Antibióticos para la bacteriuria asintomática en los receptores de trasplante renal
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011357.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 febrero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Riñón y trasplante
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Draft the protocol: JC, AS, DA, EVN, AW
-
Study selection: JC, AS, EVN
-
Extract data from studies: JC, AS,
-
Enter data into RevMan: JC, AS
-
Carry out the analysis: JC, AS, EVN
-
Interpret the analysis: JC, AS, DA, EVN, AW
-
Draft the final review: JC, AS, DA, EVN, AW
-
Disagreement resolution: EVN
-
Update the review: JC
Declarations of interest
-
Julien Coussement: is the coordinating investigator of a multicentre RCT comparing antibiotics versus no treatment in the prevention of symptomatic UTI in kidney transplant recipients with asymptomatic bacteriuria (BiRT Study 2013). This study was registered on Clinicaltrials.gov in May 2013 and started recruiting in April 2014. Results of this study are expected to become available from 2019. Julien Coussement received two grants from not‐for‐profit organisations for the purpose of initiating the study (David & Alice Van Buuren Research Grant 2014 & Prix 2014 du Fonds Carine Vyghen).
-
Anne Scemla: is an investigator of a multicentre RCT comparing antibiotics versus no treatment in the prevention of symptomatic UTI in kidney transplant recipients with asymptomatic bacteriuria (BiRT Study 2013). This study was registered on Clinicaltrials.gov in May 2013 and started recruiting in April 2014. Results of this study are expected to become available from 2019.
-
Daniel Abramowicz: is an investigator of a multicentre RCT comparing antibiotics versus no treatment in the prevention of symptomatic UTI in kidney transplant recipients with asymptomatic bacteriuria (BiRT Study 2013). This study was registered on Clinicaltrials.gov in May 2013 and started recruiting in April 2014. Results of this study are expected to become available from 2019.
-
Evi V Nagler: is an investigator of a multicentre RCT comparing antibiotics versus no treatment in the prevention of symptomatic UTI in kidney transplant recipients with asymptomatic bacteriuria (BiRT Study 2013). This study was registered on Clinicaltrials.gov in May 2013 and started recruiting in April 2014. Results of this study are expected to become available from 2019.
-
Angela C Webster: None known
Acknowledgements
We would like to thank Cochrane Kidney and Transplant for their support and the referees for their comments and feedback during the preparation of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Feb 01 | Antibiotics for asymptomatic bacteriuria in kidney transplant recipients | Review | Julien Coussement, Anne Scemla, Daniel Abramowicz, Evi V Nagler, Angela C Webster | |
| 2014 Oct 25 | Antibiotics for asymptomatic bacteriuria in kidney transplant recipients | Protocol | Julien Coussement, Anne Scemla, Daniel Abramowicz, Evi V Nagler, Angela C Webster | |
Differences between protocol and review
Following the 2005 IDSA guidelines (Nicolle 2005), we included an additional asymptomatic bacteriuria definition to be applied to catheterized patients. The following sentence was added: "a single catheterized urine specimen with one bacterial species isolated in a quantitative count ≥ 100 CFU/mL identifies bacteriuria in women or men".
We modified the criteria to evaluate the effect of antibiotics on the incidence of antimicrobial resistance between protocol and review. In hindsight, we considered the criteria we initially chose (incidence of bacteriuria resistant to primary antibiotic treatment) difficult to assess in studies with groups of untreated patients. We therefore decided to evaluate antimicrobial resistance using a widely accepted criterion that can easily be evaluated in both treated and untreated participants (isolation of a multidrug‐resistant bacterium, with multidrug‐resistance being defined as non‐susceptibility to at least one agent in three or more antimicrobial categories).
The following definitions have been added in the section entitled "secondary outcomes":
Relapsing and persistent asymptomatic bacteriuria were defined as follows:
-
Relapsing asymptomatic bacteriuria: recurrence of asymptomatic bacteriuria after clearance of the initial isolate
-
Persistent asymptomatic bacteriuria: persistence of an organism similar to the initial isolate (same species with similar antimicrobial‐susceptibility profile).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Kidney;
- Anti‐Bacterial Agents [therapeutic use];
- Asymptomatic Infections [mortality, *therapy];
- Bacteriuria [*drug therapy, mortality];
- Cause of Death;
- Drug Resistance, Bacterial;
- Graft Rejection [epidemiology, etiology];
- Kidney Transplantation [*adverse effects, mortality];
- Randomized Controlled Trials as Topic;
- Transplant Recipients;
- Urinary Tract Infections [complications, *prevention & control];
Medical Subject Headings Check Words
Humans;
PICO
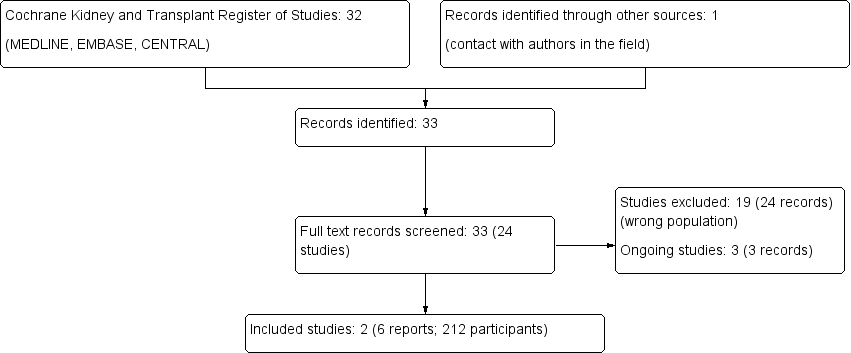
Study flow diagram.
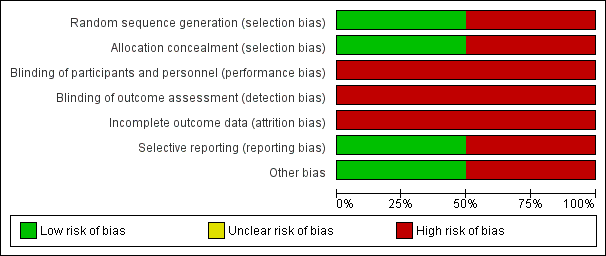
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotics versus no treatment, Outcome 1 Symptomatic urinary tract infection.
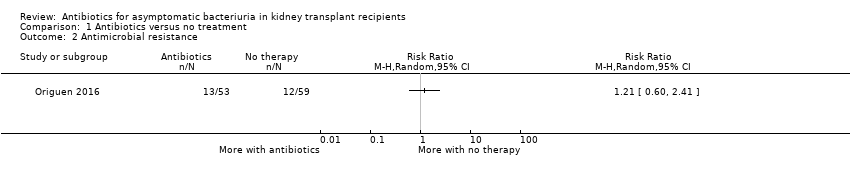
Comparison 1 Antibiotics versus no treatment, Outcome 2 Antimicrobial resistance.
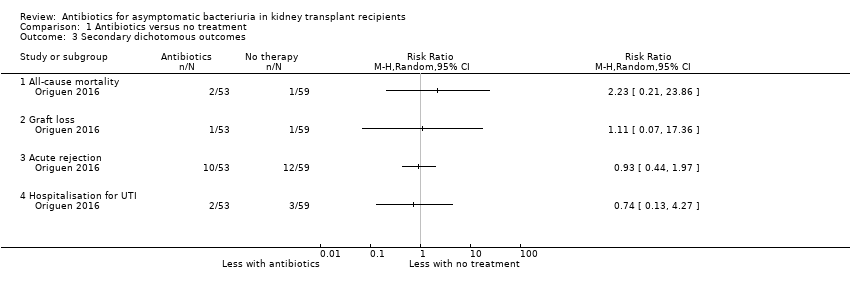
Comparison 1 Antibiotics versus no treatment, Outcome 3 Secondary dichotomous outcomes.

Comparison 1 Antibiotics versus no treatment, Outcome 4 Graft function (creatinine at end of study).
| Antibiotics versus no treatment for asymptomatic bacteriuria in kidney transplant recipients | |||||
| Patient or population: adult kidney transplant recipients | |||||
| Outcomes (follow‐up period) | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no treatment | Risk with antibiotics | ||||
| Symptomatic UTI Follow‐up: 12 to 22 months | 240 per 1,000 | 207 per 1 000 | RR 0.86 (0.51 to 1.45) | 200 2 (2 studies) | Low 3 ⊕⊕⊝⊝ |
| Antimicrobial resistance Mean follow‐up: 16.9 months | 203 per 1,000 | 245 per 1,000 | RR 1.21 (0.60 to 2.41) | 112 (1 study) | Low 4 ⊕⊕⊝⊝ |
| All‐cause mortality Mean follow‐up: 16.9 months | 17 per 1,000 | 38 per 1,000 | RR 2.23 (0.21, 23.86) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft loss Mean follow‐up: 16.9 months | 17 per 1,000 | 19 per 1,000 | RR 1.11 (0.07 to 17.36) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Acute graft rejection Mean follow‐up: 16.9 months | 203 per 1,000 | 189 per 1,000 | RR 0.93 (0.44 to 1.97) | 112 (1 study) | Low 6 ⊕⊕⊝⊝ |
| Hospitalisation for UTI Mean follow‐up: 16.9 months | 51 per 1,000 | 38 per 1,000 | RR 0.74 (0.13 to 4.27) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft function (creatinine at end of study) Follow‐up: 12 to 22 months | Mean serum creatinine in the treatment group was 0.06 mg/dL lower (0.19 mg/dL lower to 0.08 mg/dL higher) than the control group | 200 2 (2 studies) | Low 7, 8 ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; UTI: urinary tract infection 1 The two included studies compared antibiotics versus no treatment, with choice of antibiotics depending on antimicrobial susceptibility testing results. As participants could have had multiple episodes of asymptomatic bacteriuria during the follow‐up period, participants from the intervention group were retreated with antibiotics if asymptomatic bacteriuria recurred during the follow‐up period in both studies. Duration of antibiotics therapy ranged from 3 to 10 days for the first episode of asymptomatic bacteriuria. 2 212 participants included but data provided for 200 participants. 3 Neither study attempted to blind participants, personnel or data analysts. As symptoms of UTI are partly subjective, we anticipated this would put the results at risk of being biased in favour of antibiotic treatment. 4 Samples could be collected both in case of symptoms of UTI or as part of routine screening. 5 The confidence interval crosses the line of no effect but does not rule out a significant effect of antibiotics on mortality and/or graft loss. 6 No systematic graft biopsy performed during the study follow‐up. Not all episodes of allograft rejection were biopsy‐proven. 7 Graft function was evaluated using creatinine at end of study, despite different values between groups at time of inclusion. We were unable to pool the data for change in graft function from baseline to end of study (data missing for one study). 8 No significant effect of antibiotics on change in graft function from baseline to end of study in both studies. | |||||
| GRADE Working Group grades of evidence | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic urinary tract infection Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.51, 1.45] |
| 2 Antimicrobial resistance Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Graft loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Acute rejection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Hospitalisation for UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Graft function (creatinine at end of study) Show forest plot | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.18] |

