Anticoagulants oraux directs versus warfarine pour prévenir les accidents vasculaires cérébraux et les événements emboliques systémiques chez les patients atteints de maladie rénale chronique et présentant une fibrillation auriculaire.
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups according to the stratification by clinical site and prior VKA use, and the possibility that this method give the influence on the results was low |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed because participants were assigned to each group using the Interactive Voice Response System |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) | Low risk | Efficacy and safety outcomes were adjudicated on the basis of prespecified criteria by a clinical events committee whose members were unaware of study group assignments |
| Incomplete outcome data (attrition bias) | Unclear risk | Primary efficacy outcome was analysed in ITT population. Primary safety outcome was analysed in modified ITT population including all randomised patients who received least one dose of the study drug and included all events from receipt of the study drug until 2 days after the last dose of the drug. It has unclear risk because the number of participants that discontinued during study was reported, but the reason was unclear |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes were reported |
| Other bias | High risk | The study was funded by Bristol‐Myers Squibb and Pfizer |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups with the use of a central, 24‐horur, interactive, computerized response system |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed because participants were assigned to each group using a central, 24‐horur, interactive, computerized response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) | Low risk | Efficacy and safety outcomes were adjudicated by an independent clinical end‐point committee whose members were not aware of study group assignments |
| Incomplete outcome data (attrition bias) | Unclear risk | Primary efficacy outcome was reported in both ITT and modified ITT population. Primary safety outcome was analysed in modified ITT population. The number of participants that discontinued during study was reported, but the reason was unclear |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes were reported |
| Other bias | High risk | The study was funded by Daiichi Sankyo Pharma Development |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised, method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) | Low risk | Efficacy and safety outcomes were adjudicated on the basis of prespecified criteria by an independent clinical endpoint committee whose members were unaware of study group assignments |
| Incomplete outcome data (attrition bias) | Unclear risk | Primary efficacy endpoints were analysed in the per‐protocol population whose were ITT patients with no major protocol violation. Primary safety endpoints were analysed in modified ITT population. The number of participants that discontinued during study was reported, but the reason was unclear |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes were reported |
| Other bias | High risk | The study was funded by the Bayer Healthcare Pharmaceuticals Japanese subsidiary, Bayer Yakuhin |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups with means of a central, interactive, automated telephone system |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed because participants were assigned to each group using a central, interactive, automated telephone system |
| Blinding of participants and personnel (performance bias) | Unclear risk | Dabigatran was administered in a blinded fashion, but warfarin was administered in an unblinded fashion. But we judged that incomplete blinding didn't give influence for the outcomes, because the outcomes were objective measures and the outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Each primary and secondary outcome event was adjudicated by two independent investigators who were unaware of the treatment assignments |
| Incomplete outcome data (attrition bias) | Unclear risk | All outcomes were analyses in the ITT population. The information about discontinuation during study was unclear |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes were reported |
| Other bias | High risk | The study was funded by Boehringer Ingelheim Pharmaceuticals |
| Methods |
| |
| Participants |
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups with the use of a central 24‐hour, computerized, automated voice‐response system |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed because participants were assigned to each group using a central 24‐hour, computerised, automated voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) | Low risk | Efficacy and safety outcomes were adjudicated by an independent clinical end‐point committee whose members were unaware of study group assignments |
| Incomplete outcome data (attrition bias) | Unclear risk | Primary efficacy outcome was reported in both ITT and modified ITT population. Primary and secondary safety outcome was analysed in the modified ITT population |
| Selective reporting (reporting bias) | Low risk | All outcomes were analyses in the ITT population. The number of participants that discontinued during study was reported, but the reason was unclear |
| Other bias | High risk | The study was funded by Johnson & Johnson Pharmaceutical Research & Development L.L.C. (Raritan, NJ) and Bayer HealthCare Pharmaceuticals (Berlin, Germany) |
AF ‐ atrial fibrillation; CNS ‐ central nervous system; CrCl ‐ creatinine clearance; DM ‐ diabetes mellitus; GI ‐ gastrointestinal; INR ‐ international normalised ratio; IQR ‐ interquartile range; ITT ‐ intention to treat; M/F ‐ male/female; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; TIA ‐ transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong intervention: rivaroxaban versus rivaroxaban plus vitamin K2 versus vitamin K antagonist | |
| Pharmacokinetic/pharmacodynamic RCT of ximelagatran and melagatran | |
| Pharmacokinetic/pharmacodynamic RCT of 3 treatment regimens of edoxaban | |
| Pharmacokinetic/pharmacodynamic cross‐over RCT of 3 treatment regimens of argatroban |
RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Efficacy of rivaroxaban on renal function in patients with non‐valvular atrial fibrillation and chronic kidney disease: The X‐NOAC study |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date |
|
| Contact information | Makoto Suzuki, Department of Cardiology, Kameda Medical centre, 929 Higashi‐chou, Kamogawa‐city, Chiba 296‐8602, Japan. |
| Notes |
AF ‐ atrial fibrillation; eGFR ‐ estimated glomerular filtration rate; RCT ‐ randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12545 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.00] |
| Analysis 1.1 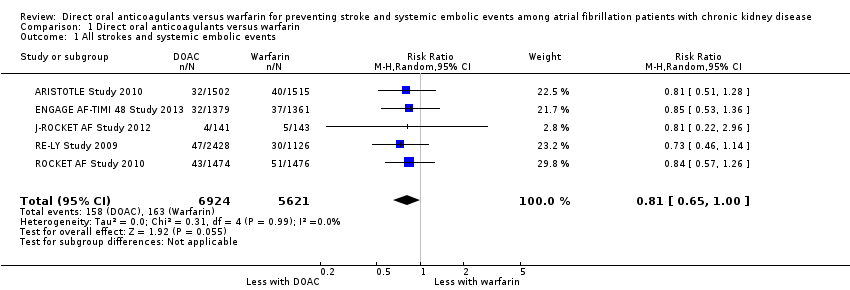 Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 1 All strokes and systemic embolic events. | ||||
| 2 Ischaemic stroke Show forest plot | 4 | 8991 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.75, 1.36] |
| Analysis 1.2  Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 2 Ischaemic stroke. | ||||
| 3 Haemorrhagic stroke Show forest plot | 4 | 8991 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| Analysis 1.3  Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 3 Haemorrhagic stroke. | ||||
| 4 Major bleeding Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.04] |
| Analysis 1.4 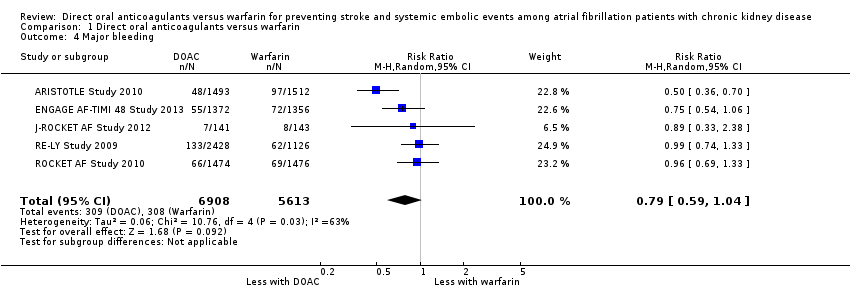 Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 4 Major bleeding. | ||||
| 5 Myocardial infarction Show forest plot | 1 | 2740 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.45, 1.90] |
| Analysis 1.5  Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 5 Myocardial infarction. | ||||
| 6 Minor bleeding Show forest plot | 2 | 3012 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.61] |
| Analysis 1.6 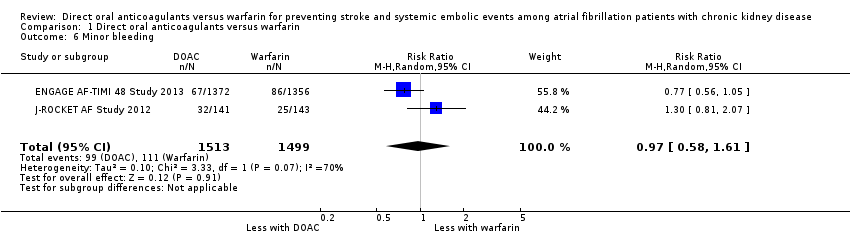 Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 6 Minor bleeding. | ||||
| 7 Gastrointestinal bleeding Show forest plot | 2 | 5678 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.97, 2.01] |
| Analysis 1.7 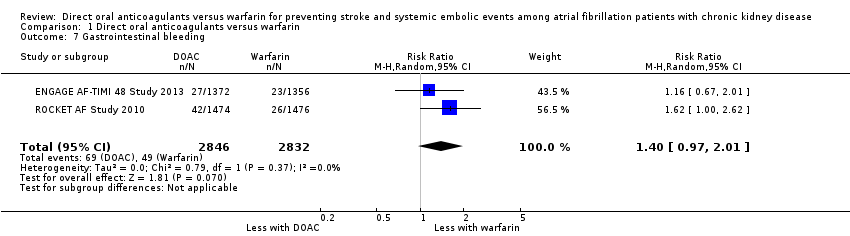 Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 7 Gastrointestinal bleeding. | ||||
| 8 Intracranial haemorrhage Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.69] |
| Analysis 1.8  Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 8 Intracranial haemorrhage. | ||||
| 9 All‐cause mortality Show forest plot | 4 | 9595 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| Analysis 1.9 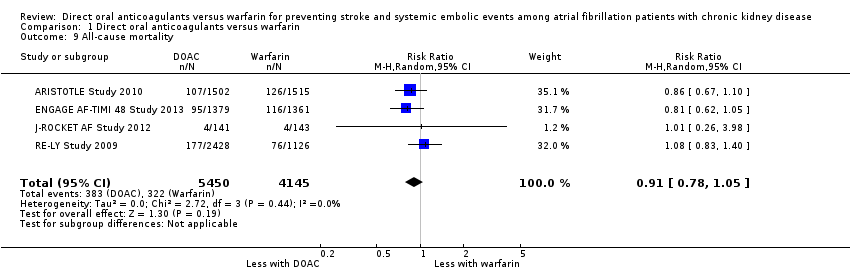 Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 9 All‐cause mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12155 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.02] |
| Analysis 2.1 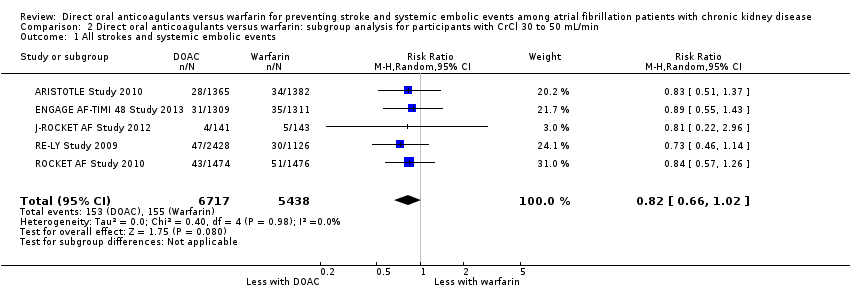 Comparison 2 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 30 to 50 mL/min, Outcome 1 All strokes and systemic embolic events. | ||||
| 2 Major bleeding Show forest plot | 5 | 12132 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| Analysis 2.2  Comparison 2 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 30 to 50 mL/min, Outcome 2 Major bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.23, 2.00] |
| Analysis 3.1  Comparison 3 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 15 to 30 mL/min, Outcome 1 All strokes and systemic embolic events. | ||||
| 2 Major bleeding Show forest plot | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.11, 0.80] |
| Analysis 3.2 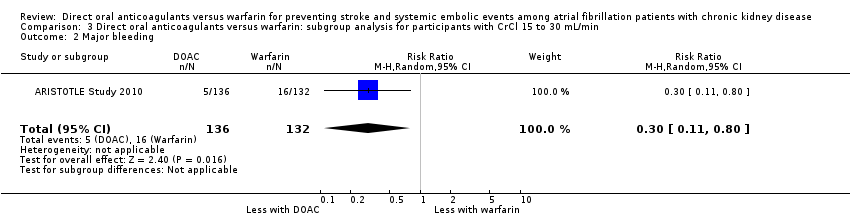 Comparison 3 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 15 to 30 mL/min, Outcome 2 Major bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12545 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.00] |
| Analysis 4.1 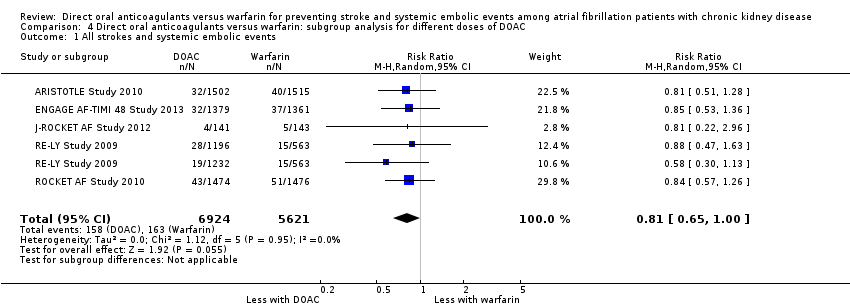 Comparison 4 Direct oral anticoagulants versus warfarin: subgroup analysis for different doses of DOAC, Outcome 1 All strokes and systemic embolic events. | ||||
| 2 Major bleeding Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.03] |
| Analysis 4.2  Comparison 4 Direct oral anticoagulants versus warfarin: subgroup analysis for different doses of DOAC, Outcome 2 Major bleeding. | ||||
| 3 All‐cause mortality Show forest plot | 4 | 9595 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| Analysis 4.3 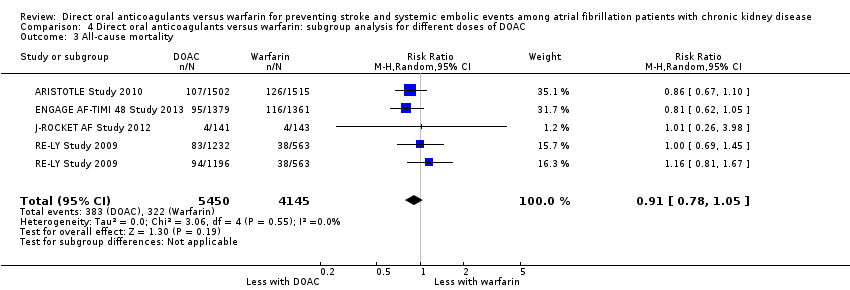 Comparison 4 Direct oral anticoagulants versus warfarin: subgroup analysis for different doses of DOAC, Outcome 3 All‐cause mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Epistaxis Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.22, 0.11] |
| Analysis 5.1  Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 1 Epistaxis. | ||||
| 2 Nasopharyngitis Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.06, 0.11] |
| Analysis 5.2 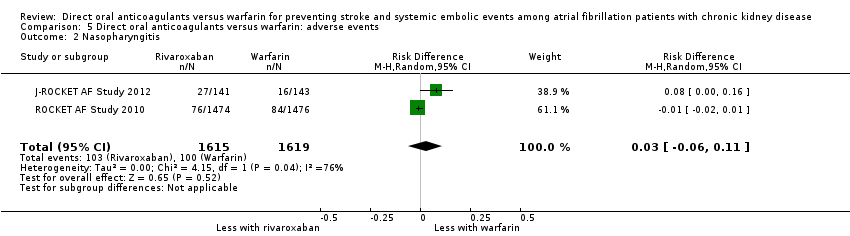 Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 2 Nasopharyngitis. | ||||
| 3 Diarrhoea Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| Analysis 5.3  Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 3 Diarrhoea. | ||||
| 4 Upper respiratory tract inflammation Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| Analysis 5.4  Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 4 Upper respiratory tract inflammation. | ||||
| 5 Back pain Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| Analysis 5.5  Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 5 Back pain. | ||||
| 6 Cardiac failure Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| Analysis 5.6  Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 6 Cardiac failure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.01] |
| Analysis 6.1  Comparison 6 Direct oral anticoagulants versus warfarin: fixed‐effect model, Outcome 1 All strokes and systemic embolic events. | ||||
| 2 Major bleeding Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.92] |
| Analysis 6.2  Comparison 6 Direct oral anticoagulants versus warfarin: fixed‐effect model, Outcome 2 Major bleeding. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
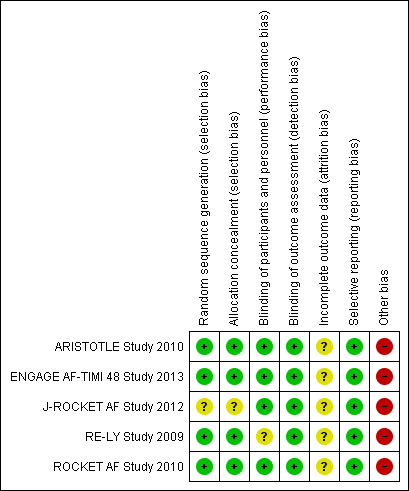
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 1 All strokes and systemic embolic events.
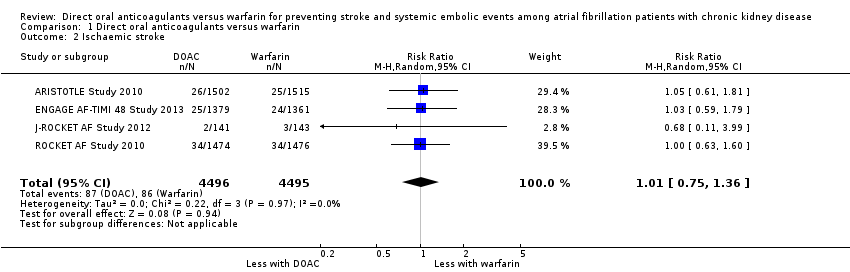
Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 2 Ischaemic stroke.

Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 3 Haemorrhagic stroke.

Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 4 Major bleeding.
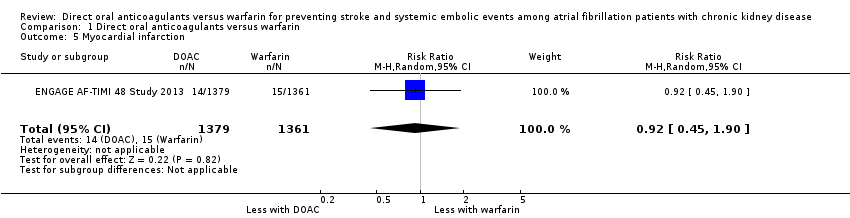
Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 5 Myocardial infarction.

Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 6 Minor bleeding.
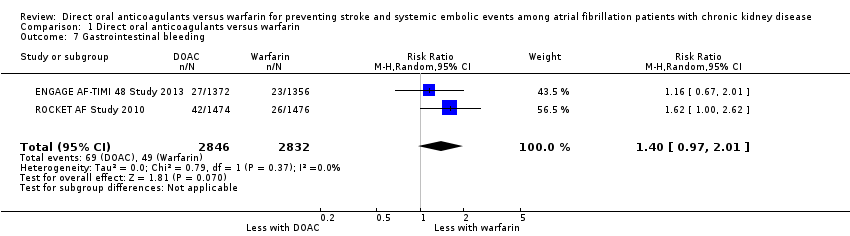
Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 7 Gastrointestinal bleeding.

Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 8 Intracranial haemorrhage.

Comparison 1 Direct oral anticoagulants versus warfarin, Outcome 9 All‐cause mortality.

Comparison 2 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 30 to 50 mL/min, Outcome 1 All strokes and systemic embolic events.
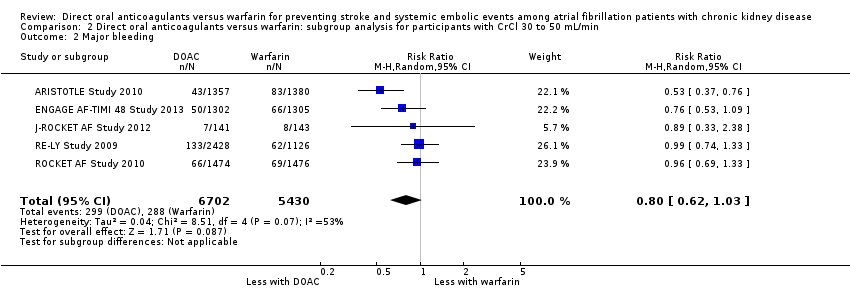
Comparison 2 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 30 to 50 mL/min, Outcome 2 Major bleeding.
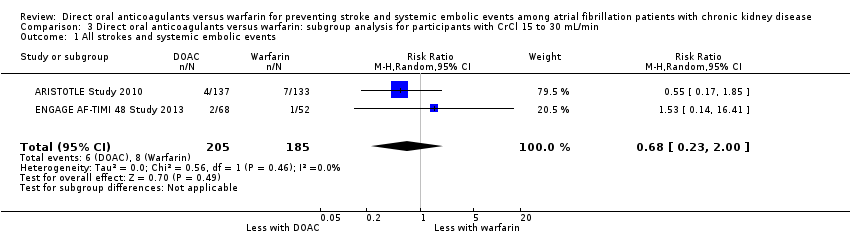
Comparison 3 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 15 to 30 mL/min, Outcome 1 All strokes and systemic embolic events.
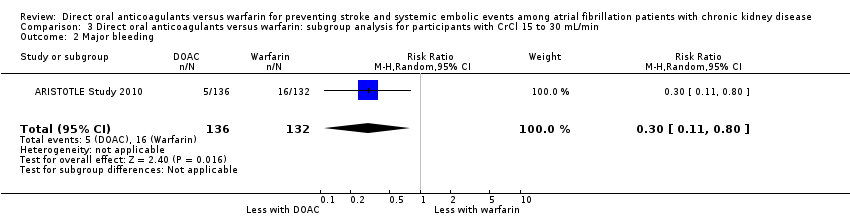
Comparison 3 Direct oral anticoagulants versus warfarin: subgroup analysis for participants with CrCl 15 to 30 mL/min, Outcome 2 Major bleeding.
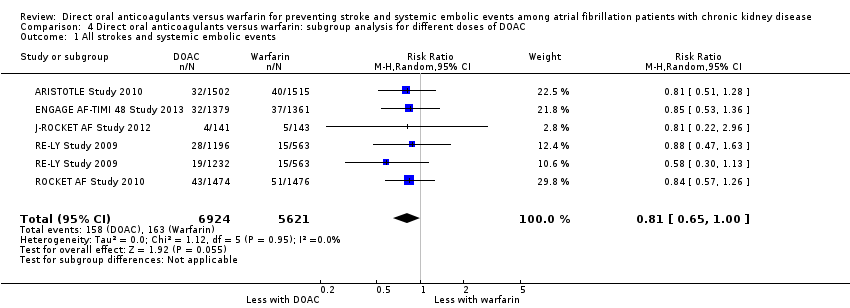
Comparison 4 Direct oral anticoagulants versus warfarin: subgroup analysis for different doses of DOAC, Outcome 1 All strokes and systemic embolic events.

Comparison 4 Direct oral anticoagulants versus warfarin: subgroup analysis for different doses of DOAC, Outcome 2 Major bleeding.

Comparison 4 Direct oral anticoagulants versus warfarin: subgroup analysis for different doses of DOAC, Outcome 3 All‐cause mortality.
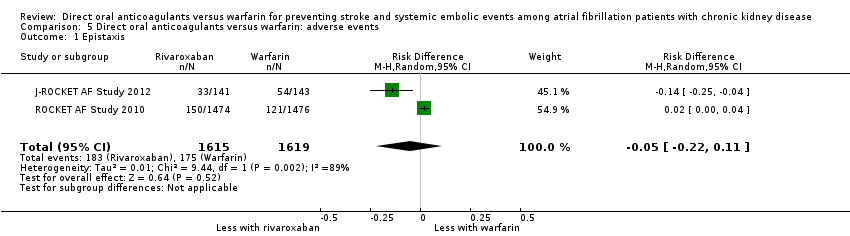
Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 1 Epistaxis.
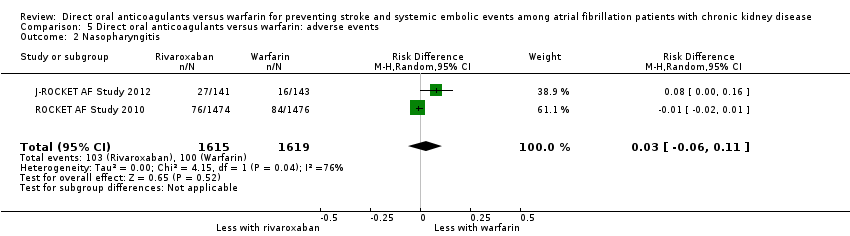
Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 2 Nasopharyngitis.
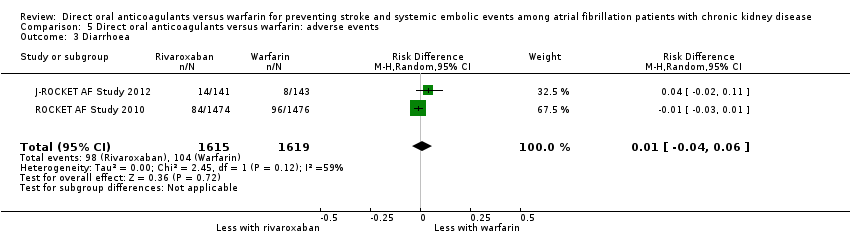
Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 3 Diarrhoea.

Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 4 Upper respiratory tract inflammation.

Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 5 Back pain.

Comparison 5 Direct oral anticoagulants versus warfarin: adverse events, Outcome 6 Cardiac failure.

Comparison 6 Direct oral anticoagulants versus warfarin: fixed‐effect model, Outcome 1 All strokes and systemic embolic events.
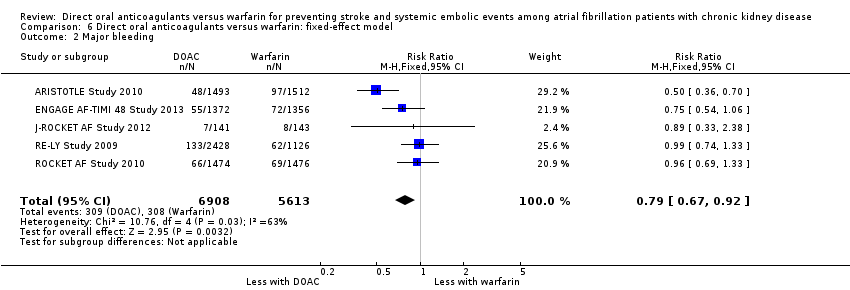
Comparison 6 Direct oral anticoagulants versus warfarin: fixed‐effect model, Outcome 2 Major bleeding.
| DOAC versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with CKD | |||||
| Patient or population: atrial fibrillation patients with CKD Setting: Hospital‐based setting Intervention: DOAC Comparison: Dose‐adjusted warfarin | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Warfarin | DOAC | ||||
| All strokes and systemic embolic events Follow up: 1.8 years to 2.8 years | 29 per 1,000 | 23 per 1,000 | RR 0.81 (0.65 to 1.00) | 12,545 (5) | ⊕⊕⊕⊝¹ |
| Major bleeding Follow up: 1.8 years to 2.8 years | 55 per 1,000 | 43 per 1,000 | RR 0.79 (0.59 to 1.04) | 12,521 (5) | ⊕⊕⊝⊝¹ ² |
| Myocardial infarction Follow up: 2.8 years | 11 per 1,000 | 10 per 1,000 (5 to 21) | RR 0.92 (0.45 to 1.90) | 2,740 (1) | ‐ |
| Minor bleeding Follow up: 2.5 years to 2.8 years | 74 per 1,000 | 72 per 1,000 (43 to 119) | RR 0.97 (0.58 to 1.61) | 3,012 (2) | ⊕⊕⊝⊝¹ ² |
| Gastrointestinal bleeding Follow up: 1.9 years to 2.8 years | 17 per 1,000 | 24 per 1,000 (17 to 35) | RR 1.40 (0.97 to 2.01) | 5,678 (2) | ⊕⊕⊕⊝¹ |
| Intracranial haemorrhage Follow up: 1.8 years to 2.8 years | 14 per 1,000 | 6 per 1,000 | RR 0.43 (0.27 to 0.69) | 12,521 (5) | ⊕⊕⊕⊝¹ |
| All‐cause mortality Follow up: 1.8 years to 2.8 years | 78 per 1,000 | 71 per 1,000 | RR 0.91 (0.78 to 1.05) | 9,595 (4) | ⊕⊕⊕⊝¹ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AF: atrial fibrillation; CI: confidence interval; DOAC: direct oral anticoagulants; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Some concerns with imprecision because of the uncertain effect estimate 2 Some concerns with inconsistency because of medium heterogeneity | |||||
| Dabigatran | Apixaban | Rivaroxaban | Edoxaban | |
| 150 mg twice daily for CKD stage G3 (CrCl 30 to 50 mL/min) No recommendation for CKD stage G4 | 2.5 mg twice daily in patients with at least two of the following characteristics: ‐ age ≥ 80 years ‐ body weight ≤ 60 kg ‐ SCr > 1.5 mg/dL | 15 mg daily for CKD stage G3 and G4 (CrCl 15 to 50 mL/min) | 30 mg once daily for CKD stage G3 and G4 (CrCl 15 to 50 mL/min) | |
| 150 mg twice daily for CKD stage G3 (CrCl > 30 mL/min) 75 mg twice daily for CKD stage G4 (CrCl 15 to 30 mL/min) | 2.5 mg twice daily in patients with at least two of the following characteristics: ‐ age ≥ 80 years ‐ body weight ≤ 60 kg ‐ SCr > 1.5 mg/dL | 15 mg daily for CKD stage G3 and G4 (CrCl 15 to 50 mL/min) | 30 mg once daily for CKD stage G3 and G4 (CrCl 15 to 50 mL/min) | |
| 110 or 150 mg twice daily for CKD stage G3 (CrCl 30 to 50 mL/min) No recommendation for CKD stage G4 | 2.5 mg twice daily in patients with at least two of the following characteristics: ‐ age ≥ 80 years ‐ body weight ≤ 60 kg ‐ SCr > 1.5 mg/dL | 15 mg daily for CKD stage G3 (CrCl 30 to 50 mL/min) No recommendation for CKD stage G4 | 30 mg once daily for CKD stage G3 (CrCl 30 to 50 mL/min) | |
| CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; SCr ‐ serum creatinine | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12545 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 2 Ischaemic stroke Show forest plot | 4 | 8991 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.75, 1.36] |
| 3 Haemorrhagic stroke Show forest plot | 4 | 8991 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 4 Major bleeding Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.04] |
| 5 Myocardial infarction Show forest plot | 1 | 2740 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.45, 1.90] |
| 6 Minor bleeding Show forest plot | 2 | 3012 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.61] |
| 7 Gastrointestinal bleeding Show forest plot | 2 | 5678 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.97, 2.01] |
| 8 Intracranial haemorrhage Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.69] |
| 9 All‐cause mortality Show forest plot | 4 | 9595 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12155 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.02] |
| 2 Major bleeding Show forest plot | 5 | 12132 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 2 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.23, 2.00] |
| 2 Major bleeding Show forest plot | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.11, 0.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12545 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 2 Major bleeding Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.03] |
| 3 All‐cause mortality Show forest plot | 4 | 9595 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Epistaxis Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.22, 0.11] |
| 2 Nasopharyngitis Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.06, 0.11] |
| 3 Diarrhoea Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 4 Upper respiratory tract inflammation Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 5 Back pain Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 6 Cardiac failure Show forest plot | 2 | 3234 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All strokes and systemic embolic events Show forest plot | 5 | 12545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.01] |
| 2 Major bleeding Show forest plot | 5 | 12521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.92] |

