نقش آنتیبیوتیکها برای باکتریوری بدون نشانه در گیرندگان پیوند کلیه
چکیده
پیشینه
باکتریوری بدون نشانه (asymptomatic bacteriuria)، که به صورت باکتریوری بدون علائم یا نشانههای عفونت مجاری ادراری (urinary tract infection; UTI) تعریف شده، در 17% تا 51% از گیرندگان پیوند کلیه رخ میدهد و به نظر میرسد خطر ابتلا به UTI متعاقب را افزایش میدهد. هیچ توافقی در مورد نقش آنتیبیوتیکها در درمان باکتریوری بدون نشانه در پیوند کلیه وجود ندارد.
اهداف
ارزیابی مزایا و آسیبهای درمان باکتریوری بدون نشانه در گیرندههای پیوند کلیه با عوامل آنتیباکتریال برای پیشگیری از نشانههای UTI، مورتالیتی به هر علتی و تاثیرات غیر‐مستقیم UTI (رد حاد، از دست دادن پیوند، بدتر شدن عملکرد پیوند).
روشهای جستوجو
پایگاه ثبت تخصصی گروه کلیه و پیوند در کاکرین را تا 1 سپتامبر 2017 از طریق تماس با متخصص اطلاعات با استفاده از کلیدواژههای جستوجوی مرتبط به این مرور جستوجو کردیم. مطالعات موجود در مرکز ثبت از طریق جستوجو در CENTRAL؛ MEDLINE؛ و EMBASE؛ مجموعه مقالات کنفرانس؛ پورتال جستوجوی پایگاه ثبت بینالمللی کارآزماییهای بالینی (ICTRP) و ClinicalTrials.gov شناسایی شدند.
معیارهای انتخاب
تمام کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و شبه‐RCTها به هر زبان که به ارزیابی درمان باکتریوری بدون نشانه در گیرندگان پیوند کلیه در هر نقطه زمانی پس از پیوند پرداختند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم واجد شرایط بودن مطالعه را تعیین کرده، به ارزیابی کیفیت مطالعه و استخراج دادهها پرداختند. پیامدهای اولیه بروز نشانههای UTI و بروز مقاومت آنتیباکتریالی بود. پیامدهای دیگر عبارت بود از مورتالیتی به هر علتی، از دست دادن پیوند، رد پیوند، عملکرد پیوند، بستری شدن به دلیل UTI، واکنشهای جانبی به عوامل آنتیباکتریال و عود یا مقاومت باکتریوری بدون نشانه. پیامدهای دو‐حالتی را به صورت تفاوت خطر (RD) مطلق یا خطر نسبی (RR) با 95% فواصل اطمینان (CI) و دادههای پیوسته را به صورت تفاوت میانگین (MD) با 95% فاصله اطمینان (CI) بیان کردیم. دادهها با استفاده از مدل اثرات تصادفی تجمیع شد.
نتایج اصلی
دو مطالعه (212 شرکتکننده) را وارد کردیم که به مقایسه آنتیبیوتیکها در برابر عدم درمان پرداختند، و سه مطالعه در حال انجام را شناسایی کردیم. بهطور کلی، میزان بروز UTI علامتدار بین گروههایی که برای باکتریوری بدون نشانه درمان نشوند بین 19% تا 31% متغیر بود. درمان آنتیبیوتیکی دارای تاثیرات نامطمئنی بر پیشگیری از UTI علامتدار بود (2 مطالعه؛ 200 شرکتکننده: RR: 0.86؛ 95% CI؛ 0.51 تا 1.45). خطر برای انتخاب ارگانیسمهای مقاوم در برابر چندین دارو با درمان آنتیبیوتیکی نامطمئن بود (1 مطالعه؛ 112 شرکتکننده: RR: 1.21؛ 95% CI؛ 0.60 تا 2.41). مقاومت باکتریوری بدون نشانه بدون توجه به درمان بالا بود. آنتیبیوتیکها تاثیرات نامطمئنی بر سایر پیامدهای مهم بیمار و پیوند دارند، به عنوان مثال در مورد مورتالیتی به هر علتی (1 مطالعه؛ 112 شرکتکننده: RR: 2.23؛ 95% CI؛ 0.21 تا 23.86)، از دست دادن پیوند (1 مطالعه؛ 112 شرکتکننده: RR: 1.11؛ 95% CI؛ 0.07 تا 17.36)، رد حاد (1 مطالعه؛ 112 شرکتکننده: RR: 0.93؛ 95% CI؛ 0.44 تا 1.97)، بستری شدن به دلیل UTI (1 مطالعه؛ 112 شرکتکننده: RR: 0.74؛ 95% CI؛ 0.13 تا 4.27)، عملکرد پیوند (2 مطالعه؛ 200 شرکتکننده: MD در غلظت سرمی کراتینین: 0.06‐ میلیگرم/دسیلیتر؛ 95% CI؛ 0.19‐ تا 0.08) و واکنشهای جانبی (1 مطالعه؛ 112 شرکتکننده: بدون حادثه جانبی شدید ناشی از درمان آنتیبیوتیکی). کیفیت شواهد برای تمام پیامدها پائین بود.
نتیجهگیریهای نویسندگان
در حال حاضر، شواهد کافی برای حمایت از درمان معمول برای گیرندگان پیوند کلیه با آنتیبیوتیکها در مورد باکتریوری بدون نشانه پس از پیوند وجود ندارد، اما دادهها نادر هستند. مطالعات بیشتر که به ارزیابی درمان آنتیبیوتیک معمول بپردازند اطلاعاتی را برای بهکارگیری عملی فراهم خواهند کرد و ما منتظر نتایج سه مطالعه تصادفیسازی شده در حال انجام هستیم، که ممکن است به حلوفصل عدم قطعیتهای موجود کمک کند.
PICOs
خلاصه به زبان ساده
استفاده از آنتیبیوتیکها برای درمان عفونت باکتریایی در ادرار در گیرندگان پیوند کلیه هنگامی که نشانهای وجود ندارد.
موضوع چیست؟
باکتریهای موجود در ادرار گیرندگان پیوند کلیه بدون وجود نشانهای از عفونت ادراری، باکتریوری بدون نشانه نامیده میشود. یک فرد از هر دو فرد گیرنده پیوند کلیه، زمانی پس از دریافت پیوند دچار عفونت باکتریایی در ادرار (باکتریوری) خواهند شد. باکتریوری با نشانههایی مانند تب، لرز، ادرار کردن دردناک، درد شکم و دیده شدن خون در ادرار، عفونت مجاری ادراری (urinary tract infection; UTI) است. باکتریوری اغلب بدون نشانه است و اغلب با آنتیبیوتیکهایی درمان میشود که ممکن است به پیشگیری از UTI بعدی کمک کنند. اجتناب از UTI ممکن است بیمار و بقای پیوند را بهبود ببخشد. با این حال، مشخص نیست که چگونه نشانههای UTI در بسیاری از افراد مبتلا به باکتریوری بدون نشانه پیشرفت میکند؛ یا اینکه درمان با آنتیبیوتیکها به راستی از UTI پیشگیری میکند یا خیر؛ یا اینکه هنگامی که نشانهای وجود ندارد درمان باعث بهبودی بقای بیمار و کلیه میشود یا خیر. همچنین، ممکن است که استفاده از آنتیبیوتیکها دارای مضراتی باشند. مصرف منظم آنتیبیوتیکها ممکن است به این معنی باشد که باکتریها نسبت به آنتیبیوتیکها مقاوم میشوند و مصرف آنتیبیوتیکها ممکن است منجر به اسهال و سایر حوادث جانبی شود. هزینههای آنتیبیوتیک نیز باید در نظر گرفته شوند. این مرور به بررسی این موضوع پرداخت که درمان با آنتیبیوتیکها مفید است یا مضر.
ما چه کاری را انجام دادیم؟
منابع علمی را تا سپتامبر 2017 جستوجو کردیم و دو مطالعه (212 شرکتکننده) را که در این مرور ارزیابی شدند، شناسایی کردیم. این مطالعات آنتیبیوتیکها را در برابر عدم درمان مقایسه کردند.
ما چه چیزی یافتیم؟
عفونت باکتریایی اغلب در ادرار باقی میماند، چه آنتیبیوتیکها مصرف شوند، چه مصرف نشوند. به دلیل دادههای بسیار کم و محدودیتهای متعدد موجود در مطالعات وارد شده، اینکه آنتیبیوتیکها از نشانههای عفونت ادراری پیشگیری میکنند یا خیر، یا خطر انتخاب باکتریهای مقاوم به آنتیبیوتیکها را افزایش میدهند یا خیر، نامطمئن است. همچنین، مشخص نیست که استفاده از آنتیبیوتیکها در مورد عفونت ادراری بدون نشانه، خطر رد پیوند، نیاز به بستری شدن در اثر نشانههای عفونت ادراری یا مرگومیر را کاهش میدهد، یا اینکه آنتیبیوتیکها باعث بهبود عملکرد پیوند کلیه میشوند یا خیر. یک مطالعه با 112 شرکتکننده نشان داد که هیچ واکنش شدید مضر ناشی از درمان آنتیبیوتیکی وجود نداشت و حوادث جانبی نهچندان شدید به ندرت دیده شد.
نتیجهگیریها
این موضوع که آنتیبیوتیکها در گیرندگان پیوند کلیه که در ادرار آنها باکتریهایی وجود دارد اما هیچ نشانهای ندارند، مفید است یا خیر، نامطمئن است. در یک مطالعه، شرکتکنندگان با استفاده از روشی که تصادفیسازی نبود (یعنی بر اساس کد پیوند بیمار) به دریافت آنتیبیوتیک یا عدم درمان اختصاص داده شدند. در هر دو مطالعه، شرکتکنندگان میدانستند که چه درمانهایی دریافت میکنند (یعنی آنتیبیوتیک یا عدم درمان)، که این موضوع ممکن بود نتایج را تحت تاثیر قرار دهد. در نهایت، ما دادههای کافی برای را تخمین دقیق برخی از تاثیرات آنتیبیوتیکها در اختیار نداشتیم. پژوهشهای بیشتری مورد نیاز است.
Authors' conclusions
Summary of findings
| Antibiotics versus no treatment for asymptomatic bacteriuria in kidney transplant recipients | |||||
| Patient or population: adult kidney transplant recipients | |||||
| Outcomes (follow‐up period) | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no treatment | Risk with antibiotics | ||||
| Symptomatic UTI Follow‐up: 12 to 22 months | 240 per 1,000 | 207 per 1 000 | RR 0.86 (0.51 to 1.45) | 200 2 (2 studies) | Low 3 ⊕⊕⊝⊝ |
| Antimicrobial resistance Mean follow‐up: 16.9 months | 203 per 1,000 | 245 per 1,000 | RR 1.21 (0.60 to 2.41) | 112 (1 study) | Low 4 ⊕⊕⊝⊝ |
| All‐cause mortality Mean follow‐up: 16.9 months | 17 per 1,000 | 38 per 1,000 | RR 2.23 (0.21, 23.86) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft loss Mean follow‐up: 16.9 months | 17 per 1,000 | 19 per 1,000 | RR 1.11 (0.07 to 17.36) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Acute graft rejection Mean follow‐up: 16.9 months | 203 per 1,000 | 189 per 1,000 | RR 0.93 (0.44 to 1.97) | 112 (1 study) | Low 6 ⊕⊕⊝⊝ |
| Hospitalisation for UTI Mean follow‐up: 16.9 months | 51 per 1,000 | 38 per 1,000 | RR 0.74 (0.13 to 4.27) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft function (creatinine at end of study) Follow‐up: 12 to 22 months | Mean serum creatinine in the treatment group was 0.06 mg/dL lower (0.19 mg/dL lower to 0.08 mg/dL higher) than the control group | 200 2 (2 studies) | Low 7, 8 ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; UTI: urinary tract infection 1 The two included studies compared antibiotics versus no treatment, with choice of antibiotics depending on antimicrobial susceptibility testing results. As participants could have had multiple episodes of asymptomatic bacteriuria during the follow‐up period, participants from the intervention group were retreated with antibiotics if asymptomatic bacteriuria recurred during the follow‐up period in both studies. Duration of antibiotics therapy ranged from 3 to 10 days for the first episode of asymptomatic bacteriuria. 2 212 participants included but data provided for 200 participants. 3 Neither study attempted to blind participants, personnel or data analysts. As symptoms of UTI are partly subjective, we anticipated this would put the results at risk of being biased in favour of antibiotic treatment. 4 Samples could be collected both in case of symptoms of UTI or as part of routine screening. 5 The confidence interval crosses the line of no effect but does not rule out a significant effect of antibiotics on mortality and/or graft loss. 6 No systematic graft biopsy performed during the study follow‐up. Not all episodes of allograft rejection were biopsy‐proven. 7 Graft function was evaluated using creatinine at end of study, despite different values between groups at time of inclusion. We were unable to pool the data for change in graft function from baseline to end of study (data missing for one study). 8 No significant effect of antibiotics on change in graft function from baseline to end of study in both studies. | |||||
| GRADE Working Group grades of evidence | |||||
Background
Description of the condition
Asymptomatic bacteriuria is generally defined as bacteriuria without signs or symptoms of urinary tract infection (UTI; e.g. dysuria, frequency, suprapubic pain or fever). The Infectious Diseases Society of America (IDSA) defines bacteriuria in men as one bacterial species isolated from a single, clean‐catch voided urine specimen in a quantitative count ≥ 105 colony forming units (CFU)/mL (Nicolle 2005). In asymptomatic women, diagnosis of bacteriuria requires a second urine specimen with isolation of the same bacterial strain in a quantitative count ≥ 105 CFU/mL. If a urine sample is collected through catheterization, a single urine specimen with isolation of a single bacterial species in a quantitative count ≥ 100 CFU/mL is enough to identify bacteriuria in women or men.
Observational studies from the 1970s and 1980s reported high incidences of asymptomatic bacteriuria in kidney transplant recipients, especially in the first six months after transplantation (Nicolle 2005). Many patients also developed symptomatic UTI with subsequent ramifications for graft function (Tolkoff‐Rubin 1982). This prompted many clinicians to screen for asymptomatic bacteriuria and treat with antibiotics on the presumption it would reduce the incidence of symptomatic episodes and improve graft and patient outcomes in the longer term (Abbott 2004).
The past two decades have seen several changes in the management of transplant recipients including the introduction of routine perioperative antibiotic prophylaxis, earlier removal of indwelling urethral catheters, and long‐term antibiotic prophylaxis for preventing Pneumocystis jirovecii pneumonia and other opportunistic infections (KDIGO 2009; Nicolle 2005). These interventions are also expected to prevent UTI and asymptomatic bacteriuria.
At present, asymptomatic bacteriuria is estimated to occur in 17% to 51% of kidney transplant recipients, estimates largely depending on definition of asymptomatic bacteriuria, follow‐up period, and frequency of urine sampling (El Amari 2011; Fiorante 2010; Green 2013). The limited retrospective data available seem to indicate that few asymptomatic episodes lead to symptomatic or severe UTI, and that graft function is not affected (El Amari 2011; Green 2013). Most transplant physicians still, however, treat asymptomatic bacteriuria after transplantation. Reasons include the possibility that denervation of the kidney graft and the use of immunosuppressive medications mask the clinical features of UTI, and the fear that kidney transplant recipients may be at higher risk for developing severe infections (Parasuraman 2013).
Description of the intervention
Treatment of asymptomatic bacteriuria involves the detection of bacteria in urine through routine processing of urine cultures. Once diagnosis of asymptomatic bacteriuria has been established, treatment with antibiotics may be started with the aim to prevent progression to symptomatic UTI (e.g. acute graft pyelonephritis).
How the intervention might work
Antibiotics are given under the assumption they are effective in improving individual patient outcomes by eliminating infection, reducing recurrence and preventing long‐term kidney damage.
Why it is important to do this review
Screening for and treatment of asymptomatic bacteriuria may be beneficial if asymptomatic bacteriuria has negative effects that could be reduced with antibiotics. There is consensus that the benefits of screening and treatment outweigh the harms in patients awaiting transurethral resection of the prostate (Nicolle 2005; Zani 2011). In the general population though, the available data do not support the use of antibiotics to treat asymptomatic bacteriuria. In pregnant women, the routine screen‐treat‐policy for asymptomatic bacteriuria has recently been called into question (Kazemier 2015; Smaill 2015). In healthy, non‐pregnant young women it may even increase the risk of symptomatic UTI (Cai 2012).
No consensus exists on the role of antibiotics for asymptomatic bacteriuria in kidney transplantation (Nicolle 2014a). The 2005 IDSA guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults refrained from making a recommendation in kidney transplant recipients for want of evidence (Nicolle 2005). In 2013, the American Society of Transplantation Infectious Diseases Community of Practice suggested not treating asymptomatic bacteriuria that occurs beyond three months after kidney transplantation, unless in case of an accompanying rise in serum creatinine (SCr) concentration (Parasuraman 2013). However, the authors underlined that the recommendation was not based on randomised controlled studies (RCTs) and that general adoption of such a strategy could lead to over‐treatment and selection of resistant micro‐organisms. Indeed, there is some concern that treating kidney transplant recipients with asymptomatic bacteriuria with antibiotics leads to selection of resistant strains (El Amari 2011). Aside from the possible consequences for the individual, there may be ramifications for society at large (Goossens 2005). In addition, treatment may have direct and very harmful side‐effects (e.g. fluoroquinolone‐induced Achilles tendon rupture), cause severe allergic complications (Chang 2012) and promote Clostridium difficile‐associated diarrhoea (Shah 2013).
Objectives
To assess the benefits and harms of treating asymptomatic bacteriuria in kidney transplant recipients with antimicrobial agents to prevent symptomatic UTI, all‐cause mortality and the indirect effects of UTI (acute rejection, graft loss, worsening of graft function).
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at treatment of asymptomatic bacteriuria in kidney transplant recipients.
Types of participants
Inclusion criteria
-
Adults and children with end‐stage kidney disease, who are recipients of a first or subsequent cadaveric or living donor kidney transplant, including combined grafts (e.g. kidney‐pancreas).
-
Asymptomatic bacteriuria defined according to the IDSA definitions or as defined by the authors, at any time‐point after transplantation.
IDSA definition of asymptomatic bacteriuria:
-
In men: a single, clean‐catch voided urine specimen with one bacterial species isolated in a quantitative count ≥ 105 CFU/mL in the absence of symptoms or signs of UTI.
-
In women: two consecutive voided urine specimens with isolation of the same bacterial strain in quantitative counts ≥ 105 CFU/mL in the absence of symptoms or signs of UTI.
-
A single catheterized urine specimen with one bacterial species isolated in a quantitative count ≥ 100 CFU/mL identifies bacteriuria in women or men.
Exclusion criteria
-
Pregnant women, as antibiotic treatment of asymptomatic bacteriuria in pregnancy effectively reduces the risk of pyelonephritis in the mother and possibly reduces the chance a baby will be born too early or have a low birthweight. This question has been addressed in a Cochrane review (Smaill 2015).
-
Transplant recipients awaiting transurethral resection of the prostate or any other urologic procedure during which mucosal bleeding is anticipated, as antibiotic treatment of asymptomatic bacteriuria is recommended in this setting (Nicolle 2005).
Types of interventions
We included studies of any antibiotic medication and investigated the following comparisons:
-
Any antibiotic medication versus placebo or no treatment
-
Any antibiotic medication versus any other antibiotic medication
-
Low dose versus high dose of the same antibiotic medication
-
Short‐course versus long‐course antibiotic therapy
-
Oral versus intravenous (IV) administration of the same or different antibiotic medication.
Types of outcome measures
Primary outcomes
-
Incidence of symptomatic UTI (isolation of a bacterial species from a patient with signs or symptoms of UTI, i.e. cystitis, pyelonephritis, prostatitis)
-
Incidence of antimicrobial resistance (isolation of multidrug‐resistant bacteria, with multidrug‐resistance being defined as non‐susceptibility to at least one agent in three or more antimicrobial categories).
Secondary outcomes
-
All‐cause mortality
-
Graft loss including death with a functioning graft
-
Graft rejection (classified as clinically suspected and treated, or biopsy proven)
-
Graft function as measured by SCr concentration, estimated or measured glomerular filtration rate
-
Hospitalisation for UTI
-
Adverse reactions to antimicrobial agents (i.e. allergic reactions, toxicity)
-
Relapse or persistent asymptomatic bacteriuria.
Relapsing and persistent asymptomatic bacteriuria were defined as follows:
-
Relapsing asymptomatic bacteriuria: recurrence of asymptomatic bacteriuria after clearance of the initial isolate
-
Persistent asymptomatic bacteriuria: persistence of an organism similar to the initial isolate (same species with similar antimicrobial‐susceptibility profile).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 1 September 2017 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney and transplant‐related journals and the proceedings of major kidney and transplant conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney and transplant journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov
Studies contained in the Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the "Specialised Register" section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used for this review.
Searching other resources
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies possibly relevant to the review. The titles and abstracts were screened independently by two authors who discarded studies that were not applicable. However, studies and reviews that possibly included relevant data or information on studies were retained initially. The same two authors independently assessed retrieved abstracts, and if necessary the full text of these studies, to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (symptomatic UTI, death, graft loss, allograft rejection, hospitalisation for UTI, adverse reactions to antimicrobial agents, asymptomatic bacteriuria relapse and persistence, antimicrobial resistance), results were expressed as absolute risk difference (RD) or risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement are used to assess the effects of treatment (graft function), the mean difference (MD) was used.
Unit of analysis issues
The unit of analysis within each study was the individual patient. All the studies included used a simple parallel group design.
Dealing with missing data
Any further information required from the original author were requested by written correspondence (e.g. emailing corresponding author) and any relevant information obtained in this manner were included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
We planned to assess the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). A guide to the interpretation of I2 values is as follows.
-
0% to 40%: might not be important
-
30% to 60%: may represent moderate heterogeneity
-
50% to 90%: may represent substantial heterogeneity
-
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011). Lack of data prevented informative formal heterogeneity analysis.
Assessment of reporting biases
We planned to assess the possibility of publication bias for every outcome studied, but there were too few studies to allow meaningful evaluation.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis to explore possible sources of heterogeneity.
Heterogeneity among participants could be related to age, sex, time from transplantation to asymptomatic bacteriuria, and possible presence of a ureteric stent. Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose and duration of antibiotic therapy.
Heterogeneity among bacterial strains could be related to the following conditions:
-
species involved (Escherichia coli versus other strains),
-
degree of resistance (multidrug‐resistant strains versus non‐multidrug‐resistant, with multidrug‐resistance being defined as non‐susceptibility to at least one agent in three or more antimicrobial categories)
The paucity of data precluded us from assessing heterogeneity.
Sensitivity analysis
We planned to perform sensitivity analysis in order to examine the stability of the results in relation to the quality of the included studies. As only two studies with available results were included in the review, it was not feasible to perform a sensitivity analysis
'Summary of findings' tables
We presented the main results of the review in the 'Summary of findings' table. This table presents key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' table.
-
Symptomatic UTI
-
Antimicrobial resistance
-
All‐cause mortality
-
Graft loss
-
Acute graft rejection
-
Hospitalisation for UTI
-
Graft function
Results
Description of studies
Results of the search
We identified 32 reports through electronic searches. We added one additional report by contacting authors of an identified study (Origuen 2016). We reviewed these 33 reports in detail and identified 24 studies. Two studies (six reports) were included and 19 studies (24 reports) were excluded. Three ongoing studies were identified and will be assessed in a future update of this review (NCT01771432; BiRT Study 2013; NCT02113774) (Figure 1).
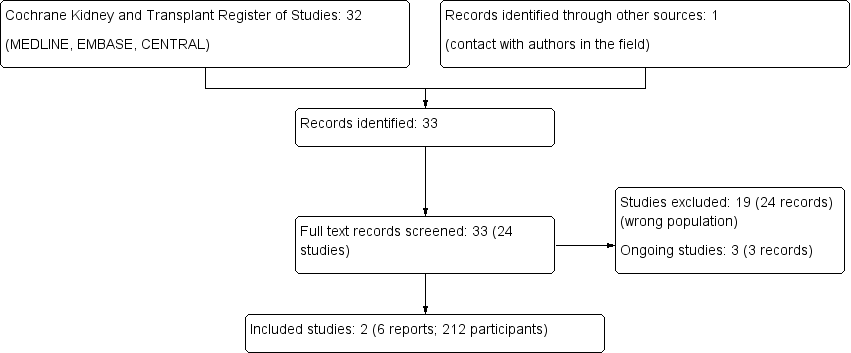
Study flow diagram.
Included studies
The two studies (212 participants enrolled, data provided for 200 participants) compared antibiotics versus no treatment, with the choice of antibiotics depending on antimicrobial susceptibility testing results (Moradi 2005; Origuen 2016). In both studies, participants from the intervention group were retreated with antibiotics if asymptomatic bacteriuria recurred during the follow‐up period. In Moradi 2005, participants took oral antibiotics for 10 days; we were unable to obtain details regarding choice of antibiotics or dosing. In Origuen 2016, participants received antibiotics for three to seven days during the first episode of asymptomatic bacteriuria, and for two and six weeks during the second and subsequent episodes. The choice of antibiotic, dosing and route of administration were left to the discretion of the treating physician (protocol not provided). In 94% of cases, participants took one of eight different antibiotics orally; IV antibiotics were given when considered appropriate by the treating physician.
Both studies exclusively enrolled adult kidney transplant recipients.
Moradi 2005 enrolled 100 participants with asymptomatic bacteriuria occurring at least one year after transplantation, who did not have ureteral stents, indwelling urethral catheters and/or a Proteus species isolated from the urine culture. Asymptomatic bacteriuria was defined as the joint presence of pyuria and bacteriuria in urinalysis; a colony count greater than 100,000 CFU/mL of a single organism after urine culture; and the absence of irritative voiding symptoms, fever, or chills. Specific strategies to obtain good quality urine samples were not mentioned. Half the participants were women (50/100), aged 45 ± 13 years. E. coli was the most common isolate (65%, 57/88 episodes); there was no information on the level of antimicrobial resistance regarding baseline episodes of bacteriuria. The investigators reported outcomes up to 9 to 12 months after randomisation.
Origuen 2016 enrolled 112 outpatients with asymptomatic bacteriuria occurring at least two months after transplantation. They excluded people with a simultaneous kidney‐pancreas transplant, a ureteral stent, an indwelling urethral catheter, pregnant women, or people who had lost the graft during the first two months after transplantation. Also, patients who had at least one episode of asymptomatic bacteriuria between the end of the second month after transplantation and the trial screening were excluded (n = 30). Asymptomatic bacteriuria was defined according to IDSA guidelines (Nicolle 2005) and dedicated nurses educated patients in order to obtain good quality urine samples. Participants were about 10 years older than in Moradi 2005 (mean age 54 ± 15 years), just under half were women (53/112; 47%). E. coli was the most commonly isolated micro‐organism (43% of episodes); there was no information on the level of antimicrobial resistance regarding baseline episodes of bacteriuria. Of the participants enrolled, 92% were within the first year after transplantation, with a median time from transplantation to study inclusion of 83 days. Outcomes were recorded until two years after transplantation or until acute pyelonephritis, graft loss or death occurred during the study period. Median duration of follow‐up was 16.9 months (range 0.43 to 22).
Both studies evaluated the incidences of symptomatic UTI and persistent asymptomatic bacteriuria, as well as graft function (Origuen 2016, Moradi 2005). Origuen and co‐workers also evaluated the incidences of acute pyelonephritis (which was selected as their primary outcome), lower UTI, hospital admission due to UTI, antimicrobial resistance, C. difficile‐associated diarrhoea, acute allograft rejection, graft loss, adverse events and all‐cause mortality (Origuen 2016).
Excluded studies
We excluded 19 studies. All studies enrolled kidney transplant recipients but participants were included independently of the presence or absence of asymptomatic bacteriuria. Nine studies evaluated the effect of pre‐ or perioperative antimicrobial prophylaxis (Castelao 1993; Cohen 1988; Ferreira 1990; Matteucci 1998; Robles 1990; Salehipour 2010; Salmela 1990; Townsend 1980; Wilms 1986), nine studies evaluated the role of antimicrobial prophylaxis of bacterial infection after transplantation (Fox 1990; Hibberd 1992; Khosroshahi 2006; Maddux 1989; Melchor 1996; Moyses‐Neto 1997; NCT01820897; Tegzess 1986; Tolkoff 1982) and one study evaluated the safety of cotrimoxazole in kidney transplant recipients treated with azathioprine (Hall 1974).
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3.
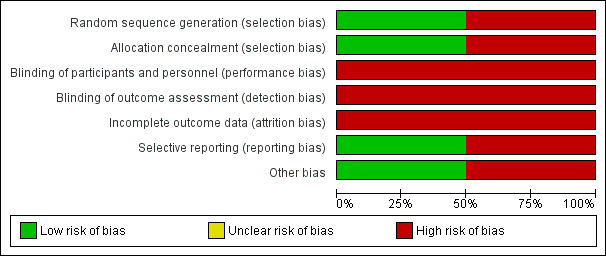
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Origuen 2016 used a computer to generate the randomisation sequence and consecutively numbered sealed envelopes to mask the allocation such that we considered the risk of selection bias to be low. Moradi 2005 used the transplantation code to determine the allocation sequence, entailing a high risk of selection bias.
Blinding
Neither study attempted to blind participants, personnel or data analysts. As symptoms of UTI are partly subjective, we anticipated this would put the results at risk of being biased in favour of antibiotic treatment. Moreover, there were differences between groups in how persistence of asymptomatic bacteriuria was determined in Origuen 2016. In the antibiotics group, investigators asked the participants to do a urinalysis two weeks after completing the antimicrobial therapy. In the control group, no systematic urinalysis occurred at two weeks but subsequent cultures were used to evaluate this outcome.
Incomplete outcome data
We considered both studies to be at high risk of attrition bias. Moradi 2005 excluded 12/100 participants from the analysis after loss to follow‐up (11) or the occurrence of acute graft pyelonephritis (1). Baseline characteristics and outcomes were only reported for the remaining 88 participants. Origuen 2016 included all 112 participants into the intention‐to‐treat analyses. However, little more than half reached the end of the two year study period (54.5%, 61/112 patients). This is partly attributed to the fact that participants were withdrawn after they developed acute pyelonephritis (9) or graft loss (2), which could have biased results for the other outcomes.
Selective reporting
Origuen 2016 had a registered protocol which was published on Clinicaltrials.gov after the end of the recruitment period. That said, the authors reported all expected outcomes related to both benefits and harms and we considered the risk of reporting bias to be low. Moradi 2005 failed to report outcomes such as incidence of pyelonephritis, antimicrobial resistance, graft rejection and graft loss, all‐cause mortality, hospitalisation for UTI, and adverse reactions to antimicrobial agents. As such, we considered it at high risk of reporting bias.
Other potential sources of bias
Moradi 2005 did not provide a specific definition of the term "symptomatic UTI" and there were no details on the episodes of symptomatic UTI. Attempts to contact the corresponding author were unsuccessful. Because the incidence of symptomatic UTI was one of our primary outcomes, we considered it at high risk of bias.
We considered the risk of sponsorship bias to be low due to the nature of the research question.
Effects of interventions
Symptomatic UTI
Overall, incidence of symptomatic UTI varied between 19% and 31% in the groups not treated for asymptomatic bacteriuria. Antibiotics had uncertain effects on the subsequent occurrence of symptomatic UTI (Analysis 1.1 (2 studies, 200 participants): RR 0.86, 95% CI 0.51 to 1.45; I2 = 0%). Origuen 2016 distinguished pyelonephritis from symptomatic UTI and found no little or no difference between the ones who were treated for asymptomatic bacteriuria and those who were not (1 study, 112 participants: RD ‐0.01, 95% CI ‐0.12 to 0.10).
Antimicrobial resistance
Origuen 2016 assessed the incidence of antimicrobial resistance as the number of study participants in whom bacteria with acquired non‐susceptibility to at least one agent in three or more antimicrobial categories were isolated during follow up. Samples could be collected both in case of symptoms of UTI or as part of routine screening. Even if numerically more people had a multidrug‐resistant bacteria in the treatment group as compared with the group not treated for asymptomatic bacteriuria (13/53 versus 12/59), the results were very uncertain (1 study, 112 participants: RR 1.21, 95% CI 0.60 to 2.41).
Secondary outcomes
All‐cause mortality, graft loss, (mostly) biopsy‐proven acute rejection, and hospitalisation for UTI were only reported by Origuen 2016. In the group not treated for asymptomatic bacteriuria, 1 of the 59 participants died (1.7%), graft loss occurred in 1/59 participants (1.7%) and acute rejection in 12/59 participants (20.3%). There was hospitalisation for UTI in 3 of the 59 untreated participants (5.1%). Overall the investigators reported little or no difference between the two groups for any of these outcomes (Analysis 1.3).
Graft function
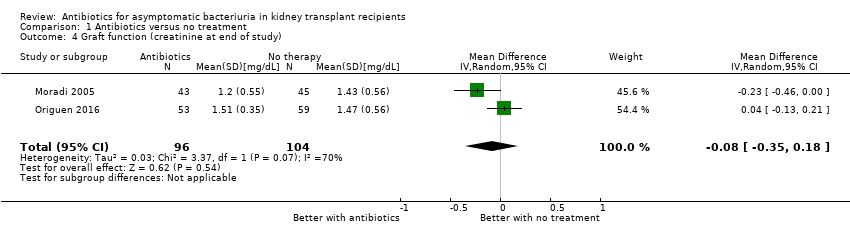
Both included studies assessed the effect of antibiotics on graft function. Antibiotics had uncertain effects on graft function, as measured by SCr (Analysis 1.4 (2 studies, 200 participants): MD ‐0.08 mg/dL, 95% CI ‐0.35 to 0.18; I2 = 70%).
Also, there was no significant effect of antibiotics on change in graft function from baseline to end of study. In Moradi 2005, mean plasma creatinine concentrations rose during the study period from 1.16 ± 0.27 mg/dL to 1.2 ± 0.55 mg/dL in the antibiotics group versus 1.42 ± 0.67 mg/dL to 1.43 ± 0.56 mg/dL in the no treatment group. In Origuen 2016, mean change in eGFR from baseline to end of study was 0.53 ± 7.6 mL/min/1.73 m2 in the antibiotics group (data available for 26/53 participants, 49.1%), as compared with 0.11 ± 15.8 mL/min/1.73 m2 in the untreated group (data available for 37/59 participants, 62.7%)
Persistence or relapse of asymptomatic bacteriuria
Both studies noted high frequencies of persisting asymptomatic bacteriuria in both groups.
In Moradi 2005, bacteriuria recurred in 25/43 treated participants (58.1%) and 33/45 untreated participants (73.3%). The difference was not statistically significant (RD ‐0.15, 95% CI ‐0.33 to 0.05). Of note, the authors did not provide a specific definition of the term "recurrence". Attempts to contact the corresponding author were unsuccessful.
In Origuen 2016, investigators took a sample for urinalysis in the patients treated with antibiotics, two weeks after completing the treatment. Analysis of data obtained from 90% of the participants revealed that persisting asymptomatic bacteriuria (as defined in our systematic review) was common despite the use of antibiotics, occurring in 46/131 episodes (35.1%). In addition, a different uropathogen was cultured in 18 episodes (13.7%). As a consequence, microbiological cure was achieved in 51.1% (67/131) of the episodes treated with antibiotics. In the control group, no systematic urinalysis occurred at two weeks, but subsequent cultures were used to evaluate the outcome. Under these conditions, asymptomatic bacteriuria persisted after 59% (175/296) of the untreated episodes, and more frequently in the control group (RD ‐0.24, 95% CI ‐0.33 to ‐0.14).
Adverse reactions to antimicrobial agents
Only Origuen 2016 assessed incidence of adverse reactions to antimicrobial agents. The investigators did not compare the incidence of adverse events between the two study groups. However, there was no severe adverse event attributable to the use of antibiotics and non‐severe adverse events appeared to be rare (two patients experienced mild diarrhoea in relation with a course of amoxicillin‐clavulanate and one patient experienced nausea).
Other outcomes
Additionally, one study evaluated incidence of C. difficile‐associated diarrhoea (Origuen 2016). In this study, C. difficile‐associated diarrhoea occurred in 3/53 participants from the antibiotics group (5.7%) and 5/59 participants from the control group (8.5%). There was no statistically significant difference between the two groups (RD ‐0.03, 95% CI ‐0.13 to 0.08).
Discussion
Summary of main results
Based on two studies treating kidney transplant recipients with asymptomatic bacteriuria had uncertain effects on preventing symptomatic UTI, and entailed uncertain risks for selecting resistant strains. Persistence of asymptomatic bacteriuria was high regardless of treatment and although the available data were limited, so far, there is no evidence to suggest antibiotic treatment of asymptomatic bacteriuria would improve patient and graft outcomes such as all‐cause mortality, graft loss, acute rejection, hospitalisation for UTI or graft function. Data on adverse reactions were very limited, but there seemed to have been no severe adverse event attributable to the antibiotic treatment, and non‐severe adverse events appeared to be rare.
Overall completeness and applicability of evidence
The two studies contributing to this review included both male and female adult kidney transplant recipients.
As kidney transplant recipients with asymptomatic bacteriuria in the first few months after transplantation (> two months in Origuen 2016, > one year in Moradi 2005) were not included in these studies, the applicability of our findings to the early post‐transplant phase is unclear. First, these exclusions may reflect the belief that asymptomatic bacteriuria increases susceptibility to subsequent UTI specifically in the first few months after transplantation due to the degree of immunosuppression, urologic manipulations and mucosal bleeding, compelling physicians to start antibiotics even when kidney transplant recipients are asymptomatic. Second, establishing the diagnosis of UTI may be difficult early after transplantation, with typical signs and symptoms of UTI being both common and often due to non‐infectious causes. Third, the incidence of asymptomatic bacteriuria and UTI is highest in the first months after transplantation (Parasuraman 2013). Despite these specificities, the usefulness of screening for and treating asymptomatic bacteriuria in the early post‐transplant period have not been evaluated in a RCT and this should be subject to further study.
We need to be careful when extrapolating the results of this review to patients with ureteral stents or indwelling urethral catheters, as the two included studies excluded these patients. People with urinary devices develop symptomatic UTI more frequently than non‐catheterized people in the general population (Hooton 2010). The use of such devices is associated with biofilm formation, where asymptomatic bacteriuria is universal and persistent (Nicolle 2014b). While screening for and treatment of catheter associated‐asymptomatic bacteriuria is not recommended in the general population, very little is known about what to do in kidney transplant recipients with urinary devices.
Because both studies exclusively enrolled kidney transplant recipients, caution is required when managing people with combined transplants (e.g. kidney and pancreas). Even if very little is known on the effect of asymptomatic bacteriuria in combined transplant recipients, there is no reason to assume antimicrobial agents would be more effective for asymptomatic bacteriuria in these patients, as compared with kidney transplant recipients. To the best of our knowledge, there is no evidence to support strategies of screening for and treatment of asymptomatic bacteriuria in recipients of non‐kidney organ transplants (Parasuraman 2013).
Last, Origuen 2016 excluded pregnant patients. Screening for and treatment of asymptomatic bacteriuria have historically been considered to effectively reduce the risk of pyelonephritis in the mother and possibly complications in the child (Smaill 2015). Even if this approach has recently been questioned (Kazemier 2015), our systematic review does not provide any additional information as pregnant women were excluded from the review.
Quality of the evidence
First, the estimates of the effect of antibiotics for preventing symptomatic UTI were very imprecise, and consistent with either important benefits or harms (Analysis 1.1 (2 studies, 200 participants): RR 0.86, 95% CI 0.51 to 1.45; I2= 0%). Regarding the absence of significant difference between study groups, we estimated that these two studies lacked power to detect a potential effect of antibiotics for preventing symptomatic UTI. In fact, neither study had an adequate sample size calculation. No sample size calculation was reported in Moradi 2005. In Origuen 2016, a sample size calculation was conducted based on the assumption of a risk reduction of pyelonephritis from 23% in the control group to 3% in the antibiotics group. Under these conditions, the study investigators estimated that 55 patients per arm were required to have a 90% chance of detecting the risk reduction expected between study groups, as significant at the 5% level. However, the incidence of pyelonephritis was much lower in the control group than expected (8.4% versus 23%) and we estimated that the sample size calculation was based on an overly optimistic effect of antimicrobial agents.
Secondly, the included studies were at high risk of bias from various sources. Regrettably, neither of the included studies attempted to blind participants, personnel or data analysts. As symptoms of UTI are partly subjective, we anticipated this would put the results at risk of being biased in favour of antibiotic treatment. As a consequence however, chances are slim that blinding would have been associated with greater effect of antibiotics on the incidence of symptomatic UTI. Nonetheless, the studies were also considered at high risk of attrition bias (Moradi 2005; Origuen 2016), selection bias, and reporting bias (Moradi 2005).
These limitations suggest that additional studies are likely to change our confidence in the effect estimates (GRADE 2008).
Potential biases in the review process
Although this review was conducted by two or more independent authors, used a comprehensive search of the published and unpublished research designed by a specialist librarian, and examined all potentially relevant clinical outcomes, potential biases exist in the review process. The single most important reservation is that four authors of this systematic review are involved in an investigator‐led multicentre ongoing RCT comparing antibiotics versus no treatment for asymptomatic bacteriuria in kidney transplant recipients (BiRT Study 2013). No results were available at the time this systematic review was written, and as a consequence no results were included in this review. None of the authors have any commercial conflict of interest related to this review, and although every care was taken to interpret the data as objective as possible, it is difficult to rule out a subconscious intellectual conflict that may have influenced the conclusions.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, there has been no previous attempt to specifically and systematically review the evidence for treating asymptomatic bacteriuria in kidney transplant recipients with antibiotics. In 2005, the IDSA guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults refrained from making a recommendation in kidney transplant recipients for want of evidence (Nicolle 2005). In 2013, the American Society of Transplantation Infectious Diseases Community of Practice suggested not treating asymptomatic bacteriuria that occurs beyond three months after kidney transplantation, unless in case of an accompanying rise in SCr concentration (Parasuraman 2013). However, this recommendation was not based on a systematic review but on expert opinion, and this group of expert acknowledged that such a strategy may be too aggressive and lead to emergence of antimicrobial resistance. Based on the evidence currently available, our review do not support treating asymptomatic bacteriuria before or after three months post‐transplantation.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
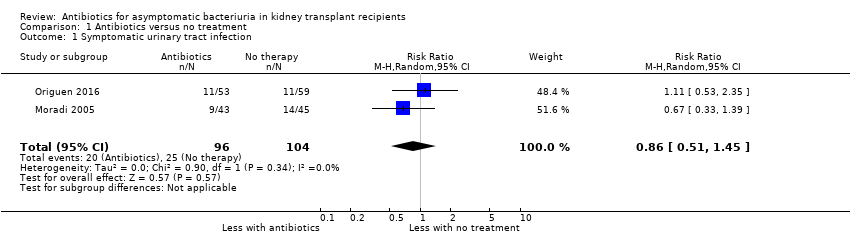
Comparison 1 Antibiotics versus no treatment, Outcome 1 Symptomatic urinary tract infection.
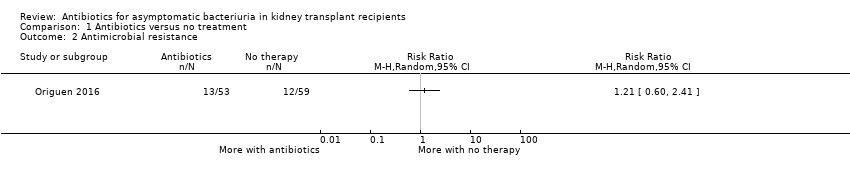
Comparison 1 Antibiotics versus no treatment, Outcome 2 Antimicrobial resistance.

Comparison 1 Antibiotics versus no treatment, Outcome 3 Secondary dichotomous outcomes.
Comparison 1 Antibiotics versus no treatment, Outcome 4 Graft function (creatinine at end of study).
| Antibiotics versus no treatment for asymptomatic bacteriuria in kidney transplant recipients | |||||
| Patient or population: adult kidney transplant recipients | |||||
| Outcomes (follow‐up period) | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with no treatment | Risk with antibiotics | ||||
| Symptomatic UTI Follow‐up: 12 to 22 months | 240 per 1,000 | 207 per 1 000 | RR 0.86 (0.51 to 1.45) | 200 2 (2 studies) | Low 3 ⊕⊕⊝⊝ |
| Antimicrobial resistance Mean follow‐up: 16.9 months | 203 per 1,000 | 245 per 1,000 | RR 1.21 (0.60 to 2.41) | 112 (1 study) | Low 4 ⊕⊕⊝⊝ |
| All‐cause mortality Mean follow‐up: 16.9 months | 17 per 1,000 | 38 per 1,000 | RR 2.23 (0.21, 23.86) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft loss Mean follow‐up: 16.9 months | 17 per 1,000 | 19 per 1,000 | RR 1.11 (0.07 to 17.36) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Acute graft rejection Mean follow‐up: 16.9 months | 203 per 1,000 | 189 per 1,000 | RR 0.93 (0.44 to 1.97) | 112 (1 study) | Low 6 ⊕⊕⊝⊝ |
| Hospitalisation for UTI Mean follow‐up: 16.9 months | 51 per 1,000 | 38 per 1,000 | RR 0.74 (0.13 to 4.27) | 112 (1 study) | Low 5 ⊕⊕⊝⊝ |
| Graft function (creatinine at end of study) Follow‐up: 12 to 22 months | Mean serum creatinine in the treatment group was 0.06 mg/dL lower (0.19 mg/dL lower to 0.08 mg/dL higher) than the control group | 200 2 (2 studies) | Low 7, 8 ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD: risk difference; RR: risk ratio; UTI: urinary tract infection 1 The two included studies compared antibiotics versus no treatment, with choice of antibiotics depending on antimicrobial susceptibility testing results. As participants could have had multiple episodes of asymptomatic bacteriuria during the follow‐up period, participants from the intervention group were retreated with antibiotics if asymptomatic bacteriuria recurred during the follow‐up period in both studies. Duration of antibiotics therapy ranged from 3 to 10 days for the first episode of asymptomatic bacteriuria. 2 212 participants included but data provided for 200 participants. 3 Neither study attempted to blind participants, personnel or data analysts. As symptoms of UTI are partly subjective, we anticipated this would put the results at risk of being biased in favour of antibiotic treatment. 4 Samples could be collected both in case of symptoms of UTI or as part of routine screening. 5 The confidence interval crosses the line of no effect but does not rule out a significant effect of antibiotics on mortality and/or graft loss. 6 No systematic graft biopsy performed during the study follow‐up. Not all episodes of allograft rejection were biopsy‐proven. 7 Graft function was evaluated using creatinine at end of study, despite different values between groups at time of inclusion. We were unable to pool the data for change in graft function from baseline to end of study (data missing for one study). 8 No significant effect of antibiotics on change in graft function from baseline to end of study in both studies. | |||||
| GRADE Working Group grades of evidence | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic urinary tract infection Show forest plot | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.51, 1.45] |
| 2 Antimicrobial resistance Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary dichotomous outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Graft loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Acute rejection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Hospitalisation for UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Graft function (creatinine at end of study) Show forest plot | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.18] |

