
| Interventions | Group 1: aprotinin (n = 22), 20 million KIU in 7 lavages over 30 h | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, sepsis, hospital stay, ICU stay Follow‐up: 1 month | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he Bayer . . . and was also responsible for coding the bottles." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "prospective double‐blind randomized multicenter trial" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "prospective double‐blind randomized multicenter trial" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[t]his study was supported by grants from Bayer AG". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Netherlands | |
| Interventions | Group 1: probiotics (n = 152): ecologic 641 (maximum of 28 days or until development of pancreatic necrosis or fluid collection) | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, organ failure, infected pancreatic necrosis, hospital stay, ICU stay Follow‐up: 3 months | |
| Notes | Reasons for postrandomisation dropouts: did not receive drug, wrong diagnosis of acute pancreatitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[r]andomisation was done with a computer‐generated permuted‐block sequence.". |
| Allocation concealment (selection bias) | Low risk | Quote: "[b]oth the probiotic and placebo preparations were packaged in identical, numbered sachets that were stored in identical, numbered containers." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[a]ll doctors, nurses, research staff , and patients involved remained unaware of the actual product administered during the entire study period." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[a]ll doctors, nurses, research staff , and patients involved remained unaware of the actual product administered during the entire study period." |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "HMT is an employee of Winclove Bio Industries, Amsterdam". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany | |
| Interventions | Group 1: antioxidants (n = 10): sodium selenite 600 μg/day for 8 days | |
| Outcomes | None of the outcomes of interest were reported. Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias. |
| Methods | Randomised clinical trial | |
| Participants | Country: Denmark Number randomised: 66 Postrandomisation dropouts: 9 (13.6%) Revised sample size: 57 Average age: not stated Women: 26 (45.6%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria:
| |
| Interventions | Group 1: NSAID (n = 27): indomethacin 100 mg rectal for 7 days | |
| Outcomes | The outcomes reported were: hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: chronic pancreatitis, wrong diagnosis, death | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany Number randomised: 223 Postrandomisation dropouts: not stated Revised sample size: 223 Average age: 50 years Women: 87 (39%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with moderate or severe acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: gabexate mesilate (n = 115), 53 mg/kg/day for 7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, sepsis, hospital stay Follow‐up: 3 months | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[a] randomization list was applied to get a random sequence of GM and placebos for increasing package numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he drug packages for each hospital were numbered sequentially and the package number was used as patient number" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomized, double‐blind trial" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "randomized, double‐blind trial" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Taiwan | |
| Interventions | Group 1: gabexate mesilate (n = 26), 100 mg/h for 7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery Follow‐up: 3 months | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Unclear risk | Comment: this information was not available. |
| Methods | Randomised clinical trial | |
| Participants | Country: China | |
| Interventions | Group 1: ulinastatin (n = 48), 50,000 IU twice daily for 3 days followed by once daily for 5 days | |
| Outcomes | Serious adverse events, adverse events Follow‐up: not stated (probably 2 weeks) | |
| Notes | Reasons for postrandomisation dropouts: recent or current treatment with other drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China | |
| Interventions | Group 1: ulinastatin (n = 14), 100,000 IU twice daily for 3 days followed by 50,000 IU once daily for 5‐10 days | |
| Outcomes | Adverse events Follow‐up: not stated (probably 2 weeks) | |
| Notes | Reasons for postrandomisation dropouts: death after starting treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Hong Kong, China Exclusion criteria: people with acute pancreatitis caused by trauma, iatrogenic, or malignancy | |
| Interventions | Group 1: somatostatin (n = 35), 250 μg bolus followed by 100 μg/h for 48 h | |
| Outcomes | Mortality, serious adverse events, adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[r]andomisation was done by drawing sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Ukraine | |
| Interventions | Group 1: antioxidants (N‐acetyl cysteine, unspecified dose and duration) plus corticosteroids (dexamethasone, unspecified dose and duration) (n = 16) | |
| Outcomes | None of the outcomes of interest were reported. Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Unclear risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Canada | |
| Interventions | Group 1: glucagon (n = 33), 1 mg every 3 h (duration not stated) | |
| Outcomes | Mortality, serious adverse events, adverse events, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[o]nce we decided to enter a patient into the study, the hospital pharmacy randomly assigned…" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[p]rospective randomized double‐blind study" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[p]rospective randomized double‐blind study" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: France Number randomised: 23 Postrandomisation dropouts: 0 (0%) Revised sample size: 23 Average age: 43 years Women: 2 (8.7%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: 23 (100%) Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 11), ceftazidime 2 g IV 3 times daily; amikacin 7.5 mg/kg IV BD; and metronidazole 0.5 g IV 3 times daily for 10 days | |
| Outcomes | Mortality, serious adverse events, requirement for surgery, requirement for endoscopic or radiological drainage, organ failure, infected pancreatic necrosis, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random‐number table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: France Number randomised: 81 Postrandomisation dropouts: not stated Revised sample size: 81 Average age: 47 years Women: 14 (17.3%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 81 (100%) Mild pancreatitis: 0 (0%) Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 53): ciprofloxacin for 7 days or 21 days (random choice); dose not stated | |
| Outcomes | Mortality, serious adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: multicentric, international Number randomised: 100 Postrandomisation dropouts: 0 (0%) Revised sample size: 100 Average age: 50 years Women: 30 (30%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 100 (100%) Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 100 (100%) Persistent organ failure: not stated Infected pancreatitis: 0 Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 50): meropenem 1 g IV 3 times daily for 7‐21 days (recommended duration: 14 days) | |
| Outcomes | Mortality, serious adverse events, adverse events, infected pancreatic necrosis Follow‐up: 1.5 months | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[t]he treatment given to each patient was determined by a random scheme prepared by the Biostatistics group at AstraZeneca (Wilmington, DE), using computer software that incorporates a standard procedure for generating random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he treatment given to each patient was determined by a random scheme prepared by the Biostatistics group at AstraZeneca (Wilmington, DE), using computer software that incorporates a standard procedure for generating random numbers" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled study" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled study" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[s]upported by a grant from AstraZeneca Pharmaceuticals" |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany | |
| Interventions | Group 1: glucagon (n = 33), 10 mg daily until surgery or at least 5 days in those who did not undergo surgery | |
| Outcomes | Mortality, requirement for surgery, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Denmark | |
| Interventions | Group 1: NSAID (n = 14), indomethacin 50 mg PR twice daily for 7 days | |
| Outcomes | Hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[c]ontrolled double‐blind trial". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[c]ontrolled double‐blind trial". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "[i]ndomethacin (Confortid) and placebo were generously supplied by Dumex Ltd, Denmark". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 62 Postrandomisation dropouts: 4 (6.5%) Revised sample size: 58 Average age: 36 years Women: 24 (41.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 31): ampicillin 500 mg to 1 g 4 times daily for 7 days (keflin 1 g 4 times daily for 7 days in people allergic to penicillin) | |
| Outcomes | Mortality, adverse events, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: required surgery, developed pneumonia, went home against medical advice, malignancy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[o]n a randomized pre‐selected basis a card was drawn to determine in which group (antibiotic treatment or non‐antibiotic treatment) the patient was to be included." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany Number randomised: 50 Postrandomisation dropouts: not stated Revised sample size: 50 Average age: not stated Women: 17 (34%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: gabexate mesilate (n = 25), 150 mg IV 3 times daily for 7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, organ failure, sepsis Follow‐up: not stated | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Comment: the drug code was concealed by third party. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Number randomised: 116 Postrandomisation dropouts: not stated Revised sample size: 116 Average age: 57 years Women: 49 (42.2%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 116 (100%) Mild pancreatitis: 0 (0%) Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: gabexate mesilate (n = 65), 3 g/day for 7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, sepsis Follow‐up: 3 months | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain | |
| Interventions | Group 1: antibiotics (n = 22): ciprofloxacin 300 mg twice daily for 10 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, organ failure, infected pancreatic necrosis, hospital stay, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: 3 ‐ no confirmed necrosis; 2 fulminant pancreatitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[p]rospective, randomized, placebo‐controlled, double‐blind study" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[p]rospective, randomized, placebo‐controlled, double‐blind study" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Low risk | Quote: "[t]his study was promoted by the “Bellvitge Hospital” and has not received any grant or payment from the pharmaceutical industry". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain Number randomised: 62. Postrandomisation dropouts: not stated Revised sample size: 62 Average age: 52 years Women: 44 (71%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: 48 (77.4%) Severe pancreatitis: 14 (22.6%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: glucagon (n = 31), 1 mg IV every 4 h (duration ‐ not stated) | |
| Outcomes | Mortality, adverse events, requirement for surgery Follow‐up: 24 months | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelope" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Denmark Number randomised: 63 Postrandomisation dropouts: not stated Revised sample size: 63 Average age: 49 years Women: 22 (34.9%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: somatostatin (n = 33), 250 μg/h for 3 days | |
| Outcomes | Mortality, serious adverse events, adverse events, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "by selecting sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blinded trial" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blinded trial" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: multicentric, international Number randomised: 94 Postrandomisation dropouts: not stated Revised sample size: 94 Average age: 55 years Women: 37 (39.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 29 (30.9%) Moderate pancreatitis: 49 (52.1%) Severe pancreatitis: 16 (17%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: calcitonin (n = 50), synthetic salmon calcitonin 20 μg 3 times daily for 6 days | |
| Outcomes | Mortality, adverse events, requirement for surgery, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany | |
| Interventions | Group 1: gabexate mesilate (n = 76), 150 mg every 2 h followed by 0.5 mg/kg/h for 7 days | |
| Outcomes | Mortality, serious adverse events, requirement for surgery Follow‐up: 3 months | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain | |
| Interventions | Group 1: somatostatin (n = 30), 250 μg/h for 5 days | |
| Outcomes | Mortality Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: did not complete the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled, multi‐centre trial across 15 centres in India" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled, multi‐centre trial across 15 centres in India" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China | |
| Interventions | Group 1: octerotide plus ulinastatin (n = 60), 0.1 mg SC 3 times daily for 7‐14 days | |
| Outcomes | Mortality, serious adverse events, adverse events, length of hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Australia | |
| Interventions | Group 1: iniprol (n = 15), single IV dose of 1 million units, followed by 500,000 units IV 4 times daily for 4‐8 days depending upon clinical course | |
| Outcomes | Mortality, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]he drug was not evaluated in a double‐blind manner". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[t]he drug was not evaluated in a double‐blind manner". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "I am grateful to Difrex (Australia) laboratories for supplying . . ." |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: not stated | |
| Interventions | Group 1: antibiotics (n = 20): meropenem 500 mg 3 times daily for 10 days | |
| Outcomes | Mortality, adverse events, requirement for surgery, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 161 Postrandomisation dropouts: not stated Revised sample size: 161 Average age: 51 years Women: 92 (57.1%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: 60 (37.3%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: aprotinin (n = 80), 500 000 KIU bolus followed by 200 000 KIU 4 times daily for 5 days | |
| Outcomes | Mortality, serious adverse events, adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelope". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind trial". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind trial". |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[i]n addition to providing both Trasylol and placebo, Bayer Pharmaceuticals contributed the financial support of a research assistant". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK | |
| Interventions | Group 1: aprotinin (n = 25), 2 million units KIU bolus followed by 400,000 KIU 4 h later | |
| Outcomes | Mortality Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind trial" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind trial" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany | |
| Interventions | Group 1: antibiotics (n = 58): metronidazole 500 mg twice daily and ciprofloxacin 400 mg twice daily (duration not reported) | |
| Outcomes | Serious adverse events, adverse events, requirement for surgery, infected pancreatic necrosis, hospital stay, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: lost to follow‐up, withdrawn from study prior to medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[s]tudy medication for each patient (verum or placebo) was packed in identical vials and labelled with consecutive patient numbers according to the randomization sequence". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind trial" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind trial" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "[s]upported by study medication provided from Bayer Vital and Ratiopharm as well as a financial grant from Bayer Vital" |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 291 Postrandomisation dropouts: 1 (0.3%) Revised sample size: 290 Average age: 63 years Women: 124 (42.8%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria: age < 18 or > 80 years | |
| Interventions | Group 1: lexipafant (n = 151), 100 mg daily for 7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, organ failure, sepsis, hospital stay, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: withdrew from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind, placebo controlled, randomised, parallel group" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind, placebo controlled, randomised, parallel group" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[t]his study was funded by British Biotech Pharmaceuticals Ltd, Oxford, UK". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Finland | |
| Interventions | Group 1: glucagon (n = 32), 7.5 mg twice daily for 4‐5 days | |
| Outcomes | Mortality, serious adverse events, adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: underwent surgery, wrong diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although placebo was used, there was no mention of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although placebo was used, there was no mention of blinding. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported |
| For profit‐bias | Unclear risk | Comment: this information was not available |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 83 Postrandomisation dropouts: not stated Revised sample size: 83 Average age: 59 years Women: 41 (49.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 54 (65.1%) Moderate pancreatitis: not stated Severe pancreatitis: 29 (34.9%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis within 48 h of onset of symptoms Exclusion criteria
| |
| Interventions | Group 1: lexipafant (n = 42), 15 mg 4 times daily for 3 days | |
| Outcomes | Mortality, adverse events Follow‐up: 1 week | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "S.W.G. was supported by British Biotech, Oxford, UK" |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany | |
| Interventions | Group 1: glucagon (n = 75), 10 mg/day for 4 days | |
| Outcomes | Mortality, serious adverse events, adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Denmark Number randomised: 22 Postrandomisation dropouts: not stated Revised sample size: 22 Average age: not stated Women: 4 (18.2%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: 11 (50%) Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: glucagon (n = 10), 1 mg IV followed by 6 mg/day for 3 days | |
| Outcomes | Mortality, adverse events Follow‐up: until discharge | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: although authors stated they did not exclude any participants for wrong diagnosis, it was not clear whether they excluded participants for other reasons. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Albania | |
| Interventions | Group 1: antibiotics (n = 40): imipenem 750 mg IV twice daily for 7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, infected pancreatic necrosis Follow‐up: 1 month | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: although authors stated they did not exclude any participants for wrong diagnosis, it was not clear whether they excluded participants for other reasons. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain Number randomised: 100 Postrandomisation dropouts: not stated Revised sample size: 100 Average age: 55 years Women: 39 (39%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 78 (78%) Moderate pancreatitis: not stated Severe pancreatitis: 22 (22%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: somatostatin (n = 50), 250 μg/h for 48 h following a 250 μg bolus | |
| Outcomes | Mortality, requirement for surgery, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[p]atients were randomly divided by means of the sealed‐envelope method and grouped according to therapy". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: although authors stated they did not exclude any participants for wrong diagnosis, it was not clear whether they excluded participants for other reasons. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: the Netherlands | |
| Interventions | Group 1: antibiotics (n = 50): selective digestive decontamination using colistin 200 mg, amphotericin 500 mg, and norfloxacin 50 mg 4 times daily orally and as rectal enema along with short course of cefotaxime 500 mg IV 3 times daily until gram‐negative bacteria were eliminated from oral cavity and rectum. Total duration of treatment: until patient was extubated and taking oral feeds | |
| Outcomes | Mortality, adverse events, requirement for surgery, hospital stay Follow‐up: until discharge | |
| Notes | Reasons for postrandomisation dropouts: perioperatively proven infected pancreatic necrosis or wrong clinical diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[a] 24‐hour randomization service was available to randomize patients with stratification per center". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Poland | |
| Interventions | Group 1: antioxidants (n = 35): vitamin C 500 mg IV 3 times daily for 5 days | |
| Outcomes | None of the outcomes of interest were reported. Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain | |
| Interventions | Group 1: calcitonin (n = 14), synthetic salmon calcitonin 100 MRC units (equivalent to 100 IU) IV 3 times daily for 5 days or more | |
| Outcomes | Mortality, requirement for surgery, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: although some participants were excluded from hospital stay, they were included for mortality and requirement of surgical intervention. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 58 Postrandomisation dropouts: 0 (0%) Revised sample size: 58 Average age: 69 years Women: 32 (55.2%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 0 (0%) Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with moderate or severe pancreatitis Exclusion criteria
| |
| Interventions | Group 1: octreotide (n = 28), 1 mg/day IV for 5 days | |
| Outcomes | Mortality, serious adverse events, adverse events, organ failure, infected pancreatic necrosis, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[r]andomization was by the use of sequentially numbered treatment packs containing either octreotide or placebo as determined by a computer‐generated random code." |
| Allocation concealment (selection bias) | Low risk | Quote: "[r]andomization was by the use of sequentially numbered treatment packs containing either octreotide or placebo as determined by a computer‐generated random code." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[p]atients, investigators, and medical staff were blinded regarding the nature of the trial infusion". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[p]atients, investigators, and medical staff were blinded regarding the nature of the trial infusion". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 51 Postrandomisation dropouts: 1 (2%) Revised sample size: 50 Average age: 65 years Women: 21 (42%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with predicted severe pancreatitis Exclusion criteria
| |
| Interventions | Group 1: lexipafant (n = 26), 4 mg bolus IV followed by 4 mg/h by continuous infusion for 5‐7 days | |
| Outcomes | Mortality, organ failure, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: incorrect diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[p]acks were numbered sequentially and prepared in advance by British Biotech (Oxford, UK)". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[i]nvestigators and patients were unaware of the nature of the trial infusion." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[i]nvestigators and patients were unaware of the nature of the trial infusion." |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "[t]his study was supported by a grant from British Biotech". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: France Number randomised: 87 Postrandomisation dropouts: 3 (3.4%) Revised sample size: 84 Average age: not stated Women: not stated Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: somatostatin (n = 44), 400 μg for first 3 days, tapered and stopped on 4th day | |
| Outcomes | None of the outcomes of interest were reported. Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "Sonafi, kindly donated" |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK | |
| Interventions | Group 1: aprotinin (n = 66), 500,000 IU IV followed by 300,000 units every 6 h for 5 days | |
| Outcomes | Mortality, requirement for surgery Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: initial amylase was too low | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled, multi‐centre trial across 15 centres in India" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[r]andomized, double‐blind, placebo‐controlled, multi‐centre trial across 15 centres in India" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Comment: the drugs and placebo were supplied by the pharmaceutical company. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Finland Number randomised: 90 Postrandomisation dropouts: 32 (35.6%) Revised sample size: 58 Average age: 46 years Women: 7 (12.1%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 58 (100%) Mild pancreatitis: 0 (0%) Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: 0 (0%) Infected pancreatitis: not stated Inclusion criteria: people with acute necrotising pancreatitis Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 25): imipenem 1 g plus cilastatin IV 3 times daily; duration not stated | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: older than 70 years of age, did not begin antibiotic as scheduled, criteria for pancreatic necrosis not fulfilled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: USA | |
| Interventions | Group 1: octreotide (n = 90), 100 μg 3 times daily SC for duration of hospital stay | |
| Outcomes | Requirement for surgery, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Hungary | |
| Interventions | Group 1: probiotics (n = 33): Synbiotic 2000 once daily for at least 1 week | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, organ failure, sepsis, infected pancreatic necrosis, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: because they were not severe acute pancreatitis after 48 h, did not tolerate jejunal feeding, participant removed the feeding tube | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Israel | |
| Interventions | Group 1: octreotide (n = 19), 01. mg SC 3 times daily for 14 days | |
| Outcomes | Mortality, serious adverse events, adverse events, sepsis, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: failure to meet inclusion criteria, incomplete data, incorrect diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[a]s placebo vials were not available to us, the study was double blinded". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[a]s placebo vials were not available to us, the study was double blinded". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Italy | |
| Interventions | Group 1: antibiotics (n = 41): imipenem 0.5 g every 8 h for 2 weeks | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, organ failure, infected pancreatic necrosis Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "casual numbers table". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Italy | |
| Interventions | Group 1: gabexate mesilate (n = 91), 3 g/day for 7 days | |
| Outcomes | Mortality, adverse events, requirement for surgery Follow‐up: 3 months for mortality; all other complications ‐ 2 weeks | |
| Notes | Reasons for postrandomisation dropouts: major protocol violations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain Number randomised: 40 Postrandomisation dropouts: not stated Revised sample size: 40 Average age: 56 years Women: 24 (60%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: cimetidine (n = 20), 1200 mg IV for 4‐5 days followed by 1000 mg oral for 10 days | |
| Outcomes | Mortality Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[r]andomisation code was held by pharmacy" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Finland Number randomised: 32 Postrandomisation dropouts: 0 (0%) Revised sample size: 32 Average age: 45 years Women: 3 (9.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 32 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: activated protein C (n = 16): drotrecogin alpha activated 24 μg/kg/h for 96 h | |
| Outcomes | Mortality, hospital stay Follow‐up: not stated (probably 2 weeks) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he code for study medication was concealed using sealed envelopes." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "Eli Lilly in part provided the study drug for this investigator‐initiated study". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Latvia | |
| Interventions | Group 1: probiotics (n = 30): 4 bioactive lactic acid bacteria | |
| Outcomes | None of the outcomes of interest were reported. Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Croatia Number randomised: 43 Postrandomisation dropouts: 0 (0%) Revised sample size: 43 Average age: not stated Women: not stated Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 23): imipenem 500 mg IV 3 times daily for 10 days | |
| Outcomes | Mortality, serious adverse events, adverse events, infected pancreatic necrosis, and organ failure Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Norway Number randomised: 73 Postrandomisation dropouts: 0 (0%) Revised sample size: 73 Average age: 58 years Women: 24 (32.9%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 73 (100%) Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 73 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 36): imipenem 0.5 g every 8 h for 5‐7 days | |
| Outcomes | Mortality, adverse events, requirement for surgery, organ failure, infected pancreatic necrosis, hospital stay, ICU stay Follow‐up: not stated (probably 2 weeks) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]he study was unblinded to all attending physicians". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[t]he study was unblinded to all attending physicians". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[w]e are grateful to the pharmaceutical company MSD for economic support in organizing meetings for the Steering Committee". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Finland Number randomised: 60 Postrandomisation dropouts: 0 (0%) Revised sample size: 60 Average age: 41 years Women: 7 (11.7%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 60 (100%) Mild pancreatitis: 0 (0%) Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with alcohol‐induced necrotising pancreatitis Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 30): cefuroxime 1.5 g IV 3 times daily continued until clinical recovery and fall to normal level of C‐reactive protein, after which oral administration of 250 mg twice daily until 14 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, sepsis, hospital stay, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: India Number randomised: 56 Postrandomisation dropouts: 3 (5.4%) Revised sample size: 53 Average age: 39 years Women: 33 (62.3%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: 10 (18.9%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: antioxidants (n = 23): vitamin C 500 mg once daily, N‐acteyl cysteine 200 mg 3 times daily, Antoxyl Forte 1 capsule 3 times daily); duration not stated | |
| Outcomes | Mortality, adverse events, organ failure, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: did not receive allocated treatment, discontinued medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a computer generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]he study was unblinded". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[t]he study was unblinded". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: India Number randomised: 50 Postrandomisation dropouts: 0 (0%) Revised sample size: 50 Average age: 41 years Women: 27 (54%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 28 (56%) Moderate pancreatitis: not stated Severe pancreatitis: 22 (44%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: probiotics (n = 24): 2.5 billion bacteria per sachet and 25 mg of fructo‐oligosaccharide every day for 7 days | |
| Outcomes | Hospital stay, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he method of allocation concealment was sequentially numbered sealed opaque envelopes technique". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "[t]he authors disclose that Alkem provided the probiotics and placebo on complimentary basis." |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain | |
| Interventions | Group 1: cimetidine (n = 30): 1200 mg IV for 4 days followed by 1000 mg oral for 10 days | |
| Outcomes | Serious adverse events, adverse events, requirement for surgery Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: although a placebo was used, it was not clear blinding was performed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 43 Postrandomisation dropouts: 0 (0%) Revised sample size: 43 Average age: 67 years Women: 28 (65.1%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: antioxidants (n = 22) selenium started with 1000 mg and then tapered to 200 mg/day for a total duration of 7 days; vitamin C started with 2000 mg and then tapered to 1000 mg/day for a total duration of 7 days; N‐acetyl cysteine started with 300 mg and then tapered to 75 mg/day for a total duration of 7 days | |
| Outcomes | Mortality, serious adverse events, organ failure, hospital stay, ICU stay Follow‐up: until discharge | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number generation" |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he pharmacy administered the randomisation and storage of therapeutics for all participating centres". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "the costs of antioxidants and placebo were met by Pharmanord UK". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Czech Republic Number randomised: 63 Postrandomisation dropouts: not stated Revised sample size: 63 Average age: 55 years Women: 25 (39.7%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 63 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 33): metronidazole 500 mg 3 times daily and ciprofloxacin 200 mg twice daily for 2 weeks | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, infected pancreatic necrosis, hospital stay, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Czech Republic Number randomised: 41 Postrandomisation dropouts: not stated Revised sample size: 41 Average age: 58 years Women: 10 (24.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 0 (0%). Moderate pancreatitis: 0 (0%) Severe pancreatitis: 41 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 20): meropenem 0.5 mg 3 times daily for 10 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, infected pancreatic necrosis, hospital stay Follow‐up: not stated | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | This information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | This information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | This information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | This information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | This information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Sweden | |
| Interventions | Group 1: aprotinin (n = 21), first half of the trial ‐ 50,000 to 100,000 units per day and then dose doubled for an average of 12 days | |
| Outcomes | Mortality Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[s]ealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: UK Number randomised: 105 Postrandomisation dropouts: not stated Revised sample size: 105 Average age: not stated Women: not stated Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: aprotinin (n = 53), 200,000 units IV stat followed by 200,000 units IV 4 times daily for 5 days | |
| Outcomes | Mortality Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he envelopes of allotment were placed in a recognized position in each hospital together with the packs of Trasylol". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | High risk | Quote: "[w]e are particularly indebted to Dr Brian Allen of Bayer Pharmaceuticals for the supplies of Trasylol and the preparation of the A and B ampoules". |
| Other bias | Unclear risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Finland Number randomised: 64 Postrandomisation dropouts: 0 (0%) Revised sample size: 64 Average age: 51 years Women: 23 (35.9%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: EDTA (n = 33), dose and duration not reported Follow‐up: not stated (probably until discharge) | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[w]e are also grateful for the drugs and support from Sinclair Pharmaceutical Limited, England." |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany Number randomised: 302 Postrandomisation dropouts: 0 (0%) Revised sample size: 302 Average age: 50 years Women: 104 (34.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: 108 (35.8%) Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: octreotide (n = 199), 100 μg or 200 μg (randomised) SC 3 times daily for 7 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, sepsis, hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he packages were used sequentially as the patients were enrolled in the study". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[t]he preparation, randomisation, and delivery of the study medication, as well as the monitoring of the study centres by checking the information in the CRFs, were carried out by Novartis (formerly Sandoz), Nuremberg (Germany)". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Germany | |
| Interventions | Group 1: somatostain (n = 36), 250 ng/h for 7 days | |
| Outcomes | Mortality Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: Spain | |
| Interventions | Group 1: gabexate mesilate (n = 51), 12 mg/kg/day continuous IV for 4‐12 days based on disappearance of abdominal pain or requirement for surgery | |
| Outcomes | Mortality, serious adverse events, adverse events, sepsis Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: protocol violations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered boxes containing FOY or placebo" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | High risk | Quote: "[t]he authors thank Laboratorio Dr Esteve SA for supplies of gabexate mesylate (FOY)". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: USA Number randomised: 28 Postrandomisation dropouts: not stated Revised sample size: 28 Average age: not stated Women: not stated Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: antioxidant (n = 14): pentoxifylline 400 mg oral 3 times daily for 3 days | |
| Outcomes | Mortality, serious adverse events, organ failure, hospital stay, ICU stay Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China | |
| Interventions | Group 1: thymosin alpha (n = 12), 3.2 mg twice daily for 7 days | |
| Outcomes | Mortality, hospital stay, ICU stay Follow‐up: 1 month | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 183 Postrandomisation dropouts: not stated Revised sample size: 183 Average age: 42 years Women: 89 (48.6%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: 159 (86.9%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: somatostatin plus ulinastatin (n = 62) | |
| Outcomes | Mortality, serious adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Low risk | Quote: "[t]he authors have no direct relationship with any of the companies mentioned in this article, either by employment or by receiving research grants". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China | |
| Interventions | Group 1: octreotide plus NSAID (n = not reported) | |
| Outcomes | None of the outcomes of interest were reported. Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 372 Postrandomisation dropouts: not stated Revised sample size: 372 Average age: 45 years Women: 174 (46.8%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: 0 (0%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria: people with alcohol dependence | |
| Interventions | Group 1: octreotide (n = 157), 50 μg/h for first 3 days followed by 25 μg/h for next 4 days or 25 μg/h for 7 days (randomised) | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, requirement for endoscopic or radiological drainage, organ failure, hospital stay Follow‐up: some outcomes were measured on 8th day and others at 1 month | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]he physicians and nurses who managed the patients were blinded so that they did not know the patient has been allocated to and what treatment they had received". |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[t]he physicians and nurses who managed the patients were blinded so that they did not know the patient has been allocated to and what treatment they had received". |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Low risk | Quote: "[t]his study was supported by a Key Grant #30330270 from the Natural Science Fund of China and the National Ministry of Health Fund for the Public Welfare 2‐13". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 492 Postrandomisation dropouts: not stated Revised sample size: 492 Average age: 41 years Women: 238 (48.4%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 492 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria: people with severe acute pancreatitis Exclusion criteria
| |
| Interventions | Group 1: somatostatin plus ulinastatin plus gabexate (n = 116) | |
| Outcomes | Mortality, adverse events, organ failure, length of hospital stay Follow‐up: not stated (probably until discharge) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[a]ccording to a computerized random number generation . . ." |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[t]his was a prospective and double‐blind study" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[t]his was a prospective and double‐blind study" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Low risk | Quote: "[t]his work was supported by National Natural Science Foundation of China, China (81360080, 81071594) and the Science Foundation of Science and Technology Hall of Jiangxi Province, China (20091391308000)." |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 140 Postrandomisation dropouts: not stated Revised sample size: 140 Average age: 43 years Women: 48 (34.3%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: not stated Moderate pancreatitis: not stated Severe pancreatitis: 140 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
| |
| Interventions | Group 1: somatostatin (3 mg IV twice daily for 7 days) plus omeprazole (40 mg IV twice daily for 7 days) (n = 70) | |
| Outcomes | Mortality, serious adverse events, adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 59 Postrandomisation dropouts: 3 (5.1%) Revised sample size: 56 Average age: 48 years Women: 28 (50%) Acute interstitial oedematous pancreatitis: 0 (0%) Necrotising pancreatitis: 56 (100%) Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 56 (100%) Persistent organ failure: not stated Infected pancreatitis: 0 Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: antibiotics (n = 29): imipenem‐cilastatin 0.5 g every 8 h for 7‐14 days | |
| Outcomes | Mortality, serious adverse events, adverse events, requirement for surgery, hospital stay Follow‐up: 1 month | |
| Notes | Reasons for postrandomisation dropouts: death after starting treatment, transferred to operation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐derived random number sequence" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: mortality and adverse events were reported. |
| For profit‐bias | Low risk | Quote: "[w]e thank Sichuan Province Science and Technology Tackling Key Project (no. 05SG011‐021‐1) for providing financial support for the trial and the publication of the paper". |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China | |
| Interventions | Group 1: somatostatin (n = 25), 250 μg/h for 3‐4 days | |
| Outcomes | Serious adverse events, adverse events Follow‐up: not stated (probably until discharge) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 163 Postrandomisation dropouts: 6 (3.7%) Revised sample size: 157 Average age: 46 years Women: 71 (45.2%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 157 (100%) Moderate pancreatitis: not stated Severe pancreatitis: not stated Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: octreotide (n = 80), 50 μg/h for 3 days | |
| Outcomes | Mortality, hospital stay Follow‐up: 1 month | |
| Notes | Reasons for postrandomisation dropouts: loss to follow‐up; lack of data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) | High risk | Comment: there were postrandomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Low risk | Quote: "[t]his study was supported by Key Grant #30330270 of the Natural Science Fund of China and the National Ministry of Health Fund for Public Welfare 2‐13." |
| Other bias | Low risk | Comment: no other risk of bias |
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 39 Postrandomisation dropouts: not stated Revised sample size: 39 Average age: 43 years Women: 18 (46.2%) Acute interstitial oedematous pancreatitis: not stated Necrotising pancreatitis: not stated Mild pancreatitis: 0 (0%) Moderate pancreatitis: 0 (0%) Severe pancreatitis: 39 (100%) Persistent organ failure: not stated Infected pancreatitis: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Group 1: probiotics (n = 20), 2 tablets twice daily for 14 days (Japanese preparation) | |
| Outcomes | Serious adverse events, adverse events, requirement for endoscopic or radiological drainage, infected pancreatic necrosis Follow‐up: not stated (probably 2 weeks) | |
| Notes | Reasons for postrandomisation dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: either mortality or adverse events were not reported. |
| For profit‐bias | Unclear risk | Comment: this information was not available. |
| Other bias | Low risk | Comment: no other risk of bias |
ERCP: endoscopic retrograde cholangiopancreatography; ICU: intensive care unit; IU: international unit; IV: intravenous; KIU: kallikrein inhibitor units; MRC: Medical Research Council (1 MRC = 1 IU); PR: per rectum; SC: subcutaneous.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not an RCT | |
| Not an RCT | |
| Comparison of 2 different antioxidants | |
| Comparison of 2 different antioxidants | |
| Comparison of 2 different antioxidants | |
| Comparison of 2 different antioxidants | |
| Comparison of 2 different antioxidants | |
| Comparison of 2 different antioxidants | |
| Comparison of 2 different antioxidants | |
| Not conducted in humans | |
| Not a primary research study (commentary) | |
| Quasi‐RCT (allocation based on birth date) comparing 2 different preparations of aprotinin | |
| Quasi‐RCT (allocation based on birth date) comparing 2 different preparations of aprotinin | |
| Not an RCT | |
| Comparison of 2 different antibiotic regimens | |
| Not an RCT | |
| Not an RCT | |
| Not a primary research study (commentary) | |
| Not an RCT | |
| Comparison of different doses of octreotide | |
| Comparison of different doses of octreotide | |
| Not a primary research study (editorial) | |
| Not an RCT | |
| Quasi‐randomised study (allocation by patient number) | |
| There was no control group for pharmacological intervention | |
| Not a primary research study (letter to editor) | |
| Not a primary research study (review) | |
| Not an RCT | |
| Not a primary research study (commentary) | |
| Not a primary research study (systematic review) | |
| Not a primary research study (commentary) | |
| Not an RCT | |
| Comparison of 2 doses of vitamin C | |
| Comparison of 2 doses of vitamin C | |
| Quasi‐RCT (allocation by alternation) | |
| Not an RCT | |
| Not a primary research study (review) | |
| Not in humans | |
| Not an RCT | |
| Not a pharmacological intervention | |
| Comparison of 2 variations of probiotics | |
| Not a primary research study (review) | |
| Comparison of different doses of octreotide | |
| Variations in nutritional supplementation | |
| Prophylactic intervention (not in people with acute pancreatitis) | |
| Not a primary research study (review) | |
| Not a pharmacological intervention | |
| Not a primary research study (comment) | |
| Not a primary research study (letter to editor) | |
| Not a primary research study (letter to editor) | |
| Not a primary research study (editorial) | |
| Quasi‐RCT (allocation by hospital number) | |
| Variations in different types of nutritional supplementation | |
| No suitable control (3 groups were: probiotics + fibre versus inactivated lactobacilli + fibre versus standard nutrition; it is not possible to obtain the effect estimate of probiotics alone from this comparison) | |
| Not an RCT | |
| Not an RCT | |
| Not a pharmacological intervention (fibre supplementation only) | |
| Not an RCT | |
| Not an RCT | |
| Variations in fatty acids used in enteral nutrition | |
| Variations in fatty acids used in enteral nutrition | |
| Variations in fatty acids used in enteral nutrition | |
| Not an RCT | |
| This started as a RCT but was converted to a cohort study after publication of negative results | |
| Not a primary research study (review) | |
| Not a pharmacological intervention (variations in parenteral nutrition) | |
| Intervention includes a non‐pharmacological treatment in addition to antioxidant | |
| Comparison of 2 different antibiotics | |
| Comparison of 2 different antibiotic regimens | |
| Not a primary research study (editorial) | |
| Not an RCT | |
| Comparison of 2 different fats | |
| Variations in composition of enteral feeds | |
| Not primary research (review) | |
| Comparison of two doses of gabexate mesilate | |
| Comparison of 2 doses of gabexate mesilate | |
| Comparison of 2 doses of gabexate mesilate | |
| In addition to the difference in the groups in terms of whether the patients received protease inhibitor, the antibiotic regimen differed between the groups | |
| Not an RCT | |
| Not a primary research study (letter to editor) | |
| Not an RCT | |
| Not a primary research study (letter to editor) | |
| Not primary research (letter to editor) | |
| No mention about randomisation | |
| No mention about randomisation | |
| There were 2 trials reported in this publication. Of these, 1 was a quasi‐RCT (alternate allocation) and it was not clear whether the second trial was an RCT | |
| Only the control group received Chinese medicines | |
| Not an RCT | |
| Not a primary research study (review) | |
| Not a primary research study (letter to editor) | |
| Not a primary research study (commentary) | |
| Not primary research (review) | |
| Variations in composition of parenteral nutrition | |
| Variations in composition of parenteral nutrition | |
| Not a primary research study (commentary) | |
| Not a primary research study (letter to editor) | |
| Variations in parenteral nutrition | |
| Variations in parenteral nutrition | |
| Not an RCT | |
| Variations in total parenteral nutrition | |
| Chinese medicines were given to the control group but not the intervention group | |
| The co‐interventions in the groups varied apart from the drug being evaluated (nasogastric suction was used only in the control group) |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Awaiting full text |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Awaiting full text |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effect of proton pump inhibitors on acute pancreatitis‐‐a randomly prospective control study |
| Methods | Randomised controlled trial |
| Participants | Adults with acute pancreatitis |
| Interventions | Proton pump inhibitor (omeprazole) versus placebo |
| Outcomes | Duration of hospital stay, gastrointestinal bleeding, and hospital costs |
| Starting date | September 2016 |
| Contact information | Xiao Ma ([email protected]) |
| Notes | — |
| Trial name or title | Trial of indomethacin in pancreatitis |
| Methods | Randomised controlled trial |
| Participants | Adults with acute pancreatitis |
| Interventions | Non‐steroidal anti‐inflammatory drugs (indomethacin) versus placebo |
| Outcomes | Mortality and organ failure |
| Starting date | May 2015 |
| Contact information | Enrique de Madaria Pascual ([email protected]) |
| Notes | ChiCTR‐IPR‐16008301, NCT02692391 |
| Trial name or title | Ulinastatin in severe acute pancreatitis |
| Methods | Randomised controlled trial |
| Participants | Adults with severe acute pancreatitis |
| Interventions | Ulinastatin versus placebo |
| Outcomes | mortality, organ failure, requirement for additional invasive intervention, hospital stay, intensive care unit stay |
| Starting date | June 2010 |
| Contact information | Chunyou Wang (Wuhan Union Hospital, China) |
| Notes | The study is currently suspended. |
| Trial name or title | DP‐b99 in the treatment of acute high‐risk pancreatitis |
| Methods | Randomised controlled trial |
| Participants | Adults with predicted severe acute pancreatitis |
| Interventions | DP‐b99 versus placebo |
| Outcomes | Complications |
| Starting date | December 2013 |
| Contact information | Gilad Rosenberg (Wuhan Union Hospital, China) |
| Notes | The University Hospital Brno, Gastroenterology Clinic, Brno, Czech Republic, 62500 |
| Trial name or title | Comparing the outcome in patients of acute pancreatitis, with and without prophylactic antibiotics |
| Methods | Randomised controlled trial |
| Participants | Adults with acute pancreatitis |
| Interventions | Antibiotics (meropenem) versus no intervention |
| Outcomes | Infections and hospital stay |
| Starting date | Jan 2013 |
| Contact information | Fazal H Shah (Benazir Bhutto Hospital, Rawalpindi, Punjab, Pakistan, 46000) |
| Notes | — |
| Trial name or title | A randomized controlled pilot trial of indomethacin in acute pancreatitis |
| Methods | Randomised controlled trial |
| Participants | Adults with acute pancreatitis |
| Interventions | Non‐steroidal anti‐inflammatory drugs (indomethacin) versus placebo |
| Outcomes | Mortality and organ failure |
| Starting date | April 2014 |
| Contact information | Georgios I Papachristou ([email protected]) |
| Notes | — |
| Trial name or title | Treatment of acute pancreatitis with ketorolac |
| Methods | Randomised controlled trial |
| Participants | Adults with predicted severe acute pancreatitis |
| Interventions | Non‐steroidal anti‐inflammatory drugs (ketorolac) versus placebo |
| Outcomes | New onset organ failure, pancreatic necrosis, and duration of hospital stay |
| Starting date | September 2016 |
| Contact information | Shaahin Shahbazi ([email protected]) |
| Notes | — |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Acute pancreatitis, Outcome 1 Short‐term mortality. | ||||
| 1.1 Antibiotics versus control | 17 | 1058 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.15] |
| 1.2 Antioxidants versus control | 4 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.53, 7.56] |
| 1.3 Aprotinin versus control | 7 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.14] |
| 1.4 Calcitonin versus control | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 2.00] |
| 1.5 Cimetidine versus control | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.18] |
| 1.6 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.12, 7.08] |
| 1.7 Gabexate versus control | 5 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.30] |
| 1.8 Glucagon versus control | 5 | 409 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.87] |
| 1.9 Iniprol versus control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 1.67] |
| 1.10 Lexipafant versus control | 3 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 1.11 Octreotide versus control | 5 | 927 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.47, 1.23] |
| 1.12 Probiotics versus control | 2 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.87, 3.30] |
| 1.13 Activated protein C versus control | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.56 [0.41, 180.52] |
| 1.14 Somatostatin versus control | 6 | 493 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.10] |
| 1.15 Somatostatin plus omeprazole versus control | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 1.11] |
| 1.16 Somatostatin plus ulinastatin versus control | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.23] |
| 1.17 Thymosin versus control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.18 Ulinastatin versus control | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.72] |
| 1.19 Gabexate versus aprotinin | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.20] |
| 1.20 Glucagon versus aprotinin | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.44, 4.08] |
| 1.21 Glucagon versus atropine | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.45, 38.21] |
| 1.22 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.60] |
| 1.23 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 1.24 Somatostatin plus ulinastatin versus somatostatin | 2 | 369 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.34, 1.56] |
| 1.25 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.21, 1.74] |
| 1.26 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 1.95] |
| 1.27 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.86] |
| 1.28 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.30, 2.80] |
| 2 Serious adverse events (proportion) Show forest plot | 17 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 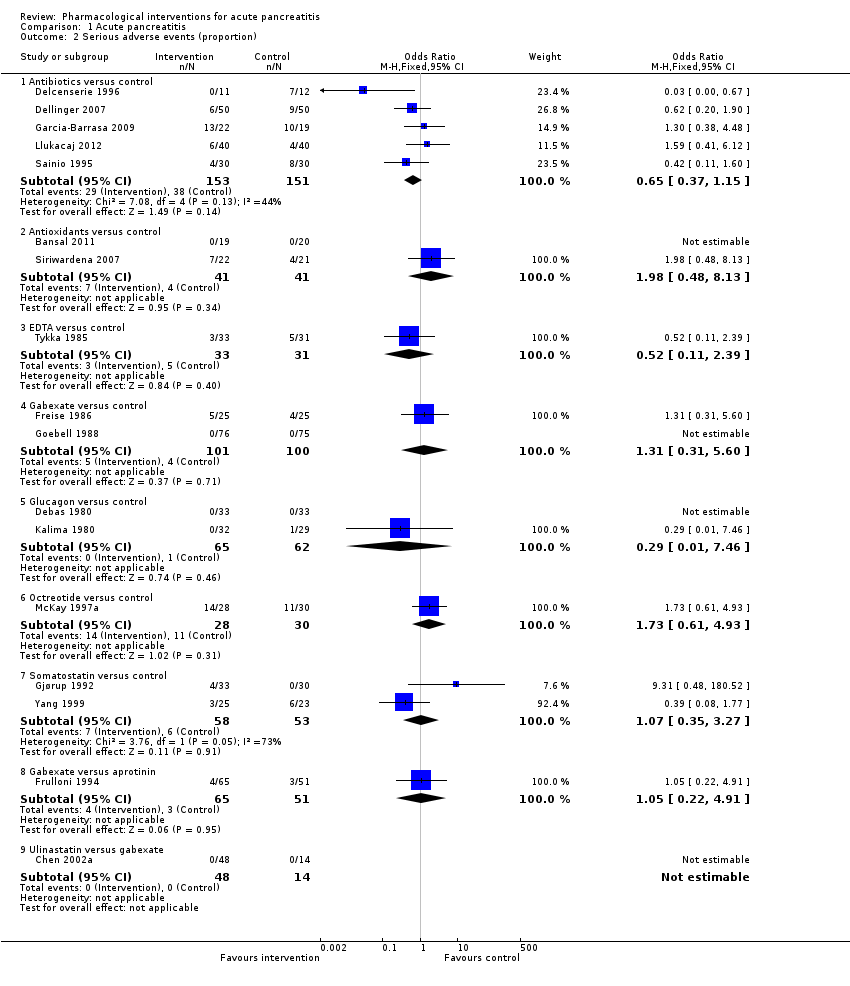 Comparison 1 Acute pancreatitis, Outcome 2 Serious adverse events (proportion). | ||||
| 2.1 Antibiotics versus control | 5 | 304 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.37, 1.15] |
| 2.2 Antioxidants versus control | 2 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.48, 8.13] |
| 2.3 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.11, 2.39] |
| 2.4 Gabexate versus control | 2 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.31, 5.60] |
| 2.5 Glucagon versus control | 2 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.46] |
| 2.6 Octreotide versus control | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.61, 4.93] |
| 2.7 Somatostatin versus control | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.35, 3.27] |
| 2.8 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.22, 4.91] |
| 2.9 Ulinastatin versus gabexate | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious adverse events (number) Show forest plot | 37 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 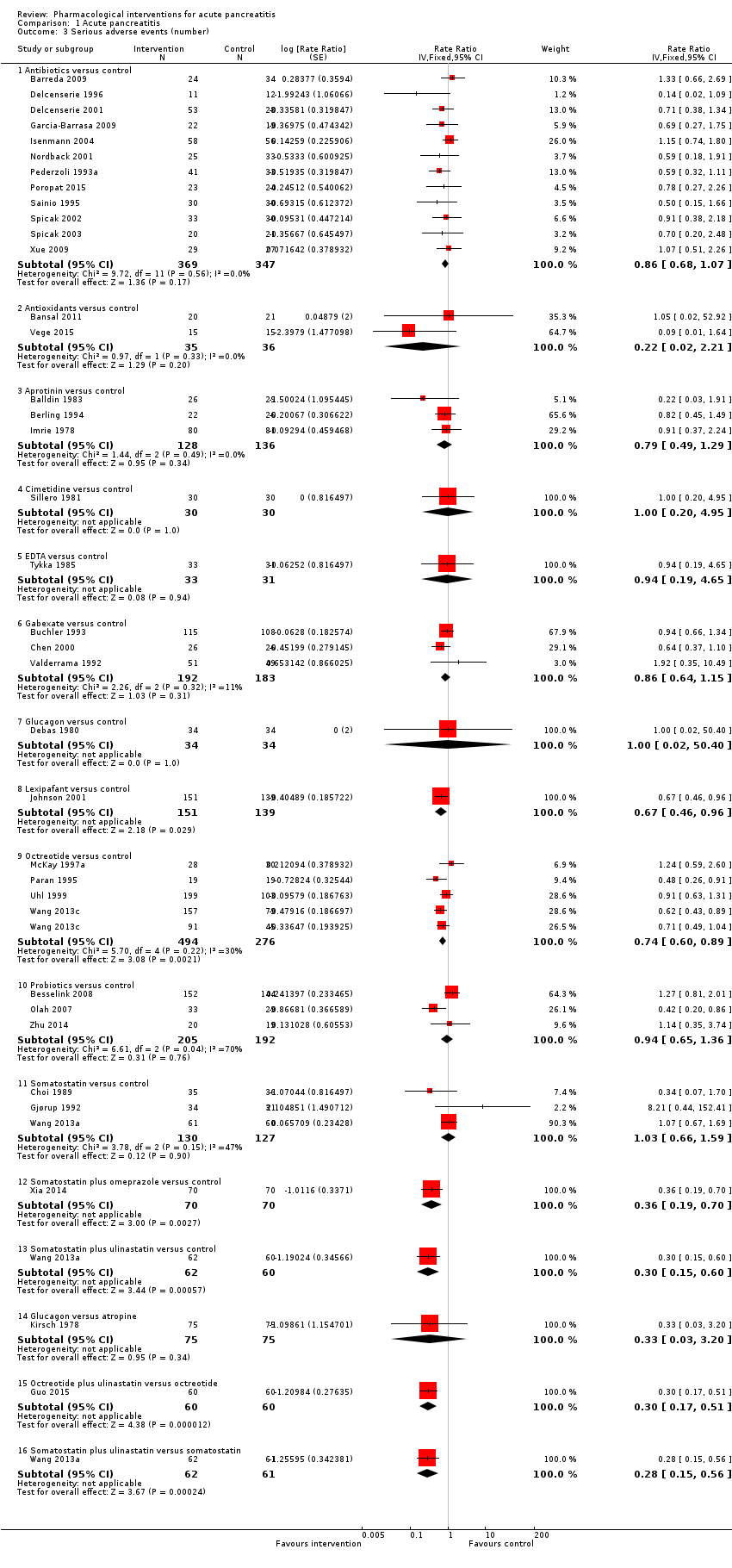 Comparison 1 Acute pancreatitis, Outcome 3 Serious adverse events (number). | ||||
| 3.1 Antibiotics versus control | 12 | 716 | Rate Ratio (Fixed, 95% CI) | 0.86 [0.68, 1.07] |
| 3.2 Antioxidants versus control | 2 | 71 | Rate Ratio (Fixed, 95% CI) | 0.22 [0.02, 2.21] |
| 3.3 Aprotinin versus control | 3 | 264 | Rate Ratio (Fixed, 95% CI) | 0.79 [0.49, 1.29] |
| 3.4 Cimetidine versus control | 1 | 60 | Rate Ratio (Fixed, 95% CI) | 1.0 [0.20, 4.95] |
| 3.5 EDTA versus control | 1 | 64 | Rate Ratio (Fixed, 95% CI) | 0.94 [0.19, 4.65] |
| 3.6 Gabexate versus control | 3 | 375 | Rate Ratio (Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 3.7 Glucagon versus control | 1 | 68 | Rate Ratio (Fixed, 95% CI) | 1.0 [0.02, 50.40] |
| 3.8 Lexipafant versus control | 1 | 290 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.46, 0.96] |
| 3.9 Octreotide versus control | 4 | 770 | Rate Ratio (Fixed, 95% CI) | 0.74 [0.60, 0.89] |
| 3.10 Probiotics versus control | 3 | 397 | Rate Ratio (Fixed, 95% CI) | 0.94 [0.65, 1.36] |
| 3.11 Somatostatin versus control | 3 | 257 | Rate Ratio (Fixed, 95% CI) | 1.03 [0.66, 1.59] |
| 3.12 Somatostatin plus omeprazole versus control | 1 | 140 | Rate Ratio (Fixed, 95% CI) | 0.36 [0.19, 0.70] |
| 3.13 Somatostatin plus ulinastatin versus control | 1 | 122 | Rate Ratio (Fixed, 95% CI) | 0.30 [0.15, 0.60] |
| 3.14 Glucagon versus atropine | 1 | 150 | Rate Ratio (Fixed, 95% CI) | 0.33 [0.03, 3.20] |
| 3.15 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Rate Ratio (Fixed, 95% CI) | 0.30 [0.17, 0.51] |
| 3.16 Somatostatin plus ulinastatin versus somatostatin | 1 | 123 | Rate Ratio (Fixed, 95% CI) | 0.28 [0.15, 0.56] |
| 4 Organ failure Show forest plot | 18 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Acute pancreatitis, Outcome 4 Organ failure. | ||||
| 4.1 Antibiotics versus control | 5 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.44, 1.38] |
| 4.2 Antioxidants versus control | 4 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.12] |
| 4.3 Gabexate versus control | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.25] |
| 4.4 Lexipafant versus control | 2 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.27] |
| 4.5 Octreotide versus control | 2 | 430 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 4.6 Probiotics versus control | 2 | 358 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.26, 2.47] |
| 4.7 Ulinastatin versus control | 1 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 6.67] |
| 4.8 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.33, 1.80] |
| 4.9 Somatostatin plus ulinastatin versus somatostatin | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.45] |
| 4.10 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.17, 1.25] |
| 4.11 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.92] |
| 4.12 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.65] |
| 4.13 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.27, 2.35] |
| 5 Infected pancreatic necrosis Show forest plot | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Acute pancreatitis, Outcome 5 Infected pancreatic necrosis. | ||||
| 5.1 Antibiotics versus control | 11 | 714 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.25] |
| 5.2 Octreotide versus control | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.04, 6.06] |
| 5.3 Probiotics versus control | 3 | 397 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.62, 1.96] |
| 6 Sepsis Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Acute pancreatitis, Outcome 6 Sepsis. | ||||
| 6.1 Antibiotics versus control | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.60] |
| 6.2 Aprotinin versus control | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.49, 6.96] |
| 6.3 Gabexate versus control | 3 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.55, 2.19] |
| 6.4 Lexipafant versus control | 1 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.83] |
| 6.5 Octreotide versus control | 2 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 3.53] |
| 6.6 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.36] |
| 6.7 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.22, 4.91] |
| 7 Adverse events (proportion) Show forest plot | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7 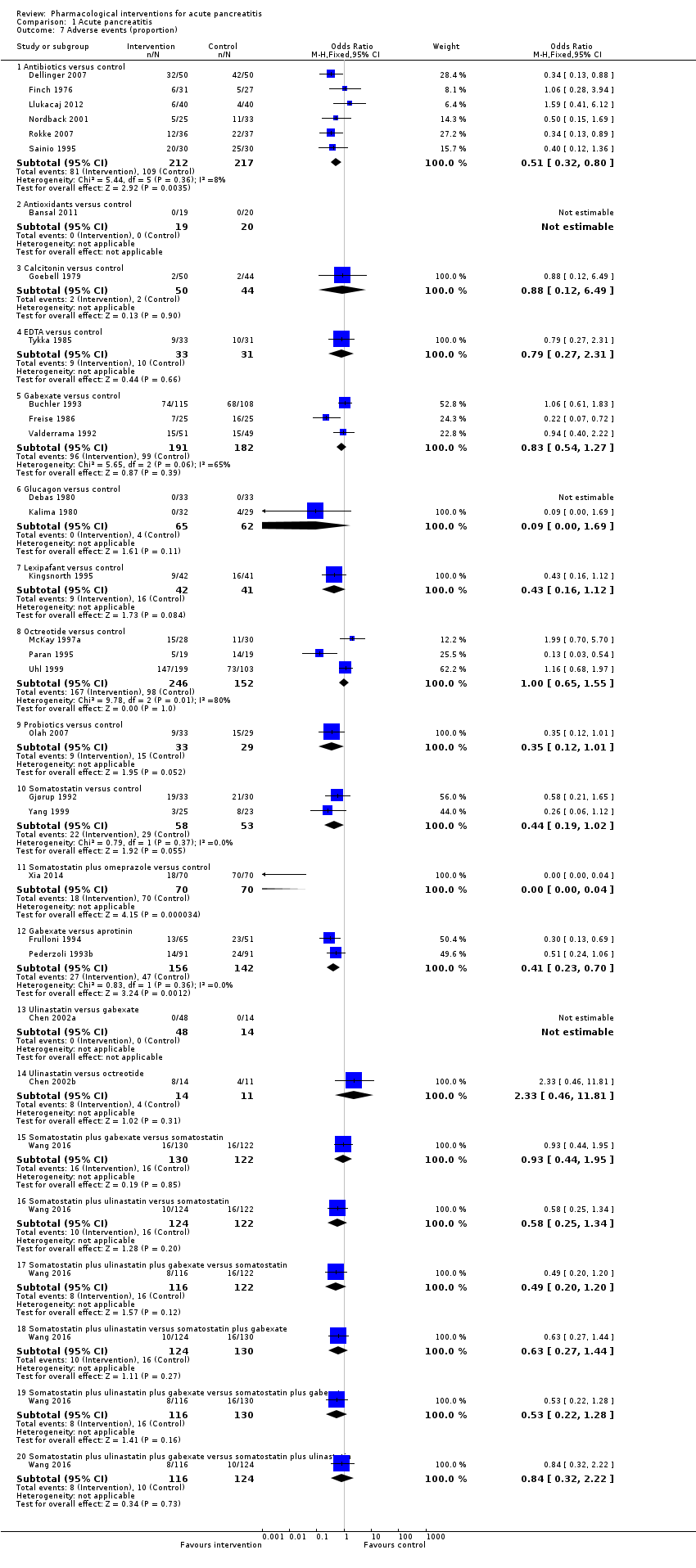 Comparison 1 Acute pancreatitis, Outcome 7 Adverse events (proportion). | ||||
| 7.1 Antibiotics versus control | 6 | 429 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.80] |
| 7.2 Antioxidants versus control | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcitonin versus control | 1 | 94 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.12, 6.49] |
| 7.4 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.27, 2.31] |
| 7.5 Gabexate versus control | 3 | 373 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.27] |
| 7.6 Glucagon versus control | 2 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.69] |
| 7.7 Lexipafant versus control | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.16, 1.12] |
| 7.8 Octreotide versus control | 3 | 398 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.65, 1.55] |
| 7.9 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.12, 1.01] |
| 7.10 Somatostatin versus control | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.19, 1.02] |
| 7.11 Somatostatin plus omeprazole versus control | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.00 [0.00, 0.04] |
| 7.12 Gabexate versus aprotinin | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.70] |
| 7.13 Ulinastatin versus gabexate | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.14 Ulinastatin versus octreotide | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.46, 11.81] |
| 7.15 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.44, 1.95] |
| 7.16 Somatostatin plus ulinastatin versus somatostatin | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.34] |
| 7.17 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.20, 1.20] |
| 7.18 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.27, 1.44] |
| 7.19 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.28] |
| 7.20 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.32, 2.22] |
| 8 Adverse events (number) Show forest plot | 40 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.8 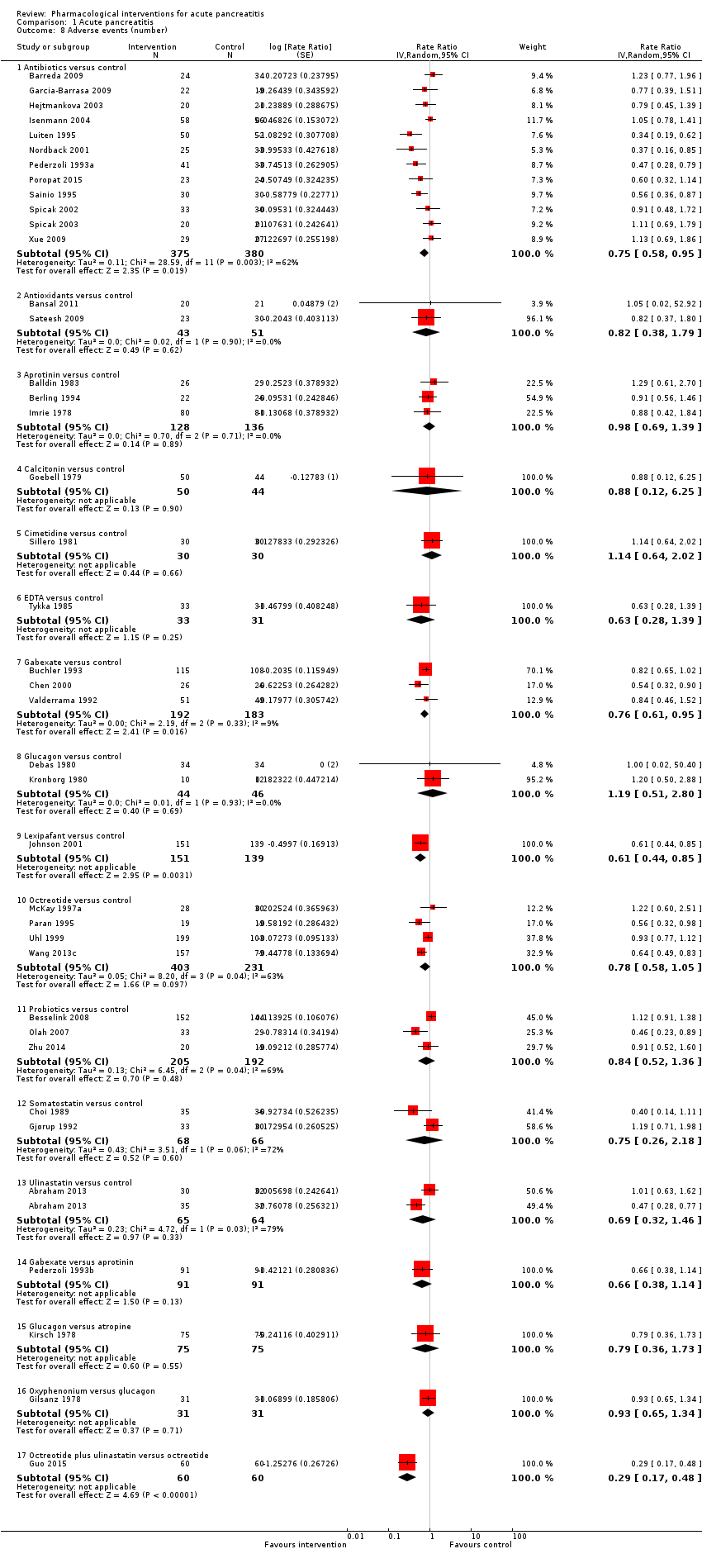 Comparison 1 Acute pancreatitis, Outcome 8 Adverse events (number). | ||||
| 8.1 Antibiotics versus control | 12 | 755 | Rate Ratio (Random, 95% CI) | 0.75 [0.58, 0.95] |
| 8.2 Antioxidants versus control | 2 | 94 | Rate Ratio (Random, 95% CI) | 0.82 [0.38, 1.79] |
| 8.3 Aprotinin versus control | 3 | 264 | Rate Ratio (Random, 95% CI) | 0.98 [0.69, 1.39] |
| 8.4 Calcitonin versus control | 1 | 94 | Rate Ratio (Random, 95% CI) | 0.88 [0.12, 6.25] |
| 8.5 Cimetidine versus control | 1 | 60 | Rate Ratio (Random, 95% CI) | 1.14 [0.64, 2.02] |
| 8.6 EDTA versus control | 1 | 64 | Rate Ratio (Random, 95% CI) | 0.63 [0.28, 1.39] |
| 8.7 Gabexate versus control | 3 | 375 | Rate Ratio (Random, 95% CI) | 0.76 [0.61, 0.95] |
| 8.8 Glucagon versus control | 2 | 90 | Rate Ratio (Random, 95% CI) | 1.19 [0.51, 2.80] |
| 8.9 Lexipafant versus control | 1 | 290 | Rate Ratio (Random, 95% CI) | 0.61 [0.44, 0.85] |
| 8.10 Octreotide versus control | 4 | 634 | Rate Ratio (Random, 95% CI) | 0.78 [0.58, 1.05] |
| 8.11 Probiotics versus control | 3 | 397 | Rate Ratio (Random, 95% CI) | 0.84 [0.52, 1.36] |
| 8.12 Somatostatin versus control | 2 | 134 | Rate Ratio (Random, 95% CI) | 0.75 [0.26, 2.18] |
| 8.13 Ulinastatin versus control | 1 | 129 | Rate Ratio (Random, 95% CI) | 0.69 [0.32, 1.46] |
| 8.14 Gabexate versus aprotinin | 1 | 182 | Rate Ratio (Random, 95% CI) | 0.66 [0.38, 1.14] |
| 8.15 Glucagon versus atropine | 1 | 150 | Rate Ratio (Random, 95% CI) | 0.79 [0.36, 1.73] |
| 8.16 Oxyphenonium versus glucagon | 1 | 62 | Rate Ratio (Random, 95% CI) | 0.93 [0.65, 1.34] |
| 8.17 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Rate Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 9 Requirement for additional invasive intervention Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Acute pancreatitis, Outcome 9 Requirement for additional invasive intervention. | ||||
| 9.1 Antibiotics versus control | 14 | 884 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.13] |
| 9.2 Aprotinin versus control | 2 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.23, 1.47] |
| 9.3 Calcitonin versus control | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.08, 1.16] |
| 9.4 Cimetidine versus control | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.61] |
| 9.5 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.14, 3.29] |
| 9.6 Gabexate versus control | 3 | 426 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.90] |
| 9.7 Glucagon versus control | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.58, 2.77] |
| 9.8 Octreotide versus control | 3 | 854 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.21] |
| 9.9 Probiotics versus control | 2 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.83, 2.71] |
| 9.10 Somatostatin versus control | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.11, 1.38] |
| 9.11 Gabexate versus aprotinin | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.19, 1.32] |
| 9.12 Glucagon versus aprotinin | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.44, 4.08] |
| 9.13 Oxyphenonium versus glucagon | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.59] |
| 10 Endoscopic or radiological drainage of collections Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10 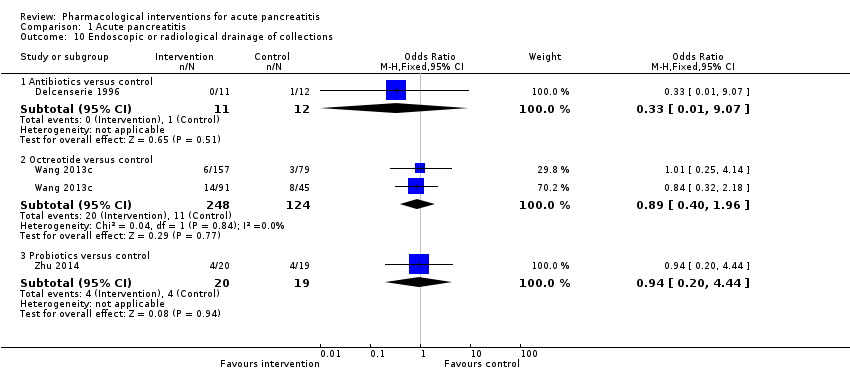 Comparison 1 Acute pancreatitis, Outcome 10 Endoscopic or radiological drainage of collections. | ||||
| 10.1 Antibiotics versus control | 1 | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 9.07] |
| 10.2 Octreotide versus control | 1 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.96] |
| 10.3 Probiotics versus control | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.20, 4.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Acute necrotising pancreatitis, Outcome 1 Short‐term mortality. | ||||
| 1.1 Antibiotics versus control | 10 | 683 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.52, 1.30] |
| 1.2 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.20, 1.36] |
| 2 Serious adverse events (proportion) Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 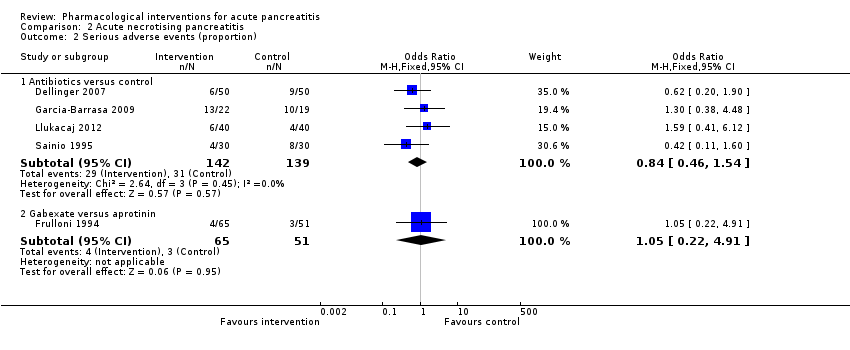 Comparison 2 Acute necrotising pancreatitis, Outcome 2 Serious adverse events (proportion). | ||||
| 2.1 Antibiotics versus control | 4 | 281 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.54] |
| 2.2 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.22, 4.91] |
| 3 Serious adverse events (number) Show forest plot | 7 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Acute necrotising pancreatitis, Outcome 3 Serious adverse events (number). | ||||
| 3.1 Antibiotics versus control | 7 | Rate Ratio (Fixed, 95% CI) | 0.79 [0.59, 1.06] | |
| 4 Organ failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Acute necrotising pancreatitis, Outcome 4 Organ failure. | ||||
| 4.1 Antibiotics versus control | 4 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.42, 1.45] |
| 5 Infected pancreatic necrosis Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5 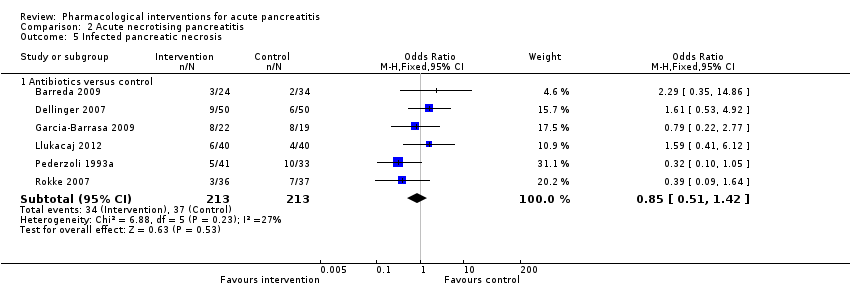 Comparison 2 Acute necrotising pancreatitis, Outcome 5 Infected pancreatic necrosis. | ||||
| 5.1 Antibiotics versus control | 6 | 426 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.42] |
| 6 Sepsis Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6 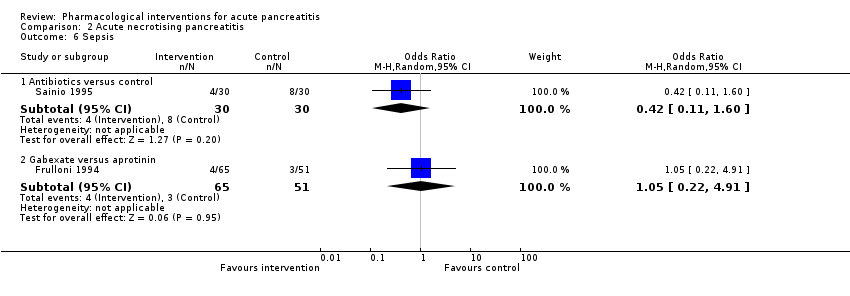 Comparison 2 Acute necrotising pancreatitis, Outcome 6 Sepsis. | ||||
| 6.1 Antibiotics versus control | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.60] |
| 6.2 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.22, 4.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1 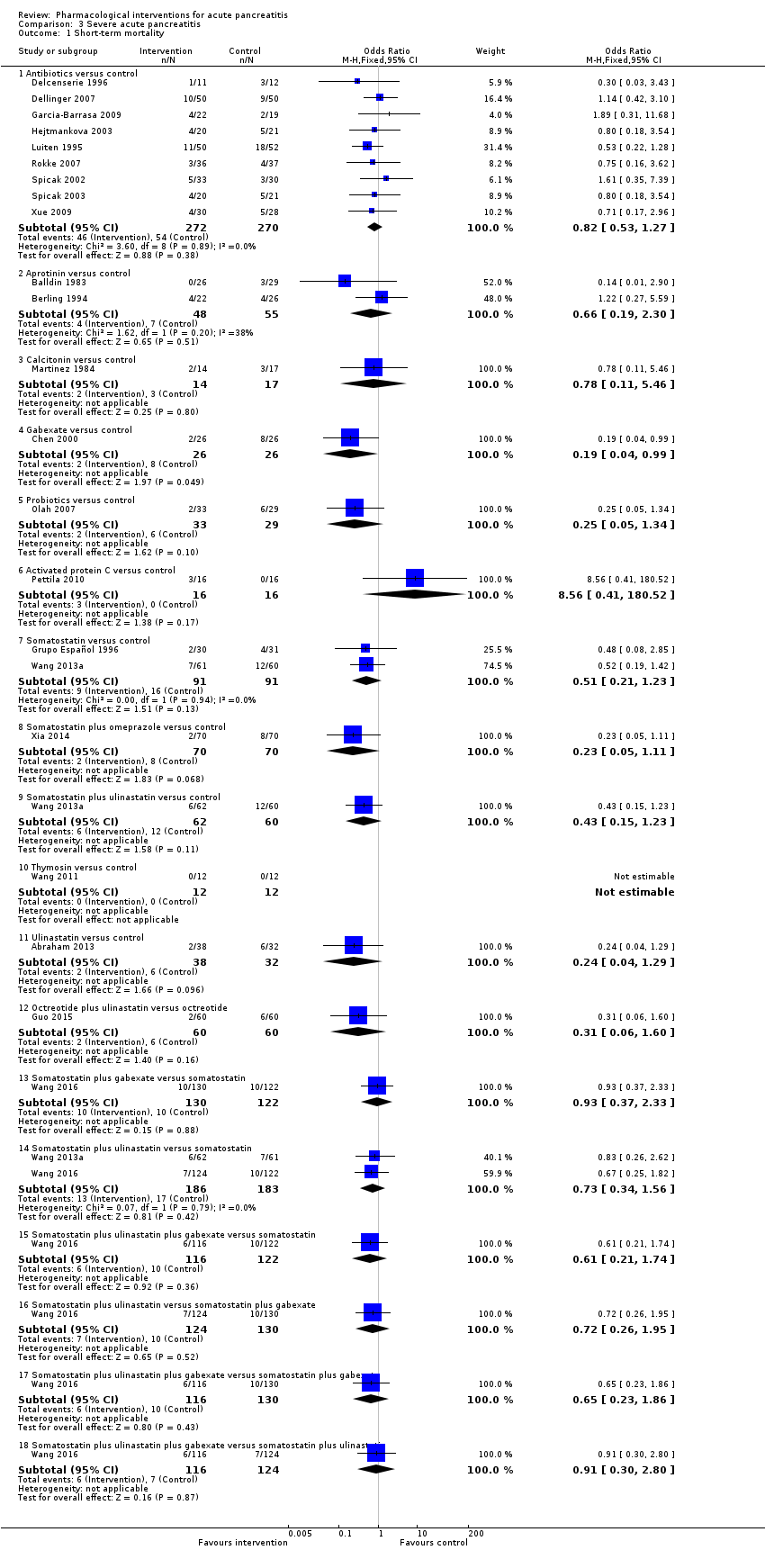 Comparison 3 Severe acute pancreatitis, Outcome 1 Short‐term mortality. | ||||
| 1.1 Antibiotics versus control | 9 | 542 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.27] |
| 1.2 Aprotinin versus control | 2 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.30] |
| 1.3 Calcitonin versus control | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.11, 5.46] |
| 1.4 Gabexate versus control | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.99] |
| 1.5 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.34] |
| 1.6 Activated protein C versus control | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.56 [0.41, 180.52] |
| 1.7 Somatostatin versus control | 2 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.21, 1.23] |
| 1.8 Somatostatin plus omeprazole versus control | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 1.11] |
| 1.9 Somatostatin plus ulinastatin versus control | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.23] |
| 1.10 Thymosin versus control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.11 Ulinastatin versus control | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.04, 1.29] |
| 1.12 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.60] |
| 1.13 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 1.14 Somatostatin plus ulinastatin versus somatostatin | 2 | 369 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.34, 1.56] |
| 1.15 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.21, 1.74] |
| 1.16 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 1.95] |
| 1.17 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.86] |
| 1.18 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.30, 2.80] |
| 2 Serious adverse events (proportion) Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Severe acute pancreatitis, Outcome 2 Serious adverse events (proportion). | ||||
| 2.1 Antibiotics versus control | 3 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 3 Serious adverse events (number) Show forest plot | 13 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Severe acute pancreatitis, Outcome 3 Serious adverse events (number). | ||||
| 3.1 Antibiotics versus control | 5 | Rate Ratio (Random, 95% CI) | 0.81 [0.52, 1.25] | |
| 3.2 Aprotinin versus control | 2 | Rate Ratio (Random, 95% CI) | 0.65 [0.25, 1.71] | |
| 3.3 Gabexate versus control | 1 | Rate Ratio (Random, 95% CI) | 0.64 [0.37, 1.10] | |
| 3.4 Probiotics versus control | 2 | Rate Ratio (Random, 95% CI) | 0.62 [0.24, 1.59] | |
| 3.5 Somatostatin versus control | 1 | Rate Ratio (Random, 95% CI) | 1.07 [0.67, 1.69] | |
| 3.6 Somatostatin plus omeprazole versus control | 1 | Rate Ratio (Random, 95% CI) | 0.36 [0.19, 0.70] | |
| 3.7 Somatostatin plus ulinastatin versus control | 1 | Rate Ratio (Random, 95% CI) | 0.30 [0.15, 0.60] | |
| 3.8 Octreotide plus ulinastatin versus octreotide | 1 | Rate Ratio (Random, 95% CI) | 0.30 [0.17, 0.51] | |
| 3.9 Somatostatin plus ulinastatin versus somatostatin | 1 | Rate Ratio (Random, 95% CI) | 0.28 [0.15, 0.56] | |
| 4 Organ failure Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4 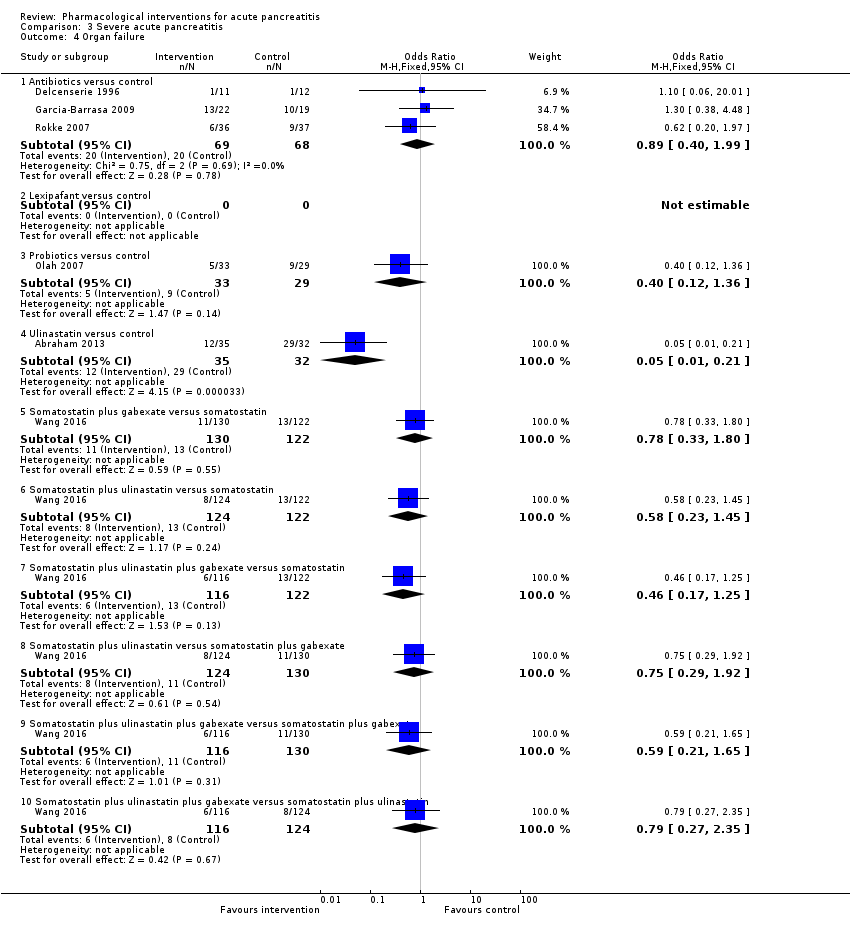 Comparison 3 Severe acute pancreatitis, Outcome 4 Organ failure. | ||||
| 4.1 Antibiotics versus control | 3 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.99] |
| 4.2 Lexipafant versus control | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.36] |
| 4.4 Ulinastatin versus control | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.21] |
| 4.5 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.80] |
| 4.6 Somatostatin plus ulinastatin versus somatostatin | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.45] |
| 4.7 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
| 4.8 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.29, 1.92] |
| 4.9 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.21, 1.65] |
| 4.10 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.27, 2.35] |
| 5 Infected pancreatic necrosis Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Severe acute pancreatitis, Outcome 5 Infected pancreatic necrosis. | ||||
| 5.1 Antibiotics versus control | 6 | 341 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.33] |
| 5.2 Probiotics versus control | 2 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.22, 1.68] |
| 6 Sepsis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Severe acute pancreatitis, Outcome 6 Sepsis. | ||||
| 6.1 Aprotinin versus control | 2 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.50, 6.98] |
| 6.2 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.36] |
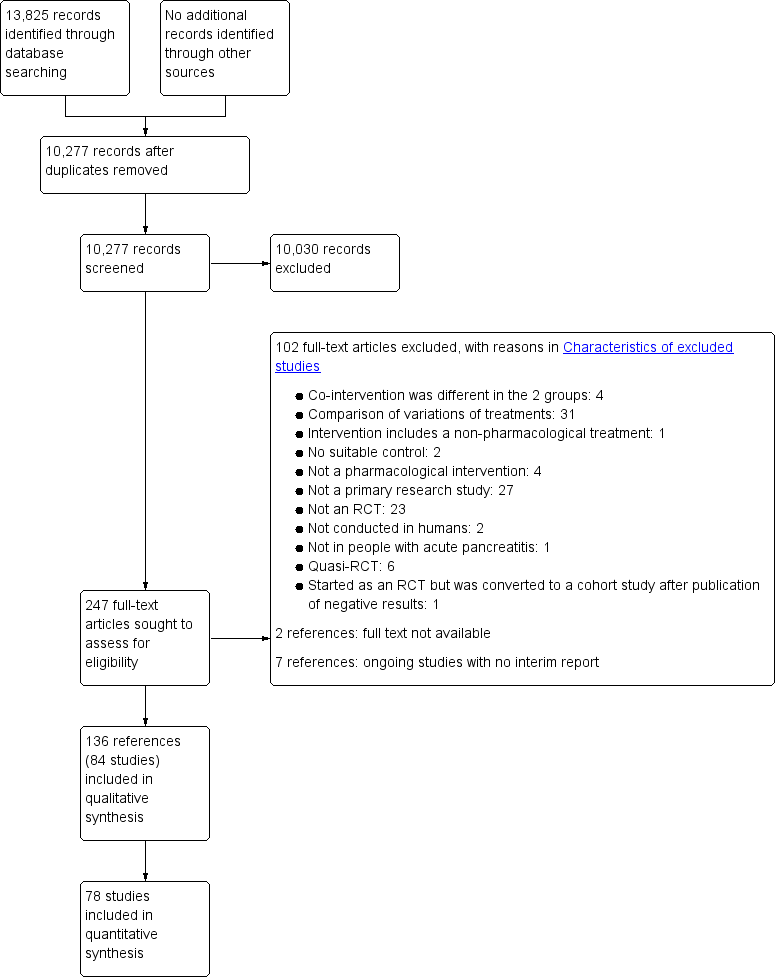
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of short‐term mortality indicating no evidence of reporting bias.

Funnel plot of infected pancreatic necrosis indicating no evidence of reporting bias.

Funnel plot of requirement for additional invasive intervention indicating no evidence of reporting bias.
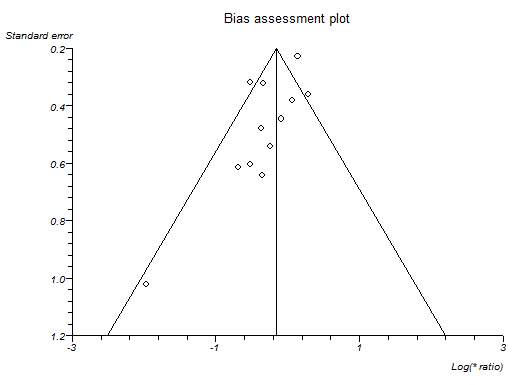
Funnel plot of serious adverse events (number) indicating that trials with lower precision favoured antibiotics without matching trials with lower precision which showed no effect or favouring control.

Funnel plot of adverse events (number) indicating that trials with lower precision favoured antibiotics while trials with greater precision favoured control.

Network plot showing the treatment comparisons that included short‐term mortality. The circles represent treatments while the lines represent the comparisons between the treatments.
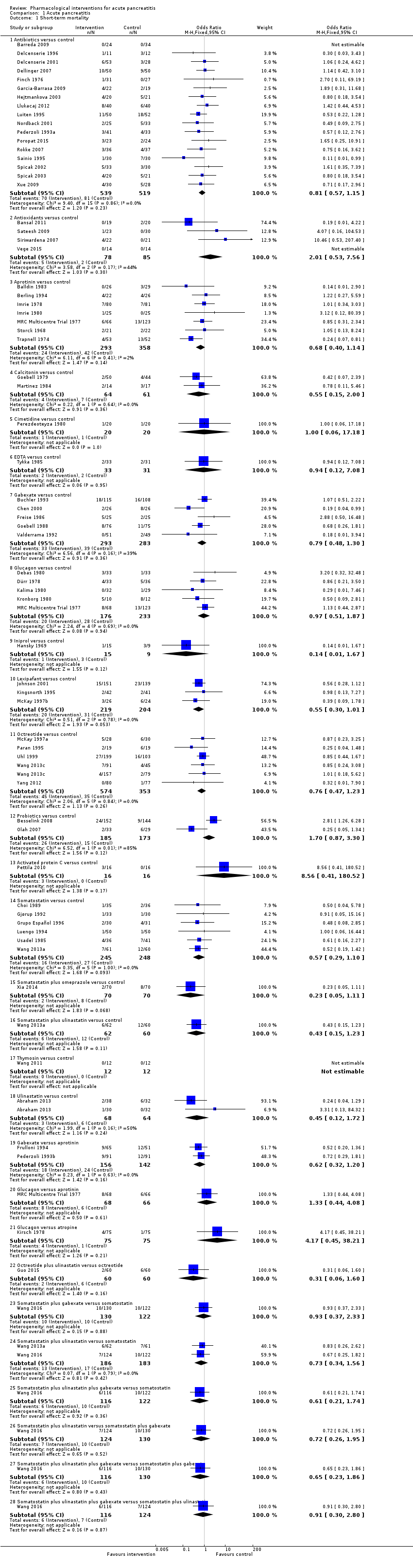
Comparison 1 Acute pancreatitis, Outcome 1 Short‐term mortality.
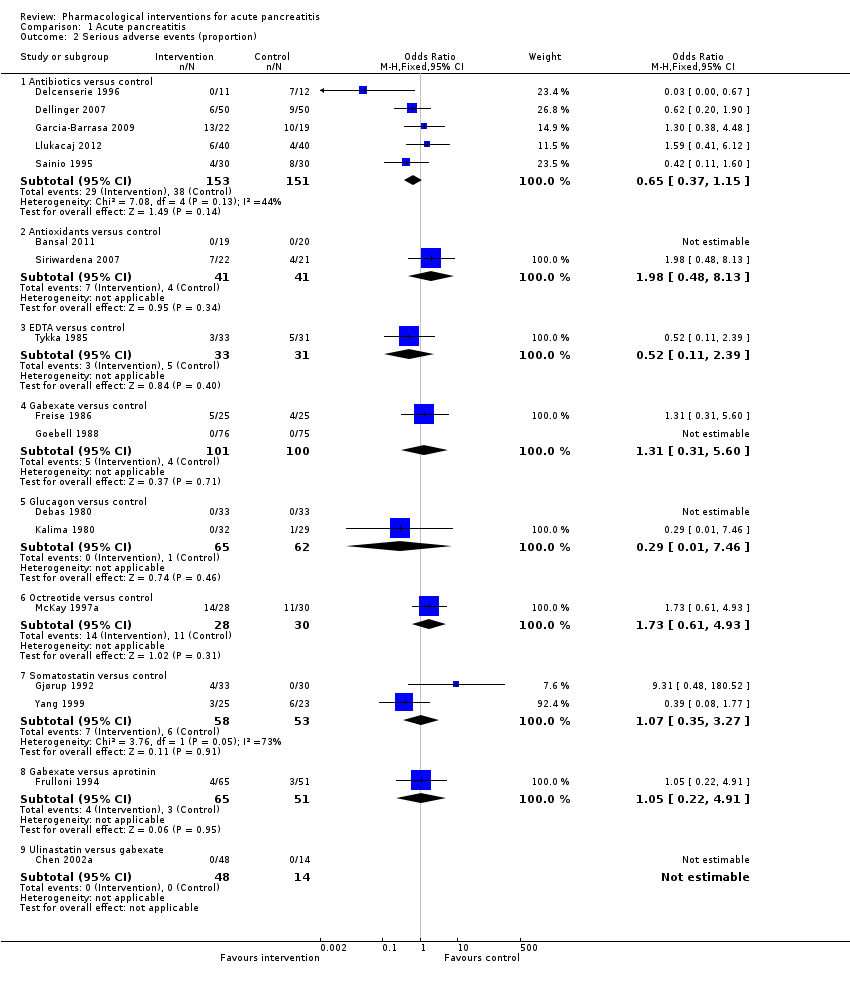
Comparison 1 Acute pancreatitis, Outcome 2 Serious adverse events (proportion).

Comparison 1 Acute pancreatitis, Outcome 3 Serious adverse events (number).

Comparison 1 Acute pancreatitis, Outcome 4 Organ failure.
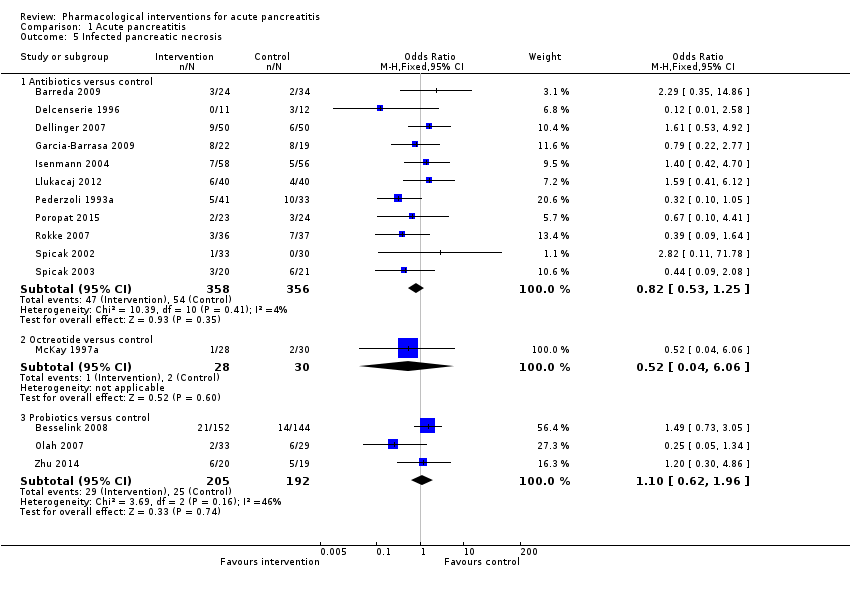
Comparison 1 Acute pancreatitis, Outcome 5 Infected pancreatic necrosis.

Comparison 1 Acute pancreatitis, Outcome 6 Sepsis.
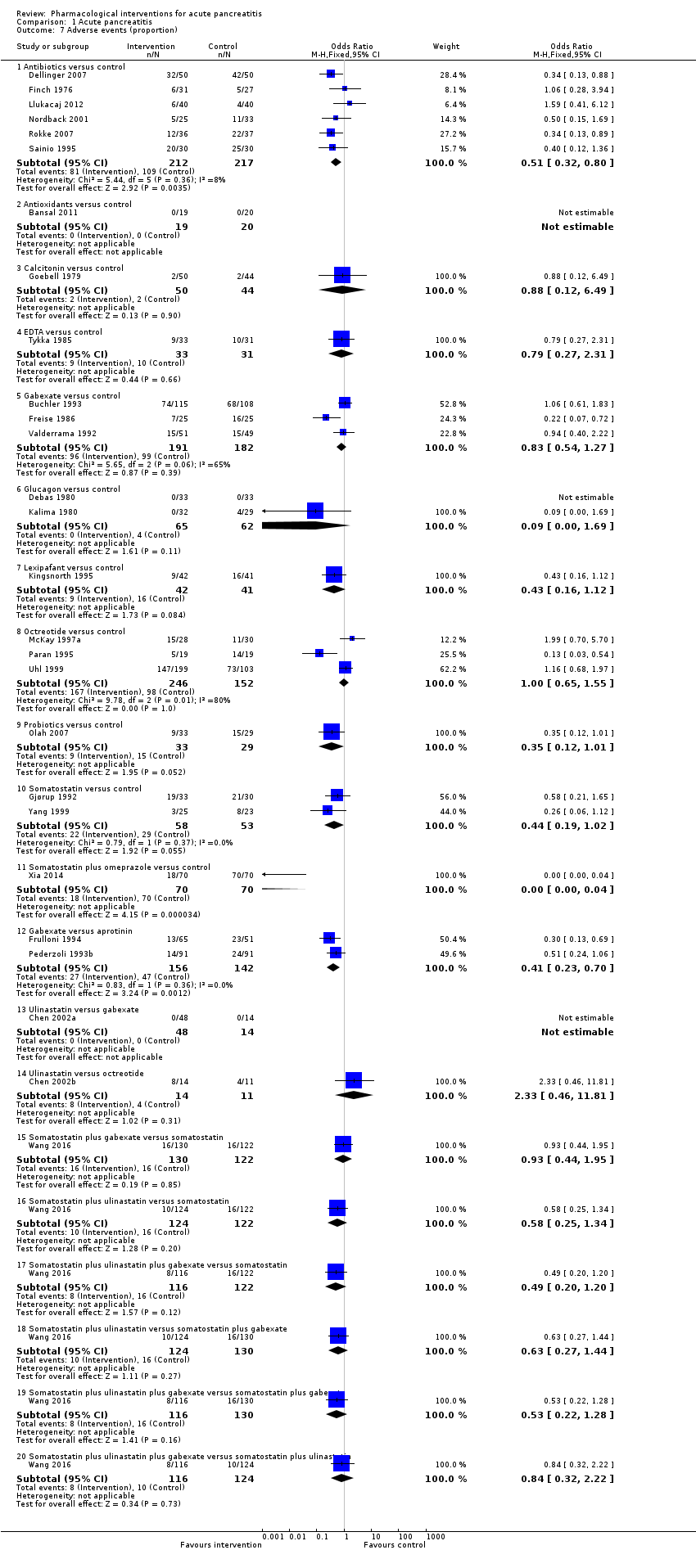
Comparison 1 Acute pancreatitis, Outcome 7 Adverse events (proportion).

Comparison 1 Acute pancreatitis, Outcome 8 Adverse events (number).

Comparison 1 Acute pancreatitis, Outcome 9 Requirement for additional invasive intervention.
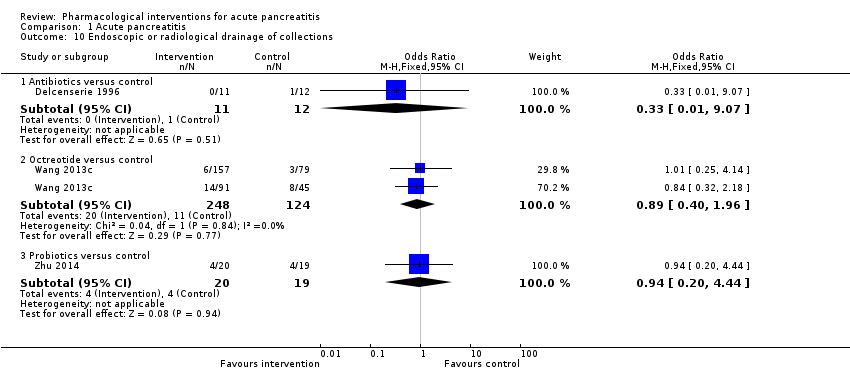
Comparison 1 Acute pancreatitis, Outcome 10 Endoscopic or radiological drainage of collections.
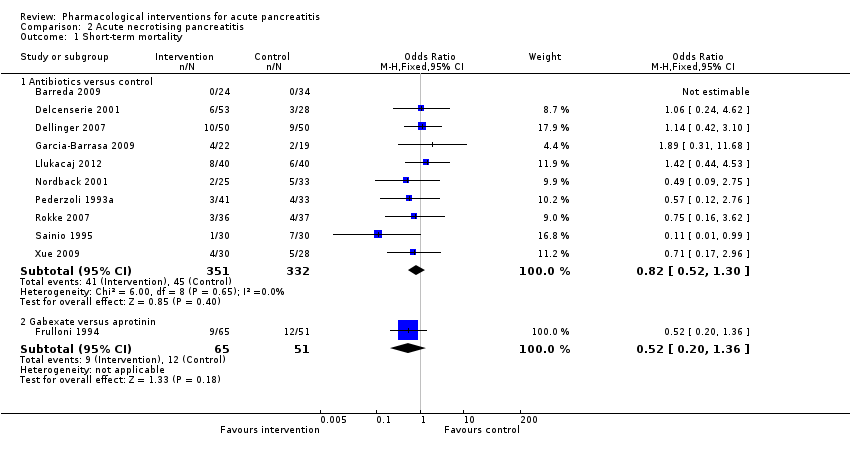
Comparison 2 Acute necrotising pancreatitis, Outcome 1 Short‐term mortality.

Comparison 2 Acute necrotising pancreatitis, Outcome 2 Serious adverse events (proportion).

Comparison 2 Acute necrotising pancreatitis, Outcome 3 Serious adverse events (number).

Comparison 2 Acute necrotising pancreatitis, Outcome 4 Organ failure.

Comparison 2 Acute necrotising pancreatitis, Outcome 5 Infected pancreatic necrosis.
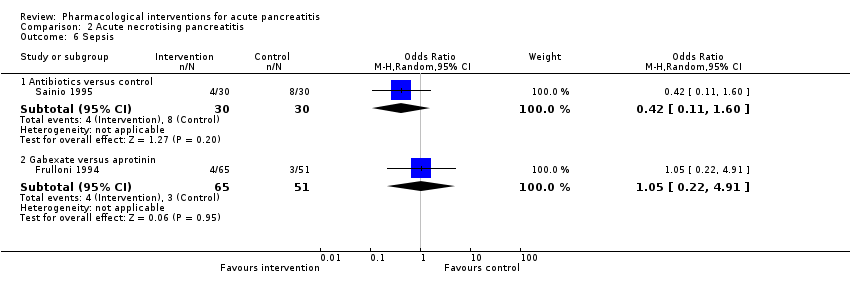
Comparison 2 Acute necrotising pancreatitis, Outcome 6 Sepsis.
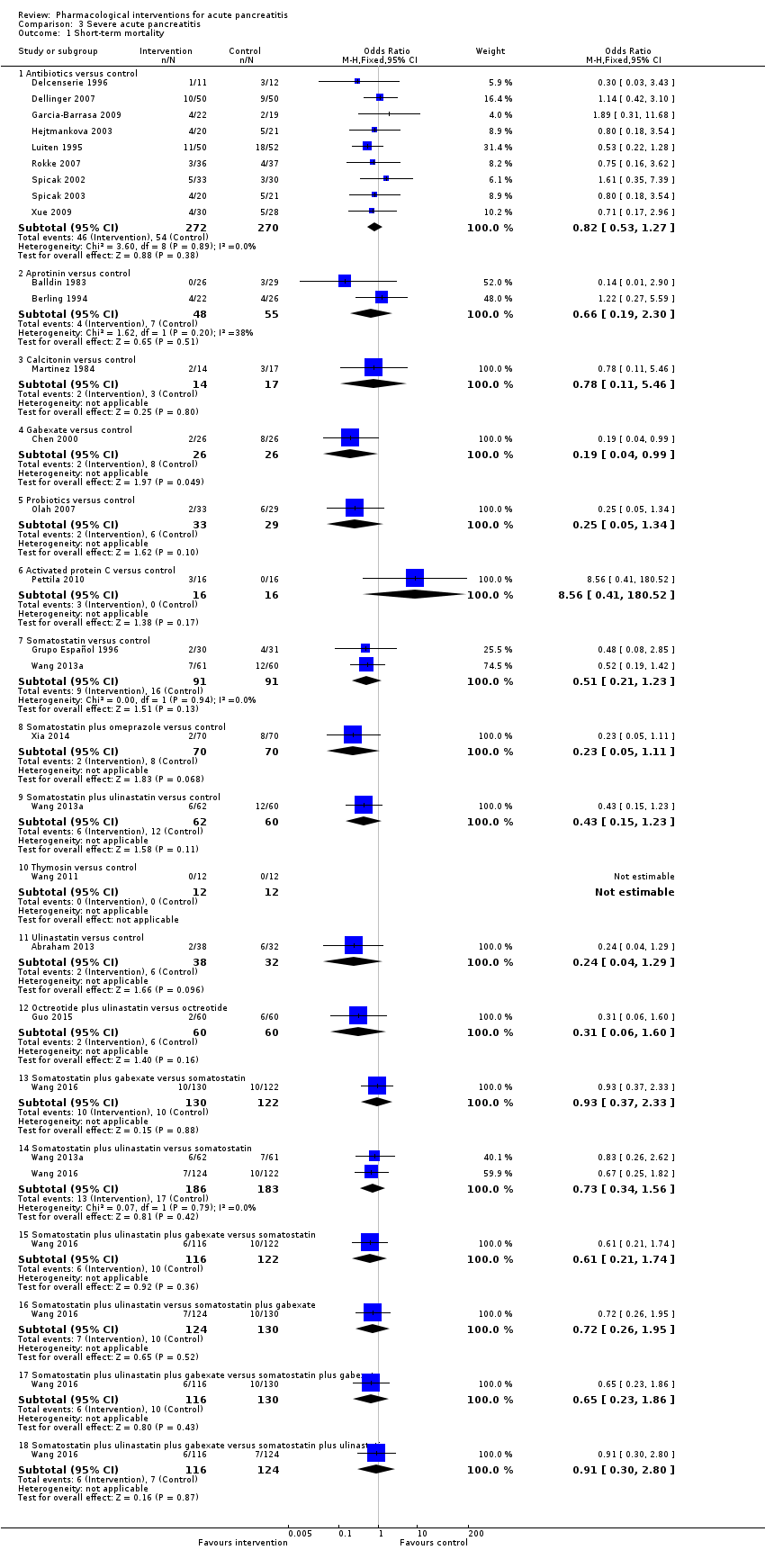
Comparison 3 Severe acute pancreatitis, Outcome 1 Short‐term mortality.
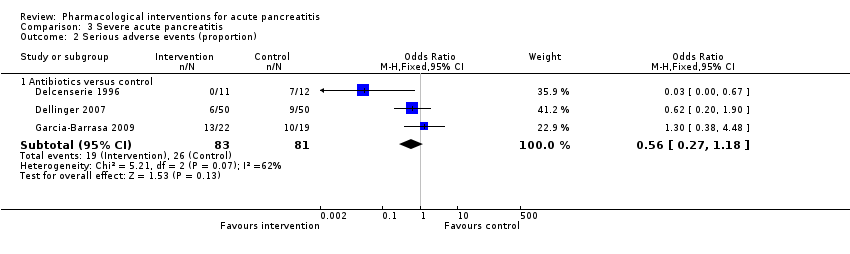
Comparison 3 Severe acute pancreatitis, Outcome 2 Serious adverse events (proportion).
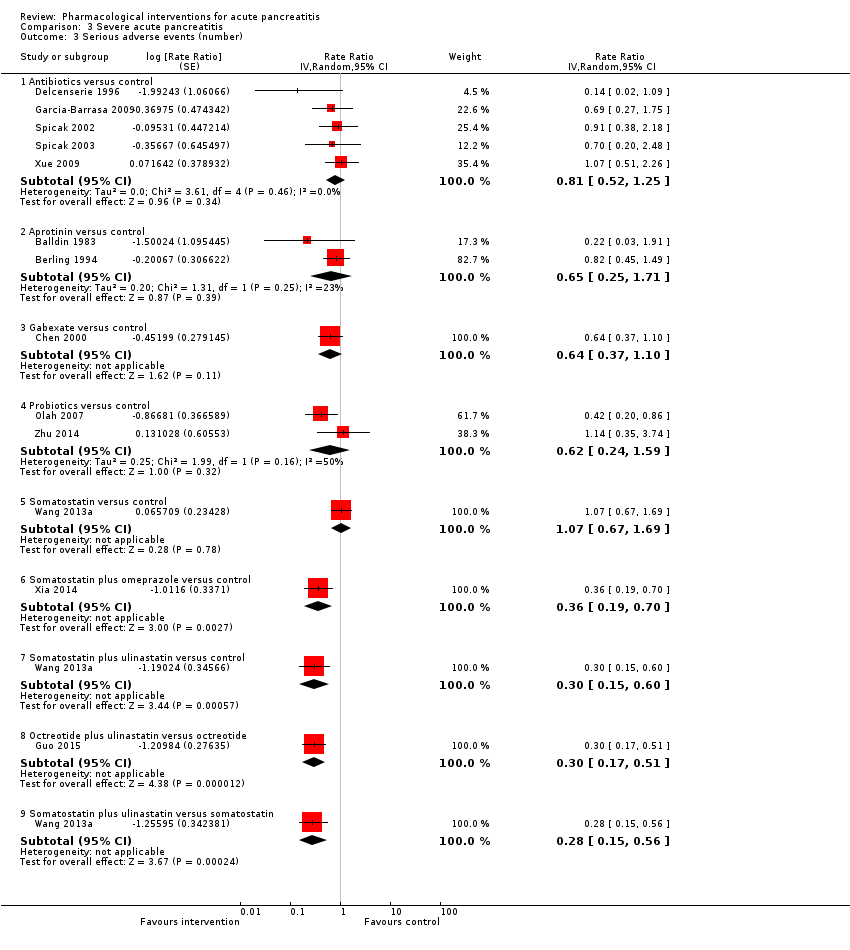
Comparison 3 Severe acute pancreatitis, Outcome 3 Serious adverse events (number).

Comparison 3 Severe acute pancreatitis, Outcome 4 Organ failure.

Comparison 3 Severe acute pancreatitis, Outcome 5 Infected pancreatic necrosis.
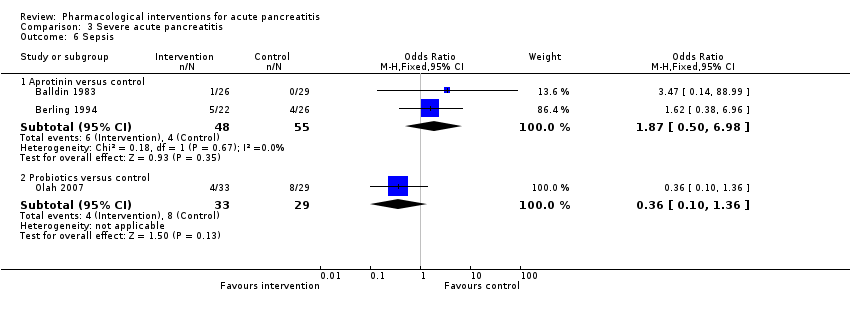
Comparison 3 Severe acute pancreatitis, Outcome 6 Sepsis.
| Pharmacological interventions for treatment of acute severe pancreatitis (mortality) | |||||
| Patient or population: people with acute pancreatitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk Inactive control | Corresponding risk Various treatments | ||||
| Short‐term mortality Follow‐up: up to 3 months | Antibiotics | OR 0.81 | 1058 | ⊕⊝⊝⊝ | |
| 120 per 1000 | 99 per 1000 | ||||
| Antioxidants | OR 2.01 | 163 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 215 per 1000 | ||||
| Aprotinin | OR 0.68 | 651 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 85 per 1000 | ||||
| Calcitonin | OR 0.55 | 125 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 69 per 1000 | ||||
| Cimetidine | OR 1.00 | 40 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 120 per 1000 | ||||
| EDTA | OR 0.94 | 64 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 113 per 1000 | ||||
| Gabexate | OR 0.79 | 576 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 98 per 1000 | ||||
| Glucagon | OR 0.97 | 409 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 117 per 1000 | ||||
| Iniprol | OR 0.14 | 24 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 19 per 1000 | ||||
| Lexipafant | OR 0.55 | 423 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 70 per 1000 | ||||
| Octreotide | OR 0.76 | 927 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 94 per 1000 | ||||
| Probiotics | OR 1.70 | 358 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 188 per 1000 | ||||
| Activated protein C | OR 8.56 | 32 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 539 per 1000 | ||||
| Somatostatin | OR 0.57 | 493 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 72 per 1000 | ||||
| Somatostatin plus omeprazole | OR 0.23 | 140 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 30 per 1000 | ||||
| Somatostatin plus ulinastatin | OR 0.43 | 122 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 55 per 1000 | ||||
| Thymosin | Not estimable | 24 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | not estimable | ||||
| Ulinastatin | OR 0.45 | 132 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 58 per 1000 | ||||
| Long‐term mortality | None of the trials with inactive treatment in the control group reported long‐term mortality. | ||||
| *The basis for the assumed risk is the average control group proportion across all comparisons. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence intervals; OR: odds ratio; EDTA: ethylenediaminetetraacetic acid. | |||||
| GRADE Working Group grades of evidence | |||||
| aRisk of bias: downgraded by one level. | |||||
| Pharmacological interventions for treatment of acute severe pancreatitis (other outcomes) | |||||
| Patient or population: people with acute pancreatitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Inactive control | Various treatments | ||||
| Serious adverse events (proportion) Follow‐up: up to 3 months | Antibiotics | OR 0.65 | 304 | ⊕⊝⊝⊝ | |
| 147 per 1000 | 101 per 1000 | ||||
| Antioxidants | OR 1.98 | 82 | ⊕⊝⊝⊝ | ||
| 147 per 1000 | 255 per 1000 | ||||
| EDTA | OR 0.52 | 64 | ⊕⊝⊝⊝ | ||
| 147 per 1000 | 83 per 1000 | ||||
| Gabexate | OR 1.31 | 201 | ⊕⊝⊝⊝ | ||
| 147 per 1000 | 185 per 1000 | ||||
| Glucagon | OR 0.29 | 127 | ⊕⊝⊝⊝ | ||
| 147 per 1000 | 48 per 1000 | ||||
| Octreotide | OR 1.73 | 58 | ⊕⊝⊝⊝ | ||
| 147 per 1000 | 230 per 1000 | ||||
| Somatostatin | OR 1.07 | 111 | ⊕⊝⊝⊝ | ||
| 147 per 1000 | 156 per 1000 | ||||
| Serious adverse events (number) Follow‐up: up to 3 months | Antibiotics | Rate ratio0.86 | 716 | ⊕⊝⊝⊝ | |
| 437 per 1000 | 374 per 1000 | ||||
| Antioxidants | Rate ratio0.22 | 71 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 94 per 1000 | ||||
| Aprotinin | Rate ratio0.79 | 264 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 345 per 1000 | ||||
| Cimetidine | Rate ratio1.00 | 60 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 437 per 1000 | ||||
| EDTA | Rate ratio0.94 | 64 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 411 per 1000 | ||||
| Gabexate | Rate ratio0.86 | 375 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 375 per 1000 | ||||
| Glucagon | Rate ratio1.00 | 68 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 437 per 1000 | ||||
| Lexipafant | rate ratio0.67 | 290 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 292 per 1000 | ||||
| Octreotide | Rate ratio0.74 | 770 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 321 per 1000 | ||||
| Probiotics | Rate ratio0.94 | 397 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 412 per 1000 | ||||
| Somatostatin | Rate ratio1.03 | 257 | ⊕⊝⊝⊝ | ||
| 437 per 1000 | 449 per 1000 | ||||
| Somatostatin plus omeprazole | Rate ratio0.36 | 140 | ⊕⊕⊝⊝ | ||
| 437 per 1000 | 159 per 1000 | ||||
| Somatostatin plus ulinastatin | Rate ratio0.30 | 122 | ⊕⊕⊝⊝ | ||
| 437 per 1000 | 133 per 1000 | ||||
| Organ failure Follow‐up: up to 3 months | Antibiotics | OR 0.78 | 258 | ⊕⊝⊝⊝ | |
| 289 per 1000 | 241 per 1000 | ||||
| Antioxidants | OR 0.92 | 163 | ⊕⊝⊝⊝ | ||
| 289 per 1000 | 271 per 1000 | ||||
| Gabexate | OR 0.32 | 50 | ⊕⊝⊝⊝ | ||
| 289 per 1000 | 115 per 1000 | ||||
| Lexipafant | OR 0.68 | 340 | ⊕⊝⊝⊝ | ||
| 289 per 1000 | 216 per 1000 | ||||
| Octreotide | OR 0.51 | 430 | ⊕⊝⊝⊝ | ||
| 289 per 1000 | 173 per 1000 | ||||
| Probiotics | OR 0.80 | 358 | ⊕⊝⊝⊝ | ||
| 289 per 1000 | 246 per 1000 | ||||
| Ulinastatin | OR 0.27 | 129 | ⊕⊝⊝⊝ | ||
| 289 per 1000 | 100 per 1000 | ||||
| Infected pancreatic necrosis Follow‐up: up to 3 months | Antibiotics | OR 0.82 | 714 | ⊕⊝⊝⊝ | |
| 140 per 1000 | 118 per 1000 | ||||
| Octreotide | OR 0.52 | 58 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 78 per 1000 | ||||
| Probiotics | OR 1.10 | 397 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 152 per 1000 | ||||
| Sepsis Follow‐up: up to 3 months | Antibiotics | OR 0.42 | 60 | ⊕⊝⊝⊝ | |
| 122 per 1000 | 56 per 1000 | ||||
| Aprotinin | OR 1.84 | 103 | ⊕⊝⊝⊝ | ||
| 122 per 1000 | 204 per 1000 | ||||
| Gabexate | OR 1.10 | 373 | ⊕⊝⊝⊝ | ||
| 122 per 1000 | 133 per 1000 | ||||
| Lexipafant | OR 0.26 | 290 | ⊕⊝⊝⊝ | ||
| 122 per 1000 | 35 per 1000 | ||||
| Octreotide | OR 0.40 | 340 | ⊕⊝⊝⊝ | ||
| 122 per 1000 | 53 per 1000 | ||||
| Probiotics | OR 0.36 | 62 | ⊕⊝⊝⊝ | ||
| 122 per 1000 | 48 per 1000 | ||||
| Health‐related quality of life | None of the trials reported this outcome. | ||||
| *The basis for the assumed risk is the average control group proportion across all comparisons. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence intervals; OR = odds ratio; EDTA = ethylenediaminetetraacetic acid. | |||||
| GRADE Working Group grades of evidence | |||||
| aRisk of bias: downgraded by one level. | |||||
| Study name | No of participants randomised | Postrandomisation dropouts | No of participants for whom outcome was reported | Treatment 1 | Treatment 2 | Selection bias | Performance and detection bias | Attrition bias | Selective reporting bias | Other bias |
| 32 | 0 | 32 | Activated protein C | Placebo | Unclear | Low | Low | High | High | |
| 80 | 22 | 58 | Antibiotics | No active intervention | Unclear | Unclear | High | Low | Unclear | |
| 23 | 0 | 23 | Antibiotics | No active intervention | Unclear | Unclear | Low | Low | Unclear | |
| 81 | Not stated | 81 | Antibiotics | No active intervention | Unclear | Unclear | Unclear | Low | Unclear | |
| 100 | 0 | 100 | Antibiotics | Placebo | Low | Low | Low | Low | High | |
| 62 | 4 | 58 | Antibiotics | No active intervention | Unclear | Unclear | High | Low | Unclear | |
| 46 | 5 | 41 | Antibiotics | Placebo | Unclear | Low | High | Low | Low | |
| 41 | Not stated | 41 | Antibiotics | No active intervention | Unclear | Unclear | Unclear | Low | Unclear | |
| 119 | 5 | 114 | Antibiotics | Placebo | Unclear | Low | High | High | High | |
| 80 | Not stated | 80 | Antibiotics | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 109 | 7 | 102 | Antibiotics | No active intervention | Unclear | Unclear | High | Low | Unclear | |
| 90 | 32 | 58 | Antibiotics | Placebo | Unclear | Unclear | High | Low | Unclear | |
| 47 | 0 | 47 | Antibiotics | No active intervention | Unclear | Unclear | Low | Low | Unclear | |
| 74 | Not stated | 74 | Antibiotics | No active intervention | Unclear | Unclear | Low | Low | Unclear | |
| 73 | 0 | 73 | Antibiotics | No active intervention | Unclear | High | Low | Low | High | |
| 60 | 0 | 60 | Antibiotics | No active intervention | Unclear | Unclear | Low | Low | Unclear | |
| 63 | Not stated | 63 | Antibiotics | No active intervention | Unclear | Unclear | Unclear | Low | Unclear | |
| 41 | Not stated | 41 | Antibiotics | No active intervention | Unclear | Unclear | Unclear | Low | Unclear | |
| 59 | 3 | 56 | Antibiotics | No active intervention | Unclear | Unclear | High | Low | Low | |
| 44 | 5 | 39 | Antioxidants | No active intervention | Unclear | High | High | Low | Low | |
| 20 | Not stated | 20 | Antioxidants | No active intervention | Unclear | Unclear | Unclear | High | Unclear | |
| 73 | 0 | 73 | Antioxidants | Placebo | Unclear | Unclear | Low | High | Unclear | |
| 56 | 3 | 53 | Antioxidants | No active intervention | Unclear | High | High | Low | Unclear | |
| 43 | 0 | 43 | Antioxidants | Placebo | Low | Low | Low | Low | High | |
| 28 | Not stated | 28 | Antioxidants | Placebo | Unclear | Low | Low | Low | Unclear | |
| 34 | Not stated | 34 | Antioxidants plus Corticosteroids | No active intervention | Unclear | Unclear | Unclear | High | Unclear | |
| (this is a 3‐armed trial; the numbers stated included all 3 arms) | 264 | 7 | 257 | Aprotinin | Placebo | Unclear | Low | High | High | High |
| 55 | Not stated | 55 | Aprotinin | No active intervention | Unclear | Unclear | Unclear | Low | High | |
| 48 | Not stated | 48 | Aprotinin | No active intervention | Unclear | Low | Low | Low | High | |
| 161 | Not stated | 161 | Aprotinin | Placebo | Unclear | Low | Unclear | Low | High | |
| 50 | Not stated | 50 | Aprotinin | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 43 | Not stated | 43 | Aprotinin | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 105 | Not stated | 105 | Aprotinin | Placebo | Low | Low | Unclear | High | High | |
| (this is a 3‐armed trial; the numbers stated included all 3 arms) | 264 | 7 | 257 | Aprotinin | Glucagon | Unclear | Low | High | High | High |
| 94 | Not stated | 94 | Calcitonin | Placebo | Unclear | Low | Unclear | Low | Unclear | |
| 31 | 0 | 31 | Calcitonin | Placebo | Unclear | Unclear | Low | High | Unclear | |
| 40 | Not stated | 40 | Cimetidine | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 60 | Not stated | 60 | Cimetidine | Placebo | Low | Unclear | Unclear | High | Unclear | |
| 64 | 0 | 64 | EDTA | Placebo | Unclear | Low | Low | Low | High | |
| 116 | Not stated | 116 | Gabexate | Aprotinin | Unclear | Unclear | Unclear | Low | Unclear | |
| 199 | 17 | 182 | Gabexate | Aprotinin | Unclear | Low | High | Low | Unclear | |
| 223 | Not stated | 223 | Gabexate | Placebo | Low | Low | Low | Low | Unclear | |
| 52 | Not stated | 52 | Gabexate | Placebo | Unclear | Unclear | Unclear | Low | Unclear | |
| 50 | Not stated | 50 | Gabexate | Placebo | Unclear | Low | Unclear | Low | Unclear | |
| 162 | 11 | 151 | Gabexate | Placebo | Unclear | Low | High | Low | Unclear | |
| 105 | 5 | 100 | Gabexate | Placebo | Low | Low | High | Low | High | |
| 150 | Not stated | 150 | Glucagon | Atropine | Unclear | Unclear | Unclear | Low | Unclear | |
| (this is a 3‐armed trial; the numbers stated included all 3 arms) | 264 | 7 | 257 | Glucagon | Placebo | Unclear | Unclear | Unclear | Low | High |
| 66 | Not stated | 66 | Glucagon | Placebo | Unclear | Low | Unclear | Low | Unclear | |
| 69 | Not stated | 69 | Glucagon | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 80 | 9 | 71 | Glucagon | Placebo | Unclear | Unclear | High | Low | Unclear | |
| 22 | Not stated | 22 | Glucagon | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 62 | Not stated | 62 | Glucagon | Oxyphenonium | Unclear | Low | Unclear | Low | Unclear | |
| 24 | Not stated | 24 | Iniprol | No active intervention | Unclear | High | Unclear | High | High | |
| 291 | 1 | 290 | Lexipafant | Placebo | Unclear | Low | High | Low | High | |
| 83 | Not stated | 83 | Lexipafant | Placebo | Unclear | Low | Unclear | High | High | |
| 51 | 1 | 50 | Lexipafant | Placebo | Unclear | Low | High | High | High | |
| 66 | 9 | 57 | NSAID | Placebo | Unclear | Unclear | Unclear | High | Unclear | |
| 30 | 0 | 30 | NSAID | Placebo | Unclear | Low | Low | High | High | |
| 58 | 0 | 58 | Octreotide | Placebo | Low | Low | Low | Low | Unclear | |
| 180 | Not stated | 180 | Octreotide | Placebo | Unclear | Unclear | Unclear | High | Unclear | |
| 51 | 13 | 38 | Octreotide | No active intervention | Unclear | High | High | Low | Unclear | |
| 302 | 0 | 302 | Octreotide | Placebo | Unclear | Low | Low | Low | High | |
| 372 | Not stated | 372 | Octreotide | No active intervention | Unclear | Unclear | High | Low | Low | |
| 163 | 6 | 157 | Octreotide | No active intervention | Unclear | Unclear | High | High | Low | |
| 354 | Not stated | 354 | Octreotide plus NSAID | Octreotide | Unclear | Unclear | Unclear | High | Unclear | |
| 120 | Not stated | 120 | Octreotide plus ulinastatin | Octreotide | Unclear | Unclear | Unclear | Low | Unclear | |
| 298 | 2 | 296 | Probiotics | Placebo | Low | Low | High | Low | High | |
| 83 | 21 | 62 | Probiotics | No active intervention | Unclear | Low | High | High | Unclear | |
| 90 | Not stated | 58 | Probiotics | No active intervention | Unclear | Low | Unclear | High | Unclear | |
| 50 | 0 | 50 | Probiotics | Placebo | Unclear | Low | Low | High | High | |
| 39 | Not stated | 39 | Probiotics | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 70 | 9 | 61 | Somatostatin | Placebo | Unclear | Low | High | High | Unclear | |
| 71 | Not stated | 71 | Somatostatin | No active intervention | Unclear | Unclear | Unclear | Low | Unclear | |
| 63 | Not stated | 63 | Somatostatin | Placebo | Unclear | Low | Unclear | Low | Unclear | |
| 100 | Not stated | 100 | Somatostatin | No active intervention | Unclear | Low | Unclear | High | Unclear | |
| 87 | 3 | 84 | Somatostatin | Placebo | Unclear | Low | Unclear | High | High | |
| 77 | Not stated | 77 | Somatostatin | Placebo | Unclear | Low | Unclear | High | Unclear | |
| Wang 2013a (this is a 3‐armed trial; the numbers stated included all 3 arms) | 183 | Not stated | 183 | Somatostatin | No active intervention | Unclear | Low | Unclear | Low | Low |
| 48 | Not stated | 48 | Somatostatin | No active intervention | Unclear | Unclear | Unclear | High | Unclear | |
| 140 | Not stated | 140 | Somatostatin plus omeprazole | No active intervention | Unclear | Unclear | Unclear | Low | Unclear | |
| Wang 2013a (this is a 3‐armed trial; the numbers stated included all 3 arms) | 183 | Not stated | 183 | Somatostatin plus ulinastatin | Placebo | Unclear | Unclear | Unclear | High | Unclear |
| Wang 2013a (this is a 3‐armed trial; the numbers stated included all 3 arms) | 183 | Not stated | 183 | Somatostatin plus ulinastatin | Somatostatin | Unclear | Low | Unclear | Low | Low |
| Wang 2016 (this is a 4‐armed trial; the numbers stated included all 4 arms) | 492 | 0 | 492 | Somatostatin plus ulinastatin | Somatostatin | Low | Low | Low | Low | Low |
| Wang 2016 (this is a 4‐armed trial; the numbers stated included all 4 arms) | 492 | 0 | 492 | Somatostatin plus gabexate | Somatostatin | Low | Low | Low | Low | Low |
| Wang 2016 (this is a 4‐armed trial; the numbers stated included all 4 arms) | 492 | 0 | 492 | Somatostatin plus ulinastatin plus gabexate | Somatostatin | Low | Low | Low | Low | Low |
| Wang 2016 (this is a 4‐armed trial; the numbers stated included all 4 arms) | 492 | 0 | 492 | Somatostatin plus ulinastatin | Somatostatin plus gabexate | Low | Low | Low | Low | Low |
| Wang 2016 (this is a 4‐armed trial; the numbers stated included all 4 arms) | 492 | 0 | 492 | Somatostatin plus ulinastatin plus gabexate | Somatostatin plus gabexate | Low | Low | Low | Low | Low |
| Wang 2016 (this is a 4‐armed trial; the numbers stated included all 4 arms) | 492 | 0 | 492 | Somatostatin plus ulinastatin plus gabexate | Somatostatin plus ulinastatin | Low | Low | Low | Low | Low |
| 24 | Not stated | 24 | Thymosin | Placebo | Unclear | Low | Unclear | High | Unclear | |
| 135 | 6 | 129 | Ulinastatin | Placebo | Unclear | Low | High | Low | Unclear | |
| 68 | 6 | 62 | Ulinastatin | Gabexate | Unclear | Unclear | High | High | Unclear | |
| 26 | 1 | 25 | Ulinastatin | Octreotide | Unclear | Unclear | High | High | Unclear |
| Study name | Treatment 1 | Treatment 2 | Severe pancreatitis | Necrotising pancreatitis | Organ failure | Infection |
| Activated protein C | Placebo | yes | not stated | not stated | not stated | |
| Antibiotics | No active intervention | not stated | yes | not stated | not stated | |
| Antibiotics | No active intervention | yes | not stated | not stated | not stated | |
| Antibiotics | No active intervention | not stated | yes | not stated | not stated | |
| Antibiotics | Placebo | yes | yes | not stated | no | |
| Antibiotics | No active intervention | not stated | not stated | not stated | not stated | |
| Antibiotics | Placebo | yes | yes | not stated | not stated | |
| Antibiotics | No active intervention | yes | not stated | not stated | not stated | |
| Antibiotics | Placebo | not stated | not stated | not stated | not stated | |
| Antibiotics | Placebo | not stated | yes | not stated | no | |
| Antibiotics | No active intervention | yes | not stated | not stated | no | |
| Antibiotics | Placebo | not stated | yes | no | not stated | |
| Antibiotics | No active intervention | not stated | yes | not stated | not stated | |
| Antibiotics | No active intervention | yes | yes | not stated | not stated | |
| Antibiotics | No active intervention | not stated | yes | not stated | not stated | |
| Antibiotics | No active intervention | yes | not stated | not stated | not stated | |
| Antibiotics | No active intervention | yes | not stated | not stated | not stated | |
| Antibiotics | No active intervention | yes | yes | not stated | no | |
| Antioxidants | No active intervention | not stated | not stated | not stated | not stated | |
| Antioxidants | No active intervention | yes | not stated | not stated | not stated | |
| Antioxidants | Placebo | not stated | not stated | not stated | not stated | |
| Antioxidants | No active intervention | not stated | not stated | not stated | not stated | |
| Antioxidants | Placebo | not stated | not stated | not stated | not stated | |
| Antioxidants | Placebo | not stated | not stated | not stated | not stated | |
| Antioxidants plus corticosteroids | No active intervention | yes | not stated | not stated | not stated | |
| Aprotinin | No active intervention | yes | not stated | not stated | not stated | |
| Aprotinin | No active intervention | yes | not stated | not stated | not stated | |
| Aprotinin | Placebo | not stated | not stated | not stated | not stated | |
| Aprotinin | Placebo | not stated | not stated | not stated | not stated | |
| Aprotinin | Placebo | not stated | not stated | not stated | not stated | |
| Aprotinin | Placebo | not stated | not stated | not stated | not stated | |
| Aprotinin | Placebo | not stated | not stated | not stated | not stated | |
| Calcitonin | Placebo | not stated | not stated | not stated | not stated | |
| Calcitonin | Placebo | yes | not stated | not stated | not stated | |
| Cimetidine | Placebo | not stated | not stated | not stated | not stated | |
| Cimetidine | Placebo | not stated | not stated | not stated | not stated | |
| EDTA | Placebo | not stated | not stated | not stated | not stated | |
| Gabexate | Placebo | not stated | not stated | not stated | not stated | |
| Gabexate | Placebo | yes | not stated | yes | not stated | |
| Gabexate | Placebo | not stated | not stated | not stated | not stated | |
| Gabexate | Placebo | not stated | not stated | not stated | not stated | |
| Gabexate | Placebo | not stated | not stated | not stated | not stated | |
| Glucagon | Placebo | not stated | not stated | not stated | not stated | |
| Glucagon | Placebo | not stated | not stated | not stated | not stated | |
| Glucagon | Placebo | not stated | not stated | not stated | not stated | |
| Glucagon | Placebo | not stated | not stated | not stated | not stated | |
| Glucagon | Placebo | not stated | not stated | not stated | not stated | |
| Iniprol | No active intervention | not stated | not stated | not stated | not stated | |
| Lexipafant | Placebo | not stated | not stated | not stated | not stated | |
| Lexipafant | Placebo | not stated | not stated | not stated | not stated | |
| Lexipafant | Placebo | not stated | not stated | not stated | not stated | |
| NSAID | Placebo | not stated | not stated | not stated | not stated | |
| NSAID | Placebo | not stated | not stated | not stated | not stated | |
| Octreotide | Placebo | not stated | not stated | not stated | not stated | |
| Octreotide | Placebo | not stated | not stated | not stated | not stated | |
| Octreotide | No active intervention | not stated | not stated | not stated | not stated | |
| Octreotide | Placebo | not stated | not stated | not stated | not stated | |
| Wang 2013c (mild pancreatitis) | Octreotide | No active intervention | no | not stated | not stated | not stated |
| Wang 2013c (severe pancreatitis) | Octreotide | No active intervention | yes | not stated | not stated | not stated |
| Octreotide | No active intervention | no | not stated | not stated | not stated | |
| Probiotics | Placebo | not stated | not stated | not stated | not stated | |
| Probiotics | No active intervention | yes | not stated | not stated | not stated | |
| Probiotics | No active intervention | yes | not stated | not stated | not stated | |
| Probiotics | Placebo | not stated | not stated | not stated | not stated | |
| Probiotics | Placebo | yes | not stated | not stated | not stated | |
| Somatostatin | No active intervention | not stated | not stated | not stated | not stated | |
| Somatostatin | Placebo | not stated | not stated | not stated | not stated | |
| Somatostatin | Placebo | yes | not stated | not stated | not stated | |
| Somatostatin | No active intervention | not stated | not stated | not stated | not stated | |
| Somatostatin | Placebo | not stated | not stated | not stated | not stated | |
| Somatostatin | Placebo | not stated | not stated | not stated | not stated | |
| Somatostatin | No active intervention | yes | not stated | not stated | not stated | |
| Somatostatin | No active intervention | not stated | not stated | not stated | not stated | |
| Somatostatin plus omeprazole | No active intervention | yes | not stated | not stated | not stated | |
| Somatostatin plus ulinastatin | No active intervention | yes | not stated | not stated | not stated | |
| Thymosin | Placebo | yes | not stated | not stated | not stated | |
| Abraham 2013 (mild pancreatitis) | Ulinastatin | Placebo | no | not stated | not stated | no |
| Abraham 2013 (severe pancreatitis) | Ulinastatin | Placebo | yes | not stated | not stated | not stated |
| Gabexate | Aprotinin | not stated | yes | not stated | not stated | |
| Gabexate | Aprotinin | not stated | not stated | not stated | not stated | |
| Glucagon | Atropine | not stated | not stated | not stated | not stated | |
| Ulinastatin | Gabexate | no | no | no | not stated | |
| Aprotinin | Glucagon | not stated | not stated | not stated | not stated | |
| Octerotide plus ulinastatin | Octreotide | yes | not stated | not stated | not stated | |
| Octreotide plus NSAID | Octreotide | not stated | not stated | not stated | not stated | |
| Ulinastatin | Octreotide | yes | yes | not stated | not stated | |
| Glucagon | Oxyphenonium | not stated | not stated | not stated | not stated | |
| Antibiotics | No active intervention | not stated | not stated | not stated | no | |
| Somatostatin plus gabexate | Somatostatin | yes | not stated | not stated | not stated | |
| Somatostatin plus ulinastatin | Somatostatin | yes | not stated | not stated | not stated | |
| Somatostatin plus ulinastatin | Somatostatin | yes | not stated | not stated | not stated | |
| Somatostatin plus ulinastatin plus gabexate | Somatostatin | yes | not stated | not stated | not stated | |
| Somatostatin plus ulinastatin | Somatostatin plus gabexate | yes | not stated | not stated | not stated | |
| Somatostatin plus ulinastatin plus gabexate | Somatostatin plus gabexate | yes | not stated | not stated | not stated | |
| Somatostatin plus ulinastatin plus gabexate | Somatostatin plus ulinastatin | yes | not stated | not stated | not stated |
| Study name | Intervention | Comparator | Number of participants in intervention | Number of participants in control | Mean or median (standard deviation or interquartile range, if reported) hospital stay in intervention group | Mean or median (standard deviation or interquartile range, if reported) hospital stay in control group | Difference | Statistical significance (P‐value if reported) |
| Antibiotics | No active intervention | 24 | 34 | 54 | 45 | 9 | Not significant | |
| Antibiotics | No active intervention | 11 | 12 | 27.8 | 22 | 5.8 | Not significant | |
| Antibiotics | No active intervention | 31 | 27 | 10.4 | 11.3 | −0.9 | Not significant | |
| Antibiotics | Placebo | 22 | 19 | 21 | 19 | 2 | Not significant (0.80) | |
| Antibiotics | No active intervention | 20 | 21 | 18 (7.2) | 25 (14.8) | −7 | Not significant | |
| Antibiotics | Placebo | 58 | 56 | 21 | 18 | 3 | Not significant | |
| Antibiotics | No active intervention | 50 | 52 | 30 | 32 | −2 | Not significant | |
| Antibiotics | No active intervention | 36 | 37 | 18 | 22 | −4 | Not significant (0.32) | |
| Antibiotics | No active intervention | 30 | 30 | 33.2 (22.1) | 43.8 (43.1) | −10.6 | Not significant (0.24) | |
| Antibiotics | No active intervention | 33 | 30 | 18.9 (8.1) | 23.8 (19.3) | −4.9 | Not significant | |
| Antibiotics | No active intervention | 20 | 21 | 18 (7.2) | 25 (14.8) | −7 | Not significant | |
| Antibiotics | No active intervention | 29 | 27 | 28.3 | 30.7 | −2.4 | Not significant | |
| Antioxidants | No active intervention | 19 | 20 | 12.8 | 15.1 | −2.3 | Not significant | |
| Antioxidants | No active intervention | 23 | 30 | 7.2 (5) | 10.3 (7) | −3.1 | Not significant (0.07) | |
| Antioxidants | Placebo | 22 | 21 | 20.4 (24.4) | 14.3 (15.7) | 6.1 | Not significant (0.34) | |
| Antioxidants | Placebo | 14 | 14 | 3 | 5 | −2 | Not significant (0.06) | |
| Aprotinin | No active intervention | 26 | 29 | 17.3 | 16.5 | 0.8 | Not significant | |
| Aprotinin | No active intervention | 22 | 26 | 25 (15‐32) | 33 (17‐38) | −8 | Not significant (0.24) | |
| Calcitonin | Placebo | 50 | 44 | 18.3 (6.4) | 20.2 (7.5) | −1.9 | Not significant | |
| Calcitonin | Placebo | 14 | 17 | 24 (20.2) | 30 (21.7) | −6 | Not significant | |
| Gabexate | Placebo | 115 | 108 | 26 (20‐43) | 23 (28‐34) | 3 | Not significant | |
| Glucagon | Placebo | 33 | 33 | 26 (28.7) | 20 (19.2) | 6 | Not significant | |
| Glucagon | Placebo | 33 | 36 | 32.6 | 26.9 | 5.7 | Not significant | |
| Iniprol | No active intervention | 15 | 9 | 14.7 (9.3) | 18.7 (10.2) | −4 | Not significant | |
| Lexipafant | Placebo | 151 | 139 | 9 | 10 | −1 | Not significant | |
| Lexipafant | Placebo | 26 | 24 | 13.3 | 14.9 | −1.6 | Not significant | |
| NSAID | Placebo | 27 | 30 | 9 | 10 | −1 | Not significant | |
| NSAID | Placebo | 14 | 16 | 13 | 15 | −2 | Not significant | |
| Octreotide | Placebo | 28 | 30 | 10 | 10 | 0 | Not significant | |
| Octreotide | Placebo | 90 | 90 | 7.3 | 8.2 | −0.9 | Not significant | |
| Octreotide | No active intervention | 19 | 19 | 17.9 (13.2) | 34.1 (22.7) | −16.2 | Significant (0.02) | |
| Octreotide | Placebo | 199 | 103 | 21.5 | 21 | 0.5 | Not significant | |
| (mild acute pancreatitis) | Octreotide | No active intervention | 157 | 79 | 14.4 | 15.37 | −0.97 | Not significant |
| (severe acute pancreatitis) | Octreotide | No active intervention | 91 | 45 | 16 | 16 | 0 | Not significant |
| Octreotide | No active intervention | 80 | 77 | 7.4 (2) | 11.8 (4) | −4.4 | Significant | |
| Probiotics | Placebo | 152 | 144 | 28.9 (41.5) | 23.5 (25.9) | 5.4 | Not significant (0.98) | |
| Probiotics | No active intervention | 33 | 29 | 14.9 | 19.7 | −4.8 | Not significant | |
| Probiotics | Placebo | 24 | 26 | 13.23 (18.19) | 9.69 (9.69) | 3.54 | Not significant (0.76) | |
| Activated protein C | Placebo | 16 | 16 | 17.1 | 34.4 | −17.3 | Significant (P < 0.05) | |
| Somatostatin | Placebo | 33 | 30 | 12 | 10 | 2 | Not significant | |
| Somatostatin | No active intervention | 50 | 50 | 14.92 (11.46) | 20.28 (15) | −5.36 | Significant | |
| Thymosin | Placebo | 12 | 12 | 37.1 (22.7) | 60.6 (32.9) | −23.5 | Not significant (0.06) | |
| (mild acute pancreatitis) | Ulinastatin | Placebo | 30 | 32 | 7 (5‐22) | 8 (5‐15) | −1 | Not significant (0.07) |
| (severe acute pancreatitis) | Ulinastatin | Placebo | 35 | 32 | 9 (6‐22) | 10 (6‐22) | −1 | Not significant (0.21) |
| Octerotide plus ulinastatin | Octreotide | 60 | 60 | 11.8 (3.9) | 23.7 (16.3) | −11.9 | Significant | |
| Somatostatin plus ulinastatin plus gabexate | Somatostatin | 116 | 122 | 17.7 (32.1) | 31.3 (37.6) | ‐13.6 | Significant | |
| Somatostatin plus ulinastatin | Somatostatin | 124 | 122 | 22.6 (34.5) | 31.3 (37.6) | ‐8.7 | Significant | |
| Somatostatin plus gabexate | Somatostatin | 130 | 122 | 23.2 (29.6) | 31.3 (37.6) | ‐8.1 | Significant | |
| Somatostatin plus ulinastatin plus gabexate | Somatostatin plus gabexate | 116 | 130 | 17.7 (32.1) | 23.2 (29.6) | −5.5 | Significant | |
| Somatostatin plus ulinastatin | Somatostatin plus gabexate | 124 | 130 | 22.6 (34.5) | 23.2 (29.6) | −0.6 | Significant | |
| Somatostatin plus ulinastatin plus gabexate | Somatostatin plus ulinastatin | 116 | 124 | 17.7 (32.1) | 22.6 (34.5) | −4.9 | Significant | |
| NSAID: non‐steroidal anti‐inflammatory drug. | ||||||||
| Study name | Intervention | Control | Number of participants in intervention | Number of participants in control | Mean or median (standard deviation or interquartile range, if reported) intensive care stay in intervention group | Mean or median (standard deviation or interquartile range, if reported) intensive care stay in control group | Difference | Statistical significance (P‐value, reported) |
| Antibiotics | Placebo | 22 | 19 | 17 | 18 | ‐1 | Not significant (P‐value = 0.83) | |
| Antibiotics | Placebo | 58 | 56 | 8 | 6 | 2 | Not significant | |
| Antibiotics | Placebo | 25 | 33 | 8 | 8 | 0 | Not significant | |
| Antibiotics | No active intervention | 36 | 37 | 8 | 7 | 1 | Not significant (P‐value = 0.78) | |
| Antibiotics | No active intervention | 30 | 30 | 12.7 (10.7) | 23.6 (28.7) | ‐10.9 | Not significant (P‐value = 0.06) | |
| Antibiotics | No active intervention | 33 | 30 | 11.4 (5.4) | 15.9 (12) | ‐4.5 | Not significant | |
| Antioxidants | Placebo | 22 | 21 | 4 (10.3) | 0 (0) | 4 | Not significant (P‐value = 0.08) | |
| Antioxidants | Placebo | 14 | 14 | 0 | 0 | 0 | Significant (P‐value = 0.03) | |
| Aprotinin | No active intervention | 22 | 26 | 9.5 (4 ‐ 10) | 12 (3‐20) | ‐2.5 | Not significant (P‐value = 0.47) | |
| Lexipafant | Placebo | 151 | 139 | 9.5 | 11 | ‐1.5 | Not significant | |
| Probiotics | Placebo | 152 | 144 | 6.6 (17.1) | 3 (9.3) | 3.6 | Not significant (P‐value = 0.08) | |
| Probiotics | Placebo | 24 | 26 | 4.94 (9.54) | 4 (5.86) | 0.94 | Not significant (P‐value = 0.94) | |
| Thymosin | Placebo | 12 | 12 | 24.6 (19.6) | 50.5 (25.7) | ‐25.9 | Significant (P‐value = 0.01) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Antibiotics versus control | 17 | 1058 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.15] |
| 1.2 Antioxidants versus control | 4 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.53, 7.56] |
| 1.3 Aprotinin versus control | 7 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.14] |
| 1.4 Calcitonin versus control | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 2.00] |
| 1.5 Cimetidine versus control | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.18] |
| 1.6 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.12, 7.08] |
| 1.7 Gabexate versus control | 5 | 576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.30] |
| 1.8 Glucagon versus control | 5 | 409 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.87] |
| 1.9 Iniprol versus control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 1.67] |
| 1.10 Lexipafant versus control | 3 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 1.11 Octreotide versus control | 5 | 927 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.47, 1.23] |
| 1.12 Probiotics versus control | 2 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.87, 3.30] |
| 1.13 Activated protein C versus control | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.56 [0.41, 180.52] |
| 1.14 Somatostatin versus control | 6 | 493 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.10] |
| 1.15 Somatostatin plus omeprazole versus control | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 1.11] |
| 1.16 Somatostatin plus ulinastatin versus control | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.23] |
| 1.17 Thymosin versus control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.18 Ulinastatin versus control | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.72] |
| 1.19 Gabexate versus aprotinin | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.20] |
| 1.20 Glucagon versus aprotinin | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.44, 4.08] |
| 1.21 Glucagon versus atropine | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.17 [0.45, 38.21] |
| 1.22 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.60] |
| 1.23 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 1.24 Somatostatin plus ulinastatin versus somatostatin | 2 | 369 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.34, 1.56] |
| 1.25 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.21, 1.74] |
| 1.26 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 1.95] |
| 1.27 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.86] |
| 1.28 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.30, 2.80] |
| 2 Serious adverse events (proportion) Show forest plot | 17 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Antibiotics versus control | 5 | 304 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.37, 1.15] |
| 2.2 Antioxidants versus control | 2 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.48, 8.13] |
| 2.3 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.11, 2.39] |
| 2.4 Gabexate versus control | 2 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.31, 5.60] |
| 2.5 Glucagon versus control | 2 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 7.46] |
| 2.6 Octreotide versus control | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.61, 4.93] |
| 2.7 Somatostatin versus control | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.35, 3.27] |
| 2.8 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.22, 4.91] |
| 2.9 Ulinastatin versus gabexate | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious adverse events (number) Show forest plot | 37 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Antibiotics versus control | 12 | 716 | Rate Ratio (Fixed, 95% CI) | 0.86 [0.68, 1.07] |
| 3.2 Antioxidants versus control | 2 | 71 | Rate Ratio (Fixed, 95% CI) | 0.22 [0.02, 2.21] |
| 3.3 Aprotinin versus control | 3 | 264 | Rate Ratio (Fixed, 95% CI) | 0.79 [0.49, 1.29] |
| 3.4 Cimetidine versus control | 1 | 60 | Rate Ratio (Fixed, 95% CI) | 1.0 [0.20, 4.95] |
| 3.5 EDTA versus control | 1 | 64 | Rate Ratio (Fixed, 95% CI) | 0.94 [0.19, 4.65] |
| 3.6 Gabexate versus control | 3 | 375 | Rate Ratio (Fixed, 95% CI) | 0.86 [0.64, 1.15] |
| 3.7 Glucagon versus control | 1 | 68 | Rate Ratio (Fixed, 95% CI) | 1.0 [0.02, 50.40] |
| 3.8 Lexipafant versus control | 1 | 290 | Rate Ratio (Fixed, 95% CI) | 0.67 [0.46, 0.96] |
| 3.9 Octreotide versus control | 4 | 770 | Rate Ratio (Fixed, 95% CI) | 0.74 [0.60, 0.89] |
| 3.10 Probiotics versus control | 3 | 397 | Rate Ratio (Fixed, 95% CI) | 0.94 [0.65, 1.36] |
| 3.11 Somatostatin versus control | 3 | 257 | Rate Ratio (Fixed, 95% CI) | 1.03 [0.66, 1.59] |
| 3.12 Somatostatin plus omeprazole versus control | 1 | 140 | Rate Ratio (Fixed, 95% CI) | 0.36 [0.19, 0.70] |
| 3.13 Somatostatin plus ulinastatin versus control | 1 | 122 | Rate Ratio (Fixed, 95% CI) | 0.30 [0.15, 0.60] |
| 3.14 Glucagon versus atropine | 1 | 150 | Rate Ratio (Fixed, 95% CI) | 0.33 [0.03, 3.20] |
| 3.15 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Rate Ratio (Fixed, 95% CI) | 0.30 [0.17, 0.51] |
| 3.16 Somatostatin plus ulinastatin versus somatostatin | 1 | 123 | Rate Ratio (Fixed, 95% CI) | 0.28 [0.15, 0.56] |
| 4 Organ failure Show forest plot | 18 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Antibiotics versus control | 5 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.44, 1.38] |
| 4.2 Antioxidants versus control | 4 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.12] |
| 4.3 Gabexate versus control | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.25] |
| 4.4 Lexipafant versus control | 2 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.27] |
| 4.5 Octreotide versus control | 2 | 430 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.97] |
| 4.6 Probiotics versus control | 2 | 358 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.26, 2.47] |
| 4.7 Ulinastatin versus control | 1 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 6.67] |
| 4.8 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.33, 1.80] |
| 4.9 Somatostatin plus ulinastatin versus somatostatin | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.45] |
| 4.10 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.17, 1.25] |
| 4.11 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.92] |
| 4.12 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.65] |
| 4.13 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.27, 2.35] |
| 5 Infected pancreatic necrosis Show forest plot | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Antibiotics versus control | 11 | 714 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.25] |
| 5.2 Octreotide versus control | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.04, 6.06] |
| 5.3 Probiotics versus control | 3 | 397 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.62, 1.96] |
| 6 Sepsis Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Antibiotics versus control | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.60] |
| 6.2 Aprotinin versus control | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.49, 6.96] |
| 6.3 Gabexate versus control | 3 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.55, 2.19] |
| 6.4 Lexipafant versus control | 1 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.83] |
| 6.5 Octreotide versus control | 2 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 3.53] |
| 6.6 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.36] |
| 6.7 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.22, 4.91] |
| 7 Adverse events (proportion) Show forest plot | 27 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Antibiotics versus control | 6 | 429 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.32, 0.80] |
| 7.2 Antioxidants versus control | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcitonin versus control | 1 | 94 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.12, 6.49] |
| 7.4 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.27, 2.31] |
| 7.5 Gabexate versus control | 3 | 373 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.27] |
| 7.6 Glucagon versus control | 2 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.69] |
| 7.7 Lexipafant versus control | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.16, 1.12] |
| 7.8 Octreotide versus control | 3 | 398 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.65, 1.55] |
| 7.9 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.12, 1.01] |
| 7.10 Somatostatin versus control | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.19, 1.02] |
| 7.11 Somatostatin plus omeprazole versus control | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.00 [0.00, 0.04] |
| 7.12 Gabexate versus aprotinin | 2 | 298 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.70] |
| 7.13 Ulinastatin versus gabexate | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.14 Ulinastatin versus octreotide | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.46, 11.81] |
| 7.15 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.44, 1.95] |
| 7.16 Somatostatin plus ulinastatin versus somatostatin | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.34] |
| 7.17 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.20, 1.20] |
| 7.18 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.27, 1.44] |
| 7.19 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.28] |
| 7.20 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.32, 2.22] |
| 8 Adverse events (number) Show forest plot | 40 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 8.1 Antibiotics versus control | 12 | 755 | Rate Ratio (Random, 95% CI) | 0.75 [0.58, 0.95] |
| 8.2 Antioxidants versus control | 2 | 94 | Rate Ratio (Random, 95% CI) | 0.82 [0.38, 1.79] |
| 8.3 Aprotinin versus control | 3 | 264 | Rate Ratio (Random, 95% CI) | 0.98 [0.69, 1.39] |
| 8.4 Calcitonin versus control | 1 | 94 | Rate Ratio (Random, 95% CI) | 0.88 [0.12, 6.25] |
| 8.5 Cimetidine versus control | 1 | 60 | Rate Ratio (Random, 95% CI) | 1.14 [0.64, 2.02] |
| 8.6 EDTA versus control | 1 | 64 | Rate Ratio (Random, 95% CI) | 0.63 [0.28, 1.39] |
| 8.7 Gabexate versus control | 3 | 375 | Rate Ratio (Random, 95% CI) | 0.76 [0.61, 0.95] |
| 8.8 Glucagon versus control | 2 | 90 | Rate Ratio (Random, 95% CI) | 1.19 [0.51, 2.80] |
| 8.9 Lexipafant versus control | 1 | 290 | Rate Ratio (Random, 95% CI) | 0.61 [0.44, 0.85] |
| 8.10 Octreotide versus control | 4 | 634 | Rate Ratio (Random, 95% CI) | 0.78 [0.58, 1.05] |
| 8.11 Probiotics versus control | 3 | 397 | Rate Ratio (Random, 95% CI) | 0.84 [0.52, 1.36] |
| 8.12 Somatostatin versus control | 2 | 134 | Rate Ratio (Random, 95% CI) | 0.75 [0.26, 2.18] |
| 8.13 Ulinastatin versus control | 1 | 129 | Rate Ratio (Random, 95% CI) | 0.69 [0.32, 1.46] |
| 8.14 Gabexate versus aprotinin | 1 | 182 | Rate Ratio (Random, 95% CI) | 0.66 [0.38, 1.14] |
| 8.15 Glucagon versus atropine | 1 | 150 | Rate Ratio (Random, 95% CI) | 0.79 [0.36, 1.73] |
| 8.16 Oxyphenonium versus glucagon | 1 | 62 | Rate Ratio (Random, 95% CI) | 0.93 [0.65, 1.34] |
| 8.17 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Rate Ratio (Random, 95% CI) | 0.29 [0.17, 0.48] |
| 9 Requirement for additional invasive intervention Show forest plot | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Antibiotics versus control | 14 | 884 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.13] |
| 9.2 Aprotinin versus control | 2 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.23, 1.47] |
| 9.3 Calcitonin versus control | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.08, 1.16] |
| 9.4 Cimetidine versus control | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.61] |
| 9.5 EDTA versus control | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.14, 3.29] |
| 9.6 Gabexate versus control | 3 | 426 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.90] |
| 9.7 Glucagon versus control | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.58, 2.77] |
| 9.8 Octreotide versus control | 3 | 854 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.21] |
| 9.9 Probiotics versus control | 2 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.83, 2.71] |
| 9.10 Somatostatin versus control | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.11, 1.38] |
| 9.11 Gabexate versus aprotinin | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.19, 1.32] |
| 9.12 Glucagon versus aprotinin | 1 | 134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.44, 4.08] |
| 9.13 Oxyphenonium versus glucagon | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.59] |
| 10 Endoscopic or radiological drainage of collections Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Antibiotics versus control | 1 | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 9.07] |
| 10.2 Octreotide versus control | 1 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.96] |
| 10.3 Probiotics versus control | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.20, 4.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Antibiotics versus control | 10 | 683 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.52, 1.30] |
| 1.2 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.20, 1.36] |
| 2 Serious adverse events (proportion) Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Antibiotics versus control | 4 | 281 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.54] |
| 2.2 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.22, 4.91] |
| 3 Serious adverse events (number) Show forest plot | 7 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Antibiotics versus control | 7 | Rate Ratio (Fixed, 95% CI) | 0.79 [0.59, 1.06] | |
| 4 Organ failure Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Antibiotics versus control | 4 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.42, 1.45] |
| 5 Infected pancreatic necrosis Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Antibiotics versus control | 6 | 426 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.42] |
| 6 Sepsis Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Antibiotics versus control | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.60] |
| 6.2 Gabexate versus aprotinin | 1 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.22, 4.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality Show forest plot | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Antibiotics versus control | 9 | 542 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.27] |
| 1.2 Aprotinin versus control | 2 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.30] |
| 1.3 Calcitonin versus control | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.11, 5.46] |
| 1.4 Gabexate versus control | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.99] |
| 1.5 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.34] |
| 1.6 Activated protein C versus control | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.56 [0.41, 180.52] |
| 1.7 Somatostatin versus control | 2 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.21, 1.23] |
| 1.8 Somatostatin plus omeprazole versus control | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 1.11] |
| 1.9 Somatostatin plus ulinastatin versus control | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.23] |
| 1.10 Thymosin versus control | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.11 Ulinastatin versus control | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.04, 1.29] |
| 1.12 Octreotide plus ulinastatin versus octreotide | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.60] |
| 1.13 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 1.14 Somatostatin plus ulinastatin versus somatostatin | 2 | 369 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.34, 1.56] |
| 1.15 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.21, 1.74] |
| 1.16 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 1.95] |
| 1.17 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.86] |
| 1.18 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.30, 2.80] |
| 2 Serious adverse events (proportion) Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Antibiotics versus control | 3 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 3 Serious adverse events (number) Show forest plot | 13 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Antibiotics versus control | 5 | Rate Ratio (Random, 95% CI) | 0.81 [0.52, 1.25] | |
| 3.2 Aprotinin versus control | 2 | Rate Ratio (Random, 95% CI) | 0.65 [0.25, 1.71] | |
| 3.3 Gabexate versus control | 1 | Rate Ratio (Random, 95% CI) | 0.64 [0.37, 1.10] | |
| 3.4 Probiotics versus control | 2 | Rate Ratio (Random, 95% CI) | 0.62 [0.24, 1.59] | |
| 3.5 Somatostatin versus control | 1 | Rate Ratio (Random, 95% CI) | 1.07 [0.67, 1.69] | |
| 3.6 Somatostatin plus omeprazole versus control | 1 | Rate Ratio (Random, 95% CI) | 0.36 [0.19, 0.70] | |
| 3.7 Somatostatin plus ulinastatin versus control | 1 | Rate Ratio (Random, 95% CI) | 0.30 [0.15, 0.60] | |
| 3.8 Octreotide plus ulinastatin versus octreotide | 1 | Rate Ratio (Random, 95% CI) | 0.30 [0.17, 0.51] | |
| 3.9 Somatostatin plus ulinastatin versus somatostatin | 1 | Rate Ratio (Random, 95% CI) | 0.28 [0.15, 0.56] | |
| 4 Organ failure Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Antibiotics versus control | 3 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.99] |
| 4.2 Lexipafant versus control | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.36] |
| 4.4 Ulinastatin versus control | 1 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.21] |
| 4.5 Somatostatin plus gabexate versus somatostatin | 1 | 252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.80] |
| 4.6 Somatostatin plus ulinastatin versus somatostatin | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.45] |
| 4.7 Somatostatin plus ulinastatin plus gabexate versus somatostatin | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
| 4.8 Somatostatin plus ulinastatin versus somatostatin plus gabexate | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.29, 1.92] |
| 4.9 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus gabexate | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.21, 1.65] |
| 4.10 Somatostatin plus ulinastatin plus gabexate versus somatostatin plus ulinastatin | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.27, 2.35] |
| 5 Infected pancreatic necrosis Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Antibiotics versus control | 6 | 341 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.33] |
| 5.2 Probiotics versus control | 2 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.22, 1.68] |
| 6 Sepsis Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Aprotinin versus control | 2 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.50, 6.98] |
| 6.2 Probiotics versus control | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.36] |

