自体结膜移植术治疗翼状胬肉
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 57 Per group: conjunctival limbal autograft 29; amniotic membrane transplant 28 Exclusions after randomization: 1 did not receive surgery (in the limbal conjunctival autograft group) Number analyzed (total and per group): Total: 51 Per group: conjunctival limbal autograft 25; amniotic membrane transplant 26 Unit of analysis (individuals vs eyes): individual (1 eye per individual) Losses to follow‐up: 5 lost to follow‐up: conjunctival limbal autograft group 3; amniotic membrane transplant group 2 How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Cuba Age: median age – limbal conjunctival autograft 41.93 years, amniotic membrane transplant 44.81 years Gender (percent): limbal conjunctival autograft 35.7% men, 64.3% women; amniotic membrane transplant 39.3% men, 60.7% women Inclusion criteria: presenting with recurrent pterygium, older than 15, signed informed consent Exclusion criteria: younger than 15, presenting with primary pterygium of any grade Equivalence of baseline characteristics: unclear | |
| Interventions | Intervention 1: limbal conjunctival autograft Intervention 2: amniotic membrane transplant Length of follow‐up: Planned: not reported Actual: not reported | |
| Outcomes | Primary outcome, as defined in study reports: recurrence Secondary outcomes, as defined in study reports: time without recurrence Adverse events reported: yes Intervals at which outcomes assessed: 24 hours, 72 hours, 1 week, every 15 days during the first 3 months and then monthly | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: not reported Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Five participants were reported to have been lost to follow‐up, yet they were included in the analysis. No mention of whether loss to follow‐up was due to a treatment effect |
| Selective reporting (reporting bias) | High risk | The authors collected data on time without recurrence, but it is not reported in the results |
| Other bias | Unclear risk | Design of study and data analysis is not clear. Funding sources are not reported |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 50 eyes of 50 participants Per group: 24 eyes of 24 participants into the conjunctival autograft group; 26 eyes of 26 participants in the amniotic membrane transplantation group Exclusions after randomization: 10 eyes in total because of difficulties in participants’ follow‐up Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individual (1 eye per participant was enrolled) Losses to follow‐up: not reported How was missing data handled?: excluded Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Iran Age (mean ± SD): 49.3 ± 15.5 years, range 24 to 77 years old Gender (percent): 80% men; 20% women Inclusion criteria: primary and secondary pterygium Exclusion criteria: diabetes, collagen vascular disease, dry eye, glaucoma Equivalence of baseline characteristics: no, there was a difference in the cosmetic appearance of the eyes between the two groups (considered a clinical sign of pterygium) | |
| Interventions | Intervention 1: conjunctival autograft transplantation Intervention 2: amniotic membrane transplantation Length of follow‐up: Planned: 24 months Actual: 24 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrences of 2 mm in the area of original pterygium; complications Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: 1 week and 1, 3, 6, and 24 months | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: April 2004 to February 2006 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | High risk | 10/50 (20%) eyes were "excluded from the analysis because of difficulties in patients' follow up" |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Some outcomes are described at 1, 3, and 6 months but not at 2 years despite the fact that recurrence is described at 2 years |
| Other bias | Unclear risk | Funding source was not reported |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 127 eyes Per group: conjunctival autograft: 40; amniotic membrane graft: 45; amniotic membrane graft combined with conjunctival autograft: 42 Exclusions after randomization: none reported Number analyzed (total and per group): Total: 127 eyes Per group: conjunctival autograft: 40; amniotic membrane graft: 45; amniotic membrane graft combined with conjunctival autograft: 42 Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design: none | |
| Participants | Country: China Age: not reported Gender (percent): not reported Inclusion criteria: people with recurrent pterygium, with history of 1 to 3 surgical excisions, and relapse after surgery Exclusion criteria: not reported Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: limbal stem cell autograft transplant Intervention 2: amniotic membrane transplant Intervention 3: combined amniotic membrane transplant and limbal stem cell autograft transplant Length of follow‐up: Planned: 12 to 24 months Actual: 12 to 24 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrence Secondary outcomes, as defined in study reports: lack of recurrence, proportion of participants with clinical improvement Adverse events reported: no Intervals at which outcomes assessed: every 3 months up to 12 to 24 months | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: March 2002 to March 2007 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “… were randomly divided into three groups… .” but does not describe how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up was reported |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | Unclear risk | Data for baseline characteristics not reported, only stated that groups were comparable, funding sources not reported |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 90 persons (90 eyes) Per group: limbal conjunctival transplantation 45; amniotic membrane transplant 45 Exclusions after randomization: N/A Number analyzed (total and per group): Total: 90 Per group: limbal conjunctival transplantation 45; amniotic membrane transplant 45 Unit of analysis (individuals vs eyes): individual (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: not reported Reported power calculation: no Unusual study design: no | |
| Participants | Country: China Age: limbal conjunctival transplantation 47 ± 8.5; amniotic membrane transplant 49 ± 9.5 Gender (percent): limbal conjunctival transplantation 55.6% men, 44.4% women; amniotic membrane transplant 51.1% men, 48.8% women Inclusion criteria: people with primary pterygium in 1 eye, 2 to 3 mm Exclusion criteria: not reported Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: limbal conjunctival transplantation Intervention 2: amniotic membrane transplant Length of follow‐up: Planned: 2 years Actual: 20.6 ± 2.5 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrence Secondary outcomes, as defined in study reports: the proportion of participants with clinical improvement; healing time Adverse events reported: yes Intervals at which outcomes assessed: 2 years | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: March 2007 to March 2010 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) | Low risk | There was no missing data |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | Unclear risk | Funding sources were not reported |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 80 Per group: conjunctival autograft 40; amniotic membrane transplant 40 Exclusions after randomization: none reported Number analyzed (total and per group): Total: 80 Per group: conjunctival autograft 40; amniotic membrane transplant 40 Unit of analysis (individuals vs eyes): individuals (1 eye per participant) Losses to follow‐up: none reported How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Cuba Age: 20 to 59 years Gender (percent): overall 57.5% men, 42.5% women Inclusion criteria: people with primary pterygium between 20 and 59 years old Exclusion criteria: people with recurrent pterygium and ocular pathologies Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: conjunctival autograft Intervention 2: amniotic membrane transplant Length of follow‐up: Planned: 6 months Actual: 6 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrence of pterygium Secondary outcomes, as defined in study reports: none reported Adverse events reported: yes, authors report no surgical or visual complications Intervals at which outcomes assessed: 24 hours, 1 week, 1 month, 3 months, and 6 months | |
| Notes | Type of study: published Funding sources: none reported Disclosures of interest: none reported Study period: September 2009 to September 2010 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not reported whether there was any missing data |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | High risk | Funding sources were not reported. The study design is not clear and the only outcome or data that is reported per group is the recurrence of pterygium |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 55 participants Per group: 30 eyes of 30 participants in the conjunctival autograft transplantation group; 30 eyes of 25 participants in the amniotic membrane transplantation group Exclusions after randomization: none Number analyzed (total and per group): Total: 55 eyes of 55 participants Per group: 30 eyes of 30 participants in the conjunctival autograft transplantation group; 25 eyes of 25 participants in the amniotic membrane transplantation group Unit of analysis: individual Losses to follow‐up: 5 eyes of 5 participants in the amniotic membrane transplantation group How was missing data handled?: excluded Reported power calculation: no Unusual study design?: participants with minimum follow‐up of 12 months were included | |
| Participants | Country: Turkey Age (mean ± SD): 57.1 ± 12.6 years, range 32 to 81 years old in total; 55.4 ± 12.9 years in the conjunctival autograft transplantation group; 59.1 ± 12.1 years in the amniotic membrane transplantation group Gender (percent): total 61.8% men, 38.1% women; conjunctival autograft transplant 60% men, 40% women; amniotic membrane transplantation 64% men, 36% women Inclusion criteria: 1) people with recurrent pterygium; 2) minimum follow‐up of 12 months Exclusion criteria: presence of major eye diseases such as dry eye, cicatricial pemphigoid, and glaucoma, and vitreoretinal disease and intercurrent severe systemic disease, or any condition affecting follow‐up or documentation Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: conjunctival autograft transplantation + MMC Intervention 2: amniotic membrane transplantation + MMC Length of follow‐up: Planned: not reported Actual: overall 27.2 ± 20.8 months (range 12 to 94 months); conjunctival autograft 25.9 ± 24.4 years; amniotic membrane transplant 28.8 ± 15.7 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrences (fibrovascular tissue growth; published rating system by Prabhasawat [Prabhasawat 1997]) Secondary outcomes, as defined in study reports: best spectacle corrected visual acuity; epithelial defect lasting more than 5 days; presence of intraoperative severe pain Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 1 week, 1 month, 3 months, 6 months, and every 12 months thereafter | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “The authors report no conflicts of interest.” Study period: May 2004 to July 2006 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | High risk | 5 eyes were excluded due to loss to follow‐up, and their data were excluded |
| Selective reporting (reporting bias) | High risk | Unclear reason for not describing outcomes at designated time points. The authors state that participants were seen at: “1, 3, 6, every 12 months” but results are only reported as being at the “final” point |
| Other bias | Low risk | The authors report no conflicts of interest |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 94 eyes of 94 participants Per group: 32 eyes of 32 participants in the conjunctival limbal autograft transplantation group; 30 eyes of 30 participants in the amniotic membrane transplantation group; 32 eyes of 32 participants in the topical MMC group (MMC applied to sclera area instead of a tissue graft) Exclusions after randomization: none Number analyzed (total and per group): Total: 94 eyes of 94 participants Per group: 32 eyes of 32 participants in the conjunctival limbal autograft transplantation group; 30 eyes of 30 participants in the amniotic membrane transplantation group; 32 eyes of 32 participants in the topical MMC group Unit of analysis (individuals vs eyes): individual Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Turkey Age (mean ± SD): conjunctival limbal autograft transplantation group 39.84 ± 11.69, range 15 to 55 years; amniotic membrane transplantation group 41.83 ± 13.41, range 19 to 68 years; topical MMC group 44.72 ± 11.21, range 20 to 65 years Gender (percent): conjunctival limbal autograft transplantation 46.9% men, 53.1% women; amniotic membrane transplant 53.3% men, 46.7% women; topical MMC group 56.2% men, 43.8% women Inclusion criteria: people with primary pterygium Exclusion criteria: not reported Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: conjunctival limbal autograft transplantation Intervention 2: amniotic membrane transplantation Intervention 3: MMC 0.2 mg/mL to the sclera area beyond the limbus, using a 2 × 2 mm sponge applied for 2 minutes Length of follow‐up: Planned: 36 months Actual: conjunctival limbal autograft group 24.38 ± 7.93 (range 12 to 36 months); amniotic membrane transplant group 23.63 ± 7.30 (range 12 to 36 months); topical MMC group 23.44 ± 7.24 (range 12 to 36 months) | |
| Outcomes | Primary outcome, as defined in study reports: recurrences (fibrovascular tissue from limbus onto cornea); complication Secondary outcomes, as defined in study reports: none Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 3 days, 1 week, 1 month, 3 months, and every 3 months thereafter | |
| Notes | Type of study: published Funding sources and disclosures of interest: “The authors have stated that they do not have a significant financial interest or other relationship with any product manufacturer or provider of services discussed in this article. The authors do not discuss the use of off‐label products, which includes unlabeled, unapproved, or investigative products or devices” Study period: January 2001 to January 2003 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported; “they were randomly assigned (by UEA) using an adaptive randomization procedure to receive CA combined with intraoperative MMC or AMT combined with intraoperative MMC treatment.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | High risk | 5/60 (8.3%) eyes were lost to follow‐up, and they were excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | Unclear risk | Funding source was not reported |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 42 eyes of 42 participants Per group: 21 eyes of 21 participants each group Exclusions after randomization: 3 eyes of 3 participants (not distinguished between exclusion and lost to follow‐up) Number analyzed (total and per group): Total: 39 eyes of 39 participants Per group: conjunctival autograft transplantation 20 eyes of 20 participants; amniotic membrane transplant 19 eyes of 19 participants Unit of analysis (individuals vs eyes): individual (1 eye per participant was enrolled) Losses to follow‐up: 3 eyes of 3 participants (not distinguished between exclusion and lost to follow‐up) How was missing data handled?: excluded Reported power calculation: no Unusual study design?: participants with less than 12‐month follow‐up were excluded from the analysis | |
| Participants | Country: Iran Age (mean ± SD): overall 45.6 ± 13.9 years, range 19 to 83 years; conjunctival autograft 47.7 ± 15.7 years; amniotic membrane transplant 42.8 ± 13.2 years Gender (percent): overall 56.4% men, 43.6% women; conjunctival autograft transplantation group 60% men, 40% women; amniotic membrane transplantation 52.6% men, 47.4% women Inclusion criteria: primary nasal pterygium Exclusion criteria: not reported Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: conjunctival autograft transplantation Intervention 2: amniotic membrane transplantation Length of follow‐up: Planned: 12 months Actual: 12 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrences; complications Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks, and 1, 3, 6, 9, and 12 months | |
| Notes | Type of study: published Funding sources: “The authors indicate no financial support or financial conflict of interest involved in conception and design of study.” Disclosures of interest: “The authors indicate no financial support or financial conflict of interest involved in conception and design of study.” Study period: not reported Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | High risk | 3/42 (7.1%) eyes were excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 27 eyes of 27 participants Per group: conjunctival autograft 14 eyes of 14 participants; amniotic membrane transplant 13 eyes of 13 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 27 eyes of 27 participants Per group: conjunctival autograft 14 eyes of 14 participants; amniotic membrane transplant 13 eyes of 13 participants Unit of analysis (individuals vs eyes): individual (1 eye per participant was enrolled) Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Turkey Age (mean ± SD): limbal conjunctival autograft 42.95 years (range 28 to 59 years); amniotic membrane transplant 44.92 years (range 32 to 65 years) Gender (percent): total 55.6% men and 44.4% women; limbal conjunctival autograft 57.1% men and 42.9% women; amniotic membrane transplant group 53.8% men and 46.2% women Inclusion criteria: primary pterygium, aged between 28 and 65 years old Exclusion criteria:
Equivalence of baseline characteristics: the size of defects measured was not statistically significantly different between the 2 groups; other baseline demographics were reported but were not evaluated for statistically significant differences so we cannot judge whether these characteristic are equivalent between the two groups | |
| Interventions | Intervention 1: limbal conjunctival autograft transplantation Intervention 2: amniotic membrane transplantation Length of follow‐up: Planned: 1 month Actual: limbal conjunctival autograft 13.66 ± 5.23 (range 6 to 24 months); amniotic membrane transplant 14.40 ± 3.25 (range 6 to 26 months) | |
| Outcomes | Primary outcome, as defined in study reports: graft vascularization Secondary outcomes, as defined in study reports: recurrence Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 7 days, 30 days, then monthly intervals | |
| Notes | Type of study: published Funding sources: “The authors indicate no financial support or financial conflict of interest.” Disclosures of interest: “The authors indicate no financial support or financial conflict of interest.” Study period: not reported Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished by a list created by a random‐number generator" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | "Patients were not informed of the surgical procedure they received. We aimed to prevent intraobserver (surgeon) bias caused by the preoperative status (for example, size, fleshiness, vascularization) of the pterygium.” |
| Masking of outcome assessment (detection bias) | Low risk | Outcomes assessors were masked to the type of surgery |
| Incomplete outcome data (attrition bias) | Low risk | Authors reported no missing data |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 78 eyes of 78 participants Per group: conjunctival autograft 40 eyes of 40 participants; amniotic membrane transplant 38 eyes of 38 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 78 eyes of 78 participants Per group: conjunctival autograft 40 eyes of 40 participants; amniotic membrane transplant 38 eyes of 38 participants Unit of analysis (individuals vs eyes): individual (1 eye per participant was enrolled) Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Turkey Age (mean ± SD): conjunctival autograft 52.4 ± 12.40 years (range 37 to 94 years); amniotic membrane transplant 57.1 ± 9.91 years (range 40 to 73 years) Gender (percent): total 50% men and 50% women; conjunctival autograft 52.5% men and 47.5% women; amniotic membrane transplant 47.4% men and 52.6% women Inclusion criteria: primary or recurrent pterygium Exclusion criteria:
Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: conjunctival autograft transplantation Intervention 2: amniotic membrane transplantation Length of follow‐up: Planned: at least 6 months Actual: conjunctival autograft 16.6 ± 3.52 (range 9 to 19 months); amniotic membrane transplant 13.4 ± 2.08 (range 10 to 16 months) | |
| Outcomes | Primary outcome, as defined in study reports: recurrence of pterygium Secondary outcomes, as defined in study reports: none Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks, and monthly thereafter | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: “The authors state that they have no proprietary interest in the products named in this study.” Study period: January 2002 to September 2003 Trial registration: none reported Reported subgroup analyses: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished by using a list created by a random‐number generator" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Masking of personnel was not reported, though the surgeon would not be masked due to the nature of the surgery. ”Patients were randomized to either of the 2 treatment groups in a masked manner, with patients being informed neither of the surgical procedure they received nor of their pterygium grade status.” |
| Masking of outcome assessment (detection bias) | Low risk | Outcome assessors were masked to the type of surgery |
| Incomplete outcome data (attrition bias) | Low risk | The authors reported no missing data |
| Selective reporting (reporting bias) | Unclear risk | A protocol was unavailable |
| Other bias | Low risk | "The authors state that they have no proprietary interest in the products named in this study." |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 133 eyes of 118 participants Per group: conjunctival autograft 81 eyes of 81 participants; amniotic membrane transplant 52 eyes of 52 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 133 eyes of 118 participants Per group: conjunctival autograft 81 eyes of 81 participants; amniotic membrane transplant 52 eyes of 52 participants Unit of analysis (individuals vs eyes): eye Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design?: both eyes of some participants were independently assigned to 2 intervention groups without taking account non‐independence of eyes | |
| Participants | Country: China Age (mean ± SD): conjunctival autograft range 32 to 85 years old; amniotic membrane transplant group range 30 to 81 years old Gender (percent): total 38.3% men and 61.7% women; conjunctival autograft 39.5% men and 60.5% women; amniotic membrane transplant 38.5% men and 61.5% women Inclusion criteria: pterygium Exclusion criteria: no ocular surface disease or systemic illnesses Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: limbal conjunctival autograft transplantation Intervention 2: amniotic membrane transplantation Length of follow‐up: Planned: 12 months Actual: 12 months | |
| Outcomes | Primary outcome, as defined in study reports: foreign body sensation or discomfort; eyelid edema and conjunctival hyperemia edema; recurrence Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1 week and 12 months | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: July 2008 to July 2010 Trial registration: none reported Reported subgroup analyses: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | High risk | Both eyes of single participant were independently assigned to the 2 intervention groups without taking account non‐independence of eyes; funding source not reported |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 346 eyes of 346 participants Per group: not reported Exclusions after randomization: 1 (died) Number analyzed (total and per group): Total: 287 eyes of 287 participants Per group: conjunctival autograft 120 eyes of 120 participants; amniotic membrane transplant 167 eyes of 167 participants Unit of analysis (individuals vs eyes): individual (1 eye per participant was enrolled) Losses to follow‐up: 58 participants How was missing data handled?: excluded Reported power calculation: yes (80%) Unusual study design?: none | |
| Participants | Country: Thailand Age (mean ± SD): conjunctival autograft 45.42 ± 11.47 years (range 19 to 72 years); amniotic membrane transplant 46.49 ± 13.63 years (range 18 to 80 years) Gender (percent): total 34.8% men and 65.2% women; limbal conjunctival autograft transplantation group 36.7% men and 63.3% women; amniotic membrane transplant 33.5% men and 66.5% women Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: amniotic membrane transplantation Intervention 2: limbal conjunctival autograft transplantation Length of follow‐up: Planned: 6 months Actual: 6 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrence; complications Secondary outcomes, as defined in study reports: none Adverse events reported: yes Intervals at which outcomes assessed: 6 weeks and 6 months | |
| Notes | Type of study: published Funding sources: “This study was supported by an invitation grant from the Faculty of Medicine, Khon Kaen University.” Disclosures of interest: none Study period: 2000 to 2001 Trial registration: protocol #I44034 approved by the Medical Science Subcommittee for the protection of Human Subjects in Research of Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand Reported subgroup analyses: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomised into two groups by a simple randomisation technique." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | High risk | Only the outcomes assessor was masked, participants and surgeons were not. “This study was designed as a single‐blind randomised control trial." |
| Masking of outcome assessment (detection bias) | Low risk | “This study was designed as a single‐blind randomised control trial”; “The results at 6 weeks and 6 months were examined by the same investigator in a blind assessment to grade the final appearance” |
| Incomplete outcome data (attrition bias) | High risk | 59/346 (17.1%) participants were lost to follow‐up, and they were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Authors report a protocol was written but do not provide a reference or a way to access the protocol |
| Other bias | Low risk | None |
| Methods | Study design: parallel‐group RCT (for the participants in the amniotic membrane graft and limbal conjunctival autograft transplantation groups) Number randomized (total and per group): Total: 163 eyes of 163 participants Per group: limbal conjunctival autograft 63 eyes of 63 participants; amniotic membrane transplant 52 eyes of 52 participants; bare sclera 48 eyes of 48 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 163 eyes of 163 participants Per group: limbal conjunctival autograft 63 eyes of 63 participants; amniotic membrane transplant 52 eyes of 52 participants; bare sclera 48 eyes of 48 participants Unit of analysis (individuals vs eyes): individual (1 eye per participant was enrolled in the study) Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design?: participants in the bare sclera technique group were not randomized | |
| Participants | Country: Turkey Age (mean ± SD): limbal conjunctival autograft 49.63 ± 14.42 years; amniotic membrane transplant 47.92 ± 15.52 years; bare sclera 47.88 ± 14.21 years Gender (percent): limbal conjunctival autograft 49.2% men and 50.8% women; amniotic membrane transplant 51.9% men and 48.1% women; bare sclera technique 41.7% men and 58.3% women Inclusion criteria: people with primary pterygium between the ages of 22 and 74 years Exclusion criteria: people with recurrent pterygium Equivalence of baseline characteristics: yes; “The age and sex distribution in all of the groups showed no significant differences.” | |
| Interventions | Intervention 1: conjunctival limbal autograft transplantation Intervention 2: amniotic membrane transplantation Intervention 3: bare sclera technique Length of follow‐up: Planned: not reported Actual: conjunctival limbal autograft 69.91 ± 12.41 (range 59 to 82 months); amniotic membrane transplant 61.43 ± 9.83 (range 53 to 74 months); bare sclera technique 72.39 ± 11.03 (range 61 to 77 months) | |
| Outcomes | Primary outcome, as defined in study reports: corneal epithelialization; recurrences; complication Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: 2, 5, 7, 15, and 30 days, and every month thereafter | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: from June 1995 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants in the bare sclera technique group were not randomized, but participants in the groups of interest to this review were randomized. The randomization method was not reported. “The first 45 consecutive patients underwent BST because LCAT and AMGT were not previously used by the surgeons at Eskişehir Osmangazi University. Once the preliminary preparations had been completed, the randomization procedure was begun, starting with the 46th patient. We chose the patients for LCAT and AMGT randomly, rather than in consideration of certain criteria. The reason we excluded BCT from our randomization process was due to the high risk of recurrence of pterygium in cases reported not only in the literature but also in our clinic. However, 3 patients refused to undergo either LCAT or AMGT after these new techniques had been explained to them in order to obtain their informed consent. For this reason, these 3 patients were placed in the group undergoing BST.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | Unclear risk | Funding source was not reported; results in recurrence risk were different in texts and table |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: not reported Per group: not reported Exclusions after randomization: participants lost to follow‐up were excluded, but this number was not reported Number analyzed (total and per group): Total: 228 Per group: conjunctival autograft 102; amniotic membrane transplant 126 Unit of analysis (individuals vs eyes): individual (1 eye per participant) Losses to follow‐up: number was not reported, but those lost to follow‐up were not included in the report How was missing data handled?: N/A Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Brazil Age: mean age 35 years (range 20 to 53) Gender (percent): conjunctival autograft 50.98% men, 49.02% women; amniotic membrane transplant 60.31% men, 39.69% women Inclusion criteria: people with primary pterygium grade II (2 to 4 mm corneal invasion), aged 18 to 60 years, absence of infection in ocular surface Exclusion criteria: age younger than 18 or older than 60, primary pterygium grade I or III, recurrent pterygium, presence of ocular surface infection, loss to follow‐up Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: conjunctival autograft Intervention 2: amniotic membrane transplant Length of follow‐up: Planned: 12 months Actual: 12 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrence Secondary outcomes, as defined in study reports: satisfaction with surgery, postoperative pain Adverse events reported: no Intervals at which outcomes assessed: baseline, daily for one week, on the 15th day then 1, 6, and 12 months postoperatively | |
| Notes | Type of study: published (doctoral thesis) Funding sources: not reported Disclosures of interest: not reported Study period: January 2001 to October 2005 Trial registration: none reported Reported subgroup analyses: yes, by gender | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors made use of a random number table; when the sum of digits was an even number, the participants were put into 1 group, and when the sum of digits was an odd number, participants were put into the other surgical group |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not performed |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported |
| Incomplete outcome data (attrition bias) | High risk | Participants who were lost to follow‐up were not included in the study report, therefore it is unclear to which group they were randomized or what data were missing for them |
| Selective reporting (reporting bias) | Unclear risk | Authors report a protocol was presented to the Committee of Ethics in Research; however authors do not provide a reference or a way to access the protocol |
| Other bias | Unclear risk | Funding and other disclosures not provided |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 16 eyes of 8 participants Per group: conjunctival autograft 8; amniotic membrane transplant 8 Exclusions after randomization: none Number analyzed (total and per group): Total: 16 eyes of 8 participants Per group: conjunctival autograft 8; amniotic membrane transplant 8 Unit of analysis (individuals vs eyes): eyes (each participant had amniotic membrane transplant in 1 eye and conjunctival autograft in the other eye) Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: participants had bilateral symptomatic pterygium and had 1 type of surgery in each eye | |
| Participants | Country: not reported Age: not reported Gender (percent): not reported Inclusion criteria: bilateral symptomatic pterygium Exclusion criteria: none reported Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: conjunctival autograft Intervention 2: amniotic membrane transplantation Length of follow‐up: Planned: not reported Actual: mean 12.5 months (range 9 to 22 months) | |
| Outcomes | Primary outcome, as defined in study reports: visual acuity, recurrence Secondary outcomes, as defined in study reports: adverse events Adverse events reported: yes Intervals at which outcomes assessed: not reported | |
| Notes | Type of study: published conference abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not reported whether or not there was missing data |
| Selective reporting (reporting bias) | Unclear risk | In this abstract, it is unclear if there was selective reporting bias |
| Other bias | Unclear risk | Funding sources and other disclosures were not reported |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 90 eyes of 60 participants Per group: conjunctival autograft 45 eyes of 30 participants; amniotic membrane transplant 45 eyes of 30 participants Exclusions after randomization: none reported Number analyzed (total and per group): Total: 90 eyes of 60 participants Per group: conjunctival autograft 45 eyes of 30 participants; amniotic membrane transplant 45 eyes of 30 participants Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: some participants had two eyes included in the study without taking into account non‐independence of eyes | |
| Participants | Country: Cuba Age: mean 47 years (range 23 to 74) Gender (percent): 60% male, 40% female Inclusion criteria: over 20 years of age, vascular and symptomatic primary pterygium Exclusion criteria: history of ophthalmic disease, systemic disease such as diabetes mellitus, vascular diseases and collagenopathies Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: conjunctival autograft Intervention 2: amniotic membrane transplant Length of follow‐up: Planned: 12 months Actual: 12 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrence of pterygium Secondary outcomes, as defined in study reports: none reported Adverse events reported: yes Intervals at which outcomes assessed: 24 hours, 7 days, 1, 6, 12 months | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January 2006 to January 2007 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing data was reported |
| Selective reporting (reporting bias) | Unclear risk | It is unclear if selective outcome reporting occurred; a protocol was not available |
| Other bias | High risk | Funding sources were not described; some participants had surgery in both eyes while others had surgery in only 1 eye |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 48 participants Per group: conjunctival autograft 20 eyes; amniotic membrane transplant 20 eyes; topical MMC plus AMT 20 eyes Exclusions after randomization: none Number analyzed (total and per group): Total: 60 eyes of 48 participants Per group: conjunctival autograft 20 eyes; amniotic membrane transplant 20 eyes; topical MMC plus AMT 20 eyes Unit of analysis (individuals vs eyes): eye Losses to follow‐up: none How was missing data handled?: N/A Reported power calculation: no Unusual study design?: both eyes of single participant were independently assigned to intervention groups without taking into account non‐independence of eyes; participants with follow‐up less than 6 months were excluded from the study | |
| Participants | Country: Egypt Age (mean ± SD): overall 45.2 ± 3.7 (range 28 to 52 years); limbal conjunctival autograft 45.2 ± 7.3 (range 30 to 50 years); amniotic membrane transplant 49.1 ± 2.3 (range 29 to 52 years); topical MMC plus AMT 39.1 ± 13.1 (range 30 to 52 years) Gender (percent): overall 80% men and 20% women; conjunctival limbal autograft 80% men and 20% women; amniotic membrane transplant 90% men and 10% women; topical MMC plus AMT group 70% men and 30% women Inclusion criteria: people with recurrent pterygium, primary pterygium excision performed 6 to 15 months before selection into study Exclusion criteria:
Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: conjunctival limbal autograft transplantation Intervention 2: amniotic membrane transplantation Intervention 3: MMC 0.05% for 3 min followed by amniotic membrane transplantation Length of follow‐up: Planned: 2 years Actual: 2 years | |
| Outcomes | Primary outcome, as defined in study reports: recurrences Secondary outcomes, as defined in study reports: complications: conjunctival irritation, chemosis, transient increase in intraocular pressure, conjunctival granuloma, transient superficial keratitis Adverse events reported: yes Intervals at which outcomes assessed: not reported | |
| Notes | Type of study: published Funding sources: “Fund of Ophthalmology Department, Ain Shams University.” Disclosures of interest: “No financial interest of authors for any of used materials” Study period: not reported Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported. “The cases were divided randomly into three equal groups” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | A protocol was not available |
| Other bias | High risk | Both eyes of single participant were independently assigned to intervention groups without taking into account non‐independence of eyes; participants with less than 6 months' follow‐up were excluded from the study |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 Per group: conjunctival autograft 30, amniotic membrane transplant 30 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 60 Per group: conjunctival autograft 30, amniotic membrane transplant 30 Unit of analysis (individuals vs eyes): individuals (1 eye per participant) Losses to follow‐up: not reported How was missing data handled?: not reported Reported power calculation: no Unusual study design?: none | |
| Participants | Country: Venezuela Age: not reported Gender (percent): not reported Inclusion criteria: recurrent pterygium Exclusion criteria: people under 20 years of age, people with primary pterygium, external eye diseases such as conjunctivitis, dry eye, blepharitis Equivalence of baseline characteristics: not reported | |
| Interventions | Intervention 1: conjunctival autograft Intervention 2: amniotic membrane transplant Length of follow‐up: Planned: 5 months Actual: 5 months | |
| Outcomes | Primary outcome, as defined in study reports: recurrence of pterygium Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: 24 hours, 72 hours, 1 week, 1 month, 5 months | |
| Notes | Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January to June 2004 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not described |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described |
| Incomplete outcome data (attrition bias) | Unclear risk | It is unclear if there was any missing data or loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | It is unclear if there was selective reporting of outcomes; a protocol was not available |
| Other bias | High risk | No details about the type of analysis were provided, funding sources and disclosures were not included |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 92 eyes of 83 participants Per group: not reported Exclusions after randomization: total 6 participants; conjunctival autograft 5 participants; amniotic membrane transplantation 1 participant Number analyzed (total and per group): Total: 86 eyes of 78 participants Per group: 42 eyes of 41 participants in the conjunctival autograft transplantation group; 44 eyes of 39 participants in the amniotic membrane transplantation group Unit of analysis (individuals vs eyes): eye Losses to follow up: 6 due to follow‐up < 6 months How was missing data handled?: discarded Reported power calculation: no Unusual study design?: for 9 participants, both eyes of single participant were independently randomized to intervention groups without taking into account non‐independence of eyes; participants with a follow‐up period of less than 6 months were excluded | |
| Participants | Country: Thailand Age (mean ± SD): conjunctival autograft 44.81 ± 8.77 years (range 21 to 59 years); amniotic membrane transplant 41.93 ± 9.0 years (range 27 to 60 years) Gender (percent): total 42.5% men and 57.5% women; conjunctival autograft 43.9% men and 56.1% women; amniotic membrane transplant 41.0% men and 59.0% women Inclusion criteria: primary pterygium; age between 20 and 60 years Exclusion criteria:
Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: conjunctival autograft transplantation Intervention 2: amniotic membrane transplantation Length of follow‐up: Planned: 12 months Actual: 12.4 ± 3.1 months in the conjunctival autograft transplantation group; 14.4 ± 5.4 months in the amniotic membrane transplantation group | |
| Outcomes | Primary outcome, as defined in study reports: recurrence risk Secondary outcomes, as defined in study reports: complication Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 1 week, and 1, 3, 6, and 12 months | |
| Notes | Type of study: published Funding sources: "Supported by the Faculty of Medicine Endowment Fund, Faculty of Medicine, Chiang Mai University.” Disclosures of interest: "The authors did not have a financial interest in any product investigated in this study.” Study period: not reported Trial registration: none reported Reported subgroup analyses: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | High risk | 6/83 (7.2%) participants were excluded because the follow‐up period was less than 6 months, and they were not included in the analysis. Authors say that the exclusion of these participants may be the reason why the follow‐up for the AMT group was “significantly longer” than for CA group |
| Selective reporting (reporting bias) | High risk | Except for recurrences, outcomes in results were not described beyond “clinical outcome” (Intro) or “other complications” (Materials and Methods). Three such outcomes are pyogenic granuloma (complication), high intraocular pressure, and “loss of uncorrected visual acuity of more than one line" (authors report “none”; however, unclear if some eyes lost one line of visual acuity) |
| Other bias | High risk | For 9 participants, both eyes of single participant were independently randomized to intervention groups without taking into account non‐independence of eyes; “Eyes, rather than people, were used as a unit for statistical analysis because there were only 9 patients who had both eyes operated on, and each eye was independently randomized to treatment” |
| Methods | Study design: parallel‐group RCT Number randomized (total and per group): Total: 82 eyes of 74 participants Per group: conjunctival autograft 43 eyes of 40 participants; amniotic membrane transplant 39 eyes of 34 participants Exclusions after randomization: not reported Number analyzed (total and per group): Total: 73 eyes of 65 participants Per group: conjunctival autograft 37 eyes of 34 participants; amniotic membrane transplant 36 eyes of 31 participants Unit of analysis (individuals vs eyes): baseline characteristics by individual; outcomes by eyes Losses to follow‐up: conjunctival autograft group 6 eyes of 6 participants; amniotic membrane transplant group 3 eyes of 3 participants How was missing data handled?: excluded Reported power calculation: no Unusual study design?: the study initially randomized 74 participants total, but they only included analyses for 65 participants because they excluded outcome data for the 9 participants who did not complete the 1‐year follow‐up | |
| Participants | Country: Turkey Age (mean ± SD): conjunctival autograft 52 ± 13.7; amniotic membrane transplant 49.8 ± 14.1 Gender (percent): conjunctival autograft 52.9% male, 47.1% female; amniotic membrane transplant 51.6% male, 48.4% female Inclusion criteria: people with primary pterygium, cosmetically significant pterygium or clinically significant pterygium presenting with ocular irritation, inflammation, or reduced vision Exclusion criteria: < 18 years of age, other concurrent ocular or lid pathology, glaucoma, ocular hypertension, pregnancy, and known hypersensitivity to any component of fibrin glue Equivalence of baseline characteristics: yes | |
| Interventions | Intervention 1: conjunctival autograft fixed with fibrin glue Intervention 2: amniotic membrane transplant fixed with fibrin glue Length of follow‐up: Planned: 12 months Actual: 12 months | |
| Outcomes | Primary outcome, as defined in study reports: rate of recurrence Secondary outcomes, as defined in study reports: complications Adverse events reported: yes Intervals at which outcomes assessed: 1, 7, and 14 days, 1, 2, 3, 4, 6, 8, and 10 months, 1 year | |
| Notes | Type of study: published Funding sources: none reported Disclosures of interest: “The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper. The authors have no financial interest on any of the materials mentioned in the study.” Study period: February 2008 to January 2011 Trial registration: none reported Reported subgroup analyses: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | In communication with a study author, we learned that participants were masked to which surgery they received, but masking of personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | In communication with a study author, we learned that the doctors who performed the postoperative examinations were not masked |
| Incomplete outcome data (attrition bias) | High risk | 9 eyes were not followed up for the full year and not discussed |
| Selective reporting (reporting bias) | Unclear risk | It is unclear if there was selective reporting of outcomes; a protocol was not available |
| Other bias | High risk | The study did not specify funding resources. The study did not have complete outcome data and secondary outcome data for the treatment groups. The study did not specify in which groups adverse events occurred |
AMT: amniotic membrane transplant
CA: conjunctival autograft
MMC: mitomycin C
N/A: not applicable
RCT: randomized controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Unclear whether participants were randomized | |
| Unclear whether participants were randomized | |
| No conjunctival autograft arm, only amniotic membrane transplant alone or combined with conjunctive reverse transplantation | |
| Compared conjunctival flap technique to amniotic membrane transplant | |
| Not a randomized controlled trial | |
| Ex vivo study of mucins found in tear film after pterygium surgeries | |
| Unclear whether participants were randomized | |
| Unclear whether participants were randomized | |
| Compared corneal limbal stem cell transplant with conjunctival flap technique to amniotic membrane transplant | |
| Compared conjunctival flap technique to amniotic membrane transplant | |
| Not a randomized controlled trial | |
| Compared corneal limbal stem cell transplant to amniotic membrane transplant |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: parallel‐group randomized controlled trial Target sample size: 31 participants |
| Participants | Country: India Inclusion criteria: people with double pterygium with operable features Exclusion criteria: children under age 18 years, previous history of trauma or chemical injury, pregnancy |
| Interventions | Intervention 1: conjunctival autograft Intervention 2: amniotic membrane transplant Length of follow‐up: Planned: 3 years |
| Outcomes | Primary outcome, as defined in study reports: recurrence of pterygium nasally and temporally Secondary outcomes, as defined in study reports: recurrence based on site of replacement Intervals at which outcomes assessed: planned to 1 year |
| Notes | Type of study: not yet published Funding sources: reported as self funded Disclosures of interest: not reported Study period: recruitment began May 2011 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pterygium at 3 months Show forest plot | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| Analysis 1.1  Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 1 Recurrence of pterygium at 3 months. | ||||
| 1.1 Participants with primary pterygium | 5 | 488 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.37, 2.30] |
| 1.2 Participants with primary or recurrent pterygium | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.85] |
| 2 Recurrence of pterygium at 6 months Show forest plot | 10 | 1021 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.85] |
| Analysis 1.2  Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 2 Recurrence of pterygium at 6 months. | ||||
| 2.1 Participants with primary pterygium | 7 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.27] |
| 2.2 Participants with recurrent pterygium | 3 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.99] |
| 2.3 Participants with primary or recurrent pterygium | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.95] |
| 3 Adverse events Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 3 Adverse events. | ||||
| 3.1 Pyogenic granuloma during the study | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 3.2 Pyogenic granuloma at 6 months | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.67, 5.90] |
| 3.3 Granuloma during study | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 3.4 Increased IOP at 6 months | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.91, 7.00] |
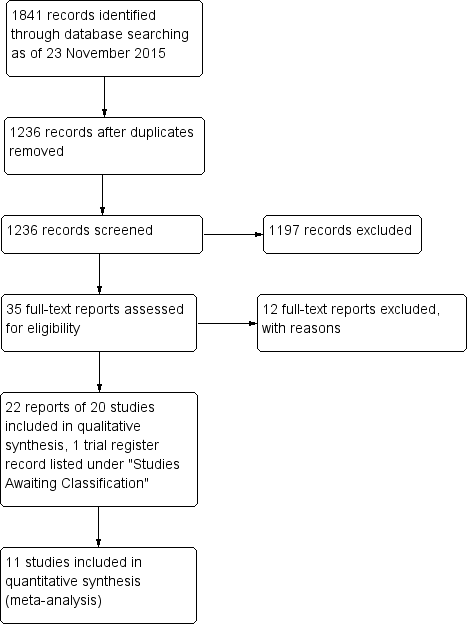
Study flow diagram.
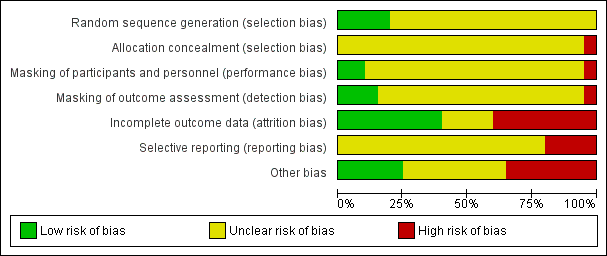
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.1 Recurrence of pterygium at 3 months.
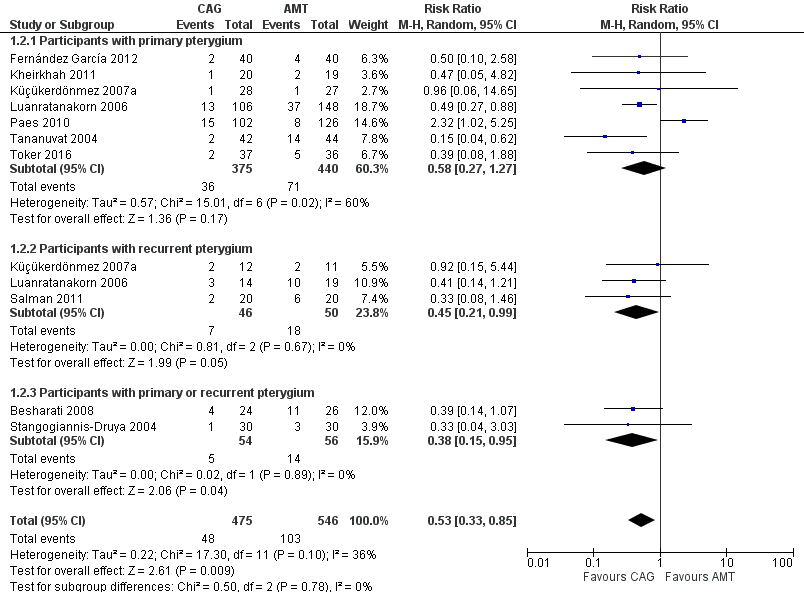
Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.2 Recurrence of pterygium at 6 months.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.3 Adverse events.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 1 Recurrence of pterygium at 3 months.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 2 Recurrence of pterygium at 6 months.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 3 Adverse events.
| Conjunctival autograft compared to amniotic membrane transplant for pterygium | ||||||
| Patient or population: people with primary or recurrent pterygium | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of eyes | Quality of the evidence | Comment | |
| Risk with amniotic membrane transplant | Risk with conjunctival autograft | |||||
| Recurrence of pterygium | Study population | RR 0.87 (0.43 to 1.77) | 538 | ⊕⊝⊝⊝ | ||
| 89 per 1000 | 77 per 1000 | |||||
| Recurrence of pterygium | Study population | RR 0.53 (0.33 to 0.85) | 1021 | ⊕⊕⊕⊝ | ||
| 189 per 1000 | 100 per 1000 | |||||
| Clinical improvement (non‐recurrence risk) follow‐up: 3 months | See comment | One study reported the risk of non‐recurrence as 93.8% for participants in the conjunctival limbal autograft group and 93.3% in the amniotic membrane transplant group at 3 months after surgery | ||||
| Need for repeat surgery | See comment | 2 studies reported the need for repeat surgery but did not provide time points. In 1 study, 1 participant in the amniotic membrane transplant group developed suture lysis, and amniotic membrane revision was performed. In the other study, 1 participant in each surgical group had surgery again | ||||
| Mean change in visual acuity | See comment | No study reported mean change. 1 study reported the logMAR at baseline and postoperatively, and there was no difference (mean difference 0.00, 95% CI ‐0.66 to 0.66) | ||||
| Quality of life | None of the included studies reported on quality of life measures after the 2 surgeries | |||||
| Direct and indirect costs | None of the included studies reported on direct or indirect costs after the 2 surgeries | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Allocation concealment unclear in every study and evidence of possible attrition bias. | ||||||
| Estimates Including Paes 2010 Study | Estimates Not Including Paes 2010 Study | |||
| Outcome | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) |
| Recurrence of pterygium at 3 months | 6 (538) | 0.87 [0.43, 1.77] | 5 (310) | 0.62 [0.30, 1.27] |
| Participants with primary pterygium | 5 (488) | 0.92 [0.37, 2.30] | 4 (260) | 0.62 [0.24, 1.60] |
| Participants with primary or recurrent pterygium | 1 (50) | 0.62 [0.21, 1.85] | 1 (50) | 0.62 [0.21, 1.85] |
| Recurrence of pterygium at 6 months | 10 (1,021) | 0.53 [0.33, 0.85] | 9 (793) | 0.43 [0.30, 0.62] |
| Participants with primary pterygium | 7 (815) | 0.58 [0.27, 1.27] | 6 (587) | 0.43 [0.27, 0.69] |
| Participants with recurrent pterygium | 3 (96) | 0.45 [0.21, 0.99] | 3 (96) | 0.45 [0.21, 0.99] |
| Participants with primary or recurrent pterygium | 2 (110) | 0.38 [0.15, 0.95] | 2 (110) | 0.38 [0.15, 0.95] |
| Event | Time point | Number of studies (reference) | Conjunctival autograft, n (%) | Amniotic membrane transplant, n (%) | Risk ratio (95% CI) |
| Chemosis | 6 months | 1 (Salman 2011) | 4 (20.0) | 2 (10.0) | 2.00 (0.41 to 9.71) |
| Conjunctival contraction | 1 month | 1 (Besharati 2008) | 3 (12.5) | 4 (15.4) | 0.81 (0.20 to 3.26) |
| 3 months | 1 (Besharati 2008) | 1 (4.2) | 3 (11.5) | 0.36 (0.04 to 3.24) | |
| 6 months | 1 (Besharati 2008) | 2 (8.5) | 5 (19.2) | 0.43 (0.09 to 2.03) | |
| Conjunctival inflammation ‐ grade 1 to 3 | During study | 1 (Kheirkhah 2011) | 3 (15) | 16 (84.2) | 0.18 (0.06 to 0.51) |
| Corneal scar | 1 month | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) |
| 3 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| 6 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| Conjunctivitis | Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | |
| Diplopia | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 2 (7.7) | 0.22 (0.01 to 4.28) | |
| Epithelial defect (lasting more than 5 days) | During study | 1 (Katircioglu 2014) | 1 (3.3) | 0 (0) | 0.67 (0.20 to 2.22) |
| Eye movement restriction | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) | |
| Eyelid edema and conjunctival hyperemia edema | 12 months | 1 (Liang 2012) | 8 (9.9) | 12 (23.1) | 0.43 (0.19 to 0.98) |
| Foreign body sensation or discomfort | 12 months | 1 (Liang 2012) | 11 (13.6) | 17 (32.7) | 0.42 (0.21 to 0.81) |
| Graft dehiscence/suture dehiscence | 1 month | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) |
| 3 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| 6 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| During study | 1 (Toker 2016) | 2 (5.4) | 2 (5.6) | 0.97 (0.14 to 6.54) | |
| Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | ||
| Graft reaction | During study | 1 (7.1) | 0 (0) | 2.80 (0.12 to 63.20) | |
| Granuloma | During study | 4 (4.3) | 6 (6.5) | 0.71 (0.23 to 2.18) | |
| Increased intraocular pressure | 6 months | 8 (6.7) | 5 (3.0) | 2.52 (0.91 to 7.00) | |
| During study | 1 (Tananuvat 2004) | 5 (11.9) | 4 (9.1) | 1.31 (0.38 to 4.55) | |
| Infection | 1 month | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| Inflammation | During study | 1 (Pérez Parra 2008) | 2 (4.4) | 0 (0) | 5.00 (0.25 to 101.31) |
| Graft edema | During study | 0 (0) | 2 (15.4) | 0.19 (0.01 to 3.56) | |
| Other defect taking > 10 days to heal | During study | 1 (Katircioglu 2014) | 4 (13.4) | 5 (20) | 2.52 (0.11 to 59.18) |
| Pyogenic granuloma | 1 month | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) |
| 3 months | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) | |
| 6 months | 8 (5.6) | 5 (2.6) | 1.99 (0.67 to 5.90) | ||
| During study | 1 (1.3) | 5 (7.0) | 0.33 (0.07 to 1.55) | ||
| Severe pain | During study | 1 (Katircioglu 2014) | 4 (13.4) | 2 (8.0) | 1.67 (0.33 to 8.36) |
| Subconjunctival hemorrhage | Overall | 4 (14.2) | 0 (0) | 9.00 (0.51 to 159.70) | |
| Superficial punctate keratitis | Overall | 3 (10.7) | 3 (10.7) | 1.00 (0.22 to 4.54) | |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 2 (4.4) | 0.50 (0.05 to 5.32) | |
| Symblepharon | 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 1 (2.2) | 1.00 (0.06 to 15.50) | |
| During study | 1 (Perry 2000) | 1 (12.5) | 0 (0) | 3.00 (0.14 to 64.26) | |
| Wound healing | 2 years | 1 (Chen 2012) | 3 (6.7) | 3 (6.7) | 1.00 (0.21 to 4.69) |
| CI: confidence interval | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pterygium at 3 months Show forest plot | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| 1.1 Participants with primary pterygium | 5 | 488 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.37, 2.30] |
| 1.2 Participants with primary or recurrent pterygium | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.85] |
| 2 Recurrence of pterygium at 6 months Show forest plot | 10 | 1021 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.85] |
| 2.1 Participants with primary pterygium | 7 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.27] |
| 2.2 Participants with recurrent pterygium | 3 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.99] |
| 2.3 Participants with primary or recurrent pterygium | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.95] |
| 3 Adverse events Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pyogenic granuloma during the study | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 3.2 Pyogenic granuloma at 6 months | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.67, 5.90] |
| 3.3 Granuloma during study | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 3.4 Increased IOP at 6 months | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.91, 7.00] |

