Esteroides anabolizantes para el tratamiento de las úlceras por presión
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011375.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 20 junio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Cho Naing: conceived and designed the review; extracted data; checked the quality of data extraction; analysed and interpreted data; performed statistical analysis; produced the first draft of the review; contributed to writing and editing the review; approved the final review prior to submission; secured funding; wrote to study author/experts/companies; provided data; and is a guarantor of the review.
Maxine Whittaker: conceived, designed and coordinated the review; checked quality assessment; produced the first draft of the review; contributed to writing or editing the review; approved the final review prior to submission; secured funding; and is a guarantor of the review.
Contributions of editorial base:
Nicky Cullum (Editor): edited the protocol; advised on methodology, interpretation and protocol content.
Jo Dumville (Editor): edited the review; advised on methodology, interpretation and review content.
Gill Rizzello and Sally Bell‐Syer (Managing Editors): co‐ordinated the editorial process. Advised on interpretation and content; edited the review.
Reetu Child and Naomi Shaw (Information Specialists): designed the search strategy, ran the searches and edited the search methods section.
Ursula Gonthier (Editorial Assistant): Edited the plain language summary and reference sections of the review.
Sources of support
Internal sources
-
School of Postgraduate Studies, International Medical University, Malaysia.
support to CN
-
College of Public Health, Medical and Veterinary Sciences, Division of Tropical Health and Medicine, James Cook University, Australia.
support to MAW and CN
External sources
-
Swiss Tropical and Public Health Institute, Basel, Switzerland.
Support to CN
-
The National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
Cho Naing: none known
Maxine A Whittaker: none known
Acknowledgements
We would like to thank Dr Helene Moustgaard (Nordic Cochrane Centre) for her Danish translation services. We are grateful to the peer reviewers David Margolis, Gill Worthy, Anne‐Marie Glenny, Ross Atkinson, Audrey Demetriou and Janet Wale, for the comments provided and valuable input; and to Evan Kontopantelis and Gero Langer who provided feedback on the protocol. We would also like to thank copy‐editors Elizabeth Royle and Denise Mitchell for their contribution. Dr Vanessa Racloz provided valuable assistance in designing the review and providing content; Dr Kyan Aung also provided us with papers. We are grateful to the participants and researchers who took part in the trial, and offer our special thanks to Professor William A. Bauman for giving us additional information related to the trial.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jun 20 | Anabolic steroids for treating pressure ulcers | Review | Cho Naing, Maxine A Whittaker | |
| 2014 Nov 12 | Anabolic steroids for treating pressure ulcers | Protocol | Cho Naing, Maxine A Whittaker, Kyan Aung, Vanessa Racloz | |
Differences between protocol and review
Only one study was included in this review. Thus, the planned data pooling, assessment of heterogeneity and sensitivity analysis were not possible.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Male; Middle Aged;
PICO
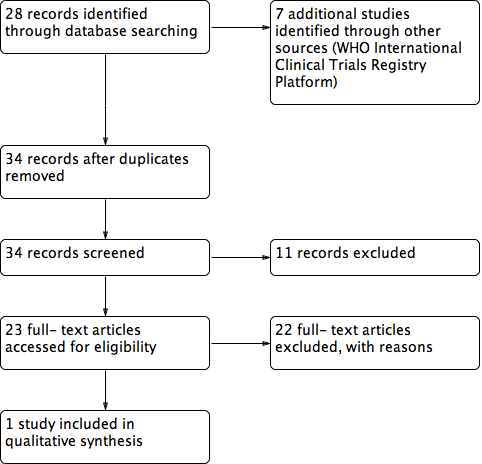
Study flow diagram
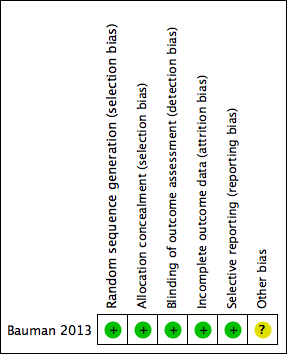
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
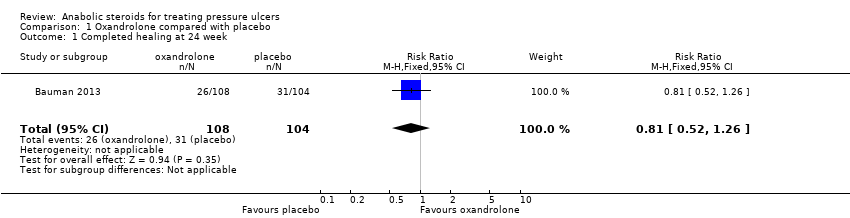
Comparison 1 Oxandrolone compared with placebo, Outcome 1 Completed healing at 24 week.

Comparison 1 Oxandrolone compared with placebo, Outcome 2 Non‐serious adverse events.
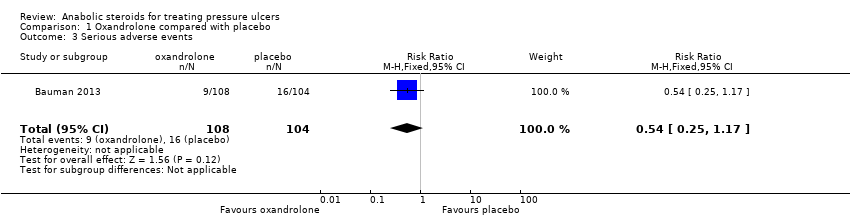
Comparison 1 Oxandrolone compared with placebo, Outcome 3 Serious adverse events.
| Anabolic steroids for treating pressure ulcers | |||||
| Patient or population: people with pressure ulcers Intervention: anabolic steroids Comparison: placebo or no anabolic steroids | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Anabolic steroids | ||||
| Proportion of wounds completely healed at 24 weeks | 298 per 1000 | 241 per 1000 | RR 0.81 (0.52 to 1.26) | 212 | ⊕⊝⊝⊝ |
| Non‐serious adverse events | 29 per 1000 | 131 per 1000 | RR 3.85 (1.12 to 13.26) | 212 | ⊕⊕⊝⊝ low2,3,4 |
| Serious adverse events | 154 per 1000 | 83 per 1000 | RR 0.54 (0.25 to 1.17) | 212 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1A wide 95% CI, which spanned both benefit and harm. | |||||
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Elevated liver enzyme levels | 5 | 1 |
| Deep venous thrombosis | 3 | 0 |
| Elevated prostate specific antigen | 0 | 1 |
| Severe osteomyelitisa | 1 | 0 |
| Sepsis, secondary cellulitisa | 1 | 0 |
| Medical illnessa | 2 | 1 |
| aIn the trial investigators' judgement these events were not associated with oxandrolone | ||
| Description | Oxandrolone group n = 108 | Placebo group n = 104 |
| Deatha | 3 | 5 |
| Myocutaneous flap surgerya | 5 | 9 |
| Elevated bladder stone removal liver enzyme levelsa | 1 | 0 |
| Small bowel obstruction, renal failurea | 0 | 1 |
| Oral cancera | 0 | 1 |
| aIn the trial investigators' judgement these events were not associated with oxandrolone | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completed healing at 24 week Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.26] |
| 2 Non‐serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [1.12, 13.26] |
| 3 Serious adverse events Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.17] |

